Abstract
Seven autosomal recessive genes associated with juvenile and young-onset Levodopa-responsive parkinsonism have been identified. Mutations in PRKN, DJ-1, and PINK1 are associated with a rather pure parkinsonian phenotype, and have a more benign course with sustained treatment response and absence of dementia. On the other hand, Kufor-Rakeb syndrome has additional signs, which distinguish it clearly from Parkinson’s disease including supranu-clear vertical gaze palsy, myoclonic jerks, pyramidal signs, and cognitive impairment. Neurodegeneration with brain iron accumulation type I (Hallervorden-Spatz syndrome) due to mutations in PANK2 gene may share similar features with Kufor-Rakeb syndrome. Mutations in three other genes, PLA2G6 (PARK14), FBXO7 (PARK15), and Spatacsin (SPG11) also produce clinical similar phenotypes in that they presented with rapidly progressive parkinsonism, initially responsive to Levodopa treatment but later, developed additional features including cognitive decline and loss of Levodopa responsiveness. Here, using homozygosity mapping and sequence analysis in families with complex parkinsonisms, we identified genetic defects in the ATP13A2 (1 family), PLA2G6 (1 family) FBXO7 (2 families), and SPG11 (1 family). The genetic heterogeneity was surprising given their initially common clinical features. On careful review, we found the FBXO7 cases to have a phenotype more similar to PRKN gene associated parkinsonism. The ATP13A2 and PLA2G6 cases were more seriously disabled with additional swallowing problems, dystonic features, severe in some, and usually pyramidal involvement including pyramidal weakness. These data suggest that these four genes account for many cases of Levodopa responsive parkinsonism with pyramidal signs cases formerly categorized clinically as pallido-pyramidal syndrome. 3 2010 Movement Disorder Society
Keywords: parkinsonism, recessive, ATP13A2, PLA2G6, FBXO7, Spatacsin
In 1954, Davison described five cases of juvenile parkinsonism with associated upper motor neuron signs. Post mortem examination revealed lesions in the pallidum, the substantia nigra, the ansa lenticularis, and the corticospinal tract, thus termed pallido-pyramidal disease. Since then some similar cases have been reported, characterized by autosomal recessive inheritance, normal neuroimaging (although usually without T2* assessment), and L-dopa responsiveness.1–3
Over the last 10 years numerous autosomal recessive genes causing L-dopa-responsive parkinsonism have been identified.4–6 Parkin (PRKN) gene mutations are associated with hyperreflexia,7 however, there is only one report of a pallido-pyramidal phenotype.8 Pyramidal signs are also infrequent in DJ-1 and PINK1 mutations.9 Dopa-responsive dystonia (DRD) can often mimic early-onset parkinsonism (EOPD), sensitive to low L-dopa doses, and carriers of GTP cyclohydrolase mutations do usually not develop dyskinesias. Tyrosine hydroxylase,10 sepiapterin reductase deficiency,11 and spasticity have been reported in DRD,12 but these are clinically distinct from pallido-pyramidal disease.
Furthermore, mutations in ATP13A2, PLA2G6, FBXO7, and SPG11 have recently been identified in cases similar to Davison’s seminal report.13–16 Here, we summarize the phenotypic and genotypic characteristics of cases with homozygous mutations in these four genes. This case series represent the cases of this syndrome, in which we have mapped the lesions by homozygosity mapping. We restrict our discussion of the literature to findings in cases with homozygous and compound heterozygous changes because only in such circumstances, we can be certain of their pathogenic nature (Table 1).
TABLE 1.
