Cellular senescence has emerged as a potent tumor suppressor mechanism in numerous human neoplasias. Senescent cells secrete a distinct set of factors, collectively termed the senescence-associated secretory phenotype (SASP), which has been postulated to carry both pro- and antitumorigenic properties depending on tissue context.
KEYWORDS: cancer, inflammation, interleukin-1, senescence
ABSTRACT
Cellular senescence has emerged as a potent tumor suppressor mechanism in numerous human neoplasias. Senescent cells secrete a distinct set of factors, collectively termed the senescence-associated secretory phenotype (SASP), which has been postulated to carry both pro- and antitumorigenic properties depending on tissue context. However, the in vivo effect of the SASP is poorly understood due to the difficulty of studying the SASP independently of other senescence-associated phenotypes. Here, we report that disruption of the interleukin-1 (IL-1) pathway completely uncouples the SASP from other senescence-associated phenotypes such as cell cycle exit. Transcriptome profiling of IL-1 receptor (IL-1R)-depleted senescent cells indicates that IL-1 controls the late arm of the senescence secretome, which consists of proinflammatory cytokines induced by NF-κB. Our data suggest that both IL-1α and IL-1β signal through IL-1R to upregulate the SASP in a cooperative manner. Finally, we show that IL-1α inactivation impairs tumor progression and immune cell infiltration without affecting cell cycle arrest in a mouse model of pancreatic cancer, highlighting the protumorigenic property of the IL-1-dependent SASP in this context. These findings provide novel insight into the therapeutic potential of targeting the IL-1 pathway in inflammatory cancers.
INTRODUCTION
Cellular senescence is a stable form of cell cycle arrest distinct from quiescence or terminal differentiation. First described by Hayflick et al., who reported that primary cells in culture cease division after a discrete number of population doublings, senescence in cultured human cells has since been attributed to telomere attrition and the associated DNA damage response (1–3). Senescence also occurs in response to harmful stresses, such as exposure to DNA-damaging agents and oncogene activation, preventing the proliferation of cells with altered genetic content (4, 5). In vivo, senescent cells accumulate in preneoplastic lesions, including early pancreatic intraepithelial neoplasias (PanINs), but are absent from malignant tumors (6–9). Moreover, inactivating the senescence response correlates with the acceleration of tumor progression, while its subsequent restoration causes tumor regression (10–12). Based on these observations, cellular senescence has long been hypothesized to act as a barrier to malignant transformation.
Additionally, senescent cells have been observed in aged tissues (13–15). Recent studies have demonstrated that ablation of senescent cells results in both prolonged health span and life span in mice (16). In conjunction with the observation that critically short telomeres trigger cellular senescence, these studies suggest that senescence contributes to aging (17, 18). Therefore, cellular senescence is considered beneficial (antitumorigenic) in some contexts and detrimental (proaging) in others. This is consistent with the theory of antagonistic pleiotropy, where traits that are selected for early in life become harmful as organisms age (19). Sterile inflammation has recently emerged as a facet of senescence that may contribute to the aging process. Indeed, senescent cells remain metabolically active and secrete inflammatory proteins that in part make up the senescence-associated secretory phenotype (SASP).
The SASP consists of cytokines, growth factors, and proteases that vary slightly depending on cell type and mode of senescence induction. Studies have suggested that the SASP reinforces senescence in an autocrine manner, induces neighboring cells to senesce, and modulates immune-mediated clearance of senescent and potentially cancerous cells (11, 20–24). As such, the SASP participates in the tumor-suppressive function of senescence. Conversely, it has been suggested that the SASP has protumorigenic effects in vivo. Some SASP factors are known to stimulate cell proliferation and motility and create an inflammatory environment that ultimately benefits tumor growth (25–27). Of note, previous studies that have aimed at deciphering the contribution of the SASP in tumorigenesis have relied on models where all aspects of senescence are abrogated (28, 29). Therefore, our understanding of the precise impact of senescence and, more specifically, the SASP on tumorigenesis remains fragmentary.
The cytokines interleukin-1α (IL-1α) and IL-1β are well-established components of the SASP (26). Both are synthesized as precursor proteins that are cleaved into an N-terminal propiece and a C-terminal mature fragment. The IL-1α propiece contains a nuclear localization signal (NLS) allowing its transport to the nucleus where it binds chromatin, but its function remains largely unknown (30, 31). Mature IL-1α, along with its full-length form, can be secreted into the extracellular milieu and bind and activate IL-1R, a receptor it shares with IL-1β (32, 33). In contrast, IL-1β requires cleavage into its mature form to become active, as the full-length protein cannot bind the receptor (34). Binding of IL-1R by either IL-1α or mature IL-1β initiates a signaling cascade that ultimately leads to the nuclear translocation of the transcription factor NF-κB and subsequent transcriptional activation of numerous inflammatory genes, including the SASP factors IL-6 and IL-8 (35). IL-6 and IL-8 are secreted at very high levels during senescence in most cell types and make up a major portion of the SASP (20, 26, 36). NF-κB also controls the transcription of both IL-1α and IL-1β, leading to SASP amplification via a feed-forward loop (37).
Previous studies have implicated IL-1α as an upstream regulator of the SASP (32). However, its impact on other senescence-associated phenotypes has not been fully explored. Furthermore, whether IL-1β, which shares the same receptor, can compensate for IL-1α in the generation of the SASP remains controversial (21, 32). Here, we show that disruption of the IL-1 signaling pathway leads to uncoupling of the SASP from senescence-associated cell cycle exit, thereby unveiling a unique approach to study the specific function of the SASP independently of other senescence features. We demonstrate here that the IL-1 pathway controls a vast majority of the SASP. We also show that inhibition of either IL-1α or IL-1β affects SASP production, but increased levels of either IL-1α or mature IL-1β can induce SASP expression. Our results also indicate that specific abrogation of the SASP in oncogene-induced senescence results in delayed cancer progression, unequivocally unveiling a protumorigenic function of the SASP. Altogether, these findings elucidate the mechanisms engaged by the IL-1 pathway in SASP activation and highlight the IL-1 pathway as a potential therapeutic target to blunt cancer progression.
RESULTS
The IL-1 pathway is required for SASP expression but not for senescence-associated cell cycle exit.
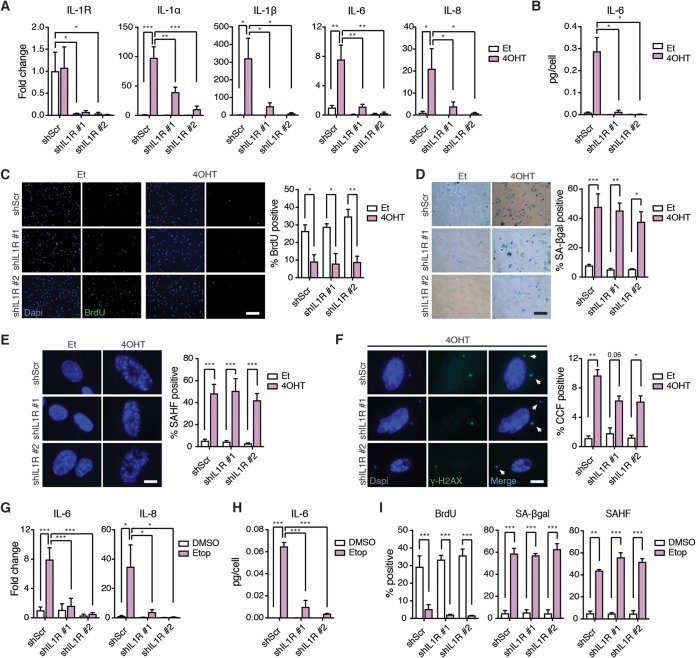
Previous studies suggested that the IL-1 pathway acts as an upstream regulator of the SASP, but whether this signaling pathway is necessary for senescence-associated cell cycle exit has not been fully explored. To address this question, we introduced a tamoxifen (4OHT)-inducible oncogenic H-RasG12V transgene (here referred to as the Ras gene) into telomerase reverse transcriptase (hTERT)-expressing human IMR90 lung embryonic fibroblasts (IMR90T). We also introduced two independent doxycycline-inducible short hairpin RNAs (shRNAs) into these cells, which efficiently decreased IL-1R expression, as evidenced by quantitative PCR (qPCR) analysis (Fig. 1A). After 10 days of Ras activation, we observed a strong induction of the SASP factors IL-1α, IL-1β, IL-6, and IL-8 in control (shScr) cells but not in IL-1R knockdown cells, consistent with previous studies (21) (Fig. 1A). Enzyme-linked immunosorbent assays (ELISAs) confirmed the dampened Ras-induced secretion of IL-6 in IL-1R knockdown cells compared to levels in control cells (Fig. 1B). A similar IL-1R-dependent expression of SASP factors was observed when IMR90T cells were induced to senesce by exposure to etoposide (Etop), a DNA damage-inducing agent (Fig. 1G and H). These results confirm the requirement for IL-1 signaling in SASP induction in different senescence settings.
