Abstract
Background
Vocimagene amiretrorepvec (Toca 511) is an investigational gamma-retroviral replicating vector encoding cytosine deaminase that, when used in combination with extended-release 5-fluorocytosine (Toca FC), results preclinically in local production of 5-fluorouracil, depletion of immune-suppressive myeloid cells, and subsequent induction of antitumor immunity. Recurrent high-grade glioma (rHGG) patients have a high unmet need for effective therapies that produce durable responses lasting more than 6 months. In this setting, relapse is nearly universal and most responses are transient.
Methods
In this Toca 511 ascending-dose phase I trial (NCT01470794), HGG patients who recurred after standard of care underwent surgical resection and received Toca 511 injected into the resection cavity wall, followed by orally administered cycles of Toca FC.
Results
Among 56 patients, durable complete responses were observed. A subgroup was identified based on Toca 511 dose and entry requirements for the follow-up phase III study. In this subgroup, which included both isocitrate dehydrogenase 1 (IDH1) mutant and wild-type tumors, the durable response rate is 21.7%. Median duration of follow-up for responders is 35.7+ months. As of August 25, 2017, all responders remain in response and are alive 33.9+ to 52.2+ months after Toca 511 administration, suggesting a positive association of durable response with overall survival.
Conclusions
Multiyear durable responses have been observed in rHGG patients treated with Toca 511 + Toca FC in a phase I trial, and the treatment will be further evaluated in a randomized phase III trial. Among IDH1 mutant patients treated at first recurrence, there may be an enrichment of complete responders.
Keywords: durable response rate, gene therapy, immuno-oncology, immunotherapy, recurrent high grade glioma
Importance of the study
Patients with rHGG have a high unmet need for effective therapies. Response to current standard treatment is uncommon and durable responses even less so. Newer therapies that engage the immune system to break tumor tolerance likely require consideration of novel endpoints, including durability of responses. We report on several multiyear complete responses in rHGG patients treated with Toca 511 + Toca FC, including 21.7% durable complete response rate in 23 patients who received maximum feasible doses of Toca 511. All responding patients are still alive, with a median duration of follow-up of >35 months. Molecular profiling shows responding patients have tumors with low level of DNA mutations, which differentiates this type of immunotherapy from others that require high mutagenic burden for response. The maturing phase I trial observations demonstrate that a replicating gene therapy vector combined with a prodrug may act as an anticancer immunotherapeutic in malignant glioma.
Vocimagene amiretrorepvec (Toca 511) + extended-release 5-fluorocytosine (Toca FC) are currently in development as a novel combination treatment for recurrent high-grade glioma (rHGG), and clinical trials have shown anticancer activity and a favorable safety profile to date.1 Since the initial published report on 45 patients, data on 11 additional patients, and 2 years of additional safety and efficacy follow-up on the 56 total patients, including, new detailed molecular profiling are now reported. The durable objective responses and stable disease along with delayed onset of responses are consistent with an immunologic mechanism of action.
The 2017 estimated incidence and prevalence of adult glioblastoma (GBM; World Health Organization [WHO] grade IV astrocytoma) and anaplastic astrocytoma (AA; WHO grade III astrocytoma) are approximately 16081 and 49407, respectively, in the United States2 and approximately 36104 and 110922, respectively, in Europe.3 AA and GBM are collectively classified as HGG. Following treatment in surgery settings for newly diagnosed disease, radiation, and chemotherapy with temozolomide, patients with GBM have a median survival of 14.5 months, and patients with AA have a median survival of 3.9 years. These tumors inevitably relapse within approximately 7 months in patients with GBM and 21 months in patients with AA.4,5 Patients with rHGG have a high unmet need due to the limited efficacy of available treatments.6 While surgical resection in newly diagnosed settings is thought to improve outcomes, prospective studies to demonstrate surgical-resection benefit in the recurrent setting are lacking. However, analyses of patients enrolled in clinical studies indicate that surgery for recurrence is not associated with improved survival.7,8 Surgery is not curative for these highly infiltrative tumors, and patients with GBM and AA invariably recur after resection with measurable or nonmeasurable residual tumor.9
Among available therapies, temozolomide was granted accelerated approval for patients with recurrent AA (rAA), who experienced disease progression while on a nitrosourea and procarbazine, based on a response rate of 22% and median duration of response of 11.6 months. These patients were temozolomide naïve in the newly diagnosed setting, and the drug is now used with radiation and surgery in first-line treatment for GBM. However, after failure of first-line temozolomide treatment, durable responses (meaning partial response [PR] or complete response [CR] observed for ≥24 wk) with available therapies is uncommon, with rates ranging from 0 to 2.15% in rGBM (Supplementary Table S1).10 While objective responses require confirmation at least 4 weeks later, they are often transient and may not translate into overall survival benefit.11 In contrast, durable responses in the current study appear to indicate a lasting treatment effect with potential to translate into an improvement in overall survival.12,13
Cancer immunotherapy presents challenges in the central nervous system (CNS) due to the unique regulation of immunologic activity within the brain compared with most other tissues and the immune suppression associated with HGGs.14 The nervous system has historically been considered an immune privileged organ, with limited immunologic function, as initially observed by the lack of graft rejection in the brain.15,16 Classically, this “privileged” site was described with selective blood–brain barrier entry of immune cells from peripheral blood into the brain parenchyma,17 a lack of lymphatic vessels and lymph nodes within the CNS, and low numbers of circulating T cells and human leukocyte antigen expression.18 However, current understanding shows that the brain hosts a lymphatic system, several populations of cells with immune function, including microglia, which when activated can recruit other immune cells to sites of inflammation.19–21 Nevertheless, to date, immunotherapies have had poor to limited success in HGG clinical trials.22,23 The lack of success with immunotherapy strategies against tumors within the brain suggests that multimodal approaches that induce immune activation against tumor antigens as well as diminish immune suppression are likely required to generate clinically meaningful durable responses. We suggest here that such a multimodal strategy, implemented by the treatment regime described here, leads to the durable responses observed in the reported study.
