Abstract
DSL1 was identified through its genetic interaction with SLY1, which encodes a t-SNARE-interacting protein that functions in endoplasmic reticulum (ER)-to-Golgi traffic. Conditional dsl1 mutants exhibit a block in ER-to-Golgi traffic at the restrictive temperature. Here, we show that dsl1 mutants are defective for retrograde Golgi-to-ER traffic, even under conditions where no anterograde transport block is evident. These results suggest that the primary function of Dsl1p may be in retrograde traffic, and that retrograde defects can lead to secondary defects in anterograde traffic. Dsl1p is an ER-localized peripheral membrane protein that can be extracted from the membrane in a multiprotein complex. Immunoisolation of the complex yielded Dsl1p and proteins of ∼80 and ∼55 kDa. The ∼80-kDa protein has been identified as Tip20p, a protein that others have shown to exist in a tight complex with Sec20p, which is ∼50 kDa. Both Sec20p and Tip20p function in retrograde Golgi-to-ER traffic, are ER-localized, and bind to the ER t-SNARE Ufe1p. These findings suggest that an ER-localized complex of Dsl1p, Sec20p, and Tip20p functions in retrograde traffic, perhaps upstream of a Sly1p/Ufe1p complex. Last, we show that Dsl1p interacts with the δ-subunit of the retrograde COPI coat, Ret2p, and discuss possible roles for this interaction.
INTRODUCTION
The endomembrane system of eucaryotic cells comprises an elaborate trafficking pathway for the transport and processing of proteins and lipids. Communication between organelles of the pathway often occurs via transport vesicles that bud from the “donor” compartment and then specifically tether to and fuse with the appropriate “target” compartment (Rothman, 1994; Waters and Hughson, 2000). For most, if not all, trafficking steps, transport between organelles is bidirectional; membrane traffic in the anterograde direction (that is, away from the endoplasmic reticulum, ER), is counterbalanced by retrograde traffic (Pelham, 1996). Bidirectional traffic is necessary to maintain the balance of lipid in the communicating compartments, to recover proteins that cycle between the compartments, and to return wayward proteins that have escaped from their normal site of residence.
Numerous polypeptides participate in transport from the ER to the Golgi. Vesicle budding from the ER begins with the activation of the small GTPase Sar1p by its guanine nucleotide exchange factor Sec12p, an ER integral membrane protein (Barlowe and Schekman, 1993). Sar1p-GTP then recruits the COPII coat, which assembles via the ordered addition of the Sec23/24p and then the Sec13/31p heterodimers, perhaps using Sec16p as a scaffold for the process (Barlowe, 2000). After vesicles are generated from the ER they are thought to uncoat and migrate to the Golgi, where they undergo an initial tethering event (Cao et al., 1998).
Like budding, vesicle tethering to the Golgi also requires a GTPase, in this case, the Rab GTPase Ypt1p (Cao et al., 1998), and its guanine nucleotide exchange factor, a large protein complex called TRAPP (Sacher et al., 1998; Barrowman et al., 2000; Jones et al., 2000; Wang et al., 2000). Also required is Uso1p (Cao et al., 1998), a homodimeric protein with two globular heads and a long coiled-coil tail (Nakajima et al., 1991; Yamakawa et al., 1996), as well as a large protein complex that contains both Sec34p and Sec35p (VanRheenen et al., 1998; Kim et al., 1999; VanRheenen et al., 1999). At the present time it is not clear why so many proteins are required or exactly how they function to bind vesicles to the target membrane.
The next set of important factors that comes into play is the SNAREs, integral membrane proteins that reside predominantly on the vesicle (v-SNARE) or target (t-SNARE) membranes (Söllner et al., 1993). In yeast, anterograde vesicle fusion with the Golgi requires the t-SNARE Sed5p (Hardwick and Pelham, 1992) and the v-SNAREs Bet1p, Bos1p, and perhaps Sec22p (Newman et al., 1990; Rexach et al., 1994; Søgaard et al., 1994; Spang and Schekman, 1998). An atomic structure of this particular SNARE complex is not yet available, but based on structural studies of the neuronal plasma membrane SNARE complex (Hanson et al., 1997; Lin and Scheller, 1997; Poirier et al., 1998; Sutton et al., 1998), it is likely to consist of a parallel four α-helix bundle with all the SNARE transmembrane domains situated at one end. Thus, assembly of a membrane-spanning (trans) SNARE complex results in close apposition of the vesicle and target membranes, perhaps providing the means by which SNAREs directly mediate membrane fusion in vitro (Weber et al., 1998; Nickel et al., 1999; Parlati et al., 1999). However, whether SNAREs are solely responsible for membrane fusion in vivo is currently under debate because several studies suggest that components downstream of the SNAREs facilitate the membrane fusion process (Peters and Mayer, 1998; Peters et al., 1999; Peters et al., 2001).
Sly1p (Dascher et al., 1991), a protein that tightly binds to the t-SNARE Sed5p, and the Rab Ypt1p (Schmitt et al., 1986) may mechanistically link the tethered state with subsequent trans-SNARE complex formation (Sapperstein et al., 1996; Lupashin and Waters, 1997; Cao et al., 1998). Genetic and biochemical studies indicate that Sly1p and Ypt1p function downstream of the tethering factors Uso1p, Sec34p, and Sec35p and upstream of the SNAREs (Dascher et al., 1991; Sapperstein et al., 1996; Cao et al., 1998; VanRheenen et al., 1998, 1999).
Retrograde vesicle formation from the Golgi is, in many ways, analogous to anterograde vesicle formation, but it involves a heptameric coat protein complex called coatomer (Waters et al., 1991; Stenbeck et al., 1993; Letourneur et al., 1994; Barlowe, 2000) and a small GTPase termed Arf (encoded by ARF1 or ARF2). Activation of Arf1/2p by GTP-binding leads to recruitment of coatomer from the cytosol, forming the COPI coat (Serafini et al., 1991). After vesicle formation, hydrolysis of GTP on Arf, which leads to vesicle uncoating (Tanigawa et al., 1993), is facilitated by an Arf GTPase-activating protein (GAP) in a reaction that, under certain circumstances, can be stimulated by coatomer itself (Goldberg, 1999; Szafer et al., 2000).
In contrast to the mechanisms outlined above, little is known about the targeting and fusion of retrograde vesicles to the ER. Two novel proteins have been described, however, that may act upstream of, or in conjunction with, the retrograde t-SNARE and, as such, might be involved in a tethering process. One of these proteins is Sec20p, an essential ∼50-kDa type II ER integral membrane protein that projects into the cytoplasm (Sweet and Pelham, 1992). The second protein is Tip20p (formerly known as Tip1p; Sweet and Pelham, 1993), which is an essential ∼81-kDa peripheral membrane protein that interacts with the cytosolic domain of Sec20p (Sweet and Pelham, 1993; Lewis et al., 1997). Both Sec20p and Tip20p function in retrograde Golgi-to-ER traffic (Lewis and Pelham, 1996; Cosson et al., 1997; Lewis et al., 1997; Ballensiefen et al., 1998), and they can be found in a protein complex along with the ER t-SNARE Ufe1p (Lewis and Pelham, 1996).
We have recently used a novel genetic screen to identify a protein, called Dsl1p (YNL258c), that is involved in membrane traffic at the ER/Golgi interface (VanRheenen et al., 2001). Dsl1p encodes an ∼88-Da protein with no apparent homologs in higher organisms and no readily discernible motifs. Dsl1p is essential for viability and conditional mutants incubated at the restrictive temperature exhibit defective ER-to-Golgi protein traffic. In this article, we show that Dsl1p is an ER protein that has a role in retrograde Golgi-to-ER traffic, raising the possibility that the previously described anterograde defects are secondary consequences of compromised retrograde traffic. Interestingly, we find that Dsl1p interacts with proteins of the ER target site as well as a component of the COPI coat.
MATERIALS AND METHODS
Media, Strains, Plasmids, and Antibodies
The Escherichia coli strain XL1-Blue (Stratagene, La Jolla, CA), which was used throughout this work, was maintained on standard media (Miller, 1972) and transformed by the Hanahan method (Hanahan, 1983). Saccharomyces cerevisiae strains, described in Table 1, were maintained either on rich media (YPD) containing 1% Bacto-yeast extract, 2% Bacto-peptone, 2% glucose, and 20 μg/ml adenine sulfate or on synthetic complete media (SC) containing 0.67% yeast nitrogen base without amino acids, 2% glucose, and the appropriate amino acid supplement (Rose et al., 1990), unless otherwise noted. Yeast transformations were by the method of Schiestl and Gietz (1989). Yeast strains were maintained at 30°C unless otherwise noted, and all incubations designated as 23°C were completed at room temperature, which ranged from ∼19°C to 23°C. All experiments used log phase cultures with an OD600 between 0.5 and 1.0. Diploid strains were sporulated by incubation for 2 to 4 d at 23°C in liquid media consisting of 1% KOAc and 0.02% glucose.
Table 1.
