Abstract
Hepatocellular cancer (HCC) is the fourth leading cause of cancer-related deaths worldwide and the fastest growing cause of cancer deaths in the United States. The overall prognosis of HCC remains dismal, except for the subset of patients who are diagnosed at early stage and receive potentially curative therapies, such as surgical resection and liver transplantation. Given this, expert society guidelines recommend HCC surveillance every 6 months in at-risk individuals. Despite these recommendations, the effectiveness of HCC surveillance remains a subject of debate. We discuss current best practices for HCC surveillance and the evidence that support these recommendations. We also describe several initiatives that are underway to improve HCC surveillance and outline areas that may serve as high-yield targets for future research. Overall, we believe these efforts will help the field move toward precision surveillance, where surveillance tests and intervals are tailored to individual HCC risk. Doing so can maximize surveillance benefits, minimize surveillance harms, and optimize overall value for all patients.
Hepatocellular cancer (HCC) is the fourth leading cause of cancer-related death worldwide and the fastest growing cause of cancer deaths in the United States.1 Between 1975 and 2009, HCC incidence in the United States increased 3-fold, with increases observed across demographic groups and all 50 states, and its incidence continues to rise.2,3 Recent epidemiological trends show some improvements in cancer stage at the time of HCC diagnosis as well as disease survival. The proportion of HCC cases diagnosed at early stage increased from 27% between 1992 and 1999 to 44% between 2006 and 2012 (63% relative increase), although some of this increase might be due to improved diagnosis and documentation of HCC stage. Concomitantly, between 1992–1999 and 2006–2012, early HCC stage (local disease) demonstrated improved survival. Specifically, 5-year survival rates for local disease improved from 16.3% between 1992 and 1999 to 31.0% in 2006 to 2012 (90% relative increase).4 Despite these trends, the overall prognosis of HCC remains dismal, except for the subset of patients who are diagnosed at early stage and receive potentially curative therapies, such as surgical resection and liver transplantation.
Most HCC cases occur in patients with established risk factors for chronic liver disease, including hepatitis C virus (HCV) infection, heavy alcohol drinking, hepatitis B virus (HBV) infection, and nonalcoholic fatty liver disease (NAFLD). These HCC risk factors lead to cirrhosis, which is present in 90% of patients with HCC in the Western world.5 Two large randomized controlled trials in patients with HBV and several observational cohort studies in patients with cirrhosis have shown that patients who undergo HCC surveillance have earlier-stage HCC, are more likely to receive potentially curative treatment, and have improved survival than those who presented symptomatically or had HCC detected incidentally.6–8 Based on available data, the American Association for the Study of Liver Diseases, European Association for the Study of the Liver, Asian Pacific Association for the Study of the Liver, and National Comprehensive Cancer Network recommend HCC surveillance every 6 months in at-risk individuals, including all patients with cirrhosis from any etiology and subgroups of patients with HBV in the absence of cirrhosis (Table 1).9–12 Surveillance is not recommended in patients with Child–Pugh class C cirrhosis unless they are awaiting liver transplantation, given the low probability of treatment eligibility and limited benefits.
Table 1.
High-Risk Groups for Hepatocellular Carcinoma Surveillance
| Groups |
|---|
| Cirrhosis |
| Chronic hepatitis B (including patients with viral suppression) |
| Chronic hepatitis C (including patients post-SVR) |
| Alcohol-related |
| Genetic hemochromatosis |
| Primary biliary cirrhosis |
| Non-alcoholic steatohepatitis |
| Autoimmune hepatitis |
| Cirrhosis from other etiologies |
| Patients with chronic hepatitis B without cirrhosis |
| Asian males ≥40 y |
| Asian females ≥50 y |
| Family history of HCC |
| African persons ≥20 ya |
SVR, sustained virologic response.
The recommendation about Africans does not apply to US-born African Americans with chronic HBV. Please see text for more details.
Despite these recommendations, the effectiveness of HCC surveillance remains a subject of debate, largely related to concerns regarding the quality of existing evidence.6,13 In this review, we discuss current best practices for HCC surveillance and the evidence that support these recommendations. We also describe gaps in the existing evidence base; ongoing initiatives to improve HCC surveillance; and identify future areas that need further research to fulfill the promise of HCC surveillance in improving patient outcomes.
Value of Hepatocellular Carcinoma Surveillance
HCC surveillance refers to screening of patients at increased risk of HCC at regular intervals, with the immediate goal of detecting HCC at an early stage. Surveillance strategies provide the best value when the balance between benefits and harms is optimal.14 Indeed, data for both benefits and harms are needed to determine the value of any cancer surveillance program. In the following sections, we describe published studies on the benefits and harms of HCC surveillance.
Hepatocellular Carcinoma Surveillance Benefits
The overarching goal of HCC surveillance is to reduce HCC-related mortality and improve quality-adjusted life-years gained. However, several conditions are necessary for HCC surveillance to result in HCC-related mortality reduction. First, HCC risk must be high enough in the at-risk groups to justify surveillance. Second, surveillance tests must accurately detect HCC at an earlier stage than it would otherwise present because of symptoms, signs, or incidental imaging. Third, effective treatments, which improve outcomes in screen-detected HCC compared to treatments for non–screen-detected HCC, must be applied.
Two large randomized controlled trials, conducted in China, have demonstrated benefits of HCC surveillance in patients with HBV infection. The first study, including 17,820 HBV-infected persons, reported that patients randomized to surveillance were more likely to have HCC detected at an early stage and undergo curative therapy.7 The 1- and 2-year survival rates for HCC patients in the surveillance group were 88.1% and 77.5%, respectively, compared to 0% at 1 year for HCC patients in the no-surveillance group (P < .01). The second trial among 18,816 HBV carriers similarly reported higher early tumor detection and curative treatment receipt among patients randomized to HCC surveillance.8 HCC-related mortality of patients undergoing surveillance was significantly lower than that of the no-surveillance arm (83.2 vs 131.5 per 100,000; P < .01), with a hazard ratio of 0.63 (95% confidence interval [CI], 0.41–0.98). Taken together, these data provide level I evidence that HCC surveillance improves early tumor detection and reduces cancer-related mortality in HBV-infected individuals.
