Abstract
Over the last two decades, improved access to human islets and the development of human islet distribution networks have enabled the use of millions of human islets in hundreds of scientific research projects, leading to a dramatic increase in our understanding of human islet biology. Here we discuss recent scientific advances as well as methodological and experimental challenges that impact human islet quality, experimental outcomes and the reporting of human islets used in scientific publications. In a survey of over 200 scientific publications with human islet experimentation, we found that the reporting of critical information was quite variable, sometimes obscure, and often failed to adequately outline the experiments and results using human islets. As the complexity of human islet research grows, we propose that members of the human islet research ecosystem work together to develop procedures and approaches for accessible and transparent collecting and reporting of crucial human islet characteristics and, through this, enhance collaboration, reproducibility and rigour, leading to further advances in our understanding of human islet biology.
Keywords: Diabetes, Human, Insulin, Islets, Pancreas, Reporting, Research, Resource, Review, Rigour, Transparency, Unique identifier
Introduction
The islets of Langerhans, comprising only ~2% of the adult human pancreas, regulate nutrient homeostasis via secretion of hormones such as insulin, glucagon and somatostatin. Islet failure through destruction and/or dysfunction underlies all forms of diabetes. Our knowledge of islet development, endocrine cell stimulus–secretion coupling, gene expression and islet cell signatures in healthy human islets and in islets in type 1 diabetes and type 2 diabetes has improved but is still inadequate. The reasons are multiple, but include the inability to safely and effectively biopsy the human pancreas and islets, the difficulty and cost of obtaining high quality donor pancreas and isolating human islets, and the limitation that pancreas begins to autodigest soon after death. Because of these challenges, until recently, much islet experimentation has been on islets from other species. However, over the past two decades, increased availability of human islets for research has enabled exciting advances in our understanding of human islet biology. This review will focus on three aspects of human islet research: a summary of recent research progress, an outline of research challenges and, finally, suggestions for future research with human islets. As human islet research continues to expand there is a need for accessible, standardised and transparent collecting and reporting of human islet information.
Increased availability of human islets has led to progress in understanding human islet biology
Improved access to human islets for research
Initially, efforts to isolate human islets focused primarily on isolation of a sufficient number and quality for transplantation into individuals with type 1 diabetes. With the support of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in the USA, the JDRF, the Special Diabetes Program and others, clinical human islet isolation centres in the USA, Canada, Europe and Australia began to isolate and distribute human islets for research-specific purposes through programmes like the Islet Cell Resource Center Consortium [1, 2]. These research-supporting islet isolation and distribution programmes have continued to evolve worldwide and now include the Integrated Islet Distribution Program (IIDP), the European Consortium for Islet Transplantation (ECIT), the Nordic Network for Clinical Islet Transplantation, the Australian Islet Transplant Consortium, and the IsletCore Program and the Alberta Islet Distribution Program (both at the University of Alberta in Canada). Over the past 15 years, these programmes and commercial sources have supplied millions of human islets to hundreds of scientists, leading to a dramatic increase in the number of scientific publications describing research with human islets.
Improved understanding of human islet biology
Greater availability of human islets and expanded research efforts have led to an improved understanding of numerous facets of human islet biology. Regrettably, space limitations prevent more than a partial listing of selected advances and references (see Text box 1 and Table 1, Human column).
Tex box 1: Areas of recent advances in human islet biology.
Islet structure, cell composition, cell arrangement, vasculature and innervation
Gene expression and regulation in beta cells and other islet endocrine cells
Islet secretory function and islet cell electrical activity
Islet response to injury and stress
Islet cell proliferation and identity
Table 1.
