Abstract
Plants sense positional changes relative to the gravity vector. To date, the signaling processes by which the perception of a gravistimulus is linked to the initiation of differential growth are poorly defined. We have investigated the role of inositol 1,4,5-trisphosphate (InsP3) in the gravitropic response of oat (Avena sativa) shoot pulvini. Within 15 s of gravistimulation, InsP3 levels increased 3-fold over vertical controls in upper and lower pulvinus halves and fluctuated in both pulvinus halves over the first minutes. Between 10 and 30 min of gravistimulation, InsP3 levels in the lower pulvinus half increased 3-fold over the upper. Changes in InsP3 were confined to the pulvinus and were not detected in internodal tissue, highlighting the importance of the pulvinus for both graviperception and response. Inhibition of phospholipase C blocked the long-term increase in InsP3, and reduced gravitropic bending by 65%. Short-term changes in InsP3 were unimpaired by the inhibitor. Gravitropic bending of oat plants is inhibited at 4°C; however, the plants retain the information of a positional change and respond at room temperature. Both short- and long-term changes in InsP3 were present at 4°C. We propose a role for InsP3 in the establishment of tissue polarity during the gravitropic response of oat pulvini. InsP3 may be involved in the retention of cold-perceived gravistimulation by providing positional information in the pulvini prior to the redistribution of auxin.
The growth of a plant is governed in part by environmental cues such as light and gravity. In response to changes in a plant's spatial orientation, roots and shoots exhibit differential growth, resulting in downward and upward curvature, respectively. Gravitropic responses of plants are mediated by a cascade of biophysical and biochemical events. The settling of dense particles such as starch-containing amyloplasts (Sack, 1991; Kaufman et al., 1995) or the pressure exerted by protoplast settlement (Staves, 1997) may be primary events in graviperception. Intra- and inter-cellular signaling events subsequently initiate downstream metabolic changes including a redistribution of auxin that results in asymmetric growth (Lomax et al., 1995; Kaufman et al., 1995). A variety of second messengers have been implicated in gravisignaling, including Ca2+, pH, and InsP3 (for review, see Chen et al., 1999; Rosen et al., 1999).
In the gravisensitive columella cells of Arabidopsis roots, changes in intracellular pH within the first minutes of gravistimulation have been reported to result in a pH gradient across the root cap (Scott and Allen, 1999). In cress roots, changes in intracellular ionic currents have been demonstrated in response to gravistimulation (Behrens et al., 1985; Sievers et al., 1995). Although to date rapid changes in [Ca2+]i in response to gravistimulation have not been detected in a multicellular plant tissue (Legue et al., 1997) there is much indirect evidence implicating Ca2+ at the initial or at later stages of gravitropic signaling (for review, see Belyavskaya, 1996; Sinclair and Trewavas, 1997). The gravistimulus may be transduced and amplified by cascades involving Ca2+/calmodulin (Sinclair et al., 1996; Lu and Feldman, 1997) and protein phosphorylation (Chang and Kaufman, 2000). However, at present we have a limited understanding of the interaction of the various players and the sequence of signaling events that lead to a biochemical asymmetry between the upper and lower halves of the gravistimulated tissue and ultimately result in a gravitropic response.
The phosphoinositide (PI) pathway is involved in the responses of plants to a variety of external stimuli (for review, see Munnik et al., 1998). We have previously shown that changes in the PI pathway, including a biphasic increase in InsP3, correlated positively with the gravitropic bending response of mature maize plants (Perera et al., 1999). An up-regulation of PI metabolism might reflect the need for an increase in membrane biogenesis and cytoskeletal restructuring, as well as an increase in InsP3-mediated Ca2+ release to initiate and sustain cell elongation (Stevenson et al., 2000).
It was the goal of this study to determine whether changes in InsP3 are required for plant gravitropism and, furthermore, to dissect the functions of the transient and sustained changes in InsP3 associated with gravistimulation. The phospholipase C (PLC) inhibitor, U73122, and cold temperature were used to interfere with the plants' gravitropic response. Oat (Avena sativa) explants were chosen over whole plants for their small size, fast gravitropic response, and to facilitate uptake and delivery of pharmacological compounds to the pulvinus tissue.
We first show that both transient and sustained increases in InsP3 occur prior to gravitropic curvature of excised oat leaf-sheath pulvini. Application of U73122 eliminated the sustained increase in InsP3 in the lower half of the pulvinus and attenuated gravitropic curvature by 65%. This suggests that PLC-mediated generation of InsP3 on the lower side of the pulvinus is required during the gravitropic response. Although gravitropic bending is inhibited by cold temperature, the perception of a gravistimulus, and subsequent signaling events must occur because plants gravistimulated in the cold will bend when returned to room temperature (Brauner and Hager, 1958; Fukaki et al., 1996; SE Wyatt, A Rashotte, G Muday, D Robertson, unpublished data). We, similarly, have found that the gravitropic curvature response of oat shoots was inhibited by cold temperature, and a cold-perceived gravistimulus elicited a bending response when plants were returned to room temperature. Both short-term and long-term InsP3 changes occurred in the cold with a magnitude similar to room temperature controls, indicating that an InsP3 gradient may set positional cues in the pulvinus tissue prior to differential growth. The data presented in this study indicate that changes in InsP3 may play significant roles in gravisignaling and in the establishment of tissue polarity in the pulvinus.
