Abstract
Integrin-based adhesions play critical roles in cell migration. Talin activates integrins and flexibly connects integrins to the actomyosin cytoskeleton, thereby serving as a ‘molecular clutch’ that transmits forces to the extracellular matrix to drive cell migration. Here we identify the evolutionarily conserved Kank protein family as novel components of focal adhesions (FAs). Kank proteins accumulate at the lateral border of FAs, which we term the FA belt, and in central sliding adhesions, where they directly bind the talin rod domain through the Kank amino-terminal (KN) motif and induce talin and integrin activation. In addition, Kank proteins diminish the talin–actomyosin linkage, which curbs force transmission across integrins, leading to reduced integrin–ligand bond strength, slippage between integrin and ligand, central adhesion formation and sliding, and reduced cell migration speed. Our data identify Kank proteins as talin activators that decrease the grip between the integrin–talin complex and actomyosin to regulate cell migration velocity.
Mesenchymal cell migration on the extracellular matrix (ECM) is crucial for embryonic development, wound healing and tumour metastasis. It commences with the formation of a lamellipodium at the cell leading edge, which is stabilized by numerous small and short-lived integrin-containing nascent adhesions (NAs)1. As cells migrate, a few NAs mature into larger focal adhesions (FAs) in the lamella, which associate with the actomyosin cytoskeleton and apply traction forces against the ECM necessary to move the cell forward2.
Integrins are core components of cell–matrix adhesion sites. They are α/β heterodimers that undergo a conformational change before they bind ligands (termed integrin activation), cluster and recruit numerous proteins to their cytoplasmic domains. A hallmark of integrin-mediated adhesion is that the lifetime of integrin–ligand bonds can be flexibly increased when forces are applied to integrins3. This property, called force-induced adhesion strengthening, depends on a dynamic association between integrins and F-actin, and is essential for FAs to withstand traction forces that pull the cell forward during cell migration4. Consequently, the destabilization of the integrin–F-actin connection or a decline in myosin II activity decreases force transmission across FAs and shortens the lifetime of integrin–ligand bonds, resulting in slippage between integrin and ligand, adhesion sliding, reduced traction forces and a drop in migration speed5. The association between integrins and F-actin occurs indirectly through integrin- and/or F-actin-binding proteins such as talin and vinculin6, which serve as a ‘molecular clutch’ that couples traction and actin-driven forces in space and time7–11.
Talin consists of an amino-terminal FERM (protein 4.1, ezrin, radixin, moesin) domain, also called the talin head domain (THD), and a carboxy-terminal rod domain with 13 helical bundles (R1–R13)12. The THD binds β-integrin tails and mediates integrin activation. The rod domain contains binding sites for F-actin, vinculin and the Rap1–GTP-interacting adapter molecule (RIAM)6. Talin cycles between cytosol, where it remains in an auto-inhibited form13, and plasma membrane, where it activates integrins and links integrins to F-actin14,15. Talin activation can be induced by RIAM binding to the R8 domain16–18, phosphatidylinositol 4,5-bisphosphate synthesized by a FA-associated splice variant of phosphatidylinositol-4-phosphate 5-kinase type Iγ (PIPKIγ90)15,19 and the actin retrograde flow, probably by unleashing the talin rod from the THD20,21. The cell-type-restricted integrin activation defects in RIAM-deficient mice and normal integrin activation in PIPKIγ90-deficient mice22–24 suggest that additional talin activators probably exist.
In the present paper, we identified the evolutionarily conserved Kank protein family as novel FA proteins. They consist of four members (Kank1–4) that are characterized by a Kank N-terminal motif (KN), several central coiled-coil domains and C-terminal ankyrin (Ank) repeats25. The single Kank orthologue in worms, VAB19, controls epidermis–muscle attachment26, neuronal migration27 and basement membrane remodelling28. Mutations in mammalian Kanks have been associated with cerebral palsy type 2, spastic quadriplegia (CPSQ2)29 and nephrotic syndrome30. Kank1 can bind liprin-β1 through a coiled-coil domain and Kif21a through the Ank repeats, and restrict microtubule (MT) outgrowth at the cell cortex and suppress stress fibre formation31,32. We report here that Kank2 localizes to the lateral border of FAs (termed the FA belt) and central adhesions, binds the talin rod through the KN motif, promotes talin and integrin activation, and interferes with F-actin binding to the talin rod, which suppresses mechanical force transmission across activated integrins, leading to adhesion sliding and reduced cell migration.
RESULTS
Kank2 is a novel FA protein
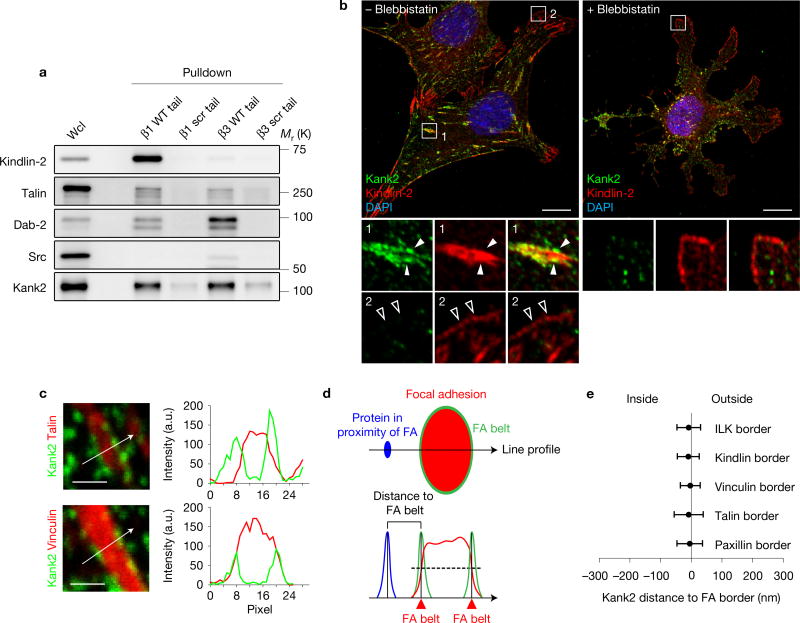
To identify novel, β1 integrin tail-associated adhesome proteins, we compared our published FA-enriched proteome (adhesome)33 with the β1 integrin tail peptide interactome34 (Supplementary Fig. 1a). Talin, kindlin-2 and the ILK, PINCH, parvin (IPP) complex were enriched in the adhesome and the β1 tail interactome, whereas Dab-2 and SNX1735, which bind integrin tails during endosomal trafficking, were not enriched in the adhesome. Interestingly, Kank2 was also enriched in the adhesome and β1 tail interactome (Supplementary Fig. 1a). Unlike kindlin-2, which preferentially bound the β1 tail, or Src and Dab-2, which preferentially bound the β3 tail, Kank2 and talin bound β1 and β3 tails equally well (Fig. 1a). Immunostaining of fibronectin (FN)-seeded, immortalized mouse fibroblasts revealed that endogenous Kank2 localized to puncta at the outer border of mature kindlin-2- and talin-positive FAs behind the lamella (Fig. 1b, arrowheads, Supplementary Fig. 1c) and to thin, elongated central adhesions (Fig. 1b and Supplementary Fig. 1b), and was absent from NAs and small FAs of the lamella (Fig. 1b, open arrowheads). Blebbistatin-treated cells also lacked Kank2 in kindlin-2-positive NAs of protruding cell membranes (Fig. 1b). The recruitment of Kank2 to the FA border was pronounced in cells cultured on FN-coated crossbow-shaped micropatterns (Supplementary Fig. 1d). Line profile analysis revealed that the Kank2 puncta peaked at the outer FA border, where canonical FA proteins (talin, kindlin-2, ILK, paxillin and vinculin) showed ~50% of their plateau intensity (Fig. 1c–e). We termed this unrecognized FA compartment the ‘FA belt’.
Figure 1.
Kank2 is a novel FA protein. (a) Western blot showing kindlin-2, talin-1, Dab-2, Src and Kank2 binding to biotinylated β1 and β3 integrin tail peptides. Peptides with scrambled amino-acid sequences (β1 scr tail; β3 scr tail) were used as negative controls. WT, wild type. Wcl, whole cell lysate. (b) Mouse fibroblasts seeded on FN for 3 h in the presence or absence of blebbistatin and immunostained for Kank2 (green) and kindlin-2 (red). Full arrowheads indicate Kank2-positive puncta along the FA border; open arrowheads indicate NAs. Scale bar, 10 µm. (c) Line profile analysis of cells immunostained for Kank2 and talin or vinculin along depicted line scans. Scale bar, 1 µm. (d) Definition of the FA belt at the lateral FA border and the distance from the FA border to proteins in proximity to the FA (outside of FAs) along the depicted line scan. (e) Kank2-positive puncta localize to the FA border defined by ILK, kindlin-2, vinculin, talin and paxillin (mean ± s.d.; n = 8 FAs for each marker, data pooled from eight cells). Unprocessed original scans of blots are shown in Supplementary Fig. 9.
Co-immunostaining for β1 and β3 integrins revealed that β3 integrins co-localized with paxillin in the core of mature FAs, whereas total and active (labelled with the activation epitope-reporting 9EG7 antibody) β1 accumulated in FA belts and central adhesions (Supplementary Fig. 1e–g). These data show that mature FAs are surrounded by a Kank2- and β1 integrin-enriched belt.
Kank2 is targeted to FAs through the KN motif
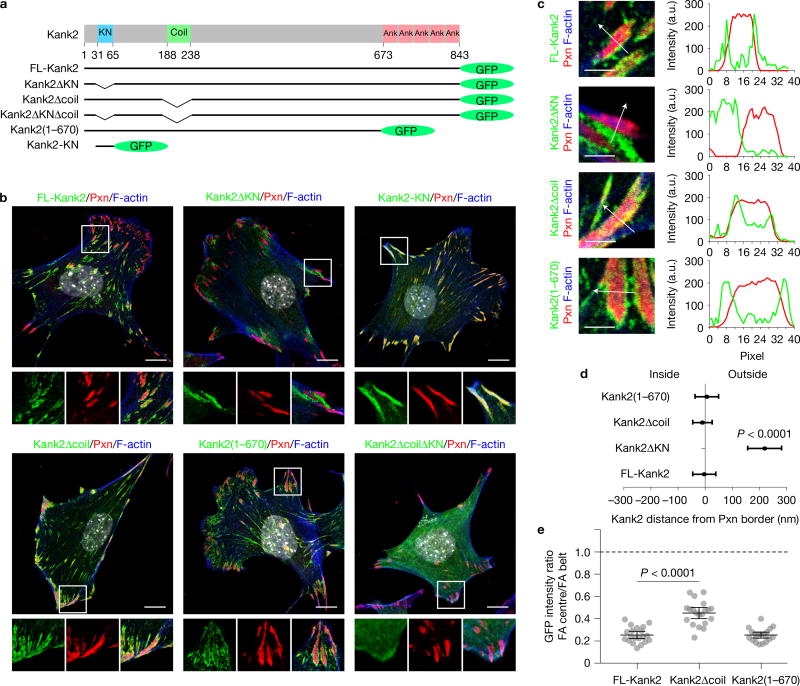
To determine how Kank2 is recruited to the FA belt, we generated green fluorescent protein (GFP)-tagged full length (FL)- Kank2 and deletion constructs of Kank2 lacking evolutionarily conserved domains (Fig. 2a): Kank2ΔKN-GFP lacking the KN motif; Kank2Δcoil-GFP lacking the liprin-β1-binding coiled-coil domain31; Kank2(1–670)-GFP lacking the Kif21a-binding Ank repeats31 and Kank2ΔKNΔcoil-GFP lacking both the KN motif and the coiled-coil domain. The Kank2 constructs were stably expressed in Kank2-depleted mouse fibroblasts (Supplementary Fig. 2b), which expressed mainly Kank2 (Supplementary Fig. 2a–c).
