Abstract
Upon plating on basement membrane Matrigel, NIH3T3 cells formed an anastomosing network of cord-like structures, inhibitable by anti-α6β1 integrin antibodies. For NIH3T3 cells transfected with human CD151 protein, the formation of a cord-like network was also inhibitable by anti-CD151 antibodies. Furthermore, CD151 and α6β1 were physically associated within NIH3T3 cells. On removal of the short 8-amino acid C-terminal CD151 tail (by deletion or exchange), exogenous CD151 exerted a dominant negative effect, as it almost completely suppressed α6β1-dependent cell network formation and NIH3T3 cell spreading on laminin-1 (an α6β1 ligand). Importantly, mutant CD151 retained α6β1 association and did not alter α6β1-mediated cell adhesion to Matrigel. In conclusion, the CD151–α6β1 integrin complex acts as a functional unit that markedly influences cellular morphogenesis, with the CD151 tail being of particular importance in determining the “outside-in” functions of α6β1-integrin that follow ligand engagement. Also, antibodies to α6β1 and CD151 inhibited formation of endothelial cell cord-like networks, thus pointing to possible relevance of CD151–α6β1 complexes during angiogenesis.
INTRODUCTION
Studies of integrin-dependent adhesion, migration, and signaling have focused largely on integrin ligand binding sites (Plow et al., 2000) and on cytoplasmic domains (Liu et al., 2000). Cytoplasmic domain perturbations alter ligand binding (“inside-out” signaling) and ligand binding triggers long-range alterations in cytoplasmic domain interactions (“outside-in” signaling). Integrin functions are modulated also by lateral associations with other transmembrane proteins (Hemler, 1998; Woods and Couchman, 2000). As shown here, outside-in signaling through α6β1 integrin is markedly influenced by its lateral association with CD151, a transmembrane-4 superfamily (TM4SF, tetraspanin) protein.
Tetraspanin proteins contain two extracellular loops, four hydrophobic transmembrane domains, and two short cytoplasmic tails. Tetraspanins regulate membrane fusion, trafficking, cell motility, and tumor metastasis (Wright and Tomlinson, 1994; Maecker et al., 1997). Tetraspanins form multimolecular complexes with many other transmembrane proteins, including integrins (Hemler et al., 1996; Hemler, 1998). Despite several reports of tetraspanin–protein complexes, only a few have documented functional relevance. For example, results from CD81-null mice support the relevance of CD81–CD19 association (Maecker and Levy, 1997; Miyazaki et al., 1997; Tsitsikov et al., 1997), CD9 influences the activity of associated HB-EGF (Iwamoto et al., 1994), and antibodies to CD81 and CD151 inhibit the functions of associated integrins (Domanico et al., 1997; Yánez-Móet al., 1998; Yauch et al., 1998; Stipp and Hemler, 2000). Notably, anti-CD81 and anti-CD151 antibodies inhibited neurite outgrowth only when associated α3β1 integrin was engaged with ligand (Stipp and Hemler, 2000).
Among tetraspanin complexes, the CD151–α3β1 integrin complex has unusually high stoichiometry, proximity, and stability. A specific site in CD151's large extracellular loop is required for α3β1 integrin interaction (Yauch et al., 2000). CD151 also may use the same site to form complexes with α6β1, α6β4, and other integrins, while influencing cell motility, hemidesmosome formation, and other functions (Yánez-Móet al., 1998; Fitter et al., 1999; Sincock et al., 1999; Sterk et al., 2000). However, the functional roles of CD151–α6 integrin complexes have not been specifically demonstrated.
We have proposed a “transmembrane linker” model for tetraspanins (Hemler, 1998). In this model, tetraspanin extracellular domains link to integrins, whereas cytoplasmic domains link to intracellular signaling enzymes such as phosphatidylinositol 4-kinase and PKC (Hemler, 1998; Yauch and Hemler, 2000; Zhang et al., 2001a, 2001b). However, the functional relevance of specific tetraspanin cytoplasmic domains has not been shown. Here we demonstrate that the CD151–α6β1 integrin complex acts as a functional unit supporting organization of NIH3T3 cells into a network of cord-like structures when cultured on Matrigel. Although the extracellular (and/or transmembrane) region of CD151 mediates α6β1 integrin association, the CD151 C terminus is of particular importance for modulating α6 integrin–dependent functions. These results provide perhaps the clearest support to date for a tetraspanin transmembrane linker model in which CD151 links to an integrin (α6β1), while using its C-terminal tail to link with intracellular pathways involved in Matrigel morphogenesis and cell spreading.
When plated on basement membrane matrix (Matrigel), collagen, or other substrates, endothelial cells often form an anastomosing cellular network, which may be a model for angiogenesis (Vernon and Sage, 1995). The Matrigel model may not fully mimic in vivo angiogenesis or branching morphogenesis, because the network of cord-like cells contains few if any lumens (Bikfalvi et al., 1991). However, advantages of the Matrigel model are that 1) it can show dramatic morphological changes that reveal useful information about morphogenic processes; 2) it is somewhat permissive, such that a variety of nonendothelial cell types may form cord-like networks (Vernon and Sage, 1995); and 3) in general, three-dimensional models are more physiological and may often reveal much more than classical 2-dimensional cell culture models. Finally, Matrigel contains an abundance of laminin-1, thus providing an opportunity to study functions of α6β1 integrin and associated proteins such as CD151.
