Abstract
Background
Chitosan was used as an alternative to promote the growth of weaned piglets. And low-molecular-weight chitosan (LC) is one of chitosan derivatives and maintain beneficial biological properties of chitoson. The present experiment was carried out to examine the effects of LC on the growth performance, intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets.
Results
A total of 40 piglets weaned at 21 d of age, with average body weight 6.37 ± 0.08 kg, were randomly assigned (5 pens/diet; 4 pigs/pen) to 2 treatments (a basal diet and the basal diet supplemented with 50 mg/kg LC) and were fed for 28 d. Compared with the control group, average daily feed intake (ADFI), and the expression of intestinal barrier protein ZO-1 was increased (P < 0.05) when the piglets fed the diet supplemented with LC. No significant differences were found in average daily gain (ADG, P > 0.05), gain-to-feed ratio (G:F, P > 0.05), the incidence of diarrhea (P > 0.05), or the antioxidant capacity (P > 0.05) between two groups. The expression of IL-1β and TNF-α in jejunal mucosa were significantly decreased (P < 0.05) in piglets fed the LC-supplemented diet in comparison to the control.
Conclusion
The results of this study indicate that dietary supplementation with LC at 50 mg/kg was effective for enhancing the growth performance in weaned piglets, improving intestinal barrier function and alleviating intestinal inflammation.
Keywords: Low-molecular-weight chitosan, Weaned piglet, Growth performance, Immune response, Intestinal barrier function
Background
Weaning is an important stage of gut development and may cause low feed intake, intestinal dysfunction and growth retardation in piglets [1, 2]. Antibiotics have long been used to solve problems in the weaning period and to promote the growth and health of piglets [3]. As the use of antibiotics can lead to bacterial resistance and potential antibiotic residues in animal products, thus many alternatives to antibiotics have been suggested and tested. Chitosan with the molecular weight of 1000 kDa, is the second most abundant carbohydrate polymer in nature [4, 5]. It has been reported that chitosan has been widely used as a potential alternative to in-feed antibiotics in piglets and broiler chickens due to many beneficial biological properties of it, such as promoting the growth performance [6, 7], anti-oxidative [8] and immunity modulation [9]. However, chitosan is restricted to be used in food and biomedical applications because of its difficult solubility and instability [10]. Low-molecular-weight chitosan (LC, < 150 kDa) and chito-oligosaccharide (COS, < 5 kDa) are obtained from chitosan by physical, chemical or enzymatic methods, and have much higher solubility and stability than chitosan. Chito-oligosaccharide (COS) with the properties of antimicrobial, anti-inflammatory, anti-oxidative and immunity modulate [11, 12], is widely used as a dietary additive in livestock. But it is still unclear whether dietary supplementation with LC can affect the piglets. The objective of the present experiment, therefore, was to clarify the effects of low-molecular-weight chitosan on the growth performance, incidence of diarrhea, intestinal morphology, barrier function, immune response, and antioxidant system in weaned piglets.
Methods
Ethics statements
The piglets examined in the present study were approved by the Animal Care and Use Committee of the Institute of Animal Science, Guangdong Academy of Agricultural Sciences, with the approval number of GAASISA-2016-017.
Animals and experimental treatments
A total of 40 Duroc × Landrace × Yorkshire piglets weaned at 21 d of age were blocked by BW (average 6.37 ± 0.08 kg), and randomly assigned to 2 treatments with 4 pigs per pen and 5 replicate pens per treatment. The piglets were purchased from WanHe Nongmu Co., Ltd., Guangdong, China. These were (1) a control group (CON) fed the basal diet, and (2) the basal diet supplemented with 50 mg/kg low-molecular-weight chitosan (LC); both were fed for 28 d. The LC (molecular weight 20 to 30 kDa), which was obtained from chitosan by radiation pyrolysis technology, was offered by Jiaxing Korui Biotech Co., Ltd., Zhejiang, China. The composition and content of the treatment diets were shown in Table 1. Piglets were housed in a temperature-controlled nursery and had ad libitum access to feed and water. The body weight of piglets and amount of the feed was measured to calculate the average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F). The number of pigs with diarrhea was recorded every day. Diarrhea index (%) was calculated as 100 × number of piglets that had diarrhea/total number of piglets.
