Abstract
Mesenchymal stromal cells (MSCs) have been widely investigated for their therapeutic potential in regenerative medicine, owing to their ability to home damaged tissue and serve as a reservoir of growth factors and regenerative molecules. As such, clinical applications of MSCs are reliant on these cells successfully migrating to the desired tissue following their administration. Unfortunately, MSC homing is inefficient, with only a small percentage of cells reaching the target tissue following systemic administration. This attrition represents a major bottleneck in realizing the full therapeutic potential of MSC-based therapies. Accordingly, a variety of strategies have been employed in the hope of improving this process. Here, we review the molecular mechanisms underlying MSC homing, based on a multistep model involving (1) initial tethering by selectins, (2) activation by cytokines, (3) arrest by integrins, (4) diapedesis or transmigration using matrix remodelers, and (5) extravascular migration toward chemokine gradients. We then review the various strategies that have been investigated for improving MSC homing, including genetic modification, cell surface engineering, in vitro priming of MSCs, and in particular, ultrasound techniques, which have recently gained significant interest. Contextualizing these strategies within the multistep homing model emphasizes that our ability to optimize this process hinges on our understanding of its molecular mechanisms. Moving forward, it is only with a combined effort of basic biology and translational work that the potential of MSC-based therapies can be realized.
Subject Areas: Biological Sciences, Cell Biology, Stem Cells Research
Graphical Abstract
Highlights
-
•
Mesenchymal stromal cells (MSCs) are a promising platform for regenerative medicine
-
•
A major bottleneck of MSC therapies is their efficiency in homing damaged tissue
-
•
MSC homing is a multi-step process involving specific molecular interactions
-
•
A variety of strategies have been employed to optimize MSC homing
Biological Sciences; Cell Biology; Stem Cells Research
Introduction
Mesenchymal stromal cells (MSCs) are multipotent adult progenitor cells capable of differentiating into various mesenchymal tissues, most prominently bone, cartilage, and adipose. They were first isolated in 1974 from the bone marrow by Friedenstein and colleagues (Friedenstein et al., 1968). Since then, MSCs have been isolated from a variety of other tissues, including adipose (Zuk et al., 2002), perivasculature (Crisan et al., 2008), dental pulp (Gronthos et al., 2000), muscle, dermis (Young et al., 2001), and fetal tissue (Campagnoli et al., 2001, in 't Anker et al., 2003), including the Wharton jelly of umbilical cords (Wang et al., 2004).
The International Society for Cellular Therapy (ISCT) has laid down several defining criteria for the identification of MSCs (Dominici et al., 2006): (1) they must be plastic adherent in standard culture conditions, (2) express the surface markers CD105, CD90, and CD73; (3) not express other lineage markers CD45 (panleukocyte), CD34 (hematopoietic and endothelial), CD14/CD11b (monocytic), CD79a/CD19 (B cell), or human leukocyte antigen (HLA) class II; and (4) show the classical trilineage differentiation into osteoblasts, adipocytes, and chondroblasts. Several nonclassical fates have also been demonstrated, including myoblastic (Crisan et al., 2008), hepatocytic (Snykers et al., 2009), and neural (Arthur et al., 2008), although the neural fate remains controversial. Although MSCs derived from any tissue must meet these minimal criteria, those isolated from different tissues often exhibit considerable differences in their transcriptomic profiles (Jaager et al., 2012, Kern et al., 2006, Stockmann et al., 2012). Of note, MSCs are not a pure population of stem cells; the ISCT criteria actually describe MSCs as a heterogeneous, nonclonal mix of multipotent stem cells, committed progenitors, and differentiated cells (Squillaro et al., 2016). This fact prompted a change in nomenclature from “mesenchymal stem cell” to “mesenchymal stromal cell” to better reflect their cellular heterogeneity, although the terms are still used interchangeably.
Despite these complications surrounding their identity and isolation, there has been much interest revolving around MSCs for their therapeutic potential, especially in regenerative medicine. When tissues are damaged, MSCs are naturally released into circulation, migrate to the site of injury, and secrete molecules to create a microenvironment that promotes regeneration (Chapel et al., 2003, Caplan, 2009). Thus the idea behind their therapeutic potential is that allogenically transplanted MSCs can home damaged tissue and act as a “drug store” to aid in recovery or serve as an effector for tissue regeneration (Caplan, 2009). Upon reaching the target tissue, MSCs secrete a variety of factors with powerful immune-modulating, angiogenic, and anti-apoptotic effects (Le Blanc and Mougiakakos, 2012, Singer and Caplan, 2011, Bronckaers et al., 2014). Accordingly, MSCs have been investigated for a wide breadth of clinical applications, such as immune modulation in inflammatory and autoimmune diseases, tissue protection following injury, and regenerative medicine (Frenette et al., 2013). However, a major hurdle still lies in getting MSCs to go where they are needed—that is, optimizing MSC homing efficiency. This review discusses the molecular details of the homing process and the various strategies that have been explored for optimizing it.
MSC Homing Mechanisms
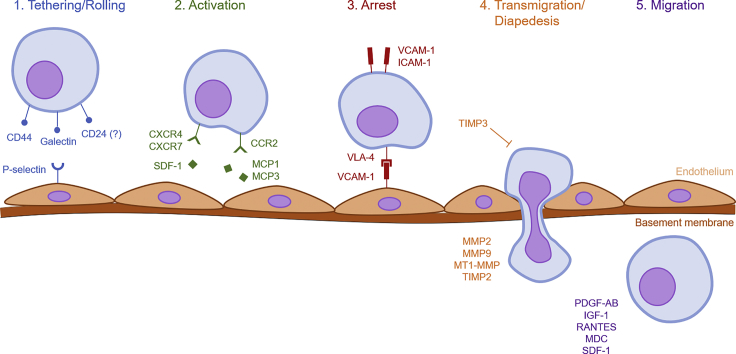
One of the key benefits of MSC-based therapies is their ability to preferentially home damaged tissues. It is worth elaborating on what exactly we mean by homing, as the term is often used vaguely in the literature. Following the recommendations of previous reviews, we take homing to encompass both non-systemic and systemic homing (Nitzsche et al., 2017). In non-systemic homing, MSCs are transplanted locally at the target tissue and are then guided to the site of injury via a chemokine gradient. In systemic homing, MSCs are administered or endogenously recruited into the bloodstream and then must undergo a multistep process to exit circulation and migrate to the injury site. The process of systemic homing can be split into five steps: (1) tethering and rolling, (2) activation, (3) arrest, (4) transmigration or diapedesis, and (5) migration (Sackstein, 2005) (Figure 1).
Figure 1.
Mesenchymal Stromal Cell Homing Mechanisms
Overview of the molecular mechanisms facilitating each step of mesenchymal stromal cell (MSC) homing.
The initial tethering is facilitated by selectins expressed by endothelial cells. MSCs express CD44, which catches onto the selectins and causes the cell to begin rolling along the vasculature wall (Sackstein et al., 2008). The exact selectin used by MSCs is still an active area of investigation, especially because they express neither the hematopoietic cell E- and L-selectin ligand (HCELL) nor the P-selectin glycoprotein ligand-1 (PSGL-1) (Sackstein et al., 2008). To model the tethering process, Rüster et al. constructed a parallel plate flow chamber seeded with endothelial cells (Ruster et al., 2006). They demonstrated that anti-P-selectin antibodies suppress MSC binding to endothelial cells, whereas immobilized P-selectin is sufficient to induce MSC rolling. However, as MSCs do not express PSGL-1, they must use a different ligand to interact with P-selectin. One study has identified galectin-1 as one such candidate (Suila et al., 2014). Another study has identified CD24 as a potential P-selectin ligand in adipose tissue-derived stromal cells, which resemble MSCs (Bailey et al., 2009).
The second step, activation, is facilitated by G protein-coupled chemokine receptors, generally in response to inflammatory signals. Indeed, mice subjected to total body or local irradiation have higher levels of MSC engraftment owing to the resulting inflammation response (Francois et al., 2006). The expression of stromal cell-derived factor (SDF)-1 on endothelial cells is considered critical for this step (Lau and Wang, 2011). SDF-1 is the ligand to the chemokine receptor CXCR4, which is thought to be expressed by MSCs (Wynn et al., 2004, Gao et al., 2009, Honczarenko et al., 2006). Indeed, overexpression of CXCR4 on MSCs increases homing to the bone marrow (Bobis-Wozowicz et al., 2011). However, some groups report that MSCs do not express CXCR4 (Von Luttichau et al., 2005), suggesting that other receptors are also involved. Indeed, it has been shown that MSCs also express CXCR7, which similarly binds SDF-1 to facilitate homing to various tissues (Li et al., 2013, Wang et al., 2014, Shao et al., 2019). Other chemokines and receptors are known to play a role as well. For example, transgenic mice expressing the inflammation marker MCP-1 on the myocardium were able to recruit MSCs expressing the corresponding receptor CCR2, due to the direct interaction between the ligand and receptor (Belema-Bedada et al., 2008). In a similar study, MCP-3 expression in the myocardium greatly increased MSC engraftment as well (Schenk et al., 2007). MSCs express a variety of other receptors, including CCR1, CCR4, CCR7, CCR9, CCR10, CXCR5, and CXCR6 (Honczarenko et al., 2006, Von Luttichau et al., 2005), although their roles remain to be elucidated in detail. Regardless of the specific receptor-ligand interaction, the role of the activation step is to increase the affinity of integrins by inducing conformational changes in their extracellular domains; these integrins are essential for cell arrest (Lin et al., 2013, Constantin et al., 2000). For example, SDF-1 stimulation recruits the signaling molecules talin and kindlin to the cytoplasmic domain of VLA-4, causing the latter to shift to a high-affinity conformation (Tadokoro et al., 2003). The expression profile of these receptors likely plays a large role in determining the tissues to which MSCs will migrate.
The third step, arrest, is facilitated by integrins. MSCs express VLA-4 (integrin α4β1), which become activated in response to chemokines like SDF-1. Following activation, the VLA-4 integrin binds to VCAM-1 on endothelial cells (Segers et al., 2006, Ruster et al., 2006, Steingen et al., 2008). Indeed, neutralizing antibodies against β1-integrin inhibit MSC homing to ischemic myocardium, although the same was not true for antibodies blocking α4-integrin (Ip et al., 2007). Overexpression of α4-integrin increases MSC homing to the bone marrow (Kumar and Ponnazhagan, 2007). Interestingly, the MSCs themselves also express integrin ligands including vascular cell adhesion molecule (VCAM)-1 and intercellular cell adhesion molecule (ICAM)-1 (Krampera et al., 2005, Krampera et al., 2006), although their importance has not been fully evaluated.
In the next step, transmigration or diapedesis, MSCs must transcellularly travel through the endothelial cell layer and basement membrane. To accomplish this, MSCs secrete matrix metalloproteinases (MMPs) to break down the endothelial basement membrane (Steingen et al., 2008). MMP maturation and activity are regulated by various other proteins, most prominently the tissue inhibitors of metalloproteinases (TIMPs). The expression of these remodeling enzymes is induced by inflammatory cytokines, which serve as a signal for migration into damaged tissue (Ries et al., 2007). Becker et al. showed that knockdown of MMP2 resulted in significantly decreased migration in vitro, as did the exogenous addition of TIMP3 to the culture media (De Becker et al., 2007). Ries et al. similarly demonstrated that knockdown of MMP2, MT1-MMP, or TIMP2 in MSCs reduces invasion, whereas knockdown of TIMP1 increases it (Ries et al., 2007). Although it may seem strange that TIMP2 facilitates transmigration, it actually plays a role in the maturation of MMP2 from its proenzyme form to its active form (Will et al., 1996). There are likely more molecular interactions involved in MSC transmigration, although they are as of yet poorly understood. Leukocyte transmigration, for example, requires homophilic interactions between platelet endothelial cell adhesion molecule expressed on both the leukocyte and endothelium as well as between CD99 (Muller, 2011). An equivalent process in MSCs, however, has not yet been described.
Finally, the MSC must migrate through the interstitium to the site of injury. This step is guided by chemotactic signals released in response to tissue damage. MSCs migrate toward various signals, including the growth factors platelet-derived growth factor-AB and insulin-like growth factor (IGF)-1, and to a lesser extent, the chemokines RANTES, MDC, and SDF-1 (Ponte et al., 2009). Preincubating the MSCs with tumor necrosis factor (TNF)-α increases their migration toward chemokines by upregulating their receptors CCR2, CCR3, and CCR4. The inflammatory chemokine interleukin (IL)-8 may promote migration of MSCs to injured sites (Bi et al., 2014, Bayo et al., 2017) and also stimulates them to secrete regenerative factors like vascular endothelial growth factor (VEGF) (Hou et al., 2014). Detailed knowledge of the molecular events facilitating MSC homing immediately presents a variety of strategies for optimizing the process for therapeutic purposes.
Improving MSC Homing
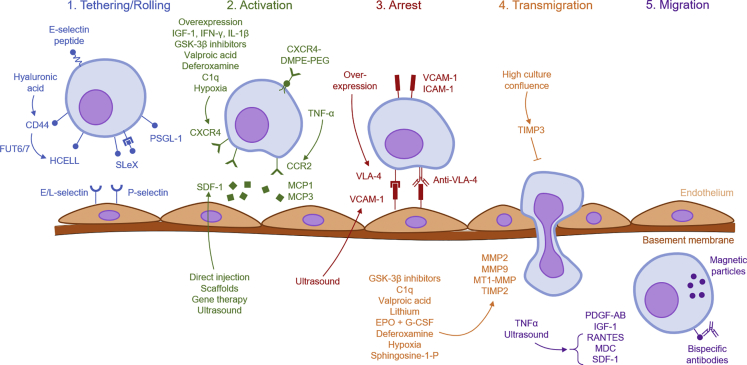
One of the biggest challenges facing MSC therapies is improving their homing efficiency. The percentage of intravenously (i.v.) administered MSCs that reach the target tissue is in the low single digits, as demonstrated by various imaging studies (Devine et al., 2003, Barbash et al., 2003, Kraitchman et al., 2005). What causes this low homing efficiency? At least part of the reason is physiological: i.v.-administered MSCs get trapped in the lung capillaries (Scarfe et al., 2018). Indeed, vasodilators and anticoagulants like heparin reduce lung trapping and increase MSC homing to other sites like the liver and bone marrow (Gao et al., 2001, Yukawa et al., 2012). The process of homing, however, is fundamentally based on specific molecular interactions, not passive dissemination. It may be the case that the expression of homing molecules, like CXCR4, is just too low on MSCs (Wynn et al., 2004, Von Luttichau et al., 2005). It has also been observed that the in vitro expansion of MSCs gradually leads to the loss in expression of homing molecules (Honczarenko et al., 2006, Rombouts and Ploemacher, 2003). To remedy these problems, a variety of approaches have been taken to improve MSC homing (Figure 2). These strategies can be broadly categorized into seven approaches: (1) targeted administration, (2) magnetic guidance, (3) genetic modification, (4) cell surface engineering, (5) in vitro priming, and (6) modification of the target tissue, and (7) radiotherapeutic techniques (Table 1).
Figure 2.
Strategies for improving Mesenchymal Stromal Cell Homing
Overview of the various strategies that have been employed to improve mesenchymal stromal cell (MSC) homing, organized by which step it targets. Arrows indicate upregulation.
Table 1.