FIG 1.
IL-1R is required for SASP activation but not for other senescence-associated phenotypes. (A) mRNA expression levels of the indicated genes in IMR90T expressing either a doxycycline-inducible scramble shRNA (shScr) or 1 of 2 independent doxycycline-inducible shRNAs against IL-1R (shIL1R) as detected by quantitative real-time PCR (qRT-PCR). Cells were treated with either ethanol (Et) as a control or tamoxifen (4OHT) to induce Ras activity. Cells were also treated with doxycycline to induce knockdown. Values are shown as fold change compared to levels for shScr treated with Et. (B) Secreted levels of IL-6 in the indicated cell lines as detected by ELISA. (C to F) Representative images and quantitation of BrdU incorporation (C), SA-βgal positivity (D), SAHF positivity (E), and CCF formation (F) in the indicated cell lines. Scale bars for panels C and D, 200 µm; scale bars for panels E and F, 12 µm. (G) mRNA expression levels of the indicated genes in the indicated cell lines. Cells were treated with either DMSO as a control or etoposide (Etop) to induce senescence. (H) Secreted levels of IL-6 in the indicated cell lines as detected by ELISA. (I) Quantitation of BrdU incorporation, SA-βgal positivity, and SAHF positivity in the indicated cell lines. Each experiment was performed at least 3 times. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether the IL-1 signaling pathway also regulates cell cycle exit, we analyzed various hallmarks of senescence. We observed similar decreases in bromodeoxyuridine (BrdU) incorporation in Ras-activated control and IL-1R knockdown cells compared to that of ethanol (Et)-treated cells, indicative of decreased proliferation under both conditions (Fig. 1C). BrdU incorporation was also decreased in both Etop-treated control and IL-1R knockdown cells compared to that of their dimethyl sulfoxide (DMSO)-treated controls (Fig. 1I). In addition, we detected increased senescence-associated beta-galactosidase (SA-βgal) positivity in Ras-activated and Etop-treated cells compared to levels in their respective controls, regardless of IL-1R status (Fig. 1D and I). Senescence-associated heterochromatin foci (SAHF) were observed in both Ras-induced control and IL-1R knockdown cells, as well as in all Etop-treated samples (Fig. 1E and I). Finally, cytoplasmic chromatin fragments (CCFs), many of which were γ-H2AX positive, were present in all Ras-activated samples (Fig. 1F). Taken together, these results indicate that IL-1R and the IL-1-dependent SASP are not required for senescence-associated cell cycle exit or for the generation of SAHF or CCFs. Therefore, IL-1R inactivation can efficiently uncouple the SASP from other senescence-associated phenotypes.
The IL-1 pathway controls at least a majority of the SASP.
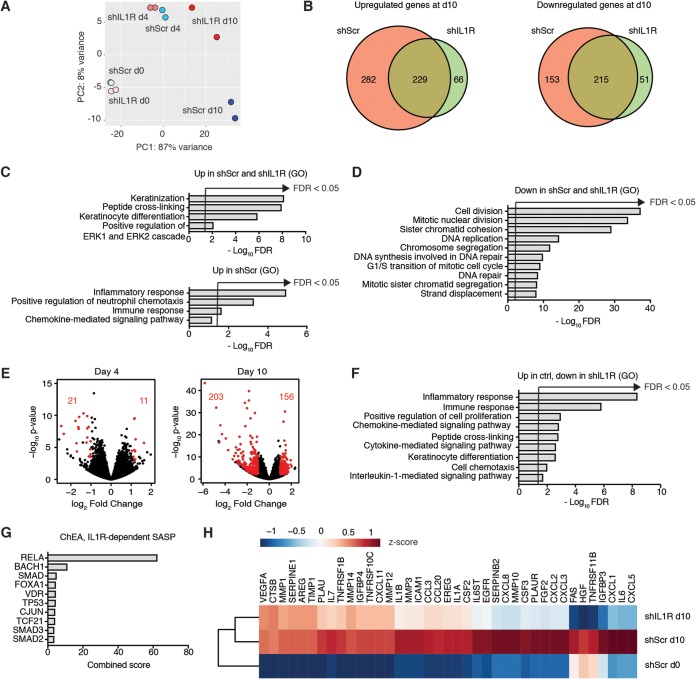
The SASP consists of a wide variety of different factors, including cytokines, growth factors, and proteases. To document the full extent of the IL-1-dependent SASP, we compared the senescence-associated transcriptome of IMR90T cells expressing shIL1R to that of control shScr cells by RNA sequencing. Cells were harvested on day 0 (d0), day 4 (d4), and day 10 (d10) of Ras induction. d0 corresponded to growing cells, d4 corresponded to the beginning of SASP induction based on a time course assay of IL-6 transcription (data not shown), and d10 corresponded to full senescence induction. As expected, the transcriptomes of shScr and shIL1R growing cells clustered close together, as evidenced by principal-component analysis (PCA) (Fig. 2A). Of note, while shScr and shIL1R transcriptomes clustered relatively close together at d4, they diverged at d10 of Ras activation, indicating that IL-1-dependent genes are expressed as part of the late arm of the senescence secretome (38). Using a log2 fold change cutoff of 3 and an adjusted P value of <0.05, we identified highly upregulated and downregulated genes in shScr and shIL1R samples at d10 compared to levels at d0 of Ras activation (Fig. 2B; see also Tables S1 to S6 in the supplemental material). Gene ontology (GO) analyses revealed that inflammatory pathways were upregulated in shScr samples at d10 versus d0, while they were not affected in shIL1R samples (Fig. 2C). Consistent with our previous results indicating that inhibiting IL-1 signaling does not impair senescence-associated cell cycle exit, mitosis and DNA replication represented commonly deregulated pathways in both shScr and shIL1R samples (Fig. 2D).
FIG 2.
IL-1 pathway controls a majority of the SASP. (A) PCA of RNA sequencing data in IMR90T cells expressing scramble shRNA or 1 of 2 shRNAs against IL-1R. RNA was harvested on days 0, 4, and 10 of Ras activation induced by addition of 4OHT. (B) Venn diagrams indicating the number of upregulated or downregulated genes in shScr and shIL1R samples at day 10 (d10) of Ras activation compared to day 0 (d0) using a log2 fold change cutoff of 3 and an adjusted P value of <0.05. (C) Gene ontology (GO) analysis of genes that are upregulated in both shScr and shIL1R d10 samples compared to d0 samples and upregulated only in shScr samples. FDR, false discovery rate. (D) GO analysis of genes that are downregulated in both shScr and shIL1R d10 samples compared to levels in d0 samples. (E) Volcano plots depicting differentially expressed genes in shIL1R samples compared to those in shScr samples at the indicated time points. Red dots represent genes where the log2 fold change was >1 and the adjusted P value was <0.05. The number of genes that pass this cutoff is indicated in red. (F and G) GO analysis (F) and ChEA (G) of genes that are downregulated in shIL1R samples at d10 compared to levels in shScr samples at d10. (H) Heatmap depicting the expression levels of the indicated genes in the indicated samples. n = 2.