MATERIALS AND METHODS
Cell Culture and CD151 Mutants and Transfectants
The NIH3T3 mouse fibroblast cell line was obtained from American Type Culture Collection (Bethesda, MD) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. CD151 mutants were generated by recombinant PCR. As a template we used wild-type human CD151 cDNA, with an HA tag linked to its C-terminus, ligated into the eukaryotic expression vector pZeoSV (Invitrogen, San Diego, CA). As shown in Figure 5, the CD151 mutants generated in this study are as follows: 1) CD151-n-A15 (N-terminal cytoplasmic domain MGEFNEKKTTCGTVCLKYLLFTY of CD151 replaced by the corresponding METKPVITCLKTLLIIYS from A15); 2) CD151-c-A15 (C-terminal cytoplasmic domain SLKLEHY of CD151 replaced with FITANQYEMV from A15); 3) CD151-nc-A15 (both N- and C-termini of CD151 replaced by corresponding domains from A15); 4) CD151-c2-NAG2 (TM4 and C-terminal tail HLRVIGAVGIGIACVQVFGMIFTCCLYRSLKLEHY of CD151 replaced by corresponding regions NLLAVGIFGLCTALVQILGLTFAMTMYCQVVKADTYCA from TM4SF protein NAG-2); and 5) CD151-Δc(GFP) (C-terminal cytoplasmic tail of CD151 LYRSLKLEHY replaced by green fluorescent protein [GFP] moiety).
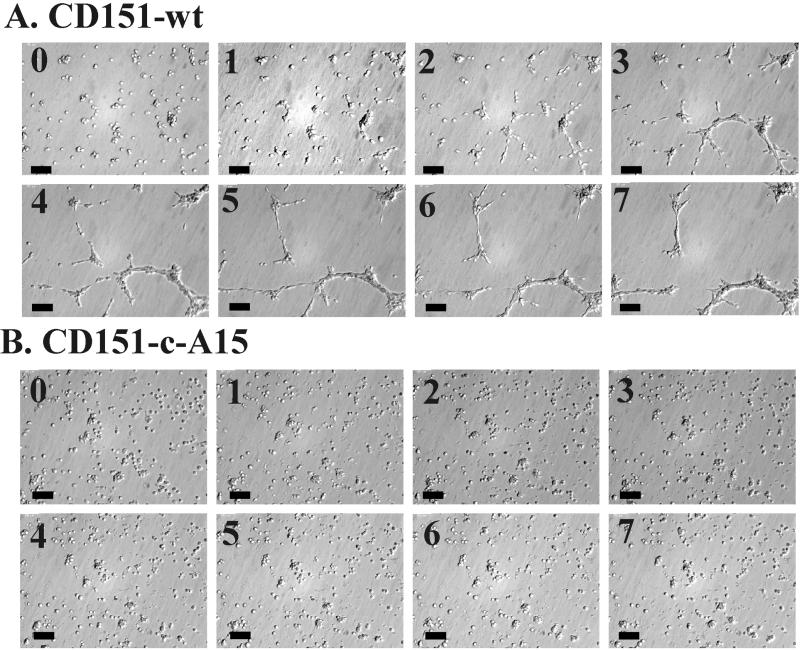
Figure 5.
Time lapse formation of reticular structures. (A) NIH3T3-CD151 wild-type cells were grown on Matrigel for 7 h, and photos were obtained from the same randomly chosen field at hourly intervals. (B) The same experiment was carried out using NIH3T3-CD151-c-A15 cells. Bar, 100 μM. (A video supplement to this figure was prepared from images recorded at 5-min intervals, over a period of 13 h).
For stable expression of CD151 mutants, plasmid DNAs were transfected into NIH3T3 cells using Lipofectamine (Life Technologies, Bethesda, MD). After 48 h, cells were then cultured in media containing Zeocin (200 μg/ml; Invitrogen) for selection. After 2 weeks of selection, colonies were pooled, and CD151-positive cells were sorted by flow cytometry. For double transfectants, human α3 cDNA in eukaryotic expression vector pRcCMV was cotransfected (into NIH3T3 cells) with CD151 mutant plasmid DNA and selected using both G418 (1 mg/ml; Life Technologies) and Zeocin. A15/TALLA1 plasmid DNA was kindly provided by Dr. Osamu Yoshie (Kinki University, Osaka, Japan), subcloned into pRcCMV vector, and selected in G418 after stable transfection into NIH3T3 cells. To assess cell surface expression, NIH3T3 transfectants were analyzed by flow cytometry as previously described (Zhang and Hemler, 1999). Cells were incubated with negative control monoclonal antibody (mAb) and specific mAbs and then with FITC-conjugated goat anti-mouse IgG and were analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Fluorescence with negative control mAb was subtracted to give specific mean fluorescence intensity (MFI) units.
Antibodies and Other Proteins
mAbs used in this study were anti-human CD151 mAbs 5C11 (Yauch et al., 1998) and 1A5 (Testa et al., 1999; provided by Dr. J Testa); anti-human integrin α3 subunit IIF5 (Weitzman et al., 1993), anti-human CD147 mAb 8G6 (Berditchevski et al., 1997), anti-human integrin αV subunit mAb P3G8 (Wayner et al., 1991), anti-mouse CD9 mAb KMC8 (PharMingen, San Diego, CA), anti-mouse integrin α6 subunit mAb GoH3 (PharMingen), anti-mouse CD44 mAb KM114 (PharMingen), and negative control mAb P3 (Lemke et al., 1978). A15 mAbs B2D and A2 M 30.3 were kindly provided by Dr. Osamu Yoshie (Kinki University, Osaka, Japan) and Dr. F. Lanza (Strasbourg, France), respectively. The rabbit polyclonal antibody 6843, against α6A integrin cytoplasmic domain, was a gift from Dr. V. Quaranta (The Scripps Research Institute, La Jolla, CA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Sigma, St. Louis, MO) were also used. Matrigel, a solubilized basement membrane matrix extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, was purchased from BD Bioscience (Bedford, MA). Other ECM proteins used in this study were human plasma fibronectin (Life Technologies), and mouse laminin 1 (Life Technologies). PDGF-BB was from BD Bioscience, and bFGF was from Roche Molecular Biochemicals, Indianapolis, IN.