Toca 511, a gamma retroviral replicating vector encoding cytosine deaminase, when used in combination with 5-FC, results in local production of concentrated 5-fluorouracil (5-FU) in the tumor without systemic 5-FU side effects due to Toca 511 cancer selectivity and 5-FU’s short half-life.24,25 In preclinical models, local 5-FU selectively causes direct cancer cell death and depletion of immune-suppressive myeloid cells such as myeloid-derived suppressor cells and tumor-associated macrophages. The resultant tumor microenvironment is permissive to establishing a durable T-cell mediated antitumor immune response and tumor shrinkage with durable systemic antitumor immunity, but without autoimmunity or surrounding healthy tissue toxicity.26–28 Based on both the clinical data, including durable objective responses, and preclinical mechanism of action, the FDA granted Breakthrough Therapy designation in rHGG, indicating that this new therapy may demonstrate substantial improvement over existing therapies. The potential for Toca 511 + Toca FC to provide relevant clinical benefit to patients is supported by the European Medicines Agency’s PRIME (PRIority MEdicines) designation in HGG.
Materials and Methods
Resection-Injection Trial
This phase I resection-injection trial (NCT01470794) was approved by the institutional review board at each site and complied with International Ethical Guidelines for Biomedical Research Involving Human Subjects, good clinical practice guidelines, the Declaration of Helsinki, and local laws. All patients provided written informed consent. Between February 2011 and October 2015, HGG patients who recurred after initial treatment with at least subtotal resection, postoperative radiation, and temozolomide were enrolled.1 Ascending doses of Toca 511 were injected into resection cavity beds in patients with rHGG who were undergoing planned surgical resection of at least 80% of the enhancing tumor, followed by oral weekly Toca FC cycles initiated approximately 6 weeks after Toca 511 and repeated every 6 weeks.
Primary Endpoint
The primary endpoint of the study was to identify dose limiting toxicities of Toca 511 and Toca FC. Clinical benefit, measured by durable objective responses and stable disease, a safety database covering over 4 years for individual patients, and extensive molecular profiling are reported here and build on previously presented data.1 Cohorts treated with Toca 511 + Toca FC plus bevacizumab or Toca 511 + Toca FC plus lomustine are also reported. In these cohorts, bevacizumab 10 mg/kg intravenously every 2 weeks or lomustine 110 mg/m2 orally every 6 weeks was administered beginning with the first cycle of Toca FC.
Response Criteria
Objective responses are determined by independent radiology review taking neurologic status and corticosteroid use into account and using modified Macdonald criteria29 for all except one cohort, in which patients received Toca 511 + Toca FC and bevacizumab, who were evaluated using modified Response Assessment in Neuro-Oncology (RANO) criteria.30 For both criteria for response assessment, evaluable and measurable disease requires a lesion at least 1 cm in 2 dimensions, which can be assessed for a partial or complete response. The modification applied to evaluable nonmeasurable disease, which can only be assessed for a complete response. For objective response assessment, baseline for radiologic assessment for all patients is the MRI scan obtained approximately 6 weeks after surgical resection and Toca 511 administration and just prior to Toca FC administration.
Statistical Analyses
Statistical analyses of phase I data are reported using summary tables, figures, and data listings. Continuous variables are summarized with means, standard deviations, medians, minimums, and maximums. Categorical variables are summarized by counts and by percentage of patients in corresponding categories. Association between durable response rate and overall survival was assessed using the Kaplan–Meier method.
For detailed material and methods including exome sequencing, immunophenotyping of blood, and RNA sequencing, see the Supplementary material.