Summary of recent advances in human islet biology and selected comparisons of human and rodent islets
| Process/property | Human | Rodent |
|---|---|---|
| Islet structure | ||
| Cellular architecture [33–38] | Intermingled endocrine cell types (adult) Young human islets resemble mouse | Beta cell core surrounded by non-beta cell mantle |
| Cellular composition (adult) [33, 34, 37] | ~55% beta, ~35% alpha, ~10% delta | ~75% beta, ~20% alpha, ~5% delta |
| Vasculature [38, 39] | Less dense | Dense |
| Innervation [38, 40] | Less dense, contacts vasculature | Dense, contacts endocrine cells |
| Basement membrane [41, 42] | Double | Single |
| Gene expression | ||
| Shared expression [43] | Core set of ~9900 beta cell transcripts | Core set of ~9900 beta cell transcripts |
| Transcription factors [38, 43–45] | UCN3 expression in beta and alpha cells MAFA and MAFB expression in beta cells | Ucn3 expression restricted to beta cells Mafa expression only in beta cells |
| Hormones, receptors, transporters in beta cells [43, 44, 46] | Single insulin gene (INS) Enriched SLC2A1 (encoding GLUT1) expression Enriched GAD2 expression | Two insulin genes (Ins1 and Ins2) Enriched Slc2a2 (encoding GLUT2) expression Enriched expression of Class I helical cytokine receptor family (Prlr, Ghr, Cntfr) and Il1r1 |
| Islet function | ||
| Insulin secretion activation threshold [46–48] | ~3 mmol/l glucose | ~ 5 mmol/l glucose |
| Insulin half-maximal secretion [46–48] | ~6 mmol/l glucose | ~8 mmol/l glucose |
| Beta cell electrical activity [15, 34, 38, 46, 49, 50] | Sub-islet synchrony, variable response Resting membrane conductance <10% of that in the mouse | Synchronous, oscillatory |
| Amount of insulin secreted/IEQ [44] | Less | More |
| Beta cell heterogeneity [19, 50–54] | Four subtypes: ST8SIA1 +/− and CD9 +/− ‘Hub’ cells | Two subtypes Fltp +/− ‘Hub’ cells |
| Response to injury/stress | ||
| Chemical (alloxan/streptozotocin) [55, 56] | Less sensitive | More sensitive |
| Reactive oxygen species [55–57] | Less sensitive | More sensitive |
| Cytokines [56, 58, 59] | Less sensitive | More sensitive |
| Elevated glucose [56, 60, 61] | More sensitive | Less sensitive |
| Insulin resistance/lipidaemia [61] | More sensitive | Less sensitive |
| Islet amyloid polypeptide [62] | Forms amyloid plaques | Does not form plaques |
| Transdifferentiation in diabetes [63–67] | Alpha and delta to beta cell conversion unclear | In vivo alpha and delta to beta cell conversion with stress |
| Dedifferentiation in diabetes [64, 66–68] | Unclear | Reversion to NGN3+, Ins-cells with stress |
| Islet endocrine cell proliferation | ||
| Beta cells [36, 69–73] | Adult cells: very low proliferation rate Juvenile islets: more prone to proliferation, responsive to exendin-4 | Proliferate in response to injury, stress and several agents |
| Alpha cells [74–77] | Amino acid-stimulated proliferation | Amino acid-stimulated proliferation |
Because of space limitations, only selected advances, comparisons and references are shown. NGN3, neurogenin 3; ST8SIA1, ST8 α-N-acetyl-neuraminide α−2,8-sialyltransferase 1
An important result of greater human islet accessibility has been a greater appreciation of the similarities and differences between human and rodent islets. Improved understanding of the characteristics of both types of islets has led to the establishment of research initiatives, such as the Human Islet Research Network (HIRN; https://hirnetwork.org), to better understand human islet biology. Table 1 summarises selected features of human and rodent islets. We do not question the use of rodent islets, but instead emphasise that to fully understand the role of the islet in human diabetes and to translate findings from commonly used models, the field needs integrated approaches incorporating multiple sources of islets and experimental designs that recognise the shared and disparate characteristics of human islets, non-human islets, and insulin-producing cells derived from pluripotent stem cells.
Challenges of using human islets to understand islet biology and human diabetes
Human islet biology research faces several challenges. These include (1) a costly, experimentally and logistically challenging organ procurement-islet isolation-islet delivery chain; (2) genetic and environmental variability of islet donors; (3) variability in human islet preparations; and (4) loss of some physiological features during islet culture. Access and cost of human islets continue to be significant obstacles to human islet research. However, these issues are beyond the scope of this review and have been discussed previously [3].
Factors affecting functional properties of human islet preparations
Several studies have identified donor, pancreas and pancreas-processing characteristics that influence islet isolation yield and transplant outcomes [4–11]. In contrast, few studies have examined factors that affect the functional quality of human islets distributed for research or clinical islet transplantation. Surprisingly, the characteristics and assays that define human islet quality are not universally accepted or standardised. Insulin secretion, assessed either in static assay or in a dynamic perifusion system, is the most commonly reported measurement of islet quality. Secretion of other islet hormones, such as glucagon, and other islet metrics (e.g. oxygen consumption) have not been assessed in a large set of human islet preparations. Thus, for this review, we considered insulin secretion as the primary in vitro measure of islet quality. This discussion about the criteria for islet quality only applies to human islets used for research; clinical islet transplantation programmes have developed quality metrics for human islets used for clinical transplantation [12].
Three recent reports have assessed the in vitro islet insulin secretory function of a large number (>50) of human islet preparations by perifusion or static culture [9, 13, 14]. Two reports described human islet preparations generated at one or two islet isolation sites (the University of Alberta or the University of Louvain and the University of Lille) [9, 13], and the third report described preparations from multiple IIDP-sponsored isolation centres [14]. These studies used different methodologies to examine the determinants of human islet functional variability. Nonetheless, they represent a strong foundation to address sources of human islet functional inconsistency that may arise from variation in donor characteristics, pancreas processing and/or isolation centres [9, 13, 14].