RESULTS
Short- and Long-Term Changes in InsP3 Precede the Gravitropic Bending Response of Oat Shoots
Oat plants respond to gravistimulation with an upward curvature of the leaf sheath pulvinus, a specialized tissue located at the base of the leaf sheath, immediately above the node (Kaufman et al., 1987). Starch-containing cells are confined to the pulvinus and are not present in the internode. The gravitropic response is conferred by differential cell elongation on the lower side of the pulvinus; the internodes of the stem do not bend.
Excised oat stem segments containing the most responsive p-1 pulvinus from 45-d-old plants (Kaufman et al., 1987) were used for our studies, and eight to 10 pulvinus halves were pooled for the measurement of InsP3 content. The parameters of gravitropic bending of the excised oat stem segments were consistent with the kinetic studies reported previously. In the oat explant system the minimum time of gravistimulation required to induce a bending response (presentation time) was 10 min. Stem segments began to bend after 30 to 45 min of gravistimulation (Dayanandan and Kaufman, 1984) and reached a curvature of 40o to 50o after 48 h (Chang and Kaufman, 2000).
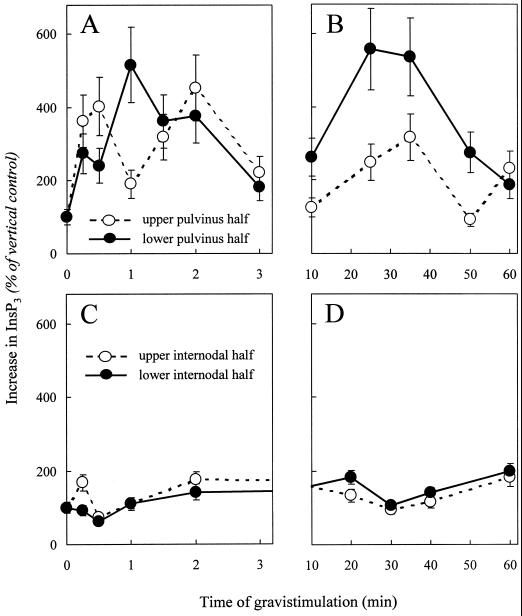
InsP3 levels were measured at various times of gravistimulation in dissected upper and lower pulvinus halves. To compare results from individual experiments, InsP3 values from upper and lower halves were standardized as a percentage of the InsP3 levels measured in vertical controls. Within 15 s of gravistimulation, InsP3 levels increased 3-fold over the vertical control in both the upper and lower halves of the gravistimulated pulvini. Over the first few min, InsP3 levels fluctuated in both halves of the pulvini (Fig. 1A).
Figure 1.
Short- and long-term changes in InsP3 precede gravitropic bending in oat pulvini and not in internodal tissue. InsP3 levels were measured in upper (dashed lines) and lower (solid lines) halves of gravistimulated oat pulvini (A and B) or internodal tissue (C and D) at the indicated times after gravistimulation. The data points represent the percentage change in InsP3 over the vertical control (set at 100%) and are the average of three independent experiments assayed in duplicate. The vertical bars indicate the range. No significant change in InsP3 levels could be detected between halves from vertical control plants (data not shown). Changes in InsP3 over the first 3 min of gravistimulation in upper and lower halves of pulvinus (A) and internodal tissue (C). Changes in InsP3 over the first 60 min of gravistimulation in upper and lower halves of pulvinus (B) and internodal tissue (D). InsP3 levels in vertical controls were in the range of 200 to 300 pmol g−1 fresh weight for both pulvinus and internode.
A sustained increase in InsP3 was detected in the lower half of the pulvinus between 10 and 30 min of gravistimulation (Fig. 1B). After 30 min of gravistimulation, InsP3 levels in the lower half were 5- to 6-fold higher than in the vertical controls and 2- to 3-fold higher than in the upper half. The onset of gravitropic bending after 30 min significantly coincided with the long-term increase in InsP3 on the lower pulvinus half. InsP3 levels returned to basal values by 50 to 60 min of gravistimulation.
The pattern of InsP3 changes preceding gravitropic bending in oats is consistent with our previous findings in maize plants (Perera et al., 1999). The initial oscillations in InsP3 have a similar period in maize and in oat. However, in the oat stem segments, the sustained increase in InsP3 occurred considerably faster than in maize and took place on a time scale of minutes (rather than hours) in keeping with the overall faster initiation of growth and bending in the oat stems.
There Are No Major Changes in InsP3 Levels in Oat Internodal Stem Tissue upon Gravistimulation
To determine whether changes in InsP3 with gravistimulation are restricted to the graviresponsive pulvinus, we monitored InsP3 levels in the upper and lower halves of discs of oat stem internodal tissue over a period of 60 min of gravistimulation (Fig. 1, C and D). In contrast to the pulvinus, no major change in InsP3 levels over the vertical control was observed in internodal tissue during the first few min (Fig. 1C) or over the first 60 min (Fig. 1D). At 15 s there was a 1.5-fold increase in InsP3 over the vertical control in the upper half of the internode. However, unlike in the pulvinus, no subsequent fluctuations or increases in InsP3 over the vertical control were detected in either the upper or lower halves of internodal tissue. The fact that both the transient and sustained increases in InsP3 are confined to the pulvinus underscores the importance of this tissue for both graviperception and response.
