Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of worldwide cancer mortality. HCC almost exclusively develops in patients with chronic liver disease, driven by a vicious cycle of liver injury, inflammation and regeneration that typically spans decades. Increasing evidence points towards a key role of the bacterial microbiome in promoting the progression of liver disease and the development of HCC. Here, we will review mechanisms by which the gut microbiota promotes hepatocarcinogenesis, focusing on the leaky gut, bacterial dysbiosis, microbe-associated molecular patterns and bacterial metabolites as key pathways that drive cancer-promoting liver inflammation, fibrosis and genotoxicity. On the basis of accumulating evidence from preclinical studies, we propose the gut-microbiota–liver axis as a promising target for the simultaneous prevention of chronic liver disease progression and HCC development in patients with advanced liver disease. We will review in detail therapeutic modalities and discuss clinical settings in which targeting the gut-microbiota–liver axis for the prevention of disease progression and HCC development seems promising.
Studies from the past decade have shed light on the important contributions of the gut microbiota to key aspects of our health. Although the gut microbiota provides substantial benefit to the host, in particular with respect to metabolism and immunity1,2, there is also increasing recognition of the involvement of the gut microbiota in disease processes 3. In addition to bacteria, the gut microbiota contains Archaea, eukaryotes such as fungi, and viruses. As the role of the commensal nonbacterial gut microbiota is not as well known, we will exclusively focus on the bacterial gut microbiota in this Review. The bacterial gut microbiota promotes disease development not only via local effects, as in chronic IBD4,5, but also at distant sites such as the liver, heart, brain and the haematopoietic system6–10. Likewise, there is accumulating evidence for an important contribution of the gut microbiota to carcinogenesis via local and long-distance effects11. Owing to its anatomic connection via the portal vein, the liver is closely linked to the gut. Not only does the liver receive nutrient-rich blood from the intestine, but it is also the first target of the intestinal microbiota, microbe-associated molecular patterns (MAMPs) – which may elicit inflammatory responses via pattern recognition receptors (PRRs) - and microbial metabolites. The multi-layer intestinal barrier ensures that hepatic exposure to pro-inflammatory MAMPs is minimal. However, a failing gut barrier and alterations of the gut microbiota in chronic liver disease (CLD) contribute to chronic inflammation and the progression of liver diseases12, and thereby increase risk for the development of hepatocellular carcinoma (HCC) as the final stage of the disease process 13–15. Here, we will review how the gut microbiota promotes the development of HCC, focusing on alterations of the gut microbiota at different disease stages and mechanisms by which it contributes to disease progression and HCC development in different types of liver diseases. We will then review therapeutic opportunities to interrupt this disease-promoting signalling axis, with a focus on the most promising drugs and clinical settings to test these therapeutic strategies.
1. THE INTESTINAL EPITHELIAL BARRIER
Strict separation of microbial entities from the host compartment forms the basis for a symbiotic relationship between host and microbiota. In the intestine, this partitioning is achieved by a well-maintained, multi-layer barrier16,17. This barrier relies on an intact epithelial lining, a mucus layer, Paneth and goblet cells, mucosa-associated lymphoid tissue, as well as a number of secreted factors such as IgA and defensins17. With constant changes in intestinal luminal contents and high epithelial cell turnover, the gut barrier is a highly dynamic system and can rapidly adjust. Continuous sampling of gut microorganisms by specialized epithelial cells, termed M cells, regulates the microbiota through the secretion of antibacterial peptides by Paneth cells; vice versa, the intestinal barrier and epithelial cell growth are regulated by the microbiota17. Moreover, the intestinal microbiota also suppresses the growth of pathobionts, as demonstrated by the protective role of the commensal microbiota against Clostridium difficile infection18 and the increased susceptibility of germ-free mice to infection with pathogens19. Bile acids represent another key factor in this complex system, regulating epithelial barrier function and the proliferation of intestinal epithelial cells via farnesoid X-activated receptor (FXR)-dependent and epidermal growth factor receptor (EGFR)-dependent pathways20–22, and controlling the growth and adhesion of intestinal bacteria16. Of note, bile acids provide an important link between the liver, bacterial microbiota and the intestine. After being synthesized in the liver, bile acids are metabolized by bacteria and sensed by FXR expressed by intestinal epithelial cells (IECs), which in turn provide feedback to the liver via the FGF19 (known as FGF15 in mice) pathway23.
Acute and chronic liver diseases exert major effects on the composition of the intestinal microbiota and intestinal barrier function, resulting in dysbiosis and a leaky gut, respectively. The majority of studies on the gut–liver axis in CLD have focused on lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria and one of the most potent inducers of inflammation via binding to the transmembrane receptor Toll-like receptor (TLR) 4 (discussed in detail later). Mean portal vein LPS levels increase in chronic liver injury from <3 pg/ml in healthy volunteers to 4.9 pg/ml, 7.9 pg/ml and 10.2 pg/ml in patients with Child–Turcotte-Pugh cirrhosis stage A, B and C, respectively24. Likewise, chronic alcohol intake increases endotoxin levels in peripheral blood from 2.5 pg/ml in healthy individuals to 14–19 pg/ml in patients with alcoholic liver disease (ALD)25. Increases in blood LPS levels reflect gut leakiness and are mirrored by a number of other alterations such as increased intestinal permeability to high molecular weight polyethylene glycol in patients with ALD26 and to FITC-dextran in mouse models of alcoholic and biliary liver disease27,28. Moreover, there is an increase in bacterial DNA , a well-established TLR9 agonist, in the peripheral blood of patients with CLD29. Together, these findings demonstrate that the chronically injured liver is subject to increased exposure to a wide range of TLR ligands as well as other bacterial products and metabolites. These pro-inflammatory mediators not only promote the development of CLD but also set the stage for the development of HCC13–15.
Mechanisms underlying the failure of the intestinal barrier and development of a leaky gut are not fully understood and are most likely multifactorial. Contributing factors include decreased bile acid secretion, bacterial dysbiosis and a subsequent increase in the expression of inflammatory cytokines in the intestine, a failing immune system and increased permeability of the gut–vascular barrier16,30,31. Although the development of a leaky gut has been demonstrated by a wide body of literature, the changes in the intestinal microbiota in patients with CLD are only beginning to be understood. Studies in the past few years have demonstrated profound alterations of the intestinal microbiota in patients with cirrhosis, showing increased Enterobacteriacea as well as strains that are typically found in the oral microbiota, such as Veillonellaceae and Streptococcaceae, consistent with an invasion of microorganisms from the mouth to the gut in liver cirrhosis32,33. At the same time, there is decreased abundance of beneficial bacteria in the gut, such as Lachnospiraceae33. These changes seem to develop progressively, as cirrhosis stage is positively correlated with Enterobacteriaceae and negatively correlated with Lachnospiraceae33,34. Likewise, a number of studies have demonstrated alterations of the gut microbiota in earlier stages of liver disease 35,36 as well as in animal models16,31. However, the current understanding of the alterations of the gut microbiota in patients with liver diseases remains incomplete and is complicated by several factors, including: changes in the gut microbiota might be disease-specific; patients with advanced liver disease often take drugs that alter the composition of the microbiota, such as antibiotics, lactulose or antacids; the faecal microbiota might not reflect some of the most characteristic alterations in CLD, such as bacterial overgrowth in the upper gastrointestinal tract; and there could be changes in the adherent microbiota that are not reflected by studying luminal microbiota. In addition, well-designed functional studies are needed to understand the contribution of dysbiotic microbiota to liver disease. Not only is it essential to confirm that dysbiosis is a driver of liver disease development and progression, but it is also important to determine whether dysbiosis contributes to gut leakiness in CLD.
2. GUT MICROBIOTA AND DISEASE PROGRESSION
HCC is typically the result of chronic disease processes in the liver and almost never occurs spontaneously in the absence of liver disease. Moreover, ~80–90% of HCCs occur in advanced fibrotic or cirrhotic livers, which translates to around one in three patients with compensated liver cirrhosis developing HCC in their lifetimes37,38. Hence, the presence of liver cirrhosis represents the most important unifying risk factor for the development of HCC. However, additional factors are involved and each type of underlying liver disease entails a specific risk for the development of HCC in cirrhosis; diseases such as chronic hepatitis B and C or haemochromatosis entail a relatively high risk and diseases such as autoimmune hepatitis or ALD a relatively low risk38–40. To dissect the contribution of the failing gut barrier and alterations of the gut microbiota to HCC development, it is not only important to understand how they might affect the development of HCC within a cirrhotic liver but also how these factors drive the progression of liver disease to advanced disease stages (which entail a significant risk for HCC development). Below, we will summarize disease-specific mechanisms by which the gut microbiota promotes progression of liver disease.
2.1. ALD
ALD contributes to about half of all cirrhosis cases41 and is a cofactor in liver disease induced by HBV, HCV and NASH. Although ALD might have a lower relative risk of causing HCC than other types of CLD39, the sheer number of patients with alcoholic cirrhosis means that the absolute number of HCCs caused by ALD is high. Moreover, subgroups of patients, such as those with cirrhosis, men, patients >55 years of age, individuals positive for antibodies against hepatitis B core protein (anti-HBc) as well as patients with high cumulative consumption of alcohol, might have extremely high risk development for HCC development, risk that may be >40% in a 10-year period42.