To further explore the transcriptomic differences between shScr and shIL1R samples, we compared differentially expressed genes using a log2 fold change cutoff of 1 and an adjusted P value of <0.05. Only 32 genes were found to be differentially expressed between shScr and shIL1R samples at d4, while 359 genes were differentially expressed between shScr and shIL1R at d10 (Fig. 2E and Tables S7 and S8). Of the 359 differentially expressed genes, 203 genes were downregulated upon IL-1R knockdown. Downregulated pathways consisted of inflammatory and immune responses, consistent with the contribution of the IL-1 signaling pathway in SASP production (Fig. 2F). Chromatin immunoprecipitation enrichment analysis (ChEA) indicated that the vast majority of the corresponding downregulated loci could be bound by RelA, the DNA-binding subunit of NF-κB (Fig. 2G). Finally, virtually all genes previously reported to be SASP factors (27) and upregulated in shScr d10 versus d0 samples were downregulated in shIL1R d10 samples, albeit at varied levels (Fig. 2H). Taken together, these results strongly support the notion that the IL-1 pathway controls the vast majority of the SASP without affecting cell cycle exit.
IL-1α signals through IL-1R to activate the SASP.
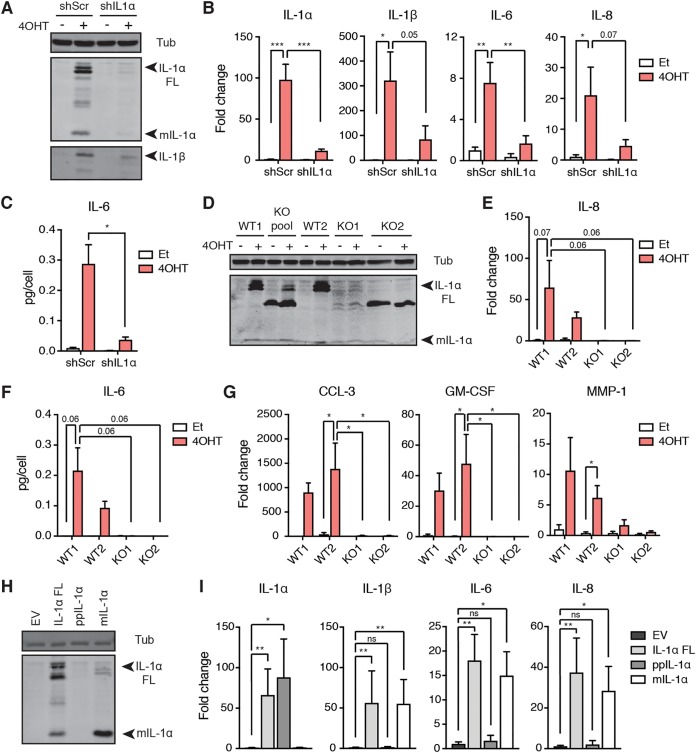
To determine the mechanism of SASP activation via the IL-1 pathway, we tested the contribution of both IL-1R ligands, IL-1α and IL-1β, to SASP expression in senescence. We first introduced a doxycycline-inducible shRNA against IL-1α into IMR90T cells and induced them to senesce via Ras activation. Western blotting and qPCR analyses validated IL-1α knockdown and indicated that IL-1α knockdown reduced IL-1β protein levels and IL-1β, IL-6, and IL-8 mRNA levels upon senescence induction compared to levels for a scramble control (Fig. 3A and B). The reduction in IL-6 transcript levels was corroborated at the protein level, as shown by ELISA (Fig. 3C). These results were further validated in IL-1α CRISPR-deleted clones, where clones were selected based on the absence of both full-length and mature IL-1α protein as detected by Western blotting (Fig. 3D). Ras-induced senescence failed to induce IL-8 expression and IL-6 secretion in these knockout cells as measured by qPCR and ELISA, respectively (Fig. 3E and F). Moreover, we validated that additional IL-1R-dependent SASP factors found in our transcriptome analysis, including CCL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and MMP-1, were also downregulated in senescent IL-1α-deleted cells compared to levels in wild-type (WT) cells (Fig. 3G).
FIG 3.
IL-1α signals through IL-1R to induce the SASP. (A) Western blot analysis of IL-1α, IL-1β, and tubulin (Tub) levels in IMR90T cells expressing either a doxycycline-inducible scramble shRNA (shScr) or shRNA against IL-1α (shIL1α). Cells were treated with either ethanol (Et) as a control or tamoxifen (4OHT) to induce Ras activity and doxycycline to induce knockdown. (B) qRT-PCR analyses of the indicated genes in the indicated cells. Values are shown as fold change compared to levels in shScr treated with Et. (C) Secreted levels of IL-6 in the indicated cell lines as detected by ELISA. (D) Western blot analysis detecting IL-1α and Tub levels in wild-type (WT) and IL-1α-deleted (KO) IMR90T cells. WT1, parental cell line; WT2, a clone that failed to delete IL-1α; KO pool, total pool of clones, where KO1 and KO2 each represent an independent clone. (E) qRT-PCR detecting IL-8 mRNA levels in the indicated cells. (F) Secreted levels of IL-6 in the indicated cell lines as detected by ELISA. (G) qRT-PCR analyses of the indicated SASP factors found via transcriptome analysis to be affected by IL-1R knockdown. Data are represented as fold change compared to levels for WT1 treated with Et. (H) Western blot analysis of IL-1α in IMR90T cells expressing an empty vector control (EV), full-length IL-1α (IL-1α FL), the N-terminal propiece (ppIL-1α), or the C-terminal mature fragment (mIL-1α). The antibody used only recognizes the C-terminal portion of IL-1α. (I) qRT-PCR analyses of the indicated genes in the indicated cell lines. Primers for IL-1α anneal to the N terminus. Values are shown as fold change compared to levels in cells expressing EV. Each experiment was performed at least 3 times. ns, not significant; *, P < 0.05; **, P < 0.01.
While both full-length and C-terminal mature IL-1α (mIL-1α) have been reported to activate IL-1R signaling, much less is known about the activity of the N-terminal propiece (ppIL-1α). ppIL-1α contains a functional NLS, but its nuclear function is elusive. Based on reported evidence that ppIL-1α may contribute to SASP activation (39), we tested the activity of full-length IL-1α, ppIL-1α, and mIL-1α upon ectopic expression in IMR90T cells (Fig. 3H and I). Expression of full-length or mature IL-1α was sufficient to induce expression of the SASP factors IL-1β, IL-6, and IL-8 (Fig. 3I). However, ectopic expression of the propiece alone failed to induce SASP factor upregulation (Fig. 3I). From these results, we concluded that nuclear ppIL-1α activity likely does not contribute to SASP activation.
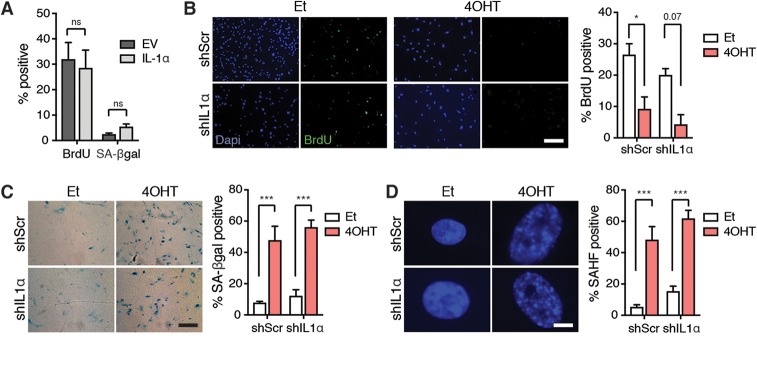
We next tested whether IL-1α levels influence other senescence-associated phenotypes. We observed that ectopic expression of IL-1α was not sufficient to induce senescence, as cells continued to incorporate BrdU and remain SA-βgal negative (Fig. 4A), while IL-1α knockdown did not affect the decreased BrdU incorporation or increased SA-βgal positivity induced by Ras (Fig. 4B and C). Similarly, SAHF were also detected in all Ras-activated cells, regardless of IL-1α status (Fig. 4D). These results further support the conclusion that the IL-1-dependent SASP is not required for cell cycle exit, and disruption of the IL-1α/IL-1R signaling pathway uncouples the SASP from senescence.