Figure 2.
Kank2 is targeted to FAs through the KN motif. (a) Domain organization of the Kank2 protein and illustration of GFP-tagged Kank2 truncation/deletion mutants. (b) Staining of paxillin (Pxn), F-actin (phalloidin), GFP-tagged Kank2 and DAPI (grey). Scale bar, 10 µm. (c) Line profile analysis of GFP-tagged FL-Kank2, Kank2ΔKN, Kank2Δcoil and Kank2(1–670) together with Pxn. Scale bar, 1 µm. (d) Line profile quantification of the distance between FL-Kank2, Kank2ΔKN, Kank2Δcoil and Kank2(1–670) to the FA border (mean ± s.d.; n = 8 FAs for each Kank2 construct, pooled from eight cells; P value calculated using Student’s t test). (e) Ratios between fluorescence intensities within the FA centre and on the FA belt for FL-Kank2, Kank2Δcoil and Kank2(1–670) (mean ± s.d.; n = 20 FAs for each Kank2 construct pooled from 10 cells; P value calculated using one-way ANOVA Tukey test).
FL-Kank2-GFP also localized to the belt of paxillin-positive mature FAs and to central adhesions (Fig. 2b). In line with a previous report31, we also found Kank2 adjacent to FAs in liprin-β1- and ELKS-enriched regions (Supplementary Fig. 3a–c), which capture MT plus ends and promote exocytosis. In contrast, Kank2ΔKN-GFP was absent from FA belts or central adhesions and instead accumulated in liprin-β1- and ELKS-positive regions adjacent to but clearly away from FA belts (Fig. 2b, and Supplementary Fig. 3a,b), as shown by line profile analyses (Fig. 2c,d). Although both Kank2Δcoil-GFP and Kank2(1–670)-GFP showed enrichment on FA belts (Fig. 2b–d), Kank2Δcoil-GFP also penetrated the FA core (Fig. 2e), indicating that the coiled-coil domain contributes to the exclusion of Kank2 from the FA core. Interestingly, Kank2 recruited liprin-β1 through the coiled-coil domain to FA belts, whereas ELKS always localized to the vicinity of the belt (Supplementary Fig. 3a–c). Kank2ΔKNΔcoil-GFP was diffusely distributed throughout the cytosol, whereas the GFP-tagged KN polypeptide completely overlapped with paxillin in all adhesions, including the small, peripheral NAs (Fig. 2b). These results indicate that the KN motif localizes Kank2 to FA belts and central adhesions and that additional protein regions exclude Kank2 from the FA core.
Peptide pulldowns revealed that FL-Kank2-GFP and Kank2-KN-GFP, but not Kank2ΔKN-GFP or GFP, associated with β1 integrin tails (Supplementary Fig. 3d,e). To investigate whether Kank1, Kank3 and Kank4 also localize to the belt, they were tagged with GFP and expressed in Kank2-depleted fibroblasts at similar levels as judged by GFP intensities. Kank1-GFP and Kank3-GFP localized to FA belts, whereas Kank4-GFP associated with FAs with additional cytoplasmic distribution (Supplementary Fig. 3f).
Kank2 inhibits cell migration by inducing adhesion sliding
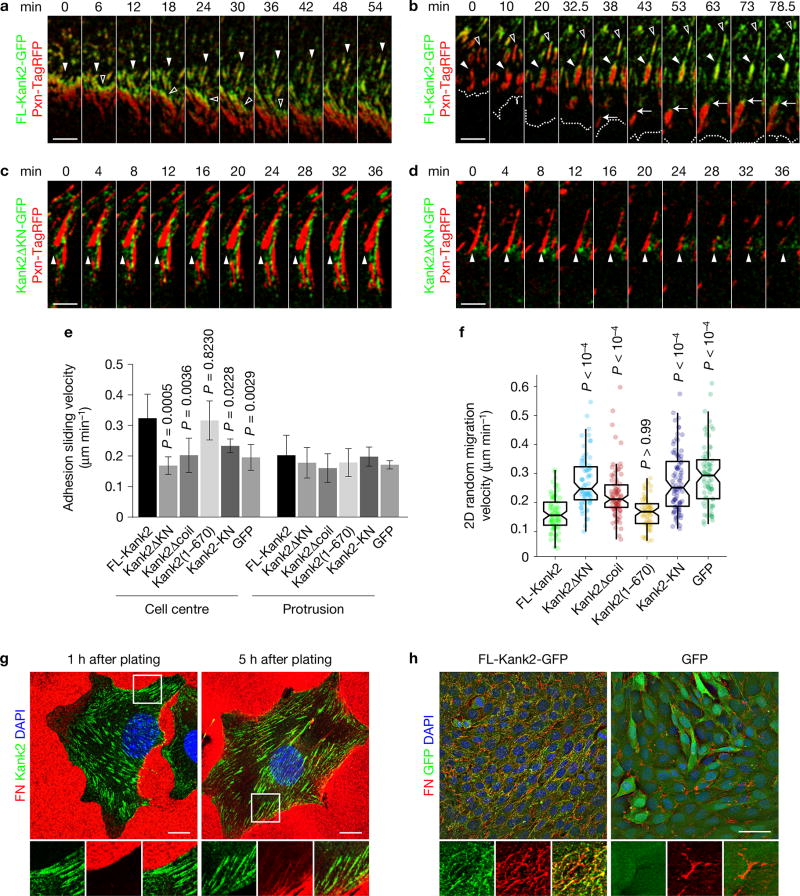
To unravel how Kank2 is recruited to FA belts, we carried out live cell imaging of paxillin-TagRFP (red fluorescent protein) and Kank2-GFP in Kank2-depleted fibroblasts. During isotropic cell spreading on FN, Kank2-containing puncta were visible in the proximity of FAs. A few minutes after the assembly of radial FAs, Kank2 puncta appeared at their proximal tips pointing to the cell centre, from where Kank2 spread along the FA belt during the following minutes (Supplementary Video 1 and Supplementary Fig. 4a). After cell polarization (45 min after plating), Kank2 puncta continued to accumulate at the proximal border of FAs (Supplementary Video 2 and Fig. 3a), from where Kank2- and paxillin-positive, thin and elongated adhesion structures developed and moved with an average speed of ~0.3 µm min−1 into the cell centre (Supplementary Video 2 and Fig. 3a). In migrating cells (4 h after plating), Kank2-GFP was recruited to the proximal tips of mature FAs and then gradually spread along the FA belt, while it remained absent from NAs in lamellipodia and FAs of the lamella (Supplementary Video 3 and Fig. 3b). The Kank2-positive FA belt formation correlated with the disassembly of the FA cores and the conversion of the FA belts into thin and motile adhesions (Supplementary Video 3 and Fig. 3b). In Kank2ΔKN-GFP-expressing cells, Kank2ΔKN-GFP-positive puncta moved to the proximity of FAs but never entered the FA belt (Supplementary Video 4 and Fig. 3c, closed arrowheads). Moreover, FAs failed to elongate and slide, and the few central adhesions that formed remained stationary (Supplementary Video 4 and Fig. 3c). The stationary FAs in Kank2ΔKN-GFP-expressing cells disassembled behind the lamella (Fig. 3d) with similar rates as in cells expressing FL-Kank2 (Supplementary Video 4 and Supplementary Fig. 4b,c), indicating that adhesion sliding is not caused by adhesion disassembly.
Figure 3.
Kank2 curbs cell migration by inducing adhesion sliding. (a) Time-lapse images of peripheral FAs in Kank2-depleted fibroblasts stably expressing FL-Kank2-GFP and paxillin-TagRFP (Pxn-TagRFP) 45 min after plating on FN. Arrowheads highlight recruitment of Kank2 to proximal borders of FAs (full arrowheads) and the developing sliding adhesions (open arrowheads). (b) Time-lapse images of cells during the migration phase 4 h after plating on FN. Full arrows highlight the recruitment of Kank2-GFP to proximal borders of mature FAs behind the lamella, and open arrowheads highlight the dynamic formation of the Kank2-positive FA belt followed by conversion into thin, elongated sliding adhesions. Dashed lines indicate the cell leading edge. (c, d) Time-lapse images of peripheral FAs in Kank2-depleted cells stably expressing Kank2ΔKN-GFP and Pxn-TagRFP 45 min after plating on FN. Arrowheads highlight the proximal border of a stable FA (c) and disassembling FA (d) behind the lamella. Scale bars in a–d, 5 µm. (e) Sliding velocities of central adhesions and FAs from indicated cells (mean ± s.d.; n = 5 cells pooled from three independent experiments, >400 central adhesions and >100 peripheral adhesions analysed for each condition). (f) 2D random migration velocities on FN (dot plot and box plot with median, 95% confidence interval (CI) notch, first–third quantile box and 5th–95th percentile whiskers; n between 60 and 90 cells for each cell line; data aggregated over four independent experiments). P values calculated using one-way ANOVA Tukey test in e and Kruskal–Wallis test in f. (g) Kank2-depleted cells re-expressing FL-Kank2-GFP were plated on FN-coated (10 µg ml−1) coverslips for 1 h or 5 h and immunostained for FN and DAPI. Scale bar, 10 µm. (h) Kank2-depleted cells re-expressing FL-Kank2-GFP or GFP control were seeded on FN-coated (10 µg ml−1) coverslips for 12 h at confluence and immunostained for FN and DAPI. Maximal intensity projection of z-stack image series. Scale bar, 50 µm.
Our data suggest that Kank2 decorates FA belts in a KN-motif-dependent manner and induces the gliding of belts into the cell centre. To confirm that Kank2 induces adhesion motility, we recorded vinculin-mCherry co-expressed in Kank2-depleted fibroblasts with either GFP-tagged FL or mutant Kank2 (Supplementary Videos 5–10). Overlay of 10 sequential colour-coded vinculin-mCherry frames revealed that central adhesions in FL-Kank-GFP- and Kank2(1–670)-expressing cells appeared as rainbows in overlay images due to their significant displacements, whereas central adhesions of cells expressing Kank2ΔKN-GFP, Kank2Δcoil-GFP, KN-GFP or GFP alone were stationary and appeared white in overlay images (Supplementary Fig. 4d). Single-adhesion tracking revealed that central adhesions in FL-Kank2-GFP- and Kank2(1–670)-expressing cells moved with a sliding velocity of ~0.3 µm min−1, whereas sliding of central adhesions in cells expressing Kank2ΔKN-GFP, Kank2Δcoil-GFP, KN-GFP or GFP alone was slower (Fig. 3e). Furthermore, over 60% of adhesions in FL-Kank2-GFP- and Kank2(1–670)-GFP-expressing cells slid with rates higher than 0.3 µm min−1, whereas the numbers of fast-sliding adhesions were significantly lower in cells expressing Kank2ΔKN-GFP, Kank2Δcoil-GFP, KN-GFP or GFP alone (Supplementary Fig. 4e). Consistent with the absence of Kank2 from adhesions in the protruding front, the sliding velocity of adhesions in protrusion was unaffected by the FL or mutant Kank2 (Fig. 3e).
Adhesion sliding correlates with reduced cell migration speed7. Indeed, both Kank2-depleted cell lines showed higher migration velocities when compared with controls (Supplementary Fig. 4f). Furthermore, expression of FL-Kank2-GFP and Kank2(1–670)-GFP but not Kank2ΔKN-GFP, Kank2Δcoil-GFP, KN-GFP or GFP alone in Kank2-depleted fibroblasts decreased migration speed (Fig. 3f). Consistent with the absence of Kank2 from the cell front, cell spreading was unaffected in Kank2-depleted cells or cells expressing Kank2 constructs (Supplementary Fig. 4g).