The key contribution of the gut microbiota to early stages of ALD has been firmly established in the past two decades. Even a single binge of alcohol is sufficient to increase bacterial translocation, as evidenced by an increase of LPS in portal blood rats from undetectable levels to 30–80 pg/ml after ethanol administration 16. Likewise, serum LPS levels are increased in patients with chronic alcohol abuse16. The ability of ethanol and its metabolite acetaldehyde to disrupt tight junctions contributes to the high levels of bacterial translocation in ALD43. Moreover, mice receiving intragastric alcohol feeding show perturbations of the intestinal microbiota, with reduced synthesis of long-chain fatty acids44. A number of functional studies have shown a key contribution of the gut-microbiota–TLR4 axis to ALD45: Global TLR4 deficiency in mice as well as gut sterilization with nonabsorbable antibiotics in rats reduces hepatic steatosis, oxidative stress and inflammation46–48.
Owing to difficulties in modelling advanced stages of ALD in rodents, the functional contribution of the intestinal-microbiota–TLR4 axis in advanced liver disease, such as in the development of cirrhosis and HCC, is not well known. In one study, ethanol-fed transgenic mice with global TLR4 deficiency, which additionally expressed the NS5A HCV protein, were protected from HCC development, suggesting that TLR4 signalling synergizes with HCV to promote HCC49. This finding fits well with the well-established clinical observation that alcohol abuse is an important cofactor in promoting liver disease development and HCC in patients with chronic HCV infection 50 and suggests a potential role for the LPS–TLR4 axis in the synergy between alcohol and HCV.
2.2. NAFLD
Although recognized as a disease only about two decades ago, NAFLD represents the most prevalent liver disease, and is projected to become the leading contributor to CLD and the development of HCC51. In comparison to other CLDs, NAFLD carries a low relative individual risk for HCC development, but makes a big population-wide contribution to HCC development owing to its high prevalence51. Studies in germ-free and gnotobiotic mice have revealed a key contribution of the gut microbiota to metabolism and energy harvest. As such, germ-free mice display decreased body weight despite increased food intake52. Metagenomic and microbiota transplantation studies have shown that the gut microbiota from obese individuals is more efficient at energy extraction and thereby contributes to obesity53,54. Hence, treatment with antibiotics ameliorates high-fat-diet-induced NAFLD in mice55. Moreover, patients with NAFLD display dysbiosis. However, bacterial abundance patterns were not consistent between studies, with levels of Bacteroidetes increased in some studies36,56 and decreased in other studies57,58, and a substantial overlap with healthy individuals59. Interestingly, dysbiotic microbiota from mice fed a high-fat diet metabolize and convert dietary choline into methylamines, resulting in low circulating levels of plasma phosphatidylcholine60. These low levels of phosphatidylcholine impaired secretion of VLDL, thereby reducing hepatic lipid export and contributing to fatty liver61. Thus, alterations in choline metabolism might link dysbiosis to the development of NAFLD.
The contribution of the gut microbiota to NASH is not as well documented as its role in earlier disease stages. High-fat diet increases intestinal permeability in mice with a two-to-three-fold increase in systemic LPS levels62. Likewise, intestinal permeability is increased in patients with NAFLD63. In a model of NASH triggered by high-fat and high-cholesterol diet given to ApoE-deficient mice, TLR4 deficiency reduced hepatic inflammation and injury64. To date, the functional role of pathways in NASH development has often been studied in mouse models such as the methionine-choline-deficient (MCD) diet — this diet results in a NASH phenotype strongly resembling human NASH but lacks other essential features of NASH, such as adiposity and insulin resistance, and therefore lacks clinical relevance. In the MCD diet model, the microbiota has a key role in NASH exacerbation as demonstrated by experiments, in which co-housing transmitted NASH risk and antibiotics reduced NASH risk65. Conversely, fecal microbiota transplantation from healthy mice attenuated steatohepatitis in high fat diet treated mice 66. The gut microbiota also has an important part in promoting HCC development in a mouse model in which HCC is driven by the carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and subsequent high-fat diet15. However, this model does not incorporate key features of NASH such as liver fibrosis and insulin resistance. Hence, further studies are needed to determine the functional role of dysbiosis in the progression of NASH and HCC in mouse models that incorporate adiposity and insulin resistance.
2.3. Chronic viral hepatitis
In contrast to ALD and NAFLD, there is little information on the role of the gut microbiota in chronic viral hepatitis. Current data suggest that dysbiosis and alterations of the gut–liver axis in patients with end-stage viral hepatitis and cirrhosis are similar to alterations in patients with cirrhosis from other causes67. However, it is not known whether the gut microbiota contributes to the pathophysiology of chronic viral hepatitis and its progression to more advanced stages. A recent study demonstrated that the gut microbiota controls immune responses and tolerance to HBV in adult mice, with 6 weeks of antibiotic treatment preventing the clearance of HBV68. Whether the impaired response to HBV is mediated by specific bacteria or the result of broad suppression of the bacterial microbiota remains an important unanswered question. Notably, HBV titres in patients positively correlate with risk for disease progression and HCC development69. Hence, the gut microbiota might control antiviral responses that affect disease progression and HCC development.
2.4. Liver fibrosis
Liver fibrosis is part of the hepatic wound healing response and common to all types of advanced CLD. Notably, there is a strong correlation between hepatic fibrosis and HCC development with 80–90% of HCCs developing in fibrotic or cirrhotic livers. Thus, fibrosis represents a risk factor for HCC development70. There is strong evidence for an important contribution of the microbiota–TLR4 axis to liver fibrosis. Studies from the past six decades have shown that antibiotics prevent hepatic injury and fibrosis induced by CCl4 treatment, bile duct ligation or a choline-deficient diet, and that endotoxin enhances hepatic fibrosis induced by a choline-deficient diet71–73. Studies in knockout mice have highlighted a key role for TLR4 and other important mediators in the TLR4 signalling pathway, such as CD14 and lipopolysaccharide-binding protein (LBP), in experimental models of toxic and cholestatic liver fibrosis73,74. However, recent studies have demonstrated an increase in liver fibrosis in germ-free mice75,76, which seemingly contradicts the decrease of liver fibrosis seen in gut-sterilized mice. It has become apparent that the endogenous commensal microbiota provides hepatoprotective signals and that complete absence of the gut microbiota results in increased liver injury — probably owing to an absence of TLR4-mediated activation of anti-apoptotic NF-κB signalling — and a subsequent increase in liver fibrosis, as demonstrated in several models13,75,76. Nonetheless, the bacterial microbiota has an important role in promoting HCC in the setting of liver fibrosis, as demonstrated by reduced HCC formation in TLR4-deficient, germ-free and antibiotic-treated mice in a diethylnitrosamine (DEN) plus CCl4 model of HCC13. Consistent with previous studies71–73, treatment with nonabsorbable antibiotics resulted in a strong reduction of fibrosis despite increased liver injury13. However, further studies are required to investigate how the intestinal microbiota affects HCC development promoted by chronic inflammation, injury and fibrosis, without preceding carcinogen exposure.
3. MECHANISMS BY WHICH THE MICROBIOTA PROMOTES HCC
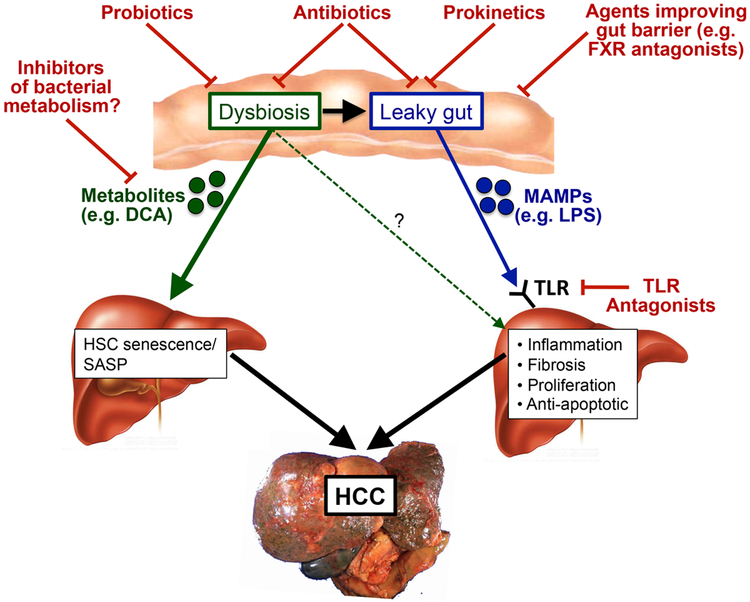
As discussed above, alterations in gut permeability and the gut microbiota are highly characteristic not only of late stages of all types of CLD, but also occur early in several types of CLD. Thus, the gut microbiota contributes to disease progression at various stages and might promote the development of HCC throughout all these stages. Here, we will discuss mechanisms through which the gut–liver axis promotes HCC development and progression, focusing on the role of the leaky gut and dysbiosis (FIG. 1).
Figure 1. Contribution of the gut microbiota to hepatocarcinogenesis: mechanisms and therapeutic targets.