However, one cannot extrapolate surveillance data from these randomized controlled trials of patients with chronic HBV infection to patients undergoing HCC surveillance in Western countries; most of which have cirrhosis from HCV, alcohol, and/or NAFLD. There are several reasons underlying the limited generalizability of findings from available randomized controlled trials. Patients with cirrhosis are older, have more comorbidities, and a higher competing risk of liver-related mortality than patients with chronic HBV. Abdominal ultrasound may have lower sensitivity for HCC detection in a nodular cirrhotic liver and in patients with obesity; the latter is prevalent in populations at risk for HCC in the West. Last, cirrhosis patients also have fewer curative treatment options for HCC than patients with chronic HBV. For example, surgical resection can be aggressively applied to many patients with non-cirrhotic HBV infection, but the presence of portal hypertension often precludes this in patients with cirrhosis. Similarly, although several locoregional treatments are available for cirrhosis patients with liver-localized HCC, liver transplantation remains the only treatment that can cure HCC and underlying liver disease.
There have not been any randomized controlled trials of HCC surveillance in patients with cirrhosis. However, several cohort studies have demonstrated an association between HCC surveillance and increased early detection, curative treatment receipt, and improved overall survival.6 A systematic review of 47 studies (including 15,158 patients with cirrhosis) demonstrated that surveillance was associated with increased early tumor detection (odds ratio [OR], 2.08; 95% CI, 1.80–2.37) and curative treatment receipt (OR, 2.24; 95% CI, 1.99–2.52).6 Surveillance was also significantly associated with improved overall survival (OR, 1.90; 95% CI, 1.67–2.17), with a pooled 3-year survival rate of 50.8% among those who underwent surveillance vs 27.9% for those who presented symptomatically or were diagnosed incidentally. Among the subset of studies that adjusted for lead-time bias, the association between HCC surveillance and improved survival was sustained (3-year survival rate of 39.7% vs 29.1%). Another systematic review of 2 trials and 18 observational studies similarly concluded HCC surveillance was associated with early tumor detection; however, the authors were unable to conclude whether there was a survival benefit associated with surveillance.13 The available cohort studies have several inherent limitations that reduce the level of confidence in their results, including unmeasured confounders, length-time bias, lead-time bias, and ascertainment bias for mortality. Yet, the overall consistency in results from the available cohort studies suggests that HCC surveillance is likely beneficial in cirrhosis patients.
In contrast, in a recent case–control study in the national US Department of Veterans Affairs (VA) health system, HCC surveillance was not associated with decreased HCC-related mortality.15 The authors found no significant difference in HCC surveillance receipt between cases who died from HCC and controls without HCC, further adding to the existing controversy surrounding the benefits of HCC surveillance.16 There are some potential aspects of the study that may account for the discrepant findings from prior cohort studies, highlighting the need for external validation of these results. Underuse of HCC surveillance and HCC therapies, which can contribute to lower surveillance effectiveness, were prevalent in this study. For example, >50% of HCCs were found at an early stage, but <15% underwent any curative therapies. Prior studies suggest higher rates of HCC surveillance and curative treatment in academic tertiary care referral centers, so it is unclear whether these results would apply to all health systems.17 Further, the authors measured exposure to ultrasound-based surveillance, but were unable to assess the quality of those examinations. Ultrasound is known to be operator-dependent, and prior studies have suggested up to 1 in 5 ultrasound examinations may be inadequate quality for evaluation of liver lesions.18 Accordingly, the American College of Radiology has recently adopted a measure of examination quality and visualization in ultrasound reporting (Liver Imaging Reporting and Data System [LI-RADS]) ranging from LI-RADS A (minimal issues with visualization) to LI-RADS C (significant issues with visualization). Regardless of the reasons underlying the discrepant results, these new data are important and highlight a need for continued evaluation of HCC surveillance effectiveness.
This ongoing debate would best be resolved with a randomized controlled trial. However, randomized data for HCC surveillance vs no surveillance are not likely to be forthcoming, mostly because patients and their clinicians strongly prefer HCC surveillance over no surveillance. In a pilot study of 205 patients, 204 (99.5%) patients declined randomization as part of a randomized controlled trial of HCC surveillance; 181 (88%) elected for a nonrandomized surveillance program, and almost all of the remaining patients chose usual care, which included ad hoc surveillance.19 Given this dilemma, expert society guidelines continue to rely on observational data to develop recommendations for HCC surveillance (see section on at-risk populations).
Hepatocellular Carcinoma Surveillance Harms
Multiple types of harms should be considered when evaluating a cancer surveillance program, including the potential for physical, financial, and psychological harms.20 Physical harms can result from surveillance or follow-up testing and extend beyond medical complications to include discomfort. Financial harms can include anticipated or actual costs of surveillance and diagnostic evaluation, plus indirect costs such as missed work. Psychological harms can occur at any step of the surveillance process and include anticipation or fear of abnormal results; reactions of depression, anxiety, or cancer-specific worry after positive results; and psychological effects of being labeled with a diagnosis. Although HCC surveillance (using ultrasound and α-fetoprotein [AFP]) has minimal discomfort and no direct physical harms, there are potential “downstream” harms associated with diagnostic evaluation protocols.
To date, few studies have quantified HCC surveillance harms. In a single-center cohort study among 680 cirrhosis patients undergoing HCC surveillance over a 3-year period, surveillance-related physical harms were reported in 187 (27.5%) patients, with 66 (9.7%) having multiple computed tomography (CT)/magnetic resonance imaging (MRI) examinations and 3 (0.4%) undergoing invasive testing, such as biopsy.21 In another single-center study among 999 patients undergoing HCC surveillance over a median follow-up of 2.2 years, 256 patients had abnormal imaging—69 were diagnosed with HCC and 187 had an indeterminate nodule, that is, lesion ≥1 cm in diameter that could not be categorized as definitely benign or definite HCC on cross-sectional imaging).22 Among those with indeterminate nodules, 18 (9.6%) did not undergo diagnostic evaluation, 132 (70.6%) returned to ultrasound surveillance after negative CT/MRI imaging, and 37 (19.8%) continued CT/MRI-based imaging. Among those who underwent diagnostic evaluation with CT/MRI, 32 (17.1%) experienced severe harms, defined as ≤4 CT/MRI examinations or invasive testing, such as biopsy.