FIG 4.
IL-1α can be used to uncouple the SASP from cell cycle exit. (A) Quantitation of BrdU incorporation and SA-βgal positivity in IMR90T cells after introduction of an empty vector (EV) or a vector containing full-length IL-1α. (B to D) Representative images and quantitation of BrdU incorporation (B), SA-βgal positivity (C), and SAHF positivity (D) in the indicated cell lines. Scale bars for panels B and C, 200 µm; scale bar for panel D, 12 µm. Each experiment was performed at least 3 times. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-1β also affects the SASP without disrupting senescence-induced cell cycle exit.
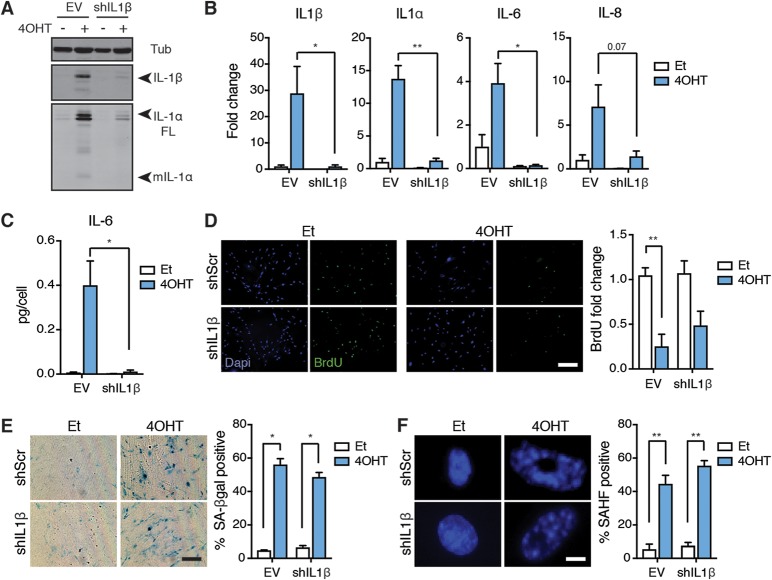
We next determined whether IL-1β, which shares the same signaling pathway as IL-1α, contributes to SASP induction. After introducing an shRNA targeting IL-1β into IMR90T cells and inducing senescence via Ras activation, we performed Western blotting and qPCR analyses to confirm IL-1β knockdown (Fig. 5A and B). Surprisingly, IL-1β depletion also dampened the Ras-induced upregulation of IL-1α, IL-6, and IL-8 transcripts and IL-1α protein levels (Fig. 5A and B). The decreased expression of IL-6 was validated at the protein level by ELISA (Fig. 5C). As with IL-1α and IL-1R, IL-1β knockdown did not affect other senescence-associated phenotypes, as evidenced by decreased BrdU incorporation, increased SA-βgal positivity, and increased SAHF emergence in Ras-activated cells, all comparable to those of control senescent cells (Fig. 5D to F). From these results, we conclude that IL-1β is also required for SASP expression and can be used to uncouple the SASP from cell cycle exit.
FIG 5.
IL-1β is also required for SASP expression but not cell cycle exit. (A) Western blot analysis of IL-1β, IL-1α, and tubulin (Tub) levels in IMR90T cells expressing either an empty vector (EV) or shRNA against IL-1β (shIL1β). Cells were treated with either ethanol (Et) as a control or tamoxifen (4OHT) to induce Ras activity. (B) mRNA expression levels of the indicated genes in the indicated cells as detected by qRT-PCR. Values are shown as fold change compared to the level for EV treated with Et. (C) Secreted levels of IL-6 in the indicated cell lines as detected by ELISA. (D to F) Representative images and quantitation of BrdU incorporation (D), SA-βgal positivity (E), and SAHF positivity (F) in the indicated cell lines. Scale bars for panels D and E, 200 µm; scale bar for panel F, 12 µm. Each experiment was performed at least 3 times. *, P < 0.05; **, P < 0.01.
Both IL-1α and mIL-1β can induce the SASP in the absence of the other.
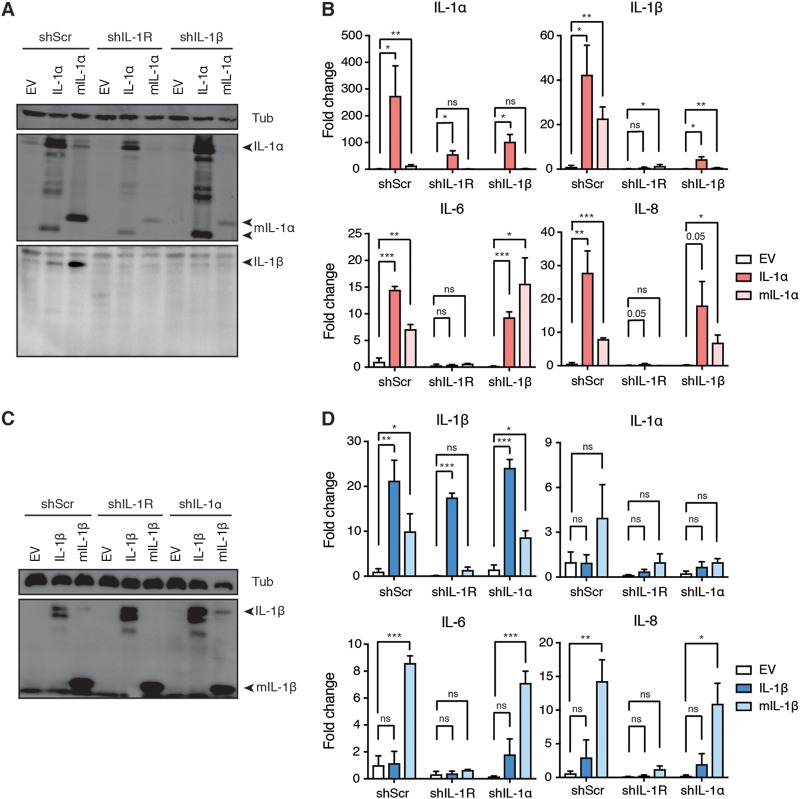
We initially hypothesized that the requirement of both IL-1α and IL-1β for SASP expression reflected a hierarchical relationship between the two cytokines. As shown in Fig. 3I, overexpression of both full-length and mature IL-1α in IMR90T cells was sufficient to upregulate the SASP factors IL-1β, IL-6, and IL-8 (Fig. 6A and B). We also observed by Western blotting that mIL-1α overexpression induced endogenous full-length IL-1α in shScr control cells, consistent with IL-1α participating in a feed-forward loop (Fig. 6A). However, overexpression of IL-1α did not induce the upregulation of IL-1β, IL-6, or IL-8 upon IL-1R knockdown, further confirming that IL-1R lies downstream of IL-1α (Fig. 6A and B). Notably, overexpression of both full-length and mature IL-1α in IL-1β knockdown cells still induced IL-6 and IL-8 expression (Fig. 6B). Therefore, we concluded that IL-1β is not required for IL-1α to induce the SASP.
FIG 6.
