Abstract
Background
During kidney fibrosis, a hallmark and promoter of CKD (regardless of the underlying renal disorder leading to CKD), the extracellular-regulated kinase 1/2 (ERK1/2) pathway, is activated and has been implicated in the detrimental differentiation and expansion of kidney fibroblasts. An ERK1/2 pathway inhibitor, trametinib, is currently used in the treatment of melanoma, but its efficacy in the setting of CKD and renal fibrosis has not been explored.
Methods
We investigated whether trametinib has antifibrotic effects in two mouse models of renal fibrosis—mice subjected to unilateral ureteral obstruction (UUO) or fed an adenine-rich diet—as well as in cultured primary human fibroblasts. We also used immunoblot analysis, immunohistochemical staining, and other tools to study underlying molecular mechanisms for antifibrotic effects.
Results
Trametinib significantly attenuated collagen deposition and myofibroblast differentiation and expansion in UUO and adenine-fed mice. We also discovered that in injured kidneys, inhibition of the ERK1/2 pathway by trametinib ameliorated mammalian target of rapamycin complex 1 (mTORC1) activation, another key profibrotic signaling pathway. Trametinib also inhibited the ERK1/2 pathway in cultured primary human renal fibroblasts stimulated by application of TGF-β1, the major profibrotic cytokine, thereby suppressing downstream mTORC1 pathway activation. Additionally, trametinib reduced the expression of myofibroblast marker α-smooth muscle actin and the proliferation of renal fibroblasts, corroborating our in vivo data. Crucially, trametinib also significantly ameliorated renal fibrosis progression when administered to animals subsequent to myofibroblast activation.
Conclusions
Further study of trametinib as a potential candidate for the treatment of chronic renal fibrotic diseases of diverse etiologies is warranted.
Keywords: renal fibrosis, ERK1/2, mTORC1, Trametinib, UUO, chronic kidney disease
CKD is a global health concern, with general population prevalence estimated to be 13.4%.1 Irrespective of the underlying cause, CKD is characterized by excessive fibrosis, defined as the deposition of extracellular matrix (ECM) proteins in the tubulointerstitium, the space between tubules and peritubular capillaries.2,3 Kidney fibrosis is a better predictor of disease progression than eGFR.4 Moreover, matrix deposition and the molecular mechanisms associated with fibrosis exacerbate kidney injury, significantly contributing to End-Stage Renal Disease (ESRD) incidence.5,6
Considerable effort has been devoted to elucidate the molecular mechanisms mediating kidney fibrosis. Detailed cell-fate studies suggest that during persistent insult, resident kidney fibroblasts and cells of hematopoietic origin differentiate into, and are the primary source of myofibroblasts.7,8 Myofibroblasts are cells of epithelial origin that, upon activation by profibrotic factors, acquire a contractile/proliferative phenotype, express markers such as α-smooth muscle actin (αSMA), and are the principal kidney collagen-producing cells.9,10
Numerous factors have been implicated in the terminal differentiation of fibroblasts into myofibroblasts, of which TGF-β1, possibly originating from injured tubules,11 is the most prominent.12,13 Ligation of TGF-β1 to its receptors results in the activation of the Smad2/3 transcription factors, mediating canonical signaling and the expression of ECM constituens.14 Concurrently, the mitogen-activated protein kinase cascade is activated, resulting in ERK1/2 signaling, further exacerbating TGF-β1–driven fibrosis.14,15 Proinflammatory cytokines,16 epithelial–mesenchymal transition,17 and impaired wound healing18 are additional key contributors to myofibroblast expansion and excessive ECM deposition in the kidney. Mechanistically, the mTORC1,19 EGF receptor,20 and NF-κB21 signaling pathways are prominently involved in myofibroblast differentiation and fibrosis progression.
Despite progress in identifying the molecular mechanisms regulating kidney fibrosis, this has not been translated into novel therapies. At the time of writing no new class of drugs has been registered for the treatment of CKD since 2001.22
A signaling intermediary shared by multiple cascades activated in myofibroblasts is the mitogen-activated protein kinase ERK1/2. ERK1/2 is activated downstream of TGF-β114 and EGF receptor20 and considerable crosstalk between ERK1/2 the mTORC123 and the NF-κB24 pathways exists. Moreover, ERK1/2 activation has been detected in αSMA-positive myofibroblasts in patient kidney biopsy specimens,25 and ERK1/2 inhibition decreased the number of myofibroblasts upon mild kidney fibrosis.26 Recently, the ERK1/2 pathway inhibitor trametinib significantly reduced mortality in clinical trials for the treatment of unresectable metastatic melanoma,27,28 and was granted accelerated approval by the US Food and Drug Administration. However, the efficacy of trametinib in the setting of renal fibrosis and CKD has not yet been explored.
Here, we investigated whether trametinib has antifibrotic effects, and if so, we aimed to elucidate the underlying molecular mechanism(s) in two complimentary rodent models of renal fibrosis.
Methods
Materials
Antibodies to vimentin, pAKT, AKT, pERK1/2, ERK1/2, pP70S6K, pS6, S6, p4E-BP1, pSTAT3, STAT3, pP65, and P65 were obtained from Cell Signaling Technology. Antibodies to β-actin, αSMA, and tubulin were obtained from Sigma. The antibody for NADPH oxidase-4 (Nox4) was obtained from Santa Cruz Biotechnology, the antibody for Ki-67 was obtained from Abcam, the F4/F80 antibody was obtained from Serotec, and TGF-β1 was obtained from R&D Systems. Trametinib was obtained from Selleck Chemicals. All other chemicals were obtained from Sigma, unless otherwise stated.
Animal Procedures and Trametinib Treatment
All animal experiments were conducted in accordance with the United Kingdom Home Office Animals 1986 Scientific Procedures, with local ethical committee approval (Project License 70/8356).
Male C57BL/6J mice (Charles River UK Ltd.) weighing between 20 and 25 g at 6–8 weeks of age were used. UUO was established according to published methods.29,30 Briefly, mice were anesthetized with isoflurane and the abdominal cavity was exposed using midline laparotomy. Subsequently, the right ureter was isolated and tied off 0.5 cm from the pelvis, using a sterile 5–0 silk-braided suture (Pearsalls). The left ureter was left unclamped and served as the sham-operated control. All incisions were closed using 5–0 Proline suture (Ethicon). The following day and for 6 days in total, trametinib (3 mg/kg) was administered once daily by oral gavage (100 μl per animal). The final trametinib dose was given 2 hours before euthanizing. Trametinib was initially dissolved in DMSO and diluted into an aqueous pooled dose containing a final concentration of 0.5% hypromellose (Sigma) and 2% Tween-80 (Sigma), according to published methods.31 Control animals received the vehicle. Final DMSO concentration was 0.2% v/v, and trametinib or vehicle were administered in a blinded fashion. In a parallel study, trametinib (3 mg/kg) or vehicle were administered 4 days after UUO once daily for 6 days. At day 10 the kidneys were harvested. Both the kidneys from each animal (UUO and sham) were cut in half longitudinally. One half of each kidney was snap-frozen in liquid nitrogen and subsequently stored at −80°C for Western blotting. The other half was fixed with 10% Formalin (Sigma) for 16 hours at 4°C, and then transferred to a 70% v/v ethanol solution for a further 24 hours before embedding in paraffin for immunohistochemistry.
In a second set of experiments, mice were fed a rodent diet (824534 RM1) containing 0.15%w/w adenine (SDS diets; LBS-Biotech, Hookwood, UK) for 2 months. Excessive adenine is enzymatically modified and eventually precipitates in the tubules, resulting in progressive tubular injury culminating in necrosis, accompanied by macrophage infiltration, kidney fibrosis, and diminished renal function.32 We used 0.15% w/w adenine-containing diet because this was found to result in progressive kidney failure over time (up to 4 months) without the excessive weight loss observed with higher adenine-enriched diets (J. Kieswich, personal communication). Animals were administered trametinib (0.3 mg/kg) or vehicle once daily for 2 months by oral gavage, and kidneys were harvested as described for the UUO model.
