Abstract
Poly (ADP-ribose) polymerase (PARP) inhibitors were developed with the intention of treating patients with homologous recombination repair deficiency (HRD), specifically for patients with tumours that harbour a BRCA mutation (BRCAm). Evidence from clinical trials to date has demonstrated that patients with a BRCAm derive the greatest benefit from PARP inhibitors. However, clinical studies have also shown that PARP inhibitors provide benefit to women with ovarian cancer who do not have a BRCAm. The recent updated approvals of olaparib, niraparib and rucaparib by the US Food and Drug Administration and the European Medicines Agency for the treatment of all platinum-sensitive relapsed (PSR) ovarian-cancer populations, regardless of their BRCAm status, support this. Long-term tolerability and efficacy of olaparib have been demonstrated in patients both with and without a BRCAm, with 13% of patients receiving maintenance olaparib for at least 5 years in one study, which is unprecedented in the relapsed ovarian-cancer setting (versus 1% on placebo). Further studies should be performed to elucidate which non-BRCAm patients are deriving benefit and what molecular processes are enabling this, so that patients continue to receive optimal treatment for their disease. Here, we review clinical and molecular markers of HRD, the long-term clinical safety and efficacy of PARP inhibitors in ovarian cancer, with a focus on olaparib and the current approved indications for PARP inhibitors, as well as guidance on treatment decisions for patients with PSR ovarian cancer.
Keywords: niraparib, olaparib, PARP inhibitor, platinum-sensitive relapsed ovarian cancer, rucaparib, targeted therapy
Introduction
The poly (ADP-ribose) polymerase (PARP) inhibitor olaparib (Lynparza™) was the first personalized treatment for patients with high-grade serous ovarian cancer, initially receiving regulatory approval in Europe for the treatment of those patients with a germline or somatic BRCA1 or BRCA2 mutation (BRCAm).1
The activity of PARP inhibitors is based on the concept of synthetic lethality, where an underlying homologous recombination repair deficiency (HRD) in tumour cells makes the cells highly susceptible to PARP inhibition.2 PARP inhibitors bind to and trap PARP1 and PARP2 on DNA at the sites of single-strand breaks, which results in the generation of a double-strand breaks. In cancer cells with HRD, double-strand DNA breaks are repaired by error-prone pathways (i.e. nonhomologous end joining), ultimately leading to cell death.2–5
Indeed, the mechanisms of action of PARP inhibitors are distinct from other targeted agents where drugs are designed to target specific driver mutations (oncogenes) or products thereof (such as tyrosine kinase inhibitors that directly inhibit mutated, constitutively activated tyrosine kinases).6 In such instances, physicians can select for patients who are most likely to respond by directly screening for the genetic aberration synonymous with the mechanism of action (e.g. osimertinib treatment for patients with an EGFR T790M mutation).7 In contrast, the antitumour effects of olaparib and other PARP inhibitors are not dependent on a direct interaction with a mutated gene/protein, but rather on an underlying defect in the DNA damage repair mechanism of cancer cells.
Platinum sensitivity and high-grade histology predict patients with homologous recombination repair deficiency
HRD is a key determinant of platinum sensitivity in high-grade serous ovarian cancer,8 and sensitivity to platinum agents is reported to correlate with sensitivity to olaparib.9
The most profound deficit in the homologous recombination repair (HRR) pathway is seen in tumours with a BRCAm. Many of the preclinical studies undertaken during the development of PARP inhibitors targeted cells or murine tumour models deficient in BRCA as a marker of HRD.10–12 However, clinical studies have demonstrated that sensitivity to PARP inhibitors occurs in tumours beyond those harbouring a BRCAm.5,13,14 Until recently, hereditary epithelial ovarian cancer was thought to be almost exclusively the result of mutations in the BRCA1 and BRCA2 genes, with a minimal proportion resulting from DNA mismatch repair gene mutations.15 BRCAm, germline or somatic, have been reported to occur in up to 18–25% of patients with newly diagnosed serous ovarian cancer.16,17 In addition to BRCAm, the HRR pathway may be compromised by other mechanisms, examples of which include loss-of-function mutations in other HRR genes, epigenetic inactivation of BRCA1 or methylation of RAD51C promoters.18,19 Further investigation of the homologous recombination repair pathway in ovarian cancers has highlighted multiple other protein cofactors that are necessary for successful HRR, including TP53, ATM, MRE11, RAD51, H2AX, PALB2, RPA, BRIP1, BARD1, RAD52 and proteins of the Fanconi anaemia pathway, in addition to potentially unknown molecular targets.15,20–23 Data from The Cancer Genome Atlas suggest that approximately 50% of high-grade serous ovarian cancers (the most common histologic subtype) have a deficiency in HRR.23
The relationship between sensitivity to PARP inhibitors and DNA repair deficiency is therefore likely to be more akin to a continuous than a discrete variable; for example, patients with a BRCAm are highly sensitive to PARP inhibition, but lack of a BRCAm does not preclude sensitivity to olaparib. As such, PARP inhibitors have the potential to be beneficial in a much wider proportion of ovarian-cancer patients than was originally proposed.
Olaparib for the treatment of platinum-sensitive relapsed ovarian cancer
Olaparib (Lynparza capsule formulation) received approval based on the results from Study 19 [ClinicalTrials.gov identifier: NCT00753545]. Study 19 was a randomized, placebo-controlled, phase II trial enrolling 265 patients who were clinically enriched for markers associated with a response to PARP-inhibitor treatment [i.e. patients with high-grade serous platinum-sensitive relapsed (PSR) ovarian cancer who had received at least two platinum-based chemotherapy regimens and were in complete or partial response to their most recent regimen]. Patients were randomized to olaparib maintenance treatment [capsules; 400 mg twice daily (b.i.d.)] or matching placebo. Median follow up for the primary analysis of progression-free survival (PFS) was 5.6 months in Study 19 and no further tumour assessments were performed following the primary data cut-off (DCO). Olaparib treatment improved PFS in Study 19 in the overall population [hazard ratio (HR) 0.35, 95% confidence interval (CI) 0.25–0.49; p < 0.0001 for olaparib versus placebo]. A planned, retrospective analysis of BRCAm status was performed and information was obtained for 96% of the overall population. In the BRCAm population, the benefit of olaparib versus placebo was even greater (HR 0.18, 95% CI 0.10–0.31; p < 0.0001) than in the non-BRCAm population (HR 0.54, 95% CI 0.34–0.85; p = 0.0075;24,25 Table 1).
Table 1.
