Innate immune signaling is a critical defense against viral infection and represents a central host-virus interaction that frequently determines the outcomes of infections. NF-κB signaling is an essential component of innate immunity that is extensively modulated by HCMV, a significant cause of morbidity in neonates and immunosuppressed individuals. However, the roles that various facets of NF-κB signaling play during HCMV infection have remained elusive. We find that the two major regulatory kinases in this pathway, IKKα and IKKβ, limit the initiation of infection, viral replication, and cell-to-cell spread. In addition, our results indicate that these kinases contribute differently to the host cell response to infection in the absence of a virally encoded NF-κB inhibitor, UL26. Given the importance of NF-κB in viral infection, elucidating the contributions of various NF-κB constituents to infection is an essential first step toward the possibility of targeting this pathway therapeutically.
KEYWORDS: IKK, NF-Kβ, cytomegalovirus, innate immunity
ABSTRACT
Human cytomegalovirus (HCMV) is a ubiquitous herpesvirus that causes disease in immunosuppressed populations. HCMV has a complex relationship with innate immune signaling pathways. Specifically, HCMV has been found to block some aspects of inflammatory signaling while benefiting from others. Through analysis of knockout cell lines targeting the NF-κB regulatory kinases IκB kinase α (IKKα) and IKKβ, we find that the IKKs are host restriction factors that contribute to cytokine-mediated resistance to viral infection, limit the initiation of HCMV infection, and attenuate viral cell-to-cell spread. The HCMV UL26 protein is a viral immune modulator important for HCMV infection that has been shown to inhibit host cell NF-κB signaling, yet it has remained unclear how UL26-mediated NF-κB modulation contributes to infection. Here, we find that UL26 modulation of NF-κB signaling is separable from its contribution to high-titer viral replication. However, we find that IKKβ is required for the induction of cytokine expression associated with ΔUL26 infection. Collectively, our data indicate that the IKKs restrict infection but HCMV targets their signaling to modulate the cellular inflammatory environment.
IMPORTANCE Innate immune signaling is a critical defense against viral infection and represents a central host-virus interaction that frequently determines the outcomes of infections. NF-κB signaling is an essential component of innate immunity that is extensively modulated by HCMV, a significant cause of morbidity in neonates and immunosuppressed individuals. However, the roles that various facets of NF-κB signaling play during HCMV infection have remained elusive. We find that the two major regulatory kinases in this pathway, IKKα and IKKβ, limit the initiation of infection, viral replication, and cell-to-cell spread. In addition, our results indicate that these kinases contribute differently to the host cell response to infection in the absence of a virally encoded NF-κB inhibitor, UL26. Given the importance of NF-κB in viral infection, elucidating the contributions of various NF-κB constituents to infection is an essential first step toward the possibility of targeting this pathway therapeutically.
INTRODUCTION
Cells express a variety of sensors that are capable of triggering a diverse array of innate immune responses during viral infection. These immune mechanisms stimulate the production of antiviral effectors and induce inflammatory responses that can attenuate productive viral replication. Consequently, viruses have evolved viral effectors that inhibit, co-opt, or otherwise evade host cell immune responses to facilitate their proliferation and transmission. The various interactions between host cell immune signaling pathways and viral immune modulators are key determinants that can dictate the outcomes of infections. One of the viruses known to have a complex relationship with the host innate immune response is human cytomegalovirus (HCMV), a prevalent opportunistic betaherpesvirus that establishes latent infection and persists in the host indefinitely (1, 2). HCMV, the largest human herpesvirus, has an ∼235-kb genome encoding over 200 viral genes (3). A significant portion of this viral coding potential is dedicated to targeted manipulation of the host cell innate immune response to support infection (reviewed in references 4 and 5).
One of the most prominent immune pathways targeted by HCMV during infection is NF-κB signaling. The NF-κB proinflammatory pathway is a broad signaling architecture that can be induced by various stimuli, including irradiation, infection, and cytokine exposure. The canonical form of NF-κB signaling is induced by the cytokine tumor necrosis factor alpha (TNF-α) and results in the phosphorylation and activation of the kinase IκB kinase β (IKKβ) (Fig. 1A), which targets the IκB repressor protein for proteasomal degradation and frees the NF-κB transcription factors RelA and p50 to translocate to the nucleus and to alter cellular gene transcription (6). Noncanonical NF-κB signaling is controlled by IKKα activation and relies on the RelB and p52 transcription factors to alter transcription (Fig. 1A) (7). HCMV has a nuanced relationship with the NF-κB pathway, and multiple reports indicate that HCMV can both induce and inhibit NF-κB activity in various contexts and at different times during infection. Early HCMV infection is characterized by activated NF-κB signaling (8, 9). Furthermore, pharmacological inhibition or expression of dominant negative forms of IKKβ restrict productive replication (10–12). Conversely, HCMV strongly inhibits canonical NF-κB signaling at later stages of infection via multiple mechanisms, including the inhibition of IKK phosphorylation and receptor-interacting protein kinase 1 (RIP1) deubiquitination (13–15). The contributions that various aspects of NF-κB signaling make to early and late stages of viral infection remain poorly understood.
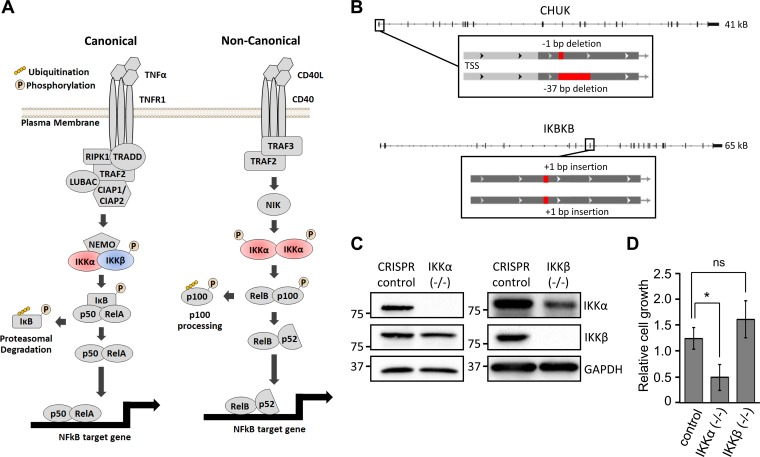
FIG 1.
CRISPR-mediated inactivation of IKKα and IKKβ. (A) The schematic demonstrates canonical and noncanonical NF-κB signaling. (B) BJ/hTert cells were transduced with either an untargeted CRISPR control plasmid or a CRISPR construct targeting either IKKα (encoded by the CHUK gene) or IKKβ (encoded by the IKBKB gene). Monoclonal cell lines with frameshift mutations in both IKKα alleles (position −1 and −37 deletions) or both IKKβ alleles (position +1 and +1 insertions) were isolated via clonal selection. (C) Total cell lysates from CRISPR control, IKKα−/−, and IKKβ−/− cells were Western blotted with the indicated antibodies. (D) A known number of CRISPR control, IKKα−/−, and IKKβ−/− cells were seeded and allowed to grow for 3 days, at which point viable cell counts were assessed by trypan blue exclusion staining (n ≥ 3). *, P < 0.05; ns, not significant.
The UL26 protein was found previously to be a viral inhibitor of NF-κB signaling (13). The UL26 open reading frame has two in-frame start codons, resulting in both large (27-kDa) and small (21-kDa) isoforms, which are both delivered with the tegument upon initial infection and expressed de novo during the viral life cycle, with early expression kinetics (16, 17). Early during infection, UL26 is required for maximal transcriptional activation of the viral major immediate early promoter and localizes to the nucleus of the host cell (16, 18). As infection progresses, UL26 exits the nucleus and is recruited to cytoplasmic virion assembly centers, where it has been shown to be required for the formation of stable virions with properly phosphorylated tegument constituents (18). Studies utilizing HCMV mutant strains lacking the UL26 open reading frame have shown that loss of UL26 during infection results in growth defects, including an ∼90% reduction in productive viral replication and significantly reduced cell-to-cell spread (18, 19). UL26 also plays a crucial role in attenuating host cell innate immune pathways. Previous work in our laboratory showed that UL26 is necessary and sufficient for the inhibition of TNF-α-induced NF-κB signaling during infection and the absence of UL26 during viral infection is linked to multiple NF-κB phenotypes, including inhibition of IKK phosphorylation, stabilization of the IκB repressor, and increased nuclear accumulation of the noncanonical transcription factor RelB (13).
