ABSTRACT
Cells of transporting epithelia are characterized by the presence of abundant F-actin-based microvilli on their apical surfaces. Likewise, auditory hair cells have highly reproducible rows of apical stereocilia (giant microvilli) that convert mechanical sound into an electrical signal. Analysis of mutations in deaf patients has highlighted the critical components of tip links between stereocilia, and related structures that contribute to the organization of microvilli on epithelial cells have been found. Ezrin/radixin/moesin (ERM) proteins, which are activated by phosphorylation, provide a critical link between the plasma membrane and underlying actin cytoskeleton in surface structures. Here, we outline recent insights into how microvilli and stereocilia are built, and the roles of tip links. Furthermore, we highlight how ezrin is locally regulated by phosphorylation, and that this is necessary to maintain polarity. Localized phosphorylation is achieved through an intricate coincidence detection mechanism that requires the membrane lipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and the apically localized ezrin kinase, lymphocyte-oriented kinase (LOK, also known as STK10) or Ste20-like kinase (SLK). We also discuss how ezrin-binding scaffolding proteins regulate microvilli and how, despite these significant advances, it remains to be discovered how the cell polarity program ultimately interfaces with these processes.
KEY WORDS: Actin, Cell polarity, Cytoskeleton, ERM proteins, Microvilli, Stereocilia
Summary: Apical surfaces of epithelial cells are characterized by the presence of abundant F-actin-based microvilli. We outline recent insights into regulation and maintenance of microvilli and stereocilia.
Introduction
Cells throughout the vertebrate body are polarized. By regulating its internal organization and plasma membrane composition in a polarized manner, the cell ensures organism viability and the execution of central biological functions, such as cell division, proliferation, signaling and migration. One critical aspect of cell polarity is how cells build and restrict morphological features to one domain of a polarized cell. The epithelial cell serves as the archetypal example of a polarized cell. Its apical membrane domain is decorated with finger-like protrusions called microvilli (Granger and Baker, 1950). Microvilli expand the interface between the cell and extracellular milieu, and accommodate membrane proteins that take part in signaling as well as ion and nutrient transport. By contrast, the basolateral membrane harbors a different composition of membrane proteins and displays a membrane morphology that is very distinct from the apical brush border membrane (Bryant and Mostov, 2008). In this Review, we discuss recent insights into the regulation and assembly of the apical microvilli and stereocilia – related finger-like protrusions that are found on the apical membrane of hair cells. We highlight the contribution of bundled actin filaments to apical organization, and the contribution of tip-linking to the structure and function of microvilli and stereocilia. Furthermore, we describe an emerging mechanism of how bundled actin filaments are connected to the plasma membrane in a way that restricts their presence to the apical membrane.
Morphological features and arrangement of actin-based surface structures
Shaping the plasma membrane into a finger-like structure requires a core of bundled cytoskeletal filaments with lateral attachments to the plasma membrane. In the case of cilia, the filaments are microtubules, whereas filaments of actin support filopodia, microvilli and stereocilia (DeRosier and Tilney, 2000; Lewis and Bridgman, 1992). Here, we restrict our discussion to microvilli and stereocilia, which are found on the apical domain of epithelial cells.
The plasma membrane of microvilli is supported by a bundle of ∼30–40 parallel, inherently polarized actin filaments with their barbed ends at the microvillus tip (Mooseker and Tilney, 1975). Early morphological studies showed that the F-actin core is bundled and attached to the plasma membrane by lateral cross-bridges of ∼30 nm length, indicating that the actin filaments are both crosslinked to one another and to the plasma membrane (Brunser and Luft, 1970; Matsudaira and Burgess, 1979; Mooseker and Tilney, 1975). On intestinal epithelia and the epithelium of the kidney proximal tubule, the microvilli are of uniform length and generally hexagonally packed. Despite over 40 years of research, it is still not clear what drives the assembly of actin filaments in microvilli, but much has been learned about their morphogenesis by studying microvillar components. Today, we appreciate that events such as cytoskeletal remodeling, membrane–cytoskeleton crosslinking and extracellular adhesion are parts of the cellular program that shapes the apical brush border domain (Crawley et al., 2014b). We discuss some of these cellular programs in this Review.
Stereocilia are found on the apical aspect of hair cells of the vertebrate cochlea and vestibular systems. Early studies of bird cochlea revealed that the length of hair cell stereocilia range from 1.5 to 5 µm, increasing in length from the proximal to distal end (Tilney and Saunders, 1983). However, in contrast to what is seen in birds, stereocilia on individual cells of the mammalian cochlea exhibit a staircase-like pattern with a predetermined graded increase in stereociliary length between rows (Fig. 1A, see Box 1). The mammalian cochlea contains two types of hair cells: a single row of inner hair cells with a linear array of stereocilia measuring ∼5 µm in length, and three rows of outer hair cells with stereocilia arranged in a V-shaped pattern and measuring 2–3 µm in length (Dallos, 1996; Kaltenbach et al., 1994). Vestibular hair bundles in the utricle of adult mouse range from ∼2 µm for the shortest stereocilia in the staircase to up to ∼15 µm (Li et al., 2008; Rzadzinska et al., 2004). Each mature stereocilium contains 400–3000 actin filaments with their barbed ends at the tip and pointed ends associated with the base, where it tapers and inserts into the cuticular plate of the hair cell (Tilney et al., 1980, 1989; Zheng et al., 2000). In an extended series of studies, Tilney et al. have documented the assembly of stereocilia during development in ultrastructural detail (Tilney et al., 1992). Stereocilia on mature hair cells are linked by tip links that activate mechano-electrical transduction channels when the stereocilia are bent at their base in response to sound. A prerequisite for proper mechano-electrical transduction is a directional mechanosensitivity based on hair cells being arranged so that their axis of sensitivity is aligned with the direction of stimulation (Shotwell et al., 1981). Recently, a series of studies has revealed the molecular mechanisms that instruct the organization of the characteristic V-shaped staircase-like architecture of stereocilia in cochlear hair cells (see Box 1).
Fig. 1.
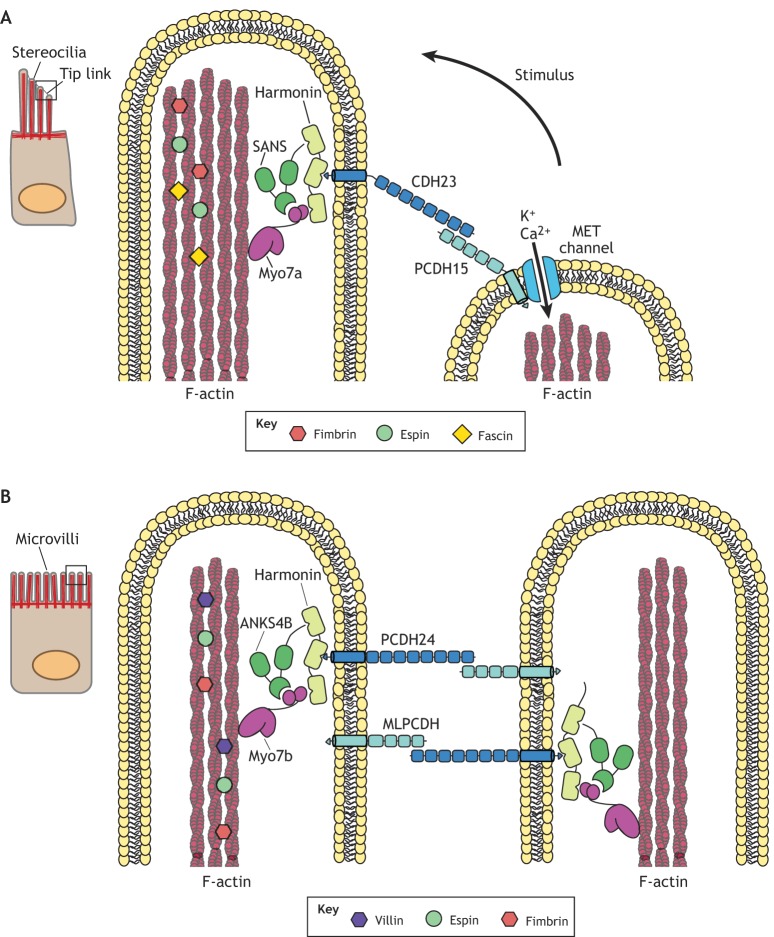
Organization and regulation of stereocilia and microvilli. (A) Stereocilia of auditory hair cells are arranged in a staircase configuration. Actin bundles are assembled and stabilized through actin-binding proteins such as fimbrin, espin and fascin. Stereocilia of each row are connected to stereocilia of adjacent rows through tip links. Tip links require a ternary complex of Myo7a, USH1C (harmonin) and SANS that binds to the cadherin CDH23. CDH23 forms a heteromeric interstereociliary link with protocadherin PCDH15 on the adjacent stereocilium. The cytoplasmic region of PCDH15 interacts with the mechano-electrical transduction (MET) channels. Auditory stimulus causes deflection towards taller rows, which generates an influx of K+ and Ca2+ ions through the MET channels. (B) Microvilli covering the apical aspects of intestinal epithelial cells require heterotypic extracellular linkages that are mediated by the protocadherin PCDH24 and mucin-like protocadherin MLPCDH. By analogy with stereocilia, the cytoplasmic tail of PCDH24 binds the ternary complex of USH1C (harmonin), the SANS homolog ANKS4B and Myo7b. The actin cytoskeleton is assembled and stabilized by villin, espin and fimbrin.
Box 1. Emerging mechanism for the staircase-like architecture of stereocilia.
