Abstract
Polycystic ovary syndrome (PCOS) is a continuum of endocrine and reproductive disorders characterized by hyperandrogenism, antral follicle growth arrest, and chronic inflammation. Macrophages play key role in inflammation, and the balance between M1 (inflammatory) and M2 (anti-inflammatory) macrophages determines physiological/pathological outcomes. Here, we investigated if hyperandrogenism increases ovarian chemerin altering the balance of M1 and M2 macrophages and the granulosa cell death. Ovarian chemerin was upregulated by 5α-dihydrotestosterone (DHT) in lean and overweight rats; while increased serum chemerin levels were only evident in overweight rats, suggesting that the serum chemerin may be reflective of a systemic response and associated with obesity, whereas increased ovarian chemerin expression is a localized response independent of the metabolic status. DHT altered follicle dynamics while increased the M1: M2 macrophages ratio in antral and pre-ovulatory follicles. While ovarian M1 macrophages expressing chemokine-like receptor 1 (CMKLR1) were increased, CMKLR1+ monocytes, which migrated toward chemerin-rich environment, were markedly decreased after 15 days of DHT. Androgen-induced granulosa cell apoptosis was dependent on the presence of macrophages. In humans, chemerin levels in follicular fluid, but not in serum, were higher in lean PCOS patients compared to BMI-matched controls and were associated with increased M1: M2 ratio. Our results support the concept that in PCOS, hyperandrogenemia increases chemerin expression while promotes CMKLR1+ monocytes recruitment and deregulates the immunological niche of ovaries. This study established a new immunological perspective in PCOS at the ovarian level. Hyperandrogenism is associated with upregulation of chemerin and macrophage unbalance in the ovaries.
Keywords: androgens, chemotaxis, female infertility, granulosa cells, immunology, macrophage, ovary, reproductive immunology
Hyperandrogenism increases chemerin inducing the migration of CMKLR1+ monocytes to the ovaries where they become CMKLR1+ M1 macrophages. Macrophages induce granulosa cell apoptosis in response to the androgen.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy amongst women of reproductive age, with prevalence rates reported to be up to 15% [1]. It is a heterogeneous continuum of disorders characterized by hyperandrogenism, oligo- or anovulation, and polycystic ovaries [2]. Chronic inflammatory markers are elevated systemically in PCOS women [3–7] and are positively correlated with body mass index (BMI) and hyperandrogeanemia [3–7]. Although systemic low-grade inflammation in PCOS has gained increased interest due its association with increased in risk of cardiovascular diseases [8, 9], whether the ovaries in PCOS patients are influenced by the systemic or local inflammation is unknown. Macrophages are key players in the inflammatory immune response and the balance between M1 (inflammatory) and M2 (anti-inflammatory) macrophages can determine the immunological milieu [10, 11] and the ovarian cell fate. Macrophages are the most abundant leukocytes in the ovaries [12, 13], but whether hyperandrogenism alters the number and function of macrophages in the ovaries, and if they contribute to the reproductive disorder in PCOS is unknown.
Chemerin is an adipokine with immunological activity [14]. It is systemically elevated in PCOS patients and although the origin of elevated chemerin levels is not fully understood, its association with the metabolic status of the patient [15, 16] together with increased chemerin mRNA levels in adipose tissue from PCOS subjects [17] strongly suggests the fat tissue as the main source of serum chemerin. Chemerin activity is dependent on its differential proteolytic cleavage at the carboxyl-terminal regions and is mediated by binding to its receptors chemokine-like receptor 1 (CMKLR1), G protein-coupled receptor 1 (GPR1), and chemokine CC motif receptor-like 2 (CCRL2) [14, 18]. CMKLR1 is responsible for the chemotactic action of chemerin, while GPR1 acts as a partial agonist of chemerin and CCRL2 lacks the DRYLAIV intracellular motif required for classical downstream signaling by G protein-coupled receptors (GPCRs) [19]. Human and mouse macrophages, plasmacytoid and myeloid dendritic cells, and natural killer cells have been shown to express CMKLR1 and migrate toward chemerin in chemotaxis assays ex vivo [20–23]. To date, there is no report on the expression of CMKLR1 in rat ovarian leukocytes or its role in the regulation of ovarian follicle growth and function.
Androgen excess is considered critical in the pathogenesis of PCOS and is one of its diagnostic criteria [24, 25]. Chronically androgenized rats [treated 3 months with 5α-dihydrotestosterone (DHT)] exhibit features that resemble ovarian and metabolic phenotypes of human PCOS [26–29], including elevated serum chemerin levels and increased ovarian chemerin and CMKLR1 expression [27]. In the present studies, we hypothesized that hyperandrogenism increases the levels of chemerin and the recruitment of CMKLR1+ monocytes. It also alters the relative abundance of macrophage subsets leading to a proinflammatory condition in the ovary and to compromised ovarian follicular growth. Using hyperandrogenic female rats and human PCOS samples, we have established that androgen excess increases ovarian chemerin independent of the metabolic status and increases M1: M2 ratio, leading to ovarian follicular growth arrest. Taken together, our findings support the hypothesis that PCOS is associated with the dysregulation at the reproductive–immunological interface and contribute to the understanding of the pathophysiology of PCOS.
Materials and methods
Animals
Female Sprague Dawley rats (Charles River, Montreal, Canada) were maintained on 12 h cycle (light and dark) and given food and water ad libitum. All procedures were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals, Canadian Council on Animal Care, and were approved by University of Ottawa Animal Care Committee.
In vivo DHT treatment
Female rats at 21 days of age were implanted subcutaneously with silicone capsules without (sham control) or with DHT (Steraloids Inc., Newport, USA), as previously described [27–29] and continuously releasing (83 μg DHT/day) for 3, 7, 15, and 28 days. Animals were weighed prior to the DHT implant and at the termination of experiments. Sham control rats were sacrificed at the diestrous stage of the estrous cycle.
Serum and ovary collection in DHT-treated rats
The rats were anesthetized by isoflurane (3%–4%; Baxter Corporation, Mississauga, Canada), and blood was collected by heart puncture. Serum was separated by centrifugation (2060 g, 5 min, 4°C) and stored at –80°C pending analysis. Whole ovaries were fixed in 4% paraformaldehyde (PFA; Sigma Aldrich, Oakville, Canada) for 24 h at 4°C and embedded in paraffin. Ninety serial sections (5 μm/section; 450 μm of tissue) were obtained per ovary. Histological sections were used to quantify ovarian follicles (H&E staining), to determine in situ apoptosis (TUNEL assay) and in the quantification of ovarian macrophages (immunofluorescence). At least three animals and nine sections per animal were used in each experiment, and a range of 5–20 ovarian structures were analyzed per section (45–120 ovarian structures per rat).
Human samples
All patient samples were selected according to the Rotterdam criteria by which two of the three phenotypes were met: (1) oligo- or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, or (3) polycystic ovaries. Exclusion criteria include the presence of congenital adrenal hyperplasia, Cushing's syndrome, androgen-secreting tumors, 21-hydroxylase deficient nonclassic adrenal hyperplasia, hypogonadotropic hypogonadism, premature ovarian failure, or syndromes of severe insulin resistance [2]. Ovarian sections (5 μm) from 9 non-PCOS and 16 PCOS subjects were obtained from the Department of Pathology, Queen Mary Hospital, University of Hong Kong. The use of archived paraffin-embedded tissue blocks for research purpose was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB UW UW 14–469). The requirement for individual consent was waived since residual formalin fixed paraffin-embedded samples after diagnosis was used.
Serum and follicular fluid from 55 non-PCOS and 25 PCOS subjects were obtained at Department of Obstetrics and Gynaecology, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan in accordance with Institutional Review Board of Taipei Medical University, Taipei (#: TMU-JIRB 201410033). All subjects (non-PCOS and PCOS) had BMI ≤ 30 and they were age-matched (non-PCOS: 35.47 ± 0.47 vs PCOS: 34.79 ± 0.67). Hormonal information for the non-PCOS and PCOS subjects includes serum levels of estradiol-17β (3051 ± 269.1 pg/ml vs 3589 ± 546.5 pg/ml), progesterone (0.98 ± 0.11 ng/ml vs 0.95 ± 0.11 ng/ml), and anti-Mullerian hormone (4.11 ± 0.34 vs 9.65 ± 1.08 ng/ml; P < 0.001). Testosterone-related measurements were also taken and were significantly different between non-PCOS and PCOS subjects: total testosterone (95.92 ± 5.35 ng/dL vs 127.40 ± 8.97 ng/dL; P < 0.01), free testosterone (0.46 ± 0.03 ng/dL vs 0.73 ± 0.10 ng/dL; P < 0.05), bioavailable testosterone (10.93 ± 0.75 ng/dL vs 17.49 ± 2.63 ng/dL; P < 0.05), and free androgen index (FAI; 1.87 ± 0.14 vs 3.04 ± 0.53 ng/dL; P < 0.05), where FAI = total testosterone/sex hormone binding globulin × 100.