Table 1.
Ingredient and chemical composition of the basal diet (as-fed basis)
| Ingredient (%) | Control | LC |
|---|---|---|
| Corn | 60.43 | 60.43 |
| Extruded soybean meal | 20.00 | 20.00 |
| Fish meal | 5.00 | 5.00 |
| Soybean protein concentrate | 2.00 | 2.00 |
| Whey powder | 7.50 | 7.50 |
| Soybean oil | 1.50 | 1.50 |
| L-Lysine.HCl | 0.25 | 0.25 |
| DL-Met | 0.10 | 0.10 |
| CaHPO4 | 1.05 | 1.05 |
| Limestone | 0.75 | 0.75 |
| 50% Choline Chloride | 0.12 | 0.12 |
| NaCl | 0.30 | 0.30 |
| Premixa | 1.00 | 1.00 |
| Low-molecular-weight chitosan | 0.00 | 0.005 |
| Calculated analysis | ||
| DE, MJ/kg | 14.10 | 14.10 |
| CP (%) | 19.55 | 19.55 |
| Lys (%) | 1.17 | 1.17 |
| Met (%) | 0.50 | 0.50 |
| Met+Cys (%) | 0.74 | 0.74 |
| Thr (%) | 0.77 | 0.77 |
| Ca (%) | 0.86 | 0.86 |
| Available P (%) | 0.68 | 0.68 |
aPremix supplied per kg: 11000 IU of vitamin A; 1100 IU of vitamin D3; 80 IU of vitamin E; 2.5 mg of vitamin K3; 17.5 mg of vitamin B; 20 mg of vitamin B2; 10 mg of vitamin B6; 220μg of vitamin B12; 150 mg of nicotinamide; 1.5 mg of D-calcium pantothenate; 1.5 mg of folic acid; 3 mg of biotin; 150 mg of Fe (FeSO4); 10 mg of Cu (CuSO4); 10 mg of Mn (MnSO4); 150 mg of Zn (ZnO); 0.2 mg of I (KIO3); 0.3 mg of Se (Na2SeO3) and 0.15 mg of Co (LCO4)
Sample collection
The BW of each piglet was recorded at the end of the experiment. After 12 h fasting, five pigs (1 per pen) were randomly selected from each treatment and anaesthetised with pentobarbital sodium (50 mg/kg, i.v.). After sedation, serum was obtained by centrifuging at 2000×g for 10 min and stored at − 20 °C for subsequent measurements of activities of catalase (CAT), glutathione peroxidase (GSH-Px), and total superoxide dismutase (T-SOD), and contents of malondialdehyde (MDA) and total antioxidant capacity (T-AOC), using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The gastrointestinal tract was immediately removed after slaughter, washed with cold PBS. And then 2–3 cm segments of duodenum, jejunum and ileum were removed and fixed in 4% formaldehyde for histometric analysis. Mucosa was scraped from a 10–15 cm segment of jejunum and stored at − 80 °C for gene expression analyses.
Analysis of small intestinal morphology
The fixed intestinal segments were dehydrated and embedded using low-melt paraffin wax by routine methods. Three cross-sections (5 μm) of each segment were dewaxed then stained with hematoxylin and eosin. Villus height and crypt depth of each intestinal segment were measured at 10 × magnification using an image processing and analysis system. At least 10 well-oriented intact villi and their associated crypts were examined in each intestinal segment of each piglet. The mean villus height and crypt depth of each section were calculated per piglet and used for further analysis.
