Abstract
Nanomaterials that respond to externally applied physical stimuli such as temperature, light, ultrasound, magnetic field and electric field have shown great potential for controlled and targeted delivery of therapeutic agents. However, the body of literature on programming these stimuli-responsive nanomaterials to attain the desired level of pharmacologic responses is still fragmented and has not been systematically reviewed. The purpose of this review is to summarize and synthesize the literature on various design strategies for simple and sophisticated programmable physical stimuli-responsive nanotherapeutics.
Keywords: nanomaterial, physical stimuli, programmability, stimuli-responsive, targeting
Introduction
The ever-increasing prevalence of cancer, metabolic disorders and neurodegenerative diseases as well as the quest for efficient treatments of these and other diseases have intensified the need for new, alternative, and novel drug delivery systems that can release the loaded drugs at the target site “on-demand”. Among the various novel drug delivery approaches investigated, nanotechnology has been playing increasingly important roles for the needed targeted drug delivery. Nanomaterials including polymeric [1], lipidic [2] inorganic [3], and inorganic-organic hybridnanoparticles [4]; liposomes [5,6]; nanocrystals [7]; micelles [8]; microemulsions [9]; polymersomes [10]; dendrimers [11]; nanogels [12]; nanofibers [13]; nanowires [14]; nanoscaffolds [15]; nanopatterned surfaces [16]; nanorods [17]; nanocomposites [18]; nanofluidic devices [19]; carbon nanotubes [20]; nanosheets [21]; and nanomembranes [22] have been developed and evaluated for controlled drug delivery. These nanocarriers can be designed to assume variety of bulk and surface chemistry, sizes, shapes, and architectures, for improved drug release, targeting, and blood circulation time. For instance, positively charged surfaces generally enhance nanoparticle cellular uptake [23–25]. PEGylation (the process of attaching polyethylene glycol (PEG) chains) of nanocarriers induces steric repulsion of blood opsonins and significantly increases the circulation time of nanomaterials [26]. The size of nanomaterials affects the biodistribution and cellular uptake of the nanomaterials. In general, it is postulated that nanomaterials with sizes 10 to 100 nm can easily be taken up by cells through endocytosis. However, larger nanomaterials can also enter into cells at slower rates through different endocytosis pathways [27]. For example, Oh et al. (2009) [28] showed layered double hydroxide nanoparticles were taken up by human osteosarcoma (MNNG/HOS) cells in the order of 50 > 100/200 > 350 nm, where 50 to 200 nm nanoparticles were selectively internalized by clathrin-mediated endocytosis. Nanomaterials with sizes > 150 nm have a much greater chance of being entrapped in the liver and spleen, and nanomaterials with sizes < 5 nm are highly likely to be filtered out by the kidneys [29,30]. Ascribed to their enhanced permeability and retention into various tumors, nanoparticles with sizes in the range of 100–200 nm have shown great tumor targeting potentials. Nonetheless, the desired level of drug targeting and release is yet to be achieved using traditional nano-formulations and, despite decades of efforts, only few nano-formulations have reached the market [30,31]. There is unmet need to program nanomaterials with more appropriate structures and properties for effective therapeutic effects.
Stimuli-responsive nanomaterials can take advantage of the specific microenvironmental changes in some disease conditions such as ischemia, inflammatory diseases, infections, and tumor, which have served as the bases for designing most of the chemical stimuli-responsive nanomaterials. Alternatively, they can be designed to respond to various externally applied physical stimuli such as temperature, light, ultrasound, magnetic field, electric field and X-ray. Generally, unlike the internal stimuli, external triggers are easier to control and are associated with less variability. The choice of a specific stimuli-responsive nanocarrier can be made based on several factors like the intended application, the target site, the cost of treatment and the safety concerns. In addition, there have been many attempts to enhance the programmability of various stimuli-responsive nanomaterials for improved therapeutic effects. For instance, functionalization of the surfaces of nanomaterials using specific ligands and targeting agents such as antibodies, peptides, nucleotide aptamers, and other small molecules may significantly improve drug targeting. Another possibility is introduction of linkers or groups that are responsive to different exogenous or endogenous stimuli, which possibly render the nanoparticles responsive to multiple stimuli and provide improved platforms for advanced programmability. In this review, the design strategies for simple and sophisticated programmable physical stimuli-responsive nanotherapeutics are systematically discussed.
Thermoresponsive Nanomaterials
Thermoresponsive nanomaterials are a class of “smart” materials that undergo phase transition in response to temperature change. The temperature at which the phase transition occurs is called critical solution temperature (CST). If thermoresponsive materials change from a hydrophilic and highly swollen state to a hydrophobic and collapsed state at CST when temperature is increased, the CST is called a lower CST or LCST. If thermoresponsive materials change from a hydrophobic and collapsed state to a hydrophilic and highly swollen state at CST when temperature is increased, the CST is called an upper CST or UCST. The thermoresponsive materials that have been investigated for biomedical applications usually have a LCST. Through tailoring their chemistry, LCST, architecture, and targeting moiety, thermoresponsive nanomaterials can be programmed for different biomedical applications. The strategies for the programming are discussed below.
Programming with different basic chemistry that is thermoresponsive
Various types of thermoresponsive polymers have been used to design thermoresponsive nanomaterials. One type of thermoresponsive polymers are poly(N-substituted acrylamide)s including poly(N-isopropylacrylamide) (PNIPAAM) and poly(N,N-diethylacrylamide). PNIPAAM is the first and most investigated thermoresponsive polymer and has a LCST of 32 °C, which is close to the physiological temperature 37 °C. The LCST of PNIPAAM is not dependent on its molecular weight, concentration, or other environmental conditions [32,33]. Unlike PNIPAAM, the LCST of poly(N,N-diethylacrylamide) depends on the tacticity of the polymer [34], which limits its use. The second type of thermoresponsive polymers are poly(N-vinyl-alkyl-amide)s like poly(N-vinylcaprolactam) and poly(N-vinylisobutyramide) polymers that have LCST of 30 – 50 °C [35]. Poly(N-vinylcaprolactam) was well-tolerated by human intestinal Caco-2 and bronchial Calu-3 cell lines but it is less investigated than PNIPAAM as a thermoresponsive polymer [36]. It exhibits a “classical” Flory–Huggins thermoresponsive phase behavior in water with LCST decreasing with increasing polymer chain length and concentration [36]. It is used to form thermoresponsive nanogels for controlled drug delivery or for polymer surface grafting. For example, chitosan was grafted by N-vinylcaprolactam and crosslinked by sodium tripoly-phosphate to form chitosan-g-poly(N-vinylcaprolactam) nanoparticles [35]. The nanoparticles released 5% and 40% of the loaded 5-fluoreuracil over 3 days below and above its LCST, respectively. The third type of thermoresponsive polymers are the block copolymers of poly(ethylene oxide) and poly(propylene oxide) called Pluronics®. They have LCSTs between 20–85 °C, which can be tailored by the lengths of the hydrophilic poly(ethylene oxide) and the hydrophobic poly(propylene oxide) segments and their ratios. They are amphiphilic polymers approved by US FDA for use as food additives and pharmaceutical ingredients [37]. Due to their amphiphilic nature they are commonly used to form thermoresponsive vesicles or surface grafting agents [37,38]. Poly(oligo ethylene glycol methacrylate)s having oligo ethylene glycol grafted to a poly(methacrylate) backbone are the fourth type of thermoresponsive polymers. Their LCSTs can be tuned from 22 to 90 °C by varying the length and density of the oligo ethylene glycol graft. The higher and longer the oligo ethylene glycol density and chain length, respectively, the higher is their LCST [33,39]. For example, Tian et al. (2016) [40] fabricated doxorubicin-loaded dual thermo- and redox-responsive nanogels using poly(oligo ethylene glycol methacrylate) and 2-(2-methoxyethoxy) ethyl methacrylate using the disulfide-containing crosslinker N,N’-bis(acryloyl)cystamine. When the mass ratio of poly(oligo ethylene glycol methacrylate) and 2-(2-methoxyethoxy) ethyl methacrylate was varied from 0/100 to 15/85, their LCST changed from 25.7 to 42.8 °C. Poly(N-alkyloxazolines) (polyoxazolines) made of pseudo-polypeptide backbone and alkyl side chains are the fifth type of thermoresponsive polymers. Polyoxazolines have a broad water solubility and reactivity depending on the alkyl chain length, and thus tunable LCST [41–43]. They were reported to have low immunogenicity [44], biodegradability, [45] and good penetration through porcine gastric mucosa [46]. It is worthy to point out that poly(2-isopropyl-2-oxazoline) is a structural isomer of PNIPAAM with a LCST close to the physiological temperature [41,42]. Polyoxazolines are commonly used as nanostructure surface grafting agents [41–43]. For example, Kurzhals et al. (2017) [42] grafted the surfaces of magnetic nanoparticles using poly(2-isopropyloxazoline) (LCST in cell culture medium = 32.5 °C) and poly(2-ethyloxazoline) (LCST in cell culture medium = 37 °C) to form core-shell magnetic nanoparticles. The permeability of poly(2-isopropyloxazoline)-grafted nanoparticles was about 4-fold greater than the permeability of poly(2-ethyloxazoline)-grafted nanoparticles in HeLa cells at 37 °C. The difference is attributed to the hydrophobicity of the former, with LCST below 37 °C.
Thermoresponsive polymers are not only made of the synthetic polymers discussed above, but also polypeptides or lipids. Elastin-like polypeptides composed of multiple repeating pentapeptide units of Val–Pro–Gly–Xaa–Gly (Xaa is any amino acid except proline) exhibit sharp transition temperature within 2–3 °C [47–49]. Their LCSTs can be tuned by both internal factors such as amino acid composition and polymer molecular weight, and external factors such as ionic strength and concentration. The more hydrophobic the amino acid and the higher the molecular weight, the lower is the LCST [49]. Elastin-like polypeptides can be used to form composite nanoparticles and vesicular nanostructures [47–49]. For example, Bessa et al. (2010) [50] prepared bone morphogenetic proteins-2 and −14-loaded nanoparticles by thermoresponsive self-assembly of the elastin-like polypeptide (VPAVG)220 (transition temperature = 33 °C) at 37 °C. Following an initial burst release for 24 h, the nanoparticles slowly released the loaded cytokines for 14 days in vitro at 37 °C. The synthetic N-substituted linear homopolypeptoids like poly(N-C3 glycine)s and the random copolypetoids like poly(N-methylglycine)-poly(N-butylglycine) are another type of thermoresponsive polypeptides with LCSTs 27 to 71 °C depending on the type and degree of monomer substitution [51]. For example, Kurzhals et al. (2017) [51] grafted magnetic nanoparticles using poly(N-methylglycine)-poly(N-butylglycine) polypeptoid with different percentages of N-methylglycine and N-butylglycine and the aggregation temperature of the nanoparticles increased from 33 to 58 °C when the percentage of N-methylglycine increased from 61% to 73%. Poly(N-substituted asparagines) are the third type of biodegradable thermoresponsive polypeptides with LCSTs between 28–78 °C [30,52]. They are amphiphilic and biodegradable. Liposomes made of dipalmitoyl phosphocholine or myristoyl stearoyl phosphatidylcholine have thermoresponsive property with UCST (note: not LCST) between 40 and 45 °C [6,53]. Above the UCSTs, the liposomes undergo gel-to-sol transition and the lipid bilayer will be transformed from a solid state to a fully liquid state rendering the membrane highly permeable for the loaded drugs [53]. Thermoresponsive liposomes are among the pioneering stimuli-responsive nanocarriers of which few have advanced to clinical trial stages [5,6]. For example, the doxorubicin-loaded thermoresponsive liposome ThermoDox® has reached phase III clinical trial for the treatment of various solid tumors and it allowed 25 times greater concentration of the drug in cancerous tissues as compared to intravenous doxorubicin [6,53].
