The Env protein of HIV is highly glycosylated, and the sites of glycosylation can change as the virus mutates during immune evasion. Due to these changes, the glycan location and heterogeneity of surrounding N-glycosylation sites can be altered, resulting in exposure of different glycan or proteoglycan surfaces while still producing a viable HIV variant. These changes present a need for vaccine developers to identify Env variants with epitopes most likely to induce durable protective responses. Here we describe a means of anticipating HIV-1 immune evasion by dividing Env into N-glycan microdomains that have a limited number of N-glycan sequon combinations.
KEYWORDS: HIV-1 envelope, N-glycosylation, human immunodeficiency virus, mass spectrometry
ABSTRACT
The HIV-1 envelope (Env) glycans shield the surface of Env from the immune system and form integral interactions important for a functional Env. To understand how individual N-glycosylation sites (NGS) coordinate to form a dynamic shield and evade the immune system through mutations, we tracked 20 NGS in Env from HIV-transmitted/founder (T/F) and immune escape variants and their mutants involving the N262 glycan. NGS were profiled in a site-specific manner using a high-resolution mass spectrometry (MS)-based workflow. Using this site-specific quantitative heterogeneity profiling, we empirically characterized the interdependent NGS of a microdomain in the high-mannose patch (HMP). The changes (shifts) in NGS heterogeneity between the T/F and immune escape variants defined a range of NGS that we further probed for exclusive combinations of sequons in the HMP microdomain using the Los Alamos National Laboratory HIV sequence database. The resultant sequon combinations, including the highly conserved NGS N262, N448, and N301, created an immune escape map of the conserved and variable sequons in the HMP microdomain. This report provides details on how some clustered NGS form microdomains that can be identified and tracked across Env variants. These microdomains have a limited number of N-glycan-sequon combinations that may allow the anticipation of immune escape variants.
IMPORTANCE The Env protein of HIV is highly glycosylated, and the sites of glycosylation can change as the virus mutates during immune evasion. Due to these changes, the glycan location and heterogeneity of surrounding N-glycosylation sites can be altered, resulting in exposure of different glycan or proteoglycan surfaces while still producing a viable HIV variant. These changes present a need for vaccine developers to identify Env variants with epitopes most likely to induce durable protective responses. Here we describe a means of anticipating HIV-1 immune evasion by dividing Env into N-glycan microdomains that have a limited number of N-glycan sequon combinations.
INTRODUCTION
A combination of interconnected N-glycans covers the surface of HIV-1 envelope (Env) spike (1), the sole surface component of HIV-1 virions (2). This glycan shield serves as an interface between the virus and the host immune system, often eliciting an antibody response (3). Recent structural studies of the closed prefusion Env trimer have characterized the interface of the glycan shield with broadly neutralizing antibodies (BnAbs) that either penetrate the shield or target specific clusters of glycans on the surface of Env (4–6).
Several studies have examined the composition of the glycan shield by using mass spectrometry (MS) to determine the multiple glycoforms observed at a single glycosylation site, sometimes referred to as site-specific heterogeneity (7–9). These studies have helped guide the search for in vitro constructs that most closely mimic the native Env trimer in terms of N-glycosylation and conformation in order to produce a priming vaccine antigen (10). One outcome of these analyses has been the realization of how different conformations of the assembled trimer can influence the site-specific heterogeneity of different N-glycosylation sites (NGS) (11). This is especially the case for NGS in close proximity to each other, such as those in the densely glycosylated gp120 outer domain known as the high-mannose patch (HMP) (12) and NGS adjacent to subunit-subunit interactions in the folded trimer, such as those found at the apex of the closed prefusion trimer (13). Recent atomic level structures of Env trimers with oligomannose glycans have also laid the groundwork for interpreting the glycan-glycan interactions that occur within the glycan shield, including branch-branch, stem-stem, and forked-N-glycan interactions (1, 14). One inference from these glycosylated structures has been that the Env glycan shield consists of microdomains of interdependent NGS (15). Other studies have begun to highlight specific structural and functional roles of individual glycans (16, 17).
The mutability of HIV-1 due to the low fidelity of its reverse transcriptase (18) produces immune escape viral variants. This process can produce Env variants with altered NGS that could change the surface of the Env trimer and potentially nullify the binding and neutralization by the host’s Abs (19). Such mutations that add or remove NGS have a potential to enhance or lessen the virulence of a given strain of HIV. Thus, there is a need to understand how the virus balances immune evasion with the functional integrity of the Env trimer in the context of the glycan shield. Several NGS are highly conserved (12, 20), but there is still extensive variability in the combinations of NGS across variants. The extent to which site-specific quantitative profiles of N-glycan heterogeneity can reflect differences in Env variants at the sequon level has not been fully explored.
In this study, we tracked the site-specific N-glycan quantitative profiles of WEAU transmitted/founder (T/F) and chronic-stage (CS) viruses that differ in seven NGS (19). The observed differences were further assessed using two corresponding mutants that had added or removed the N262 glycosylation site. This glycan influences gp120 folding (21) due to its unique interactions with amino acids in a cleft between the inner and outer domains of gp120 (16). Our results demonstrated how differences in the glycan shield between variants lead to reproducible quantitative shifts in glycan heterogeneity of some NGS, thus identifying interdependency of some adjacent glycans and empirically defining N-glycan microdomains. By following the heterogeneity patterns coupled with modeling sequons onto the Env structure, we identified a specific glycan cluster that involves the N262 and N295 glycans. Based on these results, we used the Los Alamos National Laboratory (LANL) HIV sequence database and created a series of NGS combinatorial databases that allowed us to parameterize this N-glycan sequon microdomain. This analysis represented the frequency of NGS combinations that reflect both the highly conserved and variable sites of N-glycosylation within the cluster. Overall, this work suggests that N-glycan microdomains can be parameterized by combining tracked quantitative profiles of N-glycan heterogeneity with structurally mapped NGS mutations and bioinformatic analysis of HIV Env sequon combinations. These results have broad implications for defining NGS microdomains that can be targeted across HIV-1 clades, providing a means for anticipating immune escape and a better understanding of the dynamic HIV-1 Env glycan shield.
RESULTS
Site-specific N-glycan heterogeneity profiles of WEAU gp120 trimers.
Two distinct Env variants were chosen for site-specific N-glycan heterogeneity analysis: Env from a T/F virus (WEAU#3) isolated from a patient 16 days after onset of symptoms of acute retroviral syndrome and a CS virus (WEAU#4) isolated 391 days after onset (19). Env gp120 sequences of these two variants differ in seven potential NGS (N141, N295, and N398, unique in WEAU#3, and N234, N262, N386, and N406, unique in WEAU#4). For analysis of each recombinant trimeric gp120 glycoprotein, a panel of protease- and/or glycosidase-treated preparations was generated to (i) identify specific sites of glycosylation, (ii) determine the elution profiles of sets of N-glycopeptides that correspond to a single NGS, and (iii) serve as overlapping heterogeneity profiles of the various sites (Fig. 1). Together, the different preparations for each gp120 trimer provided corroboration of observed changes and quantifiable shifts in the heterogeneity across samples and preparations, as detailed below.
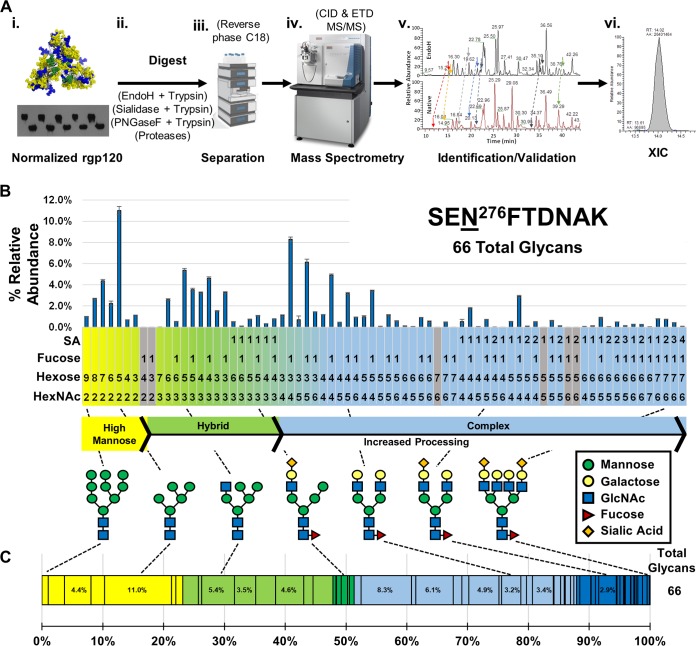
FIG 1.
N-glycan site-specific heterogeneity profiling of WEAU#4 rgp120 trimer determined by LC-MS. (A) Workflow scheme for the analysis of rgp120 N-glycopeptides. Normalized protein amounts of each rgp120 preparation (i) were digested to produce glycopeptides (ii) that were further separated by reversed-phase C18 chromatography (iii) followed by MS and MS/MS analysis (iv). Sets of glycopeptides with the same base peptide were identified based on their retention times and validated (v). Extracted ion chromatograms (XICs) were obtained for identified glycopeptides and used for site-specific N-glycan profiles (vi). (B) N-glycan heterogeneity profile for N276. This site has a high proportion of hybrid and complex N-glycans. All XICs from observed glycopeptides with the base peptide SENFTDNAK were represented for N276 site-specific quantitative analysis as a bar graph. Each glycan, from left to right, is organized in the order of N-glycan biosynthesis. Gray-shaded glycans at the bottom of the chart indicate potential glycopeptide compositions that were not observed. (C) A simplified representation of the site-specific quantitative analysis data from panel B, emphasizing the weighted distribution of high-mannose (yellow), hybrid (green), and complex (blue) oligosaccharides, arranged left to right as in panel B (Man9→Man5→hybrid→complex glycans with increasing number of antennas). Darker shading represents N-glycans that contain sialic acid (SA) residue(s). The dashed lines between panels C and B connect specific glycopeptides depicted in the two representations of the quantitative heterogeneity data with the corresponding glycan structures. The total number of glycans at each NGS is shown to the right of the bar.