In situ quantification of ovarian M1 and M2 macrophages in rats and humans
Ovarian sections were deparaffinized and hydrated. Antigen retrieval in rat sections was performed using 0.1% trypsin (Sigma-Aldrich, Oakville, Canada) in 0.1% of CaCl2 (Sigma-Aldrich) solution pH 7.8 (30 min, 37°C). Sections were washed in phosphate-buffered saline (PBS; Sigma-Aldrich) with 0.05% tween-20 (Sigma-Aldrich) (PBS-T), and nonspecific bindings were blocked with nonfat dry milk 5% (30 min). The slides were incubated (overnight, 4°C) with mouse anti-rat CD163 or mouse IgG1 (isotype control had the same concentration as the antibody) followed by anti-mouse conjugated with alexa-fluor 594 [1 h, room temperature (RT)]. Sections were incubated with biotinylated anti-rat CD68 or mouse IgG1 as negative control (2 h, RT; isotype control had the same concentrations as antibody) followed by FITC-conjugated streptoavidin (1 h, RT).
Antigen retrieval in human ovarian sections was achieved with citrate buffer (Sodium citrate-Fisher, Nepean, Canada) (10 mM; pH 6.0, 95°C, 20 min). Nonspecific binding was blocked with nonfat dry milk (5%, 30 min). M1 and M2 macrophage phenotypes were confirmed by the expression of two receptors: CD86 and MHC II for M1 macrophages and CD206 and CD163 for M2 macrophages (Supplemental Figure S1). Quantitative analyses were conducted using anti-MHCII and anti-CD163 antibodies. Mouse anti-human MHC II and rabbit anti-human CD163, and their respective isotype controls (matched concentration with antibodies) were incubated overnight at 4°C. Slides were washed with PBS-T and incubated with anti-mouse conjugated with alexa-fluor 594 and anti-rabbit conjugated with alexa-fluor 488 (1 h, RT).
Rat and human sections were washed and mounted with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Waltham, USA). Ovarian sections were observed by fluorescence microcopy (Zeiss Axioplan 2, Zeiss, North York, Canada), and images were recorded and analyzed using the Axion Vision program (Axion Vision software, Zeiss). In rats, all ovarian follicles with an oocyte in a section were considered. In human biopsies, 4 to 50 images of the stroma were taken per section (number of images was dependent on the size of the biopsy). Antibodies used in this study are described in Supplemental Table S1.
TUNEL assay
In situ localization of apoptosis was achieved using the TUNEL assay, according to manufacturer's instructions (Sigma-Aldrich; cat# 11767291910).
Flow cytometric analysis of ovarian, splenic, and peritoneal macrophages in rats
Ovarian and splenic cell suspensions were obtained by purging the tissue through a 70-μm cell strainer (Fisher Scientific, Ottawa, Canada). Cell suspension was washed with PBS (320 g, 5 min, 4°C) and leukocytes were isolated by percoll gradient centrifugation (40% and 80%; Sigma-Aldrich, St Louis, USA; 350 g, 25 min, RT), as reported by Liu et al [30]. To prevent blood leukocytes contamination, abdominal artery was perfused with PBS prior to the tissue harvest. Total peritoneal leukocytes were obtained by peritoneal lavage (PBS/EDTA 50 mM; RT). Total peritoneal cells were filtered through a 70-μm cell strainer, washed with PBS, and re-suspended in 1–2 ml of RBC lysis buffer (2 min, RT; eBioscience Thermo Fisher) and immediately washed with PBS.
Ovarian, splenic, and peritoneal leukocytes (105 cells) were stained with viability dye eFluor 450 in PBS (Biolegend, San Diego, USA; 30 min, 4°C) and washed with FACS buffer (PBS with 1% BSA, 0.1% NaN3 and 2% FBS; Gibco- Thermo Fisher). Cell surface staining (anti-CD163 and anti-CMKLR1) was performed (45 min, 4°C) followed by the intracellular staining (anti-CD68) using leucoperm kit (Bio-Rad Laboratories, Inc, Mississauga, Canada) as per the manufacturer's instructions. Cells were washed and re-suspended in 1% of PFA. Flow cytometry acquisition was performed using the Cyan ADP 9 analyzer (Beckman Coulter Inc., Mississauga, Canada) and the data were analyzed using Kaluza software (Beckman Coulter Inc.). Antibodies and isotypes used in this study are described in Supplemental Table S1. Three experimental replicates were performed (6 rats per replicate; total of 18 rats per group).
Flow cytometric analysis of blood monocytes in rats
Blood was collected through heart puncture, and K2EDTA-coated blood collection tubes (BD Vacutainer Blood collection, Mississauga, Canada) were used. Blood mononuclear cells were separated using Histopaque- 1077 (Sigma-Aldrich), washed (PBS- 320 g, 10 min, 4°C), and stained with viability dye eFluor 450 (30 min, 4°C). The cells were then stained with mouse anti-rat CD3, mouse anti-rat CD4, mouse anti-rat CD11b, mouse anti-rat CD43, and rabbit anti-rat CMKLR1, and their respective isotype controls (45 min, 4°C; Supplemental Table S1), washed, and resuspended in 1% of PFA for flow cytometry analysis.
Assessment of ovarian and serum levels of chemerin by western blotting in rats and humans
Ovaries (10 rats per group) were homogenized using tissue extraction reagent I (Thermo Fisher) supplemented with the protease inhibitors cocktail mini cOmplete (Roche, Mississauga, Canada) and centrifuged (12,000 g; 30 min; 4°C). Ovarian (20 μg of protein) and serum (5 μl) proteins were separated electrophoretically by SDS-PAGE and electrotransferred onto a nitrocellulose membrane. Nonspecific binding was blocked with nonfat dry milk (5%; 1 h, RT). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was assayed as a loading control in ovarian lysates. The membranes were washed and incubated with goat anti-mouse chemerin (1:1000) overnight at 4°C, and with rabbit anti-goat (1 h, RT). Signal intensity was assessed using Pierce ECL Western Blotting Substrate kit (Thermo Fisher) and Molecular Imager VersaDoc MP imaging system (Bio-Rad Laboratories, Inc). Blots were densitometrically analyzed using the ImageJ software. Chemerin levels in human follicular fluid and serum (5 μl) from 20 non-PCOS and 16 PCOS subjects were similarly assessed, except by the use of goat anti-human chemerin (1:1000).
Single cultures and co-cultures of granulosa cells and macrophages in rats
Peritoneal leukocytes were collected from six noncycling female rats (35 day old; 6 rats per replicate). One million cells/well (12-well plate) were plated for 4 h in 10% FBS M199 medium (Gibco Thermo Fisher, Burlington, Canada). Nonadherent immune cells were excluded by washing with fresh M199 medium. Adherent macrophages were co-cultured with granulosa cells. Of the 1 × 106 leukocytes plated, 0.4 × 106 cells remained attached on the plate after washes, 93% of which were macrophages [M1 (39%), M2 (54%)]) (Supplemental Figure S2A).
Granulosa cells were isolated from eCG (Sigma, St Louis)-treated female rats (21 day old; 10 rats per replicate) 48 h prior to sacrifice, as previously described [31]. 0.6 × 106 (single culture) or 0.2 × 106 granulosa cells (co-cultured with macrophages) were cultured in 12-well plates. Granulosa cells were stained with live cell tracker (CellTracker Orange CMRA Thermo Fisher; 2.5 μM; 30 min) prior to the co-culture, as per the manufacturer’s recommendation, washed, and laid onto macrophages (four macrophages: two granulosa cells). Cells were cultured in 10% FBS M199 (24 h) following by FBS-free M199 (16 h) prior to DHT treatment (1 μM in FBS-free M199; 12 and 24 h). Macrophage contamination in granulosa cell preparations was <0.37% (Supplemental Figure S2B).
Early and late apoptosis in rat granulosa cells: annexin V and 7AAD staining
Granulosa cells from single culture or co-culture were stained with annexin V and 7-AAD, according to the manufacturer's instructions (Biolegend cat# 640911). A total of 105 cells were washed in cell staining buffer, re-suspended in annexin V binding buffer, and incubated (15 min, RT, in dark) with annexin V conjugated with alexa Fluor 647 and 7-AAD.
Blood mononuclear chemotaxia assay
Transwell permeable supports (polycarbonate membrane pore size of 3 μm; Corning, New York, USA) were used to assess blood mononuclear cell migration toward chemerin gradient. Enriched blood mononuclear cells (Histopaque; 1077 separation) were resuspended in 10% FBS RPMI and added to the upper chamber (105 cells in 100 μl) while 600 μl of chemerin-containing medium (100 ng/ml) was placed in the lower chamber. Cells were cultured (37°C, 5% CO2) for 6, 12, and 24 h. Migrated cells were immediately fixed with 4% PFA (30 min, RT). Nonmigrating cells (top of the membrane) were removed using cotton swabs, while migrating cells (bottom of the membrane) were stained (10 min, RT) with Hoechst 33342 dye (1:1000; Thermo Fisher). The membranes were washed in PBS-T and placed on glass slides for microscopic analysis. Using ×40 magnification, 20–25 photos were taken in random areas of each membrane (n = 6 replicates; 4 animals per replicate). Hoechst-positive cells (nucleus DNA) were quantified and the results expressed as mean number of cells per 0.3795 mm2.