Strains used in this work
| Strain | Genotype | Source/reference |
|---|---|---|
| CKY100 | MATαsec27-1 ura3-52 leu2-3,-112 | C. Kaiser (MIT, Cambridge MA) |
| EGY101 | MATaret1-1 ura3-52 leu2-3,-112 his3-Δ200 trp1-Δ901 suc2-Δ9 | S. Emr (Univ. of California, San Diego CA) |
| GWY167 | MATadsl1-1, ura3-52, leu2-3,-112 ade2-3,-101 ade3-24 | This laboratory (VanRheenen et al., 2001) |
| GWY199 | MATadsl1∷LEU2 ura3-52, leu2-3,112, trpl-Δ63 +pSV61 | This study |
| GWY230 | MATα dsl1-4 ura3-52 leu2-3,-112 | This laboratory (VanRheenen et al., 2001) |
| GWY233 | MATα dsl1-7 ura3-52 leu2-3,-112 | This laboratory (VanRheenen et al., 2001) |
| GWY270 | MATasec17-1, ura3-52, his 4-612 | R. Schekman (Univ. of California, Berkeley CA) |
| GWY379 | MATadsl1-4 ste2∷LEU2 STE2-WBP∷URA3 ura3-52 leu2-3,-112 his3-Δ200 lys2-801 | This study |
| GWY380 | MATa dsl1-7 ste2∷LEU2 STE2-WBP∷URA3 ura3-52 leu2-3,-112 his3-Δ200 lys2-801 | This study |
| GWY414 | MATadsl1-7 ura3-52 leu2-3,112 | This study |
| GWY415 | MATadsl1-4 ura3-52 leu2-3,112 | This study |
| GWY416 | MATa/α ura3-1/ura3-1 leu2-3,112/LEU2 his3-11,15/his3-11,15 trpl-1/trpl-1 ade2-1/ADE2; pSV75 | This study |
| KRY8 | MATaade2-101 ade3-24 ura3-52 leu2-3,-112 | C. Kaiser (MIT, Cambridge MA) |
| MS1554 | MATaura3-5, leu2-3,112, ade2-101, pep4∷LEU2 his3-Δ200 | M. Rose (Princeton, Princeton NJ) |
| MS7512 | MATaufe1∷HIS3 ura3-52 leu2-3,-112 ade2-1; pufe1-1 | M. Rose (Princeton, Princeton NJ) |
| PC13 | MATaste2∷LEU2 STE2-WBP∷URA3 ura3-52 leu2-3,-112 his3-Δ200 lys2-801 | P. Cosson (Basel Switzerland) |
| PC75 | MATaret1-1 ste2∷LEU2 STE2-WBP∷URA3 ura3-52 leu2-3,-112 his3-Δ200 lys2-801 | P. Cosson (Basel Switzerland) |
| PC133 | MATaret2-1 ura3-52 leu2-3,-112 his3-Δ200 lys2-801 suc2-Δ9 | P. Cosson (Basel Switzerland) |
| PC137 | MATα tip20-5 ura3-52 leu2-3,-112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | G. Friggerio (Univ. of Cambridge, Cambridge, UK) |
| PJ-694A | MATaura3-52 leu2-3,112 trp1-901 his3-200 gal4Δ gal80 Δ LYS2∷GAL1-HIS3 GAL2-ADE2 met2∷GAL7-lacZ | P. James (Univ. of Wisconsin, Madison WI) |
| RSY255 | MATα ura3-52 leu2-3,-112 | R. Schekman (Univ. of California, Berkeley CA) |
| RSY275 | MATα sec20-1 ura3-52 his4-619 | M. Lewis (MRC, Cambridge, UK) |
| RSY277 | MATasec21-1 ura3-52 | R. Schekman (Univ. of California, Berkeley CA) |
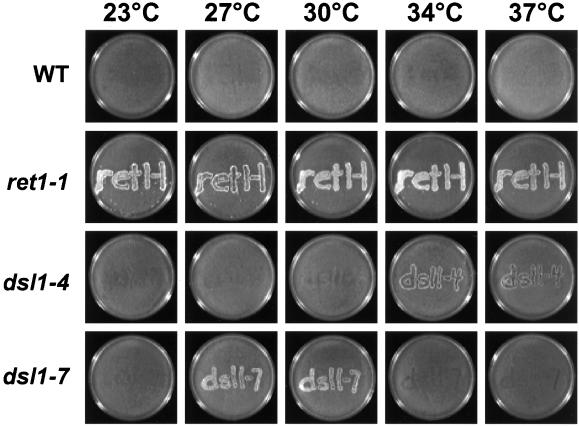
To obtain dsl1 mutant strains used in the mating assay (Figure 3), strains GWY379 and GWY380 were constructed by mating GWY230 and GWY233, respectively, with PC13 containing pBR15. After sporulation of diploids and dissection of tetrads, the temperature-sensitive MATα, Ura+, Leu+, His−, Lys− spores were isolated. The strains were backcrossed once again to obtain the temperature-sensitive MATa, Ura+, Leu+, His−, Lys− segregants used in the mating assay.
Figure 3.
Retrograde transport is inhibited in dsl1 mutants. MATa Ura+ Lys− wild-type (PC13), ret1-1 (PC75), dsl1-4 (GWY379), dsl1-7 (GWY380) strains were grown on nonselective medium for 2 h at 23, 27, 30, 34, or 37°C and then replica plated to a lawn of wild-type cells of the opposite mating type (α Ura−Lys+) and incubated at the same temperature for 6 h. Mating was then analyzed by growth on medium selective for diploids (SC-Ura-Lys). Note that although 30°C is a restrictive temperature for the dsl1-7 strain, mating is detected at this temperature. Perhaps the cells die slowly at this temperature, giving them an opportunity to mate and then survive as heterozygous diploids.
Plasmid pBR4 was constructed by excising the 3.0-kb XhoI/NotI fragment containing full-length DSL1 from pSC2 and ligating it into similarly digested pRS415. Plasmid pBR15 was generated by inserting a 4.3-kb BamHI fragment containing STE2 from MR3264 (kindly provided by M. Rose, Princeton University, Princeton, NJ) into BamHI digested pRS413. To generate the plasmid expressing GST-Dsl1p, pSV59, the complete DSL1 open reading frame was amplified by polymerase chain reaction (PCR) placing EcoRI sites upstream of the start codon and downstream of the termination codon with the use of primers D1 (5′ CGG-AAT-TCA-TGG-AGT-CTC-TTT-TTC-C 3′) and D2 (5′ CGG-AAT-TCA-TGT-AAC-CTA-TCC-TAC-G 3′). The PCR product was then digested with EcoRI and ligated into a similarly digested vector, pGEX4T-1 (Amersham Pharmacia Biotech, Piscataway, NJ). To generate pBR2, pSV59 was digested with EcoRI and the liberated fragment was ligated into similarly digested pGBDU-C1 (kindly provided by P. James, University of Wisconsin, Madison WI) (Table 2). pSV91 was generated by digestion of pSV59 with BamHI and SmaI and ligation of the liberated fragment into a similarly digested pQE31 (QIAGEN, Santa Clarita, CA). Because the BamHI site used is 51 base pairs downstream from the first base pairs in the open reading frame, this construct expresses a His6-tagged Dsl1p fragment that lacks its first 17 amino acid residues. Plasmid pSV57 contains full-length DSL1 with a NheI site before the termination codon and was generated by PCR with primers that place EcoRI sites upstream of the start codon and downstream of a primer-encoded NheI site and termination codon (D1 as described above and D3 [5′ CG-GAA-TTC-TTA-GCT-AGC-ATC-ATC-TAG-AGC-AGT-GC]). The PCR product was digested with EcoRI and ligated into similarly digested pRS416. To generate pSV61, a 100-base pair fragment encoding the triple hemagglutinin (HA) epitope sequence was removed from plasmid MR2654 (kindly provided by M. Rose) by digestion with XbaI and ligated into NheI digested pSV57. pSV66 was constructed by subcloning the HindIII/SalI fragment containing OCH1-3xHA under the control of the OCH1 promoter from pOH (Harris and Waters, 1996) into similarly digested pRS415.
Table 2.
Plasmids used in this work
| Name/alias | Relevant features | Source/reference |
|---|---|---|
| pBR2 | Gal4-DNA binding domain-Dsl1p URA3 | This study |
| pBR4 | CEN DSL1 LEU2 (pRS415)* | This study |
| pBR15 | CEN STE2 HIS3 (pRS413) | This study |
| pOH | CEN OCH1-3XHA URA3 (pRS416) | This laboratory (Harris and Waters, 1996) |
| pGBDU | Gal4-DNA binding domain URA3 | P. James (Univ. of Wisconsin, Madison WI) |
| pGEX-4T | GST AmpR | Amersham Pharmacia Biotech |
| pRS413 | CEN HIS3 | P. Hieter (Johns Hopkins, Baltimore, MD) |
| pRS414 | CEN TRP1 | P. Hieter |
| pRS415 | CEN LEU2 | P. Hieter |
| pRS416 | CEN URA3 | P. Hieter |
| pQE31 | 6xHis AmpR | QIAGEN (Valencia, CA) |
| pSC2 | CEN DSL1 URA3 (pRS416) | This laboratory (VanRheenen et al., 2001) |
| pSC10 | 2 μm DSL1 URA3 (pRS426) | This laboratory (VanRheenen et al., 2001) |
| pSTM20 | 2 μm SEC20 URA3 | G. Friggerio (Univ. of Cambridge, Cambridge, UK (Sweet and Pelham, 1992)) |
| pSV12 | CEN SLY1-20 URA3 ADE3 (pRS416) | This laboratory (VanRheenen et al., 2001) |
| pSV57 | CEN DSL1 3′ NheI URA3 (pRS416) | This study |
| pSV59 | GST-DSL1 (pGEX4T-1) | This study |
| pSV61 | CEN DSL1-HA URA3 (pRS416) | This study |
| pSV66 | CEN OCH1-HA LEU2 (pRS415) | This study |
| pSV67 | CEN DSL1 TRP1 (pRS414) | This laboratory (VanRheenen et al., 2001) |
| pSV75 | CEN DSL1-HA TRP (pRS414) | This study |
| pSV91 | 6xHis DSL1 (pQE31) | This study |
| pTM2 | 2 μm TIP20-myc URA3 | M. Lewis (MRC, Cambridge, UK (Sweet and Pelham, 1993) |
The plasmid backbone is indicated in parentheses.
GST-Dsl1p was expressed from plasmid pSV59, purified as per Amersham Pharmacia Biotech instructions, and used to inoculate rabbits by standard procedures (Harlow and Lane, 1988). The His6-Dsl1p fragment was expressed from pSV91, purified according to standard procedures under nondenaturing conditions (QIAGEN), and covalently linked to cyanogen bromide-activated Sepharose according to the manufacturer's instructions (Amersham Pharmacia Biotech). The resulting His6-Dsl1p-conjugated Sepharose was used to affinity purify antibodies against Dsl1p by standard procedures (Harlow and Lane, 1988). Yeast extracts used for the characterization of the affinity-purified α-Dsl1p antibody were generated as described (Ohashi et al., 1982).