PFS (investigator assessed), TFST, TSST and OS in the Study 19 trial of olaparib-capsule maintenance treatment.
| Median, months |
HR (95% CI) | p value | ||
|---|---|---|---|---|
| Olaparib | Placebo | |||
| PFS, overall population (58% maturity)24 |
8.4 | 4.8 | 0.35 (0.25–0.49) |
<0.0001 |
| PFS BRCAm population) (53% maturity)25 |
11.2 | 4.3 | 0.18 (0.10–0.31) |
<0.0001 |
| TFST, overall population (87% maturity)26 |
13.3 | 6.7 | 0.39 (0.30–0.52) |
<0.00001 |
| TFST, BRCAm population (84% maturity)26 |
15.6 | 6.2 | 0.33 (0.22–0.49) |
<0.00001 |
| TSST, overall population (85% maturity)26 |
19.1 | 14.8 | 0.53 (0.40–0.69) |
<0.00001 |
| TSST, BRCAm population (80% maturity)26 |
21.4 | 15.3 | 0.43 (0.29–0.64) |
<0.00003 |
| OS, overall population (79% maturity)26 |
29.8 | 27.8 | 0.73 (0.55–0.95) |
0.021 |
| OS, BRCAm population (73% maturity)26 |
34.9 | 30.2 | 0.62 (0.42–0.93) |
0.021 |
Blinded, independent, central review assessment of PFS endpoints supported the investigator-assessed primary analysis of PFS.
BRCAm, BRCA mutation; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TFST, time to first subsequent treatment; TSST, time to second subsequent treatment.
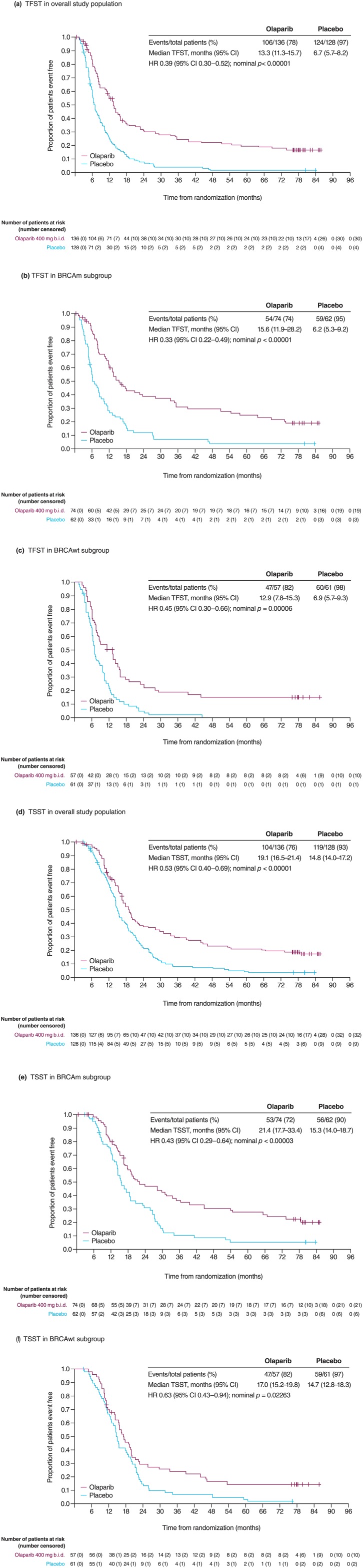
Novel intermediate endpoints between PFS and overall survival (OS) have been included in ovarian cancer clinical trials. One of these is the time from randomization to first subsequent therapy or death (TFST), which considers any differences between the time of radiological progression and the start of the next line of treatment, as well as providing important information for the patient, who is interested in knowing when they are likely to receive their next line of chemotherapy. In the case of Study 19, TFST has also provided significantly more mature efficacy information beyond the initial PFS DCO analysis, which occurred when the median follow up was only 5.6 months, with significant data immaturity in the olaparib arm, compared with a median follow up of 77.4 months for TFST. TFST in Study 19 was shown to be significantly longer in the olaparib arm than for placebo-treated patients in the overall study population, BRCAm and non-BRCAm subgroups (Figure 1; Table 1).
Figure 1.
Study 19: TFST and TSST in all patients and according to BRCAm status.
(a) TFST in the overall study population; (b) TFST in the BRCAm subgroup; (c) TFST in the non-BRCAm subgroup; (d) TSST in the overall study population; (e) TSST in the BRCAm subgroup; and (f) TSST in the non-BRCAm subgroup.
BRCAm, BRCA mutation; BRCAwt, BRCA wild type; CI, confidence interval; HR, hazard ratio; TFST, time to first subsequent therapy; TSST, time to second subsequent therapy.
Figure reproduced from Friedlander et al.26 (https://creativecommons.org/licenses/by/4.0/).