Overview of Strategies Targeting Each Step of Mesenchymal Stromal Cell Homing
Targeted Administration
Perhaps the most conceptually simple method to improve MSC homing is a physiological one: administer the cells at or near the target tissue, instead of infusing them through standard i.v. routes—that is, utilizing non-systemic rather than systemic homing. It may seem intuitive that direct delivery of MSCs to a tissue may result in greater retention, for example, intramyocardial for heart disease, intratracheal (IT) for lung disease, or intracerebral for neurological diseases.
Considerable work has gone into developing the medical technology needed for targeted administration. Indeed, several clinical trials investigating MSC therapies for ischemic cardiomyopathy use transcatheter injections directly to the myocardium (Williams et al., 2011, Heldman et al., 2014), although none have directly compared the efficacy of different routes of administration. Dick et al. took targeted delivery to the extreme by using magnetic resonance fluoroscopy to identify infarct borders real time in a porcine model (Dick et al., 2003). Here, the authors safely navigated catheters to the infarcted region to deliver MSCs at this site. The injected MSCs were then visible upon delivery and still detectable postmortem using magnetic resonance imaging. Although this study confirmed the successful physical delivery of cells, no attempt was made to quantify the engraftment efficiency, the percentage of MSCs retained in the tissue.
Nevertheless, there is decent evidence that the route of administration has a significant effect on treatment efficacy, presumably due to increased homing. Unfortunately, although many studies administer MSCs directly to the tissue of interest, very few internally compare the targeted administration against standard i.v. administration, so the evidence for the optimal route of administration largely comes from meta-analyses. In ischemic stroke, intracerebral administration of MSCs seems to result in the best improvements in the neurological severity score, followed by intra-arterial, and then i.v. routes (Vu et al., 2014). For myocardial infarction in swine models, transendocardial administration of MSCs seems to reduce infarct size, whereas intramyocardial, intracoronary, and i.v. administration showed no significant improvement (Kanelidis et al., 2017). However, in a different meta-analysis of human trials, MSCs were found to improve left ventricular ejection fraction via intracoronary, i.v., and intramyocardial routes, in descending order of effect size (Jeong et al., 2018). For bronchopulmonary dysplasia, the most effective routes of administration are, from best to worst, i.v., IT, intraperitoneal, and intranasal, with the last showing no significant improvement (Augustine et al., 2017). In acute lung injury, IT and i.v. administration both seem effective, whereas intraperitoneal is not (McIntyre et al., 2016). However, the route of administration does not appear to affect MSC therapy outcomes for traumatic brain injury (Peng et al., 2015). Evidently, it should not always be presumed that direct administration of MSCs to the target organ will yield the best outcomes. In one of the few studies that internally compared different administration routes, Antunes et al. administered MSCs into a porcine model of emphysema via either the i.v. or IT route (Antunes et al., 2014). Both routes led to decreased lung inflammation and cell damage and restored elastic fiber content. However, only the i.v. route improved cardiovascular function and shifted lung macrophage phenotypes from M1 to M2. This may be a special case for the lung, given that i.v.-administered MSCs will naturally get trapped in the lung capillaries.
Several groups have explored transplantation of MSC sheets rather than suspensions. MSC sheets are monolayers of cells that are grown in culture that then spontaneously detach with a decrease in temperature. Using a rat model of chronic myocardial infarction, Ishikane et al. transplanted MSC sheets directly onto scarred myocardium (Ishikane et al., 2013). Compared with untreated controls, the treated mice showed significantly improved cardiac function, reduced myocardial fibrosis, and greater capillary density in the infarcted region. The transplantation also elicited an M2 macrophage response. Kawamura et al. showed a similar result using a xenotransplantation model of human MSC sheets into a porcine model of ischemic cardiomyopathy (Kawamura et al., 2015), demonstrating improved cardiac performance, attenuated ventricular remodeling, and increased vascularization in pigs transplanted with MSC sheets, compared with controls. Unlike these previous two studies, which did not compare MSC sheet transplant against intraorgan injection, Tano et al. directly assessed the efficacy of epicardial MSC sheet placement versus intramyocardial injection in a rat model of ischemic cardiomyopathy (Tano et al., 2014), showing that the MSC sheets improved myocardial repair more so than MSC suspensions injected intramyocardially. Using a rat model of colitis, Pak et al. endoscopically transplanted MSC sheets into the colon (Pak et al., 2018). The MSC sheets successfully attached to the inflamed mucosa and led to significantly reduced ulcer size and inflammation compared with sham-treated controls, although no comparisons were made to other routes of administration. Kaibuchi et al. tested MSC sheet transplantation as a treatment for osteonecrosis of the jaw (Kaibuchi et al., 2016). The MSC sheet group showed significantly improved wound healing (12.5% occurrence of bone exposure) compared with both the control and the i.v. injection groups (80% and 100% occurrence of bone exposure, respectively). The directly transplanted sheets also resulted in greater vascularization (106 vessels/mm2) compared with both the control and i.v. injection groups (40 and 62 vessels/mm2, respectively). MSC sheet transplantation may be superior to injection of MSC suspensions because of (1) a better safety profile (no risk of embolism), (2) improved donor cell survival, and (3) improved secretion of regenerative factors. Although such studies strongly indicate the importance of targeted administration, the results are of course highly dependent on the tissue and disease model.
Magnetic Guidance
Another physical approach to targeting MSCs comes in the form of magnetic guidance, wherein cells labeled with magnetic particles are directed to the organ of interest using an external magnetic field. Arbab et al. labeled MSCs with iron oxide (ferumoxide) particles, which were injected i.v. into rats with or without an external magnet positioned over the liver (Arbab et al., 2004). Rats that wore the external magnet had around 2-fold the number of labeled MSCs in the liver 15 days post-administration. In the rats not wearing magnets, the MSCs mostly localized around the portal triad, but in the rats that did wear magnets, the MSCs infiltrated deeper into the liver parenchyma. This study did not explore which particular step of homing was enhanced, but it is presumed that the slowed intravascular movement of the MSCs played an essential role. Using an ex vivo system, Kobayashi et al. were able to target magnetically labeled MSCs onto an osteochondral defect in the knee joint, with the use of an external magnetic field (Kobayashi et al., 2008). Using an in vivo rat model, Yanai et al. were able to target magnetically labeled MSCs to the retina following both intravitreal or i.v. administration, with the assistance of an external magnet placed in the orbit of the rat (Yanai et al., 2012). The rats with the external magnet had significantly higher retinal levels of anti-inflammatory molecules (IL-10) and growth factors (hepatocyte growth factor [HGF]) compared with those without the magnet, suggesting a therapeutic benefit as well. Yun et al. successfully targeted magnetically labeled MSCs to damaged olfactory bulbs using a magnet implanted in the scalp (Yun et al., 2018). Interestingly, they noted an upregulation of CXCR4 resulting from internalization of the magnetic particles, which would facilitate MSC activation. Much work has been done exploring different types of magnetic particles, which have varying effects on MSC viability, proliferation, differentiation, and gene expression (Wang et al., 2017). In most studies, however, there has been no observed effect of magnetic labeling on MSC function (Hsiao et al., 2007, Song and Ku, 2007). Of course, in a real clinical setting, the placement of a permanent magnet must also be practical and not excessively invasive, limiting the target sites for which this technique might be useful.
Genetic Modification
Permanent Overexpression
Many early studies exploring genetic modification of MSCs utilized the permanent overexpression of homing factors via viral transduction. The overexpression of CXCR4 in MSCs increases their activation and engraftment to ischemic myocardium in rats, which expresses the CXCR4 ligand SDF-1. This enhanced homing has been shown to improve post-myocardial infarction recovery (Cheng et al., 2008, Zhang et al., 2008). CXCR4-overexpressing MSCs also show increased homing to the bone marrow in mice (Bobis-Wozowicz et al., 2011, Chen et al., 2013), as well as to damaged intestinal mucosa in a mouse model of colitis (Chen et al., 2018b). CXCR7 overexpression has been demonstrated to promote MSC homing to injured lung tissue (Shao et al., 2019). However, it seems that neither CXCR4 nor CXCR7 overexpression improves homing to the kidney in mouse models of acute kidney injury (AKI) (Gheisari et al., 2012).
At the step of cell arrest, overexpression of the α4-integrin, a component of the VLA-4 integrin, increases MSC homing to the bone marrow (Kumar and Ponnazhagan, 2007). However, α4-integrin overexpression did not increase homing to the heart in a rat model of stroke, although it did decrease the formation of potentially harmful cell aggregates (Cui et al., 2017). As with any type of gene therapy, such approaches come with inherent safety concerns; the integration of viral DNA within a tumor suppressor gene, for example, can result in insertional oncogenesis. Viral transduction is also a lengthy and relatively costly process.
Transient Overexpression
Owing to the potential safety concerns of viral transduction, several groups have instead opted for mRNA transfection because of its simplicity, rapid protein expression, and transience. Levy et al. sought to improve MSC tethering by transfecting mRNA for PSGL-1 and SLeX, the ligands for P- and E-/L-selectin, respectively (Levy et al., 2013). Transfected cells successfully rolled on inflamed endothelium in vitro and in vivo and showed enhanced homing to the bone marrow and the inflamed ear in a mouse model. Liao et al. used a similar transfection strategy, showing that the modified MSCs significantly increased rolling and adherence on inflamed brain microvascular endothelial cells and enhanced homing to inflamed spinal cord in a mouse model of encephalomyelitis, where they were better able to increase myelination and decrease inflammation (Liao et al., 2016). Ryser et al. transfected CXCR4 mRNA into MSCs, which increased migration in transwell migration experiments toward an SDF-1 gradient (Ryser et al., 2008). However, Wiehe et al. were unable to replicate such results; although they successfully overexpressed CXCR4 following mRNA nucleofection, the modified MSCs showed no improvement in cell migration (Wiehe et al., 2013). The authors use their results to emphasize the importance of other factors responsible for MSC activation, outside of the SDF-1/CXCR4 axis.
Cell Surface Engineering
Enzymatic Modification
Given the potential safety concerns of genetic modification, many groups have attempted to directly chemically engineer the cell surface of MSCs. Unlike viral transduction, these modifications are temporary. However, as transmigration occurs just a few hours post-administration (Teo et al., 2012), even a transient modification should be sufficient to improve homing. MSCs naturally express the selectin ligand CD44, but not HCELL, the ligand for E- and L-selectin that hematopoietic stem cells use to home to the bone marrow. In a seminal study, Sackstein et al. were able to enzymatically convert CD44 into HCELL via sugar modifications (specifically, an α(1,3)-exofucosylation), allowing MSCs to utilize E- and L-selectin to tether and home to the bone marrow (Teo et al., 2012). In a mouse model of diabetes, this modification increased infiltration of MSCs to pancreatic islets by 3-fold following i.v. administration and durably reversed hyperglycemia (Abdi et al., 2015). These sugar modifications can also be exploited via genetic modification: expression of the α(1,3)-fucosyltransferase FUT6 or FUT7 in MSCs converts CD44 to HCELL, allowing for E-selectin binding (Lo et al., 2016, Dykstra et al., 2016, Chou et al., 2017).
Ligand Conjugation
Instead of modifying existing surface glycoproteins, cell surface engineering methods can be used to directly conjugate desired ligands. Sarkar et al. developed a platform that can theoretically attach any ligand to the MSC surface. They used biotinylated lipid vesicles to coat MSCs in biotin. They then attached a streptavidin adaptor, followed by biotinylated SLeX, the active site of the P-selectin ligand PSGL-1 (although any biotinylated ligand could theoretically be attached). The modification was able to improve adhesion to a P-selectin-coated surface (Sarkar et al., 2008).
Cheng et al. conjugated an E-selectin-binding peptide to MSCs via an NHS-PEG2-maleimide linker molecule (Cheng et al., 2012). The preparation time was quick (30 min), and the modified MSCs adhered better than controls to an in vitro model of inflamed endothelium.
The attachment of antibodies to the MSC cell surface is another popular strategy. One such technique uses palmitated protein G: the palmityl group serves as an anchor to the cell membrane, whereas protein G serves as a dock for binding antibodies. Lo et al. used this method to attach a fragment of PSGL-1 bound to an IgG tail, which successfully facilitated tethering and rolling at high shear stresses (Lo et al., 2013). Ko et al. used the same system to attach anti-ICAM-1 antibodies, which facilitated cell arrest in a flow chamber (Ko et al., 2009). The same group also attached anti-VCAM-1 antibodies to MSCs, which they tested in a mouse model of inflammatory bowel disease. Compared with untreated MSCs, the anti-VCAM-1-coated MSCs showed improved homing to the inflamed lymph nodes (1.3-fold) and colon (1.8-fold), as well as dramatically improved survival rates and clinical endpoints (Ko et al., 2010).
Bispecific antibodies have also been investigated, with one end recognizing a native MSC marker and the other end recognizing a target. Lee et al. generated bispecific antibodies, one end of which attached to CD44 on the MSC surface and the other end of which recognized myosin light chain expressed by infarcted myocardium. The armed MSCs were able to localize to the infarcted region of the heart (Lee et al., 2007). Gundlach et al. synthesized a bispecific antibody that recognized CD90 expressed by MSCs on one end and MLC expressed by ischemic myocardium on the other end (Gundlach et al., 2011). The construct improved MSC adhesion to immobilized MLC in vitro, although it still has not been tested in vivo. Both these studies seeked to enhance the migration of MSCs to the damaged tissue following extravasation by targeting injury markers at the final destination.
Unlike the previous studies that focused on the initial tethering, Won et al. sought to optimize the activation step. They conjugated recombinant CXCR4 to DMPE-PEG, a phospholipid that provides cell membrane anchoring. These modified MSCs were better able to migrate toward an SDF-1 gradient, in a CXCR4-dose-dependent manner (Won et al., 2014).
In nearly all these studies, cell viability, proliferation, adhesion, and differentiation were not affected. However, these cell surface engineering methods tend to be complex, requiring complicated reactions and optimization for each specific ligand or receptor.
In Vitro Priming
Priming methods avoid genetic and chemical modifications entirely by simply altering culture conditions to affect gene expression. These methods have been used to improve the tethering, activation, and transmigration steps of systemic homing.
Culture Media Supplementation
At the tethering step, coating MSCs with hyaluronic acid leads to the upregulation of CD44, the selectin ligand. This treatment doubled in vitro invasion and increased targeting to an LPS-induced inflamed ear murine model, subsequently reducing inflammation (Corradetti et al., 2017).
The greatest amount of attention has been focused at the activation step by increasing CXCR4 expression. Several combinations of soluble factors have been found to increase its expression, including (1) a combination of FLT3LG, stem cell factor (SCF), IL-3, IL-6, and HGF (Shi et al., 2007); (2) IGF-1 (Li et al., 2007); (3) interferon (IFN)-γ (Duijvestein et al., 2011); (4) IL-1β (Fan et al., 2012b); (5) glycogen synthase kinase (GSK)-3β inhibitors (Kim et al., 2013); (6) the mood stabilizer drug valproic acid (Tsai et al., 2010, Tsai et al., 2011); and (7) the iron chelator deferoxamine (Najafi and Sharifi, 2013). Hypoxic cell culture conditions induce hypoxia-inducible factor (HIF)-1α, which increases the expression of CXCR4 (Annabi et al., 2003) as well as CX3CR1 (Hung et al., 2007) and CXCR7 (Liu et al., 2010, Meng et al., 2018). Deferoxamine seems to stabilize HIF-1α even under normal oxygen levels (Chu et al., 2008), increasing the expression of various homing genes (Najafi and Sharifi, 2013). Exposure to complement 1q (C1q) enhances MSC migration toward SDF-1, partly by increasing surface expression of CXCR4 (Qiu et al., 2012). Other homing receptors can be upregulated by culture conditions as well. Treatment with TNF-α was found to increase MSC migration toward chemokines by upregulating expression of several chemokine receptors including CCR2, CCR3, and CCR4, but not CXCR4 (Ponte et al., 2009).