Hair cells display two integrated levels of polarity. The cells are systematically and uniformly oriented in the cochlea, thus they display organ-wide planar cell polarity (PCP) (Goodrich and Strutt, 2011). Additionally, hair cells have an intrinsic planar axis, outlined by the direction of the staircase-like gradient of stereocilia height towards the tallest hair cell, the kinocilium, at the edge of the V-shaped bundle of stereocilia (Pickles et al., 1984). Initially, epithelial cells of sensory epithelium are covered by microvillar precursors. During apical morphogenesis, a lateral ‘bare zone’ emerges, uniquely devoid of microvilli. The bare zone forms through apical recruitment of a complex of the mammalian adaptor protein inscuteable (mInsc), Leu-Gly-Asn repeat-enriched protein (LGN, also known as GPSM2) and the inhibitory heteromeric G protein Gαi (Tarchini et al., 2013). The complementary medial side is enriched in atypical PKC, which is excluded from the bare zone. The mInsc–LGN–Gαi complex expands the bare zone, thus relocalizing the kinocilium from the lateral junction towards the cell center and shaping the stereocilia bundle (Tarchini et al., 2013). Mice lacking mInsc display impaired lateromedial extension of the bare zone and exhibit abnormal stereociliary sub-bundles. Deletion of LGN results in disorganized or even missing bundles, displacement of kinocilium and a generally disrupted apical morphology, indicating that LGN is responsible for the inward relocalization of the kinocilium along the lateromedial axis and asymmetric positioning of stereocilia (Tarchini et al., 2013). Deletion of Gαi causes delocalization of LGN and results in misorientation of the kinocilia, suggesting that Gαi regulates the lateral hair cell orientation through the movement of the kinocilium. LGN and Gαi, but not mInsc, are also enriched in the tip of stereocilia in the tallest row at the medial boundary of the bare zone, and LGN mutants display shorter stereocilia and an increased number of rows in the architecture of the staircase (Tarchini et al., 2016). Consequently, it has been suggested that the mInsc–LGN–Gαi first orchestrates the formation of the bare zone and the V-shaped stereocilia, before LGN and Gαi are localized to stereociliary tips in order to define the height of the tallest first row (Tarchini et al., 2016). A fourth component, dishevelled-associating protein with a high frequency of leucine residues (Daple, encoded by CCDC88C), couples the PCP pathway to the formation of the cell-intrinsic planar axis by connecting the PCP protein dishevelled to the cell-instrinsic signaling component Gαi. Mice lacking Daple exhibit misoriented and misshapen staircases of stereocilia, caused by uncoupling of Gαi from the kinocilium (Siletti et al., 2017).
It is now clear that assembly of actin-based protrusions requires the assembly of protein complexes, the timing and spatial distribution of which are beginning to be elucidated. In the next section, we focus on how membrane traffic provides support for assembly of distinct membrane domains in epithelial cells.
Apical membrane morphology: the cell polarity program and membrane traffic
Microvilli and stereocilia are restricted to the apical domain of their respective cells (Fig. 1A,B). This apical-basolateral polarity is directed by an epithelial polarity program that involves intrinsic polarity proteins as well as signals from adjacent cells and the extracellular matrix (Rodriguez-Boulan and Macara, 2014). This program was elucidated mostly from studies in C. elegans and Drosophila (Campanale et al., 2017) and, in the fly, it consists of Bazooka, Partitioning defective-6 and Atypical protein kinase C (Baz–Par-6–aPKC), which function antagonistically through aPKC kinase to exclude the basolateral complex Lethal giant larvae, Discs large 1 and Scribble (Lgl–Dlg1–Scrib). Basolaterally, in turn, Par-1 kinase antagonizes the apical complex. aPKC and Par-1 establish phosphorylation-dependent feedback loops in order to exclude trespassing of proteins and to maintain membrane domain identity (Benton and St Johnston, 2003). Crumbs (Crb), a large transmembrane protein that forms the so-called Crumbs complex, participates in an essential epithelial polarity program (Bachmann et al., 2001). The Crumbs complex localizes to the subapical compartment of the cell (Tepass, 1996), and its deletion results in complete loss of the apical membrane domain, whereas Crb overexpression expands the size of apical domain (Wodarz et al., 1995). The regulatory machinery of membrane polarity overlaps with trafficking of cargos to specific membrane domains. For example, the GTPases Rab11 and, downstream, Rab8 cooperate with the apical Par3–aPKC complex to deliver vesicles with early apical identity markers, such as podocalyxin, to membranes for initiation of lumenogenesis (Bryant et al., 2010). Misregulation of the intracellular trafficking machinery that underlies cell polarity leads to disease such as microvillous inclusion disease (MVID), a severe neonatal enteropathy that affects villus enterocytes (Cutz et al., 1989; Davidson et al., 1978). Lack of absorptive channels, exchangers and nutrient transporters in apical membranes of enterocytes leads to diarrhea, life-threatening dehydration and metabolic disorders (Ruemmele et al., 2006). Neonates with MVID exhibit increased numbers of subapical vesicles, a sporadic presence of microvilli on the basolateral surface, loss of microvilli and microvillous inclusions, characterized by intracellular vesicle-like structures luminally lined by microvilli (Sherman et al., 2004). MVID has been attributed to mutations in the genes MYO5B and STX3, encoding the motor protein Myo5b and the SNARE protein syntaxin 3, which are involved in regulated apical transport and membrane fusion, respectively (Müller et al., 2008; Wiegerinck et al., 2014). A prominent feature in patients with defects in Myo5b is the accumulation of apical tubules and vesicles that appear to represent Rab8 and Rab11 double-positive recycling compartments (Vogel et al., 2017). Myo5b interacts with Rab8 and Rab11, which are both required for transport of cargos from the trans-Golgi network to the plasma membrane (Chen et al., 1998; Roland et al., 2007). Rab8a-deficient mice exhibit a marked decrease in apical proteins in intestinal cells, a phenotype that leads to nutrient malabsorption and death (Sato et al., 2007). These mice also display subapical microvillus inclusion bodies produced by endocytosis from the apical membrane. Myo5b–Rab11 interaction is required for delivery of cargo to plasma membranes in order to establish an apical membrane domain (Wakabayashi et al., 2005). Mice that are deficient in both Rab8a and Rab11a show a severe enteropathy and extensive formation of basolateral microvilli (Feng et al., 2017). Syntaxin 3 is also known to localize apically in epithelial cells where it facilitates fusion of vesicles to the plasma membrane (Delgrossi et al., 1997; Sharma et al., 2006). In summary, appropriate apical morphology and physiology requires an intimate relationship between cargo trafficking, recycling and local assembly of delivered cargo into a functional membrane domain. Defects in any of these systems result in disease.
Tip-links in the structure and function of stereocilia and microvilli
Analyses of the molecular basis of deafness in patients with hereditary Usher Syndrome 1 have been instrumental in defining a protein complex that links the tip of one stereocilium to the side of the adjacent one (Fig. 1A) (Pickles et al., 1984). Collectively, this work has shown that the MyTH4-FERM tail domain of the motor protein Myo7a is essential to form a stable ternary complex with the PDZ-domain-containing scaffolding proteins USH1C (harmonin) and scaffold protein containing ankyrin repeats and SAM domain (SANS, also called USH1G) to bind the cytoplasmic tail of the cadherin CDH23 (Caberlotto et al., 2011; Grati and Kachar, 2011; Li et al., 2017). In addition, the extracellular region of the cadherin dimer interacts with the extracellular region of the protocadherin PCDH15 dimer on a neighboring stereocilium (Siemens et al., 2004). There, the cytoplasmic region of PCDH15 interacts with the mechano-electrical transduction (MET) channels located on the tip of the adjacent stereocilium. When sound induces the stereocilia to bend, the tip-link transmits tension that allows for the influx of K+ and Ca2+ (Beurg et al., 2006, 2009). The identity of MET channels that are linked to PCDH15 has not been firmly established. However, sensory transduction signals require the transmembrane channel-like proteins (TMC) 1 and 2, which form heteromeric complexes at stereociliary tips and allow Ca2+ permeability (Kurima et al., 2015; Pan et al., 2013). In addition, studies in frogs have shown that Pcdh15a interacts with Tmc1 and Tmc2a, orthologs of mammalian TMC1 and TMC2 (Maeda et al., 2014).
Remarkably, a similar type of linking system has been discovered between microvilli on epithelial cells (Fig. 1B). Here, heterotypic extracellular linkages occur near the tips of microvilli between protocadherin PCDH24 (encoded by CDHR2) and mucin-like protocadherin MLPCDH (encoded by CDHR5) to form links of 50 nm width (Crawley et al., 2014a, 2016; Li et al., 2016; Yu et al., 2017). The cytoplasmic tail of PCDH24 binds USH1C (harmonin) and forms a ternary linkage with the SANS homolog ANKS4B to link to the MyTH4-FERM domains of Myo7b. Since USH1C is a common component of tip-linkages between stereocilia and microvilli, and is abundant in the intestine (Crawley et al., 2014a), this explains why a fraction of patients with mutations in the USH1C gene, besides suffering from hearing defects, are also affected by intestinal disease (Bitner-Glindzicz et al., 2000), including protracted diarrhea (Kobayashi et al., 1998, 1999; Powell et al., 1982). The membrane proteome of intestinal epithelial cells includes proteins that are involved in ion transport, nutrient uptake and bacterial sensing (van der Post and Hansson, 2014) and Ush1c-knockout mice display loss of microvilli in a fraction of intestinal epithelial cells (Crawley et al., 2014a), which explains the observed enteropathy. Loss of other components of the inter-microvillar linkage also results in irregular microvillar lengths and loss of the hexagonal packing that is typical of fully differentiated cells in culture or in the mouse intestine (Crawley et al., 2014a). Interestingly, the Drosophila ortholog of PCDH15, Cad99C, is found on the microvilli of follicle cells, and its loss results in shortened and disordered microvilli (D'Alterio et al., 2005). Additionally, the adhesion molecule Fasciclin 2 has been found to localize to the brush border of the renal tubules in Drosophila, and interference studies indicate that it is necessary for the regulation of microvilli length and organization (Halberg et al., 2016). Thus, these data suggest an emerging principle showing that correct length regulation and organizational packing of microvilli requires appropriate inter-microvillar links.
