Abstract
HIV-1 spreads through both the release of cell-free particles and by cell-to-cell transmission. Mounting evidence indicates that cell-to-cell transmission is more efficient than cell-free transmission of particles and likely influences the pathogenesis of HIV-1 infection. This mode of viral transmission also influences the generation and maintenance of the latent reservoir, which represents the main obstacle for curing the infection. In this review we will discuss general cell contact-dependent mechanisms that HIV-1 utilizes for its spread and the evidence pointing to cell-to-cell transmission as a mechanism for the establishment and maintenance of latent infection.
Keywords: HIV cell-to-cell transmission, HIV latency, Virological synapse, Infectious synapse, Dendritic cells, CD4 T cells, Resting CD4 T cells
Introduction
Animal viruses reproduce mainly through the production and release of cell-free particles for infecting new host cells and for spreading to new individuals. However, cell-free spread exposes viral particles to the challenges of surviving the extracellular environment. In addition, viral particles must survive a number of innate and adaptive immune defenses if the virus is to successfully establish a population in a new animal host. To circumvent these challenges, some viruses have evolved ways of exploiting normal cellular processes to protect progeny particles. An example of such a strategy is transmission across cell-cell contacts. This mode of virus spread, known as virus cell-to-cell transmission, is employed by several enveloped viruses, including HIV-1, and provides important advantages for viral survival, which in turn influences viral pathogenesis.
By concentrating the release of viral particles at the site of cell-cell contact, HIV-1 cell-to-cell transmission increases the efficiency of viral spread several orders of magnitude compared to the dissemination of cell-free particles (Carr et al., 1999; Chen et al., 2007; Dimitrov et al., 1993; Iwami et al., 2015; Martin et al., 2010; Zhong et al., 2013a), it provides protection from neutralizing antibodies (Abela et al., 2012; Chen et al., 2007; Dufloo et al., 2018; Ganesh et al., 2004; Gombos et al., 2015; Gupta et al., 1989; Li et al., 2017; Malbec et al., 2013; Massanella et al., 2009; McCoy et al., 2014; Reh et al., 2015; Zhong et al., 2013a) and it overcomes the inhibitory effects of some anti-viral restriction factors such as tetherin and TRIM5α under certain conditions (Casartelli et al., 2010; Coleman et al., 2011; Giese and Marsh, 2014; Jolly et al., 2010; Kuhl et al., 2010; Richardson et al., 2008; Zhong et al., 2013a). This mode of viral transmission also influences the treatment and pathogenesis of the infection. For example, some anti-retroviral drugs poorly inhibit HIV-1 cell-to-cell transmission when administered as mono-therapies (Agosto et al., 2014; Duncan et al., 2013; Kim et al., 2018; Sigal et al., 2011; Titanji et al., 2013). Another example is that HIV-1 cell-to-cell transmission to lymph node-derived resting CD4+ T cells induces a highly inflammatory form of apoptosis, known as pyroptosis, which has been suggested to contribute to depletion of CD4+ T cells in lymphoid tissues. (Doitsh et al., 2010; Doitsh et al., 2014; Galloway et al., 2015; Monroe et al., 2014; Munoz-Arias et al., 2015). Importantly, HIV-1 cell-to-cell transmission is a route that leads to the establishment of latent infection.
Latent infection in CD4+ T cells represents the main obstacle to the eradication of HIV-1 from infected individuals (Margolis et al., 2016; Mbonye and Karn, 2017; Siliciano and Greene, 2011). The best characterized mechanism for the generation of latent infection is the return of productively infected activated CD4+ T cells to a resting state, a cell state that is restrictive for proviral gene transcription (Han et al., 2007; Pace et al., 2011; Schiralli Lester and Henderson, 2012). However, direct infection of resting CD4+ T cells also results in the generation of latent infection and may play an important role for the generation of latent infection in vivo (Agosto et al., 2007; Cameron et al., 2010; Chan et al., 2016; Lassen et al., 2012; Swiggard et al., 2005; Tabler et al., 2014; Vatakis et al., 2007; Zerbato et al., 2016; Zhang et al., 1999; Zhang et al., 2004). Since cell-to-cell transmission of HIV-1 facilitates efficient infection of CD4+ T cells, several laboratories are investigating how contact with virus-carrying cells supports infection of resting CD4+ T cells and the cellular and molecular mechanisms that are involved in the generation of proviral latency in this context (Agosto et al., 2018; Evans et al., 2013; Kumar et al., 2015; Schilthuis et al., 2018; Shen et al., 2013). Cell signaling mediated by cell-cell contacts and cytokine release, and the transfer of large numbers of particles to target CD4+ T cells, could have profound effects on the establishment of latent infection and will likely impact the design of therapeutic approaches that target the latent reservoir. How these mechanisms mediate HIV-1 cell-to-cell transmission and their influence on the generation of latent infection in resting CD4+ T cells are critical questions that need to be addressed.
Mechanisms of cell-to-cell transmission
Several modes of cell-to-cell transmission have been described for HIV-1 (Bracq et al., 2018; Chen, 2012; Sattentau, 2008; Zhong et al., 2013b). The best described of these utilize direct cell-cell contacts that resemble the immunological synapse (IS) and are known as infectious or virological synapses (Figure 1). Similar to the IS, cell-cell contacts involved in viral transmission result in signal transduction and biological changes in both the virus-donor and the virus-target cells, which in turn influence viral spread and pathogenesis.
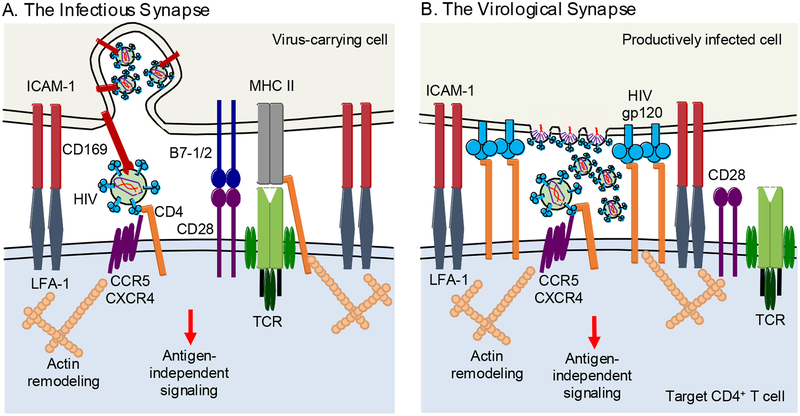
Figure 1. Cell-cell synapse-dependent transmission of HIV-1.