Antibodies that recognize carboxypeptidase Y (CPY), Kar2p, and Sec61p were the kind gifts of Tom Stevens (University of Oregon, Eugene, OR), Mark Rose (Princeton University, Princeton, NJ), and Randy Schekman (University of California, Berkeley, CA), respectively. Anti-invertase and anti-Sed5p (Lupashin and Waters, 1997) were generated in this laboratory by standard methods. Anti-phosphoglycerate kinase (PGK) was from Molecular Probes (Eugene, OR).
Metabolic Labeling and Immunoprecipitation of CPY and Invertase
For pulse-chase experiments, log phase strains (RSY255, GWY270, MS1554, GWY230, and GWY233) were shifted to SC-methionine media that contained 2% raffinose and 0.1% glucose (to induce the expression of invertase) for 1 h before the temperature shift. One OD600 unit of cells was shifted to the appropriate temperature by centrifugation and resuspension in 0.5 ml of the same prewarmed media. After incubation at the same temperature for 20 min, cells were metabolically labeled with 50 μCi of Tran35S-label (ICN Pharmaceuticals, Irvine, CA) for 10 min. For the chase, 10 μl of 250 mM cysteine, 250 mM methionine was added and incubation was continued for 20 min. Processing was stopped by placing the cells on ice and adding 20 μl of 10 mg/ml cycloheximide, 500 mM NaN3. CPY or invertase was immunoprecipitated as previously described (Sapperstein et al., 1996) with 2 μl of α-CPY antibody or 5 μl of α-invertase antibody (this laboratory) per OD600 unit of cells. The immunoprecipitated material was analyzed by SDS-7% PAGE and autoradiography.
Analysis of Kar2p Secretion
Log phase strains (RSY255, GWY230, and GWY233) containing vector (pRS416) or CEN DSL1 (pSC2) were spotted onto YPD plates that, after the media were adsorbed into the plates, were overlayed with nitrocellulose prewet in sterile water. After a 16-h incubation, the nitrocellulose was removed and the levels of Kar2p were analyzed by immunoblotting with a 1:100,000 dilution of α-Kar2p antibody and ECL PLUS detection (Amersham Pharmacia Biotech).
Mating Assay
The mating assay is essentially as described by Letourneur et al. (1994). Briefly, log phase PC13, PC75, GWY379, and GWY380 strains were grown on YPD plates, each strain was replica plated to five YPD plates, and the replicas were incubated overnight at 23°C. One plate from each strain was then incubated for 2 h at 23, 27, 30, 34, or 37°C before replica plating onto lawns of RSY255, the mating tester strain, which had been grown on YPD. After 6 h at the same temperature to allow mating, cells were replica plated to SC-Ura-Lys and incubated at 23°C for 2 d to allow growth of diploids.
Synthetic Lethality
The appropriate mating type of strains dsl1-4 (GWY230 or GWY415) and dsl1-7 (GWY233 or GWY414) were mated with EGY101 (ret1-1), CKY100 (sec27-1), PC133 (ret2-1), RSY277 (sec21-1), MY7512 (ufe1-1), PC137 (tip20-5), and RSY275 (sec20-1). Resulting diploids were sporulated and dissected as described above. Segregants of each tetrad were replica plated to YPD and plates were incubated at 23, 27, 30, 34, or 37°C for 2 d. Putative tetratypes were identified as those sets of spores displaying 1:3 viable:inviable ratio at 37°C, because all single temperature-sensitive mutants are inviable at 37°C. Genotypes of all the spore-derived colonies in the putative tetratypes were determined by complementation of temperature sensitivity with plasmids containing DSL1, SEC20, or TIP20 (pSC2, 2 μm SEC20, or pTM2, respectively). Cotransformation with plasmids pBR4 and 2 μm SEC20 were used for complementation of the dsl1-7 sec20-1 double mutant. The dsl1-7 tip20-5 double mutant was inviable at all temperatures; therefore, its genotype was inferred from the other segregants.
Subcellular Fractionation and Membrane Extraction
Subcellular fractionation and membrane extraction were performed as previously described (VanRheenen et al., 1998). Briefly, a log phase 250 ml RSY255 culture was grown in YPD, harvested, and resuspended at 30 OD600/ml in Buffer 88 [20 mM HEPES, pH 7.0, 150 mM KOAc, 5 mM Mg(OAc)2] containing 1 mM dithiothreitol (DTT) and 1× protease inhibitor mix (2 μg/ml aprotinin, 0.5 μg/ml leupeptin, 4 mM PEFABLOC, 2 μM pepstatin A, and 0.5 mM phenanthroline), and lysed by vortexing in the presence of 0.3 g of 425–600 μm acid-washed glass beads (Sigma, St. Louis, MO). This crude lysate was centrifuged twice at 1000 × g and the second supernatant was diluted to a protein concentration of ∼5 mg/ml with Buffer 88. This clarified lysate was then centrifuged at 175,000 × g for 60 min in a TLA100.2 rotor (Beckman Instruments, Palo Alto CA), generating the supernatant (S) or pellet (P) fractions. Supernatant and pellet fractions, derived from equivalent amounts of starting material, were resolved by SDS-12%PAGE and analyzed by immunoblotting with antibodies against Dsl1p, Sed5p, Sec61p, and PGK (Figure 5B).
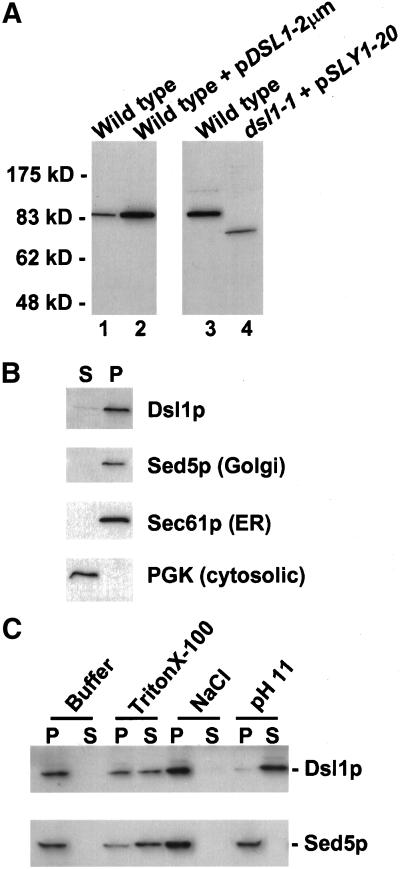
Figure 5.
Dsl1p is a peripheral membrane protein. (A) Characterization of the anti-Dsl1p antibody. Total yeast lysates prepared from a wild-type strain (RSY255) and an identical strain expressing DSL1 from a high-copy plasmid (pSC10) (left) or a wild-type strain (KRY8) and the dsl1-1 strain (GWY167) containing pSV12 (right) were separated by SDS-7% PAGE and immunoblotted with antibodies against Dsl1p. The migration of molecular mass standards (New England Biolabs, Beverly, MA) is shown on the left. Note that the dsl1-1 strain harbors the pSV12 SLY1-20-bearing plasmid because it suppresses the inviability of the dsl1-1 mutation (VanRheenen et al., 2001). (B) Subcellular fractionation of Dsl1p. A yeast lysate was centrifuged at 175,000 × g and separated into supernatant (S) and pellet (P) fractionation, which were resolved by SDS-12% PAGE and immunoblotted with antibodies against Dsl1p, Sed5p, Sec61p, or PGK, as indicated. (C) Dsl1p is a tightly bound peripheral membrane protein. Total yeast membranes were isolated on a step gradient and incubated for 45 min in either buffer, 1% Triton X-100, 1 M NaCl, or 100 mM Na2CO3, pH 11. Reaction mixtures were separated into supernatant (S) and pellet (P) fractions by centrifugation at 175,000 × g for 60 min and analyzed by SDS-12% PAGE and immunoblotting with antibodies against Dsl1p (top) or Sed5p (bottom).
Extractions were performed on Dsl1p-containing total yeast membranes that had been isolated on a step gradient. Lysate (2 ml), generated as described above, was layered onto a step gradient composed of 2 ml of Buffer 88, 1 mM DTT, 50% sucrose, and 8 ml of Buffer 88, 1 mM DTT, 10% sucrose. The gradient was centrifuged at 40,000 × g for 2 h at 4°C in a Beckman SW41 rotor and membranes were collected from the 10%/50% sucrose interface. Membranes (100 μl/reaction) were mixed with an equal volume of 2× extraction buffer and then diluted to 1 ml in 1× extraction buffer, resulting in the following final concentrations of extraction reagent: 1% Triton X-100; 1 M NaCl; or 0.1 M Na2CO3, pH 11. In addition, a mock extraction was performed in which 100 μl of membranes was diluted to 1 ml in Buffer 88. After a 45-min incubation on ice, 800 μl of each sample was layered over a 200-μl 10% sucrose cushion made in the appropriate 1× extraction buffer and centrifuged at 175,000 × g for 60 min at 4°C in a Beckman TLA100.2 rotor. The top 900 μl of the solution was removed as the supernatant fraction and the remaining 100 μl, including a visible pellet, was resuspended in an equivalent volume (900 μl total) of Buffer 88. Samples were then resolved by SDS-10% PAGE and analyzed by immunoblotting with affinity-purified anti-Dsl1p and anti-Sed5p antibodies (Figure 5C).