It is attractive to think that tip links might be a simple mechanism to automatically give rise to the beautiful hexagonal packing characteristic of microvilli on intestinal epithelial cells. As shown in both the vertebrate and fly models, tethering microvilli to each other could also be an effective physical mechanism for regulation of microvilli length on individual cells. Whereas the length of microvilli between adjacent cells is usually quite uniform, occasionally this can be variable, but remains strikingly uniform on individual cells, which is consistent with this model (Crawley et al., 2014a). However, how hair cells generate rows of stereocilia with the same length adjacent to rows with longer lengths is not yet fully understood. For a discussion of a potential shared evolutionary origin of microvilli and stereocilia, see Box 2.
Box 2. Evolutionary origins of microvilli and stereocilia.
The closely related structural and tip-link components of microvilli and stereocilia suggest they could share a common evolutionary origin. As the gut is an evolutionary older organ than the auditory system, it is intriguing to think of microvilli as the primordial actin-based structure and a precursor to stereocilia that appeared later in evolution. In support of this concept, stereocilia typically first emerge as small, short microvilli of uniform length, before growing into the classic pattern of rows with increasing heights (Tilney et al., 1992). The specialized tip links most likely arose through gene duplication followed by genetic divergence to encode tissue-specific complexes (Peña et al., 2016). The conservation of tip linkages in microvilli and stereocilia might be associated with membrane remodeling and mechanosensing in each respective organ. Fluid shear stress has been shown to trigger microvilli formation through Ca2+ influx through mechanosensitive activation of the Ca2+-selective transient receptor potential cation channel vanilloid 6 (TRPV6), which is expressed in intestinal epithelial cells (Bianco et al., 2006; Miura et al., 2015). Likewise, mechanical stimuli to deflect stereocilia result in opening of MET channels, giving rise to an electrical output (Ohmori, 1985). Finally, both microvilli and stereocilia have to maintain tension generated by the formation of actin-based protrusions on the apical cell membrane. The closely related ERM protein family that crosslink the actin core to the plasma membrane appear to contribute to this function in hair cell stereocilia and intestinal microvilli (Rouven Brückner et al., 2015).
Actin filament crosslinkers
The length of microvilli varies depending on cell type. In enterocytes, each microvillus measures ∼1–2 µm and contains a core of ∼30–40 unipolar actin filaments (Mooseker, 1985). Stereocilia can be considered an exaggerated form of microvilli with variable lengths based on hair cell type and localization in the cochlea or vestibular system, as outlined above (Tilney and Saunders, 1983; Tilney et al., 1988). The length of microvilli and stereocilia is, at least in part, regulated by the growth rate of the core actin bundle, where longer actin protrusions have faster rates of actin polymerization (Narayanan et al., 2015; Rzadzinska et al., 2004).
The actin-crosslinking proteins espin, fimbrin and villin each play a part in regulation of microvillar length (Fig. 1B) (Grimm-Günter et al., 2009; Loomis et al., 2003; Ubelmann et al., 2013). Mice carrying mutations in the Espn gene, so-called jerker mice (Grüneberg et al., 1941; Zheng et al., 2000), which exhibit characteristic head-jerking movements and rapid circling, show gradual shortening and thinning of stereocilia followed by hair cell degeneration and vestibular dysfunction (Anniko et al., 1989; Sekerková et al., 2011). The role of espin in length regulation has also been highlighted in epithelial cells in which espin overexpression results in lengthening of microvilli (Loomis et al., 2003). However, whether espin is directly involved in length regulation or whether shortened actin protrusions are an indirect effect of diminished crosslinking and smaller actin bundle diameter remains to be determined. Fimbrin acts as an F-actin bundler in stereocilia as well as in microvilli (Hanein et al., 1998; Tilney et al., 1989), whereas villin is a multifunctional protein that acts as an F-actin-severing and -capping protein, as well as an actin bundler and nucleator that regulates the distribution of actin filaments (Bretscher and Weber, 1980; Khurana and George, 2008).
Remarkably, mice that lack espin and villin display no particular microvillar phenotype, whereas fimbrin-knockout mice exhibit shorter microvilli that lack rootlets (Ferrary et al., 1999; Grimm-Günter et al., 2009; Zheng et al., 2000). Nevertheless, triple knockout mice lacking espin, fimbrin and villin are not only viable, but still develop microvilli. However, microvilli are sparse, disorganized and shorter in these animals (Revenu et al., 2012). Consequently, it has been proposed that one or more additional bundling proteins participate in regulating actin dynamics and microvillus length; however, none have so far been identified (Revenu et al., 2012). A protein that has recently been implicated in regulation of microvillar length is cordon bleu, a WH2 domain-containing protein localized at the base of microvilli (Wayt and Bretscher, 2014). Overexpression of cordon bleu results in shorter microvilli in human syncytiotrophoblasts in a WH2 domain-dependent manner (Wayt and Bretscher, 2014), thus suggesting a role for the reported F-actin-severing features of these WH2 domains (Jiao et al., 2014). Other studies have shown that cordon bleu usually localizes to the base of microvilli, and that overexpression of cordon bleu in intestinal epithelial cells generates more and longer microvillar actin bundles in a WH2-dependent manner (Grega-Larson et al., 2016). It is likely that the inconsistent data on the function of cordon bleu reflects cell-specific effects. In fact, cordon bleu interacts with PACSIN-2 (also known as Syndapin-2) in both syncytiotrophoblasts and intestinal epithelial cells (Grega-Larson et al., 2016; Wayt and Bretscher, 2014), but whereas this interaction is required for targeting PACSIN-2 to microvilli in syncytiotrophoblasts, in intestinal epithelial cells, PACSIN-2 acts upstream of cordon bleu for its localization at the base of microvilli.
Less is known about the actin-crosslinking proteins of stereocilia. However, stereocilia lack villin, but utilize at least fimbrin, espin and fascin as F-actin crosslinkers (Fig. 1A). Fimbrin was the first crosslinking protein described (Flock et al., 1982; Tilney et al., 1989) and recent work indicates it is the most abundant one (Krey et al., 2016). Espin was initially identified by analysis of the deaf jerker mouse (Zheng et al., 2000), and fascin was the most recently described (Chou et al., 2011). A recent study points at the important role of barbed-end-capping proteins in stabilizing actin filaments by preventing depolymerization. Here, the heterodimeric capping protein CAPZ is recruited to growing actin filaments in order to prevent depolymerization, especially when the concentration of free actin monomers is low (Avenarius et al., 2017). Deletion of CAPZ in mouse utricles results in both shorter and narrower stereocilia, and diminished auditory and vestibular function.
In conclusion, microvilli and stereocilia are dynamic structures that require a diverse set of actin-regulating proteins to provide accurate control over actin filament assembly, disassembly and stability. In the next section, we discuss another aspect of stereociliary and microvillar dynamics, namely the physical coupling of the actin bundle of microvilli and stereocilia by phosphoregulated crosslinkers.
Linking the actin core to the plasma membrane
The ERM proteins belong to the 4.1 superfamily of proteins that share a 4.1 protein, ezrin, radixin and moesin (FERM) domain as a common feature. The ERM proteins, each encoded by a single gene in mammals, act as tissue-specific crosslinkers between the plasma membrane and the underlying cortical F-actin cytoskeleton in many different cellular contexts (Fehon et al., 2010). Most epithelial cells only express ezrin; stereocilia on hair cells of the cochlea contain some ezrin, but predominantly express radixin (Kikuchi et al., 2002; Kitajiri et al., 2004). Consequently, ezrin-knockout mice die soon after birth with short microvilli and disorganized rootlets (Saotome et al., 2004) and the intestinal defects are even more severe upon tissue-specific depletion of ezrin in adult animals (Casaletto et al., 2011). The mouse radixin knockout is viable, but stereocilia degenerate after formation, resulting in deafness (Kitajiri et al., 2004). Thus, ERM proteins have central roles in the structure and function of microvilli and stereocilia.
ERM proteins consist of an N-terminal FERM domain, a central α-helical domain and a C-terminal domain (CTD) in which the F-actin-binding site lies (Turunen et al., 1994). In their inactive (closed) form, the FERM domain binds with high affinity to the CTD with the α-helical region forming an anti-parallel coiled coil (Gary and Bretscher, 1995; Li et al., 2007). In this closed state, both the sites on the FERM domain for binding membrane proteins and the F-actin-binding site in the CTD are masked (Li et al., 2007). Phosphorylation of a conserved threonine residue (T567 in ezrin) in the CTD lowers the affinity between the FERM domain and CTD, resulting in ezrin adopting a more open configuration (Nakamura et al., 1995). Open ezrin can now act as a crosslinker by engaging plasma membrane proteins through its N-terminal FERM domain, whereas the CTD binds F-actin in the bundled filaments (Gary and Bretscher, 1995) (Fig. 2B). Oscillation of ezrin between the open and closed states is regulated by rapid cycles of phosphorylation and dephosphorylation (phosphocycling), exhibiting a turnover time of ∼1 min in cultured cells (Viswanatha et al., 2012). If the cycle of ezrin phosphorylation and dephosphorylation is decoupled – either by inhibiting ezrin dephosphorylation or by expressing a constitutively active phosphomimetic ezrin mutant (T567E) – the polarized distribution of ezrin in the apical membrane is lost (Viswanatha et al., 2012). Consequently, restricting ezrin function to the apical domain requires local phosphorylation at the apical membrane, followed by dephosphorylation in the cytoplasm (Fig. 2A). Remarkably, the basal rate of ezrin phosphocycling correlates with the turnover rate of fluorescently tagged ezrin in the apical membrane (∼1–2 min) and is significantly faster than the turnover of the microvillus itself (∼12 min) (Garbett and Bretscher, 2012; Gorelik et al., 2003). The fast cycling of ezrin activation reflects its role as a crosslinker between the continuously treadmilling actin filaments and the numerous membrane proteins in the overlying plasma membrane.
