Abstract
Background
The present systematic review and meta-analysis was performed to explore the possible effect of bariatric surgery on semen parameters.
Material/Methods
Studies on the effect of bariatric surgeries on semen parameters were collected by searching Cochrane Library, PUBMED, EMBASE, MEDLINE, and CNKI databases. We extracted information on essential data and outcome measures, including study design, bariatric surgery, and semen parameters at baseline and after the surgery from the included studies, and STATA 12.0 software was applied to conduct the meta-analysis. Predefined subgroup analyses were also conducted by study design and bariatric surgical procedures. The standard mean difference (SMD) was calculated to estimate the effect on semen parameters.
Results
After the literature search, 6 articles that fulfilled the inclusion criteria were included in the present meta-analysis. The results revealed that patients who had undergone gastric bypass surgery had an increase in semen volume (SMD (95%CI)=0.583 (0.121–1.045), p=0.013). However, the seminal concentration (overall, SMD (95%CI)=−0.123 (−0.418–0.173), p=0.416) and the semen progressive motility (overall SMD (95%CI)=0.148 (−0.148–0.444), p=0.328) remained unchanged after the bariatric surgery. Nevertheless, semen normal morphology experienced an increase in the subgroup of prospective design and sleeve gastrectomy (prospective study, SMD (95%CI)= 0.385 (0.074–0.697), p=0.015, sleeve gastrectomy, SMD (95%CI)=0.880 (0.465–1.296), p=0.000; overall, SMD (95%CI)=0.372 (0.068–0.677), p=0.017).
Conclusions
In conclusion, based on the limitations of the present meta-analysis, definite conclusions cannot be reached regarding the possible effect of bariatric surgery on semen parameters.
MeSH Keywords: Bariatric Surgery, Gastric Bypass, Meta-Analysis, Semen Analysis
Background
Obesity is an increasingly serious public health problem worldwide, and is associated with multiple health problems, including increased risk for cardiovascular disorders, diabetes, and reduced life expectancy [1]. In the USA, about one-third of adult men are obese and 3% are morbidly obese [2]. Obesity is also reported to be associated with reproductive abnormalities, which included hypogonadism [3], impaired sperm quality [4], and diminished sexual quality of life [5].
Although behavioral and pharmacologic weight-loss therapies are well-established, bariatric surgery has proven to be an effective treatment strategy in treating obesity, and was associated with improved quality of life and comorbidities [6]. Based on data from the American Society of Metabolic and Bariatric Surgery, approximately 19 600 bariatric surgeries were conducted in 2015 in the USA [7]. The most commonly conducted bariatric surgical procedures are Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic adjustable gastric band [8].
There have been several investigations reporting abnormalities in semen parameters associated with obesity [9], and a higher risk of subfertility is demonstrated among couples in which the male is obese [10]. Several hypotheses have also been established to interpret the deleterious effects of obesity on male fertility potential and seminal parameters, including the abnormal release of reproductive hormones, sleep apnea, and the abnormal adipose-derived hormone releases [10]. Although the impact of life modification, including diet and vitamin supplementation, may be beneficial in weight loss and restoring male fertility potential, there is still controversy regarding the effect of excess weight loss, especially via bariatric surgeries, on male fertility and semen quality. Several studies have been published to evaluate its clinical effect on male fertility preservation and seminal parameters, but the results were conflicting. Therefore, we performed the present study using systematic evaluation and meta-analysis to provide a more precise and comprehensive estimation of the effect of bariatric surgery on male fertility restoration, while focusing on conventional seminal parameters postoperatively.
Material and Methods
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) was applied to conduct the present meta-analysis [11].
Literature search
We performed a literature search from database inception before March 31, 2018 from Cochrane Library, PUBMED, EMBASE, MEDLINE, and CNKI databases. The keywords used for searching were “bariatric surgery,” “semen,” “sperm”, “obesity”, alone or with different combinations. The titles and abstracts of articles were evaluated for possible enrollment after acquiring the preliminary results. Then full-text articles were evaluated for the final inclusion. A cited reference search was also conducted from the eligible articles obtained via the prior keyword search. Articles identified via the reference search were then assessed using the study selection criteria for possible enrollment. The reference search was also conducted on all newly obtained articles until no new articles were identified. Two investigators (Yong Wei and Quanbing Chen) conducted the screening process independently, and any possible disagreement was resolved by discussion. The patient, intervention, comparison, outcome (PICO) question for this study is as follows: in males who underwent bariatric surgeries (P), do the surgeries (I) affect semen quality (O) compared with the baseline data (C)?