The study that examined IIDP-derived islet preparations found that most human islet preparations secreted insulin in response to increased extracellular glucose and increased glucose with cAMP stimulation during islet perifusion. However, nearly a quarter of human islet preparations were dysfunctional (i.e. unstable basal insulin secretion and/or impaired stimulated insulin secretion) [14]. This, and other studies [9, 13, 15], suggest that the following characteristics affected in vitro islet insulin secretion (i.e. basal and/or stimulated insulin secretion): isolation centre [14], cause of death (COD) [14], estimated islet purity [13, 14], BMI [13, 14], islet size [13], pancreas cold ischaemia time (CIT) [9], age [9, 15] and HbA1c [9]. The Alberta-based study noted several similar factors impacted human islet function but concluded that these made a modest contribution to the overall human islet functional heterogeneity observed [9].
Overall, these studies emphasise important considerations for research using human islets, including (1) considerable functional heterogeneity exists between human islet preparations; (2) a significant percentage of human islet preparations are dysfunctional as defined by insulin secretion; (3) explanations for this heterogeneity or dysfunction are not clear, and multiple biological, donor, transport, isolation and as-yet unknown characteristics likely influence islet quality; and (4) the functional state of an islet preparation cannot be predicted or assumed a priori. In light of these challenges, we believe that investigators, journals and funding agencies must develop more holistic, nuanced and sophisticated standards and criteria for assessing and reporting critical characteristics of human islets used for research.
Characteristics of human islet preparations reported in recent scientific publications
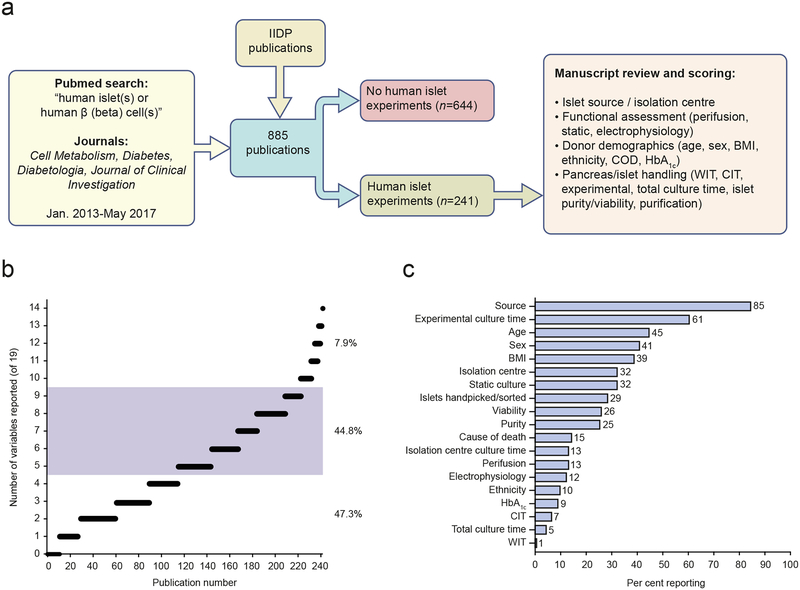
Since human islet quality is a critical consideration for research and may be influenced by various characteristics, we assessed which characteristics of human islet preparations have been reported in recent publications. We conducted a PubMed literature search of four leading journals that publish human islet research (Cell Metabolism, Diabetes, Diabetologia and Journal of Clinical Investigation) from 2013 to mid-2017, and combined these search results with the IIDP publication list (Fig. 1a). We identified 241 publications that contained studies of isolated human islets. We then assessed whether each publication reported a selected set of 19 islet characteristics related to islet origin, islet function, donor demographics and pancreatic/islet handling that have been reported to, or would be predicted to, influence islet quality (Fig. 1b, c). Review of articles published in other journals would be expected to produce similar findings.
Fig. 1.
Reporting of pancreas donor and human islet information in research publications. (a) Schematic of literature review of publications describing research with human islets. (b) Number of variables reported in each manuscript (individual manuscripts are represented by open black circles; multiple, overlapping circles create a black line with the length reflected by the number of circles). ~47% of manuscripts report four islet variables or fewer. ~45% report between five and nine variables (shaded grey) and ~8% of manuscripts report ten or more islet variables. (c) Percentage of manuscripts (n=241 in total) that reported selected variables of islet donor/islet function/islet handling information (listed on y-axis). Functional measurement was considered as having performed tests of islet responsiveness to glucose using static culture, perifusion or electrophysiology. CIT, cold ischaemia time; WIT, warm ischaemia time. This figure is available as part of a downloadable slideset
In these 241 publications, the information about the human islet preparations was often difficult to find, quite variable and in many cases did not adequately report the nature of the human islets used. Several studies reported none of the 19 selected islet characteristics examined (Fig. 1b). The most detailed publication reported 14 of the 19 characteristics (Fig. 1b). Almost 50% of human islet studies described four or fewer of the selected characteristics (Fig. 1b). The islet source and experimental culture time were the only two characteristics reported in more than 50% of the reviewed publications (Fig. 1c). We noted that ~15% of publications did not report where the investigators obtained the human islets from and ~70% did not report the islet isolation centre. Furthermore, the majority of publications did not report details of human islet donor demographic information. Age, sex and BMI were reported most commonly (reported in 45%, 41%, 39%, respectively), then COD, ethnicity and HbA1c (15%,10%, and 9%, respectively). In addition, the vast majority of studies did not mention warm ischaemia time (1%) or CIT (7%) or the total time of islet culture (5%).