Relative quantification of mRNA expression
Total RNA from jejuna mucosa was extracted according to the manufacturer’s instructions of TRIzol reagent (Invitrogen, Carlsbad, CA). The concentration of RNA was measured by NanoDrop ND-1000 (Thermo Fisher Scientific). And the integrity of RNA was checked by electrophoresis on 1% agarose gel. After removed genomic DNA with gDNA Eraser (TaKaRa, Dalian, reverse transcription was carried out using PrimeScript™RT reagent kit (TaKaRa, Dalian, China) followed the manufacturer’s instructions. The expression levels of intestinal tight junction and cytokines were analyzed using SYBR® Premix Ex Taq™ II kit (Takara, Dalian, China) and iQ™5 Real Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The sequences of the primers were listed in Table 2. The 2-ΔΔCt method was used to estimate the abundance of mRNA.
Table 2.
Primer sequences for real-time PCR analysis
| Gene | Sequence(5′ → 3′) | Product size (bp) | GenBank accession number |
|---|---|---|---|
| ZO-1 | CTCTTGGCTTGCTATTCG | 256 | XM_003353439.2 |
| AGTCTTCCCTGCTCTTGC | |||
| Occludin | GTAGTCGGGTTCGTTTCC | 167 | NM_001163647.2 |
| GACCTGATTGCCTAGAGTGT | |||
| IL-1β | CTCCAGCCAGTCTTCATTGTTC | 132 | NM_214055.1 |
| TGCCTGATGCTCTTGTTCCA | |||
| TNF-α | CACCACGCTCTTCTGCCTAC | 116 | X54859 |
| ACGGGCTTATCTGAGGTTTGAGACG | |||
| IL-10 | GGTTGCCAAGCCTTGTCAG | 202 | NM_214041 |
| AGGCACTCTTCACCTCCTC | |||
| TGF-β | GAAGCGCATCGAGGCCATTC | 162 | NM_214015 |
| GGCTCCGGTTCGACACTTTC | |||
| β-actin | CGGGACATCAAGGAGAAGC | 273 | DQ845171 |
| ACAGCACCGTGTTGGCGTAGAG |
Statistical analysis
All data were subjected to a t-tests using SAS (Version 8.1; SAS Inst. Inc., Cary, NC). Data are presented as means and SEM. P-values < 0.05 were used to indicate statistical significance.
Results
Growth performance and incidence of diarrhea
As shown in Table 3, ADG and ADFI was increased by 22.28% (P = 0.084) and 8.95% (P < 0.05) in the piglets fed with dietary supplementation with low-molecular-weight chitosan (LC). No significant changes were found in G:F and rate diarrhea rate between control group and LC group.
Table 3.
Effect of dietary supplementation with LC on growth performance of weaned piglets
| Variables | CON | LC | SEM | P-value |
|---|---|---|---|---|
| ADG (g/d) | 177.7 | 217.3 | 10.03 | 0.084 |
| ADFI (g/d) | 297.3b | 323.9a | 9.93 | 0.013 |
| G:F (g/g) | 0.63 | 0.66 | 0.02 | 0.391 |
| Diarrhea rate (%) | 26.32 | 25.97 | 1.08 | 0.882 |
ADG average daily gain, ADFI average daily feed intake, G:F gain-to-feed ratio, CON piglets fed the basal diet, LC piglets fed the basal diet with 50 mg/kg 20–30 kDa chito-oligosaccharide, SEM standard error of mean
a,bValues within a row with different superscripts differ significantly at P < 0.05. n = 5
Small intestinal morphology
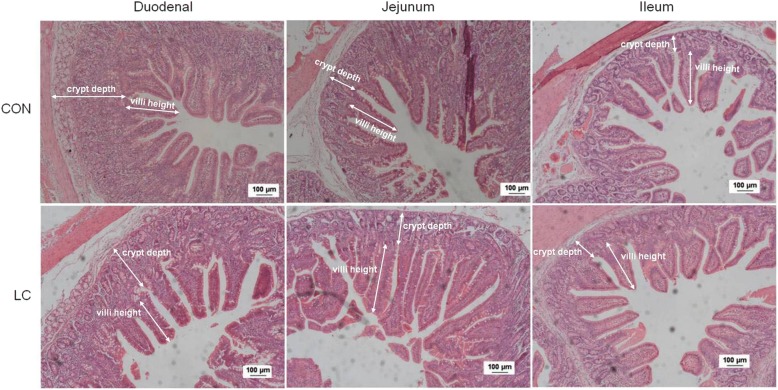
No significant differences in villus height, crypt depth, or villus height:crypt depth in duodenum, jejunum and ileum were observed between CON and LC groups (Table 4, Fig. 1).