What Is Coming Next?
A pragmatic randomized controlled trial is under way that is randomizing patients to HCC surveillance outreach with patient navigation services vs “opportunistic” surveillance at 3 diverse health systems, including an academic tertiary care referral center, a large urban VA health system, and an integrated safety-net health system (ClinicalTrials.gov ID NCT02582918). Although the primary outcome of the trial is receipt of HCC surveillance, secondary outcomes include early detection and receipt of treatment. The trial is also measuring surveillance-related physical, psychological, and financial harms. While awaiting results from this trial, we recommend following expert society guidelines for HCC surveillance.
Current efforts evaluating HCC surveillance in large cohorts should consider both benefits and harms to better characterize its overall value of surveillance. Although false-positive ultrasound and AFP results are the most common causes for HCC surveillance harms, non-guideline concordant management of indeterminate ultrasound results can also account for a substantial proportion of surveillance harms. This includes diagnostic testing, such as liver biopsy being performed for sub-centimeter lesions and/or heterogeneous echotexture on ultrasound. Guidelines currently recommend close observation with short interval ultrasound in these cases. Interventions such as provider education may reduce unnecessary evaluation of indeterminate results and improve the value of HCC surveillance in patients with cirrhosis.
At-Risk Populations
Expert society guidelines recommend HCC surveillance for all patients with cirrhosis from any etiology and subgroups of patients with non-cirrhotic HBV (Table 1). Although the latter recommendation includes Africans aged 20 years and older, it does not apply to US-born African Americans with chronic HBV infection. In a retrospective cohort study of patients with chronic HBV infection seen in the national VA from 2001 through 2013, the annual HCC incidence was highest in Asian Pacific Islanders (0.65%), followed by whites (0.57%) and then African Americans (0.40%). After adjusting for clinical and viral factors, there was no difference in HCC risk between African Americans and whites (adjusted hazard ratio, 0.77; 95% CI, 0.58–1.02).23
Most US patients with HCC have cirrhosis resulting from 1 or more established etiologic risk factors, such as HCV, heavy alcohol drinking, and HBV infection; HCC develops at an annual incidence of 2%–7% in patients with active viral (HCV or HBV) cirrhosis24–28 (Figure 1). However, virologically cured HCV and NAFLD are now the most important emerging risk factors for HCC. With the advent of highly effective and well-tolerated direct-acting antiviral agents, HCV treatment rates and number of patients cured of HCV have increased dramatically. Similarly, NAFLD is now the fastest growing cause of chronic liver disease, cirrhosis, and HCC. A simulation model reported as many as 100 million adults may have NAFLD in the United States by 2030, of which 10 million may progress to advanced fibrosis or cirrhosis and thus be at risk for HCC.29 Recent data provide a glimpse of this emerging trend. In an analysis of patients awaiting liver transplantation, the proportion of HCC accounted for by HCV or alcohol-related liver disease remained stable between 2002 and 2016 (both trend P > .10), the proportion of HBV decreased 3.1-fold (P < .0001), while the proportion of NAFLD in HCC increased 7.7-fold (from 2.1% to 16.2%; P < .0001).30
Figure 1.
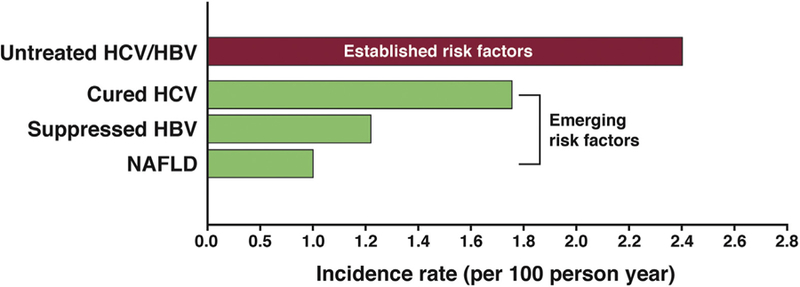
Annual risk of HCC in cirrhosis patients with established24,25,28 and emerging cohorts including patients with cured HCV infection,31,46 virologically suppressed HBV infection,40 and nonalcoholic fatty liver disease.32
This shift in at-risk population has direct relevance for the surveillance of HCC. In more than 8700 HCV-cirrhosis patients followed after virologic cure with the direct-acting antiviral agents, the annual risk of HCC was 1.8% (ie, significantly lower than the 2%–8% reported for previous cohorts).31 The annual incidence of HCC was 1.06% in a large cohort of patients with NAFLD cirrhosis followed for an average of 9 (SD 2.2) years.32 In other studies of patients with NAFLD (and nonalcoholic steatohepatitis) cirrhosis seen in tertiary referral centers, the rate of HCC ranged from 1.8% to 3.3%,33 with higher risk in patients with higher Child–Turcotte–Pugh scores. Although cirrhosis appears to be the main risk factor for HCC in NAFLD, carcinogenesis can occur in NAFLD in the absence of advanced fibrosis/cirrhosis. In a recent study, 20% of NAFLD patients with HCC had no evidence of cirrhosis based on detailed medical record review.32 Other studies have reported an even higher proportion of NAFLD-related HCC cases (10%–75%) that developed in the absence of cirrhosis.34 It is possible that some patients classified as having HCC in the absence of cirrhosis (non-cirrhotic HCC) actually do have cirrhosis and are misclassified (as non-cirrhotic) due to sampling error. However, cases of HCC have been reported in patients with mild or absent fibrosis,35–38 suggesting that HCC can develop in patients without any advanced fibrosis in NAFLD. However, the overall risk of HCC in NAFLD patients without cirrhosis is too low for HCC surveillance to be cost-effective.32
The risk of HCC in patients with alcohol-related cirrhosis is posited to be low, ranging from 1.0% in population-based studies and 2.5% in tertiary referral center–based studies.39 Most HBV patients in clinical practice are those on long-term nucleos(t)ide analog treatment with suppressed viral DNA. In a recent study of HBV patients recruited from 6 US centers and a community-based cohort in Taiwan, the annual incidence of HCC was 1.25% (95% CI, 0.4%–3.8%) among 774 patients treated with tenofovir, compared with 12.8% in untreated patients.40 Together, these data show that the pretest probability of HCC in the newer cohorts of cirrhosis patients will likely be different (and lower) than expected based on historic cohorts. Specifically, HCC risk estimates in the newer cohorts of cirrhosis patients might be lower than the 1.5% estimate beyond which HCC surveillance becomes cost-effective.