Centripetal α5β1 integrin translocation was proposed to mediate FN fibrillogenesis36. We noticed that Kank2-positive central adhesions formed within 1 h of plating on FN before FN fibrillogenesis occurred, whereas, 5 h after cell seeding, thin FN fibrils, frequently decorated with Kank2, extended from the cell periphery to the cell centre (Fig. 3g). After overnight culture, FL-Kank2-GFP-expressing cells assembled extensive networks of elongated and branched FN fibrils, compared with the few and thickened FN fibrils in GFP-expressing Kank2-depleted cells (Fig. 3h).
Kank2 induces integrin–ligand-bound slippage
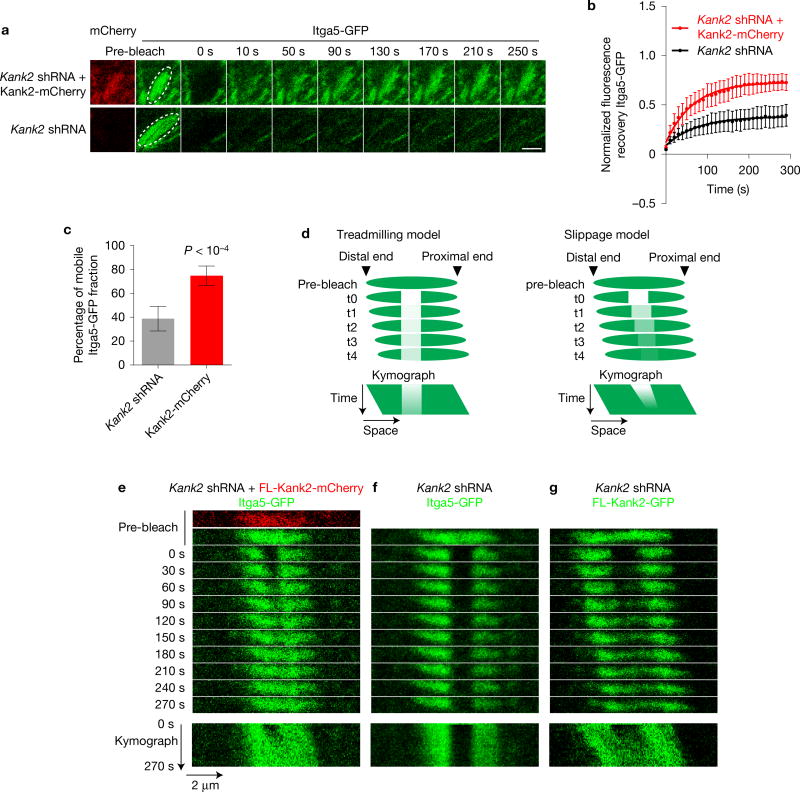
Adhesion sliding can be due to ECM cleavage by metalloproteinases (MMPs)37 or turnover of integrin–ligand complexes38. Treatment of FL-Kank2-GFP-expressing cells with the pan-MMP inhibitor Gm6001 did not affect adhesion sliding (Supplementary Fig. 4h). To analyse integrin turnover, we photobleached the C-terminally GFP-tagged α5 integrin (Itga5-GFP) in entire adhesions of Kank2-depleted cells expressing FL-Kank2-mCherry or an empty control plasmid, and found that FL-Kank2 increased the mobile fraction of α5 integrin from about 40% to about 70% (Fig. 4a–c). To distinguish slippage between integrin and ligand from adhesion treadmilling as the cause of adhesion sliding, we photobleached Itga5-GFP in the middle segment of an adhesion and measured GFP recovery. In the case of treadmilling defined by integrin recruitment to the proximal end and release from the distal ends the bleached middle segment should remain stationary (Fig. 4d, left panel), whereas in the case of slippage between integrin and ligand the bleached middle segment should move in the direction of the adhesion sliding, at least during the short time window before integrins are turned over (Fig. 4d, right panel). The experiment revealed slippage movement of integrins in Kank2-positive adhesions, but not treadmilling (Fig. 4e). Similarly, photobleaching of FL-Kank2-GFP in the middle segment of a sliding adhesion revealed the same co-sliding of bleached and unbleached areas (Fig. 4g). In contrast, photobleaching of Itga5-GFP in the middle segment of a stationary adhesion in Kank2-depleted cells revealed that neither bleached nor unbleached regions slid (Fig. 4f). These findings indicate that Kank2 destabilizes integrin–ligand complexes, leading to sliding of the Kank2–integrin complex along the ECM and the formation of FN fibrils.
Figure 4.
Adhesion sliding occurs at the interface between integrin and ligand. (a) Still images from representative time-lapse FRAP experiments with Itga5-GFP in Cherry-tagged FL-Kank2 or empty-vector-expressing fibroblasts. A pre-bleach image shows that Kank2-mCherry and Itga5-GFP co-localized in the region of interest (ROI, white circle). Scale bar, 2 µm (b) Fluorescence recovery curves of indicated FRAP experiments. FRAP of Itga5-GFP in central adhesions of Kank2-depleted fibroblasts transduced with either mCherry-tagged FL-Kank2 or empty plasmid. Mean optical intensities in the ROI are normalized to cytosolic background and plotted as percentage of initial intensity before bleaching (mean ± s.d.; n = 8 independent FRAPs in eight cells for each cell line). Fluorescence recovery curves are fitted to a one-phase association model. (c) Mobile Itga5-GFP fractions in bleached adhesions (mean ± s.d.; n=8 independent FRAPs in eight cells for each cell line; P value calculated using Students t test). (d) Adhesion treadmilling (left) and integrin slippage models predict different experimental results in time-lapse images and kymographs on photobleaching of middle segments in sliding adhesions. (e–g) Time-lapse images (upper panel) and kymograph (lower panel) of Itga5-GFP (e, f) or Kank2-GFP (g) after photobleaching of the GFP signal in the middle segments of adhesion sites in Kank2-depleted fibroblasts expressing Kank2-mCherry and a low level of Itga5-GFP (e), empty plasmid (f) or Kank2-GFP (g).
Kank2 suppresses force transmission across integrins
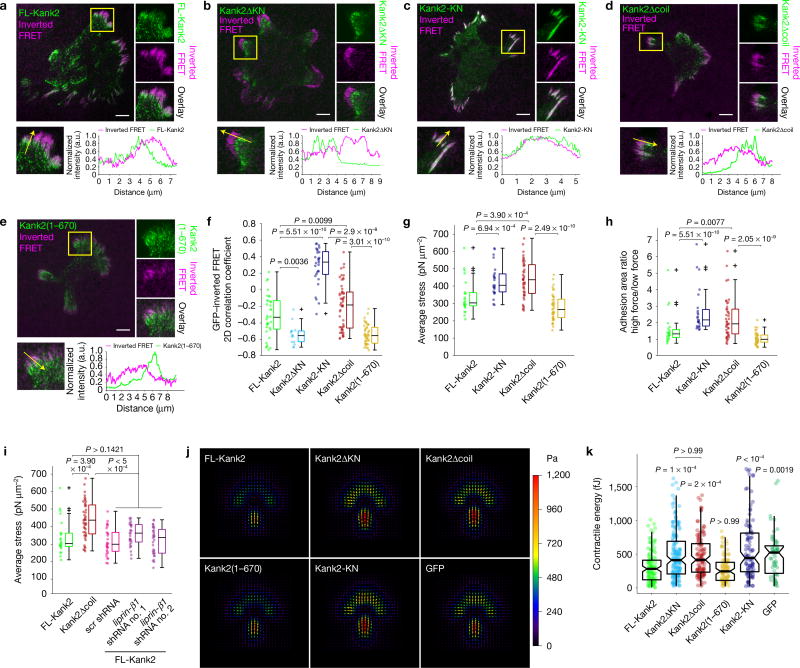
A reduced force transmission across integrins can destabilize integrin–ligand bonds. To test this possibility, we plated fibroblasts expressing GFP-tagged FL-Kank2, Kank2ΔKN, Kank2Δcoil, Kank2(1–670), Kank2-KN or GFP alone on RGD peptides conjugated with a Förster resonance energy transfer (FRET)-based molecular tension sensor, which decreases FRET efficiency under tension39. To visualize FRET maps recorded by total internal reflection fluorescence (TIRF) microscopy, we inverted the FRET ratios such that bright signals correspond to low FRET ratios and high traction. We found that FL-Kank2 was enriched at proximal borders of tensioned RGD peptide clusters in the cell periphery and in central adhesions with low inverted FRET signals. In contrast, Kank2ΔKN was absent from tensioned RGD ligand clusters in the periphery and the cell centre, whereas the KN polypeptide overlapped with tensioned RGD clusters in the cell periphery and less frequently in the cell centre (Fig. 5a–c). To determine the coincidence between the GFP-tagged proteins and locally generated tension, we calculated the 2D correlation coefficient between the GFP-tagged proteins and inverted FRET signals and found that tension had a negative correlation with FL-Kank2 (−0.33) and Kank2ΔKN (−0.55) and a positive correlation with the KN polypeptide (0.33; Fig. 5f). Moreover, FL-Kank2 significantly decreased the force at peripheral adhesions when compared with the Kank2-KN (Fig. 5g). Furthermore, the ratios between high-(nominal stress of ≥250 pN µm−2) and low-force- (nominal stress of <250 pN µm2) bearing adhesion areas were significantly decreased in FL-Kank2-expressing cells (Fig. 5h), indicating that FL-Kank2 curbs force transmission across adhesion sites. Importantly, deletion of the liprin-β1-binding coiled-coil domain (Kank2Δcoil) but not the deletion of the Kif21a-binding Ank repeats, reduced the negative correlation with the locally generated tension and failed to diminish force transmission across integrins (Fig. 5d–h).
Figure 5.
Kank2 impairs force transmission across integrins. (a–e) GFP and inverted FRET signals in Kank2-depleted cells expressing GFP-tagged FL-Kank2, Kank2ΔKN, Kank2-KN, Kank2Δcoil and Kank2(1–670) seeded on FRET-based RGD tension sensors for 5 h. Split channels of boxed regions are shown on the right-hand side and line profiles of indicated adhesions in the boxed region below. Scale bar, 10 µm. (f) 2D correlation coefficient between GFP and inverted FRET signals (dot plot and box plot; FL-Kank2, n = 35 cells; Kank2ΔKN, n = 14 cells; Kank2-KN, n = 29 cells; Kank2Δcoil, n = 45 cells; Kank2(1–670), n = 42 cells; data aggregated from three independent experiments for each condition; P values were calculated using the Wilcoxon rank sum test; crosses represent outliers). (g) Force exerted by adhesions at the cell periphery in cells expressing indicated constructs. (h) Ratios between adhesion areas with high tension (≥250 pN µm−2) and adhesion areas with low tension (<250 pN µm−2) were calculated in cells expressing indicated constructs. (i) Force exerted by adhesions at the cell periphery in Kank2-depleted cells expressing FL-Kank2 or Kank2Δcoil, or FL-Kank2 together with a scramble (scr) shRNA or two independent shRNAs against liprin-β1 (for g–i, dot plot and Tukey box plot; FL-Kank2, n = 24 cells; Kank2-KN, n = 24 cells; Kank2Δcoil, n = 45 cells; Kank2(1–670), n = 43 cells; FL-Kank2 scr shRNA, n = 30 cells; FL-Kank2 liprin-β1 shRNA no. 1, n = 31 cells; FL-Kank2 liprin-β1 shRNA no. 2, n = 33 cells; data aggregated from three independent experiments for each condition; P values were calculated using the Wilcoxon rank sum test; crosses represent outliers). (j) Average traction-force fields of indicated cells on FN-coated micropatterns with 35 kPa rigidity. Arrows indicate force orientation, and colour and length represent local stress magnitude in pascals. (k) Contractile energy of individual cells (dot plot and box plot with median, 95% CI notch, first–third quantile box and 5th–95th percentile whiskers; FL-Kank2, n = 125; Kank2ΔKN, n = 168; Kank2Δcoil, n = 124; Kank2(1–670), n = 98; Kank2-KN, n = 98; GFP only, n = 62 cells; data aggregated over three to six independent experiments for each cell line; P values calculated using Kruskal–Wallis test).