Fig. 2.
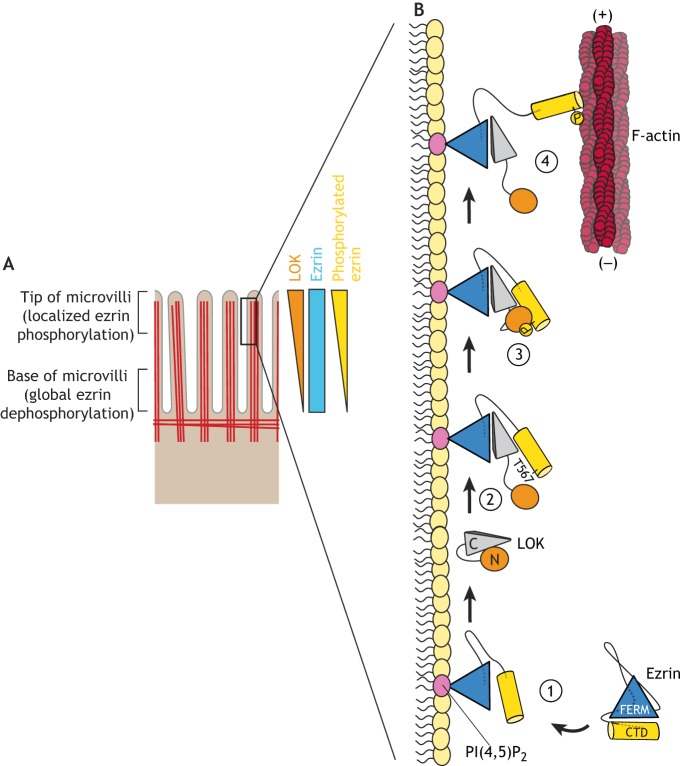
Model for spatial control of ezrin phosphocycling required for microvilli formation. (A) Ezrin undergoes continuous cycles of local phosphorylation in the tip region of microvilli where LOK resides; this is followed by global dephosphorylation, most likely at the base of microvilli where the LOK concentration is lower, before ezrin cycles back to its active, phosphorylated form. (B) Model of the coincidence detection mechanism behind LOK-mediated phosphorylation of ezrin. (1) Inactive cytoplasmic ezrin is recruited to the first apical identity code PI(4,5)P2. Priming by PI(4,5)P2 results in lower affinity between the FERM domain and the CTD. (2) The CTD of the second apical identity code, LOK, binds in a a wedge-like manner between the FERM domain and the CTD of ezrin. (3) LOK kinase domain can now phosphorylate ezrin on T567. (4) Phosphorylated active ezrin adopts an open conformation that allows crosslinking of the plasma membrane to the underlying actin cytoskeleton. Adapted from Pelaseyed et al. (2017), where it was published under a CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
A coincidence detection mechanism restricts active ezrin apically
Two closely related kinases, lymphocyte-oriented kinase (LOK, also known as STK10) and Ste20-like kinase (SLK), have been identified as the major ezrin kinases in epithelial cells (Viswanatha et al., 2012). Phosphorylation of ERM proteins by LOK and SLK is functionally conserved: in Drosophila, Slik is the only kinase that phosphorylates the single ERM protein present in flies, Moesin, on the regulatory threonine residue that corresponds to residue T567 in human ezrin (Carreno et al., 2008; Hipfner et al., 2004; Kunda et al., 2008). LOK-mediated phosphorylation of ezrin is an intricate multistep process that culminates in T567 phosphorylation (Pelaseyed et al., 2017). The FERM domain of ezrin contains a binding pocket for phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] (Niggli et al., 1995) located adjacent to the coiled-coil α-helix connecting the FERM domain to the CTD. It is likely that binding of PI(4,5)P2 to this particular binding site triggers a conformational change that lowers the affinity between the FERM domain and CTD. The lower-affinity FERM–CTD complex allows for the CTD of LOK to wedge in between the FERM and CTD, thus prying ezrin open and allowing LOK kinase domain to gain access to the phosphorylatable T567 residue (Fig. 2B). Several important features of this mechanism are highlighted below: the transition of the FERM–CTD complex from the high-affinity closed conformation to a lower-affinity conformation requires that the FERM and CTD domains are connected through the coiled-coil α-helix. In fact, a FERM–CTD complex that lacks this coiled-coil region cannot be phosphorylated by LOK, even in the presence of PI(4,5)P2 (Pelaseyed et al., 2017). Thus, binding of PI(4,5)P2 to full-length ezrin – a process we call ‘priming’ – does not simply change the conformation of the FERM domain, but requires active involvement of the α-helical region to reduce the affinity between the FERM and CTD domains (Pelaseyed et al., 2017). In this sense, the central helical region can function as a spring to reduce the affinity between the domains. Ezrin is explicitly primed by PI(4,5)P2 and not by inositol trisphosphate (IP3), phosphatidylinositol 3-phosphate [PI(3)P], phosphatidylinositol 4-phosphate [PI(4)P] or phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3] (Pelaseyed et al., 2017). The specificity of PI(4,5)P2 for ezrin is in agreement with reports showing that PI(4,5)P2 constitutes ∼1% of the phospholipids in the apical membrane, where it recruits proteins at the interface between the plasma membrane and the cytoplasm. By contrast, PI(3,4,5)P3 is found in the inner membrane leaflet on the basolateral surface (Blazer-Yost et al., 2004; van den Bogaart et al., 2011). As recruited proteins engage, through electrostatic interactions, with negatively charged phosphates in the inositol ring of phosphoinositides (Balla, 2005), our findings suggest that the specific location of negative charges in PI(4,5)P2 dictates the specificity for the ezrin FERM domain. LOK is the only known kinase to be specifically localized to the apical membrane of epithelial cells, and this is mediated through its CTD (Viswanatha et al., 2012). Since LOK and PI(4,5)P2 are both enriched on the apical membrane, and both are required for ezrin phosphorylation, this provides a powerful coincidence detection mechanism to ensure that ezrin activation only occurs on the apical domain (Pelaseyed et al., 2017).
Importantly, two additional features ensure LOK kinase specificity for ezrin. First, the LOK kinase domain requires a distal docking site, situated N-terminal to T567, in order to efficiently phosphorylate T567 (Pelaseyed et al., 2017). Second, LOK and SLK are highly specific kinases for ERM proteins, because they exhibit a strong preference for substrates with a tyrosine at position −2 from T567, a residue that is conserved in all ERM proteins (Belkina et al., 2009). Replacing the tyrosine residue with a positively charged lysine residue greatly diminishes the ability of LOK to phosphorylate ezrin (T.P., unpublished data). LOK and its relative SLK belong to the germinal center-like kinase (GCK)-V subfamily of kinases (Dan et al., 2001; Delpire, 2009). They consist of a conserved N-terminal kinase domain, a less-conserved intermediate region and a moderately conserved CTD (Sabourin et al., 2000). The LOK CTD inhibits the kinase activity of LOK, most likely through a cis interaction (Pelaseyed et al., 2017). In fact, even small amounts of the LOK CTD prevent phosphorylation of ezrin in vitro as well as in cells, where it inhibits microvilli assembly (Viswanatha et al., 2012). Remarkably, the CTD of LOK itself localizes to the distal region of microvilli (Viswanatha et al., 2012), suggesting that the CTD targets LOK to microvilli through an unidentified mechanism. Interestingly, cells expressing delocalized LOK lacking the CTD still phosphorylate ezrin, but ezrin is mislocalized to basolateral membranes. Furthermore, intentional delocalization of LOK to basolateral subdomains caused mislocalized ezrin enrichment at these basolateral sites (Viswanatha et al., 2012).
In summary, a coincidence detection mechanism controls the specificity of ezrin phosphorylation by a combination of two different identity codes – PI(4,5)P2 and LOK – both of which are spatially restricted to apical membranes (Pelaseyed et al., 2017). This high specificity, coupled with dynamic ezrin phosphocycling involving local phosphorylation and delocalized dephosphorylation (Viswanatha et al., 2012) (Fig. 2A,B), reveals that morphological polarity is not a static, but rather a dynamic and spatially controlled process.
Regulation of membrane protein composition
The microvilli of epithelial cells increase the interface between the cell and the extracellular environment ∼10-fold, whereas the protein content of the microvillar membrane controls the physiological processes within the cell. Microvillar proteins include the intermicrovillar adhesion molecules discussed previously, as well as specialized receptors that elicit intracellular signaling upon ligand recognition, channels, exchangers and transporter of ions and nutrients, and enzymes such as hydrolases that process digested nutrients (McConnell et al., 2011; van der Post and Hansson, 2014). Large, extended glycoproteins, such as transmembrane mucins, constitute a glycocalyx that protects the cell against extracellular insults (Pelaseyed et al., 2013).