Inhibition of PLC by U73122 Attenuates Gravitropic Bending of Oat Stems
InsP3 is produced by the hydrolysis of the phospholipid phosphatidylinositol 4,5-bisphosphate (PtdInsP2) by PLC (Drøbak, 1992). The aminosteroid, U73122, has been shown to inhibit PLC in both animal and plant systems (Bleasdale et al., 1990; Thompson et al., 1991; Staxen et al., 1999). U73122 blocked the activity of purified recombinant soybean phosphoinositide-specific PLC (PI-PLC) with a 50% inhibition of initial activity of 23 μm (Staxen et al., 1999). Used in conjunction with its inactive analog, U73343, U73122 is a useful tool to affect PLC-mediated turnover of PtdInsP2.
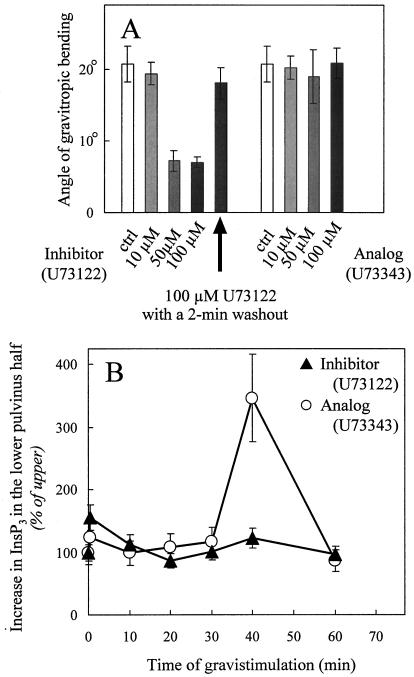
To test our hypothesis that PLC-mediated generation of InsP3 is necessary for the gravitropic response, we first examined the effect of the PLC inhibitor on gravitropic bending of oat stems (Fig. 2A). Oat stem segments were pretreated for 2 h with 10, 50, and 100 μm U73122 or U73343 prior to gravistimulation. Application of U73122 (50 and 100 μm) inhibited bending by 65%, whereas U73343-treated stems showed a normal gravitropic response. Internodal extension growth was not significantly different between dimethyl sulfoxide (DMSO)-treated, U73343-treated, and U73122-treated oat stem segments at the concentrations used (Table I). When 100-μm inhibitor was applied and subsequently removed by a 2-min washout prior to gravistimulation, gravitropic bending was restored to 87% of the control (Fig. 2A). These experiments demonstrate that the inhibition of the bending response by U73122 was not due to toxic effects of the inhibitor on the plants.
Figure 2.
The PLC inhibitor blocks the gravitropic bending response of oat-pulvini and abolishes the long-term increase in InsP3. A, Gravitropic bending of oat stems is inhibited by the PLC inhibitor, U73122. Oat stem segments were pretreated for 2 h prior to gravistimulation with the indicated concentrations of U73122, or with an inactive analog, U73343. Gravitropic bending was measured after 24 h. In one set of segments (indicated by the arrow) the inhibitor was washed out for 2 min before gravistimulation. The values plotted are the average measurements from 12 segments/treatment and the vertical bars indicate the range. Gravitropic bending of plants treated with 0.5% (v/v) DMSO in Suc buffer (ctrl) was reduced by 10% to 15% compared with plants treated with Suc buffer alone. B, The long-term increase in InsP3 is prevented by inhibitor treatment. InsP3 levels were measured over the first 60 min of gravistimulation in upper and lower halves from gravistimulated pulvini treated with 100 μm U73122 (▴) or 100 μm U73343 (○). The increase in InsP3 levels in lower pulvinus halves was plotted as a percentage of that in the upper halves. The data represent the average of two independent experiments assayed in duplicate. Vertical bars indicate the range. InsP3 values of vertical controls were 851 ± 100, 638 ± 50, and 311 ± 100 pmol g−1 fresh weight for U73122, U73343, and DMSO, respectively.
Table I.
The Effects of the PLC inhibitor U73122 are reversible and do not affect extension growth of the internodes
Application of U73122 Abolishes the Long-Term Increase in InsP3
To confirm that the attenuation of the gravitropic response of oat stems in the presence of U73122 was due to inhibition of PLC activity, we examined the effect of the inhibitor on the InsP3 increases associated with gravistimulation (Fig. 2B). Treatment of excised oat stem segments with 100 μm U73122 abolished the long-term increase in InsP3 in the lower half of the pulvini. In contrast, when oat stem segments were treated with the inactive analog, U73343, within 40 min of gravistimulation, InsP3 levels in the lower half of the pulvinus increased 3-fold over those in the upper half. The increase in InsP3 was slightly delayed with U73343 compared with the DMSO control. Stems that had been treated with DMSO alone showed a 3-fold increase in InsP3 in the lower half over the upper around 30 min (data not shown). InsP3 levels in both the control and U73343-treated stems returned to basal values by 60 min (U73343-data shown in Fig. 2B). The effect of the inhibitor treatment on both the gravitropic response and the long-term InsP3 increase on the lower side of the pulvinus suggests that the gradual InsP3 increase is necessary for the gravitropic response.