Results
Patient Characteristics and Treatment
In this phase I dose escalation trial of Toca 511 + Toca FC in rHGG, patients in the newly diagnosed setting had undergone surgical resection, radiation therapy, and chemotherapy with temozolomide. Results are presented for all 56 patients, including patients treated in combination cohorts with lomustine or bevacizumab, and for a subgroup of 23 patients that matches the recommended phase III Toca 511 dose and patient population (referred to as the phase III eligible subgroup). The phase III eligible subgroup was identified as part of a post-hoc analysis based on selection of patients who received within a half-log of the recommended Toca 511 phase III dose and who had the demographic profile of patients who appeared to derive the greatest benefit. This includes patients with GBM or AA who were at first and second recurrence and had received no prior bevacizumab and whose tumor size at the longest dimension was ≤5 cm (Supplementary Fig. S1). Such inclusion criteria are commonly used in rHGG trials.
In Supplementary Table S2, demographics and baseline characteristics for all patients and the phase III eligible subgroup are reported.
Complete Responses Observed by Radiographic Evaluation
In this recurrent setting, the study entry criteria were designed to minimize pseudoprogression by enrolling rHGG patients who had an interval of at least 12 weeks after prior radiation for patients at first recurrence or, if less than 12 weeks, had histopathologic confirmation of recurrent tumor or new enhancement outside of the radiation therapy field. While enhancing tumor can be frequently resected, the non-enhancing deeply infiltrative tumor portion is typically not resected, in order to maintain neurologic function. Post resection, Toca 511 was injected into the walls of the resection cavity followed after approximately 6 weeks by cycles of oral Toca FC. In addition, the possibility of pseudo-responses was minimized by obtaining a baseline MRI brain scan at 6 weeks post resection, a time when most postsurgical changes have typically resolved,31 and when therapeutic activity is not expected, as this starts with Toca FC administration and subsequent local conversion to 5-FU.
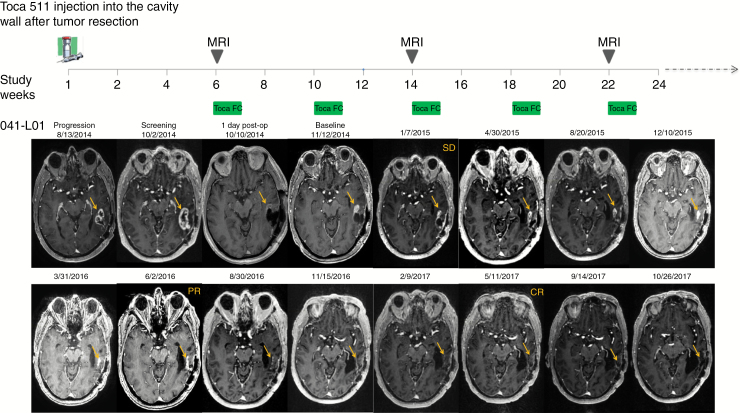
In the setting of rHGG, tumors are growing prior to surgical resection. Surgery is not a curative treatment for these highly infiltrative tumors, and patients invariably recur following re-resection. At the time of the baseline scan, all patients had evaluable disease, and a majority (55%) had measurable disease. In Fig. 1, an evaluable and measurable lesion 1 (041-LO1) grew in the area that was injected with Toca 511. Over time, the lesion regressed, resulting in a durable complete response. In addition, an evaluable and measurable lesion 1 (19-LO1) grew in the area that was injected with Toca 511, while a secondary lesion (19-LO2) was in an area that was not injected. Over a similar time, both lesions regressed, resulting in a durable complete response. Examples of responses with evaluable and either measurable or nonmeasurable disease at 6 weeks post resection and the durability of such responses are shown in Supplementary Fig. S2.
Fig. 1.
Top panel: timeline of Toca 511 delivery, Toca FC dosing, and MRI. Bottom panel: independent radiology review determined radiographic response after Toca 511 + Toca FC therapy. The left temporal tumor is shown to be progressing prior to surgical resection. After surgical resection, residual tumor remains, which continues to grow postoperatively. Following Toca FC administration, there is gradual decrease of the tumor to a partial response and then to a complete response (see Supplementary Fig. S2 for complete series).
Objective and Durable Response Rates Observed in Patients Treated with Toca 511 + Toca FC
Among the 53 efficacy-evaluable patients, the rate of objective responses, which were all complete responses, was 11.3% (6/53). In the 23-patient phase III eligible subgroup, the percentage of patients with objective response was 21.7%, with 5 complete responders, and the stable disease rate was 21.7% for an overall clinical benefit rate of 43.5% (Table 1). All responders met phase III eligibility criteria and were treated at or within a half-log of the recommended Toca 511 phase III dose. One additional complete responder was in the 5-patient bevacizumab combination treatment cohort and was treated with Toca FC in combination with bevacizumab for approximately 4.6 months and continued on bevacizumab for more than 16.2 months. No responders were observed in lower-dose cohorts or in the lomustine combination treatment cohort.