The protein composition of the microvillar membrane is primarily determined by sorting and delivery pathways during membrane trafficking (Apodaca et al., 2012; Garcia-Castillo et al., 2017). It is believed that the unmasked FERM domain of ezrin engages the cytoplasmic tails of a number of membrane proteins and this might occur directly or through additional scaffolding proteins (Viswanatha et al., 2013). Ezrin oscillates between open/active and closed/inactive conformations (Jayaraman and Nicholson, 2007). Consequently, the interaction between ezrin and specific partners that bind the FERM domain and CTD, respectively, depends on the ‘openness’ of ezrin, a feature that is controlled in part by the phosphorylation status of T567 in the CTD (Nakamura et al., 1999; Pearson et al., 2000). Studies on ezrin indicate that phosphorylation of T567 in CTD creates a partially open ezrin (Chambers and Bretscher, 2005). A fully open ezrin with an unmasked FERM domain requires a synthetic truncation in the CTD that completely abolishes the interaction between ezrin FERM and CTD (Reczek and Bretscher, 1998). A global analysis of ezrin-binding partners has identified several membrane and cytosolic proteins (Viswanatha et al., 2013) that can be classified into three groups: (1) proteins that preferentially associate with phosphorylated and/or active ezrin [e.g. LOK, SLK and dystroglycan (DAG1)], (2) proteins that associate most strongly with a synthetic hyperactive ezrin variant with a fully unmasked FERM domain [e.g. ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50)] and, (3) proteins that selectively interact with non-phosphorylated and/or inactive ezrin [e.g. the F-actin-nucleating formin FHOD-1, the Ras GTPase-activating protein RASA1, and protein-associating with the carboxyl-terminal domain of ezrin (SCYL3, also known as PACE-1) (Viswanatha et al., 2013)]. The comprehensive interactome of ezrin supports a ‘conformation activation model’ in which some, mostly cytoplasmic, proteins associate with inactive ezrin, and some microvillar proteins associate with partially active ezrin and others with fully open ezrin.
Fully active ezrin binds the PDZ domain-containing scaffolding proteins of the NHE3-regulatory factor (NHERF) family, which can interact with a host of other proteins. The family consists of four members: EBP50 (also known as NHERF1 and SLC9A3R1), NHE3 kinase A regulatory protein (E3KARP, also known as NHERF2 and SLC9A3R2), PDZ domain-containing protein 1 (PDZK1, also known as NHERF3) and intestinal and kidney-enriched PDZ protein (IKEPP, also known as NHERF4 and PDZD3). EBP50 and the paralog E3KARP contain two PDZ domains, whereas PDZK1 and IKEPP have four PDZ domains in tandem. EBP50 and E3KARP both have an ERM-binding domain in their C-terminal tails (Reczek and Bretscher, 1998; Yun et al., 1998), whereas PDZK1 and IKEPP do not. The four PDZ proteins also exhibit differences in terms of tissue distribution and subcellular localization. EBP50 and PDZK1 are expressed apically in polarized cells of the kidney, small intestine and the liver, whereas E3KARP resides apically in lungs and kidney (Donowitz et al., 2005; Ingraffea et al., 2002; Yun et al., 1998). By contrast IKEPP is a primarily cytoplasmic protein found in the intestine and kidneys (Scott et al., 2002).
EBP50 associates with the FERM domain of ezrin at a site that is fully masked by the CTD in the closed inactive conformation (Reczek and Bretscher, 1998; Reczek et al., 1997). EBP50 colocalizes with ezrin in microvilli, but is much more dynamic than ezrin, exhibiting a remarkable in vivo exchange rate of ∼10 s, compared with ∼1 min for ezrin (Garbett and Bretscher, 2012). Knockdown of EBP50 in human syncytiotrophoblasts reduces, but does not fully eliminate, the number of cells with microvilli (Garbett et al., 2010; Hanono et al., 2006). The PDZ1 domain and ezrin-binding site of EBP50 are crucial elements for restoration of microvilli in EBP50-deficient cells. Surprisingly, mutations that inactivate the PDZ1 and PDZ2 of EBP50, thus precluding ligand binding, greatly diminish its in vivo exchange rate, suggesting that proteins interacting with the PDZ domains in EBP50 influence its in vivo dynamics (Garbett and Bretscher, 2012). EBP50 undergoes phosphorylation by Cdc2 (He et al., 2001) and PKC (Fouassier et al., 2005; Li et al., 2009; Raghuram et al., 2003). In phosphorylated EBP50, ligand binding to PDZ2 renders PDZ1 inaccessible to its ligand(s), resulting in inactive EBP50. Once PDZ2 is unoccupied, phosphorylated EBP50 can function in microvilli assembly (Garbett et al., 2010). EBP50 is a scaffolding protein for numerous membrane proteins, such as cystic fibrosis transmembrane conductance regulator (CFTR) and β2-adrenergic receptor (β2AR) (Cao et al., 1999; Hall et al., 1998a,b; Viswanatha et al., 2014), which in turn are under tight regulation (Guggino and Stanton, 2006; Yang et al., 2015). Therefore, it is plausible that a highly dynamic EBP50 reflects the need for a tunable protein scaffold that can be controlled through phosphorylation as well as PDZ interactions in order to meet the demands from rapidly changing physiological cues. As noted above, EBP50 contributes in an unknown manner to the regulation of microvilli assembly, as cultured cells with reduced EBP50 expression have greatly reduced apical microvilli number (Garbett et al., 2010).
In summary, phosphorylation-dependent regulation of the interactions between ERM proteins and their binding partners plays a central role in instructing the dynamic morphological and functional changes that take place in the apical aspects of polarized epithelial cells.
Conclusions and perspectives
Ever since microvilli were first seen on intestinal epithelial cells by electron microscopy more than 60 years ago (Granger and Baker, 1950), their structure and function have fascinated cell biologists. In parallel, over the past 40 years, our understanding of the program that specifies epithelial cell polarity has advanced immensely; however, how it restricts microvilli, or stereocilia, to the apical membrane has not been fully elucidated. Furthermore, we do not know how epithelial cells regulate the precise number of microvilli or sterocilia on their surface – in one case with structures of uniform length, and in the other with precisely defined different lengths. Whereas the role of protocadherins in distal tip complexes of stereocilia and microvilli has been established, it remains to be proven whether other apical membrane proteins, such as the extended transmembrane mucins of the glycocalyx, play important roles in membrane bending and curvature when actin protrusions push through the apical plasma membrane to form tight bundles of stereocilia and microvilli (Stachowiak et al., 2012).
The proposed mechanism of ezrin phosphorylation, which involves two apical identity codes – PI(4,5)P2 and the ERM-specific kinases LOK and SLK – represents one aspect of the requirements for localized microvilli assembly at apical membranes. However, we still have little knowledge about how microvillar assembly is initiated at this specific membrane domain. Furthermore, how do LOK and SLK localize to microvilli and how does the CTD of LOK contribute to its localization? How does the presence of microvilli impact on the steady-state apical membrane proteome, and what role does the dynamics of ezrin and scaffolding proteins play in retention or regulation of membrane proteins? Addressing both general questions regarding cell polarity as well as highly specific questions regarding microvilli and stereocilia will be greatly facilitated by combining recent advances in organoid engineering with modern techniques, such as quantitative proteomics, genome editing with CRISPR/Cas9 and high-resolution in vivo imaging.
Acknowledgements
We are grateful to Dr Andrew Lombardo for valuable comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our research is support by the Wenner-Gren Foundation (T.P.), Swedish Cystic Fibrosis Foundation (Riksförbundet Cystisk Fibros; T.P.), Birgit and Hellmuth Hertz Foundation (T.P.) and National Institutes of Health (A.B., NIHGM036552). Deposited in PMC for release after 12 months.