The large number of potential NGS in each gp120 trimer creates a challenge for data processing, annotation, and informatics, as detailed by other groups (22, 23). We developed a workflow to execute cross-sample comparisons of HIV-1 Env trimer variants (Fig. 1A). Samples were normalized by Western blotting using a V5 tag-specific antibody so that the same amount of each variant was analyzed, preventing load bias (24). Each recombinant gp120 (rgp120) trimer was digested by a panel of proteases and glycosidases, each digest separately providing information about the glycosylation. For example, peptide-N-glycosidase F (PNGase F) and endoglycosidase H (endo H) digests confirmed N-glycan occupancy of potential NGS. After digestions, the peptides and glycopeptides were separated using reverse-phase C18 chromatography and analyzed by high-resolution mass spectrometry (MS) and tandem mass spectrometry (MS/MS). Both collision-induced dissociation (CID) and electron transfer dissociation (ETD) were utilized to identify specific glycopeptides. To speed up the validation process and reduce the number of false-positive results, the confirmed glycopeptides from the endo H digest were used to limit the retention time (RT) window search for each glycopeptide (Fig. 1A, panel v). Further details on the peak assignment and validation process are provided in Materials and Methods. Once the glycopeptides were identified, the area under the curve (AUC) from the extracted ion chromatogram (XIC) obtained from the mass spectra was calculated (Fig. 1A, panel vi). The AUC from each glycopeptides’s XIC was used for label-free quantitative analysis by expressing the observed N-glycoform as a percent relative abundance of the total sum of all glycoforms for an NGS (25, 26). The combined data points for each glycoform at a given NGS provide a site-specific N-glycan profile (Fig. 1B) that can then be tracked across HIV Env variants.
The site-specific glycopeptide profiles were rendered in an order that reflects N-glycan processing starting with high-mannose glycans that are remodeled by processing enzymes into hybrid and complex N-glycans in the Golgi apparatus (27) (Fig. 1B). This presentation of glycans in the order of biosynthesis/processing enables a quick visualization of the most abundant oligosaccharide(s) at a given site and links heterogeneity patterns to N-glycan processing/controlling events. For example, the most predominant oligosaccharide at N276 is Man5GlcNAc2 (11%), followed by a series of related oligosaccharides centered on the addition of saccharides to Fuc1Man3GlcNAc4 (Fuc1Hex3HexNAc4). This analysis implies that N276 is a heavily processed NGS wherein oligomannose N-glycans are converted to a mixture of bi- and triantennary oligosaccharides.
To further simplify the representation, the entire range of glycans at a single site was presented as a bar divided into the relative distributions of the broad N-glycan categories of high-mannose (yellow), hybrid (green), and complex (blue) (Fig. 1C). Each N-glycan category is subdivided into individual oligosaccharides based on their weighted distribution. Sialylated oligosaccharides of hybrid and complex glycans are identified by darker shades of green and blue, respectively. These bars are organized from left to right based on N-glycan processing in the same order as the bar graph in Fig. 1B. The iterative process led to the identification of 17 clusters of glycopeptides that corresponded to 20 NGS that we tracked and visualized across multiple samples.
General classifications of NGS heterogeneity.
Visualizing 20 NGS of gp120 trimer WEAU#4 revealed three distinct types of N-glycan heterogeneity patterns: (i) predominantly high-mannose (Fig. 2A) with dominant Man9, (ii) mixed (codominance of processed and unprocessed glycans (Fig. 2B), and (iii) predominantly processed (Fig. 2C) with Man5 dominant among high-mannose glycans. We have initially reported on this nomenclature (28) and now provide greater detail here. For each distribution, 74 possible oligosaccharides are shown. All NGS heterogeneity profiles are presented in Data Set S1 in the supplemental material.
FIG 2.
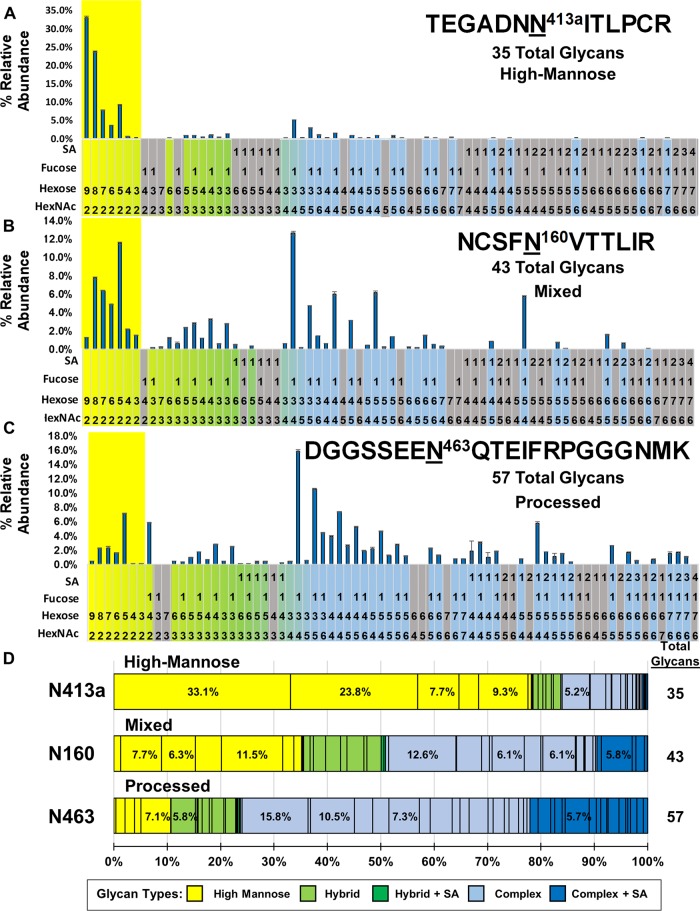
Distinct site-specific N-glycosylation classifications. (A to C) Bar graphs of the glycosylation analysis for three representative types of NGS glycan profiles from WEAU#4 rgp120 trimer. The high-mannose N-glycans are highlighted in yellow. (A) N413a has predominantly high-mannose glycans with few complex glycans. (B) N160 has a mixed population of processed and unprocessed glycans. (C) N463 contains a wide range of predominantly processed oligosaccharides. (D) The same data as for panels A to C rendered as percent weighted distribution graphs of each NGS. The total number of glycans at each NGS is shown to the right of the bar.
The first type of NGS heterogeneity reflects a predominantly high-mannose pattern and is represented by N413a (Fig. 2A). Within the quantitative distribution of glycans at N413a, Man9GlcNAc2 (33%) is the most abundant oligosaccharide, closely followed by Man8GlcNAc2 (24%), suggesting little processing at the site, which is consistent with fewer hybrid and complex glycans (29 oligosaccharides; 22% of total distribution). This single-site heterogeneity has fewer observed N-glycopeptides (i.e., 35) than other NGS. N-glycan heterogeneity profiles at N234, N241, N262, N332, N339, and N448 also fall within this category.
The opposite type of NGS heterogeneity is represented by N463, which has a broad range of N-glycans, including both high-mannose and highly branched complex glycans. Within the high-mannose glycans, there is a dominance of the Man5GlcNAc2 (7.1%) oligosaccharide, indicative of a highly processed NGS (i.e., more hybrid and complex oligosaccharides). Figure 2C shows the 58 assigned N-glycan compositions, including 51 hybrid/complex N-glycans containing several tetra-antennary glycans with up to three sialic acid residues, indicating that the site is dominated by processed glycans. When an equivalent sample pretreated with neuraminidase was analyzed, several complex N-glycopeptides were found to be consolidated into their desialylated forms with a relative abundance equal or greater than that of the Man5 glycoform of the high-mannose glycans (Data Set S1). This heterogeneity classification of predominantly processed glycans is also observed for NGS N88, N132c, N188, N197, N241, N276, and N356.
The third type of site-specific heterogeneity is represented by N160 (Fig. 2B). In this classification, there is no dominant high-mannose glycan. Instead, the distribution reflects a mixed population in which Man5GlcNAc2 (11.5%) and Man8GlcNAc2 (7.7%) are the most abundant high-mannose N-glycans. The profile reflects a bimodal distribution within the population of observed glycopeptides of unprocessed (mostly oligomannose glycans) and partially processed (hybrid and complex glycans with a low number of antennas) glycans. For example, N160 contains four individual hybrid/complex N-glycopeptides that are equal in abundance to the high-mannose glycan content. The codominance in the high-mannose glycans and the mixed distribution of the heterogeneity profile is distinct from both the high-mannose NGS (Fig. 2A) and the processed NGS (Fig. 1B and C and Fig. 2C). This mixed classification of gp120 N-glycan site-specific heterogeneity is observed at NGS N130 and N156 in gp120 trimer WEAU#4. Figure 2D provides a comparison of the three types of NGS heterogeneity represented by NGS N413a, N160, and N463.
Based on the consistent heterogeneity profiles of WEAU#4, analysis of NGS heterogeneity profiles of gp120 trimer WEAU#3 was performed. The same three classifications were observed in the WEAU#3 gp120 trimer variant (Data Set S1). However, three of the NGS observed had significant changes in their heterogeneity profiles between WEAU#4 and WEAU#3 (N448, N160, and N156). N448, a predominantly high-mannose glycan in WEAU#4, was classified as mixed in WEAU#3. These data suggest a quantitative change in N-glycan heterogeneity toward more processed oligosaccharides in these three NGS of gp120 WEAU#3. The trend toward more processed glycans was consistently observed for technical and biological replicates for all NGS when analyzed in parallel with WEAU#4. Regarding other reported gp120 trimer site-specific N-glycan heterogeneity profiles, our results were similar to those reported for the BG505 WT.SEKS Env trimer (11).