Cell Culture and Protein Extraction
Primary human adult kidney fibroblasts from a single donor were obtained from Cambridge Bioscience and cultured in I-GRO medium containing growth supplements (Cambridge Bioscience). For acute (up to 30 minutes) TGF-β1 stimulation, cells (approximately 100,000) were serum-starved for 1 hour in a physiologic serum-free buffer as previously described.33,34 For longer-term (24 hours) stimulation, fibroblasts were in complete medium including serum and growth supplements. Fibroblasts were preincubated with trametinib (10 or 20 nM) or vehicle for 30 minutes before TGF-β1 stimulation (5 ng/ml). At the end of the experiment fibroblasts were lysed and stored at −80°C until further use, as previously described.33,35
Western Blot
Immunoblot analysis of kidney samples or fibroblast cellular lysates were conducted using the NuPAGE electrophoresis and buffers system (Invitrogen), as previously described.33,35 Proteins were visualized with ECL Prime (GE Healthcare). Optical densities of bands of interest were determined using ImageJ 1.46r (National Institutes of Health) and normalized against the appropriate loading controls. The value of the normalized control sample was arbitrarily set to 1. Membranes were stripped using the Restore Plus reagent (Fisher Scientific), and reprobed for tubulin, β-actin, total ERK1/2, total STAT3, or total P65. For technical reasons, total S6 and total AKT were estimated from parallel Western blots.
Cell Proliferation Experiments
Human renal fibroblasts were seeded at a density 3×103 cells per well, in 96-well plates, (Nunc) in complete medium. The following day, fresh medium was added along with the indicated concentrations of trametinib or vehicle. After 30 minutes of incubation, 5 ng/ml TGF-β1 was added to the corresponding wells. After 24 or 48 hours of culture, the number of viable cells in each well was quantified with the MTT assay (Promega) according to the manufacturer’s instructions. Each condition was assayed in triplicate and the experiment was repeated three times. All data were normalized against the absorbance of the unstimulated (no TGF-β1) control sample at 24 hours.
Immunohistochemical Staining
Formalin-fixed kidneys were embedded in paraffin and 4-μm sections were cut by the Barts Cancer Institute Pathology Unit. Staining with Sirius red or hematoxylin and eosin were performed as previously described.36 Immunostaining was performed with the DISCOVERY XT (Ventana) automated slide processing instrument using the OmniMap reagents (Ventana), according to the manufacturer’s recommendations. Images were captured at ×20 magnification, unless otherwise stated, using a Zeiss AxioPhot microscope with an AxioCam HRc camera. Five images of kidney cortex were captured per mouse and staining was quantified as percentage of total area, using ImageJ 1.46r.37 For Ki67 staining, positive nuclei per field of view were counted instead.
Urine and Blood Biochemistry
Twenty four hours before the end of the adenine-diet experiment, animals were placed in metabolic cages for urine collection. Blood was taken by cardiac puncture into heparinized syringes, decanted into Eppendorf tubes, and placed on ice. Subsequently, the blood was centrifuged at 9900×g for 3 minutes to separate serum. Creatinine clearance (CCL, an indicator of GFR) was calculated using the following formula: CCL (ml/min)=[CU (µmol/L)×urine flow (ml/min)]/CS (µmol/L), where CU is the concentration of creatinine in the urine and CS is the creatinine concentration in the serum. Fractional excretion of sodium (FENA), an indicator of tubular function, was calculated with the following formula: FENa (%)=100×[NaU (µmol/L)×urine flow (ml/min)]/[CCL (ml/min)×NaS (µmol/L)], where NaU is the sodium concentration in the urine and NaS is the sodium concentration in the serum. All biochemical parameters in serum and urine were measured by a commercial veterinary testing laboratory (IDEXX Bioresearch, Ludwigsburg, Germany).
Statistical Analyses
Data are expressed as the means±SEM. Statistical significance was determined by one- or two-way analysis of variance and Tukey post hoc test, using GraphPad Prism v5, as appropriate. Values of P<0.05 were deemed statistically significant. Where possible, experimenters were blinded. No animals were excluded from the analysis.
Results
Trametinib Ameliorated Kidney Fibrosis after UUO
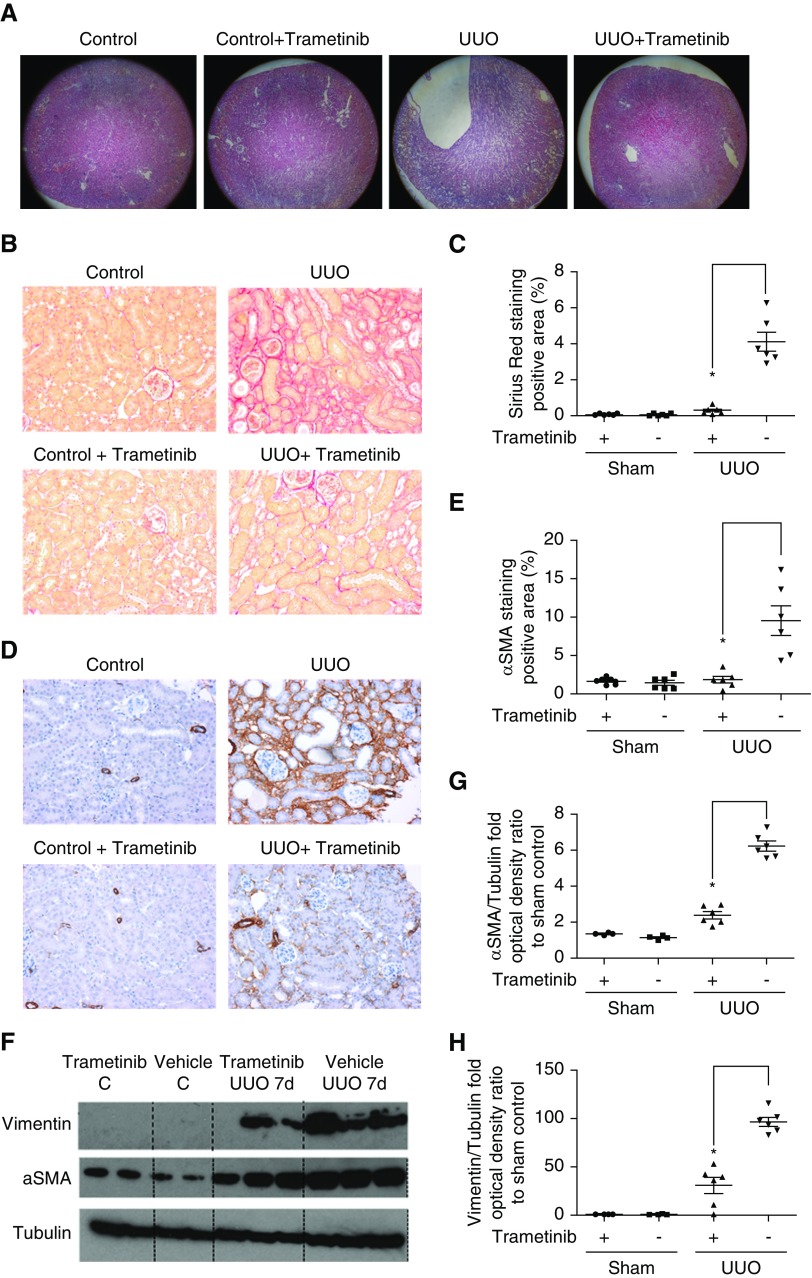
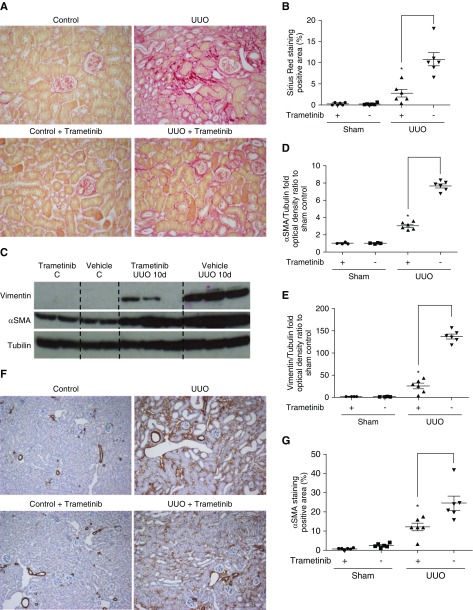
We investigated whether ERK1/2 pathway inhibition can hinder renal fibrosis progression by subjecting mice to UUO. As reported previously,29,30 UUO kidneys exhibited severe morphologic lesions characterized by tubular dilation and tubulointerstitial expansion (Figure 1A). Conversely, the kidneys of animals treated with trametinib displayed considerably less morphologic abnormalities (Figure 1A). To measure collagen deposition in the interstitium, a hallmark of fibrosis,2,3 kidneys were stained with Sirius red (Figure 1B). UUO significantly increased Sirius red staining, whereas staining was dramatically decreased by 92% in the kidneys of trametinib-treated animals (Figure 1C).