Programming the LCST for thermal targeting and release
The LCST is a unique property of thermoresponsive nanomaterials that can be utilized to localize drugs at a target site [11,54]. The thermoresponsive nanomaterials made of different thermoresponsive polymers with different chemistries have different LCSTs which are higher or lower than body temperature, 37 °C. Thermoresponsive nanocarriers with LCST lower than 37 °C can be used to increase drug retention time and permeability across biological barriers due to their sol-to-gel phase transition at 37 °C. For example, thermoresponsive self-assembled poloxamer 407 nanogels were shown to adhere on the corneal surface and increased the permeability of muscone across the cornea 3.4-fold [55]. The hydrophilic poly(ethylene oxide) segments and hydrophobic poly(propylene oxide) segments of Pluronics® and D-α-tocopheryl PEG succinate self-assembled into micelles/vesicles at 50 °C and could cross the blood brain barrier and enhance the permeability of the small-molecular model drug Rho123 in Sprague-Dawley (SD) rats after intravenous administration [56]. Pluronics® are known efflux protein inhibitors and the mixed micelles containing Pluronic® F127 and Plasdone S630 increased the oral bioavailability of biochanin A 2 16-fold in Sprague Dawley (SD) rats compared with the free drug [57].
If the LCST is designed to be slightly higher than the body temperature 37 °C, the nanomaterials are dispersible in physiological fluid and can circulate in the body at body temperature. However, if the disease site (target site) is locally heated up to 40–42 °C by ultrasound, near infrared light [58], magnetic field [58,59], radiofrequency [6], or other techniques, the thermoresponsive nanomaterials circulating in the blood become hydrophobic and are easily taken up by the surround cells and tissue so that thermally targeted drug delivery can be achieved. For example, doxorubicin [60], 17-(Allylamino)-17-demethoxygeldanamycin [61] and 5-fluorouracil [35] were loaded into cationic thermosensitive liposomes, core-shell composite thermoresponsive nanoparticles, and chitosan-g-poly(N-vinylcaprolactam) thermoresponsive nanoparticles, respectively. The nanocarriers improved the cellular uptake of the drugs in different tumor cell lines upon hyperthermia and were more cytotoxic in comparison to the free drugs alone. Furthermore, when gold nanorods that can absorb near-infrared (NIR) light at around 800 nm to generate heat or inorganic nanoparticles such as magnetic nanoparticles that can convert external alternating magnetic field into heat [58,59] can be imbedded within the core of such thermoresponsive nanoparticles, drug release can be turned “ON” or “OFF” by applying and removing NIR or magnetic field, respectively, to induce “on-demand drug release”. Such smart nanocarriers, loaded with different drugs such as doxorubicin [59,62], bupivacaine [63], vascular endothelial growth factor [64] and curcumin [65] are reported. In addition, unlike externally applied direct thermal stimulation, which heats the entire area of operation, utilizing internal heat sources may provide highly localized and remotely controlled drug release [66].
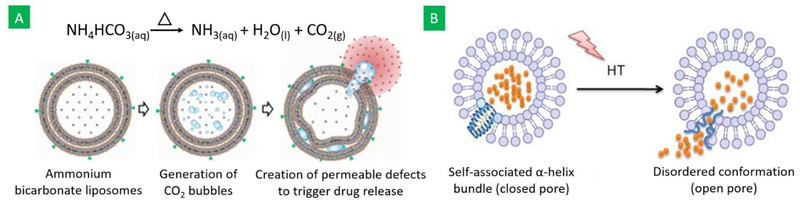
The desired LCST can be obtained by incorporating other components into basic thermoresponsive polymers through copolymerization, conjugation, and grafting [63,64,67]. In general, hydrophilic components increase the LCST, and hydrophobic components decrease the LCST [68]. For examples, the LCST of NIPAAM nanogels increased from 32 to 37, 42 and 46 °C upon copolymerizing it with 51% N-isopropylmethacrylamide and 6% acrylamide, 58% N-isopropylmethacrylamide and 7% acrylamide, and 55% N-isopropylmethacrylamide and 11% acrylamide, respectively [63]. Similarly, addition of 20% of the lipophilic monomer poly(N-alkylacrylamide) N-tert-butylacrylamide lowered the LCST of NIPAAM to 20 °C, whereas incorporation of the hydrophilic monomer poly(N-alkylacrylamide) acrylamide increased the LCST to 42.1 °C [67]. Adsorption of superparamagnetic iron oxide nanoparticles (SPIONs) on the PNIPAAM chain increased its LCST from 32 to 52 °C, depending on the amount of SPIONs added [64]. Vesicular nanostructures can also be rendered thermoresponsive for controlled drug release and diagnosis purposes using bubble generating agents. For example, ammonium bicarbonate – a CO2 bubble-generating agent – was incorporated into thermoresponsive liposomes. When heated to a little above 40 °C, CO2 bubbles were generated, which created permeable defects on the liposomes and enhanced drug release was obtained (Figure 1A) [69]. In addition, the generated CO2 bubbles are hyperechogenic and may be used as an ultrasound contrast agent in elucidating the status of the carriers and providing real-time diagnostic images [69]. The potential of using therapeutic gases such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) in such bubble-generating carrier systems for the treatment of tumors has also been assessed [70].
Figure 1:
Schematic representation of (A) thermoresponsive bubble-generating liposomes, designed by adding bubble generating agents, and (B) liposome-peptide hybrid thermoresponsive vesicles, designed by adding a thermoresponsive amphiphilic leucine zipper peptide, into thermoresponsive liposomes and their response to hyperthermia (HT) (Figures obtained from references [69] and [71]).
Programming with different architecture
The size, shape and porosity of thermoresponsive nanomaterials also affect the targeting and therapeutic efficiency of the drug-loaded nanomaterials [12,68]. Many of the thermoresponsive polymers developed have been deployed to form composite nanoparticles including crosslinked nanogels. When thermoresponsive block copolymers such as Pluronics® are used, micelles can be formed [55]. Furthermore, thermoresponsive block copolymers can self-assemble into thermoresponsive supramolecular nanostructures with different intraparticle morphologies like lamella and gyroid, which allow different drug release mechanisms. For example, 1-anilinonaphthalene-8-sulfonic acid-loaded nanoparticles of the triblock polymer polystyrene-PNIPAAM-polystyrene were prepared in three different morphological architectures: polystyrene spheres in PNIPAAM matrix, polystyrene gyroids in PNIPAAM matrix, and polystyrene—PNIPAAM lamellar structure. Dye release from the gyroidal nanoparticles (15.7% at 25 °C; 8.1% at 45 °C in 3.6 h) was higher than the sphere-forming nanoparticles (10.6% at 25 °C; 4.3% at 45 °C in 3.6 h) [32]. Micellar aggregates can also be crosslinked to give thermodynamically stable vesicular systems with thermoresponsive cores [24]. Thermoresponsive liposomes are a special type of vesicles formed by hydrophobic lipid bilayers and aqueous core.
Programming with additional functional groups
To make thermoresponsive nanomaterials more functionable, charges, cell binding ligands, and biodegradable crosslinkers have been added to the nanomaterials. Charged nanoparticles can increase drug loading and sustain the release of oppositely charged drugs. For example, incorporation of 20 mole% of the negatively charged acrylic acid to PNIPAAM nanogels significantly increased the loading capacity [72] and sustained the release of the positively charged local anesthetic bupivacaine due to ionic interactions and increased the duration of action of the drug by more than 3-fold [23]. Conversely, 2-aminoethyl methacrylamide hydrochloride rendered thermoresponsive nanoparticles cationic and improved the encapsulation efficiency, prolonging the release of the negatively charged proteins insulin, bovine serum albumin, and β-galactosidase [24]. Du et al. [25] (2010) designed special pH-responsive charge conversional thermoresponsive nanogels, which transformed from negatively charged into positively charged in the slightly acidic tumor extracellular environment. The charge conversion significantly enhanced nanogel cellular uptake and doxorubicin release from the nanogels to improve the cytotoxic effect of the drug. The surfaces of thermoresponsive nanoparticles can also be modified by cell binding ligands such as antibodies, peptides, aptamers, or small molecules, which can enhance cell targeting and nanocarrier cellular uptake by endocytosis. For instance, folate receptors are overexpressed in a wide variety of tumor cells and folic acid has been widely used as tumor targeting ligand by conjugating it to thermoresponsive nanoparticles [59,73]. In another example, surface modification of composite/hybrid core-shell thermoresponsive nanoparticles by integrin β4 increased the accumulation of the nanoparticles on the surfaces of squamous head and neck carcinoma cells, on which A9 antigen was over-expressed [59].
When nanoparticles are biodegradable, they can achieve sustained drug delivery. Crosslinkers that degrade or hydrolyze in response to different endogenous stimuli such as acidic pH (e.g. 2,2-dimethacroyloxy-1-ethoxypropane [24,74], HEMA-lactate-Dextran [75–78], poly(l-lactic acid)[79]), redox potential (e.g. bis(2-methacryloyloxyethyl) disulfide [80] and disulfide-containing crosslinker N,N’- bis(acryloyl) cystamine [40]) or enzymes (e.g. dextran-methacrylate [81]) have been introduced to thermoresponsive nanoparticles. PEGylation can help to increase circulation time and improve treatment effectiveness of nanotherapeutics. For example, PEGylation of PNIPAAM-co-polymethacrylate thermoresponsive nanogels significantly decreased the uptake of the nanogels by THP-1 human acute monocyte cells (macrophages) in vitro [82]. Hybrids of different thermoresponsive polymers and or polypeptides into a nanomaterial system can have synergetic effects on the temperature-responsiveness and consequently better therapeutic effects of the nanosystem. For example, a hybrid nanosystem containing the thermoresponsive amphiphilic leucine zipper peptide and thermoresponsive liposomes (Figure 1B), which have a phase transition temperature 42 °C, prolonged the blood circulation time of the loaded doxorubicin, leading to a 3-fold accumulation of the drug in the heated tumor site in SW480 tumor-bearing mice in comparison to lysolipid-modified thermoresponsive liposomes [71,83].
Light-responsive Nanomaterials
Light-responsive (photoresponsive) nanomaterials are a class of “smart” materials that undergo chemical and or physical changes in response to light stimuli. Light in long ultraviolet 200–400 nm, and NIR 650–900 nm (wavelength range that is minimally absorbed by skin and tissue), have been utilized as attractive exogenous stimuli for biomedical applications owing to their minimally invasive nature and possibility to be applied with high spatial and temporal precision [84,85]. Drug release from light-responsive nanomaterials can be regulated via adjustments of the chemistry of photosensitive or photocleavable compounds, light wavelength and intensity, and duration of exposure [86]. The strategies for designing these parameters to program light-responsive nanomaterials for desired therapeutic effects are discussed below.