Validation of N-glycan quantitative heterogeneity profiles.
To validate the observed differences in NGS profiles in different Env trimers and assess the significance of quantitative changes, we performed a series of site-specific quantitative profiles using preparations where the N-glycan heterogeneities of WEAU#3 and WEAU#4 were altered by two different means. First, rgp120 trimers were produced in the presence of an α-mannosidase I inhibitor (deoxymannojirimycin [DMJM]) to perturb the heterogeneity toward high-mannose glycans (24). In the subsequent glycomic analysis, there was a broad shift in all NGS to high-mannose glycans, regardless of the classification of NGS in the untreated samples. Figure 3 shows the quantitative heterogeneity shift for two representative NGS (N276 and N339) associated with a decrease in the total number of observed glycans. The same trend was seen in the other tracked NGS. Conversely, the O-glycan heterogeneity profile of T503 (13 glycopeptides) exhibited no shifts in heterogeneity between native and DMJM-treated samples, as expected (Data Set S1).
FIG 3.
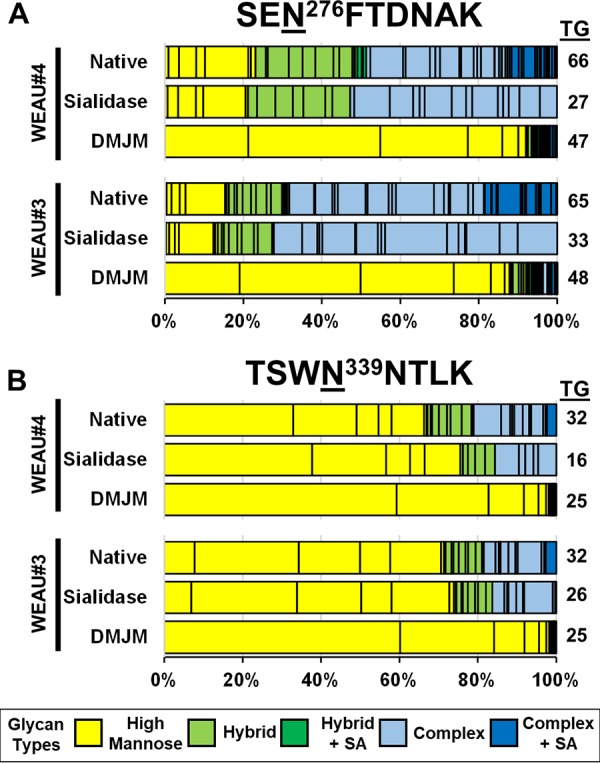
Manipulation of N-glycan heterogeneity by an α-mannosidase inhibitor or sialidase treatment. Shown are single-bar graphs of two NGS classification types (N276, predominantly processed [A], and N339, predominantly high-mannose glycans [B]) from WEAU#3 and WEAU#4 trimeric rgp120 preparations under native conditions (untreated), after treatment with sialidase, and isolated from cells treated with deoxymannojirimycin (DMJM), an α-mannosidase I inhibitor. The sialidase-treated samples show shifts within the hybrid and complex subcategories of heterogeneity and small shifts in the total distribution of high-mannose, hybrid, and complex glycans, all due to removal of sialic acid by sialidase. Trimeric rgp120 produced by cells treated with DMJM shows a shift in the overall glycan distributions to mostly high-mannose glycans at both sites. Both types of manipulation of N-glycan heterogeneity confirmed that relative quantitative assessment of site-specific rgp120 heterogeneity by LC-MS is indicative of the actual distribution of N-glycoforms within a given preparation of gp120 trimer variant. The total number of glycans (TG) at each NGS is shown to the right of the bar.
As a second test, the N-glycan heterogeneity profiles were altered by incubating the extracted tryptic peptides with sialidase to (i) determine the impact of removing negatively charged sialic acid on the heterogeneity profiles and (ii) test whether small changes in glycan heterogeneity are reproducible in our quantitative profiles. After sialidase treatment, there was no gross change in the overall abundance of the high mannose, hybrid, or complex glycopeptide categories for WEAU#3 and WEAU#4, even though the number of observed glycopeptides decreased (Fig. 3). Based on analysis of technical and biological replicates, the standard deviation of measurements of individual glycoform XICs was <0.5%, allowing us to reproducibly detect changes (shifts) in single-site heterogeneity of ≥1.5% (3 standard deviations). These experiments confirmed that observed differences between WEAU#4 and WEAU#3 rgp120 trimer variants are indicative of actual changes in heterogeneity at individual sites and that even small quantitative shifts in overall single-site heterogeneities could be detected.
Inhibition of glycan processing impacts activity of HIV-1 neutralizing antibodies.
To compare Env gp120 glycomic analysis with functional data, we produced pseudotyped WEAU#4 and WEAU#3 HIV-1 in the absence or presence of 800 μM DMJM and tested them for sensitivity to neutralization by a panel of five BnAbs with known epitopes (PG9, PG16, 2G12, PGT121, and 4E10) (Table 1). For PG9 and PG16, with epitopes in the V1/V2 loop of gp120, WEAU#4 was less sensitive to neutralization after DMJM treatment, consistent with the known requirement for sialic acid at N160 for high-affinity binding of the BnAbs (29). For 2G12 (epitope consisting of five glycans in the high-mannose glycan patch [30]), neutralization of WEAU#3 virus was enhanced after treatment with DMJM. WEAU#4, an immune escape virus resistant to 2G12 (50% inhibitory concentration [IC50] >33.3 μl/ml), became somewhat sensitive to 2G12 after treatment with DMJM. Neutralization activity of PGT121 was only marginally affected by DMJM treatment of WEAU pseudotyped viruses. This is in agreement with the observation that a related BnAb, PGT122, binds to N332 high-mannose glycans (1). A control, gp41-specific BnAb, 4E10, showed similar activity regardless of DMJM treatment, as expected. These data show good correlation with the determined glycomic heterogeneity profiles.
TABLE 1.
Neutralization activities of BnAbs against WEAU Env-pseudotyped viruses
| BnAb | Reported epitope | IC50 (μg/ml) |
|||
|---|---|---|---|---|---|
| WEAU#4 | WEAU#4-DMJMa | WEAU#3 | WEAU#3 DMJMa |
||
| PG9 | V1/V2 N156 or 173, N160 | 10.5 | >33.3 | 4.3 | >33.3 |
| PG16 | V1/V2 N156 or 173, N160 | 12.2 | >33.3 | 3.55 | >33.3 |
| 2G12 |
C2/C3/V4/CD4bsb
N295, N332, N386, N392, N448 |
>33.3 | 16.9 | 0.194 | 0.046 |
| PGT121 | C3 N332 | 0.014 | 0.022 | 0.019 | 0.007 |
| 4E10 | gp41 MPERc | 1.3 | 1.5 | 0.40 | 0.37 |
Virus stocks were produced in the presence of 800 μM DMJM.
CD4bs, CD4-binding site.
MPER, membrane-proximal external region.
Constructing a series of WEAU gp120 trimer variants.
WEAU#4 gp120 and WEAU#3 gp120 differ in seven NGS, one of them being N262. WEAU#3, a T/F virus variant, is one of 43 reported Env sequences that do not have an N262 NGS (HIV Sequence Database [http://www.hiv.lanl.gov/]). Based on the comparison of WEAU#4 and WEUA#3 single-site heterogeneities, we examined the influence of the N262 glycan on the heterogeneity of the entire gp120 trimer glycan shield. Ser264 in WEAU#4 was changed to Asn to model the WEAU#3 mutation that disrupts the consensus sequon of N262. The resultant rgp120 mutant (WEAU#4 S264N; WEAU#4M) mimics WEAU#3 in that it lacks an N262 NGS. A second mutant, with N262 NGS inserted in WEAU#3 (WEAU#3 N264S; WEAU#3M), was also constructed.
A published structure of glycosylated monomeric Env reported the N295 glycan within 3 Å of the N262 glycan (16). With N295 absent from WEAU#4, the N262 mutants would also serve to probe the influence of this nearby NGS. High-resolution MS analysis of the N-glycan heterogeneity of equivalent preparations of trimeric rgp120 (Fig. 1A, panel i) of WEAU#3 N264S, WEAU#4, WEAU#3, and WEAU#4 S264N was performed, as before. The NGS common for WEAU#4 and WEAU#3 were consistent with our previous analysis.
Cross-sample comparison of WEAU variants.
To examine the influence of the N262 glycan on individual NGS heterogeneities across the four trimeric rgp120 samples, we created a series of bar representations (using the style shown in Fig. 1C). For the sites tracked across all four constructs, the single-site heterogeneities were assessed for (i) quantitative shifts between NGS classifications, (ii) significant shifts within individual glycan subcategories, and (iii) smaller shifts between individual oligosaccharides. The tracked NGS heterogeneities fell into two broad categories of sites that shifted less than or greater than 15% among the four constructs with varied combinations of N-glycan sites.
Figure 4A shows NGS heterogeneity profiles for N88, N160, and N463, which had a small reproducible shift (<15%) but the overall character of the single-site heterogeneity did not change. N88 has a predominantly processed N-glycan heterogeneity profile that maintains ∼80% complex oligosaccharides among all gp120 trimer variants. This conclusion agrees with other reports on processed oligosaccharides at this site and identifies it as being located in a dispersed region of the gp120 structure (1). Even though there was no significant difference in the subcategories of N-glycans among the variants, a shift of ∼10% for specific oligosaccharides was observed when WEAU#4 was compared to WEAU#4 S264N. For example, the Hex6HexNAc4 hybrid oligosaccharide increased from WEAU#4 (2%) to WEAU#4 S264N (12%). The opposite was true for a complex oligosaccharide, Fuc1Hex3HexNAc4, that decreased from WEAU#4 (18%) to WEAU#4 S264N (6%). When N88 in WEAU#3 was compared to WEAU#3 N264S, no significant difference between the heterogeneity profiles was found. Therefore, while there were discernible shifts among the four analyzed rgp120 variants, the overall character of N88 glycan heterogeneity was unchanged. A similar trend was observed for most tracked sites. Based on existing trimeric models of gp120, the NGS with small to medium shifts in heterogeneity patterns would be considered distal to the N262 NGS.