References
- Anniko M., Sjöström B. and Webster D. (1989). The effects of auditory deprivation on morphological maturation of the ventral cochlear nucleus. Arch. Otorhinolaryngol. 246, 43-47. 10.1007/BF00454133 [DOI] [PubMed] [Google Scholar]
- Apodaca G., Gallo L. I. and Bryant D. M. (2012). Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 14, 1235-1243. 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius M. R., Krey J. F., Dumont R. A., Morgan C. P., Benson C. B., Vijayakumar S., Cunningham C. L., Scheffer D. I., Corey D. P., Müller U. et al. (2017). Heterodimeric capping protein is required for stereocilia length and width regulation. J. Cell Biol. 216, 3861-3881. 10.1083/jcb.201704171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Schneider M., Theilenberg E., Grawe F. and Knust E. (2001). Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414, 638-643. 10.1038/414638a [DOI] [PubMed] [Google Scholar]
- Balla T. (2005). Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 118, 2093-2104. 10.1242/jcs.02387 [DOI] [PubMed] [Google Scholar]
- Belkina N. V., Liu Y., Hao J.-J., Karasuyama H. and Shaw S. (2009). LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc. Natl. Acad. Sci. USA 106, 4707-4712. 10.1073/pnas.0805963106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R. and St Johnston D. (2003). Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691-704. 10.1016/S0092-8674(03)00938-3 [DOI] [PubMed] [Google Scholar]
- Beurg M., Evans M. G., Hackney C. M. and Fettiplace R. (2006). A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J. Neurosci. 26, 10992-11000. 10.1523/JNEUROSCI.2188-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M., Fettiplace R., Nam J.-H. and Ricci A. J. (2009). Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci. 12, 553-558. 10.1038/nn.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco S. D. C., Peng J.-B., Takanaga H., Suzuki Y., Crescenzi A., Kos C. H., Zhuang L., Freeman M. R., Gouveia C. H. A., Wu J. et al. (2006). Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 22, 274-285. 10.1359/jbmr.061110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner-Glindzicz M., Lindley K. J., Rutland P., Blaydon D., Smith V. V., Milla P. J., Hussain K., Furth-Lavi J., Cosgrove K. E., Shepherd R. M. et al. (2000). A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat. Genet. 26, 56-60. 10.1038/79178 [DOI] [PubMed] [Google Scholar]
- Blazer-Yost B. L., Vahle J. C., Byars J. M. and Bacallao R. L. (2004). Real-time three-dimensional imaging of lipid signal transduction: apical membrane insertion of epithelial Na(+) channels. Am. J. Physiol. Cell Physiol. 287, C1569-C1576. 10.1152/ajpcell.00226.2004 [DOI] [PubMed] [Google Scholar]
- Bretscher A. and Weber K. (1980). Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20, 839-847. 10.1016/0092-8674(80)90330-X [DOI] [PubMed] [Google Scholar]
- Brunser O. and Luft J. H. (1970). Fine structure of the apex of absorptive cells from rat small intestine. J. Ultrastruct. Res. 31, 291-311. 10.1016/S0022-5320(70)90133-4 [DOI] [PubMed] [Google Scholar]
- Bryant D. M. and Mostov K. E. (2008). From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887-901. 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodríguez-Fraticelli A. E., Peränen J., Martín-Belmonte F. and Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035-1045. 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E., Michel V., Foucher I., Bahloul A., Goodyear R. J., Pepermans E., Michalski N., Perfettini I., Alegria-Prévot O., Chardenoux S. et al. (2011). Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc. Natl. Acad. Sci. USA 108, 5825-5830. 10.1073/pnas.1017114108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanale J. P., Sun T. Y. and Montell D. J. (2017). Development and dynamics of cell polarity at a glance. J. Cell Sci. 130, 1201-1207. 10.1242/jcs.188599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T. T., Deacon H. W., Reczek D., Bretscher A. and von Zastrow M. (1999). A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401, 286-290. 10.1038/45816 [DOI] [PubMed] [Google Scholar]
- Carreno S., Kouranti I., Glusman E. S., Fuller M. T., Echard A. and Payre F. (2008). Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J. Cell Biol. 180, 739-746. 10.1083/jcb.200709161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto J. B., Saotome I., Curto M. and McClatchey A. I. (2011). Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc. Natl. Acad. Sci. USA 108, 11924-11929. 10.1073/pnas.1103418108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D. N. and Bretscher A. (2005). Ezrin mutants affecting dimerization and activation. Biochemistry 44, 3926-3932. 10.1021/bi0480382 [DOI] [PubMed] [Google Scholar]
- Chen W., Feng Y., Chen D. and Wandinger-Ness A. (1998). Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell 9, 3241-3257. 10.1091/mbc.9.11.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S.-W., Hwang P., Gomez G., Fernando C. A., West M. C., Pollock L. M., Lin-Jones J., Burnside B. and McDermott B. M. (2011). Fascin 2b is a component of stereocilia that lengthens actin-based protrusions. PLoS ONE 6, e14807 10.1371/journal.pone.0014807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S. W., Shifrin D. A., Grega-Larson N. E., McConnell R. E., Benesh A. E., Mao S., Zheng Y., Zheng Q. Y., Nam K. T., Millis B. A. et al. (2014a). Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157, 433-446. 10.1016/j.cell.2014.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S. W., Mooseker M. S. and Tyska M. J. (2014b). Shaping the intestinal brush border. J. Cell Biol. 207, 441-451. 10.1083/jcb.201407015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S. W., Weck M. L., Grega-Larson N. E., Shifrin D. A. and Tyska M. J. (2016). ANKS4B Is Essential for Intermicrovillar Adhesion Complex Formation. Dev. Cell 36, 190-200. 10.1016/j.devcel.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutz E., Rhoads J. M., Drumm B., Sherman P. M., Durie P. R. and Forstner G. G. (1989). Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N. Engl. J. Med. 320, 646-651. 10.1056/NEJM198903093201006 [DOI] [PubMed] [Google Scholar]
- Dallos P. (1996). Overview: Cochlear Neurobiology. pp. 1-43. New York, NY: Springer. [Google Scholar]
- D'Alterio C., Tran D. D. D., Yeung M. W. Y. A., Hwang M. S. H., Li M. A., Arana C. J., Mulligan V. K., Kubesh M., Sharma P., Chase M. et al. (2005). Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J. Cell Biol. 171, 549-558. 10.1083/jcb.200507072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan I., Watanabe N. M. and Kusumi A. (2001). The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220-230. 10.1016/S0962-8924(01)01980-8 [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Cutz E., Hamilton J. R. and Gall D. G. (1978). Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75, 783-790. [PubMed] [Google Scholar]
- Delgrossi M. H., Breuza L. and Mirre C. (1997). Human syntaxin 3 is localized apically in human intestinal cells. J. Cell Sci. 110, 2207-2214. [DOI] [PubMed] [Google Scholar]
- Delpire E. (2009). The mammalian family of sterile 20p-like protein kinases. Pflugers Arch. 458, 953-967. 10.1007/s00424-009-0674-y [DOI] [PubMed] [Google Scholar]
- DeRosier D. J. and Tilney L. G. (2000). F-actin bundles are derivatives of microvilli: what does this tell us about how bundles might form? J. Cell Biol. 148, 1-6. 10.1083/jcb.148.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cha B., Zachos N. C., Brett C. L., Sharma A., Tse C. M. and Li X. (2005). NHERF family and NHE3 regulation. J. Physiol. 567, 3-11. 10.1113/jphysiol.2005.090399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon R. G., McClatchey A. I. and Bretscher A. (2010). Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276-287. 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Bonder E. M., Engevik A. C., Zhang L., Tyska M. J., Goldenring J. R. and Gao N. (2017). Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy. J. Cell Sci. 130, 2491-2505. 10.1242/jcs.201897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrary E., Cohen-Tannoudji M., Pehau-Arnaudet G., Lapillonne A., Athman R., Ruiz T., Boulouha L., El Marjou F., Doye A., Fontaine J.-J. et al. (1999). In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 146, 819-830. 10.1083/jcb.146.4.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock Å., Bretscher A. and Weber K. (1982). Immunohistochemical localization of several cytoskeletal proteins in inner ear sensory and supporting cells. Hear. Res. 7, 75-89. 10.1016/0378-5955(82)90082-X [DOI] [PubMed] [Google Scholar]
- Fouassier L., Nichols M. T., Gidey E., McWilliams R. R., Robin H., Finnigan C., Howell K. E., Housset C. and Doctor R. B. (2005). Protein kinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp. Cell Res. 306, 264-273. 10.1016/j.yexcr.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Garbett D. and Bretscher A. (2012). PDZ interactions regulate rapid turnover of the scaffolding protein EBP50 in microvilli. J. Cell Biol. 198, 195-203. 10.1083/jcb.201204008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett D., LaLonde D. P. and Bretscher A. (2010). The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J. Cell Biol. 191, 397-413. 10.1083/jcb.201004115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castillo M. D., Chinnapen D. J.-F. and Lencer W. I. (2017). Membrane transport across polarized epithelia. Cold Spring Harb. Perspect. Biol. 9, a027912 10.1101/cshperspect.a027912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R. and Bretscher A. (1995). Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 6, 1061-1075. 10.1091/mbc.6.8.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. V. and Strutt D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik J., Shevchuk A. I., Frolenkov G. I., Diakonov I. A., Lab M. J., Kros C. J., Richardson G. P., Vodyanoy I., Edwards C. R. W., Klenerman D. et al. (2003). Dynamic assembly of surface structures in living cells. Proc. Natl. Acad. Sci. USA 100, 5819-5822. 10.1073/pnas.1030502100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. and Baker R. F. (1950). Electron microscope investigation of the striated border of intestinal epithelium. Anat. Rec. 103 Suppl., 459 10.1002/ar.1091070409 [DOI] [PubMed] [Google Scholar]