Previously reported and novel autosomal recessive parkinsonism mutations
| cDNA | Protein | References | |
|---|---|---|---|
| ATP13A2: PARK9 | c.546C>A | p.Phe182Leu | Ref. 17 |
| c.1103_1104insGA | p.Thr367ArgfsX29 | This paper | |
| c.1306+5G>A | NA | Ref. 13 | |
| c.1510G>C | p.Gly504Arg | Ref. 18 | |
| c.1632_1653dup22 | p.Leu552fsX237 | Ref. 13 | |
| c.3057delC | p.Gly1019fsX3 | Ref. 13 | |
| PLA2G6: PARK14 | c.109C>T | p.Arg37X | Unpublished data |
| c.1078−3C>A | NA | Unpublished data | |
| c.1715C>T | p.Thr572Ile | Unpublished data | |
| c.1894C>T | p.Arg632Trp | Ref. 19 | |
| c.2222G>A | p.Arg741Gln | Ref. 14 | |
| c.2239C>T | p.Arg747Trp | Ref. 14 | |
| FBXO7: PARK15 | c.65C>T | p.Thr22Met | Ref. 20 |
| c.907+1G>T | NA | Ref. 20 | |
| c.1132C>G | p.Arg378Gly | Ref. 15 | |
| c.1492C>T | p.Arg498X | Ref. 20 | |
| SPATACSIN: SPG11 | c.704_705delAT | p.His235ArgfsX12 | Ref. 16 |
| c.733_734delAT | p.Met245fsX2 | This paper |
All ATP13A2, PLA2G6, FBXO7, and Spatacsin mutations identified to date in either recessive parkinsonism or idiopathic Parkinson’s disease patients. Only homozygous or compound heterozygous mutations are included because on these have strong evidence for pathogenicity.
NA, Not Applicable.
ATP13A2 (PARK9)
Homozygous and compound heterozygous ATP13A2 (PARK9) mutations were first described in patients of Jordanian and Chilean ancestries. The main clinical features were juvenile akinetic-rigid parkinsonism, pyramidal weakness, spasticity, and Babinski signs, supranuclear gaze paresis, and cognitive impairment.13,21,22 On clinical follow-up visual hallucinations, facial-faucial-finger mini myoclonus and oculogyric dystonic spasms were added to the phenotypic spectrum.23 The Chilean and Japanese kindreds were clinically similar.13,17 An apparently sporadic Brazilian patient with a single homozygous mutation with disease onset aged 12 was also reported. However, Babinski signs, supranuclear gaze paresis or dementia18 were absent and the case closely resembled PRKN disease. This single case suggests that ATP13A2 mutations may play a role in EOPD, 24 although it has to be acknowledged that the pathogenicity remains uncertain.
PLA2G6 (PARK 14)
PLA2G6 mutations have been associated with neuro-degenerative disorders with increased basal ganglia iron accumulation, such as infantile neuroaxonal dystrophy (INAD) and neurodegeneration with brain iron accumulation (NBIA-type 2).25,26 Pathologically, both, INAD and NBIA, show axonal degeneration with spheroid bodies (distended axons) throughout the central nervous system. PLA2G6 mutations have also been found in patients without spheroids and in classical INAD.27 INAD presents in infancy and death by age 10 is usual. Typically, NBIA presents between infancy and 30 years of age with faster disease progression in infantile and juvenile onset cases.19,28 There is clinical heterogeneity as recently L-dopa responsive dystonia-parkinsonism cases with an onset age ranging from 10 to 26, whose main clinical features were severe akinesia and rigidity, generalized dystonia and cognitive impairment, however, with no evidence of brain iron accumulation on neuroimaging were described.15,19 These latest findings led to a designation of PLA2G6 as PARK14. However, the fact that identical disease-associated PLA2G6 mutations may cause NBIA, INAD, and dystonia-parkinsonism suggests that additional unknown genetic, epigenetic, or nongenetic factors may influence the PLA2G6-associated phenotype.15,19,26
FBX07 (PARK 15)
A disease-associated variant in FBXO7 causing p.Arg378Gly has recently been identified in an Iranian kindred which presented with spastic weakness and Babinski signs. Parkinsonism with bradykinesia and rigidity was developed as a late feature in some familial members.15 Three novel FBXO7 mutations, c.907+1G>T and p.Thr22Met in the compound heterozygous state and p.Arg498X in homozygous state, were later identified in Dutch and Italian families exhibiting spasticity and Babinski signs, tremor, bradykinesia, and postural instability. Dystonia was also present in the homozygous p.Arg498X mutation carriers. These families expanded the phenotypic spectrum associated with FBXO7 mutations making it another cause of recessive EOPD (PARK15).20