Depletion of liprin-β1 reduced endogenous and overexpressed Kank2 levels (Supplementary Fig. 5a). Although liprin-β1 depletion slightly affected the negative correlation between FL-Kank2-GFP and tension (Supplementary Fig. 5b), force transmission remained unaffected (Fig. 5i and Supplementary Fig. 5c), indicating that the coiled-coil domain in Kank2 regulates force transmission in a liprin-β1-independent manner.
Finally, we confirmed that FL-Kank2 impairs force transmission to ECM-bound integrins with traction-force microscopy (TFM) of our cell lines on FN-coated micropatterns with a rigidity of 35 kPa (ref. 34). In line with the molecular tension sensor experiments, FL-Kank2- and Kank2(1–670)-expressing cells generated significantly less traction force than did cells expressing Kank2ΔKN, Kank2Δcoil, the KN polypeptide or the GFP control (Fig. 5j,k).
Kank2 directly binds the talin rod
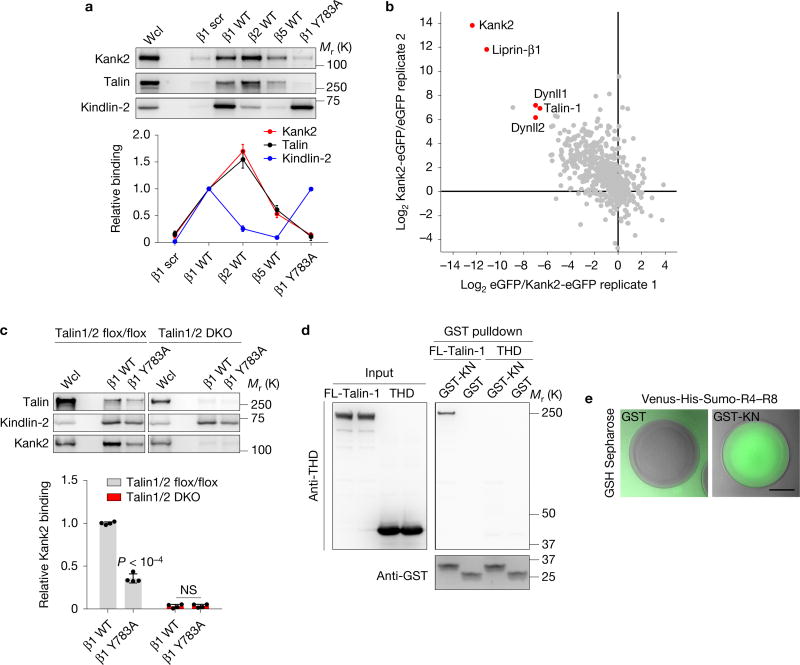
To unravel how Kank2 is recruited to the FA belt and suppresses integrin-mediated force transmission, we carried out pulldown experiments with different integrin tail peptides, which revealed that the Kank2-binding profile resembled that of talin but differed from that of kindlin-2 (Fig. 6a). Furthermore, talin-binding-deficient β1 tails (β1 Y783A) pulled down neither Kank2 nor talin, but still kindlin-2 (Fig. 6a), suggesting that talin and Kank2 are co-recruited to integrin tails.
Figure 6.
Kank2 directly binds the talin rod. (a) Western blot (upper panel) and densitometric analysis (lower panel) of Kank2, kindlin-2 and talin binding to biotinylated wild-type (WT) β1, β2, β5 integrin tails, Y783A-substituted β1 integrin tail (β1 Y783A) and scrambled peptides (β1 scr). Data is illustrated as the mean ± s.d. Wcl, whole cell lysate. (b) Scatter plot of label-free quantification (LFQ)-intensity ratios of Kank2-GFP and GFP immunoprecipitates. Specific interactions displaying high Kank2-GFP to GFP and low GFP to Kank2-GFP ratios in two independent replicates are highlighted in red. eGFP, enhanced green fluorescent protein. (c) Western blot (upper panel) and densitometric analysis (lower panel) of Kank2, kindlin-2 and talin binding to β1 wild-type and β1 Y783A tails using either talin-1/2 flox/flox or talin-1/2-deficient cells (talin-1/2 double knockout (DKO); mean ± s.d.; n = 4 independent pulldown experiments; P values were calculated using Student’s t test). NS, not significant. (d) Representative GST pulldown of recombinant GST-KN or GST pre-incubated with recombinant talin-1 or THD from two independent experiments. (e) Representative epifluorescence images of Venus-His-Sumo-tagged talin R4–R8 domain recruited by GST-KN but not GST control to GSH Sepharose beads from two independent experiments. Scale bar, 5 µm. Source data for c can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 9.
To identify Kank2-interacting proteins that mediate its recruitment to β integrin tails, we immunoprecipitated FL-Kank2-GFP in fibroblasts and determined the interacting proteins by mass spectrometry (MS). Talin-1, dynein light chain isoforms (Dynll1 and Dynll2) and liprin-β1 were identified as binding partners of Kank2 (Fig. 6b). To test whether Kank is recruited to β1 tails in a talin-dependent manner, we carried out β1 tail peptide pulldown in fibroblasts lacking the Talin-1 and Talin-2 genes40 and found that the absence of talin-1 and talin-2 expression abolished Kank2 recruitment to β1 integrin tails (Fig. 6c). Since the FA belt recruitment is KN motif dependent, we purified recombinant glutathione S-transferase (GST)-KN, full length talin-1 (FL-talin-1) and the THD (Supplementary Fig. 6a) and carried out GST pulldown experiments, which showed that the GST-KN motif pulled down FL-talin-1 but not the THD (Fig. 6d), indicating that Kank2 binds the talin-1 rod through the KN motif. Expression of different GFP-tagged domains of the talin-1 rod in HEK293 cells followed by co-immunoprecipitation of GST-KN with GFP antibodies revealed that R7R8 domains but not the R8 domain alone associated with the KN polypeptide (Supplementary Fig. 6b). Furthermore, recombinant Venus-tagged talin R4–R8 efficiently bound GST-KN-coupled glutathione beads but not control GST beads (Fig. 6e). These findings indicate that Kank2 directly binds the R7R8 domain of the talin rod through the KN motif.
Kank2 induces talin and β1 integrin activation
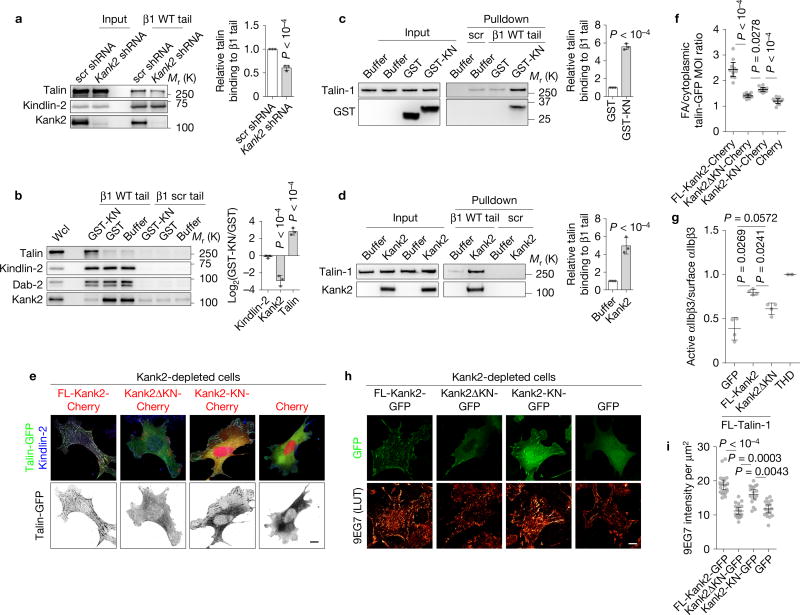
The R7R8 domains were shown to bind RIAM, actin and vinculin41, all of which can induce activation of talin, which in turn binds and activates integrins. To investigate whether Kank2 or KN binding to talin also promotes talin–integrin interactions, we carried out a series of experiments. First, short hairpin RNA (shRNA)-mediated depletion of Kank2 in fibroblasts decreased binding of endogenous talin to β1 integrin tail peptides by about 30% (Fig. 7a), whereas doxycycline-induced expression of FL-Kank2-GFP but not Kank2ΔKN-GFP increased talin binding to β1 integrin tails in a dose-dependent manner (Supplementary Fig. 7a,b). Second, incubation of normal fibroblast lysates with GST-KN or chemically synthesized KN peptide efficiently reduced endogenous Kank2 binding to β1 integrin tail peptides and increased talin binding in a dose-dependent manner, but left kindlin-2 and Dab-2 binding unchanged (Fig. 7b and Supplementary Fig. 7c–e). Third, β1 tails pulled down about five times more recombinant FL-talin in the presence of equal molar GST-KN or recombinant FL-Kank2 (Supplementary Fig. 7f) when compared with GST only (Fig. 7c,d). Fourth, the KN-peptide-mediated activation of FL-talin depended on the THD in full length talin, as it was efficiently blocked by an excess amount of THD domain (Supplementary Fig. 7g,h). Finally, co-expression of talin-GFP and Cherry-tagged FL-Kank2 in Kank2-depleted fibroblasts increased talin-GFP signals in kindlin-2-positive central adhesions and expression of the KN motif promoted talin-GFP localization to peripheral FAs (Fig. 7e,f), whereas Kank2ΔKN- or Cherry-expressing cells had the majority of talin-1-GFP in the cytosol (Fig. 7e,f). Thus Kank2 induces talin activation and binding to integrin β tails.
Figure 7.
Kank2 induces talin and integrin activation. (a) Western blot (left) and densitometric analysis (right) of Kank2, talin and kindlin-2 binding to β1 integrin tail in control (scr shRNA) cells or Kank2-depleted (Kank2 shRNA) cells (mean ± s.d.; n = 3 independent pulldown experiments; P values were calculated using Student’s t test). (b) Western blot (left) and densitometric analysis (right) of Kank2, talin, kindlin-2 and Dab-2 binding to β1 integrin tails or scrambled peptide (β1 scr) after addition of recombinant GST-KN or GST (mean ± s.d.; n = 3 independent pulldown experiments; P values calculated using Student’s t test). (c, d) Western blot (left) and densitometric analysis (right) of recombinant talin-1 binding to β1 integrin tail after addition of recombinant GST-KN (c) or FL-Kank2 (d) (mean ± s.d.; n = 3 independent pulldown experiments; P values calculated using Student’s t test). (e) Talin-GFP co-expressed with Cherry-tagged FL-Kank2, Kank2ΔKN, Kank2-KN in Kank2-depleted cells and stained for kindlin-2. (f) Quantification of talin-1-GFP mean optical intensity (MOI) ratio between kindlin-2-positive adhesion area and kindlin-2-negative cytosolic region in (e) (dot plot, mean ± 95% CI; n = 10 cells per cell line; P values calculated using one-way ANOVA Tukey test). (g) Binding of PAC1 antibody reporting active αIIbβ3 integrins normalized to binding of anti-total αIIbβ3 on CHO cells co-expressing talin-1-tagRFP with either Kank2-GFP, Kank2ΔKN-GFP or GFP, or expressing THD only (mean ± s.d.; n = 4 independent experiments; P values calculated using one-way ANOVA Tukey test). (h) Kank2-depleted fibroblasts stably transfected with GFP-tagged FL-Kank2, Kank2ΔKN, Kank2-KN or GFP only (green), seeded on FN and immune-stained with the 9EG7 antibody reporting the exposure of a β1 integrin-specific activation epitope (using orange look-up table (LUT)). (i) Signal intensities of 9EG7 staining quantified from (h) (dot plot, mean ± 95% CI; n = 30 cells per cell line; data aggregated from three independent experiments; P values calculated using Kruskal–Wallis test). Scale bars in e,h, 10 µm. Source data for a–d,g can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 9.