FIG 4.
Comparative analysis of quantitative site-specific heterogeneity profiles of rgp120 trimers from transmitted/founder (T/F; WEAU#3) and chronic-stage (CS; WEAU#4) viruses and their N262 mutant variants. (A) Heterogeneity profiles that are distal to the naturally occurring difference between the WEAU T/F and CS virus variants show very little shift in their overall site-specific quantitative profiles. (B) The presence of a glycan at N262 reduced overall processing of glycans at neighboring N-glycosylation sites (N448, N413a, N332, and N339). The total number of glycans at each NGS is shown to the right of the bar. Bar graphs of glycan heterogeneity for the remaining NGS are provided in Data Set S1. (C) NGS are mapped onto the structure of HIV-1 gp120 trimer (PDB code 5FYJ [1]) depicting sites proximal to N262 (green), sites distal to N262 (blue), and N262 oligosaccharide (red). (Top) side view; (bottom) apex view. SA, sialic acid.
A second subset of tracked site-specific N-glycan heterogeneity profiles for NGS that map closer to N262 is shown in Fig. 4B. When the data were arranged starting with the gp120 trimer variants that included the N262 glycan (WEAU#3 N264S and WEAU#4), followed by those that did not have the N262 glycan (WEAU#3 and WEAU#4 S264N), a specific shift toward more processed oligosaccharides was observed. A gross N-glycan category change was noted for N448 when the content of high-mannose glycans shifted from 74.5% (WEAU#3 N264S) to 64.5% (WEAU#4) to 56.1% (WEAU#3) to 35% (WEAU#4 S264N). This shift (approximately 39%) in high-mannose glycan content resulted in a classification change from predominantly high-mannose to predominantly processed glycans. Similarly, a trend toward more processed high-mannose glycans was observed at N413a. This trend can be tracked by following the shift of the Man9GlcNAc2 oligosaccharide from 41% of the total observed N-glycans in WEAU#3 N264S to 17% in WEAU#4 S264N. Likewise, N332 and N339 followed the same trend, although the extent of the shift was less pronounced. The pattern of the shifts for N332 mimicked the trends for N448 and N413a across all four constructs, whereas the pattern of the shifts for N339 reflected the addition or removal of N262 to each wild-type variant (WEAU#3 or WEAU#4).
Overall, the removal of N262 from gp120 trimer WEAU#4 variant altered the heterogeneity of the NGS to resemble that of the WEAU#3 gp120 trimer (more processed) and, in several cases, altered the heterogeneities beyond the profile of WEAU#3. In a corresponding fashion, the addition of N262 to the WEAU#3 variant shifted the overall heterogeneity of the glycans toward less processing, and a few sites contained a higher percentage of high-mannose N-glycans than the WEAU#4 variant. The presence or absence of the nearby N295 glycan also appeared to impact the shifts among the variants analyzed, specifically at N339. Heterogeneity profiles for all the tracked NGS across the four trimeric gp120 samples are provided in Data Set S1. When the patterns of quantitative single-site heterogeneity profiles are grouped by the extent of shifts across the analyzed constructs, those with the greatest shift appear to be in close proximity to the N262 NGS, based on the existing Env trimer models (1).
Mapping of NGS heterogeneity profiles onto Env trimeric structures.
To evaluate the N-glycosylation heterogeneity classifications in the context of Env structure, we mapped the NGS for each WEAU sequence onto the structure of an HIV-1 clade B JR-FL prefusion Env trimer (PDB 5FYK) (1). JR-FL was chosen because it has 85% sequence homology with the WEAU variants, it is in clade B (as is WEAU), and most of the N-glycans were observed in the previous structural studies.
Individual characteristics of the N-glycan heterogeneity classification were observed when the NGS were mapped onto the structure (Fig. 4C). The NGS with predominantly high-mannose glycans are located in crowded regions of the trimer surface, as previously reported (1). Of the predominantly high-mannose sites, those with the fewest observed N-glycans were on Asn residues that are part of secondary structures (i.e., N339-α-helix; N332-β-sheet). It is possible that secondary structure may play a role in limiting heterogeneity, and single-site NGS diversity is not solely defined by location in a crowded glycan region of the trimer. Conversely, the predominantly processed NGS are located in disperse loop regions of the trimer where there is more flexibility, allowing processing to readily occur (31). The NGS with mixed glycan heterogeneity are located on the edge of crowded regions and at the apex of the gp120 trimer (N160). This may imply that subpopulations of differentially folded/assembled trimers have more access to processing enzymes, creating a bimodal distribution of populations. By mapping each NGS onto the gp120 Env trimer, we gain insight into the differential N-glycan heterogeneity profiles observed for each NGS.
To further assess the NGS differences observed between WEAU#4 and WEAU#3, we examined the cluster of NGS that had the most prominent changes in N-glycan heterogeneity and sequon occurrence. This included N262 (only present in WEAU#4), N295 (present only in WEAU#3), N448, N413a, and N332, which are all located in the HMP. In the context of our WEAU gp120 trimer variants, we examined existing structures of the gp120 monomers and Env trimers that had resolved glycans for this region. These structures included glycosylated gp120 monomer (16) and three glycosylated Env trimers (1). From these structures, we observed an alignment of Asn residues (ranging from 5 to 9 Å apart) with N-glycan stems/cores of N332, N295, and N448 projecting out as spines from β13, β12, and β22, respectively, of the outer domain β-sheet as modeled in Fig. 5A. The N262 N-glycan is wedged in the cleft between the inner and outer domains of gp120 and is somewhat aligned with β22. As reported by Kong et al., the glycan cores of N448 and N295 interact with the N262 glycan (16). The proximity of N448, located between N295 and N262, as well as the glycan-glycan interactions that occur between these NGS likely contribute to the N-glycan heterogeneity shift between our WEAU gp120 trimer variants. Additionally, the N295 core is in close proximity to N332. N332 maintains high-mannose glycans in all WEAU gp120 trimer variants, but we observed a shift toward more processing in the absence of N295 (in the WEAU#4 variants) and/or N262. Based on the proximity of N448 and N262, it is apparent why N448 exhibited the greatest amount of glycan heterogeneity shift among the tested WEAU Env variants with and without NGS N262.
FIG 5.
High-mannose patch (HMP) N-glycan microdomain and sequon combinations. (A) Model of the HMP from the glycosylated JR-FL Env trimer (1). N262 (blue, fully resolved N-glycan), N301, and N332 (blue, showing only the first GlcNAc) glycans define the borders of this microdomain engaging in glycan-glycan interactions with N448 and N295 (red, showing only the first GlcNAc). Glycans at N442, N413, N293, and N334 (purple) are the NGS that exhibit often-occurring mutations, can interact with the highly conserved oligosaccharides in this microdomain. (B) Histogram plot of exclusive NGS combinations within the HMP microdomain based on queries of the combinatorial database that includes N262, N448, N295/N293, N332/N334, and N413 from the LANL HIV database. (C) Weighted histogram plot with the addition of the highly conserved N301 NGS. The addition of N301 shifts the distribution to center on 4, 5, and 6 sequon combinations and makes the distribution a little less symmetrical. Each bin has been subdivided to highlight the most abundant combinations to match those shown in panel D. The WEAU variants and solved glycosylated Env trimer structures (JR-FL, BG505, and X1193.c1) with HMP microdomains are designated in their appropriate bins. Colors represent grouped combinations of sequons. Sequon combinations anchored by N262, N301, and N332/N334 are in green. Sequon combinations that also include N448 and N295 are in red. Sequon combinations that substitute N442 and/or N413 for N295 are in blue. Sequon combinations that lack either N301 or N332/N334 but maintain two NGS adjacent to N262 are in purple. Less common combinations are not color-coded.
To understand the shift in the N413a quantitative heterogeneity profile, we examined the glycosylated Env structure of the clade G HIV (X1193.c1; PDB code 5FYJ) (1). In this structure, the N-glycan at N413 engages in a stem-stem interaction with N332 with one branch reaching down the β-sheet to form a branch-stem interaction with N442, thus filling the space that N295 occupies in the other glycosylated Env structures. N293 appears to serve as a surrogate for the N448 position in the X1193.c1 crystal structure. N413a could play a similar role in WEAU#4, a variant that lacks N295, potentially explaining why a more distant NGS’s N-glycan heterogeneity (compared to N448) is considerably influenced by the presence or absence of N262. Although there is no interaction between N262 and N413, the loss of N262 lowers the glycan density of the HMP and allows more access for glycan processing.
Assessing high-mannose patch microdomain sequon combinations.
Based on the differences of NGS sequons within the HMP in WEAU gp120 trimers and the reported structures (Table 2), we wanted to determine if there was a preference for a set number of N-glycans within close proximity of N262. A database of 6,223 Env sequences was obtained from the Los Alamos National Laboratory (LANL) HIV-1 sequence database to determine the frequency at which NGS sequons in the proximity of N262 occur in combination with each other within the HMP. We first queried pairs of sequons and observed that N262 and N301 showed the highest common occurrence (in 93% of the 6,223 sequences), followed by the pairing of N262 and N448 (87%). Two pairs of NGS sequons never occur together (N295/N293 and N332/N334), and are, in fact, mutually exclusive, as they have overlapping N-glycan consensus sites, as previously reported (32–34). When N332/N334 is counted as a single position within the HMP and is paired with N262, the combination is found to occur 93% of the time. The remaining pairs with other HMP NGS sequons occur with lower frequency (<60%), corroborating the idea that there is a set number of N-glycans within the HMP that contain both highly conserved sequons (N262, N448, N332/N334, and N301) and less conserved sequons (N295/N293, N413, and N442). The results for the HMP NGS sequon pair frequency are provided in Data Set S2.