SPATACSIN (SPG11)
Spatacsin (SPG11) is the major mutated gene in autosomal recessive spastic paraplegia with thin corpus callosum (ARHSP-TCC). To date, more than 50 different SPG11 mutations, including nonsense, spice-site, and frameshift variants, have been reported in familial and idiopathic cases presenting with complicated HSP.29–31 In addition, an unusual parkinsonism presenting with resting tremor, akinesia and with either weak or no L-dopa response has recently been described in two SPG11 patients from a consanguineous Turkish family. Both showed mental retardation, characteristic of the complex HSP, and bilateral Babinski signs. An 123I-ioflupane SPECT scan revealed dopaminergic denervation in one of the probands. They carried a homozygous frameshift SPG11 mutation (p.His235ArgfsX12).16
SUBJECTS AND METHODS
Subjects
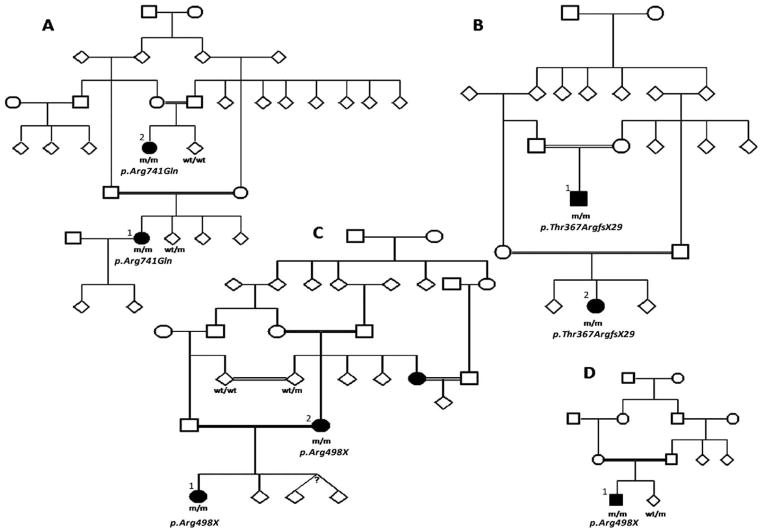
Patients from five unrelated consanguineous families with L-dopa-responsive EOPD gave informed consent to this study approved by the local ethics committee. Different cases were clinically examined by the clinicians involved in the patients’ care and video footage of all cases was retrospectively reviewed by HH, KPB, and AJL. Clinical details are partly given below and summarized in Table 2. For full information see supplements. We also compare the clinical features of two previously published PLA2G6 mutation families (Table 2) and the video of Iranian FBX07 mutation family E. The family trees for families reported here, with the exception of family E where only one proband was available for study, are shown in Figure 1.
TABLE 2.
Summary of the clinical details of the families reported here (families A–D)
| Table | Family A PLA2G6 |
Family B ATP13A2 |
Family C FBX07 |
Family D FBX07 |
Family E SPG11 |
Family DP Sina et al.27 PLA2G6 |
Family 2 from Paisan-Ruiz et al.14 PLA2G6 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family/case | 1 | 2 | 1 | 2 | 1 | 2 | 3 | 1 | 1 | Cases 1–3 | 1 |
| Current age (yr) | 35 | 41 | 41 | 44 | 22 | 44 | 22 | Died 27 | 27 | 23, 25, 31 | 21 |
| Age of onset (yr)/first symptom | 26 Cognitive | 29 Falls | 16 Psychosis | 18 Gait | 17 Eyelid dyspraxia | 24 Bradykinesia | 22 Bradykinesia | 17 | 14 | 21, 22, 25 Dragging feet | 18 Dragging foot |
| Cognitive decline | ++ | +++ | ++ | +++ | + | + | + | − | + | ++ | + |
| Psychiatric features | + | + | ++ | ++ | + | ++ | ++ | + | (+) | + | + |
| Extrapyramidal signs | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ |
| Pyramidal features | + | ++ | + | ++ | + | + | + | − | +++ | ++ | ++ |
| L-dopa response | ++ | ++ | ++PT | ++PT | +++ | ++ | ++ | ++ | ++PT* | ++ | ++ |
| L-dopa-induced dyskinesias | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | NA | ++ | + |
| Dystonia | +++ | +++ | ++ | + | − | − | + | − | + | +++ | ++ |
| Eye movement abnormalities | + | ++ | ++ | ++ | ++ | + | + | − | + | + | + |
| Imbalance/impaired postural reflexes | ++ | ++ | + | + | + | + | + | + | + | ++ | + |
| Dysarthria/dysphonia | +++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | + | ++ | + |
| Swallowing problems | +++/PEG | +++/PEG | ++ | ++ | + | ++ | ++ | + | − | ++ | + |
| Other | Seizures pale blue sclera | Pale blue sclera | Bleph | OA | Bleph cateracts | Cateracts | Cervical dystonia | Nicotine responsive. Dopa induced dystonia and aggression | Nil | Nil | Foot dystonia with hemiparetic gait |
| MRI brain | Frontal white matter | General atrophy | General/Caudate atrophy | General/Caudate atrophy | Normal | General atrophy | General atrophy | Normal MRI. Beta-CIT SPECT, no uptake | General atrophy, thin corpus callosum | General atrophy | General atrophy |
We have previously reported two other families with PLA2G6 mutations and their clinical features are also shown for comparison. +++ = severe, ++ = moderate, + = mild, (+) = related to treatment, − = absent. PEG = Percutaneous endoscopic gastrostomy, Bleph = blepharospasm/clonus, OA = optic atrophy. PT = poorly tolerated:
treated with ropinerole.