More than 50% of publications did not report an assessment of islet function. Thus, the quality and function of the human islets used in those publications were not relayed to the readership and were potentially not known. The most common functional islet assessment method was static islet culture (32%), followed by perifusion (13%) and electrophysiology (12%), with some studies reporting more than one measure of islet function. Estimated islet viability and purity and efforts to purify islets after arrival in the user’s laboratory (e.g. handpicked or sorted) were reported in 26%, 25% and 29% of studies, respectively. Since additional islet purification was not reported in most studies, and human islet preparation purity and determination of purity can vary considerably, the possibility of inclusion of non-islet cells (acinar, ductal, etc.) in RNA and protein studies is quite likely and could be problematic.
We also observed that in publications that studied numerous islet preparations, it was often difficult to discern which islet preparations were used for a particular experiment, highlighting the need for a clear description of human islets used in specific experiments within publications. Finally, we observed that human islet experiments were often a minor aspect of the manuscript. For example, many manuscripts had a single figure or figure panel with human islets that contained limited experiments. We appreciate that human islet scarcity and cost are substantial challenges and likely to be responsible for these experimental limitations (addressed in [3]). Nevertheless, if human islets are used in research studies, key islet characteristics should be reported and the experimental design should be transparent and rigorous to optimise data interpretation.
One can readily see how different interpretations or conclusions related to human islet biology could arise from different laboratories performing human islet experiments at different times with different islet preparations. Thus, standardised and more detailed assessment and reporting of biological and non-biological variables such as pancreas processing, donor and islet characteristics are needed. There is also certainly biological variability between human islet preparations and future efforts are needed to define these sources of this variability.
Suggestions for the human islet research ecosystem
Human islet research relies on a complex infrastructure to rapidly separate a small group of viable cells from a heterogeneous gland and subsequently distribute these under time constraints to national/international investigators. For the purposes of this review, we term this complex network the ‘human islet research ecosystem’, which consists of organ procurement organisations, islet isolation centres, islet distribution programmes, centralised islet phenotyping centres, scientific investigators, funding agencies and the scientific publication infrastructure (see Text box 2). As suggested by our literature search, our own experience and discussions with various entities of this ecosystem, communication, reporting and data sharing are often less than optimal. Here, we propose that members of the human islet research ecosystem enhance the current systems of human islet assessment and distribution, experimental approaches and reporting of methods in scientific publications. This will improve the human islet research infrastructure and further advance our understanding of human islet biology. These suggested enhancements and modifications will require the involvement of all members of the human islet research ecosystem (discussed below; Table 2). We hope this review stimulates discussion, ideas, and action that lead to improvements in this critical research ecosystem. In Fig. 2 and Table 2, we suggest actions by members of the human islet research ecosystem and briefly discuss these in the text that follows.
Tex box 2: The human islet research ecosystem.
Organ procurement organisations
Islet isolation centres
Islet distribution programmes
Islet phenotyping centres
Scientific investigators
Funding agencies
Scientific journals
Table 2.
Responsibilities of members of the human islet research ecosystem
| Key human islet characteristics | Islet centre/distribution network (collection and reporting of information) | Centralised islet phenotyping centre (generation and reporting of information) | Investigator (collection, generation and reporting of information) | Journal (ensuring reporting of data) |
|---|---|---|---|---|
| Pancreas donor demographics | ||||
| Unique identifiera | X | X | ||
| Agea, sexa, BMIa, ethnicitya | X | X | ||
| HbA1ca | X | X | ||
| CODa | X | X | ||
| Pancreas for islet isolation | ||||
| Origin/source | X | X | ||
| WITa | X | X | ||
| CITa | X | X | ||
| Islets distributed for research | ||||
| Origin/source (IIDP, ECIT, Alberta IsletCore, etc.) | X | X | ||
| Isolation centrea | X | X | ||
| Estimated puritya | X | X | ||
| Estimated viabilitya | X | X | ||
| Total culture timea | X | X | ||
| Functional measurement | X | X | ||
| Compilation of human islet preparation information | X | X | ||
| Description of human islet handling, purification and quantification | X | X | ||
| Description of experimental islet use | X | X |
Characteristics currently reported by IIDP
WIT, warm ischaemia time
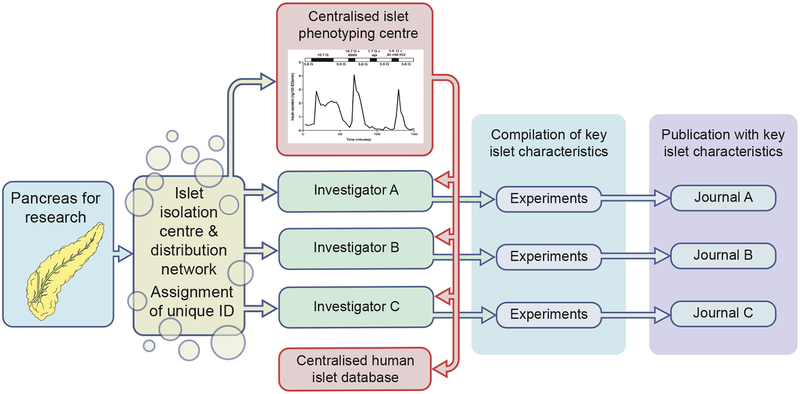
Fig. 2.