Here, to assess the contributions of canonical and noncanonical NF-κB signaling to HCMV infection, we employed clustered regularly interspaced short palindromic repeat (CRISPR) methodology to generate cell lines with targeted knockouts of the key NF-κB kinases IKKα and IKKβ. We report that the IKKs act as antiviral restriction factors that limit HCMV infection. In addition, we explored the contributions that IKKα and IKKβ make to the growth defect associated with a UL26 deletion mutant. Analysis of various UL26 mutants suggests that UL26 inhibition of NF-κB signaling is insufficient for wild-type (WT) levels of viral replication, indicating that UL26 possesses distinct functions that contribute to productive infection. With respect to NF-κB signaling, we find that IKKβ mediates the induction of NF-κB target genes during ΔUL26 infection. Taken together, these results highlight antiviral roles for the kinases IKKα and IKKβ that limit HCMV infection, and we identify IKKβ as a substantial contributor to the inflammatory transcriptional state inhibited by UL26.
RESULTS
Loss of IKKα and IKKβ attenuates HCMV sensitivity to TNF-α treatment.
The IKKs play central roles in the signal transduction cascades of canonical and noncanonical NF-κB signaling (Fig. 1A). We employed CRISPR to generate monoclonal cell lines with knockouts of either IKKα or IKKβ, to determine the contributions that the IKKs make to HCMV infection. Frameshift mutations were introduced into both alleles of either the conserved helix-loop-helix ubiquitous kinase (CHUK) gene encoding IKKα (position −1 and −37 deletions) or the IKBKB gene encoding IKKβ (position +1 and +1 insertions) (Fig. 1B). In both cases, we observed total loss of the targeted IKK protein in each cell line. There was an observable decrease in the accumulation of the nontargeted IKK protein (e.g., reduced IKKα was evident upon knockout of IKKβ) (Fig. 1C). Cell viability of the IKKα and IKKβ knockout cell lines was assessed via the trypan blue exclusion cell viability assay, and we observed ∼2-fold growth reduction in cells lacking IKKα expression, whereas no discernible defect was observed in IKKβ−/− cells.
RelA and RelB are prominent transcription factors that are strongly associated with canonical and noncanonical NF-κB signaling, respectively. To analyze how loss of the IKKs perturbs the expression of downstream NF-κB pathway constituents, we assessed the accumulation of these transcription factors in our CRISPR control and IKK knockout cell lines. We found that loss of IKKα strongly limited the accumulation of RelA and RelB in both mock-infected and HCMV-infected cells, whereas both RelA and RelB still accumulated to WT levels in the absence of IKKβ (Fig. 2A). These observations suggest that IKKα is required for optimal expression of the RelA and RelB NF-κB transcription factors.
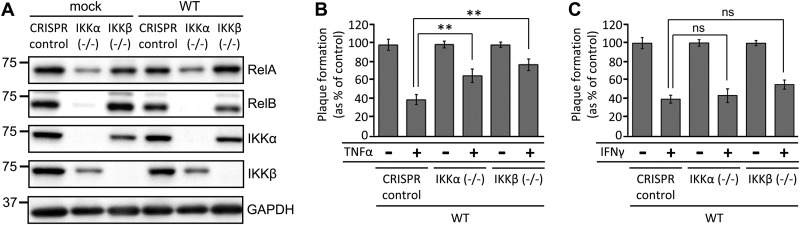
FIG 2.
IKK inactivation alters the accumulation of NF-κB transcription factors and reduces viral sensitivity to TNF-α. (A) Serum-starved CRISPR control, IKKα−/−, and IKKβ−/− cells were mock infected or infected with WT HCMV at a MOI of 3.0. Cell lysates were collected at 48 hpi and Western blotted with the indicated antibodies. (B and C) Confluent, serum-starved CRISPR control, IKKα−/−, or IKKβ−/− cell lines were mock treated or treated with either 10 ng/ml TNF-α (B) or 5 U/ml IFN-γ (C) for 4 h prior to infection with 40 PFU of WT-GFP HCMV. Relative plaque formation was measured by dividing the cytokine-treated plaque number for each virus by the number of plaques for that virus on the mock-treated cell line (CRISPR control, IKKα−/−, or IKKβ−/−) and plotted as a percentage. Error bars indicate standard errors (n ≥ 4). **, P < 0.01; ns, not significant.
TNF-α pretreatment of cells has been shown to negatively influence the ability of HCMV to establish initial lytic infection and to form plaques in human fibroblasts (20). To determine the extent to which the IKKs contribute to this viral sensitivity to TNF-α treatment, we assessed the impact of TNF-α treatment on viral plaque formation efficiencies in our CRISPR control and IKK knockout cell lines. We observed that treatment of CRISPR control cells with10 ng/ml TNF-α reduced viral plaque formation to 40% of the rate seen in nontreated cells (Fig. 2B). In IKKα−/− cells, the rate of plaque formation increased to 60% of that in nontreated controls. Further, this ratio increased to 80% in IKKβ−/− cells (Fig. 2B). These results suggest that both IKKα and IKKβ contribute to the antiviral effects of TNF-α treatment. However, the plaque formation efficiency in both IKK knockout cell lines after TNF-α challenge was still reduced, relative to non-TNF-α-treated cells (Fig. 2B), suggesting that, in each case, the presence of the reciprocal IKK could contribute to the TNF-α-induced antiviral state. Interferon gamma (IFN-γ) has also been shown to inhibit the establishment of HCMV lytic infection in human fibroblasts, putatively through JAK/STAT signaling (21). In contrast to the findings for TNF-α challenge, we found that knockout of each IKK had little impact on the plaque formation efficiency of HCMV on cells pretreated with 5 U/ml IFN-γ (Fig. 2C). Collectively, these data indicate that the IKKs are important for HCMV-associated sensitivity to TNF-α treatment but are largely dispensable for the antiviral signaling associated with IFN-γ treatment.
IKKα and IKKβ restrict HCMV viral replication.
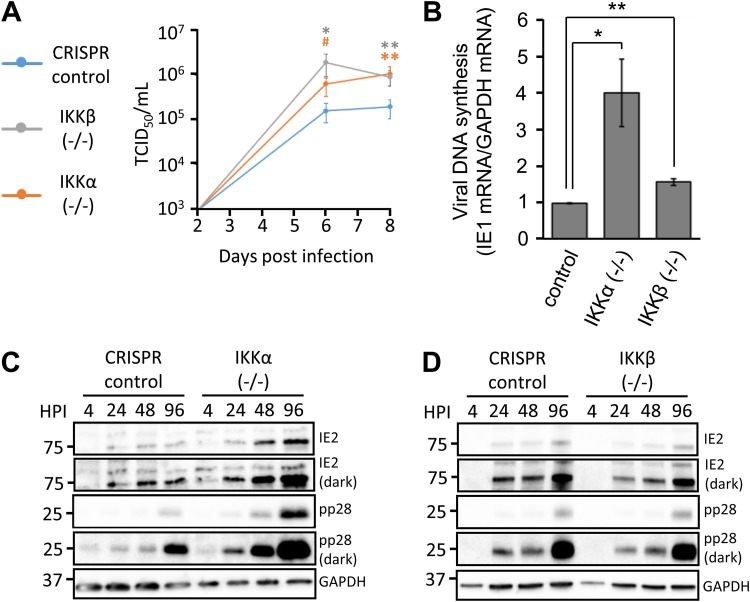
The relationship between NF-κB signaling and the HCMV life cycle is complex. HCMV induces IKK activity, which is important for viral replication in certain contexts (11, 12, 22). However, NF-κB activation has been found to inhibit HCMV infection in other contexts (21, 23, 24). Therefore, we wanted to explore how IKKα and IKKβ contribute to HCMV viral growth. We found that knockout of either IKK increased low-multiplicity of infection (MOI) viral replication by ∼1 log unit, compared to growth in CRISPR control cells, as assessed by 50% tissue culture infective dose (TCID50) analysis (Fig. 3A). Analysis of viral DNA accumulation during low-MOI infection showed an ∼4-fold increase in viral DNA replication in the absence of IKKα (Fig. 3B). Upon inactivation of IKKβ, a much smaller but still statistically significant increase in viral DNA abundance was also evident (Fig. 3B). The increased viral DNA abundance observed in the absence of IKKα correlated with an increase in the accumulation of viral gene products observed in these cells (Fig. 3C), most notably in the true late protein pp28. In contrast, IKKβ-deficient cells did not display a substantial change in the accumulation of viral immediate early or late proteins (Fig. 3D). Notably, this difference in viral gene expression did not affect the production of infectious viral progeny, as the loss of either IKKβ or IKKα increased production of infectious virions to similar extents, relative to CRISPR control cells. These results demonstrate that IKKα and IKKβ each play a role in restricting HCMV infection and reducing the viable progeny produced during viral replication but each kinase affects viral gene expression differently.