Several of these treatments also facilitate transmigration by increasing the expression of MMPs. GSK-3β inhibitors increase MMP2 and MT1-MMP expression (Kim et al., 2013). C1q treatment increases secretion of MMP2 (Qiu et al., 2012). Two mood stabilizer drugs, valproic acid and lithium, increase MMP2 and MMP9 activity, respectively (Tsai et al., 2010). The combination of erythropoietin and granulocyte colony-stimulating factor also increases MMP2 expression, improving MSC migration (Yu et al., 2011). The iron chelator deferoxamine increases expression of MMP2 and MMP9 through HIF-1α signaling (Najafi and Sharifi, 2013). Hypoxic conditions seem to have a mixed effect on MMP expression: MMP2 is downregulated, whereas MT1-MMP is upregulated, although the overall effect is still significantly increased transmigration (Annabi et al., 2003). Treatment with sphingosine-1-phosphate has been found to enhance MSC migration in in vitro transwell assays (Kang et al., 2015) and increase homing to infarcted myocardium, possibly by upregulating MMP9 (Chen et al., 2018a).
Culture Confluence
Interestingly, the cell culture confluence also seems to affect MSC migratory ability, although there are conflicting results in the literature. De Becker et al. found that MSCs cultured to high confluence secrete more TIMP3, an inhibitor of MMPs, resulting in decreased migration compared with MSCs cultured at low confluence (De Becker et al., 2007). Kim et al. conducted thorough gene expression profiling of MSCs grown at low or high density. Low-confluence MSCs expressed more proliferation-related genes, whereas high-confluence MSCs expressed more genes linked to activation (CXCL1, CXCL2, CXCL5, CXCL6, CXCL8, CXCL16), immune modulation (IL-1B, IL-6), migration, and regeneration (VEGFA, FGF9, PDFGD, A2M, MDK, WISP2, GDF15) (Kim et al., 2014). Thus it is unclear whether low- or high-density MSC cultures are preferable for therapeutic applications.
Coculture
Coculture with other cell populations also influences MSC migration. Sertoli cells are found in the testes and are generally thought of as “nurse cells” to developing germ cells. Coculturing MSCs with Sertoli cells upregulates proliferation genes as well as homing genes in the former, including CXCR4 (Zhang et al., 2012) and MMP-2 (Luo et al., 2018). Coculture of amniotic MSCs with amniotic epithelial cells enhances proliferation and upregulates CXCR4 (Ran et al., 2018). Treating MSCs with conditioned media from endothelial cells increases their migration in vitro, possibly due to the presence of cytokines like IL-6 and IL-8 (Zhidkova et al., 2018).
Modification of Target Tissue
All previous methods have sought to modify the MSCs to potentiate their homing ability. Instead of modifying the MSCs, however, perhaps the target tissue can be modified to make them a more attractive destination.
Direct Injection of Homing Factors
The direct injection of SDF-1 into ischemic tissue has been demonstrated to enhance neovascularization in both skeletal muscle (Yamaguchi et al., 2003) and myocardium (Sasaki et al., 2007, Saxena et al., 2008). Although the study did not use MSCs, Yamaguchi et al. found that SDF-1 injection into ischemic hindlimb muscle improved the homing of i.v.-infused endothelial progenitor cells 1.8-fold in mice (Yamaguchi et al., 2003). Sasaki et al. directly injected SDF-1 into ischemic myocardium and observed increased endogenous homing of bone marrow cells, although these were not confirmed to be MSCs (Sasaki et al., 2007). One critical drawback to this technique is the short half-life of SDF-1 because of its quick degradation by proteolytic enzymes. To overcome this, Segers et al. bioengineered a protease-resistant version of SDF-1 that was still capable of binding CXCR4, improve cardiac function following myocardial infarction, and improve blood flow following peripheral artery disease (Segers et al., 2007, Segers et al., 2011). To our knowledge, no study has combined direct SDF-1 injection with MSC administration.
Genetic Modification of Target Tissue
Other groups have attempted to actually transfect the target tissue with constructs encoding for chemokines (Penn et al., 2012). Fujii et al. used a method known as ultrasound-mediated microbubble destruction (UMMD) to deliver an SDF-1- or SCF-containing plasmid into the myocardium. The plasmids are injected alongside microbubbles, which cavitate in response to ultrasound and create shear stress that facilitates uptake of the plasmid. This technique successfully increased SDF-1 expression in the myocardium and increased homing of endogenous CXCR4-expressing progenitor cells (Fujii et al., 2011). Sundararaman et al. achieved similar results without the use of UMMD, instead injecting the SDF-1 plasmid directly into the heart (Sundararaman et al., 2011). Treated mice showed improved angiogenesis and cardiac function. This therapy was taken into a phase I clinical trial, where 17 patients with ischemic cardiomyopathy were administered the SDF-1 plasmid directly do the infarcted region (Penn et al., 2013). After 12 months, the patients demonstrated improvements in walk distance and quality of life, in a dose-dependent manner. This initial human study did not include a control group. In the follow-up phase II placebo-controlled study, there was no significant difference between placebo and treatment groups based on the primary endpoints of 6-min walk distance or the Minnesota Living with Heart Failure Questionnaire score (Chung et al., 2015). However, by limiting the analysis to the sickest one-third of patients, there was significant improvement in ejection fraction, attenuation of left ventricle size, and stroke volume. As is the case with the genetic modification of MSCs, though, transfection of the target tissue comes with concerns regarding immunogenicity and insertional mutagenesis, as well as cost.
Scaffold Implantation
A final method of cytokine delivery comes in the form of scaffolds. Many of the scaffolds being studied release SDF-1 after they are implanted at the target site. Kimura and Tabata developed a gelatin hydrogel that slowly releases SDF-1. Subcutaneous implantation of the hydrogel was superior to injection of SDF-1, as measured by angiogenesis and CXCR4 expression at the implantation site (Kimura and Tabata, 2010). Similarly, He et al. synthesized a poly(lactide ethylene oxide fumarate) hydrogel loaded with SDF-1, which was able to increase bone marrow stromal cell migration in vitro in a dose-dependent manner (He et al., 2010). Goncalves et al. developed a chitosan/poly(γ-glutamic acid) complex incorporated with SDF-1, which increased MSC migration in vitro (Goncalves et al., 2012). Shen et al. engineered a bioactive knitted silk-collagen sponge scaffold that could be incorporated with SDF-1. In a rat Achilles tendon injury model, the scaffold successfully enhanced migration of endogenous progenitor cells and enhanced tendon regeneration (Shen et al., 2010). In a slightly more involved system, Schantz et al. developed a cellular polycaprolactone scaffold, from which SDF-1 could be constantly delivered using a microneedle apparatus (Schantz et al., 2007). Similarly, Thevenot et al. designed a poly(lactic-co-glycolic acid) scaffold that releases SDF-1 via a miniosmotic pump. After subcutaneous implant in mice, these scaffolds resulted in a 3-fold increase in homing (Thevenot et al., 2010). Although such scaffolds may serve as a “homing beacon” for MSCs, they may be difficult to optimize, and come with safety and cost concerns.
Radiotherapeutic Techniques
When target tissues are irradiated, they show a higher rate of MSC engraftment (Chapel et al., 2003, Mouiseddine et al., 2007). This increase is due to upregulated expression of SDF-1, thus promoting the activation step of homing (Ponomaryov et al., 2000). Of course, radiation therapy comes with inherent safety concerns in human patients, and so although these results are conceptually interesting, it may not be an option that can be implemented clinically. This is especially true with safer alternative techniques that achieve similar improvements in MSC homing—namely, the use of sound waves.
Ultrasound Techniques
In addition to its diagnostic applications, ultrasound has been adopted for a variety of therapeutic applications (Miller et al., 2012). Unlike diagnostic ultrasound, therapeutic ultrasound is selectively focused on the tissue of interest. Sophisticated image guidance technology allows for the targeting of deep structures without affecting intervening tissue. At the target site, the mechanical pressure exerted by sound waves induces various biological effects that aid in regeneration.
Many studies have investigated UMMD for improving MSC homing. In this technique, microbubbles are administered systemically alongside the MSCs. Ultrasound is focused on the target tissue, which cavitates the microbubbles, causing shock waves and shear stress that exert various biological effects. These effects include changes in gene expression, and the disruption of endothelial linings, which increases vascular permeability. Several studies have utilized UMMD in combination with MSCs to increase cardiac recovery following myocardial infarction (Zen et al., 2006, Ghanem et al., 2009, Xu et al., 2010). This effect likely arises from both the increased vascular permeability, as well as changes in gene expression. Li et al. found that UMMD both elevates secretion of SDF-1 on the target tissue and increases the proportion of MSCs that express CXCR4 (Li et al., 2015). In the kidney, UMMD has been shown to promote MSC homing by the upregulation of cytokines, integrins, selectins, and other trophic factors (Wang et al., 2015). UMMD to the brain has been shown to induce a sterile inflammatory response that upregulates a variety of inflammatory and trophic factors, including MMPs (Kovacs et al., 2017), although MSC homing has not yet been assessed in this system. However, as microbubble cavitation is known to be damaging to the host tissue (Burgess et al., 2011, Chonpathompikunlert et al., 2012, Fan et al., 2012a), some investigators have begun looking into focused ultrasound without microbubbles.
Pulsed focused ultrasound (pFUS), as its name implies, administers focused pulses of high-intensity sound waves. The non-continuous administration of the waves prevents extreme temperatures and tissue damage (Burks et al., 2013). Instead, pFUS exerts its effects solely by mechanical pressure, activating mechanotransduction pathways that upregulate inflammatory and chemoattractive molecules (Ziadloo et al., 2012). There has been a quickly growing body of literature studying the molecular changes induced by pFUS. In the muscle, Burks et al. demonstrated that pFUS creates a transient local chemical gradient. Upregulated molecules include cytokines, growth factors, and cell adhesion molecules, like ICAM-1 and VCAM-1 (Burks et al., 2011). This chemical gradient enhances homing of MSCs to the muscle and induces an anti-inflammatory M2 macrophage response (Burks et al., 2013). To establish this local gradient, pFUS seems to induce an initial activation of TNF-α, which drives cyclooxygenase-2 (COX2) signaling that upregulates various cytokines and homing factors (Tebebi et al., 2015). In a mouse model of limb ischemia, pFUS + MSCs increased MSC homing 4-fold compared with MSCs alone, resulting in improved clinical outcomes (Tebebi et al., 2017). Parallel results have been achieved in the kidney, where pFUS created a similar chemical gradient without any physiological damage to the host tissue, and dramatically increased MSC homing (Ziadloo et al., 2012). Similar to muscle, pFUS causes initial activation of TNFα and IL-1α in the kidney, which drive signaling through the nuclear factor-κB and COX2 pathways (Burks et al., 2017). In a mouse model of cisplatin-induced AKI, pFUS + MSCs significantly enhanced kidney homing and clinical outcomes compared with MSCs alone (Burks et al., 2015). In the AKI model, pFUS was found to upregulate IFN-γ in the kidney, which signals MSCs to produce the anti-inflammatory IL-10 (Burks et al., 2018). Jang et al. used pFUS on the heart and observed a similar initial increase in TNF-α followed by the upregulation of cytokines and growth factors, although MSC homing was not assessed (Jang et al., 2017). Although further studies are needed on its long-term effects, ultrasound appears to be a promising avenue for enhancing MSC homing.
Conclusions
The effort to improve MSC homing has taken on a wide breadth of strategies. Some of these strategies focus on non-systemic homing, such as the targeted administration and magnetic guidance. Most of the other techniques discussed here focus on systemic homing, a multistep process governed by specific molecular interactions. Unlike our relatively comprehensive knowledge on the homing of blood cells (Muller, 2011), MSC homing mechanisms remain poorly understood at several steps, such as tethering or rolling and transmigration. It is also unclear which of these steps is the major bottleneck for MSC attrition and would thus be the most fruitful for further investigation. Understanding the underlying biology has provided a wealth of optimization strategies at each step of the homing process. Each approach comes with its own drawbacks. Targeted administration may not always be feasible or may be highly invasive, depending on the target tissue. Most modifications to the MSCs do not prevent them from distributing to non-targeted organs. On the other hand, modifying the target tissue via chemical or genetic means raises safety concerns. These limitations present formidable barriers to applying them in the clinic. Although still an active area of study, the use of ultrasound to enhance MSC homing seems a promising avenue, with easy targeting of both deep and superficial organs and, to our current knowledge, a lack of significant safety concerns.
Although homing is perhaps the major impediment to realizing MSC-based therapies, other hurdles lie in the way as well. MSCs used in clinical settings are derived from healthy donors, and although these cells may be homogeneous in their expression of defining surface markers, they are not necessarily homogeneous in their function. Notably, the activity of IFN-γ-induced Indoleamine 2,3-dioxygenase (IDO) has been shown to be critical for MSC-facilitated immunosuppression, but donor variation gives rise to substantial differences in IDO responsiveness (Francois et al., 2012b). Furthermore, large-scale in vitro expansion of MSCs, which is necessary for clinical applications, results in senescence that impairs their therapeutic effect (Capasso et al., 2015). Contrary to conventional wisdom, which states that MSCs are immune privileged, studies have shown that MSCs may in fact undergo immune rejection due to HLA mismatches (Nauta et al., 2006, Eliopoulos et al., 2005). Finally, clinical trials almost universally use freshly thawed MSC stocks; however, cryopreservation appears to impair the immunosuppressive properties of MSCs and shorten their persistence in vivo (Francois et al., 2012a, Chinnadurai et al., 2016). These difficulties may well be the cause of conflicting results in both basic science and clinical settings (Galipeau, 2013). Moving forward, it will be critical to be cognizant of these sources of variation. Although much work still needs to be done, tackling the basic biological mechanisms underlying MSC biology will reveal new paths for optimization. Such investigations will continue to push forward the field of cell-based therapies, broadly boosting the effectiveness of therapeutics in applications ranging from immune modulation to regeneration.
Acknowledgments
This work was supported by the Akiko Yamzaki and Jerry Yang Faculty Scholar Fund in Pediatric Translational Medicine and the Stanford Maternal and Child Health Research Institute and the SIR Foundation Ring Development Grant.