A. The infectious synapse. HIV-1 is captured by cell surface molecules such as CD169 (SIGLEC-1) and sequestered as intact particles in non-lysosomal compartments. Upon cell-cell contact and attachment via LFA-1 and ICAM-1, bound virus is brought to the site of contact where it is brought into close proximity with CD4, CXCR4 and CCR5 on the uninfected target CD4+ T cell, facilitating efficient transmission of virus. B. The virological synapse. A productively infected donor cell establishes contact with an uninfected CD4+ T cell in a gp120-CD4-dependent manner. The interaction is strengthened by binding of the attachment proteins LFA-1 and ICAM-1, and the HIV-1 co-receptors CCR5 and CXCR4 are trafficked to the site. Polarization of the infected donor cell towards the target cell results in the directed release of de novo viral particles across the synapse towards the uninfected target cell. Both forms of cell-to-cell transmission generate antigen-independent cell signaling likely impacting the outcome of HIV-1 infection in the target CD4+ T cell.
HIV-1 Infectious Synapses
The infectious synapse is formed when HIV-1 is captured by a cell without itself becoming infected and the virus-carrying cell subsequently directs the intact particles to a target cell during cell-cell contact (Kijewski and Gummuluru, 2015; McDonald, 2010; McDonald et al., 2003). This mechanism, also known as HIV-1 trans-infection, is typically associated with viral transmission from myeloid antigen-presenting cells (APCs) to CD4+ T cells, such as macrophages and dendritic cells (DCs), but can occur between other cell types as well. For example, mucosal fibroblasts and mammary epithelial cells have been suggested to capture and transmit HIV-1 to CD4+ T cells in a trans-infection-like manner (Dorosko and Connor, 2010; Neidleman et al., 2017). HIV-1 has evolved to take advantage of the normal cell-cell contacts and immune functions of myeloid APCs for this mechanism of dissemination.
HIV-1 is captured by APC surface molecules, such as the C-type lectin SIGLEC-1, and retained within non-lysosomal compartments where it avoids degradation and antibody neutralization (Gummuluru et al., 2014; Izquierdo-Useros et al., 2012; Puryear et al., 2013; Puryear et al., 2012; Waki and Freed, 2010). The infectious synapse is formed following initial interactions between virus-carrying APCs and target cells mediated by the attachment proteins ICAM-1 and LFA-1 (Rodriguez-Plata et al., 2013; Sanders et al., 2002). Following attachment, the APC recruits a variety of molecules to the site of contact including those found in an IS, as well as HIV-1 bound to SIGLEC-1 (Akiyama et al., 2015). Meanwhile in the CD4+ T cell, a complimentary process is occurring with the reorganization of the local cytoskeleton, and a recruitment of a variety of molecules to the site of contact including CD4, CXCR4 and CCR5, the receptors required by HIV-1 for entry. This process is thought to benefit HIV-1 by concentrating particles and receptors in a discrete area of close contact. The generation of a synaptic structure demonstrates how HIV-1 is capable of co-opting the normal APC function to enable viral dissemination.
HIV-1 Virological Synapses
HIV-1 virological synapses depend on the interaction between HIV-1 envelope on infected donor cells and CD4 on uninfected target cells (Chen et al., 2007; Jolly et al., 2004; Vasiliver-Shamis et al., 2009; Vasiliver-Shamis et al., 2008) (Figure 1). These cell-cell contacts can take place between adjacent cells or over relatively long distances such as in the case of filopodia (Sherer et al., 2007; Sowinski et al., 2008). These cell-cell contacts initiate signaling cascades in both the HIV-1 producer cells and the uninfected target cells (Len et al., 2017). In uninfected target cells, this interaction leads to the recruitment of LFA-1 to the site of cell-cell contact and it interacts with ICAM-1 on the HIV-1-producer cell, which strengthens the cell-cell junction (Jolly et al., 2007; Rudnicka et al., 2009; Starling and Jolly, 2016). Additional cell membrane molecules such as GM1 and other lipid raft-associated molecules (Jolly and Sattentau, 2005), and tetraspannins (Jolly and Sattentau, 2007; Krementsov et al., 2009) to the synaptic junction in an actin- and filamin-A-dependent fashion (Agosto et al., 2013; Jimenez-Baranda et al., 2007; Jolly et al., 2004). This interaction results in the polarization of HIV-1 assembly, and transit of CD4, CXCR4 and CCR5 to the site of cell-cell contact. This re-organization of viral assembly results in the delivery of many particles to target cells effectively increasing the probability of target cell infection with multiple viruses (Del Portillo et al., 2011; Law et al., 2016; Russell et al., 2013; Zhong et al., 2013a).
Synapse-independent cell-cell transmission
Alternative modes of HIV-1 cell-to-cell transmission are being investigated and each may have its own repercussions on the spread and pathogenesis of the virus. While these observations are certainly intriguing, further validation, a clearer understanding of the molecular mechanisms involved and additional evidence of their impact on viral spread in vivo are required.
Phagocytosis.
Work from the Sattentau laboratory proposes that macrophages phagocytosing dying HIV-1-infected CD4+ T cells subsequently become infected (Baxter et al., 2014). Since phagocytosis of infected cells occurs in an HIV-1 envelope-CD4-independent manner, infection of the macrophage is unlikely to result from virological synapse formation. Further work will reveal the precise mechanism for infection of the macrophage during phagocytosis.
Syncytia.
Syncytium formation was one of the earliest observations of HIV-1 infection of cells in culture, and occurs as a consequence of HIV-1-gp120 on infected cells engaging CD4 on uninfected target cells resulting in the fusion of the two cell membranes (Bracq et al., 2018; Lifson et al., 1986). However, the relevance of this mechanism for the pathogenesis of HIV-1 in vivo is less clear. Recent evidence conducted in humanized mice and 3D cultures suggest that multi-nucleated cells resulting from HIV-1-mediated cell-cell fusion are viable and may contribute to the spread of HIV-1 (Bracq et al., 2017; Compton and Schwartz, 2017; Law et al., 2016; Murooka et al., 2012; Symeonides et al., 2015).
Tunneling nanotubes.