Inclusion criteria
Study design: Randomized controlled trials and non-randomized controlled trials, prospective or retrospective cohort and case-control studies, case series comparing the seminal parameters after the bariatric and before surgeries were included in our meta-analysis, with quantitative data available on related outcome measures.
Type of participants: Male patients who underwent bariatric surgeries (Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic adjustable gastric band) were included in the present study.
Type of interventions: Semen parameters for bariatric surgeries postoperatively versus semen parameters preoperatively.
Type of outcome measures: Semen parameters including semen volume, semen concentration, semen progressive motility, and semen normal morphology.
Exclusion criteria: Studies with (1) incomplete data; (2) reviews, animal experiments, comments, editorials, letters, and congresses; (3) data not available.
Evidence quality assessment: Two reviewers (Yong Wei and Quanbing Chen) applied the Newcastle-Ottawa Scale (NOS) [12] independently to evaluate the article quality of the enrolled studies. Studies with a quality score of more than 5 in the NOS 9-scale system were considered high quality.
Study enrollment and data extraction: Two individuals (Yong Wei and Quanbing Chen) evaluated articles for possible enrollment independently according to the inclusion and exclusion criteria and extracted the related article data, including study design, year of publication, first author name, and related data in the 2 approaches on outcome measures. Any discrepancy was resolved by discussion between the 2 authors.
Statistical analysis
STATA software (version 12.0; Stata corporation, College Station, TX, USA) was used to conduct the systematic review and meta-analysis. Heterogeneity among the included studies was calculated and defined as Q test or I2 value. The heterogeneity was considered non-significant if P>0.1 or I2 <40%. The fixed-effects model was applied if there was no heterogeneity among the studies; otherwise, we used the random-effects model. The standardized mean difference (SMD) was calculated for outcome measures. Publication bias was calculated using Begg’s funnel plots and Egger’s test. A value of “ Pr>|z|” less than 0.05 for Begg’s funnel plots or a value of „P>|t|” less than 0.05 for Egger’s test was defined as positive publication bias. We predefined subgroup analysis by study design and bariatric surgery procedures. Sensitivity analysis was conducted by sequentially omitting each individual study at a time to evaluate the effect of each study on the pooled SMDs. We checked this meta-analysis using the PRISMA 2009 Checklist.
Results
Characteristics of the included studies and evidence quality assessment
A total of 104 published articles were acquired based on the publication search strategy. A total of 97 articles defined as reviews or fundamental researches or unrelated publications were then excluded. Based on the exclusion criteria, 1 study with insufficient data extracted was also excluded [13]. We were unable to identify any additional studies after manually screening of the reference lists of the included studies. In total, 6 publications were included that fulfilled the inclusion criteria [14–19]. Of the included studies, 3 were from Europe [14,15,18], 1 was from Brazil [16], 1 was from the USA [17], and 1 was from Qatar [19]. The general information of these studies, as well as the differential study design and outcome measures among the included studies are demonstrated in Table 1. The patient source of 2 studies was from obesity clinics [15,16], 3 studies were from infertility clinics [14,18,19], and 1 was not available [17]. Four studies were of prospective study design [15–17,19] and 2 studies were case series [14,18]. All but 1 study [17] failed to report the power calculation method. Of the 4 studies with prospective study design, 2 had no control group [17,19]. Moreover, 2 studies failed to report adequate outcome measures [14,18]. One study [14] was grouped as low quality, and the 5 studies were grouped as high-quality studies. The flow diagram is shown in Figure 1.
Table 1.
The essential information of selected studies.
