Abstract
Kidney fibrosis is a histological hallmark of chronic kidney disease and arises in large part through extracellular matrix deposition by activated fibroblasts. The signaling protein complex mTOR complex 2 (mTORC2) plays a critical role in fibroblast activation and kidney fibrosis. Protein kinase Cα (PKCα) is one of the major sub-pathways of mTORC2, but its role in fibroblast activation and kidney fibrosis remains to be determined. Here, we found that transforming growth factor β1 (TGFβ1) activates PKCα signaling in cultured NRK-49F cells in a time-dependent manner. Blocking PKCα signaling with the chemical inhibitor Go6976 or by transfection with PKCα siRNA largely reduced expression of the autophagy-associated protein lysosomal-associated membrane protein 2 (LAMP2) and also inhibited autophagosome–lysosome fusion and autophagic flux in the cells. Similarly to chloroquine, Go6976 treatment and PKCα siRNA transfection also markedly inhibited TGFβ1-induced fibroblast activation. In murine fibrotic kidneys with unilateral ureteral obstruction (UUO) nephropathy, PKCα signaling is activated in the interstitial myofibroblasts. Go6976 administration largely blocked autophagic flux in fibroblasts in the fibrotic kidneys and attenuated the UUO nephropathy. Together, our findings suggest that blocking PKCα activity may retard autophagic flux and thereby prevent fibroblast activation and kidney fibrosis.
Keywords: kidney, fibroblast, signal transduction, cell signaling, fibrosis
Introduction
Kidney fibrosis is one of the histological hallmarks of chronic kidney diseases (1, 2). Although many cell types are involved in kidney fibrosis, fibroblast is the major cell type that contributes to interstitial myofibroblast accumulation as well as excessive extracellular matrix (ECM)3 deposition (3). Deciphering the molecular mechanisms that regulate the conversion of fibroblast into ECM-producing myofibroblasts and exploring the efficient therapeutic strategy for retarding kidney fibrosis are necessary.
Autophagy is an important degradation system to maintain cellular homeostasis via the formation of autophagosomes followed by autolysosomes (4), which may be involved in regulating many cellular functions, including cell survival, differentiation, and metabolism (5, 6). Microtubule-associated protein 1 light chain 3 (LC3) is the most widely monitored autophagy-related protein that functions as a structural component in the formation of autophagosomes (7). LC3B is the best characterized form and the most widely used as an autophagic marker. The conversion of the cytosolic form of LC3B (LC3B-I) to the lipidated form (LC3B-II) indicates autophagosome formation. LC3B-binding protein SQSTM1/p62 (herein referred to as p62) regulates the formation of protein aggregates and is removed by autophagy, thus serving as an index of autophagic degradation (8, 9). Lysosomal membrane proteins (LMPs) are involved in lysosomal acidification, cytoplasmic protein import, membrane fusion, and degraded product transportation to the cytoplasm (10). The best-known and most abundant LMPs are the lysosome associated proteins 1 (LAMP-1) and 2 (LAMP-2), which are both involved in maintaining lysosomal membrane integrity and phagolysosome formation (11, 12).
Evidence is mounting that dysregulation of autophagy is implicated in the pathogenesis of various types of renal disease such as glomerulosclerosis, diabetic nephropathy, acute kidney injury, and kidney cystic disease (13). It has been well demonstrated that autophagy is induced in the obstructed renal tubule after UUO (14). Also, TGFβ1 may activate autophagy in cultured renal tubular and mesangial cells (15–17). Several studies found that activation of autophagy inhibits kidney fibrosis through promoting collagen degradation (16, 18). Baisantry et al. (19) demonstrated that specific ablation of Atg5 from the proximal tubule exacerbated ischemic acute kidney injury at earlier time points but suppressed renal fibrosis during kidney recovery or repair. Furthermore, Livingston et al. (20) found that obstructive nephropathy was ameliorated in renal proximal tubule Atg7-knockout mice, which was associated with the less production of pro-fibrotic cytokines regulated by autophagy (21). Autophagy is a highly dynamic, multistep process and is modulated at many levels. Among them, activation of mTORC1, a serine/threonine protein kinase, may suppress autophagy in many cell types (22, 23). Our previous studies found that Rictor/mTORC2 promoted kidney tubular cell survival through induction of autophagy (24). However, whether PKCα, one of the important downstream targets of mTORC2, can regulate autophagy in kidney fibroblast and its contribution to kidney fibrosis remains largely unknown.
In this study, we found that PKCα signaling activation mediates TGFβ1-induced fibroblast activation and contributes to kidney fibrosis by promoting autophagic flux. Go6976, a specific PKCα signaling inhibitor, may act as a novel therapeutic strategy for retarding kidney fibrosis.
Results
Inhibition of PKCα reduces LAMP2 expression and induces lysosomal dysfunction in NRK-49F cells
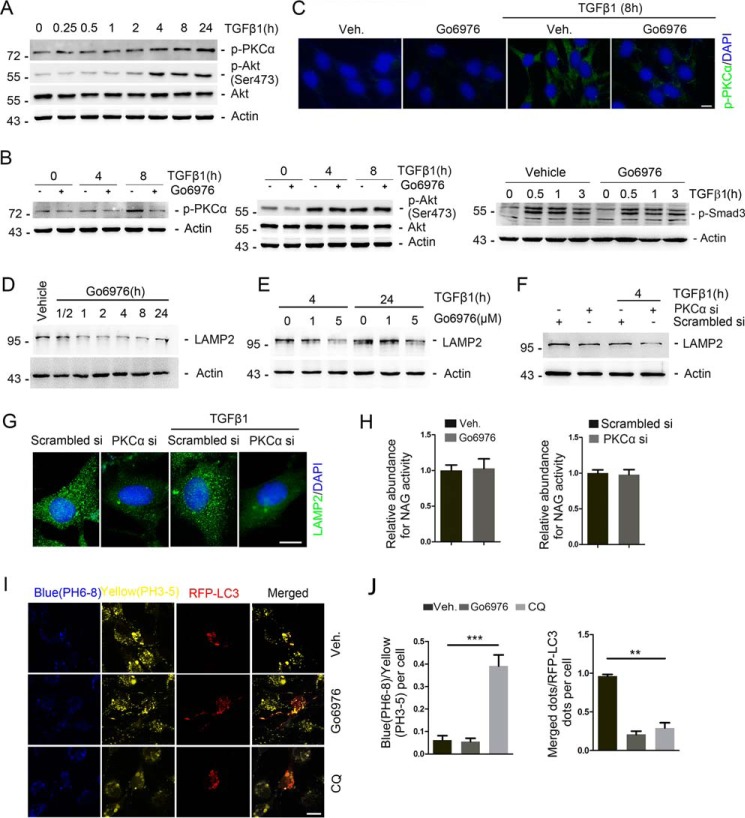
To investigate the role of PKCα signaling in fibroblast activation, NRK-49F cells, a rat kidney interstitial fibroblast cell line, were treated with TGFβ1 (2 ng/ml) for different time points as indicated. As shown in Fig. 1A, the amount of p-PKCα and p-Akt (Ser-473) was increased at 4 h and maintained at a high level within 24 h after TGFβ1 treatment. NRK-49F cells were pretreated with the PKCα inhibitor Go6976 for 30 min, followed by TGFβ1 incubation, and harvested at 4 h and 8 h later. Western blotting analyses revealed that the abundance of p-PKCα but not p-Akt (Ser-473) was diminished after Go6976 treatment (Fig. 1B). Immunofluorescent staining further confirmed Western blotting results (Fig. 1C). In addition, Smad3 phosphorylation was not markedly decreased in NRK-49F cells pretreated with Go6976 compared with TGFβ1 treatment alone (Fig. 1B).
Figure 1.