In contrast to the inhibitory effects of U73122 on the long-term increase in InsP3, the early changes in InsP3 during the initial minutes of gravistimulation did not appear to be affected by the inhibitor treatment. When stem segments were treated with either U73122, U73343, or DMSO alone, the initial fluctuations in InsP3 were observed with all three treatments (Table II). Because increases in InsP3 were detected in both the upper and the lower half of the pulvinus during the first minutes of gravistimulation, the entire pulvinus was analyzed for InsP3 content at two time points (15 and 30 s). To compare data from independent experiments, changes in InsP3 upon gravistimulation measured in stems pretreated with U73122 or U73343 are presented as a percentage of the increase observed in gravistimulated DMSO-treated controls (Table II). These data indicate that the short-term changes were unaffected by the inhibitor treatment, and thus, that the short-term and long-term increases in InsP3 were differentially sensitive to the inhibitor.
Table II.
Early changes in InsP3 are not significantly affected by treatment with the PLC inhibitor U73122
| Treatment | Gravistimulation for:
|
|
|---|---|---|
| 15 s | 30 s | |
| % | % | |
| Control | 100 | 100 |
| Inactive analog U73343 | 122 | 74 |
| PLC inhibitor U73122 | 107 | 82 |
The change in InsP3 in gravistimulated intact pulvinus tissue was determined after treatment with 0.5% (w/w) DMSO, 100 μm U73343 in DMSO, or 100 μm U73122 in DMSO. Changes are given as the percentage of the DMSO control. Data are the average of four independent experiments; the error is approximately 20%.
Cold Temperature Inhibits the Gravitropic Response But Not Graviperception
The gravitropic bending response of plants is inhibited in the cold (Brauner and Hager, 1958; Fukaki et al., 1996; SE Wyatt, A Rashotte, G Muday, D Robertson, unpublished data). However, the perception of the stimulus and some or all of the downstream signaling events are not blocked by cold temperature although statolith sedimentation may be delayed (Philosoph-Hadas et al., 2000). Fukaki et al. (1996) have shown that the presentation time of Arabidopsis inflorescence stems is not increased in the cold, and the “memory” of a gravistimulus perceived in the cold is retained by the plants for up to 60 min after the discontinuation of the gravistimulus. Arabidopsis stems gravistimulated for 30 min at 4°C and then incubated in a vertical orientation for 60 min at 4°C, still exhibited a full response to the cold-perceived gravistimulation upon being returned to room temperature (Fukaki et al., 1996).
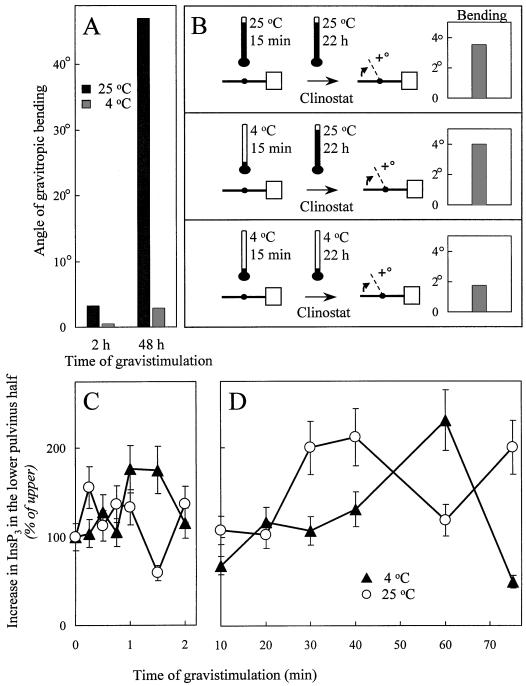
Consistent with the data reported for other systems, the gravitropic bending response of oat plants was inhibited by cold temperature (Fig. 3A). At 4°C, gravitropic bending of oat stem segments was inhibited by 80% after 2 h and by 94% after 48 h of gravistimulation compared with control segments gravistimulated for equivalent times at room temperature. In contrast, the extension growth of internodal stem segments at 4°C was reduced by 46% in vertical plants and by 60% in gravistimulated plants compared with plants monitored at room temperature. Therefore, the inhibition of gravitropic bending by cold temperature is more severe than the inhibition of internodal extension. Despite the cold-inhibition of growth, oat stems that have been gravistimulated in the cold exhibited full gravitropic bending when returned to room temperature.
Figure 3.
Cold temperature inhibits the gravitropic bending response but does not block graviperception or increases in InsP3. A, The gravitropic response is blocked in the cold. Oat segments were gravistimulated at 4°C and 25°C, and bending was measured after 2 and 48 h. Data shown are the average of 20 segments/treatment. B, Graviperception is not blocked in the cold. Oat stems were gravistimulated for 15 min at 4°C or at 25°C, and then transferred to a clinostat at 4°C or at 25°C. Gravitropic bending was measured after 22 h of clinorotation. The data presented are the average of 20 segments/treatment. C and D, Changes in InsP3 levels are unaffected by the cold. InsP3 levels were measured in oat stem segments gravistimulated at 4°C or at 25°C during the initial minutes of gravistimulation (C) and over the first 75 min of gravistimulation (D). The increases in InsP3 levels in lower pulvinus halves were plotted as a percentage of the InsP3 levels in the upper halves. The data represent the average of two independent experiments assayed in duplicate and vertical bars indicate the range.