TABLE 2.
HMP microdomain combinations
| HIV env variant | N-glycosylation sitea
|
||||||
|---|---|---|---|---|---|---|---|
| N262 | N448 | N442 | N295 | N332 | N413a | N301 | |
| WEAU#3 | X | X | X | X | X | ||
| WEAU#4 | X | X | X | X | X | ||
| WEAU#3 N264S | X | X | X | X | X | X | |
| WEAU#4 S264N | X | X | X | X | |||
| Clade A BG505 | X | X | X | X | X | ||
| Clade B JR-FL | X | X | X | X | X | ||
| Clade G X1193.L | X | X | X | X | X | X | |
Numbering is based on alignments with HXB2. X denotes the presence of NGS in the gp120 sequence.
To further examine the idea of preferred sets of N-glycans within the HMP, we queried higher combinations of sequons using the same LANL database of 6,223 sequences. One such combinatorial database, that included N262, N448, N295/N293, N332/N334, N413, and N442, was created and mined systematically for mutually exclusive combinations of sequons from one to six total. The 10 most abundant sequon combinations (out of 66 possible) account for 90% of the sequences (Table 3). The N262, N448, N295/N293, and N332/N334 combination accounted for 2,323 (37.33%) of all sequences in the database. The next top nine combinations reflected either an addition or loss to these four core sequons, with a high preference for potential NGS at N262, N332/N334, and N448. By analyzing these combinations, we observed an interchangeability between three NGS sequons at N295, N413, and N442. The results from mining five different combinatorial N-glycan sequon databases, including a control database, are provided in Data Set S2.
TABLE 3.
Sequon combinations in the N262 HMP microdomain
| Combinationa | Env proteins |
No. of sequons | |
|---|---|---|---|
| No. | % | ||
| N262-N448-N295/N293-N332/N334 | 2,323 | 37.3 | 4 |
| N262-N448-N295/N293-N332/N334-N413 | 724 | 11.6 | 5 |
| N262-N448-N332/N334-N442 | 706 | 11.4 | 4 |
| N262 -N448-N332/N334 | 458 | 7.4 | 3 |
| N262-N448-N295/N293-N332/N334-N442 | 317 | 5.1 | 5 |
| N262-N448-N332/N334-N413 | 299 | 4.8 | 4 |
| N262-N295/N293-N332/N334 | 257 | 4.1 | 3 |
| N262-N448-N332/N334-N413-N442 | 194 | 3.1 | 5 |
| N262-N332/N334-N442 | 187 | 3.0 | 3 |
| N262-N448-N295/N293 | 134 | 2.1 | 3 |
| Total | 5,599 | 89.9 | |
Combinations queried from 6,223 total sequences in the Los Alamos National Laboratory HIV sequence database. The sequons in bold denote where NGS (N413 and or N442) are added to or substituted for N295.
To visualize these database results, we created a series of histogram plots (Fig. 5B and C). Figure 5B shows a weighted histogram of the NGS sequon combinatorial database that includes N262, N448, N295/N293, N332/N334, N442, and N413 among the eight possible NGS that make up the highest-density region of the HMP surrounding N262. The resultant histogram centers on exclusive use of combinations of four sequons with an equal distribution of three and five sequon combinations on each side. The numbers continue to taper in both directions for combinations of two and six sequons to produce a Gaussian-like distribution of sequon density within the HMP. When other NGS combinatorial databases were constructed to include sequons that are part of the HMP and those outside this range of interdependent high-density glycans, the normal distribution does not empirically emerge from the data (Data Set S2). This evaluation of gp120 NGS sequon combinations reflects a histogram of preferred sequon combinations in this HMP microdomain similar to mutation cluster analysis of oncogenes (35). When N301, a site with long-range glycan-glycan interactions with N295 and N413 (1), is added to the combinatorial database, the histogram distribution shifts to center on four, five, and six sequons. Interestingly, the same combinations in Table 2 plus N301 still represent 88% of all sequences (Fig. 5C).
Together, the NGS sequon frequency analysis and histogram plots of sequon combinations showed a microdomain of the HMP built around the highly conserved sequons at N262, N301, and N332/N334 (green bins). The remaining adjacent glycan density of this microdomain is comprised of a limited number of sequon combinations that follow a rank order based on their location with respect to the three highly conserved positions and the amount of total glycan density in this region. These adjacent glycans are most often comprised of the predominant pair of N448 and N295 (Fig. 5C and D, red bins) for an optimal density of five glycans. Both NGSs can be replaced, but N448 is much more conserved. N295 can be replaced by N442 or N413 (blue bins), in that order. In microdomains with six sequons, the inclusion of N413 with N295 occurs more often than N442. The histogram also indicates that the microdomain can suffice with four glycans as long as the glycan(s) adjacent to N262 are maintained. In these combinations, N262, N301, and N332/N334 are most often present, with N448, N295, or N442 serving as the 4th glycan, in that order (green bins). In each case, the 4th glycan has some type of previously reported glycan-glycan interaction with N262 (1). In fact, the next three combinations of four glycans lack either N301 or N332/N334 in favor of N448 and either N295 or N442 (purple bins). This observation suggests that the glycan-glycan interactions with N262 are of great importance. Correspondingly, N413 is only a small contributor to this bin of four sequon combinations. These data suggest that the microdomain of the HMP is a definable, limited-parameter set of sequons maintaining the number of glycans in the proximity of N262.
DISCUSSION
Changes in the Env gp120 glycan sequons play a role in HIV-1 immune escape (19). These sequon changes lead to differences in the site-specific and overall glycan heterogeneity of the Env trimer. In this study, a combination of naturally occurring Env variants and their mutants created a unique series of HMP sequon combinations. Through our analyses, we examined how glycan density, mutations in NGS sequons, and the need to maintain Env function work together in this region. We saw a consistent pattern of glycan arrangements within the HMP that involved the highly conserved N262 NGS as well as a set of adjacent NGS in the proximity of this sequon. One inference from the Env trimeric structures with resolved glycans was that the glycan shield consists of microdomains of interdependent glycans that interact in various ways, as has recently been proposed on the basis of molecular dynamics simulations (15). Our quantitative profiles revealed shifts in heterogeneity among the WEAU variants that empirically demonstrated this interdependence of glycans around N262 that had also been implicated in other structural analyses (1, 15). These findings led us to seek interpretation in the context of the trimeric Env glycoprotein structure. Specifically, we parameterized a key microdomain of interdependent glycans that are part of the HMP via a series of sequon combination queries of the LANL HIV database. Twelve related combinations of four, five, or six sequons in the HMP represent 88% of all deposited sequences to date (Fig. 5). We identified the most common sequon combinations, several of which were corroborated by our quantitative heterogeneity profiling.
We performed a series of site-specific quantitative N-glycosylation heterogeneity analyses using two naturally occurring Env gp120 variants and two corresponding single NGS mutants (Fig. 1). The results revealed three broad types of NGS heterogeneity in all four Env variants (Fig. 2), in agreement with findings from other Env trimers (11). Several NGS profiles had clear shifts in heterogeneity among WEAU variants, suggesting a quantitative change in the broad categories of high-mannose, hybrid, and complex glycans. Other NGS demonstrated consistent shifts within individual categories (i.e., Man5 to Man9) (Fig. 4). Based on our confidence in the site-specific quantitative analysis, we ranked the shifts in heterogeneity and then mapped the tracked NGS onto the gp120 trimer structure.
With the combination of single-site glycan heterogeneity analysis, modeling WEAU gp120 sequences onto existing Env structures, and independently querying the LANL HIV database, we began to ask if selective pressure influences N-glycan heterogeneity and sequon clustering. We assessed how Env function and immune evasion select for sequon clusters from the many mutations that occur due to the low fidelity of HIV-1 reverse transcriptase (18), just as other groups have mined somatic mutations in oncogenes to identify mutation clusters based on their location in protein tertiary structure (35). The HMP microdomain consists of four highly conserved NGS (N262, N301, N448, and N332/N334) and a second set of less conserved sites (N295/N293, N413, and N442). Of the less conserved sites, our data suggest that when immune pressure results in the loss of the N295 glycan, another glycan will be substituted to maintain a functional trimer. For WEAU variants, N413 is likely a surrogate in waiting in higher sequon combinations (WEAU#3M [Table 2]), as this NGS is on the periphery of this microdomain. Although N442 was not in WEAU Env variants we tested, its presence and location in the X1193.c1 structure suggested that it provides needed glycan mass to this microdomain in many Env variants. The originating position of the glycan, however, can affect the epitope presentation and thus BnAb neutralization, as we saw with the 2G12 antibody (Table 1). Other groups have reported that some BnAbs, such as PGT128, are more promiscuous and can neutralize Env proteins that have altered glycan positions (32, 33). We propose that not only a single site but also the surrounding context of NGS in this HMP microdomain may play a role in determining if a given BnAb can be promiscuous. Because Env function needs to be maintained for virus viability, selective pressure creates a finite number of viable sequon combinations within the HMP microdomain, as is suggested by our bioinformatics analysis, which reported that 12 sequon combinations in the HMP account for 88% of the 6,223 Env sequence database.