FIG 3.
IKKα and IKKβ restrict WT HCMV infection. (A) Confluent, serum-starved CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were infected with WT-GFP HCMV at a MOI of 0.1. Viral supernatants were harvested at 2, 6, and 8 dpi, and infectious viral progeny were quantified via TCID50 assay. The indicated statistical comparisons were made using Student's t test, and results are color coded for each cell line relative to the control cells. *, P < 0.05; **, P < 0.01; #, P = 0.056. (B) Viral DNA replication of WT HCMV was measured at 6 dpi in CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts infected as in panel A (n ≥ 3). *, P < 0.05; **, P < 0.01. (C and D) Confluent CRISPR control and IKKα−/− fibroblasts (C) or IKKβ−/− fibroblasts (D) were infected with WT HCMV as in panel A. Cells were harvested at the indicated time points and Western blotted with antibodies directed against IE2, pp28, and GAPDH. IE2 and pp28 blots are displayed with both light and dark exposures.
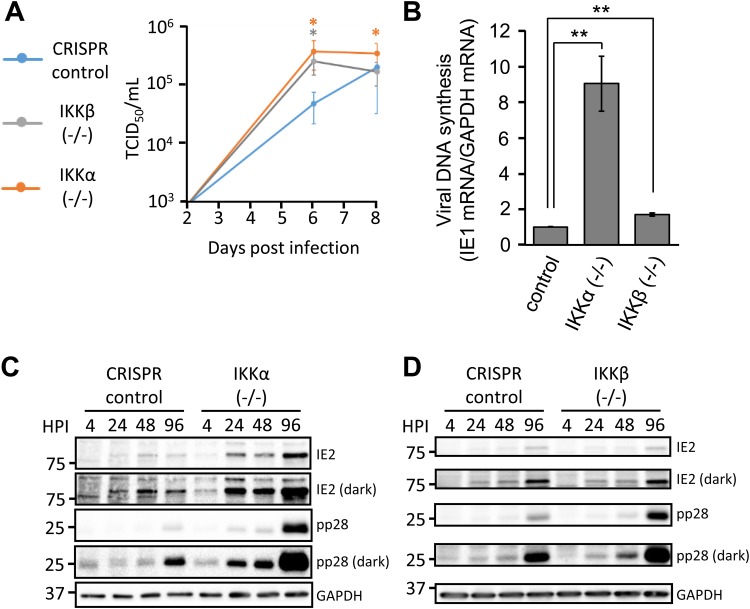
Previously, we found that the UL26 protein is necessary and sufficient to attenuate TNF-α-induced NF-κB activity and that UL26-deficient HCMV (ΔUL26) is more susceptible to challenge with TNF-α treatment (13). To determine whether either of the IKKs was functioning in a UL26-specific manner during viral infection, we assessed viral growth of a UL26-deficient green fluorescent protein (GFP)-positive HCMV mutant in our CRISPR IKK knockout cell lines (Fig. 4A). We found that loss of either IKK increased ΔUL26 infectious virion production by approximately 1 log unit at 6 days postinfection (dpi) (Fig. 4A), which was similar to the effect observed for GFP-positive WT (WT-GFP) HCMV (Fig. 3A). The difference in infectious virion production between IKK-deficient and control cells was reduced by day 8 (Fig. 4A). Viral DNA accumulation was also affected somewhat similarly by the loss of the IKKs, with an ∼9-fold increase in the amount of viral DNA present in IKKα−/− cells and an almost 2-fold increase in IKKβ−/− cells. Concomitantly, during ΔUL26 infection of the IKKα−/− cells, we observed significantly more production of the late viral protein pp28 (Fig. 4C). Levels of the pp28 protein were also elevated in IKKβ knockout cells during ΔUL26 infection (Fig. 4D), albeit to a lesser extent. The accumulation of the immediate early viral protein IE2 is enhanced in IKKα−/− cells but is unaffected in IKKβ-deficient cells (Fig. 4C and D). The elevated expression of pp28 at later time points during ΔUL26 infection in the IKKβ−/− cells differs from WT infection of the same cells (Fig. 3D), i.e., IKKβ inactivation did not substantially increase pp28 accumulation. This suggests the possibility that, in the absence of UL26, IKKβ may be limiting DNA replication and the expression of viral late genes. However, the observation that the loss of the IKKs increased WT and ΔUL26 viral replication to similar extents (∼10-fold at 6 dpi) suggests that the IKKs influence overall viral replication in a largely UL26-independent manner.
FIG 4.
IKKα and IKKβ restrict ΔUL26 infection. (A) Confluent, serum-starved CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were infected with ΔUL26-GFP HCMV at a MOI of 0.1. Viral supernatants were harvested at 2, 6, and 8 dpi, and infectious viral progeny were quantified via TCID50 assay. The indicated statistical comparisons were made using Student's t test, and results are color coded for each cell line. *, P < 0.05. (B) Viral DNA replication of ΔUL26-GFP HCMV was measured at 6 dpi in CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts infected as in panel A. Values are means ± standard errors (n ≥ 3). **, P < 0.01. (C and D) Confluent CRISPR control and IKKα−/− fibroblasts (C) or IKKβ−/− fibroblasts (D) were serum starved and infected with ΔUL26 HCMV as in panel A. Cells were harvested at the indicated time points and Western blotted with antibodies directed against IE2, pp28, and GAPDH. IE2 and pp28 blots are displayed with both light and dark exposures.
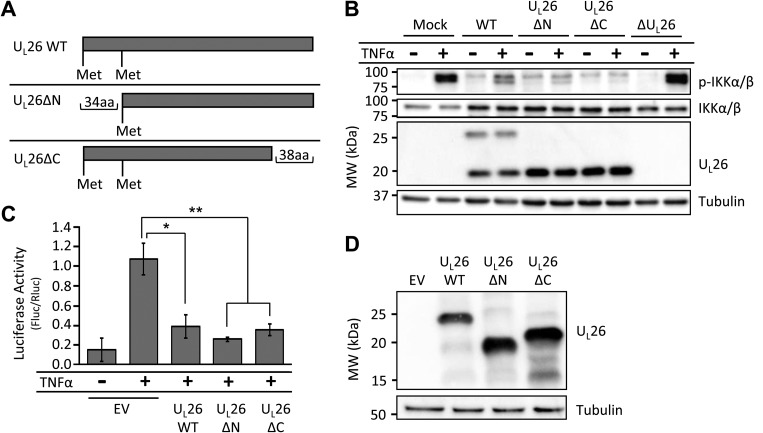
The UL26 domains required for high-titer viral replication are dispensable for inhibition of TNF-α-induced NF-κB activation.
Previously, we generated a panel of HCMV mutant strains encoding truncated forms of the UL26 protein and examined the contributions of the various UL26 domains to infection (Fig. 5A) (19). To determine whether specific UL26 domains are required for both NF-κB modulation and high-titer viral replication, we analyzed these mutants with respect to their ability to inhibit NF-κB signaling. In contrast to ΔUL26-infected cells, cells infected with recombinant viruses encoding N-terminal- and C-terminal-truncated UL26 isoforms were sufficient to inhibit TNF-α-induced phosphorylation of the IKK complex during infection (Fig. 5B). Similarly, in the absence of infection, transient expression of either the N-terminal or the C-terminal UL26 truncation mutant was sufficient to inhibit TNF-α-induced NF-κB-dependent luciferase activity (Fig. 5C and D). Collectively, these data suggest that the N- and C-terminal domains of UL26 are not necessary for attenuation of TNF-α-induced NF-κB activation. Further, given that the UL26ΔC mutant inhibits NF-κB signaling but has a replication defect similar in magnitude to that of the ΔUL26 null mutant (19), these data indicate that the UL26 sequences necessary for high-titer replication are separable from those necessary for prevention of TNF-α-induced NF-κB activation.
FIG 5.
The UL26 domains required for viral growth are separable from those required for TNF-α-induced NF-κB activation. (A) The diagram shows the truncations made to the UL26 open reading frame to generate the UL26ΔN and UL26ΔC mutants. (B) Serum-starved, confluent fibroblasts were infected with WT, UL26ΔN, UL26ΔC, or ΔUL26 HCMV at a MOI of 3.0. At 48 hpi, cells were subjected to mock treatment or 10 ng/ml TNF-α treatment for 10 min. Cell protein was then harvested and analyzed via Western blotting with the indicated antibodies. (C) HEK 293T cells were transiently transfected with an NF-κB-dependent luciferase expression construct, a Renilla luciferase expression construct, and either an empty vector (EV) or an expression vector encoding the UL26WT, UL26ΔN, or UL26ΔC isoform. At 24 hpi, cells were either mock treated or treated with 10 ng/ml TNF-α for 24 h prior to being harvested and subjected to a luciferase assay. Values are means ± standard errors (n = 3). Firefly luciferase activity was normalized to Renilla luciferase activity. *, P < 0.05; **, P < 0.01. (D) HEK 293T cells transfected with UL26 mutant expression constructs as in panel C were harvested at 48 hpi and Western blotted with antibodies specific to UL26 or tubulin.