References
- Abdi R., Moore R., Sakai S., Donnelly C.B., Mounayar M., Sackstein R. HCELL expression on murine MSC licenses pancreatotropism and confers durable reversal of autoimmune diabetes in NOD mice. Stem Cells. 2015;33:1523–1531. doi: 10.1002/stem.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abdi, R., Moore, R., Sakai, S., Donnelly, C.B., Mounayar, M. & Sackstein, R. 2015. HCELL expression on murine MSC licenses pancreatotropism and confers durable reversal of autoimmune diabetes in NOD mice. Stem Cells, 33, 1523-1531. [DOI] [PMC free article] [PubMed]
- Annabi B., Lee Y.T., Turcotte S., Naud E., Desrosiers R.R., Champagne M., Eliopoulos N., Galipeau J., Beliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]; Annabi, B., Lee, Y.T., Turcotte, S., Naud, E., Desrosiers, R.R., Champagne, M., Eliopoulos, N., Galipeau, J. & Beliveau, R. 2003. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells, 21, 337-347. [DOI] [PubMed]
- Antunes M.A., Abreu S.C., Cruz F.F., Teixeira A.C., Lopes-Pacheco M., Bandeira E., Olsen P.C., Diaz B.L., Takyia C.M., Freitas I.P. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir. Res. 2014;15:118. doi: 10.1186/s12931-014-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Antunes, M.A., Abreu, S.C., Cruz, F.F., Teixeira, A.C., Lopes-Pacheco, M., Bandeira, E., Olsen, P.C., Diaz, B.L., Takyia, C.M., Freitas, I.P., et al. 2014. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir. Res., 15, 118. [DOI] [PMC free article] [PubMed]
- Arbab A.S., Jordan E.K., Wilson L.B., Yocum G.T., Lewis B.K., Frank J.A. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum. Gene Ther. 2004;15:351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]; Arbab, A.S., Jordan, E.K., Wilson, L.B., Yocum, G.T., Lewis, B.K. & Frank, J.A. 2004. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum. Gene Ther., 15, 351-360. [DOI] [PubMed]
- Arthur A., Rychkov G., Shi S., Koblar S.A., Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]; Arthur, A., Rychkov, G., Shi, S., Koblar, S.A. & Gronthos, S. 2008. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26, 1787-1795. [DOI] [PubMed]
- Augustine S., Avey M.T., Harrison B., Locke T., Ghannad M., Moher D., Thebaud B. Mesenchymal stromal cell therapy in bronchopulmonary Dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med. 2017;6:2079–2093. doi: 10.1002/sctm.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]; Augustine, S., Avey, M.T., Harrison, B., Locke, T., Ghannad, M., Moher, D. & Thebaud, B. 2017. Mesenchymal stromal cell therapy in bronchopulmonary Dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med., 6, 2079-2093. [DOI] [PMC free article] [PubMed]
- Bailey A.M., Lawrence M.B., Shang H., Katz A.J., Peirce S.M. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput. Biol. 2009;5:e1000294. doi: 10.1371/journal.pcbi.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bailey, A.M., Lawrence, M.B., Shang, H., Katz, A.J. & Peirce, S.M. 2009. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput. Biol., 5, e1000294. [DOI] [PMC free article] [PubMed]
- Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]; Barbash, I.M., Chouraqui, P., Baron, J., Feinberg, M.S., Etzion, S., Tessone, A., Miller, L., Guetta, E., Zipori, D., Kedes, L.H., et al. 2003. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation, 108, 863-868. [DOI] [PubMed]
- Bayo J., Real A., Fiore E.J., Malvicini M., Sganga L., Bolontrade M., Andriani O., Bizama C., Fresno C., Podhajcer O. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2017;8:80235–80248. doi: 10.18632/oncotarget.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bayo, J., Real, A., Fiore, E.J., Malvicini, M., Sganga, L., Bolontrade, M., Andriani, O., Bizama, C., Fresno, C., Podhajcer, O., et al. 2017. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget, 8, 80235-80248. [DOI] [PMC free article] [PubMed]
- Belema-Bedada F., Uchida S., Martire A., Kostin S., Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2:566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]; Belema-Bedada, F., Uchida, S., Martire, A., Kostin, S. & Braun, T. 2008. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell, 2, 566-575. [DOI] [PubMed]
- Bi L.K., Zhou N., Liu C., Lu F.D., Lin T.X., Xuan X.J., Jiang C., Han J.L., Huang H., Zhang C.X. Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urol. Oncol. 2014;32:607–612. doi: 10.1016/j.urolonc.2013.10.018. [DOI] [PubMed] [Google Scholar]; Bi, L.K., Zhou, N., Liu, C., Lu, F.D., Lin, T.X., Xuan, X.J., Jiang, C., Han, J.L., Huang, H., Zhang, C.X., et al. 2014. Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urol. Oncol., 32, 607-612. [DOI] [PubMed]
- Bobis-Wozowicz S., Miekus K., Wybieralska E., Jarocha D., Zawisz A., Madeja Z., Majka M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011;39:686–696.e4.. doi: 10.1016/j.exphem.2011.03.004. [DOI] [PubMed] [Google Scholar]; Bobis-Wozowicz, S., Miekus, K., Wybieralska, E., Jarocha, D., Zawisz, A., Madeja, Z. & Majka, M. 2011. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol., 39, 686-696.e4. [DOI] [PubMed]
- Bronckaers A., Hilkens P., Martens W., Gervois P., Ratajczak J., Struys T., Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014;143:181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]; Bronckaers, A., Hilkens, P., Martens, W., Gervois, P., Ratajczak, J., Struys, T. & Lambrichts, I. 2014. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther., 143, 181-196. [DOI] [PubMed]
- Burgess A., Ayala-Grosso C.A., Ganguly M., Jordao J.F., Aubert I., Hynynen K. Targeted delivery of neural stem cells to the brain using mri-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burgess, A., Ayala-Grosso, C.A., Ganguly, M., Jordao, J.F., Aubert, I. & Hynynen, K. 2011. Targeted delivery of neural stem cells to the brain using mri-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One, 6.e27877 [DOI] [PMC free article] [PubMed]
- Burks S.R., Nagle M.E., Bresler M.N., Kim S.J., Star R.A., Frank J.A. Mesenchymal stromal cell potency to treat acute kidney injury increased by ultrasound-activated interferon-gamma/interleukin-10 axis. J. Cell Mol. Med. 2018;22:6015–6025. doi: 10.1111/jcmm.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burks, S.R., Nagle, M.E., Bresler, M.N., Kim, S.J., Star, R.A. & Frank, J.A. 2018. Mesenchymal stromal cell potency to treat acute kidney injury increased by ultrasound-activated interferon-gamma/interleukin-10 axis. J. Cell Mol. Med., 22, 6015-6025. [DOI] [PMC free article] [PubMed]
- Burks S.R., Nguyen B.A., Bresler M.N., Nagle M.E., Kim S.J., Frank J.A. Anti-inflammatory drugs suppress ultrasound-mediated mesenchymal stromal cell tropism to kidneys. Sci. Rep. 2017;7:8607. doi: 10.1038/s41598-017-08887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burks, S.R., Nguyen, B.A., Bresler, M.N., Nagle, M.E., Kim, S.J. & Frank, J.A. 2017. Anti-inflammatory drugs suppress ultrasound-mediated mesenchymal stromal cell tropism to kidneys. Sci. Rep., 7, 8607. [DOI] [PMC free article] [PubMed]
- Burks S.R., Nguyen B.A., Tebebi P.A., Kim S.J., Bresler M.N., Ziadloo A., Street J.M., Yuen P.S., Star R.A., Frank J.A. Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice. Stem Cells. 2015;33:1241–1253. doi: 10.1002/stem.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burks, S.R., Nguyen, B.A., Tebebi, P.A., Kim, S.J., Bresler, M.N., Ziadloo, A., Street, J.M., Yuen, P.S., Star, R.A. & Frank, J.A. 2015. Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice. Stem Cells, 33, 1241-1253. [DOI] [PMC free article] [PubMed]
- Burks S.R., Ziadloo A., Hancock H.A., Chaudhry A., Dean D.D., Lewis B.K., Frenkel V., Frank J.A. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One. 2011;6:e24730. doi: 10.1371/journal.pone.0024730. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burks, S.R., Ziadloo, A., Hancock, H.A., Chaudhry, A., Dean, D.D., Lewis, B.K., Frenkel, V. & Frank, J.A. 2011. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One, 6, e24730. [DOI] [PMC free article] [PubMed]
- Burks S.R., Ziadloo A., Kim S.J., Nguyen B.A., Frank J.A. Noninvasive pulsed focused ultrasound allows spatiotemporal control of targeted homing for multiple stem cell types in murine skeletal muscle and the magnitude of cell homing can be increased through repeated applications. Stem Cells. 2013;31:2551–2560. doi: 10.1002/stem.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]; Burks, S.R., Ziadloo, A., Kim, S.J., Nguyen, B.A. & Frank, J.A. 2013. Noninvasive pulsed focused ultrasound allows spatiotemporal control of targeted homing for multiple stem cell types in murine skeletal muscle and the magnitude of cell homing can be increased through repeated applications. Stem Cells, 31, 2551-2560. [DOI] [PMC free article] [PubMed]
- Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]; Campagnoli, C., Roberts, I.A., Kumar, S., Bennett, P.R., Bellantuono, I. & Fisk, N.M. 2001. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood, 98, 2396-2402. [DOI] [PubMed]
- Capasso S., Alessio N., Squillaro T., di Bernardo G., Melone M.A., Cipollaro M., Peluso G., Galderisi U. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget. 2015;6:39457–39468. doi: 10.18632/oncotarget.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]; Capasso, S., Alessio, N., Squillaro, T., di Bernardo, G., Melone, M.A., Cipollaro, M., Peluso, G. & Galderisi, U. 2015. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget, 6, 39457-39468. [DOI] [PMC free article] [PubMed]
- Caplan A.I. Why are MSCs therapeutic? New data: new insight. J. Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]; Caplan, A.I. 2009. Why are MSCs therapeutic? New data: new insight. J. Pathol., 217, 318-324. [DOI] [PMC free article] [PubMed]
- Chapel A., Bertho J.M., Bensidhoum M., Fouillard L., Young R.G., Frick J., Demarquay C., Cuvelier F., Mathieu E., Trompier F. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]; Chapel, A., Bertho, J.M., Bensidhoum, M., Fouillard, L., Young, R.G., Frick, J., Demarquay, C., Cuvelier, F., Mathieu, E., Trompier, F., et al. 2003. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med., 5, 1028-1038. [DOI] [PubMed]
- Chen R., Cai X., Liu J., Bai B., Li X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018;215:31–42. doi: 10.1016/j.lfs.2018.10.047. [DOI] [PubMed] [Google Scholar]; Chen, R., Cai, X., Liu, J., Bai, B. & Li, X. 2018a. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci., 215, 31-42. [DOI] [PubMed]
- Chen W., Li M., Cheng H., Yan Z.L., Cao J., Pan B., Sang W., Wu Q.Y., Zeng L.Y., Li Z.Y., Xu K.L. Overexpression of the mesenchymal stem cell Cxcr4 gene in irradiated mice increases the homing capacity of these cells. Cell Biochem. Biophys. 2013;67:1181–1191. doi: 10.1007/s12013-013-9632-6. [DOI] [PubMed] [Google Scholar]; Chen, W., Li, M., Cheng, H., Yan, Z.L., Cao, J., Pan, B., Sang, W., Wu, Q.Y., Zeng, L.Y., Li, Z.Y. & Xu, K.L. 2013. Overexpression of the mesenchymal stem cell Cxcr4 gene in irradiated mice increases the homing capacity of these cells. Cell Biochem. Biophys., 67, 1181-1191. [DOI] [PubMed]
- Chen Z., Chen Q., Du H., Xu L., Wan J. Mesenchymal stem cells and CXC chemokine receptor 4 overexpression improved the therapeutic effect on colitis via mucosa repair. Exp. Ther. Med. 2018;16:821–829. doi: 10.3892/etm.2018.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, Z., Chen, Q., Du, H., Xu, L. & Wan, J. 2018b. Mesenchymal stem cells and CXC chemokine receptor 4 overexpression improved the therapeutic effect on colitis via mucosa repair. Exp. Ther. Med., 16, 821-829. [DOI] [PMC free article] [PubMed]
- Cheng H., Byrska-Bishop M., Zhang C.T., Kastrup C.J., Hwang N.S., Tai A.K., Lee W.W., Xu X.Y., Nahrendorf M., Langer R., Anderson D.G. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials. 2012;33:5004–5012. doi: 10.1016/j.biomaterials.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheng, H., Byrska-Bishop, M., Zhang, C.T., Kastrup, C.J., Hwang, N.S., Tai, A.K., Lee, W.W., Xu, X.Y., Nahrendorf, M., Langer, R. & Anderson, D.G. 2012. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials, 33, 5004-5012. [DOI] [PMC free article] [PubMed]
- Cheng Z., Ou L., Zhou X., Li F., Jia X., Zhang Y., Liu X., Li Y., Ward C.A., Melo L.G., Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]; Cheng, Z., Ou, L., Zhou, X., Li, F., Jia, X., Zhang, Y., Liu, X., Li, Y., Ward, C.A., Melo, L.G. & Kong, D. 2008. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther., 16, 571-579. [DOI] [PubMed]
- Chinnadurai R., Copland I.B., Garcia M.A., Petersen C.T., Lewis C.N., Waller E.K., Kirk A.D., Galipeau J. Cryopreserved Mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNgamma licensing. Stem Cells. 2016;34:2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chinnadurai, R., Copland, I.B., Garcia, M.A., Petersen, C.T., Lewis, C.N., Waller, E.K., Kirk, A.D. & Galipeau, J. 2016. Cryopreserved Mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNgamma licensing. Stem Cells, 34, 2429-2442. [DOI] [PMC free article] [PubMed]
- Chonpathompikunlert P., Fan C.H., Ozaki Y., Yoshitomi T., Yeh C.K., Nagasaki Y. Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine (Lond.) 2012;7:1029–1043. doi: 10.2217/nnm.12.2. [DOI] [PubMed] [Google Scholar]; Chonpathompikunlert, P., Fan, C.H., Ozaki, Y., Yoshitomi, T., Yeh, C.K. & Nagasaki, Y. 2012. Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine (Lond.), 7, 1029-1043. [DOI] [PubMed]
- Chou K.J., Lee P.T., Chen C.L., Hsu C.Y., Huang W.C., Huang C.W., Fang H.C. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp. Cell Res. 2017;350:91–102. doi: 10.1016/j.yexcr.2016.11.010. [DOI] [PubMed] [Google Scholar]; Chou, K.J., Lee, P.T., Chen, C.L., Hsu, C.Y., Huang, W.C., Huang, C.W. & Fang, H.C. 2017. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp. Cell Res., 350, 91-102. [DOI] [PubMed]
- Chu K., Jung K.H., Kim S.J., Lee S.T., Kim J., Park H.K., Song E.C., Kim S.U., Kim M., Lee S.K., Roh J.K. Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1alpha stabilization in the host brain. Brain Res. 2008;1207:182–192. doi: 10.1016/j.brainres.2008.02.043. [DOI] [PubMed] [Google Scholar]; Chu, K., Jung, K.H., Kim, S.J., Lee, S.T., Kim, J., Park, H.K., Song, E.C., Kim, S.U., Kim, M., Lee, S.K. & Roh, J.K. 2008. Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1alpha stabilization in the host brain. Brain Res., 1207, 182-192. [DOI] [PubMed]
