Abstract
Mutations in the human genes encoding hepatocyte nuclear factors (HNF) 1α, 1β, 4α, and IPF1(PDX1/IDX1/STF1) result in pancreatic β cell dysfunction and diabetes mellitus. In hepatocytes, hnf4α controls the transcription of hnf1α, suggesting that this same interaction may operate in β cells and thus account for the common diabetic phenotype. We show that, in pancreatic islet and exocrine cells, hnf4α expression unexpectedly depends on hnf1α. This effect is tissue-specific and mediated through direct occupation by hnf1α of an alternate promoter located 45.6 kb from the previously characterized hnf4α promoter. Hnf1α also exerts direct control of pancreatic-specific expression of hnf4γ and hnf3γ. Hnf1α dependence of hnf4α, hnf4γ, hnf3γ, and two previously characterized distal targets (glut2 and pklr) is established only after differentiated cells arise during pancreatic embryonic development. These studies define an unexpected hierarchical regulatory relationship between two genes involved in human monogenic diabetes in the cells, which are relevant to its pathophysiology. Furthermore, they indicate that hnf1α is an essential component of a transcription factor circuit whose role may be to maintain differentiated functions of pancreatic cells.
Terminally differentiated pancreatic β cells possess a highly specialized apparatus designed to sense extracellular concentrations of glucose, other metabolites, and hormones. This information is processed to couple the synthesis and secretion of insulin to the demands of the organism (1). Despite growing knowledge derived from mouse genetic studies indicating which transcriptional regulators are required to complete discrete steps in pancreatic development (2, 3), much less is known regarding the transcription factors needed to ensure the specialized functions of differentiated β cells once these are formed. Recent human genetic studies (reviewed in ref. 4) have pointed to a set of genes whose role may be relevant to this function. Thus, humans with heterozygous mutations in the genes encoding hepatocyte nuclear factors (HNF) 1α, 1β, and 4α, as well as pancreatic islet duodenum homeobox PDX1/IDX1/IPF1 develop a diabetic phenotype resulting from β cell dysfunction [maturity onset diabetes of the young (MODY)] (5–8).
Perhaps the best characterized MODY defect is that resulting from mutations in the gene encoding HNF1α, an atypical homeodomain protein (9). A hallmark of the pathophysiology of human HNF1α deficiency (MODY3) and hnf1α-null mutant mice is defective β cell glucose sensing (10, 11). This results at least in part from reduced aerobic glycolysis and possibly mitochondrial metabolism (12, 13) although the precise molecular defects are unknown. It is likely that several islet cell-enriched genes are involved, including the glut2 glucose transporter, liver type pyruvate kinase (pklr), and aldolase B (13, 14). Mice lacking hnf1α are nevertheless capable of forming islet structures without a conspicuous decrease of β cell mass or insulin content (11, 14).
Decreased glucose-induced insulin release has also been shown to underlie hnf4α-deficient diabetes (MODY1) (15). The related phenotype of MODY subtypes has been linked to a regulatory hierarchy whereby hnf4α controls the transcription of hnf1α in embryonic liver cells and cultured hepatocyte cell lines (16, 17). Because the diabetogenic defect in MODY lies in β cells, rather than in hepatocytes (10, 15), it is possible that similar interactions between hnf4α and hnf1α occur in β cells, although this has not yet been experimentally addressed.
It is likely that hnf1α- and hnf4α-dependent transcriptional regulation in β cells has distinct properties relative to other tissues, because human heterozygous mutations result in selective defective β cell function, with only minor or no conspicuous abnormalities in other tissues where these genes are expressed (4, 10). Furthermore, we have shown in earlier work (14) that two target promoters, glut2 and pklr, are occupied in vivo by hnf1α in diverse mouse tissues but only require hnf1α for gene activity in islet cells.
In this study, we have identified a unique transcription factor circuit that is controlled by hnf1α specifically in differentiated pancreatic endocrine and exocrine cells. These studies place hnf4α downstream of hnf1α, in sharp contrast to what is known to occur in liver (16), and reveal that hnf1α dependence is mediated through a tissue-specific alternate hnf4α promoter. Hnf1α is also shown to control pancreatic expression of hnf4γ and hnf3γ. Chromatin immunoprecipitations (ChIPs) were used to map transcription factor-promoter interactions in vivo in mouse tissues, providing a partial structure of the regulatory circuit. Finally, the circuit is shown to be switched on as differentiated pancreatic cells arise in embryonic development. The results uncover a regulatory strategy used by hnf1α in pancreatic cells and reveal that pancreatic exocrine and endocrine cells do not only share common cellular precursors, but also a tissue-specific genetic program that is likely to be involved in the control of differentiated cellular functions.
Methods
Animal Breeding and Tissue Isolation.
A colony of hnf1α−/− mice, generated in the laboratory of F. Gonzalez (National Institutes of Health), was established locally and maintained as described (14, 18). Embryos from timed pregnancies were collected and used for pancreas dissection. Mouse hepatocytes, pancreatic islets, and exocrine tissue were isolated as described (14).