Recent epidemiologic evidence supports the association between smoking and HCC risk.41,42 Using data from the EPIC (European Prospective Investigation Into Cancer and Nutrition) study from 1992 to 2006, Trichopolous et al42 found that although chronic infection with HBV or HCV was the strongest risk factor for HCC (adjusted ORs, 9.10 and 13.36, respectively), obesity (OR, 2.13; 95% CI, 1.06– 4.29) and tobacco smoking (former smoking OR, 1.98; current smoking OR, 4.55) were responsible for more cases of HCC than HBV or HCV, given the higher prevalence of smoking in the study population. Although these data have implications for primary prevention of HCC, current expert society guidelines do not make any recommendations regarding HCC surveillance in former or current smokers who do not meet other indications for surveillance shown in Table 1.
What Is Coming Next?
There is consensus regarding the benefit of HCC surveillance after sustained virologic response in HCV patients with cirrhosis. However, there is uncertainty whether HCC risk will sufficiently decrease over time, such that HCC surveillance can be discontinued. Recent data suggest that the risk may remain high enough in the intermediate term to require ongoing HCC surveillance.43
Although HCC has been reported increasingly in some patients with non-cirrhotic NAFLD, HCC surveillance is not recommended in NAFLD patients in the absence of cirrhosis until future studies identify biomarkers that can reliably identify at-risk subgroups among patients with non-cirrhotic NAFLD.
HCC surveillance is currently recommended in all patients with cirrhosis; however, HCC risk varies widely between patients. There are currently only a few existing risk stratification tools for HCC. Most were derived from cohorts of mostly untreated (ie, active) HBV or HCV patients44,45—virtually all are obsolete because of recent changes in the treatment paradigm for viral hepatitis and the growth of NAFLD and metabolic syndrome. A recent study developed and validated models predicting HCC risk after antiviral treatment in HCV patients and may be useful to HCC risk prediction in this specific subgroup.46 Studies are needed that evaluate and incorporate new as well as established factors to create novel risk-stratification indices that are applicable to patients with cirrhosis from all etiologies. These will allow personalized estimation of the downstream risk of HCC in patients with cirrhosis across diverse etiologies. A novel tissue-based gene signature has been shown to accurately risk stratify patients in terms of HCC risk and may identify a subgroup of low-risk patients who do not require HCC surveillance47; however, it requires a blood-based surrogate to facilitate adoption in clinical practice. Recent epidemiologic evidence supports the association between current smoking and HCC risk. Incorporating this information, along with other emerging risk factors, may enhance the predictive ability of risk-stratification indices.
Differences in society recommendations for at-risk individual populations require further study. For example, European Association for the Study of the Liver and American Association for the Study of Liver Diseases–Infectious Disease Society of America guidelines include HCV-infected persons with F3 fibrosis among at-risk patients, but this subgroup is not included in other guidelines. Similarly, there is uncertainty regarding cost-effectiveness of HCC surveillance in all patients with F3 fibrosis after sustained virologic response.
Last, updated cost-effectiveness models are needed to allow long-term comparative effectiveness analyses of HCC surveillance and to determine the circumstances when HCC surveillance is cost-effective in the newer cohorts of patients with virologically cured HCV, suppressed HBV, and NAFLD.
Surveillance Tests
An ideal surveillance test would be highly sensitive and specific for early HCC and undetectable in premalignant liver disease. Both radiographic and serologic tests are used for HCC surveillance, although both have notable limitations and there is an urgent need for more accurate surveillance tests.
Radiographic Surveillance Tests
Liver ultrasound has been long regarded as a standard surveillance test for HCC and is one of the most commonly used. Its advantages include being non-invasive and relatively inexpensive, and it poses no risk of contrast or radiation exposure. In one of the randomized trials evaluating surveillance in HBV patients, the sensitivity of ultrasound was 84% for any stage HCC and 63% for early-stage HCC.48 However, the performance of ultrasound appears to be suboptimal in patients with cirrhosis. A meta-analysis of cohort studies performed in patients with cirrhosis reported a pooled sensitivity and specificity of 84% (95% CI, 76%–92%) and 91% (95% CI, 86%–94%), respectively, for detection of HCC at any stage. In studies that examined early-stage HCC detection, the pooled sensitivity of ultrasound was only 47% (95% CI, 33%–61%) for detection of early-stage HCC.49
Detection of HCC in the background of a nodular cirrhotic liver is particularly challenging due to the presence of fibrous septa and regenerative nodules, which appear as a coarse pattern on ultrasound and may mask the presence of a small tumor. In a retrospective analysis of the HALT-C (Hepatitis C Antiviral Long-Term Treatment against Cirrhosis) trial data, the most common reason for not diagnosing HCC at an early stage was “absence of detection,” that is, ultrasound failing to detect HCC lesions.50 The operator dependency of ultrasound and the importance of high-quality equipment for good performance has been emphasized in consensus guidelines.51 To maximize ultrasound efficacy, special training for those performing ultrasounds for HCC surveillance has been advocated.
The performance of ultrasound can also be impacted by several patient-level factors, with particularly suboptimal performance in obese patients (such as those with NAFLD) and those with more advanced cirrhosis. In a retrospective analysis of 941 cirrhotic patients undergoing HCC surveillance, >20% of ultrasound examinations were determined to be of inadequate quality for surveillance. Factors correlated with ultrasound inadequacy included male sex, obesity and morbid obesity, alcohol or nonalcoholic steatohepatitis etiology of cirrhosis, advanced liver disease (Child–Pugh class B cirrhosis), inpatient status, and elevated alanine transaminase.18 Likewise, another study found male sex, Child–Pugh class B cirrhosis, and elevated AFP levels were significantly correlated with surveillance failure in patients with cirrhosis, which was defined as a tumor detected beyond an early stage or missed on ultrasound and later identified by CT or MRI.52 These data highlight a need for better HCC surveillance tools to improve detection of early-stage tumors.