| Author (publication year) | Study design | Country or region | Patient source | Fertility status | Follow-up duration | Bariatric surgery performed | Sample size | Outcome reported | limitations | Quality score/total score |
|---|---|---|---|---|---|---|---|---|---|---|
| Samavat (2017) | Prospective case-control study | Italy | Obese clinic | 26% of the patients had normal seminal analysis | 6 months | gastric bypass | 23 | Sperm motility, morphology, number, volume, DNA fragmentation and interlekin-8 | No power calculation | 7/9 |
| Reis (2012) | Prospective case-control study | Brazil | Obese clinic | Only percentage of the patients had normal seminal analysis | 24 months | Gastric bypass | 10 | Semen volume, PH, motility, concentration, leukocytes, vitality, normal morphology | No power calculation, | 7/9 |
| Lazaros (2012) | Case series | Greece | Infertility clinic | Wife previously pregnant with assisted reproductive technology | One patient 24 months, one patient 12 month | Gastric bypass | 2 | Semen concentration, motility, normal morphology, percentage of mature spermatozoa | No power calculation, outcome measures not enough | 5/9 |
| Bardisi (2016) | Prospective cohort study | Qatar | Infertility clinic | Azoospermia, oligospermia, normal semen | 12 months | Sleeve gastrectomy | 46 | Semen volume, concentration, motility, normal morphology | No power calculation, odds ratio not provided, no control group designed | 5/9 |
| Sermondade (2012) | Case series | France | Infertility clinic | One patient terato-zoospermia, 2 patients oligoasthe-noterato-zoospermia | One patient 24 months, one patient 15 months, one patient 6 months | Sleeve gastrectomy | 3 | Semen concentration, motility, normal morphology, assisted reproductive treatment | No power calculation, outcome measures not enough | 4/9 |
| Legro (2015) | Prospective cohort study | USA | Not available | Only percentage of the patients had normal seminal analysis | 12 months | Gastric bypass | 6 | Semen volume, concentration, motility, normal morphology | odds ratio not provided, no control group designed | 6/9 |
Figure 1.
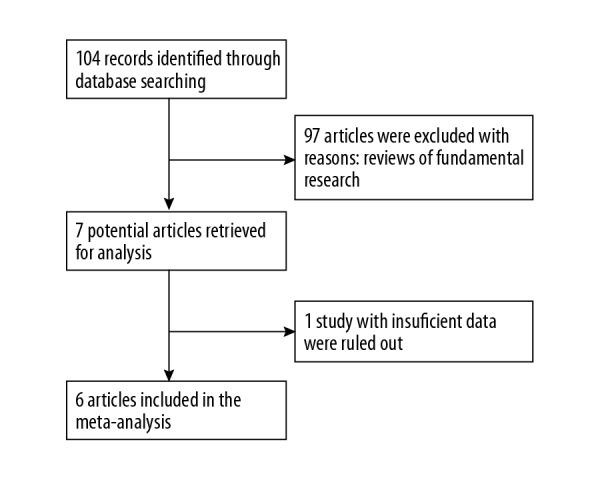
The flow gram of the identification and selection of the studies.
Meta-analysis results
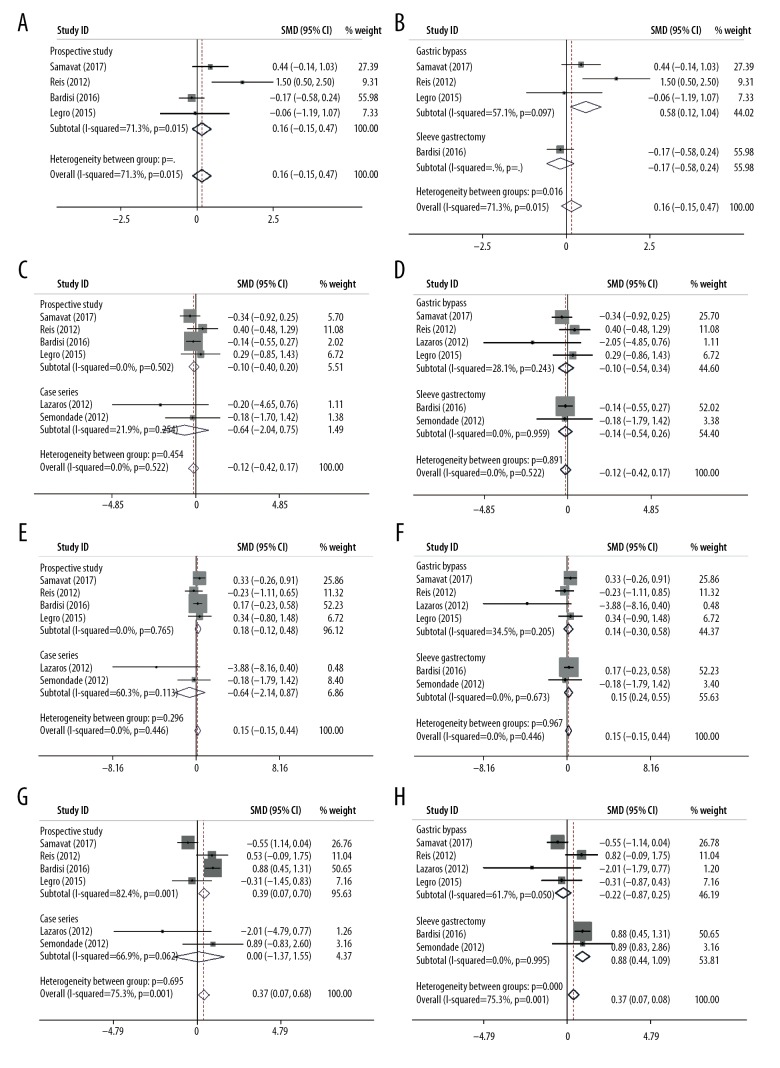
Semen volume
Four studies reporting semen volume were included in the meta-analysis. The pooled SMD based on 3 studies demonstrated that there was an evident increase (0.583 ml) in semen volume after the gastric bypass surgery (after the surgery versus before the surgery, SMD (95%CI)=0.583 (0.121–1.045), p=0.013), yet it was not confirmed in the overall analysis and subgroup sleeve gastrectomy (after the surgery vs. before the surgery, overall SMD (95%CI)=0.159 (−0.147–0.466), p=0.308, sleeve gastrectomy, 1 study, SMD (95%CI)=−0.174 (−0.583–0.236), p=0.406). All included studies reporting semen volume were prospective (after the surgery vs. before the surgery, overall SMD (95%CI)=0.159 (−0.147–0.466), p=0.308). The heterogeneity in the gastric subgroup (I-squared ranged 57.1%) was relatively lower than the overall heterogeneity (I-squared 71.3%), revealing the surgical method partly explained the source of heterogeneity (Figure 2A, 2B, and Table 2).