Although OS remains an accepted and important clinical trial endpoint, it is becoming increasingly difficult to detect an OS benefit for investigational treatments because of confounding factors such as crossover to the experimental drug, the use of multiple lines of chemotherapy and investigational treatments following disease progression.27 As such, observation of significant improvement in PFS can be supported by other intermediate endpoints between PFS and OS, which also have value in indicating whether investigational treatments can provide extended benefit, beyond initial disease progression. Such intermediate endpoints include the time from randomization to second subsequent therapy or death (TSST), and time from randomization to second disease progression or death (PFS2), which are being introduced into oncology clinical trials.28,29
Consistent with the PFS and TFST benefit observed in patients receiving olaparib, TSST was also significantly longer in the olaparib arm than the placebo arm in the overall study population, BRCAm and non-BRCAm subgroups (Figure 1; Table 1), demonstrating a clinically meaningful increase in time between chemotherapy regimens, while also providing evidence of a sustained benefit of olaparib treatment in both BRCAm and non-BRCAm populations after long-term follow up.26,30
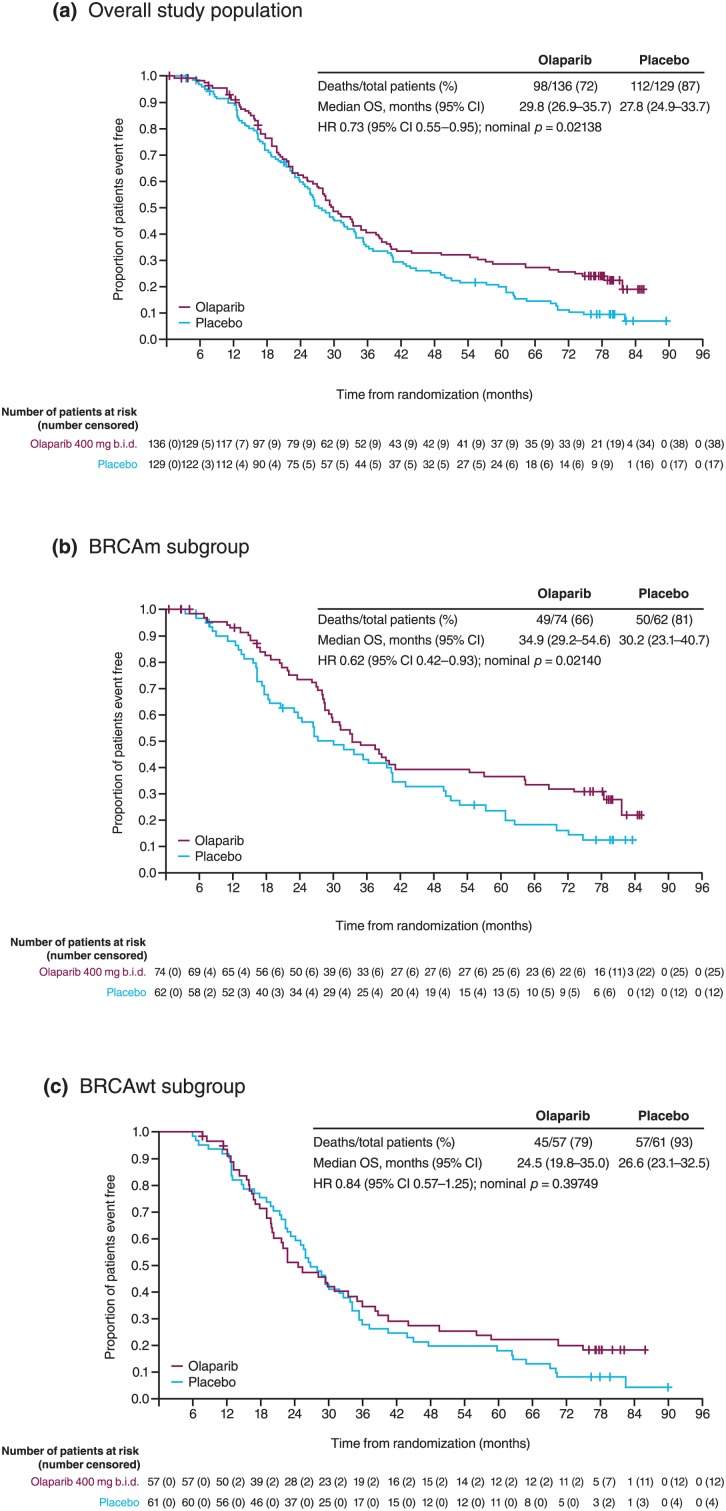
After a median follow up of 6.5 years (79% data maturity), there was a clinically significant improvement in OS in the overall population of Study 19 (HR 0.73, 95% CI 0.55–0.95; nominal p = 0.021) and in patients with a BRCAm (HR 0.62, 95% CI 0.42–0.93; p = 0.021; Figure 2).26 Although the criterion for statistical significance (p < 0.0095) was not reached for OS because of the alpha spending approach used during interim analyses of the trial, these data demonstrate continued treatment benefit. A separation in favour of olaparib treatment was seen in the Kaplan–Meier (KM) curves for the overall study population and the BRCAm subgroup as follow-up duration increased (Figure 2). The separation of the KM curves became more apparent after 36 months of follow up, reflecting the long-term benefit derived for olaparib-treated patients.26 Furthermore, the HRs reported are unadjusted for patient crossover, and 13% of placebo-receiving patients received postdiscontinuation PARP-inhibitor treatment in other clinical trials, whereas no patients in the olaparib arm received retreatment with olaparib or any other subsequent PARP-inhibitor treatment.26
Figure 2.
Study 19: final overall survival in all patients and according to BRCAm status.
(a) OS in overall study population; (b) OS in BRCAm subgroup; (c) OS in non-BRCAm subgroup.
BRCAm, BRCA mutation; CI, confidence interval; HR, hazard ratio; OS, overall survival.
Figure reproduced from Friedlander et al.26 (https://creativecommons.org/licenses/by/4.0/).
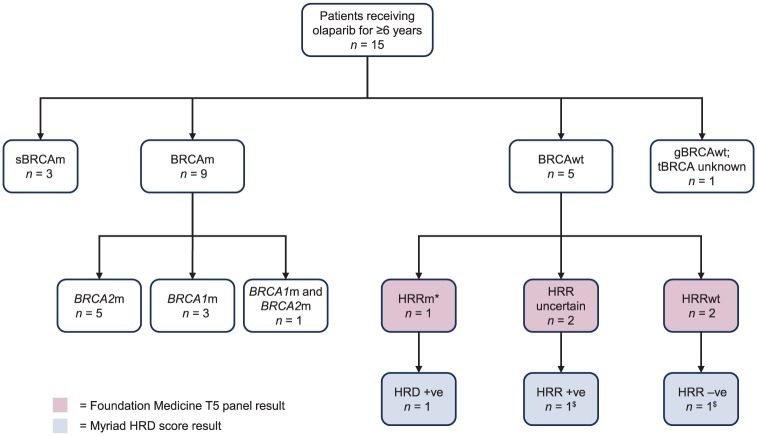
The long-term exposure seen in Study 19 is unprecedented for therapy in recurrent ovarian cancer. Eighteen patients in the olaparib arm remained on study treatment for at least 5 years (13%), of whom 11 had a BRCAm and seven were non-BRCAm; in the placebo arm, only one patient (1%) stayed on treatment for at least 5 years.26 Consistent across BRCAm and non-BRCAm subgroups, approximately 10% of patients experienced a durable benefit from olaparib maintenance monotherapy for at least 6 years.26 Over a third of patients who had received olaparib for at least 6 years did not harbour a BRCAm. Of the 15 patients who received olaparib for at least 6 years, nine had a BRCAm, three of whom had a somatic BRCAm, and a slight preponderance of BRCA2 mutations was observed (BRCA2m, n = 5; BRCA1m, n = 3; BRCA1m or BRCA2m, n = 1); five patients were non-BRCAm, one of whom was found to have a RAD51B mutation, the HRR status (determined by Foundation Medicine T5 panel test)31,32 was unknown for two patients, one of whom was HRD positive (determined by Myriad myChoice® HRD (Myriad Genetics, Inc. UT, USA) HRR deficiency test),31,33 and two patients had no HRR mutations, one of whom was HRD negative.13 One patient, who was germline BRCA wild type, had no available tumour test results (Figure 3).13 These data demonstrate that there are patients beyond those with a BRCAm who derive a long-term benefit from PARP-inhibitor treatment in line with the US Food and Drug Administration (FDA) and the updated European indication for olaparib (all PSR ovarian cancer patients who are in complete or partial response to their last platinum treatment).34
Figure 3.