FIG. 1.
Pedigrees of families reported here. Manifesting members are shown in bold. A: PLA2G6 family, B: ATP13A2 family, C and D: FBXO7 families. m/m: homozygous mutation carriers, wt/m: heterozygous mutation carriers, wt/wt: homozygous carriers for the wild type sequence.
Family A (PLA2G6)
This patient was described by Paisan-Ruiz et al.14 without video documentation.
In summary, onset was at age 26 with progressive cognitive decline, slow movements (video segment 1), clumsiness, progressive imbalance, hand tremor, and slurred speech, followed by the development of dystonia. There was an excellent L-dopa response. However, after 1 year she developed prominent dyskinesias and improvement declined considerably over the next few years. By age 34 years, she was bed-bound (Video segment 2) and started to have epileptic seizures.
Additional data is also provided now on the previously undescribed cousin, a 36-year-old North Indian female. Her cousin had a foot drag dystonia at age 10. At age 26, she developed arm and leg tremor, followed by infrequent falls from age 29. In view of the findings of ankle clonus and extensor planters on examination a diagnosis of spastic quadriplegia was initially made (Video segment 3). She later developed bradykinesia and extreme rigidity. On examination at age 33, she had a supranuclear vertical gaze palsy, eyelid opening apraxia, a positive glabellar tap sign, and facial hypomimia (Video segment 4). She had developed a pill-rolling tremor, limb bradykinesia, brisk reflexes, and bilateral Babinski signs. She was severely dysarthic with slow tongue movements. L-dopa treatment was beneficial but caused prominent early dyskinesias.
Family B (ATP13A2)
This case was first described in 1995.32 At age 16 he developed an L-dopa-responsive akinetic rigid syndrome. He developed dyskinesias at high doses and secondary nonresponsiveness to dopaminergic therapy. Over the next 15 years he deteriorated. On examination at age 40 years he was anarthric. He had normal fundi but reduced up- and down-gaze with broken pursuit and slow saccades. There was marked dystonia, brisk reflexes, ankle clonus, and bilateral Babinski signs (Video segment 5).
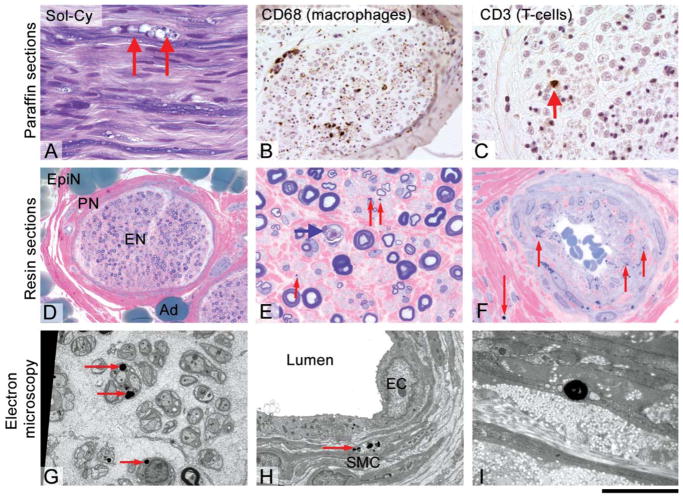
Brain MRI showed general involutional change involving the cerebral cortex, basal ganglia, and cerebellum with presence of basal ganglia iron on T2* sequences33. A sural nerve biopsy performed at age 40 showed acute axonal degeneration, some regeneration, and a very mild chronic inflammatory response with endoneurial and epineurial T-cells. There were numerous cytoplasmic inclusion bodies (1–5 μm in diameter) within the Schwann cells, perineurial and epineurial cells but not in axons. Electron microscopy showed the inclusions to be membrane-bound, irregular, and occasionally folded. Overall they resembled irregular primary lysosomes (Fig. 2).