IL-1α can induce the SASP in the absence of IL-1β and vice versa. (A) Western blot analysis of IL-1α, IL-1β, and tubulin (Tub) levels in IMR90T cells expressing either an empty vector (EV), full-length IL-1α, or myc-tagged mature IL-1α (mIL-1α). IMR90T cells were also expressing either a scramble shRNA (shScr), a doxycycline-inducible shRNA against IL-1R (shIL-1R), or a constitutively active shRNA against IL-1β (shIL-1β). (B) mRNA expression levels of the indicated genes in the indicated cell lines as detected by qRT-PCR. Values are expressed as fold change compared to levels for shScr cells expressing EV. (C) Western blot analysis of IL-1β and Tub levels in IMR90T cells expressing either EV, full-length IL-1β, or mature IL-1β (mIL-1β). Cells were also expressing either a doxycycline-inducible scramble shRNA (shScr) or a doxycycline-inducible shRNA against IL-1R (shIL-1R) or IL-1α (shIL-1α). (D) mRNA expression levels of the indicated genes in the indicated cell lines as detected by qRT-PCR. Values are expressed as fold change compared to levels in shScr cells expressing EV. Each experiment was repeated at least 3 times. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next introduced full-length and mature IL-1β (mIL-1β) into IMR90T cells (Fig. 6C). Interestingly, we observed that although overexpression of mIL-1β resulted in the upregulation of IL-1α, IL-6, and IL-8 in shScr control cells, overexpression of the full-length form did not (Fig. 6D). This is consistent with previous studies demonstrating that IL-1β requires a cleavage event before it can activate IL-1R (34). Indeed, ectopic expression of full-length IL-1β does not result in the spontaneous generation of its mature form, unlike IL-1α (Fig. 6A and C). Similar to IL-1α, overexpression of IL-1β in IL-1R knockdown cells was not sufficient to induce IL-1α, IL-6, or IL-8 upregulation, confirming that IL-1R acts downstream of both IL-1α and IL-1β (Fig. 6D). Surprisingly, overexpression of mIL-1β is sufficient to induce the upregulation of IL-6, IL-8, and IL-1β even in IL-1α knockdown cells (Fig. 6C and D). From these results, we concluded that, once cleaved, IL-1β does not require IL-1α to induce the SASP.
Taken together, these results argue against the hypothesis that a hierarchical relationship between IL-1α and IL-1β dictates SASP production. Rather, our observations point to a model where a specific amount of either IL-1α or mIL-1β is sufficient to signal through IL-1R and activate the SASP. Thus, reducing the levels of either IL-1α or IL-1β would impact SASP activation.
The IL-1-dependent SASP drives pancreatic cancer progression.
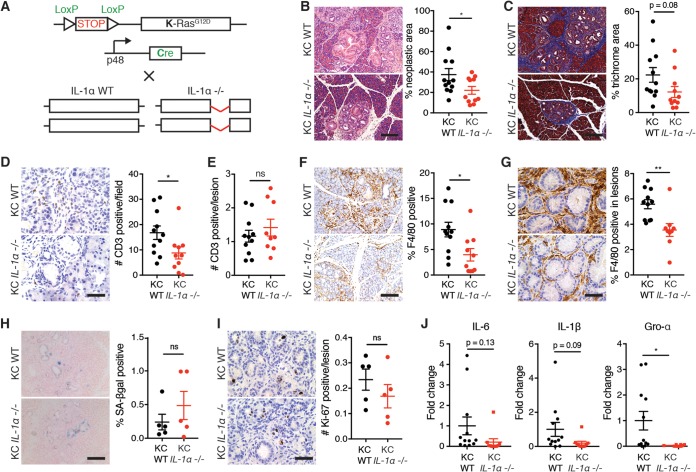
Using a mouse model of pancreatic cancer, we previously demonstrated that inactivation of the senescence pathway through genetic deletion of the chromatin modifier Sin3B correlates with impaired SASP production and delayed tumor progression (28). Thus, we investigated whether ablation of the SASP through the genetic inactivation of IL-1α could recapitulate the delayed cancer progression in this mouse model of pancreatic cancer. Mice harboring a Lox-STOP-Lox-K-RasG12D allele were crossed with mice expressing Cre under the control of the pancreas-specific p48 promoter (here referred to as KC) (Fig. 7A). These mice develop PanINs, a precursor to malignancy, as early as 2 weeks of age, and senescent cells can be detected within low-grade PanIN lesions (40, 41). KC mice were bred in an IL-1α-deleted background and sacrificed at 12 weeks of age (Fig. 7A). Upon examination of pancreata from KC WT mice, we confirmed the presence of PanIN lesions among normal acini by H&E staining (Fig. 7B). Strikingly, KC IL-1α-deleted pancreata displayed a significantly lower percentage of neoplastic lesions (Fig. 7B). We also observed decreased desmoplastic tissue, a hallmark of pancreatic lesions, in KC IL-1α-deleted pancreata compared to that in KC WT tissue, as measured by Masson trichrome staining (Fig. 7C).
FIG 7.
IL-1α deletion delays pancreatic cancer progression. (A) Schematic of crosses in the mouse model used in this study. Mice containing a Lox-Stop-Lox K-RasG12D allele were crossed with mice containing a p48-Cre allele to create KC WT mice. KC mice were crossed to mice that have both IL-1α alleles disrupted to create KC IL-1α−/− mice. (B) Representative H&E images of pancreata and quantitation of neoplastic area from 12-week-old KC WT and KC IL-1α−/− mice. Scale bar, 200 µm. (C) Representative images and quantitation of Masson trichrome staining. Scale bar, 200 µm. (D) Representative images and quantitation of CD3 staining in total pancreas tissue in the indicated samples. Scale bar, 50 µm. (E) Quantitation of CD3 staining within lesions in the indicated samples. (F and G) Representative images and quantitation of F4/80 staining in total pancreas tissue (F) and within lesions in the indicated samples (G). Scale bars, 200 µm in panel F and 50 µm in panel G. (H) SA-βgal positivity and quantitation in the indicated samples. Scale bar, 200 µm. (I) Ki-67 staining and quantitation in the indicated samples. Scale bar, 50 µm. (J) mRNA expression levels of the indicated genes in the indicated samples. n was at least 10 in panels B to G and J, and n was 5 in panels H and I. ns, not significant; *, P < 0.05.
We next assessed whether immune cell infiltration was affected in KC pancreata by the absence of IL-1α. Correlating with the decrease in PanIN lesions, we observed a reduction in the number of infiltrating T cells and macrophages, as evidenced by decreased CD3 and F4/80 staining, respectively, in KC IL-1α-deleted pancreata compared to that of their KC WT counterparts (Fig. 7D and F). However, when we compared the numbers of immune cells specifically within or surrounding low-grade lesions, we did not detect any difference in CD3 staining, suggesting that the reduced amount of T cells in KC IL-1α−/− pancreas reflected the decreased number of lesions and not reduced T cell recruitment (Fig. 7E). Strikingly, similar analyses revealed decreased amounts of macrophages per lesion in KC IL-1α−/− pancreas, suggesting that IL-1α inactivation leads to reduced macrophage recruitment in senescent precancerous lesions (Fig. 7G).
Furthermore, we assessed whether IL-1α affected cell cycle exit and the SASP in an in vivo setting. We detected a slight but nonsignificant increase in the percentage of SA-βgal-positive senescent cells in PanINs of IL-1α-deleted mice, indicating that IL-1α deletion does not affect all senescence-associated phenotypes, consistent with our in vitro results (Fig. 7H). This increase correlated with a trending decrease in the number of proliferating cells in low-grade lesions of KC IL-1α-deleted mice compared to that of KC WT mice as evidenced by Ki-67 staining, although it failed to reach statistical significance (Fig. 7I). These results suggest that decreased proliferation in IL-1α-deleted pancreatic lesions contributes to the delayed cancer progression we observed (Fig. 7B). Lastly, we detected a decrease of the SASP factors IL-6, IL-1β, and Gro-α in KC IL-1α-deleted whole pancreas extracts compared to levels in KC WT pancreas, suggesting that SASP production requires IL-1α in this in vivo model, consistent with our in vitro data (Fig. 7J).
Together, these observations indicate that the presence of IL-1α correlates with the development of PanIN lesions and immune cell infiltration and suggest that IL-1α and the associated SASP serve as a therapeutic target to delay pancreatic cancer progression.