Inhibition of PKCα reduces LAMP2 expression and induces lysosomal dysfunction in NRK-49F cells. NRK-49F cells were treated with TGFβ1 (2 ng/ml) with or without the PKCα inhibitor Go6976 for different times as indicated. A, Western blotting analyses show the induction of PKCα and Akt phosphorylation after TGFβ1 treatment in a time-dependent manner. Cell lysates were immunoblotted with Abs against p-Akt (Ser-473), p-PKCα, and actin, respectively. B, Western blotting analyses revealing the remarkable reduction of PKCα but not Akt or Smad3 phosphorylation after TGFβ1 and Go6976 treatment compared with TGFβ1 treatment alone. Cell lysates were immunoblotted with Abs against p-PKCα, p-Akt (Ser-473), Akt, p-Smad3, and actin, respectively. C, representative micrographs show the immunostaining for p-PKCα at 8 h after TGFβ1 (2 ng/ml) without Go6976 (5 μm) treatment. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm. D, NRK-49F cells were treated with Go6976 for different time as indicated. Western blotting assay shows the down-regulation of LAMP2 expression after Go6976 treatment in a time-dependent manner. E, Western blotting analyses reveal the LAMP2 protein expression after TGFβ1 treatment and PKCα inhibitor Go6976 treated for different times or dosages as indicated. F, Western blotting analyses showing that knocking down PKCα reduced LAMP2 protein expression in NRK-49F cells. Cell lysates were immunoblotted with Abs against LAMP2 and actin, respectively. G, representative images showing the immunostaining for LAMP2 after various treatments as indicated. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm. H, relative lysosomal NAG activity of Go6976-treated and PKCα siRNA-transfected NRK-49F cells. n = 4 independent experiments. I, representative confocal micrographs of lysosomes detected with the pH indicator LysoSensor yellow/blue after transfected RFP-LC3 plasmid for 24 h and then treated with vehicle, Go6976(5 μm), or CQ (50 μm) for 4 h; scale bar, 5 μm. J, blue/yellow ratio and merged dots/RFP-LC3 ratio are presented. **, p < 0.01; ***, p < 0.001 compared with vehicle (Veh)-treated cells, n = 5.
PKCα was reported to play a key role for lysosome biogenesis (25). We then examined the intracellular intensity of lysosomes in cultured NRK-49F cells treated with Go6976 (5 μm) for a different time duration as indicated. As shown in Fig. 1D, LAMP2 expression was continuously down-regulated within 24 h after Go6976 treatment. We pretreated cells with Go6976 at different dosages as indicated for 30 min before TGFβ1 treatment. The Western blotting analyses revealed that LAMP2 abundance was significantly decreased after Go6976 treatment (Fig. 1E). To further investigate the role of PKCα in regulating lysosomal function, NRK-49F cells transfected with scrambler or PKCα siRNA were treated with TGFβ1 for 4 h. PKCα siRNA transfection could markedly down-regulate PKCα mRNA expression, whereas the other isoforms of PKC remained unchanged. As shown in Fig. 1F, PKCα siRNA transfection markedly down-regulates LAMP2 protein expression. Immunofluorescent staining further confirmed Western blotting results (Fig. 1G). The lysosomal protease activity, measured by β-N-acetylglucosaminidase (NAG) assays, was not affected by Go6976 or PKCα siRNA transfection in NRK-49F cells (Fig. 1H). Furthermore, we utilized the LysoSensor Yellow/Blue reagent, a pH-sensitive probe that emits predominantly yellow fluorescence in acidic organelles and blue fluorescence in less acidic organelles. NRK-49F cells were pre-transfected with RFP-LC3 expression plasmid and then incubated with LysoSensor (Fig. 1I). The blue/yellow ratio was not changed in the acidic vesicular organelles after Go6976 treatment, whereas chloroquine (CQ) administration significantly increased the blue/yellow ratio, reflecting an increase in pH values. The co-localization of the yellow/blue/autophagosome was inhibited in NRK-49F cells after CQ and Go6976 treatment (Fig. 1J).
Inhibition of PKCα retards autophagic flux in NRK-49F cells
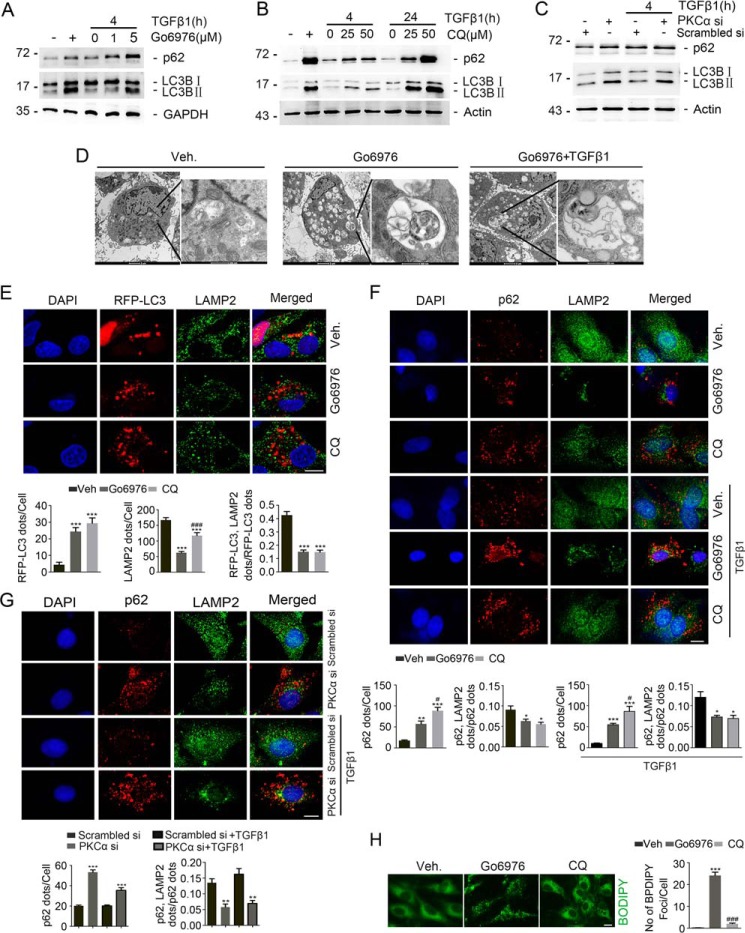
Inspired by the above observations, we postulated that Go6976 may affect autophagosome–lysosome fusion and/or autophagy activity in NRK-49F cells. As shown in Fig. 2A, Western blotting analyses showed that Go6976 induced the accumulation of both MAP1LC3B-I (LC3B-I) and MAP1LC3B-II (LC3B-II) in a dose-dependent manner. Interestingly, SQSTM1/p62 protein abundance was also increased after Go6976 treatment in a dose-dependent manner at 4 h after TGFβ1 treatment, indicating the retardation of autophagic flux in NRK-49F cells. We then treated NRK-49F cells with CQ, and similar results were observed (Fig. 2B). To further explore the role of PKCα signaling in regulating autophagic flux, NRK-49F cells were transfected with scramble and PKCα siRNA. Western blotting analyses revealed that PKCα siRNA transfection significantly increased LC3B-II and SQSTM1/p62 abundance in NRK-49F cells (Fig. 2C). Electronic microscopic examination revealed an increased organized cytoplasmic structure typical of lysosomes and autophagosomes and/or autolysosomes and massive vacuolization containing undigested proteins and organelles in NRK-49F cells after Go6976 with or without TGFβ1 treatment (Fig. 2D).
Figure 2.