FIG. 2.
Histological and ultrastructural analysis of the sural nerve biopsy of Family B (ATP13A2): Paraffin sections (A, B, C) show a reduction of myelinated fibre density with frequent formation of myelin digestion chambers (arrows) (A). Immunohistochemical staining for CD68 on a transverse section shows frequent endoneurial macrophages (B), a characteristic finding in florid axonal neuropathies. Very occasionally, there were endoneurial and scattered epineurial T-cells (CD3 immunohistochemistry; C). Resin semi thin sections (D, E, F) show a mild generalized axon loss, subperineurial, and endoneurial oedema (D) and significant numbers of degenerating axons (E, blue arrow). Strikingly, there are numerous small cytoplasmic inclusion (E, red arrows and F, red arrows). These inclusions were found in the endoneurium, in smooth muscle cells of vessels and in the perineurium. Electron microscopy (G, H, I) confirms the presence of electron dense inclusions of circa 1 μm size, which are always located intracellularly, and are most frequently seen in the cytoplasm of Schwann cells (G, red arrows) and in smooth muscle cells (H, I). Scale bar 40 μm (E, F), 60 μm (A, C), 120 μm (B), 230 μm (D).
The proband’s cousin was phenotypically very similar. Onset was at age when aged 18 she developed gait difficulty with frequent falls backwards. She developed arm tremor and urine incontinence. Video segment 6 shows her at age 26 years. L-dopa treatment produced significant improvement; however, with the emergence of early drug-induced dyskinesias and the L-dopa effect reduced within 5 years.
Family C (FBX07)
The proband originating from Pakistan from a family with multiple consanguineous loops presented at age 17 years with difficulty opening her eyes, generalized stiffness and bradykinesia. Over 5 years she developed dysarthria, hypophonic speech, frequent respiratory sighs, and urinary problems.
On examination, she had cataracts, prominent apraxia of eye opening, and supranuclear gaze palsy. She had slow saccades with prominent blepharospasm. There was upper and lower limb rigidity, bradykinesia but no tremor. Reflexes were brisk and the plantars were extensor (Video segment 7).
An L-dopa challenge was strongly positive (UPDRS score 42 pre- and 20 post-treatment). For aggression and mood she later required Olanzapine.
The proband’s mother was similarly affected by L-dopa-responsive parkinsonism without tremor and onset at age 24. She had difficulty with upgaze and abnormal respiration with sighs. Aged 40 she had cataracts, was very rigid and slow, incomprehensible speech, cognitive problems, and swallowing difficulties. For details of the proband’s aunt and investigational results see supporting information.
Family D (FBX07)
This family with multiple consanguineous loops originated from southeast Turkey. Clinical details and a video of the proband have previously been reported by Hanagasi et al.34 before the gene was identified. The 26-year-old male proband developed walking difficulties at age 17, followed by L-dopa-responsive limb rigidity and marked bradykinesia. Because L-dopa caused psychosis it had to be withdrawn. See supplements for further clinical details and Ref. 33 for a video. The patient died at age 28.
The patient had four paternal cousins, who were said to have had severe gait difficulty and bradykinesia. Their symptoms had also started before the age of 20, and they had died within a few years in a bedridden state.
Family E (SPG11)
The symptoms of this 27-year-old Asian from a consanguineous family began at age 14 with postural and writing tremor. Aged 17 he developed walking difficulties with imbalance, speech problems, and slowness. His gait became progressively stiff and he complained of leg weakness and falls. Pharmacological treatment (i.e., baclofen, tizanidine, clonazepam) was either ineffective or produced intolerable side-effects. At age 24 (Video segment 8) he presented with mild gynaecomastia, facial hypomimia, laryngeal dystonia, upgaze skew deviation with slowed upward eye movements, hand dystonia and writing tremor, marked spastic paraplegia, bradykinesia, axial rigidity, and imbalance. Reflexes were brisk bilaterally with bilateral ankle clonus. Routine and genetic testing for SCAs 1,2,3,7,17, DRPLA, and SPG4 were normal. An MRI brain scan revealed generalized atrophy with a thin corpus callosum. A DaT-SPECT scan showed decreased bilateral putaminal and caudate uptake. Motor symptoms improved on ropinirole but caused confusion and hallucination.