Preservation of key human islet information throughout the human islet research. Proposed responsibilities of members of the human islet ecosystem for the dissemination of human islet characteristics from organ procurement to publication, as outlined in Table 2. The red line represents communication of islet quality between a centralised phenotyping centre and investigators, as well as a centralised human islet database. Individual investigators should be responsible for compiling the characteristics of human islet preparations, and journals should ensure these characteristics are reported in a clear, accessible manner. This figure is available as part of a downloadable slideset
Need for a unique identifier for each islet preparation
We propose that each islet preparation be assigned a unique identifier, something equivalent to a Globally Unique Identifier (GUID) or Universally Unique Identifier (UUID) that is used to identify information in computer systems (https://en.wikipedia.org/wiki/Universally_unique_identifier). The IIDP has recently adopted an approach to assign each human islet preparation a Research Resource Identifier (RRID) based on the US National Institutes of Health (NIH) Resource Identification Portal created as part of their initiative to authenticate key biological resources (https://scicrunch.org/resources). The creation, use, and reporting of an RRID for each human islet preparation would allow investigators to identify which islet preparations were used in particular publications and if other investigators used the same islet preparation. A unique research identifier for each human islet preparation would also create an organisational framework, allowing establishment of centralised databases in which multidisciplinary human islet data from multiple sources and investigators could be stored, referenced, accessed and harmonised.
Infrastructure support
Funding agencies and academic medical centres directly or indirectly support several components of the human islet research ecosystem. Funding agencies should continue to provide critical financial and organisational support for human islet production and distribution. Funding agencies are also vital in ensuring that different components of the human islet research ecosystem work cooperatively and meet their responsibilities.
Islet isolation, phenotyping, and distribution
As part of their regular activities, islet isolation centres and islet distribution programmes should continue to gather information on donor demographics, document pancreatic handling before and during the isolation procedures, assign a unique identifier to islet preparations, and make this information available to recipient investigators. Because the islet isolation centres are dispersed and function largely autonomously, phenotyping of human islets used for research has been performed at each isolation site and/or has been left to individual laboratories. As evident from our literature review, most publications have not reported the function and health of islet preparations and there is a lack of agreement on a standardised way of assessing human islet health and function. Investigators must establish and report the function and health of islets used in their studies. We support efforts to establish centralised islet phenotyping centres and/or standardised approaches to assess the health, function, number and purity of all research islets. To this end, the IIDP has recently developed the Human Islet Phenotyping Program (HIPP), which will provide phenotypic data on all IIDP-associated islet shipments. In this programme, a small aliquot of each IIDP-distributed islet preparation is evaluated in a centralised phenotyping centre using standardised protocols, allowing for enhanced comparison of experimental outcomes with different islet preparations. The HIPP is assessing human islet insulin and glucagon secretion, estimated viability, estimated purity and morphological characteristics. These data will be made available to investigators using IIDP islet preparations. The University of Alberta IsletCore also characterises each of their islet preparations and makes this information available upon request. By informing investigators in a timely manner about the function and health of islet preparations, centralised phenotyping centres will ensure that investigators know the quality and function of the islets used in an experiment. Additionally, centralised phenotyping centres could also reduce net experimental cost by eliminating the need for extensive islet phenotyping by individual laboratories. Furthermore, such efforts may foster collaboration between investigators using the same human islet preparation and lay the foundation for human islet data repositories, such as is being done in the Nordic Network for Clinical Islet Transplantation (https://nordicislets.medscinet.com/en/islet-isolation/nics-database.aspx). Since human islets are being distributed for research in the USA, Canada, Europe and Australia, we suggest, as a first step, that the human islet ecosystem work collaboratively to standardise islet assessment through workshops and other efforts, as has been done for standardisation of other assays (e.g. HbA1c and islet cell autoantibodies). In this way, centralised phenotyping centres could also determine which assays are optimal for assessing human islet health and function. For example, whether additional assays beyond insulin secretion, such as glucagon secretion and oxygen consumption, should be considered for assessing human islet health. Unfortunately, it is not known whether the same assessment of islet quality is needed for islets used in very different experimental approaches (e.g. genetics, protein expression, RNA isolation).