Table 4.
Effect of LC supplementation on morphology of the small intestine of weaned piglets
| Group | Villus heigh (μm) | Crypt depth (μm) | VH:CD (μm: μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Duodenal | Jejunum | Ileum | Duodenal | Jejunum | Ileum | Duodenal | Jejunum | Ileum | |
| CON | 376.0 | 375.1 | 330.8 | 337.1 | 245.2 | 182.8 | 1.16 | 1.57 | 1.88 |
| LC | 424.0 | 373.8 | 330.7 | 295.9 | 218.4 | 213.1 | 1.45 | 1.71 | 1.58 |
| SEM | 15.22 | 20.81 | 0.09 | 9.83 | 10.74 | 0.07 | 14.34 | 13.98 | 0.12 |
| P-value | 0.118 | 0.351 | 0.106 | 0.952 | 0.232 | 0.380 | 0.768 | 0.349 | 0.247 |
CON piglets fed the basal diet, LC piglets fed the basal diet with 50 mg/kg 20–30 kDa low-molecular-weight chitosan, VH:CD villus height: crypt depth, SEM standard error of mean, n = 5
Fig. 1.
Effects of LC on intestinal morphology of duodenum, jejunum and ileum. CON: piglets fed the basal diet; LC: piglets fed the basal diet with 50 mg/kg 20–30 kDa low-molecular-weight chitosan. The scale bars in each image indicate 100 μm
Intestinal cytokines
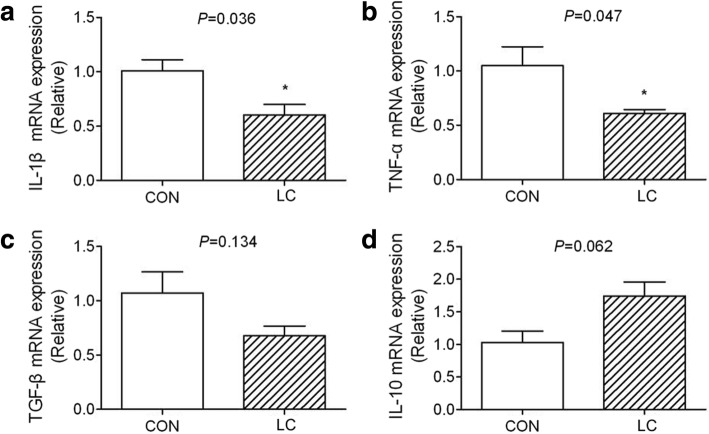
Dietary supplementation with low-molecular-weight chitosan (LC) significantly decreased (P < 0.05) the expression of IL-1β and TNF-α in the jejunal mucosa of piglets in comparison to the CON group (Fig. 2a-b). There were no significant differences in the jejunal mucosal expression of TGF-β and IL-10 (Fig. 2c-d).
Fig. 2.
The pro-inflammatory cytokine gene expression was inhibited by LC. IL-1β (a), TNF-α (b), TGF-β(c) and IL-10 (d) were quantified by RT-PCR. CON: piglets fed the basal diet; LC: piglets fed the basal diet with 50 mg/kg 20–30 kDa low-molecular-weight chitosan. Five piglets per treatment. *P < 0.05
Anti-oxidation indices
The effect of LC on the activity of serum antioxidant enzymes and indices in weaned piglets are shown in Table 5. While there were no significant differences, activities of antioxidant enzymes, T-AOC, CAT, GSH-Px and T-SOD were slightly higher in the piglets fed LC-supplemented diets, and the concentration of MDA in serum was reduced.
Table 5.