Figure 1.
Blockade of the ERK1/2 pathway ameliorated collagen deposition and αSMA expression in UUO kidneys. (A) Staining with hematoxylin and eosin of kidney sections from obstructed (UUO; 7 days after surgery) or contralateral sham-operated kidneys (control) at ×5 magnification. Animals received trametinib (3 mg/kg for 6 days) or vehicle as indicated. n=6 per group. (B) Sirius red staining of kidney sections from the animals in (A) at ×20 magnification, and (C) quantification of stained area as percentage of total area. (D) Immunostaining of kidney sections for αSMA at ×20 magnification and (E) quantification of positive αSMA staining. (F) Western blot of whole-kidney lysates for αSMA and vimentin expression. Membranes were subsequently stripped and reprobed for tubulin, as loading control. A representative photomicrograph from n=2 Western blots with n=4–6 animals in each group is shown. OD of the (G) αSMA and (H) vimentin bands in (F) were normalized against tubulin. The normalized density of the sham-vehicle treated samples was arbitrarily set to 1. For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group. *P<0.05 versus the UUO control.
Next, the effect of trametinib on the expression of the myofibroblast marker αSMA8–10 was investigated. UUO resulted in considerably increased αSMA-positive staining, primarily in the tubulointerstitium (Figure 1D). In animals that received trametinib, αSMA staining was reduced by 75% (Figure 1, D and E). Western blotting also confirmed this, revealing 62% reduction of αSMA expression in trametinib-treated UUO kidneys (Figure 1, F and G).
The cytoskeletal protein vimentin is upregulated in myofibroblasts5 and its expression can be modulated by ERK1/2.38 Thus, we investigated whether ERK1/2 inhibition could suppress vimentin upregulation. No vimentin bands were detected in sham-operated kidneys by Western blotting, whereas dramatic upregulation of vimentin was observed in UUO samples (Figure 1F). Treatment with trametinib significantly attenuated vimentin expression by 68% (Figure 1H).
Trametinib Suppressed ERK1/2 and AKT Activation and Myofibroblast Expansion in UUO
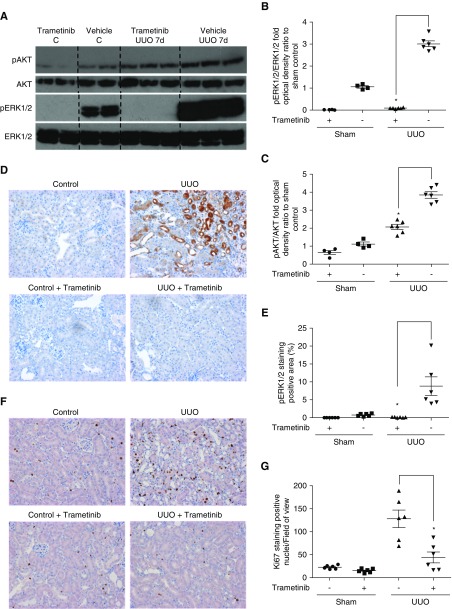
As previously reported,20,26 phospho-ERK1/2 was significantly upregulated in UUO kidneys, as determined by Western blot (Figure 2A) and immunostaining (Figure 2D). Trametinib administration completely suppressed ERK1/2 phosphorylation in both sham- and UUO-operated animals (Figure 2, A and B), as confirmed by immunohistochemistry (Figure 2, D and E). AKT is phosphorylated during UUO and contributes to injury.26 Western blotting revealed augmented AKT phosphorylation after UUO and trametinib application reduced phospho-AKT levels by 46% (Figure 2, A and C).
Figure 2.
Trametinib suppressed UUO-induced ERK1/2 activation and myofibroblast proliferation. (A) Representative Western blot (n=2) from kidney lysates of animals subjected to UUO and treated with trametinib as indicated. Membranes were probed for phospho-AKT and phospho-ERK1/2. Protein loading was determined by probing for total ERK1/2 and total AKT. Densitometric analyses of blots from (A) for normalized (B) phospho-ERK1/2 and (C) phospho-AKT, as described in Figure 1. (D) Immunostaining of kidney sections for phospho-ERK1/2 at ×20 magnification and (E) quantification of positive pERK1/2 staining. (F) Immunostaining for positive Ki67 nuclei in kidney sections from our experimental groups at ×20 magnification. (G) Quantification of Ki67 positive nuclei per field of view from the experiment in (F). For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group. *P<0.05 versus the UUO control.
Myofibroblast proliferation is a major driver of fibrosis2,3 and ERK1/2 modulates renal fibroblast expansion.39 Accordingly, we tested the effect of trametinib on myofibroblast proliferation by staining for Ki67, a marker of proliferating cells. Hardly any Ki67-positive nuclei were detected in sham kidneys (Figure 2F). UUO resulted in a dramatic increase in the detected Ki67-positive nuclei primarily in the interstitium, whereas trametinib significantly reduced the number of proliferating cells by 65% (Figure 2G).
Trametinib Blocked UUO-Induced mTORC1 Activation and Nox4 Overexpression
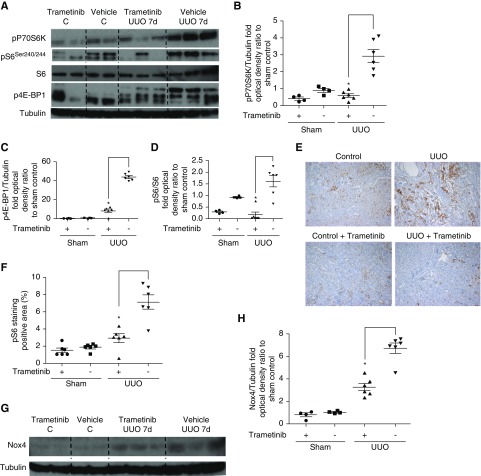
mTORC1 activation downstream of TGF-β1 promotes kidney fibrosis19 and contributes to injury by upregulating Nox4.40–42 As previously reported,19 significant phosphorylation of the key downstream mTORC1 effectors p70S6 kinase (P70S6K) and eIF4E binding protein-1 (4E-BP1)43 was observed upon UUO (Figure 3, A–C). In contrast, in trametinib-treated animals mTORC1 activation was significantly suppressed, as exemplified by the reduction of phospho-P70S6K and phospho-4E-BP1 levels by 80% and 81%, respectively (Figure 3, A–C). Increased phosphorylation of the ribosomal protein S6, a direct target of P70S6K43 (Figure 3, A and D), was also attenuated by 85% by trametinib administration (Figure 3D). This was further corroborated by decreased phospho-S6 immunostaining observed in the kidneys of trametinib-treated animals (Figure 3, E and 3F). In agreement with previous studies reporting Nox4 upregulation downstream of mTORC1 and ERK1/2,40–42 Western blotting revealed marked induction of Nox4 protein in UUO kidneys (Figure 3G). Trametinib significantly blunted Nox4 upregulation by 51% (Figure 3, G and H).