Biomarker characterization in patients receiving olaparib for ⩾6 years in Study 19.
*This patient was found to have a RAD51B mutation.
$Two of five BRCAwt patients had no available Myriad HRD score result.
BRCAm, BRCA mutation; BRCAwt, BRCA wild type; gBRCAwt, germline BRCA wild type; HRD, homologous recombination repair deficiency; HRRm, homologous recombination repair mutation; HRRwt, HRR wild type; sBRCAm, somatic BRCA mutation; tBRCA, tumour BRCA.
The full dose for the originally approved olaparib formulation was 400 mg b.i.d. administered as eight 50 mg capsules;35,36 because of the high administration burden of the capsules, an alternative tablet formulation was developed. The capsule and tablet formulations are not bioequivalent, therefore an adaptive study, Study 24 [ClinicalTrials.gov identifier: NCT00777582] enrolling patients with advanced solid tumours, including patients with BRCAm ovarian and breast cancer, was performed to determine the optimal dose of the tablet formulation for use in phase III trials. This study determined that the olaparib 300 mg b.i.d. (2 × 150 mg) tablet formulation matched or exceeded the exposure of 400 mg b.i.d. capsules; the 300 mg b.i.d. tablet regimen was also shown to be non-inferior to 400 mg b.i.d. capsules in terms of tumour shrinkage,37 which led to 300 mg b.i.d. tablets being assessed in phase III trials of olaparib.
The SOLO2 [ClinicalTrials.gov identifier: NCT01874353] study of olaparib was designed to evaluate the tablet formulation (300 mg b.i.d.) and to confirm the efficacy of olaparib in a phase III trial of patients with BRCAm, PSR ovarian cancer who were in complete or partial response to their most recent platinum-based regimen. It confirmed the efficacy of olaparib in this patient population with a significant improvement in both the investigator-assessed (primary endpoint) and blinded, independent central review (BICR; sensitivity analysis) PFS following olaparib treatment versus placebo (investigator-assessed median PFS: 19.1 versus 5.5 months, respectively; HR 0.30, 95% CI 0.22–0.41; p < 0.0001; Table 2; BICR median PFS: 30.2 versus 5.5 months; HR 0.25, 95% CI 0.18–0.35; p < 0.0001).38 Of note, the BICR median PFS following olaparib treatment was over 11 months longer than the investigator-assessed median PFS. Differences in median PFS between investigator- and BICR-assessed PFS have also been observed in clinical trials of the PARP inhibitors niraparib and rucaparib in patients with ovarian cancer. When only local review shows progression, no further assessments are performed, and patients are censored in the BICR analysis. BICR medians are shown to be exaggerated if there is a positive correlation between BICR and local evaluation PFS times, especially when patients are assessed many times prior to progression. When efficacy is substantial, it can lead to exaggeration of the difference in medians between arms. However, the HR is much less susceptible to bias and is therefore a more reliable indicator of the true treatment effect and the most valid measure to describe the benefit of a PFS analysis.39
Table 2.
PFS (investigator assessed), TFST, PFS2 and TSST in the SOLO2 trial of olaparib tablet maintenance treatment in patients with a BRCAm38.
| Median, months |
HR (95% CI) | p value | ||
|---|---|---|---|---|
| Olaparib | Placebo | |||
| PFS (63% maturity) |
19.1 | 5.5 | 0.30 (0.22–0.41) |
<0.0001 |
| TFST (58% maturity) |
27.9 | 7.1 | 0.28 (0.21–0.38) |
<0.0001 |
| PFS2*
(40% maturity) |
NR | 18.4 | 0.50 (0.34–0.72) |
<0.0002 |
| TSST*
(43% maturity) |
NR | 18.2 | 0.37 (0.26–0.53) |
0.0001 |
Blinded, independent, central review assessment of PFS endpoints supported the investigator-assessed primary analysis of PFS.
The analysis of TSST included more events than did analysis of PFS2 (128 versus 119) because at the time of data cut-off, some patients who had received a second subsequent therapy were not yet classed as having investigator-assessed disease progression following their first subsequent therapy.
BRCAm, BRCA mutation; CI, confidence interval; HR, hazard ratio; NR, not reached; PFS, progression-free survival; PFS2, time to second progression or death; TFST, time to first subsequent treatment; TSST, time to second subsequent treatment.
In the SOLO2 trial, TFST, PFS2 and TSST were included as prospective secondary endpoints rather than exploratory endpoints (TFST and TSST) as in Study 19. In SOLO2, at the time of the primary PFS analysis, median TFST was 27.9 months (95% CI 22.6–not calculable) in the olaparib group versus 7.1 months (6.3–8.3) for placebo; median PFS2 was not reached in patients receiving olaparib (24.1–not calculable) and was 18.4 months (15.4–22.8) in the placebo group; and median TSST in the olaparib group was not reached (95% CIs not calculable) and was 18.2 months (15.0–20.5) in the placebo group;38 these data further demonstrated the prolonged efficacy of olaparib in patients with ovarian cancer beyond completion of their treatment with olaparib. OS data for SOLO2 are not yet mature.38
Study 19 allowed patients to continue to receive their randomized treatment beyond Response Evaluation Criteria in Solid Tumours (RECIST) progression (Table 3). Although the majority of patients who had progressed at the time of DCO discontinued their olaparib treatment within 2 weeks of their progression date, a proportion of patients continued with olaparib treatment for >2 weeks after progression. Similarly, in SOLO2, patients could continue with treatment beyond their RECIST progression. Of interest in Study 19, seven (41%) patients continued treatment with olaparib for more than 3 months following disease progression [versus four (24%) on placebo] and in SOLO2, 20 (48%) women continued with olaparib for more than 3 months following their disease progression [versus three (13%) on placebo], with five (12%) patients continuing olaparib treatment for more than 12 months following progression [versus one (4%) on placebo; AstraZeneca, Cambridge, UK, 2019; Table 3]. Although these exploratory data should be interpreted with caution, they highlight that patients are tolerating olaparib treatment well and are the first data to demonstrate that the investigators believe patients are still deriving benefit from PARP-inhibitor treatment, even in the context of progressive disease.
Table 3.