Investigators using human islets for research
We propose that investigators using human islets should be responsible for ensuring that the appropriate donor and pancreas characteristics are compiled and recorded (Table 2). In addition, investigators should report how and when human islets were used, including the number of islets used in particular experiments and whether the islets were further purified in any manner. We suggest that investigators studying human islets (1) report stimulated hormone secretion; (2) determine at the receiving institution the quantity of human islets used in experiments (i.e. in-house calculation of islet equivalents/amount); (3) if conducting studies of islet proteins or nucleic acids, use purified islets or islet cells or report the relative contributions of various cell populations; and (4) record and report key islet characteristics and experimental use.
Scientific journals
Scientific journals publishing reports on human islet research should ensure that key human islet characteristics are reported and presented in a clear and accessible manner. To facilitate the reporting of key information about human islet preparations, we propose a checklist detailing human islet characteristics that would be completed by investigators and submitted with the publication, as is now required by several journals for methods and reagents (ESM Table 1). We hope the proposed checklist will stimulate discussion and will be modified and improved by investigators and journals moving forward.
Other considerations for handling and reporting human islet samples
In addition to human islets from non-diabetic individuals, improved pancreatic procurementisolation-delivery chains have increased the availability of pancreases and islets from individuals with diabetes (type 1, type 2, pancreatogenic, cystic fibrosis-related, monogenic) for use in research [16–26]. These valuable islets present their own unique set of challenges, including the cellular composition of the islet, which may differ from normal islets and affect their function. We suggest that investigators using isolated islets from individuals with diabetes collect and report as much clinical and research information as possible without compromising the anonymity of the islet donor. This is especially true for islets from individuals with type 2 diabetes, where clinical information (stage of disease, treatment, etc.) is often unavailable or not reported. Furthermore, recent studies highlight that clear and correct clinical diagnosis of type 1 and 2 diabetes, monogenic diabetes or pancreatogenic diabetes is more difficult than previously appreciated [27–32], emphasising the need for holistic investigation and integration of donor information and research with pancreatic tissue and islets.
Conclusions
The recent expansion of human islet research has led to significant gains in our understanding of human islet biology. However, the increased availability of human islets has simultaneously revealed several experimental and reporting challenges, including crucial information about human islet preparations not being available, collected or reported. We have suggested ways that the members of the human islet research ecosystem can work in partnership to improve how human islet research is conducted and communicated. We advocate for adoption of a unique research identifier for each human islet preparation and centralised and standardised islet phenotyping to ensure that human islet preparations from different facilities can be compared and integrated into experimental results arising from human islet research. To build on recent momentum in human islet research, we propose that investigators and journals adopt clear, rigorous and standardised experimental approaches and report key islet characteristics and experimental details. We also provide a standardised template that can be used to report key characteristics of human islets and human islet use.
Preparation of this review generated many questions and issues that should be addressed, such as the availability and reportability of donor clinical information and the contribution of biology or islet isolation technique to human islet variability. New efforts and paradigms are needed to address these questions and issues. Clearly, more discussion and consensus are needed among members of the human islet research ecosystem, which will hopefully lead to new ideas, policies and procedures related to human islet research. For example, we suggest a workshop or consensus conference where members from each arm of the human islet research ecosystem discuss challenges and opportunities and identify ways to improve procedures, protocols and communication related to human islets. As new technologies enable new experimental approaches, improvements and modifications in the human islet research ecosystem will further expand our knowledge of human islet biology in health and disease.
Supplementary Material
Acknowledgements
We thank numerous scientists for their helpful suggestions related to the manuscript, but the opinions are those of the authors. We especially thank G. Poffenberger and J. Kimber (Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA) for their assistance in the literature review. We highlight that publications from our research group at Vanderbilt were included in the 241 publications mentioned in this review.
Funding
Work in ACP’s laboratory is supported by resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID: SCR014393; https://hirnetwork.org; UC4 DK104211, DK108120 and DK112232), by DK106755, DK89572, DK97829, DK94199, the Vanderbilt Diabetes Research and Training Center (DK20593), and by grants from the JDRF, The Leona M. and Harry B. Helmsley Charitable Trust and the Department of Veterans Affairs. NJH was supported by the Vanderbilt Research Training in Diabetes and Endocrinology Program (grant 5T32 DK007061).
Abbreviations
- CIT
Cold ischaemia time
- COD
Cause of death
- ECIT
European Consortium for Islet Transplantation
- HIPP
Human Islet Phenotyping Program
- HIRN
Human Islet Research Network
- IIDP
Integrated Islet Distribution Program
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- RRID
Research Resource Identifier
Footnotes
Tweet (250 character max.-*Figure 2 best represents the paper): Use and reporting of human islets is crucial for diabetes research #diabetes #humanislets @VUMChealth
Duality of interest
The authors declare that there is not duality of interest associated with this manuscript. The HIPP of the IIDP is located at Vanderbilt University Medical Center and directed by M. Brissova, a collaborator of the authors. Our research group at Vanderbilt has received human islets from the IIDP and the Alberta islet isolation facility.