Figure 3.
Trametinib treatment ameliorated mTORC1 activation and Nox4 upregulation in UUO kidneys. (A) Western blot analysis of kidney lysates for the activation of the downstream mTORC1 effectors pP70S6K, p4E-BP1, and pS6. (B–D) Densitometric analysis of Western blots in (A). Protein loading was normalized with tubulin or total S6 as appropriate. (E) Kidney sections from our experimental groups were immunostained with a specific antibody recognizing phospho-S6. Representative images at ×10 magnification are shown. (F) Quantification of positive pS6 staining in (E). (G) Kidney lysates were subjected to Western blot and membranes were probed with a specific anti-Nox4 antibody. (H) Densitometric analysis of the normalized Nox4 protein bands in (G). For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group. *P<0.05 versus the UUO control.
Trametinib Attenuated UUO-Induced Phosphorylation of STAT3, NF-κB, and Smad2/3 and Macrophage Infiltration
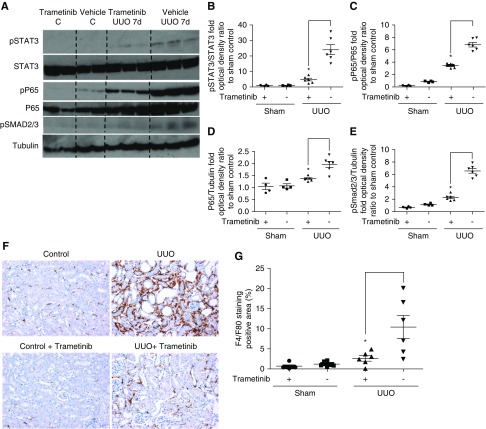
Renal fibroblast differentiation requires STAT3 phosphorylation and phospho-STAT3 is upregulated in myofibroblasts during UUO.44 Moreover, STAT3 is activated downstream of mTORC1.45 Negligible phospho-STAT3 was observed in sham-operated kidneys (Figure 4A), whereas UUO resulted in a striking increase of phospho-STAT3, which was significantly attenuated by trametinib administration by 80% (Figure 4, A and B).
Figure 4.
Trametinib inhibited STAT3, NF-κB, and Smad2/3 activation and macrophage infiltration in the UUO kidney. (A) Western blot analysis of kidney lysates for the activation of STAT3, P65 (the main subunit of NF-κB), and Smad2/3 in UUO kidneys. A representative photomicrograph of n=2 Western blots is shown. Membranes were stripped and protein loading was normalized against total STAT3, total P65, or tubulin as appropriate. (B–E) Densitometric analysis of the Western blot shown in (A). (F) Immunostaining of kidney sections for macrophage infiltration with F4/F80 at ×20 magnification. (G) Quantification of positively stained area as percentage of total area from the section in (F). For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group. *P<0.05 versus the UUO control.
Genes transcribed downstream of NF-κB prominently contribute to myofibroblast expansion and differentiation.21 As shown in Figure 4A, phosphorylation of the NF-κB subunit P65, was barely detectable in sham-operated kidneys, whereas considerable upregulation of phospho-P65 was observed upon UUO. Trametinib significantly suppressed phospho-P65 by 51% when normalized by total P65 levels (Figure 4, A and C). Total P65 was also significantly upregulated by 83% in the UUO kidneys when compared with controls, whereas trametinib administration blunted P65 expression, although the difference was still significant (Figure 4, A and D).
Phosphorylation of the main effectors of canonical TGF-β1 signaling, Smad2/3,14 was also enhanced in control UUO kidneys, and treatment with trametinib suppressed phospho-Smad2/3 levels by 65% (Figure 4, A and E).
Persistent inflammation is a major contributor to kidney fibrosis and infiltration of macrophages in UUO kidneys exacerbates injury.46 Thus, we tested whether trametinib treatment reduced macrophage infiltration during UUO. A dramatic increase in F4/F80 staining (a marker of macrophages) was observed in the interstitium of UUO control animals, whereas significantly less staining (75% reduction) was detected in UUO kidneys from trametinib-treated animals (Figure 4, F and G).
Delayed Administration of Trametinib Suppressed Kidney Fibrosis and Profibrotic Signaling upon UUO
We determined whether ERK1/2 pathway inhibition could slow kidney fibrosis subsequent to injury by administering trametinib or vehicle 4 days post-UUO surgery for 6 days. Significant myofibroblast activation and phospho-ERK1/2 upregulation was observed in kidneys after 4 days of UUO (Supplemental Figure 1). Considerable Sirius red staining was observed in the tubulointerstitium after 10 days of UUO (Figure 5, A and B). Treatment with trametinib 4 days postsurgery decreased Sirius red staining by 74% (Figure 5, A and B). Western blotting revealed that in animals that received trametinib, αSMA and vimentin expression were significantly reduced by 60% and 77%, respectively (Figure 5, C–E). Decreased αSMA-positive immunostaining was also detected in the obstructed kidneys of these animals (Figure 5, F and G).
Figure 5.
Delayed administration of trametinib suppressed collagen deposition and αSMA expression in the UUO kidney. (A) Representative images of Sirius red staining from UUO (10 days postsurgery) or contralateral sham-operated kidneys (control) at ×20 magnification. Animals received trametinib (3 mg/kg daily for six consecutive days, 4 days after surgery) or vehicle as indicated; n=6 in each group. (B) Quantification of positively Sirius red stained in (A), expressed as percentage of total area. (C) Representative image of Western blot analysis of whole kidney lysates probed for αSMA and vimentin. (D and E) Densitometric analysis of band intensities for αSMA and vimentin normalized for protein loading against tubulin. The normalized optical intensity of the sham control samples was arbitrarily set to 1. (F) Representative images of immunostaining of kidney sections at ×10 magnification from our experimental animals for αSMA. (G) Densitometric analysis of αSMA-positive staining from the sections in (F). For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group. *P<0.05 versus the UUO control.
Significant upregulation of ERK1/2 and AKT phosphorylation was observed in kidneys 10 days post-UUO (Supplemental Figure 2, A–C). However, in animals that received trametinib 4 days postinjury, ERK1/2 and AKT activation were suppressed by 92% and 52%, respectively (Supplemental Figure 2, A–C). Immunohistochemical analysis confirmed almost complete phospho-ERK1/2 suppression in trametinib-treated animals (Supplemental Figure 2, D and E).
Immunoblotting revealed that delayed administration of trametinib resulted in significant reduction in mTORC1 pathway activation as judged by the reduction of the phosphorylated levels of P70S6K, 4E-BP1 and S6 by 68%, 88%, and 33%, respectively (Supplemental Figure 2, F–I).
Immunoblotting showed significant kidney Nox4 overexpression upon 10 days of UUO, which was blunted by trametinib administration (Supplemental Figure 3, A and B).