Table 1.
Objective response, durable response, and clinical benefit rates
| Response Category1 | All Patients,2 n = 53 (%) |
Phase III Eligible Subgroup, n = 23 (%) |
|---|---|---|
| Complete response | 6 (11.3)3 | 5 (21.7) |
| Partial response | 0 | 0 |
| Stable disease [SD] | 10 (18.9) | 5 (21.7) |
| Progressive disease | 37 (69.8) | 13 (56.6) |
| Clinical benefit rate (CR+PR+SD ≥6 wk) | 16 (30.2) | 10 (43.5) |
| Durable response rate (PR or CR ≥24 wk) | 6 (11.3) | 5 (21.7) |
| Median duration of follow-up for responders (mo) | 35.1+ (9.2−44.9) | 35.7+ (14.1−44.9) |
1Determined by independent radiology review using modified Macdonald criteria for all patients, except for a cohort that received Toca 511 + Toca FC and bevacizumab that used modified RANO criteria, taking into account corticosteroid and clinical data.
2Of 56 safety evaluable patients, 53 patients who received Toca 511 + Toca FC are efficacy evaluable.
3Includes patient treated with Toca 511 and Toca FC and bevacizumab had a CR, which began more than 10 months after administration of Toca 511. Because responses in the bevacizumab setting typically occur within a few months of treatment, and even then CRs are rare, the response in this patient is more likely consistent with an immunologic mechanism of the Toca 511 and Toca FC therapy.
Tumors which responded were progressing prior to surgical resection and continued to grow after re-resection. Baseline MRI obtained prior to start of Toca FC showed all responders had either measurable disease of 1.7 to 2.8 cm2 or nonmeasurable but evaluable tumor (Supplementary Table S3).
In this trial, there was a delayed time to response (PR or CR) onset of more than 6 months and responses occurred gradually over time, such that they were not representative of a rapid decrease following surgery, which would be indicative of resolution of postoperative changes or ischemia. A majority of patients were using ≤2 mg/day systemic corticosteroids with dexamethasone at last follow-up. Among patients with a response, only 1 was on corticosteroids at varying doses of dexamethasone of 1 to 4 mg (Supplementary Table S3 and Supplementary Table S4).
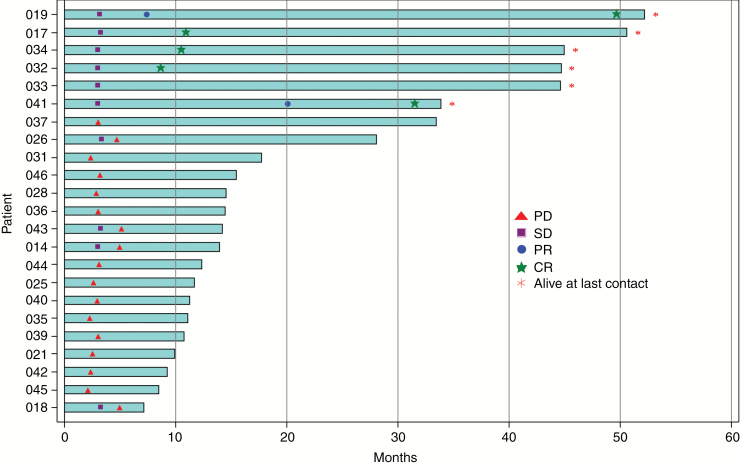
In the phase III eligible subgroup, swim lanes (Fig. 2) show onset and durability of responses. Complete and partial responses begin about 6 to 19 months after Toca 511 administration, consistent with an immunologic-based response (Supplementary Fig. S2). Among the phase III eligible subgroup, median time to initial response is 9.2 months and the median duration of response has not been reached after a median follow-up of 35.7+ months (range, 14.1+ to 44.9+ mo). All partial responders improved to complete responders and, as of August 25, 2017, all responders are in complete response and remain alive (range, 33.9+ to 52.2+ mo) after Toca 511 administration, suggesting a positive association of durable response with overall survival (Fig. 2). Typically in HGG, the duration of each successive recurrence is usually shorter. In the phase III eligible subgroup, the patients’ disease trajectory from initial diagnosis for newly diagnosed HGG is summarized (Supplementary Fig. S3) and supports that their disease course may be altered by Toca 511 + Toca FC. Median survival post-progression for the 23 patients was 9.1 months (95% CI: 7.5, 11.4), suggesting that many patients who did not have a response may have still received some benefit from treatment, since overall survival in this setting is typically around 5 months (Supplementary Table S1).32
Fig. 2.