Cross-sectional imaging modalities, such as CT or MRI, would be anticipated to have high accuracy based on data for HCC diagnosis. However, there have been few data evaluating CT or MRI for surveillance purposes, with only 2 studies evaluating CT-based surveillance and 2 evaluating MRI-based surveillance. In a single-center randomized controlled trial comparing ultrasound- and CT-based surveillance in patients with cirrhosis, the sensitivity and specificity of CT for any stage detection were 87.5% (95% CI, 50.8%–99.9%) and 87.5% (95% CI, 77.7%– 93.5%), respectively; however, the sensitivity of CT for early HCC detection was only 62.5% (95% CI, 30.4%–86.5%) and did not differ significantly from that of ultrasound.53 Further, CT-based surveillance is also likely limited by potential harms, including radiation exposure and contrast-induced nephrotoxicity. The 2 studies evaluating MRI had a pooled sensitivity and specificity for any HCC detection of 83.1% (95% CI, 72.0%–90.5%) and 89.1% (95% CI, 86.5%–91.3%), respectively. In the PRIUS (Gadoxetic Acid-MRI Versus Ultrasonography for the Surveillance of Hepatocellular Carcinoma in High-Risk Patients) study, a prospective cohort study of 407 cirrhotic patients comparing MRI- and ultrasound-based surveillance, MRI-based surveillance resulted in significantly higher early-stage HCC detection compared with ultrasound (83.7% vs 25.6%; P < .001).54 In addition, MRI had significantly fewer false-positive findings (3.0% vs 5.6%; P = 0.004). However, MRI is too time-consuming and costly for widespread adoption among all cirrhosis patients, with prior cost-effectiveness analyses demonstrating MRI is not cost-effective for HCC surveillance in all patients with cirrhosis.55 Abbreviated MRI had been proposed as a shorter version to reduce in-scanner time, significantly save costs, and make it a more cost-effective strategy.56 In a study among 174 patients, abbreviated MRI had a sensitivity of 81% and specificity of 96%.57 In a subsequent pilot study among 19 patients at University of California–San Diego, abbreviated MRI had a sensitivity of 90% for early HCC and specificity of 89% compared to only 50% and 67%, respectively, for ultrasound.
Serologic Surveillance Tests
The best-studied biomarker to date is AFP, with a level of 20 ng/mL being the most commonly used cutoff to trigger further evaluation in clinical practice. A systematic review of 5 studies evaluating AFP at a cutoff value of 20 ng/mL in cirrhotic patients showed sensitivities ranging from 41% to 65% and specificities ranging from 80% to 94% for HCC at any stage.58 However, the sensitivity of AFP for early-stage tumors is lower, at only 32%–49%. AFP may be elevated in the setting of chronic liver disease, particularly in patients with significant elevations of transaminases, and in some patients with non-HCC malignancies, such as cholangiocarcinoma.59 Although current serologic biomarkers are insufficiently accurate when used alone, the addition of biomarkers to ultrasound for surveillance has been proposed as a means of increasing the sensitivity of surveillance, particularly with regard to early tumor detection. A meta-analysis of studies comparing the accuracy of ultrasound with or without AFP found ultrasound with AFP had significantly higher sensitivity than ultrasound alone (relative risk, 1.23; 95% CI, 1.08–1.41).49 The pooled sensitivities of ultrasound with and without AFP for early-stage HCC were 63% (95% CI, 48%–75%) and 45% (95% CI, 30%–62%), respectively (P = .002). The benefit of AFP as an adjunct test to ultrasound was consistent across subgroups, including prospective studies, studies conducted in the United States, and studies conducted after the year 2000. These data suggest that, among currently available tests, ultrasound in combination with AFP may be the most effective strategy for HCC surveillance in patients with cirrhosis.
There have been several methods proposed to further improve AFP accuracy for HCC detection. Gopal and colleagues60 suggested tailoring AFP cutoffs by liver disease etiology may maximize accuracy, including cutoffs of 59 ng/ mL for HCV-positive patients and 11 ng/mL for HCV-negative patients. Another proposed method to improve AFP accuracy is incorporating other patient factors to develop AFP-adjusted algorithms. In another study among 11,721 patient with HCV-related cirrhosis, an AFP-adjusted algorithm incorporating platelet count, alanine transaminase level, and patient age was developed to improve predictive value of AFP for identifying patients likely to develop HCC within 6 months.61 Finally, longitudinal AFP measurements have been evaluated to discriminate benign changes in AFP from changes that reflect true development of HCC and improve accuracy compared to single-threshold measurements.62 In a secondary analysis of the HALT-C trial, a parametric empirical Bayes screening algorithm was similarly shown to be more effective at detecting HCC in patients with cirrhosis than the single threshold method (77.1% vs 60.4%; P < .005).63
In combination with AFP, 2 biomarkers, lens culinaris agglutinin-reactive AFP (AFP-L3) and des-γ-carboxy prothrombin are widely used in Asia for early detection of HCC. Both have been approved by the US Food and Drug Administration for risk stratification for HCC. In a US cohort, the sensitivity and specificity of AFP-L3% for detecting HCC at a cutoff of 1.7% were 37% and 94%, respectively. The combination of AFP with AFP-L3% or des-γ-carboxy prothrombin improved AFP performance for early HCC diagnosis. However, the sensitivity for early HCC for each biomarker individually has remained low in studies to date.64 A model including sex, age, AFP-L3, AFP, and des-γ-carboxy prothrombin (GALAD) was evaluated in a multinational study with 2430 HCC patients and 4404 patients with chronic liver disease, demonstrating sensitivity >70% for overall HCC detection and >60% for early HCC detection.65
There is a dearth of other sensitive and specific HCC biomarkers that are well-validated in prospective studies. Although the hope is that better imaging and serologic biomarkers will be available in the future, these strategies still require extensive evaluation before routine adoption in clinical practice. In the interim, we will continue to depend on ultrasound-based surveillance, with or without AFP, and efforts should focus on maximizing ultrasound quality and surveillance utilization. In our centers, we routinely use ultrasound and AFP as our surveillance strategy in patients with cirrhosis. For those in whom ultrasound-based surveillance is not effective, for example, obese patients in whom ultrasound has a visualization score of C, alternative surveillance tests, such as MRI, can be considered; however, there are few data evaluating the effectiveness of this strategy.