Programming with different basic chemistry that is light-responsive
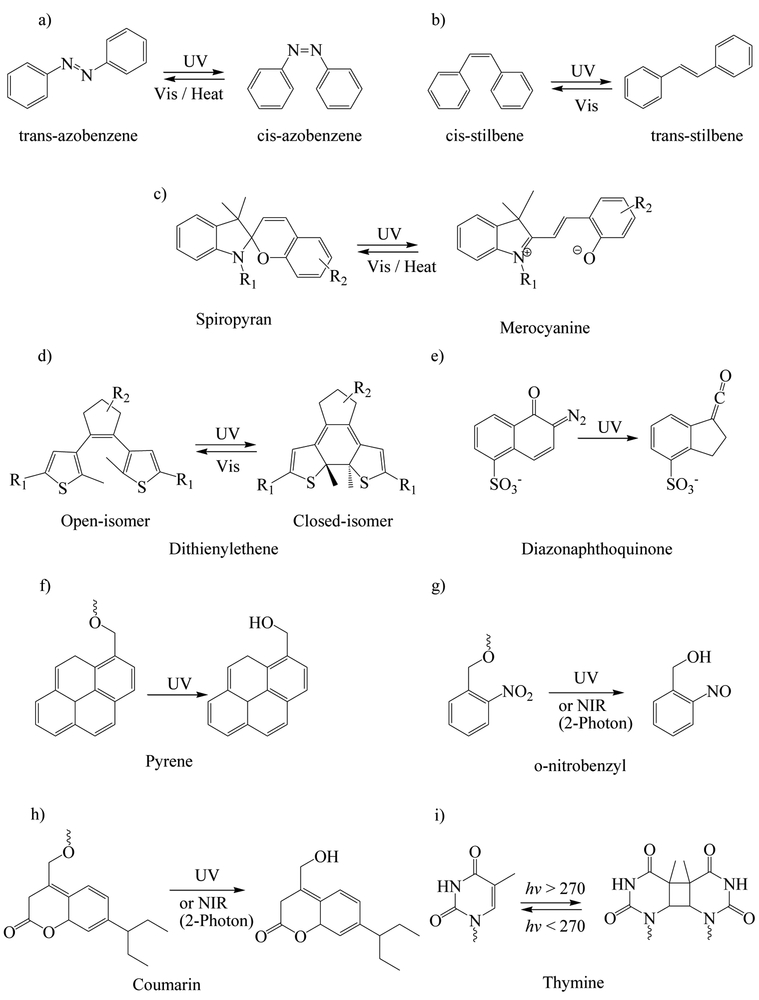
Photosensitive compounds that are commonly used for designing light-responsive nanomaterials are azobenzene, stilbene, spiropyran, dithienylethene, diazonaphthoquinone, and Pheophorbide A; these undergo reversible or irreversible photoisomerization upon exposure to light (Figure 2). They are usually doped or covalently bound to various nanostructures. Azobenzene and stilbene undergo reversible trans—cis isomerization when exposed to 300–380 nm, where the cis isomers have much higher dipole moment than the trans-isomer. Patnaik et al. (2007) [87] conjugated the hydrophobic azobenzene to the hydrophilic dextran and then obtained self-assembled micelles. These micelles could dissociate and rapidly release the loaded acetylsalicylic acid and rhodamine upon UV irradiation due to the photoisomerization of the hydrophobic trans-azobenzene into the hydrophilic cis-azobenzene. Spiropyran is neutral and can isomerize to charged merocyanine. Dithienylethene can undergo a reversible transition from the ring-open isomer to ring-closed isomer. Diazonaphthoquinone undergoes irreversible photoinduced Wolff rearrangement when exposed to UV light [88]. Pheophorbide A is a photosensitizer, which upon exposure to longer excitation wavelengths generates reactive oxygen species (mainly singlet oxygen) that can rupture endo/lysosomes to induce photochemical internalization. Photochemical internalization is a process by which macromolecules and other compounds that are entrapped in endocytic vesicles formed after endocytosis are released to the cytosol by light [89]. Pheophorbide A-labelled polyethylenimine nanoparticles enhanced the cellular uptake of FITC-labeled ovalbumin by murine dendritic cells by approximately 2.8-fold, and after irradiation of the cells by a 670 nm laser, a more diffused pattern of the protein was observed in the cytoplasm indicating protein release from the endocytic vesicles to the cytoplasm [89].
Figure 2.
Commonly used photosensitive (a-e) and photocleavable (f-i) compounds/functionalities used for the preparation of light-responsive nanomaterials and their reaction to light.
The commonly used photocleavable groups include pyrene, o-nitrobenzyl, coumarin, and thymine (Figure 2). Pyrene undergoes photosolvolysis in the presence of water or other protonic solvents. The o-nitrobenzyl group is sensitive to far UV light and undergoes photolysis or intramolecular rearrangement even in the absence of water and can also be activated by NIR light through two-photon absorption [88,90]. Azagarsamy et al. (2012) [91] used hydroxyethyl acrylate and o-nitrobenzyl-containing crosslinker to synthesize photodegradable nanogels. When the nanogel was irradiated with 365 nm UV light, it degraded to release the loaded protein alkaline phosphatase. Huu et al. (2015) [90] prepared nintedanib-loaded, light-responsive nanoparticles using a preformed polymer that contains o-nitrobenzyl groups. The nanoparticles remained stable for 10 weeks post-intravitreal injection but rapidly released nintedanib when exposed to 365 nm light to suppress the choroidal neovascularization in Brown Norway rats. Coumarin has a more efficient two-photon absorption of NIR light than o-nitrobenzyl derivatives [88]. Thymine photodimerizes upon irradiation above 270 nm and reverts back to its monomeric form when irradiated below 270 nm [92]. He et al. (2012) [93] grafted thymine derivatives on the surfaces of mesoporous silica nanoparticles as gatekeepers. When the nanoparticles were irradiated with 240 nm UV light, thymine was cleaved to open the gate and then the loaded model compound tris(bipyridine)ruthenium(II) dichloride was released. Afterwards, the gate could be closed by applying 365 nm UV light to induce photodimerization of thymine.
Some metals or metallic oxides like TiO2, ZnO, CuO, and Au have also been utilized to prepare light-responsive nanomaterials. For example, Wang et al. (2015) [94] fabricated paclitaxel-loaded porous TiO2 nanoparticles and grafted their surfaces using polyethylenimine by amide linkage to close the pores. The nanoparticles were further modified by folic acid for tumor targeting. The cumulative amount of paclitaxel release from the nanoparticles after 3 h was 3.2%. However, upon UV irradiation of the nanoparticles for 5 min, 10 min and 15 min, the polyethylenimine molecules on the surface were cleaved by the free radicals (OH· and O2·) generated by TiO2 and released 20.1%, 37.2%, and 73.4% of the paclitaxel over 3 h, respectively. Nanoparticles made of gold in rod, shell, or hollow sphere shape, and carbon nanotubes can absorb NIR light and generate heat for photothermally targeted drug delivery [26]. This technology was also mentioned in the above Thermoresponsive Nanomaterials Section, and can be used to deliver drugs in deep tissues because NIR can penetrate through 10 cm with minimal absorption or scattering by water and tissues [26,95–97]. Doxorubicin-loaded hollow gold nanospheres were administered intravenously to mice bearing Hey tumor and irradiation of the tumor area 24 h after injection using 808 nm NIR laser light resulted in rapid release and distribution of the doxorubicin in the treated area [96].
Programming with additional functional groups
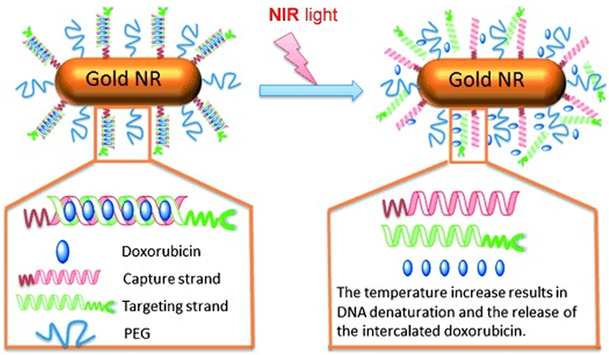
The programmability of light-responsive nanomaterials can be enhanced by attaching additional functional groups such as folic acid [73], antibodies [34], aptamer [98], PEG [73], and thermoresponsive materials [85,98] for targeted and efficient drug delivery. Xiao et al. (2012) [85] developed interesting light-responsive nanocarriers based on complementary DNA strands, which contained sequential CG base pairs to provide a loading platform for doxorubicin (Figure 3). One end of one of the DNA strands (capturing strand) was thiolated and attached to gold nanorods, whereas the opposite end of the other complementary DNA strand (targeting strand) was conjugated with folic acid ligand for cell-specific targeting. Upon 808 nm NIR irradiation, the gold nanorods served as NIR light-to-heat transducers and the heat generated by the gold nanorods dehybridized the DNA strands to release the loaded doxorubicin in BALB/c nude mice xenograft tumor site. Furthermore, the nanoparticles were PEGylated to improve their blood circulation half-life. The folic acid-targeted nanoparticles showed greater cytotoxicity than the non-targeted nanoparticles in human nasopharyngeal epidermoid carcinoma cell lines (34.37±3.03 versus 56.37±0.69 cell viability). In cancerous mice models, induced by injection of human nasopharyngeal epidermoid carcinoma cells, the relative tumor volume growth after 14 days of administration of the targeted nanoparticles was 35% less than the non-targeted nanoparticles due to targeted photothermal ablation. Doxorubicin loading decreased tumor growth rate by a further 28%. In another study, Lee et al. (2011) [99] conjugated herceptin, an antihuman epidermal growth factor receptor 2 (HER2) antibody, to poly(lactic-co-glycolic acid) (PLGA)-gold half-shell nanoparticles, to have dual receptor binding and NIR irradiation effects and to increase the accumulation of the nanoparticles. This technology allowed slow release of doxorubicin at breast cancer cells in mice. When the mice were treated with doxorubicin alone or the targeted nanoparticles without NIR, the tumor grew continuously, but at a slower rate than the control groups. When they were treated with the non-targeted nanoparticles or targeted nanoparticles without doxorubicin and irradiated with NIR for 10 min, tumor growth was reduced by 75% and 65% in 10 and 18 days, respectively, and afterwards the the tumor started to grow rapidly. Treatment with the targeted doxorubicin-loaded nanoparticles followed by 10 min NIR irradiation resulted in complete tumor destruction within 7 days with no tumor recurrence.
Figure 3.
Doxorubicin loaded and folic acid modified DNA nanoaggregates that are attached to gold nanorods (gold NR) to form NIR-responsive nanotherapeutics. Upon NIR exposure, the gold NR generate heat that dehybridizes the DNA aggregates and releases the loaded doxorubicin (Figure taken from reference [85]).