Swim lane demonstrating responses are durable (≥24 wk) and associated with long-term survival.
Overall Survival Improved with Toca 511 + Toca FC Treatment
In the phase III eligible subgroup, median survival was 14.4 months; landmark survival shows durability of response with key landmarks at OS12 at 65.2% (15/23), OS24 at 34.8% (8/23), and, although the sample size is small, Kaplan–Meier estimate of the probability of survival at 3 years or beyond was 26.1% (6/23) (Table 2). Details regarding responding patients and a patient with durable stable response for more than 3 years are summarized (Supplementary Table S3). The safety profile is consistent with that previously reported1 with related treatment-emergent serious adverse events at 7.1% (Supplementary Table S5). With additional follow-up since the previous report1 and with survival of up to 52.2 months, no secondary malignancies or lymphoproliferative disorders have been reported with this retroviral replicating vector treatment.
Table 2.
Median and landmark survival as of August 15, 2017
| All Efficacy- Evaluable Patients, n = 53 |
Phase III Eligible Subgroup, n = 23 |
|
|---|---|---|
| Median Survival, mo (95% CI) | 11.9 (10.7, 15.1) | 14.4 (11.3, 28.1) |
| Landmark Survival* n (%) | ||
| OS 6 mo | 47 (88.7) | 23 (100) |
| OS 9 mo | 38 (71.7) | 21 (91.3) |
| OS 12 mo | 26 (49.1) | 15 (65.2) |
| OS 18 mo | 14 (26.4) | 8 (34.8) |
| OS 24 mo | 13 (24.5) | 8 (34.8) |
| OS 36 mo | 7 (13.4) | 6 (26.1) |
| OS 42 mo | 7 (13.4) | 6 (26.1) |
| OS 48 mo | 7 (13.4) | 6 (26.1) |
*OS, overall survival; Kaplan–Meier estimate of the probability of survival.
Durable Response Rate and Overall Survival Are Associated
Durable response rate and overall survival outcomes are similar comparing patients with baseline evaluable and measurable disease with those with evaluable and nonmeasurable disease (Supplementary Table S6). The Kaplan–Meier curve suggests a clear relationship between durable response rate and overall survival (Supplementary Fig. S4). Median survival for patients with a durable response has not been reached, and for nonresponders is 13.2 months (95% CI: 10.8, 14.6).
Responding Patients Have Low Genomic Mutational Burden
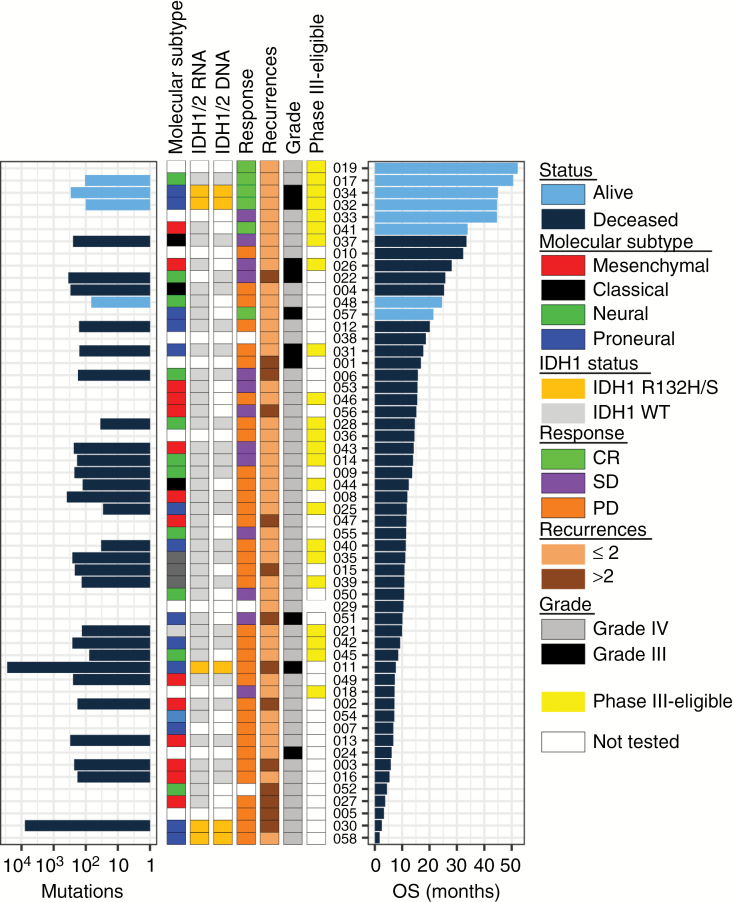
Response to checkpoint inhibitors correlates with tumor mutational burden, which is a proxy for the presence of “neoantigens” that can be recognized by tumor-infiltrating lymphocytes.33 As it appears that responses to Toca 511 treatment have an immunologic basis, we tested whether mutational burden correlates with response, by “exome” sequencing of all available patient tumors resected immediately prior to Toca 511 treatment. Consistent with previous characterization of HGG,34 most patient tumors had relatively few total mutations (or nonsynonymous mutations)—of approximately 100 or about 2 per/Mb sequenced using blood DNA as baseline (Fig. 3). Two patients (011 and 030) displayed hallmarks of temozolomide-induced hypermutation with about 100-fold more mutations and a strong bias for CG to TA. Neither patient responded and both patients had isocitrate dehydrogenase 1 (IDH1) R132H/S driver mutations. Patient 011 did not take Toca FC and Patient 030 was on fifth recurrence. Neither patient lived longer than 7 months, whereas most responding patients took 6 to 19 months to respond. All responding patients had few mutations and thus responses observed were not associated with tumor mutation load determined by exome sequencing. No patients were reported to have 1p/19q mutation.