- Chung E.S., Miller L., Patel A.N., Anderson R.D., Mendelsohn F.O., Traverse J., Silver K.H., Shin J., Ewald G., Farr M.J. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur. Heart J. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chung, E.S., Miller, L., Patel, A.N., Anderson, R.D., Mendelsohn, F.O., Traverse, J., Silver, K.H., Shin, J., Ewald, G., Farr, M.J., et al. 2015. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur. Heart J., 36, 2228-2238. [DOI] [PMC free article] [PubMed]
- Constantin G., Majeed M., Giagulli C., Piccio L., Kim J.Y., Butcher E.C., Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]; Constantin, G., Majeed, M., Giagulli, C., Piccio, L., Kim, J.Y., Butcher, E.C. & Laudanna, C. 2000. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity, 13, 759-769. [DOI] [PubMed]
- Corradetti B., Taraballi F., Martinez J.O., Minardi S., Basu N., Bauza G., Evangelopoulos M., Powell S., Corbo C., Tasciotti E. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci. Rep. 2017;7:7991. doi: 10.1038/s41598-017-08687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Corradetti, B., Taraballi, F., Martinez, J.O., Minardi, S., Basu, N., Bauza, G., Evangelopoulos, M., Powell, S., Corbo, C. & Tasciotti, E. 2017. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci. Rep., 7.7991. [DOI] [PMC free article] [PubMed]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]; Crisan, M., Yap, S., Casteilla, L., Chen, C.W., Corselli, M., Park, T.S., Andriolo, G., Sun, B., Zheng, B., Zhang, L., et al. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 3, 301-313. [DOI] [PubMed]
- Cui L.L., Nitzsche F., Pryazhnikov E., Tibeykina M., Tolppanen L., Rytkonen J., Huhtala T., Mu J.W., Khiroug L., Boltze J., Jolkkonen J. Integrin alpha4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017;48:2895–2900. doi: 10.1161/STROKEAHA.117.017809. [DOI] [PubMed] [Google Scholar]; Cui, L.L., Nitzsche, F., Pryazhnikov, E., Tibeykina, M., Tolppanen, L., Rytkonen, J., Huhtala, T., Mu, J.W., Khiroug, L., Boltze, J. & Jolkkonen, J. 2017. Integrin alpha4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke, 48, 2895-2900. [DOI] [PubMed]
- De Becker A., van Hummelen P., Bakkus M., Broek I.V., De Wever J., De Waele M., Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]; De Becker, A., van Hummelen, P., Bakkus, M., Broek, I.V., De Wever, J., De Waele, M. & Van Riet, I. 2007. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica, 92, 440-449. [DOI] [PubMed]
- Devine S.M., Cobbs C., Jennings M., Bartholomew A., Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]; Devine, S.M., Cobbs, C., Jennings, M., Bartholomew, A. & Hoffman, R. 2003. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood, 101, 2999-3001. [DOI] [PubMed]
- Dick A.J., Guttman M.A., Raman V.K., Peters D.C., Pessanha B.S., Hill J.M., Smith S., Scott G., Mcveigh E.R., Lederman R.J. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation. 2003;108:2899–2904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dick, A.J., Guttman, M.A., Raman, V.K., Peters, D.C., Pessanha, B.S., Hill, J.M., Smith, S., Scott, G., Mcveigh, E.R. & Lederman, R.J. 2003. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation, 108, 2899-2904. [DOI] [PMC free article] [PubMed]
- Dominici M., le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]; Dominici, M., le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D. & Horwitz, E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315-317. [DOI] [PubMed]
- Duijvestein M., Wildenberg M.E., Welling M.M., Hennink S., Molendijk I., Van Zuylen V.L., Bosse T., Vos A.C., De Jonge-Muller E.S. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]; Duijvestein, M., Wildenberg, M.E., Welling, M.M., Hennink, S., Molendijk, I., Van Zuylen, V.L., Bosse, T., Vos, A.C., De Jonge-Muller, E.S., et al. 2011. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells, 29, 1549-1558. [DOI] [PubMed]
- Dykstra B., Lee J., Mortensen L.J., Yu H., Wu Z.L., Lin C.P., Rossi D.J., Sackstein R. Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells. Stem Cells. 2016;34:2501–2511. doi: 10.1002/stem.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dykstra, B., Lee, J., Mortensen, L.J., Yu, H., Wu, Z.L., Lin, C.P., Rossi, D.J. & Sackstein, R. 2016. Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells. Stem Cells, 34, 2501-2511. [DOI] [PMC free article] [PubMed]
- Eliopoulos N., Stagg J., Lejeune L., Pommey S., Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]; Eliopoulos, N., Stagg, J., Lejeune, L., Pommey, S. & Galipeau, J. 2005. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood, 106, 4057-4065. [DOI] [PubMed]
- Fan C.H., Liu H.L., Huang C.Y., Ma Y.J., Yen T.C., Yeh C.K. Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood-brain barrier disruption by ultrasound imaging. Ultrasound Med. Biol. 2012;38:1372–1382. doi: 10.1016/j.ultrasmedbio.2012.03.013. [DOI] [PubMed] [Google Scholar]; Fan, C.H., Liu, H.L., Huang, C.Y., Ma, Y.J., Yen, T.C. & Yeh, C.K. 2012a. Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood-brain barrier disruption by ultrasound imaging. Ultrasound Med. Biol., 38, 1372-1382. [DOI] [PubMed]
- Fan H.Y., Zhao G.F., Liu L., Liu F., Gong W., Liu X.Q., Yang L., Wang J.J., Hou Y.Y. Pre-treatment with IL-1 beta enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell. Mol. Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fan, H.Y., Zhao, G.F., Liu, L., Liu, F., Gong, W., Liu, X.Q., Yang, L., Wang, J.J. & Hou, Y.Y. 2012b. Pre-treatment with IL-1 beta enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell. Mol. Immunol., 9, 473-481. [DOI] [PMC free article] [PubMed]
- Francois M., Copland I.B., Yuan S., Romieu-Mourez R., Waller E.K., Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]; Francois, M., Copland, I.B., Yuan, S., Romieu-Mourez, R., Waller, E.K. & Galipeau, J. 2012a. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy, 14, 147-152. [DOI] [PMC free article] [PubMed]
- Francois M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]; Francois, M., Romieu-Mourez, R., Li, M. & Galipeau, J. 2012b. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther., 20, 187-195. [DOI] [PubMed]
- Francois S., Bensidhoum M., Mouiseddine M., Mazurier C., Allenet B., Semont A., Frick J., Sache A., Bouchet S., Thierry D. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]; Francois, S., Bensidhoum, M., Mouiseddine, M., Mazurier, C., Allenet, B., Semont, A., Frick, J., Sache, A., Bouchet, S., Thierry, D., et al. 2006. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells, 24, 1020-1029. [DOI] [PubMed]
- Frenette P.S., Pinho S., Lucas D., Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]; Frenette, P.S., Pinho, S., Lucas, D. & Scheiermann, C. 2013. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol., 31, 285-316. [DOI] [PubMed]
- Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]; Friedenstein, A.J., Petrakova, K.V., Kurolesova, A.I. & Frolova, G.P. 1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation, 6, 230-247. [PubMed]
- Fujii H., Li S.H., Wu J., Miyagi Y., Yau T.M., Rakowski H., Egashira K., Guo J., Weisel R.D., Li R.K. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur. Heart J. 2011;32:2075–2084. doi: 10.1093/eurheartj/ehq475. [DOI] [PubMed] [Google Scholar]; Fujii, H., Li, S.H., Wu, J., Miyagi, Y., Yau, T.M., Rakowski, H., Egashira, K., Guo, J., Weisel, R.D. & Li, R.K. 2011. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur. Heart J., 32, 2075-2084. [DOI] [PubMed]
- Galipeau J. The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]; Galipeau, J. 2013. The mesenchymal stromal cells dilemma-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy, 15, 2-8. [DOI] [PubMed]
- Gao H., Priebe W., Glod J., Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]; Gao, H., Priebe, W., Glod, J. & Banerjee, D. 2009. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells, 27, 857-865. [DOI] [PubMed]
- Gao J., Dennis J.E., Muzic R.F., Lundberg M., Caplan A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]; Gao, J., Dennis, J.E., Muzic, R.F., Lundberg, M. & Caplan, A.I. 2001. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs, 169, 12-20. [DOI] [PubMed]
- Ghanem A., Steingen C., Brenig F., Funcke F., Bai Z.Y., Hall C., Chin C.T., Nickenig G., Bloch W., Tiemann K. Focused ultrasound-induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J. Mol. Cell Cardiol. 2009;47:411–418. doi: 10.1016/j.yjmcc.2009.06.008. [DOI] [PubMed] [Google Scholar]; Ghanem, A., Steingen, C., Brenig, F., Funcke, F., Bai, Z.Y., Hall, C., Chin, C.T., Nickenig, G., Bloch, W. & Tiemann, K. 2009. Focused ultrasound-induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J. Mol. Cell Cardiol., 47, 411-418. [DOI] [PubMed]
- Gheisari Y., Azadmanesh K., Ahmadbeigi N., Nassiri S.M., Golestaneh A.F., Naderi M., Vasei M., Arefian E., Mirab-Samiee S., Shafiee A. Genetic modification of mesenchymal stem cells to overexpress CXCR4 and CXCR7 does not improve the homing and therapeutic potentials of these cells in experimental acute kidney injury. Stem Cells Dev. 2012;21:2969–2980. doi: 10.1089/scd.2011.0588. [DOI] [PubMed] [Google Scholar]; Gheisari, Y., Azadmanesh, K., Ahmadbeigi, N., Nassiri, S.M., Golestaneh, A.F., Naderi, M., Vasei, M., Arefian, E., Mirab-Samiee, S., Shafiee, A., et al. 2012. Genetic modification of mesenchymal stem cells to overexpress CXCR4 and CXCR7 does not improve the homing and therapeutic potentials of these cells in experimental acute kidney injury. Stem Cells Dev., 21, 2969-2980. [DOI] [PubMed]
- Goncalves R.M., Antunes J.C., Barbosa M.A. Mesenchymal stem cell recruitment by stromal derived factor-1-delivery systems based on chitosan/poly(gamma-glutamic acid) polyelectrolyte complexes. Eur. Cell Mater. 2012;23:249–260. doi: 10.22203/ecm.v023a19. discussion 260–261. [DOI] [PubMed] [Google Scholar]; Goncalves, R.M., Antunes, J.C. & Barbosa, M.A. 2012. Mesenchymal stem cell recruitment by stromal derived factor-1-delivery systems based on chitosan/poly(gamma-glutamic acid) polyelectrolyte complexes. Eur. Cell Mater., 23, 249-260; discussion 260-261. [DOI] [PubMed]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gronthos, S., Mankani, M., Brahim, J., Robey, P.G. & Shi, S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U S A, 97, 13625-13630. [DOI] [PMC free article] [PubMed]
- Gundlach C.W.T., Caivano A., Cabreira-Hansen Mda G., Gahremanpour A., Brown W.S., Zheng Y., Mcintyre B.W., Willerson J.T., Dixon R.A. Synthesis and evaluation of an anti-MLC1 x anti-CD90 bispecific antibody for targeting and retaining bone-marrow-derived multipotent stromal cells in infarcted myocardium. Bioconjug. Chem. 2011;22:1706–1714. doi: 10.1021/bc200309h. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gundlach, C.W.T., Caivano, A., Cabreira-Hansen Mda, G., Gahremanpour, A., Brown, W.S., Zheng, Y., Mcintyre, B.W., Willerson, J.T., Dixon, R.A., et al. 2011. Synthesis and evaluation of an anti-MLC1 x anti-CD90 bispecific antibody for targeting and retaining bone-marrow-derived multipotent stromal cells in infarcted myocardium. Bioconjug. Chem., 22, 1706-1714. [DOI] [PMC free article] [PubMed]
- He X., Ma J., Jabbari E. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1alpha from poly(lactide ethylene oxide fumarate) hydrogels. Int. J. Pharm. 2010;390:107–116. doi: 10.1016/j.ijpharm.2009.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]; He, X., Ma, J. & Jabbari, E. 2010. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1alpha from poly(lactide ethylene oxide fumarate) hydrogels. Int. J. Pharm., 390, 107-116. [DOI] [PMC free article] [PubMed]
- Heldman A.W., Difede D.L., Fishman J.E., Zambrano J.P., Trachtenberg B.H., Karantalis V., Mushtaq M., Williams A.R., Suncion V.Y., Mcniece I.K. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heldman, A.W., Difede, D.L., Fishman, J.E., Zambrano, J.P., Trachtenberg, B.H., Karantalis, V., Mushtaq, M., Williams, A.R., Suncion, V.Y., Mcniece, I.K., et al. 2014. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA, 311, 62-73. [DOI] [PMC free article] [PubMed]
- Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]; Honczarenko, M., Le, Y., Swierkowski, M., Ghiran, I., Glodek, A.M. & Silberstein, L.E. 2006. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells, 24, 1030-1041. [DOI] [PubMed]
- Hou Y., Ryu C.H., Jun J.A., Kim S.M., Jeong C.H., Jeun S.S. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int. 2014;38:1050–1059. doi: 10.1002/cbin.10294. [DOI] [PubMed] [Google Scholar]; Hou, Y., Ryu, C.H., Jun, J.A., Kim, S.M., Jeong, C.H. & Jeun, S.S. 2014. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int., 38, 1050-1059. [DOI] [PubMed]
- Hsiao J.K., Tai M.F., Chu H.H., Chen S.T., Li H., Lai D.M., Hsieh S.T., Wang J.L., Liu H.M. Magnetic nanoparticle labeling of mesenchymal stem cells without transfection agent: cellular behavior and capability of detection with clinical 1.5 T magnetic resonance at the single cell level. Magn. Reson. Med. 2007;58:717–724. doi: 10.1002/mrm.21377. [DOI] [PubMed] [Google Scholar]; Hsiao, J.K., Tai, M.F., Chu, H.H., Chen, S.T., Li, H., Lai, D.M., Hsieh, S.T., Wang, J.L. & Liu, H.M. 2007. Magnetic nanoparticle labeling of mesenchymal stem cells without transfection agent: cellular behavior and capability of detection with clinical 1.5 T magnetic resonance at the single cell level. Magn. Reson. Med., 58, 717-724. [DOI] [PubMed]
- Hung S.C., Pochampally R.R., Hsu S.C., Sanchez C., Chen S.C., Spees J., Prockop D.J. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hung, S.C., Pochampally, R.R., Hsu, S.C., Sanchez, C., Chen, S.C., Spees, J. & Prockop, D.J. 2007. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One, 2.e416. [DOI] [PMC free article] [PubMed]
- in 't Anker P.S., Noort W.A., Scherjon S.A., Kleijburg-van der Keur C., Kruisselbrink A.B., van Bezooijen R.L., Beekhuizen W., Willemze R., Kanhai H.H., Fibbe W.E. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]; in 't Anker, P.S., Noort, W.A., Scherjon, S.A., Kleijburg-van der Keur, C., Kruisselbrink, A.B., van Bezooijen, R.L., Beekhuizen, W., Willemze, R., Kanhai, H.H. & Fibbe, W.E. 2003. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica, 88, 845-852. [PubMed]
- Ip J.E., Wu Y.J., Huang J., Zhang L.N., Pratt R.E., Dzau V.J. Mesenchymal stem cells use integrin beta 1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ip, J.E., Wu, Y.J., Huang, J., Zhang, L.N., Pratt, R.E. & Dzau, V.J. 2007. Mesenchymal stem cells use integrin beta 1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell, 18, 2873-2882. [DOI] [PMC free article] [PubMed]