Figure 2.
Forest plots for the effect of bariatric surgery on semen parameters. (A) semen volume (subgroup study design); (B) semen volume (subgroup surgical procedures); (C) semen concentration (subgroup study design); (D) semen concentration (subgroup surgical procedures); (E) semen progressive motility (subgroup study design); (F) semen progressive motility (subgroup surgical procedures); (G) semen normal morphology (subgroup study design); (H) semen normal morphology (subgroup surgical procedures).
Table 2.
Main results of the meta-analysis.
| Subgroup | I2 (%) | SMD (95%CI) | P value | Begg (Pr>|z|) | Egger (P>|t|) | |
|---|---|---|---|---|---|---|
| Semen volume | Prospective study | 71.3% | 0.159 (−0.147–0.466) | 0.308 | 0.734 | 0.475 |
| Case series | – | – | – | |||
| Gastric bypass | 57.1% | 0.583 (0.121–1.045) | 0.013 | |||
| Sleeve gastrectomy | – | −0.174 (−0.583–0.236) | 0.406 | |||
| Overall | 71.3% | 0.159 (−0.147–0.466) | 0.308 | |||
| Semen concentration | Prospective study | 0.0% | −0.098 (−0.400–0.204) | 0.524 | 0.707 | 0.865 |
| Case series | 21.9% | −0.642 (−2.036–0.751) | 0.366 | |||
| Gastric bypass | 28.1% | −0.100 (−0.542–0.342) | 0.659 | |||
| Sleeve gastrectomy | 0.0% | −0.141 (−0.538–0.255) | 0.486 | |||
| Overall | 0.0% | −0.123 (−0.418–0.173) | 0.416 | |||
| Semen progressive motility | Prospective study | 0.0% | 0.179 (−0.123–0.481) | 0.244 | 0.260 | 0.071 |
| Case series | 60.3% | −0.638 (−2.141–0.865) | 0.406 | |||
| Gastric bypass | 34.5% | 0.141 (−0.304–0.585) | 0.535 | |||
| Sleeve gastrectomy | 0.0% | 0.153 (−0.244–0.550) | 0.449 | |||
| Overall | 0.0% | 0.148 (−0.148–0.444) | 0.328 | |||
| Semen normal morphology | Prospective study | 82.4% | 0.385 (0.074–0.697) | 0.015 | 0.452 | 0.278 |
| Case series | 66.9% | 0.088 (−1.371–1.546) | 0.906 | |||
| Gastric bypass | 61.7% | −0.219 (−0.668–0.229) | 0.338 | |||
| Sleeve gastrectomy | 0.0% | 0.880 (0.465–1.296) | 0.000 | |||
| Overall | 75.3% | 0.372 (0.068–0.677) | 0.017 |
Semen concentration
In the analysis of the 6included articles evaluating the possible seminal concentration improvement after the bariatric surgery, the overall and subgroup SMDS indicated that seminal concentration remained unchanged after the bariatric surgery (overall, SMD (95%CI)=−0.123 (−0.418–0.173), p=0.416), which showed a similar trend in the subgroup analysis by study design and bariatric surgery methods (prospective study, SMD (95%CI)=−0.098 (−0.400–0.204), p=0.524; case series, SMD (95%CI)=−0.642 (−2.036–0.751), p=0.366; gastric bypass, SMD (95%CI)=−0.100 (−0.524–0.342), p=0.659; sleeve gastrectomy, SMD (95%CI)=−0.141 (−0.538–0.255) (Figure 2C, 2D, and Table 2). Moreover, the heterogeneity examination showed relative homogeneity among the studies, both in the overall and subgroup analysis (I2 ranged from 0.0% to 28.1%).