Fig. 3.
Summary of RNA and DNA sequencing results from patient tumors. (Left to right) The barplot shows the total number of high confidence mutations called from exome sequencing data by MuSE (Mutation calling using a Markov Substitution model for Evolution). The first 3 left columns summarize results from RNA sequencing: molecular subtype (mesenchymal—red, classical—black, neural—green, proneural—blue), and IDH1 R132H/S mutation (orange). The next 4 columns show response (CR—green, stable disease—purple, progressive disease—orange), clinical features, including eligibility for phase III trial (phase III eligible subgroup—yellow), tumor grade at study entry as determined by clinical site pathologist (WHO grade IV = gray, grade III = black), and number of recurrences (1 or 2—light brown, >2—dark brown). Patients are ordered by duration of survival post resection and Toca 511 treatment. Patients alive at last contact are indicated by light blue bars.
HGG is a heterogeneous disease with diverse underlying driver mutations and consequent molecular profiles. We tested if response correlated with specific mutations, molecular subtype, or expression of key immune cell markers. Responding patients did not show significant bias for molecular subtype (Supplementary Fig. S5). Levels of mRNA of T-cell genes—PD-L1, CD8A, IDO1, ICOS, OX40, and CTLA-4—did not differ between tumors from patients who lived more than 24 months compared with those who lived less than 24 months, suggesting that baseline resident T cells are not likely to be predictors of survival to this therapy (Supplementary Fig. S6).
We performed longitudinal flow cytometric profiling of peripheral blood mononuclear cells from combination therapy cohorts. We observed a sustained treatment-associated increase in Ki-67 positive (proliferating) T lymphocytes (particularly the CD8+ cytotoxic T-cell subset) in 6 patients who did not have disease progression compared with 7 patients who progressed at time of analysis (Supplementary Fig. S7).
Discussion
Multiyear durable responses were observed in rHGG patients receiving Toca 511 + Toca FC. Responders were a diverse group across ages, KPS, and molecular subtypes. Whether patients had evaluable measurable tumors or evaluable nonmeasurable tumors, similar efficacy outcomes of durable response rate and overall survival were observed. While these data were obtained from uncontrolled nonrandomized studies, durable responses lasting more than 24 weeks are rare in rGBM patients receiving approved treatments, with durable response rates ranging from 0 to 2.15% for rGBM.10,32 Also, patients with rGBM and IDH1 mutation have the same objective response rate as patients with wild-type (wt) phenotype.35 Treatment with Toca 511 + Toca FC compares favorably, demonstrating a durable response rate in rHGG of 21.7% and in rGBM of 15.8%. Among efficacy-evaluable patients (n = 53), the estimated median duration of response for rHGG of at least 35.1+ months is substantially longer than the median duration of response observed for existing therapies, ranging from 2.79 to 11.6 months (Supplementary Table S1). In our dataset, there was positive association of durable response rate and overall survival. The delayed time to response, responses in some patients, and prolonged duration of response are consistent with other immuno-oncology drugs.36,37 Common considerations for immuno-oncology drugs include objective response rates in a select subset of patients with prolonged duration of response, a delayed separation, and a late plateau of survival curves, as well as a median overall survival that may obscure the long-term benefits in the minority of patients with responses.38 Therefore durable response rate may represent a new and clinically meaningful surrogate endpoint for overall survival in brain cancer trials, to more accurately assess clinical benefit of novel immune-oncology drugs for the treatment of brain tumors. In postsurgical settings, appropriately timed baseline measurement provides confidence that outcomes of this novel treatment differentiate the treatment from standard of care. The reproducibility of the observed results, validity of durable response rate, and relationship between durable response rate and overall survival will be further explored in the currently ongoing phase III trial in recurrent glioblastoma and recurrent anaplastic astrocytoma (NCT02414165).