In Vitro Morphogenesis Assay
The spontaneous formation of interconnecting web-like structures by NIH3T3 or HUVEC cells on Matrigel was used to assess morphogenic potential. Matrigel was plated into 24-well plates (0.4 ml/well) and allowed to solidify for 2 h at 37°C. NIH3T3 or HUVEC cells were then seeded into each well at a concentration of 1 × 105 cells/well in a 0.6 ml volume of DMEM. The final concentration of fetal calf serum was 5%. Cells on Matrigel were cultured at 37°C in 10% CO2 and photographed after ∼8, 10, or 24 h or 7 d, as indicated. Images were captured using Scion Image 1.60 (Scion Corp.. Frederick, MD) software through a video camera (TM-7AS; PULNiX America, Inc., Sunnyvale, CA) attached to an inverted phase contrast microscope. All results were obtained in at least two independent experiments.
Immunoprecipitation and Western Blot
Immuoprecipitations were carried out as described (Zhang and Hemler, 1999). Briefly, NIH3T3 transfectants were lysed in 1% Brij 99 lysis buffer (containing 150 mM NaCl, 25 mM HEPES, 2 mM phenylmethylsulfonylfluoride, 20 μg/ml leupeptin, 20 μg/ml aprotinin, 2 mM sodium vanadate, and 2 mM sodium fluoride), at 4°C for 1 h. Lysates were preincubated (2 times) with a combination of protein A– and protein G–Sepharose beads (Pharmacia Amersham Biotech, Uppsala, Sweden) at 4°C and each time were clarified by centrifugation at 10,000 rpm centrifugation. Next, mAb preabsorbed protein A– and protein G–Sepharose beads were incubated with cell lysate at 4°C overnight. Beads were washed with 1% Brij 99 lysis buffer three times, dissolved in Laemmli sample buffer and heated at 95°C for 5 min, and then proteins were resolved by 10% SDS-PAGE. After electrophoretic transfer, nitrocellulose membranes (Schleicher & Schuell, Keene, NH) were sequentially blotted with primary antibody and HRP-conjugated anti-mouse IgG (Sigma) and then visualized with chemiluminescence reagent (New England Nuclear Life Science, Boston, MA).
DIC Time-lapse Video Microscopy
As described elsewhere (Zhang et al., 2001a, 2001b), acid-washed glass coverslips were affixed to a 60-mm Petri dish, covering a 12-mm hole. Coverslips were coated 2 h at 37°C with 100 μl Matrigel. Immediately before image acquisition, NIH3T3 transfectants were detached with 2 mM EDTA in PBS, washed once with PBS, and plated onto coverslips in complete DMEM medium. Image acquisition was achieved using a Zeiss Axiovert 135 microscope (Thornwood, NY) with a VS25 shutter controlled by a Uniblitz D122 driver (Vincent Associates, Rochester, NY) and a video camera (TM-7AS; PULNiX America, Inc.) connected to a Power Macintosh 6500 equipped with a VG-5 frame grabber (Scion Corp., Frederick, MD) through a focusing monitor (PVM-137; Sony Corp., Parkridge, NJ). A macro written for Scion Image 1.60 (Scion Corp.) controlled the shutter driver and image acquisition. Images were captured every 5 min for 13 h, as cells were maintained in a humidified, 37°C, 10% CO2 environment in a custom-built stage incubator. DIC images were obtained using a Hoffman Modulation Contrast system (Modulation Optics Inc., Greenvale, NY) consisting of a contrast objective, a condenser, and a contrast control polarizer.
RESULTS
CD151–α6β1-dependent Network of Cord-like NIH3T3 Cell Structures on Matrigel
Upon plating on basement membrane Matrigel, NIH3T3 cells assembled into a network of cord-like structures visible after 8 h (Figure 1A) and 30 h (Figure 1B). The process began at ∼5 h after cell plating, and the cord-like network pattern sometimes lasted more than 7 days (Figure 1C), depending on the batch of Matrigel. Typically, 1–1.5 × 105 cells (in area = 2 cm2) were sufficient to yield cord-like structures, whereas 5 × 104 cells was often insufficient. Cell network formation was diminished in the absence of serum but was strongly promoted in the presence of either PDGF-BB (40 ng/ml) or bFGF (40 ng/ml). For time-lapse images of cellular network formation and accompanying video, see Figure 5A, below.
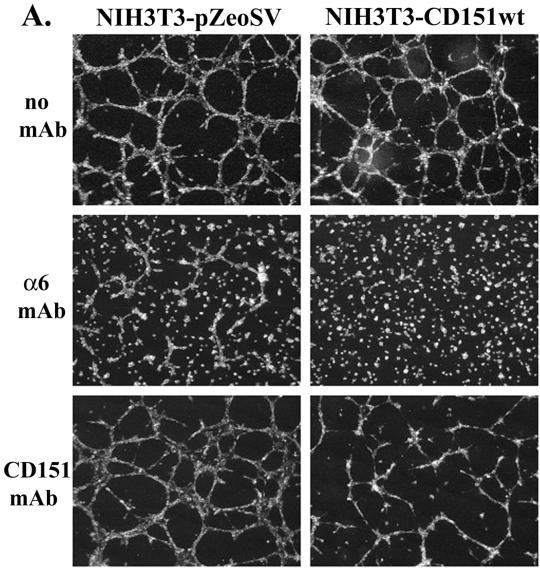
Figure 1.