RNA Analysis.
RNA extraction and reverse transcription (RT) were performed as described (14). PCR was carried out with oligonucleotides designed to span an intron. Test products were coamplified with an internal control (β-actin, hprt, or tbp). At least two cycle numbers were tested in each experiment, and conditions were adjusted so that both products were in the exponential phase of amplification. Primer sequences and reaction conditions are available on request. All results were verified in at least five control and mutant mice. The 5′ rapid amplification of cDNA ends was performed by using the Marathon kit (CLONTECH), using nested PCR amplification with gene-specific oligonucleotides complementary to HNF4α exon 2 cDNA (5′-GGTCCCCGCAGATGGCACAC-3′ and 5′-CTGTTGGGCGCGTTGAGGTTGGT-3′).
Computer Genome Analysis.
Human genome and trace mouse genome sequences were analyzed with ensembl (http://www.ensembl.org/). Promoter prediction was performed with tssw and nppw programs by using the bcm search launcher (19). Alignment was performed with clustal v (DNAstar, Madison, WI).
Immunohistochemistry.
Immunofluorescence analysis was performed as described (14), using 3-μM sections from paraffin-embedded embryos dissected from embryonic day (E) 13.5, E15.5, E18.5, or 2-week-old mice.
Electrophoretic Mobility-Shift Assays.
The hnf1α-binding site from the human HNF4α P2 promoter was analyzed with oligonucleotides P2 (5′-AGTGACTGGTTACTCTTTAACGTATCCAC-3′) and P2m (5′-AGTGACTGGTTcCTCTTgAACGTATCCAC-3′, mutated bases in small case). 32P-labeled oligonucleotides were incubated for 20 min at 22°C with 5 μg of nuclear extracts from mouse pancreas, isolated islets, hepatocytes, and MIN6 cells or 1 μl of in vitro-synthesized hnf1α in 20 mM Hepes (pH 7.9), 90 mM KCl, 5 mM MgCl2, and 0.05% Nonidet P-40. Synthesis of hnf1α was carried out with a TNT kit (Promega) from full-length mouse hnf1α inserted in EcoRI–SalI sites of pCMVTag2c. For supershifts, 1 μl of rabbit polyclonal serum raised against hnf1α peptides GLIEEPTGDELPTK and EASSEPGLHEPPSPA or preimmune serum was added to the reaction and incubated for 15 min at 22°C before addition of the labeled probe. Samples were electrophoresed on a 5% acrylamide gel in 0.5 × TBE buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3) and autoradiographed.
ChIP.
ChIPs were performed as described (14), except that fixed cells were resuspended in denaturing buffer (2 M NaCl/5 M urea), incubated on ice 10 min, and washed twice in PBS before resuspension in sonication buffer. Multiplex PCR conditions were adjusted to ensure nonsaturation kinetics and similar amplification efficiencies for all amplicons within a reaction. Primers were designed to amplify segments either encompassing hnf1-binding sites and/or located <50 bp from the transcription initiation region of selected genes. Primer sequences and amplification conditions are available on request. Each PCR was performed at least twice with samples resulting from three immunoprecipitation experiments.
Results
Hnf1α Controls Expression of hnf4α Selectively in Pancreatic Cells.
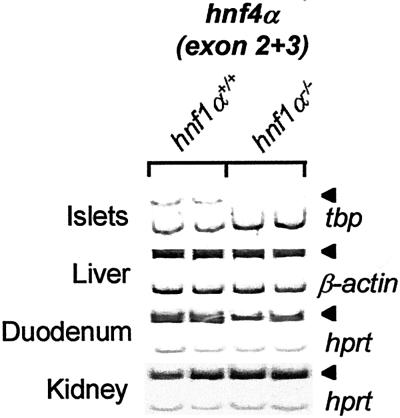
Purified pancreatic islet RNA from 3-wk-old hnf1α−/− and hnf1α+/+ mice was analyzed to search for β cell transcription factor genes downstream of hnf1α. Hnf4α, previously reported to be upstream of hnf1α in an hepatocyte transcriptional regulatory hierarchy (16, 17), exhibited >10-fold decreased mRNA levels in pancreatic islets of hnf1α-deficient mice (Fig. 1). Note nevertheless that low levels of hnf4α mRNA can be elicited in islets of hnf1α−/− mice at high amplification cycles (Fig. 2C). In keeping with previous findings (20), hnf4α mRNA was not affected by loss of hnf1α in liver and kidney, although it was partially inhibited in duodenum (Fig. 1). Immunohistochemical and EMSA analysis of hnf4α using two different C terminus-specific antisera in hnf1α−/− and hnf1α+/+ adult and E15.5 embryonic pancreatic tissue revealed very weak, hnf1α-dependent hnf4α immunoreactivity under conditions that indicated strong expression in hepatocytes and kidney (not shown). Thus, despite genetic evidence for its relevant role in pancreatic islet cells (7, 15), the abundance of hnf4α protein in mouse pancreas is low.
Figure 1.