Patients who continued randomized treatment for more than 2 weeks following RECIST disease progression in Study 19 and SOLO2.
| Olaparib | Placebo | |
|---|---|---|
| Study 19 full analysis set, n | 136 | 128 |
| Patients, who continued treatment >2 weeks following RECIST progression, n (%) | 17 (12.5) | 17 (13.3) |
| Time to treatment discontinuation following RECIST progression date, n (%) | ||
| ⩽3 months | 10 (58.8) | 13 (76.5) |
| >3–⩽6 months | 2 (11.8) | 2 (11.8) |
| >6–⩽12 months | 3 (17.6) | 1 (5.9) |
| >12 months | 2 (11.8) | 1 (5.9) |
| SOLO2 full analysis set, n | 195 | 99 |
| Patients who continued treatment >2 weeks following RECIST progression, n (%) | 42 (21.5) | 23 (23.2) |
| Time to treatment discontinuation following RECIST progression date, n (%) | ||
| ⩽3 months | 22 (52.4) | 20 (87.0) |
| >3–⩽6 months | 7 (16.7) | 1 (4.3) |
| >6–⩽12 months | 8 (19.0) | 1 (4.3) |
| >12 months | 5 (11.9) | 1 (4.3) |
For SOLO2, the progression date is based on that determined by investigator assessment.
RECIST, Response Evaluation Criteria in Solid Tumours.
The median PFS difference for patients with a BRCAm between the two olaparib trials [11.2 versus 19.1 months for patients treated with olaparib in Study 19 (capsule formulation) versus SOLO2 (tablet formulation), as assessed by the investigator] might be interpreted as a difference in the efficacy of the formulations; however, there are several other factors that could explain the observed variance. First, differences were observed for the median number of prior chemotherapies; patients in Study 19 had a median of three prior lines of chemotherapy and in SOLO2, patients had a median of two prior lines of chemotherapy (44% and 56% of patients receiving olaparib in Study 19 and SOLO2 had received two prior lines of chemotherapy, respectively). It is known that with each additional line of chemotherapy a patient with ovarian cancer receives, their PFS interval is reduced.40 Therefore, patients in SOLO2 would be expected to perform better than those from Study 19. Secondly, the HRs observed for PFS indicate efficacy in both trials (0.18 and 0.30, respectively). It should also be noted that methodological bias can affect PFS data, including the median. As noted previously, the HR is currently the most valid measure to describe the benefit of a PFS analysis and is therefore a more reliable indicator of the true treatment effect.39 Other factors that could contribute to the variance in PFS between the studies are the nonequivalent bioavailability of the two olaparib formulations,37 and the data maturity for PFS events between the two trials, with the data from Study 19 being less mature than those from SOLO2 (53% and 63%, respectively).
The safety and tolerability of olaparib capsule and tablet formulations have been well document-ed.24,25,37,38,41 The most common adverse events experienced following olaparib treatment are fatigue or gastrointestinal symptoms, or haematological side effects, which are mostly of low-grade severity (grade 1–2) and manageable with supportive treatment with or without dose modifications.25,38 Importantly, in both Study 19 and SOLO2, olaparib maintenance therapy did not have a significant detrimental effect on health-related quality of life (HRQoL) compared with placebo.42,43 SOLO2 was the first trial to report the impact of maintenance therapy with a PARP inhibitor on predefined HRQoL and patient-centred endpoints to help interpret the benefits of prolongation of PFS in the patient population. This is particularly important in maintenance therapy trials given that most patients do not have symptoms associated with ovarian cancer at randomization. In the prespecified primary HRQoL analysis for SOLO2, the mean change from baseline in the Functional Assessment of Cancer Therapy: Ovarian Cancer (FACT-O) Trial Outcome Index score during the first 12 months of the study did not significantly differ between olaparib and placebo groups.38 Furthermore, secondary planned quality of life (QoL) analyses demonstrated a significantly longer quality-adjusted PFS (QAPFS; mean QAPFS 13.96 versus 7.28 months) and time without symptoms or toxicity (TWiST; 15.03 versus 7.70 months) in patients randomized to olaparib compared with placebo, respectively.44 TWiST and QAPFS use validated measures and are well-developed methods of describing the duration of ‘good QoL’ in clinical trials for patients with a wide range of malignancies.45–48 These results support the primary outcome of SOLO2 and indicate that the significant prolongation of PFS with olaparib in this patient population was achieved with no appreciable detrimental effect on patients’ QoL, supported by additional patient-centred benefits.44
Several ongoing trials are evaluating olaparib (tablet formulation) in patients with ovarian cancer who do not have a BRCAm, including the phase IIIb trial OPINION [ClinicalTrials.gov identifier: NCT03402841 (olaparib maintenance monotherapy in PSR non-germline BRCAm ovarian cancer patients)], the phase III trials L-MOCA [ClinicalTrials.gov identifier: NCT03534453; olaparib maintenance monotherapy in ovarian cancer patients after complete or partial response to platinum (a Chinese study)] and OReO [ClinicalTrials.gov identifier: NCT03106987 (olaparib retreatment in patients with or without a BRCAm)], and the phase II LIGHT study [ClinicalTrials.gov identifier: NCT02983799 (olaparib monotherapy treatment in ovarian cancer patients with different HRD tumour status)].