Semen progressive motility
Pooled analyses were also performed in the enrolled articles with the evaluation of seminal progressive motility. Pooled analysis of the 4 prospective studies showed that no heterogeneity existed (I-squared 0.0%) and indicated that there was no statistical increase in semen progressive motility after the bariatric surgery intervention (SMD (95%CI)=0.179 (−0.123–0.481), p=0.244). A similar trend was also observed in the overall and case series subgroup (overall SMD (95%CI)=0.148 (−0.148–0.444), p=0.328, case series subgroup, SMD (95%CI)=−0.638 (−2.141–0.865), p=0.406); nevertheless, a moderate heterogeneity was observed in the case series subgroup (I-squared 60.3%). No association was observed between bariatric surgery and seminal progressive motility improvement in the pooled analyses (gastric bypass subgroup, SMD (95%CI)=0.141 (−0.304–0.585), p=0.535, sleeve gastrectomy subgroup, SMD (95%CI)=0.153 (−0.244–0.550), p=0.449) (Figure 2E, 2F, and Table 2).
Semen normal morphology
In the pooled analysis of semen normal morphology, the meta-analysis based on the studies of prospective study design and sleeve gastrectomy subgroup revealed that there was a significant increase in semen normal morphology after the surgery (prospective study, SMD (95%CI)=0.385 (0.074–0.697), p=0.015, sleeve gastrectomy, SMD (95%CI)=0.880 (0.465–1.296), p=0.000, overall, SMD (95%CI)=0.372 (0.068–1677), p=0.017). However, this trend was not observed in other subgroup analyses (case series, SMD (95%CI)=0.088 (−1.371–1.546), p=0.906, gastric bypass, SMD (95%CI)=−0.219 (−0.668–0.229), p=0.338). Furthermore, a relatively high heterogeneity also existed among the studies (I-squared from 0.0% to 82.4%) (Figure 2G, 2H, and Table 2).
Assessment of publication bias and sensitivity analyses
Sensitivity analyses was conducted to determine the influence of any individual study on the overall estimate, and no significant changes occurred on the corresponding SMDs. Additionally, Begg’s funnel plots and Egger’s test revealed no publication bias (Begg’s funnel plots: semen volume, Pr>|z|=0.734; semen concentration, Pr>|z|=0.707; semen progressive motility, Pr>|z|=0.260; semen normal morphology, Pr>|z|=0.452; Egger’s test: semen volume, P>|t|=0.475; semen concentration, P>|t|=0.865; semen progressive motility, P>|t|=0.071; semen normal morphology, P>|t|=0.071=0.278) (Table 1).
Discussion
Bariatric surgery, including gastric bypass and sleeve gastrectomy, is an effective method to achieve weight loss. The purpose of the present study was to evaluate the influence of the operation on improving seminal parameters. Bariatric surgery is highly successful in inducing weight loss and treating morbid obesity, but only a few small studies have investigated the possible beneficial effect of bariatric surgery on male fertility and seminal parameters, and the results were controversial. Thus, we performed this quantitative analysis based on a meta-analysis to pool the limited studies for a more comprehensive pooled estimation.