Long distance cell-cell connections, such as tunneling nanotubes, have been described for some myeloid cells and T cells. These thin cell-cell junctions have been suggested to mediate cell-cell communication in the form of cytoplasmic and plasma membrane components, vesicles, endosomes and some organelles (Buszczak et al., 2016). These structures were originally suggested to enable the transfer of extracellular viral particles between cells (Sowinski et al., 2008), but nanotubes generated by macrophages have also been proposed to allow the transfer of intracellular infectious particles contained within endosomes (Kadiu and Gendelman, 2011a; Kadiu and Gendelman, 2011b).
Transcytosis.
Mucosal epithelial cells likely play an important role during sexual transmission of HIV-1 (Anderson, 2014). These cells are capable of internalizing viral particles into vesicles at the apical surface, transport the vesicles to the basal layer and transmit the particles to CD4+ T cells in a process known as transcytosis (Bomsel, 1997; Kinlock et al., 2014). Transcytosis naturally mediates the transport of large molecules across epithelial barriers such as some vitamins and immunoglobulins (Tuma and Hubbard, 2003). Transcytosis of HIV-1 transport provides a mechanism for viral particles to cross the epithelial barrier and reach CD4+ T cells during mucosal transmission.
HIV-1 cell-to-cell transmission involves cell signaling
It has been hypothesized that synapses facilitate HIV-1 infection by activating cell signaling cascades similar to the IS. IS formation leads to the activation of signaling molecules downstream of the T cell receptor (TCR) such as Lck, CD3ζ, ZAP70, LAT, SLP-76, ITK, and PLCγ in response to strong antigen-dependent MHC/TCR and B7/CD28 interactions between APCs and CD4+ T cells (Dustin and Choudhuri, 2016; Smith-Garvin et al., 2009). These signaling cascades results in the activation of a series of transcription factors, most notably AP-1, NFAT and NFκB, which lead to the regulation of effector molecules, such as cytokines and chemokines, and the stimulation of cell division. Infectious and virological synapse formation stimulates similar signaling cascades with the important exception that the signaling cascades are antigen-independent (Deng et al., 2016; Hioe et al., 2011; Len et al., 2017; Readinger et al., 2008; Schiralli Lester et al., 2013; Sol-Foulon et al., 2007; Strasner et al., 2008; Vasiliver-Shamis et al., 2009). The strength and outcome of the signaling cascades stimulated during infectious and virological synapse formation on HIV-1 replication is less clear, but recent work by the Jolly laboratory suggests that virological synapses between T cells generate TCR/CD28 signaling cascades in an antigen-independent fashion and that these cascades are important for efficient viral spread (Len et al., 2017). Len, et al. conducted phosphoproteomic analysis of T cells during HIV-1 cell-to-cell transmission and confirmed the activation of signaling molecules and transcriptional regulators downstream of TCR/CD28 including Lck, ITK, MAP kinases, NFκB and NFAT. Pathways involved in actin reorganization, Rac1 signaling and Cdc42 signaling were also observed to be activated. Interestingly, these signaling cascades took place in both the HIV-1-infected T cells and the uninfected target T cells. These studies suggests that TCR signaling cascades appear to be particularly important in HIV-1-infected T cells as cells lacking TCR and downstream signaling molecules are less efficient at generating virological synapses with uninfected cells resulting in less efficient viral transmission. How signal transduction during cell-to-cell transmission influences target cell transcriptional regulation and how changes in transcriptional regulation affect subsequent HIV-1 replication also remains unclear. The activation of AP-1, NFκB, NFAT and other transcription factors are well known to facilitate HIV-1 transcription and replication (Kaczmarek et al., 2013; Karn and Stoltzfus, 2012). Given that infectious and virological synapse formation activate these transcription factors in target cells, it seems probable that signals transduced during cell-cell contact could contribute to transcriptional changes in the target cells that in turn increase susceptibility to infection, have repercussions on cell behavior, and impact anti-viral immune responses and pathogenesis. For example, ITK-mediated signaling influences multiple steps of HIV-1 replication including proviral transcription and release of HIV-1 likely through the activity of AP-1, NFκB, and NFAT (Readinger et al., 2008; Schiralli Lester et al., 2013). Another potential contribution of cell signaling during HIV-1 cell-to-cell transmission is through the generation of latent infection.
Cell-to-cell transmission and the establishment of the latent HIV-1 reservoir
The study of HIV-1 cell-to-cell transmission has largely focused on investigating its contribution to viral spread. However, several laboratories, including our own, have begun investigating whether this process influences the establishment and maintenance of latent infection (Agosto et al., 2018; Evans et al., 2013; Kumar et al., 2015). Latent infection is largely found among resting memory CD4+ T cells in vivo (Brenchley et al., 2004; Chomont et al., 2009; Chun et al., 1997; Cohn et al., 2018; Maldarelli et al., 2014; Ostrowski et al., 1999; Reeves et al., 2018; Wagner et al., 2014). These latently infected resting cells do not support efficient viral production due to the absence of significant levels of positive transcriptional regulators, the presence of transcriptional repressors, and repressive epigenetic modifications (Agosto et al., 2015; Karn and Stoltzfus, 2012; Siliciano and Greene, 2011). The main mechanism for the formation the latent reservoir in resting CD4+ T cells remains unclear, but one mechanism is through direct infection of resting cells despite the relative resistance of these cells to HIV-1 compared to activated CD4+ T cells (Agosto and Henderson, 2018). “Gentle” stimulation such as from the chemokines CCL19 and CCL21, and the cytokines IL-4, IL-7 have been suggested to increase the susceptibility of resting CD4+ T cells to direct infection with HIV-1 without the induction of significant T cell activation (Cameron et al., 2010; Chan et al., 2016; Dardalhon et al., 2001; Moutsopoulos et al., 2006; Saleh et al., 2007; Unutmaz et al., 1999; Verhoeyen et al., 2003).