Indications for PARP inhibitors in patients with recurrent ovarian cancer
The initial approval of olaparib in December 2014 by the FDA was as monotherapy in patients with deleterious or suspected deleterious germ-line BRCAm (as detected by an FDA-approved test) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy;49 and by the European Medicines Agency (EMA) as monotherapy for the maintenance treatment of adult patients with PSR BRCAm (germline or somatic) high-grade serous epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial response) to platinum-based chemotherapy.50 Although originally approved for BRCAm ovarian cancer patients only, the unprecedented long-term efficacy observed from Study 19 in both BRCAm and non-BRCAm patients, the phase III SOLO2 results and those observed from other PARP-inhibitor ovarian cancer trials, led to expanding the FDA label of olaparib (tablet formulation) in August 2017 to include the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy, irrespective of their BRCAm status and the number of prior lines of platinum-based chemotherapy received.35 In May 2018, the EMA also updated the olaparib (tablet formulation) indication for use as a maintenance therapy for patients with PSR high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy, regardless of their BRCAm status.34
Trials with two other PARP inhibitors, niraparib and rucaparib, have confirmed the efficacy of these drugs as maintenance therapy for patients with PSR ovarian cancer who are in response to their most recent platinum regimen, with or without a BRCAm. NOVA [ClinicalTrials.gov identifier: NCT01847274] was a phase III trial of niraparib maintenance treatment originally evaluated in platinum-sensitive ovarian cancer patients who have either a germline BRCAm or a tumour with high-grade histology and no germline BRCAm. Following a protocol amendment, the non-germline BRCAm cohort was hierarchically evaluated, first in a subgroup of patients positive for HRD (somatic BRCAm and HRD positive/BRCA wild type)51 and then in all non-germline BRCAm patients.52 Niraparib treatment improved PFS in all three cohorts versus placebo: in patients with a germline BRCAm (HR 0.27, 95% Cl 0.17–0.41), in the HRD-positive non-germline BRCAm cohort (HR 0.38, 95% CI 0.24–0.59) and in the overall non-germline BRCAm population (HR 0.45, 95% CI 0.34–0.61, versus placebo).14 The results from the NOVA trial led to the FDA approval of niraparib (Zejula®) in March 2017 for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer in a complete or partial response to platinum-based chemotherapy;53 and by the EMA in November 2017 as monotherapy for the maintenance treatment of adult patients with PSR high-grade serous epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy.54
Rucaparib has also been investigated as maintenance treatment in patients with PSR ovarian cancer in the phase III ARIEL3 study [ClinicalTrials.gov identifier: NCT01968213].55 PFS was significantly improved across all primary analysis cohorts: in patients with a tumour BRCAm (HR 0.23, 95% CI 0.16–0.34), in the HRD-positive cohort (HR 0.32, 95% CI 0.24–0.42) that included BRCAm and BRCA wild-type patients with high loss of heterozygosity (LOH) scores56 and in the overall population (HR 0.36, 95% CI 0.30–0.45, versus placebo).55 Rucaparib (Rubraca®) was initially approved by the FDA in December 2016 for the treatment of adult patients with BRCAm (germline or somatic) epithelial ovarian cancer who have been treated with at least two prior lines of chemotherapy.57 Following the results of the ARIEL3 study, in April 2018, the rucaparib FDA approval was updated as a maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.57 In May 2018, rucaparib received approval in Europe for the monotherapy treatment of adult patients with PSR or progressive, BRCAm (germline or somatic), high-grade epithelial ovarian cancer, who have been treated with at least two prior lines of platinum-based chemotherapy, and are unable to tolerate further platinum-based chemotherapy.58 Most recently, in January 2019, rucaparib was approved in Europe as monotherapy for the maintenance treatment of adult patients with PSR high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy.
PARP inhibitors and precision medicine
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines for ovarian cancer recommend olaparib or niraparib maintenance therapy after partial or complete response to platinum chemotherapy, irrespective of BRCAm testing.59 These recommendations for the use of PARP inhibitors in a broad ovarian cancer indication have led to some concern of a shift away from patient-focused, personalized medicines, with a proportion of patients potentially receiving treatments that may not provide a benefit.60 However, the use of mutation detection and HRD tests to direct PARP-inhibitor therapy has also caused concern, as cancers may undergo evolution in response to treatment. Indeed, in the tumours of patients with a BRCAm, reversion mutations that can restore gene function have been reported, resulting in loss of PARP-inhibitor sensitivity.60 Although HRD tests help to define patients with sensitivity to PARP inhibitors, the genomic damage caused by HRD persists even if the original cause is no longer present. Therefore, tests to predict PARP-inhibitor sensitivity may become less reliable as the disease progresses60 and have therefore not been widely incorporated into clinical trials. Furthermore, genomic testing has not been able to identify women who have a poor response to olaparib treatment from those who demonstrate long-term responses following olaparib treatment.61 Platinum status (i.e. response to platinum-based therapy) observed in patients has outperformed novel HRD tests used in clinical studies of PARP inhibitors in patients with ovarian cancer. This may be because such HRD tests (Myriad myChoice® HRD test51 used in the phase III NOVA study of niraparib,14 Foundation Medicine FoundationFocusTM CDxBRCA LOH assay56 used in the phase III ARIEL3 study of rucaparib55 and HRD evaluation used in the phase II Study 19 of olaparib)62 do not investigate currently unknown genes that are involved in HRR.63 Platinum sensitivity therefore remains an important determinant of PARP-inhibitor sensitivity.
Another concern of the broad labels for PARP inhibitors in the treatment of ovarian cancer is the risk that physicians will not perform BRCA testing. Assessing for a BRCAm not only identifies patients who are most likely to respond to treatment with PARP inhibitors and platinum agents but also identifies patients with a germline BRCAm, which enables their relatives to be informed and undergo testing and risk-reduction treatments if required.
Treatment decision making for patients with platinum-sensitive relapsed ovarian cancer
PARP inhibitors have provided PSR ovarian cancer patients in response to their most recent platinum-based regimen with a new maintenance treatment option that has been shown to extend PFS and therefore delay the requirement for additional lines of platinum-based chemotherapy. New definitions of platinum sensitivity based on response to platinum, rather than platinum-free interval of ⩾6 months from prior therapy, may influence clinical decision making for selecting patients for maintenance therapy with a PARP inhibitor.64 PARP inhibitors are shown to be well tolerated and maintain patient QoL. Olaparib, niraparib and rucaparib have all demonstrated a significant benefit as maintenance therapies for patients with PSR ovarian cancer. The unprecedented maintenance treatment with olaparib in PSR ovarian cancer patients for at least 6 years, with long-term tolerability and efficacy, demonstrates that this treatment has the potential to enable patients to be in long-term remission of their disease.
Second-line maintenance treatment options for patients with PSR ovarian cancer also include treatment with the antiangiogenic monoclonal antibody bevacizumab,65,66 and therefore treatment algorithms are required (Figure 4). For example, it may be favourable to consider bevacizumab in patients with malignant ascites, as it has been reported to improve control of ascites,69 and in patients with symptomatic relapse, particularly with effusions where rapid control of disease is needed. However, for all other patients with PSR ovarian cancer who are in response (complete or partial) to their second-line platinum-based chemo-therapy regimen, treatment with a PARP inhibitor should be initiated regardless of their BRCAm status. PARP-inhibitor treatment is not only convenient for patients, being an oral treatment, but has demonstrated long-term survival benefit, which has not been shown with bevacizumab.30,67
Figure 4.