Pancreatic-specific hnf1α dependence of hnf4α. RT-PCR analysis of hnf4α mRNA (encompassing exons 2 and 3) in purified tissues of hnf1α−/− and hnf1α+/+ mice. β-actin, tbp, or hprt was coamplified as internal control. Arrowheads indicate expected position of hnf4α PCR products.
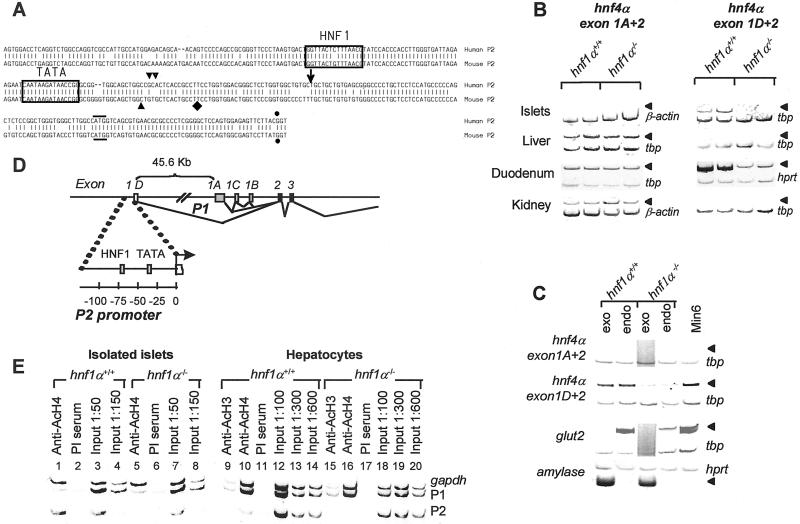
Figure 2.
Pancreatic hnf4α mRNA is transcribed from a tissue-specific alternate promoter. (A) Alignment of human and mouse genomic sequences encoding the 5′ end of pancreatic hnf4α mRNA (exon 1D) and its 5′ flanking region; ↓, 5′ end of human pancreatic islet RNA rapid amplification of cDNA ends product; ▾ and ▿, prediction of transcription initiation by using tssw and nppw programs (19); ⧫, 5′ end of mouse hnf4α7 cDNA (21); and ●, exon-intron boundary. A horizontal line indicates the predicted initiator codon. The hnf1-binding site is boxed. (B) RT-PCR analysis of hnf4α transcripts containing either exon 1A or exon 1D sequences in tissues from hnf1α−/− and hnf1α+/+ mice. Arrowheads indicate expected position of hnf4α PCR products. (C) RT-PCR analysis of hnf4α mRNA in pancreatic endocrine (endo) and exocrine (exo) fractions from hnf1α−/− and hnf1α+/+ mice. Glut2 and amylase mRNA were assayed to indicate tissue purity. (D) Schematic indicating the genomic position of exon 1D and P2 promoter relative to the known (P1) HNF4α promoter. (E) ChIP analysis of acetylated histones H3 and H4 (AcH3 and AcH4), indicating tissue-specific, hnf1α-dependent hyperacetylation of P2 in islets. The gapdh promoter represents a control hyperacetylated chromatin region, which is unaffected by hnf1α. PI, preimmune serum. Input 1:50–600, diluted DNA purified before ChIP to indicate expected results in the absence of enrichment of specific DNA fragments.
Pancreatic hnf4α mRNA Is Transcribed from a Tissue-Specific Alternative Promoter.
One potential mechanism to diversify transcriptional control of a gene in different tissues is the use of alternative promoters. Although only a single hnf4α promoter has been described to date, 5′ rapid amplification of cDNA ends in human pancreatic islets revealed a previously unreported sequence that is 82% identical to the 5′ end of an alternatively spliced mouse cDNA known as hnf4α7 (21). Analysis of human and mouse genome sequences revealed that this alternate 5′ leader sequence constitutes a single conserved exon in both species, which we refer to as exon 1D (Fig. 2A).
Transcripts containing the mouse exon 1D leader sequence were previously shown to be the prevalent species in early embryonic cells, with only low levels detected in several adult tissues (21). Strikingly, most adult mouse islet hnf4α transcripts contain this alternative 5′ mRNA sequence (Fig. 2B). In contrast, hnf4α transcripts in liver or kidney almost exclusively contain the exon 1A leader sequence, whereas both forms are expressed in duodenum. Interestingly, hnf4α transcripts containing exon 1D are present and depend on hnf1α in both endocrine and exocrine pancreatic compartments (Fig. 2C, compare to expression patterns of islet and exocrine-specific markers). Pancreatic hnf4α transcripts containing exon 1D include three possible known 3′ end variants (unpublished results), and therefore constitute not solely hnf4α7 but also an unique combination of known splice variations, which can be referred to as hnf4α8 and hnf4α9, following an existing nomenclature (22).