If there is an optimal glycan density, such as five sequons with attached glycans, in this HMP microdomain (Fig. 5C), it raises questions as to how the site-specific heterogeneity for these NGS changes in the four- and six-sequon combinations. Presumably, combinations of four sequons would allow better access for glycan-processing enzymes. Our analyses of WEAU seemed to confirm these conclusions, as even changes in the distributions within the high-mannose glycan category were detected among WEAU variants. A change from Man9 to Man5 will likely produce distinct surfaces and glycan-glycan interactions, especially for branch-branch interactions. For six- and even seven-sequon combinations, such as WEAU#3 mutant with added N262, the issue of glycan density and glycan site occupancy in this confined space arises. Do these variants truly have six sequons with attached glycans, or are they mixtures of differentially glycosylated combinations of five? Quantitative heterogeneity profiles that include site-occupancy analysis could answer these questions and may explain why only partially occupied NGS exist.
Behrens and colleagues have previously used quantitative site-specific heterogeneity profiles to provide insight into the glycan shield of differentially formed Env trimers (11). Go et al. used this type of analysis to identify Env production variables that may impact overall glycosylation (36). Recently, Struwe et al. have demonstrated that there are soluble trimeric immunogen constructs that closely mimic the virion-derived HIV-1 (37). The N-glycan heterogeneity profile for the tested WEAU variants is similar to that of the native flexibly linked trimer constructs. Regardless of construct, there will also be a need to address the process of HIV-1 Env immune escape, as it is targeted for vaccine development. The goal of this work was to interrogate the glycan-glycan interactions of the Env glycan shield through quantitative site-specific heterogeneity analysis. By tracking N-glycan heterogeneity profiles from a related sequence of Env variants, we identified the extent of interdependency among some NGS that are in agreement with other structural studies (1, 15). Overall, a lower content of oligomannose glycans at each NGS was found when comparing WEAU#3 to WEAU#4. This finding may imply that WEAU#3 has a less compact fold that may increase the traffic time through the trans-Golgi network, as previously proposed for Env gp120 variants without N262 (21). Moreover, shifts in quantitative N-glycan heterogeneity profiles among the variants provide empirical evidence for the interdependency among NGS implied from the glycosylated Env structures.
Given the central role of N262 and the HMP in the formation of functional trimers (12, 16, 31), it would be of interest to compare degrees of virulence of representative HIV-1 strains in the context of our proposed optimal sequon combinations. We expect that virulence would be associated with certain combinations of sequon density and sequon preferences. However, it is difficult to predict where the combinations of four sequons will fall in the spectrum of virulence phenotype compared to those with six sequons. Are there certain low-number NGS combinations that are favored over high-number ones? Similar questions can be answered by testing neutralizing activities of different BnAbs that target the HMP. Of note, in our validation process, we tested the activity of BnAb 2G12 against HIV-1 pseudotyped with WEAU#4 Env and produced it in the presence or absence of the α-mannosidase inhibitor DMJM. Despite WEAU#4 being a 2G12 escape variant, DMJM treatment partially rescued the neutralization of WEAU#4 by 2G12. This observation indicated that despite the absence of N295 (mechanism of the immune escape), the 2G12 epitope was partially reconstituted on the surface of the HMP with a different sequon combination.
Through site-specific quantitative heterogeneity profiling, structural modeling, and bioinformatic analysis, we have mapped the sequon parameters of a microdomain of the HMP. Recent breakthroughs in HIV-1 vaccine design have created the possibility that several conserved epitopes can be targeted to counter the frequency of immune escape (4, 38). Our results provide a map of how a critical glycan microdomain in the HMP can be altered in immune escape variants. Wei et al. reported on longitudinal samples from the original WEAU patient (19). The reported sequences over time show the loss and return of N262 through mutation of the Env sequence as reflecting the importance of this glycan. The less conserved N295 is lost and never returns in the reported sequences over 1 year. However, the adjacent N413 glycan is present throughout and, based on our modeling and database analysis, serves as a sufficient functional surrogate for N295. Other longitudinal sample cohorts could be examined in the context of our proposed immune escape map. Our results also suggest that other regions of the HMP and Env glycan surface can be parameterized in terms of sequon combinations. The number of combinations at the sequon level is limited, illustrating an important role for glycan-glycan interactions with N262, as recently reported for the Env trimer in open and closed conformations (39). Vaccine designs that target highly conserved combinations of N-glycan sequons will also have to account for the selective pressure that targeting may produce. Based on the limited number of sequon combinations we report here, our results provide a methodology to assess the likely routes of immune escape for different glycan shield microdomains.
MATERIALS AND METHODS
Reagents.
All chemicals, unless otherwise specified, were purchased from Sigma (St. Louis, MO). Tissue culture media and medium supplement were purchased from Invitrogen (Carlsbad, CA).
HIV-1 env gp160 and gp120 constructs.
WEAU#3 d16 gp160 DNA corresponded to the sequence of WEAU16-02 SGA direct amplicon from plasma HIV-1 RNA 16 days after acute infection. WEAU#4 gp160 was based on the amino acid backbone of WEAU#3 d16. The potential N-glycosylation sites (NGS) were mutated to resemble chronic-stage WEAU391-03 gp160 identified from plasma HIV-1 RNA 391 days after acute infection (19, 40). Both DNA sequences coding for respective gp160 protein were codon optimized, synthesized by a commercial supplier (ATG:biosynthetics, Merzhausen, Germany), and cloned in frame into expression plasmid pcDNA3.1. Both gp160 amino acid sequences are numbered according to the alignment with the HIV-1 HXB2 sequence (GenBank number K03455). The Env mutants in which Ser was replaced with Asn at position 264 by site-directed mutagenesis were designated WEAU#3 N264S and WEAU#4 S264N. To produce recombinant Env glycoproteins, gp120 DNA of WEAU#3, WEAU#4, WEAU#3 N264S, and WEAU#4 S264N was each fused at the 5′ end with cDNA encoding the first 62 amino acids of the nonglycosylated fragment of human mannan-binding lectin (MBL) (GenBank accession number EU596574; fragment consisting of nucleotides 66 to 252) to drive gp120 trimerization and secretion (41, 42). The resultant WEAU gp120 and MBL DNA were then cloned into pcDNA3.1D/V5-His for purification and detection purposes (24). Plasmid DNA used in transfection of 293F cells (see below) was purified with the Qiagen MaxiPrep kit (Qiagen, Valencia, CA). The entire env gene of each plasmid construct was confirmed by DNA sequencing.
Cells.
FreeStyle 293-F cells (293F; Invitrogen) derived from human embryonic kidney (HEK) cells were cultured in serum-free FreeStyle 293 expression medium at 37°C in a humidified atmosphere with 8% CO2 on an orbital shaker platform rotating at 135 rpm. 293F cells were subcultured at a 1:10 dilution when the density reached between 1.5 × 106 and 3 × 106 viable cells per ml. The TZM-bl cell line (NIH AIDS Research and Reference Reagent Program [ARRRP]; catalog no. 8129) and HEK cell line 293T/17 (ATCC CRL-11268) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 µg/ml). Cell cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. Cell monolayers were split at 1:10 confluence by treatment with 0.25% trypsin–1 mM EDTA solution. The TZM-bl cell line is a genetically engineered HeLa cell clone that expresses CD4, CXCR4, and CCR5 and contains Tat-responsive reporter genes for firefly luciferase (Luc) under the regulatory control of an HIV-1 long terminal repeat (LTR) (43).
Expression and purification of recombinant gp120.
293F cells were transfected by plasmids with WEAU gp120 using 293fectin according to the manufacturer’s instructions (Invitrogen). Briefly, plasmid DNA was mixed with 293fectin at a ratio of 1:2 and after a 20-min incubation, DNA-293fectin complex was added to 1 × 106 viable 293F cells per ml. After 4 days of incubation at 37°C with 8% CO2, cell culture supernatants were collected and cell cultures were continued after adding fresh serum-free FreeStyle 293 expression medium. Cell culture supernatants were collected again on day 7 posttransfection. The trimerized form of recombinant gp120 (rgp120) was assessed in the cell culture supernatants by Western blotting using horseradish-labeled anti-V5 antibody for detection. Then the rgp120 was isolated under native conditions on a nickel-nitrilotriacetic acid (Ni-NTA)-agarose column according to the manufacturer’s instructions (Qiagen). The elution buffer was exchanged for phosphate-buffered saline (PBS; pH 7.2) using an Amicon Ultra-4 centrifugal filter unit (Millipore, Billerica, MA). The concentration of rgp120 was calculated by dividing optical density at 280 nm (NanoDrop 2000/2000c) by 1.21, a molar extinction coefficient determined for WEAU gp120. To produce rgp120 in the presence of the α-mannosidase inhibitor deoxymannojirimycin (DMJM; Tocris Bioscience, Minneapolis, MN), fresh medium containing the desired concentration of DMJM (800 µM) was added at the time of transfection. All isolated rgp120 trimers were kept at −80°C until use.
Preparation of recombinant gp120 trimers for high-resolution mass spectrometry analysis.
Recombinant gp120 trimer preparations were normalized based on densitometric analysis of Western blotting-detected protein bands obtained after N-glycans of rgp120 were removed by peptide-N-glycosidase F (PNGase F) (Prozyme, Hayward, CA). For glycomic analyses, 10 µg of each rgp120 was loaded onto a 4% to 15% Mini-PROTEAN TGX precast gel (Bio-Rad Laboratories Inc., Hercules, CA) under denaturing and reducing conditions. The bands (∼130 kDa) were excised, digested with trypsin (Promega), and used for high-resolution mass spectrometry analysis. For initial amino acid site-specific glycan assignment, rgp120 trimers samples were prepared using either endoglysidase H (endo H) (New England BioLabs; 5 µl added to trypsin-digested rgp120 and incubated for 18 h at 37°C), PNGase F, or sialidase (Prozyme) (24). For assessment of total N-glycan heterogeneity, an additional sample of DMJM-treated rgp120 was digested with trypsin.
LC-MS and MS/MS analysis of gp120.