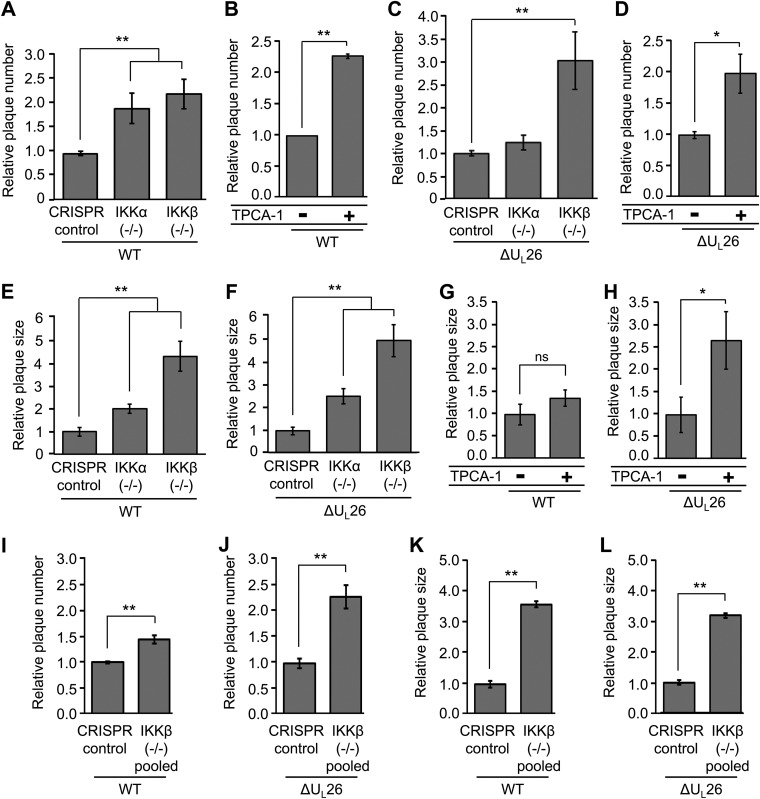
IKKα and IKKβ restrict initiation of infection and cell-to-cell spread.
To further explore the roles the IKKs play during viral infection, we analyzed how the loss of IKKα or IKKβ activity affected various plaque phenotypes. We found that knockout of either IKKα or IKKβ resulted in an ∼2-fold increase in the incidence of WT plaque formation (Fig. 6A). Similarly, treatment with 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide (TPCA-1), a pan-IKK inhibitor at the concentrations employed (25), also induced WT plaque formation by ∼2-fold (Fig. 6B). Plaque formation of the ΔUL26 virus was affected differentially in response to individual IKK deletions. First, knockout of IKKβ induced a 3-fold increase in ΔUL26 plaque formation efficiency, relative to control cells; this was higher than the level observed for the WT virus (Fig. 6A and C), suggesting that, in the absence of UL26, IKKβ restricts plaque formation to a greater extent. A more notable UL26-specific difference was that the knockout of IKKα did not increase the plaque formation of the ΔUL26 virus (Fig. 6C), which suggests that, in this context, UL26 is necessary for HCMV to benefit from the absence of IKKα. When both IKKs were inhibited via TPCA-1 treatment, the plaque formation efficiency of the ΔUL26 virus increased 2-fold (Fig. 6D), similar to WT infection.
FIG 6.
IKKα and IKKβ limit the initiation and cell-to-cell spread of HCMV infection. (A) CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were grown to confluence, serum starved, and infected with 40 PFU of WT-GFP HCMV for analysis of plaque phenotypes. The average efficiencies of plaque formation in IKK knockout fibroblasts were normalized to CRISPR control cell values (n ≥ 4). (B) Confluent, serum-starved MRC5 fibroblasts were either mock treated or treated with 10 μM TPCA-1 for 2 h before being infected with 25 PFU of WT-GFP HCMV. The average plaque formation efficiency under each condition was normalized to the number of plaques in mock-treated cells (n ≥ 4). (C) CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were infected as in panel A with ΔUL26-GFP HCMV. The average efficiencies of plaque formation in IKK knockout fibroblasts were normalized to CRISPR control cell values (n ≥ 4). (D) MRC5 fibroblasts were treated with TPCA-1 and infected as in panel B with ΔUL26-GFP HCMV. Values were normalized as in panel B (n ≥ 4). (E and F) CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were infected as in panel A with either WT-GFP (E) or ΔUL26-GFP (F) HCMV. Individual plaque sizes were quantified using ImageJ software, averaged, and normalized to the average plaque size in CRISPR control cells (n = 40). (G and H) MRC5 fibroblasts were infected as in panel B with WT-GFP (G) or ΔUL26-GFP (H) HCMV. Plaques for each condition were quantified using ImageJ software, averaged, and normalized to the average plaque size in untreated cells (n ≥ 19). (I, J, K, and L) The CRISPR control and a heterogeneous pool composed of multiple CRISPR IKKβ−/− cell lines were serum starved and infected as in panel A for plaque formation (I and J) and plaque size (K and L) analyses. Plaque formation efficiencies were normalized as in panel A (n ≥ 4). Plaque size was measured and normalized as in panel E (n ≥ 20). All values are plotted as means ± standard errors, and all statistical comparisons were made using Student's t test. *, P < 0.05; **, P < 0.05; ns, not significant.
In addition to measuring the impact on plaque formation efficiency, we measured the role that the IKKs play in cell-to-cell spread via an analysis of plaque size. WT HCMV plaques increased in size in the absence of the IKKs but to differing degrees. The size of WT viral plaques was enhanced ∼2-fold in the IKKα knockout cell line, while the IKKβ knockout cell line exhibited a plaque size increase of almost 5-fold (Fig. 6E). Similar increases in ΔUL26 plaque size were observed in both IKKα and IKKβ knockout cells, which exhibited ∼2-fold and ∼5-fold increases, respectively (Fig. 6F). These results suggest that, regardless of the presence of UL26, IKKβ restricts HCMV cell-to-cell spread to a greater extent than does IKKα and their individual contributions to plaque initiation can largely be separated from their contributions to cell-to-cell spread. In contrast to the knockout of either IKK alone, treatment with TPCA-1, which inhibits both IKKs, did not affect WT cell-to-cell spread, as evidenced by equal plaque sizes (Fig. 6G). Notably, ΔUL26 plaque sizes increased by ∼2.5-fold in the presence of TPCA-1 (Fig. 6H). These results suggest the possibility that, during WT infection, the potential benefits associated with losing one IKK are masked by the simultaneous loss of the other IKK. This is not seen in the context of ΔUL26 infection, however, where dual inhibition of the IKKs in the absence of the NF-κB regulator UL26 still increases the extent of cell-to-cell spread.
To rule out the possibility that the increased plaque formation and size phenotypes observed in the original IKKβ−/− cell line were a result of a clonal artifacts, we assessed these phenotypes in a polyclonal pool of newly generated IKKβ clones in which we combined six individual monoclonal IKKβ−/− clones in equal numbers. Upon assessment of WT and ΔUL26 plaque formation (Fig. 6I and J), we observed similar increases in plaque formation efficiency, with plaque formation increases of 1.5-fold and 2.5-fold for HCMV WT and ΔUL26 viruses, respectively (Fig. 6I and J). Similarly, cell-to-cell spread for both WT and ΔUL26 viruses was also increased ∼3- to 4-fold in this pooled population, only a slight reduction in comparison with the ∼4- to 5-fold increases observed in the monoclonal IKKβ−/− cell line (Fig. 6K and L). These findings imply that clonal selection effects are not a likely explanation for the IKKβ-related plaque formation phenotypes we observe. Collectively, our results indicate that the IKKs restrict the initiation of infection and restrict cell-to-cell spread.