Sucrose Density Gradients
Subcellular fractionation (Figure 6A) was based on the method of Antebi and Fink (1992) with the following modifications. Log phase RSY255 containing pSV66 cells were grown in 2 liters of SC-Leu, harvested by centrifugation, resuspended at 100 OD600 units/ml in 100 mM Tris-Cl, pH 9.4, 10 mM DTT, and incubated at room temperature for 10 min. Cells were centrifuged and resuspended at 100 U/ml in 0.7 M sorbitol, 10 M Tris-Cl, pH 7.4, 0.75% yeast extract, 1.5% Bacto peptone, 0.5% glucose, and incubated at 30°C for 45 min in the presence of 0.5 mg/ml Zymolyase 100T (Seikagaku, Tokyo Japan). Spheroplasts were centrifuged at 4000 rpm for 5 min at 4°C in an SA600 rotor and then resuspended in 1 ml of lysis buffer 1 (10 mM HEPES, pH 7.4, 12.5% sucrose, 1 mM EDTA, 1× protease inhibitor mix). Spheroplasts were gently lysed by 10 hand strokes with a 5-ml Dounce homogenizer. Lysate was centrifuged at 3000 rpm and the supernatant was transferred to a fresh tube. The unlysed cell pellet was resuspended in 1 ml of lysis buffer 1 and homogenization and centrifugation was repeated. Supernatants were pooled and 1 ml of lysate was layered onto gradients containing 1-ml steps of 22, 26, 30, 34, 38, 42, 46, 50, 54, 58, and 60% sucrose (wt/vol) in 10 mM HEPES, pH 7.4, 1 mM MgCl2. Gradients were centrifuged in an SW41 rotor (Beckman Instruments) at 38,000 rpm (174,000 × g) for 2.5 h at 4°C after which twelve 0.8-ml fractions were collected from the top of the gradient. Aliquots from each fraction (0.5% total) were analyzed by SDS-12% PAGE, immunoblotted with anti-HA, anti-Kar2p, or anti-Dsl1p, and visualized by ECL PLUS.
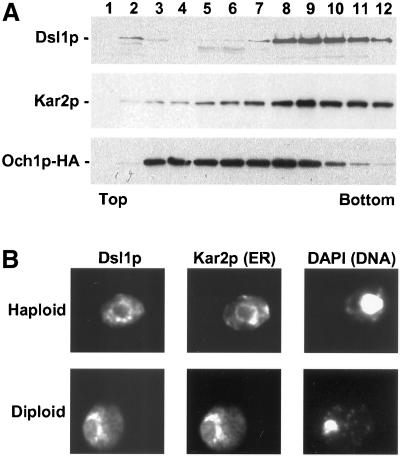
Figure 6.
Dsl1p is an ER protein. (A) Dsl1p comigrates with Kar2p and not with Och1p-HA by buoyant density centrifugation. Membranes from an Och1p-HA-expressing strain (RSY255 bearing pSV66) were separated by buoyant density centrifugation. Gradient fractions were analyzed by immunoblotting with anti-Dsl1p, anti-Kar2p, and anti-HA antibodies as indicated. (B) Dsl1p localizes to perinuclear regions similar to Kar2p. Haploid or diploid strains expressing Dsl1p-HA (pSV61) were examined by indirect immunofluorescence for Dsl1p and Kar2p, as indicated. Diamidophenylindole (DAPI) staining of the nucleus is shown on the right.
Indirect Immunofluorescence
Log phase GWY199 and GWY416 cells (8 ml) were centrifuged at 15,000 rpm for 5 min and the cell pellet was resuspended in 2 ml of 4% paraformaldehyde, 0.1 M KH2PO4, pH 6.5, 1 mM MgCl2 (prepared as follows: 2 g of paraformaldehyde was heated to 55°C in 45 ml of water, 210 μl of 10 N NaOH was added to solubilize the paraformaldehyde, 0.68 g KH2PO4 and 50 μl 1 M MgCl2 were added, the volume was brought to 50 ml, and the solution was cooled to room temperature). Cells were incubated in fixation solution at 23°C overnight with rocking. Fixed cells were washed twice with 1-ml aliquots of spheroplasting buffer (0.1 M KH2PO4, pH 6.5, 1.2 M sorbitol) and then spheroplasts were generated in 1 ml of 0.5% β-mercaptoethanol, 2.5% glusulase (NEN), 0.15 mg/ml Zymolyase 100T in spheroplasting buffer for 1 h at 30°C followed by washing with 1-ml aliquots of 1.2 M sorbitol in phosphate-buffered saline (PBS). Cells were then incubated in 2% SDS, 1.2 M sorbitol for 10 min at 23°C, and then washed twice in 1.2 M sorbitol in PBS. Subsequent, methanol/acetone fixation, blocking, incubation with 1:2000 anti-HA or anti-Kar2p, incubation with 1:2000 Alexa Fluor 568 goat anti-mouse or Alexa Fluor 488 goat anti-rabbit secondary antibodies (Molecular Probes), diamidophenylindole staining, and fixation were done as described (Adams et al., 1998).
Size Exclusion Chromatography and Complex Immunoprecipitation
Log phase RSY255 cells, grown in 500 ml of YPD, were harvested by centrifugation, resuspended at 100 OD600 units/ml in 100 mM Tris-Cl, pH 9.4, 10 mM DTT and incubated at room temperature for 10 min. Cells were centrifuged and resuspended at 100 OD600 units/ml in 0.7 M sorbitol, 10 mM Tris-Cl, pH 7.4, 0.75% yeast extract, 1.5% Bacto peptone, 0.5% glucose, and incubated at 30°C for 45 min in the presence of 0.5 mg/ml Zymolyase 100T. Spheroplasts were centrifuged at 4000 rpm for 5 min in an SA600 rotor and then resuspended at 10 OD600 units/ml in lysis buffer 2 (20 mM HEPES, pH 7.4, 50 mM KOAc, 2 mM EDTA, 0.1 M sorbitol, and 1× protease inhibitor mix). Spheroplasts were lysed on ice with 20 strokes of a Sorvall Omni-mixer set at the maximum speed. The lysate was cleared twice by centrifugation at 3000 rpm for 5 min in an SA600 rotor. The cleared supernatant was transferred to a new tube that was centrifuged at 13,500 rpm in an SA600 rotor for 15 min. The membrane pellet was washed twice in 1 ml of Buffer 88 through gentle pipetting and resuspended in 1 ml of Buffer 88 with a 2-ml Dounce homogenizer. For size exclusion chromatography (Figure 7A), aliquots (150 μl) of washed membranes were incubated on ice in Buffer 88 containing either 1% n-dodecyl-β-d-maltoside or 1% Triton X-100H in a total volume of 1 ml for 1 h. An 800-μl sample was overlayed on a 200-μl cushion of Buffer 88 and either 10% (wt/vol) sucrose, or 1% n-dodecyl-β-d-maltoside and 10% (wt/vol) sucrose, or 1% Triton X-100H and 10% (wt/vol) sucrose, and then centrifuged in a TLA100.2 rotor at 100,000 × g (48,000 rpm) for 1 h at 4°C. The supernatant was removed and 50 μl was chromatographed on a Superose 6 PC3.2/30 column at a flow rate of 50 μl/min with the use of a SMART System (Amersham Pharmacia Biotech). Fractions (75 μl) were collected starting just before the void volume, precipitated with trichloroacetic acid, and analyzed by SDS-10% PAGE and immunoblotting with anti-Dsl1p antibodies and ECL PLUS development.
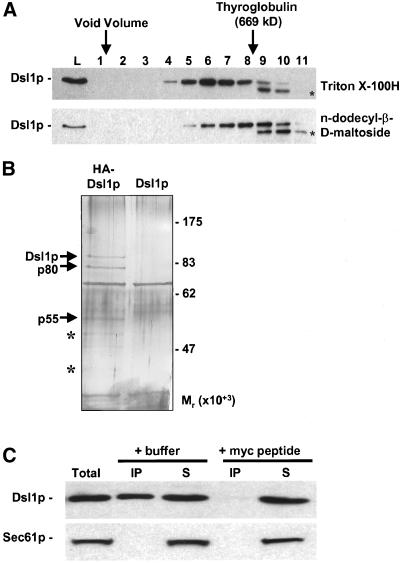
Figure 7.
Dsl1p is present in a large complex that contains Tip20p. (A) Dsl1p is part of a large complex. Size exclusion chromatography of Triton X-100H- or n-dodecyl-β-d-maltoside-extracted membranes was followed by immunoblotting fractions with anti-Dsl1p. The asterisks indicate a potential proteolytic product generated during chromatography. L represents a sample of the material loaded on to the column. (B) Dsl1p interacts with proteins of ∼80 and ∼50 kDa. Triton X-100H-solubilized membranes from a strain expressing Dsl1p-HA (pSV61, left lane) or wild-type Dsl1p (pSV67, right lane) were immunoprecipitated with anti-HA, and the harvested proteins were subjected to SDS-12% PAGE and silver staining. Proteolytic fragments of Dsl1p are indicated with an asterisk. (C) Dsl1p coimmunoprecipitates with Tip20p-myc. Lysate from a log phase wild-type strain (RSY255) bearing TIP20-myc (pTM2) was incubated in the presence of 1% Triton X-100. Precleared supernatant (Total, lane 1) was subjected to immunoprecipitation with anti-myc antibody (9E10)-conjugated protein A-Sepharose beads in the presence of buffer (lanes 2 and 3) or 1 μg/ml myc peptide (lanes 4 and 5). The immunoprecipitated (IP) and nonbound protein (S) fractions were probed for Dsl1p and Sec61p by immunoblotting followed by ECL PLUS detection.
For immunoprecipitation of the Dsl1p complex (Figure 7B), RSY255 and GWY199 membranes were prepared as described for size exclusion chromatography, except that the starting volume was 2 liters and the membrane pellet was resuspended in 1 ml of Buffer 88 containing 3% Triton X-100H and incubated on ice for 45 min to extract Dsl1p from membranes. Aliquots (800 μl) were placed on 200-μl cushions composed of Buffer 88, 10% (wt/vol) sucrose, 3% Triton X-100H and centrifuged in a TLA100.2 rotor at 100,000 × g for 60 min at 4°C. Immunoprecipitation reactions consisted of 750 μl of the supernatant, 250 μl of IP buffer 1 (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% TX-100H, 2 mM NaN3) and 50 μl of 50% anti-HA.11-conjugated protein G-Sepharose beads (Covance, Denver, PA). After an overnight incubation with rocking at 4°C, the beads were washed four times with 1-ml aliquots of IP buffer 1. Thirty-five microliters of 2% SDS was added to the beads, bound protein was eluted by incubation at 100°C for 5 min, and 30% of the eluates were resolved by SDS-12% PAGE and visualized by silver staining.