Effect of LC supplementation on serum anti-oxidation indices of weaned piglets
| Group | CON | LC | SEM | P-value |
|---|---|---|---|---|
| CAT (U/mL) | 101.3 | 107.3 | 7.13 | 0.704 |
| GSH-Px (U/mL) | 44.1 | 45.4 | 0.69 | 0.357 |
| T-SOD (U/mL) | 111.6 | 114.2 | 4.17 | 0.779 |
| MDA (nmol/mL) | 3.10 | 1.74 | 0.55 | 0.233 |
| T-AOC (U/mL) | 1.45 | 1.82 | 0.10 | 0.064 |
CON piglets fed the basal diet, LC piglets fed the basal diet with 50 mg/kg 20–30 kDa low-molecular-weight chitosan, CAT activities of catalase, GSH-Px glutathione peroxidase, T-SOD total superoxide dismutase, MDA contents of malondialdehyde, T-AOC total antioxidant capacity, SEM standard error of mean, n = 5
Indices of intestinal barrier function
Compared with the piglets in control group, the relative expression of jejuna mucosa ZO-1 transcripts was dramatically increased (P = 0.025) in piglets from LC group, while no significant change was found in the expression of Occludin-1 (Fig. 3).
Fig. 3.
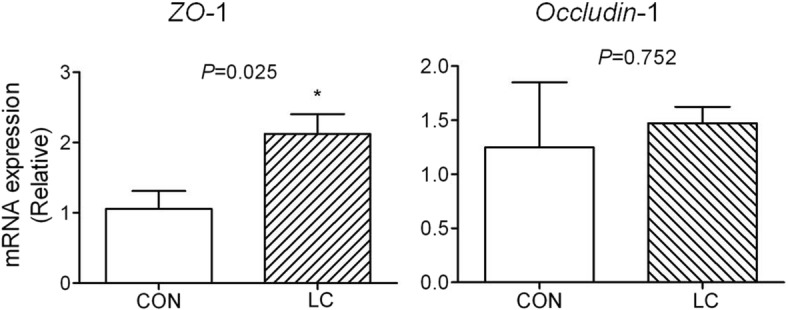
Effect of dietary LC on jejunal expression of intestinal barrier genes. CON: piglets fed the basal diet; LC: piglets fed the basal diet with 50 mg/kg 20–30 kDa low-molecular-weight chitosan. Five piglets per treatment. *P < 0.05
Discussion
Chitosan, one of the most abundant polymers in nature, is an alkaline polysaccharide with positive charges [13]. Because of its characteristics of nontoxic [14], and the biological activities, such as antimicrobial activity and anti-inflammatory [15, 16], chitosan is widely used as a dietary supplement in livestock industry to promote the growth [13, 17]. However, the influences of low-molecular-weight chitosan (LC) on the growth of livestock remain unknown. In the present study, the effect of dietary supplementation with 50 mg/kg LC (20–30 kDa) on growth performance, incidence of diarrhea, intestinal morphology, intestinal barrier, cytokines expression and anti-oxidation indices were examined in weaned piglets. The results demonstrated that, after 4 wk. of post-weaning feeding, there were no significant differences in ADG and G:F between piglets fed the LC-containing and control diets. When compared with the piglets fed the CON diet, ADFI was significantly increased by about 9% and ADG increased 22% in the piglets fed LC. While the latter was not significant, it was of greater magnitude than the change in ADFI, suggesting that the effects of LC on nutrient metabolism might mediate part of the growth response. It has been demonstrated that low molecular weight chitosans prevented the increases in bodyweight by decreasing the absorption of dietary lipids [18] and intestinal disaccharidase activities [19]. Many studies showed positive effects of another small molecular weight COS on the growth performance of weaned pigs; other studies found that there were no significant differences between piglets fed COS-supplemented and CON diets [20, 21]. Walsh et al. [22] demonstrated that 5 to 10 kDa COS possessed antibacterial activity and the 10 to 50 kDa preparation was optimum for enhancing intestinal structure. It can be speculated that the different effects of the LC on growth performance of weaned piglets may result from the molecular weight, dosage, solubility or the duration of LC supplementation.