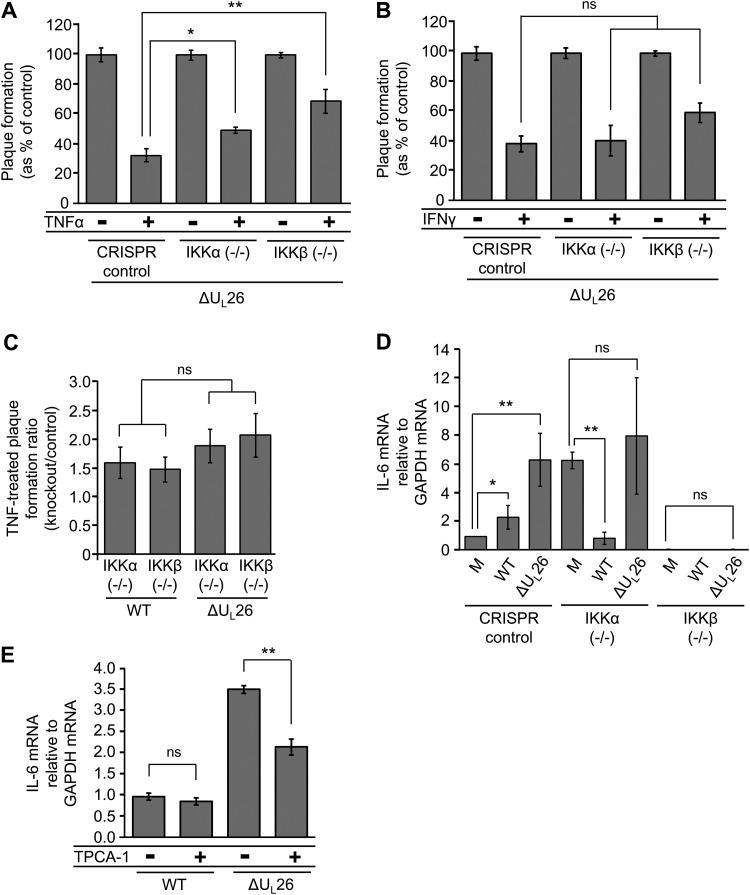
Contributions of IKKα and IKKβ to ΔUL26 innate immune phenotypes.
The ΔUL26 virus is more vulnerable to TNF-α challenge than WT HCMV (13). To assess the extent to which the IKKs contribute to viral TNF-α sensitivity in a UL26-dependent manner, we assessed viral plaque formation in our IKK knockout cell lines in the face of TNF-α or IFN-γ challenge (Fig. 7A and B). We found that loss of either of the IKKs modulated ΔUL26 sensitivity to TNF-α treatment, with the IKKβ knockout having a larger effect than the IKKα knockout and exhibiting approximately twice the number of plaques formed upon TNF-α treatment, relative to the control cells (Fig. 7A). In contrast, knockout of either IKKα or IKKβ had little effect on the plaque formation efficiency after treatment with IFN-γ (Fig. 7B). The increase in plaque formation efficiency observed in the IKKα or IKKβ knockout cells, relative to control cells, was slightly higher in the context of ΔUL26 infection, compared to WT HCMV infection (Fig. 7C). However, the effect was very modest and not statistically significant, suggesting that IKK activity does not have a substantially larger inhibitory effect on plaque formation efficiency in the absence of UL26.
FIG 7.
IKKβ is required for ΔUL26-mediated induction of IL-6 expression. (A and B) Confluent, serum-starved CRISPR control, IKKα−/−, or IKKβ−/− cell lines were mock treated or treated with either 10 ng/ml TNF-α (A) or 5 U/ml IFN-γ (B) for 4 h prior to infection with 40 PFU of ΔUL26-GFP HCMV. Relative plaque formation was measured by dividing the cytokine-treated plaque number for each virus by the number of plaques for that virus on the mock-treated cell line (CRISPR control, IKKα, or IKKβ) and plotted as percentage ± standard error (n ≥ 4). (C) The plaque formation efficiencies of TNF-α-challenged WT-GFP (Fig. 2B) and ΔUL26-GFP (Fig. 6A) HCMV in IKKα−/− and IKKβ−/− cell lines were divided by the plaque formation efficiency of each virus in TNF-α-challenged CRISPR control cells to determine the ratio representing the extent to which loss of each IKK rescued viral sensitivity to TNF-α. Error bars indicate standard errors (n ≥ 4). (D) CRISPR control, IKKα−/−, and IKKβ−/− fibroblasts were grown to confluence, serum starved, and either mock infected (M) or infected with WT or ΔUL26 HCMV at a MOI of 3. Cellular RNA was harvested at 48 hpi, and the abundance of IL-6 transcript was measured by real-time PCR and normalized to that of GAPDH. Values are plotted as means ± standard errors (n ≥ 3). (E) Confluent, serum-starved MRC5 fibroblasts were either mock treated or treated with 10 μM TPCA-1 for 2 h before being infected with WT or ΔUL26 HCMV at a MOI of 3. Cellular RNA was harvested and assayed for IL-6 expression at 48 hpi as in panel D (n ≥ 4). All statistical comparisons were made using Student's t test. *, P < 0.05; **, P < 0.05; ns, not significant.
Previously, we found that ΔUL26 infection induces noncanonical NF-κB activation, including inducing the nuclear translocation of RelB, which is typically mediated by IKKα (Fig. 1A) (reviewed in reference 26). In addition, expression and secretion of the antiviral cytokine interleukin 6 (IL-6), a canonical NF-κB target gene, are induced during ΔUL26 infection (Fig. 7D) (13). To elucidate how IKKα and IKKβ contribute to ΔUL26-mediated induction of IL-6, we measured the accumulation of IL-6 mRNA during WT and ΔUL26 infection. IKKα knockout cells showed notably higher levels of IL-6 transcription during mock infection. This large induction of IL-6 transcription was preserved during infection in the absence of UL26 but not WT infection. Loss of IKKβ, however, entirely ablated the transcription of IL-6 across all mock infection and infection conditions (Fig. 7D). To corroborate these findings, we utilized the IKK inhibitor TPCA-1 to impede IKK signaling during infection with either WT or ΔUL26 HCMV (MOI of 3.0), and we measured the IL-6 mRNA abundance at 48 h postinfection (hpi). We observed that TPCA-1 inhibition had little effect on the transcription of IL-6 during WT infection but significantly reduced IL-6 mRNA abundance, almost 2-fold, during ΔUL26 infection (Fig. 7E). These results indicate that the IKKs both regulate the accumulation of the downstream NF-κB target gene IL-6 and that IKKβ in particular is required for the upregulation of IL-6 during infection in the absence of UL26.
DISCUSSION
The NF-κB pathway has a complex relationship with HCMV infection. Studies have shown that HCMV can induce NF-κB activation (9, 27, 28), and viral replication in certain cellular contexts is reduced by pharmaceutical inhibition or expression of dominant negative IKK constructs (11, 12, 22). Conversely, aspects of the NF-κB response are downregulated by HCMV infection (15, 29), and various gene products, such as UL26, have been found to inhibit NF-κB activation (13, 30, 31). Further, stimulation of NF-κB signaling with cytokines such as TNF-α decreases viral replicative success (20). Collectively, these data suggest that monolithic declarations about the impact of NF-κB signaling and HCMV infection are insufficient. This insufficiency arises in part from the associated complexity of the NF-κB signaling architecture, which includes various related but distinct pathways that can result in substantially different transcriptional responses (32). Another contributing factor is the complexity of HCMV biology. In all likelihood, the virus tailors its interactions with the NF-κB pathway to favor a lytic or latent infection, depending on the cellular context and both external and internal cues.
To begin to deconvolute some of the issues raised above, we utilized CRISPR-mediated mutagenesis to assess how the key NF-κB regulatory kinases IKKα and IKKβ contribute to HCMV replication in fibroblasts. In total, our results indicate that both IKKs restrict lytic HCMV infection, although they functionally diverge with respect to several viral phenotypes. Our results indicate that inactivation of either IKKα or IKKβ increases low-MOI HCMV replication by ∼10-fold (Fig. 3A). In addition to viral replication, inactivation of either IKK increased the ability of HCMV to initiate infection and to form a plaque, increasing plaque formation efficiency to a similar extent (Fig. 6A). In these contexts, our results indicate that both IKKα and IKKβ restrict HCMV infection. Notably, the accumulation of viral progeny during high-MOI infection (MOI of 3.0) in IKKα−/− or IKKβ−/− fibroblasts was not increased, relative to CRISPR control cells (data not shown), suggesting that the antiviral contributions of the IKKs are MOI dependent.