Short-term gravistimulation followed by clinorotation was used to study the effects of cold-treatment on graviperception and response. Oat stem segments gravistimulated for 15 min at 4°C and then placed on a clinostat at room temperature for 22 h exhibited angles of gravitropic curvature equal to or higher than those of control plants gravistimulated and clinorotated at room temperature (Fig. 3B, middle and upper panels). In contrast, bending was inhibited by >60% in stem segments that remained at 4°C for both the 15 min gravistimulation and subsequent 22-h clinorotation (Fig. 3B, lower panel). These data indicate that graviperception and the signaling cascade necessary for the commitment to differential growth of the oat pulvinus occur in the cold.
Cold Temperature Does Not Prevent Changes in InsP3
If InsP3 signals are necessary for the perception of a gravistimulus and commitment to differential growth, the pattern of InsP3 changes upon gravistimulation should be unaffected by the cold. To test whether cold temperature affects changes in InsP3 during gravistimulation, oat stem segments were gravistimulated at 4°C, and InsP3 levels were monitored in upper and lower halves of the pulvini in time course experiments. Over the first few minutes of gravistimulation, InsP3 levels fluctuated between the upper and lower halves of the pulvinus similar to control segments gravistimulated at room temperature (Fig. 3C).
Figure 3D depicts the long-term increases in InsP3 on the lower side of the pulvinus over the upper side at room temperature and at 4°C. As described in Figure 1B, at room temperature InsP3 values were 2- to 3-fold increased on the lower side of the pulvinus around 30 min of gravistimulation. InsP3 values in the lower half of the pulvinus decreased with the onset of gravitropic bending. The increased InsP3 level in the lower pulvinus half after 75 min of gravistimulation may reflect changes in PI metabolism associated with elongation growth. At 4°C, the timing of the InsP3 increase on the lower half was delayed compared with the room temperature control. In the cold, InsP3 levels increased in the lower half of the pulvinus up to 3-fold over those in the upper half after 50 to 60 min of gravistimulaton (Fig. 3D). The magnitude and the duration of the InsP3 increases were similar between the segments in the cold and at room temperature.
DISCUSSION
Rapid, transient increases in InsP3 were detected in both pulvinus halves of gravistimulated oats, followed by a slower, gradual increase in InsP3 only in the lower half of the pulvinus. This biphasic pattern of InsP3 increases appears to be necessary for the initiation of differential growth. Changes in InsP3 were confined to the pulvinus and did not occur in internodal tissue. These results highlight the importance of the pulvinus as the graviresponsive organ. It is striking that the biphasic pattern of InsP3 changes prior to differential growth is conserved between the leaf sheath pulvinus of oats and the internodal pulvinus of maize and, importantly, also between whole maize plants and excised oat stem segments. Whereas the sustained increase in InsP3 in excised stem segments of oats is considerably faster than the long-term InsP3 generation reported previously in mature maize plants (Perera et al., 1999), the gravitropic bending is also correspondingly more rapid. Gravitropic bending of oat shoot segments was detected after 30 to 45 min of gravistimulation, and a sustained increase in InsP3 occurred in the lower half of the pulvinus during this period of time. Gravitropic bending of maize stems was in contrast first visible after 8 h of gravistimulation and the long-term increase in InsP3 manifested over a period of 3 to 7 h. These results are indicative of a mechanism for gravisignaling conserved among cereal grasses, which involves InsP3.
The PLC inhibitor, U73122, severely impaired the gravitropic response of oat stem segments. Application of U73122, importantly, also abolished the sustained increase in InsP3, which precedes differential growth. The dual effects of U73122 on both the growth response and the increase in InsP3 suggest a causative connection between the two, implying that PLC-mediated turnover of PtdInsP2, and the generation of InsP3 are involved in the cellular processes necessary for initiating gravitropic growth.
In contrast to the effect of U73122 on the long-term increase in InsP3, the rapid initial changes in InsP3 were not affected by the inhibitor. Differential inhibition of short-term and long-term InsP3 increases suggests that these increases are generated from spatially or functionally distinct pools of PtdInsP2. It is possible that the different pools of PtdInsP2 and the associated PLC enzymes may not be equally susceptible or accessible to the inhibitor. The replenishment of PtdInsP2 pools, alternatively, may be affected by the inhibitor. The presence of distinct pools of PtdInsP2 has been proposed previously in several plant systems (Heilmann et al., 1999; Kost et al., 1999).
As a note of caution, it has been reported that the aminosteroid U73122 can have effects on metabolism other than inhibition of PLC. These effects include the activation of Ca2+ influx or the release of Ca2+ from internal stores (De Moel et al., 1995; Mogami et al., 1997; Jan et al., 1998), which may potentially affect Ca2+ homeostasis and interfere with a signaling cascade involving InsP3 and Ca2+. We have observed that basal InsP3 levels of vertical oat shoot segments treated with the inhibitor were elevated compared with DMSO-treated segments. For this reason we have focused on the difference in InsP3 levels between the upper and lower halves to document effects of the inhibitor treatment on the production of InsP3 (Fig. 2B). Our results clearly indicate that the long-term increase in InsP3 was abolished in oat pulvini, consistent with an inhibition of PLC activity. It should be noted that, although the concentration of the inhibitor in the media effective at blocking the gravitropic response was 50 to 100 μm, the actual inhibitor concentration within the tissue will be considerably lower. When the inhibitor (100 μm) was washed out of the oat stems, gravitropic bending was restored to near control values. Furthermore, extension growth of the internode was not affected by the inhibitor treatment. These results indicate that at the concentrations used, the inhibitor was not toxic and did not cause irreversible damage to the plants.