References
- 1.Kaddis JS, Olack BJ, Sowinski J, et al. (2009) Human pancreatic islets and diabetes research. JAMA 301:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niland JC, Stiller T, Cravens J, et al. (2010) Effectiveness of a web-based automated cell distribution system. Cell Transplant 19:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni RN, Stewart AF (2014) Summary of the Keystone islet workshop (April 2014): the increasing demand for human islet availability in diabetes research. Diabetes 63:3979–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaddis JS, Danobeitia JS, Niland JC, et al. (2010) Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am J Transplant 10:646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaddis JS, Hanson MS, Cravens J, et al. (2013) Standardized transportation of human islets: an islet cell resource center study of more than 2,000 shipments. Cell Transplant 22:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balamurugan AN, Naziruddin B, Lockridge A, et al. (2014) Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am J Transpant 14:2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilling DE, Bouwman E, Terpstra OT, Marang-Van De Mheen PJ (2014) Effects of donor, pancreas-, and isolation-related variables on human islet isolation outcome: a systematic review. Cell Transplant 23:921–928. [DOI] [PubMed] [Google Scholar]
- 8.Nano R, Clissi B, Melzi R, et al. (2005) Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia 48:906–912. [DOI] [PubMed] [Google Scholar]
- 9.Lyon J, Manning Fox JE, Spigelman AF, et al. (2016) Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 157:560–569. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro AMJ, Pokrywczynska M, Ricordi C (2016) Clinical pancreatic islet transplantation. Nat Rev Immunol 13:268–277. [DOI] [PubMed] [Google Scholar]
- 11.Ponte GM, Pileggi A, Messinger S, et al. (2007) Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell Transplant 16:595–607. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Goldstein JS, Balamurugan AN, et al. (2016) National Institutes of HealthSponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a complex cellular product at eight processing facilities. Diabetes 65:3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henquin JC (2018) Influence of organ donor attributes and preparation characteristics on the dynamics of insulin secretion in isolated human islets. Physiol Rep 6:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayton NS, Poffenberger G, Henske J, et al. (2015) Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab 308:E592–E602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westacott MJ, Farnsworth NL, St Clair JR, et al. (2017) Age-dependent decline in the coordinated [Ca2+] and insulin secretory dynamics in human pancreatic islets. Diabetes 66:2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brissova M, Haliyur R, Saunders D, et al. (2018) α Cell function and gene expression are compromised in type 1 diabetes. Cell Rep 22:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart NJ, Aramandla R, Poffenberger G, et al. (2018) Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 3:e98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babon JAB, DeNicola ME, Blodgett DM, et al. (2016) Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 22:1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YJ, Golson ML, Schug J, et al. (2016) Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab 24:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YJ, Schug J, Won K-J, et al. (2016) Single-cell transcriptomics of the human endocrine pancreas. Diabetes 65:3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segerstolpe Å, Palasantza A, Eliasson P, et al. (2016) Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 24:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels AW, Landry LG, McDaniel KA, et al. (2017) Islet-derived cd4 t cells targeting proinsulin in human autoimmune diabetes. Diabetes 66:722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ediger BN, Lim H-W, Juliana C, et al. (2017) LIM domain-binding 1 maintains the terminally differentiated state of pancreatic β cells. J Clin Invest 127:215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raum JC, Soleimanpour SA, Groff DN, et al. (2015) Tshz1 regulates pancreatic β-cell maturation. Diabetes 64:2905–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delong T, Wiles TA, Baker RL, et al. (2016) Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351:711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyoura M, Jacobsen L, Carmody D, et al. (2018) Pancreatic histopathology of human monogenic diabetes due to causal variants in KCNJ11, HNF1A, GATA6, and LMNA. J Clin Endocrinol Metab 103:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodmansey C, McGovern AP, McCullough KA, et al. (2017) Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care 40:1486–1493. [DOI] [PubMed] [Google Scholar]
- 28.Oram RA, Patel K, Hill A, et al. (2016) A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care 39:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmer A, Fox R (2011) Diagnosis, classification, and treatment of diabetes. BMJ 342:d3319. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd M, Shields B, Hammersley S, et al. (2016) Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care 39:1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hope SV, Wienand-Barnett S, Shepherd M, et al. (2016) Practical Classification Guidelines for Diabetes in patients treated with insulin: a cross-sectional study of the accuracy of diabetes diagnosis. Br J Gen Pract 66:e315–e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brissova M, Fowler MJ, Nicholson WE, et al. (2005) Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087–1097. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera O, Berman DM, Kenyon NS, et al. (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103:2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan FC, Wright C (2011) Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 240:530–565. [DOI] [PubMed] [Google Scholar]