Although the increases in viral replication and plaque formation efficiency in CRISPR IKKα−/− or IKKβ−/− cells are similar in magnitude, the IKKs have divergent effects on viral protein accumulation during infection. Loss of IKKα increased the accumulation of viral immediate early and late proteins, relative to control cells, which contrasts with the IKKβ knockout cells, in which there was no detected difference in viral protein accumulation (Fig. 3C and D). The similar magnitudes of increased replication between the IKKβ and IKKα knockout cells suggest that this increase in viral protein expression in the IKKα knockout cells does not functionally contribute to the production of viral progeny in this context. In addition to differentially affecting viral gene expression, the knockout of IKKα and IKKβ appears to disparately influence viral spread between cellular neighbors, as plaques in IKKβ−/− cells were substantially larger than those observed in IKKα−/− cells (Fig. 6E). These differential effects on viral protein accumulation and cell-to-cell spread point to IKKα and IKKβ contributing to aspects of viral infection via different mechanisms. It is well established that IKKα and IKKβ can direct different NF-κB transcriptional programs via differential activation of the RelA and RelB transcription factors (Fig. 1A) (reviewed in reference 32), raising the possibility that these unequal contributions to HCMV infection could be mediated through divergent NF-κB transcriptional programs regulated by IKKα or IKKβ.
Deletion of the UL26 reading frame is associated with an ∼10-fold defect in viral replication (18). More recently, we found that the UL26 protein inhibits NF-κB signaling and that the ΔUL26 virus induces the nuclear translocation of RelB and is sensitive to treatment with TNF-α (13). Here, we explore the contributions of IKKα and IKKβ to the ΔUL26 replication defect. We find that inactivation of either IKKα or IKKβ increases ΔUL26 viral replication, although the extent of the increase is similar to that observed for WT HCMV, i.e., ∼10-fold (Fig. 3A and Fig. 4A). This similarity suggests that inactivation of IKKα or IKKβ is not sufficient to rescue the replication defect specifically associated with the absence of UL26. Our current analysis of various UL26 mutants also provides evidence that the modulatory role of UL26 in NF-κB signaling is independent of its contributions to viral replication. Specifically, we find that a C-terminal UL26 deletion mutant that replicates with the same kinetics as a ΔUL26 virus (19) is still capable of blocking TNF-α-induced IKK phosphorylation and activation of NF-κB (Fig. 5B and C). Collectively, these data suggest that UL26-mediated modulation of NF-κB activity is likely separable from its contribution to high-titer viral replication.
Despite the likely independence of the NF-κB modulatory activities of UL26 from its contributions to in vitro viral replication, the interplay between UL26 and NF-κB is still of particular interest, given the critical roles that NF-κB and innate immunity play in determining the outcome of infection in a natural host. In this regard, the impact of IKK knockout on UL26-associated NF-κB phenotypes can provide insight into this interaction. Previously, we found that the ΔUL26 mutant induces the expression and secretion of IL-6, a well-described NF-κB target (33). Here, we find that the inactivation of IKKβ, but not IKKα, blocks the induction of IL-6 expression that is associated with ΔUL26 infection (Fig. 7D). These data suggest that IKKβ and, by extension, canonical NF-κB signaling are critical mediators of the aberrant NF-κB transcriptional environment induced by ΔUL26 infection, and they provide further evidence that UL26 targets canonical NF-κB signaling for inhibition during infection. Further, the accumulation of pp28 was significantly increased in IKKβ−/− cells (Fig. 4D), a phenomenon not observed during WT infection of the same cells. This suggests that an interaction between IKKβ signaling and UL26 can alter the kinetics of late gene expression during infection.
We found previously that, in contrast to WT infection, ΔUL26 infection induces the nuclear accumulation of RelB (13), consistent with activation of noncanonical NF-κB signaling. In combination with the ability of UL26 to inhibit TNF-α-induced NF-κB activity, these results suggest that UL26 can inhibit both canonical and noncanonical NF-κB signaling. It remains to be determined whether UL26-mediated modulation of both canonical and noncanonical NF-κB signaling occurs via targeting of an NF-κB component capable of modulating both pathways or whether UL26 affects the activity of more than one NF-κB-associated signaling factor. Our current results indicate that inactivation of IKKα increases the efficiency with which WT HCMV forms plaques but UL26 is necessary for this increase, i.e., the ΔUL26 mutant does not display this increased plaque formation efficiency in IKKα−/− cells (Fig. 6C). Given our results linking UL26-mediated NF-κB gene expression with IKKβ, the simplest explanation for this could be that IKKβ-activated NF-κB signaling in the absence of UL26, e.g., leading to increased IL-6 expression, compensates for the absence of IKKα during ΔUL26 infection in this context. Generally speaking, potential compensation by one IKK for the loss of the other must be considered when making conclusions about the role that the IKKs play during HCMV infection.
In summary, our results indicate that IKKα and IKKβ are important host determinants of viral growth, infection initiation, and cell-to-cell spread. We report that the IKKs contribute differently to WT and ΔUL26 infection, with IKKβ playing a specific role in shaping the transcriptional response to ΔUL26 viral infection, whereas IKKα has an independent role in downregulating the accumulation of viral immediate early and late gene products. Moving forward, it will be important to elucidate the role that the HCMV–NF-κB interaction plays in other infectious contexts, including other cell types important to pathogenesis, as well as during latency and reactivation. Viral targeting of innate immune pathways is a critical contributor to successful viral infection. Elucidating the mechanisms though which innate immune signaling limits viral replication, as well as the means by which HCMV subverts these mechanisms, will likely continue to provide novel avenues for antiviral therapeutic intervention.
MATERIALS AND METHODS
Cell culture, viruses, and viral infection.
MRC5 fibroblasts (ATCC CCL-171), human embryonic kidney (HEK) 293T cells, and telomerase-expressing BJ fibroblasts (generated via lentiviral transduction, as described previously [34]) were cultured at 37°C in Dulbecco’s modified Eagle serum (DMEM) (Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 4.5 g/liter glucose, and 1% penicillin-streptomycin (Life Technologies), in a 5% (vol/vol) CO2 atmosphere. For seeding and cell growth assays, cell viability was determined via the trypan blue exclusion method, with a TC10 automated cell counter (Bio-Rad). The WT strain of HCMV used in this work was BADwt, a bacterial artificial chromosome (BAC) clone of AD169 (35). The ΔUL26-DBmetΔ (referred to here as ΔUL26), UL26ΔN, and UL26ΔC recombinant viruses were previously generated from this BAC as described (19). GFP-expressing AD169 (BADsubUL21.5, referred to as WT-GFP) and ΔUL26 with a transposon insertion (ΔUL26-TI, referred to as ΔUL26-GFP) were used for plaque formation efficiency and plaque size experiments and have been described previously (19, 36). Unless otherwise indicated, viral infection was performed as follows: cells were grown to a confluence of ∼3.2 × 104 cells per cm2 in a 6-well plate and were serum starved for 24 h prior to infection, after which viral inocula were added for an adsorption period of 1.5 h and then replaced with fresh serum-free medium. Cells were infected at a MOI of 3.0 unless otherwise indicated. Culturing in serum-free medium prior to infection was employed to minimize the effects of serum on cellular signaling, as well as to synchronize and to control for cell cycle progression in the cell population. Viral stocks were propagated in MRC5 fibroblasts. For titering of viral stocks, as well as experiments involving the assessment of viral replication in CRISPR cell lines, viral titers were determined via TCID50 analysis.
Lentiviral plasmids.
CRISPR-targeted IKK knockout in BJ/hTert cells was mediated by the following guide RNAs: IKKα, 5′-ACAGACGTTCCCGAAGCCGC-3′; IKKβ, 5′-ACCACCGCTCTCGGTTCCGG-3′. These sequences were determined by inserting early segments of the CHUK (IKKα) and IKBKB (IKKβ) open reading frames into the Zhang Lab online generator (http://crispr.mit.edu). Guide RNAs were appended with flanking homology arms and cloned into the LentiCRISPRv2 Cas9 expression platform according to the Zhang Lab single guide RNA protocol (37, 38). CRISPR control cells were generated via lentiviral transduction of the unmodified LentiCRISPRv2 plasmid.
Lentiviral transduction and cell line generation.