Substantial activation of STAT3, NF-κB, and Smad2/3 and upregulation of total P65 was also detected in UUO kidneys (Supplemental Figure 3, C–G). As shown in Supplemental Figure 3, D–G, trametinib administration significantly ameliorated STAT3 and Smad2/3 phosphorylation by 73% and 83%, respectively. Phospho-P65 was reduced significantly by 33% even when corrected for total P65, which was upregulated in UUO. Additionally, trametinib decreased macrophage infiltration by 74%, as demonstrated by immunostaining with F4/F80 (Supplemental Figure 3, H and I).
Trametinib Reduced Kidney Fibrosis in a Chronic Model of CKD Induced by Adenine-Rich Diet
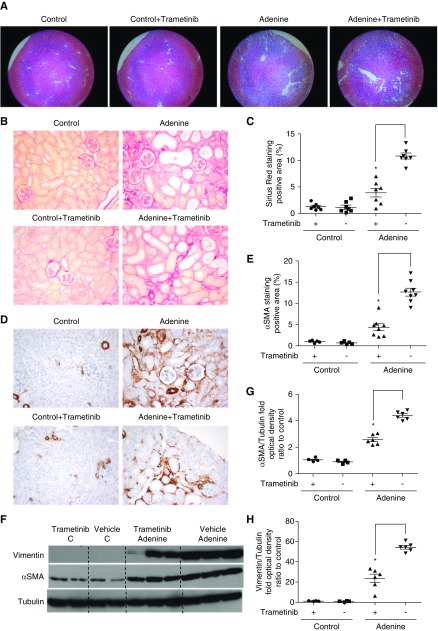
We next evaluated whether trametinib could ameliorate renal fibrosis in a chronic model of CKD. Mice fed a diet containing 0.15% w/w adenine for 2 months32 received trametinib (0.3 mg/kg), a dose that results in approximately 75% ERK1/2 pathway inhibition,37 or vehicle for the duration of the experiment.
As previously reported,32 gross morphologic lessons were observed in the kidneys of adenine-fed animals. Unlike UUO, trametinib did not appear to reduce tubular dilation (Figure 6A). Considerable Sirius red staining, an indicator of collagen deposition, was observed in the tubulointerstitium of adenine-diet fed animals. Treatment with trametinib (0.3 mg/kg) significantly reduced staining by 63% (Figure 6, B and C). Decreased αSMA immunostaining (by 66%) was also detected in the kidneys of adenine-fed animals that received trametinib (Figure 6, D and E). Western blotting revealed that trametinib treatment suppressed αSMA and vimentin expression by 41% and 57%, respectively (Figure 6, F–H).
Figure 6.
Blockade of the ERK1/2 pathway ameliorated collagen deposition and αSMA expression in the kidneys of adenine-fed animals. (A) Staining with hematoxylin and eosin of kidney sections from adenine-fed (Adenine) or control kidneys (Control) at ×5 magnification. Animals received trametinib (0.3 mg/kg once daily for the duration of the experiment) or vehicle as indicated; n=7–8 per group. (B) Sirius red staining of kidney sections from the animals in (A) at ×20 magnification, and (C) quantification of stained area as percentage of total area. (D) Immunostaining of kidney sections for αSMA at ×20 magnification and (E) quantification of positive αSMA staining. (F) Western blot of whole-kidney lysates for αSMA and vimentin expression. Membranes were subsequently stripped and reprobed for tubulin, as loading control. A representative photomicrograph from n=2 Western blots with n=4–6 animals in each group is shown. OD of the (G) αSMA and (H) vimentin bands in (F) were normalized against tubulin. The normalized density of the sham-vehicle treated samples was arbitrarily set to 1. For all graphs, error bars represent the means±SEM of data from n=4–6 animals per group for Western blots, and n=7–8 for immunostaining experiments. *P<0.05 versus the control.
In the kidneys of adenine-fed animals significant upregulation of ERK1/2 and AKT phosphorylation was observed (Supplemental Figure 4, A–C). ERK1/2 and AKT activation was suppressed by 84% and 56%, respectively, in the trametinib-treated animals (Supplemental Figure 4, A–C). Immunohistochemical analysis showed phospho-ERK1/2 suppression by 80% (Supplemental Figure 4, D and E), accompanied with a reduction of the number of proliferating, Ki67-positive cells (Supplemental Figure 4, F and G) in the kidneys of animals that received trametinib.
Increased mTORC1 pathway activation was observed by immunoblotting in the kidneys of adenine-fed animals and this was blunted by trametinib administration, as exemplified by the reduction of phospho-P70S6K, phospho-4E-BP1, and phospho-S6 by 64%, 73%, and 85%, respectively (Supplemental Figure 5, A–D). Additionally, considerable macrophage infiltration was observed in the kidneys of adenine-fed animals. Conversely, trametinib reduced F4/F80-positive immunostaining by 78% (Supplemental Figure 6, A and B).
Mice that were fed an adenine-rich diet exhibited a significant reduction in kidney function, as exemplified by a four-fold increase in plasma creatinine and urea, a two-fold increase in urine protein, and a significantly increased fractional excretion of sodium and creatinine clearance ratio (Table 1). Trametinib treatment, despite significantly reducing kidney fibrosis, did not improve kidney function in adenine-fed mice (Table 1).
Table 1.
Blood and urine biochemical parameters from animals treated with trametinib and fed a control or an adenine-rich diet as indicated over 2 months
| Treatment | Control | Control and Trametinib | Adenine | Adenine and Trametinib |
|---|---|---|---|---|
| Animals | 6 | 6 | 8 | 8 |
| Serum | ||||
| Serum urea, mmol/L | 8.03±0.54 | 8.61±0.27 | 30.65±3.81a | 31.22±2.61 |
| Serum creatinine, µmol/L | 7.61±1.59 | 8.13±0.73 | 32.66±7.16a | 31.12±4.47 |
| Serum sodium, mmol/L | 152.86±0.43 | 152.27±1.30 | 157.51±0.23a | 156.52±1.01 |
| Urine | ||||
| Urine volume, ml | 0.82±0.07 | 0.90±0.09 | 5.17±0.60a | 4.42±0.33 |
| Urine creatinine, µmol/L | 6402.3±290.9 | 6749.3±366.4 | 1022.0±95.4a | 1144.17±113.9 |
| Urine sodium, mmol/L | 164.0±5.63 | 159.86±19.68 | 62.67±6.43a | 63.50±7.21 |
| Proteinuria, mg/24 h | 4.36±1.49 | 4.26±0.56 | 9.34±1.40a | 10.05±1.30 |
| Kidney functional parameters | ||||
| Creatinine clearance, ml/min | 0.216±0.038 | 0.207±0.025 | 0.058±0.014a | 0.053±0.013 |
| FE Na+, % | 0.13±0.03 | 0.13±0.02 | 1.39±0.40a | 1.18±0.24 |
Creatinine clearance and fractional excretion of sodium (FE Na+) were calculated as described in the Methods section; n=6–8 animals per group.
P<0.05 versus the control.