In the study described here, tumor shrinkage was observed in areas that had not been directly injected with Toca 511, suggesting viral spread or abscopal antitumor immune effects (Supplementary Fig. S2; Patient 019). The impact of brain cancer on quality of life has been well documented in the literature.39 One component of quality of life is being able to resume one’s professional life. Historically, less than a third of patients with rHGG return to work, highlighting the pharmacoeconomic impact of this disease. Anecdotally, the majority of patients who responded in our study have been reported to return to work.
In newly diagnosed HGG, initial growth of IDH1 mutant (mt) tumors may be slow and IDH1 mt is a significant prognostic marker of overall survival.35 However, in the recurrent setting, particularly among patients with rGBM, the biology of IDH1 mt and wt tumors converge, with neither conferring a survival advantage.35 Additionally, IDH1 mt tumors do not spontaneously regress.35 Objective response rates in patients with recurrent IDH1 mt tumors are similar to IDH1 wt tumors and IDH1 mutation does not correlate with objective response.35,39,40 However, the observed data suggest an enrichment of durable complete responses in IDH1 mt patients at first or second recurrence treated with Toca 511 + Toca FC.
In rHGG, complete responses are uncommon and typically occur in ≤1% of treated GBM patients.32,35 We observed complete responses in patients with IDH1 mt and wt tumors, suggesting a benefit across the rHGG setting. Following treatment with Toca 511 + Toca FC, there were 6 complete responses (2 IDH1 mt and 3 IDH1 wt) and 1 also receiving bevacizumab (IDH1 wt). Interestingly, of 5 patients with IDH1 mutation, the 2 patients who entered the study at first recurrence had durable complete responses (Supplementary Table S3).
Patients with hypermutated tumors seem more likely to respond to immunotherapies.41 Case reports of hypermutated GBMs have observed clinical responses to checkpoint inhibitors.42,43 While HGG tumors, especially those treated with temozolomide, are known to have higher mutational loads than low-grade gliomas,44 an increased tumor mutational burden in responding patients was not observed here. This observation held true for the responding IDH1 mutant tumor patients,1 even though 50%–60% of IDH1 mt tumors have been reported to be hypermutated due to hypermethylation and inactivation of DNA repair genes.45 Tumors with IDH1 mutations may be more susceptible to our immunotherapy for reasons other than hypermutation, including increased sensitivity to 5-FU.46 Also, as previously reported, methylation status of O6-methylguanine-DNA methyltransferase does not appear to be a significant marker of survival in the recurrent setting.1
Given that long lead times to achieve responses can be 6 to 19 months and that responses occurred in patients at first and second recurrence in rHGG settings, patients with newly diagnosed HGG who typically live longer and may have a more intact immune system may derive benefit from treatment with Toca 511 + Toca FC. The newly diagnosed setting also provides an opportunity to leverage Toca 511 + Toca FC activity with radiation therapy and temozolomide as reported in preclinical models.47,48
The ability of Toca 511 + Toca FC therapy to alter immune-suppressive networks within the tumor microenvironment and cause cancer cell death may provide a mechanism for turning tumors with low immunogenic potential and immune activity into tumors actively destroyed by the immune system. Toca 511 + Toca FC likely breaks immune tolerance to the notoriously recalcitrant HGG by increasing both immunogenicity and immune activity within the tumor microenvironment. These data support further testing of Toca 511 + Toca FC in newly diagnosed HGG as well as a general modality for cancer immunotherapy in other indications.
Supplementary Material
Supplementary material is available at Neuro-Oncology online
Funding
The authors thank the ABC2 Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musella Foundation, and Voices Against Brain Cancer for their support and collaborations. N.K. was also supported in part by U01NS059821 from the National Institute of Neurological Diseases and Stroke.
Acknowledgments
We thank Nick Boyle PhD, Amanda M Richter MS RAC, and John Wood RAC for critical readings of the manuscript and Sean Mitchell for preparation of selected figures. We also wish to thank Dewen Yang MD PhD, Head of Quantitative Imaging at ICON Medical Imaging, Inc for independent radiology review.
Conflict of interest statement. D.O., W.A., O.R.D., D.J.H., D.G., T.K., D.J.J., H.E.G., and A.D. are employees and/or shareholders of Tocagen. N.K. is a consultant, has ownership interest in, and is the recipient of a research grant from Tocagen. T.W. and J.L. are advisers for Novocure. L.M.L. is an adviser for Genentech/Roche; M.A.V. is an adviser with Infuseon Therapeutics. T.F.C. is a paid consultant for Tocagen.