Coimmunoprecipitation of Tip20p-myc and Dsl1p
Log phase RSY255 cells containing pTM2 (75 OD600 units), grown in SC-Ura, were washed once with 20 mM Tris-Cl, pH 7.5, 20 mM NaN3 and resuspended in 5 ml of IP buffer 2 (50 mM HEPES, pH 7.4, 150 mM KCl, 1 mM EDTA, 1 mM DTT, 1× protease inhibitor mix). The cultures (1.5-ml aliquots) were transferred to 2-ml screw cap conical tubes containing 1 g of 425–600-μm acid washed glass beads and lysed in a Biospec Products Mini-beadbeater-8 at full power for 4 min at 4°C. The supernatant was collected after a 15-min centrifugation at 15,000 rpm at 4°C and incubated with 50 μl of 50% slurry of protein A-Sepharose/IP buffer 2 containing 1% Triton X-100 in a final volume of 1.2 ml for 1 h at 4°C with rocking. This served to remove components that nonspecifically bind to protein A-Sepharose, and to detergent extract Dsl1p from membranes. After a 15-min centrifugation at 15,000 rpm at 4°C in a microcentrifuge, 1-ml aliquots of the supernatant were transferred to 1.5-ml tubes containing 60 μl 50% protein A-Sepharose beads conjugated with anti-myc antibody (9E10) and either 5 μl of PBS or 5 μl of 200 μg/ml myc peptide (EQKLISEEDL) in PBS. A separate 1-ml aliquot was incubated without beads to serve as a control for total protein. After 2 h with rocking at 4°C, samples were centrifuged at 15,000 rpm for 30 s. Beads were washed four times with 1 ml of IP buffer 2, and the supernatants and unfractionated control sample were precipitated with trichloroacetic acid. Samples were analyzed by SDS-10% PAGE, immunoblotted with anti-Dsl1p and anti-Sec61p antibodies, and visualized with ECL PLUS.
Yeast Two-Hybrid Screen
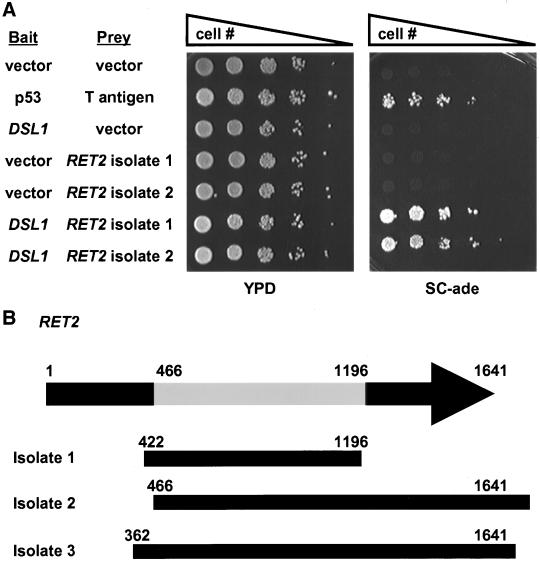
The yeast two-hybrid screen (Figure 8) was exactly as described by James et al. (1996) except that PJ69–4A yeast strain carried the bait plasmid pBR2, which encodes a Gal4p-DNA binding domain-Dsl1p fusion protein.
Figure 8.
Dsl1p interacts with Ret2p/δ-COP in a two-hybrid system. (A) Log phase two-hybrid strain (PJ69–4A) bearing bait (DNA-binding domain) and prey (activation domain) vectors as indicated were serially diluted and spotted onto YPD medium to assess viability, or onto synthetic complete medium lacking adenine to assay the two-hybrid interaction. Coexpression of p53 fused to the Gal4p-BD and T-antigen fused to the Gal4p-AD was used as a positive control. (B) Dsl1p-interacting domain of Ret2p. The RET2 open reading frame is 1641 base pairs in length. RET2 isolate 1 contains a library insert corresponding to bases 422-1196 and RET2 isolates 2 and 3 start at bases 466 and 362, respectively, and continue past the stop codon. Thus, the central portion (bases 466-1196) of Ret2p encodes a Dsl1p-interacting region.
RESULTS
DSL1 Mutants Exhibit Defects Characteristic of Retrograde Trafficking under Conditions Where No Anterograde Trafficking Defects Are Apparent
In our previous work (VanRheenen et al., 2001) we showed that some dsl1 mutants could be genetically suppressed by overexpression of SEC21, which encodes the γ-subunit of the COPI retrograde coat. This finding stimulated us to further explore the possibility that Dsl1p is involved in retrograde Golgi-to-ER traffic. The phenotypes we had previously observed, including the block in ER-to-Golgi traffic, were consistent with this possibility. Indeed, many of the components that are known to function in retrograde Golgi-to-ER traffic were originally characterized as mutants that impact on anterograde ER-to-Golgi traffic (Novick et al., 1980). It is presumed that these anterograde defects are secondary consequences of a defect in retrograde traffic (Cosson et al., 1997; Gaynor and Emr, 1997).
Several mutants that block retrograde traffic exhibit a defect in ER-to-Golgi traffic of CPY under conditions where invertase traffics normally (Gaynor and Emr, 1997). Although the reason for this is not clear it has been proposed that the cause may be failure to recycle a component(s) required for ER egress of CPY. To determine whether dsl1-4 and/or dsl1-7 mutants exhibit a similar phenotype we performed pulse-chase analysis of CPY and invertase traffic at a range of temperatures from 23 to 37°C (Figure 1). CPY undergoes an ordered series of glycosylation and proteolytic events en route to the vacuole that results in characteristic changes in molecular mass: the p1 form (67 kDa) has yet to enter the Golgi, the p2 form (69 kDa) has received Golgi-specific glycosyl modifications, and the mature form (61 kDa) has been proteolytically processed in the vacuole (Stevens et al., 1982). Similarly, invertase is post-translationally modified as it moves from the ER, where it is present in distinct core-glycosylated forms, to the Golgi, where the oligosaccharides are extended by the addition of mannose residues leading to a population of molecules with varying molecular masses that migrate as a “smear” on SDS-PAGE.
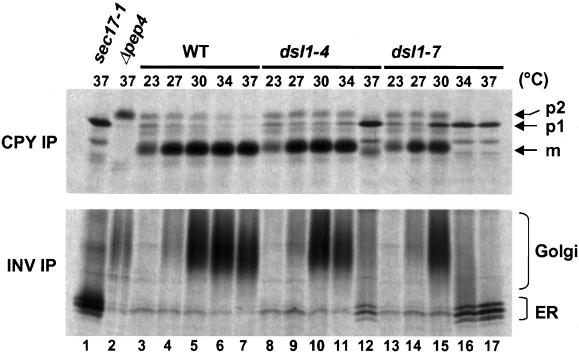
Figure 1.
dsl1-7 shows a CPY trafficking block in the absence of an invertase block. Log phase wild-type (RSY255), dsl1-4 (GWY230), and dsl1-7 (GWY233) strains were grown on media lacking methionine and containing 2% raffinose and 0.1% glucose for 1 h before a shift to the indicated temperature for 20 min. Strains were labeled with 35S-amino acids for 10 min, chased for 20 min at the same temperature, and lysed. CPY (top) or invertase (bottom) was then immunoprecipitated from each sample, separated by SDS-7% PAGE, and visualized by autoradiography. The migration of the pre-Golgi (p1), Golgi (p2), and mature (m) forms of CPY, and of the ER and Golgi forms of invertase is indicated on the right. Immunoprecipitations from lysates of sec17-1 (GWY270) and pep4Δ (MS1554) strains incubated at 37°C were used as controls for the p1 and p2 forms of CPY, and the ER and mature form of invertase, respectively. INV, invertase; IP, immunoprecipitation.
We found (Figure 1) that both CPY and invertase traffic ceased simultaneously between 34 and 37°C in the dsl1-4 strain (compare lanes 7 and 12), providing no support for a retrograde defect. However, in the dsl1-7 strain, CPY traffic was compromised at a lower temperature (30°C, compare lanes 5 and 15) than invertase traffic (34°C, compare lanes 6 and 16). Thus, under at least one condition, dsl1-7 exhibits a defect in CPY traffic, whereas invertase traffic is unaffected. This is not the expectation for a component involved (exclusively) in anterograde traffic and has been reported previously for other retrograde trafficking mutants (Gaynor and Emr, 1997).
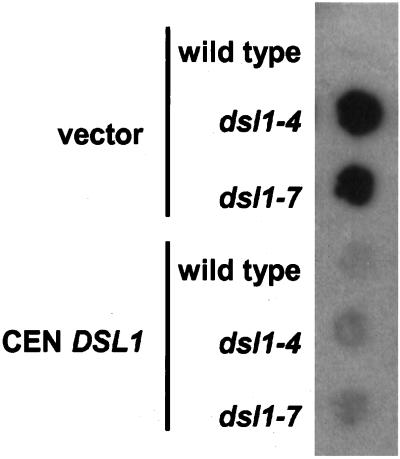
One of the hallmarks of mutants in Golgi-to-ER retrograde traffic is the inability to recycle ER-resident proteins, such as the chaperone Kar2p, that have escaped from the ER. To examine whether dsl1 mutants exhibit this phenotype, wild-type, dsl1-4, and dsl1-7 strains containing a control low-copy vector, or the same vector bearing DSL1, were grown at 37°C for 16 h in contact with nitrocellulose, which binds secreted Kar2p and allows subsequent immunodetection (Figure 2). We found that neither dsll1 mutant secretes Kar2p at the permissive temperature (our unpublished results), but that both mutants secrete the protein at the restrictive temperature. Control experiments indicate that Kar2p is not released due to cell lysis (our unpublished results). Last, as a control to address the possibility that Kar2p secretion in the dsl1 mutants might be due to increased cellular levels of Kar2p, perhaps exceeding the capacity of the Golgi-ER recycling system (Belden and Barlowe, 2001), intracellular levels of Kar2p were examined by immunoblotting total cell extracts. We found that, compared with wild-type cells, Kar2p levels in the mutants were not affected significantly at the permissive or restrictive temperature (our unpublished results); thus, Kar2p induction is unlikely to account for the Kar2p secretion.