Diarrhea in the post-weaning period is always due to intestinal dysfunction. The decrease of villus height and the increase of crypt depth associated with dysfunction have been found in previous studies [23–25]. Liu et al. [20] observed that 160 mg/kg supplemental COS reduced the incidence of diarrhea and increased the villus:crypt ratio in weaned piglets challenged with Escherichia coli. Supplementation with 200, 400, or 600 mg/kg COS in diets of weaned piglets not influencing the villus:crypt ratio in duodenum, jejunum, or ileum [7]. In the results of the present experiment, basic dietary-supplemented with LC at 50 mg/kg did not influence incidence of diarrhea, villus height, crypt depth and villus:crypt ratio in the duodenum, jejunum and ileum. Supplementation with LC at 50 mg/kg tended to increase the villus:crypt ratio in duodenum and jejunum of the piglets. Tight junctions are the important determinants of epithelial barrier functions [26]. When the piglets feed with 30 mg/kg COS, the expression of epithelial tight junction, such as occluding-1 and ZO-1 was decreased [21]. The present study found that the jejuna mucosa expression of ZO-1 was significantly enhanced by 50 mg/kg dietary LC (20–30 kDa), thought it did not affect the incidence of diarrhea in weaned piglets. Further researches were needed to be carried out to explore the effects of low dosage of COS on intestinal barrier function.
The balance between pro-inflammatory and anti-inflammatory cytokines is of great importance for the health of weaned piglets [27]. It has been reported that the intestinal expression of pro-inflammatory cytokine genes, such as IL-1β, IL-6, and TNF-α, is up-regulated in weaned piglets [28] and the present study found that dietary supplementation with 50 mg/kg LC (20–30 kDa) significantly reduced the jejunal mucosal expression of pro-inflammatory cytokines IL-1β and TNF-α while not influencing that of anti-inflammatory cytokines IL-10 and TGF-β. The data suggest that dietary supplementation with 50 mg/kg LC modulated immune responsivity by inhibiting the expression of pro-inflammatory cytokines in weaned piglets.
Oxidative stress accompanies weaning and plays a very important role in intestinal health [29]. The present study found that dietary supplementation with 50 mg/kg LC tended to increase the concentration of the total anti-oxidant capacity and decrease the concentration of MDA in serum, while all the indices showed no significant difference. Previous study reported that dietary supplementation with 30 mg/kg COS had dramatically inhibited T-AOC and T-SOD [21]. In contrast, no significant differences in the antioxidant enzymes and MDA were observed here in piglets supplemented with 50 mg/kg LC. It is widely known that diarrhea usually activates the antioxidant system [30], so the very slight changes in activities of antioxidant enzyme and the concentration of MDA were consistent with the incidence of diarrhea compared the control and LC-groups.
Conclusions
These observations suggested that 50 mg/kg low-molecular-weight chitosan (LC) supplements enhanced the growth of weaned piglets, improved intestinal barrier and inhibited intestinal inflammation. The findings will contribute to the guidance on LC supplements.
Acknowledgments
We would like to thank W. B. Currie, Emeritus Professor, Cornell University, Ithaca, NY for suggestions on the manuscript.
Authors’ contribution
SH and YW helped in experiment studies, interpretation of data and preparation of the manuscript. XW, LW and ZJ helped in statistical analysis and manuscript revision. CZ designed the experiment and overall coordinated the project. All authors read and approved the final manuscript.
Funding
The design of this work, samples collection and analysis were supported by National Natural Science Foundation of China (No. 31601955), the Start-up Fund of the Natural Science Foundation of Guangdong Province (No. 2016A030310322), National Science and Technology Support Program (2012BAD39B01–5) and China Agriculture Research System (CARS-35). The interpretation of data, the modification of manuscript was get the financial support by Science and Technology Planning Project of Guangzhou (201607020035), Science and Technology Program of Guangdong Province (2016A020210041) and Tianjin Science Technology Program (15ZXZYNC00100, ITTPRS2017006).