Based on evidence of an alternative hnf4α mRNA initiation site in mouse and human islets, we refer to the 5′ flanking region of exon 1D as the P2 promoter region. This region is located 45.6 kb upstream of the previously known promoter (P1) and exon 1A (Fig. 2 A and D). Computer analysis of human and mouse genomic sequences using two different algorithms predicted a putative transcription start site in this region (Fig. 2A). In vivo supportive evidence that P2 represents the active hnf4α-promoter region in pancreatic cells was obtained from ChIP analysis of nucleosomal histone acetylation. Acetylation of histone tails is thought to represent a key event in the activation of many eukaryotic genes (23). In previous work (14), we showed that hnf1α-dependent tissue-specific gene activity is tightly linked to the requirement for hnf1α to maintain localized hyperacetylation of nucleosomal histones. As shown in Fig. 2E, nucleosomal histones of the P2 region are hyperacetylated in pancreatic islets (lane 1), whereas P1 histones are hypoacetylated. A similar pattern is seen in MIN6 β cells (data not shown). In contrast, P1 rather than P2 chromatin is hyperacetylated in liver (lanes 9 and 10), in parallel with the observed tissue-specific transcription initiation patterns. P2 nucleosomes in islets of hnf1α-deficient mice are hypoacetylated (lane 5), in keeping with the selective reduction of hnf4α mRNA in islets of hnf1α−/− mice. In aggregate, these results suggest that in vivo P2 chromatin is hyperacetylated and transcriptionally active selectively in pancreatic cells, and this depends on hnf1α.
Hnf1α Occupies the hnf4α P2 Promoter in a Tissue-Specific Manner.
We next investigated whether control of hnf4α by hnf1α in islet cells is direct. A consensus hnf1 site present in the P2 5′ flanking region binds native and in vitro synthesized hnf1α (Figs. 2A and 3A). ChIP analysis of mouse islet chromatin by using anti-hnf1α Abs revealed enrichment of hnf4α P2 chromatin as compared to P1 chromatin, indicating that hnf1α occupies the hnf4α P2 promoter in vivo in pancreatic islets (Fig. 3B, lane 1). In contrast, in liver hnf1α predominantly occupies P1, but not P2 chromatin (Fig. 3B, lane 5). The P1 promoter contains a previously identified high-affinity hnf1-binding site (24), which perhaps underlies dependence on hnf1β observed in early endodermal cells (25). Thus, hnf1α directly occupies P2 chromatin in pancreatic islets, rather than acting exclusively through intermediary factors. Furthermore, occupancy by hnf1α of either P1 or P2 regions in liver or pancreas is tightly linked to regional chromatin acetylation and transcriptional activity status, suggesting that chromatin configuration modulates access of hnf1α to its cognate DNA-binding sites in vivo.
Figure 3.
Hnf1α occupies hnf4α P2 promoter DNA in vitro and in vivo. (A, lanes 1–9) Electrophoretic mobility-shift assay showing interaction of a pancreatic nuclear complex to an oligonucleotide containing the site boxed in Fig. 2A. Binding is blocked by excess (×2–×200) unlabeled probe (P2) but not by an oligonucleotide with two single base substitutions (P2m). Anti-hnf1α Ab but not preimmune (PI) serum supershifts the complex (arrowhead). Analogous results are observed by using synthetic hnf1α (lanes 11–15), whereas no retardation is seen with an in vitro translation reaction performed with a control plasmid (lane 10). Similar results are seen with gluthathione S-transferase hnf1α, purified islet, and mouse hepatocyte nuclear extracts (not shown). (B) ChIP assays indicate that hnf1α occupies the endogenous hnf4α P2 promoter in islets (lane 1, compare to preimmune serum in lane 2 and input DNA), whereas in liver it contacts the P1 promoter (lane 5). Input 1:50–300, diluted input DNA.
Hnf1α Directly Controls hnf4γ and hnf3γ mRNA Specifically in the Pancreas.
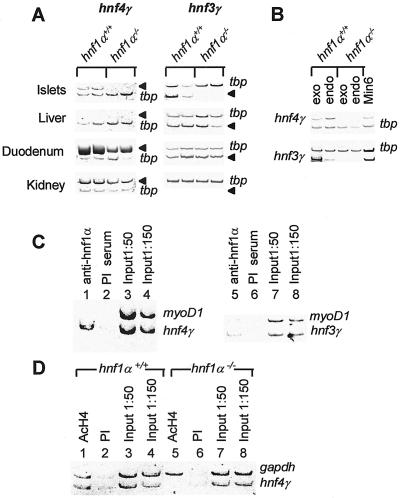
Further analysis by RT-PCR of candidate transcription factors expressed in pancreatic cells revealed an essential role of hnf1α in maintaining the expression of hnf4γ and hnf3γ mRNA (Fig. 4A, and not shown). Hnf4γ is a nuclear receptor structurally related to hnf4α but expressed at only very low levels in liver (26, 27). Hnf4γ mRNA is enriched in islet cells within the pancreas, but is present and exhibits hnf1α dependence in both endocrine and exocrine compartments (Fig. 4B). It also displays partial hnf1α dependence in duodenum, but not in kidney cells (Fig. 4A). The reported mouse 5′ untranslated mRNA region of hnf4γ is readily detected and depends on hnf1α in islets (not shown). Analysis of the mouse genome identified a consensus hnf1- binding site located 120 bp 5′ of the reported murine cDNA sequence, which differs by a single nucleotide from the hnf4α P2 hnf1 site (not shown). As shown in Fig. 4C, hnf1α directly interacts with 5′ flanking hnf4γ chromatin in mouse islets. In liver, where hnf4γ mRNA expression is virtually undetectable by RT-PCR, we did not detect hnf1α association with this promoter region and only trace levels of nucleosomal acetylation (not shown). In contrast, hnf4γ promoter chromatin is hyperacetylated in wild-type islets, where hnf4γ is expressed, but hypoacetylated in hnf1α−/− islets, which do not express hnf4γ mRNA (Fig. 4D).