Figure 2.
dsl1ts strains secrete ER resident Kar2p. Log phase wild-type (RSY255), dsl1-4 (GWY230), and dsl1-7 (GWY233) strains bearing pRS416 (vector) or pSC2 (CEN DSL1) were spotted onto rich medium, overlayed with nitrocellulose, and allowed to grow at 37°C for 16 h. Kar2p that had been secreted and bound to the nitrocellulose was detected by immunoblotting with α-Kar2p antibody and ECL PLUS detection.
To further probe this possibility that Dsl1p plays a role in retrograde Golgi-to-ER traffic, we examined the effect of dsl1 mutations in an established system that is sensitive to defects in retrograde Golgi-to-ER traffic (Letourneur et al., 1994). In this assay, the α-factor receptor Ste2p, which must be on the cell surface to allow mating, is retained in the ER by virtue of its fusion to the ER-resident protein Wbp1p, which displays a cytoplasmic dilysine retention signal. The ste2Δ STE2-WBP1 strain is sterile due to retrieval of Ste2p-Wbp1p to the ER, but it can mate if certain retrograde Golgi-to-ER transport components are mutated, thereby allowing the α-factor receptor to be transported to the cell surface (Letourneur et al., 1994; Cosson et al., 1997). We constructed dsl1-4 ste2Δ STE2-WBP and dsl1-7 ste2Δ STE2-WBP strains and tested them, along with positive and negative control strains, for their ability to mate at a range of temperatures. The strains (which are Ura+Lys−) were grown for 2 h, replica plated to lawns of wild-type cells of the opposite mating type (which are Ura−Lys+), and incubated for 6 h at temperatures ranging from 23 to 37°C to allow an opportunity to mate. These plates were then replica plated to medium selective for diploids (Ura+Lys+) and incubated for 2 d.
As expected, the ste2Δ STE2-WBP control strain does not mate at any temperature (Figure 3, first row). In contrast, the ret1-1 ste2Δ STE2-WBP strain, which bears a mutation in the α-COP subunit of the COPI coat that disrupts recognition of the retrieval signal without compromising retrograde traffic in general (Letourneur et al., 1994), mates at all temperatures (Figure 3, second row). Interestingly, both the dsl1 strains display temperature-dependent mating. Robust mating ability was conferred to dsl1-4 at its semipermissive temperature of 34°C (Figure 3, third row). At the restrictive temperature of 37°C, only a small population of dsl1-4 cells is able to mate. Similarly, dsl1-7 was able to mate at the lowest restrictive temperatures of 30°C, but not at the restrictive temperatures of 34 or 37°C (Figure 3, fourth row).
These findings suggest that the Ste2p-Wpb1p fusion protein is maintained in the ER in dsl1 mutants at the permissive temperature, which precludes mating. As the temperature is increased to the semipermissive temperature, enough fusion protein escapes to enable mating. Further increases in temperature into the restrictive range may preclude mating due to the fact that anterograde transport, which is required to deliver the fusion protein to the cell surface, is blocked (Figure 1).
Taken together, the biochemical data (Figures 1 and 2) and the mating assay (Figure 3) suggest that Dsl1p is required for retrograde Golgi-to-ER traffic. An additional role in ER-to-Golgi traffic remains a possibility.
Genetic Interactions of DSL1 with Genes Encoding Retrograde Transport Components
Because Dsl1p appeared to be involved in retrograde transport, we sought to identify synthetic lethal interactions between dsl1 mutants and mutants in other genes that play a role in this process. We therefore examined the growth phenotypes of dsl1-4 and dsl1-7 strains bearing mutations in retrograde COPI coat components α-COP, β′-COP, γ-COP, or δ-COP (ret1-1, sec27-1, sec21-1, or ret2-1, respectively), the retrograde t-SNARE Ufe1p (ufe1-1), or the Ufe1p-associated proteins Sec20p and Tip20p (sec20-1 and tip20-5).
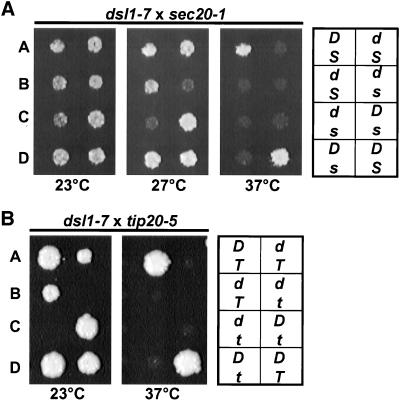
We found no synthetic interactions between the dsl1 mutants and ret1-1, sec27-1, ret2-1, sec21-1, or ufe1-1 (our unpublished results). In contrast, both dsl1-4 and dsl1-7 exhibited synthetic lethal interactions with sec20-1 and tip20-5. Two representative tetratypes from dissection of the dsl1-7/DSL1 sec20-1/s20 diploids (Figure 4A) and dsl1-7/DSL1 tip20-5/TIP20 diploids (Figure 4B) grown at 23, 27, and 37°C are shown. The dsl1-7 sec20-1 double mutant is inviable at the dsl1-7 semipermissive temperature of 27°C (Figure 4A) and dsl1-7 tip20-5 double mutant is inviable at all temperatures tested (Figure 4B). This synthetic lethality provides further support for a retrograde role for Dsl1p, and suggests that it may act at the ER target site in conjunction with Sec20p and Tip20p.
Figure 4.
dsl1-7 exhibits synthetic lethal interactions with both sec20-1 and tip20-5. dsl1-7 (GWY414) was mated to sec20-1 (RSY275) (A) or tip20-5 (PC137) (B), and the resulting diploid strains were sporulated, dissected onto YPD plates, and incubated at 23°C for 2 d. Spores were then replica plated to YPD and incubated at the indicated temperatures for 2 d. Two representative tetratypes are shown. Genotypes (indicated on the right; D, DSL1; d, dsl1-7; S, SEC20, s, sec20-1; T, TIP20; t, tip20-5) were determined by complementation of the temperature sensitivity with plasmid-borne SEC20, TIP20, and/or DSL1.
In contrast to the clear synthetic lethal interactions, we found no suppression of dsl1-4 (GWY230) or dsl1-7 (GWY233) by overexpression of TIP20 or SEC20. Similarly, we found no suppression of sec20-1 (RSY275) or tip20-5 (PC137) by overexpression of DSL1 (our unpublished results).
Dsl1p Is a Peripheral Membrane Protein of Endoplasmic Reticulum
To initiate biochemical analyses of Dsl1p, we generated polyclonal α-Dsl1p antisera in rabbits. After affinity purification, the antibodies recognized a single protein in crude yeast extracts that migrated at ∼88 kDa (Figure 5A, lanes 1 and 3), the predicted molecular mass of Dsl1p. Transformation of a wild-type strain with a high-copy plasmid bearing DSL1 resulted in the overexpression of this protein (Figure 5A, lane 2). Examination of the original mutant found in the DSL screen (VanRheenen et al., 2001), dsl1-1, revealed that the full-length protein was absent; rather, a protein migrating at ∼75 kDa was observed, and this truncated protein was less abundant than wild-type Dsl1p (Figure 5A, lane 4). These data demonstrate that the antibody specifically recognizes Dsl1p.
Many cytosolic proteins that function in the secretory pathway have membrane-associated pools. To investigate whether this is the case for Dsl1p, we centrifuged a yeast lysate at 175,000 × g to separate cytosolic from membrane-associated proteins, and probed the supernatant (S) and pellet (P) fractions for Dsl1p by immunoblotting. Almost all of the Dsl1p was contained in the pellet fraction, as were the integral membrane proteins Sed5p and Sec61p (Figure 5B); very little Dsl1p was found in the supernatant with the cytosolic marker protein PGK.
Membrane extractions were performed to test whether Dsl1p is a peripheral membrane protein, as would be expected from the data mentioned above and the lack of an apparent transmembrane domain in the predicted primary sequence of the protein (VanRheenen et al., 2001). Crude yeast membranes were isolated on a buoyant density step gradient and then incubated either with buffer, Triton X-100, 1 M NaCl, or pH 11 sodium carbonate. After centrifugation at 175,000 × g the supernatant (S) and pellet (P) fractions were analyzed by immunoblotting with antibodies against Dsl1p (Figure 5C). Buffer alone did not remove Dsl1p, again indicating that it tightly associates with membrane. On incubation with Triton X-100, Dsl1p is partially released into the supernatant fraction, confirming its membrane association. Dsl1p was not extracted from the membrane by high salt treatment, which is unusual for a peripheral membrane protein and suggests that Dsl1p's interaction with the membrane is not solely through ionic interactions. Last, Dsl1p is efficiently removed upon exposure to pH 11 sodium carbonate. This extraction behavior indicates that Dsl1p is a tightly associated peripheral membrane protein.
Because Dsl1p functions at the ER/Golgi interface, we investigated through subcellular fractionation whether Dsl1p associates with the membranes of either of these organelles. Thus, membranes from a wild-type strain bearing the Golgi resident protein Och1p tagged with an HA-epitope tag (Harris and Waters, 1996) were subjected to sucrose density centrifugation followed by fractionation and immunoblotting with α-Dsl1p, α-Kar2p, and α-HA antibodies (Figure 6A). Dsl1p cofractionated with the ER resident Kar2p, and overlapped only to a limited extent with peak of Och1p-HA. To confirm that the cofractionation of Dsl1p and an ER marker was due to association with the ER rather than another cofractionating organelle we also performed the centrifugation in the presence of EDTA, which causes a reduction in the density of the ER due to dissociation of ribosomes (Antebi and Fink, 1992). We found that the localization of Och1p-HA in the gradient was unchanged, but that both Dsl1p and Kar2p were shifted to less dense fractions (our unpublished results). This fractionation behavior is consistent with Dsl1p residence on the ER membrane.