In women, the effects of obesity on reproductive function are readily evident and extensively studied. On the contrary, the possible negative effects of obesity on reproductive function in males have been relatively less studied. The biologic basis for the association between weight loss and changes in sperm parameters is likely multifactorial, and different hypotheses have been proposed established. First, the hormonal changes associated with obesity likely play an important role. The hormonal profile in obese males is often characterized by decreased testosterone levels and increased estrogen levels. The increased estrogen levels act on the hypothalamus, negatively affecting the release of gonadotrophin-releasing hormone (GnRH) and gonadotrophin (FSH and LH) at the pituitary level, which results in the reduction of testosterone and reduced testicular function. In addition, excess estrogen has a direct deleterious effect on spermatogenesis [20]. Second, several environmental toxic substances and liposoluble endocrine disruptors present a preferential accumulation in the fatty tissue, thus impairing male fertility potential [21]. The increased testicular local heat associated with obesity can severely affect sperm production, and altered sexual health and genetic abnormalities also seem to play a role [22]. Of the 6 enrolled studies, only a percentage of the surgical patients had a normal baseline seminal analysis, which further indicates the possible negative effect of obesity on male fertility.
Between 1990 and 2000, the US national annual rate of bariatric surgical interventions increased 6-fold, from about 2.4 to 14.1 per 100 000 adults [23]. Accumulating evidence suggests that patients with a preoperative BMI of 40 kg/m2 are expected to lose 20 to 40 kg within 2 years after bariatric surgery and maintain their reduced weight for up to 10 years [24]. In China, approximately 7800 patients that underwent bariatric surgeries were reported from 2001 to 2015, mostly in the most recent 5 years. Although bariatric surgery is still at an early stage, it is now experiencing explosive growth in China [25]. Thus, the possible effect of bariatric surgeries on semen quality and male fertility is of clinical importance, but the results in the literature are inconsistent; therefore, the present meta-analysis was performed. Although the final positive impact is expected, some negative consequences of bariatric procedures on male fertility were also highlighted in anecdotal reports. A series of 6 patients were reported to experiencing azoospermia, presenting secondary infertility after Roux-en-Y gastric bypass interventions [13]. Another series of 3 cases reported a worsening of semen parameters after the bariatric surgery, including oligoasthenoteratozoospermia [14]. The negative effect of weight loss from bariatric surgery and impairment of semen parameters is likely multifactorial, and several explanations have been proposed. A popular hypothesis is that the rapid weight loss secondary to bariatric surgery, especially for Roux-en-Y gastric bypass, may lead to a relative under-nutrition status, with deficiencies of iron, calcium, and vitamins B1, B9, and B12, which causes diverse deficiencies in spermatogenesis after the bariatric surgery, although vitamin and mineral supplementation is recommended after bariatric surgery [26]. Additionally, the surgery could possibly cause the release of toxic and liposoluble substances in the fatty tissue after the surgery, leading to impaired spermatogenesis [27]. The rapid weight loss could also interrupt the normal gonadotrophin-releasing hormone secretion and cause reproductive disorders [28]. In the present meta-analysis, the main outcome measures, including semen progressive motility and semen concentration, remained unaltered after the bariatric interventions, both in the overall and subgroup analysis stratified by study design and surgical methods. Semen normal morphology showed a slight but statistically significant increase after sleeve gastrectomy, but this was not observed after gastric bypass surgery. Nevertheless, owing to the high variability of sperm morphologic criteria in different hospitals and the relative sample size of sleeve gastrectomy (2 studies, 49 participants) and the high heterogeneity among the included studies, the positive effect of bariatric surgery on sperm normal morphology was not confirmed and needs further investigation.