DISCUSSION
We report here that disruption of the IL-1 signaling pathway efficiently uncouples SASP production from cell cycle exit in senescent cells. More specifically, we demonstrate that while engagement of the IL-1 pathway by IL-1α is essential for the upregulation of the SASP, it is dispensable for all other senescence-associated phenotypes investigated here, including cell cycle exit and nuclear reorganization. Additionally, ectopic expression of IL-1α is insufficient to promote cell cycle exit despite the upregulation of inflammatory SASP factors. Surprisingly, ablation of IL-1β also downregulates the SASP without affecting cell cycle exit. We report that IL-1α does not require the presence of IL-1β to induce the SASP, and similarly, cleaved IL-1β also does not require IL-1α to induce the same SASP factors. Finally, we demonstrate that genetic inactivation of IL-1α alters pancreatic cancer progression and immune cell infiltration in a well-characterized mouse model. Altogether, our study suggests that targeting the IL-1 signaling pathway to uncouple the SASP from cell cycle exit in senescent lesions proves beneficial to prevent tumor progression in inflammatory cancers.
Senescence is a process by which cells that have been exposed to DNA-damaging insults stably exit the cell cycle, thereby preventing the proliferation of potentially tumorigenic cells. In stark contrast to the antitumorigenic nature of senescence-associated cell cycle exit, the presence of SASP-producing senescent cells in preneoplastic lesions may contribute to cancer progression. Indeed, the emergence of preneoplastic lesions often correlates with the generation of a proinflammatory environment, which can be detrimental to tissue homeostasis and stimulate cancer progression (42). Abrogating the SASP in preneoplastic lesions without affecting cells’ ability to withdraw from the cell cycle may serve as a promising therapeutic approach to dampen tumor progression in cancers that are fueled by inflammation.
However, the means to uncouple the SASP from cell cycle exit remained elusive. Previous studies had suggested that at least some components of the SASP (including IL-6, IL-8, IL-1R, and the cGAS-STING cytosolic DNA sensing pathway) reinforced senescence-associated cell cycle exit (20, 21, 43, 44). Based on such observations, it was anticipated that uncoupling cell cycle exit from SASP production could not be achieved in senescent cells. However, recent reports argue that the abrogation of the SASP via the disruption of wide-ranging molecular pathways (for example, mTOR signaling, the transcriptional coactivator MLL1, and the cGAS-STING pathway) does not prevent senescence-induced cell cycle exit (45–48). In addition, overexpression of the CDK inhibitor p16INK4A or 21CIP1 induced senescence without inducing the SASP, indicating that the SASP is dispensable for senescence-associated cell cycle exit, at least in some contexts (49). The reasons for the discrepancy between these studies remain unclear but are likely to lie in the experimental settings used to induce senescence, the cellular context, and tools used to document senescence-associated phenotypes. The results presented here, however, unequivocally indicate that inactivation of the IL-1 pathway specifically and efficiently abrogates the SASP while allowing senescent cells to exit the cell cycle. Moreover, we observed that overexpression of IL-1α does not induce cell cycle exit despite the strong upregulation of SASP factors. Therefore, our results firmly demonstrate that the SASP and senescence-associated cell cycle withdrawal can be uncoupled. In doing so, we observed delayed pancreatic cancer progression, correlating with SASP-mediated immune cell recruitment. The impact of immune cell recruitment on the progression of pancreatic preneoplastic and neoplastic lesions has been well established (50–52). Our observations point to a potential molecular basis for the modulation of this recruitment during early stages of cancer progression, when senescent cells are present.
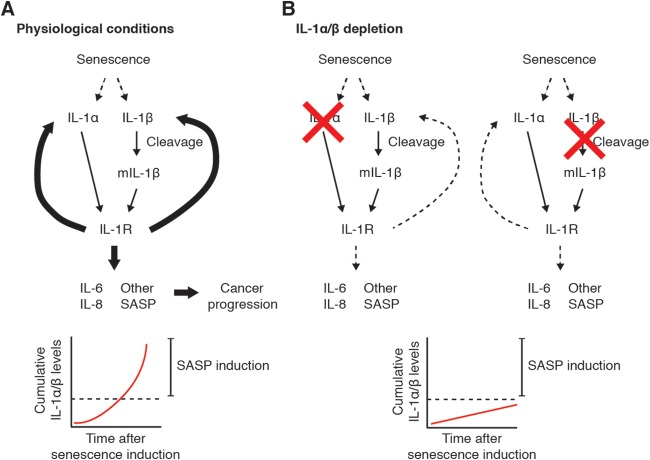
Previous observations argue against a role of IL-1β in the induction of the SASP (32). We report here that IL-1β does in fact contribute to SASP expression, similar to IL-1α. Inhibition of either cytokine results in SASP abrogation. Based on our observations, we hypothesize that there exists a threshold at which a specific cumulative amount of IL-1α and mature IL-1β must be present in order to effectively encounter, bind, and activate IL-1R to induce the SASP (Fig. 8A). Inactivation of either cytokine brings the amount of total existing IL-1α and IL-1β down below the threshold needed to activate IL-1 signaling and amplify the SASP (Fig. 8B). This is supported by our overexpression experiments, where addition of nonphysiological amounts of IL-1α is sufficient to induce the SASP even in the absence of IL-1β and vice versa. Previous experiments may have been performed under conditions in which the amount of IL-1α induced in senescent cells was sufficient to amplify the SASP even without the presence of IL-1β. This discrepancy may be due to different cell types or modes of senescence induction.
FIG 8.
Model of senescence-induced SASP activation under physiological conditions (A) or conditions where either IL-1α or IL-1β is depleted (B).
We and others have demonstrated that senescent cells are found in preneoplastic lesions in the pancreas. We argue that the SASP produced by these cells supports cancer progression by promoting an inflamed environment. Importantly, we observed a significant reduction in the recruitment of macrophages, but not T cells, to preneoplastic lesions upon IL-1α inactivation, suggesting that the SASP contributes to the recruitment of specific subsets of immune cells to senescent lesions in vivo. In light of previous studies suggesting that tissue-resident macrophages promote pancreatic adenocarcinoma (PDAC) progression (53), it is tempting to speculate that at least one of the protumorigenic properties of the SASP in pancreatic cancer results from its ability to recruit macrophages. Further studies can be completed to classify whether the recruited macrophages in IL-1α-deleted KC pancreas are more anti-inflammatory in nature.
Other studies observed that chronic inflammation caused by constitutively active NF-κB signaling promotes PDAC development in the same mouse model as the one used in our study (54). Interestingly, NF-κB activation in pancreatic cancer cells correlates with increased aggressiveness and worse prognosis independent of immune cell activity. Inhibition of the IL-1 pathway dampens NF-κB signaling, rendering PDAC cells less invasive and more susceptible to chemotherapy (55). Therefore, in addition to dampening the tumor-promoting inflammatory environment in the pancreas, inhibition of the IL-1 pathway could also render PDAC less aggressive and more responsive to treatment in a cell-autonomous manner.
Aside from senescent cells within PanINs, stromal cells in the tumor microenvironment may contribute a protumorigenic SASP as well. We utilized a mouse model where IL-1α was deleted in the whole animal, and the SASP produced by both senescent preneoplastic and stromal cells is likely equally impaired. If senescent cells develop in the absence of preexisting tumors, for example, in aged individuals, can the accompanying SASP contribute to de novo emergence? More studies detailing the senescent status and SASP contribution of each cell type within the microenvironment need to be completed to address this question. The notion of “inflamm-aging” (56) supports this possibility, and targeting the IL-1 pathway may serve as a promising approach to mitigate age-associated inflammatory diseases.
As mentioned above, we utilized mice with global IL-1α gene deletion to perform our pancreatic cancer studies. Therefore, we also cannot rule out the effect of impaired IL-1 signaling within immune cells on the phenotypes presented here. Further studies of pancreatic cancer progression involving bone marrow transplants of wild-type cells into lethally irradiated IL-1α-deleted mice could mitigate any confounding factors. Alternatively, generation and analysis of pancreas-specific IL-1α-deleted animals could elucidate the effect of pancreatic cell-produced SASP on immune cell recruitment and pancreatic cancer progression.