- Grati M. and Kachar B. (2011). Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc. Natl. Acad. Sci. USA 108, 11476-11481. 10.1073/pnas.1104161108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega-Larson N. E., Crawley S. W. and Tyska M. J. (2016). Impact of cordon-bleu expression on actin cytoskeleton architecture and dynamics. Cytoskeleton 73, Spc1 10.1002/cm.21343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm-Günter E.-M. S., Revenu C., Ramos S., Hurbain I., Smyth N., Ferrary E., Louvard D., Robine S. and Rivero F. (2009). Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol. Biol. Cell 20, 2549-2562. 10.1091/mbc.e08-10-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüneberg H., Burnett J. B. and Snell G. D. (1941). The origin of Jerker, a new gene mutation of the house mouse, and linkage studies made with it. Proc. Natl. Acad. Sci. USA 27, 562-565. 10.1073/pnas.27.12.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino W. B. and Stanton B. A. (2006). New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat. Rev. Mol. Cell Biol. 7, 426-436. 10.1038/nrm1949 [DOI] [PubMed] [Google Scholar]
- Halberg K. A., Rainey S. M., Veland I. R., Neuert H., Dornan A. J., Klämbt C., Davies S.-A. and Dow J. A. T. (2016). The cell adhesion molecule Fasciclin2 regulates brush border length and organization in Drosophila renal tubules. Nat. Commun. 7, 11266 10.1038/ncomms11266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. A., Premont R. T., Chow C.-W., Blitzer J. T., Pitcher J. A., Claing A., Stoffel R. H., Barak L. S., Shenolikar S., Weinman E. J. et al. (1998a). The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392, 626-630. 10.1038/33458 [DOI] [PubMed] [Google Scholar]
- Hall R. A., Ostedgaard L. S., Premont R. T., Blitzer J. T., Rahman N., Welsh M. J. and Lefkowitz R. J. (1998b). A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc. Natl. Acad. Sci. USA 95, 8496-8501. 10.1073/pnas.95.15.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein D., Volkmann N., Goldsmith S., Michon A.-M., Lehman W., Craig R., DeRosier D., Almo S. and Matsudaira P. (1998). An atomic model of fimbrin binding to F-actin and its implications for filament crosslinking and regulation. Nat. Struct. Biol. 5, 787-792. 10.1038/1828 [DOI] [PubMed] [Google Scholar]
- Hanono A., Garbett D., Reczek D., Chambers D. N. and Bretscher A. (2006). EPI64 regulates microvillar subdomains and structure. J. Cell Biol. 175, 803-813. 10.1083/jcb.200604046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Lau A. G., Yaffe M. B. and Hall R. A. (2001). Phosphorylation and cell cycle-dependent regulation of Na+/H+ exchanger regulatory factor-1 by Cdc2 kinase. J. Biol. Chem. 276, 41559-41565. 10.1074/jbc.M106859200 [DOI] [PubMed] [Google Scholar]
- Hipfner D. R., Keller N. and Cohen S. M. (2004). Slik Sterile-20 kinase regulates Moesin activity to promote epithelial integrity during tissue growth. Genes Dev. 18, 2243-2248. 10.1101/gad.303304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraffea J., Reczek D. and Bretscher A. (2002). Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur. J. Cell Biol. 81, 61-68. 10.1078/0171-9335-00218 [DOI] [PubMed] [Google Scholar]
- Jayaraman B. and Nicholson L. K. (2007). Thermodynamic dissection of the Ezrin FERM/CERMAD interface. Biochemistry 46, 12174-12189. 10.1021/bi701281e [DOI] [PubMed] [Google Scholar]
- Jiao Y., Walker M., Trinick J., Pernier J., Montaville P. and Carlier M.-F. (2014). Mutagenetic and electron microscopy analysis of actin filament severing by Cordon-Bleu, a WH2 domain protein. Cytoskeleton 71, 170-183. 10.1002/cm.21161 [DOI] [PubMed] [Google Scholar]
- Kaltenbach J. A., Falzarano P. R. and Simpson T. H. (1994). Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J. Comp. Neurol. 350, 187-198. 10.1002/cne.903500204 [DOI] [PubMed] [Google Scholar]
- Khurana S. and George S. P. (2008). Regulation of cell structure and function by actin-binding proteins: villin's perspective. FEBS Lett. 582, 2128-2139. 10.1016/j.febslet.2008.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S., Hata M., Fukumoto K., Yamane Y., Matsui T., Tamura A., Yonemura S., Yamagishi H., Keppler D. and Tsukita S. (2002). Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat. Genet. 17, 17 10.1038/ng905 [DOI] [PubMed] [Google Scholar]
- Kitajiri S., Fukumoto K., Hata M., Sasaki H., Katsuno T., Nakagawa T., Ito J., Tsukita S. and Tsukita S. (2004). Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J. Cell Biol. 166, 559-570. 10.1083/jcb.200402007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Imamura K., Yamada M., Okano M., Yara A., Ikema S. and Ishikawa N. (1998). A 75-kD autoantigen recognized by sera from patients with X-linked autoimmune enteropathy associated with nephropathy. Clin. Exp. Immunol. 111, 527-531. 10.1046/j.1365-2249.1998.00523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Imamura K., Kubota M., Ishikawa S., Yamada M., Tonoki H., Okano M., Storch W. B., Moriuchi T., Sakiyama Y. et al. (1999). Identification of an autoimmune enteropathy–related 75-kilodalton antigen. Gastroenterology 117, 823-830. 10.1016/S0016-5085(99)70340-9 [DOI] [PubMed] [Google Scholar]
- Krey J. F., Krystofiak E. S., Dumont R. A., Vijayakumar S., Choi D., Rivero F., Kachar B., Jones S. M. and Barr-Gillespie P. G. (2016). Plastin 1 widens stereocilia by transforming actin filament packing from hexagonal to liquid. J. Cell Biol. 215, 467-482. 10.1083/jcb.201606036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda P., Pelling A. E., Liu T. and Baum B. (2008). Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18, 91-101. 10.1016/j.cub.2007.12.051 [DOI] [PubMed] [Google Scholar]
- Kurima K., Ebrahim S., Pan B., Sedlacek M., Sengupta P., Millis B. A., Cui R., Nakanishi H., Fujikawa T., Kawashima Y. et al. (2015). TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep. 12, 1606-1617. 10.1016/j.celrep.2015.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. K. and Bridgman P. C. (1992). Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J. Cell Biol. 119, 1219-1243. 10.1083/jcb.119.5.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Nance M. R., Kulikauskas R., Nyberg K., Fehon R., Karplus P. A., Bretscher A. and Tesmer J. J. G. (2007). Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J. Mol. Biol. 365, 1446-1459. 10.1016/j.jmb.2006.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Xue J. and Peterson E. H. (2008). Architecture of the mouse utricle: macular organization and hair bundle heights. J. Neurophysiol. 99, 718-733. 10.1152/jn.00831.2007 [DOI] [PubMed] [Google Scholar]
- Li J., Callaway D. J. E. and Bu Z. (2009). Ezrin induces long-range interdomain allostery in the scaffolding protein NHERF1. J. Mol. Biol. 392, 166-180. 10.1016/j.jmb.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., He Y., Lu Q. and Zhang M. (2016). Mechanistic basis of organization of the Harmonin/USH1C-mediated brush border microvilli tip-link complex. Dev. Cell 36, 179-189. 10.1016/j.devcel.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Li J., He Y., Weck M. L., Lu Q., Tyska M. J. and Zhang M. (2017). Structure of Myo7b/USH1C complex suggests a general PDZ domain binding mode by MyTH4-FERM myosins. Proc. Natl. Acad. Sci. USA 114, E3776-E3785. 10.1073/pnas.1702251114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis P. A., Zheng L., Sekerkova G., Changyaleket B., Mugnaini E., Bartles J. R., Sekerková G., Changyaleket B., Mugnaini E. and Bartles J. R. (2003). Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163, 1045-1055. 10.1083/jcb.200309093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R., Kindt K. S., Mo W., Morgan C. P., Erickson T., Zhao H., Clemens-Grisham R., Barr-Gillespie P. G. and Nicolson T. (2014). Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc. Natl. Acad. Sci. USA 111, 12907-12912. 10.1073/pnas.1402152111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T. and Burgess D. R. (1979). Identification and organization of the components in the isolated microvillus cytoskeleton. J. Cell Biol. 83, 667-673. 10.1083/jcb.83.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. E., Benesh A. E., Mao S., Tabb D. L. and Tyska M. J. (2011). Proteomic analysis of the enterocyte brush border. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G914-G926. 10.1152/ajpgi.00005.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Sato K., Kato-Negishi M., Teshima T. and Takeuchi S. (2015). Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nat. Commun. 6, 8871 10.1038/ncomms9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S. (1985). Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol 1, 209-241. 10.1146/annurev.cb.01.110185.001233 [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. and Tilney L. G. (1975). Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 67, 725-743. 10.1083/jcb.67.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Hess M. W., Schiefermeier N., Pfaller K., Ebner H. L., Heinz-Erian P., Ponstingl H., Partsch J., Röllinghoff B., Köhler H. et al. (2008). MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163-1165. 10.1038/ng.225 [DOI] [PubMed] [Google Scholar]
- Nakamura F., Amieva M. R. and Furthmayr H. (1995). Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J. Biol. Chem. 270, 31377-31385. 10.1074/jbc.270.52.31377 [DOI] [PubMed] [Google Scholar]
- Nakamura F., Huang L., Pestonjamasp K., Luna E. J. and Furthmayr H. (1999). Regulation of F-actin binding to platelet moesin In vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol. Biol. Cell 10, 2669-2685. 10.1091/mbc.10.8.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P., Chatterton P., Ikeda A., Ikeda S., Corey D. P., Ervasti J. M. and Perrin B. J. (2015). Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat. Commun. 6, 6855 10.1038/ncomms7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V., Andréoli C., Roy C. and Mangeat P. (1995). Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 376, 172-176. 10.1016/0014-5793(95)01270-1 [DOI] [PubMed] [Google Scholar]
- Ohmori H. (1985). Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol. 359, 189-217. 10.1113/jphysiol.1985.sp015581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Géléoc G. S., Asai Y., Horwitz G. C., Kurima K., Ishikawa K., Kawashima Y., Griffith A. J. and Holt J. R. (2013). TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79, 504-515. 10.1016/j.neuron.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. A., Reczek D., Bretscher A. and Karplus P. A. (2000). Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259-270. 10.1016/S0092-8674(00)80836-3 [DOI] [PubMed] [Google Scholar]