Since active, integrin-tail-bound talin induces integrin activation, we next tested whether Kank2 promotes integrin activation. The expression of talin-1 together with FL-Kank2-GFP in αIIbβ3-integrin-expressing CHO cells increased binding of the αIIbβ3 integrin activation epitope-reporting PAC1 antibody when compared with cells expressing talin-1 and GFP (Fig. 7g). Importantly, expression of talin-1 and Kank2ΔKN failed to increase PAC1 binding over the talin-1/GFP control (Fig. 7g). Similarly, immunostaining with 9EG7 antibody of FN-adherent Kank2-depleted fibroblasts expressing GFP-tagged FL-Kank2, Kank2ΔKN, Kank2-KN or GFP alone revealed that FL-Kank2-GFP and Kank2-KN-GFP co-localized with active, 9EG7-positive β1 integrins and increased the number of 9EG7-positive adhesion sites, whereas Kank2ΔKN-GFP or GFP alone had no effect on 9EG7 binding (Fig. 7h,i). Altogether, these findings indicate that Kank2 activates talin and integrins in a KN-motif-dependent manner.
Kank2 interferes with F-actin binding to the ABS2 of talin
How does Kank2 binding to talin decrease force transmission across integrins? Kank2 was shown to regulate RhoA and myosin II activities through an association with RhoGDIα in kidney podocytes30,32. In contrast to podocytes, however, RhoGDIα was neither identified as a binding partner of FL-Kank2-GFP in the MS-based Kank2 interactome screen nor co-localized with FL-Kank2 in fibroblasts (Supplementary Fig. 8a). Furthermore, similar GTP-bound RhoA and phospho-Ser 19-myosin light chain (pMLC) levels in cells expressing FL-Kank2, Kank2ΔKN, the KN polypeptide or GFP (Supplementary Fig. 8b,c) excluded a role for Kank2 in regulating myosin II activity.
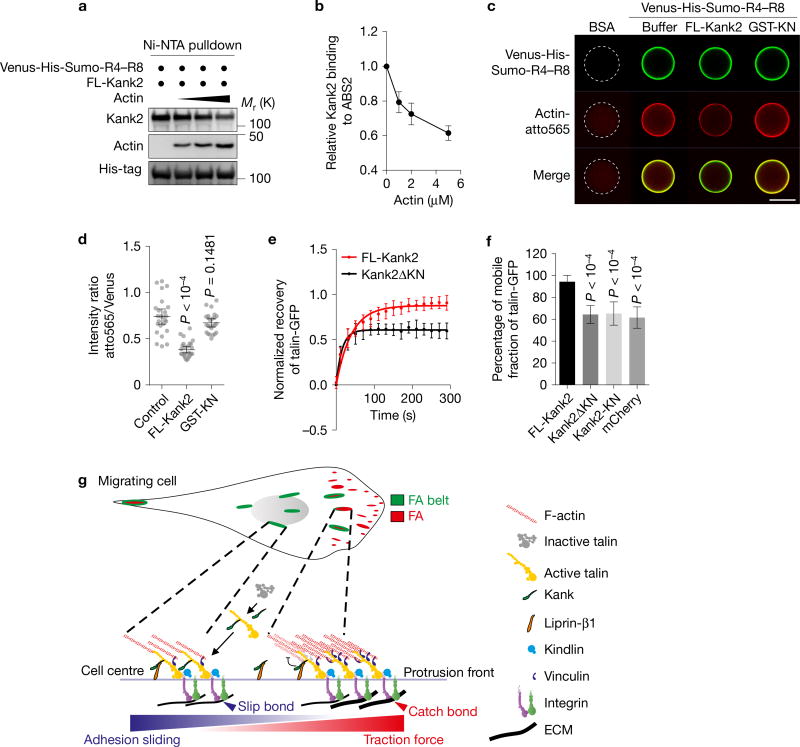
The destabilization of the Talin–F-actin linkage can also decrease force transmission across integrins. Kank2 binds talin in the vicinity of the F-actin-binding site 2 (ABS2). To test whether Kank2 and actin binding to the talin rod is mutually regulated, we incubated recombinant His-tagged talin R4–R8-coated Ni2+-NTA (nitrilotriacetate) beads with FL-Kank2 and actin under polymerization-permissive conditions and observed a dose-dependent inhibition of Kank2 binding to talin R4–R8 by actin (Fig. 8a,b). Consistently, recombinant FL-Kank2 but not GST-KN significantly decreased actin binding to His-tagged talin R4–R8-coated Ni2+-NTA beads (Fig. 8c,d). These results suggest that Kank2 interferes with actin binding to ABS2.
Figure 8.
Kank2 decreases F-actin binding to talin-ABS2. (a, b) Western blot (a) and densitometric analysis (b) of Kank2 binding to the talin R4–R8 domain in the presence of increasing concentrations of actin (0 µM, 1 µM, 2 µM and 5 µM, mean ± s.d.; n = 3 independent pulldown experiments) under polymerization-permissive conditions. (c) Representative images of atto565-labelled actin recruited to Ni2+NTA beads coated with bovine serum albumin (BSA) (control) or Venus-His-Sumo-tagged talin R4–R8 domain in the presence of the recombinant GST-KN motif or FL-Kank2 under polymerization-permissive conditions. Scale bar, 100 µm. (d) Fluorescence intensity ratios between atto565 and Venus on bead surfaces quantified on the basis of experiments in c (dot plot, mean ± 95% CI; n > 25 beads per condition; data aggregated from three independent experiments; P value calculated using one-way ANOVA Tukey test). (e) Fluorescence recovery curves of indicated FRAP experiments. FRAP of talin-1-GFP in central adhesions of Kank2-depleted fibroblasts co-expressing talin-1-GFP and either mCherry-tagged FL-Kank2 or mCherry-tagged Kank2ΔKN. Mean optical intensities in the ROI are normalized to cytosolic background and plotted as percentages of the initial intensity before bleaching (mean ± s.d.). Fluorescence recovery curves are fitted to a one-phase association model. (f) Mobile fractions of talin-1-GFP in the bleached adhesions (mean ± s.d.; n = 10 independent FRAPs from 10 cells for each condition; P value calculated using one-way ANOVA Tukey test). (g) Model depicting Kank function in FAs. In migrating cells, Kank2 is absent from adhesion sites of the protrusion front. Behind the lamella, Kank2 is first recruited to the proximal tips of mature FAs, from where it gradually spreads over the entire FA belt. The recruitment of Kank2 to the FA belt is mediated by a direct interaction between the KN motif of Kank2 and the R7 domain in the talin rod. Kank2 displaces F-actin from the talin-ABS2 while simultaneously promoting and/or maintaining talin activation. This dual function of Kank2 permits a partial decoupling of talin-bound, activated integrins from the actomyosin cytoskeleton, leading to diminished force transmission across FAs, reduced traction force, formation of slip bonds between integrins and ligands and the conversion of FA belts into sliding central adhesions. Unprocessed original scans of blots are shown in Supplementary Fig. 9.
The association of talin with F-actin was shown to immobilize talin in FAs42,43. Fluorescence recovery after photobleaching (FRAP) experiments in Kank2-depleted fibroblasts expressing FL-Kank2 or mutant versions of Kank2 showed that FL-Kank2 increased the mobile fraction of talin-GFP from 63.8 ± 9.0% to 92.4 ± 7.5% in a 5 min time window (Fig. 8e,f and Supplementary Fig. 8d), whereas neither Kank2ΔKN nor Kank2-KN affected the mobile fraction of talin. These findings confirm that Kank2 destabilizes the talin–F-actin linkage.
DISCUSSION
Kank proteins are defined by a unique KN motif. While a recent study reported that Kank proteins in HeLa cells localize to liprin-β1- and ELKS-containing clusters at the plasma membrane that capture MTs in close proximity to, but always outside FAs31, we found Kank highly enriched in the β1 integrin tail interactome and the integrin-induced adhesome, pointing to the possibility that Kank proteins also directly function inside FAs. We tested this hypothesis and found that Kank proteins are indeed present in FAs, where they play a dual role: on the one hand they control integrin activity through activating talin, and on the other hand they reduce the talin linkage with the actomyosin, which leads to reduced force transmission across FAs, increased adhesion sliding and reduced cell migration speed.
Our analysis shows that Kank recruitment to β integrin tails is indirect and occurs through a direct interaction between the KN motif of Kank and the talin R7 domain. The consequence of the Kank–talin interaction is the activation of talin, the binding of the Kank/talin to β integrin tails, integrin activation and integrin–ligand binding. The activation of talin and integrins by Kank is KN motif dependent. In line with this finding, the KN polypeptide is sufficient to induce talin activation and recruitment to β1 integrin tails in vitro and to FAs ex vivo, and to activate αIIbβ3 and β1 integrins in cells.
Our findings also demonstrate that Kank reduces the cell migration speed by converting FAs at the lamella border into thin and elongated sliding adhesions that glide into the cell centre (Fig. 7). Kank is absent from NAs and becomes recruited to the proximal tip of FAs at the lamella border either by binding to tensed, integrin-bound talin or as a de novo assembled Kank2–talin complex. From the proximal FA tips Kank gradually expands around the entire outer FA border, which is followed by the disassembly of the FA core and the gliding of the thin Kank2-positive FA border into the cell centre. We term the outer FA border, to which Kank concentrates during FA conversion, the FA belt. The exclusive localization of the KN-motif-deficient Kank2ΔKN to the previously described liprin-β1- and ELKS-containing clusters and the inability of Kank2ΔKN to induce FA sliding indicate that both FA belt recruitment and FA sliding depend on talin binding. Furthermore, the KN polypeptide also penetrates into the FA centre but fails to induce FA sliding, pointing to the functional importance of additional region(s) in Kank such as the coiled-coil domain.
We also found that Kank binding to talin destabilizes the integrin–actomyosin linkage, leading to decreased force transmission across adhesion sites, which diminishes adhesion strength and traction force, causing integrin–ligand bond slippage, FA sliding and a reduction in migration speed. The destabilization of the integrin–actomyosin linkage occurs with Kank2 binding to the talin rod. Since the KN motif is not sufficient to compete with actin for talin rod binding and for curbing force transmission across integrins, additional region(s) in Kank2 are required to execute these tasks. Such a region could be the coiled-coil domain, whose absence enabled a partial intrusion of the mutant Kank2Δcoil protein into the FA core, impaired the negative correlation with locally produced tension and failed to reduce force transmission across integrins. Mechanistically, the coiled-coil domain could hinder F-actin binding to talin-ABS2 either by oligomerizing Kank244 or by recruiting proteins such as liprin-β1. However, the localization of Kank2 to the FA belt and the normal force transduction after liprin-β1 depletion exclude liprin-β1 as potential binding partner of the coiled-coil domain for this function. Alternatively, Kank may bind as-yet-unidentified protein(s) that abrogate(s) talin–integrin tail binding on the FA belt. Such talin binding partners, however, would probably disassemble rather than convert the FA belt into a sliding central adhesion.
The reduced F-actin binding to talin by Kank2 provides a reasonable explanation for the reduced force transmitted across talin–Kank2 complexes to ligand-bound integrins, increased integrin turnover and slippage of integrin ligand bonds, which altogether leads to adhesion sliding and reduced migration speed (Fig. 8g). Consistent with our findings, cells carrying mutations in the ABS2 of talin also show reduced actomyosin coupling to integrin adhesion sites, an increase of the mobile fraction of talin and a decrease of the traction force without affecting cell adhesion or spreading11. Kank2 was also shown to bind RhoGDIα, which can potentially inhibit RhoA activity at FA belts and suppress formin-dependent F-actin filament assembly and Rock-mediated myosin II activities30,32,45. However, we did not detect RhoGDIα in our MS-based Kank2 interactome or colocalized with Kank2 at FA belts. Furthermore, the normal levels of active RhoA and pMLC in cells expressing FL-Kank2 suggest that RhoA-dependent myosin II activity is not grossly altered, although subtle changes cannot be excluded with our assays.