What Is Coming Next?
Although MRI-based surveillance could improve early tumor detection, this is likely not cost-effective if implemented in all at-risk patients, and might best be reserved for the subset of patients who are prone to ultrasound failure or have sufficiently high risk of HCC in which this strategy may be cost-effective. Other strategies, such as abbreviated MRI, may reduce cost while maintaining sufficient sensitivity for early detection but still require further evaluation.
One efficient strategy may be to improve existing blood-based biomarkers for early HCC detection. For example, platforms using combinations of biomarkers with other clinical data as well as changes over time may achieve acceptable test performance (similar to AFP algorithm and GALAD).66,67 This framework (biomarker + demographic and clinical data + surveillance history) is likely to be an essential platform for future application of biomarkers for early HCC detection, including novel biomarkers.
Several HCC-related biomarkers, including DNA, messenger RNAs, non-coding RNAs, proteins, and posttranslational protein modifications have been identified or tested in preclinical (phase 1) studies. However, few have been evaluated in case–control (phase 2) studies, so their utility as HCC surveillance markers is not yet known. Glypican 3, GP73, osteopontin, squamous cell carcinoma antigen, human hepatocyte growth factor, and insulin growth factor-1 are examples of biomarkers that are currently being evaluated. More biomarkers are likely to be identified using proteomics-based approaches. Plasma-based methylation markers found in the cell-free DNA (ie, liquid biopsy) are also promising markers for detection of HCC. In a case– control (phase 2 biomarker) study including 95 HCC cases, 51 cirrhosis controls, and 98 healthy controls, a panel of 6 methylation markers had a sensitivity of 95% overall, 75% (3 of 4) for stage 0, and 93% (39 of 42) stage A, 93% (13 of 14) stage B, 96% (27 of 28) stage C, and 100% (7 of 7) sensitivity for stage D HCC at a specificity of 86% for cirrhotic controls.68 Further studies, based on guidelines for biomarker development, are necessary to better evaluate the potential role of these biomarkers during surveillance.
There are at least 2 large cohorts of cirrhosis patients in the United States, including National Cancer Institute–funded Early Detection Research Network and Cancer Prevention and Research Institute of Texas–funded Texas HCC Consortium that will allow phase 3 biomarker evaluation studies. Additionally, the National Cancer Institute recently funded the Consortium on Translational Research in Early Detection of Liver Cancer to accelerate testing and validation of promising biomarkers for HCC detection. Eventually, randomized studies that assess the clinical utility of the newer biomarkers and biomarker combinations for HCC surveillance are needed.
In the era of precision medicine, we may witness programs that tailor HCC surveillance, precisely matching surveillance intensity and/or type with patients’ underlying risk and their potential for a survival benefit. This approach can maximize benefits of surveillance by shifting resource-intensive efforts toward high-risk patients, while reducing harms of testing (including false-positive results) in patients at low risk for HCC.
Surveillance Interval
HCC surveillance should be performed every 6 months. A retrospective analysis of a large prospectively maintained multi-center Italian database showed that patients who received surveillance every 6 months had tumors detected at an earlier stage and significantly better overall survival than patients receiving annual surveillance, even after correcting for lead-time bias.69 The median corrected survival among the 510 patients in the 6-month surveillance group was 40.3 months, compared to 30 months in the 139 patients in the 12-month surveillance group (P = .03). A subsequent multi-center randomized controlled trial among 1340 patients with cirrhosis evaluated whether further shortening the surveillance interval to 3 months results in better detection of early-stage tumors and improves survival.70 The majority of patients in both groups were detected at an early stage (79% vs 71%; P = .40) and similar proportions received curative therapies (62% vs 58%; P = .88). Furthermore, the 3-month surveillance group had a higher incidence of non-malignant lesions, leading to a higher number of unnecessary recall procedures. Overall, these data provide support to the 6-month surveillance interval.
What Is Coming Next?
With the introduction of newer surveillance tests with higher sensitivity for very early-stage HCC detection, studies will be needed to see if surveillance intervals can be prolonged.
Surveillance Utilization
Despite demonstrated benefits, including early tumor detection, <20% of patients with cirrhosis undergo surveillance.71,72 Among those receiving regular hepatology care by a specialist, surveillance rates are higher at 52%, but almost one-third receive inconsistent HCC surveillance. HCC surveillance underuse can be attributed to several failures in the surveillance process, including provider failure to identify liver disease, provider failure to identify the silent transition to cirrhosis, provider failure to order HCC surveillance, and patient failure to adhere with surveillance recommendations.73 In a single-center study of patients with HCC, the most common reason for surveillance underuse was failure of providers to order HCC surveillance in patients with recognized cirrhosis. Survey studies among primary care providers found several barriers to providers ordering HCC surveillance, including insufficient knowledge regarding professional society guidelines for HCC surveillance, insufficient time in clinic, and competing clinical demands.74,75 One study conducted at a safety-net health system suggested patients may also experience potential barriers to HCC surveillance, including difficulty navigating the scheduling process, costs of surveillance tests, uncertainty where to complete surveillance, and transportation barriers; however, these results still require validation in other settings.76 Recent data continue to highlight systemic underutilization of HCC surveillance. In a study of 26,577 patients with cirrhosis followed for a median of 4.7 years in the national VA, the mean percentage of time patients were up-to-date with HCC surveillance was 17.8% ± 21.5% (for ultrasounds) and 23.3% ± 24.1% when any liver imaging modality was included.77 In this study, the strongest predictor of adequate surveillance (measured as percentage of time up-to-date with surveillance) was the number of visits to a specialist (gastroenterologist/hepatologist and/or infectious diseases) in the first year after cirrhosis diagnosis. Increasing distance to the closest hospital and longer times between the date an ultrasound was ordered and the requested examination date were inversely associated with adequate surveillance.