Statistical analysis
Parametric unpaired t-test was used to determine significant differences between two experimental groups in rats (control and DHT) in regard to (1) ovarian and body weight; (2) ovarian and serum chemerin in rats; (3) ovarian, splenic and peritoneal macrophages, and blood monocytes expressing CMKLR1 (flow cytometry). One-way ANOVA was used to compare cell migration in response to chemerin (three time points). Two-way ANOVA was used to compare (1) ovarian follicle quantification (time and DHT treatment), (2) in situ quantification of ovarian M1 and M2 macrophages (ovarian follicle stage and DHT treatment), (3) the in situ TUNEL assay in the ovarian sections (ovarian follicle stage and DHT treatment), and (4) the in vitro apoptosis rate in rats (annexin 5 and 7-AAD assay; time and DHT treatment). In humans, the continuous variables were tested for normality using the Kolmogorov–Smirnov test and formed the basis for the choice of nonparametric test. The parametric unpaired t-test was used to compare follicular fluid and serum chemerin (normally distributed), while the nonparametric Mann–Whitney U-test was used to compare the human stromal macrophages (M1 and M2 quantification and CMKLR1+ M1 macrophages by immunofluorescence) in non-PCO and PCO ovaries (data not normally distributed). Data are expressed as mean ± SEM, unless otherwise stated. Significant differences were considered at P ≤ 0.05.
Results
The ovarian changes following chronic DHT treatment in vivo
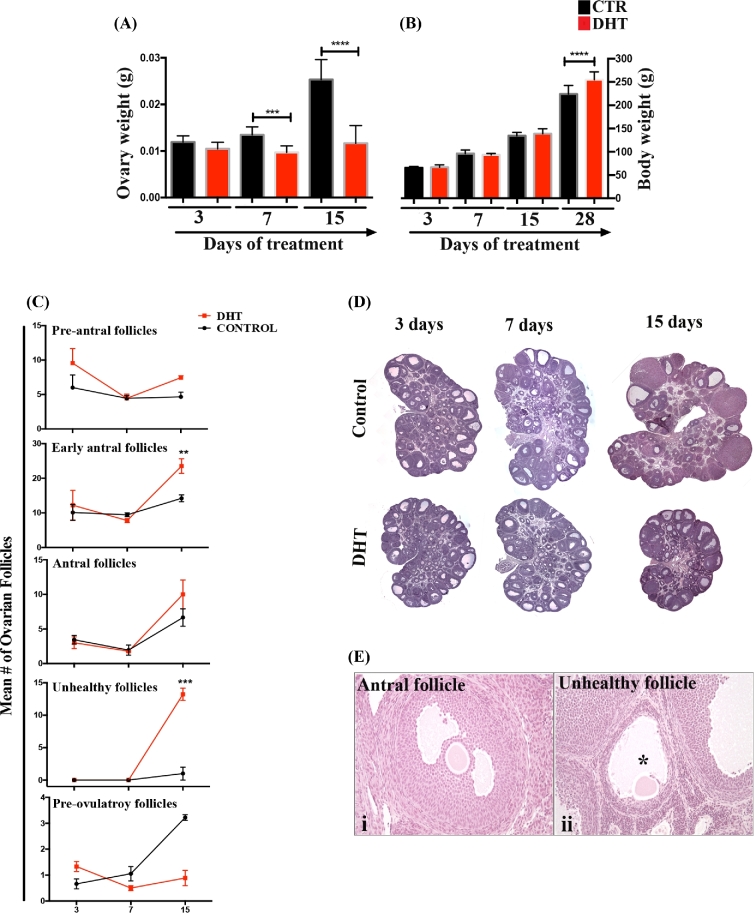
The ovaries were primarily affected by DHT during early phase of treatment. Ovarian weight was reduced by DHT after 7 days of treatment (Figure 1A; P < 0.001 and P < 0.001, for 7 and 15 days respectively), while changes in body weight were only observed after 28 days (P < 0.001; Figure 1B).
Figure 1.
DHT-induced changes in the ovarian morphology. (A and B) Ovarian and body weight of DHT-treated and control rats at 3, 7, 15, and 28 days. Significant changes in the ovarian weight (A) were observed in 7 and 15 days after DHT treatment (P < 0.001), while changes in body weight (B) were only detected after 28 days of DHT (P < 0.001). (C) Quantification of specific ovarian follicles (pre-antral, early antral, antral, unhealthy, and pre-ovulatory follicles) overtime in control and DHT-treated rats. Early antral and unhealthy follicles were more numerous in DHT-treated ovaries after 15 days. Pre-ovulatory follicles’ number was reduced in 15 days DHT-treated ovaries but it was not significant different. (D) Panoramic view ovaries from control and DHT treated rats after 3, 7 and 15 days. (E) shows examples of a healthy early antral follicle (i) and an unhealthy follicle (ii) in DHT-treated ovary. Unhealthy follicles had evident oocyte, antral space (*), and an intact layer of theca cells, while the thickness of the granulosa cell layer was reduced.
Folliculogenesis was affected with 15 days after DHT treatment (Figure 1C and D). The number of early antral follicles (Figure 1C; P < 0.05) and unhealthy follicle (P < 0.001) was significantly elevated in the DHT-treated ovaries compared to controls. Unhealthy follicles were morphologically distinctive from the atretic ones (also present in control ovaries) presenting reduced layer of granulosa cells with infiltrated macrophages but intact theca cell layer. A representative healthy antral follicle and an unhealthy follicle are shown in Figure 1E (i–ii). The frequency of pre-antral, antral, or pre-ovulatory follicles did not differ between control and DHT-treated ovaries after 15 days (Figure 1C).
Ovarian follicle stage-specific M1 and M2 macrophages in DHT-treated rats and human PCOS ovaries
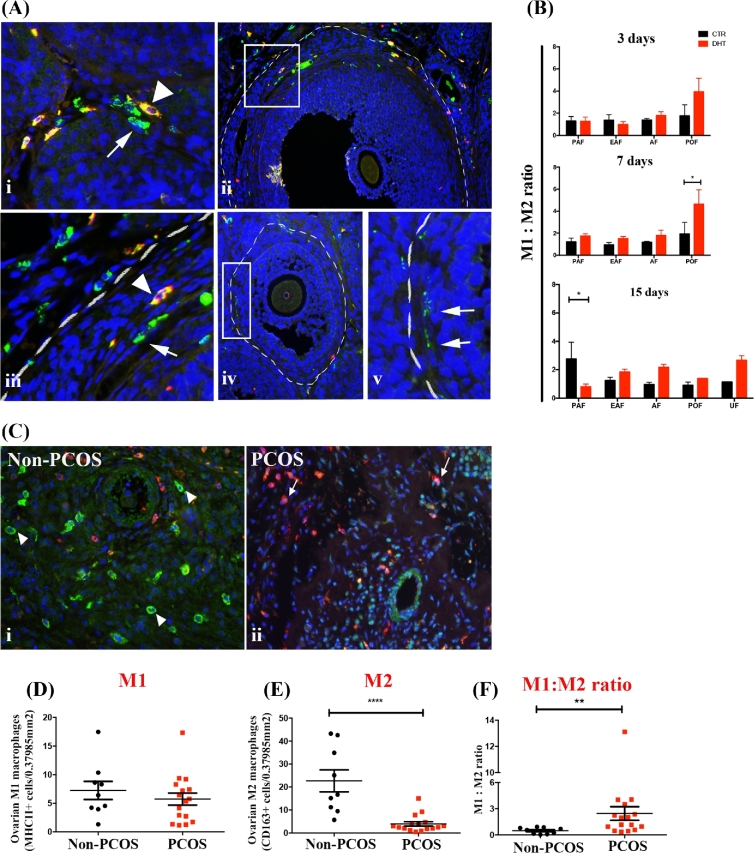
Ovarian M1 and M2 macrophages were assessed in the ovaries from androgenized rats and in humans (non-PCOS or PCOS subjects). In rats, M1 (CD68+CD163–) and M2 (CD68+CD163+) macrophages were quantified in a follicle stage-specific manner (Figure 2Ai–v), including theca, granulosa cell layers, and the antrum of individual ovarian follicles following 3, 7, and 15 days of DHT treatment. The number of M1 and M2 macrophages present was dependent on the stage of ovarian follicular development, as well as was regulated by DHT (Table 1). Three days of DHT treatment failed to influence ovarian M1 macrophages frequency in any of the follicle stages (Table 1), while significantly reduced M2 macrophages in pre-ovulatory follicles (P < 0.05; Table 1). Seven and 15 days of DHT treatment significantly increased M1 macrophages in antral follicles (P < 0.05). While 7 days of DHT significant increased the number of M1 macrophages in pre-ovulatory follicles (P < 0.001; Table 1), rats treated for 15 days with DHT had the number of pre-ovulatory follicles markedly reduced preventing the statistical comparison of macrophage phenotypes in these follicles. The frequency of M2 macrophages in antral and pre-ovulatory follicles from 7 and 15 days DHT-treated ovaries was slightly but not significantly reduced (Table 1). Unhealthy follicles were predominant in DHT-treated ovaries and rich in M1 macrophages. A single structure resembling unhealthy follicle was found in control ovaries, which prevented comparisons between control and DHT-treated ovaries. The most prominent change in M1: M2 macrophage ratio was evident in pre-ovulatory follicles at 7 days after DHT treatment (Figure 2B), as pre-ovulatory follicles were not longer observed in the ovaries from rats treated for 15 days.