Figure 4.
Hnf1α dependence of hnf4γ and hnf3γ in pancreatic cells (A) RT-PCR analysis of hnf4γ and hnf3γ mRNA in hnf1α−/− and hnf1α+/+ mice. Arrowheads indicate expected position of hnf PCR products. (B) RT-PCR analysis of hnf4γ and hnf3γ mRNA in pancreatic endocrine and exocrine tissues from hnf1α−/− and hnf1α+/+ mice (see Fig. 2C for more details and tissue-specific markers). (C) ChIP analysis indicating that hnf1α directly occupies the hnf4γ promoter and hnf3γ enhancer in vivo in pancreatic islets. See Figs. 2D and 3B and text for further details. (D) ChIP analysis indicating that islet hnf4γ promoter chromatin is enriched in acetylated H4 and depends on hnf1α function. See also legend of Fig. 2E.
The analysis of the forkhead homolog hnf3γ in hnf1α−/− pancreas was prompted by a previous study identifying an enhancer containing a functional hnf1-binding site in the 3′ region of the gene encoding hnf3γ (28). Hnf3γ mRNA is clearly enriched in the exocrine pancreatic compartment but is also represented in islet and MIN6 β cells (Fig. 4B, see also Fig. 2B). Hnf3γ mRNA exhibits hnf1α dependence in pancreatic cells but not in liver or duodenum where it is also expressed (Fig. 4A). As shown in Fig. 4C, hnf1α likely exerts this effect through occupation of the hnf3γ enhancer.
The Pancreatic hnf1α-Dependent Genetic Program Operates in Differentiated Cells.
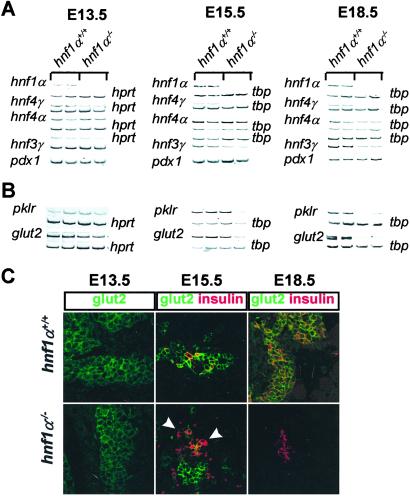
A fundamental question concerning the obligate role of hnf1α in pancreatic β cell transcription lies in determining the time in development at which it is established. Hnf1α is broadly expressed in most cells of the developing pancreas as early as E13.5 (M.A.M. and J.F., unpublished results and Fig. 5A), a stage in which the pancreas is populated primarily by undifferentiated epithelial cells (3). Hnf4α transcripts containing predominantly exon 1D are expressed in E13.5 pancreas, but similar steady-state levels were found in hnf1α−/− and hnf1α+/+ E13.5 embryos. Decreased hnf4α mRNA levels are not elicited in hnf1α−/− embryos until E15.5–E18 (Fig. 5A). Although a moderate reduction of hnf4γ mRNA is already observed in E13.5 hnf1α−/− embryos, this becomes much more pronounced at later stages of development (Fig. 5A). Hnf3γ exhibits a nearly identical pattern of hnf1α dependence as hnf4α (Fig. 5A). The finding that hnf4α, hnf4γ, and hnf3γ mRNA is reduced in hnf1α−/− embryos suggests that (i) the defect is not secondary to the postnatal diabetic environment, and (ii) hnf1α dependence is elicited in parallel with the major surge of differentiated endocrine and exocrine pancreatic cells occurring between E14 and E17 (29, 30).
Figure 5.
Ontogeny of the pancreatic hnf1α-dependent genetic program. (A and B) RT-PCR analysis of dissected pancreatic tissue at indicated embryonic ages reveals that hnf1α dependence of hnf4α (P2), hnf4γ, hnf3γ, glut2, and pklr is established in parallel with the surge of differentiated pancreatic cells at ≈E15. (C) Immunofluorescence analysis of glut2 (green) in timed embryonic pancreas. Loss of glut2 in hnf1α−/− embryos is first elicited at E15.5 in some insulin-positive cells (red) (arrowheads). At E18.5 all insulin-positive cells lack glut2 in hnf1α−/− embryos.