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADFI
Average daily feed intake
- ADG
Average daily gain
- BW
Body weight
- CAT
Activities of catalase
- CON
Control
- COS
Chito-oligosaccharide
- G:F
Gain-to-feed ratio
- GSH-Px
Glutathione peroxidase
- LC
Low-molecular-weight chitosan
- MDA
Contents of malondialdehyde
- T-AOC
Total antioxidant capacity
- T-SOD
total superoxide dismutase
Ethics approval and consent to participate
The experimental design were approved by Animal Care and Use Committee of the Institute of Animal Science, Guangdong Academy of Agricultural Sciences (Permit Number: GAASISA-2016-017). The collection of specimens in this work was strictly complied the approved guidelines and regulations of Animal Care and Use Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Shenglan Hu and Yu Wang contributed equally to this work.
Contributor Information
Shenglan Hu, Email: hushenglan@gdaas.cn.
Yu Wang, Email: wangyu199002@163.com.
Xiaolu Wen, Email: wenxl2013@qq.com.
Li Wang, Email: wangli1@gdaas.cn.
Zongyong Jiang, Email: jiangz28@qq.com.
Chuntian Zheng, Email: zhengct@163.com.
References
- 1.Kang P, Wang M, Hou YQ, Yin YL, Ding BY, Zhu HL, et al. Effects of oral administration of spermine on the development of small intestine and growth performance of weaned pigs. J Anim Vet Adv. 2012;11(15):2782–2787. doi: 10.3923/javaa.2012.2782.2787. [DOI] [Google Scholar]
- 2.Lalles JP, Bosi P, Smidt H, Stokes CR. Nutritional management of gut health in pigs around weaning. Proc Nutr Soc. 2007;66(2):260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- 3.Gong J, Yin F, Hou Y, Yin Y. Review: chinese herbs as alternatives to antibiotics in feed for swine and poultry production: potential and challenges in application. Can J Anim Sci. 2014;94(2):223–241. doi: 10.4141/cjas2013-144. [DOI] [Google Scholar]
- 4.Knaul JZ, Hudson SM, Creber KA. Crosslinking of chitosan fibers with dialdehydes: proposal of a new reaction mechanism. J Polym Sci Pol Phys. 1999;37(11):1079–1094. doi: 10.1002/(SICI)1099-0488(19990601)37:11<1079::AID-POLB4>3.0.CO;2-O. [DOI] [Google Scholar]
- 5.Kim S, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohyd Polym. 2005;62(4):357–368. doi: 10.1016/j.carbpol.2005.08.012. [DOI] [Google Scholar]
- 6.Chen YJ, Kim IH, Cho JH, Yoo JS, Wang Y, Huang Y, et al. Effects of chitooligosaccharide supplementation on growth performance, nutrient digestibility, blood characteristics and immune responses after lipopolysaccharide challenge in weanling pigs. Livest Sci. 2009;124(1–3):255–260. doi: 10.1016/j.livsci.2009.02.006. [DOI] [Google Scholar]
- 7.Yang CM, Ferket PR, Hong QH, Zhou J, Cao GT, Zhou L, et al. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J Anim Sci. 2012;90(8):2671–2676. doi: 10.2527/jas.2011-4699. [DOI] [PubMed] [Google Scholar]
- 8.Anandan R, Ganesan B, Obulesu T, Mathew S, Kumar RS, Lakshmanan PT, et al. Dietary chitosan supplementation attenuates isoprenaline-induced oxidative stress in ratmyocardium. Int J Biol Macromol. 2012;51(5):783–787. doi: 10.1016/j.ijbiomac.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Terashima Y, Itoh H. Effects of dietary chitosan on fat deposition and lipase activity in digesta in broiler chickens. Br Poult Sci. 2002;43(2):270–273. doi: 10.1080/00071660120121490. [DOI] [PubMed] [Google Scholar]
- 10.Hemantaranjan AA. Future perspective in crop protection: chitosan and its oligosaccharides. Adv Plants Agric Res. 2014;1(1):00006. [Google Scholar]
- 11.Holappa J, Hjalmarsdottir M, Masson M, Runarsson OV, Asplund T, Soininen P, et al. Antimicrobial activity of chitosan N-betainates. Carbohyd Polym. 2006;65(1):114–118. doi: 10.1016/j.carbpol.2005.11.041. [DOI] [Google Scholar]
- 12.Swiatkiewicz S, Swiatkiewicz M, Arczewskawlosek A, Jozefiak D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol Anim Nutr (Berl) 2015;99(1):1–12. doi: 10.1111/jpn.12222. [DOI] [PubMed] [Google Scholar]