Dysbiosis and the leaky gut promote the progression of liver disease and the development of hepatocellular carcinoma (HCC) via multiple mechanisms, including the release of cancer-promoting and senescence-promoting metabolites such as deoxycholic acid (DCA) from the dysbiotic microbiota, and increased hepatic exposure to gut-derived microbe-associated molecular patterns (MAMPs) such as lipopolysaccharide (LPS), which in turn promote hepatic inflammation, fibrosis, proliferation and the activation of anti-apoptotic signals. These cancer-promoting signalling pathways can be interrupted at several levels: using probiotics to restore eubiosis; using antibiotics to eliminate disease-promoting bacteria and decrease the release of MAMPs and metabolites from the leaky gut; using agents to improve the gut barrier; and potentially using inhibitors of bacterial metabolism to reduce the production of cancer-promoting metabolites by the gut microbiota. HSC, hepatic stellate cell; TLR, Toll-like receptor; SASP, senescence-associated secretory phenotype; FXR, farnesoid X receptor.
3.1. HCC promotion via a leaky gut and the MAMP–TLR axis
High circulating LPS levels in mice and patients with CLD as well as in HCC14,24,77,78 demonstrate the presence of a leaky gut during multiple stages of CLD and hepatocarcinogenesis (FIG. 1). Functional experiments in germ-free, gut-sterilized, TLR-deficient and LPS-treated mice have provided evidence that the leaky gut, via LPS and its receptor TLR4, makes essential contributions to hepatocarcinogenesis. As such, HCC development induced by the combination of DEN and CCl4 was attenuated in gut-sterilized and germ-free mice compared with their specific pathogen-free counterparts13. In addition to causing characteristic infectious complications in end-stage liver disease, increased bacterial translocation also generates a chronic inflammatory state in the liver. The inflammatory responses in the liver are mediated by interaction between MAMPs and host PRRs, specifically the TLRs79. Accordingly, chronic infusion of low-dose LPS via osmotic pumps promotes HCC development in mice13. Likewise, disruption of the gut barrier by administration of dextran sulfate sodium not only results in increased systemic LPS levels and increased liver fibrosis, but also promotes HCC formation in mice80,81. Conversely, inhibition of TLR4 signalling suppresses liver inflammation, fibrosis and HCC formation in mice and rats13,14,73. The majority of tumour-promoting signals from the leaky gut occur in late stages of DEN+CCl4-induced hepatocarcinogenesis, as demonstrated by strong inhibitory effects of gut sterilization on HCC formation in late stages but only mild effects in early stages13. However, the relative contribution of the leaky gut at early versus late stages of hepatocarcinogenesis has not yet been tested in other models.
TLR4 is present in multiple hepatic cell types, including Kupffer cells, hepatic stellate cells (HSCs), endothelial cells and hepatocytes. Experiments in bone-marrow-chimeric mice demonstrated that TLR4 expressed on liver-resident cells (which include hepatocytes, HSCs and Kupffer cells) is responsible for promotion of fibrogenesis and hepatocarcinogenesis13. LPS from the leaky gut seems to promote hepatocarcinogenesis via multiple cellular targets, including HSCs, the hepatocyte–tumour compartment as well as liver-resident Kupffer cells. In HSCs, TLR4 activation leads to an NF-κB-mediated upregulation of the hepatomitogen epiregulin13. Epiregulin is an epidermal growth factor family member with a potent mitogenic effect on hepatocytes82. Accordingly, epiregulin-deficient mice displayed reduced hepatocarcinogenesis when treated with DEN+CCl413. Another key mechanism by which the LPS–TLR4 axis promotes HCC formation is via NF-κB-mediated prevention of hepatocyte apoptosis. Accordingly, expression of the apoptosis marker cleaved caspase 3 in TLR4-deficient and gut-sterilized mice is inversely correlated with the formation of tumours13. However, due to the lack of studies in mice with conditional TLR4 ablation, it remains unclear whether this survival pathway is directly activated in the hepatocyte–tumour cell compartment, or whether it might involve paracrine signals from neighboring TLR4-expressing cells such as HSC or Kupffer cells. Moreover, it has been demonstrated that activation of the LPS–TLR4 signalling pathway in Kupffer cells leads to TNF- and IL6-dependent compensatory hepatocyte proliferation as well as reduced oxidative stress and apoptosis14. In addition, TLR4 activation in HCC cell lines by LPS enhances their invasive potential and induces the epithelial–mesenchymal transition83. In order to delineate the contribution of TLR4 on specific cell types in the liver, further experiments in mice with conditional TLR4 ablation are required.
Together, these data clearly show that the leaky gut, via MAMP–TLR-mediated signals, contributes to hepatocarcinogenesis. Dysbiosis (discussed below) and the leaky gut are probably intimately linked; it is likely that intestinal dysbiosis contributes to a leaky gut by multiple mechanisms, such as dysbiosis-induced alterations of the intestinal barrier as well as a shift to bacterial species with increased propensity to translocate.
3.2. HCC promotion via dysbiosis, bacterial metabolites and immunosuppression
Increasing evidence supports a key role for dysbiosis in the development of CLD and HCC (FIG. 1). Metagenomic studies have revealed substantial alterations in the composition of the gut microbiota in a range of CLD as well as in patients with cirrhosis12,32. The gut microbiomes of patients with advanced liver disease and cirrhosis are characterized by an increase in potentially pathogenic bacteria, along with reduced numbers of bacteria with beneficial properties32,84,85. Studies conducted so far on the gut microbiota in liver cirrhosis have pooled patients with different underlying liver diseases32, indicating that at least some of the microbial alterations in cirrhosis are common to different aetiologies, and suggesting that alterations are driven by characteristic features of end-stage liver disease, such as reduced bile output and changes to the intestinal secretion of antimicrobial peptides and IgA . Key changes in the composition of the intestinal microbiota in cirrhosis include enrichment of Veillonella or Streptococcus as well as decreased bacteria from the order Clostridiales32. Of note, the majority of the patient-enriched species were of buccal origin, suggesting an invasion of the gut from the mouth in liver cirrhosis32. The finding that the intestinal microbiota of patients with compensated cirrhosis differs from that of patients with decompensated cirrhosis34 suggests that cirrhosis stage, rather than the underlying liver disease, drives gut microbiota changes. However, a recent small-scale study described differences in the gut microbiota between different types of underlying liver disease86. Therefore, sufficiently powered studies in large cohorts are needed determine disease-specific alterations of the gut microbiota in liver cirrhosis. In addition to alterations in bacterial composition, there is evidence for bacterial overgrowth in the upper gastrointestinal tract, which in turn is associated with increased circulating LPS levels 87. Bacterial translocation in the upper gastrointestinal tract is relevant for the development of liver disease owing to the anatomic connection of the small intestine to the liver. Recent studies have demonstrated differences in the duodenal and salivary microbiota between healthy controls and patients with cirrhosis86,88, suggesting that there are also qualitative and quantitative changes in the upper gastrointestinal tract that might be linked to changes in the more distal microbiota and contribute to the pathophysiology of CLD as well as the development of HCC.
Functional studies utilizing co-housing and faecal transplantation have provided evidence that dysbiosis is a transmissible driver of liver disease development and progression 65,89. In one study, high-fat diet feeding in mice resulted in dysbiosis, with increased abundance of Gram-negative bacteria and a reduced ratio of Bacteroidetes to Firmicutes . Transplantation of these dysbiotic microbiota into control-diet-fed mice that had undergone bile duct ligation increased liver damage and fibrosis in the recipient89. Similarly, dysbiosis represented a transmissible risk factor in a genetic NASH model in which NASH was triggered by inflammasome deficiency; co-housing of dysbiotic inflammasome-deficient mice with control mice resulted in the development of NASH in control mice65. Although studies demonstrating a transmissible HCC risk by dysbiotic microbiota are still missing, several functional studies point towards a contribution of dysbiosis. As such, perturbation of the gut eubiosis by penicillin increased HCC formation in rats77, which could be suppressed by probiotics77.
Recent evidence suggests that the effects of dysbiosis on the development of liver disease and HCC are mediated by bacterial metabolites, possibly in a disease-specific manner. In a mouse model of NASH-induced HCC, triggered by the combination of DMBA and high-fat diet, there was a strong increase in Gram-positive bacterial strains, in particular of specific Clostridium clusters15. At the same time, this treatment led to increased serum levels of deoxycholic acid (DCA), a secondary bile acid whose production depends on 7α-dehydroxylation of primary bile acids by the bacterial microbiota, notably Clostridium clusters. The key role of DCA in hepatocarcinogenesis was further demonstrated in experiments that showed increased HCC development in mice after supplementing diets with DCA, and decreased HCC formation after inhibition of 7α-dehydroxylation15. In concert with TLR2 agonist lipoteichoic acid, DCA promoted a senescence-associated secretory phenotype in hepatic stellate cells, which in turn suppressed anti-tumor immunity through a prostaglandin E2-dependent mechanisms90. Together, these studies link bacterial dysbiosis to altered immune responses via bacterial metabolites and MAMPs. Further studies are required to determine whether the procarcinogenic effects of dysbiotic microbiota may be mediated by additional pathways. The gut microbiota exerts a key role in a number of other metabolic pathways, including overall energy extraction from the diet as well as the generation of a wide range of important metabolites with beneficial effects for the host91. One example is the production of short-chain fatty acids (SCFAs), which are a primary energy source for intestinal epithelial cells91, and might provide a link between dysbiosis and alterations of the intestinal barrier that lead to a leaky gut and increased risk for HCC development, as discussed above.