In a meta-analysis consisting of 24 retrieved studies, bariatric surgery was associated with an 8.7 increase in total testosterone levels, a decrease in estradiol, and an increase in gonadotropins [29]. In our 6 enrolled studies, the 4 prospective studies reported an increase in total testosterone level and a decrease in estradiol level, suggesting a causal relationship between bariatric surgery and hormonal profile normalization. However, we found that semen quality does not change with bariatric surgical procedures. This is in contrast to the hormonal profile normalization, suggesting that a developmental block of spermatogenesis occurred at a point not related to the release of sex hormones in the testis. However, the conclusion that bariatric surgery is unable to reverse semen quality could also partially be interpreted as the inner shortcomings of the present meta-analysis, which are related to the different study designs and methods. Extended Table 1 summarizes the differences among the included studies that could possibly affect the outcome measures, including baseline BMI, time to perform seminal analysis after the surgery, and postoperative BMI, as well as whether physical activity or pharmacological or diet weight loss therapies performed after the bariatric surgery. The level of baseline BMI is positively correlated with the severity of the reproductive and seminal parameters impairment, and this was further evidenced by the fact that only a percentage of the surgical patients had a normal baseline seminal analysis. The gap and difference of baseline BMI associated with possible selection bias could possibly partially interpret the heterogeneity source among the included studies. Regular physical activity, on the other hand, can improve semen quality in obese males [30]. The heterogeneity in study design further weakened the findings of the present meta-analysis. Thus, the homogeneity of study design, including BMI, and other cofactors that may interfere with the seminal parameters among the differential studies in pooled analysis should be addressed in future studies. The present studies included the results selected for semen parameters before and after the surgery, and there was no comparison with another patient who underwent another intervention. Moreover, another possible bias is the relatively small sample size and the differences in study design and length of follow-up among the included studies. The present study fails to provide a direct link between bariatric surgery and other sperm features, including number of spermatozoa and sperm DNA fragmentation index, and change in capacity to impregnate a woman either in a natural process or in an assisted reproductive program. For infertile couples in which the male is obese that seek medical advice in an infertility clinic, the primary concern is the possible improvement of chances of pregnancy associated with bariatric surgery, when compared to the improvement of seminal parameters. Thus, the results of the present meta-analysis should be interpreted with caution and studies with better study design and high-quality studies, like RCTs, with larger sample size and more outcome measures involved, including conventional and unconventional semen parameters, are warranted for a more precise estimation.
Extended Table 1.
| Author (publication year) | Semen analysis criteria | Motility and morphology evaluation | Number of semen specimens analyzed prior to surgery | Period of abstinence | Initial BMI | Time to seminal analyses after the surgery | Number of semen specimens analyzed after the surgery | Posto-perative BMI | If physical activity or pharmacologic or diet weight loss therapies performed besides bariatric surgery | If inflamm-atory markers analyzed |
|---|---|---|---|---|---|---|---|---|---|---|
| Samavat (2017) | 4th WHO edition of laboratory manual for human semen | Motility using optical microscopy checking, morphology checking after Diff-Quick staining | Once | Not available | 45.8±7.4 | 6 months | Once | 34.7±5.3 | No | Interle-ukin-8 in seminal plasma showed a decrease after the surgery |
| Reis (2012) | 4th WHO edition of laboratory manual for human semen | Not available | Once | 2–3 days | 55.7±7.8 | 24 months | Once | 31.0±5.3 | Physical activity and low energy diet | No |
| Lazaros (2012) | 4th WHO edition of laboratory manual for human semen | Motility not available, morphology checking after Papanicolaou staining | Twice | 2–3 days | One patient 40.1, one 38.2 | One patient 24 months, one patient 12 month | Twice | One patient 26.2, one 29.4 | No | No |
| Bardisi (2016) | 4th WHO edition of laboratory manual for human semen | Not available | Once | 3–5 days | 71.4 (42.9–96.2) | 12 months | Once | 46.9 (32.2–76.9) | No | No |
| Sermondade (2012) | Not available | Not available | Not available | Not available | One 65.7, one 53.5, one 38.6 | One patient 24 months, one patient 15 months, one patient 6 months | Not available | One 33.4, one 30.4, one 27.5 | No | No |
| Legro (2015) | Not available | Motility not available, morphology checking after Spermac staining | Once | 2–7 days | 48±7 | 12 months | Once | 32±7 | No | No |
The main advantages of our meta-analysis are the following. First, this was the first systematic review and meta-analysis to evaluate seminal response after bariatric surgery. Second, we performed stratified analyses by study design and surgical methods, which partially explains the source of this heterogeneity among the studies. Third, we performed Begg’s funnel plots and Egger’s test to evaluate the publication bias, which offers a better understanding of the current status of studies in this field. Thus, we consider the results of the present meta-analyses to be reliable.