Inhibition of PKCα retards autophagic flux in NRK-49F cells. A and B, Western blotting assay showing the accumulation of LC3-I, LC3-II and SQSTM1/p62 protein after Go6976 or CQ treatment without TGFβ1 incubation. C, Western blotting assay revealing the accumulation of LC3-I, LC3-II, and SQSTM1/p62 after the transfection of PKCα siRNA without TGFβ1 treatment. D, representative transmission electron microscopic images of NRK-49F cells showing the accumulation of autophagosome in cells treated with Go6976 without TGFβ1 treatment. Cells were pretreated with vehicle or Go6976 (5 μm) for 30 min and then incubated without TGFβ1 for 4 h. E, representative images showing autophagosomes and lysosomes in NRK-49F cells. Yellow dots in merged images represent autolysosomes. Cells were transfected with RFP-LC3 for 24 h and then incubated with vehicle, Go6976 (5 μm), or CQ (50 μm) and TGFβ1 (2 ng/ml) for 4 h. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm. Quantification of RFP-LC3 (red) puncta, LAMP2 (green) puncta, and merged dots/RFP-LC3 ratio (lower part) is shown. ***, p < 0.001 compared with vehicle-treated cells (n = 5–7); ###, p < 0.01 compared with Go6976-treated cells. F, representative images for SQSTM1/p62 and LAMP2 among different groups as indicated. Yellow dots in merged images represent colocalization of SQSTM1/p62 and LAMP2. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm. Quantitative analysis for SQSTM1/p62 puncta/cell and merged dots/p62 in NRK-49F cells after various treatments is as indicated (lower part). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with vehicle (Veh)-treated cells (n = 5–7); #, p < 0.05 compared with cells treated with Go6976. G, representative images for p62 and LAMP2 among different groups as indicated. Yellow dots in merged images represent colocalization of p62 and LAMP2. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm. Quantitative analysis for p62 puncta/cell and merged dots/p62 in NRK-49F cells after various treatments as indicated (lower part). **, p < 0.01; ***, p < 0.001 compared with vehicle-treated cells (n = 5–7). H, lipid droplet clearance assays were performed with vehicle, Go6976, or CQ. Representative images of BODIPY staining of lipid droplets in NRK-49F cells at 4 h post-treatment with vehicle, Go6976, or CQ; scale bar, 5 μm. Quantification of BODIPY staining. n = 3 independent experiments. ***, p < 0.001 compared with vehicle-treated cells (n = 5–7); ###, p < 0.01 compared with Go6976-treated cells.
We also transfected NRK-49F cells with RFP-LC3 expression plasmid and then treated with Go6976 or CQ. As shown in Fig. 2E, Go6976 and CQ treatment could similarly increase RFP-LC3–positive and decrease LAMP2-positive dot numbers in NRK-49F cells. Interestingly, RFP-positive puncta were rarely co-localized with LAMP2 in NRK-49F cells treated with Go6976 or chloroquine. The accumulation of p62 represents the amount of substrate requiring degradation that accumulated in chloroquine and Go6976 administration cells. CQ and Go6976 treatment decreased the p62/LAMP2 merged dots/p62 puncta ratio in NRK-49F cells with or without TGFβ1 treatment (Fig. 2F). Furthermore, as shown in Fig. 2G, the number of p62 positive dots was increased, whereas the number of p62/LAMP2 merged dots/p62 dots ratio was decreased in cells transfected with PKCα siRNA compared with those transfected with scrambler siRNA with or without TGFβ1 treatment. In NRK-49F cells, Go6976 but not CQ administration obviously inhibited the clearance of lipid droplets (Fig. 2H). Together, these results demonstrate that PKCα signaling is indispensable for maintaining autophagic flux in NRK-49F cells.
Blockade of PKCα signaling diminishes TGFβ1-induced fibroblast activation
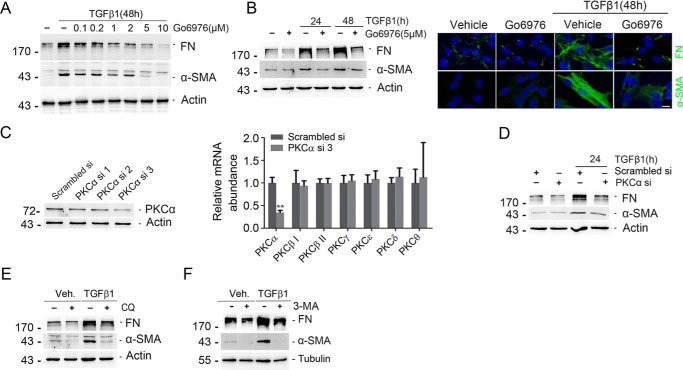
We then treated NRK-49F cells with the PKCα inhibitor Go6976, followed 30 min later by TGFβ1 treatment for 24 and 48 h. Go6976 could remarkably inhibit TGFβ1-induced fibronectin (FN) and α-smooth muscle actin (α-SMA) expression in a time- and dose-dependent manner (Fig. 3, A and B). Immunofluorescent staining further confirmed Western blotting results (Fig. 3B, right). NRK-49F cells were also transfected with PKCα siRNAs as indicated. PKCα siRNA transfection could markedly down-regulate PKCα protein and mRNA expression (Fig. 3C). Down-regulation of PKCα could markedly reduce TGFβ1-induced FN and α-SMA expression (Fig. 3D). To further clarify the role for autophagic flux retardation in fibroblast activation, we treated NRK-49F cells with CQ and 3-MA, and we found that CQ and 3-MA could remarkably inhibit TGFβ1-induced FN and α-SMA expression (Fig. 3, E and F). Thus, it is clear that PKCα signaling mediates TGFβ1-induced fibroblast activation through autophagy induction.
Figure 3.
Inhibition of PKCα diminishes TGFβ1-induced fibroblast activation. A and B, Western blotting analyses showing that blocking PKCα could inhibit TGFβ1-induced FN and α-SMA) expression in a dose-dependent (A) and time-dependent (B, left) manner. Cell lysates were immunoblotted with Abs against FN, α-SMA, and actin, respectively. Representative micrographs showing the immunostaining for FN and α-SMA after various treatments are as indicated. NRK-49F cells were treated Go6976 and incubated with TGFβ1 for 48 h. Cells were co-stained with DAPI to visualize the nuclei; scale bar, 5 μm (B, right). C, NRK-49F cells were transfected with scrambled or PKCα siRNA for 24 h, followed by TGFβ1 treatment for 24 h. Western blotting analyses revealed the reduction of PKCα protein in NRK-49F cells at 24 h after PKCα siRNA transfection compared with scrambled siRNA transfection (left). Real time PCR analysis showing the reduction of PKCα mRNA abundance after PKCα siRNA-3 transfection. **, p < 0.01 compared with Scrambled siRNA transfection (n = 4). D, Western blotting analyses showed that knocking down PKCα blocked TGFβ1-induced FN and α-SMA expression. E, Western blot analysis revealed that CQ inhibited TGFβ1-induced FN and α-SMA expression. F, Western blot analysis revealed that 3-MA (10 mm) could inhibit TGFβ1-induced FN and α-SMA expression. Veh, vehicle.