Authorship statement. T.C., J.L., S.B., B.C., C.C.C., J.B.E., S.N.K., S.K., A.L., I.Y.L., L.M.L., T.M., P.N., D.P., M.A.V., and T.W. ran the clinical trial at their respective sites, provided clinical samples, and reviewed and provided insight to the manuscript. D.J.H., T.K., A.D., D.G., D.O., W.A., and O.R.D. performed experiments and analyzed the data. H.E.G., D.J.J., A.D., and D.O. designed the study and supervised the overall project. A.D. and D.O. wrote the manuscript. N.K. developed the founding technology on which Toca 511 is based. All authors reviewed the final manuscript.
References
- 1. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferlay J, SI, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013. http://globocan.iarc.fr. Accessed February 12, 2018. [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Chang S, Zhang P, Cairncross JG, et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol. 2017;19(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—are we there yet?Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortega A, Sarmiento JM, Ly D, et al. Multiple resections and survival of recurrent glioblastoma patients in the temozolomide era. J Clin Neurosci. 2016;24:105–111. [DOI] [PubMed] [Google Scholar]
- 10. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wick W, Brandes AA, Gorlia T, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016;34(15_suppl):2001. [Google Scholar]
- 12. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macdonald DR. Temozolomide for recurrent high-grade glioma. Semin Oncol. 2001;28(4 Suppl 13):3–12. [DOI] [PubMed] [Google Scholar]
- 14. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 16. Sehgal A, Berger MS. Basic concepts of immunology and neuroimmunology. Neurosurg Focus. 2000;9(6):e1. [DOI] [PubMed] [Google Scholar]
- 17. de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49(2):143–155. [PubMed] [Google Scholar]
- 18. John B, Hunter CA, Harris TH.. Immune Cell Trafficking in the Central Nervous System. New York: Springer Science+Business Media; 2014. [Google Scholar]
- 19. Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol. 1992;2(4):277–284. [DOI] [PubMed] [Google Scholar]
- 20. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2017;24(13):379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perry VH, Andersson PB. The inflammatory response in the CNS. Neuropathol Appl Neurobiol. 1992;18(5):454–459. [DOI] [PubMed] [Google Scholar]
- 22. Polivka J Jr, Polivka J, Holubec L, et al. Advances in experimental targeted therapy and immunotherapy for patients with glioblastoma multiforme. Anticancer Res. 2017;37(1):21–33. [DOI] [PubMed] [Google Scholar]
- 23. Reardon DA, Omuro A, Brandes AA, et al. Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017;19(Supp 3):iii21. [Google Scholar]
- 24. Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dixit K, Kumthekar P. Gene delivery in neuro-oncology. Curr Oncol Rep. 2017;19(11):69. [DOI] [PubMed] [Google Scholar]
- 26. Hiraoka K, Inagaki A, Kato Y, et al. Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro Oncol. 2017;19(7):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell LA, Lopez Espinoza F, Mendoza D, et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19(7):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yagiz K, Huang TT, Lopez Espinoza F, et al. Toca 511 plus 5-fluorocytosine in combination with lomustine shows chemotoxic and immunotherapeutic activity with no additive toxicity in rodent glioblastoma models. Neuro Oncol. 2016;18(10):1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 30. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 31. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 33. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colli LM, Machiela MJ, Myers TA, Jessop L, Yu K, Chanock SJ. Burden of nonsynonymous mutations among TCGA cancers and candidate immune checkpoint inhibitor responses. Cancer Res. 2016;76(13):3767–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mandel JJ, Cachia D, Liu D, et al. Impact of IDH1 mutation status on outcome in clinical trials for recurrent glioblastoma. J Neurooncol. 2016;129(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hazarika M. Regulatory perspective on endpoints for immuno-oncology drug products. Office of New Center for Drug Evaluation and Research, FDA. Bethesda MD: AAADV Regulatory Science Session; 2017. [Google Scholar]
- 39. Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden—and should be considered when allocating research funds. Br J Cancer. 2005;92(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 41. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 42. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 43. Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Draaisma K, Wijnenga MM, Weenink B, et al. PI3 kinase mutations and mutational load as poor prognostic markers in diffuse glioma patients. Acta Neuropathol Commun. 2015;3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim J, Lee IH, Cho HJ, et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28(3):318–328. [DOI] [PubMed] [Google Scholar]
- 46. Zhu H, Zhang Y, Chen J, et al. IDH1 R132H mutation enhances cell migration by activating AKT-mTOR signaling pathway, but sensitizes cells to 5-FU treatment as NADPH and GSH are reduced. PLoS One. 2017;12(1):e0169038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang TT, Hlavaty J, Ostertag D, et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20(10):544–551. [DOI] [PubMed] [Google Scholar]
- 48. Takahashi M, Valdes G, Hiraoka K, et al. Radiosensitization of gliomas by intracellular generation of 5-fluorouracil potentiates prodrug activator gene therapy with a retroviral replicating vector. Cancer Gene Ther. 2014;21(10):405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.