Figure 2.
DHT-induced changes in ovarian M1 and M2 macrophage balance. Immunolocalization and quantification of M1 and M2 macrophages in the ovaries from DHT-treated rats (A, B) and from humans (non-PCOS vs PCOS; C–F). (A) Rat ovarian macrophages were identified using anti-CD68 (green) and anti-CD163 (red). (A, i) M1 (arrows; green) and M2 (arrowheads; yellow) macrophages in the theca layer from an ovarian follicle. (A, ii) An antral follicle delineated by white broken line; macrophages are located in the theca cells layer. (A, iii) A digital magnification of (A, ii) demonstrating the presence of M1 (arrow) and M2 macrophages (arrowhead). (A, iv) An early antral follicle delineated by white dashes; (A, v) a digital magnification of Aiv shows the presence of M1 macrophages (arrow) in the theca cells layer. (B) M1: M2 ratio of individual ovarian follicles in control and rats treated with DHT for 3, 7, and 15 days. The M1: M2 ratio in pre-ovulatory follicles was significantly higher after 7 days of DHT treatment. (C) Human stromal macrophages were identified using anti-human MCH II (red; arrows—M1) and CD163 (green; arrowheads—M2) in non-PCOS (C, i) and PCOS (C, ii) ovaries. The quantification of stromal M1 (D) and M2 (E) macrophages (per area of ×20 magnification - 0.37985 mm2) showed that M2 were significantly reduced in PCOS ovaries compared to control (P < 0.001). No difference was found with M1 macrophages, but the M1: M2 ratio (F) demonstrated to be significantly higher in PCOS subjects compared to control (P < 0.01). DAPI (nucleus; blue). Magnification of images (Ai): ×40 (Aii, Aiv, Ci and Cii): ×20, (Aiii and v) are digital magnification of Aii and Aiv. PAF: pre-antral follicle, EAF: early antral follicle, AF: antral follicle, POF: pre-ovulatory follicle and UF: unhealthy follicle.
Table 1.
Ovarian follicle stage-specific regulation of M1 and M2 macrophages by DHT.
| 3 days | 7 days | 15 days | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | M1 | M2 | |||||||||||||
| Follicle Stage | CTR | DHT | P | CTR | DHT | P | CTR | DHT | P | CTR | DHT | P | CTR | DHT | P | CTR | DHT | P |
| Pre-antral | 0.94 ± 0.21 | 0.52 ± 0.07 | >0.05 | 0.80 ± 0.22 | 0.47 ± 0.16 | >0.05 | 0.80 ± 0.19 | 1.35 ± 0.48 | >0.05 | 0.80 ± 0.49 | 0.77 ± 0.21 | >0.05 | 0.50 ± 0.05 | 0.34 ± 0.26 | >0.05 | 0.31 ± 0.27 | 0.51 ± 0.12 | >0.05 |
| Early antral | 0.61 ± 0.19 | 0.38 ± 0.03 | >0.05 | 0.59 ± 0.35 | 0.43 ± 0.17 | >0.05 | 0.62 ± 0.25 | 1.23 ± 0.68 | >0.05 | 0.69 ± 0.33 | 0.78 ± 0.30 | >0.05 | 0.78 ± 0.10 | 0.91 ± 0.25 | >0.05 | 0.67 ± 0.21 | 0.49 ± 0.09 | >0.05 |
| Antral | 2.59 ± 0.36 | 2.09 ± 0.18 | >0.05 | 1.93 ± 0.63 | 1.33 ± 0.69 | >0.05 | 2.50 ± 0.21* | 3.85 ± 0.93* | <0.05 | 2.12 ± 0.37 | 2.30 ± 0.49 | >0.05 | 3.01 ± 0.23** | 4.26 ± 0.92** | <0.05 | 3.32 ± 0.50 | 2.01 ± 0.37 | >0.05 |
| Unhealthy | – | – | – | – | – | – | – | – | – | – | – | – | 6.13 (n = 1) | 7.65 ± 0.49 | N/A | 5.42 (n = 1) | 2.91 ± 0.47 | N/A |
| Pre-ovulatory | 2.90 ± 0.27 | 3.68 ± 0.93 | >0.05 | 2.71 ± 1.66** | 1.06 ± 0.38* | <0.05 | 4.18 ± 0.50*** | 6.45 ± 0.47*** | <0.001 | 3.33 ± 2.08 | 1.30 ± 1.25 | >0.05 | 5.10 ± 0.42 | 3.75 (n = 1) | N/A | 5.94 ± 0.96 | 2.75 (n = 1) | N/A |
Mean of ovarian follicle stage-specific M1 and M2 macrophages (CD68+CD163– and CD68+CD163+ cells) identified through immunofluorescence and quantified using Image ProPlus Premier program. Mean ± SD.
In humans, M1 (MHC II+CD86+ cells) and M2 (CD163+CD206+ cells) macrophages were identified (liver as positive control, Supplemental Figure S1A–C) and quantified in the stroma of ovaries from PCOS and non-PCOS subjects (Figure 2Bi–ii). A lower number of M2 (P < 0.001; Figure 2D) but not M1 (Figure 2C) macrophages were observed in the stroma from PCOS ovaries compared to control. The M1: M2 ratio was higher in PCOS subjects than controls (Figure 2E; P < 0.01), suggesting an altered immunological balance in the ovary.
Granulosa cells apoptosis in mature ovarian follicles under androgenic stimulus is dependent on the presence of macrophages
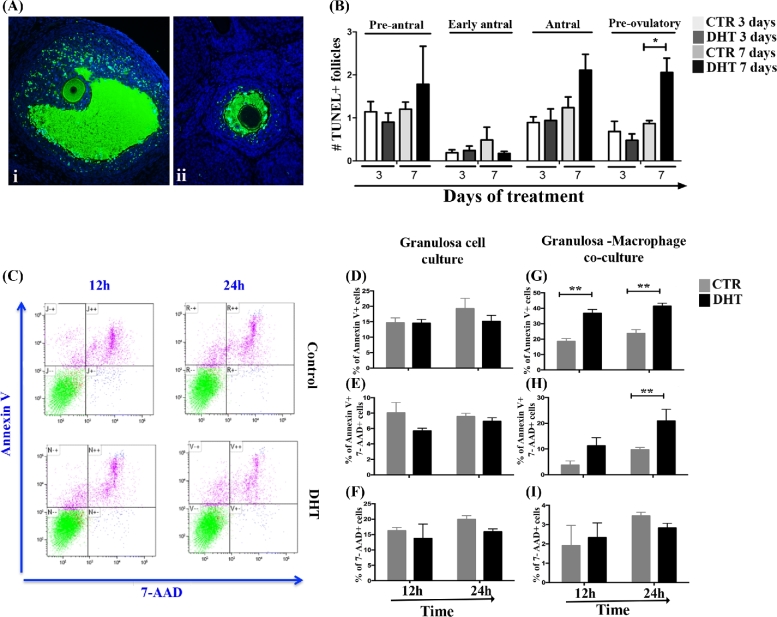
To elucidate the outcomes of the abnormal increase of macrophages in mature ovarian follicles, we determined whether macrophages are involved in the androgenic induction of apoptosis in the ovarian follicles. In situ apoptosis assay (TUNEL) was performed in ovaries of rats treated with DHT for 3 and 7 days (Figure 3A), and ovarian follicles with a large number of apoptotic granulosa cells were considered apoptotic. ANOVA indicates that DHT treatment (P = 0.001) and ovarian follicle stage (P = 0.001) significantly affected the follicle apoptotic rate but with no apparent significant interaction (P > 0.05). Seven days of DHT treatment significantly increased the incidence of TUNEL + pre-ovulatory follicles compared to control (P < 0.05; Figure 3B). No significant differences were observed in pre-antral, early antral, or antral follicles. Moreover, to investigate whether granulosa cell apoptosis under androgenic influence is dependent on the presence of macrophages, the effect of DHT (12 and 24 h) in vitro was studied in single culture of granulosa cells derivate from mature follicles (isolated from rats treated with eCG for 48 h) and in granulosa cells co-cultured with macrophages, and apoptosis was evaluated (Figure 3C). Although DHT was ineffective in inducing apoptosis in granulosa cells in single culture (Figure 3D–F), granulosa cells co-cultured with macrophages in the presence of DHT exhibited significant increase in apoptosis at early stage (12 and 24 h, P < 0.05) and late stage (24 h, P < 0.05) apoptosis when compared to control (Figure 3G and H). Necrosis (7AAD + cells) was not affected by DHT (Figure 2I).
Figure 3.