Blockade of PKCα inhibits autophagic flux in the interstitial myofibroblasts from UUO kidneys
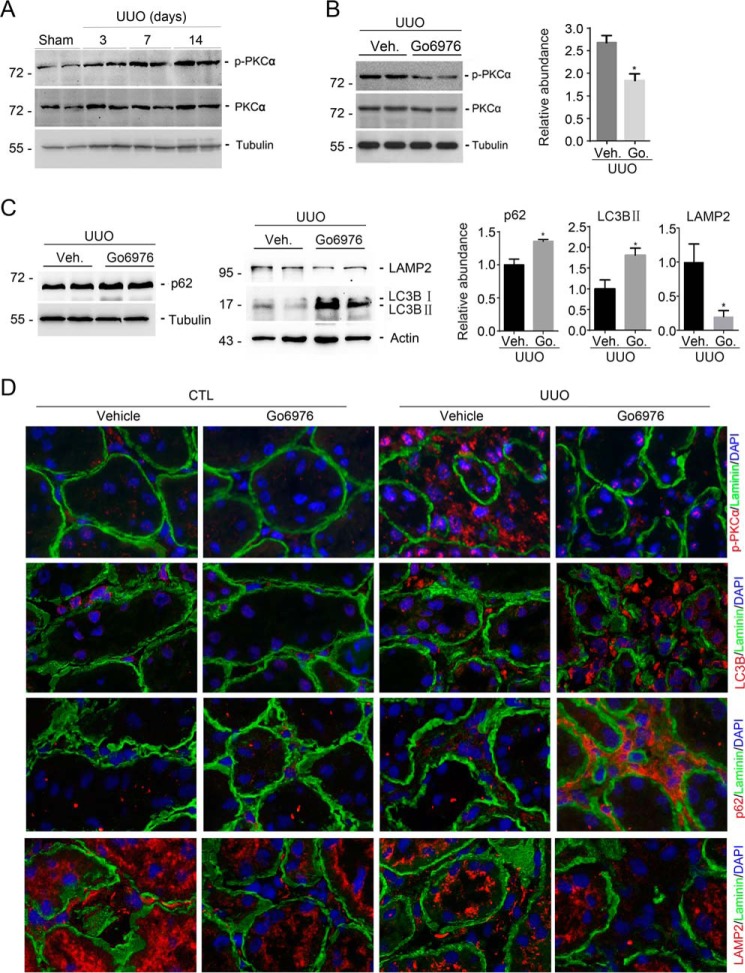
Western blotting assays revealed that phosphorylated protein kinase Cα (p-PKCα) was increased at day 3 and reached peak at days 7 and 14 after UUO (Fig. 4A). Go6976 markedly decreased the abundance for p-PKCα at day 7 after UUO (Fig. 4B). Immunofluorescent staining confirmed the results of Western blotting assay (Fig. 4D).
Figure 4.
Go6976 attenuates PKCα signaling activation and impairs interstitial myofibroblast autophagy in the UUO kidneys. A, Western blotting analyses showing the induction of phosphorylated protein kinase Cα (p-PKCα) in the kidneys with UUO nephropathy compared with sham control. B, Western blotting analyses showing the abundance of p-PKCα in the kidney lysates from mice after Go6976 (Go.) treatment. Graphic presentation shows the results of semi-quantitative analysis for p-PKCα protein abundance among groups. *, p < 0.05 compared with vehicle (Veh)-treated UUO kidneys (n = 5). C, Western blotting analyses showing the abundance of SQSTM1/p62, LC3, and LAMP2 in the kidneys at day 7 after UUO from mice after Go6976 treatment. Graphic presentation showing the semi-quantitative analysis for p62, LC3-II, and LampP2 protein abundance between groups as indicated. *, p < 0.05 compared with vehicle-treated UUO kidneys (n = 5). D, representative micrographs showing immunostaining results for p-PKCα and laminin, LC3, and laminin, SQSTM1/p62 and laminin, and LAMP2 and laminin in the kidneys at day 7 after UUO. Slides were counterstained with DAPI to visualize cell nuclei.
In NRK-49F cells, we found that PKCα signaling activation is required for fibroblast activation through facilitating autophagic flux. We then investigated autophagic flux in kidneys with UUO nephropathy. On day 7 after UUO, Go6976 treatment increased the abundance of SQSTM1/p62 and LC3B-II, and it decreased LAMP2 abundance compared with the abundance from mice treated with vehicle (Fig. 4C). Immunofluorescent co-staining of anti-laminin and anti-SQSTM1/p62, or anti-laminin and anti-LC3B-II, or anti-laminin and anti-LAMP2, respectively, showed that the interstitial cells from UUO kidneys treated with Go6976 expressed more p62 and LC3B-II and less LAMP2 than from mice treated with vehicle (Fig. 4D). These results suggest blocking PKCα signaling inhibits autophagic flux and lysosome function in the interstitial myofibroblasts from kidneys with UUO nephropathy, which confirmed the results from NRK-49F cells.
Blockade of PKCα signaling ameliorates UUO nephropathy in mice
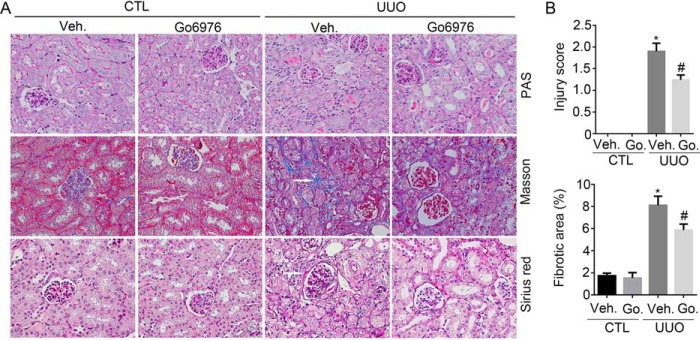
To further explore the role of PKCα signaling in kidney fibrosis in vivo, we created a mouse model with kidney fibrosis by UUO in male CD1 mice, and Go6976, a specific PKCα signaling inhibitor, was administered intraperitoneally once daily at 2 mg/kg/day from 2 days before the operation. The mice were sacrificed, and the kidneys were harvested at 1 and 2 weeks after UUO surgery. Periodic Acid-Schiff (PAS), Sirius red, and Masson staining showed that renal fibrotic lesions were significantly ameliorated, and interstitial matrix production was decreased in the Go6976-treated group at day 7 after UUO, compared with the vehicle-treated group (Fig. 5A). Kidney injury and fibrotic area were also assessed. Vehicle-treated UUO group exhibited a dramatic induction of kidney injury in the kidneys at 7 days after surgery, whereas Go6976 significantly ameliorated kidney injury. In addition, kidney fibrotic area in Go6976-treated group was also decreased compared with UUO control (Fig. 5B).
Figure 5.
Blocking PKCα signaling diminishes kidney fibrosis after UUO. A, representative micrographs for PAS, Masson, and Sirius red staining in the kidney from different groups as indicated. White arrows indicate the fibrotic area. B, graphic presentation showing the kidney injury score and the percentage of fibrotic area in the kidney from different groups as indicated. *, p < 0.05 compared with vehicle (Veh.)-treated contralateral kidneys (n = 3–5); #, p < 0.05 compared with vehicle-treated UUO kidneys (n = 5). Go., Go6976.
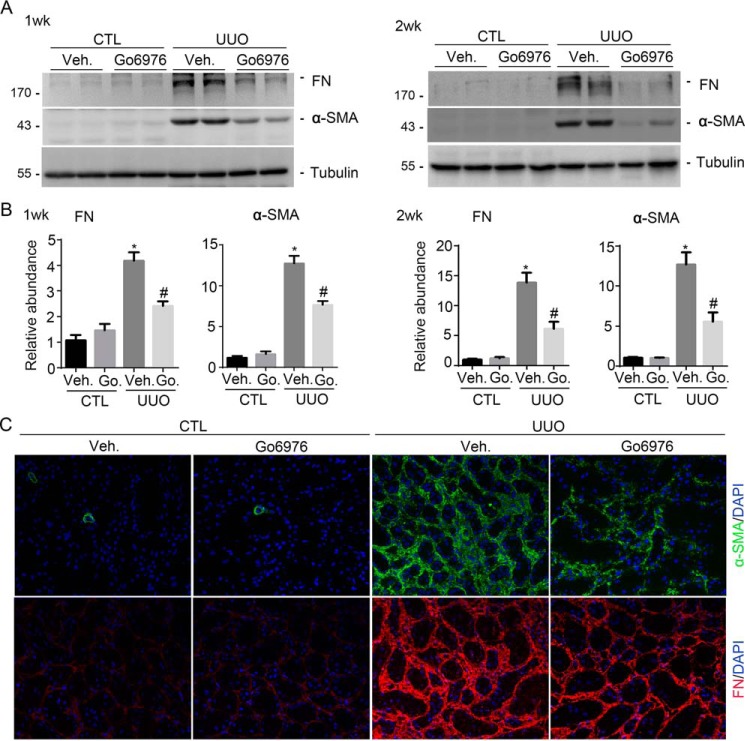
We then detected α-SMA and FN expression by Western blotting analyses. FN and α-SMA protein abundance were largely induced on days 7 and 14 in mice after UUO. Go6976 could markedly down-regulate their expression in the UUO kidneys (Fig. 6, A and B). Immunofluorescent staining confirmed the results of Western blotting analyses (Fig. 6C).