Kank proteins are also found in liprin-β1- and ELKS-enriched clusters at the plasma membrane where MT plus ends are captured to allow focal exocytosis of MT1-MMP and other cargo31,46–48, which could also contribute to adhesion sliding and cell migration velocity control. However, Kank2-induced adhesion sliding was not altered when the activities of MMPs were inhibited or MT targeting to FAs was compromised by depleting CLASP46, indicating that talin–Kank2 complex on FA belts and the ELKS–liprin-β1–Kank2 complex function separately. It is conceivable, however, that Kank proteins coordinate the properties of both compartments and thereby adjust MT dynamics and ECM remodelling with adhesion strengthening.
METHODS
Mass spectrometry
To measure Kank1–4 levels in FA-enriched fractions and whole cell lysates, cells were serum-starved for 4 h, plated for 90 min in serum-free medium on FN-coated, BSA-blocked culture dishes, and FAs were isolated34 for quantitative MS analysis on an LTQ Orbitrap analyser (Thermo Electron). Data were processed using the label-free quantification (LFQ) algorithm embedded in the MaxQuant software49.
For the MS analysis of anti-GFP pulldowns, the samples were run 1 cm into a 12% SDS–PAGE. The entire lane up to the front was cut into 1 mm × 1 mm slices and subjected to standard in-gel digestions as previously described50. Briefly, the gel slices were destained in ethanol followed by sequential reduction and alkylation with 10 mM dithiothreitol (DTT) and 40 mM chloroacetamide (CAA), then dried and incubated with digestion buffer containing 12.5 ng µl−1 trypsin in 25 mM Tris buffer (pH 8.5) overnight. Peptides were extracted, purified in StageTips, analysed in an LTQ Orbitrap XL analyser and processed as described above. MS data were further analysed in Perseus and manually annotated for data illustration.
Antibodies
Plasmids, constructs and expression and purification of recombinant proteins
Mouse Kank1 and Kank2 complementary DNAs were cloned into peGFP-N1 vector (Clontech) using XhoI and EcoRI sites, mouse Kank3 using BglII and EcoRI sites and mouse Kank4 using XhoI and AgeI sites. For retrovirus-mediated overexpression, cDNAs of FL-Kank2, Kank2ΔKN lacking amino acids 31–56, Kank2Δcoil lacking amino acids 181–240, Kank2ΔKNΔcoil lacking amino acids 31–56 and 181–240, Kank2(1–670) encoded by amino acids 1–670 and Kank2-KN encoded by amino acids 29–72 were inserted between XhoI and EcoRI sites of the pRetroQ-AcGFP-N1 (Clontech) vector. Expression plasmids for talin-AcGFP and talin-TagRFP were generated from pLPCXmod-Talin1-Ypet (gift from C. Grashoff, Max Planck Institute for Biochemistry) by replacing Ypet with AcGFP or TagRFP-T. The talin rod truncations were inserted between XhoI and EcoRI of the peGFP-N1 vector. The expression plasmid of paxillin-TagRFP was generated by inserting the mouse paxillin cDNA between XhoI and SacI sites of pRetroQ-AcGFP-N1, in which the AcGFP was replaced by TagRFP-T, and the vinculin-mCherry by inserting the mouse vinculin cDNA between NheI and EcoRI sites of the pmCherry-N1 vector.
For stable depletion of Kank2 and liprin-β1 mRNA, two shRNAs targeting 5′-A TACTGTATTCTTGAGTCA-3′ (Kank2 shRNA no 1) and 5′-AGCCAGAAAGCCAAGCTAC-3′ (Kank2 shRNA no 2) of the Kank2 3′ untranslated region and two shRNAs targeting 5′-GTGGATTGTTGGAGATGAT-3′ (liprin-β1 shRNA no 1) and 5′-GAAGCTCAAGTCAACTAAA-3′ (liprin-β1 shRNA no 2) in the liprin-β1 coding region were cloned into pSuper.Retro.puro or pSuper.Retro.hygro vector (Oligoengine) for retrovirus production. For recombinant Kank2-KN production, the cDNA encoding amino acids 29–72 was cloned between EcoRI and BamHI sites of pGEX-6P1 (GE Healthcare). To produce the THD cDNA was cloned into the pCoofy vector, and to produce FL-talin-1 the pET101-talin-FL construct was used as previously described51.
The GST-tagged Kank2-KN and GST alone were expressed in BL21 (DE3) overnight at 37 °C. Biomass was lysed in the presence of protease inhibitors (AEBSF-HCl 1 mM, aprotinin 2 µg ml−1, leupeptin 1 µg ml−1, pepstatin 1 µg ml−1) and nuclease (Benzonase) with a high-pressure homogenizer in GST binding buffer (Tris-HCl 50 mM, NaCl 150 mM, EDTA 1 mM, DTT 1 mM, pH 7.5), clarified, incubated with glutathione Sepharose 4 Fast Flow (GE Healthcare) for 3.5 h at 4 °C, washed three times with GST binding buffer and eluted with 50 mM glutathione in glutathione binding buffer. Eluted fractions were desalted (Sephadex G-25 in Hi Prep 26/10) in desalting buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1 mM DTT) and further purified with size-exclusion chromatography (Superdex 75 PC 3.2/30) in desalting buffer.
His-tagged talin-1 production was optimized in BL21 (DE3) on the basis of previously published protocols51. The expression was induced with 1 mM isopropylthiogalactoside at 18 °C overnight. After lysis with high-pressure homogenizer in lysis buffer (50 mM Tris-HCl at pH 7.8, 500 mM NaCl, 30 mM imidazole, 1 mM DTT) and clarification of the supernatant, talin-1 was purified by Ni-NTA affinity chromatography (Ni Sepharose High Performance, GE Healthcare) and anion exchange (HiTrap Q HP, GE Healthcare) in MES buffer (20 mM MES at pH 6.3, 1 mM DTT, gradient from 500 mM KCl to 100 mM KCl). The eluted fractions were concentrated (Amicon Ultra-15, molecular weight cutoff 100 kDa) and further purified by size-exclusion chromatography (Superdex 200 10/300 GL, GE Healthcare) in 50 mM Tris-HCl at pH 7.8, 150 mM KCl, 1 mM DTT. Purified fractions were stored in the presence of 50% glycerol at −80 °C.
The THD was produced in Escherichia coli Rosetta cells (Merck Millipore). After cell lysis and clarification of the supernatant, the THD was purified by Ni-NTA affinity chromatography (Qiagen), cleaved with SenP2 protease and purified by size-exclusion chromatography (Superdex 200 26/600, GE Healthcare). For the production of recombinant talin R4–R8 domain, mouse talin R4–R8 (corresponding to residues 913–1655) was amplified from pLPCXmod-Talin1-Ypet and subcloned into a vector containing a cleavable N-terminal Venus-His8-Sumo tag (gift from C. Biertümpfel, Max Planck Institute for Biochemistry). Talin R4–R8 was transformed in E. coli BL21 (DE3) and expressed with the ZY auto-induction system. Cells were lysed by sonication in 50 mM Tris-HCl at pH 7.8, 500 mM NaCl, 1 mM β-mercaptoethanol, 5 mM EDTA in the presence of protease inhibitor cocktail (Roche) and cleared by centrifugation, and the supernatant was injected into a His-affinity column (Roche), washed with 50 mM Tris-HCl at pH 7.8, 500 mM NaCl, 1 mM β-mercaptoethanol, 5 mM EDTA, 10 mM imidazole and the protein was eluted with 50 mM Tris-HCl at pH 7.8, 500 mM NaCl, 1 mM β-mercaptoethanol, 1 mM EDTA, 500 mM imidazole. Eluted products were further purified using a Superdex 20016/60 column (GE Healthcare) with 20 mM HEPES at pH 7.5, 500 mM NaCl, 1 mM EDTA and 1 mM DTT.
For expression of FL-Kank2, mouse Kank2 was expressed with a cleavable C-terminal 3C-Venus-His8 tag (gift from C. Biertümpfel, Max Planck Institute for Biochemistry) in HEK293S cells. HEK293S cells were grown to 1.5 × 106, transfected with 1 µg plasmid per millilitre of cells and grown for 2 days at 32 °C in the presence of 3.75 µM valproic acid. Cells were lysed by sonication in 50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 3 mM DTT, 1 mM EDTA 1 mM pepstatin, 1 mM AEBSF, 1 mM leupeptin. Soluble fractions were purified by affinity purification, washed (50 mM Tris-HCl at pH 7.5, 1 M NaCl, 3 mM DTT, 1 mM EDTA, 5% glycerol) and eluted by tag cleavage with 3C protease in 50 mM Tris-HCl at pH 7.5, 500 mM NaCl, 3 mM DTT, 1 mM EDTA, 5% glycerol. Proteins were purified using Superdex 200 16/60 column (GE Healthcare) in 20 mM HEPES at pH 7.5, 150 mM KCl, 3 mM DTT, 5% glycerol. All purified proteins were stored at −80 °C.
Viral transduction and transient transfection of cell lines
SV40 large T-immortalized mouse kidney-derived fibroblasts have been previously described6 and were used to analyse the Kank proteins. For the analysis of integrin α5-GFP turnover, mouse kidney fibroblasts were transduced with lentivirus expressing human integrin α5-GFP, and cells with low expression were FACS-sorted. HEK293 cells were used for GFP pulldown of different talin rod domains. Transient transfections were carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transduction of VSV-G pseudotyped retroviral vectors produced by transient transfection of HEK293T were used to generate stable cell lines as previously described52. The cell lines used here were not found in the database of commonly misidentified cell lines maintained by ICLAC and NCBI BioSample. The cell lines were tested for mycoplasma contamination and were found to be negative.
Immunoprecipitation and GST pulldown
For immunoprecipitation of GFP-tagged proteins, cells were lysed in M-PER (Thermo Scientific, no 78501) buffer and immunoprecipitated using the µMACS GFP Isolation Kit (Miltenyi Biotec) following the manufacturer’s protocol. Elutes were separated in SDS–PAGE for Western blotting or for in-gel digestion and mass spectrometry analysis.
For GST pulldown experiments, recombinant talin-1 and THD, respectively, were re-buffered in GST binding buffer (50 mM Tris-HCl at pH 7.5, 137 mM NaCl, 13 mM KCl, 0.05% Tween-20) with Zebra Desalt Spin Columns (Thermo Scientific). 200 nM GST or GST-Kank2-KN fusion proteins were incubated with 100 nM recombinant talin-1 or 300 nM THD for 30 min at 4 °C and incubated with glutathione Sepharose (GE Healthcare) for another 1.5 h at 4 °C. The resin was washed three times with the GST binding buffer and eluted in ×2 Laemmli buffer at 95 °C for 2 min. Samples were analysed by Western blot using antibodies against THD or GST.
In vitro F-actin competition assay
24 µM purified alpha skeletal muscle actin (no 8010-01, HYPERMOL) and Atto565-labelled alpha skeletal muscle actin (no 8162-1, HYPERMOL) were polymerized in PolyMix buffer (100 mM KCl, 10 mM imidazole at pH 7.4, 1 mM ATP and 2 mM MgCl2) according to the manufacturer’s instructions and stored on ice. The percentage of polymerized actin was estimated to be about 95% by F-actin sedimentation assay at 100,000g for 1 h at 25 °C.