4. TARGETING MICROBIOTA TO PREVENT HCC
Currently, there are no therapeutic options for HCC prevention besides treating the underlying disease. On the basis of its important contribution to CLD progression and hepatocarcinogenesis in particular, the gut-microbiota–liver axis represents a promising target for preventative approaches (FIG. 1). With a complete lack of clinical studies testing this strategy, targeting the gut-microbiota–liver axis represents an exciting and understudied clinical opportunity, supported by a large number of studies preclinical studies showing a drastic (≈80%) reduction of HCC development in murine models13–15. Moreover, several small-scale clinical studies have suggested that antibiotics such as norfloxacin and rifaximin increase survival in patients with liver cirrhosis92–95. Targeting the gut microbiota axis for HCC prevention is particularly attractive as it may utilize currently FDA approved drugs with high safety profile in CLD patients, such as the nonabsorbable antibiotic Rifaximin, or other approaches with low risk for severe adverse effects, such as probiotics or faecal microbiota transplantation (FMT). Moreover, the gut-microbiota–liver axis has a key involvement in many complications of CLD and could be targeted to ‘kill several birds with one stone’: In addition to potentially reducing the risk for HCC development, targeting the gut-microbiota–liver axis has been shown to reduce liver fibrosis 73,96 and portal hypertension97 in rodents, and spontaneous bacterial peritonitis98 and hepatic encephalopathy99 in patients. As the strongest effects of antibiotics on HCC and complications of cirrhosis in mice and patients, respectively, have been observed in advanced disease stages, preventative strategies that target the gut–liver axis seem most promising in patients with cirrhosis and at high risk for HCC development, which would also reduce the number of patients that would be unnecessarily subjected to such treatments. Moreover, targeting the gut microbiota-liver axis is unlikely to have a major effect on patients in which the gut–liver axis is not a dominant driver of disease progression, HCC development and mortality, for example those with perinatal HBV infection, high HBV titres and minimal liver fibrosis. Although there is accumulating evidence that the gut microbiota modulates responses to chemotherapy100,101 and immunomodulatory therapies102,103, there is currently no data supporting the concept of targeting the gut-microbiota–liver axis for the treatment of HCC. With increased understanding of the underlying pathophysiology, the number of clinically feasible approaches to target the gut-microbiota–liver axis is continuously growing (Table 1).
Table 1.
Drugs targeting the gut-liver axis for prevention of CLD progression and HCC development.
| Class of drug/treatment |
Drug | Mechanism of action | Effects in mouse models | Effects in patients | Ref |
| Antibiotics | Rifaximin | A minimally absorbed oral antimicrobial agent when administrated orally; broad-spectrum activity against enteric bacteria by inhibiting the bacterial protein synthesis; low risk of inducing bacterial resistance | Reduced HCC development in the DEN+CCl4 model of HCC Rifaximin reduces fibrosis, angiogenesis and portal hypertension in mice following bile duct ligation |
Reduced the development of spontaneous bacterial peritonitis and portal hypertension, suggesting that it effectively targets the gut-liver axis in advanced liver disease Improved survival in Rifaximin-treated patients with chronic liver disease in several small-scale studies |
13,93-96,113-115 |
| Norfloxacin | A poorly absorbable quinolone when administrated orally; selectively eliminates the intestinal gram-negative microbiota; low activity against anaerobic bacteria | Suppressed numbers of cecal aerobic and anaerobic bacteria in ob/ob mice and ameliorated glucose tolerance when combined with ampicillin | Long-term use reduced the 1-year probability of developing SBP and hepatorenal syndrome Improves 3-month survival in advanced cirrhotic patients Reduces the levels of bacterial translocation and proinflammatory cytokines in serum and ascites |
92,109,117,160,161 | |
| Probiotics | VSL#3 | Mechanisms not conclusively determined; possibly acting via modulation of the host’s microbiota, improvement of gut barrier function and modulation of the immune system | Mitigated enteric dysbacteriosis, ameliorated intestinal inflammation, and decreased liver tumor growth and multiplicity in a DEN-induced rat HCC model |
Reduced the risk of hospitalization for HE Reduced Child-Turcotte -Pugh and model for end-stage liver disease (MELD) scores, in patients with cirrhosis in one study Improved NAFLD in children |
77,121,122 |
| Prohep | In a subcutaneous transplant model, it reduced tumor size and weight by 40% | Not studied. | 120 | ||
| TLR4 antagonists | E5564 (eritorin) |
Binds to the hydrophobic pocket of MD-2, competitively inhibits the lipid A component of endotoxin from binding to the same site, and thereby prevents dimerization of TLR4 and intracellular signaling | Protective in animal models of sepsis No data on liver disease or HCC development. |
In healthy volunteers, blocked symptoms of endotoxmia in a dose-dependent manner No data on liver disease or HCC development. |
129 |
| TAK-242 | Binds to the intracellular domain of TLR4 and inhibits interaction with TLR adapter molecules TIRAP and TRAM, thereby blocking TLR4 signaling | Protected mice against LPS-induced lethality No data on liver disease or HCC development. |
Failed to suppress cytokine levels in patients with severe sepsis and septic shock or respiratory failure No data on liver disease or HCC development. |
129 | |
| FXR agonists | GW4064 | Activating the FXR signaling in the ileum | Attenuated mucosal injury, ileal barrier permeability, bacterial overgrowth and bacterial translocation | Not studied. | 20,21 |
| Obeticholic acid | Activating the FXR signaling in the ileum | Attenuated mucosal injury, ileal barrier permeability, bacterial overgrowth and bacterial translocation; improved portal hypertension (which might contribute to BT in cirrhosis) in TAA and bile duct ligation-induced cirrhosis models | Improved histological features of NASH | 21,141-144 | |
| Prokinetics | Cisapride | Ameliorates gut dysmotility | Inhibited intestinal bacteria overgrowth and bacterial translocation in animal models | Inhibition of intestinal bacterial overgrowth and translocation in cirrhotic patients | 150-152 |
| Betablockers | Propranolol | Non-selective beta-adrenergic receptor antagonist that ameliorates gut dysmotility, reduces portal pressure and thereby might reduce bacterial translocation | Increased intestinal transit and reduced intestinal bacterial overgrowth, intestinal permeability and bacterial translocation in experimental models | Decreased HCC occurrence in patients with HGV-related cirrhosis | 153-158 |
| FMT | Restores in patients with antibiotics-induced and C.difficile infection | FMT improves NASH Cohousing and transplantation studies in mice have also shown worsening of NASH and liver fibrosis by dysbiotic microbiomes |
Restoration of eubiosis and significant clinical improvements in patients with C.difficile infection Improved hepatic and peripheral insulin resistance |
18,65,66,89,126 |
4.1. Antibiotics
Because antibiotics target several pathways through which the gut microbiota promote HCC development (Table 1), they could represent one of the most efficient strategies to interrupt the tumor-promoting gut liver axis in CLD: Decreasing the overall number of bacteria in the gut and eliminating bacteria that have a high ability to translocate will reduce bacterial translocation and thereby inhibit proinflammatory signals coming from leaky gut. At the same time, selective antibiotics might also block the production of HCC-promoting bacterial metabolites, such as DCA15, by reducing the number of bacteria that produce specific metabolites. Continuous gut sterilization by a cocktail of oral antibiotics, consisting of ampicillin, neomycin, metronidazole and vancomycin, effectively reduced the number and size of HCC induced by DEN+CCl4 or DMBA+HFD in mice13,15. Moreover, this antibiotic cocktail also reduced liver fibrosis 73, which often precedes HCC and represents a risk factor for HCC development in CLD70. Of note, administration of antibiotics at late stages of carcinogenesis, when microscopic tumours already existed, was more efficient at reducing HCC in mice than administration at earlier stages13. These data support the concept that HCC prevention by antibiotic treatment could be applied even at late stages, i.e. in patients with advanced cirrhosis and high risk for HCC development. However, findings from mice cannot be translated directly to patients as long-term administration of the employed antibiotic cocktail would be deleterious due to the depletion of almost all detectable commensal microbiota (>99.5%)73,104 and the inclusion of nephrotoxic drugs, such as neomycin. Moreover, HCC prevention with antibiotics would require long-term, possibly life-long administration. Therefore, the use of single antibiotics with a high safety profile in patients with CLD represents the only clinically feasible approach.