Figure 6.
Blocking PKCα signaling reduces FN and α-SMA expression in the UUO kidneys. A, Western blotting analyses showing the abundance of FN and α-SMA in the kidneys at 1 and 2 weeks after UUO without Go6976 (Go.) treatment. B, graphic presentation showing FN and α-SMA protein abundance from A in the different groups as indicated. *, p < 0.05 compared with contralateral kidneys (n = 3–5); #, p < 0.05 compared with UUO kidneys (n = 5). C, representative micrographs showing immunostaining for FN and α-SMA expression in various groups as indicated. Veh, vehicle.
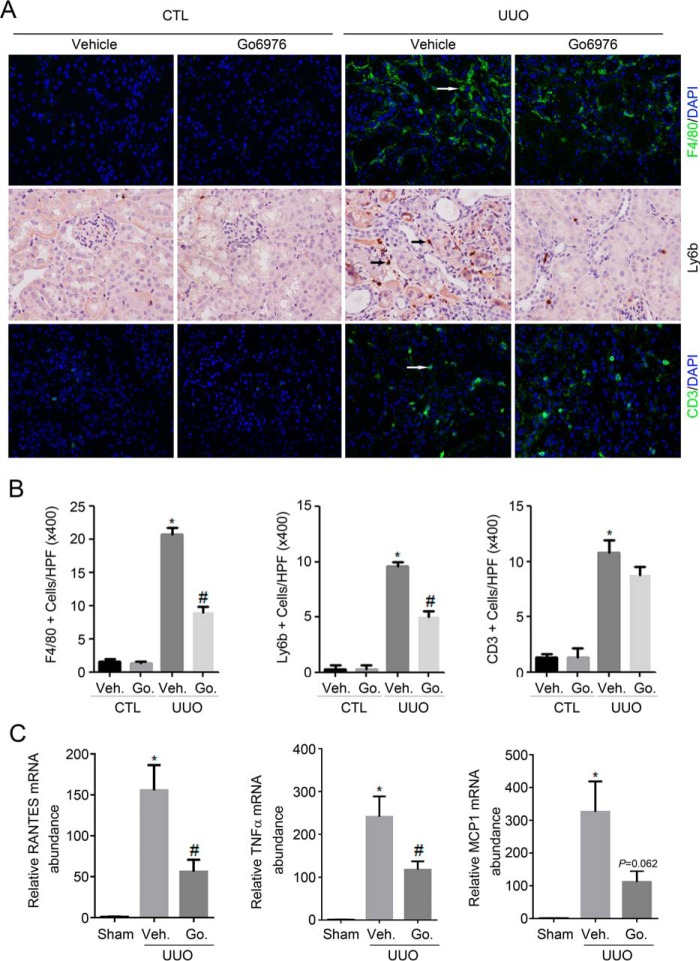
Previous studies revealed that inflammation contributes to the progression of kidney fibrosis. We also examined inflammatory cell accumulation and cytokine expression in kidney tissues. Macrophage, neutrophils, and T lymphocytes are identified by immunostaining of F4/80, ly6b, and CD3, respectively. A few F4/80-positive cells were detected in the sham kidneys from vehicle-treated group. In UUO kidneys, F4/80-positive cell number was largely increased compared with sham kidneys. Blocking PKCα signaling activation by Go6976 diminished the accumulation of F4/80-positive inflammatory cells in the UUO kidneys (Fig. 7, A and B). The ly6b-positive cell accumulation was also diminished in the Go6976-treated group compared with the vehicle-treated group, although the CD3-positive cell accumulation was not altered. We then determined the mRNA abundance of several proinflammatory cytokines, including TNFα, monocyte chemotactic protein-1 (MCP1), and RANTES (regulation on activation normal T cell expressed and secreted) in kidney tissues by real-time PCR assay. As shown in Fig. 7C, on day 14 after UUO, TNFα, MCP1, and RANTES mRNA abundances were markedly up-regulated. Administration of Go6976 could significantly down-regulate RANTES, TNFα, and MCP1 mRNA expression.
Figure 7.
Blocking PKCα signaling diminishes inflammatory response in the UUO kidneys. A, representative micrographs showing the immunostaining for F4/80, ly6b, and CD3 among different groups as indicated. Kidney sections were counterstained with DAPI to visualize the cell nuclei. Arrows indicate the inflammatory cells. B, graphic presentation showing quantitative analysis for F4/80, ly6b, and CD3-positive cells in the kidney tissues from various groups. *, p < 0.05 compared with sham kidneys (n = 3–5); #, p < 0.05 compared with UUO kidneys with vehicle (Veh) treatment (n = 5). C, graphic presentation showing the real-time PCR analysis results for RANTES, TNFα, and MCP1 in the kidney tissues among groups. *, p < 0.05 compared with sham kidneys (n = 3–5); #, p < 0.05 compared with UUO kidneys with vehicle treatment (n = 5). Go., Go6976.
Discussion
In cultured NRK-49F cells, TGFβ1 treatment could induce both mTORC1 and mTORC2 signaling activation at a similar pattern, and blocking either one of them could partially interfere with TGFβ1-induced fibroblast activation (26). Our previous studies found that mTORC2/Akt signaling is involved in TGFβ1-induced fibroblast activation and kidney fibrosis (27). In addition to Akt, we demonstrated here that mTORC2/PKCα signaling activation may also contribute to TGFβ1-induced fibroblast activation and kidney fibrosis by promoting autophagic flux.
A growing body of evidence showed that autophagy induction may be an adaptive response against various stress stimuli and linked with the fibrotic diseases. Dysregulated autophagy has been implicated in disorders characterized by fibrosis in various tissues, including idiopathic pulmonary fibrosis, liver fibrosis, cardiac fibrosis, and renal fibrosis (28–30). Autophagy may either promote kidney fibrosis via the induction of tubular atrophy and decomposition or attenuate kidney fibrosis via the intracellular degrading of excessive collagen. In the UUO rat model, autophagy was induced in a time-dependent manner, and inhibition of autophagy by 3-MA enhanced tubular cell apoptosis and tubule-interstitial fibrosis in the obstructed kidney (14). Collagen deposition was increased in the kidneys of mice deficient in the autophagic protein beclin1 as compared with littermate mice. Genetic disruption of beclin1 led to increased type I collagen abundance in primary mouse mesangial cells (MMC) (16). Ding et al. (18) found that deletion of LC3B (LC3−/− mice) resulted in increased collagen deposition and beclin1 heterozygous (beclin1+/−) mice also displayed increased collagen deposition in the obstructed kidneys after UUO. However, other researchers found that autophagy induction may promote fibrogenesis. Livingston et al. (20) reported that persistent activation of autophagy in kidney proximal tubules promotes UUO nephropathy in mice. Baisantry et al. (19) found that lacking autophagy in proximal tubular S3 segments attenuates early survival mechanisms but prevents subsequent maladaptive repair and the development of chronic kidney diseases in mice. In addition, Hernandez-Gea et al. (29) found that animals with HSC-specific deletion of Atg7 display attenuated activation following liver injury, leading to reduced fibrosis in vivo. Consistently, in this study we found that the inhibition of autophagy with chloroquine could decrease NRK-49F cell activation after TGFβ1 treatment, suggesting a profibrotic role for autophagy induction in kidney fibroblasts.