To test the effects of F-actin on the interaction between the talin R4–R8 domain and FL-Kank2, 0.4 µM Venus-His-Sumo-tagged R4–R8 domain was incubated with 0.2 µM FL-Kank2 in PolyMix buffer for 1 h on ice before supplementing with 1 µM, 2 µM and 5 µM polymerized actin or empty buffer, and then incubated with Ni2+-NTA beads at 4 °C for 1 h. Beads were washed with PolyMix buffer at 4 °C three times, and bound proteins were analysed by Western blotting.
To visualize actin recruitment onto the talin R4–R8 domain, 5% BSA or 2 µM Venus-His-Sumo-tagged R4–R8 domain were diluted in 30 µl KMEI buffer (50 mM KCl, 10 mM imidazole at pH 7.1, 1 mM EDTA, 2 mM MgCl2) and incubated with Ni2+-NTA beads at 4 °C for 30 min. Then 10 µl buffer (20 mM HEPES at pH 7.5, 150 mM KCl, 3 mM DTT, 5% glycerol) containing 5 °M FL-Kank2, re-buffered 5 µM GST-KN motif or control buffer was added and incubated for 30 min at 4 °C. 10 µl polymerized Atto565-labelled actin was then added and incubated for 30 min at 4 °C. Before visualization under a confocal microscope, beads were briefly washed twice with ice-cold KMEI buffer and a 20 µl mixture was then injected into a flow chamber made with glass slides and cover slips spaced by one thin layer of Parafilm.
Integrin tail peptide pulldowns
Peptide pulldowns were carried out as previously described35 with the β1 wild-type cytoplasmic tail peptide (HDRREFAKFEKEKMNAKWDTGENPIYKSAVTTVVNPKYEGK-OH), β1 Y795A tail peptide (HDRREFAKFEKEKMNAKWDTGENPIYKSAVTTVVNPKAEGK-OH), β3 wild-type tail peptide (HDRKEFAKFEEERARAKWDTANNPLYKEATSTFTNITYRGT-OH), β5 wild-type tail peptide (HDRREFAKFQSERSRARYEMASNPLYRKPISTHTVDFAFNKFNKSYNGSVD-OH), β2 wild-type tail peptide (TDLREYRRFEKEKLKSQWNN-DNPLFKSATTTVMNPKFAES-OH) and a scrambled peptide (EYEFEPDKVDTGAKGTKMAKNEKKFRNYTVHNIWESRKVAP-OH).
The tail peptides were synthesized de novo with a desthiobiotin on the N-terminus, coupled to Dynabeads MyOne streptavidin C1 (10 mg ml−1, Invitrogen), incubated with cell lysates and after a mild wash with washing buffer containing 137 mM NaCl, 13 mM KCl, 50 mM Tris-HCl at pH 7.4, 0.05% Tween-20, supplemented with 1% BSA and then with M-PER buffer. Bound proteins were eluted in ×2 Laemmli buffer at 95 °C for 5 min.
For the in vitro talin activation assay, 10 nM recombinant talin-1 was diluted in the washing buffer and then incubated with 10 nM GST or GST-Kank2-KN together with β1-integrin-tail-peptide-coupled Dynabeads at 4 °C. Beads were washed three times with washing buffer and eluted with ×2 Laemmli buffer at 95 °C for 5 min. The chemically synthesized KN peptide (DPPYSVETPYGYRLDLDFLKYVDDIEKGHTLRRVAVQRRPRLGS) used for talin activation assays was N-terminally acetylated and C-terminally amide-protected. For competition assay between THD and FL-talin in the presence of KN peptide, 10 nM FL-talin-1 was activated with 50 nM chemically synthesized KN peptide and competed with 20 nM and 60 nM THD.
CHO-based αIIbβ3 integrin activation assay
The αIIbβ3 integrin activation was assessed in CHO cells stably expressing human αIIbβ3 integrin (A5 cells)53 by flow cytometry using the ligand-mimetic antibody PAC1 (BD)54. CHO cells were lipofected with 1 µg of plasmids expressing eGFP-tagged Kank2 constructs and/or 3 µg TagRFP-tagged talin-1 or GFP-tagged THD. Cells were trypsinized 24 h after transfection and incubated with the PAC1 antibody (BD) in Tyrode’s buffer (pH 7.35) for 30 min on ice, washed and stained with a secondary IgM for 30 min on ice followed by a final wash and staining with a streptavidin-Cy5 for 30 min on ice. PAC1 binding was measured with a FACS Canto, gated for living cells, using 7AAD (Thermo) exclusion as well as GFP–RFP double-positive cells. Total αIIbβ3 integrin surface levels were determined with an anti-αIIb integrin antibody (HIP8, BioLegend). Data evaluation was carried out with the FlowJo software. Surface antigen binding was expressed as geometric mean fluorescence intensity. αIIbβ3 integrin activation levels were defined as the ratio of PAC1 and HIP8 binding in cells positive for eGFP and/or RFP and were normalized with that of the cell expressing THD only.
Polyacrylamide gel micropatterning
Micropatterns were first produced on glass coverslips as previously described55. Briefly, 20 mm square glass coverslips were oxidized through oxygen plasma (FEMTO, Diener Electronic) for 10 s at 30 W before incubating with 0.1 mg ml−1 poly-l-lysine (PLL)–PEG (polyethylene glycol) (PLL20K-G35-PEG2K, JenKem) in 10 mM HEPES at pH 7.4 for 30 min. After drying, coverslips were exposed to 165 nm UV (UVO-Cleaner, Jelight) through a photomask (Toppan) for 5 min. Then, coverslips were incubated with 20 mg ml−1 of FN (FF1141, Sigma) and 20 µg ml−1 fluorescently labelled fibrinogen (Alexa Fluor 647 conjugate, no F35200, Invitrogen) in 100 mM sodium bicarbonate solution at pH 8.3 for 30 min.
The polyacrylamide solution was prepared by mixing acrylamide and bisacrylamide (Sigma) in a respective ratio of 8%/0.264%, resulting in a measured Young’s modulus of 35 kPa. The polyacrylamide solution was de-gassed for around 30 min and mixed with passivated fluorescent beads (red fluorescent FluoSpheres carboxylate-modified microspheres, 0.2 µm F8810, Thermo Fisher) by sonication before adding ammonium persulfate and tetramethylethylenediamine. 25 µl of the solution was immediately deposited on the ROI on the photomask and incubated for 21 min under a silanized coverslip. The silanized coverslip was then carefully removed in the presence of sodium bicarbonate and the gels stored overnight at 4 °C in sodium bicarbonate. Coverslips were washed in sterile PBS before seeding the cells and then mounted in a magnetic chamber (Chamlide, LCI) for TFM processing.
Traction-force microscopy and image analysis
Images of cells, beads and patterns were acquired with a confocal spinning disk system (Eclipse Ti-E Nikon inverted microscope) equipped with a CSUX1-A1 Yokogawa confocal head, an Evolve EMCCD camera (Roper Scientific, Princeton Instrument) and a Nikon CFI Plan-APO VC ×60–numerical aperture 1.4 oil immersion objective). The system was controlled by MetaMorph software (Universal Imaging).
TFM and image analysis were carried out as described. All processing was carried out using ImageJ (http://rsb.info.nih.gov/ij). Plugins and macros are available at https://sites.google.com/site/qingzongtseng/tfm. First, bead images are aligned to correct experimental drift. Displacement field was calculated using particle image velocimetry on the basis of a normalized cross-correlation algorithm following an iterative scheme. The final grid size for the displacement fields was 0.267 µm × 0.267 µm. Subsequently, the traction-force field was calculated by means of Fourier transform traction cytometry with a regularization parameter set to 2 × 10−10.
FN fibrillogenesis assay
Cells were seeded at 90% confluence onto coverslips coated with 10 µg ml−1 FN (Calbiochem) at 37 °C and cultured for 12 h in normal culture medium before paraformaldehyde (PFA) fixation and immunostaining.
Immunofluorescence microscopy
For immunostaining, cells were cultured on glass coated with 10 µg ml−1 FN (Calbiochem) for 5 h, then fixed with cold methanol:acetone (1:1) for 5 min at −20 °C (for endogenous talin, vinculin and kindlin-2 staining) followed by 15 min rehydration in PBS at room temperature, or with 2% PFA–PBS for 15 min at room temperature (for other immunostaining). PFA-fixed cells were permeabilized when indicated with 0.1% Triton X-100–PBS for 30 min at room temperature. Fixed cells were blocked with 5% BSA in PBS for 1 h at room temperature followed by incubation with the primary antibodies in 5% BSA in PBS overnight at 4 °C and then with secondary antibodies for 1 h at room temperature. Images were collected at room temperature on a Zeiss (Jena) LSM780 confocal laser scanning microscope equipped with a Zeiss Plan-APO ×63–numerical aperture 1.46 oil immersion objective.
Fluorescence recovery after photobleaching
FRAP and fluorescence live cell imaging were carried out on a Zeiss (Jena) LSM780 confocal laser scanning microscope equipped with a Zeiss Plan-APO ×63–numerical aperture 1.46 oil immersion objective with environmental control (5% CO2 and humidification). Cells were cultured on 10 µg ml−1 FN-coated glass-bottom live cell imaging chambers (ibidi). For FRAP experiments, the ROI was bleached with full laser power at 488 nm for 30 iterations and fluorescence recovery was monitored for 5 min with 20 s intervals and 1% laser power for talin-GFP turnover, or was monitored for 5 min with 10 s intervals and 1% laser power for integrin α5-GFP turnover. No significant photobleaching was observed during the post-bleaching phase. FRAP data were extracted with ImageJ. Due to the rapid diffusion of cytosolic talin into the bleached area immediately after bleaching, the mean optical intensity outside adhesion was subtracted from the mean optical intensity in FAs to correct the background. FRAP curves were fitted to a one-phase association model to calculate the mobile fraction.
Confocal microscopy live cell imaging
For confocal microscopy live cell imaging, we used the same microscopic setting as for FRAP. Cells were imaged for the indicated time with 20 s or 30 s intervals with 1–3% laser power depending on the fluorophores. To block MMP activities, cells were plated for 5 h with or without 50 µM GM6001 (no sc-203979, Santa Cruz) before live cell imaging. For data illustration, fluorescent images were background-subtracted and passed through low-pass filters followed by Unsharp Mask in ImageJ.
For the tracking of sliding adhesions, a time lapse of 20 min with 2 min intervals was extracted from the full Supplementary Videos. Raw images were background-subtracted. Single adhesions were identified by thresholding and tracked with the TrackMate plugin in ImageJ. Structures with sizes below 3 pixels (0.04 µm per pixel) were excluded. Adhesion sites with lifetimes above 6 min were tracked to measure their sliding velocities. Average adhesion sliding velocities were calculated on the basis of all analysed adhesions for each cell. For the determination of sliding velocities of central adhesions, vinculin-mCherry-positive adhesions were analysed within 20 µm distance from nuclei in the first frame. For the FAs in the cell protrusions, vinculin-mCherry-positive adhesions were analysed within 10 µm behind the protruding leading edge in the first frame.
Intensity-based immunofluorescence image analysis
For line profile analysis, the fluorescence intensity along a transverse line across elongated FAs was analysed with ImageJ. The pixel position of the intensity peak for the Kank2 puncta was defined as the position of Kank2. Pixel positions corresponding to 50% of the average plateau intensity for conventional adhesion markers were manually annotated.
Kindlin-2 was used to analyse the localization of talin-1-GFP at adhesion sites. GFP intensities outside kindlin-2-positive areas were considered cytosolic. Mean optical intensities of GFP within and outside kindlin-2-positive areas were measured in ImageJ.
For quantification of active β1 integrin levels on the ventral cell surface of adherent cells, PFA-fixed cells were stained with the rat monoclonal antibody 9EG7 without permeabilization. The cellular borders were manually inspected and defined. Total intensities corresponding to 9EG7 signals and total cell areas were then measured in ImageJ.