- 13.Li LL, Wu X, Peng HZ, Fan MZ, Kong XF, Yin YL, et al. The effect of dietary addition of a polysaccharide from Atractylodes macrophala Koidz on growth performance, immunoglobulin concentration and IL-1β expression in weaned piglets. J Agr Sci. 2009;147(5):625–631. doi: 10.1017/S002185960999013X. [DOI] [Google Scholar]
- 14.Goiri I, Oregui LM, Garcia-Rodriguez A. Use of chitosans to modulate ruminal fermentation of a 50:50 forage-to-concentrate diet in sheep. J Anim Sci. 2010;88(2):749–755. doi: 10.2527/jas.2009-2377. [DOI] [PubMed] [Google Scholar]
- 15.Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MF, Mameri N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012;29(1):48–56. doi: 10.1016/j.foodhyd.2012.02.013. [DOI] [Google Scholar]
- 16.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53(2):393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao DF, Wang YF, Liu G, He JH, Qiu W, Hu XG, et al. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli. PLoS One. 2014;9(8):e104192. doi: 10.1371/journal.pone.0104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumiyoshi M, Kimura Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J Pharm Pharmacol. 2006;58(2):201–207. doi: 10.1211/jpp.58.2.0007. [DOI] [PubMed] [Google Scholar]
- 19.Chiu CY, Feng SA, Liu SH, Chiang MT. Functional comparison for lipid metabolism and intestinal and fecal microflora enzyme activities between low molecular weight chitosan and chitosan oligosaccharide in high-fat-diet-fed rats. Mar Drugs. 2017;15(7):E234. doi: 10.3390/md15070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Piao XS, Kim SW, Wang LX, Shen YB, Lee HS, et al. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and lactobacillus in weaning pigs. J Anim Sci. 2008;86(10):2609–2618. doi: 10.2527/jas.2007-0668. [DOI] [PubMed] [Google Scholar]
- 21.Xiong X, Yang HG, Wang XC, Hu Q, Liu CX, Wu X, et al. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J Anim Sci. 2015;93(3):1089–1097. doi: 10.2527/jas.2014-7851. [DOI] [PubMed] [Google Scholar]
- 22.Walsh AM, Sweeney T, Bahar B, Flynn B, O'Doherty JV. The effect of chitooligosaccharide supplementation on intestinal morphology, selected microbial populations, volatile fatty acid concentrations and immune gene expression in the weaned pig. Animal. 2012;6(10):1620–1626. doi: 10.1017/S1751731112000481. [DOI] [PubMed] [Google Scholar]
- 23.Montagne L, Boudry G, Favier C, Le Huërou-Luron I, Lallès JP, Sève B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Brit J Nutr Br J Nutr. 2007;97(1):45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- 24.Verdonk JM, Bruininx EM, Meulen JV, Verstegen MW. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest Sci. 2007;108(1):146–149. doi: 10.1016/j.livsci.2007.01.093. [DOI] [Google Scholar]
- 25.Hu CH, Xiao K, Luan ZS, Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91(3):1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- 26.Ren W, Yin J, Wu M, Liu G, Yang G, et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One. 2014;9(2):e88335. doi: 10.1371/journal.pone.0088335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren W, Zou L, Li N, Wang Y, Liu G, Peng Y, et al. Dietary arginine supplementation enhances immune responses to inactivated pasteurella multocida vaccination in mice. Br J Nutr. 2013;109(5):867–872. doi: 10.1017/S0007114512002681. [DOI] [PubMed] [Google Scholar]
- 28.Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134(3):641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Chen L, Li P, Li X, Zhou H, Wang F, et al. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138(6):1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- 30.Granot E, Kohen R. Oxidative stress in childhood-in health and disease states. Clin Nutr. 2004;23(1):3–11. doi: 10.1016/S0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.