We next assessed whether the loss of intermediary transcription factors during pancreatic development correlates with the establishment of defective expression of two genes, glut2 and pklr, which in adult tissues depend on hnf1α selectively in pancreatic islet cells (14). Although glut2 expression is largely restricted to β cells within the adult pancreas, it is known that early multipotent pancreatic epithelial cells also express glut2 (29). Pancreatic mRNA levels of glut2 and pklr are similar in control and hnf1α−/− E13.5 embryos but then decrease between E15.5 and E18.5 (Fig. 5B). Moreover, glut2 immunostaining is unaltered in hnf1α−/− early pancreatic epithelial cells (Fig. 5C Left). At E15.5, shortly after the major surge of insulin-producing cells, most glut2-positive cells in control mice still represent a precursor pool not expressing endocrine differentiation markers, but all insulin-positive cells exhibit glut2 staining (Fig. 5C Center Upper and data not shown). In E15.5 hnf1α−/− embryos, only some insulin-positive cells express glut2 at varying degrees, whereas many others do not exhibit glut2 immunoreactivity (Fig. 5C Center Lower). From this point on, glut2 expression is lost in a progressively larger fraction of β cells and is uniformly undetectable in hnf1α−/− mice by E18.5 (Fig. 5C Right and data not shown for E16.5). These findings indicate that although hnf1α is coexpressed with glut2 in multiple early pancreatic cell types, it is indispensable for glut2 expression only after insulin-producing cells initiate terminal differentiation, in parallel with the requirement to maintain pancreatic expression of hnf4α, hnf4γ, and hnf3γ.
Discussion
Pancreatic hnf4α mRNA Is Transcribed from a Distant Upstream Promoter That Is Directly Controlled by hnf1α.
Studies performed primarily in hepatocyte lineages have revealed a transcriptional regulatory hierarchy whereby hnf4α controls hnf1α transcription (16, 17). Because mutations in both of these genes result in pancreatic β cell dysfunction (10, 15), it has been inferred that the same regulatory hierarchy is operative in β cells (4). The studies presented here prove that in pancreatic cells hnf1α occupies an alternate hnf4α promoter located >45 kb away from the one previously characterized in liver cells and acts as an obligate factor to induce nucleosomal hyperacetylation and activity of this transcription unit. Moreover, the results indicate that the pancreas represents the sole organ where P2 is the predominant hnf4α promoter throughout embryonic and postnatal development. These findings have several interesting implications. Because this epistatic interaction occurs in the cells that are involved in the pathogenesis of MODY, it is likely to be fundamental to the common insulin secretory phenotype occurring in hnf1α and hnf4α deficiency. The fact that hnf4α is downstream of hnf1α in these cells offers a potential mechanism to bypass the hnf1α block to correct the insulin secretory defects of hnf1α-deficient diabetes. Importantly, attempts to manipulate the expression of hnf4α in pancreatic cells should be directed at a site located >45 kb away from the previously known hnf4α promoter. Furthermore, the finding that hnf4α is transcribed from a distinct promoter in pancreatic cells provides a new candidate sequence to search for variants causing MODY1 diabetes or underlying the chromosome 20q susceptibility region for polygenic type 2 diabetes identified in several genome scans (reviewed in ref. 31).
Hnf1α Controls a Pancreatic-Specific Transcription Factor Circuit.
At least two more transcriptional activators, hnf4γ and hnf3γ, are downstream of hnf1α in pancreatic cells. The precise role of these activators in pancreatic β cells is currently unknown. For hnf3γ, genetic inactivation has revealed no pancreatic phenotype (32). However, the consequences of hnf3γ deficiency in hnf1α−/− islets cannot be predicted from the hnf3γ−/− phenotype if there is functional redundancy between hnf1α-dependent transcriptional regulators. Perhaps most important at this time is the finding that hnf1α orchestrates a broad transcription factor circuit, which is specific for pancreatic cells. Recent data (33) supports the notion that regulation of subsidiary transcription factors may be a modus operandi of hnf1α function. Thus, hnf1α control of cholesterol metabolism enzymes in the liver was shown to be mediated through regulation of a transcriptional hierarchy whereby hnf1α activates the nuclear receptor fxr1 gene, which in turn transactivates shp1, a repressor of cholesterol 7-hydroxylase transcription (33).
The results presented here suggest that the structure of the pancreatic hnf1α-dependent regulatory circuit is complex. ChIP analysis indicates that hnf1α directly occupies the promoter regions of subsidiary transcription factors. In previous work (14), we have shown that hnf1α also directly occupies the promoter regions of nontranscription factor genes (e.g., glut2 and pklr). Based on these findings, we postulate a circuit model whereby rather than a simple lineal hierarchy in which hnf1α acts either as the sole essential activator binding to a distal target promoter, or exclusively in an indirect manner through regulation of a single intermediary activator, transcription of targets such as glut2 and pklr could depend on hnf1α inasmuch as they require direct occupation by both hnf1α and its downstream transcription factors. In support for this proposal, ChIP results using two different hnf4α antisera indicate co-occupation of glut2 and pklr promoter region chromatin by hnf4α along with hnf1α (M.P. and J.F., unpublished observations). Previous data (34) has already indicated binding of hnf4 to the pklr promoter in pancreas. Furthermore, inhibition of hnf4α in cultured tumor β cells results in reduced expression of glut2 and pklr (35), and hnf4α−/− embryoid bodies fail to express glut2 despite only partial reduction of hnf1α mRNA (36). Thus, silencing of glut2 and pklr genes selectively in islet cells of hnf1α−/− mice could result from the aggregate tissue-specific failure of a set of transcriptional regulators required for its expression, including hnf1α, hnf4α, and plausibly others.