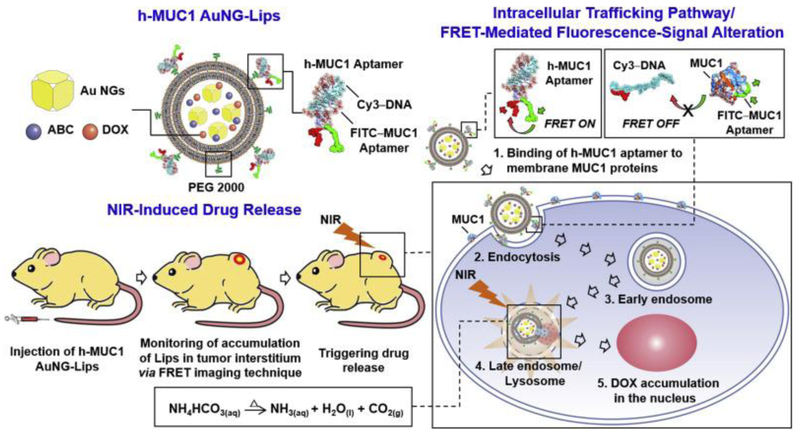
To further enhance drug delivery at the targeted site, ammonium bicarbonate-loaded bubble-generating and mucin-1 aptamer surface-modified thermoresponsive liposomes were used together with gold nanocages [98]. Upon irradiation, the gold nanocages converted the NIR into localized heat and decomposed the loaded ammonium bicarbonate to generate CO2 bubbles, which created permeable defects on the lipid membrane and rapidly triggered doxorubicin release (Figure 4). The mucin-1 aptamer that was hybridized on the surfaces of the thermoresponsive liposomes not only functioned for drug targeting, but also acted as a molecular beacon signaling the optimal timing of photothermal heating. Administration of the loaded liposomal systems in tumorigenic rat models reduced the relative tumor volume to about 25% and 60% over 12 days when administered with and without NIR, respectively. Administration of free doxorubicin did not significantly reduce the tumor volume. Drug release from UV/visible light-responsive nanomaterials can also be modulated in deep tissues by introduction of upconversion luminescent materials such as lanthanide ions, ytterbium and erbium, which convert low energy NIR light to higher energy radiation UV/visible light via multiple absorption or energy transfer. For example, Liang et al. (2017) [73] fabricated folic acid-functionalized, doxorubicin-loaded, hollow mesoporous multifunctional upconversion luminescent ytterbium- and erbium-codoped sodium yttrium fluoride nanoparticles. The nanosystem showed more cytotoxicity in folate receptor-positive KB cells due to increased nanoparticle uptake by receptor-mediated endocytosis in comparison to the folate receptor-negative A549 cells, and the nanoparticles converted the 980 NIR light to three emission lower wavelength peaks centered at 521, 541, and 656 nm, which can be used for cell imaging.
Figure 4.
Selective endocytosis of Mucin-1 aptamer and PEG 2000 modified and gold nanocages (AuNG), ammonium bicarbonate (ABC), and doxorubicin (Dox), loaded bubble-generating thermoresponsive liposomes (Lips) by cancerous cells. Upon NIR exposure, the AuNGs convert the NIR to heat, which heats the ABC and generates bubble that disrupts the liposome to releases the Dox at the target site (Figure taken from reference [98]).
Ultrasound-responsive Nanomaterials
Ultrasound-responsive nanomaterials are a class of “smart” materials that undergo chemical and physical changes in response to ultrasound stimulus. Ultrasound, especially high-intensity focused ultrasound, has been utilized as a promising exogenous stimulus for biomedical applications due to its noninvasiveness, ease of accessibility, cost effectiveness, lack of ionizing radiation residues, controllable spatiotemporal effect, and high patient acceptability [100–102]. In this Section, the design strategies for programmable ultrasound-responsive nanomaterials having desired therapeutic effects are discussed.
Programming with different basic chemistry that is ultrasound-responsive
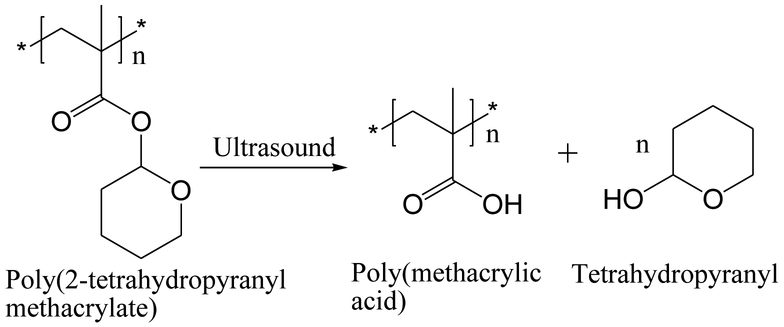
Ultrasound-responsive nanomaterials can be designed by introduction of ultrasound-labile moieties – called mechanophores – to polymeric nanoparticles. Tetrahydropyranyl is the most commonly used ultrasound-labile compound and is usually conjugated to methacrylic monomer via an ester bond for synthesizing ultrasound-responsive polymers. Upon insonation, the hydrophobic tetrahydropyranyl group is cleaved from the polymer and leaves the hydrophilic acidic group on the polymer (Figure 5) [8,103]. The transition of the polymer from the hydrophobic to the hydrophilic state upon ultrasound stimulus can be used for controlling drug delivery. For example, Paris et al. (2015) [103] grafted the surface of mesoporous silica nanoparticles with 2-tetrahydropyranyl methacrylate copolymerized with a thermoresponsive monomer 2-(2-methoxyethoxy)ethyl methacrylate to obtain a polymeric gatekeeper that released the loaded model dye fluorescein in response to ultrasound stimulus. Xuan et al. (2012) [8] copolymerized a small amount of 2-tetrahydropyranyl methacrylate with an amphiphilic diblock copolymer comprising poly(ethylene oxide) and poly(2-(2-methoxyethoxy)ethyl methacrylate), which formed micelles at 25 °C. The micelles dissociated upon insonation due to the cleavage of the tetrahydropyranyl group and subsequently released the loaded model hydrophobic compound Nile Red.
Figure 5.
Cleavage of 2-hydroxytetrahydropyranyl group to from poly(2-tetrahydropyranyl methacrylate) by the action of ultrasound.
Ultrasound-created strong acoustic cavitation can also disrupt several drug loaded lipidic or polymeric nanoaggregates such as liposomes [104], Pluronic micelles [105], nanobubbles [106], and nanodroplets [101] for ultrasound trigger drug release at the target site. Marin et al. (2001) [105] showed that continuous wave and pulsed 20-kHz ultrasound significantly enhanced the uptake of doxorubicin from Pluronic micelles by HL-60 cells due to the disruption of the Pluronic micelle as well as perturbation of the cell membrane by the action of the ultrasound. Xin et al. (2017) [104] wrapped PLGA nanoparticles in liposomes and upon insonation the liposomes immediately vibrated and broken down to release the PLGA nanoparticles and the loaded mitoxantrone. Encapsulation of the drug increased its half-life 6.7-fold in adult Sprague-Dawley rats, which again decreased to 1.7-fold upon insonation. Yildirim et al. (2017) [102] showed that ultrasound could even disrupt solid inelastic polymeric nanoparticles made by 3,4-dihydro-2H-pyran-co-2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl methacrylate-co-2-(dimethylamino) ethyl methacrylate copolymer. Apart from its vesicular nanocarrier destabilizing effect, the mechanical cavitation applied to the tissue by ultrasound could also enhance nanoparticle extravasation across blood capillaries and penetration across cell membranes [100,107].
Furthermore, ultrasound-responsive nanomaterials can also be designed by incorporating drugs into various ultrasound contrast agents [107,108]. The ultrasound-induced hyperthermia can also be used to generate gas bubbles for vascular occlusion and ablation of cancer cells [109]. For example, Wang et al. (2012) [109] incorporated doxorubicin into perfluorocarbon nano-droplets which remain stable in the blood stream. Upon ultrasound insonation, the ultrasound-induced hyperthermia caused the perfluorocarbon droplets to undergo an instant phase transition into gas bubbles, a phenomenon described as acoustic droplet vaporization effect, which resulted in a 12.5 ± 5.6% decrease in human acute lymphoblastic leukemia cell viability in vitro after 6 h of incubation [109].
Programming with additional functional groups
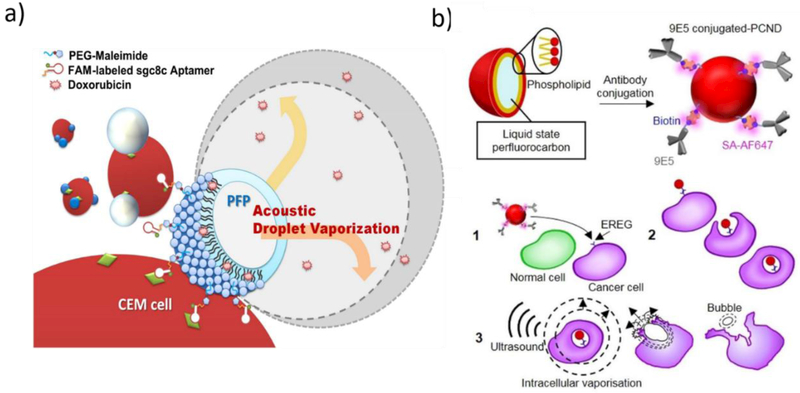
Drug release from ultrasound-responsive nanoparticles can be well controlled by the action of the ultrasound. The biodistribution and targeting of ultrasound-responsive nanoparticles can, however, be enhanced through the introduction of active ligands like antibodies, peptides, and aptamers to the nanoparticles. For example, Wang et al. (2012) [109] designed sgc8c aptamer-conjugated, doxorubicin-loaded acoustic droplets consisting of liquid perfluoropentane core and lipid shell for tumor theranostic purposes (Figure 6a). High-intensity focused ultrasound insonation of the aptamer-conjugated droplets resulted in 56.8% decrease in cell viability in vitro, which was 4.5-fold higher than that of the non-conjugated analogs. Recently, anticancer monoclonal antibody 9E5-conjugated phase-change nanodroplets, which contained a perfluorocarbon liquid core (a mixture of perfluoropentane and perfluorohexane) and a phospholipid shell, were designed for intracellular vaporization and drug release (Figure 6b). The conjugated antibody bound to epiregulin receptors, which are overexpressed on human colonic adenocarcinoma cell line DLD1 and caused 97.8 ± 0.5% accumulation of the nanoparticles into the DLD1 cells, which was significantly higher than the 1.4 ± 0.3% accumulation of the nanodroplets without the antibody. Furthermore, upon insonation, intracellular vaporization generated by the perfluorocarbon liquid in the nanodroplets killed 57% of the targeted DLD1 cells [110]. In a different approach, placental mesenchymal stem cells were used as cell-targeting vectors for the ultrasound-responsive nanoparticles into tumor cells. The ultrasound-responsive nanoparticles were prepared by grafting porous silica nanoparticles using the ultrasound-responsive copolymer, poly(2-(2methoxyethoxy)ethyl methacrylate-co-2-tetrahydropyranyl methacrylate) as a gatekeeper. The ultrasound-responsive nanoparticles were loaded with doxorubicin and were coated with polyethylenimine to enhance their permeation into the mesenchymal stem cells. The ultrasound-responsive nanoparticle-loaded mesenchymal stem cells were then co-cultured with N-nitroso-N-methylurea-induced tumor cells obtained from Sprague-Dawley female rats. Stem cell migration did not significantly change due to nanoparticle loading and insonation of the stem cells decreased tumor cell viability by about 60% due to doxorubicin release by insonation [108]. In another strategy, magnetic nanoparticles were introduced into an ultrasound-responsive protein-polymer nanodroplets core to achieve trio magnetic field, receptor and ultrasound mediated targeted drug delivery and 40% increase of the cancer cell killing effect of paclitaxel was obtained [101].
Figure 6:
(a) aptamer- and (b) antibody-conjugated ultrasound-responsive nanodroplets designed for tumor targeted therapy and their interaction with cancerous cells and subseqent degradation by ultrasound (Figures taken from references [109] and [110], respectively).