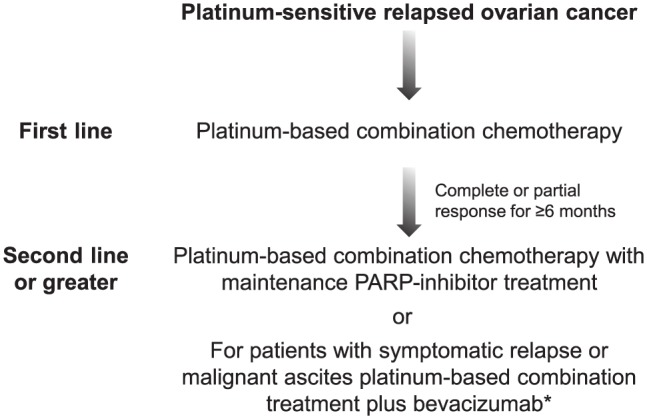
Treatment algorithm for patients with platinum-sensitive relapsed ovarian cancer.
*There are limited data on the efficacy of bevacizumab in the recurrence therapy setting for patients with platinum-sensitive disease previously treated with bevacizumab.
PARP, poly (ADP-ribose) polymerase inhibitor.
There is now a choice of PARP inhibitors available for PSR ovarian cancer patients as maintenance therapy following platinum-based treatment. For olaparib, the dosing and bioavailability of tablets and capsules is different, and the two formulations should not be used interchangeably; if the capsule posology is used for the tablets, there is a risk of overdose and increased adverse events, and if the tablet posology is used for the capsules, there is a risk of lack of efficacy. Healthcare professionals should inform patients that olaparib capsules and tablets are not interchangeable and refer patients to the information provided in the package leaflet. There is no guidance about the choice of drug. The side-effect profiles are similar, although there are differences between the drugs. No comparative activity data exist, and selection is often based on familiarity with the drug and its availability.
Rechallenge with PARP-inhibitor maintenance treatment after relapse and subsequent platinum-based chemotherapy may be a viable treatment option and is currently under investigation [(OReO) ClinicalTrials.gov identifier: NCT03106987 (olaparib retreatment in patients with or without a BRCAm)], as is PARP-inhibitor combination treatment with other targeted agents including immuno-oncology drugs. The results of these trials will provide further direction of the treatment approach for patients with PSR ovarian cancer.
Conclusion
In summary, PARP inhibitors were developed with the intention of treating patients with HRD, specifically for patients with tumours that harbour a BRCAm. Evidence from clinical trials to date has demonstrated that patients with a BRCAm derive the greatest benefit from PARP inhibitors. However, there is a clear body of evidence showing that PARP inhibitors also benefit ovarian cancer patients without a BRCAm and the approval of olaparib, niraparib and rucaparib by the FDA and EMA in all PSR ovarian cancer populations who are in response to platinum supports this. Long-term tolerability and efficacy of olaparib have been demonstrated in patients both with and without a BRCAm through Study 19, with patients receiving maintenance treatment for 6 years or more, which is unprecedented in the relapsed ovarian-cancer setting. Further studies should be performed to elucidate which non-BRCAm patients are deriving benefit, and what HRD molecular processes are enabling this, so that patients continue to receive optimal treatment for their disease.
Acknowledgments
Jonathan Ledermann is a senior National Institute for Health Research (NIHR) investigator and receives funding from the NIHR Biomedical Research Centre at University College London/ University College London Hospitals. The authors acknowledge Claire Routley, PhD, of Mudskipper Business Ltd., who provided medical writing assistance funded by AstraZeneca and Merck & Co., Inc.
Footnotes
Funding: This work was supported by AstraZeneca and Merck & Co., Inc.
Conflict of interest statement: Jonathan A. Ledermann has attended AstraZeneca, Clovis Oncology, Pfizer and Roche Advisory Board Meetings and received lecture fees from these companies. He has received research funding from AstraZeneca and Merck/MSD.
Eric Pujade-Lauraine has attended AstraZeneca, Clovis, Pfizer, Roche and Tesaro Advisory Board Meetings, has received travel support from AstraZeneca, Roche and Tesaro, is Chair for ARCAGY Research and is a consultant for Menarini.
ORCID iD: Jonathan A. Ledermann  https://orcid.org/0000-0003-3799-3539
https://orcid.org/0000-0003-3799-3539
Contributor Information
Jonathan A. Ledermann, UCL Cancer Institute, University College London, 90 Tottenham Court Road, London W1T 4TJ, UK.
Eric Pujade-Lauraine, Hôpital Hôtel-Dieu, Université Paris Descartes, Paris, France.
References
- 1. European Medicines Agency. Lynparza recommended for approval in ovarian cancer. Press release, http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/10/news_detail_002196.jsp&mid=WC0b01ac058004d5c1 (2014, accessed 3 July 2018).
- 2. Evans T, Matulonis U. PARP inhibitors in ovarian cancer: evidence, experience and clinical potential. Ther Adv Med Oncol 2017; 9: 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov 2015; 5: 1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012; 72: 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 2016; 8: 362ps17. [DOI] [PubMed] [Google Scholar]
- 6. Paul MK, Mukhopadhyay AK. Tyrosine kinase - role and significance in cancer. Int J Med Sci 2004; 1: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowtell DD, Bohm S, Ahmed AA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015; 15: 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010; 28: 2512–2519. [DOI] [PubMed] [Google Scholar]
- 10. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434: 913–917. [DOI] [PubMed] [Google Scholar]
- 11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921. [DOI] [PubMed] [Google Scholar]
- 12. Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 2008; 105: 17079–17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gourley C, Friedlander M, Matulonis UA, et al. Clinically significant long-term maintenance treatment with olaparib in patients with platinum-sensitive relapsed serous ovarian cancer. J Clin Oncol 2017; 35: 5533. [Google Scholar]
- 14. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016; 375: 2154–2164. [DOI] [PubMed] [Google Scholar]
- 15. Pennington KP, Swisher EM. Hereditary ovarian cancer: beyond the usual suspects. Gynecol Oncol 2012; 124: 347–353. [DOI] [PubMed] [Google Scholar]
- 16. Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol 2011; 121: 353–357. [DOI] [PubMed] [Google Scholar]
- 17. Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012; 30: 2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer 2016; 60: 49–58. [DOI] [PubMed] [Google Scholar]
- 19. Kondrashova O, Topp M, Nesic K, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun 2018; 9: 3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2016; 2: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Randall LM, Pothuri B. The genetic prediction of risk for gynecologic cancers. Gynecol Oncol 2016; 141: 10–16. [DOI] [PubMed] [Google Scholar]
- 22. Frey MK, Pothuri B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: a review of the literature. Gynecol Oncol Res Pract 2017; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366: 1382–1392. [DOI] [PubMed] [Google Scholar]
- 25. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014; 15: 852–861. [DOI] [PubMed] [Google Scholar]
- 26. Friedlander M, Matulonis U, Gourley C, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer 2018; 119: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matulonis UA, Oza AM, Ho TW, et al. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015; 121: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 28. European Medicines Agency. Appendix I to the guideline on the evaluation of anticancer medicinal products in man. EMA/CHMP/27994-2008/Rev.1, https://www.ema.europa.eu/en/documents/scientific-guideline/appendix-1-guideline-evaluation-anticancer-medicinal-products-man-methodological-consideration-using_en.pdf (2013, accessed 7 May 2019).