Existing drugs that target the IL-1 pathway are currently used to avert the deleterious phenotypes associated with autoinflammatory diseases. Anakinra, an IL-1R antagonist, is currently used in clinical settings to treat inflammation associated with rheumatoid arthritis (57). It is also used to treat neonatal-onset multisystem inflammatory disease (NOMID), a disease of chronic systemic inflammation (58). Given its efficacy in the disruption of IL-1 signaling, Anakinra may prove to have promising effects in cancers driven by inflammation, such as pancreatic cancer. Arguably, the effect of IL-1 inhibition may have a significant negative impact on the immune response in general. As such, new technologies can be utilized to increase specificity of drug delivery. For example, the GalNP drug delivery system would only release the encapsulated drug in cells with high levels of β-galactosidase (59). This system could be paired with Anakinra to inhibit the IL-1 pathway specifically in senescent cells. As we observed that IL-1α is also required for SASP maintenance (data not shown), targeting the IL-1 signaling pathway may be efficacious even after senescence onset, making it an ideal candidate to mitigate progression of early-stage tumor lesions.
MATERIALS AND METHODS
Cells.
IMR90 primary lung embryonic fibroblasts expressing hTERT (IMR90T) were obtained from S. Smith (NYU School of Medicine, New York, NY). Cells were cultured in minimum essential medium Eagle (MEM; Corning) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% penicillin-streptomycin (Cellgro). HEK293T cells (ATCC) were used to generate retro- and lentiviruses and were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning) supplemented with 10% donor calf serum (Atlanta Biologicals) and 1% penicillin-streptomycin. IMR90T cells were maintained in 6% O2 and 5% CO2 at 37°C, while 293T cells were maintained in 5% CO2 at 37°C.
Viruses and infections.
Oncogenic ERT2-RasG12V was cloned into pQCXIN. Inducible shRNAs against IL-1α and IL-1R were purchased from Dharmacon, and shRNA against IL-1β was purchased from Sigma. The IL-1α coding sequence (Origene) was cloned into either MSCV or pLB(N)CX. The IL-1β coding sequence was cloned into pLB(N)CX. ppIL-1α, myc-tagged mIL-1α, and mIL-1β constructs were generated using Q5 site-directed mutagenesis (NEB) by following the manufacturer’s protocol. Retro- and lentiviruses expressing the constructs described above were generated via calcium phosphate transfection using a standard procedure. Cells were infected for 2 consecutive days and selected with 400 µg/ml G418 (ERT2-Ras) for 1 week, 1.5 µg/ml puromycin (IL-1α constructs, shRNAs) for 3 days, or 10 µg/ml blasticidin (IL-1α and IL-1β constructs) for 3 days.
CRISPR/Cas9 editing.
Lentiviral Cas9 WT construct was a gift from E. Hernando (NYU School of Medicine, New York, NY). A guide RNA against IL-1α (5′GGTAGTAGCAACCAACGGGA-3′) was cloned in pLKO-GFP. Both constructs were infected into IMR90T cells, and clones were generated via single-cell cloning in 96-well plates. Successful deletion was confirmed via Western blotting.
Senescence induction.
For Ras-induced senescence, cells were treated with 200 nM tamoxifen (Sigma) continuously for 10 days. Fresh media and tamoxifen were added every 2 to 3 days. Cells were treated with an equal volume of ethanol as a control. For etoposide-induced senescence, cells were treated with 50 µM etoposide (Sigma) or an equal volume of DMSO as a control for 48 h. Etoposide-containing medium was then replaced by normal culture medium for 7 days before harvest.
shRNA knockdown.
To induce IL-1α (no. V2THS_111503; Dharmacon) or IL-1R (no. V2THS_131081 and V2THS_131083; Dharmacon) knockdown, cells were cultured in the presence of 0.5 µg/ml doxycycline (Sigma) for 24 h before the start of senescence induction. Medium was changed and fresh doxycycline was added every 48 h until the end of the experiment to maintain knockdown. shIL-1β (no. TRCN0000058384; Sigma) was constitutively active. The levels of knockdown were determined by Western blot analysis and qPCR.
Western blot analysis.
Western blotting was performed as previously described (28). The following primary antibodies were used: mouse antitubulin (T9026; Sigma) at 1:2,000 dilution, rabbit anti-IL-1α (ab9614; Abcam) at 1:500 dilution, and anti-mouse IL-1β (MAB201; R&D) at 1:1,000 dilution.
Real-time qPCR.
Total RNA was extracted using TRIzol (Life Technologies) according to the manufacturer’s instructions. One microgram of total RNA was used to generate cDNA with oligo(dT). Real-time qPCR was performed using Maxima SYBR green (Fisher Scientific), and samples were run on a Bio-Rad iCycler MyiQ. Expression levels were normalized to those for tubulin. Primer sequences are available upon request.
BrdU incorporation.
Cells were plated in duplicate on coverslips and were pulsed with 30 µM bromodeoxyuridine (BD Biosciences) for 2 h. Cells were fixed for 10 min with 4% paraformaldehyde–phosphate-buffered saline (PBS) and incubated in 4 N HCl for 10 min to denature DNA. Cells were neutralized with 0.1 M sodium tetraborate, pH 8.5, for 7 min and then permeabilized with 0.1% Triton X-100–3% bovine serum albumin (BSA)–0.1% Tween 20–PBS for 5 min. Cells were incubated with 1:100 mouse anti-BrdU (BD Biosciences) for 75 min and 1:500 Alexa Fluor 488–anti-mouse secondary antibody (Life Technologies) for 1 h. Coverslips were then mounted on slides using mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vectashield). Slides were examined on a Zeiss AxioImager A2 microscope. A total of 200 cells per coverslip were counted.
SA-βgal assay and SAHF quantification.
Cells were plated in duplicate on coverslips 48 h before harvest. Cells were fixed with 2% formaldehyde–0.2% glutaraldehyde in PBS for 10 min and stained at 37°C overnight in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) solution (1 mg/ml X-Gal, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2, 25.2 mM sodium phosphate, 7.36 mM citric acid in H2O at pH 6.0). Coverslips were washed and mounted on slides as described above. Slides were examined on a Zeiss AxioImager A2. UV light was used to visualize SAHF, and bright-field microscopy was used to visualize SA-βgal positivity. A total of 200 cells per coverslip were counted.
Immunofluorescence.
Cells were plated in duplicate on coverslips 48 h before harvest. Cells were fixed with 4% paraformaldehyde–PBS for 15 min and blocked with 0.3% Triton X-100–5% goat serum–PBS for 1 h. Cells were then incubated with rabbit anti-γ-H2AX (9718; CST) at 1:400 dilution in 0.3% Triton X-100–1% BSA–PBS overnight at 4°C. The following day, cells were incubated with 1:500 Alexa Fluor 488–anti-rabbit secondary antibody (Life Technologies) in 0.3% Triton X-100–1% BSA–PBS for 1 h. Coverslips were then washed and mounted as described above. Slides were examined on a Zeiss AxioImager A2 microscope. A total of 100 cells per coverslip were counted.
ELISA.
Conditioned medium (CM) was prepared by washing cells once in PBS and incubating in serum-free MEM. CM was collected after 24 h, and cell numbers were determined. ELISA kits for detecting IL-6 were purchased from BioLegend, and ELISAs were performed according to the manufacturer’s instructions.
RNA sequencing.
RNA was harvested from 2 biological replicates using the RNeasy microkit (Qiagen). RNA quality assessment, library preparation, and sequencing were performed at the NYU School of Medicine Genome Technology Center. Strand-specific libraries were prepared using the TruSeq RNA Library Prep kit, and libraries were sequenced on an Illumina HiSeq2500 using 50-bp paired-end reads. Sequences were mapped to the hg19 genome, and analysis was done as previously described (60).
Mice.
LSL-KRasG12D mice were gifts from T. Jacks (Massachusetts Institute of Technology, Cambridge, MA). D. Bar-Sagi (NYU School of Medicine, New York, NY) provided the p48-Cre mice. IL-1α−/− mice were generated as described previously (61). The strains were mated to obtain mice with the correct genotypes. All animals were maintained on a C57BL/6 background, and both males and females were used. All experiments were approved by the IACUC.
Histological and immunohistochemical staining.