Magnetic field-responsive Nanomaterials
Magnetic field-responsive (magnetic) nanomaterials are a class of “smart” materials that respond to magnetic field stimulus and have emerged as attractive nanotherapeutics for diagnostic and therapeutic applications [111]. Generally, magnetic field frequency below 400 Hz is hardly absorbed by the body and can be remotely directed to the desired tissue [112]. Magnetic nanoparticles are easy to be synthesized, are biocompatible, and can be remotely controlled via magnetic fields. When exposed to an alternating magnetic field, they are capable of generating local hyperthermia, which can be used to increase blood vessel permeability, induce drug release, or kill cancerous cells [113]. In this Section, the design strategies for programming magnetic field-responsive nanomaterials for desired therapeutic effects are discussed.
Programming with different basic chemistry that is magnetic field-responsive
Generally, magnetic-field-responsive nanomaterials are core-shell systems containing magnetite (Fe3O4) or maghemite (Fe2O3) in the core [3]. Various materials such as polymers, mesoporous silica, squalenoyl-gemcitabine [83], and lipids have been used to form the shell of the magnetic field-responsive nanomaterials [114]. Superparamagnetic iron oxide nanoparticles (SPIONs) are the predominantly studied magnetic field-responsive nanomaterials because they can be guided to the target site without retaining any residual magnetism, which is attributed to quantum effects at the nanometer scale. SPIONs coated with polyethylenimine have been used for gene transfection and DNA vaccine delivery (magnetofection). Polyethylenimine is positively charged and can interact with the negatively charged sugar phosphate backbone of the nucleic acid to form a stable complex. It also provides a proton sponge effect to the nanoparticles, which enables release of the nanoparticles from endolysosomes into cytoplasm. Prijic et al. (2012) [112] loaded a cytokine interleukin 12A encoded plasmid DNA in polyethylenimine and acrylic acid-coated SPIONs. The nanoparticles stimulated an immune response and delayed tumor growth in murine mammary adenocarcinoma-transfected female BALB/c mice by 0.6 ± 0.5 and 7.8 ± 1.3 days without or in the presence of Nd-Fe-B generated magnetic field, respectively. The free plasmid and gene electro-transfer delayed tumor growth by −0.3 ± 0.00 and 6.6 ± 1.1 days, respectively, showing that gene magnetofection is equally effective as gene electro-transfer. Furthermore, Park et al. (2010) [115] reported that when 3,4-dihydroxy-L-phenylalanine conjugated, branched polyethylenimine was coated on SPIONs, the SPIONs formed clusters and showed better magneto-responsive properties than individual magnetite nanoparticles, and efficiently delivered siRNA into cancer cells.
Magnetic nanoparticles can also be designed to generate localized hyperthermia and control drug release from thermoresponsive and lipid nanomaterials [116,117]. For example, alternating magnetic field-induced localized hyperthermia caused DNA dehybridization and released the loaded model compound fluorescein on-demand from mesoporous silica nanoparticles, which were designed by using complementary DNA strands as gatekeepers (Figure 7) [118]. In another study, SPIONs and ethosuximide were loaded in thermoresponsive Pluronics® F127 micelles, which were stabilized by poly(vinyl acetate) (Pluronics® F127: poly vinyl acetate; 3:2). The LCST of the nanocarrier was about 38 °C and when a magnetic field of 2.5 kA m−1 at a frequency of 44.2 kHz was applied, heat was rapidly generated, which broke the hydrogen bonds between the PVA and F127 to irreversibly deform and rupture the micelle-like structure and trigger drug release [116]. Katagiri et al. (2011) [117] designed hybrid thermoresponsive liposomes loaded with pyranine dye and iron oxide nanoparticles using phosphatidylcholine, PEG-modified phosphatidylethanolamine, and a thermosensitive block copolymer of (2-ethoxy)ethoxyethyl vinyl ether and octadecyl vinyl ether at a molar ratio of 84:7:4. The phase transition temperature of the hybrid liposome was about 35 °C. At 10 °C and 30 °C the liposomes released negligible amount of the loaded pyranine over 10 minutes. However, at 45 °C, the magnetic nanoparticle-loaded and the unloaded hybrid liposomes released more than 95% and 70% of the dye in 1 minute, respectively. Similarly, when the nanoparticles were exposed to an alternating magnetic field (360 kHz and 234 Oe) for about 60 minutes, the hybrid liposomes that contained no iron oxide nanoparticles released negligible amount of the dye, whereas the iron oxide-loaded nanoparticles released more than 80% of the dye, in vitro.
Figure 7:
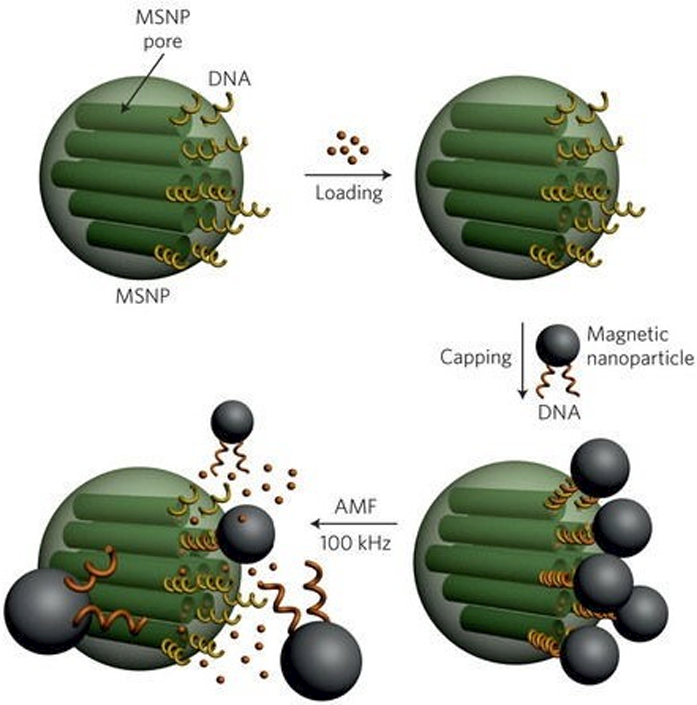
DNA modified drug loaded mesoporous silica nanoparticles (MSNP) that are hybridized with magnetic nanoparticles as gatekeepers. Upon exposure to alternating magnetic field, the nanoparticles generated hyperthermia caused DNA dehybridization, pore opening, and on-demand drug release from the mesoporous silica nanoparticles (Figure taken from reference [118]).
Besides their application in drug delivery and gene therapy discussed above, SPIONs can be used to localize micelles at target tissues and induce drug release. Qin et al. (2009) [119] encapsulated SPIONs in ferrogel-based Pluronic-F127 micelles, along with the lipophilic drug indomethacin, to form injectable ferrogels. Upon magnetic field application, the indomethacin release half-life decreased from 3195 to 1500 min, in vitro. This was attributed to the tendency of the SPIONs to orient and approach each other by the action of the externally applied magnetic field, which squeezed the hydrophobic core and pumped the drug out. In addition, SPIONs can be used as cores to form layer-by-layer assembled magnetic nano-formulations. Jayant1 et al. (2017) [120] were successful in depositing nelfinavir and rimcazole dihydrochloride layer-by-layer on SPIONs with the help of dextran sulfate sodium polyelectrolytes. The assembled nanocarriers were able to cross an in vitro blood brain barrier model with the aid of magnetic force and released the loaded drugs for over 8 days.
Programming with additional functional groups
Magnetic fields localize magnetic nanoparticles within a certain area of the body, and additional cell-targeting ligands and other stimuli-responsive materials like aptamers can be added to the surface of magnetic nanoparticles to achieve better targeting. For example, Wang et al. (2008) [121] conjugated A10 RNA aptamer, which binds to the extracellular domain of the prostate-specific membrane antigen, to thermally crosslinked SPIONs for prostate cancer therapy and imaging. The A10 RNA aptamer contained a CG sequence in which doxorubicin was encapsulated. Unlike the non-conjugated SPIONs, the aptamer-conjugated nanoparticles were taken up by prostate-specific membrane antigen-expressing prostate cancer cells, in vitro. In addition, the aptamer-conjugated nanoparticles were not taken up by non-prostate-specific membrane antigen-expressing prostate cancer cells.
Electric-field-responsive Nanomaterials
Electric-field-responsive (Electro-responsive) nanomaterials are a class of “smart” materials that respond to weak electric field to attain pulsed or controlled diagnostic and therapeutic effects [111]. Electrical stimulus is relatively easy to generate, control, and remotely apply without the need for sophisticated instruments, which makes electro-responsive nanocarriers very attractive drug delivery systems. Drug release from electro-responsive nanomaterials can be regulated via adjustments of the chemistry of electro-erodible or electro-conductive materials, and electric voltage, current, and exposure duration. In this Section, the strategies for designing these parameters to program electro-responsive nanomaterials for desired therapeutic effects are discussed.
Programming with different basic chemistry that is electro-responsive
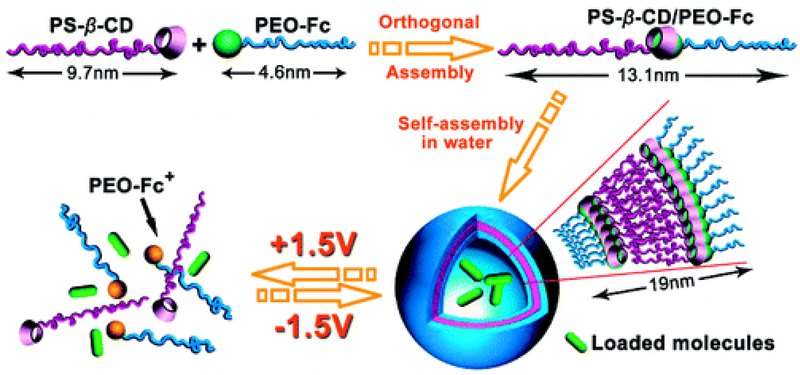
Electro-responsive nanomaterials can be designed by using the common electro-erodible or electro-conductive materials such as polypyrrole, multiwalled carbon nanotubes, polyelectrolytes, montmorillonite, ferrocene, and tetraaniline [122,123]. Samanta et al. (2016) [122] designed fluorescein-, piroxicam- and insulin-loaded electro-responsive nanoparticles using polypyrrole. Fluorescein release from the nanoparticles increased linearly when the applied current increased from 0 to −300 µA, the duration of exposure increased from 0 to 75 seconds, and the applied voltage increased from 0 to −1 V, and in each case dye release increased by at least 50%. Besides this result, the release of piroxicam and insulin from the nanoparticles increased linearly from about 1.5 to about 2.2 and 7.0 µg mL−1 when the number of pulses increased from 0 to 3 (−100 µA for 25 seconds) and 2 (−1 V for 4 min), respectively. Ying et al. (2014) [124] synthesized phenytoin sodium-loaded electro-responsive nanogels using sodium 4-vinylbenzene sulfonate-based polyelectrolyte that swelled from 102.3 ± 16.8 to 388.0 ± 20.4 nm when exposed to 500 µA for 1 min. Phenytoin sodium release from the nanogels also increased from 34.6% to 60.8% and 87.3% upon exposure to 100 and 200 µA current for 4 h, respectively. Yan et al. (2010) [125] reported interesting electro-responsive self-assembled micellar nanostructures based on an amphiphilic block copolymer comprising two end-functionalized polymers, PEG-ferrocene and polystyrene-β-cyclodextrin (Figure 8). The amphiphilic block copolymer was formed due to inclusion of the hydrophobic ferrocene on the hydrophilic end of the PEG to the β-cyclodextrin cavity of the hydrophobic styrene polymer, which spontaneously self-assembled into micelle-like vesicles. Upon application of an external electric field, the ferrocene became hydrophilic and left the β-cyclodextrin cavity to reversibly disassemble the micelle-like vesicle and release the encapsulated model compound Rhodamine B. Rhodamine B release was highly dependent on the applied voltage and it took about 32, 120 and 450 min to release the loaded compound at +4, +2, and +1 V, respectively, and in the absence of electric stimuli less than 25% of the loaded dye was released in 600 min.