NIH3T3 cells form cord-like structures when plated on Matrigel. (A) Mock or human C0151-NIH3T3 cell CD151-NIH3T3 cell transfectants were grown in 5% FBS-DMEM on the surface of Matrigel for 8 h and then photographed. Monoclonal antibodies to mouse α6 integrin (GoH3), and human CD151 (1A5) were added at 5 μg/ml at the beginning of the experiment. CD151 was well expressed on the surface of NIH3T3-CD151 cells (∼100 MFI units, see Figure 4). (B) Cells were grown as in A but for 30 h. (C) NIH3T3-CD151-α3 double-transfected cells were grown in 5% FBS-DMEM on the surface of Matrigel for 7 d and then photographed. Monoclonal antibodies to mouse α6 integrin, human α3 integrin (IIF5), human CD151 (5C11), mouse CD9 (KMC9), and mouse CD44 (KM114) were each added at 10 μg/ml at the beginning of the experiment. Levels of transfected α3 (∼55 MFI) were somewhat higher than that of endogenous α6 (∼26 MFI). As seen elsewhere, CD44 (Kawano et al., 2000) and CD9 (unpublished data) are highly expressed on the surface of NIH3T3 cells. Magnification, ×10.
Because a major component of Matrigel is laminin-1, we considered that integrin α6β1 (α6β4 is not present in NIH3T3 cells) and associated CD151 may be involved in network formation. Indeed, anti-murine α6 function–blocking mAb GoH3 dramatically inhibited the 8 h (Figure 1A) and 30 h (Figure 1B) formation of cord-like structures by NIH3T3-pZeo mock transfectants. Network formation by NIH3T3-CD151 wt cells (transfected with wild-type human CD151) was inhibited not only by anti-α6 mAb GoH3 but also by anti-CD151 mAb 1A5 (Figure 1, A and B). Semiquantitative RT-PCR revealed that transfected human CD151 was present at a level two- to threefold greater than endogenous murine CD151. In the absence of human CD151, mAb 1A5 was not inhibitory (Figure 1, A and B, bottom left panels). In a separate experiment, after 7 d in culture, NIH3T3-CD151-α3β1 cotransfectants showed again a dramatic inhibition of cord-like networks by anti-α6 and anti-CD151 antibodies (Figure 1C). In contrast, antibodies to CD44, to another tetraspanin protein (CD9), or to α3 integrin had minimal effect after 7 d (Figure 1C) or after 30 h (our unpublished results), even though each of those molecules was present in these NIH3T3 cells at a level higher than either endogenous α6β1 integrin or transfected CD151.
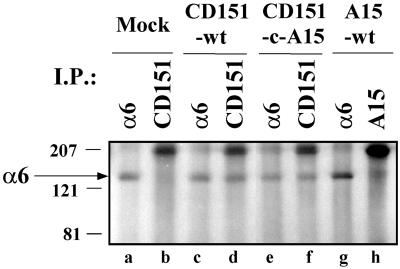
As seen elsewhere, CD151 may physically associate with α6β1 integrin (Serru et al., 1999). To test for CD151–α6 integrin complex formation in NIH3T3 cells, cells were lysed, and then human CD151 immunoprecipitates were blotted for integrin α6 subunit. Levels of α6 associated with human CD151 (Figure 2, lane d) were comparable to the levels of total detectable α6 (lanes a and c), thus indicating a high stoichiometry interaction. These results are consistent with human CD151 being more prevalent than endogenous murine CD151 (which would also be expected to associate with α6 integrin). In a control experiment, anti-CD151 antibody failed to coimmunoprecipitate α6 from cells that did not contain human CD151 (lane b). Immunoprecipitation of the A15 tetraspanin protein yielded minimal associated α6 integrin (lane h), even though both the α6 integrin (lane g) and the A15 molecule (see Figure 4, below) were well expressed in NIH3T3-A15 transfectants. Exchange of the CD151 C-terminal cytoplasmic tails with the tail of A15 (see Figure 4 below) did not result in loss of α6 association (lane f). Thus, specificity for α6 association does not reside in the cytoplasmic tails of CD151. Importantly, expression of endogenous α6 was not perturbed upon transfection of mutant or wild-type CD151 (Figure 2, lanes a, c, and e) and as seen by flow cytometry.
Figure 2.
Association of wild-type and mutant CD151 with α6 integrin. NIH3T3 transfectants were lysed in 1% Brij 99, and then immunoprecipitations were carried out using anti-human CD151 mAb 5C11 (lanes b, d, and f), anti-human A15 mAb A2 M 30.3 (lane h), and anti-mouse α6 integrin mAb GoH3 (lanes a, c, e, and g). Proteins were resolved by nonreducing 10% SDS-PAGE and blotted with anti-α6 integrin polyclonal antibody 6843. The arrow indicates the position of the integrin α6 subunit. Expression levels for wild-type and mutant CD151 are indicated in Figure 4 below.
Figure 4.
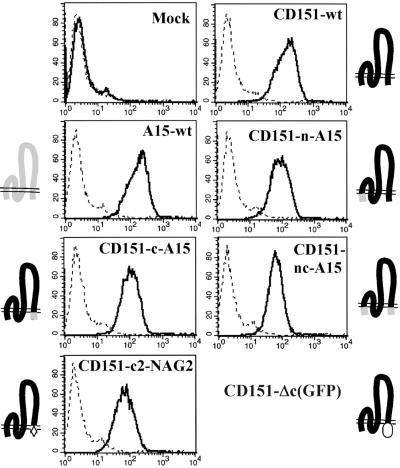
Cell surface expression of wild-type and mutant CD151 in NIH3T3 cells. Stable NIH3T3 transfectants were stained with negative control mAb P3 (dashed line), anti-human A15 mAb B2D (A15-wt transfectants) or anti-human CD151 mAb 5C11 (for all other transfectants, including mock) and analyzed by flow cytometry. Schematic diagrams indicate regions from CD151 (black), A15 (gray), NAG2 (hatched), and green fluorescent protein (oval).