The kinase mTOR is a critical regulator of autophagy. There is a tight, inverse coupling of autophagy induction and mTORC1 activation (31). Autophagy induction by genetic or pharmacologic inhibition of mTORC1 (TORC1 in yeast) was first demonstrated in yeast (32). In mammalian cells, mTORC1 and AMP-activated protein kinase regulate by inhibiting activation of UKL1, an autophagy-initiating UNC-5–like autophagy-activating kinase (ULK) complex (22). In addition, another layer of ULK1 regulation by mTORC1 has been suggested in which mTORC1 inhibits ULK1 stability by inhibitory phosphorylation of autophagy/beclin1 regulator 1 (AMBRA1) (33). Furthermore, mTORC1 also regulates autophagy at the transcriptional level by modulating localization of transcription factor EB (TFEB), a master transcriptional regulator of lysosomal and autophagy genes (34). However, how mTORC2 regulates autophagy is not fully understood. MTORC2 phosphorylates AKT (Ser-473), which can lead to the activation of the AKT/mTORC1 signaling axis (35). Therefore, mTORC2 may indirectly suppress autophagy by activating mTORC1. Our previous study found that Rictor/mTORC2 has a critical role in autophagy induction in kidney tubular cells (22).
Recent studies reported conflicting regulation of autophagy by protein kinase C (PKC) signaling. It has been shown that activation of PKC suppressed starvation- or rapamycin-induced autophagy, whereas PKC inhibitors dramatically induced autophagy (36). Deleting the Prkcα gene, which encodes PKCα, reverses diabetes-induced autophagy impairment, and PKCα increases the expression of miR-129-2, which is a negative regulator of autophagy and mediates the inhibitory effect of maternal diabetes on autophagy (37). Other researchers found that PKCθ activation induced the Epstein-Barr virus lytic cycle through the activation of p38 MAPK and autophagy induction, which resulted in a prosurvival effect, as indicated by p38 or ATG5 knockdown experiments (38). Tan et al. (39) found that inhibition of classical PKC isoforms (PKCα) was able to effectively suppress palmitic acid-induced autophagy, which found to be independent of mTOR regulation. In agreement with the positive regulation of PKCα on autophagy, our results showed that the PKCα-signaling pathway may promote autophagy flux in kidney fibroblast.
Lysosomes are degradation and signaling centers that coordinate cellular metabolism with clearance (34, 40). Activated PKCα and PKCδ cause inactivation of GSK3β, which in turn causes TFEB nuclear translocation and then promotes lysosome biogenesis (25). In agreement with the positive effect of PKCα on lysosome, our findings in cultured cells demonstrated that blocking PKCα activity decreased the abundance of LAMP-2, a principal lysosomal membrane protein. Lysosomal dysfunction leads to lower autophagic clearance, resulting in autophagosome accumulation (41). In this study, the impaired autophagosome–lysosome fusion after PKCα inhibition may due to the lysosomal dysfunction. It was previously shown that mutation of LAMP-2 causes X-linked vacuolar cardiomyopathy and myopathy (also called Danon disease) (42). In our study, transmission electron microscopy showed that lysosomal accumulation was observed after PKCα inhibitor treatment. However, how PKCα regulates LAMP2 expression remains to be further determined.
In conclusion, this study demonstrated that PKCα signaling contributes to TGFβ1-induced fibroblast activation and development of kidney fibrosis by promoting autophagy. Targeting this signaling pathway may inhibit kidney fibrosis in patients with chronic kidney diseases.
Experimental procedures
Mice
Male CD1 mice aged 6–8 weeks and weighing ∼18–20 g were acquired from the Specific Pathogen-Free Laboratory Animal Center of Nanjing Medical University and were maintained according to the guidelines of the Institutional Animal Care and Use Committee at Nanjing Medical University. UUO was performed as reported previously. The animals were divided into three groups: 1) sham control; 2) UUO mice treated with vehicle (5% DMSO); and 3) UUO treated with Go6976. Mice were treated with Go6976 (2 mg/kg·day) from 2 days before UUO surgery to 1 or 2 weeks after surgery. Go6976 (catalog no. S7119, Selleck, Boston) was prepared in a 5% DMSO solution and injected into mice intraperitoneally. The mice were euthanized on day 7 or 14 after UUO. The UUO as well as the contralateral kidneys were removed. One portion of the kidney was fixed in 10% phosphate-buffered formalin followed by paraffin embedding for histological and immunohistochemical staining. Another portion was immediately frozen in Tissue-Tek optimum cutting temperature compound (Sakura Finetek, Torrance, CA) for cryosection. The remaining kidney tissue was snap-frozen in liquid nitrogen and stored at −80 °C for extraction of RNA and protein. All experiments were performed in accordance with the approved guidelines and regulations of the Animal Experimentation Ethics Committee at Nanjing Medical University.
Cell culture
NRK-49F cells were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco's modified Eagle's medium/F-12 medium supplemented with 10% fetal bovine serum (Invitrogen). The cells were seeded on 6-well culture plates to 60–70% confluence in complete medium containing 10% fetal bovine serum for 16 h and then changed to serum-free medium after washing twice with serum-free medium. Recombinant human TGFβ1 (catalog no. 100-B-010-CF, R&D Systems, Minneapolis, MN) was added to the serum-free medium for various periods of time. Go6976 (catalog no. ab141413, Abcam) or CQ (catalog no. C6628, Sigma) dissolved in DMSO or 3-MA (catalog no. s2767, Selleck) dissolved in double-distilled H2O was added at 30 min before TGFβ1 stimulation. PKCα siRNA (GenePharma, Shanghai, China) and RFP-LC3 plasmid DNA was transfected into NRK-49F cells using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instruction.
Histology and immunohistochemistry
Mouse kidney samples were fixed in 10% neutral formalin and embedded in paraffin. Three-μm thickness sections were used for PAS, Masson, and Sirius red staining. Kidney tubular injury was scored by percentage of morphological changes in the tubule, such as dilation, distortion of tubular basement membranes, and atrophy as follows: 0 = normal; 1 = <10%; 2 = 10–25%; 3 = 26–50%; 4 = 51–75%, and 5 = >75%. Ten random fields per section within the cortical area were selected for counting. For immunohistochemical staining, paraffin-embedded kidney sections were processed as per routine protocol. Sections were blocked with 10% normal donkey serum, followed by incubation with anti-Ly-6b (catalog no. MCA771G, AbD Serotec, Raleigh, NC) overnight at 4 °C. After incubation with secondary antibody for 1 h at room temperature, sections were incubated with ABC reagents for 1 h at room temperature before subjected to 3,3′-diaminobenzidine staining (Vector Laboratories, Burlingame, CA). Slides were viewed with a Nikon Eclipse 80i microscope equipped with a digital camera (DS-Ri1, Nikon, Shanghai, China).