Confirming the fractionation behavior, double-label immunofluorescence microscopy of Dsl1p-HA revealed a perinuclear staining similar to that of Kar2p (Figure 6B). Together, the cell fractionation and immunofluorescence data strongly suggest that Dsl1p is peripheral membrane protein localized predominantly to the ER at steady state.
Immunoisolation of Dsl1p-interacting Proteins
Because Dsl1p is tightly associated with the ER membrane, we used an affinity approach in an effort to isolate membrane-associated Dsl1p-interacting proteins. First, we screened a number of nonionic detergents and found that Triton X-100H (the hydrogenated form of Triton X-100, which is UV-transparent) and n-dodecyl-β-d-maltoside fairly efficiently (∼50%) extract Dsl1p from microsomal membranes (our unpublished results). Next, to examine whether the extracted Dsl1p associates with other proteins, we subjected the detergent extracts to size exclusion chromatography followed by immunoblotting with α-Dsl1p antibodies (Figure 7A). For both detergents Dsl1p was found to chromatograph at an inordinately large size, comparable with an ∼700–800-kDa globular protein. This size, however, is only an estimate due to the presence of detergent and because the shape of the complex is not known. Nevertheless, this chromatographic behavior suggested that detergent-extracted Dsl1p may stably associate with other proteins.
To isolate Dsl1p interacting proteins, membranes from a dsl1Δ strain bearing DSL1 or DSL1-HA on a low-copy number plasmid were extracted with Triton X-100H, the extracts were clarified by centrifugation to remove aggregated material, and the supernatants were immunoprecipitated with α-HA antibody. Visualization of the proteins in the immunoprecipitates after SDS-PAGE and silver staining revealed that proteins of ∼90-, 80-, and 55-kDa apparent molecular mass were specifically and reproducibly harvested from the Dsl1p-HA strain (Figure 7B). Minor proteins of ∼40 and 50 kDa were also reproducibly obtained. Immunoblotting of the immunoprecipitates with α-Dsl1p and α-HA antibodies showed that the 90-kDa protein was Dsl1p-HA, and the minor 40- and 50-kDa polypeptides were proteolytic fragments of Dsl1p-HA (our unpublished results). The 80- and 55-kDa proteins were not recognized by α-Dsl1p or α-HA antibodies and therefore represent Dsl1p-interacting proteins.
As shown above (Figure 4), DSL1 interacts genetically with both SEC20 and TIP20. These genes encode proteins with apparent molecular masses on SDS-PAGE of ∼50 and 80 kDa, respectively (Sweet and Pelham, 1992, 1993), very similar to those of the Dsl1p-interacting proteins (Figure 7B). In addition, Tip20p interacts with Sec20p, and both proteins are localized to the ER (Sweet and Pelham, 1993) and function in retrograde traffic (Cosson et al., 1997). Additional evidence suggesting that the Dsl1p-interacting proteins might be Sec20p and Tip20p has been provided by a recent genome-wide two-hybrid screen of yeast protein–protein interactions (Ito et al., 2001), which revealed interactions between Dsl1p and Tip20p. The interaction seems reliable because an interaction was observed irrespective of whether the proteins are fused to the DNA-binding or activation domains, and because each protein has few other partners.
To address whether the 80-kDa Dsl1p-interacting protein is Tip20p, we sought to coimmunoprecipiate Dsl1p from a strain bearing Tip20p with a C-terminal triple-myc epitope tag (Figure 7C). Indeed we found that Dsl1p associates with Tip20p-myc and that the immunopreciptiation could be blocked by inclusion of competitor myc peptide, showing that the immunoprecipitation is specific (Figure 7C, lane 4). As another control, we probed the immunoprecipitate for the presence of Sec61p, but were unable to detect this ER-resident protein (Figure 7C, bottom), again suggesting that the Dsl1p-Tip20p interaction is specific. The immunoprecipitation was also quite efficient, with ∼30–40% of the Dsl1p coimmunoprecipitating with the Tip20p-myc.
Two-Hybrid Interactions of DSL1 with Retrograde Transport Components
In a parallel effort to identify proteins that physically interact with Dsl1p we performed a yeast two-hybrid screen with Dsl1p fused to the Gal4p-DNA binding domain as bait. The strain used in this screen tends to have fewer false positives than previous designs because it uses three Gal4p-driven genes with different promoter regions (James et al., 1996). We isolated three Dsl1p-interacting components in the screen, each one multiple times. Two were represented by uncharacterized open reading frames and the third was the Ret2p/δ-COP component of the retrograde COPI coat (Figure 8A). We obtained three distinct but overlapping fragments of Ret2p/δ-COP (Figure 8B); the region common to all the isolates encodes approximately the central one-half of the protein. This finding, in conjunction with our previous demonstration of genetic interactions between DSL1 and genes encoding subunits of the COPI coat (VanRheenen et al., 2001), suggests that Dsl1p physically interacts with Ret2p/δ-COP and raises interesting possibilities about the molecular function of Dsl1p in retrograde traffic.
DISCUSSION
In this study we show that Dsl1p plays a role in retrograde Golgi-to-ER traffic by examining the trafficking of a number of proteins in dsl1 mutant strains. Dsl1p resides predominantly on the ER, and it forms a tight complex with at least two other proteins, one of which we have identified as Tip20p. Tip20p physically interacts with Sec20p (Lewis et al., 1997), and we show here that Sec20p genetically interacts with Dsl1p. Thus, the evidence supports the existence of an ER-localized complex of Dsl1p, Tip20p, and Sec20p. This complex is likely to impact on the function of the ER t-SNARE Ufe1p, because Tip20p and Sec20p have previously been shown to bind tightly to Ufe1p.
Our study has been complemented by the recent work of Andag et al. (2001), who independently isolated DSL1 in a genetic screen for mutants that are synthetically lethal with a deletion of the v-SNARE Sec22p. The authors also find a genetic interaction between dsl1-22 and either sec20-1 or tip20-5 and demonstrate that dsl1-22 allele is defective for Golgi-to-ER retrograde traffic of a number of proteins. Their localization studies confirm ours, indicating that Dsl1p resides on the ER membrane.
At the outset of our work we expected that Dsl1p would function in anterograde traffic, not in retrograde traffic. This model was based on the genetic interaction of DSL1 with SLY1–20 (VanRheenen et al., 2001), a dominant allele of SLY1, which encodes a protein that regulates the activity of the cis-Golgi t-SNARE Sed5p (Søgaard et al., 1994; Lupashin and Waters, 1997). In retrospect, however, it is perhaps not surprising that we identified a retrograde component by screening for mutants that can be suppressed by SLY1-20. It is clear that SLY1-20 can strongly suppress mutations in many anterograde vesicle-tethering components (Dascher et al., 1991; Sapperstein et al., 1996; VanRheenen et al., 1998, 1999), but it can also weakly suppress mutations in some retrograde trafficking components. For example, SLY1-20 can weakly suppress a mutation in SEC21/γ-COP (Ossig et al., 1991). These findings raise the possibility that Sly1p functions at the ER as well as at the Golgi. This is a reasonable postulate because there are nine t-SNAREs in yeast and only four Sec1p/Sly1p homologs, suggesting that some Sec1p/Sly1p proteins may bind to more than one t-SNARE. Because the ER and Golgi t-SNAREs Ufe1p and Sed5p, respectively, are very closely related (Lewis and Pelham, 1996), it is possible that Sly1p binds to both. Indeed, Sly1p has been shown to interact with both Sed5p and Ufe1p by two-hybrid analysis (Fred Hughson, Princeton University, personal communication, and S. Fields Web site, http://depts.washington.edu/sfields/yplm/data/new2h.html). Taken together, the available data suggest that the ER target site may be comprised of the t-SNARE Ufe1p in association with Sly1p, and/or a Dsl1p/Tip20p/Sec20p complex. Further studies are required to biochemically test the postulate that Sly1p interacts with Ufe1p, and, if this is confirmed, to determine whether all these proteins can exist in the same t-SNARE complex, or whether the interactions are mutually exclusive.
What might the role Dsl1p/Tip20p/Sec20p complex be? One possibility is that the complex acts in retrograde vesicle tethering. In this model, Sly1p may perform a role similar to its role at the Golgi, where it binds to Sed5p (Lupashin et al., 1996; Sapperstein et al., 1996; Lupashin and Waters, 1997; Cao et al., 1998; VanRheenen et al., 1998, 1999). Thus, by analogy to many studies on anterograde traffic (Dascher et al., 1991; Sapperstein et al., 1996; VanRheenen et al., 1998, 1999), binding of Sly1-20p to Ufe1p may bypass the requirement for upstream components that may be involved in a putative retrograde tethering step. The Dsl1p/Sec20p/Tip20p complex may be one of these tethering components.
Other potential roles for Dsl1p are suggested by the genetic interactions of DSL1 (Andag et al., 2001; VanRheenen et al., 2001) and TIP20 (Frigerio, 1998) with retrograde coat components, by our isolation of the COPI component Ret2p/δ-COP in a two-hybrid screen with Dsl1p, and by the recent demonstration by Andag et al. (2001) that GST-Dsl1p can harvest the COPI complex from yeast extracts. One possibility is that the Dsl1p complex cycles between the ER and Golgi and interacts with the COPI coat on the Golgi, perhaps during the budding process. This would require that Dsl1p/Tip20p/Sec20p complex, or at least its integral membrane component Sec20p, be present on COPII vesicles leaving the ER. To date, however, there is no evidence for any of these proteins on ER-to-Golgi vesicles, which have been very extensively characterized (Otte et al., 2001).