The gravitropic bending responses of various plants are attenuated in the cold (Brauner and Hager, 1958; Fukaki et al., 1996). Plants gravistimulated in the cold are, however, capable of retaining the information of the spatial re-orientation, to respond when returned to room temperature. The fact that graviperception occurs in the cold suggests that some or all of the components of the gravisignaling cascade can operate at low temperature. In the present study, we show that changes in InsP3 were largely unaffected by cold treatment, except for a slight delay in the long-term increase in InsP3. We suggest that for the plant to retain the cold-perceived gravistimulation, a biochemical asymmetry is created and maintained between the upper and lower pulvinus halves, which involves a gradient of InsP3.
Based on our results we propose a three-phase model to describe the role of InsP3 changes in the gravity signal transduction cascade in cereal grass pulvini. Phase 1 involves early signaling events, including initial changes in pressure exerted by statoliths and rapid, transient changes in InsP3. The rapid changes in InsP3 could be part of an initiation signal, namely an “all purpose wake up call,” common to the perception of numerous stimuli and could be triggered by tension and compression of pulvinus tissue upon re-orientation. Rapid and transient increases in InsP3 in response to stimulation have been reported from many plant systems (for review, see Munnik et al., 1998). Although the recurring generation of transient InsP3 signals may be necessary for the perception of the gravistimulus in maize and oat shoot pulvini, it is not sufficient to induce a differential growth response. Instead the plant is committed to a growth response only after a minimum time of gravistimulation (presentation time) is exceeded. Repetitive signals, (e.g. InsP3 spikes) along with other second messengers such as Ca2+ and pH may be involved in setting the presentation time.
Once the presentation time is met, the plant is committed to differential growth (phase 2). In phase 2 the extent of the response can still be modulated, and the duration of the gravistimulation influences the extent of the bending response. The extent of the bending response is proportional to the magnitude of the long-term increase in InsP3. We have shown previously that the discontinuation of a gravistimulus subsequent to the presentation time results in the termination of the long-term InsP3 increase and in a reduced gravitropic bending response (Perera et al., 1999). The fact that the plants can sense the sudden cessation of the gravistimulus is suggestive of feedback regulation between a gravisensory element and the generation of the long-term increase in InsP3, which would allow for modulation of the response. In our previous work, the long-term increase in InsP3 was accompanied by an up-regulation of the PI pathway (Perera et al., 1999). Consistent with an involvement of PLC-mediated turnover of PtdInsP2, the PLC inhibitor, U73122, blocked the generation of the long-term increase in InsP3, leading to an attenuation of the gravitropic response.
Gravitropic bending (phase 3) results from differential elongation on the lower side of the pulvinus. According to the Cholodny-Went hypothesis, an auxin asymmetry is a prerequisite for differential growth. Auxin redistribution in the oat shoot is first detectable after 30 min of gravistimulation (Brock et al., 1991). Over a period of 1 to 3 h, a gradient of auxin builds up in the lower half of the pulvinus to a maximum ratio of 1.5:1 (lower:upper). The velocity of auxin transport is retarded by low temperature (Morris, 1979; S.E. Wyatt, A. Rashotte, G. Muday, and D. Robertson, unpublished data), which could interfere with auxin redistribution. Although the gravitropic response was attenuated in the cold, the InsP3 changes were not cold sensitive. We propose that the cold treatment may interrupt the sequence of events between the commitment to differential growth and the establishment of an auxin asymmetry.
Elongation growth of plant cells is mainly turgor driven and involves increased water and solute uptake. Consistent with increased demand for solute uptake, Philippar et al. (1999) showed that the K+-channel ZMK1 mRNA levels on the lower side of gravistimulated maize coleoptiles increase correlated with the redistribution of auxin. Invertase gene expression, similarly, increased up to 5-fold on the lower side of oat pulvini after 1 h of gravistimulation (Wu et al., 1993), providing support for osmotically driven differential cell elongation in phase 3.
In summary, the gravity signal transduction cascade linking graviperception and the onset of differential growth is a complex multistep process. The gravitropic signal may be propagated by gradients of signaling factors, such as pH, InsP3, and possibly Ca2+, and by gradients of hormones, such as auxin and ethylene (Philosoph-Hadas et al., 1996; Friedman et al., 1998). An integration of pathways may help to amplify and distribute the signal within the tissue and confer the specificity of the differential growth response. Our data indicate that biphasic changes in InsP3 play a major role in the gravitropic signaling cascade. The initial changes in InsP3 could be part of a general signal initiation, and the long-term increase in InsP3 on the lower side may contribute to the generation of the biochemical asymmetry between the upper and lower sides preceding differential growth. It has been shown that gradients of InsP3 may be involved in the specification of the dorso-ventral axis in vertebrate embryos (Ault et al., 1996; Berridge et al., 1998), and we suggest that InsP3 may play a comparable role in positional signaling in plants.