DHT-induced granulosa cell apoptosis is dependent on the presence of macrophages. (A) In situ TUNEL assay in ovaries from rats treated with DHT for 3 and 7 days. (Ai and Aii) TUNEL + (green) mature and immature ovarian follicles, respectively. (B) Mean number of TUNEL + ovarian follicles per section at 3 and 7 days of DHT treatment. The quantification of TUNEL + ovarian follicles was normalized by the total mean number of specific ovarian follicles per histological section. Apoptotic pre-ovulatory follicles were higher after 7 days of DHT treatment (P < 0.05). (C) Flow cytometric analysis of early (annexin V+) and late (annexin V+7-AAD+) apoptotic cells following 12 and 24 h of DHT treatment. After DHT treatment, early and late apoptosis in granulosa cells were assessed in single culture (D–F) and in co-culture with macrophages (G–I). DHT did not affect the apoptosis or necrosis of granulosa cells in single culture (D–F), while the presence of macrophages increased early (12 and 24 h; P < 0.05, G) and late apoptosis (24 h; P < 0.05; H) of granulosa cells, but not necrosis (I).
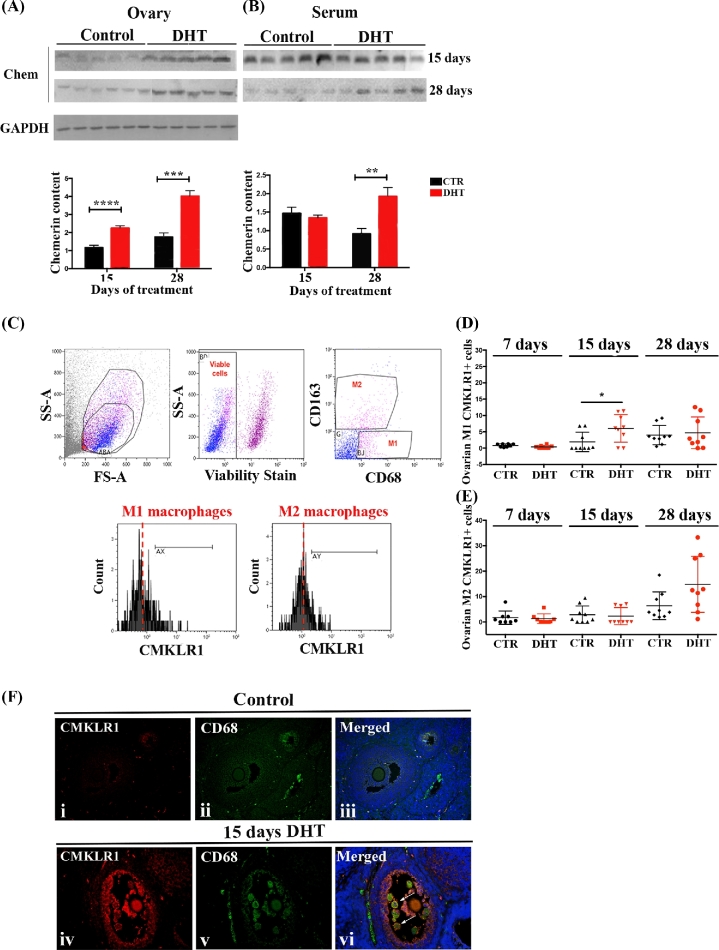
Chemerin is upregulated in ovaries from PCOS women and DHT-treated rats
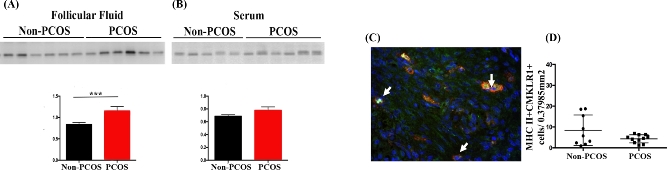
Chemerin levels in the follicular fluid (P < 0.05) but not in the serum were significantly higher in lean PCOS patients (BMI ≤ 30) compared to their matched control (Figure 4A and B) suggesting that ovarian and serum chemerin may have distinct cellular origins and are differentially regulated. Similar to these observations in the human, serum and ovarian chemerin also appeared to be differently regulated in rats treated with DHT without (15 days-normal weight) or with symptoms of metabolic changes (28 days-overweight rats). Only ovarian chemerin level was significantly higher in rats treated with DHT for 15 days (P < 0.001; Figure 5A), and no differences were detected at the serum level (Figure 5B). In contrast, in rats treated with DHT for 28 days, which exhibit an overweight phenotype, both ovarian and serum chemerin levels were significantly higher (P < 0.001 and P < 0.05, respectively; Figure 5A and B).
Figure 4.
Chemerin and CMKLR1+ ovarian macrophages in PCOS women. (A and B) Chemerin content in human follicular fluid (A) and serum (B) from non-PCOS and PCOS subjects. Chemerin level was higher in follicular fluid (P < 0.05) but not in serum from PCOS patients compared to control. (C, D) Human stromal M1 macrophages expressing CMKLR1 (arrows) were identified (C) and quantified (D) through immunofluorescence using anti-human MCH II (red) and anti-human CMKLR1 (green). No significant differences were found in the incidence of ovarian MHC II+CMKLR1+ cells between PCOS and non-PCOS subjects. DAPI (nucleus; blue). Magnification of image (C): ×20.
Figure 5.
Chemerin and CMKLR1+ ovarian macrophages in DHT-treated rats. (A and B) Chemerin content in rat ovaries (A) and serum (B) after15 and 28 days of treatment with DHT. DHT-treated ovaries had higher chemerin content (P < 0.001 and P < 0.001, respectively) compared to control in both treatments (A). Serum chemerin (B) was significant higher in rats treated with DHT only after 28 days (P < 0.05), but not after 15 days. (C) Gating strategy used to identify ovarian M1 and M2 macrophages expressing CMKLR1. Side scatter (SS) and forward scatter (FS) were used to gate total ovarian leukocytes. M1 (CD68+CD163–) and M2 (CD68+CD163+ cells) macrophages were gated from viable leukocytes. The frequency of M1 and M2 macrophages expressing CMKLR1 was measured in comparison to the isotype control. M1 macrophages expressing CMKLR1 at 15 days of DHT treatment were significantly higher compared to control (P < 0.05), but not at 7 (P = 0.070) or 28 (P = 0.715) days (D). No differences were found in CMKLR1+ M2 macrophages in any day of DHT treatment [7 days (P < 0.713), 15 days (P < 0.715) 28 days (P < 0.057)] (E). (F) Double immunofluorescence using anti-CD68 (green; macrophages) and anti-CMKLR1 (red) on ovarian sections of 15 days DHT-treated rats. Low expression of CMKLR1 was observed in control ovaries (F, i–iii). The intensity of CMKLR1 staining was higher in the granulosa cells and CD68+ macrophages (arrows) from DHT-treated ovaries (F, iv-vi) compared to control. DAPI (nucleus; blue). Magnification of image (F): ×40.
A transient influx of ovarian CMKLR1+ M1 macrophages in unhealthy follicles after 15 days of DHT treatment
Chemerin may be involved in the recruitment of M1 macrophages to the ovaries under androgenic conditions. To test this hypothesis, we first investigated whether the number of ovarian CMKLR1+ macrophages was upregulated in vivo by DHT. The frequency of ovarian macrophages (M1 and M2) expressing CMKLR1 was measured by flow cytometry and the gating strategy is demonstrated in Figure 5C. Fifteen days of DHT treatment significant increased ovarian CMKLR1+ M1 macrophages (P < 0.05; Figure 5D) without significant changes in ovarian CMKLR1+ M2 macrophage numbers (Figure 5E). The frequency of ovarian CMKLR1+ M1 macrophages returned to levels comparable to control with longer duration of DHT treatment (28 days of treatment; P > 0.05; Figure 5D). In situ analysis demonstrated that CMKLR1+CD68+ cells were mainly localized in unhealthy follicles (Figure 5F).
Human stromal M1 macrophages expressing CMKLR1 (MHC II+CMKLR1+ cells) in the ovaries were also quantified by immunofluorescence but no differences between PCOS and non-PCOS patients were evident (Figure 4C and D).
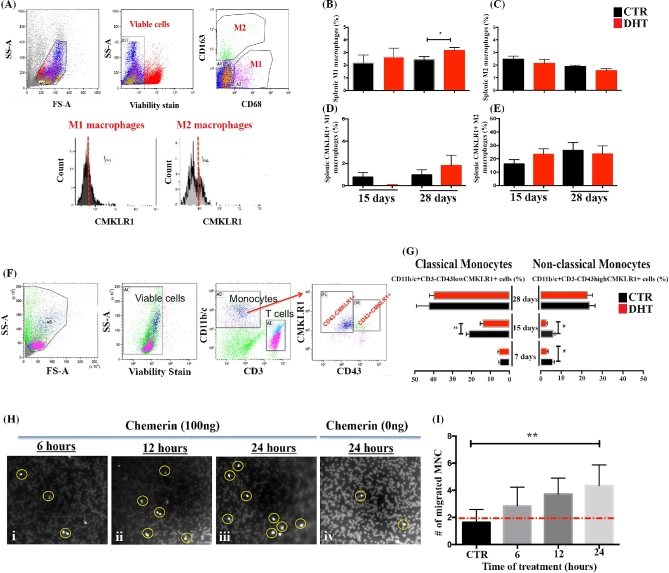
Increased splenic M1 macrophages after 28 days of DHT is independent of CMKLR1
In contrast to ovarian macrophages, changes in the frequency of splenic M1 macrophages were noted only after 28 days of DHT treatment (P < 0.05; Figure 6A and B) and were independent of CMKLR1 expression (Figure 6D). The frequency of splenic M2 macrophages was not altered by DHT (Figure 6C). Very few or no splenic M1 macrophages expressed CMKLR1 compared to M2 macrophages and were not altered by DHT (Figure 6D and E). Indeed, peritoneal macrophages from rats treated for 15 days with DHT were also evaluated (Supplemental Figure S3A). DHT failed to influence the incidence of peritoneal M1 or M2 macrophages (Supplemental Figure S3B).