Currently, two antibiotics, norfloxacin and rifaximin, have shown beneficial effects in patients with CLD or murine HCC models and fulfill these criteria. Vancomycin, another antibiotic that has shown effectiveness as monotherapy in the prevention of HCC in the combined DMBA and high-fat diet mouse model15, is rarely used for long-term therapy in patients and may cause a number of potentially severe adverse effects. Gram-negative bacteria have been found to be the most adept at translocating to the mesenteric lymph nodes and are the most frequent cause of spontaneous bacterial infections in patients with cirrhosis105–107. Norfloxacin, a poorly absorbed quinolone, is currently one of the drugs of choice for the primary or secondary prophylaxis of spontaneous bacterial peritonitis and infections in high-risk patients with cirrhosis 108. Clinical trials in patients with advanced cirrhosis have shown that long-term use of orally administered norfloxacin is safe, produces a marked reduction of gram-negative bacteria in the faecal microbiota109, reduces the 1-year probability of developing spontaneous bacterial peritonitis and hepatorenal syndrome and improves 3-month survival92. Although these data show that norfloxacin can effectively reduce small intestinal bacteria overgrowth and bacterial translocation in patients with advanced cirrhosis, the effects of norfloxacin on HCC development in patients with liver cirrhosis are not known. A major problem with the use of norfloxacin is the development of antibiotic resistance110–112, suggesting that it might be suitable for treatment lasting weeks to months but not for long-term or life-long application in patients with cirrhosis. Rifaximin is a nonabsorbable antibiotic with broad-spectrum antimicrobial activity and an excellent safety profile113 that was initially approved for the treatment of traveller’s diarrhoea but is increasingly used for the prevention of hepatic encephalopathy99. Moreover, rifaximin appears to reduce the development of spontaneous bacterial peritonitis and may improve portal hypertension, suggesting that it effectively targets the gut–liver axis in advanced liver disease93,114,115. Similar to norfloxacin, rifaximin has been noted to increased survival in patients with advanced liver cirrhosis in several small-scale trials92–95. Of note, rifaximin reduces HCC development in the DEN–CCl4 model of HCC, albeit less efficiently than the quadruple antibiotics cocktail described above13. Despite the large number of patients receiving rifaximin for the prevention of hepatic encephalopathy, the effects of rifaximin on HCC development remain unknown. Therefore, studies that determine the effects of long-term rifaximin treatment on HCC development are urgently needed. In contrast to norfloxacin, clinically relevant development of resistance to rifaximin has not been reported, suggesting that it is well-suited for long-term or even life-long treatment. As data from murine studies show that non-absorbable antibiotics and norfloxacin improve ALD48,116 and insulin resistance in NAFLD117, they might be particularly attractive for HCC prevention in these patient groups.
4.2. Probiotics
Probiotics have been proposed as a means of re-equilibrating the gut microbiota in CLD by restoring beneficial bacteria. Although a large number of studies have demonstrated the effectiveness of probiotics in treating liver diseases both in animal models and in patients (reviewed elsewhere118,119), substantial controversy remains on the basis of: the inability of most probiotics to permanently colonize the gut; largely unknown mechanisms of action, in particular given the lack of permanent colonization; the large number of different combinations of bacteria within different probiotics that have not been systemically evaluated and compared for their efficacy in CLD; and in view of a lack of large-scale studies, potential publication bias towards studies reporting positive results . So far, probiotics have only been investigated in murine HCC models and data in patients are lacking (Table 1). In a rat model of DEN-induced hepatocarcinogenesis, administration of VSL#3 (containing Streptococcus thermophiles, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. Bulgaricus) mitigated enteric dysbiosis, ameliorated intestinal inflammation and decreased liver tumour growth and multiplicity77. In a subcutaneous transplant mouse model, the probiotic mixture Prohep (comprising Lactobacillus rhamnosus GG, Escherichia coli Nissle 1917 and heat inactivated VSL#3) reduced tumour size and weight120. The authors suggested that a shift of the gut microbiota composition towards beneficial bacteria such as Prevotella and Oscillibacter, and production of anti-inflammatory mediators by these bacteria, decreased Th17 cell levels within tumors and thereby limited tumour growth. However, the effects of probiotics on endogenously arising tumours are not known. To date, there are several clinical trials on probiotics in patients with CLD but none in patients with HCC. A double-blind trial showed that daily intake of VSL#3 reduced the risk of hospitalization for hepatic encephalopathy, as well as Child–Turcotte–Pugh and model for end-stage liver disease (MELD) scores, in patients with cirrhosis121. Another randomized trial showed that 4-month supplementation with VSL#3 improves NAFLD in children122. The possible mechanisms of action include modulation of the host microbiota123, improvement of gut barrier function and modulation of the immune system. However, further studies are required to confirm these data, extend human studies and investigate mechanisms of action.
4.3. FMT
FMT has successfully been used in patients with C. difficile infection, resulting in restoration of eubiosis and clinical improvements that were superior to standard antibiotic therapy18. Currently, FMT is being evaluated in clinical trials for a number of additional diseases including NASH124 and cirrhosis125. A randomized controlled trial demonstrated amelioration of hepatic and peripheral insulin resistance in patients with metabolic syndrome who had received microbiota from lean donors126. However, one needs to keep in mind that patients receiving FMT for recurrent C. difficile infection have usually undergone multiple courses of antibiotic treatment, and present with a marked reduction of microbial diversity 18. Thus, these patients not only represent an ideal ‘breeding ground’ for transplanted microbiota but also suffer from a disease that is clearly linked to reduced bacterial diversity and overgrowth of single and measurable pathogenic strain. Nonetheless, it is conceivable that FMT might also restore eubiosis in patients with CLD, similar to effect seen in the trial of Vrieze et al.126, and that FMT might reduce or delay the development of HCC (Table 1). However, there are currently no data supporting this premise and a number of hurdles have to be overcome. Most importantly, it is not clear whether the severe alterations of the gastrointestinal ecology in cirrhosis would allow permanent restoration of the microbiota by FMT. It is possible that effects will be transient and the microbiota will ultimately revert to the pre-FMT state. Moreover, there is substantial concern that viral infections and other pathogens might be transmitted via FMT, which would be particularly harmful to patients with advanced liver disease owing to their immunosuppression. In the future, faeces might be substituted in favour of defined mixtures of cultured bacteria that resemble the human microbiota transplanted via FMT and confer the same beneficial effects. This approach will not only alleviate concerns regarding the inadvertent transmission of disease-causing pathogens through FMT, but also make intestinal microbiota therapy more acceptable to patients and physicians127. Once this goal has been achieved, patients with advanced liver disease should be considered as potential candidates to study effects on disease progression and HCC development.
4.4. TLR antagonists
Several studies have shown a key role for the TLR4 pathway as a mediator of the disease-promoting effects of the gut–liver axis in CLD and hepatocarcinogenesis13,14,73. On the basis of these findings, blocking the TLR4 pathway might represent another avenue for HCC prevention (Table 1). With detailed knowledge about mechanisms by which LPS activates TLR4, a variety of TLR4 antagonist have been developed, which can be clustered into several groups: compounds binding and sequestering LPS, such as polymyxin B; compounds antagonizing LBP and CD14–LPS interactions128; compounds targeting LPS–MD-2 or LPS–MD-2–TLR4 interactions, such as E5531 and eritoran (E5564); compounds directly targeting TLR4, such as resatorvid (TAK-242); and molecules inhibiting TLR4 activity such as thalidomide (reviewed elsewhere129). Eritoran130 and resatorvid131 improved survival in animal models of sepsis, but did not reduce mortality in patients with severe sepsis132,133. So far, none of these agents have been tested in clinical trials in patients with CLD or HCC. Although TLR antagonists represent an exciting opportunity, long-term inhibition of TLR4 could result in immunosuppression, which might be deleterious due to the severely immunocompromised state of CLD patients. Therefore, the safety profile of TLR4 antagonists needs to be carefully evaluated before long-term studies for prevention of HCC and other complications of CLD can be considered.
4.5. Targeting the gut barrier
On the basis that the leaky gut is a major driver of liver disease progression and HCC development (FIG. 1), targeting the gut barrier seems an attractive therapeutic approach (Table 1) that might avoid some of the complications of targeting the microbiota (such as development of resistance and/or decreased microbial diversity) or receptors that mediate the disease-promoting effects of a leaky gut (such as immunosuppression resulting from TLR4 antagonism. Moreover, therapies that target the gut barrier could potentially be combined with other approaches that directly target the gut microbiota or liver. With improved understanding of the gut barrier and mechanisms that disrupt the gut barrier in cirrhosis, targeting the gut barrier via specific pharmacologic approaches seems to be realistic.
Bile acids are an important regulator of the gut barrier. Decreased bile secretion in rodents by either ligation of the common bile duct or induction of cirrhosis contributes to bacterial translocation, which is not only caused by intestinal bacterial overgrowth but also by increased gut permeability20,28,134. Notably, these effects are attenuated after oral administration of bile acids in different experimental cirrhotic models20,134,135. FXR is a receptor for bile acids that mediates their effects on the intestinal epithelial barrier as well as multiple effects on the liver, such as suppression of bile acid synthesis, inhibition of liver inflammation, promotion of liver regeneration and tumour suppression (reviewed elsewhere136). Many of the hepatic effects of FXR activation are mediated by intestinal FXR receptors, resulting in the release of FGF19, which then acts on targets in the liver136–139. Fxr-deficient mice exhibit compromised intestinal integrity, with further deterioration after bile duct ligation20, and a high incidence of HCC140. Accordingly, FXR activation by agonists GW4064 or obeticholic acid (OCA) attenuates mucosal injury, ileal barrier permeability, bacterial overgrowth and bacterial translocation in mice and rats 20,21,141,142. Moreover, OCA improves portal hypertension (which might contribute to bacterial translocation in cirrhosis) in thioacetamide- or bile duct ligation-treated rats143. OCA has a high safety profile in patients with NASH as demonstrated in the FLINT trial, with the major adverse effects being pruritus and alterations of serum lipid profiles144. Thus, OCA seems to be a promising candidate for HCC prevention therapies, and could be particularly effective by correcting multiple abnormalities in the gut–liver axis that promote the development of chronic inflammation and HCC in patients with cirrhosis.