CD151 C-terminal Tail Involvement
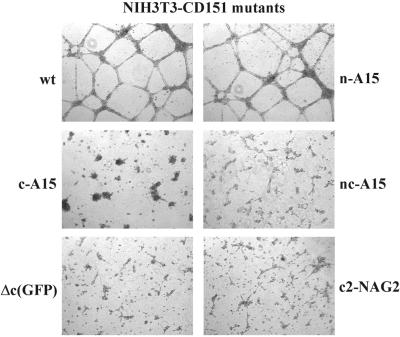
Next we discovered that the C-terminal tail of CD151 is clearly involved during CD151–α6β1-dependent network formation. A tail exchange mutation (CD151-c-A15) caused an almost complete loss of cellular network formation (Figure 3) without altering integrin association (see Figure 2 above). In contrast, the N-terminal tail exchange mutation (CD151-n-A15) had no discernible effect, whereas exchange of both tails (CD151-nc-A15) did again abolish the cord-like structures. Expression of wt A15 itself had no effect. To confirm the role of CD151 C-terminal tail of CD151, additional mutants were generated, including another exchange mutant (CD151-c-NAG2) and a deletion mutant (CD151-Δc-GFP). Again, CD151-dependent network formation among NIH3T3 cells was essentially abolished (Figure 3, bottom panels). Wild-type CD151, A15, and the various mutants were all stably expressed at comparable levels on the surface of NIH-3T3 cells (Figure 4). Surface expression of the CD151-Δc-GFP mutant was not analyzed by flow cytometry using FITC-conjugated second antibody (due to excessive GFP fluorescence) but instead was confirmed by immunoprecipitation (our unpublished results). Mutant human CD151 molecules were each present at levels two- to threefold greater than endogenous murine CD151, as indicated by semiquantitative RT-PCR.
Figure 3.
Role of the CD151 C-terminal cytoplasmic domain during morphogenesis on Matrigel. The indicated transfectants were cultured in 5% FBS-DMEM on the surface of Matrigel for 24 h. Magnification, ×10. Wild-type CD151 and all mutant CD151 proteins were expressed at comparable levels (see Figure 4).
In a time-lapse video microscopy study, wild-type CD151 transfectants initially showed a directional migration and alignment of cells. Next, there was cell–cell contact among aligned cells, and finally the cells merged into elongated rod-like structures, before condensing into thicker cellular cables (Figure 5A and attached video). In sharp contrast, CD151-c-A15 cells were relatively motile but showed no directional cell migration and no cell–cell alignment (Figure 5B and attached video).
CD151 C-terminal Tail-spreading Functions
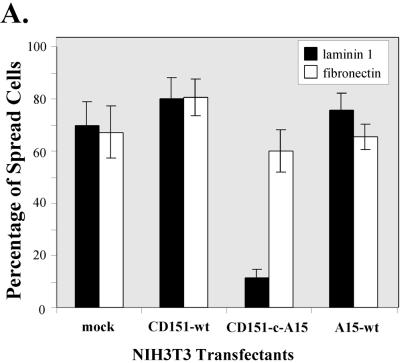
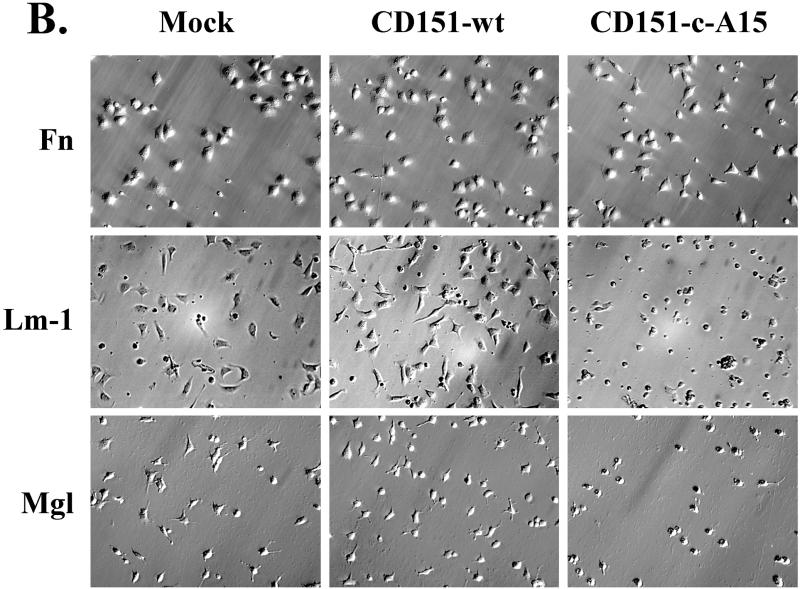
We hypothesized that within a functional CD151–α6β1 complex, effects of CD151 tail mutation should be best seen when α6β1 is engaged with ligand. To address this, we carried out cell-spreading assays on laminin-1 (to engage α6β1) and on fibronectin (to engage α5β1). As shown in Figure 6A, a high percentage of all NIH3T3 transfectants showed abundant spreading after a 30-min incubation on fibronectin. On laminin 1, the majority of mock, CD151 wild-type, and A15 transfectants were well spread after 30 min, but the CD151-c-A15 mutant showed severely impaired spreading. Photographs of representative spread cells are presented in Figure 6B. Cell spreading on laminin-1 and a coating of Matrigel yielded comparable results (Figure 6B, bottom row), consistent with laminin-1 being a major component of Matrigel. These results emphasize that functional effects of CD151 C-terminal domain mutations are obvious only when the α6β1 integrin is engaged. Previous results illustrate that CD151 has little effect on integrin-dependent cell adhesion (Yauch et al., 1998). Consistent with this, static cell adhesion to polymerized Matrigel (at levels identical to that used in morphogenesis experiments) was not markedly different among NIH3T3 transfectants (mock, wild-type CD151, CD151-c-A15, wild-type A15). Each transfectant showed ∼950–1100 adherent cells/mm2, corresponding to ∼70–81% of 50,000 input cells, in a standard cell adhesion assay (Pujades et al., 1997). Consistent with the presence of laminin 1 in the Matrigel, adhesion of NIH3T3 transfectants was strongly inhibited (∼80%) by anti-α6 integrin antibody GoH3 (our unpublished results).