Figure 6.
DHT does not alter splenic CMKLR1+ macrophages, but decreases the number of CMKLR1+ monocytes while chemerin functions as chemoattractant. (A) Gating strategy used to identify splenic M1 and M2 macrophages expressing CMKLR1. Side scatter (SS) and forward scatter (FS) were used to gate total leukocytes, while M1 (CD68+CD163+) and M2 (CD68+CD163+) macrophages were gated from viable leukocytes. The frequency of M1 and M2 macrophages expressing CMKLR1 was measured in comparison to isotype control. (B, C) Quantification of M1 (B) and M2 (C) macrophages revealed that splenic M1 macrophages were significantly higher in rats treated with DHT for 28 days (P < 0.05), but not for 15 days. No differences were found comparing the incidence of M2 macrophages. (D, E) DHT did not alter the incidence of CMKLR1+M1 or CMKLR1+M2 macrophages in the spleen. (F) Gating strategy used to identify monocytes expressing CMKLR1. Total mononuclear cells were gated based on their SS and FS properties and on viability. (G) Monocytes were identified as CD3–CD11b/c+CD43 high (nonclassical monocytes) and CD3–CD11b/c+CD43low (classical monocytes) cells expressing or not CMKLR1. Quantification of classical CMKLR1+ and nonclassical CMKLR1+ monocytes showed that both subsets were affected in a manner dependent on the duration of DHT treatment. Classical monocytes expressing CMKLR1 was reduced at 15 days after DHT treatment compared to control rats (P < 0.05), while nonclassical monocytes expressing CMKLR1 were significantly reduced in rats treated for 7 and 15 days (P < 0.05). (H–I) Time-course study showing the migration of mononuclear cells in response to chemerin (100 ng/ml; 6 h, 12 h, and 24 h). (H) Representative images used to quantify the migrations of mononuclear cells after 6 h (H, i), 12 h (H, ii), 24 h (H, iii), and 24 h control (H, iv). Increased mononuclear cells migration was observed after 24 h of chemerin incubation compared to control group (P < 0.05). No significant differences were observed with 6 or 12 h.
Transient decrease of blood monocytes expressing CMKLR1 at 15 days
Our aforementioned results suggest that the incidence of ovarian CMKLR1+ M1 macrophages is tissue specific and regulated by androgen in a time-dependent manner. Therefore, we investigated whether the recruitment of blood monocytes expressing CMKLR1 would be involved in the increase of ovarian CMKLR1+ M1 macrophages after DHT treatment. Blood monocytes were identified as CD3–CD11b/c+CD43– (classical monocytes) or as CD3–CD11b/c+CD43+ (nonclassical monocytes), and both monocyte subsets expressed CMKLR1 (Figure 6F). Lower incidence of classical monocytes expressing CMKLR1 was detected within 15 days of DHT treatment (P < 0.05; Figure 6G), while nonclassical monocytes expressing CMKLR1 were lower at 7 and 15 days after DHT treatment (P < 0.05; Figure 6G). The number of monocytes expressing CMKLR1 return to levels similar to control rats by 28 days of DHT.
Blood monocytes migrate toward chemerin gradient
To test the function of CMKLR1 on monocytes, a migration assay was performed using transwell plate inserts. Mononuclear cells challenged with chemerin for 6, 12, and 24 h exhibited higher migration rate over time compared to control groups (Figure 6H and I). Migrating mononuclear cells were significant higher after 24 h compared to control (P < 0.05; Figure 6I). No differences were found at 6 and 12 h.
Discussion
This communication represents the first report on immunological alterations at the ovarian and systemic levels associated with acute and chronic hyperandrogenic states. We have demonstrated that ovarian chemerin is upregulated in hyperandrogenic rats and functions as a chemoattractant for blood monocytes expressing CMKLR1. Ovarian CMKLR1+ M1 macrophages are abundantly present in unhealthy follicles and contribute to androgen-induced proinflammatory ovarian state as evident by increased ovarian M1: M2 ratio and granulosa cell apoptosis. Our findings support the hypothesis that monocyte-derived CMKLR1+ macrophages participate in the antral growth arrest in hyperandrogenic state and provide pathophysiological basis for anovulatory infertility observed in PCOS.
M1 and M2 macrophages play important role in various physiologic processes, including tissue development, immune response to pathogens by generating and resolving inflammatory reactions, surveillance and monitoring of tissue changes, and clearance of apoptotic and senescent cells [10]. The balance between M1 and M2 macrophages determines the nature of each process. Dysregulation of both M1 and M2 macrophages has been reported in many pathological situations, such as cancer, inflammatory and autoimmune disorders, and chronic infections [32], and the M1: M2 ratio is considered a determinant of the inflammatory state of the tissue. In the ovary, macrophages are the most abundant immune cells in the ovaries and contribute to ovarian homeostasis and function [13]. Depletion of macrophages decreases ovulation rate and disrupts estrous cyclicity [33]. In this study, M1 and M2 macrophages are present in rat ovarian follicles at different stages of development and DHT treatment diminished the frequency of ovarian M2 macrophages while M1 macrophages leading to a higher M1: M2 ratio. These responses appeared to be follicle stage-specific and critical for normal follicle growth. DHT treatment disrupted the M1: M2 ratio and therefore the inflammatory versus anti-inflammatory signaling in the ovaries [34, 35] and potentially contributing to the apoptosis in granulosa cells [36, 37].
Our present observation of a coincidental increased apoptosis and M1: M2 ratio and the appearance of unhealthy follicles in DHT-treated rats support the notion that macrophages play a role in the process of granulosa cell apoptosis. This is further supported by our in vitro findings demonstrating that granulosa cells derived from rat mature follicles exhibited early and late apoptosis when co-cultured with macrophages in the presence of DHT. Our results are consistent with the report that luteal macrophages are capable of modulating granulosa cell viability and steroidogenesis during gonadotropic stimulation [38, 39]. Since the presence of macrophages was required for androgen-induced granulosa cell apoptosis, it is possible that the action of androgen may be mediated by macrophage-derived factors. The influence of androgens on macrophages is controversial, although evidences suggest that it may involve proinflammatory responses [40–43]. In males, castration or blockage of DHT synthesis significant decreases inflammation during acute wound healing [40]. Indeed, DHT enhances the production of the proinflammatory cytokines IL1-β and TNFα while suppresses IL6 secretion by LPS-stimulated mouse macrophages treated with DHT [44]. Proinflammatory cytokines were not evaluated in the medium of our co-cultures and further investigations will be necessary to determine the cellular mechanism by which macrophages participate in the androgen-induced granulosa cell apoptosis.
The recruitment of inflammatory monocyte-derived macrophages is under control of several cytokines and chemokines, including chemerin [45, 46]. Since chemerin is upregulated in the ovaries of DHT-treated rats, we propose that ovarian chemerin functions as chemoattractant for inflammatory monocytes/macrophages expressing CMKLR1. DHT treatment modulated specifically ovarian CMKLR1+ M1 macrophages, but not splenic or peritoneal CMKLR1+ macrophages. The frequency of ovarian CMKLR1+ M1 macrophages was transient and time dependent, as CMKLR1+ M1 macrophages were more abundant within 15 days of DHT but returned to levels similar to control rats thereafter (28 days of DHT). It infers that perhaps M1 macrophages are involved in the development of PCO symptom in the ovaries, and once the pathological condition is established they would no longer be recruited. The role of chemerin-CMKLR1 in the inflammation is unclear, and controversial results from CMKLR1-deficient mice have been reported [47–49]. In experimental autoimmune encephalomyelitis and chronic obstructive pulmonary disease, CMKLR1-deficient mice developed less severe clinical symptoms, including reduced leukocytes infiltration [47, 48]. In contrast, in an infectious mouse model of viral pneumonia, CMKLR1-deficient mice developed more severe pulmonary inflammation associated with increased polymorphonuclear cell, myeloid dendritic cell, and macrophage infiltration into the lung [49]. Thus, timing and the type of stimulus may also determine the function of chemerin and CMKLR1+ cells.