- Pelaseyed T., Gustafsson J. K., Gustafsson I. J., Ermund A. and Hansson G. C. (2013). Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am. J. Physiol. Cell Physiol. 305, C457-C467. 10.1152/ajpcell.00141.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T., Viswanatha R., Sauvanet C., Filter J. J., Goldberg M. L., Bretscher A., Sabatini D., Chen I., Hahn W., Sharp P. et al. (2017). Ezrin activation by LOK phosphorylation involves a PIP2-dependent wedge mechanism. eLife 6, 35437-35451. 10.7554/eLife.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña J. F., Alié A., Richter D. J., Wang L., Funayama N. and Nichols S. A. (2016). Conserved expression of vertebrate microvillar gene homologs in choanocytes of freshwater sponges. Evodevo 7, 13 10.1186/s13227-016-0050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D. and Osborne M. P. (1984). Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103-112. 10.1016/0378-5955(84)90041-8 [DOI] [PubMed] [Google Scholar]
- Powell B. R., Buist N. R. M. and Stenzel P. (1982). An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J. Pediatr. 100, 731-737. 10.1016/S0022-3476(82)80573-8 [DOI] [PubMed] [Google Scholar]
- Raghuram V., Hormuth H. and Foskett J. K. (2003). A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc. Natl. Acad. Sci. USA 100, 9620-9625. 10.1073/pnas.1633250100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D. and Bretscher A. (1998). The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J. Biol. Chem. 273, 18452-18458. 10.1074/jbc.273.29.18452 [DOI] [PubMed] [Google Scholar]
- Reczek D., Berryman M. and Bretscher A. (1997). Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169-179. 10.1083/jcb.139.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C., Ubelmann F., Hurbain I., El-Marjou F., Dingli F., Loew D., Delacour D., Gilet J., Brot-Laroche E., Rivero F. et al. (2012). A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol. Biol. Cell 23, 324-336. 10.1091/mbc.e11-09-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. and Macara I. G. (2014). Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225-242. 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. T., Kenworthy A. K., Peranen J., Caplan S. and Goldenring J. R. (2007). Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol. Biol. Cell 18, 2828-2837. 10.1091/mbc.e07-02-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouven Brückner B., Pietuch A., Nehls S., Rother J. and Janshoff A. (2015). Ezrin is a major regulator of membrane tension in epithelial cells. Sci. Rep. 5, 14700 10.1038/srep14700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruemmele F. M., Schmitz J. and Goulet O. (2006). Microvillous inclusion disease (microvillous atrophy). Orphanet J. Rare Dis. 1, 22 10.1186/1750-1172-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska A. K., Schneider M. E., Davies C., Riordan G. P. and Kachar B. (2004). An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol. 164, 887-897. 10.1083/jcb.200310055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin L. A., Tamai K., Seale P., Wagner J. and Rudnicki M. A. (2000). Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol. Cell. Biol. 20, 684-696. 10.1128/MCB.20.2.684-696.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I., Curto M. and McClatchey A. I. (2004). Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6, 855-864. 10.1016/j.devcel.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A. et al. (2007). The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366-369. 10.1038/nature05929 [DOI] [PubMed] [Google Scholar]
- Scott R. O., Thelin W. R. and Milgram S. L. (2002). A novel PDZ protein regulates the activity of Guanylyl Cyclase C, the heat-stable enterotoxin receptor. J. Biol. Chem. 277, 22934-22941. 10.1074/jbc.M202434200 [DOI] [PubMed] [Google Scholar]
- Sekerková G., Richter C.-P. and Bartles J. R. (2011). Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet. 7, e1002032 10.1371/journal.pgen.1002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Low S. H., Misra S., Pallavi B. and Weimbs T. (2006). Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J. Cell Biol. 173, 937-948. 10.1083/jcb.200603132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Mitchell D. J. and Cutz E. (2004). Neonatal enteropathies: defining the causes of protracted diarrhea of infancy. J. Pediatr. Gastroenterol. Nutr. 38, 16-26. 10.1097/00005176-200401000-00007 [DOI] [PubMed] [Google Scholar]
- Shotwell S. L., Jacobs R. and Hudspeth A. J. (1981). Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann. N. Y. Acad. Sci. 374, 1-10. 10.1111/j.1749-6632.1981.tb30854.x [DOI] [PubMed] [Google Scholar]
- Siemens J., Lillo C., Dumont R. A., Reynolds A., Williams D. S., Gillespie P. G. and Müller U. (2004). Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950-955. 10.1038/nature02483 [DOI] [PubMed] [Google Scholar]
- Siletti K., Tarchini B. and Hudspeth A. J. (2017). Daple coordinates organ-wide and cell-intrinsic polarity to pattern inner-ear hair bundles. Proc. Natl. Acad. Sci. USA 114, E11170-E11179. 10.1073/pnas.1716522115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak J. C., Schmid E. M., Ryan C. J., Ann H. S., Sasaki D. Y., Sherman M. B., Geissler P. L., Fletcher D. A. and Hayden C. C. (2012). Membrane bending by protein–protein crowding. Nat. Cell Biol. 14, 944-949. 10.1038/ncb2561 [DOI] [PubMed] [Google Scholar]
- Tarchini B., Jolicoeur C. and Cayouette M. (2013). A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev. Cell 27, 88-102. 10.1016/j.devcel.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Tarchini B., Tadenev A. L. D., Devanney N. and Cayouette M. (2016). A link between planar polarity and staircase-like bundle architecture in hair cells. Development 143, 3926-3932. 10.1242/dev.139089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. (1996). Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177, 217-225. 10.1006/dbio.1996.0157 [DOI] [PubMed] [Google Scholar]
- Tilney L. G. and Saunders J. C. (1983). Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J. Cell Biol. 96, 807-821. 10.1083/jcb.96.3.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Derosier D. J. and Mulroy M. J. (1980). The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol. 86, 244-259. 10.1083/jcb.86.1.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S. and Cotanche D. A. (1988). Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J. Cell Biol. 106, 355-365. 10.1083/jcb.106.2.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney M. S., Tilney L. G., Stephens R. E., Merte C., Drenckhahn D., Cotanche D. A. and Bretscher A. (1989). Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J. Cell Biol. 109, 1711-1723. 10.1083/jcb.109.4.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S. and DeRosier D. J. (1992). Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257-274. 10.1146/annurev.cb.08.110192.001353 [DOI] [PubMed] [Google Scholar]
- Turunen O., Wahlstrom T. and Vaheri A. (1994). Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J. Cell Biol. 126, 1445-1453. 10.1083/jcb.126.6.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelmann F., Chamaillard M., El-Marjou F., Simon A., Netter J., Vignjevic D., Nichols B. L., Quezada-Calvillo R., Grandjean T., Louvard D. et al. (2013). Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. Proc. Natl. Acad. Sci. USA 110, E1380-E1389. 10.1073/pnas.1218446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G., Meyenberg K., Risselada H. J., Amin H., Willig K. I., Hubrich B. E., Dier M., Hell S. W., Grubmüller H., Diederichsen U. et al. (2011). Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552-555. 10.1038/nature10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Post S. and Hansson G. C. (2014). Membrane protein profiling of human colon reveals distinct regional differences. Mol. Cell. Proteomics 13, 2277-2287. 10.1074/mcp.M114.040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha R., Ohouo P. Y., Smolka M. B. and Bretscher A. (2012). Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199, 969-984. 10.1083/jcb.201207047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha R., Wayt J., Ohouo P. Y., Smolka M. B. and Bretscher A. (2013). Interactome analysis reveals ezrin can adopt multiple conformational States. J. Biol. Chem. 288, 35437-35451. 10.1074/jbc.M113.505669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha R., Bretscher A. and Garbett D. (2014). Dynamics of ezrin and EBP50 in regulating microvilli on the apical aspect of epithelial cells. Biochem. Soc. Trans. 42, 189-194. 10.1042/BST20130263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. F., Janecke A. R., Krainer I. M., Gutleben K., Witting B., Mitton S. G., Mansour S., Ballauff A., Roland J. T., Engevik A. C. et al. (2017). Abnormal Rab11-Rab8-vesicles cluster in enterocytes of patients with microvillus inclusion disease. Traffic 18, 453-464. 10.1111/tra.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y., Dutt P., Lippincott-Schwartz J. and Arias I. M. (2005). Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA 102, 15087-15092. 10.1073/pnas.0503702102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayt J. and Bretscher A. (2014). Cordon Bleu serves as a platform at the basal region of microvilli where it regulates microvillar length through its WH2 domains. Mol. Biol. Cell 25, 2817-2827. 10.1091/mbc.e14-06-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegerinck C. L., Janecke A. R., Schneeberger K., Vogel G. F., van Haaften-Visser D. Y., Escher J. C., Adam R., Thöni C. E., Pfaller K., Jordan A. J. et al. (2014). Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147, 65-68.e10. 10.1053/j.gastro.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M. and Knust E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67-76. 10.1016/0092-8674(95)90053-5 [DOI] [PubMed] [Google Scholar]
- Yang L., Zheng J., Xiong Y., Meng R., Ma Q., Liu H., Shen H., Zheng S., Wang S. and He J. (2015). Regulation of β2-adrenergic receptor cell surface expression by interaction with cystic fibrosis transmembrane conductance regulator-associated ligand (CAL). Amino Acids 47, 1455-1464. 10.1007/s00726-015-1965-6 [DOI] [PubMed] [Google Scholar]
- Yu I.-M. M., Planelles-Herrero V. J., Sourigues Y., Moussaoui D., Sirkia H., Kikuti C., Stroebel D., Titus M. A. and Houdusse A. (2017). Myosin 7 and its adaptors link cadherins to actin. Nat. Commun. 8, 15864 10.1038/ncomms15864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.-H. C., Lamprecht G., Forster D. V. and Sidor A. (1998). NHE3 kinase a regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J. Biol. Chem. 273, 25856-25863. 10.1074/jbc.273.40.25856 [DOI] [PubMed] [Google Scholar]
- Zheng L., Sekerková G., Vranich K., Tilney L. G., Mugnaini E. and Bartles J. R. (2000). The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377-385. 10.1016/S0092-8674(00)00042-8 [DOI] [PMC free article] [PubMed] [Google Scholar]