Adenine-fed animals exhibited a 5% reduction in body weight after 2 months. Trametinib-treated mice displayed a modest, albeit significant, reduction in weight by further 7% (Supplemental Figure 7). This was not due to reduced food consumption because once weight differences became apparent (after 5–6 weeks), we measured food consumption for the remainder of the experiment and no significance differences were found (data not shown). Trametinib-treated animals did not display any signs of discomfort or reduced physical activity. A similar trend was observed in the control arm of the study. Given that ERK1/2 is known to regulate metabolism in mice,31 it is probable that the observed weight differences were due to altered basal metabolism. No excessive weight loss has been reported in humans that received trametinib.27,28
Trametinib Ameliorated TGF-β1–Induced Renal Fibroblast Activation
We next investigated the effect of trametinib on renal fibroblasts expansion and signaling by stimulating primary human fibroblasts with TGF-β1, the predominant profibrotic cytokine.13,15
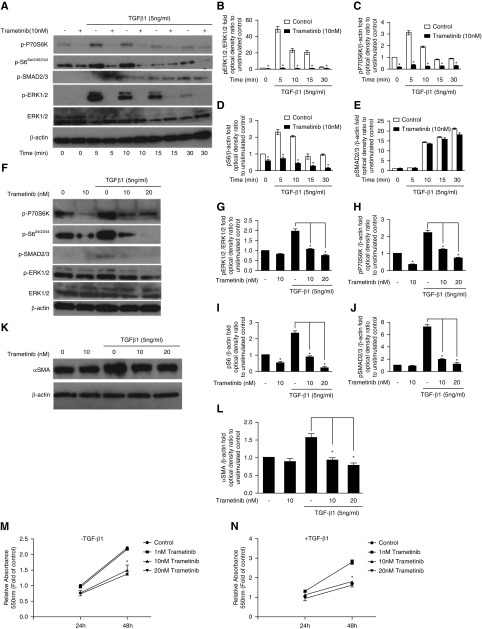
Acute application of TGF-β1 resulted in significantly increased mTROC1 and ERK1/2 pathways activation, as previously reported19,39 (Figure 7A). Trametinib (10 nM) completely inhibited ERK1/2 activation at all time points tested (Figure 7, A and B). Concurrently, at 5 minutes poststimulation, phosphorylation of P70S6K and S6 was attenuated by 88% and 75%, respectively (Figure 7, A, C, and D). Trametinib also significantly blunted mTORC1 pathway activation for all of the other time points tested (Figure 7A). Conversely, Smad2/3 phosphorylation was not significantly affected by trametinib, suggesting no direct interference with TGF-β1 receptor signaling (Figure 7, A and E).
Figure 7.
Trametinib inhibits ERK1/2 and mTORC1 pathway activation, αSMA expression, and proliferation of primary human renal fibroblasts in response to TGF-β1. (A) Serum-starved human renal fibroblasts were preincubated with trametinib (10 nM) for 30 minutes before stimulation with TGF-β1 (5 ng/ml) for the indicated times. ERK1/2, P70S6K, S6, and Smad2/3 activation were probed with Western blotting. Membranes were subsequently stripped and reprobed for total ERK1/2 and β-actin to ensure equal protein loading. A representative image from n=3 independent experiments is shown. (B–E) Normalized optical densities of the phospho-P70S6K, phospho-S6, phospho-Smad2/3, and phospho-ERK1/2 bands against the appropriate loading controls. The value of the unstimulated control at time 0 was arbitrarily set to 1. Bars represent the means±SEM from n=3 experiments; *P<0.05. (F) Primary human renal fibroblasts in complete medium were preincubated for 30 minutes with trametinib (10 or 20 nM) or vehicle and then stimulated with TGF-β1 (5 ng/ml) for 24 hours. The activation of ERK1/2, Smad2/3, P70S6K, and S6 was determined by Western blotting as in (A). (G–J) Densitometric analysis of the Western blots in (F). Band intensity was normalized against total ERK1/2 or β-actin as appropriate, and expressed as folds of the unstimulated control. Bars represent the means±SEM from n=3 experiments; *P<0.05. (K) Human renal fibroblast were stimulated with TGF-β1 (5 ng/ml) for 24 hours in the presence or absence of trametinib as indicated and αSMA expression was determined by Western blotting. (L) Densitometric analysis of the experiments shown in (K). Bars represent the means±SEM from n=3 independent experiments; *P<0.05. (M and N) Human renal fibroblasts in complete medium were preincubated with vehicle or the indicated concentrations of trametinib for 30 minutes. Subsequently, some fibroblasts were stimulated with TGF-β1 (5 ng/ml) as appropriate and their proliferation was estimated by the MTT assay after 24 or 48 hours. Each condition was assayed in triplicate. The absorbance at 550 nm of the control unstimulated sample after 24 hours was arbitrarily set to 1. Bars represent the means±SEM from n=3 experiments; *P<0.05.
Likewise, trametinib suppressed TGF-β1–induced ERK1/2 and mTORC1 activation in the presence of serum over 24 hours. Fibroblasts preincubated with 20 nM trametinib exhibited reduced phospho-ERK1/2 levels by 58% at 24 hours after TGF-β1 treatment (Figure 7, F and G). Phospho-P70S6K and phospho-S6 were also significantly suppressed by 67% and 91%, respectively, in these cells (Figure 7, F, H, and I). In contrast to short-term stimulation, trametinib also ameliorated TGF-β1–induced Smad2/3 phosphorylation after 24 hours (Figure 7, F and J). TGF-β1 significantly increased αSMA expression by 1.57-fold and trametinib (20 nM) resulted in 51% reduction in αSMA expression (Figure 7, K and L). Additionally, trametinib dose-dependently suppressed both TGF-induced and basal fibroblast proliferation, with a more prominent effect for 20 nM after 48 hours in culture (Figure 7, L and M). Specifically, at 48 hours, 20 nM trametinib reduced fibroblast expansion at basal or TGF-β1–stimulated conditions by 39% and 41%, respectively.
Considerable αSMA expression was detected in proliferating fibroblasts at basal conditions (no TGF-β1), suggesting that in the presence of serum, human fibroblasts acquired some myofibroblast properties. This is in agreement with studies using murine fibroblasts.39,44 Nonetheless, TGF-β1 had an additive effect, significantly upregulating αSMA and increasing fibroblast proliferation (Figure 7, K and N).
Discussion
We report that ERK1/2 pathway inhibition with the clinically available MEK-specific inhibitor trametinib significantly ameliorated collagen deposition and myofibroblast expansion during kidney fibrosis. Mechanistically, MEK-ERK1/2 inhibition by trametinib also significantly suppressed downstream mTORC1, STAT3, Smad2/3, and NF-κB pathway activation (Figures 1–4). Work with human fibroblasts corroborated our findings and revealed that trametinib suppressed TGF-β1–induced ERK1/2 and mTORC1 pathway activation, and αSMA upregulation and proliferation (Figure 7). Trametinib also significantly attenuated renal fibrosis progression and signaling when administered to animals subsequent to injury (Figure 5, Supplemental Figures 2 and 3). Moreover, trametinib alleviated kidney fibrosis in adenine-fed animals, a diet-induced model of CKD, over a 2-month period (Figure 6). This is the first study showing that the clinically available ERK1/2 pathway inhibitor trametinib could ameliorate kidney fibrosis in vivo.
ERK1/2 is activated during renal fibroblast differentiation in vitro,39 both in preclinical models of kidney fibrosis in vivo20,47 and in patient myofibroblasts.25 Moreover, ERK1/2 is phosphorylated downstream of TGF-β1, EGF, vascular endothelial growth factor, and PDGF receptors, all of which have been detrimentally implicated in the progression of renal fibrosis.20,39,47 However, whether ERK1/2 inhibition alone in the fibrotic kidney would be sufficient to prevent disease progression has not been previously comprehensively tested.
Trametinib dramatically decreased collagen deposition (Figure 1B) and significantly reduced αSMA expression (Figure 1, D–H) in obstructed kidneys. Additionally, trametinib ameliorated αSMA upregulation and proliferation in TGF-β1–stimulated human fibroblasts (Figure 7, K–N). Vimentin expression, often used alongside αSMA as a marker of myofibroblasts and known to be upregulated during UUO,48 was also significantly attenuated by trametinib (Figure 1F).