Immunofluorescent staining
Three-μm thickness kidney cryosections were fixed for 15 min in 4% paraformaldehyde, followed by permeabilization with 0.2% Triton X-100 in 1× phosphate-buffered saline (PBS) for 5 min at room temperature. After blocking with 2% donkey serum for 60 min, the slides were immunostained with the following: anti-FN (catalog no. F3648, Sigma); anti-α-SMA (catalog no. A5228, Sigma); anti-p-PKCα (Thr-638/641) (catalog no. 9375, Cell Signaling Technology); anti-LAMP2 (catalog no. L0668, Sigma); anti-SQSTM1/p62 (catalog no. ab56416, Abcam); anti-LC3B (catalog no. L7543, Sigma); and anti-laminin (catalog no. ab44941, Abcam). Cells cultured on coverslips were washed twice with cold 1× PBS and fixed with cold methanol/acetone (1:1) for 10 min at −20 °C. After three times of extensive washing with 1× PBS, the cells were treated with 0.1% Triton X-100 for 5 min, blocked with 2% normal donkey serum in 1× PBS buffer for 40 min at room temperature, and incubated with antibodies against p-PKCα, FN, α-SMA, LAMP2, SQSTM1/p62, or LC3 followed by staining with FITC or tetramethylrhodamine-conjugated secondary antibody. Cells were also stained with 4′,6-diamidino-2-phenylindole to visualize the nuclei. Slides were viewed with a Nikon Eclipse 80i epi-fluorescence microscope equipped with a digital camera or Zeiss LSM710 (Zeiss).
Assessment of kidney fibrosis
Briefly, kidney sections (3 μm thickness) were stained with a Masson Trichrome kit (catalog no. HT15–1KT; Sigma) according to the manufacturer's instruction. Images were taken from 10 fields of one section under high magnification (×400) with a combination of cortex and medulla (including cortex and medulla with six and four fields selected, respectively). The percentage of interstitial fibrotic area relative to the selected field was analyzed with Image Pro Plus 6.0 software. An average percentage of kidney fibrotic area for each section was calculated.
Western blot analysis
Cultural NRK-49F cells were lysed in 1× SDS sample buffer. The kidneys were lysed with radioimmunoprecipitation assay solution containing 1% Nonidet P-40, 0.1% SDS, 100 mg/ml phenylmethanesulfonyl fluoride, 1% protease inhibitor mixture, and 1% phosphatase I and II inhibitor mixture (Sigma) on ice. The supernatants were collected after centrifugation at 13,000 × g at 4 °C for 30 min. The primary antibodies were as follows: anti-p-PKCα (Thr-638/641) and anti-PKCα (catalog no. 2056, Cell Signaling Technology); anti-p-Akt (Ser-473) (catalog no. 3868, Cell Signaling Technology); anti-Akt (catalog no. 4691, Cell Signaling Technology); anti-β-actin (C4) (catalog no. sc-47778, Santa Cruz Biotechnology, Dallas, TX); and anti-LAMP2, anti-SQSTM1/p62, anti-LC3B, anti-FN, anti-α-SMA, and anti-tubulin (catalog no. sc53646, Santa Cruz Biotechnology). Quantification was performed by measuring the intensity of the signals with the aid of ImageJ software package (National Institutes of Health).
Lipid droplet clearance assay
NRK-49F cells grown on coverslips of 6-well culture plates to 60–70% confluence and then changed to serum-free medium with Go6976 or CQ for 4 h. Lipid droplets were stained with BODIPY493/503 (2 μg/ml) for 30 min prior to the time of examination. Slides were viewed with a Nikon Eclipse 80i epi-fluorescence microscope equipped with a digital camera.
NAG assay
NAG assays were performed using a kit from Nanjing Jiancheng Bioengineering Institute (catalog no. A031, Nanjing, China), based on the principle that NAG hydrolyzes the substrate to generate free p-nitrophenol that can be measured colorimetrically at 400 nm following ionization at basic pH. Briefly, NRK-49F cells treated with Go6976 (5 μm) or CQ (50 μm) for 4 h or transfected with PKCα or Scramble siRNA for 24 h were lysed in RIPA buffer (100 μl). Ten micrograms of cell lysates from each sample were normalized to equal volume and measured in quadruplicate for NAG activity following the protocol provided by the supplier.
EM assay
Lysosomes and autophagosomes and/or autolysosome structures were evaluated by EM. NRK-49F cells were seeded in 6-well plates. Go6976 (5 μm) was added to the wells and then treated with or without TGFβ1. After 4 h, the cells were collected and fixed with pentanediol and observed by transmission electron microscopy (Tecnai G2 Spirit Bio TWIN).
RNA isolation and real-time quantitative reverse transcriptase-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized with 1 μg of total RNA, ReverTra Ace (Vazyme, Nanjing, China), and oligo(dT)12–18 primers. Gene expression was measured by real-time PCR assay (Vazyme) and the 7300 real-time PCR system (Applied Biosystems, Foster City, CA). The relative amount of mRNA or gene to internal control was calculated using the equation 2ΔCT, in which ΔCT = CTgene − CTcontrol.
Assessment of lysosome acidification
To assess lysosome acidification, we used LysoSensor Yellow/Blue DND-160 (PDMPO) (Invitrogen). We diluted the 1 mm probe stock solution in the 1 μm working concentration in the growth medium. When NRK-49F cells reached the desired confluence, we removed the medium from the dish and added the pre-warmed (37 °C) probe-containing medium. We incubated the cells for 30 min under growth conditions and then replaced the loading solution with fresh medium, and the fluorescence images were collected at the wavelength range from 510 to 641 nm (yellow) and at the wavelength range from 404 to 456 nm (blue) with the Zeiss LSM710 (Zeiss). The mean fluorescence intensity in the fluorescence-positive area was measured using ImageJ, for yellow and blue fluorescence, respectively. The blue/yellow ratio was calculated by division process in each section. The average blue/yellow ratio of a given sample was calculated from five sections. Intensity was measured using at least three independent experiments.
Statistical analysis
All data examined are presented as mean ± S.E. Statistical analyses of the data were performed using the SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way analysis of variance, followed by the Student-Newman-Keuls test. p < 0.05 was considered statistically significant.
Author contributions
X. X., J. R., and C. D. investigation; X. X., J. R., X. S., Y. G., Y. F., B. S., W. W., Q. L., Y. L., W. H., and J. Y. methodology; X. X. and J. R. writing-original draft; C. D. conceptualization; C. D. resources; C. D. data curation; C. D. formal analysis; C. D. supervision; C. D. funding acquisition; C. D. project administration; C. D. writing-review and editing.
This work was supported by National Science Foundation of China Grants 81570611/H0503 and 81770675/H0503 and Science Foundation of Jiangsu Province Grant BK20140048 (to C. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ECM
- extracellular matrix
- UUO
- unilateral ureteral obstruction
- TGFβ1
- transforming growth factor β1
- PKCα
- protein kinase Cα
- RANTES
- regulation on activation normal T cell expressed and secreted
- TNFα
- tumor necrosis factor-α
- DAPI
- 4,6-diamidino-2-phenylindole
- CQ
- chloroquine
- NAG
- β-N-acetylglucosaminidase
- 3-MA
- 3-methyladenine
- FN
- fibronectin
- Ab
- antibody
- α-SMA
- α-smooth muscle actin
- PAS
- periodic acid-Schiff
- RFP
- red fluorescent protein
- mTOR
- mammalian target of rapamycin
- TFEB
- transcription factor EB.