Cell-cell contacts between infected and uninfected cells, in combination with cytokine release by the cells involved, could influence the susceptibility of a target resting CD4+ T cell to HIV-1 infection and influence the generation of latent infection. Some of the initial evidence that implicated cell-cell contact for enhancing the susceptibility of resting CD4+ T cells to infection came from experiments conducted with endothelial cells (Choi et al., 2005a; Choi et al., 2005b). These experiments showed that infecting resting cells in co-culture with endothelial cells improved the susceptibility of resting CD4+ T cells and subsequent replication of HIV-1. This process was dependent on signaling through MHCII-TCR and LFA3-CD2 interactions. A prior study conducted using macrophage-resting T cell co-cultures also showed an enhancement of the susceptibility of resting cells to HIV-1 infection, but the study proposed a cell-contact independent mechanism for the increased susceptibility of resting cells including the secretion of soluble CD23 and soluble ICAM (Swingler et al., 2003). Evidence that endothelial cells not only increase the susceptibility of resting CD4+ T cells to HIV-1 infection but also mediate the establishment of latent infection was reported more recently. Two studies by Shen, et al. revealed that a proportion of resting CD4+ T cells infected in co-cultures with lymphatic endothelial cells become latently infected and implicated the secretion of IL-6 by endothelial cells as a contributing factor for increasing the susceptibility of resting cells to infection (Schilthuis et al., 2018; Shen et al., 2013).
Additional evidence for the generation of latent infection in resting cells through cell-cell contact came from experiments conducted in APC-T cell co-cultures (Evans et al., 2013; Kumar et al., 2015). Evans, et al. observed that these cell-cell interactions during infection of resting CD4+ T cells led to the upregulation of factors involved in interferon responses such as ISG15 and RIG-I and the negative regulation of the NFκB pathway through down-regulation of PRKCA. They also observed upregulation of KLF6, a factor involved in T cell quiescence and ATF3, a factor that negatively regulates AP-1. Altogether, cell-cell contacts between APCs and resting CD4+ T cells during HIV-1 infection appear to increase the susceptibility of resting CD4+ T cells to HIV-1 infection while simultaneously restrict viral replication and promote latent infection. Interestingly, these experiments showed that not all APCs are capable of generating latent infection to the same extent. The studies found that CD14+ monocytes and myeloid DC subsets were all able to generate latent infection in resting cells while B cells, CD16+ monocytes and plasmacytoid DCs were less efficient. They also found that those APCs able to generate latent infection in resting cells shared a common expression of certain cell surface molecules such as ICAM-3, CLEC-7A, SIGLEC-10 and CD1d. Although studies conducted with endothelial cell and APC co-cultures implicate cell-cell contacts in the establishment of latent infection in resting CD4+ T cells, it was still unclear whether the process of HIV-1 cell-to-cell transmission across infectious or virological synapses contributed to the infection of target resting cells.
Our laboratory investigated HIV-1 cell-to-cell transmission across virological synapses from productively infected activated CD4+ T cells to resting CD4+ T cells and found that this process leads to the generation of latently infected cells (Agosto et al., 2018). These cell-cell interactions do not induce significant target T cell activation similar to cell-cell contacts with endothelial cells (Schilthuis et al., 2018; Shen et al., 2013), but in contrast to cell-cell contacts with APCs (Evans et al., 2013; Kumar et al., 2015). Interestingly, while a proportion of latent proviruses generated through HIV-1 cell-to-cell transmission were inducible to produce HIV-1 Gag, this proportion was smaller compared to proviruses generated by cell-free infection of resting CD4+ T cells. This observation suggests that HIV-1 cell-to-cell transmission influences the subsequent regulation and maintenance of latent infection.
Outcomes of cell signaling during cell-to-cell transmission on the maintenance and regulation of latent infection
Our laboratory has preliminary evidence suggesting that the strength of activating signal through the TCR plays a major role in biasing the infected cell towards either a transcriptionally latent or transcriptionally active state. The strength of signaling through the signaling domains of CD3 and CD28 at the time of HIV-1 infection directly impacts the generation of latent infection. We found that strong signaling at the time of HIV-1 infection leads to more HIV-1 expression compared to weaker signaling despite similar levels of HIV-1 integration. Furthermore, we observed that latent proviruses generated during stronger signaling were reversible compared to latent proviruses generated during weak signals suggesting that weaker signaling during HIV-1 infection promotes deep seated latent infection. It is possible that a similar principle is at play during HIV-1 cell-to-cell transmission (Gagne, et al. unpublished observations).
In preliminary experiments using APCs as HIV-1 carriers, we find that DC-mediated infection of T cells appears to reduce the signaling threshold required for generating reversible latent infection. This lowering of the signaling threshold is likely due to other signals provided by the DC in addition to signaling associated with the TCR. From this, we might hypothesize that infection mediated by APCs, such as DCs, would be more likely to establish reversible latent infection (Pedro, et al. unpublished observations). This hypothesis is consistent with the observations by Evans, et al. that latent infection generated in DC-T cell co-cultures stimulate interferon-dependent signaling cascades while simultaneously stimulating responses that promote cell quiescence (Evans et al., 2013). T cell-T cell co-cultures appear have the opposite effect compared to APC-T cell co-cultures (Agosto et al., 2018). We found that latent infection generated after transmission of HIV-1 from activated T cells to resting cells was more difficult to reverse compared to latent infection generated by cell-free infection of resting cells as determined by HIV-1 protein expression relative to the level of HIV-1 integration per cell. The mechanism behind this observation is unknown, but we hypothesize that cell-to-cell transmission between T cells generates weaker cell signaling compared to APC-T cell synapses and thus promotes deeper seated latent infection. This observation suggests that the mechanism through which latent infection is generated in resting CD4+ T cells will influence its potential to be reversed (Figure 2) (Kumar et al., 2018; Rezaei et al., 2018). The ease to reverse latent infection generated by cell-to-cell transmission could be dependent on the specific signaling cascades activated during cell-cell contact and to unique transcriptional programs generated in target cells. Further investigation of the signaling cascades generated during HIV-1 cell-to-cell transmission and the transcriptional changes created in target resting cells will likely reveal novel factors involved in the generation and maintenance of latent infection.