Another possibility is that Dsl1p interacts with Ret2p/δ-COP at the ER. Although most models for vesicular transport suggest that the vesicle coat is removed soon after the vesicle is formed, this has not been directly demonstrated. Perhaps the COPI coat remains on the vesicle until it arrives at the ER target site where it interacts with Dsl1p. This Dsl1p–COPI interaction might help to tether the coated vesicle to the ER. A similar proposal has been put forth for ER-localized Tip20p based in its numerous genetic interactions with the COPI coat (Frigerio, 1998).
A related hypothesis is that COPI vesicle uncoating occurs after arrival of the vesicle at the ER (Frigerio, 1998; Andag et al., 2001) and is facilitated by interaction with Dsl1p. In this regard, one of the long-standing questions about the uncoating process is how it is regulated; that is, what determines when and where uncoating will occur? Uncoating requires GTP hydrolysis on ARF (Tanigawa et al., 1993; Teal et al., 1994), which is stimulated by a GAP (Dogic et al., 1999; Poon et al., 1999; Eugster et al., 2000). In vitro studies suggest that under certain conditions COPI can contribute to the catalysis of this reaction (Goldberg, 1999; Szafer et al., 2000). Perhaps the Dsl1p complex facilitates uncoating through interaction with COPI, stimulating its GAP-enhancing activity.
If the Dsl1p complex proves to function in the tethering or uncoating of COPI-coated retrograde vesicles it would provide a simple mechanism for maintaining transport directionality at the ER/Golgi interface. Indeed, the question of directionality is relevant at all transport steps because v-SNAREs cycle between compartments, and therefore should be present on both anterograde and retrograde vesicles. Perhaps part of the mechanism for maintaining directionality at the ER/Golgi interface is that retrograde vesicles remain coated until they interact with the Dsl1p complex on the ER, where they can specifically tether and/or uncoat, and fuse. This mechanism need not be limited to the retrograde direction, it could also occur in the anterograde direction; Gallwitz and coworkers have previously shown that Sed5p, the cis-Golgi t-SNARE, can interact with the anterograde COPII coat component Sec24p (Peng et al., 1999), a situation analogous to the one reported here. Further studies of Dsl1p and its collaborators at the ER target site should shed light on the mechanism of retrograde Golgi-to-ER traffic
ACKNOWLEDGMENTS
We are grateful to P. Cosson, P. James, R. Duden, S. Emr, G. Friggerio, M. Lewis, H. Pelham, M. Rose, R. Schekman, T. Stevens, and members of their laboratories for generously supplying reagents and strains. We thank F. Hughson and A. Betz (Princeton University) for communicating unpublished results, D. Ungar and N. Erdeniz (Princeton University) for advice and assistance with chromatography and immunofluorescence and S. Barrett and D. Hasara (Princeton University) for assistance in antibody production. We are grateful to A. Chan (Princeton University) for technical laboratory assistance. This work was supported the American Cancer Society (RPG-98-050-01-CSM). B.A.R, B.A.K., and S.M.V.R. were supported, in part, by training grants Public Health Service GM-07388 and Public Health Service CA-09528 and GM-07312, respectively.
Abbreviations used:
- CPY
carboxypeptidase Y
- ER
endoplasmic reticulum
- GAP
GTPase-activating protein
- HA
hemagglutinin
- TX-100H
hydrogenated Triton X-100
REFERENCES
- Adams A, Gottschling DE, Kaiser DE, Stearns T. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- Andag U, Neuman T, Schmitt HD. The coatomer interacting protein is required for Golgi-to-ER retrieval in yeast. J Biol Chem. 2001;276:39150–39160. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- Antebi A, Fink GR. The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballensiefen W, Ossipov D, Schmitt HD. Recycling of the yeast v-SNARE Sec22p involves COPI-proteins and the ER transmembrane proteins Ufe1p and Sec20p. J Cell Sci. 1998;111:1507–1520. doi: 10.1242/jcs.111.11.1507. [DOI] [PubMed] [Google Scholar]
- Barlowe C. Traffic COPs of the early secretory pathway. Traffic. 2000;1:371–377. doi: 10.1034/j.1600-0854.2000.010501.x. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- Barrowman J, Sacher M, Ferro-Novick S. TRAPP stably associates with the Golgi and is required for vesicle docking. EMBO J. 2000;19:862–869. doi: 10.1093/emboj/19.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Deletion of yeast p24 genes activates the unfolded protein response. Mol Biol Cell. 2001;12:957–969. doi: 10.1091/mbc.12.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Schroder-Kohne S, Sweet DS, Demolliere C, Hennecke S, Frigerio G, Letourneur F. The Sec20/Tip20p complex is involved in ER retrieval of dilysine-tagged proteins. Eur J Cell Biol. 1997;73:93–97. [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogic D, de Chassey B, Pick E, Cassel D, Lefkir Y, Hennecke S, Cosson P, Letourneur F. The ADP-ribosylation factor GTPase-activating protein Glo3p is involved in ER retrieval. Eur J Cell Biol. 1999;78:305–310. doi: 10.1016/s0171-9335(99)80064-8. [DOI] [PubMed] [Google Scholar]
- Eugster A, Frigerio G, Dale M, Duden R. COPI domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio G. The Saccharomyces cerevisiae early secretion mutant tip20 is synthetic lethal with mutants in yeast coatomer and the SNARE proteins Sec22p and Ufe1p. Yeast. 1998;14:633–646. doi: 10.1002/(SICI)1097-0061(199805)14:7<633::AID-YEA267>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Harris SL, Waters MG. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J Cell Biol. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci USA. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Sacher M, Scarpa A, Quinn AM, Ferro-Novick S. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol Biol Cell. 1999;10:3317–3329. doi: 10.1091/mbc.10.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Hamamoto S, Schekman RW. Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J Cell Biol. 1996;132:277–289. doi: 10.1083/jcb.132.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a Rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1972. [Google Scholar]
- Nakajima H, Hirata A, Ogawa Y, Yonehara T, Yoda K, Yamasaki M. A cytoskeleton-related gene, uso1, is required for intracellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991;113:245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AP, Shim J, Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Weber T, McNew JA, Parlati F, Sollner TH, Rothman JE. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Gibson J, Gregor I, Schatz G. Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J Biol Chem. 1982;257:13042–13047. [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, Gallwitz D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte S, Belden W, Heidtman M, Liu J, Jensen O, Barlowe C. Erv41p and Erv46p. New components of COPII vesicles involved in transport between the ER and Golgi complex. J Cell Biol. 2001;152:503–518. doi: 10.1083/jcb.152.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, Weber T, McNew JA, Westermann B, Sollner TH, Rothman JE. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc Natl Acad Sci USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. The dynamic organization of the secretory pathway. Cell Struct Funct. 1996;21:413–419. doi: 10.1247/csf.21.413. [DOI] [PubMed] [Google Scholar]
- Peng R, Grabowski R, De Antoni A, Gallwitz D. Specific interaction of the yeast cis-Golgi syntaxin Sed5p and the coat protein complex II component Sec24p of endoplasmic reticulum-derived transport vesicles. Proc Natl Acad Sci USA. 1999;96:3751–3756. doi: 10.1073/pnas.96.7.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Andrews PD, Stark MJ, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 1999;285:1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–588. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA, Johnston GC. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach MF, Latterich M, Schekman RW. Characteristics of endoplasmic reticulum-derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Winston F, Hieter P. Methods in Yeast Genetics. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates IJ, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acid as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schmitt HD, Wagner P, Pfaff E, Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986;47:401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A Rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spang A, Schekman R. Reconstitution of retrograde transport from the Golgi to the ER in vitro. J Cell Biol. 1998;143:589–599. doi: 10.1083/jcb.143.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbeck G, Harter C, Brecht A, Herrmann D, Lottspeich F, Orci L, Wieland FT. β′-COP, a novel subunit of coatomer. EMBO J. 1993;12:2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Sweet DJ, Pelham HR. The Saccharomyces cerevisiae SEC20 gene encodes a membrane glycoprotein which is sorted by the HDEL retrieval system. EMBO J. 1992;11:423–432. doi: 10.1002/j.1460-2075.1992.tb05071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DJ, Pelham HR. The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J. 1993;12:2831–2840. doi: 10.1002/j.1460-2075.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafer E, Pick E, Rotman M, Zuck S, Huber I, Cassel D. Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J Biol Chem. 2000;275:23615–23619. doi: 10.1074/jbc.M003171200. [DOI] [PubMed] [Google Scholar]
- Tanigawa G, Orci L, Amherdt M, Ravazzola M, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1672. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal SB, Hsu VW, Peters PJ, Klausner RD, Donaldson JG. An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J Biol Chem. 1994;269:3135–3158. [PubMed] [Google Scholar]
- VanRheenen SM, Cao X, Lupashin VV, Barlowe C, Waters MG. Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J Cell Biol. 1998;141:1107–1119. doi: 10.1083/jcb.141.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen SM, Cao X, Sapperstein SK, Chiang EC, Lupashin VV, Barlowe C, Waters MG. Sec34p and Sec35p are components of a protein complex required for vesicle tethering the yeast Golgi. J Cell Biol. 1999;147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen SM, Reilly BA, Chamberlain SJ, Waters MG. Dsl1p, an essential protein required for membrane traffic at the endoplasmic reticulum/Golgi interface in yeast. Traffic. 2001;2:212–231. doi: 10.1034/j.1600-0854.2001.020307.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–296. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Hughson FM. Membrane tethering and fusion in the secretory and endocytic pathways. Traffic. 2000;1:588–597. doi: 10.1034/j.1600-0854.2000.010802.x. [DOI] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Seog D-H, Yoda K, Yamasaki M, Wakabayashi T. Uso1 protein is a dimer with two globular heads and a long coiled-coil tail. J Struct Biol. 1996;116:356–365. doi: 10.1006/jsbi.1996.0053. [DOI] [PubMed] [Google Scholar]