MATERIAL AND METHODS
Culture and Gravistimulation of Oat Plants
Oat (Avena sativa) stem segments containing the p-1 pulvinus were excised from whole plants and cultivated according to Chang and Kaufman (2000). During culture and experimental procedures, care was taken to minimize handling and movement of the stem segments. Gravistimulation was carried out by positioning the segments horizontally between two paper towels saturated with 0.1 m Suc, 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-NaOH, pH 7.5, according to Chang and Kaufman (2000). Two 5-mm glass plates were placed above and below the paper towels to maintain the stem segments in a horizontal position during gravistimulation and to prevent the segments from rotating during upward bending. The “sandwich” was kept in a Plexiglas chamber containing 1 cm of water to ensure uniform humidity. The chamber was placed inside an incubator (Dual Programmed Illuminated Incubator 818, Precision Scientific, Chicago) in continuous darkness at 25°C for the gravistimulation times indicated. Pulvini were excised from the stem segments with a single-edge razor blade (either as intact pulvini or “top” and “bottom” halves). The excision was carried out as quickly as possible, and the cut pulvini or pulvinus-halves were placed immediately on dry ice. For vertical controls, the sandwiches were kept in a vertical position in the Plexiglas chambers, then removed at the same times as gravistimulated segments for harvest of pulvini into “left” and “right” halves. All harvested tissue was stored at −80°C until analyzed.
Application of Pharmacological Compounds
Stem segments were kept in vertical position in 0.1 m Suc, 50 mm HEPES-NaOH, pH 7.5, for 24 h at 4°C. The site of the p-l pulvinus was gently abraded by rotating three to four times between thumb and forefinger in an aqueous paste of silicic acid to remove cuticular wax and to improve the uptake of compounds into the pulvinus tissue. The segments were thoroughly washed in distilled water to remove the silicic acid. The PLC-inhibitor U73122 (Calbiochem, San Diego) and the inactive analog U73343 (Calbiochem) were dissolved in 2 mL of DMSO by heating for 10 min at 37°C. The abraded stem segments were placed in 0.5% (v/v) DMSO, 100 mm Suc, 100 mm MES [2-(N-morpholino)ethanesulfonic acid], pH 5.5, containing varying concentrations of the active or inactive compound (1, 10, 50, or 100 μm). The stem segments were positioned vertically so that the basal 3-cm stem portions, p-l pulvini, and 2 cm of sheath tissue above the pulvini were covered by the respective inhibitor/analog and DMSO-containing solutions. Uptake was facilitated by application of a mild vacuum for 2 min, using a sink water tap aspirator. The segments were returned to atmospheric pressure and incubated in the dark at 25°C for 2 h. Following pretreatment with the inhibitor, analog, or DMSO, stem segments were gravistimulated and harvested as described above.
Quantification of InsP3 Content
For analysis of InsP3 content, 8 to 10 pulvinus or internodal samples were harvested for each time point and frozen at −80°C. The tissue was ground to a fine powder in liquid N2 and added to a preweighed tube containing 500 μL of ice-cold 20% (v/v) perchloric acid. After incubation on ice for 20 min, proteins were precipitated by centrifugation at 2,000g for 15 min at 4°C. The supernatant was transferred to a new tube and adjusted to pH 7.5, using ice-cold 1.5 m KOH in 60 mm HEPES containing 5% (v/v) of universal pH indicator dye (Fisher Scientific, Loughborough, Leicestershire, UK). The neutralized samples were assayed for InsP3, using a [3H]Ins(1, 4, 5) P3 receptor-binding assay kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). Assays were carried out along with controls for complete and non-specific binding according to the manufacturer's instructions by using 50 μL of sample per assay in a total assay volume of 200 μL. The InsP3 content of each sample was determined by interpolation from a standard curve generated with commercial InsP3. The presence of Ins(1, 4,5) P3 in the samples was verified by pretreatment with recombinant inositol polyphosphate 5-phosphatase I according to Perera et al. (1999). The phosphatase treatment eliminated >90% of the InsP3 from the oat samples.
Footnotes
This work was supported by the National Aeronautics and Space Administration Specialized Center of Research and Training (grant no. NAGW–4984 to W.F.B.), by the Binational Agricultural Research and Development Fund (grant no. IS2434–94 to P.B.K.), and by a Deutscher Akademischer Austauschdienst (fellowship HSPIII to I.H.) financed by the German Federal Ministry of Education, Science, Research, and Technology.