- Ishikane S., Hosoda H., Yamahara K., Akitake Y., Kyoungsook J., Mishima K., Iwasaki K., Fujiwara M., Miyazato M., Kangawa K., Ikeda T. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96:697–706. doi: 10.1097/TP.0b013e31829f753d. [DOI] [PubMed] [Google Scholar]; Ishikane, S., Hosoda, H., Yamahara, K., Akitake, Y., Kyoungsook, J., Mishima, K., Iwasaki, K., Fujiwara, M., Miyazato, M., Kangawa, K. & Ikeda, T. 2013. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation, 96, 697-706. [DOI] [PubMed]
- Jaager K., Islam S., Zajac P., Linnarsson S., Neuman T. RNA-seq analysis reveals different dynamics of differentiation of human dermis- and adipose-derived stromal stem cells. PLoS One. 2012;7:e38833. doi: 10.1371/journal.pone.0038833. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jaager, K., Islam, S., Zajac, P., Linnarsson, S. & Neuman, T. 2012. RNA-seq analysis reveals different dynamics of differentiation of human dermis- and adipose-derived stromal stem cells. PLoS One, 7, e38833. [DOI] [PMC free article] [PubMed]
- Jang K.W., Tu T.W., Nagle M.E., Lewis B.K., Burks S.R., Frank J.A. Molecular and histological effects of MR-guided pulsed focused ultrasound to the rat heart. J. Transl. Med. 2017;15:252. doi: 10.1186/s12967-017-1361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jang, K.W., Tu, T.W., Nagle, M.E., Lewis, B.K., Burks, S.R. & Frank, J.A. 2017. Molecular and histological effects of MR-guided pulsed focused ultrasound to the rat heart. J. Transl. Med., 15, 252. [DOI] [PMC free article] [PubMed]
- Jeong H., Yim H.W., Park H.J., Cho Y., Hong H., Kim N.J., Oh I.H. Mesenchymal stem cell therapy for ischemic heart disease: systematic review and meta-analysis. Int. J. Stem Cells. 2018;11:1–12. doi: 10.15283/ijsc17061. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jeong, H., Yim, H.W., Park, H.J., Cho, Y., Hong, H., Kim, N.J. & Oh, I.H. 2018. Mesenchymal stem cell therapy for ischemic heart disease: systematic review and meta-analysis. Int. J. Stem Cells, 11, 1-12. [DOI] [PMC free article] [PubMed]
- Kaibuchi N., Iwata T., Yamato M., Okano T., Ando T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016;42:400–410. doi: 10.1016/j.actbio.2016.06.022. [DOI] [PubMed] [Google Scholar]; Kaibuchi, N., Iwata, T., Yamato, M., Okano, T. & Ando, T. 2016. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater., 42, 400-410. [DOI] [PubMed]
- Kanelidis A.J., Premer C., Lopez J., Balkan W., Hare J.M. Route of delivery modulates the efficacy of Mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ. Res. 2017;120:1139–1150. doi: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kanelidis, A.J., Premer, C., Lopez, J., Balkan, W. & Hare, J.M. 2017. Route of delivery modulates the efficacy of Mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ. Res., 120, 1139-1150. [DOI] [PMC free article] [PubMed]
- Kang H., Kim K.H., Lim J., Kim Y.S., Heo J., Choi J., Jeong J., Kim Y., Kim S.W., Oh Y.M. The therapeutic effects of human Mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 2015;24:1658–1671. doi: 10.1089/scd.2014.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kang, H., Kim, K.H., Lim, J., Kim, Y.S., Heo, J., Choi, J., Jeong, J., Kim, Y., Kim, S.W., Oh, Y.M., et al. 2015. The therapeutic effects of human Mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev., 24, 1658-1671. [DOI] [PMC free article] [PubMed]
- Kawamura M., Miyagawa S., Fukushima S., Saito A., Toda K., Daimon T., Shimizu T., Okano T., Sawa Y. Xenotransplantation of bone marrow-derived human Mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng. Part A. 2015;21:2272–2280. doi: 10.1089/ten.tea.2014.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kawamura, M., Miyagawa, S., Fukushima, S., Saito, A., Toda, K., Daimon, T., Shimizu, T., Okano, T. & Sawa, Y. 2015. Xenotransplantation of bone marrow-derived human Mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng. Part A, 21, 2272-2280. [DOI] [PMC free article] [PubMed]
- Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]; Kern, S., Eichler, H., Stoeve, J., Kluter, H. & Bieback, K. 2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 24, 1294-1301. [DOI] [PubMed]
- Kim D.S., Lee M.W., Yoo K.H., Lee T.H., Kim H.J., Jang I.K., Chun Y.H., Kim H.J., Park S.J., Lee S.H. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014;9:e83363. doi: 10.1371/journal.pone.0083363. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim, D.S., Lee, M.W., Yoo, K.H., Lee, T.H., Kim, H.J., Jang, I.K., Chun, Y.H., Kim, H.J., Park, S.J., Lee, S.H., et al. 2014. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One, 9, e83363. [DOI] [PMC free article] [PubMed]
- Kim Y.S., Noh M.Y., Kim J.Y., Yu H.J., Kim K.S., Kim S.H., Koh S.H. Direct GSK-3beta inhibition enhances mesenchymal stromal cell migration by increasing expression of beta-PIX and CXCR4. Mol. Neurobiol. 2013;47:811–820. doi: 10.1007/s12035-012-8393-3. [DOI] [PubMed] [Google Scholar]; Kim, Y.S., Noh, M.Y., Kim, J.Y., Yu, H.J., Kim, K.S., Kim, S.H. & Koh, S.H. 2013. Direct GSK-3beta inhibition enhances mesenchymal stromal cell migration by increasing expression of beta-PIX and CXCR4. Mol. Neurobiol., 47, 811-820. [DOI] [PubMed]
- Kimura Y., Tabata Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010;21:37–51. doi: 10.1163/156856209X410193. [DOI] [PubMed] [Google Scholar]; Kimura, Y. & Tabata, Y. 2010. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed., 21, 37-51. [DOI] [PubMed]
- Ko I.K., Kean T.J., Dennis J.E. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–3710. doi: 10.1016/j.biomaterials.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ko, I.K., Kean, T.J. & Dennis, J.E. 2009. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials, 30, 3702-3710. [DOI] [PMC free article] [PubMed]
- Ko I.K., Kim B.G., Awadallah A., Mikulan J., Lin P., Letterio J.J., Dennis J.E. Targeting improves MSC treatment of inflammatory bowel disease. Mol. Ther. 2010;18:1365–1372. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ko, I.K., Kim, B.G., Awadallah, A., Mikulan, J., Lin, P., Letterio, J.J. & Dennis, J.E. 2010. Targeting improves MSC treatment of inflammatory bowel disease. Mol. Ther., 18, 1365-1372. [DOI] [PMC free article] [PubMed]
- Kobayashi T., Ochi M., Yanada S., Ishikawa M., Adachi N., Deie M., Arihiro K. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24:69–76. doi: 10.1016/j.arthro.2007.08.017. [DOI] [PubMed] [Google Scholar]; Kobayashi, T., Ochi, M., Yanada, S., Ishikawa, M., Adachi, N., Deie, M. & Arihiro, K. 2008. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy, 24, 69-76. [DOI] [PubMed]
- Kovacs Z.I., Kim S., Jikaria N., Qureshi F., Milo B., Lewis B.K., Bresler M., Burks S.R., Frank J.A. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. U S A. 2017;114:E75–E84. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kovacs, Z.I., Kim, S., Jikaria, N., Qureshi, F., Milo, B., Lewis, B.K., Bresler, M., Burks, S.R. & Frank, J.A. 2017. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. U S A, 114, E75-E84. [DOI] [PMC free article] [PubMed]
- Kraitchman D.L., Tatsumi M., Gilson W.D., Ishimori T., Kedziorek D., Walczak P., Segars W.P., Chen H.H., Fritzges D., Izbudak I. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kraitchman, D.L., Tatsumi, M., Gilson, W.D., Ishimori, T., Kedziorek, D., Walczak, P., Segars, W.P., Chen, H.H., Fritzges, D., Izbudak, I., et al. 2005. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation, 112, 1451-1461. [DOI] [PMC free article] [PubMed]
- Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]; Krampera, M., Cosmi, L., Angeli, R., Pasini, A., Liotta, F., Andreini, A., Santarlasci, V., Mazzinghi, B., Pizzolo, G., Vinante, F. 2006. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells, 24, 386-398. [DOI] [PubMed]
- Krampera M., Pasini A., Rigo A., Scupoli M.T., Tecchio C., Malpeli G., Scarpa A., Dazzi F., Pizzolo G., Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]; Krampera, M., Pasini, A., Rigo, A., Scupoli, M.T., Tecchio, C., Malpeli, G., Scarpa, A., Dazzi, F., Pizzolo, G. & Vinante, F. 2005. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood, 106, 59-66. [DOI] [PubMed]
- Kumar S., Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]; Kumar, S. & Ponnazhagan, S. 2007. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J., 21, 3917-3927. [DOI] [PubMed]
- Lau T.T., Wang D.A. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011;11:189–197. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]; Lau, T.T. & Wang, D.A. 2011. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther., 11, 189-197. [DOI] [PubMed]
- Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]; Le Blanc, K. & Mougiakakos, D. 2012. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol., 12, 383-396. [DOI] [PubMed]
- Lee R.J., Fang Q., Davol P.A., Gu Y., Sievers R.E., Grabert R.C., Gall J.M., Tsang E., Yee M.S., Fok H. Antibody targeting of stem cells to infarcted myocardium. Stem Cells. 2007;25:712–717. doi: 10.1634/stemcells.2005-0602. [DOI] [PubMed] [Google Scholar]; Lee, R.J., Fang, Q., Davol, P.A., Gu, Y., Sievers, R.E., Grabert, R.C., Gall, J.M., Tsang, E., Yee, M.S., Fok, H., et al. 2007. Antibody targeting of stem cells to infarcted myocardium. Stem Cells, 25, 712-717. [DOI] [PubMed]
- Levy O., Zhao W., Mortensen L.J., Leblanc S., Tsang K., Fu M., Phillips J.A., Sagar V., Anandakumaran P., Ngai J. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122 doi: 10.1182/blood-2013-04-495119. e23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; Levy, O., Zhao, W., Mortensen, L.J., Leblanc, S., Tsang, K., Fu, M., Phillips, J.A., Sagar, V., Anandakumaran, P., Ngai, J., et al. 2013. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood, 122, e23-32. [DOI] [PMC free article] [PubMed]
- Li L., Wu S., Liu Z., Zhuo Z., Tan K., Xia H., Zhuo L., Deng X., Gao Y., Xu Y. Ultrasound-targeted microbubble destruction improves the migration and homing of mesenchymal stem cells after myocardial infarction by upregulating SDF-1/CXCR4: a pilot study. Stem Cells Int. 2015;2015:691310. doi: 10.1155/2015/691310. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, L., Wu, S., Liu, Z., Zhuo, Z., Tan, K., Xia, H., Zhuo, L., Deng, X., Gao, Y. & Xu, Y. 2015. Ultrasound-targeted microbubble destruction improves the migration and homing of mesenchymal stem cells after myocardial infarction by upregulating SDF-1/CXCR4: a pilot study. Stem Cells Int., 2015, 691310. [DOI] [PMC free article] [PubMed]
- Li Q., Zhang A., Tao C., Li X., Jin P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2013;441:675–680. doi: 10.1016/j.bbrc.2013.10.071. [DOI] [PubMed] [Google Scholar]; Li, Q., Zhang, A., Tao, C., Li, X. & Jin, P. 2013. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun., 441, 675-680. [DOI] [PubMed]
- Li Y., Yu X., Lin S., Li X., Zhang S., Song Y.H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]; Li, Y., Yu, X., Lin, S., Li, X., Zhang, S. & Song, Y.H. 2007. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun., 356, 780-784. [DOI] [PubMed]
- Liao W., Pham V., Liu L., Riazifar M., Pone E.J., Zhang S.X., Ma F., Lu M., Walsh C.M., Zhao W. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials. 2016;77:87–97. doi: 10.1016/j.biomaterials.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liao, W., Pham, V., Liu, L., Riazifar, M., Pone, E.J., Zhang, S.X., Ma, F., Lu, M., Walsh, C.M. & Zhao, W. 2016. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials, 77, 87-97. [DOI] [PMC free article] [PubMed]
- Lin T.H., Liu H.H., Tsai T.H., Chen C.C., Hsieh T.F., Lee S.S., Lee Y.J., Chen W.C., Tang C.H. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim. Biophys. Acta. 2013;1830:4917–4927. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]; Lin, T.H., Liu, H.H., Tsai, T.H., Chen, C.C., Hsieh, T.F., Lee, S.S., Lee, Y.J., Chen, W.C. & Tang, C.H. 2013. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim. Biophys. Acta, 1830, 4917-4927. [DOI] [PubMed]
- Liu H., Xue W., Ge G., Luo X., Li Y., Xiang H., Ding X., Tian P., Tian X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem. Biophys. Res. Commun. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]; Liu, H., Xue, W., Ge, G., Luo, X., Li, Y., Xiang, H., Ding, X., Tian, P. & Tian, X. 2010. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem. Biophys. Res. Commun., 401, 509-515. [DOI] [PubMed]
- Lo C.Y., Antonopoulos A., Dell A., Haslam S.M., Lee T., Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 2013;34:8213–8222. doi: 10.1016/j.biomaterials.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lo, C.Y., Antonopoulos, A., Dell, A., Haslam, S.M., Lee, T. & Neelamegham, S. 2013. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials, 34, 8213-8222. [DOI] [PMC free article] [PubMed]
- Lo C.Y., Weil B.R., Palka B.A., Momeni A., Canty J.M., Jr., Neelamegham S. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs): pilot validation in porcine ischemia-reperfusion model. Biomaterials. 2016;74:19–30. doi: 10.1016/j.biomaterials.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lo, C.Y., Weil, B.R., Palka, B.A., Momeni, A., Canty, J.M., Jr. & Neelamegham, S. 2016. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs): pilot validation in porcine ischemia-reperfusion model. Biomaterials, 74, 19-30. [DOI] [PMC free article] [PubMed]
- Luo Y., Mohsin A., Xu C., Wang Q., Hang H., Zhuang Y., Chu J., Guo M. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology. 2018;70:1409–1422. doi: 10.1007/s10616-018-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luo, Y., Mohsin, A., Xu, C., Wang, Q., Hang, H., Zhuang, Y., Chu, J. & Guo, M. 2018. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology, 70, 1409-1422. [DOI] [PMC free article] [PubMed]
- McIntyre L.A., Moher D., Fergusson D.A., Sullivan K.J., Mei S.H., Lalu M., Marshall J., Mcleod M., Griffin G., Grimshaw J. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS One. 2016;11:e0147170. doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]; McIntyre, L.A., Moher, D., Fergusson, D.A., Sullivan, K.J., Mei, S.H., Lalu, M., Marshall, J., Mcleod, M., Griffin, G., Grimshaw, J., et al. 2016. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS One, 11, e0147170. [DOI] [PMC free article] [PubMed]