Increased production of TNF by monocytes in mesenteric lymph nodes constitutes one of the main factors increasing tight junction permeability145,146. TNF increases tight junction permeability by decreasing expression of tight junction proteins as well as by activating myosin light chain kinase (MLCK)147. Treatment with an anti-TNF monoclonal antibody decreases the incidence of bacterial translocation in experimental cirrhosis in rats148. However, translating these findings to patients might be difficult because of strong immunosuppressive effects of TNF inhibitors and increased rates of severe infection. Owing to these adverse effects, long-term anti-TNF therapy might confer more harm than benefit, and further efforts need to be made to develop therapies that act locally to improve gut barrier function without negatively affecting systemic immune responses.
4.6. Prokinetics
Another factor that contributes to intestinal bacteria overgrowth in liver cirrhosis is gut dysmotility149. The prokinetic drug cisapride not only decreases intestinal transit time but also inhibits intestinal bacterial overgrowth and bacterial translocation, both in animal models150,151 and in patients with cirrhosis150,152. However, the long-term benefits of prokinetics such as cisapride have yet to be determined in patients with CLD (Table 1). One of the purported mechanisms for altered motility in cirrhosis is increased adrenergic activity. Accordingly, nonselective β-adrenergic blockers decrease intestinal transit time and reduce intestinal bacterial overgrowth, intestinal permeability and bacterial translocation in experimental models of cirrhosis as well as in patients153–157. Interestingly, a retrospective long-term observational study suggests that propranolol treatment might decrease HCC occurrence in patients with HCV cirrhosis158, suggesting a potential role for HCC prevention.
Malnutrition is common in patients with CLD and is associated with increased morbidity and mortality (reviewed elsewhere149). Of note, malnutrition increases intestinal permeability and facilitates bacterial translocation159. Therefore, nutritional support represents an important aspect to correct dysbiosis in patients with CLD.
5. CLINICAL TRANSLATION
Current data from mouse models suggest that targeting the gut–liver axis has a potential role for the primary or secondary prevention of HCC, but not for the treatment of HCC (Table 2). Primary prevention seems the most appealing approach, and could be tested prospectively in large cohorts of patients with liver cirrhosis and at high risk for HCC development. Alternatively, the efficacy of primary prevention could be evaluated retrospectively in cohorts of patients that already received treatment, for example rifaximin for the prevention of hepatic encephalopathy. Primary prevention most likely requires long-term, if not life-long treatment. As discussed in the previous sections, good safety and beneficial effects on non-HCC complications of CLD are the most important selection criteria for the best-suited drug candidates. In this regard, rifaximin is probably the candidate with the best safety profile, and there is strong evidence that it positively affects additional complications of CLD such as hepatic encephalopathy, portal hypertension and liver fibrosis, and possibly even improves overall survival. The efficacy of targeting the gut–liver axis for secondary prevention could be tested in patients who have undergone curative HCC resection. Although this strategy would require a well-defined cohort of patients with high risk for HCC relapse, one would still have to carefully distinguish between tumour recurrence and de novo tumour formation, as therapies such as rifaximin might positively affect one but not the other.
Table 2.
Clinical setting to studying therapeutic interventions in the gut-liver axis in HCC patients
| Study design and participants |
Treatment | Primary outcomes |
Primary outcomes |
Advantages | Disadvantages |
|---|---|---|---|---|---|
| Retrospective studies | |||||
| Patients with liver cirrhosis treated with rifaximin for the primary or secondary prevention of HE | Rifaximin | Reduction of HCC development | Difficult to study as available data might not be sufficient | Large number of patients that have received treatment with rifaximin | • Untreated control group needs to be well-matched in regards to disease stage • HCC surveillance might not have been ideal in the majority of patients • Additional treatments and interventions might not be recorded and could represent confounders |
| Prospective, primary HCC prevention trials | |||||
| Patients with liver cirrhosis and high risk of HCC development | Long-term or life-long treatment with drugs such as antibiotics, probiotics or FXR agonists | HCC development and HCC-related mortality | • Reduction of overall and liver-related mortality • Reduction of liver disease progression, for example, MELD score, synthetic liver function, portal hypertension |
Allows for best study design and patient selection criteria | • Would probably require a very long treatment and observation period • Expensive owing to long treatment and observation period and large number of patients required to detect small differences in risk reduction |
| Prospective, secondary HCC prevention trials | |||||
| Patients that have undergone curative HCC resection | Long-term or life-long treatment with drugs such as antibiotics, probiotics or FXR agonists | HCC development, in particular late recurrence, and HCC-related mortality | Difficult to study as many patients will not have advanced liver disease | Patients are at high risk and many recurrences are early | • Not clear if targeting the microbiota or gut–liver axis can prevent recurrences or only de novo tumour formation • For the latter, long-term treatment and large patient numbers might be required |
6. CONCLUSIONS
Overwhelming evidence from the past three decades support a key contribution of the gut microbiota to multiple aspects of liver disease progression, thereby contributing to a hepatic environment that promotes that development and progression of HCC. The mechanisms by which the gut microbiota promotes the development of liver disease and HCC include dysbiosis — which results in altered bacterial metabolites such as the cancer-promoting secondary bile acid DCA — as well as a leaky gut, which promotes chronic hepatic inflammation via TLR-mediated signals. Currently, it is not clear whether chronic inflammation driven by the translocation of MAMPs from the leaky gut is the dominant contributor to hepatocarcinogenesis, whether alterations of bacterial metabolites are restricted to specific diseases such as NAFLD, or whether both mechanisms work hand-in-hand to synergistically promote the development of HCC in most settings. Some alterations of the gut microbiota are probably disease-specific, and therefore some mechanisms by which the gut microbiota promotes the progression of liver disease and HCC could be — at least in part — disease-specific. Hence, better understanding of disease-specific alterations and thorough determination of their functional contributions to liver disease development are needed. Detailed knowledge about key pathways through which the gut microbiota affect CLD and HCC development could allow the development of broad or tailored therapeutic approaches that block the disease-promoting gut–liver signalling axis. Moreover, our current understanding of the contribution of the gut microbiota is largely based on animal models and faecal microbiota samples from patients. As many of the key changes in the gut–liver axis occur in the small intestine and possibly also within mucosa-adherent microbiota, better analysis of the human microbiome at different anatomic sites is needed. Finally, many types of CLD that confer a high risk for HCC development cannot be perfectly modeled in mice. Therefore, more effort should be put into translating our current knowledge on the HCC-promoting role of the gut–liver axis into well-designed trials in patients.
Intestinal dysbiosis and increased bacterial translocation contribute to the pathophysiology of chronic liver disease (CLD) and hepatocarcinogenesis
A large body of literature has demonstrated that targeting the gut microbiota-liver axis can inhibit the development of hepatocellular carcinoma in mice and rats
Translation of preclinical studies in mice and rats to clinical settings is missing and present a therapeutic opportunity
Targeting the gut-liver axis by non-absorbable antibiotics such as Rifaximin may not only prevent the development of HCC in CLD patients, but additionally reduce other complications, and improve survival.
Acknowledgements:
The authors’ research is supported by U01AA021912, R01CA200597, and R01CA190844 (all to R.F.S).
Footnotes
Competing interests:
The authors declare no competing interests.