Pancreata from 3-month-old mice were fixed for 48 h in zinc fixative (BD Biosciences) and processed for paraffin embedding. Sectioning and histological, trichrome, and immunohistochemical staining were performed at the NYU Langone Experimental Pathology Research Laboratory as previously described (28). The following antibodies were used: anti-F4/80 (clone ND; Cell Signaling), anti-CD3 (clone SP7; Spring Biosciences), and anti-Ki-67 (clone SP6; Abcam). Slides were scanned into SlidePath and examined there. The percentage of neoplastic lesions was calculated by dividing the number of pixels that constituted normal acinar area by the total number of pixels that constituted tissue and subtracting this number from 1. The percentage of trichrome area was calculated by dividing the number of blue pixels by the total number of tissue pixels. The number of CD3-positive cells was calculated by counting the horseradish peroxidase (HRP)-positive brown cells per field of view and averaging the number over 15 fields or by counting the HRP-positive brown cells in low-grade lesions and dividing by the total number of lesions. The percentage of F4/80-positive cells was calculated by dividing the number of HRP-positive brown pixels by the total number of tissue pixels or by dividing the number of brown pixels in or surrounding low-grade lesions by the total number of lesion pixels. Representative images were taken with a Zeiss AxioImager A2.
Tissue SA-βgal staining.
Pancreata from 3-month-old mice were freshly frozen in optimal cutting temperature (OCT) compound. Frozen sections of pancreatic tissue were fixed with 2% formaldehyde–0.2% glutaraldehyde in PBS for 5 min, washed with PBS, and stained at 37°C overnight in X-Gal solution as described above. After counterstaining with Nuclear Fast Red solution (Ricca), slides were subjected to an alcohol dehydration series and mounted with Permount (Fisher). Slides were examined on a Zeiss AxioImager A2. Percent SA-βgal positivity was calculated by dividing the number of blue pixels by the total number of tissue pixels and averaging the number over 10 fields.
Statistical analysis.
Results were compared statistically using GraphPad Prism software. Values were subjected to unpaired two-tailed t tests, multiple t tests, one-way analysis of variance (ANOVA) followed by Dunnett’s multiple-comparison test, or two-way ANOVA followed by Tukey’s multiple-comparison test. All data are presented as means ± standard errors of the means.
Accession number(s).
Raw transcriptomic data were deposited at GEO and are available under accession number GSE108278.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all members of the David laboratory for helpful discussions during the preparation of the manuscript. We thank J. C. Acosta for helpful discussions. We thank the NYU Genome Technology Center for help with RNA sequencing, R. Raviram from the laboratory of J. A. Skok for help with bioinformatic analysis, and the NYU HPC for their support. We thank the NYU Langone Experimental Pathology Research Laboratory for help with tissue sectioning and staining. Finally, we thank G. Miller and C. Hajdu for their expertise.
This work was funded by the National Institutes of Health (R01CA148639, R21CA206013, and R21CA155736 to G.D.) and the Samuel Waxman Cancer Research Foundation (G.D.). L.L. was supported by a predoctoral NIH/NCI NRSA (F31CA206387). The NYU Genome Technology Center and Experimental Pathology Research Laboratory are partially supported by Cancer Center support grant P30CA016087 at NYU Langone’s Laura and Isaac Perlmutter Cancer Center.
L.L. and G.D. designed research, L.L., A.P., and A.Y. performed research and analyzed data, Y.I. generated and provided reagents, and L.L. and G.D. wrote the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00586-18.
REFERENCES
- 1.Hayflick L, Moorhead PS. 1961. The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Futcher AB, Greider CW. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 4.Robles SJ, Adami GR. 1998. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 5.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 6.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. 2005. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 7.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. 2005. Tumour biology: senescence in premalignant tumours. Nature 436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 8.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 9.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. 2005. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, Lowe SW. 2013. Non-cell-autonomous tumor suppression by p53. Cell 153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. 2006. Cellular senescence in aging primates. Science 311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. 2007. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campisi J. 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Campisi J, d'Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 19.Campisi J. 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. 2008. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L. 2011. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 24.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. 2013. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. 2001. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A 98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppe JP, Desprez PY, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rielland M, Cantor DJ, Graveline R, Hajdu C, Mara L, Diaz BDD, Miller G, David G. 2014. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J Clin Investig 124:2125–2135. doi: 10.1172/JCI72619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bainor AJ, Deng FM, Wang Y, Lee P, Cantor DJ, Logan SK, David G. 2017. Chromatin-Associated Protein SIN3B Prevents Prostate Cancer Progression by Inducing Senescence. Cancer Res 77:5339–5348. doi: 10.1158/0008-5472.CAN-16-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. 2010. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A 107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamostna B, Novak J, Vopalensky V, Masek T, Burysek L, Pospisek M. 2012. N-terminal domain of nuclear IL-1alpha shows structural similarity to the C-terminal domain of Snf1 and binds to the HAT/core module of the SAGA complex. PLoS One 7:e41801. doi: 10.1371/journal.pone.0041801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. 2009. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A 106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rider P, Carmi Y, Voronov E, Apte RN. 2013. Interleukin-1a. Semin Immunol 25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. 1987. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem 262:2941–2944. [PubMed] [Google Scholar]
- 35.Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 36.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz ML, Weber A, Roxlau T, Gaestel M, Kracht M. 2011. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim Biophys Acta 1813:2165–2175. doi: 10.1016/j.bbamcr.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, Lehner PJ, Zender L, Narita M. 2016. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. 2013. Redox control of the senescence regulator interleukin-1alpha and the secretory phenotype. J Biol Chem 288:32149–32159. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. 2003. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 41.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. 2011. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Greten FR, Karin M. 2010. Immunity, inflammation, and cancer. Cell 140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gluck S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A. 2017. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19:1061–1070. doi: 10.1038/ncb3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Wang H, Ren J, Chen Q, Chen ZJ. 2017. cGAS is essential for cellular senescence. Proc Natl Acad Sci U S A 114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martinez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ, Gil J. 2015. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J. 2015. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capell BC, Drake AM, Zhu J, Shah PP, Dou Z, Dorsey J, Simola DF, Donahue G, Sammons M, Rai TS, Natale C, Ridky TW, Adams PD, Berger SL. 2016. MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev 30:321–336. doi: 10.1101/gad.271882.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jiang T, Shoshkes-Carmel M, Tanim K, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. 2017. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. 2012. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. 2017. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep 20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, Avanzi A, Tippens D, Narayanan R, Jang JE, Newman E, Pillarisetty VG, Dustin ML, Bar-Sagi D, Hajdu C, Miller G. 2016. Gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell 166:1485–1499. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG. 2017. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47:597. doi: 10.1016/j.immuni.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ. 2012. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang Z, Ju HQ, Aguilar M, Gocho T, Li H, Iida T, Lee H, Fan X, Zhou H, Ling J, Li Z, Fu J, Wu M, Li M, Melisi D, Iwakura Y, Xu K, Fleming JB, Chiao PJ. 2016. IL1 receptor antagonist inhibits pancreatic cancer growth by abrogating NF-kappaB activation. Clin Cancer Res 22:1432–1444. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franceschi C, Campisi J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 57.Furst DE. 2004. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther 26:1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Lovell DJ, Bowyer SL, Solinger AM. 2005. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum 52:1283–1286. doi: 10.1002/art.20953. [DOI] [PubMed] [Google Scholar]
- 59.Munoz-Espin D, Rovira M, Galiana I, Gimenez C, Lozano-Torres B, Paez-Ribes M, Llanos S, Chaib S, Munoz-Martin M, Ucero AC, Garaulet G, Mulero F, Dann SG, VanArsdale T, Shields DJ, Bernardos A, Murguia JR, Martinez-Manez R, Serrano M. 2018. A versatile drug delivery system targeting senescent cells. EMBO Mol Med 10:e9355. doi: 10.15252/emmm.201809355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Proudhon C, Snetkova V, Raviram R, Lobry C, Badri S, Jiang T, Hao B, Trimarchi T, Kluger Y, Aifantis I, Bonneau R, Skok JA. 2016. Active and inactive enhancers cooperate to exert localized and long-range control of gene regulation. Cell Rep 15:2159–2169. doi: 10.1016/j.celrep.2016.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. 1998. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.