Serum chemerin is reported to be elevated in PCOS patients, a phenomenon often associated with metabolic dysregulation [15, 16]. To determine if the upregulation of chemerin is dependent on the metabolic status or if DHT directly upregulates its content in the ovary, we measured ovarian and serum chemerin in normal weight (15 days of DHT) and overweight (28 days of DHT) hyperandrogenic rats. We found that normal weight DHT-treated rats exhibited increased chemerin concentration in the ovary but not in the serum, while both serum and ovarian chemerin concentrations were higher in overweight androgenized rats compared to controls. These findings suggest that serum chemerin levels are related to the systemic metabolic state, and alterations in ovarian chemerin levels may reflect a direct action of the androgen in the ovary and independent of the changes in serum chemerin or adiposity in androgenized rats. The current study does not allow us to determine the exact source of ovarian chemerin in DHT-treated rats, but support the notion that chemerin is independently regulated at the ovarian level.
Our findings indicate that blood monocytes expressing CMKLR1 were significantly reduced after 7 and 15 days of DHT treatment but returned to normal at 28 days. These changes were coincidental to the observed increase in ovarian CMKLR1+ macrophages, suggesting that CMKLR1+ monocytes may be specifically recruited to chemerin-rich ovarian follicles. Monocytes are cells circulating in the blood, bone marrow, and spleen, and are precursors of macrophages. Blood mononuclear cells from rats migrated toward a chemerin-rich ovarian region in a time-dependent manner. Classical (inflammatory) and nonclassical (anti-inflammatory) monocytes are believed to have different functions during homeostasis, immune defense/inflammation, and tissue repair [10, 50, 51]. We have demonstrated that the frequencies of classical and nonclassical monocytes expressing CMKLR1 are regulated by DHT. We observed a reduction in classical monocytes expressing CMKLR1 15 days after DHT treatment when there is a transitory elevation in ovarian CMKLR1+ M1 macrophages. Notwithstanding, it was unexpected to also observe reduced levels of nonclassical monocytes expressing CMKLR1 at 7 and 15 days but, considering the DHT may also affect different organs, the possibility of recruitment of nonclassical monocytes expressing CMKLR1 to other chemerin-rich tissues could not be excluded. Further studies are required to determine which monocyte subset(s) contributes to the increased ovarian M1 macrophages under androgenic influence.
We have previously demonstrated that immature rat chronically androgenized exhibits many of the phenotypes of the human PCOS, including dysregulated estrous cycle, ovarian follicular growth arrest and apoptosis, decreased estrogen biosynthesis and increased ovarian chemerin level, adiposity, and insulin resistance due the dysregulated expression of genes involved in steroid, cholesterol, and lipid metabolism [27–29, 52, 53]. This study does not only extend these findings to show the involvement of the immune response in the rat PCOS model but also provide preliminary validation of this experimental model. By comparing chemerin levels in non-obese PCOS subjects (BMI < 30) and normal weight androgenized rats, we have demonstrated that as in rats, the rise of chemerin in the follicular fluids, but not in the sera, is independent of changes in serum concentration and adiposity. It has been reported that serum chemerin levels are higher in obese PCOS patients compared to PCOS non-obese counterparts [54, 55] and that serum chemerin may reflect the secretion from peripheral sources, such as the fat tissue [56] and liver [57]. In addition, we have also demonstrated that, as in androgenized rats, human PCOS exhibits a proinflammatory ovarian phenotype, as evident by a higher M1: M2 ratio compared to controls. While in rats we showed an association between macrophages and granulosa cell apoptosis, in this study we could not assess apoptosis of granulosa cells in humans, and although Mikaeili and colleagues [58] have shown that granulosa cell apoptosis is higher in PCOS patients compared to controls it will be necessary additional in vitro studies of co-culture of granulosa cell and macrophages to confirm this association in humans.
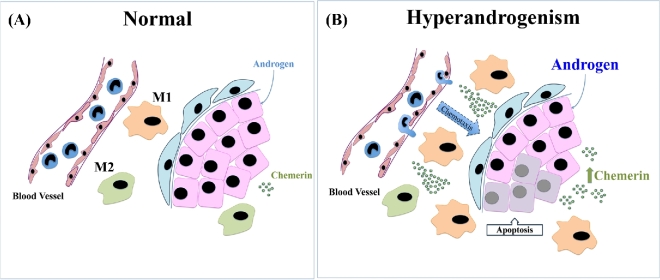
In conclusion, we have demonstrated for the first time a possible role of chemerin in the regulation of the immune system in the pathogenesis of PCOS. Hyperandrogenemia induces chemerin-mediated follicle growth arrest by regulating the monocyte-macrophage migration and function. To facilitate future investigations on the regulation of immune cells in the pathogenesis of PCOS, we propose the following working hypothesis: hyperandrogenism, as often observed in PCOS, increases ovarian chemerin, which in turn functions as a chemoattractant for blood monocytes expressing CMKLR1. Inflammatory monocytes-derived CMKLR1-expressing macrophages are attracted to chemerin-rich ovarian follicles and induce granulosa cell apoptosis (Figure 7). These studies would enhance the current understanding on the molecular and cellular basis of anovulatory infertility, including PCOS, and offer new therapeutic strategies for this complex health condition.
Figure 7.
Hypothetical model illustrating the role of chemerin in the androgenic regulation of immune cells in the ovary and granulosa cell fate. (A) Physiological levels of androgen and chemerin are important in maintaining cellular and immunological homeostasis in the ovary. M1 and M2 macrophages are present in the ovarian follicles and promote follicle growth. (B) Under hyperandrogenic conditions, as commonly observed in PCOS, androgen promotes the production of chemerin by granulosa cells which function as a chemoattractant and induces the migration of CMKLR1+ monocytes from the circulation to the ovarian tissue, where they are differentiated in CMKLR1+ M1 macrophages. Inflammatory CMKLR1+ macrophages under androgenic stimulus contribute to the granulosa cells apoptosis and follicle growth arrest.
Supplementary data
Supplemental Figure S1. M1 (CD86+MHC-II+) and M2 (CD163+CD206+) macrophages identification. Two surface markers were used to confirm the identity of human M1 (CD86 and MHC-II) and for M2 (CD163 and CD206) macrophages. (A) Co-localization of anti-CD86 (green) and anti-MHC II (red) on ovarian macrophages confirmed the M1 phenotype. (B) Co-localization of anti-CD163 (green) and anti-CD206 (red) confirmed the M2 phenotype. (C) Human liver sections were used as positive control (i) for M1 and M2 macrophages co-stained with anti-MHC II (M1 macrophages; red; white arrow) and anti-CD163 (M2 macrophages; green; yellow arrow). Liver sections were also stained with respective isotype control (negative control; ii).
Supplemental Figure S2. Frequency of enriched peritoneal M1 and M2 macrophages and macrophage contamination in granulosa cell culture. (A) Optimized method for peritoneal macrophages enrichment (described in materials and methods section) demonstrates that 38.73% and 53.84% of the viable cells were M1 (CD163–CD68+ cells) and M2 (CD163+CD68+ cells) macrophages, respectively. (B) When tested for macrophages contamination in the granulosa cells culture, 0.37% of cells were positive for CD68.
Supplemental Figure S3. The frequency of peritoneal macrophages (M1 and M2) expressing CMKLR1 was not altered by DHT treatment in vivo. (A) Analysis of peritoneal macrophages from rats treated with DHT for 15 days. Viable cells were gated according to side scatter (SS) and forward scatter (FS). M1 (CD68+CD163–) and M2 (CD68+CD163+) macrophages were identified and quantified. (B) Neither M1 nor M2 macrophage frequency was altered by DHT. Less than 10% of macrophages (M1 or M2) expressed CMKLR1 and were not affected by DHT. Unpaired t-test was used to compare control vs DHT groups. Mean ± SEM (n = 3 rats/replicate, three replicates).
Supplemental Table S1. Antibodies information.
Acknowledgments
Authors’ contributions: PDAL developed the study, designed and performed the experiments, interpreted the results and wrote the manuscript; QW helped in the design of experiments; ALN performed experiments; YAC was responsible for patient data collection and management; AL advised on the experimental design and discussion of results and modified the manuscript; AC and CRT provided human samples, designed experiment, discussed and analyzed the results, and reviewed the manuscript; BKT provided the research funding, developed the study, designed the experiments, and reviewed and modified the manuscript.
Notes
Edited by Dr. Lane K. Christenson, PhD, University of Kansas Medical Center
Footnotes
Grant Support: This work was supported by a grant from the Canadian Institutes of Health Research (MOP-119381 to BKT), the Ottawa Fertility Centre, Ministry of Science and Technology (MOST-104-2314-B-038-063-MY2), Academia Sinica (BM10501010036, BM10601010024) and from National Health Research Institute (MG-105-SP-07, MG-106-SP-07). CIHR postdoctoral fellowships from the IHDCYH-QTNPR Training Program (PDAL) and the IHDCYH-REDIH Training Program (QW, ALN).