To address these lapses, models to improve surveillance have been proposed. For example, electronic medical record clinical reminders to perform surveillance significantly improved surveillance rates from 18.2% to 27.6% (P < .001) in a study among 2884 VA patients with cirrhosis who had not received HCC surveillance in the preceding 6 months.78 A randomized study among high-risk patients showed that a mailed outreach strategy that encouraged patients to undergo ultrasound surveillance with or without patient navigation also significantly improved surveillance rates. One-time surveillance completion rates within 6 months were significantly higher in outreach/navigation (47.2%) and outreach alone (44.5%) arms than usual care (24.3%) (P < .001 for both comparisons); however, surveillance rates did not differ significantly between outreach arms (P = .25).79 Similarly, HCC surveillance every 6 months during the 18-month study period was performed in 23.3% of outreach/navigation patients, 17.8% of outreach-alone patients, and 7.3% of usual care patients.80 HCC surveillance was significantly higher in both outreach groups than usual care (P < .001 for both) and higher for outreach/ navigation than outreach alone (P = .02). Despite improvements in surveillance rates in intervention studies to date, HCC surveillance in most intervention groups have remained disappointingly low, highlighting a need for more intensive interventions, particularly in patients who would otherwise be eligible for potentially curative HCC treatments.
HCC surveillance is a process; it is not just restricted to simply performing surveillance tests (such as an ultrasound with or without AFP) and ensuring appropriate surveillance utilization, but also includes quality assurance of testing and appropriate follow-up of surveillance results. Studies have suggested failures at each step in the HCC surveillance process.17,81
What Is Coming Next?
Studies are needed to evaluate more intensive interventions to increase HCC surveillance utilization with the current imaging and AFP-based tests, ensure quality of ultrasound testing, and to optimize follow-up of surveillance results.
Summary
Despite an increased proportion of HCC being detected at a localized stage and improvement in stage-specific survival, HCC-related mortality continues to rise sharply. The upward trend in HCC incidence underscores the importance of effective HCC surveillance strategies in the United States, particularly among emerging at-risk cohorts, such as those with NAFLD and post–sustained virologic response HCV cirrhosis. Expert society guidelines recommend HCC surveillance with bi-annual abdominal ultrasound, with and without AFP, in at-risk individuals. This strategy identifies patients with early-stage HCC and increases the likelihood of receipt of curative therapy. However, the quality of the evidence supporting a mortality benefit of HCC surveillance remains limited. Further, there are few data on the harms associated with HCC surveillance. Several efforts are underway that will quantity the benefits and harms of HCC surveillance in prospective cohorts, improve risk stratification among patients with cirrhosis, identify and validate novel (imaging- and serology-based) biomarkers for early detection of HCC, and increase uptake of HCC surveillance among at-risk patients.
In this review, we outlined several areas that may serve as high-yield targets for future research to improve the overall value of HCC surveillance (Table 2). We believe these efforts will likely help the field move toward precision surveillance, where surveillance tests and intervals are tailored to individual HCC risk. Doing so can maximize surveillance benefits, minimize surveillance harms, and optimize overall value for all patients.
Table 2.
Suggested Areas for Future Research to Improve Hepatocellular Carcinoma Surveillance
| Areas for future research |
|---|
| Improve risk stratification |
| Development of tools to allow personalized assessment of HCC risk, especially in the newer cohorts of patients with virologically cured HCV, suppressed HBV, and NAFLD |
| Identification and validation of biomarkers that identify at-risk groups among patients with non-cirrhosis NAFLD |
| Identify new biomarkers for early identification of HCC |
| Development and validation of new serum-based HCC biomarkers that are accurate for early and very early HCC detection |
| Development and validation of new imaging-based HCC biomarkers that are accurate for early and very early HCC detection |
| Development and validation of platforms that use combinations of biomarkers with other clinical data, as well as longitudinal changes in biomarkers over time |
| Enhance the value of HCC surveillance |
| Characterization of benefits and harms of HCC surveillance in large prospective cohort studies, including diverse patient populations |
| Interventions to reduce physical, financial, and psychological harms of HCC surveillance |
| Development, evaluation, and implementation of interventions to increase HCC surveillance utilization |
| Development of cost-effectiveness models to examine long-term comparative effectiveness of HCC surveillance strategies, particularly in emerging at-risk populations |
Acknowledgments
Funding
Dr Kanwal’s research is supported by the US Department of Veterans Affairs Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, Texas, and the Center for Gastrointestinal Development, Infection and Injury (National Institute of Diabetes and Digestive and Kidney Diseases P30 DK 56338) and National Cancer Institute U01 CA230997. Dr Singal’s research is supported by National Cancer Institute RO1 CA222900, RO1 CA212008, and U01 CA 230694. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs or the National Institutes of Health.
The authors disclose the following: Dr Kanwal has received grant funding from Merck. Dr Singal has been on advisory boards and served as a consultant for Bayer, Wako Diagnostics, Roche, Exact Sciences, and Glycotest.