- 29. European Medicines Agency. Guideline on the evaluation of anticancer medicinal products in man. EMA/CHMP/205/95 Rev.5, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/11/WC500238764.pdf (2017, accessed 21 May 2018).
- 30. Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol 2016; 17: 1579–1589. [DOI] [PubMed] [Google Scholar]
- 31. Hodgson DR, Dougherty BA, Lai Z, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer 2018; 119: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013; 31: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Timms KM, Abkevich V, Hughes E, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 2014; 16: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. AstraZeneca. Lynparza tablets receive EU approval for the treatment of platinum-sensitive relapsed ovarian cancer [press release], https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2018/lynparza-tablets-receive-eu-approval-for-the-treatment-of-platinum-sensitive-relapsed-ovarian-cancer08052018.html (2018, accessed 3 July 2018).
- 35. US Food and Drug Administration. Lynparza prescribing information (2017. Update), https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208558s000lbl.pdf (2014, accessed 3 July 2018).
- 36. European Medicines Agency. Lynparza (olaparib); EPAR, http://www.ema.europa.eu/ema/index.jsp?curl=/pages/medicines/human/medicines/003726/human_med_001831.jsp (2015, accessed 3 July 2018).
- 37. Mateo J, Moreno V, Gupta A, et al. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol 2016; 11: 401–415. [DOI] [PubMed] [Google Scholar]
- 38. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 39. Stone A, Gebski V, Davidson R, et al. Exaggeration of PFS by blinded, independent, central review (BICR). Ann Oncol. Epub ahead of print 23 November 2018. DOI: 10.1093/annonc/mdy514. [DOI] [PubMed] [Google Scholar]
- 40. Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 2012; 23: 2605–2612. [DOI] [PubMed] [Google Scholar]
- 41. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 42. Ledermann JA, Harter P, Gourley C, et al. Quality of life during olaparib maintenance therapy in platinum-sensitive relapsed serous ovarian cancer. Br J Cancer 2016; 115: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life (HRQOL) and patient-centered outcomes with maintenance olaparib compared with placebo following chemotherapy in patients with germline (g) BRCA-mutated (m) platinum-sensitive relapsed serous ovarian cancer (PSR SOC): SOLO2 phase III trial. J Clin Oncol 2017; 35: Abstr 5507. [Google Scholar]
- 44. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol 2018; 19: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diaby V, Adunlin G, Ali AA, et al. Using quality-adjusted progression-free survival as an outcome measure to assess the benefits of cancer drugs in randomized-controlled trials: case of the BOLERO-2 trial. Breast Cancer Res Treat 2014; 146: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gelber RD, Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol 1986; 4: 1772–1779. [DOI] [PubMed] [Google Scholar]
- 47. Gelber RD, Goldhirsch A, Cavalli F. Quality-of-life-adjusted evaluation of adjuvant therapies for operable breast cancer. Ann Intern Med 1991; 114: 621–628. [DOI] [PubMed] [Google Scholar]
- 48. Glasziou PP, Cole BF, Gelber RD, et al. Quality adjusted survival analysis with repeated quality of life measures. Stat Med 1998; 17: 1215–1229. [DOI] [PubMed] [Google Scholar]
- 49. US Food and Drug Administration. Lynparza (olaparib) capsules, for oral use: initial US approval, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206162s008lbl.pdf (2014, accessed 5 December 2018).
- 50. European Medicines Agency. Lynparza summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003726/WC500180151.pdf (2014, accessed 3 July 2018).
- 51. Myriad. myChoice® HRDTM, https://myriad.com/products-services/companion-diagnostics/mychoice-hrd/ (2018, accessed 23 January 2019).
- 52. Nova. Clinical study protocol, https://www.nejm.org/doi/suppl/10.1056/NEJMoa1611310/suppl_file/nejmoa1611310_protocol.pdf (2018, accessed 11 September 2018).
- 53. US Food and Drug Administration. Zejula: prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208447lbl.pdf (2017, accessed 3 July 2018).
- 54. European Medicines Agency. Zejula (niraparib), https://www.ema.europa.eu/en/medicines/human/EPAR/zejula (2019, accessed 4 March 2019).
- 55. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foundation Medicine. FoundationFocusTM CDxBRCA LOH, https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160018S001c.pdf (2018, accessed 11 September 2018).
- 57. US Food and Drug Administration. FDA approval for RUBRACA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209115s003lbl.pdf (2016, accessed 3 July 2018).
- 58. European Medicines Agency. Rubraca SmPC, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004272/WC500249806.pdf (2018, accessed 3 July 2018).
- 59. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1, https://www.nccn.org (2019, accessed 7 May 2019).
- 60. Berchuck A, Secord AA, Moss HA, et al. Maintenance poly (ADP-ribose) polymerase inhibitor therapy for ovarian cancer: precision oncology or one size fits all? J Clin Oncol 2017; 35: 3999–4002. [DOI] [PubMed] [Google Scholar]
- 61. Lheureux S, Lai Z, Dougherty BA, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res 2017; 23: 4086–4094. [DOI] [PubMed] [Google Scholar]
- 62. Hodgson DR, Dougherty B, Lai Z, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. In: European Cancer Congress 2015 Vienna, Austria, 25–29 September 2015, abst 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caruso D, Papa A, Tomao S, et al. Niraparib in ovarian cancer: results to date and clinical potential. Ther Adv Med Oncol 2017; 9: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 2019. DOI: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 65. US Food and Drug Administration. Avastin (bevacizumab) injection, for intravenous use. Prescribing Information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125085s323lbl.pdf (2018, accessed 3 July 2018).
- 66. European Medicines Agency. Avastin 25 mg/ml concentrate SmPC, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf (2018, accessed 3 July 2018).
- 67. Rossi L, Verrico M, Zaccarelli E, et al. Bevacizumab in ovarian cancer: a critical review of phase III studies. Oncotarget 2017; 8: 12389–12405. [DOI] [PMC free article] [PubMed] [Google Scholar]