Figure 8:
Schematics representing formation of electro-responsive, drug loaded, micelle-like vesicles by self-assembly of an electro-responsive amphiphilic molecule and subsequent on-demand drug release from the vesicles by the action of applied electric field (Figure taken from reference [125]).
Electro-responsive nanoparticles can be good candidates for the treatment of epilepsy. Epilepsy is characterized by recurrent, abrupt and unpredicted seizures. Patients take prophylactic doses of antiepileptic drugs, and the prolonged use of higher doses of these drugs is associated with severe side effects. To avoid this, the epileptic seizure can be utilized as an internal stimulus to induce on-demand drug release from electro-responsive nanoparticles. Consequently, Wang et al. (2016) [126] synthesized phenytoin sodium-loaded electrical-responsive nanogels using 2-(dimethylamino)ethyl methacrylate, styrene, and the electro-responsive monomer 4-vinylbenzene sulfonate and the crosslinker N,N’-methylenebisacrylamide, which released the loaded drug in a sustained manner. Interestingly, pentylenetetrazole-induced epileptic seizure in rats triggered rapid drug release and increased the concentration of phenytoin sodium in rat hippocampus by about 150%. Electro-responsive nanocarriers have also been extensively investigated in the areas of transdermal drug delivery. Iontophoresis, which uses very low voltages to enhance the penetration of charged compounds across the skin, has been employed to enhance drug penetration from various electro-responsive nanocarriers across the skin and sclera. Electroporation, which uses relatively high transmembrane voltage to cause the formation of pores in cell membranes, has also been utilized to enhance the permeability of drugs and various nanocarriers across biological membranes. For example, PEG-coated silica nanoparticles, which were rendered positively charged (+4.06 mV) and negatively charged (−5.51 mV) by surface adsorption of 5-propylsulfonyloxyimino-5H-thiophen-2-ylidene-(2-methylphenyl)acetonitrile and poly(4-methyl-2-pentyne), respectively, were investigated as gene transporters. The nanoparticles were labeled by covalent conjugation of the fluorescent dye rhodamine B-isothiocyanate and the negatively charged pEGFP-N1 was loaded on the nanoparticles. The negatively charged nanoparticles significantly enhanced gene transfection in HeLa cells when combined with electroporation [127]. In a similar study, electroporation enhanced the permeability of antisense oligonucleotide-loaded transferrin-decorated liposomes across leukemia cells [128].
Programming with additional functional groups
Surface modification of electro-responsive nanocarriers with different active ligands has been utilized to enhance drug targeting to the target tissue. For example, Ying et al. (2014) [124] modified the surfaces of phenytoin sodium-loaded electro-responsive nanogels using brain-targeting angiopep-2 peptide, a ligand of the low-density lipoprotein receptor-related protein, to improve the blood brain barrier penetration of the nanogels for the treatment of epilepsy. In comparison to the free drug, the concentration of phenytoin sodium in the brain from the non-modified and surface-modified nanogels increased by 1.49- and 1.97-fold, respectively, in vivo in rats. Another method that can enhance the programmability of electro-responsive nanomaterials is to combine electro-stimuli nanoparticles with other stimuli-systems. Ge et al. (2012) [129] dispersed daunorubicin-loaded polypyrrole nanoparticles in the thermoresponsive and biodegradable PLGA-PEG-PLGA polymer to form an injectable, conductive hydrogel. The hydrogel was injected to the dorsal sites of FVB adult mice and upon application of 1.5 V/cm for 40 seconds, pulsatile drug release was attained.
Concluding Remarks and Future Perspectives
Physical stimuli-responsive nanomaterials are smart materials that can control drug release in response to physical stimuli including temperature, light, ultrasound, magnetic field, and electric field. Many strategies have been explored to program them to have multiple-functionality, less degree of variability, and high precession to address the unmet need of on-demand and targeted drug delivery over the past few decades. These strategies can be divided into three categories: the chemistry including the basic/core chemistry and the chemistry of surface targeting ligands (antibody, peptides, and aptamers, etc.), the architecture of the nanomaterials, and the parameters of the physical stimuli such as type, intensity, and duration, etc. All of these strategies can be utilized to control the interactions of the nanomaterials with drugs, and thus drug loading and release efficiency. Uptake of the nanotherapeutics by cells and tissues, and the permeability of the nanotherapeutics across biological barriers, which indicates targeting effect, can also be manipulated via these strategies [34]. However, there are several major hurdles that need to be overcome in order to successfully translate these physical stimuli-responsive nanomaterials into clinical practice. The first challenge is to avoid uncontrolled accumulation and/or cellular uptake of these nanomaterials by non-target tissues [130,131]. The off-target accumulation/uptake mainly occurs due to non-specific adsorption of proteins on nanomaterials surfaces (forming a protein corona) in the biological milieu. This, protein adsorption often causes protein denaturation that leads to a signaling cascade, resulting in either nanomaterial aggregation and/or phagocytosis via activated macrophages [131]. Since the protein adsorption is non-specific, it can also happen to nanomaterials targeting moieties. Consequently, the protein adsorption negatively causes more nanomaterials to reach organs involved in clearance like kidney, liver, spleen, etc. but rather the target sites [130]. The second challenge that these stimuli-responsive nanomaterials share with conventional nanotherapeutics is the lack of efficient clearance of the nanotherapeutics from the body once they have accomplished their mission. Most nanotherapeutics have sizes beyond the renal threshold and cannot be removed from the body via the kidneys, and thus if they are not biodegradable, they tend to accumulate in the body. Even for some biodegradable nanomaterials, their degraded fragments might be sequestered in lysosomal compartments to cause toxicity/side effects [130]. The third challenge is that in most cases targeting moieties conjugated on the nanomaterials are actually not specific to the target sites, because the receptors for the targeting moieties are expressed not only at the target sites but also other organs. For example, folate receptor is overexpressed in a large number of malignancies, but it is also expressed to a moderate to high level in normal organs including small intestine, placenta, and kidneys. In addition, the overexpressed folate receptor is also inhomogeneously distributed on malignant cells, resulting in non-uniform accumulation of the nanotherapeutics in the target tissue. Furthermore, some targeting moieties like antibodies and peptides may lose their activity during conjugation with the nanomaterials and may not induce the intended tissues targeting effect. Targeting ligands on the surface of the nanocarriers may also alter nanomaterials surface characteristics like the charge and hydrophobicity and lead to increased opsonization, aggregation and clearance of the nanomaterials by the mononuclear-phagocyte system. The fourth challenge is that some of the physical stimuli may not be fully tolerated by the body and their use and controlling may incur additional cost. For example, UV light cannot penetrate into tissues deeper than ~10 mm due to its absorption by endogenous chromophores such as oxy- and deoxy-hemoglobin, lipids, and water; and prolonged UV irradiation can be cytotoxic [95]. Therefore, UV-responsive nanotherapeutics should be used restrictively to the eye, skin, and other mucosal surfaces, be doped by upconversion luminescent materials, or be used along with NIR [95–97,132]. The cavitation caused by ultrasound stimulus may enhance vessel permeability of cancer cells to cause metastatic dissemination. Electrical stimulus also has low tissue penetration and can possibly cause tissue damage, and thus limit the clinical application of electro-responsive nanoparticles despite the nanoparticles’ flexibility and low-cost advantages. Magnetic field stimulus is costly due to its complexity and need of special set-up for adequate focusing and deep penetration into the disease area with sufficient strength. Thermoresponsive materials need longer duration to undergo phase transition that results in burst drug release and precise temperature control at the target site without causing tissue damage is a challenge [133]. Due to these challenges, restricted number of physical stimuli-responsive nanotherapeutic has been advanced to the level of clinical studies. Therefore, in order for physical stimuli-responsive nanotherapeutic to be developed into intelligent drug delivery systems to treat human diseases, continuous design improvements, more in vivo toxicology and efficacy evaluations, and robust stability and production scale-up studies on these nanomaterials, are expected in the future.
Acknowledgements
This work was financially supported by NIH R01EY023853.
Abbreviations:
- LCST
lower critical solution temperature
- NIPAAM
N-isopropylacrylamide
- NIR
near-infrared
- PEG
polyethylene glycol
- PLGA
poly(lactic-co-glycolic acid)
- PNIPAAM
poly(N-isopropylacrylamide)
- SPIONs
superparamagnetic iron oxide nanoparticles
- UCST
upper critical solution temperature
Footnotes
Teaser
This review presents a systematic approach to design different programmable physical stimuli-responsive nanotherapeutics intended for controlled and targeted delivery of various therapeutic agents.