Abbreviations used in this paper:
- AFP
α-fetoprotein
- AFP-L3
lens culinaris agglutinin-reactive α-fetoprotein
- CI
confidence interval
- CT
computed tomography
- HBV
hepatitis B virus
- HCC
hepatocellular cancer
- HCV
hepatitis C virus
- LI-RADS
Liver Imaging Reporting and Data System
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- VA
Veterans Affairs
References
- 1.Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812–820. e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015 Bethesda, MD: National Cancer Institute, 2018. [Google Scholar]
- 5.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123:357–360. [DOI] [PubMed] [Google Scholar]
- 8.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–422. [DOI] [PubMed] [Google Scholar]
- 9.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 10.Covey AM. Hepatocellular carcinoma: updates to screening and diagnosis. J Natl Compr Cancer Netw 2018;16(5S):663–665. [DOI] [PubMed] [Google Scholar]
- 11.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017; 11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- 13.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261–269. [DOI] [PubMed] [Google Scholar]
- 14.Harris RP, Wilt TJ, Qaseem A. A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:712–717. [DOI] [PubMed] [Google Scholar]
- 15.Moon AM, Weiss NS, Beste LA, et al. No association between screening for hepatocellular carcinoma and reduced cancer-related mortality in patients with cirrhosis. Gastroenterology 2018;155:1128–1139.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Murphy C. Hepatocellular carcinoma surveillance: an effective but complex process. Gastroenterology 2019;156:1215. [DOI] [PubMed] [Google Scholar]
- 17.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med 2017;130:1099–1106. e1091. [DOI] [PubMed] [Google Scholar]
- 18.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poustchi H, Farrell GC, Strasser SI, et al. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011; 54:1998–2004. [DOI] [PubMed] [Google Scholar]
- 20.Heleno B, Thomsen MF, Rodrigues DS, et al. Quantification of harms in cancer screening trials: literature review. BMJ 2013;347:f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma A, Konerman MA, Zhao B. Impact of a Structured Hepatocellular Carcinoma Surveillance Program in Patients With Cirrhosis: Frequency, Evaluation and Subsequent Clinical Outcomes of Patients With Abnormal Imaging Findings. Presented at: AASLD: The Liver Meeting 2017, October 20–24, 2017, Washington, DC. [Google Scholar]
- 23.Mittal S, Kramer JR, Omino R, et al. Role of age and race in the risk of hepatocellular carcinoma in veterans with hepatitis B virus infection. Clin Gastroenterol Hepatol 2018;16:252–259. [DOI] [PubMed] [Google Scholar]
- 24.Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2(8256):1129–1133. [DOI] [PubMed] [Google Scholar]
- 25.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut 2000;47:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463–472. [DOI] [PubMed] [Google Scholar]
- 27.Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996; 334:1422–1427. [DOI] [PubMed] [Google Scholar]
- 28.Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology 1998;28:1687–1695. [DOI] [PubMed] [Google Scholar]
- 29.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748–755.e3. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153:996–1005. e1001. [DOI] [PubMed] [Google Scholar]
- 32.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828–1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010; 51:1972–1978. [DOI] [PubMed] [Google Scholar]
- 34.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384–1391. [DOI] [PubMed] [Google Scholar]
- 36.Bralet MP, Regimbeau JM, Pineau P, et al. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology 2000;32:200–204. [DOI] [PubMed] [Google Scholar]
- 37.Ertle J, Dechene A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011; 128:2436–2443. [DOI] [PubMed] [Google Scholar]
- 38.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851–859. [DOI] [PubMed] [Google Scholar]
- 39.Mancebo A, Gonzalez-Dieguez ML, Cadahia V, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol 2013;11:95–101. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen MH, Yang HI, Le A, et al. Reduced incidence of hepatocellular carcinoma with tenofovir in chronic hepatitis B patients with and without cirrhosis—a propensity score matched study. J Infect Dis 2019;219:10–18. [DOI] [PubMed] [Google Scholar]
- 41.Lee YC, Cohet C, Yang YC, et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 2009;38:1497–1511. [DOI] [PubMed] [Google Scholar]
- 42.Trichopoulos D, Bamia C, Lagiou P, et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst 2011;103:1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanwal F, Kramer JR, Asch SM, et al. Longer term risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Presented at: AASLD: The Liver Meeting; 2018 San Francisco, CA. [Google Scholar]
- 44.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568–574. [DOI] [PubMed] [Google Scholar]
- 46.Ioannou GN, Green PK, Beste LA, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 2018; 69:1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen 1999;6:108–110. [DOI] [PubMed] [Google Scholar]
- 49.Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706–1718.e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 2013;108:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 52.Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1927–1933.e1922. [DOI] [PubMed] [Google Scholar]
- 53.Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography—a randomised study. Aliment Pharmacol Ther 2013;38:303–312. [DOI] [PubMed] [Google Scholar]
- 54.Kim SY, An J, Lim YS, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 2017; 3:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersson KL, Salomon JA, Goldie SJ, et al. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goossens N, Singal AG, King LY, et al. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol 2017;8(6):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdomin Radiol (NY) 2017;42:179–190. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46–50. [DOI] [PubMed] [Google Scholar]
- 59.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005; 43:434–441. [DOI] [PubMed] [Google Scholar]
- 60.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Serag HB, Kanwal F, Davila JA, et al. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014;146:1249–1255. e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee E, Edward S, Singal AG, et al. Improving screening for hepatocellular carcinoma by incorporating data on levels of alpha-fetoprotein, over time. Clin Gastroenterol Hepatol 2013;11:437–440. [DOI] [PubMed] [Google Scholar]
- 63.Tayob N, Lok AS, Do KA, et al. Improved detection of hepatocellular carcinoma by using a longitudinal alpha-fetoprotein screening algorithm. Clin Gastroenterol Hepatol 2016;14:469–475.e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol 2016;14:875–886.e876. [DOI] [PubMed] [Google Scholar]
- 66.Wang M, Sanda M, Comunale MA, et al. Changes in the glycosylation of kininogen and the development of a kininogen-based algorithm for the early detection of HCC. Cancer Epidemiol Biomark Prev 2017;26:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White DL, Richardson P, Tayoub N, et al. The Updated model: an adjusted serum alpha-fetoprotein-based algorithm for hepatocellular carcinoma detection with hepatitis C virus-related cirrhosis. Gastroenterology 2015;149:1986–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kisiel JB, Allawi HT, Giakoumopoulos M, et al. 1044-hepatocellular carcinoma detection by plasma assay of methylated dna markers: phase II clinical validation. Gastroenterology 2018;154:S-1113–S-1114. [Google Scholar]
- 69.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291–297. [DOI] [PubMed] [Google Scholar]
- 70.Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987–1997. [DOI] [PubMed] [Google Scholar]
- 71.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus–infected veterans in the United States. Ann Intern Med 2011;154:85–93. [DOI] [PubMed] [Google Scholar]
- 72.Singal AG, Yopp A, S Skinner C, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012; 27:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res 2012;5:1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simmons OL, Feng Y, Parikh ND, et al. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol 2019; 17:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalton-Fitzgerald E, Tiro J, Kandunoori P, et al. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2015; 13:791–798.e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017;65:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldberg DS, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017;65:864–874. [DOI] [PubMed] [Google Scholar]
- 78.Beste LA, Ioannou GN, Yang Y, et al. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172–179. [DOI] [PubMed] [Google Scholar]
- 79.Singal AG, Tiro JA, Marrero JA, et al. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology 2017;152:608–615.e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singal AG, Tiro JA, Murphy CC, et al. Mailed outreach invitations significantly improve HCC surveillance rates in patients with cirrhosis: a randomized clinical trial. Hepatology 2019;69:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel N, Yopp AC, Singal AG. Diagnostic delays are common among patients with hepatocellular carcinoma. J Natl Compr Cancer Netw 2015;13:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]