- Meng S.S., Xu X.P., Chang W., Lu Z.H., Huang L.L., Xu J.Y., Liu L., Qiu H.B., Yang Y., Guo F.M. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther. 2018;9:280. doi: 10.1186/s13287-018-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meng, S.S., Xu, X.P., Chang, W., Lu, Z.H., Huang, L.L., Xu, J.Y., Liu, L., Qiu, H.B., Yang, Y. & Guo, F.M. 2018. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther., 9.280. [DOI] [PMC free article] [PubMed]
- Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R., Bioeffects Committee of the American Institute of Ultrasound in Medicine Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miller, D.L., Smith, N.B., Bailey, M.R., Czarnota, G.J., Hynynen, K., Makin, I.R. & Bioeffects Committee of the American Institute of Ultrasound in Medicine. 2012. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med., 31, 623-634. [DOI] [PMC free article] [PubMed]
- Mouiseddine M., Francois S., Semont A., Sache A., Allenet B., Mathieu N., Frick J., Thierry D., Chapel A. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br. J. Radiol. 2007;80:S49–S55. doi: 10.1259/bjr/25927054. [DOI] [PubMed] [Google Scholar]; Mouiseddine, M., Francois, S., Semont, A., Sache, A., Allenet, B., Mathieu, N., Frick, J., Thierry, D. & Chapel, A. 2007. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br. J. Radiol., 80, S49-S55. [DOI] [PubMed]
- Muller W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]; Muller, W.A. 2011. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol., 6, 323-344. [DOI] [PMC free article] [PubMed]
- Najafi R., Sharifi A.M. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin. Biol. Ther. 2013;13:959–972. doi: 10.1517/14712598.2013.782390. [DOI] [PubMed] [Google Scholar]; Najafi, R. & Sharifi, A.M. 2013. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin. Biol. Ther., 13, 959-972. [DOI] [PubMed]
- Nauta A.J., Westerhuis G., Kruisselbrink A.B., Lurvink E.G., Willemze R., Fibbe W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nauta, A.J., Westerhuis, G., Kruisselbrink, A.B., Lurvink, E.G., Willemze, R. & Fibbe, W.E. 2006. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood, 108, 2114-2120. [DOI] [PMC free article] [PubMed]
- Nitzsche F., Muller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35:1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]; Nitzsche, F., Muller, C., Lukomska, B., Jolkkonen, J., Deten, A. & Boltze, J. 2017. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells, 35, 1446-1460. [DOI] [PubMed]
- Pak S., Hwang S.W., Shim I.K., Bae S.M., Ryu Y.M., Kim H.B., Do E.J., Son H.N., Choi E.J., Park S.H. Endoscopic transplantation of mesenchymal stem cell sheets in experimental colitis in rats. Sci. Rep. 2018;8:11314. doi: 10.1038/s41598-018-29617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pak, S., Hwang, S.W., Shim, I.K., Bae, S.M., Ryu, Y.M., Kim, H.B., Do, E.J., Son, H.N., Choi, E.J., Park, S.H., et al 2018. Endoscopic transplantation of mesenchymal stem cell sheets in experimental colitis in rats. Sci. Rep., 8, 11314. [DOI] [PMC free article] [PubMed]
- Peng W., Sun J., Sheng C., Wang Z., Wang Y., Zhang C., Fan R. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res. Ther. 2015;6:47. doi: 10.1186/s13287-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Peng, W., Sun, J., Sheng, C., Wang, Z., Wang, Y., Zhang, C. & Fan, R. 2015. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res. Ther., 6, 47. [DOI] [PMC free article] [PubMed]
- Penn M.S., Mendelsohn F.O., Schaer G.L., Sherman W., Farr M., Pastore J., Rouy D., Clemens R., Aras R., Losordo D.W. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res. 2013;112:816–825. doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]; Penn, M.S., Mendelsohn, F.O., Schaer, G.L., Sherman, W., Farr, M., Pastore, J., Rouy, D., Clemens, R., Aras, R. & Losordo, D.W. 2013. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res., 112, 816-825. [DOI] [PubMed]
- Penn M.S., Pastore J., Miller T., Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–587. doi: 10.1038/gt.2012.32. [DOI] [PubMed] [Google Scholar]; Penn, M.S., Pastore, J., Miller, T. & Aras, R. 2012. SDF-1 in myocardial repair. Gene Ther., 19, 583-587. [DOI] [PubMed]
- Ponomaryov T., Peled A., Petit I., Taichman R.S., Habler L., Sandbank J., Arenzana-Seisdedos F., Magerus A., Caruz A., Fujii N. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ponomaryov, T., Peled, A., Petit, I., Taichman, R.S., Habler, L., Sandbank, J., Arenzana-Seisdedos, F., Magerus, A., Caruz, A., Fujii, N., et al. 2000. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest., 106, 1331-1339. [DOI] [PMC free article] [PubMed]
- Ponte A.L., Marais E., Gallay N., Langonne A., Delorme B., Herault O., Charbord P., Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2009;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]; Ponte, A.L., Marais, E., Gallay, N., Langonne, A., Delorme, B., Herault, O., Charbord, P. & Domenech, J. 2009. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells, 25, 1737-1745. [DOI] [PubMed]
- Qiu Y., Marquez-Curtis L.A., Janowska-Wieczorek A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 2012;14:285–295. doi: 10.3109/14653249.2011.651532. [DOI] [PubMed] [Google Scholar]; Qiu, Y., Marquez-Curtis, L.A. & Janowska-Wieczorek, A. 2012. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy, 14, 285-295. [DOI] [PubMed]
- Ran L.J., Zeng Y., Wang S.C., Zhang D.S., Hong M., Li S.Y., Dong J., Shi M.X. Effect of coculture with amniotic epithelial cells on the biological characteristics of amniotic mesenchymal stem cells. Mol. Med. Rep. 2018;18:723–732. doi: 10.3892/mmr.2018.9053. [DOI] [PubMed] [Google Scholar]; Ran, L.J., Zeng, Y., Wang, S.C., Zhang, D.S., Hong, M., Li, S.Y., Dong, J. & Shi, M.X. 2018. Effect of coculture with amniotic epithelial cells on the biological characteristics of amniotic mesenchymal stem cells. Mol. Med. Rep., 18, 723-732. [DOI] [PubMed]
- Ries C., Egea V., Karow M., Kolb H., Jochum M., Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]; Ries, C., Egea, V., Karow, M., Kolb, H., Jochum, M. & Neth, P. 2007. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood, 109, 4055-4063. [DOI] [PubMed]
- Rombouts W.J.C., Ploemacher R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]; Rombouts, W.J.C. & Ploemacher, R.E. 2003. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia, 17, 160-170. [DOI] [PubMed]
- Ruster B., Gottig S., Ludwig R.J., Bistrian R., Muller S., Seifried E., Gille J., Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]; Ruster, B., Gottig, S., Ludwig, R.J., Bistrian, R., Muller, S., Seifried, E., Gille, J. & Henschler, R. 2006. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood, 108, 3938-3944. [DOI] [PubMed]
- Ryser M.F., Ugarte F., Thieme S., Bornhauser M., Roesen-Wolff A., Brenner S. mRNA transfection of CXCR4-GFP fusion - simply generated by PCR - results in efficient migration of primary human mesenchymal stem cells. Tissue Eng. Part C Methods. 2008;14:179–184. doi: 10.1089/ten.tec.2007.0359. [DOI] [PubMed] [Google Scholar]; Ryser, M.F., Ugarte, F., Thieme, S., Bornhauser, M., Roesen-Wolff, A. & Brenner, S. 2008. mRNA transfection of CXCR4-GFP fusion - simply generated by PCR - results in efficient migration of primary human mesenchymal stem cells. Tissue Eng. Part C Methods, 14, 179-184. [DOI] [PubMed]
- Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr. Opin. Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]; Sackstein, R. 2005. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr. Opin. Hematol., 12, 444-450. [DOI] [PubMed]
- Sackstein R., Merzaban J.S., Cain D.W., Dagia N.M., Spencer J.A., Lin C.P., Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]; Sackstein, R., Merzaban, J.S., Cain, D.W., Dagia, N.M., Spencer, J.A., Lin, C.P. & Wohlgemuth, R. 2008. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med., 14, 181-187. [DOI] [PubMed]
- Sarkar D., Vemula P.K., Teo G.S., Spelke D., Karnik R., Wee Le Y., Karp J.M. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug. Chem. 2008;19:2105–2109. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]; Sarkar, D., Vemula, P.K., Teo, G.S., Spelke, D., Karnik, R., Wee Le, Y. & Karp, J.M. 2008. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug. Chem., 19, 2105-2109. [DOI] [PubMed]
- Sasaki T., Fukazawa R., Ogawa S., Kanno S., Nitta T., Ochi M., Shimizu K. Stromal cell-derived factor-1 alpha improves infarcted heart function through angiogenesis in mice. Pediatr. Int. 2007;49:966–971. doi: 10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]; Sasaki, T., Fukazawa, R., Ogawa, S., Kanno, S., Nitta, T., Ochi, M. & Shimizu, K. 2007. Stromal cell-derived factor-1 alpha improves infarcted heart function through angiogenesis in mice. Pediatr. Int., 49, 966-971. [DOI] [PubMed]
- Saxena A., Fish J.E., White M.D., Yu S., Smyth J.W., Shaw R.M., Dimaio J.M., Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]; Saxena, A., Fish, J.E., White, M.D., Yu, S., Smyth, J.W., Shaw, R.M., Dimaio, J.M. & Srivastava, D. 2008. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation, 117, 2224-2231. [DOI] [PMC free article] [PubMed]
- Scarfe L., Taylor A., Sharkey J., Harwood R., Barrow M., Comenge J., Beeken L., Astley C., Santeramo I., Hutchinson C. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther. 2018;9:332. doi: 10.1186/s13287-018-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scarfe, L., Taylor, A., Sharkey, J., Harwood, R., Barrow, M., Comenge, J., Beeken, L., Astley, C., Santeramo, I., Hutchinson, C., et al. 2018. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther., 9.332. [DOI] [PMC free article] [PubMed]
- Schantz J.T., Chim H., Whiteman M. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng. 2007;13:2615–2624. doi: 10.1089/ten.2006.0438. [DOI] [PubMed] [Google Scholar]; Schantz, J.T., Chim, H. & Whiteman, M. 2007. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng., 13, 2615-2624. [DOI] [PubMed]
- Schenk S., Mal N., Finan A., Zhang M., Kiedrowski M., Popovic Z., Mccarthy P.M., Penn M.S. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]; Schenk, S., Mal, N., Finan, A., Zhang, M., Kiedrowski, M., Popovic, Z., Mccarthy, P.M. & Penn, M.S. 2007. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells, 25, 245-251. [DOI] [PubMed]
- Segers V.F., Revin V., Wu W., Qiu H., Yan Z., Lee R.T., Sandrasagra A. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease. Circulation. 2011;123:1306–1315. doi: 10.1161/CIRCULATIONAHA.110.991786. [DOI] [PubMed] [Google Scholar]; Segers, V.F., Revin, V., Wu, W., Qiu, H., Yan, Z., Lee, R.T. & Sandrasagra, A. 2011. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease. Circulation, 123, 1306-1315. [DOI] [PubMed]
- Segers V.F., Tokunou T., Higgins L.J., Macgillivray C., Gannon J., Lee R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]; Segers, V.F., Tokunou, T., Higgins, L.J., Macgillivray, C., Gannon, J. & Lee, R.T. 2007. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation, 116, 1683-1692. [DOI] [PubMed]
- Segers V.F., Van Riet I., Andries L.J., Lemmens K., Demolder M.J., De Becker A.J., Kockx M.M., De Keulenaer G.W. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]; Segers, V.F., Van Riet, I., Andries, L.J., Lemmens, K., Demolder, M.J., De Becker, A.J., Kockx, M.M. & De Keulenaer, G.W. 2006. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am. J. Physiol. Heart Circ. Physiol., 290, H1370-H1377. [DOI] [PubMed]
- Shao Y., Zhou F., He D., Zhang L., Shen J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019;109:1233–1239. doi: 10.1016/j.biopha.2018.10.108. [DOI] [PubMed] [Google Scholar]; Shao, Y., Zhou, F., He, D., Zhang, L. & Shen, J. 2019. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother., 109, 1233-1239. [DOI] [PubMed]
- Shen W.L., Chen X.A., Chen J.L., Yin Z., Heng B.C., Chen W.S., Ouyang H.W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]; Shen, W.L., Chen, X.A., Chen, J.L., Yin, Z., Heng, B.C., Chen, W.S. & Ouyang, H.W. 2010. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials, 31, 7239-7249. [DOI] [PubMed]
- Shi M., Li J., Liao L., Chen B., Li B., Chen L., Jia H., Zhao R.C. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]; Shi, M., Li, J., Liao, L., Chen, B., Li, B., Chen, L., Jia, H. & Zhao, R.C. 2007. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica, 92, 897-904. [DOI] [PubMed]
- Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]; Singer, N.G. & Caplan, A.I. 2011. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol., 6, 457-478. [DOI] [PubMed]
- Snykers S., de Kock J., Rogiers V., Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]; Snykers, S., de Kock, J., Rogiers, V. & Vanhaecke, T. 2009. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells, 27, 577-605. [DOI] [PMC free article] [PubMed]
- Song Y.S., Ku J.H. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol. Urodyn. 2007;26:584–593. doi: 10.1002/nau.20351. [DOI] [PubMed] [Google Scholar]; Song, Y.S. & Ku, J.H. 2007. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol. Urodyn., 26, 584-593. [DOI] [PubMed]
- Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]; Squillaro, T., Peluso, G. & Galderisi, U. 2016. Clinical trials with mesenchymal stem cells: an update. Cell Transplant., 25, 829-848. [DOI] [PubMed]
- Steingen C., Brenig F., Baumgartner L., Schmidt J., Schmidt A., Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell Cardiol. 2008;44:1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]; Steingen, C., Brenig, F., Baumgartner, L., Schmidt, J., Schmidt, A. & Bloch, W. 2008. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell Cardiol., 44, 1072-1084. [DOI] [PubMed]
- Stockmann P., Park J., von Wilmowsky C., Nkenke E., Felszeghy E., Dehner J.F., Schmitt C., Tudor C., Schlegel K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources. J. Craniomaxillofac. Surg. 2012;40:310–320. doi: 10.1016/j.jcms.2011.05.004. [DOI] [PubMed] [Google Scholar]; Stockmann, P., Park, J., von Wilmowsky, C., Nkenke, E., Felszeghy, E., Dehner, J.F., Schmitt, C., Tudor, C. & Schlegel, K.A. 2012. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources. J. Craniomaxillofac. Surg., 40, 310-320. [DOI] [PubMed]