References
- 1.Gil ES et al. (2012) β-Cyclodextrin-poly(β-Amino Ester) Nanoparticles for Sustained Drug Delivery across the Blood–Brain Barrier. Biomacromolecules 13 (11), 3533–3541 [DOI] [PubMed] [Google Scholar]
- 2.Müller RH et al. (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics 50 (1), 161–177 [DOI] [PubMed] [Google Scholar]
- 3.Hua MY et al. (2011) The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials 32 (2), 516–527 [DOI] [PubMed] [Google Scholar]
- 4.Poß M et al. (2017) Multimodal [GdO]+[ICG]− Nanoparticles for Optical, Photoacoustic, and Magnetic Resonance Imaging. Chemistry of Materials 29 (8), 3547–3554 [Google Scholar]
- 5.Landon CD et al. (2011) Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. The open nanomedicine journal 3, 38–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulbake U et al. (2017) Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9 (2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo M et al. (2017) In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int J Pharm 521 (1–2), 156–166 [DOI] [PubMed] [Google Scholar]
- 8.Xuan J et al. (2012) Ultrasound-Responsive Block Copolymer Micelles Based on a New Amplification Mechanism. Langmuir 28 (47), 16463–16468 [DOI] [PubMed] [Google Scholar]
- 9.Sahle FF et al. (2012) Polyglycerol fatty acid ester surfactant–based microemulsions for targeted delivery of ceramide AP into the stratum corneum: Formulation, characterisation, in vitro release and penetration investigation. European Journal of Pharmaceutics and Biopharmaceutics 82 (1), 139–150 [DOI] [PubMed] [Google Scholar]
- 10.Lorenceau E et al. (2005) Generation of Polymerosomes from Double-Emulsions. Langmuir 21 (20), 9183–9186 [DOI] [PubMed] [Google Scholar]
- 11.Stover TC et al. (2008) Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials 29 (3), 359–369 [DOI] [PubMed] [Google Scholar]
- 12.Sahle FF et al. (2017) Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale [DOI] [PubMed] [Google Scholar]
- 13.Hu X et al. (2014) Electrospinning of polymeric nanofibers for drug delivery applications. Journal of Controlled Release 185 (Supplement C), 12–21 [DOI] [PubMed] [Google Scholar]
- 14.Sharma HS et al. (2007) Drug Delivery to the Spinal Cord Tagged with Nanowire Enhances Neuroprotective Efficacy and Functional Recovery following Trauma to the Rat Spinal Cord. Annals of the New York Academy of Sciences 1122 (1), 197–218 [DOI] [PubMed] [Google Scholar]
- 15.Jafari S et al. (2017) Biomacromolecule based nanoscaffolds for cell therapy. Journal of Drug Delivery Science and Technology 37 (Supplement C), 61–66 [Google Scholar]
- 16.Yu Q et al. (2013) Nanopatterned Smart Polymer Surfaces for Controlled Attachment, Killing, and Release of Bacteria. ACS Applied Materials & Interfaces 5 (19), 9295–9304 [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz C et al. (2016) Novel Nanoprinting for Oral Delivery of Poorly Soluble Drugs. Methodist Debakey Cardiovasc J 12 (3), 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLeon VH et al. (2012) Polymer nanocomposites for improved drug delivery efficiency. Materials Chemistry and Physics 132 (2), 409–415 [Google Scholar]
- 19.Duan CH et al. (2013) Review article: Fabrication of nanofluidic devices. Biomicrofluidics 7 (2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W et al. (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Research Letters 6 (1), 555–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W et al. (2017) Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Advanced Materials 29 (5), 1603864-n/a [DOI] [PubMed] [Google Scholar]
- 22.Jeon G et al. (2012) Functional nanoporous membranes for drug delivery. Journal of Materials Chemistry 22 (30), 14814–14834 [Google Scholar]
- 23.Hoare T et al. (2012) Thermoresponsive nanogels for prolonged duration local anesthesia. Acta Biomater 8 (10), 3596–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhuchar N et al. (2012) Degradable Thermoresponsive Nanogels for Protein Encapsulation and Controlled Release. Bioconjugate Chemistry 23 (1), 75–83 [DOI] [PubMed] [Google Scholar]
- 25.Du J-Z et al. (2010) A Tumor-Acidity-Activated Charge-Conversional Nanogel as an Intelligent Vehicle for Promoted Tumoral-Cell Uptake and Drug Delivery. Angewandte Chemie International Edition 49 (21), 3621–3626 [DOI] [PubMed] [Google Scholar]
- 26.Alibolandi M et al. (2017) Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. International Journal of Pharmaceutics 519 (1), 352–364 [DOI] [PubMed] [Google Scholar]
- 27.Sahay G et al. (2010) Endocytosis of Nanomedicines. Journal of controlled release : official journal of the Controlled Release Society 145 (3), 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh JM et al. (2009) Inorganic metal hydroxide nanoparticles for targeted cellular uptake through clathrin-mediated endocytosis. Chem Asian J 4 (1), 67–73 [DOI] [PubMed] [Google Scholar]
- 29.Zhang P et al. (2017) Aptamer-coded DNA nanoparticles for targeted doxorubicin delivery using pH-sensitive spacer. Frontiers of Chemical Science and Engineering [Google Scholar]
- 30.Stejskalova A et al. (2016) Programmable biomaterials for dynamic and responsive drug delivery. Exp Biol Med (Maywood) 241 (10), 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J et al. (2013) Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Advanced Materials 25 (32), 4386–4396 [DOI] [PubMed] [Google Scholar]
- 32.Rahikkala A et al. (2015) Thermoresponsive Nanoparticles of Self-Assembled Block Copolymers as Potential Carriers for Drug Delivery and Diagnostics. Biomacromolecules 16 (9), 2750–2756 [DOI] [PubMed] [Google Scholar]
- 33.Lutz J-F et al. (2006) Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? Journal of the American Chemical Society 128 (40), 13046–13047 [DOI] [PubMed] [Google Scholar]
- 34.Gandhi A et al. (2015) Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian Journal of Pharmaceutical Sciences 10 (2), 99–107 [Google Scholar]
- 35.Rejinold NS et al. (2011) Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydrate Polymers 83 (2), 776–786 [Google Scholar]
- 36.Vihola H et al. (2005) Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 26 (16), 3055–3064 [DOI] [PubMed] [Google Scholar]
- 37.Wang N et al. (2013) Magnetic nanoparticles (MNPs) covalently coated by PEO-PPO-PEO block copolymer for drug delivery. J Colloid Interface Sci 395, 50–57 [DOI] [PubMed] [Google Scholar]
- 38.Chen S et al. (2007) Temperature-responsive magnetite/PEO-PPO-PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir 23 (25), 12669–12676 [DOI] [PubMed] [Google Scholar]
- 39.Hu Z et al. (2010) Thermoresponsive oligo(ethylene glycol)-methacrylate- based polymers and microgels. Soft Matter 6 (10), 2115–2123 [Google Scholar]
- 40.Tian Y et al. (2016) A redox-labile poly(oligo(ethylene glycol)methacrylate)-based nanogel with tunable thermosensitivity for drug delivery. Polymer Chemistry 7 (10), 1913–1921 [Google Scholar]
- 41.Diehl C and Schlaad H (2009) Thermo-responsive polyoxazolines with widely tuneable LCST. Macromol Biosci 9 (2), 157–161 [DOI] [PubMed] [Google Scholar]
- 42.Kurzhals S et al. (2017) Controlled aggregation and cell uptake of thermoresponsive polyoxazoline-grafted superparamagnetic iron oxide nanoparticles. Nanoscale 9 (8), 2793–2805 [DOI] [PubMed] [Google Scholar]
- 43.Panek J et al. (2012) Thermoresponsive nanoparticles based on poly(2-alkyl-2-oxazolines) and Pluronic F127. Macromol Rapid Commun 33 (19), 1683–1689 [DOI] [PubMed] [Google Scholar]
- 44.Viegas TX et al. (2011) Polyoxazoline: Chemistry, Properties, and Applications in Drug Delivery. Bioconjugate Chemistry 22 (5), 976–986 [DOI] [PubMed] [Google Scholar]
- 45.Ulbricht J et al. (2014) On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 35 (17), 4848–4861 [DOI] [PubMed] [Google Scholar]
- 46.Mansfield EDH et al. (2015) POZylation: a new approach to enhance nanoparticle diffusion through mucosal barriers. Nanoscale 7 (32), 13671–13679 [DOI] [PubMed] [Google Scholar]
- 47.Kowalczyk T et al. (2014) Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World J Microbiol Biotechnol 30 (8), 2141–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kracke B et al. (2015) Thermoswitchable Nanoparticles Based on Elastin-like Polypeptides. Macromolecules 48 (16), 5868–5877 [Google Scholar]
- 49.Sicilia G et al. (2014) Programmable polymer-DNA hydrogels with dual input and multiscale responses. Biomaterials Science 2 (2), 203–211 [DOI] [PubMed] [Google Scholar]
- 50.Bessa PC et al. (2010) Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. Journal of Controlled Release 142 (3), 312–318 [DOI] [PubMed] [Google Scholar]
- 51.Kurzhals S et al. (2017) Thermoresponsive Polypeptoid-Coated Superparamagnetic Iron Oxide Nanoparticles by Surface-Initiated Polymerization. Macromolecular Chemistry and Physics 218 (13), 1700116-n/a [Google Scholar]
- 52.Watanabe E et al. (2007) New Biodegradable and Thermoresponsive Polymers Based on Amphiphilic Poly(asparagine) Derivatives. Macromolecular Symposia 249-250 (1), 509–514 [Google Scholar]
- 53.Ta T and Porter TM (2013) Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. Journal of Controlled Release 169 (1), 112–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YS et al. (2006) Synthesis and characterization of thermoresponsive-co-biodegradable linear-dendritic copolymers. Macromolecules 39 (23), 7805–7811 [Google Scholar]
- 55.Wang G et al. (2016) Self-Assembled Thermoresponsive Nanogels Prepared by Reverse Micelle --> Positive Micelle Method for Ophthalmic Delivery of Muscone, a Poorly Water-Soluble Drug. J Pharm Sci 105 (9), 2752–2759 [DOI] [PubMed] [Google Scholar]
- 56.Meng X et al. (2017) Pluronic F127 and D-α-Tocopheryl Polyethylene Glycol Succinate (TPGS) Mixed Micelles for Targeting Drug Delivery across The Blood Brain Barrier. Scientific Reports 7 (1), 2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X et al. (2017) Enhancing the oral bioavailability of biochanin A by encapsulation in mixed micelles containing Pluronic F127 and Plasdone S630. Int J Nanomedicine 12, 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han H et al. (2013) Thermoresponsive nanoparticles + plasmonic nanoparticles = photoresponsive heterodimers: facile synthesis and sunlight-induced reversible clustering. Chemical Communications 49 (55), 6122–6124 [DOI] [PubMed] [Google Scholar]
- 59.Kim DH et al. (2013) Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles. Langmuir 29 (24), 7425–7432 [DOI] [PubMed] [Google Scholar]
- 60.Dicheva BM et al. (2014) Targeted and heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. Journal of Controlled Release 195 (Supplement C), 37–48 [DOI] [PubMed] [Google Scholar]
- 61.Sanyal S et al. (2011) Thermo-Responsive Core-Shell Composite Nanoparticles Synthesized via One-Step Pickering Emulsion Polymerization for Controlled Drug Delivery. Journal of Nanomedicine & Nanotechnology 2 (7), 1–7 [Google Scholar]
- 62.Yildiz I and Sizirici Yildiz B (2015) Applications of Thermoresponsive Magnetic Nanoparticles. Journal of Nanomaterials 2015, 12 [Google Scholar]
- 63.Hoare T et al. (2011) Magnetically Triggered Nanocomposite Membranes: A Versatile Platform for Triggered Drug Release. Nano Letters 11 (3), 1395–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dionigi C et al. (2014) Smart magnetic poly(N-isopropylacrylamide) to control the release of bio-active molecules. Journal of Materials Science: Materials in Medicine 25 (10), 2365–2371 [DOI] [PubMed] [Google Scholar]
- 65.Patra S et al. (2015) Dual-Responsive Polymer Coated Superparamagnetic Nanoparticle for Targeted Drug Delivery and Hyperthermia Treatment. ACS Applied Materials & Interfaces 7 (17), 9235–9246 [DOI] [PubMed] [Google Scholar]
- 66.Yassine O et al. (2016) Highly Efficient Thermoresponsive Nanocomposite for Controlled Release Applications. Scientific Reports 6, 28539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cunliffe D et al. (2003) Thermoresponsive Surface-Grafted Poly(N—isopropylacrylamide) Copolymers: Effect of Phase Transitions on Protein and Bacterial Attachment. Langmuir 19 (7), 2888–2899 [Google Scholar]