Pseudotyped lentivirus was produced in 293T cells seeded in 10-cm dishes at a density of 2 × 106 cells per cm2 and grown for 24 h prior to transfection with 2.6 μg lentiviral vector, 2.4 μg PAX2, and 0.25 μg vesicular stomatitis virus G glycoprotein expression plasmid, using Fugene 6 (Promega). The medium was aspirated after 24 h, and 4 ml fresh medium was added to the plate. After another 24 h, the supernatant was filtered through a 0.45-μm filter and applied to fibroblast cells in the presence of 5 μg/ml Polybrene (Millipore), and the cells were incubated for 24 h. The medium was refreshed and the cells were allowed to recover for 72 h prior to selection with 1 μg/ml puromycin (VWR). Following transduction with guide RNA-expressing lentivirions and subsequent puromycin selection, BJ/hTert fibroblasts were clonally selected by seeding individual cells in 96-well plates. CRISPR control cells transduced with non-guide RNA-containing LentiCRISPRv2 were retained as heterogeneous polyclonal populations. Genomic DNA was harvested from monoclonal lines, and the guide RNA-targeted region of each IKK was amplified by PCR using the following primers: IKKα forward primer, 5′-GGGGACTTAAAGAGCGGACC-3′; IKKα reverse primer, 5′-CCCCACTGATATCATATGGCCT-3′; IKKβ forward primer, 5′-TGGATGGATAGAAATGAGGTGGG-3′; IKKβ reverse primer, 5′-TAGAAGCAGCAGAGTCACCGT-3′. To verify the presence of indels in the IKKα/IKKβ reading frames of both alleles in clonally isolated cell lines, the amplified PCR product was gel purified, ligated into the pCR2.1-TOPO TA vector using a TA cloning kit (Invitrogen), according to manufacturer’s instructions, and transformed into DH10B competent cells. Bacterial colonies were grown and color selected on LB plates containing 50 μg/ml kanamycin and supplemented with 40 μl each of 100 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and 40 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), spread over the surface of the plate. Plasmids were isolated from colorless colonies containing the PCR inserts and sequenced using M13 forward (position −20) and M13 reverse primers. CRISPR-mediated indel generation in the genomic IKKα and IKKβ reading frames was also confirmed via TIDE analysis (39). In addition to the monoclonal IKKα and IKKβ cell lines, an IKKβ−/− pooled cell line was created by combining equivalent cell numbers of multiple IKKβ−/− clones with the following frameshift deletions: positions −7 and +1, positions +1 and +10, and positions +1 and +1.
Immunoblotting.
Cells were harvested in disruption buffer (50 mM Tris [pH 7.0], 2% SDS, 5% 2-mercaptoethanol, and 2.75% sucrose), and the proteins were solubilized via sonication and boiling and separated by 10% SDS-PAGE. Samples were transferred to a nitrocellulose membrane in Tris-glycine transfer buffer and stained with Ponceau S to ensure uniform protein loading, and blots were blocked with 5% milk solution in Tris-buffered saline-Tween 20 (TBST). Primary and secondary antibody incubations were followed by development with an enhanced chemiluminescence (ECL) kit (Bio-Rad) and imaging using a Molecular Imager Gel Doc XR+ system (Bio-Rad). Primary antibodies were specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (D16H11; Cell Signaling), phospho-IKKα(Ser176)/IKKβ(Ser177) (16A6; Cell Signaling), IKKα (2682; Cell Signaling), IKKβ (2684; Cell Signaling), RelA (C-20; Santa Cruz Biotechnology), RelB (C-19; Santa Cruz Biotechnology), α-tubulin (11H10; Cell Signaling), IKKα/IKKβ (H-470; Santa Cruz Biotechnology), pp28 (40), IE2 (41), and UL26 (7H19) (18, 40). Secondary antibodies were rabbit polyclonal (Santa Cruz Biotechnology) and mouse monoclonal (Abcam) anti-IgG antibodies.
Analysis of DNA and transcripts during infection.
Viral DNA accumulation was assessed by real-time quantitative PCR, as described previously (42). Briefly, CRISPR control, IKKα−/−, and IKKβ−/− cells were infected with WT or ΔUL26 virus at a MOI of 0.1 and then, at various time points, scraped in medium, washed with phosphate-buffered saline, and resuspended in lysis buffer (100 mM NaCl, 100 mM Tris [pH 8.0], 25 mM EDTA, 0.5% SDS, 0.1 mg/ml proteinase K, and 40 μg/ml RNase A). Cells were lysed overnight at 55°C, extracted with phenol/chloroform/isoamyl alcohol (25:24:1), extracted again with chloroform/isoamyl alcohol (24:1), precipitated with ethanol, and resuspended in water. The DNA abundance was assessed via quantitative PCR using Fast SYBR green master mix (Applied Biosystems), a model 7500 Fast real-time PCR system (Applied Biosystems), and Fast 7500 software (Applied Biosystems), according to the manufacturer's instructions. Viral DNA abundance was determined with a primer pair targeting the viral IE1 gene, i.e., 5′-CCATGTCCACTCGAACCTTAAT-3′ (forward) and 5′-TGAACAAGTGACCGAGGATTG-3′ (reverse). Host cell DNA was assessed using the following primers targeting GAPDH: 5′-CATGTTCGTCATGGGTGTGAACCA-3′ (forward) and 5′-ATGGCATGGACTGTGGTCATGAGT-3′ (reverse). IE1 and GAPDH gene equivalent values were determined using the 2−ΔΔCT method, and normalized viral DNA accumulation values were obtained by dividing the abundance of IE1 gene equivalents by the abundance of GAPDH gene equivalents. To measure IL-6 transcription during infection, RNA was isolated from infected cells via TRIzol (Invitrogen) extraction, according to manufacturer’s instructions, and used to synthesize cDNA with random hexamer primers and the Superscript II reverse transcriptase system (Invitrogen). Relative quantities of gene expression were measured and normalized to GAPDH levels via the 2−ΔΔCT method, using the following primers: IL-6, 5′-AAATTCGGTACATCCTCGACGGCA-3′ (forward) and 5′-AGTGCCTCTTTGCTGCTTTCACAC-3′ (reverse); GAPDH, 5′-CATGTTCGTCATGGGTGTGAACCA-3′ (forward) and 5′-ATGGCATGGACTGTGGTCATGAGT-3′ (reverse).
NF-κB luciferase assay.
The NF-κB reporter construct pNF-κB-FF was induced by TNF-α treatment, as described previously (43). Briefly, calcium phosphate (Stratagene) was used to cotransfect 50% confluent 293T cells in 96-well plates with 0.25 μg pNFκB-FF, 0.25 μg of a Renilla luciferase expression plasmid with an SV40 promoter (pRL-SV40), and 0.25 μg of either pCDNA3.1(+) empty vector (Invitrogen) or one of a panel of pCDNA vectors encoding UL26 mutants (pCDNA-UL26WT, pCDNA-UL26ΔN, and pCDNA-UL26ΔC). The following oligonucleotides were used to amplify portions of the UL26 open reading frame and to insert the sequences into the EcoRI-NotI region of the pCDNA3.1(+) vector via Gibson assembly: pCDNAUL26 short isoform, 5′-CGGATCCACTAGTCCAGTGTGGTGGAATTATGACGAGTAGGCGCGCACCCGACGGCGG-3′ (forward) and 5′-GTTTAAACGGGCCCTCTAGACTCGAGCGGCCTTACGGCAACAGCGCTGATGGCACGTTGC-3′ (reverse); pCDNAUL26ΔC isoform, 5′-CGGATCCACTAGTCCAGTGTGGTGGAATTATGTACGCCGTTTTCGGCCTCACGAGGTCG-3′ (forward) and 5′-GTTTAAACGGGCCCTCTAGACTCGAGCGGCCCTAGCGGATGACCTGGCCGTCGGCGTCGC-3′ (reverse). The UL26 WT insert was amplified using the pCDNAUL26ΔC isoform forward primer and the pCDNAUL26 short isoform reverse primer. Cells were allowed to recover from transfection for 24 h and then exposed to 10 ng/ml TNF-α treatment for 24 h prior to analysis. Luciferase activity was measured using the Dual-Glo luciferase assay system (Promega), according to the manufacturer’s instructions, and a Synergy 2 plate reader (BioTek). NF-κB reporter activity was normalized to Renilla luciferase activity as a transfection efficiency control.
Plaque formation and size assays.
Serum-starved confluent cells were infected for 1.5 h with a known number of PFU from freshly thawed stocks of indicated GFP-positive viruses. A standard agarose gel overlay was placed over the cells, and plaques were allowed to develop for 10 days before the number of plaques was counted and plaque sizes were measured using ImageJ software. The criterion for identification of a plaque was a locus of multiple GFP-positive cells displaying a cytopathic effect. For experiments involving plaque formation during cytokine treatment, cells were exposed to 10 ng/ml TNF-α (Sigma) or 5 U/ml IFN-γ (PBL Assay Science) for 4 h prior to infection. For plaque formation and size experiments using the IKKα/IKKβ inhibitor TPCA-1, cells were pretreated with 10 μM TPCA-1 (Sigma) for 2 h prior to infection.
Statistical analysis.