After peptide/glycopeptide extraction, samples were loaded onto a self-prepared 11-cm, 100-µm-diameter pulled tip packed with Jupiter 5-µm C18 reversed phase beads (Phenomenex, Torrance, CA). Digested (glyco)peptides were analytically separated via nano-liquid chromatography (nano-LC) by use of an Eksigent MicroAS autosampler and two-dimensional (2D) LC nanopump (Eksigent, Dublin, CA) at a flow rate of 650 nl/min over 90 min. Mobile phases utilized for the experiments consisted of solvent A (2.5% acetonitrile [ACN] and 0.1% formic acid) and solvent B (97.5% ACN and 0.1% formic acid). The following multistep gradient was used: 5% solvent B for 5 min, followed by a linear increase to 35% solvent B in 50 min, a linear increase to 40% solvent B in 10 min, a linear increase to 60% solvent B in 10 min, and an increase to 98% solvent B in 5 min. The column was held at 98% solvent B for 10 min before reequilibration. The eluted tryptic peptides were electrosprayed at 2 kV into a dual linear quadrupole ion trap Orbitrap Velos Pro mass spectrometer (Thermo Fisher, San Jose, CA). The mass spectrometer was set to switch between a full scan (400 < m/z < 2,000) followed by successive MS/MS (200 < m/z < 2,000) scans of the 10 most abundant precursor ions (parent ions). A dynamic exclusion setting was set to exclude ions for 2 min after a repeat count of three within a 45-s duration. Two methods were utilized to obtain MS/MS scans: collision-induced dissociation (CID) and electron transfer dissociation (ETD). This process was repeated with gp120 preparations that were digested with endo H, PNGase F, sialidase, or rgp120 prepared in the presence of the DMJM inhibitor.
Glycopeptide identification.
All LC-MS/MS data for gp120 digested with proteases/glycosidases were initially analyzed by use of the Byonic (Protein Metrics) proteomic search algorithm making use of both CID and ETD fragmentation. Data for untreated rgp120 and PNGase F- and endo H-treated preparations were searched by use of SEQUEST to confirm the amino acid sequences of potential gp120 glycopeptides, with a peptide tolerance of 10 ppm and an MS/MS tolerance of 0.5 Da. Based on the output of the search algorithms, initial glycopeptides were identified across the different gp120 preparations and assembled into a composite spreadsheet which included Byonic glycopeptide identifications (CID and ETD), corresponding retention times (RT), SEQUEST NGS identifications from endo H and PNGase F digests, and their shifted RT. PNGase F digestion resulted in 86% sequence coverage for rgp120 for both WEAU#3 and WEAU#4, which covered all potential NGS. EndoH digestion confirmed 17 glycopeptides, covering 20 NGS.
To identify the full the range of glycan heterogeneity at specific sites, MS1 features were examined in all gp120 glycopeptide preparations by use of Optys Pinnacle software (version 1.0; Optys Tech Corporation). Specifically, the search was conducted with a 10-ppm mass accuracy of the 3 most abundant isotopes with a series of custom peptide and glycopeptide workbooks generated for each NGS. Based on the initial Pinnacle MS1 search results, the search was narrowed to include only 2+, 3+, and 4+ charge states. Candidate glycopeptides for each NGS were then curated to eliminate N-glycoforms that had an area less than 1E4 and to confirm novel N-glycoforms not identified by the Byonic algorithm. Pinnacle MS1 searches were tailored to a series of retention time windows enabling fast assessment of multiple assignments of N-glycoforms at each NGS across several gp120 trimers. Newly identified N-glycopeptides were verified for assigned composition by MS/MS. For gp120 glycopeptides with two NGS, individual sites were parsed based on observed retention time clusters of glycopeptide ions with single-site and double-site occupancy. Single- and double-site occupancy was confirmed by MS/MS for selected glycopeptides in each clustered series. For each curated data set, the resultant profiles within Pinnacle provided glycopeptide molecular composition, RT, and extracted ion chromatogram area under the curve for each isotope within a mass error of 8 ppm.
Visualizing N-glycan heterogeneity profiles.
Visualizing the analysis in an order that reflects N-glycan processing provides a means to assess N-glycan heterogeneity in terms of NGS accessibility to glycan biosynthetic enzymes. Hence, we rendered our glycopeptide profiles in an order that reflects processing based on the inferred structure from the assigned glycopeptide compositions (Fig. 1B). High-mannose glycans are ordered by decreasing mannose content, i.e., from Man9GlcNAc2 to Man3GlcNAc2 oligosaccharides. This is followed by hybrid glycans that have three GlcNAc sugars, inferring one GlcNAc added to the N-glycan chain and then ordered by decreasing number of hexose (Hex) sugars. The glycoforms with sialic acid (SA) are listed in the same order, reflecting that SA could only be added to the “complex-glycan side” in hybrid N-glycans (44). Next, the oligosaccharides are ordered by increasing number of antennas of branched complex chains (+GlcNAc, HexNAc) and then within each antenna the presumed addition of galactose (increasing Hex ≥ 3). Finally, the same order with complex oligosaccharides that contain SA follows.
Generation of WEAU Env-pseudotyped viruses and neutralization assay.
Env-pseudotyped viruses (Table 1) were produced in 293F cells with or without 800 µM DMJM. HIV-1 broadly neutralizing antibodies (BnAbs) were obtained through the NIH ARRRP, Division of AIDS, NIAID, NIH: 2G12, PG9, PG16, NIH45-46G54W IgG, and 4E10. The ability of BnAbs to neutralize WEAU viruses was assessed using TZM-bl reporter cells; results are presented in Table 1.
Mapping of gp120 N-glycosylation sites.
Structural models were generated for WEAU Env based on homology modeling with JR-FL (PDB codes 5FYK and 5FYL) (1) using SWISS-MODEL (45). Man9GlucNAc2 was docked at each N-glycosylation site using GLYCAM (www.glycam.org). Further refinement of oligosaccharide side chain positions was made using WinCoot (46) to account for NGS not present in JR-FL. These were overlaid and positioned relative to their locations in either the BG505, X1193.1, or HXBc2 crystal structure (PDB code 5FYL, 5FYJ, or 3JWO).
NGS sequon combination databases.
A database of all HIV-1 Env sequences deposited in the HIV sequence database (2016; http://www.hiv.lanl.gov/) aligned to the HXB2 sequence was generated using the HIV Premade alignment tool. The database was then uploaded into the AnalyzeAlign tool and a series of either 7, 8, or 9 known potential NGS positions was queried for frequency of sequon in exclusive combinations of 1 to 7 or 1 to 8, as applicable. The potential NGS used were chosen based on their proximity to N262. The alignment databases were then mined for frequency of NGS sequons occurring together and exclusively together. Potential NGS that never occurred together are reported as single potential NGS. The data were then plotted in a histogram that visualized number of sequons that occurred together. To account for possible bias in choosing a database of 8 NGS, we have included a control database of potential NGS that are not confined to a structural regions but occur with the same single-site frequency as the HMP sequons. The results from the queried NGS sequon databases are provided in Data Set S2.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by National Institutes of Health grant GM098539 (to M.B.R.) and by NIH T32 fellowship GM008111 (to A.A.H.). This work was also supported in part by the University of Alabama (UAB) CFAR developmental grant and a pilot grant from the UAB School of Medicine. M.R. was supported in part by the Czech Republic Ministry of Education, Youth, and Sport, grants LO1304 and LH15263.
A.A.H., Q.W., Z.M., M.R., J.N., and M.B.R. designed the study. A.A.H. performed the HR MS heterogeneity analysis. Q.W., B.K., S.H., and Z.-Q.H. engineered the constructs and performed the functional experiments. A.A.H. and T.J.G. constructed the models. A.A.H. and M.B.R. performed the glycomics and database analysis. A.P. provided substantial assistance in the execution and analysis of glycomics data to A.A.H. and M.B.R. S.H., Z.-Q.H., Z.M., and M.R. provided resources. A.A.H. and M.B.R. wrote the manuscript with input from the other authors. J.N. and M.B.R. oversaw the project.
The authors declare no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01177-18.