Trametinib significantly blocked ERK1/2 activation in vivo (Figure 2, A and D) and in vitro (Figure 7, A and F), and reduced interstitial Ki67 expression in the UUO kidneys, possibly reflecting suppression of myofibroblast and macrophage expansion (Figure 2D). In addition to the interstitium, considerable ERK1/2 phosphorylation was detected in the tubules of UUO animals (Figure 2B). Tubular injury can directly induce fibroblast activation via the secretion of soluble factors.11 Additionally, injured tubules undergo epithelial–mesenchymal transition, upregulate vimentin, and promote fibrosis.17 It is probable that some of the beneficial effects of ERK1/2 inhibition are due to effects on tubular cells. Indeed, trametinib has been shown to reduce tubular damage during ischemia/reperfusion injury49,50 and sepsis.50 Whether ERK1/2 pathway inhibition ameliorated tubular injury upon UUO in addition to suppressing myofibroblast expansion merits further investigation. Trametinib also attenuated AKT phosphorylation (Figure 2A), known to be activated downstream of TGF-β151 and induced by UUO.26 AKT inhibition in trametinib-treated animals was modest (around 50%), suggesting that it could be due to indirect effects of ERK1/2 inhibition on fibroblast expansion and subsequent diminished TGF-β1 secretion.52
Blocking the ERK1/2 pathway suppressed mTORC1 activation in UUO kidneys (Figure 3, A–E) and cultured primary kidney fibroblasts in response to acute (Figure 7A) or prolonged (Figure 7F) TGF-β1 stimulation. mTORC1 becomes activated in TGF-β1–stimulated fibroblasts and during UUO,19 and its inhibition ameliorated renal fibrosis.19,53 ERK1/2 can activate mTORC1, probably by phosphorylating upstream regulators in cells,23 including kidney podocytes.40 However, whether ERK1/2 modulates mTORC1 activity in kidney fibroblasts had not been previously reported. The effect of trametinib on mTORC1 is unlikely to be due to off-target effects because it is specific for MEK1/2, with minimal activity against a panel of 180 other kinases, including mTORC1.54 Thus, trametinib in the fibrotic kidney could act as a dual-target inhibitor with potentially fewer side effects than a combination of targeted therapies. Further work is needed to identify precisely how ERK1/2 modulated mTORC1 signaling in myofibroblasts.
Nox4 is a major superoxide source during kidney pathology55 and is induced by TGF-β1 in kidney fibroblasts41 and podocytes.40,42 Trametinib ameliorated Nox4 upregulation in vivo (Figure 3G). This supports our finding that ERK1/2 inhibition suppressed mTORC1 activation in the UUO kidney because Nox4 overexpression occurs downstream of mTORC1 activation.40
Trametinib ameliorated the phosphorylation of STAT3 and NF-κB (Figure 4A), pathways also implicated in kidney fibrosis progression.21,44 Notably, STAT3 can be activated downstream of mTORC1,45 supporting our finding that trametinib ameliorates mTORC1 signaling in injured kidneys. Because both these transcription factors regulate the expression of genes intimately involved in the inflammatory response, we expected trametinib to have anti-inflammatory effects. Indeed, significantly less macrophage infiltration was observed in the obstructed kidneys of trametinib-treated animals (Figure 4F).
In UUO kidneys and kidney fibroblasts trametinib significantly reduced phosphorylation of Smad2/3, one of the main effectors of profibrotic TGF-β1 signaling14 (Figures 4A and 7F). The effect of trametinib is probably indirect because in cultured fibroblasts ERK1/2 inhibition did not affect Smad2/3 phosphorylation upon acute TGF-β1 stimulation (Figure 7A). Proliferating fibroblasts are known to secrete TGF-β1 constitutively52; hence, it is possible that suppressing fibroblast proliferation trametinib reduced bioavailable TGF-β1.
Trametinib also delayed the progression of fibrosis subsequent to injury (Supplemental Figure 1). In the kidneys of animals that received trametinib 4 days post-UUO, collagen deposition, vimentin, and αSMA expression; macrophage infiltration; and the activation of the ERK1/2, AKT, mTORC1, STAT3, and NF-κB pathways were also significantly ameliorated (Figure 5, Supplemental Figures 2 and 3). Consequently, trametinib is potentially suitable for the treatment of patients that present with established CKD and considerable myofibroblast activation.
The UUO model results in rapid myofibroblast expansion,30 thus it does not necessarily recapitulate the renal fibrosis progression observed in patients with CKD. Therefore, we tested the applicability of ERK1/2 pathway inhibition in a chronic murine model of CKD.
Mice were fed an adenine-rich diet for 2 months and trametinib, at a dose (0.3 mg/kg) that inhibits 75% of ERK1/2 signaling, was concurrently administered.56 Adenine is metabolized into 2,8-dihydroxyadenine, which precipitates in the tubules and causes obstruction, resulting in progressive tubular necrosis, macrophage infiltration, tubulointerstitial fibrosis, and diminished renal function.32 Trametinib treatment significantly decreased collagen deposition and myofibroblast expansion, as exemplified by αSMA or vimentin expression (Figure 6). Moreover, profibrotic pERK1/2 and mTORC1 signaling and proinflammatory macrophage infiltration were ameliorated by trametinib (Supplemental Figures 4–6). These findings suggest that ERK1/2 pathway inhibition could also reduce kidney fibrosis in chronic CKD.
Adenine-fed animals displayed a marked decline in renal function manifested by increased plasma creatinine and urea, decreased creatinine clearance ratio, and increased fractional excretion of sodium and proteinuria (Table 1). Despite significant reduction in kidney fibrosis, no improvement in renal function was observed after trametinib treatment (Table 1). This is not entirely surprising given the nature of the model (continuous, long-term tubular injury due to tubular obstruction and mechanical stress upon 2,8-dihydroxyadenine crystal formation). Contrary to our findings with adenine-fed mice, Liu et al.57 recently reported that application of the early MEK inhibitor U0126 (10 mg/kg) improved kidney function in addition to reducing fibrosis in hyperuricemic rats. This discrepancy could be due to species difference (mouse versus rat) and/or differences in the length and nature of the insult (2,8-dihydroxyadenine crystal formation over 2 months versus uric acid accumulation over 1 month in the Liu et al. study57). Alternatively, persistent ERK1/2 inhibition by trametinib over 2 months could result in some tubular toxicity in injured kidneys, cancelling out the positive effect of reduced fibrosis. A third possibility is that longer-term kidney functional deterioration in the adenine-diet model may become “uncoupled” from fibrosis and/or macrophage infiltration, and be driven primarily by tubular damage from 2,8-dihydroxyadenine crystal formation.
Nonetheless, even if trametinib does not improve renal function in the presence of continuous tubular injury, this does not preclude its applicability in the clinic. Up to 74% of patients with stage 4 CKD progress to ESRD, even when the initial cause of CKD has been treated,58 at least in part because of persistent renal fibrosis.5 Moreover, advances in identifying noninvasive biomarkers of renal fibrosis59 could help identify patients most expected to benefit from antifibrotic therapy.
Trametinib is well tolerated in humans, with no renal toxicity and the majority of side effects (skin rash and diarrhea) easily managed with secondary medication.27,28,60 Importantly, because 80% of trametinib is excreted in the feces,60 it is potentially suitable for patients with renal impairment. Moreover, we found that trametinib had antifibrotic effects at up to 30-fold lower doses than those used in tumor animal models,61 suggesting that lower doses could be beneficial in patients with CKD with fewer side effects.
Unlike most common diseases, mortality rates are increasing for patients with CKD.22 There has never been a greater need for the development of novel therapeutic agents for CKD. Repurposing already available drugs could significantly accelerate their transition to the renal clinic and alleviate the CKD health burden. We envisage that our work could guide future clinical trials to verify the safety and efficacy of trametinib in the treatment of renal fibrotic diseases.
Disclosures
None.
Supplementary Material
Acknowledgments
P.A., J.K., S.P., L.N., S.M.H., and C.E.O. performed experiments. P.A. and M.M.Y. designed the study. P.A., C.T., and M.M.Y. interpreted the results and wrote the paper. All authors commented on the manuscript.