Figure 6.
Comparison of cell spreading for CD151 transfectants on laminin-1 and fibronectin. NIH3T3 transfectants were seeded onto coverslips coated with either laminin-1 or fibronectin. (A) Spread cells were readily defined as cells that had increased their surface contact area by at least two- to threefold, as they began to show a flattened morphology. Each bar represents the mean ± SD from three separate experiments. (B) Photos of spread cells are shown at ×20 magnification. Coverslips were coated with laminin (Lm, 10 μg/ml), fibronectin (Fn, 10 μg/ml), or Matrigel (Mgl, diluted 1/30 from stock solution, according to manufacturer's instructions; BD Labware, Bedford, MA). Cell spreading was carried out in serum-free DMEM at 37°C for 30 min. For cells on laminin-1, PDGF (40 ng/ml) was included to enhance spreading.
Perturbation of Endothelial Cell Structures
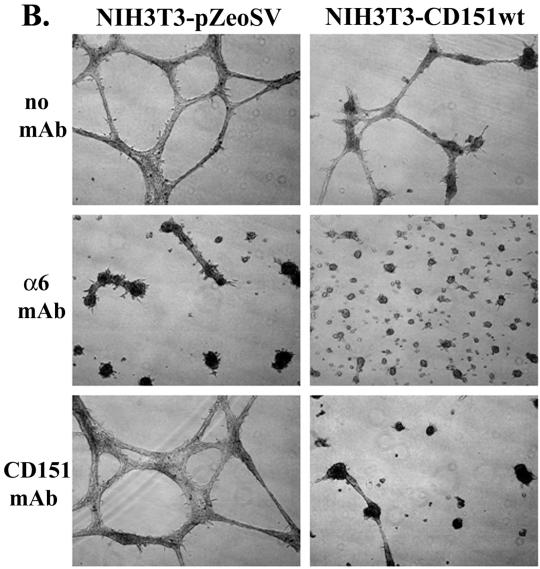
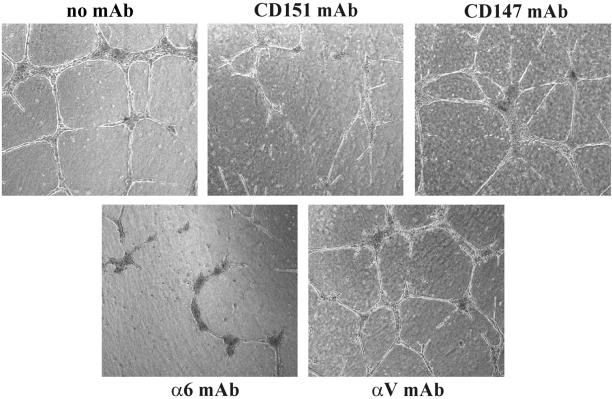
Early passage HUVEC cells also form a network of cord-like structures when cultured on Matrigel (Sincock et al., 1999). The appearance of these cord-like structures (Figure 7) was partially disrupted by antibodies to either CD151 (mAb 5C11), or anti-α6 integrin (mAb GoH3). However, anti-CD147 mAb 8G6 and anti-αV integrin mAb P3G8 had no obvious effect. These results indicate that CD151–α6β1 complexes in multiple cell types can play a critical role during morphogenesis into cellular networks on Matrigel.
Figure 7.
Perturbation of capillary-like structures formed by human endothelial cells. HUVECs were seeded on Matrigel for 24 h, in the presence of monoclonal antibodies (anti-integrin α6, GoH3; anti-CD151, 5C11; anti-CD147, 8G6; anti-integrin αV, P3G8), each at 10 μg/ml. The CD151 and CD147 molecules and the α6 and αV integrins are each very well expressed on HUVECs (Leukocyte Typing VI, 1998). Photos were obtained after 24 h. Magnification, ×10.
DISCUSSION
The CD151–α6β1 Complex as a Functional Unit
CD151 formed a complex with integrin α6β1 as shown here in NIH3T3 cells and as shown elsewhere in HUVECs and other cell types (Fitter et al., 1999; Serru et al., 1999; Sincock et al., 1999). The specificity of the CD151–α6β1 interaction is underscored by our failure to observe A15/Talla1 tetraspanin association with α6β1 integrin. Elsewhere, the CD151–α6β1 interaction was highly specific and was similarly retained under conditions in which other integrin–tetraspanin interactions were disrupted (Serru et al., 1999).
Although CD151-α6β1 complexes have been demonstrated previously, their functional relevance had not been demonstrated. Here we show that the CD151–α6β1 complex is acting as a functional unit because CD151 C-terminal tail mutation perturbed α6β1-dependent functions (network formation, cell spreading) only when α6β1 was engaged (on purified laminin-1 or on Matrigel) and not when a different integrin (α5β1) was engaged (on fibronectin). Furthermore, antibodies to both CD151 and α6β1 strongly inhibited network formation by HUVECs (Figure 7), NIH3T3 cells, NIH3T3-α3 cells (Figure 1) and by an immortalized α3-deficient murine kidney epithelial cell line (Wang et al., 1999; our unpublished results).
In contrast to CD151–α6β1 complexes, CD151–α3β1 complexes did not influence NIH3T3 cell morphogenesis on Matrigel. Presumably, although the α3β1 integrin may interact with laminin-1, it may not interact in the same manner or to the same extent as α6β1. Alternatively, CD151–α3β1 complexes may transmit different cellular signals, not conducive to network formation. Whereas anti-CD151 antibodies inhibited α6β1 integrin–dependent function when cells were plated on laminin-1 (as shown here); elsewhere, anti-CD151 antibodies inhibited α3β1 functions on laminin-5 but not on laminin-1 (Stipp and Hemler, 2000). Thus, CD151–α6β1 and CD151–α3β1 complexes are functionally distinct.