REFERENCES
- 1.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, Kong R, Thomas PV, Wagh K, Zhou T, Behrens AJ, Bylund T, Choi CW, Davison JR, Georgiev IS, Joyce MG, Kwon YD, Pancera M, Taft J, Yang Y, Zhang B, Shivatare SS, Shivatare VS, Lee CC, Wu CY, Bewley CA, Burton DR, Koff WC, Connors M, Crispin M, Baxa U, Korber BT, Wong CH, Mascola JR, Kwong PD. 2016. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 165:813–826. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 3.Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 4.Medina-Ramirez M, Garces F, Escolano A, Skog P, de Taeye SW, Del Moral-Sanchez I, McGuire AT, Yasmeen A, Behrens AJ, Ozorowski G, van den Kerkhof T, Freund NT, Dosenovic P, Hua Y, Gitlin AD, Cupo A, van der Woude P, Golabek M, Sliepen K, Blane T, Kootstra N, van Breemen MJ, Pritchard LK, Stanfield RL, Crispin M, Ward AB, Stamatatos L, Klasse PJ, Moore JP, Nemazee D, Nussenzweig MC, Wilson IA, Sanders RW. 2017. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J Exp Med 214:2573–2590. doi: 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng C, Pancera M, Bossert A, Schmidt SD, Chen RE, Chen X, Druz A, Narpala S, Doria-Rose NA, McDermott AB, Kwong PD, Mascola JR. 2015. Immunogenicity of a prefusion HIV-1 envelope trimer in complex with a quaternary-structure-specific antibody. J Virol 90:2740–2755. doi: 10.1128/JVI.02380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong L, Torrents de la Pena A, Deller MC, Garces F, Sliepen K, Hua Y, Stanfield RL, Sanders RW, Wilson IA. 2015. Complete epitopes for vaccine design derived from a crystal structure of the broadly neutralizing antibodies PGT128 and 8ANC195 in complex with an HIV-1 Env trimer. Acta Crystallogr D Biol Crystallogr 71:2099–2108. doi: 10.1107/S1399004715013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Diedrich JK, Kulp DW, Pauthner M, He L, Park SR, Sok D, Su CY, Delahunty CM, Menis S, Andrabi R, Guenaga J, Georgeson E, Kubitz M, Adachi Y, Burton DR, Schief WR, Yates Iii JR, Paulson JC. 2017. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat Commun 8:14954. doi: 10.1038/ncomms14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panico M, Bouche L, Binet D, O'Connor MJ, Rahman D, Pang PC, Canis K, North SJ, Desrosiers RC, Chertova E, Keele BF, Bess JW Jr, Lifson JD, Haslam SM, Dell A, Morris HR. 2016. Mapping the complete glycoproteome of virion-derived HIV-1 gp120 provides insights into broadly neutralizing antibody binding. Sci Rep 6:32956. doi: 10.1038/srep32956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go EP, Chang Q, Liao HX, Sutherland LL, Alam SM, Haynes BF, Desaire H. 2009. Glycosylation site-specific analysis of clade C HIV-1 envelope proteins. J Proteome Res 8:4231–4242. doi: 10.1021/pr9002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou T, Doria-Rose NA, Cheng C, Stewart-Jones GBE, Chuang GY, Chambers M, Druz A, Geng H, McKee K, Kwon YD, O'Dell S, Sastry M, Schmidt SD, Xu K, Chen L, Chen RE, Louder MK, Pancera M, Wanninger TG, Zhang B, Zheng A, Farney SK, Foulds KE, Georgiev IS, Joyce MG, Lemmin T, Narpala S, Rawi R, Soto C, Todd JP, Shen CH, Tsybovsky Y, Yang Y, Zhao P, Haynes BF, Stamatatos L, Tiemeyer M, Wells L, Scorpio DG, Shapiro L, McDermott AB, Mascola JR, Kwong PD. 2017. Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell Rep 19:719–732. doi: 10.1016/j.celrep.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens AJ, Harvey DJ, Milne E, Cupo A, Kumar A, Zitzmann N, Struwe WB, Moore JP, Crispin M. 2017. Molecular architecture of the cleavage-dependent mannose patch on a soluble HIV-1 envelope glycoprotein trimer. J Virol 91:e01894-16. doi: 10.1128/JVI.01894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchard LK, Spencer DI, Royle L, Bonomelli C, Seabright GE, Behrens AJ, Kulp DW, Menis S, Krumm SA, Dunlop DC, Crispin DJ, Bowden TA, Scanlan CN, Ward AB, Schief WR, Doores KJ, Crispin M. 2015. Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies. Nat Commun 6:7479. doi: 10.1038/ncomms8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrabi R, Su CY, Liang CH, Shivatare SS, Briney B, Voss JE, Nawazi SK, Wu CY, Wong CH, Burton DR. 2017. Glycans function as anchors for antibodies and help drive HIV broadly neutralizing antibody development. Immunity 47:1004. doi: 10.1016/j.immuni.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemmin T, Soto C, Stuckey J, Kwong PD. 2017. Microsecond dynamics and network analysis of the HIV-1 SOSIP Env trimer reveal collective behavior and conserved microdomains of the glycan shield. Structure 25:1631–1639.e2. doi: 10.1016/j.str.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Kong L, Wilson IA, Kwong PD. 2015. Crystal structure of a fully glycosylated HIV-1 gp120 core reveals a stabilizing role for the glycan at Asn262. Proteins 83:590–596. doi: 10.1002/prot.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landais E, Murrell B, Briney B, Murrell S, Rantalainen K, Berndsen ZT, Ramos A, Wickramasinghe L, Smith ML, Eren K, de Val N, Wu M, Cappelletti A, Umotoy J, Lie Y, Wrin T, Algate P, Chan-Hui PY, Karita E, IAVI Protocol C Investigators, IAVI African HIV Research Network, Ward AB, Wilson IA, Burton DR, Smith D, Pond SLK, Poignard P. 2017. HIV envelope glycoform heterogeneity and localized diversity govern the initiation and maturation of a V2 apex broadly neutralizing antibody lineage. Immunity 47:990–1003.e9. doi: 10.1016/j.immuni.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston BD, Poiesz BJ, Loeb LA. 1988. Fidelity of HIV-1 reverse transcriptase. Science 242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 19.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Nie J, Prochnow C, Truong C, Jia Z, Wang S, Chen XS, Wang Y. 2013. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 10:14. doi: 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathys L, Francois KO, Quandte M, Braakman I, Balzarini J. 2014. Deletion of the highly conserved N-glycan at Asn260 of HIV-1 gp120 affects folding and lysosomal degradation of gp120, and results in loss of viral infectivity. PLoS One 9:e101181. doi: 10.1371/journal.pone.0101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go EP, Hewawasam G, Liao HX, Chen H, Ping LH, Anderson JA, Hua DC, Haynes BF, Desaire H. 2011. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J Virol 85:8270–8284. doi: 10.1128/JVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens AJ, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, Krumm SA, Struwe WB, Cupo A, Kumar A, Zitzmann N, Seabright GE, Kramer HB, Spencer DI, Royle L, Lee JH, Klasse PJ, Burton DR, Wilson IA, Ward AB, Sanders RW, Moore JP, Doores KJ, Crispin M. 2016. Composition and antigenic effects of individual glycan sites of a trimeric HIV-1 envelope glycoprotein. Cell Rep 14:2695–2706. doi: 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, Moldoveanu Z, Elliott MC, Wilson L, Brown R, Jancova D, Barnes S, Vrbkova J, Tomana M, Smith PD, Mestecky J, Renfrow MB, Novak J. 2010. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem 285:20860–20869. doi: 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebecchi KR, Wenke JL, Go EP, Desaire H. 2009. Label-free quantitation: a new glycoproteomics approach. J Am Soc Mass Spectrom 20:1048–1059. doi: 10.1016/j.jasms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Wall SB, Suzuki H, Smith ADt, Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, Novak J, Renfrow MB. 2010. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics 9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Wang Y. 2016. Glycosylation quality control by the Golgi structure. J Mol Biol 428:3183–3193. doi: 10.1016/j.jmb.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargett A, Wei Q, Raksa M, Knoppona B, Hall S, Zachova K, Huang Z, Moldoveanu Z, Novak J, Renfrow MB. 2015. Glycosylation patterns on HIV-1 envelope glycoprotein. Glycobiology 25:1249. [Google Scholar]
- 29.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang LX, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. 2013. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol 20:804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 31.Behrens AJ, Crispin M. 2017. Structural principles controlling HIV envelope glycosylation. Curr Opin Struct Biol 44:125–133. doi: 10.1016/j.sbi.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien JP, Menis S, Wickramasinghe L, Seaman MS, Schief WR, Wilson IA, Poignard P, Burton DR. 2014. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med 6:236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doores KJ, Kong L, Krumm SA, Le KM, Sok D, Laserson U, Garces F, Poignard P, Wilson IA, Burton DR. 2015. Two classes of broadly neutralizing antibodies within a single lineage directed to the high-mannose patch of HIV envelope. J Virol 89:1105–1118. doi: 10.1128/JVI.02905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anthony C, York T, Bekker V, Matten D, Selhorst P, Ferreria RC, Garrett NJ, Karim SSA, Morris L, Wood NT, Moore PL, Williamson C. 2017. Cooperation between strain-specific and broadly neutralizing responses limited viral escape and prolonged the exposure of the broadly neutralizing epitope. J Virol 91:e00828-17. doi: 10.1128/JVI.00828-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto A, Okada Y, Boroevich KA, Tsunoda T, Taniguchi H, Nakagawa H. 2016. Systematic analysis of mutation distribution in three dimensional protein structures identifies cancer driver genes. Sci Rep 6:26483. doi: 10.1038/srep26483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Go EP, Ding H, Zhang S, Ringe RP, Nicely N, Hua D, Steinbock RT, Golabek M, Alin J, Alam SM, Cupo A, Haynes BF, Kappes JC, Moore JP, Sodroski JG, Desaire H. 2017. Glycosylation benchmark profile for HIV-1 envelope glycoprotein production based on eleven Env trimers. J Virol 91:e02428-16. doi: 10.1128/JVI.02428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Struwe WB, Chertova E, Allen JD, Seabright GE, Watanabe Y, Harvey DJ, Medina-Ramirez M, Roser JD, Smith R, Westcott D, Keele BF, Bess JW Jr, Sanders RW, Lifson JD, Moore JP, Crispin M. 2018. Site-specific glycosylation of virion-derived HIV-1 Env is mimicked by a soluble trimeric immunogen. Cell Rep 24:1958–1966.e5. doi: 10.1016/j.celrep.2018.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Do Kwon Y, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O’Dell S, Todd JP, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang ZY, Mascola JR, Nabel GJ. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, Torres JL, Copps J, Stanfield RL, Cupo A, Pugach P, Moore JP, Wilson IA, Ward AB. 2017. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547:360–363. doi: 10.1038/nature23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raska M, Czernekova L, Moldoveanu Z, Zachova K, Elliott MC, Novak Z, Hall S, Hoelscher M, Maboko L, Brown R, Smith PD, Mestecky J, Novak J. 2014. Differential glycosylation of envelope gp120 is associated with differential recognition of HIV-1 by virus-specific antibodies and cell infection. AIDS Res Ther 11:23. doi: 10.1186/1742-6405-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raska M, Moldoveanu Z, Novak J, Hel Z, Novak L, Bozja J, Compans RW, Yang C, Mestecky J. 2008. Delivery of DNA HIV-1 vaccine to the liver induces high and long-lasting humoral immune responses. Vaccine 26:1541–1551. doi: 10.1016/j.vaccine.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schachter H. 2000. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj J 17:465–483. doi: 10.1023/A:1011010206774. [DOI] [PubMed] [Google Scholar]
- 45.Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.