Conclusions
In summary, the present meta-analysis indicates that the effect of bariatric surgery on semen quality remains unclear. Better studies regarding this topic are warranted to further elucidate this issue.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Zarotsky V, Huang MY, Carman W, et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2:819–34. doi: 10.1111/andr.274. [DOI] [PubMed] [Google Scholar]
- 4.El Salam MAA. Obesity, an enemy of male fertility: A mini review. Oman Med J. 2018;33:3–6. doi: 10.5001/omj.2018.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glina FPA, de Freitas Barboza JW, Nunes VM, et al. What is the impact of bariatric surgery on erectile function? A systematic review and meta-analysis. Sex Med Rev. 2017;5:393–402. doi: 10.1016/j.sxmr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson J, Taft C, Rydén A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: The SOS intervention study. Int J Obes (Lond) 2007;31:1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 7.Ponce J, DeMaria EJ, Nguyen NT, et al. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis. 2016;12:1637–39. doi: 10.1016/j.soard.2016.08.488. [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Freeman NLB, Lee JA, et al. Early major complications after bariatric surgery in the USA, 2003–2014: A systematic review and meta-analysis. Obes Rev. 2018;19:529–37. doi: 10.1111/obr.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammoud AO, Wilde N, Gibson M, et al. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–25. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Koloszár S, Fejes I, Závaczki Z, et al. Effect of body weight on sperm concentration in normozoospermic males. Arch Androl. 2005;51:299–304. doi: 10.1080/01485010590919701. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.di Frega AS, Dale B, Di Matteo L, Wilding M. Secondary male factor infertility after Roux-en-Y gastric bypass for morbid obesity: Case report. Hum Reprod. 2005;20:997–98. doi: 10.1093/humrep/deh707. [DOI] [PubMed] [Google Scholar]
- 14.Sermondade N, Massin N, Boitrelle F, et al. Sperm parameters and male fertility after bariatric surgery: three case series. Reprod Biomed Online. 2012;24:206–10. doi: 10.1016/j.rbmo.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Samavat J, Cantini G, Lotti F, et al. Massive weight loss obtained by bariatric surgery affects semen quality in morbid male obesity: A preliminary prospective double-armed study. Obes Surg. 2018;28:69–76. doi: 10.1007/s11695-017-2802-7. [DOI] [PubMed] [Google Scholar]
- 16.Reis LO, Zani EL, Saad RD, et al. Bariatric surgery does not interfere with sperm quality – a preliminary long-term study. Reprod Sci. 2012;19:1057–62. doi: 10.1177/1933719112440747. [DOI] [PubMed] [Google Scholar]
- 17.Legro RS, Kunselman AR, Meadows JW, et al. Time-related increase in urinary testosterone levels and stable semen analysis parameters after bariatric surgery in men. Reprod Biomed Online. 2015;30:150–56. doi: 10.1016/j.rbmo.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazaros L, Hatzi E, Markoula S, et al. Dramatic reduction in sperm parameters following bariatric surgery: Report of two cases. Andrologia. 2012;44:428–32. doi: 10.1111/j.1439-0272.2012.01300.x. [DOI] [PubMed] [Google Scholar]
- 19.El Bardisi H, Majzoub A, Arafa M, et al. Effect of bariatric surgery on semen parameters and sex hormone concentrations: A prospective study. Reprod Biomed Online. 2016;33:606–11. doi: 10.1016/j.rbmo.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Akingbemi BT. Estrogen regulation of testicular function. Reprod Biol Endocrinol. 2005;3:51. doi: 10.1186/1477-7827-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez Torres M, Campoy Folgoso C, Cañabate Reche F, et al. Organochlorine pesticides in serum and adipose tissue of pregnant women in Southern Spain giving birth by cesarean section. Sci Total Environ. 2006;372:32–38. doi: 10.1016/j.scitotenv.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Brindley GS. Deep scrotal temperature and the effect on it of clothing, air temperature, activity, posture and paraplegia. Br J Urol. 1982;54:49–55. doi: 10.1111/j.1464-410x.1982.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 23.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–20. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 24.Barnett SJ, Stanley C, Hanlon M, et al. Long-term follow-up and the role of surgery in adolescents with morbid obesity. Surg Obes Relat Dis. 2005;1:394–98. doi: 10.1016/j.soard.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 25.Du X, Dai R, Zhou HX, et al. Bariatric surgery in China: How is this new concept going? Obes Surg. 2016;26:2906–12. doi: 10.1007/s11695-016-2204-2. [DOI] [PubMed] [Google Scholar]
- 26.Coupaye M, Puchaux K, Bogard C, et al. Nutritional consequences of adjustable gastric banding and gastric bypass: A 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 27.Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, et al. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005;20:208–15. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–37. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: A systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 30.Rosety MÁ, Díaz AJ, Rosety JM, et al. Exercise improved semen quality and reproductive hormone levels in sedentary obese adults. Nutr Hosp. 2017;34:603–7. doi: 10.20960/nh.549. [DOI] [PubMed] [Google Scholar]