REFERENCES
- 1.Rooks MG & Garrett WS Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16, 341–352, doi: 10.1038/nri.2016.42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremaroli V & Backhed F Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249, doi: 10.1038/nature11552 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Schroeder BO & Backhed F Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22, 1079–1089, doi: 10.1038/nm.4185 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Chu H et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 352, 1116–1120, doi: 10.1126/science.aad9948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamas B et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med, doi: 10.1038/nm.4102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeth RA et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19, 576–585, doi: 10.1038/nm.3145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothhammer V et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med, doi: 10.1038/nm.4106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WH et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368, 1575–1584, doi: 10.1056/NEJMoa1109400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63, doi: 10.1038/nature09922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 165, 111–124, doi: 10.1016/j.cell.2016.02.011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwabe RF & Jobin C The microbiome and cancer. Nat Rev Cancer 13, 800–812, doi: 10.1038/nrc3610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabl B & Brenner DA Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524, doi: 10.1053/j.gastro.2014.01.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dapito DH et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516, doi: 10.1016/j.ccr.2012.02.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu LX et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology (Baltimore, Md.) 52, 1322–1333, doi: 10.1002/hep.23845 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto S et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101, doi: 10.1038/nature12347 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Pradere JP, Troeger JS, Dapito DH, Mencin AA & Schwabe RF Toll-like receptor 4 and hepatic fibrogenesis. Seminars in liver disease 30, 232–244, doi: 10.1055/s-0030-1255353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson LW & Artis D Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14, 141–153, doi: 10.1038/nri3608 (2014). [DOI] [PubMed] [Google Scholar]
- 18.van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368, 407–415, doi: 10.1056/NEJMoa1205037 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Kamada N et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329, doi: 10.1126/science.1222195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagaki T et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 103, 3920–3925, doi: 10.1073/pnas.0509592103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadaleta RM et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472, doi: 10.1136/gut.2010.212159 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Dossa AY et al. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol 310, G81–92, doi: 10.1152/ajpgi.00065.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modica S et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142, 355–365 e351–354, doi: 10.1053/j.gastro.2011.10.028 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Lin RS et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol 22, 165–172 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Fukui H, Brauner B, Bode JC & Bode C Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12, 162–169 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Parlesak A, Schafer C, Schutz T, Bode JC & Bode C Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32, 742–747 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Yan AW et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology (Baltimore, Md.) 53, 96–105, doi: 10.1002/hep.24018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouts DE, Torralba M, Nelson KE, Brenner DA & Schnabl B Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol 56, 1283–1292, doi: 10.1016/j.jhep.2012.01.019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellot P et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology (Baltimore, Md.) 52, 2044–2052, doi: 10.1002/hep.23918 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Spadoni I et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834, doi: 10.1126/science.aad0135 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Wiest R & Garcia-Tsao G Bacterial translocation (BT) in cirrhosis. Hepatology (Baltimore, Md.) 41, 422–433 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Qin N et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64, doi: 10.1038/nature13568 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Chen Y et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology (Baltimore, Md.) 54, 562–572, doi: 10.1002/hep.24423 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Bajaj JS, Betrapally NS & Gillevet PM Decompensated cirrhosis and microbiome interpretation. Nature 525, E1–2, doi: 10.1038/nature14851 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouzaki M et al. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PloS one 11, e0151829, doi: 10.1371/journal.pone.0151829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boursier J et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology (Baltimore, Md.) 63, 764–775, doi: 10.1002/hep.28356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabrera R & Nelson DR Review article: the management of hepatocellular carcinoma. Alimentary pharmacology & therapeutics 31, 461–476, doi: 10.1111/j.1365-2036.2009.04200.x (2010). [DOI] [PubMed] [Google Scholar]
- 38.El-Serag HB Hepatocellular carcinoma. N Engl J Med 365, 1118–1127, doi: 10.1056/NEJMra1001683 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Sanyal AJ, Yoon SK & Lencioni R The etiology of hepatocellular carcinoma and consequences for treatment. The oncologist 15 Suppl 4, 14–22, doi: 10.1634/theoncologist.2010-S4-14 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Singal AG & El-Serag HB Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol 13, 2140–2151, doi: 10.1016/j.cgh.2015.08.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao B & Bataller R Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585, doi: 10.1053/j.gastro.2011.09.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi K, Kohli A, Manch R & Gish R Alcoholic Liver Disease: High Risk or Low Risk for Developing Hepatocellular Carcinoma? Clinics in liver disease 20, 563–580, doi: 10.1016/j.cld.2016.02.012 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Rao RK, Seth A & Sheth P Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286, G881–884, doi: 10.1152/ajpgi.00006.2004 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Chen P et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 148, 203–214.e216, doi: 10.1053/j.gastro.2014.09.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo G Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36, doi: 10.1053/j.gastro.2014.10.042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uesugi T, Froh M, Arteel GE, Bradford BU & Thurman RG Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology (Baltimore, Md.) 34, 101–108 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Hritz I et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology (Baltimore, Md.) 48, 1224–1231, doi: 10.1002/hep.22470 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adachi Y, Moore LE, Bradford BU, Gao W & Thurman RG Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108, 218–224 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Machida K et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A 106, 1548–1553, doi: 10.1073/pnas.0807390106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siu L, Foont J & Wands JR Hepatitis C virus and alcohol. Seminars in liver disease 29, 188–199, doi: 10.1055/s-0029-1214374 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michelotti GA, Machado MV & Diehl AM NAFLD, NASH and liver cancer. Nature reviews. Gastroenterology & hepatology 10, 656–665, doi: 10.1038/nrgastro.2013.183 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Backhed F et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101, 15718–15723, doi: 10.1073/pnas.0407076101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031, doi: 10.1038/nature05414 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484, doi: 10.1038/nature07540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang C et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. The Journal of clinical investigation 125, 386–402, doi: 10.1172/jci76738 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology (Baltimore, Md.) 57, 601–609, doi: 10.1002/hep.26093 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Del Chierico F et al. Gut microbiota profiling of pediatric NAFLD and obese patients unveiled by an integrated meta-omics based approach. Hepatology (Baltimore, Md.), doi: 10.1002/hep.28572 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Mouzaki M et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 58, 120–127, doi: 10.1002/hep.26319 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Leung C, Rivera L, Furness JB & Angus PW The role of the gut microbiota in NAFLD. Nature reviews. Gastroenterology & hepatology 13, 412–425, doi: 10.1038/nrgastro.2016.85 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Dumas ME et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A 103, 12511–12516, doi: 10.1073/pnas.0601056103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang XC et al. Phospholipid transfer protein deficiency impairs apolipoprotein-B secretion from hepatocytes by stimulating a proteolytic pathway through a relative deficiency of vitamin E and an increase in intracellular oxidants. The Journal of biological chemistry 280, 18336–18340, doi: 10.1074/jbc.M500007200 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Cani PD et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772, doi: 10.2337/db06-1491 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Miele L et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 49, 1877–1887, doi: 10.1002/hep.22848 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Ye D et al. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut 61, 1058–1067, doi: 10.1136/gutjnl-2011-300269 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Henao-Mejia J et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185, doi: 10.1038/nature10809 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou D et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Scientific reports 7, 1529, doi: 10.1038/s41598-017-01751-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajaj JS et al. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Alimentary pharmacology & therapeutics 44, 638–643, doi: 10.1111/apt.13732 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Chou HH et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 112, 2175–2180, doi: 10.1073/pnas.1424775112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen CJ et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama 295, 65–73, doi: 10.1001/jama.295.1.65 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Affo S, Yu LX & Schwabe RF The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol Mech Dis doi: 10.1146/annurev-pathol-052016-100322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luckey TD, Reyniers JA, Gyorgy P & Forbes M Germfree animals and liver necrosis. Annals of the New York Academy of Sciences 57, 932–935 (1954). [DOI] [PubMed] [Google Scholar]
- 72.Rutenburg AM et al. The role of intestinal bacteria in the development of dietary cirrhosis in rats. The Journal of experimental medicine 106, 1–14 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seki E et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13, 1324–1332, doi: 10.1038/nm1663 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Isayama F et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol 290, G1318–1328, doi: 10.1152/ajpgi.00405.2005 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Mazagova M et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29, 1043–1055, doi: 10.1096/fj.14-259515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tabibian JH et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology (Baltimore, Md.) 63, 185–196, doi: 10.1002/hep.27927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang HL et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol 57, 803–812, doi: 10.1016/j.jhep.2012.06.011 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Nolan JP The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology (Baltimore, Md.) 52, 1829–1835 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Takeuchi O & Akira S Pattern recognition receptors and inflammation. Cell 140, 805–820, doi: 10.1016/j.cell.2010.01.022 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Gabele E et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol 55, 1391–1399, doi: 10.1016/j.jhep.2011.02.035 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Achiwa K et al. DSS colitis promotes tumorigenesis and fibrogenesis in a choline-deficient high-fat diet-induced NASH mouse model. Biochemical and biophysical research communications 470, 15–21, doi: 10.1016/j.bbrc.2015.12.012 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Toyoda H et al. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. The Journal of biological chemistry 270, 7495–7500 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Jing YY et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC medicine 10, 98, doi: 10.1186/1741-7015-10-98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei X et al. Cirrhosis related functionality characteristic of the fecal microbiota as revealed by a metaproteomic approach. BMC gastroenterology 16, 121, doi: 10.1186/s12876-016-0534-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lv LX et al. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environmental microbiology 18, 2272–2286, doi: 10.1111/1462-2920.13401 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Chen Y et al. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Scientific reports 6, 34055, doi: 10.1038/srep34055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer TM et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. The American journal of gastroenterology 97, 2364–2370, doi: 10.1111/j.1572-0241.2002.05791.x (2002). [DOI] [PubMed] [Google Scholar]