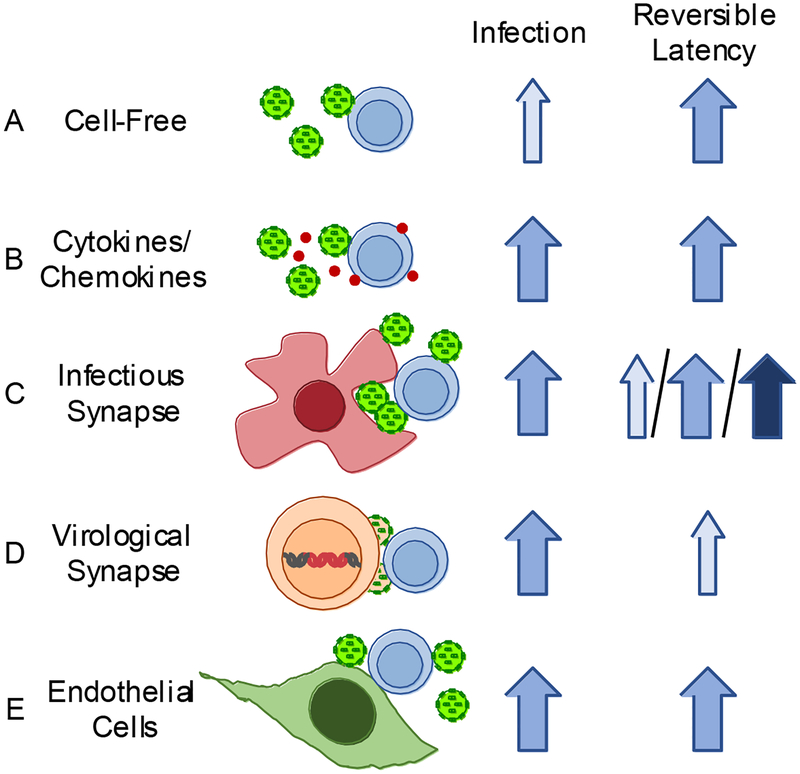
Figure 2. The efficiency of establishing and reversing latent infection is dependent on the mechanism of resting CD4+ T cell infection.
Various modes of HIV-1 infection of resting CD4+ T cells are depicted with their corresponding efficiency for establishing infection and reversible latent infection. Although no study has compared all of these mechanisms side-by-side, we estimated infection and latency reversal efficiency qualitatively based on published observations. Light blue arrows represent lower relative levels of HIV-1 infection or latency reversion and dark blue arrows correspond to higher relative levels. A. Cell-free infection results in lower relative levels of infection (light blue arrow) but the establishment of a moderately inducible latent population (medium blue)(Agosto et al., 2018; Lassen et al., 2012; Swiggard et al., 2005). B. Cell-free infections aided by chemokines, such as CCL19 and CCL21, result in increased resting cell susceptibility to infection compared to infections without added chemokines and a moderately inducible latent population (Cameron et al., 2010; Kumar et al., 2018; Saleh et al., 2007). C. An uninfected HIV-1-carrying cell contacts an uninfected CD4+ T cell. Virus is transmitted in trans via the infectious synapse. This mode of transmission promotes latent infection of resting CD4+ T cells. However, the reversion of latent infection varies widely depending on the HIV-1-transmitting cell type, indicated by the light, medium and dark blue arrows (Kumar et al., 2015). The efficiency of latency reversion also depends whether or not the target cells are proliferating during infection in APC co-cultures (Kumar et al., 2018). D. An infected donor cell establishes contact with an uninfected donor cell and transmits HIV-1 via a virological synapse. This is an efficient method of transmission and results in moderate levels of resting cell infection. However, although latent infection can be detected in target cells by HIV-1 DNA, viral re-expression is not readily induced (Agosto et al., 2018). E. The susceptibility of resting CD4+ T cells to HIV-1 infection increases when in contact with endothelial cells and enables the establishment inducible latent infection (Choi et al., 2005a; Choi et al., 2005b; Schilthuis et al., 2018; Shen et al., 2013).
Concluding remarks
Virus cell-to-cell transmission provides several advantages for the spread and survival of HIV-1. Active research is underway to determine the degree to which this mode of viral transmission contributes to the spread and pathogenesis of HIV-1 in vivo and the generation and maintenance of latent reservoirs. Continued examination of HIV-1 cell-to-cell transmission is critical for a full understanding of the latent reservoir and the discovery of novel therapeutic targets for improved treatment efficacy and prognosis of people living with HIV-1 infection.
Highlights.
HIV-1 utilizes several forms of cell-to-cell transmission for viral dissemination, a process that influences viral survival and pathogenesis.
HIV-1 cell-to-cell transmission mediates the generation of latent infection in CD4+ T cells.
HIV-1 cell-to-cell transmission affects the regulation of HIV-1 latency posing an additional challenge for the development of curative therapies.
Acknowledgements
This work was supported by the NIH-NIAID (AI138960 and AI097117) and the Providence/Boston Center for AIDS Research (P30-AI042853).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A, 2012. Cell-Cell Transmission Enables HIV-1 to Evade Inhibition by Potent CD4bs Directed Antibodies. PLoS Pathog. 8(4), e1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Gagne M, Henderson AJ, 2015. Impact of Chromatin on HIV Replication. Genes 6(4), 957–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Henderson AJ, 2018. CD4(+) T Cell Subsets and Pathways to HIV Latency. AIDS Res. Hum. Retrovir 34(9), 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Herring MB, Mothes W, Henderson AJ, 2018. HIV-1-Infected CD4+ T Cells Facilitate Latent Infection of Resting CD4+ T Cells through Cell-Cell Contact. Cell Rep. 24(8), 2088–2100. [DOI] [PubMed] [Google Scholar]
- Agosto LM, Yu JJ, Dai J, Kaletsky R, Monie D, O’Doherty U, 2007. HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology 368(1), 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Zhong P, Mothes W, 2013. Cell-to-Cell Transmission of HIV In: Freed EO (Ed.), Advances in HIV-1 assembly and release. Springer International Publishing, New York, Heidelberg, Dordrecht, London, pp. 167–184. [Google Scholar]
- Agosto LM, Zhong P, Munro J, Mothes W, 2014. Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission. PLoS Pathog. 10(2), e1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S, 2015. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 11(3), e1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, 2014. Modeling mucosal cell-associated HIV type 1 transmission in vitro. J. Infect. Dis 210(Suppl 3), S648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AE, Russell RA, Duncan CJ, Moore MD, Willberg CB, Pablos JL, Finzi A, Kaufmann DE, Ochsenbauer C, Kappes JC, Groot F, Sattentau QJ, 2014. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 16(6), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med 3(1), 42–47. [DOI] [PubMed] [Google Scholar]
- Bracq L, Xie M, Benichou S, Bouchet J, 2018. Mechanisms for Cell-to-Cell Transmission of HIV-1. Front. Immunol 9, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracq L, Xie M, Lambele M, Vu LT, Matz J, Schmitt A, Delon J, Zhou P, Randriamampita C, Bouchet J, Benichou S, 2017. T cell-macrophage fusion triggers multinucleated giant cell formation for HIV-1 spreading. J. Virol 91(24), e01237–01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA, 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol 78(3), 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Inaba M, Yamashita YM, 2016. Signaling by Cellular Protrusions: Keeping the Conversation Private. Trends Cell Biol. 26(7), 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly RP, Harman AN, Anderson JL, Jones KL, Mak J, Cunningham AL, Jaworowski A, Lewin SR, 2010. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc. Natl. Acad. Sci. U. S. A 107(39), 16934–16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JM, Hocking H, Li P, Burrell CJ, 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265(2), 319–329. [DOI] [PubMed] [Google Scholar]
- Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin AG, Guatelli J, Schwartz O, 2010. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 6(6), e1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CN, Trinite B, Lee CS, Mahajan S, Anand A, Wodarz D, Sabbaj S, Bansal A, Goepfert PA, Levy DN, 2016. HIV-1 latency and virus production from unintegrated genomes following direct infection of resting CD4 T cells. Retrovirology 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, 2012. T cell virological synapses and HIV-1 pathogenesis. Immunol. Res 54(1–3), 133–139. [DOI] [PubMed] [Google Scholar]