References
- 1. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 3. Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, Katsilambros N, Kreatsas G, Panidis D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 2006;21(6):1426–1431. [DOI] [PubMed] [Google Scholar]
- 4. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001; 86(6):2453–2455. [DOI] [PubMed] [Google Scholar]
- 5. Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, Bellastella A, Carella C, Izzo A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol 2003; 101(6):1177–1182. [DOI] [PubMed] [Google Scholar]
- 6. Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol 2011;159(1):148–150. [DOI] [PubMed] [Google Scholar]
- 7. Shen SH, Shen SY, Liou TH, Hsu MI, Chang YI, Cheng CY, Hsu CS, Tzeng CR. Obesity and inflammatory biomarkers in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2015; 192:66–71. [DOI] [PubMed] [Google Scholar]
- 8. Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF II, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; (6) 88:2562–2568. [DOI] [PubMed] [Google Scholar]
- 9. Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, Blumenfeld Z. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab 2004; (5)89:2160–2165. [DOI] [PubMed] [Google Scholar]
- 10. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014; 5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clària J, González-Périz A, López-Vicario C, Rius B, Esther Titos E. New insights into the role of macrophages in adipose tissue inflammation and fatty liver disease: modulation by endogenous Omega-3 fatty acid-derived lipid mediators. Front Immun 2011; 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner EC, Hughes J, Wilson H, Clay M, Mylonas KJ, Kipari T, Duncan WC, Fraser HM. Conditional ablation of macrophages disrupts ovarian vasculature Reproduction 2011; 141(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update 2004; 10(2):119–133. [DOI] [PubMed] [Google Scholar]
- 14. Mariani F, Roncucci L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm Res 2015; 64(2):85–95. [DOI] [PubMed] [Google Scholar]
- 15. Yang S, Wang Q, Huang W, Song Y, Feng G, Zhou L, Tan J. Are serum chemerin levels different between obese and non-obese polycystic ovary syndrome women? Gynecol Endocrinol 2016; 32(1):38–41. [DOI] [PubMed] [Google Scholar]
- 16. Huang R, Yue J, Sun Y, Zheng J, Tao T, Li S, Liu W. Increased serum chemerin concentrations in patients with polycystic ovary syndrome: Relationship between insulin resistance and ovarian volume. Clin Chim Acta 2015; 450:366–369. [DOI] [PubMed] [Google Scholar]
- 17. Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O’Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 2009; 58(9):1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du XY, Leung LL. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim Biophys Sin (Shanghai) 2009; 41(12):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshimura T, Oppenheim JJ. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); Two multifunctional receptors with unusual properties. Exp Cell Res 2011; 317(5):674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cash JL, Hart R, Russ A, Dixon JPC, Colledge WH, Doran J, Hendrick AG, Carlton MBL, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 2008; 205(4):767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, Sironi M, Mantovani A et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 2005; 201(4):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol 2009; 183(10):6489–6499. [DOI] [PubMed] [Google Scholar]
- 23. Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007; 109(9):3625–3632. [DOI] [PubMed] [Google Scholar]
- 24. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab 2006; 91(11):4237–4245. [DOI] [PubMed] [Google Scholar]
- 25. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Task force on the phenotype of the polycystic ovary syndrome -the androgen excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009; 91(2):456–488. [DOI] [PubMed] [Google Scholar]
- 26. Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007; 148(8):3781–3791. [DOI] [PubMed] [Google Scholar]
- 27. Wang Q, Kim JY, Xue K, Liu JY, Leader A, Tsang BK. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology 2012;153(11):5600–5611. [DOI] [PubMed] [Google Scholar]
- 28. Hossain MM, Cao M, Wang Q, Kim JY, Schellander K, Tesfaye D, Tsang BK. Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J Ovarian Res 2013; 6(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JY, Xue K, Cao M, Wang Q, Liu JY, Leader A, Han JY, Tsang BK. Chemerin suppresses ovarian follicular development and its potential involvement in follicular arrest in rats treated chronically with dihydrotestosterone. Endocrinology 2013; 154(8):2912–2923. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Chen K, Wang C, Gong W, Yoshimura T, Wang JM, Liu M. Isolation of mouse tumor-infiltrating leukocytes by Percoll gradient centrifugation. 2013; 3 (17), http://www.bio-protocol.org/e892. Accessed 7 January 2015. [Google Scholar]
- 31. Wang Q, Leader A, Tsang BK. Follicular stage-dependent regulation of apoptosis and steroidogenesis by prohibitin in rat granulosa cells. J Ovarian Res 2013; 8:6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA, Norman RJ. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod 2000; 62(4):1059–1066. [DOI] [PubMed] [Google Scholar]
- 33. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, Ottenhoff TH. Human Anti-Inflammatory macrophages induce Foxp3+GITR+CD25+ Regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol 2008; 181(3):2220–2226. [DOI] [PubMed] [Google Scholar]
- 35. Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukocyte Biol 2006; 79(2):285–293. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto Y, Kuwahara A, Taniguchi Y, Yamasaki M, Tanaka Y, Mukai Y, Yamashita M, Matsuzaki T, Yasui T, Irahara M. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries. Reprod Med Biol 2015; 14(3):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Figueroa F, Motta A, Acosta M, Mohamed F, Oliveros L, Forneris M. Role of macrophage secretions on rat polycystic ovary: its effect on apoptosis. Reproduction 2015; 150(5):437–448. [DOI] [PubMed] [Google Scholar]
- 38. Duda M, Knet M, Tabarowski Z, Slomczynska M. Luteal macrophage conditioned medium affects steroidogenesis in porcine granulosa cells. Reprod Biol 2011; 11(2):117–134. [DOI] [PubMed] [Google Scholar]
- 39. Shakil T, Whitehead SA. Inhibitory action of peritoneal macrophages on progesterone secretion from Co-Cultured rat granulosa cells. Biol Reprod 1994; 50(5):1183–1189. [DOI] [PubMed] [Google Scholar]
- 40. Hofer MD, Cheng EY, Bury MI, Xu W, Hong SJ, Kaplan WE, Sharma AK. Androgen supplementation in rats increases the inflammatory response and prolongs urethral healing. Urology 2015; 85(3):691–697. [DOI] [PubMed] [Google Scholar]
- 41. Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci 2006; 119(4):722–732. [DOI] [PubMed] [Google Scholar]
- 42. Crisosto N, Flores C, Maliqueo M, Echiburú B, Vásquez J, Maluenda F, Sir-Petermann T. Testosterone increases CCL-2 expression in visceral adipose tissue from obese women of reproductive age. Mol Cell Endocrinol 2017; 444:59–66. [DOI] [PubMed] [Google Scholar]
- 43. Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest 2009; 119(12):3739–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahmadi-Renani K, McCruden AB. Effect of five alpha dihydrotestosterone (5a-DHT) on cytokine production by peritoneal macrophages of NZB/BALBc mice. Med J Islam Rep Iran 1997; 11 (3):223–228. [Google Scholar]
- 45. Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003; 198(7):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol 2010; 185(6):3728–3739. [DOI] [PubMed] [Google Scholar]
- 47. Demoor T, Bracke KR, Dupont LL, Plantinga M, Bondue B, Roy MO, Lannoy V, Lambrecht BN, Brusselle GG, Joos GF. The role of ChemR23 in the induction and resolution of cigarette smoke-induced inflammation. J Immunol 2011; 186(9):5457–5467. [DOI] [PubMed] [Google Scholar]
- 48. Graham KL, Zabel BA, Loghavi S, Zuniga LA, Ho PP, Sobel RA, Butcher EC. Chemokine-Like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J Immunol 2009; 183(10):6717–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 2011; 7(11):e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5(12):953–964. [DOI] [PubMed] [Google Scholar]
- 51. Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res 2012; 53(1-3):41–57. [DOI] [PubMed] [Google Scholar]
- 52. Lim JJ, Lima PDA, Salehi R, Lee DR, Tsang BK. Regulation of androgen receptor signaling by ubiquitination during folliculogenesis and its possible dysregulation in polycystic ovarian syndrome. Sci Rep 2017; 7(1):10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salilew-Wondim D, Wang Q, Tesfaye D, Schellander K, Hoelker M, Hossain MM, Tsang BK. Polycystic ovarian syndrome is accompanied by repression of gene signatures associated with biosynthesis and metabolism of steroids, cholesterol and lipids. J Ovarian Res 2015; 8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guvenc Y, Var A, Goker A, Kuscu NK. Assessment of serum chemerin, vaspin and omentin-1 levels in patients with polycystic ovary syndrome. J Int Med Res 2016; 44(4):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang S, Wang Q, Huang W, Song Y, Feng G, Zhou L, Tan J. Are serum chemerin levels different between obese and non-obese polycystic ovary syndrome women? Gynecol Endocrinol 2016;32(1):38–41. [DOI] [PubMed] [Google Scholar]
- 56. Alfadda AA, Sallam RM, Chishti MA, Moustafa AS, Fatma S, Alomaim WS, Al-Naami MY, Bassas AF, Chrousos GP, Jo H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol Cells 2012; 33(6):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kajor M, Kukla M, Waluga M, Liszka Ł, Dyaczyński M, Kowalski G, Żądło D, Berdowska A, Chapuła M, Kostrząb-Zdebel A, Bułdak RJ, Sawczyn T et al. Hepatic chemerin mRNA in morbidly obese patients with nonalcoholic fatty liver disease. Pol J Pathol 2017;68(2):117–127. [DOI] [PubMed] [Google Scholar]
- 58. Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, Abbasi M. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet 2016; 294(1):185–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.