Family F (PLA2G6)
See Ref. 27 for genetic findings and Video segment 9 for clinical features.
Molecular Analysis
Genome-wide SNP genotyping was carried out using either HumanHap240 or HumanHap317 illumina bead-chips. Homozygosity mapping was performed as previously described35,36 and using the Homozygosity detector plug-in software within the BeadStudio 3.2 program where a minimum physical size threshold of 1Mb and at least 100 adjacent markers in length were used as limiting parameters (www.illumina.com). Gene screening analyses for ATP13A2, PLA2G6, FBXO7, and SPG11 were performed by PCR analysis using 10 picomoles of both forward and reverse primers (Supporting information 1) and FastStart Taq DNA polymerase (http://www.roche-applied-science.com). Each purified PCR product was then sequenced with Applied Biosystems BigDye terminator v3.1 sequencing chemistry as per the manufacturer’s instructions; the resulting reactions were resolved on an ABI3730 XL genetic analyzer (Applied Biosystems, Foster city, CA) and analyzed by Sequencher 4.8 software (Gene Codes Corporation, Ann Arbor, MI).
RESULTS
All families presented with EOPD that was initially L-dopa responsive. Cognitive and psychiatric features were common in all except the FBX07 mutation cases where agitation and mood problems occurred only after L-dopa treatment. Supranuclear gaze palsy, severe bulbar signs with speech and swallowing difficulties were present in all families. Pyramidal signs were perhaps most marked in the family E (SPG11) and were absent in the Turkish family D (FBX07). Dystonia was also present in the PLA2G6 and ATP13A2 families but they were not a significant feature in the FBX07 mutation families where only one individual had cervical dystonia. MRI brain scans revealed generalized involutional change in most cases. In family E (SPG11), there was a thin corpus callosum in addition to the generalized atrophy. Details of the nerve biopsy in family B are given above in the clinical description.
Comprehensive homozygosity mapping was carried out in nine individuals (eight affected and one unaffected) belonging to four families. No homozygosity mapping was performed in family E as only the proband was available for study. In first instance, we searched for autozygous segments shared among all affected individuals to locate the pathogenic loci,14 although the proximity of the FBXO7 gene (5.7 Mb upstream of PLA2G6) to PLA2G6 on chromosome 22 prevented this analysis alone from distinguishing these loci before gene sequencing (Supporting information 2). Gene sequencing identified the lesion in each family. Similarly, the proximity of the ATP13A2 gene to the established parkinsonism gene PINK1 on chromosome 1 meant that homozygosity mapping alone could not delineate the lesion in this family (Supplement 2). In conclusion, Family A carried the previously reported PLA2G6 mutation (p.Arg741Gln; c.2222G>A), Family B a novel ATP13A2 mutation (p.Thr367ArgfsX29; c.1103-1104insGA), Families C and D the same FBXO7 mutation (p.Arg498X; c.1492C>T), and Family E a 2bp SPG11 deletion (c.733_734delAT; p.Met245fsX2) previously reported in families presenting with complicated ARHSP-TCC (Table 1).29
DISCUSSION
There are now eight recessive loci, which can lead to EOPD syndromes. These are the classical recessive loci, PRKN (PARK2), PINK1 (PARK6), DJ-1 (PARK7), the four loci we present examples of here, ATP13A2 (PARK9), PLA2G6 (PARK14), FBX07 (PARK15), and SPG11 and the PANK2 genes.16,37 It is important to be able to characterize disease causing mutations and the phenotypic features associated with the mutation in the genes for clinical purposes and, also because observed distinctions may give mechanistic insights.