This work was directly funded by the Barts and the London National Institutes of Health Research Cardiovascular Biomedical Research Unit. We also acknowledge support to P.A., J.K., S.P., S.M.H., and M.M.Y. by the Barts Health Diabetic Kidney Disease Centre, funded by the Barts and the London Charity (grant 577-2348).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018020209/-/DCSupplemental.
Supplemental Material
Supplemental Figure 1. Significant myofibroblast activation is observed in kidneys subjected to UUO for 4 days.
Supplemental Figure 2. Delayed administration of trametinib attenuated ERK1/2, AKT, and mTORC1 pathway activation in UUO kidneys.
Supplemental Figure 3. Delayed trametinib administration attenuated Nox4 upregulation and suppressed STAT3, NF-κB, Smad2/3 phosphorylation, and macrophage infiltration in UUO kidneys.
Supplemental Figure 4. Trametinib suppressed ERK1/2 activation and myofibroblast proliferation in the kidneys of adenine-fed animals.
Supplemental Figure 5. Trametinib treatment ameliorated mTORC1 activation in the kidneys of adenine-fed mice.
Supplemental Figure 6. Trametinib ameliorated macrophage infiltration in the kidneys of adenine-fed mice.
Supplemental Figure 7. Animal weights for the course of the adenine-diet experiment.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al.: Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 11: e0158765, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leaf IA, Duffield JS: What can target kidney fibrosis? Nephrol Dial Transplant 32[Suppl 1]: i89–i97, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Nicholson ML, McCulloch TA, Harper SJ, Wheatley TJ, Edwards CM, Feehally J, et al.: Early measurement of interstitial fibrosis predicts long-term renal function and graft survival in renal transplantation. Br J Surg 83: 1082–1085, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Campanholle G, Ligresti G, Gharib SA, Duffield JS: Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 304: C591–C603, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddy AA, Neilson EG: Chronic kidney disease progression. J Am Soc Nephrol 17: 2964–2966, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Mack M, Yanagita M: Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int 87: 297–307, 2015 [DOI] [PubMed] [Google Scholar]
- 8.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al.: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Meran S, Steadman R: Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92: 158–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, et al.: Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393–2406, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Border WA, Noble NA: Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int 54: 1390–1391, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Meng XM, Tang PMK, Li J, Lan HY: TGF-β/Smad signaling in renal fibrosis. Front Physiol 6: 82, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derynck R, Zhang YE: Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, et al.: Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182: 118–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn TA, Ramalingam TR: Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, et al.: Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997, 2015 [DOI] [PubMed] [Google Scholar]
- 18.White ES, Mantovani AR: Inflammation, wound repair, and fibrosis: Reassessing the spectrum of tissue injury and resolution. J Pathol 229: 141–144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Xu L, Mao J, Li J, Fang L, Zhou Y, et al.: Rheb/mTORC1 signaling promotes kidney fibroblast activation and fibrosis. J Am Soc Nephrol 24: 1114–1126, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al.: Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Sun SC: NF-κB in inflammation and renal diseases. Cell Biosci 5: 63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breyer MD, Susztak K: The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 15: 568–588, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP: Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Beinke S, Robinson MJ, Hugunin M, Ley SC: Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol 24: 9658–9667, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ: Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol 15: 1835–1843, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Peña AB, Grande MT, Eleno N, Arévalo M, Guerrero C, Santos E, et al.: Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int 74: 196–209, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al.: Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372: 30–39, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al.: Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386: 444–451, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Puri TS, Shakaib MI, Chang A, Mathew L, Olayinka O, Minto AW, et al.: Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol 298: F1024–F1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, et al.: An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 517: 391–395, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura M, Aizawa R, Hori M, Ozaki H: Progressive renal dysfunction and macrophage infiltration in interstitial fibrosis in an adenine-induced tubulointerstitial nephritis mouse model. Histochem Cell Biol 131: 483–490, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Andrikopoulos P, Eccles SA, Yaqoob MM: Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF-induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell Signal 37: 12–30, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Andrikopoulos P, Baba A, Matsuda T, Djamgoz MB, Yaqoob MM, Eccles SA: Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem 286: 37919–37931, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrikopoulos P, Kieswich J, Harwood SM, Baba A, Matsuda T, Barbeau O, et al.: Endothelial angiogenesis and barrier function in response to thrombin require Ca2+ influx through the Na+/Ca2+ exchanger. J Biol Chem 290: 18412–18428, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson FL, Patel NSA, Purvis GSD, Chiazza F, Chen J, Sordi R, et al. : Inhibition of IκB kinase at 24 hours after acute kidney injury improves recovery of renal function and attenuates fibrosis. J Am Heart Assoc 6: e005092, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen EC: Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 296: 378–381, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Buonato JM, Lazzara MJ: ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res 74: 309–319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saliba Y, Karam R, Smayra V, Aftimos G, Abramowitz J, Birnbaumer L, et al.: Evidence of a role for fibroblast transient receptor potential canonical 3 Ca2+ channel in renal fibrosis. J Am Soc Nephrol 26: 1855–1876, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das R, Xu S, Nguyen TT, Quan X, Choi SK, Kim SJ, et al.: Transforming Growth factor β1-induced apoptosis in podocytes via the extracellular signal-regulated kinase-mammalian target of rapamycin complex 1-NADPH oxidase 4 axis. J Biol Chem 290: 30830–30842, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, et al.: NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eid AA, Ford BM, Bhandary B, de Cassia Cavaglieri R, Block K, Barnes JL, et al.: Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 62: 2935–2947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxton RA, Sabatini DM: mTOR signaling in growth, metabolism, and disease. Cell 169: 361–371, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, et al.: A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Meng Y, Kwiatkowski DJ, Chen X, Peng H, Sun Q, et al.: Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest 120: 103–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolic-Paterson DJ, Wang S, Lan HY: Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl (2011) 4: 34–38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F, Wang L, Qi H, Wang J, Wang Y, Jiang W, et al.: Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin Sci (Lond) 131: 2125–2143, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Yuan Y, Zhang F, Wu J, Shao C, Gao Y: Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci Rep 5: 9314, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collier JB, Whitaker RM, Eblen ST, Schnellmann RG: Rapid renal regulation of peroxisome proliferator-activated receptor γ coactivator-1α by extracellular signal-regulated kinase 1/2 in physiological and pathological conditions. J Biol Chem 291: 26850–26859, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collier JB, Schnellmann RG: Extracellular signal-regulated kinase 1/2 regulates mouse kidney injury molecule-1 expression physiologically and following ischemic and septic renal injury. J Pharmacol Exp Ther 363: 419–427, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL: Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Kelley J, Fabisiak JP, Hawes K, Absher M: Cytokine signaling in lung: Transforming growth factor-beta secretion by lung fibroblasts. Am J Physiol 260: L123–L128, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, et al.: Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7: e33626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi T, Kakefuda R, Tajima N, Sowa Y, Sakai T: Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int J Oncol 39: 23–31, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Thallas-Bonke V, Jandeleit-Dahm KA, Cooper ME: Nox-4 and progressive kidney disease. Curr Opin Nephrol Hypertens 24: 74–80, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Itamura H, Shindo T, Tawara I, Kubota Y, Kariya R, Okada S, et al.: The MEK inhibitor trametinib separates murine graft-versus-host disease from graft-versus-tumor effects. JCI Insight 1: e86331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu N, Xu L, Shi Y, Fang L, Gu H, Wang H, et al.: Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats. Oncotarget 8: 33807–33826, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong J, Yang HC, Fogo AB: A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol 312: F375–F384, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, et al.: Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 28: 598–611, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al.: Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 31: 482–489, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al.: BRAF mutation predicts sensitivity to MEK inhibition. Nature 439: 358–362, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.