All Western blot analyses are representative blots from at least two independent experiments. Statistical significance was determined using a nonpaired one-tailed homoscedastic Student's t test unless otherwise indicated. A probability value of <0.05 was considered statistically significant. For the growth curves in Fig. 3 and Fig. 4, the values produced for a given biological replicate experiment were normalized to the average amount of infectious virus produced in the control cells for the biological replicate in the same experiment before statistical comparisons between the infectious virions produced by control and IKK knockout cells were performed. This normalization serves to reduce the noise associated with the variability in the kinetics of peak viral titers between experiments, which are independent of any potential IKKα/IKKβ contributions to infectious virus production.
ACKNOWLEDGMENTS
C.M.G. was supported by an NIH training grant from the Training Program in Oral Science (grant T90-DE021985-07). The work was also supported by NIH grant AI127370 to J.M. and by a Research Scholar Grant from the American Cancer Society (grant RSG-15-049-01-MPC).
We thank Xenia Schafer for critical readings of the manuscript.
REFERENCES
- 1.Mocarski ES, Shenk T, Pass RF, Knipe DM. 2006. Cytomegaloviruses. Lippincott-Williams & Wilkins, New York, NY. [Google Scholar]
- 2.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. [DOI] [PubMed] [Google Scholar]
- 3.Stern-Ginossar N, Weisburd B, Michalski A, Le VT, Hein MY, Huang SX, Ma M, Shen B, Qian SB, Hengel H, Mann M, Ingolia NT, Weissman JS. 2012. Decoding human cytomegalovirus. Science 338:1088–1093. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin C, Ciesla J, Munger J. 2018. Who’s driving? Human cytomegalovirus, interferon, and NFκB signaling. Viruses 10:447. doi: 10.3390/v10090447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le‐Trilling V, Trilling M. 2015. Attack, parry and riposte: molecular fencing between the innate immune system and human herpesviruses. Tissue Antigens 86:1–13. doi: 10.1111/tan.12594. [DOI] [PubMed] [Google Scholar]
- 6.Napetschnig J, Wu H. 2013. Molecular basis of NF-κB signaling. Annu Rev Biophys 42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun SC. 2011. Non-canonical NF-κB signaling pathway. Cell Res 21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambucetti LC, Cherrington JM, Wilkinson GW, Mocarski ES. 1989. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J 8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurochko AD, Kowalik TF, Huong SM, Huang ES. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol 69:5391–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMeritt IB, Milford LE, Yurochko AD. 2004. Activation of the NF-κB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol 78:4498–4507. doi: 10.1128/JVI.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caposio P, Luganini A, Hahn G, Landolfo S, Gribaudo G. 2007. Activation of the virus-induced IKK/NF-κB signalling axis is critical for the replication of human cytomegalovirus in quiescent cells. Cell Microbiol 9:2040–2054. doi: 10.1111/j.1462-5822.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 12.Caposio P, Musso T, Luganini A, Inoue H, Gariglio M, Landolfo S, Gribaudo G. 2007. Targeting the NF-κB pathway through pharmacological inhibition of IKK2 prevents human cytomegalovirus replication and virus-induced inflammatory response in infected endothelial cells. Antiviral Res 73:175–184. doi: 10.1016/j.antiviral.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Mathers C, Schafer X, Martinez-Sobrido L, Munger J. 2014. The human cytomegalovirus UL26 protein antagonizes NF-κB activation. J Virol 88:14289–14300. doi: 10.1128/JVI.02552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon KM, Oh SE, Kim YE, Han TH, Ahn JH. 2017. Cooperative inhibition of RIP1-mediated NF-κB signaling by cytomegalovirus-encoded deubiquitinase and inactive homolog of cellular ribonucleotide reductase large subunit. PLoS Pathog 13:e1006423. doi: 10.1371/journal.ppat.1006423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montag C, Wagner J, Gruska I, Hagemeier C. 2006. Human cytomegalovirus blocks tumor necrosis factor alpha- and interleukin-1β-mediated NF-κB signaling. J Virol 80:11686–11698. doi: 10.1128/JVI.01168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamminger T, Gstaiger M, Weinzierl K, Lorz K, Winkler M, Schaffner W. 2002. Open reading frame UL26 of human cytomegalovirus encodes a novel tegument protein that contains a strong transcriptional activation domain. J Virol 76:4836–4847. doi: 10.1128/JVI.76.10.4836-4847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abate DA, Watanabe S, Mocarski ES. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J Virol 78:10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munger J, Yu D, Shenk T. 2006. UL26-deficient human cytomegalovirus produces virions with hypophosphorylated pp28 tegument protein that is unstable within newly infected cells. J Virol 80:3541–3548. doi: 10.1128/JVI.80.7.3541-3548.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathers C, Spencer CM, Munger J. 2014. Distinct domains within the human cytomegalovirus UL26 protein are important for wildtype viral replication and virion stability. PLoS One 9:e88101. doi: 10.1371/journal.pone.0088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, O'Malley JA. 1987. Antiviral effects of recombinant human tumor necrosis factor. Lymphokine Res 6:309–318. [PubMed] [Google Scholar]
- 21.Davignon JL, Castanie P, Yorke JA, Gautier N, Clement D, Davrinche C. 1996. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol 70:2162–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caposio P, Dreano M, Garotta G, Gribaudo G, Landolfo S. 2004. Human cytomegalovirus stimulates cellular IKK2 activity and requires the enzyme for productive replication. J Virol 78:3190–3195. doi: 10.1128/JVI.78.6.3190-3195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allan-Yorke J, Record M, de Préval C, Davrinche C, Davignon JL. 1998. Distinct pathways for tumor necrosis factor alpha and ceramides in human cytomegalovirus infection. J Virol 72:2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszinowski UH. 1993. Participation of endogenous tumour necrosis factor alpha in host resistance to cytomegalovirus infection. J Gen Virol 74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 25.Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De Alba J, Newton R, Haj-Yahia S, Pun KT, Watts CJ, Shaw RJ, Savage TJ, Belvisi MG. 2005. Iκ-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med 172:962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 26.Sun SC. 2012. The noncanonical NF-κB pathway. Immunol Rev 246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yurochko AD, Hwang ES, Rasmussen L, Keay S, Pereira L, Huang ES. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol 71:5051–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Petrovas C, Sonenshein GE. 2002. RelB-p50 NF-κB complexes are selectively induced by cytomegalovirus immediate-early protein 1: differential regulation of Bcl-xL promoter activity by NF-κB family members. J Virol 76:5737–5747. doi: 10.1128/JVI.76.11.5737-5747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis MA, Borton JA, Keech AM, Wong J, Britt WJ, Magun BE, Nelson JA. 2006. Human cytomegalovirus attenuates interleukin-1β and tumor necrosis factor alpha proinflammatory signaling by inhibition of NF-κB activation. J Virol 80:5588–5598. doi: 10.1128/JVI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor RT, Bresnahan WA. 2006. Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NFκB-dependent gene expression. J Virol 80:10763–10771. doi: 10.1128/JVI.01195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browne EP, Shenk T. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc Natl Acad Sci U S A 100:11439–11444. doi: 10.1073/pnas.1534570100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smale ST. 2012. Dimer-specific regulatory mechanisms within the NF-κB family of transcription factors. Immunol Rev 246:193–204. doi: 10.1111/j.1600-065X.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 33.Keller ET, Chang C, Ershler WB. 1996. Inhibition of NFκB activity through maintenance of IκBα levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem 271:26267–26275. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Schafer X, Munger J. 2016. Expression of oncogenic alleles induces multiple blocks to human cytomegalovirus infection. J Virol 90:4346–4356. doi: 10.1128/JVI.00179-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu D, Smith GA, Enquist LW, Shenk T. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol 76:2316–2328. doi: 10.1128/jvi.76.5.2316-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D, Silva MC, Shenk T. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A 100:12396–12401. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanjana NE, Shalem O, Zhang F. 2014. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. 2014. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkman EK, Chen T, Amendola M, van Steensel B. 2014. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva MC, Yu QC, Enquist L, Shenk T. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J Virol 77:10594–10605. doi: 10.1128/JVI.77.19.10594-10605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Shen Y, Shenk T. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol 69:7960–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terhune S, Torigoi E, Moorman N, Silva M, Qian Z, Shenk T, Yu D. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J Virol 81:3109–3123. doi: 10.1128/JVI.02124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigo WWSI, Ortiz-Riaño E, Pythoud C, Kunz S, de la Torre JC, Martínez-Sobrido L. 2012. Arenavirus nucleoproteins prevent activation of nuclear factor kappa B. J Virol 86:8185–8197. doi: 10.1128/JVI.07240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]