- 88.Bajaj JS et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology (Baltimore, Md.) 62, 1260–1271, doi: 10.1002/hep.27819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Minicis S et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology (Baltimore, Md.) 59, 1738–1749, doi: 10.1002/hep.26695 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Loo TM et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov, doi: 10.1158/2159-8290.CD-16-0932 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Lee WJ & Hase K Gut microbiota-generated metabolites in animal health and disease. Nature chemical biology 10, 416–424, doi: 10.1038/nchembio.1535 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Fernandez J et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133, 818–824, doi: 10.1053/j.gastro.2007.06.065 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Vlachogiannakos J et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. Journal of gastroenterology and hepatology 28, 450–455, doi: 10.1111/jgh.12070 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Elfert A, Abo Ali L, Soliman S, Ibrahim S & Abd-Elsalam S Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. European journal of gastroenterology & hepatology 28, 1450–1454, doi: 10.1097/meg.0000000000000724 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Sharma BC et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. The American journal of gastroenterology 108, 1458–1463, doi: 10.1038/ajg.2013.219 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Zhu Q et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol 56, 893–899, doi: 10.1016/j.jhep.2011.11.013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steib CJ et al. Intraperitoneal LPS amplifies portal hypertension in rat liver fibrosis. Lab Invest 90, 1024–1032, doi: 10.1038/labinvest.2010.60 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Lutz P et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PloS one 9, e93909, doi: 10.1371/journal.pone.0093909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bass NM et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 362, 1071–1081, doi: 10.1056/NEJMoa0907893 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Iida N et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970, doi: 10.1126/science.1240527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Viaud S et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976, doi: 10.1126/science.1240537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vetizou M et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084, doi: 10.1126/science.aad1329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sivan A et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089, doi: 10.1126/science.aac4255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S & Medzhitov R Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Steffen EK, Berg RD & Deitch EA Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis 157, 1032–1038 (1988). [DOI] [PubMed] [Google Scholar]
- 106.Navasa M et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology 111, 1011–1017 (1996). [DOI] [PubMed] [Google Scholar]
- 107.Bert F et al. Genetic diversity and virulence profiles of Escherichia coli isolates causing spontaneous bacterial peritonitis and bacteremia in patients with cirrhosis. J Clin Microbiol 48, 2709–2714, doi: 10.1128/JCM.00516-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jalan R et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 60, 1310–1324, doi: 10.1016/j.jhep.2014.01.024 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Gines P et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology (Baltimore, Md.) 12, 716–724 (1990). [DOI] [PubMed] [Google Scholar]
- 110.Fernandez J et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology (Baltimore, Md.) 35, 140–148 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Fernandez J et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology (Baltimore, Md.) 55, 1551–1561, doi: 10.1002/hep.25532 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Tandon P, Delisle A, Topal JE & Garcia-Tsao G High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol 10, 1291–1298, doi: 10.1016/j.cgh.2012.08.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koo HL & DuPont HL Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 26, 17–25, doi: 10.1097/MOG.0b013e328333dc8d (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vlachogiannakos J et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Alimentary pharmacology & therapeutics 29, 992–999, doi: 10.1111/j.1365-2036.2009.03958.x (2009). [DOI] [PubMed] [Google Scholar]
- 115.Kalambokis GN et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol 10, 815–818, doi: 10.1016/j.cgh.2012.02.025 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Chen P, Starkel P, Turner JR, Ho SB & Schnabl B Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology (Baltimore, Md.) 61, 883–894, doi: 10.1002/hep.27489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Membrez M et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22, 2416–2426, doi: 10.1096/fj.07-102723 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Natividad JM & Verdu EF Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 69, 42–51, doi: 10.1016/j.phrs.2012.10.007 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Cesaro C et al. Gut microbiota and probiotics in chronic liver diseases. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 43, 431–438, doi: 10.1016/j.dld.2010.10.015 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Li J et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A 113, E1306–1315, doi: 10.1073/pnas.1518189113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dhiman RK et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 147, 1327–1337.e1323, doi: 10.1053/j.gastro.2014.08.031 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Alisi A et al. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Alimentary pharmacology & therapeutics 39, 1276–1285, doi: 10.1111/apt.12758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arthur JC et al. VSL#3 probiotic modifies mucosal microbial composition but does not reduce colitis-associated colorectal cancer. Scientific reports 3, 2868, doi: 10.1038/srep02868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02496390. (2016). [DOI] [PubMed]
- 125.US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02862249. (2016). [DOI] [PubMed]
- 126.Vrieze A et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 143, 913–916.e917, doi: 10.1053/j.gastro.2012.06.031 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Kelly CP Fecal microbiota transplantation--an old therapy comes of age. N Engl J Med 368, 474–475, doi: 10.1056/NEJMe1214816 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Piazza M et al. Glycolipids and benzylammonium lipids as novel antisepsis agents: synthesis and biological characterization. J Med Chem 52, 1209–1213, doi: 10.1021/jm801333m (2009). [DOI] [PubMed] [Google Scholar]
- 129.Peri F & Piazza M Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv 30, 251–260, doi: 10.1016/j.biotechadv.2011.05.014 (2012). [DOI] [PubMed] [Google Scholar]
- 130.Barochia A, Solomon S, Cui X, Natanson C & Eichacker PQ Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol 7, 479–494, doi: 10.1517/17425255.2011.558190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Naugler WE et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Opal SM et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. Jama 309, 1154–1162, doi: 10.1001/jama.2013.2194 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Rice TW et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Critical care medicine 38, 1685–1694, doi: 10.1097/CCM.0b013e3181e7c5c9 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Lorenzo-Zuniga V et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology (Baltimore, Md.) 37, 551–557, doi: 10.1053/jhep.2003.50116 (2003). [DOI] [PubMed] [Google Scholar]
- 135.Ding JW, Andersson R, Soltesz V, Willen R & Bengmark S The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res 25, 11–19 (1993). [DOI] [PubMed] [Google Scholar]
- 136.Schaap FG, Trauner M & Jansen PL Bile acid receptors as targets for drug development. Nature reviews. Gastroenterology & hepatology 11, 55–67, doi: 10.1038/nrgastro.2013.151 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Thomas C, Pellicciari R, Pruzanski M, Auwerx J & Schoonjans K Targeting bile-acid signalling for metabolic diseases. Nature reviews. Drug discovery 7, 678–693, doi: 10.1038/nrd2619 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Degirolamo C et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology (Baltimore, Md.) 61, 161–170, doi: 10.1002/hep.27274 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Marschall H-U, L. V., Lovgren-Sandblom A, Benthin L, Kowdley K 4, Hirschfield G, Mason A, Lindor K, Gordon S, Vincent C, Chapman R, Mayo M, Burroughs A, Pares A, Jones D, Schramm C, Eliot L, Hofmann AF, Shapiro D The farnesoid X receptor (FXR) agonist (OCA) increases plasma FGF-19 concentrations and decreases bile acid synthesis in primary biliary cirrhosis (PBC). J. Hepatol. 56, S377 (2012). [Google Scholar]
- 140.Kim I et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28, 940–946, doi: 10.1093/carcin/bgl249 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verbeke L et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. The American journal of pathology 185, 409–419, doi: 10.1016/j.ajpath.2014.10.009 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Ubeda M et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 64, 1049–1057, doi: 10.1016/j.jhep.2015.12.010 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Verbeke L et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology (Baltimore, Md.) 59, 2286–2298, doi: 10.1002/hep.26939 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Neuschwander-Tetri BA et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet (London, England) 385, 956–965, doi: 10.1016/s0140-6736(14)61933-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Munoz L et al. Mesenteric Th1 polarization and monocyte TNF-alpha production: first steps to systemic inflammation in rats with cirrhosis. Hepatology (Baltimore, Md.) 42, 411–419, doi: 10.1002/hep.20799 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Genesca J et al. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut 52, 1054–1059 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor CT, Dzus AL & Colgan SP Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology 114, 657–668 (1998). [DOI] [PubMed] [Google Scholar]
- 148.Frances R et al. Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol 46, 797–803, doi: 10.1016/j.jhep.2006.11.018 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Kalaitzakis E Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol 20, 14686–14695, doi: 10.3748/wjg.v20.i40.14686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pardo A et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology (Baltimore, Md.) 31, 858–863 (2000). [DOI] [PubMed] [Google Scholar]
- 151.Zhang SC et al. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol 9, 534–538 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Madrid AM, Hurtado C, Venegas M, Cumsille F & Defilippi C Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am.J.Gastroenterol. 96, 1251–1255 (2001). [DOI] [PubMed] [Google Scholar]
- 153.Perez-Paramo M et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology (Baltimore, Md.) 31, 43–48 (2000). [DOI] [PubMed] [Google Scholar]
- 154.Madsen BS, Havelund T & Krag A Targeting the gut-liver axis in cirrhosis: antibiotics and non-selective beta-blockers. Adv Ther 30, 659–670, doi: 10.1007/s12325-013-0044-1 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Mookerjee RP et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol 64, 574–582, doi: 10.1016/j.jhep.2015.10.018 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Senzolo M et al. Oral propranolol decreases intestinal permeability in patients with cirrhosis: another protective mechanism against bleeding? The American journal of gastroenterology 104, 3115–3116, doi: 10.1038/ajg.2009.457 (2009). [DOI] [PubMed] [Google Scholar]
- 157.Senzolo M et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 29, 1189–1193, doi: 10.1111/j.1478-3231.2009.02038.x (2009). [DOI] [PubMed] [Google Scholar]
- 158.Nkontchou G et al. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila) 5, 1007–1014, doi: 10.1158/1940-6207.CAPR-11-0450 (2012). [DOI] [PubMed] [Google Scholar]
- 159.De Santis S, Cavalcanti E, Mastronardi M, Jirillo E & Chieppa M Nutritional Keys for Intestinal Barrier Modulation. Frontiers in immunology 6, 612, doi: 10.3389/fimmu.2015.00612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zapater P et al. Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology 137, 1669–1679.e1661, doi: 10.1053/j.gastro.2009.07.058 (2009). [DOI] [PubMed] [Google Scholar]
- 161.Frances R et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 47, 978–985, doi: 10.1002/hep.22083 (2008). [DOI] [PubMed] [Google Scholar]