A New Dimension in Integrin Signaling
It is well established that integrin cytoplasmic domains play critical roles in determining the functional consequences of ligand binding (Liu et al., 2000). For example, integrin α6 cytoplasmic domains can regulate MAP kinase activation and cell migration (Gimond et al., 1998; Wei et al., 1998). Now we demonstrate that another cytoplasmic tail, that of CD151, may be just as important for α6 integrin function as integrin tails themselves. A transmembrane linker role for tetraspanins has been proposed (Hemler, 1999) because extracellular domains of tetraspanin proteins such as CD151 provide specificity for integrin association, and intracellular domains may determine association with signaling molecules such as PtdIns 4-K (Yauch and Hemler, 2000) and PKC (Zhang et al., 2001a, 2001b). Results here support the concept of TM4SF proteins as transmembrane linkers. First, a distinct region of CD151, likely extracellular and not involving the cytoplasmic tails, is needed for α6β1 integrin association. Second, the CD151 C-terminal cytoplasmic tail makes an essential contribution to NIH3T3 morphogenesis, as evidenced by tail deletion and two different tail exchange mutations. Most likely, our CD151 C-terminal tail mutants are having a dominant negative effect on endogenous CD151. While retaining integrin association, they disrupt critical CD151 tail-dependent signaling pathways that complement α6β1 integrin–signaling pathways. Neither integrin α6β1 expression levels or α6β1-dependent cell adhesion were affected by transfection of either wild-type or mutant CD151 into NIH3T3 cells. These latter results are in agreement with previous studies showing that CD151 and other tetraspanin proteins have little or no effect on cell adhesion (Hemler et al., 1996; Yauch et al., 1998). Thus, our dominant negative CD151 is altering outside-in rather than inside-out integrin signaling.
Among tetraspanin proteins, there has been little precedent for cytoplasmic domains having clear functional relevance. Now we demonstrate that the CD151 C-terminal tail is clearly distinct from the A15 and NAG2 tails with respect to its influence on cell network formation. With the CD151 tail having only ∼9 residues, it should be readily feasible to identify the critical individual amino acids in future studies. It remains to be demonstrated whether the CD151 C-terminal tail may also play a critical role in the functioning of CD151–α6β4 complexes in hemidesmosomes (Sterk et al., 2000) or in CD151–α3β1 complexes during neurite outgrowth (Stipp et al., 2001) and in cell migration (Yauch et al., 1998).
Formation of Cord-like Networks on Matrigel
Several cell types may form cord-like networks on a variety of different extracellular matrices (Vernon and Sage, 1995). Although the role of integrins (including α6β1) during network formation has been well established (Bauer et al., 1992; Berdichevsky et al., 1994; Davis and Camarillo, 1995; Vernon and Sage, 1995; Stahl et al., 1997; Sun et al., 1998), a major role for a tetraspanin protein has not previously been observed. In a prior study of endothelial cells on Matrigel, anti-CD151 antibody inhibition effects were more subtle than shown here, perhaps because of the use of different antibodies (Sincock et al., 1999).
In several previous studies, formation of cord-like structures on Matrigel has been seen as an in vitro model of angiogenesis. Indeed, our antibody inhibition results seen in NIH3T3 cells were confirmed using HUVECs, thus suggesting that CD151 may contribute to angiogenesis in particular and to branching morphogenesis in general. For fibroblasts in particular, a network of cord formation on Matrigel may be a model also for wound healing and development (Vernon and Sage, 1995). On Matrigel, cells that exert mechanical traction forces on the matrix may then align along “matrix guidance pathways” to form cord-like structures (Davis and Camarillo, 1995; Vernon and Sage, 1995).
Our time-lapse video results suggest that CD151-dependent NIH3T3 cell morphogenesis has at least three phases: cellular alignment due to tractional forces, cell motility, and cell–cell contact. It remains to be determined which of these phases is specifically facilitated by CD151. CD151 could play a key role at cell–cell contact sites (Yánez-Móet al., 1998; Sincock et al., 1999). However, because CD151 effects on cell alignment and migration precede cell–cell contact, that seems unlikely. Instead, CD151 may promote cell movement, because tetraspanin proteins in general and CD151 in particular are well established as regulators of cell motility (Hemler et al., 1996; Maecker et al., 1997; Yauch et al., 1998). Also, CD151 may promote mechanical traction–related forces. Consistent with this, CD151 tail mutation abolished cell spreading, another event requiring tractional forces.
In conclusion, our results provide perhaps the best support to date for a tetraspanin protein (CD151) having a transmembrane linker function. The extracellular portion associates with the integrin, whereas the cytoplasmic tail determines the specific consequences of integrin outside-in signaling (without altering cell adhesion). We suggest that while α6β1 is interacting with laminin-1, α6β1-associated CD151 is optimally localized to promote cell alignment with mechanical traction forces, cell motility, cell spreading, and/or other events needed for NIH3T3 cell morphogenesis into a cellular reticulum. This points to a new functional role for CD151. Finally, CD151 and α6β1 integrin also play critical roles during endothelial cell morphogenesis, illustrating the generality of our findings.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants CA86712 and CA42368 (to M.E.H).
Abbreviations used:
- DIC
differential interference contrast
- ECM
extracellular matrix
- HUVEC
human umbilical vein endothelial cells
- TM4SF
transmembrane 4 superfamily
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–10-0481. Article and publication date are found at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–10-0481.