- Chen P, Hübner W, Spinelli MA, Chen BK, 2007. Predominant Mode of Human Immunodeficiency Virus Transfer between T Cells Is Mediated by Sustained Env-Dependent Neutralization-Resistant Virological Synapses. J. Virol 81(22), 12582–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Walker J, Boichuk S, Kirkiles-Smith N, Torpey N, Pober JS, Alexander L, 2005a. Human endothelial cells enhance human immunodeficiency virus type 1 replication in CD4+ T cells in a Nef-dependent manner in vitro and in vivo. J. Virol 79(1), 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Walker J, Talbert-Slagle K, Wright P, Pober JS, Alexander L, 2005b. Endothelial cells promote human immunodeficiency virus replication in nondividing memory T cells via Nef-, Vpr-, and T-cell receptor-dependent activation of NFAT. J. Virol 79(17), 11194–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP, 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med 15(8), 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo Y-H, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF, 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387(6629), 183–188. [DOI] [PubMed] [Google Scholar]
- Cohn LB, da Silva IT, Valieris R, Huang AS, Lorenzi JCC, Cohen YZ, Pai JA, Butler AL, Caskey M, Jankovic M, Nussenzweig MC, 2018. Clonal CD4(+) T cells in the HIV-1 latent reservoir display a distinct gene profile upon reactivation. Nat. Med 24(5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Spearman P, Wu L, 2011. Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef. Retrovirology 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton AA, Schwartz O, 2017. They Might Be Giants: Does Syncytium Formation Sink or Spread HIV Infection? PLoS Pathog. 13(2), e1006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Jaleco S, Kinet S, Herpers B, Steinberg M, Ferrand C, Froger D, Leveau C, Tiberghien P, Charneau P, Noraz N, Taylor N, 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A 98(16), 9277–9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK, 2011. Multiploid inheritance of HIV-1 during cell-to-cell infection. J. Virol 85(14), 7169–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Mitsuki YY, Shen G, Ray JC, Cicala C, Arthos J, Dustin ML, Hioe CE, 2016. HIV Envelope gp120 Alters T Cell Receptor Mobilization in the Immunological Synapse of Uninfected CD4 T Cells and Augments T Cell Activation. J. Virol 90(23), 10513–10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA, 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol 67(4), 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC, 2010. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143(5), 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC, 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505(7484), 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosko SM, Connor RI, 2010. Primary human mammary epithelial cells endocytose HIV-1 and facilitate viral infection of CD4+ T lymphocytes. J. Virol 84(20), 10533–10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufloo J, Bruel T, Schwartz O, 2018. HIV-1 cell-to-cell transmission and broadly neutralizing antibodies. Retrovirology 15(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CJ, Russell RA, Sattentau QJ, 2013. High multiplicity HIV-1 cell-to-cell transmission from macrophages to CD4+ T cells limits antiretroviral efficacy. AIDS 27(14), 2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Choudhuri K, 2016. Signaling and Polarized Communication Across the T Cell Immunological Synapse. Annu. Rev. Cell Dev. Biol 32, 303–325. [DOI] [PubMed] [Google Scholar]
- Evans VA, Kumar N, Filali A, Procopio FA, Yegorov O, Goulet JP, Saleh S, Haddad EK, da Fonseca Pereira C, Ellenberg PC, Sekaly RP, Cameron PU, Lewin SR, 2013. Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells. PLoS Pathog. 9(12), e1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC, 2015. Cell-to-Cell Transmission of HIV-1 Is Required to Trigger Pyroptotic Death of Lymphoid-Tissue-Derived CD4 T Cells. Cell Rep. 12(10), 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh L, Leung K, Lore K, Levin R, Panet A, Schwartz O, Koup RA, Nabel GJ, 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol 78(21), 11980–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese S, Marsh M, 2014. Tetherin can restrict cell-free and cell-cell transmission of HIV from primary macrophages to T cells. PLoS Pathog. 10(7), e1004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos RB, Kolodkin-Gal D, Eslamizar L, Owuor JO, Mazzola E, Gonzalez AM, Korioth-Schmitz B, Gelman RS, Montefiori DC, Haynes BF, Schmitz JE, 2015. Inhibitory Effect of Individual or Combinations of Broadly Neutralizing Antibodies and Antiviral Reagents against Cell-Free and Cell-to-Cell HIV-1 Transmission. J. Virol 89(15), 7813–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummuluru S, Pina Ramirez NG, Akiyama H, 2014. CD169-dependent cell-associated HIV-1 transmission: a driver of virus dissemination. J. Infect. Dis 210(Suppl 3), S641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Balachandran R, Ho M, Enrico A, Rinaldo C, 1989. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J. Virol 63(5), 2361–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wind-Rotolo M, Yang HC, Siliciano JD, Siliciano RF, 2007. Experimental approaches to the study of HIV-1 latency. Nat. Rev. Microbiol 5(2), 95–106. [DOI] [PubMed] [Google Scholar]
- Hioe CE, Tuen M, Vasiliver-Shamis G, Alvarez Y, Prins KC, Banerjee S, Nadas A, Cho MW, Dustin ML, Kachlany SC, 2011. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA). PLoS One 6(8), e23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami S, Takeuchi JS, Nakaoka S, Mammano F, Clavel F, Inaba H, Kobayashi T, Misawa N, Aihara K, Koyanagi Y, Sato K, 2015. Cell-to-cell infection by HIV contributes over half of virus infection. eLife 4, e08150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J, 2012. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 10(12), e1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, Martinez AC, Manes S, 2007. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat. Cell Biol 9(7), 838–846. [DOI] [PubMed] [Google Scholar]