- 68.Nykänen A et al. (2012) Thermally Sensitive Block Copolymer Particles Prepared via Aerosol Flow Reactor Method: Morphological Characterization and Behavior in Water. Macromolecules 45 (20), 8401–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen K-J et al. (2013) A Thermoresponsive Bubble-Generating Liposomal System for Triggering Localized Extracellular Drug Delivery. ACS Nano 7 (1), 438–446 [DOI] [PubMed] [Google Scholar]
- 70.Lin Y-J et al. (2017) Recent advances in CO2 bubble-generating carrier systems for localized controlled release. Biomaterials 133 (Supplement C), 154–164 [DOI] [PubMed] [Google Scholar]
- 71.Al-Ahmady ZS et al. (2012) Lipid-Peptide Vesicle Nanoscale Hybrids for Triggered Drug Release by Mild Hyperthermia in vitro and in vivo. ACS nano 6 (10), 9335–9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoare T et al. (2012) Nanogel scavengers for drugs: Local anesthetic uptake by thermoresponsive nanogels. Acta Biomaterialia 8 (4), 1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang X et al. (2017) A targeted drug delivery system based on folic acid-functionalized upconversion luminescent nanoparticles. J Biomater Appl 31 (9), 1247–1256 [DOI] [PubMed] [Google Scholar]
- 74.Banga RJ et al. (2017) Drug-Loaded Polymeric Spherical Nucleic Acids: Enhancing Colloidal Stability and Cellular Uptake of Polymeric Nanoparticles through DNA Surface-Functionalization. Biomacromolecules 18 (2), 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lanier LA and Bermudez H (2015) DNA nanostructures: a shift from assembly to applications. Curr Opin Chem Eng 7, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samanta A and Medintz IL (2016) Nanoparticles and DNA - a powerful and growing functional combination in bionanotechnology. Nanoscale 8 (17), 9037–9095 [DOI] [PubMed] [Google Scholar]
- 77.Cubero E et al. (2006) Theoretical Study of the Hoogsteen–Watson-Crick Junctions in DNA. Biophysical Journal 90 (3), 1000–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang X et al. (2008) Novel Nanogels with Both Thermoresponsive and Hydrolytically Degradable Properties. Macromolecules 41 (22), 8339–8345 [Google Scholar]
- 79.Kim YS et al. (2018) Thermoresponsive- co-Biodegradable Linear-Dendritic Nanoparticles for Sustained Release of Nerve Growth Factor To Promote Neurite Outgrowth. Mol Pharm [DOI] [PubMed] [Google Scholar]
- 80.Jiang X et al. (2009) Degradable Thermoresponsive Core Cross-Linked Micelles: Fabrication, Surface Functionalization, and Biorecognition. Langmuir 25 (23), 13344–13350 [DOI] [PubMed] [Google Scholar]
- 81.Aguirre G et al. (2013) Synthesis of new enzymatically degradable thermo-responsive nanogels. Soft Matter 9 (1), 261–270 [Google Scholar]
- 82.Motlaq VF (2017) Effect of PEGylation on Physical and Biological Behavior of Temperature-Responsive Nanogels In Department of Chemistry, Faculty of Mathematics and Natural Sciences, pp. 93, University of Oslo [Google Scholar]
- 83.Al-Ahmady ZS et al. (2015) Triggered doxorubicin release in solid tumors from thermosensitive liposome-peptide hybrids: Critical parameters and therapeutic efficacy. Int J Cancer 137 (3), 731–743 [DOI] [PubMed] [Google Scholar]
- 84.Zhou H et al. (2006) Electrochemical study of photovoltaic effect of nano titanium dioxide on hemoglobin. Bioelectrochemistry 69 (1), 34–40 [DOI] [PubMed] [Google Scholar]
- 85.Xiao Z et al. (2012) DNA Self-Assembly of Targeted Near-Infrared-Responsive Gold Nanoparticles for Cancer Thermo-Chemotherapy. Angewandte Chemie (International Ed. in English) 51 (47), 11853–11857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fomina N et al. (2012) Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev 64 (11), 1005–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patnaik S et al. (2007) Photoregulation of drug release in azo-dextran nanogels. International Journal of Pharmaceutics 342 (1), 184–193 [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y (2012) Light-Responsive Block Copolymer Micelles. Macromolecules 45 (9), 3647–3657 [Google Scholar]
- 89.Zhang C et al. (2017) A Light Responsive Nanoparticle-Based Delivery System Using Pheophorbide A Graft Polyethylenimine for Dendritic Cell-Based Cancer Immunotherapy. Molecular Pharmaceutics 14 (5), 1760–1770 [DOI] [PubMed] [Google Scholar]
- 90.Huu VAN et al. (2015) Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. Journal of Controlled Release 200 (Supplement C), 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azagarsamy MA et al. (2012) Photocontrolled Nanoparticles for On-Demand Release of Proteins. Biomacromolecules 13 (8), 2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Itoh H et al. (2004) Photochemical Assembly of Gold Nanoparticles Utilizing the Photodimerization of Thymine. Langmuir 20 (5), 1972–1976 [DOI] [PubMed] [Google Scholar]
- 93.He D et al. (2012) A Light-Responsive Reversible Molecule-Gated System Using Thymine-Modified Mesoporous Silica Nanoparticles. Langmuir 28 (8), 4003–4008 [DOI] [PubMed] [Google Scholar]
- 94.Wang T et al. (2015) Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomaterialia 13 (Supplement C), 354–363 [DOI] [PubMed] [Google Scholar]
- 95.Karimi M et al. (2016) Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chemical Society reviews 45 (5), 1457–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.You J et al. (2012) Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors. Cancer Res 72 (18), 4777–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fomina N et al. (2010) UV and Near-IR Triggered Release from Polymeric Nanoparticles. Journal of the American Chemical Society 132 (28), 9540–9542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chuang E-Y et al. (2016) A FRET-guided, NIR-responsive bubble-generating liposomal system for in vivo targeted therapy with spatially and temporally precise controlled release. Biomaterials 93 (Supplement C), 48–59 [DOI] [PubMed] [Google Scholar]
- 99.Lee S-M et al. (2011) Multifunctional Nanoparticles for Targeted Chemophotothermal Treatment of Cancer Cells. Angewandte Chemie 123 (33), 7723–7728 [DOI] [PubMed] [Google Scholar]
- 100.Sirsi SR and Borden MA (2014) State-of-the-art materials for ultrasound-triggered drug delivery. Advanced Drug Delivery Reviews 72 (Supplement C), 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JY et al. (2015) Nanoparticle-Loaded Protein–Polymer Nanodroplets for Improved Stability and Conversion Efficiency in Ultrasound Imaging and Drug Delivery. Advanced Materials 27 (37), 5484–5492 [DOI] [PubMed] [Google Scholar]
- 102.Yildirim T et al. (2017) Dual pH and ultrasound responsive nanoparticles with pH triggered surface charge-conversional properties. Polymer Chemistry 8 (8), 1328–1340 [Google Scholar]
- 103.Paris JL et al. (2015) Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 9 (11), 11023–11033 [DOI] [PubMed] [Google Scholar]
- 104.Xin Y et al. (2017) PLGA nanoparticles introduction into mitoxantrone-loaded ultrasound-responsive liposomes: In vitro and in vivo investigations. Int J Pharm 528 (1–2), 47–54 [DOI] [PubMed] [Google Scholar]
- 105.Jo H and Ban C (2016) Aptamer–nanoparticle complexes as powerful diagnostic and therapeutic tools. Experimental & Molecular Medicine 48 (5), e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie X et al. (2015) Ultrasound-responsive nanobubbles contained with peptide-camptothecin conjugates for targeted drug delivery. Drug Deliv, 1–9 [DOI] [PubMed] [Google Scholar]
- 107.Zhao YZ et al. (2013) Potential and problems in ultrasound-responsive drug delivery systems. Int J Nanomedicine 8, 1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paris JL et al. (2017) Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 9 (17), 5528–5537 [DOI] [PubMed] [Google Scholar]
- 109.Wang C-H et al. (2012) Aptamer-conjugated and drug-loaded acoustic droplets for ultrasound theranosis. Biomaterials 33 (6), 1939–1947 [DOI] [PubMed] [Google Scholar]
- 110.Ishijima A et al. (2017) Selective intracellular vaporisation of antibody-conjugated phase-change nano-droplets in vitro. Sci Rep 7, 44077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang HW et al. (2011) Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials 32 (27), 6523–6532 [DOI] [PubMed] [Google Scholar]
- 112.Prijic S et al. (2012) Surface modified magnetic nanoparticles for immuno-gene therapy of murine mammary adenocarcinoma. Biomaterials 33 (17), 4379–4391 [DOI] [PubMed] [Google Scholar]
- 113.McGill SL et al. (2009) Magnetically Responsive Nanoparticles for Drug Delivery Applications Using Low Magnetic Field Strengths. IEEE Transactions on NanoBioscience 8 (1), 33–42 [DOI] [PubMed] [Google Scholar]
- 114.Zhang F et al. (2012) Mesoporous multifunctional upconversion luminescent and magnetic “nanorattle” materials for targeted chemotherapy. Nano Lett 12 (1), 61–67 [DOI] [PubMed] [Google Scholar]
- 115.Park JW et al. (2011) Clustered Magnetite Nanocrystals Cross-Linked with PEI for Efficient siRNA Delivery. Biomacromolecules 12 (2), 457–465 [DOI] [PubMed] [Google Scholar]
- 116.Huang H-Y et al. (2012) Self-assembling PVA-F127 thermosensitive nanocarriers with highly sensitive magnetically-triggered drug release for epilepsy therapy in vivo. Journal of Materials Chemistry 22 (17), 8566–8573 [Google Scholar]
- 117.Katagiri K et al. (2011) Magnetoresponsive on-demand release of hybrid liposomes formed from Fe3 O4 nanoparticles and thermosensitive block copolymers. Small 7 (12), 1683–1689 [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Hernandez E et al. (2011) Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 5 (2), 1259–1266 [DOI] [PubMed] [Google Scholar]
- 119.Qin J et al. (2009) Injectable Superparamagnetic Ferrogels for Controlled Release of Hydrophobic Drugs. Advanced Materials 21 (13), 1354–1357 [Google Scholar]
- 120.Jayant RD et al. (2017) Novel nanoformulation to mitigate co-effects of drugs of abuse and HIV-1 infection: towards the treatment of NeuroAIDS. J Neurovirol 23 (4), 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang AZ et al. (2008) Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem 3 (9), 1311–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samanta D et al. (2016) Electroresponsive nanoparticles for drug delivery on demand. Nanoscale 8 (17), 9310–9317 [DOI] [PubMed] [Google Scholar]
- 123.Im JS et al. (2010) The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials 31 (6), 1414–1419 [DOI] [PubMed] [Google Scholar]
- 124.Ying X et al. (2014) Angiopep-conjugated electro-responsive hydrogel nanoparticles: therapeutic potential for epilepsy. Angew Chem Int Ed Engl 53 (46), 12436–12440 [DOI] [PubMed] [Google Scholar]
- 125.Yan Q et al. (2010) Voltage-responsive vesicles based on orthogonal assembly of two homopolymers. J Am Chem Soc 132 (27), 9268–9270 [DOI] [PubMed] [Google Scholar]
- 126.Wang Y et al. (2016) Electroresponsive Nanoparticles Improve Antiseizure Effect of Phenytoin in Generalized Tonic-Clonic Seizures. Neurotherapeutics 13 (3), 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim JA and Lee WG (2011) Role of weakly polarized nanoparticles in electroporation. Nanoscale 3 (4), 1526–1532 [DOI] [PubMed] [Google Scholar]
- 128.Wang S et al. (2010) Targeted nanoparticles enhanced flow electroporation of antisense oligonucleotides in leukemia cells. Biosens Bioelectron 26 (2), 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ge J et al. (2012) Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano 6 (1), 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soni KS et al. (2016) Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. Journal of controlled release : official journal of the Controlled Release Society 240, 109–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bobo D et al. (2016) Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 33 (10), 2373–2387 [DOI] [PubMed] [Google Scholar]
- 132.Yang J et al. (2009) Smart Drug-Loaded Polymer Gold Nanoshells for Systemic and Localized Therapy of Human Epithelial Cancer. Advanced Materials 21 (43), 4339–4342 [DOI] [PubMed] [Google Scholar]
- 133.Zardad A-Z et al. (2016) A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers 8 (10), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]