The Pancreatic hnf1α-Dependent Genetic Program Is Activated in Differentiated Cells.
The diabetic phenotype in humans and mice with MODY3 typically appears during the second decade of life (37). Similarly, hnf1α−/− mice do not exhibit defective pancreatic organogenesis and develop manifest hyperglycemia only several weeks after birth (11). This does not prove, however, that the requirement for hnf1α in the pancreas is restricted to postnatal cells. The studies shown here reveal that hnf1α dependence of a set of pancreatic genes is activated in early differentiated cells of the embryonic pancreas. The switch between hnf1α-independent to hnf1α-dependent states was elicited most accurately for glut2. Thus, the requirement for hnf1α to express glut2 is observed shortly after insulin-producing cells arise. The findings suggest that hnf1α is either essential to initiate glut2 expression in differentiated β cells, in which case glut2 staining in some early insulin-positive cells reflects a long residence time of proteins activating glut2 in precursor cells or of glut2 itself, or alternatively, hnf1α may be dispensable to initiate glut2 transcription in β cells but required to maintain its expression in differentiated cells.
Thus, a critical function of hnf1α in the pancreas appears to be to deploy a genetic program in cells that have already committed to a differentiated pancreatic fate. Interestingly, the program shares common downstream transcription factors in both endocrine and exocrine pancreatic cell types, but possesses cell-specific distal target readouts presumably determined by additional cell-specific regulatory factors. Although we have not examined the possible physiological role of the circuit in exocrine cells, it appears likely that the ultimate mission in insulin-producing cells is to support the expression of genes like glut2 and pklr involved in highly specialized functions such as glucose sensing. These results allow us to place the role of the hnf1α-dependent transcription circuit in pancreatic embryonic development at a discrete stage subsequent to those known to depend on other transcription factors, including those involved in early pancreatic bud development (e.g., pdx1 or hlxb9), proendocrine commitment within pluripotential pancreatic cells (e.g., ngn3), refinement to a β cell fate (e.g., pax4 or nkx6.1), or early differentiation of β cells (e.g., nkx2.2) (2, 3). This notion reconciles well with knowledge that hnf1α deficiency in mice and humans results in a selective defect of glucose-induced insulin release without gross derangement of postnatal pancreatic histology (11).
Acknowledgments
We are indebted to Frank Gonzalez for the hnf1α−/− mice; Gehardt Ryffel, Cris VanSchravendyck, Bernard Thorens, and Frances Sladeck for antisera, and M. Vallejo for reading a draft version of this manuscript. Amaya Paniagua maintained mouse colonies. This work was supported by an Eli Lilly/European Association for the Study of Diabetes award and grants to J.F. from Ministerio de Ciencia y Tecnología (SAF01–2457), Marató TV3, and the European Commission (QLRT1999–546). M.P. was supported by a contract from Comision Interministerial de Ciencia y Tecnologia.
Abbreviations
- HNF
hepatocyte nuclear factor
- MODY
maturity onset diabetes of the young
- RT-PCR
reverse transcription–PCR
- ChIP
chromatin immunoprecipitation
- E
embryonic day
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 14189.