LITERATURE CITED
- Ault KT, Durmowicz G, Galione A, Harger PL, Busa WB. Modulation of Xenopus embryo mesoderm-specific gene expression and dorsoanterior patterning by receptors that activate the phosphatidylinositol cycle signal transduction pathway. Development. 1996;122:2033–2041. doi: 10.1242/dev.122.7.2033. [DOI] [PubMed] [Google Scholar]
- Behrens HM, Gradmann D, Sievers A. Membrane-potential responses following gravistimulation in roots of Lepidium sativum L. Planta. 1985;163:463–472. doi: 10.1007/BF00392703. [DOI] [PubMed] [Google Scholar]
- Belyavskaya NA. Calcium and graviperception in plants: inhibitor analysis. Int Rev Cytol. 1996;168:123–185. [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium: a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophiles. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Brauner L, Hager A. Versuche zur Analyze der geotropischen Perzeption. I. Planta. 1958;51:115–147. doi: 10.1007/BF00392282. [DOI] [PubMed] [Google Scholar]
- Brock TG, Kapen EH, Ghosheh NS, Kaufman PB. Dynamics of auxin movement in the gravistimulated leaf-sheath pulvinus of oat (Avena sativa) J Plant Physiol. 1991;138:57–62. doi: 10.1016/s0176-1617(11)80730-3. [DOI] [PubMed] [Google Scholar]
- Chang SC, Kaufman PB. Effects of staurosporine, okadaic acid and sodium fluoride on protein phosphorylation in graviresponding oat shoot pulvini. Plant Physiol Biochem. 2000;38:315–323. doi: 10.1016/s0981-9428(00)00745-2. [DOI] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanandan P, Kaufman PB. Analysis and significance of gravity-induced asymmetric growth in the grass leaf-sheath pulvinus. Ann Bot. 1984;53:29–44. doi: 10.1093/oxfordjournals.aob.a086668. [DOI] [PubMed] [Google Scholar]
- De Moel MP, Van De Put FHMM, Vermegen TMJA, De Pont JJHHM, Willems PHGM. Effect of the aminosteroid, U73122, on Ca2+ uptake and release properties of rat liver microsomes. Eur J Biochem. 1995;234:626–631. doi: 10.1111/j.1432-1033.1995.626_b.x. [DOI] [PubMed] [Google Scholar]
- Drøbak BK. The plant phosphoinositide system. Biochem J. 1992;288:697–712. doi: 10.1042/bj2880697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, Philosoph-Hadas S. Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol. 1998;118:483–492. doi: 10.1104/pp.118.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. Gravitropic response of inflorescence stems in Arabidopsis. Plant Physiol. 1996;110:933–943. doi: 10.1104/pp.110.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann I, Perera IY, Gross W, Boss WF. Changes in phosphoinositide metabolism with days in culture affect signal transduction pathways in Galdieria sulphur-aria. Plant Physiol. 1999;119:1331–1340. doi: 10.1104/pp.119.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C-R, Ho C-M, Wu S-N, Tseng C-J. The phospholipase C inhibitor U73122 increases cytosolic calcium in MDCK cells by activating calcium influx and releasing stored calcium. Life Sci. 1998;63:895–908. doi: 10.1016/s0024-3205(98)00346-4. [DOI] [PubMed] [Google Scholar]
- Kaufman PB, Brock TG, Song I, Rho YB, Ghosheh NS. How cereal grass shoots perceive and respond to gravity. Am J Bot. 1987;74:1446–1457. [PubMed] [Google Scholar]
- Kaufman PB, Wu L-L, Brock TG, Kim D. Hormones and the orientation of growth. In: Davies PJ, editor. Plant Hormones. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 547–571. [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologs and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Lu Y-T, Feldman LJ. Light regulated root gravitropism: a role for, and characterization of a calcium/calmodulin-dependant protein kinase homolog. Planta. 1997;203:S91–S97. doi: 10.1007/pl00008121. [DOI] [PubMed] [Google Scholar]
- Mogami H, Lloyd Mills C, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5) P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. The effect of temperature on the velocity of exogenous auxin transport in intact chilling-sensitive and chilling-resistant plants. Planta. 1979;146:603–605. doi: 10.1007/BF00388839. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signaling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph-Hadas S, Friedman H, Meir S, Berkovitz-Simantov R, Rosenberger I, Halevy AH, Kaufman PB, Balk P, Woltering EJ (2000) Gravitropism in cut flower stalks of snapdragon. Adv Space Res (in press) [DOI] [PubMed]
- Philosoph-Hadas S, Meir S, Rosenberger I, Halevy AH. Regulation of the gravitropic response and ethylene biosynthesis in gravistimulated snapdragon spikes by calcium chelators and ethylene inhibitors. Plant Physiol. 1996;110:301–310. doi: 10.1104/pp.110.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E, Chen R, Masson PH. Root gravitropism: a complex response to a simple stimulus? Trends Plant Sci. 1999;4:407–412. doi: 10.1016/s1360-1385(99)01472-7. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Sondag C, Trebacz K, Hejnowicz Z. Gravity induced changes in intracellular potentials in statocytes of cress roots. Planta. 1995;197:392–398. doi: 10.1007/BF00202662. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Oliver I, Maher P, Trewavas AJ. The role of calmodulin in the gravitropic response of Arabidopsis agr-3 mutant. Planta. 1996;199:343–351. doi: 10.1007/BF00195725. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ. Calcium in gravitropism: a re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- Staves MP. Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta. 1997;203:S79–S84. doi: 10.1007/pl00008119. [DOI] [PubMed] [Google Scholar]
- Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF. Inositol signaling and plant growth. Trends Plant Sci. 2000;5:252–258. doi: 10.1016/s1360-1385(00)01652-6. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells: a role for Gp in receptor compartmentation. J Biol Chem. 1991;266:23856–23862. [PubMed] [Google Scholar]
- Wu L-L, Song I, Kim D, Kaufman PB. Molecular basis of the increase in invertase activity elicited by gravistimulation of oat-shoot pulvini. J Plant Physiol. 1993;142:179–183. doi: 10.1016/s0176-1617(11)80960-0. [DOI] [PubMed] [Google Scholar]