REFERENCES
- Bauer J, Margolis M, Schreiner C, Edgell CJ, Azizkhan J, Lazarowski E, Juliano RL. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992;153:437–449. doi: 10.1002/jcp.1041530302. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D'Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins: evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 1997;272:29174–29180. doi: 10.1074/jbc.272.46.29174. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Cramer EM, Tenza D, Tobelem G. Phenotypic modulations of human umbilical vein endothelial cells and human dermal fibroblasts using two angiogenic assays. Biol Cell. 1991;72:275–278. doi: 10.1016/0248-4900(91)90298-2. [DOI] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113–123. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- Domanico SZ, Pelletier AJ, Havran WL, Quaranta V. Integrin α6Aβ1 induces CD81-dependent cell motility without engaging the extracellular matrix migration substrate. Mol Biol Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter S, Sincock PM, Jolliffe CN, Ashman LK. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with beta 1 and alpha IIb beta 3 integrins in hemopoietic cell lines and modulates cell-cell adhesion. Biochem J. 1999;338(Pt 1):61–70. [PMC free article] [PubMed] [Google Scholar]
- Gimond C, Baudoin C, van der Neut R, Kramer D, Calafat J, Sonnenberg A. Cre-loxP-mediated inactivation of the α6A integrin splice variant in vivo: evidence for a specific functional role of α6A in lymphocyte migration but not in heart development [published erratum appears in J. Cell Biol. 1998, 143(5), following 1412] J Cell Biol. 1998;143:253–266. doi: 10.1083/jcb.143.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Integrin-associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Dystroglycan versatility. Cell. 1999;97:543–546. doi: 10.1016/s0092-8674(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as a diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which upregulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Okamoto I, Murakami D, Itoh H, Yoshida M, Ueda S, Saya H. Ras oncoprotein induces CD44 cleavage through phosphoinositide 3-OH kinase and the rho family of small G proteins [In Process Citation] J Biol Chem. 2000;275:29628–29635. doi: 10.1074/jbc.M002440200. [DOI] [PubMed] [Google Scholar]
- Lemke H, Hammerling GJ, Hohmann C, Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978;271:249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Kikutani H, von dem Borne AEGKr, Goyert SM, Mason DY, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer TA, Sugamura K, Zola H, editors. Leukocyte Typing, VI. New York: Garland Press; 1998. [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113(Pt 20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Miyazaki T, Muller U, Campbell KS. Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 1997;16:4217–4225. doi: 10.1093/emboj/16.14.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Pujades C, Alon R, Yauch RL, Masumoto A, Burkly LC, Chen C, Springer TA, Lobb RR, Hemler ME. Defining extracellular integrin α chain sites that affect cell adhesion and adhesion strengthening without altering soluble ligand binding. Mol Biol Cell. 1997;8:2635–2645. doi: 10.1091/mbc.8.12.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serru V, Naour FL, Billard M, Azorsa DO, Lanza F, Boucheix C, Rubinstein E. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J. 1999;340(Pt 1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localized to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833–844. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110(Pt 1):55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp CS, Hemler ME. Transmembrane-4-Superfamily proteins CD151 and CD81 associate with α3β1integrin, and selectively contribute to α3β1-dependent neurite outgrowth. J Cell Sci. 2000;113:1871–1882. doi: 10.1242/jcs.113.11.1871. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Orlicky D, Hemler ME. FPRP. A major, highly stoichiometric, highly specific CD81 and CD9-associated protein. J Biol Chem. 2001;276:4853–4862. doi: 10.1074/jbc.M009859200. [DOI] [PubMed] [Google Scholar]
- Sun H, Santoro SA, Zutter MM. Downstream events in mammary gland morphogenesis mediated by reexpression of the alpha2beta1 integrin: the role of the alpha6 and beta4 integrin subunits. Cancer Res. 1998;58:2224–2233. [PubMed] [Google Scholar]
- Testa JE, Brooks PC, Lin JM, Quigley JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59:3812–3820. [PubMed] [Google Scholar]
- Tsitsikov EN, Gutierrez-Ramos JC, Geha RS. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc Natl Acad Sci USA. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol. 1995;147:873–883. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Symons JM, Goldstein SL, McDonald A, Miner JH, Kreidberg JA. α3β1 integrin regulates epithelial cytoskeletal organization. J Cell Sci. 1999;112:2925–2935. doi: 10.1242/jcs.112.17.2925. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Orlando RA, Cheresh DA. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Woods A, Couchman JR. Integrin modulation by lateral association. J Biol Chem. 2000;275:24233–24236. doi: 10.1074/jbc.R000001200. [DOI] [PubMed] [Google Scholar]
- Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase and may regulate cell migration. Mol Biol Cell. 1998;9:2751–2765. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Hemler ME. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphatidylinositol 4-kinase. Biochem J. 2000;351:629–637. [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Kazarov AR, Desai B, Lee RT, Hemler ME. Direct extracellular contact between integrin α3β1 and TM4SF protein CD151. J Biol Chem. 2000;275:9230–9238. doi: 10.1074/jbc.275.13.9230. [DOI] [PubMed] [Google Scholar]
- Yánez-Mó M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Ursa MA, Ashman LK, De Landázuri MO, Sánchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3 β1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Stipp CS, Kraeft S-K, Bazzoni G, Chen LB, Hemler ME. Phosphorylation of a conserved integrin α3 chain QPSXXE motifs regulates signaling, motility, and cytoskeletal engagement. Mol Biol Cell. 2001a;12:351–365. doi: 10.1091/mbc.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Hemler ME. TM4SF proteins associate with activated PKC and Link PKC to specific beta1 integrins. J Biol Chem. 2001b;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- Zhang XA, Hemler ME. Interaction of the integrin β1 cytoplasmic domain with ICAP-1 protein. J Biol Chem. 1999;274:11–19. doi: 10.1074/jbc.274.1.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.