- Suila H., Hirvonen T., Kotovuori A., Ritamo I., Kerkela E., Anderson H., Natunen S., Tuimala J., Laitinen S., Nystedt J. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand. J. Immunol. 2014;80:12–21. doi: 10.1111/sji.12179. [DOI] [PubMed] [Google Scholar]; Suila, H., Hirvonen, T., Kotovuori, A., Ritamo, I., Kerkela, E., Anderson, H., Natunen, S., Tuimala, J., Laitinen, S., Nystedt, J., et al. 2014. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand. J. Immunol., 80, 12-21. [DOI] [PubMed]
- Sundararaman S., Miller T.J., Pastore J.M., Kiedrowski M., Aras R., Penn M.S. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18:867–873. doi: 10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sundararaman, S., Miller, T.J., Pastore, J.M., Kiedrowski, M., Aras, R. & Penn, M.S. 2011. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther., 18, 867-873. [DOI] [PMC free article] [PubMed]
- Tadokoro S., Shattil S.J., Eto K., Tai V., Liddington R.C., de Pereda J.M., Ginsberg M.H., Calderwood D.A. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]; Tadokoro, S., Shattil, S.J., Eto, K., Tai, V., Liddington, R.C., de Pereda, J.M., Ginsberg, M.H. & Calderwood, D.A. 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science, 302, 103-106. [DOI] [PubMed]
- Tano N., Narita T., Kaneko M., Ikebe C., Coppen S.R., Campbell N.G., Shiraishi M., Shintani Y., Suzuki K. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther. 2014;22:1864–1871. doi: 10.1038/mt.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tano, N., Narita, T., Kaneko, M., Ikebe, C., Coppen, S.R., Campbell, N.G., Shiraishi, M., Shintani, Y. & Suzuki, K. 2014. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther., 22, 1864-1871. [DOI] [PMC free article] [PubMed]
- Tebebi P.A., Burks S.R., Kim S.J., Williams R.A., Nguyen B.A., Venkatesh P., Frenkel V., Frank J.A. Cyclooxygenase-2 or tumor necrosis factor-alpha inhibitors attenuate the mechanotransductive effects of pulsed focused ultrasound to suppress mesenchymal stromal cell homing to healthy and dystrophic muscle. Stem Cells. 2015;33:1173–1186. doi: 10.1002/stem.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tebebi, P.A., Burks, S.R., Kim, S.J., Williams, R.A., Nguyen, B.A., Venkatesh, P., Frenkel, V. & Frank, J.A. 2015. Cyclooxygenase-2 or tumor necrosis factor-alpha inhibitors attenuate the mechanotransductive effects of pulsed focused ultrasound to suppress mesenchymal stromal cell homing to healthy and dystrophic muscle. Stem Cells, 33, 1173-1186. [DOI] [PMC free article] [PubMed]
- Tebebi P.A., Kim S.J., Williams R.A., Milo B., Frenkel V., Burks S.R., Frank J.A. Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci. Rep. 2017;7:41550. doi: 10.1038/srep41550. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tebebi, P.A., Kim, S.J., Williams, R.A., Milo, B., Frenkel, V., Burks, S.R. & Frank, J.A. 2017. Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci. Rep., 7, 41550. [DOI] [PMC free article] [PubMed]
- Teo G.S.L., Ankrum J.A., Martinelli R., Boetto S.E., Simms K., Sciuto T.E., Dvorak A.M., Karp J.M., Carman C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-alpha-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30:2472–2486. doi: 10.1002/stem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]; Teo, G.S.L., Ankrum, J.A., Martinelli, R., Boetto, S.E., Simms, K., Sciuto, T.E., Dvorak, A.M., Karp, J.M. & Carman, C.V. 2012. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-alpha-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells, 30, 2472-2486. [DOI] [PMC free article] [PubMed]
- Thevenot P.T., Nair A.M., Shen J.H., Lotfi P., Ko C.Y., Tang L.P. The effect of incorporation of SDF-1 alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thevenot, P.T., Nair, A.M., Shen, J.H., Lotfi, P., Ko, C.Y. & Tang, L.P. 2010. The effect of incorporation of SDF-1 alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials, 31, 3997-4008. [DOI] [PMC free article] [PubMed]
- Tsai L.K., Leng Y., Wang Z., Leeds P., Chuang D.M. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology. 2010;35:2225–2237. doi: 10.1038/npp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tsai, L.K., Leng, Y., Wang, Z., Leeds, P. & Chuang, D.M. 2010. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology, 35, 2225-2237. [DOI] [PMC free article] [PubMed]
- Tsai L.K., Wang Z., Munasinghe J., Leng Y., Leeds P., Chuang D.M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tsai, L.K., Wang, Z., Munasinghe, J., Leng, Y., Leeds, P. & Chuang, D.M. 2011. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke, 42, 2932-2939. [DOI] [PMC free article] [PubMed]
- Von Luttichau I., Notohamiprodjo M., Wechselberger A., Peters C., Henger A., Seliger C., Djafarzadeh R., Huss R., Nelson P.J. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]; Von Luttichau, I., Notohamiprodjo, M., Wechselberger, A., Peters, C., Henger, A., Seliger, C., Djafarzadeh, R., Huss, R. & Nelson, P.J. 2005. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev., 14, 329-336. [DOI] [PubMed]
- Vu Q., Xie K., Eckert M., Zhao W., Cramer S.C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vu, Q., Xie, K., Eckert, M., Zhao, W. & Cramer, S.C. 2014. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology, 82, 1277-1286. [DOI] [PMC free article] [PubMed]
- Wang G., Zhuo Z., Yang B., Wu S., Xu Y., Liu Z., Tan K., Xia H., Wang X., Zou L. Enhanced homing ability and retention of bone marrow stromal cells to diabetic nephropathy by microbubble-mediated diagnostic ultrasound irradiation. Ultrasound Med. Biol. 2015;41:2977–2989. doi: 10.1016/j.ultrasmedbio.2015.07.002. [DOI] [PubMed] [Google Scholar]; Wang, G., Zhuo, Z., Yang, B., Wu, S., Xu, Y., Liu, Z., Tan, K., Xia, H., Wang, X., Zou, L., et al. 2015. Enhanced homing ability and retention of bone marrow stromal cells to diabetic nephropathy by microbubble-mediated diagnostic ultrasound irradiation. Ultrasound Med. Biol., 41, 2977-2989. [DOI] [PubMed]
- Wang H.S., Hung S.C., Peng S.T., Huang C.C., Wei H.M., Guo Y.J., Fu Y.S., Lai M.C., Chen C.C. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]; Wang, H.S., Hung, S.C., Peng, S.T., Huang, C.C., Wei, H.M., Guo, Y.J., Fu, Y.S., Lai, M.C. & Chen, C.C. 2004. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells, 22, 1330-1337. [DOI] [PubMed]
- Wang W.W., Deng Z.J., Xu X., Li Z.D., Jung F., Ma N., Lendlein A. Functional nanoparticles and their interactions with mesenchymal stem cells. Curr. Pharm. Des. 2017;23:3814–3832. doi: 10.2174/1381612823666170622110654. [DOI] [PubMed] [Google Scholar]; Wang, W.W., Deng, Z.J., Xu, X., Li, Z.D., Jung, F., Ma, N. & Lendlein, A. 2017. Functional nanoparticles and their interactions with mesenchymal stem cells. Curr. Pharm. Des., 23, 3814-3832. [DOI] [PubMed]
- Wang Y., Fu W., Zhang S., He X., Liu Z., Gao D., Xu T. CXCR-7 receptor promotes SDF-1alpha-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res. 2014;1575:78–86. doi: 10.1016/j.brainres.2014.05.035. [DOI] [PubMed] [Google Scholar]; Wang, Y., Fu, W., Zhang, S., He, X., Liu, Z., Gao, D. & Xu, T. 2014. CXCR-7 receptor promotes SDF-1alpha-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res., 1575, 78-86. [DOI] [PubMed]
- Wiehe J.M., Kaya Z., Homann J.M., Wohrle J., Vogt K., Nguyen T., Rottbauer W., Torzewski J., Fekete N., Rojewski M. GMP-adapted overexpression of CXCR4 in human mesenchymal stem cells for cardiac repair. Int. J. Cardiol. 2013;167:2073–2081. doi: 10.1016/j.ijcard.2012.05.065. [DOI] [PubMed] [Google Scholar]; Wiehe, J.M., Kaya, Z., Homann, J.M., Wohrle, J., Vogt, K., Nguyen, T., Rottbauer, W., Torzewski, J., Fekete, N., Rojewski, M., et al. 2013. GMP-adapted overexpression of CXCR4 in human mesenchymal stem cells for cardiac repair. Int. J. Cardiol., 167, 2073-2081. [DOI] [PubMed]
- Will H., Atkinson S.J., Butler G.S., Smith B., Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]; Will, H., Atkinson, S.J., Butler, G.S., Smith, B. & Murphy, G. 1996. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem., 271, 17119-17123. [DOI] [PubMed]
- Williams A.R., Trachtenberg B., Velazquez D.L., Mcniece I., Altman P., Rouy D., Mendizabal A.M., Pattany P.M., Lopera G.A., Fishman J. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ. Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]; Williams, A.R., Trachtenberg, B., Velazquez, D.L., Mcniece, I., Altman, P., Rouy, D., Mendizabal, A.M., Pattany, P.M., Lopera, G.A., Fishman, J., et al. 2011. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ. Res., 108, 792-796. [DOI] [PMC free article] [PubMed]
- Won Y.W., Patel A.N., Bull D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]; Won, Y.W., Patel, A.N. & Bull, D.A. 2014. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials, 35, 5627-5635. [DOI] [PubMed]
- Wynn R.F., Hart C.A., Corradi-Perini C., O'neill L., Evans C.A., Wraith J.E., Fairbairn L.J., Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]; Wynn, R.F., Hart, C.A., Corradi-Perini, C., O'neill, L., Evans, C.A., Wraith, J.E., Fairbairn, L.J. & Bellantuono, I. 2004. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood, 104, 2643-2645. [DOI] [PubMed]
- Xu Y.L., Gao Y.H., Liu Z., Tan K.B., Hua X., Fang Z.Q., Wang Y.L., Wang Y.J., Xia H.M., Zhuo Z.X. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int. J. Cardiol. 2010;138:182–195. doi: 10.1016/j.ijcard.2009.03.071. [DOI] [PubMed] [Google Scholar]; Xu, Y.L., Gao, Y.H., Liu, Z., Tan, K.B., Hua, X., Fang, Z.Q., Wang, Y.L., Wang, Y.J., Xia, H.M. & Zhuo, Z.X. 2010. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int. J. Cardiol., 138, 182-195. [DOI] [PubMed]
- Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S., Bosch-Marce M., Masuda H., Losordo D.W., Isner J.M., Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]; Yamaguchi, J., Kusano, K.F., Masuo, O., Kawamoto, A., Silver, M., Murasawa, S., Bosch-Marce, M., Masuda, H., Losordo, D.W., Isner, J.M. & Asahara, T. 2003. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation, 107, 1322-1328. [DOI] [PubMed]
- Yanai A., Hafeli U.O., Metcalfe A.L., Soema P., Addo L., Gregory-Evans C.Y., Po K., Shan X.H., Moritz O.L., Gregory-Evans K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012;21:1137–1148. doi: 10.3727/096368911X627435. [DOI] [PubMed] [Google Scholar]; Yanai, A., Hafeli, U.O., Metcalfe, A.L., Soema, P., Addo, L., Gregory-Evans, C.Y., Po, K., Shan, X.H., Moritz, O.L. & Gregory-Evans, K. 2012. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant., 21, 1137-1148. [DOI] [PubMed]
- Young H.E., Steele T.A., Bray R.A., Hudson J., Floyd J.A., Hawkins K., Thomas K., Austin T., Edwards C., Cuzzourt J. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]; Young, H.E., Steele, T.A., Bray, R.A., Hudson, J., Floyd, J.A., Hawkins, K., Thomas, K., Austin, T., Edwards, C., Cuzzourt, J., et al. 2001. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec., 264, 51-62. [DOI] [PubMed]
- Yu Q.A., Chen L., You Y., Zou C., Zhang Y.S., Liu Q.H., Cheng F.J. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol. Med. Rep. 2011;4:31–36. doi: 10.3892/mmr.2010.387. [DOI] [PubMed] [Google Scholar]; Yu, Q.A., Chen, L., You, Y., Zou, C., Zhang, Y.S., Liu, Q.H. & Cheng, F.J. 2011. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol. Med. Rep., 4, 31-36. [DOI] [PubMed]
- Yukawa H., Watanabe M., Kaji N., Okamoto Y., Tokeshi M., Miyamoto Y., Noguchi H., Baba Y., Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177–2186. doi: 10.1016/j.biomaterials.2011.12.009. [DOI] [PubMed] [Google Scholar]; Yukawa, H., Watanabe, M., Kaji, N., Okamoto, Y., Tokeshi, M., Miyamoto, Y., Noguchi, H., Baba, Y. & Hayashi, S. 2012. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials, 33, 2177-2186. [DOI] [PubMed]
- Yun W.S., Choi J.S., Ju H.M., Kim M.H., Choi S.J., Oh E.S., Seo Y.J., Key J. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models. Int. J. Mol. Sci. 2018;19:1376. doi: 10.3390/ijms19051376. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yun, W.S., Choi, J.S., Ju, H.M., Kim, M.H., Choi, S.J., Oh, E.S., Seo, Y.J. & Key, J. 2018. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models. Int. J. Mol. Sci., 19. [DOI] [PMC free article] [PubMed]
- Zen K., Okigaki M., Hosokawa Y., Adachi Y., Nozawa Y., Takamiya M., Tatsumi T., Urao N., Tateishi K., Takahashi T., Matsubara H. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J. Mol. Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]; Zen, K., Okigaki, M., Hosokawa, Y., Adachi, Y., Nozawa, Y., Takamiya, M., Tatsumi, T., Urao, N., Tateishi, K., Takahashi, T. & Matsubara, H. 2006. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J. Mol. Cell Cardiol., 40, 799-809. [DOI] [PubMed]
- Zhang D., Fan G.C., Zhou X., Zhao T., Pasha Z., Xu M., Zhu Y., Ashraf M., Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J. Mol. Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang, D., Fan, G.C., Zhou, X., Zhao, T., Pasha, Z., Xu, M., Zhu, Y., Ashraf, M. & Wang, Y. 2008. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J. Mol. Cell Cardiol., 44, 281-292. [DOI] [PMC free article] [PubMed]
- Zhang F., Hong Y., Liang W., Ren T., Jing S., Lin J. Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012;427:86–90. doi: 10.1016/j.bbrc.2012.09.007. [DOI] [PubMed] [Google Scholar]; Zhang, F., Hong, Y., Liang, W., Ren, T., Jing, S. & Lin, J. 2012. Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem. Biophys. Res. Commun., 427, 86-90. [DOI] [PubMed]
- Zhidkova O.V., Andreeva E.R., Buravkova L.B. Endothelial cells modulate differentiation potential and mobility of mesenchymal stromal cells. Bull. Exp. Biol. Med. 2018;165:127–131. doi: 10.1007/s10517-018-4113-y. [DOI] [PubMed] [Google Scholar]; Zhidkova, O.V., Andreeva, E.R. & Buravkova, L.B. 2018. Endothelial cells modulate differentiation potential and mobility of mesenchymal stromal cells. Bull. Exp. Biol. Med., 165, 127-131. [DOI] [PubMed]
- Ziadloo A., Burks S.R., Gold E.M., Lewis B.K., Chaudhry A., Merino M.J., Frenkel V., Frank J.A. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30:1216–1227. doi: 10.1002/stem.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ziadloo, A., Burks, S.R., Gold, E.M., Lewis, B.K., Chaudhry, A., Merino, M.J., Frenkel, V. & Frank, J.A. 2012. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells, 30, 1216-1227. [DOI] [PMC free article] [PubMed]
- Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zuk, P.A., Zhu, M., Ashjian, P., De Ugarte, D.A., Huang, J.I., Mizuno, H., Alfonso, Z.C., Fraser, J.K., Benhaim, P. & Hedrick, M.H. 2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell, 13, 4279-4295. [DOI] [PMC free article] [PubMed]