To calculate FA disassembly rates, paxillin-TagRFP intensities during the FA disassembly phase were measured within the ROI with a fixed position that covers the initial FA area. Data were fitted into a one-phase exponential decay function and the decay rate constants were used as FA disassembly rates.
Time-lapse phase contrast video microscopy of 2D random cell migration
Cells were sparsely seeded on a six-well plate coated with 5 µg ml−1 FN in the absence of serum for 2 h. Subsequently, cell migration was recorded with a Zeiss Axiovert 200 M equipped with a ×10–numerical aperture 0.3 objective, a motorized stage (Märzhäuser), an environment chamber (EMBL Precision Engineering) and a cooled CCD (charge-coupled device) camera (Roper Scientific) at 37 °C and 5% CO2 for 6 h with 5 min time intervals. Image acquisition and microscope control were carried out with MetaMorph software (Molecular Devices). The acquired images were analysed using the manual tracking plugin of ImageJ and the Chemotaxis and Migration Tool (ibidi).
RGD tension sensor measurements
The molecular tension sensor was adapted from the sensor previously described39, with slight modifications to the labelling chemistry. Briefly, the unnatural amino acid used to attach an ATTO647 dye was replaced with a second cysteine to attach an Alexa 647 dye for a higher labelling efficiency. This modified molecular tension sensor still presents the RGD ligand derived from FN and does not change the interactions with the cell. Perfusion chambers were attached to PEGylated coverslips as previously described39. Briefly, labelled molecular tension sensor (~100 nM) was then added to the flow cell and incubated for 30 min, followed by Pluronic F-127 (0.2% w/v) for ~1 min to prevent nonspecific cell attachment. All the above steps were preceded by a PBS wash to remove excess reagent from the previous step. The cells were then added and incubated for at least 5 h at 37 °C in DMEM high-glucose medium. Images were taken using TIRF microscopy on an inverted microscope (Nikon Ti-E) equipped with an Apo TIRF ×100–numerical aperture 1.49 oil immersion objective (Nikon). Data were acquired at 5 frames per second with either an EMCCD camera (Andor iXon) or an sCMOS camera (Hamamatsu Orca Flash).
For 2D correlation analyses, FL-Kank2-GFP and FRET images were normalized and median filtered to reduce background noise39. The channels were then manually thresholded to separate adhesions from the background signal and to create masks for each channel. The pixels from both masks were combined into a single mask, which was applied to the GFP and FRET images. We then used the resulting masked images to calculate the 2D correlation coefficient. Significance was calculated using the Wilcoxon rank sum test.
For comparison of average stress within adhesions between cell lines, the force per pixel was calculated using images of the entire cell taken on the Hamamatsu sCMOS camera. The GFP channel was normalized and processed using a moving boxcar average and thresholding. The adhesions were segmented using a watershed algorithm with a minimum adhesion size of 0.5 µm2. The average force per µm2 for adhesions was then calculated with an average sensor density on the basis of previous calibrations39. For the adhesion area ratio, each of the pixels from the segmented adhesions was converted to force and the ratio of the number of pixels above 1.25 pN versus below 1.25 pN was calculated. Significance was calculated using the Wilcoxon rank sum test.
RhoA GTPase activity
Indicated cells were serum-starved for 3 h and plated on FN for 1 h in the absence of serum. Active GTP-bound RhoA was affinity purified and detected with the Active Rho Pull-Down and Detection Kit (no 16116, Thermo Scientific) following manufacturer’s instructions.
Statistics and reproducibility
Statistical analysis was carried out in GraphPad Prism software (version 5.00, GraphPad Software). Statistical calculations to predetermine required sample size were not carried out. The data sets with data points above 10 were analysed with the D’Agostino and Pearson omnibus normality test. Data sets with normal distributions were analysed with either Student’s t tests to compare two conditions, or with one-way ANOVA Tukey tests to compare multiple conditions. Data sets that did not follow normal distribution in the normality test or those that should not be considered as normal distribution (for example, TFM measurements) were analysed with a Kruskal–Wallis test (multiple comparison) or Wilcoxon rank sum test (two-sample comparison). In the case of TFM measurements, we carried out outlier identification and data cleaning due to apparent defects in some individual measurements. For data with replicates below 10, we assumed normal distribution on the basis of the appearance of the data and analysed with Student’s two-tailed t test. Results are depicted as mean ± s.d., mean ± s.e.m. or mean ± 95% CI as indicated in figure legends. All experiments for quantitative analysis were reproduced at least three times.
Data availability
Source data for Figs 6c, 7a–d, g and Supplementary Figs 7b and 8b,c have been provided as Supplementary Table 2. All other data that support the conclusions are available from the authors on request.
Supplementary Material
Acknowledgments
We thank N. Nagaraj for technical support, and C. Grashoff, O. Rosslier, G. Giannone and R. Böttcher for discussions and reading of the manuscript. The work was supported by the National Institutes of Health R01GM112998 (to A.R.D.), Stanford Graduate Fellowship (to S.T.) and ERC, DFG and Max Planck Society (to R.F.).
Footnotes
Note: Supplementary Information is available in the online version of the paper
AUTHOR CONTRIBUTIONS
Z.S. and R.F. initiated the project, designed the experiments and wrote the paper; Z.S. performed the vast majority of the experiments; H.-Y.T., S.T., F.S., L.K., D.D. and A.A.W. performed experiments; Z.S., S.T., N.M., M.T., A.R.D. and R.F. analysed data; all authors read and approved the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Vicente-Manzanares M, Horwitz AR. Adhesion dynamics at a glance. J. Cell Sci. 2011;124:3923–3927. doi: 10.1242/jcs.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Critchley DR. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 2009;38:235–254. doi: 10.1146/annurev.biophys.050708.133744. [DOI] [PubMed] [Google Scholar]
- 7.Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- 8.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 9.Austen K, et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol. 2015;17:1597–1606. doi: 10.1038/ncb3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thievessen I, et al. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J. Cell Biol. 2013;202:163–177. doi: 10.1083/jcb.201303129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atherton P, et al. Vinculin controls talin engagement with the actomyosin machinery. Nat. Commun. 2015;6:10038. doi: 10.1038/ncomms10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goult BT, et al. Structural studies on full-length talin1 reveal a compact auto-inhibited dimer: implications for talin activation. J. Struct. Biol. 2013;184:21–32. doi: 10.1016/j.jsb.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, et al. A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion. Cell Res. 2012;22:1533–1545. doi: 10.1038/cr.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goksoy E, et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell. 2008;31:124–133. doi: 10.1016/j.molcel.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YC, et al. Structural and mechanistic insights into the recruitment of talin by RIAM in integrin signaling. Structure. 2014;22:1810–1820. doi: 10.1016/j.str.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J, et al. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Lafuente EM, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Legate KR, et al. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 2011;30:4539–4553. doi: 10.1038/emboj.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comrie WA, Babich A, Burkhardt JK. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J. Cell Biol. 2015;208:475–491. doi: 10.1083/jcb.201406121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015;17:955–963. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legate KR, Montag D, Bottcher RT, Takahashi S, Fassler R. Comparative phenotypic analysis of the two major splice isoforms of phosphatidylinositol phosphate kinase type Igamma in vivo. J. Cell Sci. 2012;125:5636–5646. doi: 10.1242/jcs.102145. [DOI] [PubMed] [Google Scholar]
- 23.Stritt S, et al. Rap1-GTP-interacting adaptor molecule (RIAM) is dispensable for platelet integrin activation and function in mice. Blood. 2015;125:219–222. doi: 10.1182/blood-2014-08-597542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klapproth S, et al. Loss of the Rap-1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice. Blood. 2015;126:2704–2712. doi: 10.1182/blood-2015-05-647453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakinuma N, Zhu Y, Wang Y, Roy BC, Kiyama R. Kank proteins: structure, functions and diseases. Cell. Mol. Life Sci. 2009;66:2651–2659. doi: 10.1007/s00018-009-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding M, Goncharov A, Jin Y, Chisholm AD. C. elegans ankyrin repeat protein VAB-19 is a component of epidermal attachment structures and is essential for epidermal morphogenesis. Development. 2003;130:5791–5801. doi: 10.1242/dev.00791. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Lee WS, Tang X, Wadsworth WG. Extracellular matrix regulates UNC-6 (netrin) axon guidance by controlling the direction of intracellular UNC-40 (DCC) outgrowth activity. PLoS ONE. 2014;9:e97258. doi: 10.1371/journal.pone.0097258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihara S, et al. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat. Cell Biol. 2011;13:641–651. doi: 10.1038/ncb2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerer I, et al. Deletion of the ANKRD15 gene at 9p24.3 causes parent-of-origin-dependent inheritance of familial cerebral palsy. Human Mol. Genet. 2005;14:3911–3920. doi: 10.1093/hmg/ddi415. [DOI] [PubMed] [Google Scholar]
- 30.Gee HY, et al. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J. Clin. Invest. 2015;125:2375–2384. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Vaart B, et al. CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Dev. Cell. 2013;27:145–160. doi: 10.1016/j.devcel.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Kakinuma N, Roy BC, Zhu Y, Wang Y, Kiyama R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J. Cell Biol. 2008;181:537–549. doi: 10.1083/jcb.200707022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO. Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller HB, et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 35.Bottcher RT, et al. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 36.Zamir E, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, et al. Integrin Molecular Tension within Motile Focal Adhesions. Biophys. J. 2015;109:2259–2267. doi: 10.1016/j.bpj.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimatsu M, Mekhdjian AH, Chang AC, Tan SJ, Dunn AR. Visualizing the interior architecture of focal adhesions with high-resolution traction maps. Nano. Lett. 2015;15:2220–2228. doi: 10.1021/nl5047335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theodosiou M, et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 2016;5:e10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gingras AR, et al. Central region of talin has a unique fold that binds vinculin and actin. J. Biol. Chem. 2010;285:29577–29587. doi: 10.1074/jbc.M109.095455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Himmel M, et al. Control of high affinity interactions in the talin C terminus: how talin domains coordinate protein dynamics in cell adhesions. J. Biol. Chem. 2009;284:13832–13842. doi: 10.1074/jbc.M900266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossier O, et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012;14:1057–1067. doi: 10.1038/ncb2588. [DOI] [PubMed] [Google Scholar]
- 44.Medves S, et al. Multiple oligomerization domains of KANK1-PDGFRβ are required for JAK2-independent hematopoietic cell proliferation and signaling via STAT5 and ERK. Haematologica. 2011;96:1406–1414. doi: 10.3324/haematol.2011.040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iskratsch T, et al. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell. 2013;27:545–559. doi: 10.1016/j.devcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stehbens SJ, et al. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 2014;16:561–573. doi: 10.1038/ncb2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grigoriev I, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Lansbergen G, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5β. Dev. Cell. 2006;11:21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 50.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 51.Ciobanasu C, Faivre B, LeClainche C. Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat. Commun. 2014;5:3095. doi: 10.1038/ncomms4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc. Natl Acad. Sci. USA. 2000;97:12227–12232. doi: 10.1073/pnas.220399597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frojmovic MM, O’ Toole TE, Plow EF, Loftus JC, Ginsberg MH. Platelet glycoprotein IIb-IIIa (α IIb β 3 integrin) confers fibrinogen- and activation-dependent aggregation on heterologous cells. Blood. 1991;78:369–376. [PubMed] [Google Scholar]
- 54.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J. Biol. Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 55.Azioune A, Carpi N, Tseng Q, Thery M, Piel M. Protein Micropatterns: a Direct Printing Protocol Using Deep UVs. Method Cell Biol. 2010;97:133–146. doi: 10.1016/S0091-679X(10)97008-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for Figs 6c, 7a–d, g and Supplementary Figs 7b and 8b,c have been provided as Supplementary Table 2. All other data that support the conclusions are available from the authors on request.