- 36.Gregg BE, Moore PC, Demozay D, et al. (2012) Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 97:3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steiner DJ, Kim A, Miller K, Hara M (2014) Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair G, Hebrok M (2015) Islet formation in mice and men: lessons for the generation of functional insulin-producing β-cells from human pluripotent stem cells. Curr Opin Genet Dev 32:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brissova M, Shostak A, Fligner CL, et al. (2015) Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Hitsochem Cytochem 63:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. (2011) Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otonkoski T, Banerjee M, Korsgren O, et al. (2008) Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab 10 Suppl 4:119–127. [DOI] [PubMed] [Google Scholar]
- 42.Virtanen I, Banerjee M, Palgi J, et al. (2008) Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 51:1181–1191. [DOI] [PubMed] [Google Scholar]
- 43.Benner C, van der Meulen T, Cacéres E, et al. (2014) The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 15:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai C, Brissova M, Hang Y, et al. (2012) Islet-enriched gene expression and glucoseinduced insulin secretion in human and mouse islets. Diabetologia 55:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arda HE, Li L, Tsai J, et al. (2016) Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 23:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rorsman P, Braun M (2013) Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75:155–179. [DOI] [PubMed] [Google Scholar]
- 47.Henquin JC, Dufrane D, Nenquin M (2006) Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55:3470–3477. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. (2018) Paracrine Interactions within the pancreatic islet determine the glycemic set point. Cell Metab 27:549–558.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodson DJ, Mitchell RK, Bellomo EA, et al. (2013) Lipotoxicity disrupts incretin-regulated human β cell connectivity. J Clin Invest 123:4182–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston NR, Mitchell RK, Haythorne E, et al. (2016) Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bader E, Migliorini A, Gegg M, et al. (2016) Identification of proliferative and mature βcells in the islets of Langerhans. Nature 535:430–434. [DOI] [PubMed] [Google Scholar]
- 52.Dorrell C, Schug J, Canaday PS, et al. (2016) Human islets contain four distinct subtypes of β cells. Nat Commun 7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierrez GD, Gromada J, Sussel L (2017) Heterogeneity of the pancreatic beta cell. Front Genet 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avrahami D, Wang YJ, Klochendler A, et al. (2017) β-Cells are not uniform after all – Novel insights into molecular heterogeneity of insulin-secreting cells. Diabetes Obes Metab 19(Suppl 1):147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eizirik DL, Pipeleers DG, Ling Z, et al. (1994) Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A 91:9253–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eizirik DL (1996) Beta-cell defence and repair mechanisms in human pancreatic islets. Horm Metab Res 28:302–305. [DOI] [PubMed] [Google Scholar]
- 57.Welsh N, Margulis B, Borg LA, et al. (1995) Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1:806–820. [PMC free article] [PubMed] [Google Scholar]
- 58.Kawahara DJ, Kenney JS (1991) Species differences in human and rat islet sensitivity to human cytokines. Monoclonal anti-interleukin-1 (IL-1) influences on direct and indirect IL1-mediated islet effects. Cytokine 3:117–124. [DOI] [PubMed] [Google Scholar]
- 59.Eizirik DL, Sandler S, Welsh N, et al. (1994) Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest 93:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eizirik DL, Korbutt GS, Hellerström C (1992) Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest 90:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai C, Kayton NS, Shostak A, et al. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J Clin Invest 126:1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826. [DOI] [PubMed] [Google Scholar]
- 63.Thorel F, Népote V, Avril I, et al. (2010) Conversion of adult pancreatic alpha-cells to betacells after extreme beta-cell loss. Nature 464:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talchai C, Xuan S, Lin HV, et al. (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spijker HS, Ravelli RBG, Mommaas-Kienhuis AM, et al. (2013) Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes 62:2471–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chera S, Baronnier D, Ghila L, et al. (2014) Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cinti F, Bouchi R, Kim-Muller JY, et al. (2016) Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 101:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, York NW, Nichols CG, Remedi MS (2014) Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 19:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulkarni RN, Mizrachi E-B, Ocana AG, Stewart AF (2012) Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernal-Mizrachi E, Kulkarni RN, Scott DK, et al. (2014) Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 63:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart AF (2014) Betatrophin versus bitter-trophin and the elephant in the room: time for a new normal in β-cell regeneration research. Diabetes 63:1198–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart AF, Hussain MA, García-Ocaña A, et al. (2015) Human β-cell proliferation and intracellular signaling: part 3. Diabetes 64:1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai C, Hang Y, Shostak A, et al. (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest 127:3835–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solloway MJ, Madjidi A, Gu C, et al. (2015) Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep 12:495–510. [DOI] [PubMed] [Google Scholar]
- 75.Dean ED, Li M, Prasad N, et al. (2017) Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab 25:1362–1373.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean ED, Unger RH, Holland WL (2017) Glucagon antagonism in islet cell proliferation. Proc Natl Acad Sci USA 114:3006–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Okamoto H, Huang Z, et al. (2017) Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab 25:1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.