References
- 1.Deeney J T, Prentki M, Corkey B E. Semin Cell Dev Biol. 2000;11:267–275. doi: 10.1006/scdb.2000.0175. [DOI] [PubMed] [Google Scholar]
- 2.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela C F, Schwitzgebel V, Hayes-Jordan A, German M. Development (Cambridge, UK) 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 3.Edlund H. Curr Opin Cell Biol. 1999;11:663–668. doi: 10.1016/s0955-0674(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 4.Froguel P, Velho G. Trends Endocrinol Metab. 1999;10:142–146. doi: 10.1016/s1043-2760(98)00134-9. [DOI] [PubMed] [Google Scholar]
- 5.Yamagata K, Oda N, Kaisaki P J, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox R D, Lathrop G M, Boriraj V V, et al. Nature (London) 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]
- 6.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn B N, Lindner T, Yamagata K, Ogata M, Tomonaga O, et al. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 7.Yamagata K, Furuta H, Oda N, Kaisaki P J, Menzel S, Cox N J, Fajans S S, Signorini S, Stoffel M, Bell G I. Nature (London) 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 8.Stoffers D A, Ferrer J, Clarke W L, Habener J F. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 9.Frain M, Swart G, Monaci P, Nicosia A, Stampfli S, Frank R, Cortese R. Cell. 1989;59:145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- 10.Byrne M M, Sturis J, Menzel S, Yamagata K, Fajans S S, Dronsfield M J, Bain S C, Hattersley A T, Velho G, Froguel P, et al. Diabetes. 1996;45:1503–1510. doi: 10.2337/diab.45.11.1503. [DOI] [PubMed] [Google Scholar]
- 11.Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick A J, Baldwin A, Velho G, Froguel P, et al. J Clin Invest. 1998;101:2215–2222. doi: 10.1172/JCI2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dukes I D, Sreenan S, Roe M W, Levisetti M, Zhou Y P, Ostrega D, Bell G I, Pontoglio M, Yaniv M, Philipson L, et al. J Biol Chem. 1998;273:24457–24464. doi: 10.1074/jbc.273.38.24457. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Maechler P, Hagenfeldt K A, Wollheim C B. EMBO J. 1998;17:6701–6713. doi: 10.1093/emboj/17.22.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrizas M, Maestro M A, Boj S F, Paniagua A, Casamitjana R, Gomis R, Rivera R, Ferrer J. Mol Cell Biol. 2001;21:3234–3243. doi: 10.1128/MCB.21.9.3234-3243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne M M, Sturis J, Fajans S S, Ortiz F J, Stoltz A, Stoffel M, Smith M J, Bell G I, Halter J B, Polonsky K S. Diabetes. 1995;44:699–704. doi: 10.2337/diab.44.6.699. [DOI] [PubMed] [Google Scholar]
- 16.Kuo C J, Conley P B, Chen L, Sladek F M, Darnell J E J, Crabtree G R. Nature (London) 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Ning G, Duncan S A. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y H, Sauer B, Gonzalez F J. Mol Cell Biol. 1998;18:3059–3068. doi: 10.1128/mcb.18.5.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith R F, Wiese B A, Wojzynski M K, Davison D B, Worley K C. Genome Res. 1996;6:454–462. doi: 10.1101/gr.6.5.454. [DOI] [PubMed] [Google Scholar]
- 20.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach J P, Babinet C, Yaniv M. Cell. 1996;84:575–585. doi: 10.1016/s0092-8674(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 21.Nakhei H, Lingott A, Lemm I, Ryffel G U. Nucleic Acids Res. 1998;26:497–504. doi: 10.1093/nar/26.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sladek F M, Senkel S. In: Nuclear Receptors and Genetic Disease. Burris T P, McCabe E R B, editors. San Diego: Academic; 2001. pp. 309–361. [Google Scholar]
- 23.Struhl K. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 24.Taraviras S, Monaghan A P, Schutz G, Kelsey G. Mech Dev. 1994;48:67–79. doi: 10.1016/0925-4773(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 25.Barbacci E, Reber M, Ott M O, Breillat C, Huetz F, Cereghini S. Development (Cambridge, UK) 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- 26.Drewes T, Senkel S, Holewa B, Ryffel G U. Mol Cell Biol. 1996;16:925–931. doi: 10.1128/mcb.16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taraviras S, Mantamadiotis T, Dong-Si T, Mincheva A, Lichter P, Drewes T, Ryffel G U, Monaghan A P, Schutz G. Biochim Biophys Acta. 2000;1490:21–32. doi: 10.1016/s0167-4781(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 28.Hiemisch H, Schutz G, Kaestner K H. EMBO J. 1997;16:3995–4006. doi: 10.1093/emboj/16.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang K, Mukonoweshuro C, Wong G G. Proc Natl Acad Sci USA. 1994;91:9559–9563. doi: 10.1073/pnas.91.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack J M. Development (Cambridge, UK) 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 31.Permutt M A, Hattersley A T. Trends Endocrinol Metab. 2000;11:383–393. doi: 10.1016/s1043-2760(00)00329-5. [DOI] [PubMed] [Google Scholar]
- 32.Kaestner K H, Hiemisch H, Schutz G. Mol Cell Biol. 1998;18:4245–4251. doi: 10.1128/mcb.18.7.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih D Q, Bussen M, Sehayek E, Ananthanarayanan M, Shneider B L, Suchy F J, Shefer S, Bollileni J S, Gonzalez F J, Breslow J L, et al. Nat Genet. 2001;27:375–382. doi: 10.1038/86871. [DOI] [PubMed] [Google Scholar]
- 34.Miquerol L, Lopez S, Cartier N, Tulliez M, Raymondjean M, Kahn A. J Biol Chem. 1994;269:8944–8951. [PubMed] [Google Scholar]
- 35.Wang H, Maechler P, Antinozzi P A, Hagenfeldt K A, Wollheim C B. J Biol Chem. 2000;275:35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 36.Stoffel M, Duncan S A. Proc Natl Acad Sci USA. 1997;94:13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehto M, Tuomi T, Mahtani M M, Widen E, Forsblom C, Sarelin L, Gullstrom M, Isomaa B, Lehtovirta M, Hyrkko A, et al. J Clin Invest. 1997;99:582–591. doi: 10.1172/JCI119199. [DOI] [PMC free article] [PubMed] [Google Scholar]