References
- 1. Duffield J. S. (2014) Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 124, 2299–2306 10.1172/JCI72267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y. (2011) Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 10.1038/nrneph.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. LeBleu V. S., Taduri G., O'Connell J., Teng Y., Cooke V. G., Woda C., Sugimoto H., and Kalluri R. (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 10.1038/nm.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korolchuk D. V., Carroll D. B., and Lovat P. (2016) in Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 12, 151–175 10.1080/15548627.2015.1100358 18188003 [DOI] [Google Scholar]
- 5. Das G., Shravage B. V., and Baehrecke E. H. (2012) Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4, a008813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Codogno P., and Meijer A. J. (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12, 1509–1518 10.1038/sj.cdd.4401751 [DOI] [PubMed] [Google Scholar]
- 7. Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., and Ohsumi Y. (2001) The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 20, 5971–5981 10.1093/emboj/20.21.5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., and Johansen T. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komatsu M., Waguri S., Koike M., Sou Y. S., Ueno T., Hara T., Mizushima N., Iwata J., Ezaki J., Murata S., Hamazaki J., Nishito Y., Iemura S., Natsume T., Yanagawa T., et al. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 10.1016/j.cell.2007.10.035 [DOI] [PubMed] [Google Scholar]
- 10. Pereira E. M., do Monte S. J., do Nascimento F. F., de Castro J. A., Sousa J. L., Filho H. C., da Silva R. N., Labilloy A., Monte Neto J. T., and da Silva A. S. (2014) Lysosome-associated protein 1 (LAMP-1) and lysosome-associated protein 2 (LAMP-2) in a larger family carrier of Fabry disease. Gene 536, 118–122 10.1016/j.gene.2013.11.063 [DOI] [PubMed] [Google Scholar]
- 11. Eskelinen E. L. (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 27, 495–502 10.1016/j.mam.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 12. Saftig P., Beertsen W., and Eskelinen E. L. (2008) LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy 4, 510–512 10.4161/auto.5724 [DOI] [PubMed] [Google Scholar]
- 13. De Rechter S., Decuypere J. P., Ivanova E., van den Heuvel L. P., De Smedt H., Levtchenko E., and Mekahli D. (2016) Autophagy in renal diseases. Pediatr. Nephrol. 31, 737–752 10.1007/s00467-015-3134-2 [DOI] [PubMed] [Google Scholar]
- 14. Kim W. Y., Nam S. A., Song H. C., Ko J. S., Park S. H., Kim H. L., Choi E. J., Kim Y. S., Kim J., and Kim Y. K. (2012) The role of autophagy in unilateral ureteral obstruction rat model. Nephrology 17, 148–159 10.1111/j.1440-1797.2011.01541.x [DOI] [PubMed] [Google Scholar]
- 15. Ding Y., Kim J. K., Kim S. I., Na H. J., Jun S. Y., Lee S. J., and Choi M. E. (2010) TGF-β1 protects against mesangial cell apoptosis via induction of autophagy. J. Biol. Chem. 285, 37909–37919 10.1074/jbc.M109.093724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S. I., Na H. J., Ding Y., Wang Z., Lee S. J., and Choi M. E. (2012) Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J. Biol. Chem. 287, 11677–11688 10.1074/jbc.M111.308460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koesters R., Kaissling B., Lehir M., Picard N., Theilig F., Gebhardt R., Glick A. B., Hähnel B., Hosser H., Gröne H. J., and Kriz W. (2010) Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 177, 632–643 10.2353/ajpath.2010.091012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding Y., Kim S. L., Lee S. Y., Koo J. K., Wang Z., and Choi M. E. (2014) Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol. 25, 2835–2846 10.1681/ASN.2013101068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baisantry A., Bhayana S., Rong S., Ermeling E., Wrede C., Hegermann J., Pennekamp P., Sörensen-Zender I., Haller H., Melk A., and Schmitt R. (2016) Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol. 27, 1609–1616 10.1681/ASN.2014111059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livingston M. J., Ding H. F., Huang S., Hill J. A., Yin X. M., and Dong Z. (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12, 976–998 10.1080/15548627.2016.1166317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard M., Dieudé M., Yang B., Hamelin K., Underwood K., and Hébert M. J. (2014) Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF. Autophagy 10, 2193–2207 10.4161/15548627.2014.981786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J., Kundu M., Viollet B., and Guan K. L. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mihaylova M. M., and Shaw R. J. (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 10.1038/ncb2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J., Xu Z., Jiang L., Mao J., Zeng Z., Fang L., He W., Yuan W., Yang J., and Dai C. (2014) Rictor/mTORC2 protects against cisplatin-induced tubular cell death and acute kidney injury. Kidney Int. 86, 86–102 10.1038/ki.2013.559 [DOI] [PubMed] [Google Scholar]
- 25. Li Y., Xu M., Ding X., Yan C., Song Z., Chen L., Wang X., Jian Y., Tang G., Tang C., Di Y., Mu S., Liu X., Liu K., et al. (2016) Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat. Cell Biol. 18, 1065–1077 10.1038/ncb3407 [DOI] [PubMed] [Google Scholar]
- 26. Jiang L., Xu L., Mao J., Li J., Fang L., Zhou Y., Liu W., He W., Zhao A. Z., Yang J., and Dai C. (2013) Rheb/mTORC1 signaling promotes kidney fibroblast activation and fibrosis. J. Am. Soc. Nephrol. 24, 1114–1126 10.1681/ASN.2012050476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J., Ren J., Liu X., Jiang L., He W., Yuan W., Yang J., and Dai C. (2015) Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis. Kidney Int. 88, 515–527 10.1038/ki.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Principe D., Lista P., Malorni W., and Giammarioli A. M. (2013) Fibroblast autophagy in fibrotic disorders. J. Pathol. 229, 208–220 10.1002/path.4115 [DOI] [PubMed] [Google Scholar]
- 29. Hernández-Gea V., Ghiassi-Nejad Z., Rozenfeld R., Gordon R., Fiel M. I., Yue Z., Czaja M. J., and Friedman S. L. (2012) Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 10.1053/j.gastro.2011.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel A. S., Lin L., Geyer A., Haspel J. A., An C. H., Cao J., Rosas I. O., and Morse D. (2012) Autophagy in idiopathic pulmonary fibrosis. PLoS ONE 7, e41394 10.1371/journal.pone.0041394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim Y. C., and Guan K. L. (2015) mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125, 25–32 10.1172/JCI73939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noda T., and Ohsumi Y. (1998) Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273, 3963–3966 10.1074/jbc.273.7.3963 [DOI] [PubMed] [Google Scholar]
- 33. Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M., Gretzmeier C., Dengjel J., Piacentini M., Fimia G. M., and Cecconi F. (2013) mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406–416 10.1038/ncb2708 [DOI] [PubMed] [Google Scholar]
- 34. Settembre C., Fraldi A., Medina D. L., and Ballabio A. (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 10.1038/nrm3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarbassov D. D., Guertin D. A., Ali S. M., and Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- 36. Jiang H., Cheng D., Liu W., Peng J., and Feng J. (2010) Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem. Biophys. Res. Commun. 395, 471–476 10.1016/j.bbrc.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang F., Xu C., Reece E. A., Li X., Wu Y., Harman C., Yu J., Dong D., Wang C., Yang P., Zhong J., and Yang P. (2017) Protein kinase C-α suppresses autophagy and induces neural tube defects via miR-129–2 in diabetic pregnancy. Nat. Commun. 8, 15182 10.1038/ncomms15182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonnella R., Granato M., Farina A., Santarelli R., Faggioni A., and Cirone M. (2015) PKCθ and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim. Biophys. Acta 1853, 1586–1595 10.1016/j.bbamcr.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 39. Tan S. H., Shui G., Zhou J., Li J. J., Bay B. H., Wenk M. R., and Shen H. M. (2012) Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J. Biol. Chem. 287, 14364–14376 10.1074/jbc.M111.294157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luzio J. P., Pryor P. R., and Bright N. A. (2007) Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 10.1038/nrm2217 [DOI] [PubMed] [Google Scholar]
- 41. Mizunoe Y., Sudo Y., Okita N., Hiraoka H., Mikami K., Narahara T., Negishi A., Yoshida M., Higashibata R., Watanabe S., Kaneko H., Natori D., Furuichi T., Yasukawa H., Kobayashi M., and Higami Y. (2017) Involvement of lysosomal dysfunction in autophagosome accumulation and early pathologies in adipose tissue of obese mice. Autophagy 13, 642–653 10.1080/15548627.2016.1274850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J. E., Oh S. J., Koga Y., Sue C. M., Yamamoto A., Murakami N., Shanske S., Byrne E., et al. (2000) Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406, 906–910 10.1038/35022604 [DOI] [PubMed] [Google Scholar]