- Jolly C, Booth NJ, Neil SJ, 2010. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol 84(23), 12185–12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ, 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med 199(2), 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ, 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol 81(24), 13916–13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Sattentau QJ, 2005. Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J. Virol 79(18), 12088–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Sattentau QJ, 2007. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J. Virol 81(15), 7873–7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek K, Morales A, Henderson AJ, 2013. T Cell Transcription Factors and Their Impact on HIV Expression. Virology (Auckl) 2013(4), 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Gendelman HE, 2011a. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J. Neuroimmune Pharmacol 6(4), 658–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Gendelman HE, 2011b. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J. Proteome Res 10(7), 3225–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J, Stoltzfus CM, 2012. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb. Perspect. Med 2(2), a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijewski SD, Gummuluru S, 2015. A mechanistic overview of dendritic cell-mediated HIV-1 trans infection: the story so far. Future Virol. 10(3), 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JT, Chang E, Sigal A, Baltimore D, 2018. Dendritic cells efficiently transmit HIV to T Cells in a tenofovir and raltegravir insensitive manner. PLoS One 13(1), e0189945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock BL, Wang Y, Turner TM, Wang C, Liu B, 2014. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PLoS One 9(5), e96760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov DN, Weng J, Lambele M, Roy NH, Thali M, 2009. Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BD, Sloan RD, Donahue DA, Bar-Magen T, Liang C, Wainberg MA, 2010. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology 7, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NA, Cheong K, Powell DR, da Fonseca Pereira C, Anderson J, Evans VA, Lewin SR, Cameron PU, 2015. The role of antigen presenting cells in the induction of HIV-1 latency in resting CD4(+) T-cells. Retrovirology 12, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NA, van der Sluis RM, Mota T, Pascoe R, Evans VA, Lewin SR, Cameron PU, 2018. Myeloid Dendritic Cells Induce HIV Latency in Proliferating CD4(+) T Cells. J. Immunol 201(5), 1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen KG, Hebbeler AM, Bhattacharyya D, Lobritz MA, Greene WC, 2012. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS One 7(1), e30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KM, Komarova NL, Yewdall AW, Lee RK, Herrera OL, Wodarz D, Chen BK, 2016. In Vivo HIV-1 Cell-to-Cell Transmission Promotes Multicopy Micro-compartmentalized Infection. Cell Rep. 15(12), 2771–2783. [DOI] [PubMed] [Google Scholar]
- Len ACL, Starling S, Shivkumar M, Jolly C, 2017. HIV-1 Activates T Cell Signaling Independently of Antigen to Drive Viral Spread. Cell Rep. 18(4), 1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zony C, Chen P, Chen BK, 2017. Reduced Potency and Incomplete Neutralization of Broadly Neutralizing Antibodies against Cell-to-Cell Transmission of HIV-1 with Transmitted Founder Envs. J. Virol 91(9), e02425–02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Feinberg MB, Reyes GR, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer KS, Engleman EG, 1986. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature 323(6090), 725–728. [DOI] [PubMed] [Google Scholar]
- Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, Schwartz O, 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J. Exp. Med 210(13), 2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH, 2014. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345(6193), 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DM, Garcia JV, Hazuda DJ, Haynes BF, 2016. Latency reversal and viral clearance to cure HIV-1. Science 353(6297), aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ, 2010. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol 84(7), 3516–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massanella M, Puigdomenech I, Cabrera C, Fernandez-Figueras MT, Aucher A, Gaibelet G, Hudrisier D, Garcia E, Bofill M, Clotet B, Blanco J, 2009. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS 23(2), 183–188. [DOI] [PubMed] [Google Scholar]
- Mbonye U, Karn J, 2017. The Molecular Basis for Human Immunodeficiency Virus Latency. Annu. Rev. Virol 4(1), 261–285. [DOI] [PubMed] [Google Scholar]
- McCoy LE, Groppelli E, Blanchetot C, Haard H, Verrips T, Rutten L, Weiss RA, Jolly J, 2014. Neutralisation of HIV-1 cell-cell spread by human and llama antibodies. Retrovirology 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, 2010. Dendritic Cells and HIV-1 Trans-Infection. Viruses 2(8), 1704–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ, 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300(5623), 1295–1297. [DOI] [PubMed] [Google Scholar]
- Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC, 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343(6169), 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Vazquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM, 2006. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J. Leukoc. Biol 80(5), 1145–1155. [DOI] [PubMed] [Google Scholar]
- Munoz-Arias I, Doitsh G, Yang Z, Sowinski S, Ruelas D, Greene WC, 2015. Blood-Derived CD4 T Cells Naturally Resist Pyroptosis during Abortive HIV-1 Infection. Cell Host Microbe 18(4), 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR, 2012. HIV-infected T cells are migratory vehicles for viral dissemination. Nature 490(7419), 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidleman JA, Chen JC, Kohgadai N, Muller JA, Laustsen A, Thavachelvam K, Jang KS, Sturzel CM, Jones JJ, Ochsenbauer C, Chitre A, Somsouk M, Garcia MM, Smith JF, Greenblatt RM, Munch J, Jakobsen MR, Giudice LC, Greene WC, Roan NR, 2017. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathog. 13(2), e1006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski MA, Chun T-W, Justement SJ, Motola I, Spinelli MA, Adelsberger J, Ehler LA, Mizell SB, Hallahan CW, Fauci AS, 1999. Both memory and CD45RA(+)/CD62L(+) naive CD4(+) T cells are infected in human immunodeficiency type 1-infected individuals. J. Virol 73(8), 6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace MJ, Agosto L, Graf EH, O’Doherty U, 2011. HIV reservoirs and latency models. Virology 411(2), 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S, 2013. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 9(4), e1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S, 2012. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc. Natl. Acad. Sci. U. S. A 109(19), 7475–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readinger JA, Schiralli GM, Jiang JK, Thomas CJ, August A, Henderson AJ, Schwartzberg PL, 2008. Selective targeting of ITK blocks multiple steps of HIV replication. Proc. Natl. Acad. Sci. U. S. A 105(18), 6684–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves DB, Duke ER, Wagner TA, Palmer SE, Spivak AM, Schiffer JT, 2018. A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nat. Commun 9(1), 4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, Trkola A, 2015. Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent. PLoS Pathog. 11(7), e1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei SD, Lu HK, Chang JJ, Rhodes A, Lewin SR, Cameron PU, 2018. The pathway to establishing HIV latency is critical to how latency is maintained and reversed. J. Virol 92(13), e02225–02217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J, Riley JL, 2008. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J. Virol 82(22), 11117–11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Plata MT, Puigdomenech I, Izquierdo-Useros N, Puertas MC, Carrillo J, Erkizia I, Clotet B, Blanco J, Martinez-Picado J, 2013. The infectious synapse formed between mature dendritic cells and CD4(+) T cells is independent of the presence of the HIV-1 envelope glycoprotein. Retrovirology 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka D, Feldman J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O, 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple tragets through polysynapses. J. Virol 83(12), 6234–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RA, Martin N, Mitar I, Jones E, Sattentau QJ, 2013. Multiple proviral integration events after virological synapse-mediated HIV-1 spread. Virology 443(1), 143–149. [DOI] [PubMed] [Google Scholar]
- Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR, 2007. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110(13), 4161–4164. [DOI] [PubMed] [Google Scholar]
- Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B, 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol 76(15), 7812–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q, 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol 6(11), 815–826. [DOI] [PubMed] [Google Scholar]
- Schilthuis M, Verkaik S, Walhof M, Philipose A, Harlow O, Kamp D, Kim BR, Shen A, 2018. Lymphatic endothelial cells promote productive and latent HIV infection in resting CD4+ T cells. Virol. J 15(1), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiralli Lester GM, Akiyama H, Evans E, Singh J, Gummuluru S, Henderson AJ, 2013. Interleukin 2-inducible T cell kinase (ITK) facilitates efficient egress of HIV-1 by coordinating Gag distribution and actin organization. Virology 436(1), 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiralli Lester GM, Henderson AJ, 2012. Mechanisms of HIV Transcriptional Regulation and Their Contribution to Latency. Mol. Biol. Int 2012, 614120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A, Baker JJ, Scott GL, Davis YP, Ho YY, Siliciano RF, 2013. Endothelial cell stimulation overcomes restriction and promotes productive and latent HIV-1 infection of resting CD4+ T cells. J. Virol 87(17), 9768–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W, 2007. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol 9(3), 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D, 2011. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477(7362), 95–98. [DOI] [PubMed] [Google Scholar]
- Siliciano RF, Greene WC, 2011. HIV latency. Cold Spring Harb. Perspect. Med 1(1), a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS, 2009. T cell activation. Annu. Rev. Immunol 27, 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol-Foulon N, Sourisseau M, Porrot F, Thoulouze MI, Trouillet C, Nobile C, Blanchet F, di Bartolo V, Noraz N, Taylor N, Alcover A, Hivroz C, Schwartz O, 2007. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. Embo Journal 26(2), 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM, 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol 10(2), 211–219. [DOI] [PubMed] [Google Scholar]
- Starling S, Jolly C, 2016. LFA-1 Engagement Triggers T Cell Polarization at the HIV-1 Virological Synapse. J. Virol 90(21), 9841–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasner AB, Natarajan M, Doman T, Key D, August A, Henderson AJ, 2008. The Src kinase Lck facilitates assembly of HIV-1 at the plasma membrane. J. Immunol 181(5), 3706–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, Theodosopoulos T, O’Doherty U, 2005. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J. Virol 79(22), 14179–14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M, 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424(6945), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symeonides M, Murooka TT, Bellfy LN, Roy NH, Mempel TR, Thali M, 2015. HIV-1-Induced Small T Cell Syncytia Can Transfer Virus Particles to Target Cells through Transient Contacts. Viruses 7(12), 6590–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler CO, Lucera MB, Haqqani AA, McDonald DJ, Migueles SA, Connors M, Tilton JC, 2014. CD4+ memory stem cells are infected by HIV-1 in a manner regulated in part by SAMHD1 expression. J. Virol 88(9), 4976–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titanji BK, Aasa-Chapman M, Pillay D, Jolly C, 2013. Protease inhibitors effectively block cell-to-cell spread of HIV-1 between T cells. Retrovirology 10, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma P, Hubbard AL, 2003. Transcytosis: crossing cellular barriers. Physiol. Rev 83(3), 871–932. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S, Littman DR, 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med 189(11), 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliver-Shamis G, Cho MW, Hioe CE, Dustin ML, 2009. Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J. Virol 83(21), 11341–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliver-Shamis G, Tuen M, Wu TW, Starr T, Cameron TO, Thomson R, Kaur G, Liu J, Visciano ML, Li H, Kumar R, Ansari R, Han DP, Cho MW, Dustin ML, Hioe CE, 2008. Human immunodeficiency virus type 1 envelope gp120 induces a stop signal and virological synapse formation in noninfected CD4+ T cells. J. Virol 82(19), 9445–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis DN, Bristol G, Wilkinson TA, Chow SA, Zack JA, 2007. Immediate activation fails to rescue efficient human immunodeficiency virus replication in quiescent CD4+ T cells. J. Virol 81(7), 3574–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL, 2003. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood 101(6), 2167–2174. [DOI] [PubMed] [Google Scholar]
- Wagner TA, McLaughlin S, Garg K, Cheung CY, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM, 2014. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345(6196), 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki K, Freed EO, 2010. Macrophages and Cell-Cell Spread of HIV-1. Viruses 2(8), 1603–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbato JM, Serrao E, Lenzi G, Kim B, Ambrose Z, Watkins SC, Engelman AN, Sluis-Cremer N, 2016. Establishment and Reversal of HIV-1 Latency in Naive and Central Memory CD4+ T Cells In Vitro. J. Virol 90(18), 8059–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-Q, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT, 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT, 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. U. S. A 101(15), 5640–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Agosto LM, Ilinskaya A, Dorjbal B, Truong R, Derse D, Uchil PD, Heidecker G, Mothes W, 2013a. Cell-to-Cell Transmission Can Overcome Multiple Donor and Target Cell Barriers Imposed on Cell-Free HIV. PLoS One 8(1), e53138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Agosto LM, Munro JB, Mothes W, 2013b. Cell-to-cell transmission of viruses. Curr. Opin. Virol 3(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]