Loss of function mutations at PRKN, PINK1, and DJ-1 nearly always give rise to a pure parkinsonian phenotype which has an early onset, a benign course, sleep benefit and a good and prolonged response to L-dopa. The lifespan of mutation carriers is only marginally reduced and there have been no reports of brain iron accumulation. All three proteins have functions related to mitochondrial biology and PRKN mutations are usually not associated with Lewy bodies.37
Loss of function mutations in PLA2G6 and PANK2 lead to variable and overlapping clinical features of progressive parkinsonism, dystonia, ataxia, and cognitive decline. The endophenotypes range from the aggressive INAD and Hallevorden-Spatz disease with variable brain iron inclusion and death usually before the age of 20 years, through to the patients that present with predominantly EOPD and dystonia and later develop other manifestations. The pathology of these cases is likely to include extensive Lewy bodies as seen in childhood onset neuroaxonal dystrophy,26 although the neuropathology of adult onset cases with PLA2G6 mutations has not yet been reported. Over many years there have been reports of Hallervorden-Spatz cases (PKAN/PANK2) with extensive Lewy body disease. We suggest that Kufor Rakeb syndrome may also belong to this same class of diseases, as gene products of both PLA2G6 and PANK2 impinge on ceramide metabolism. The role of ATP13A2 as a lysosomal pump fits with this suggestion, although its precise function is not known.37
Loss of function mutations in FBX07 appears to give a phenotype intermediate between the two disease classes. In some cases the phenotype resembles PRKN mutation associated phenotype,33,38 but the disease is generally less benign and has a reduced life expectancy, pyramidal signs and late cognitive problems. This overlap of phenotypes related to FBX07 and PRKN mutations is consistent with the related functions of these two genes and their likely common disease pathway. Like PRKN, F-box proteins, such as FBXO7, are components of the modular E3 ubiquitin protein ligases.39
These findings have allowed us to dissect the pal-lido-pyramidal disease described by Davison1 into at least five recessive forms of complex parkinsonism with subtle clinical differences. Although there are still typical cases of L-dopa-responsive parkinsonism with pyramidal signs where the genetic cause is yet to be identified these data suggest that these five genes account for many of these cases.
Supplementary Material
Family A (PLA2G6) (Video of the pro-band). At 29 years of age she is in a wheelchair. There is a mild postural tremor of both hands and some dystonic posturing and she is slow in her movements. On testing eye movements, pursuits are broken and there is limitation of upgaze.
At age 32 years she requires nasogastric feeding and has facial hypomimia, dystonic facial grimacing and spontaneous tongue protrusion (not related to Levodopa). There is severe dystonic posturing in her upper limbs and a bilateral resting tremor, greater on the left.
Family A (PLA2G6) (Video of the cousin). The patient is now age of 33 years and in a wheelchair. This video essentially demonstrates the bilateral brisk reflexes and the Babinski signs to show the marked pyramidal component of the syndrome.
She has facial hypomimia and square wave jerks on primary gaze with slowed pursuit movements but of full range. Eyelid opening apraxia and a supranuclear vertical gaze palsy is demonstrated (although in the video the patient’s eyes were forcefully retracted which can exaggerate this appearance). She is dysarthric with slow tongue movements. There are orolingual dyskinesias probably related to Levodopa treatment. There is a pill rolling rest tremor of her left hand, mild bilateral postural arm tremor (left more than right) and bilateral bradykinesia.
Family B (ATP13A2) (Video of the proband). The initial segment shows him at the age of 16 after he had an acute hypomanic psychotic episode associated with a phenothiazine induced akinetic rigid syndrome (June 1985), this improved after a few weeks but left him with mild parkinsonian features with cog-wheel rigidity clearly demonstrated in October 1985. The second part of the video shows him at the age of 40 when his disease had progressed. He was anarthric and confined to his bed. He has facial hypomimia and risus sardonicus. There is reduced upgaze with broken pursuit and slow saccades with occasional blepharospasm. There is marked dystonic posturing and increased tone in his upper and lower limbs with little movement, there was upper limb bradykinesia and the lower limb reflexes were brisk.
Family B (ATP13A2) (Video of the cousin). The patient showed slow, stooped, dragging gait at the age of 25 years.
Segment 7. Family C (FBX07) (Video of the proband). At the age of 22 years this girl had slow monotonous speech, facial hypomia and hirsutism. During the examination of eye movements (at the start and in between) she has blinking and difficulty opening her eyes (possible apraxia of eye opening or blepharospasm). There is slowed and limited upgaze. She has bradykinesia and a postural but no rest tremor. Planters were extensor and her gait was stooped and slow with poor arm swing.
Segment 8. Family E (SPG11). There is a stiff, spastic gait with difficulty turning. When holding the arms outstretched, there is dystonic posturing of the arms. On writing, there is micrographia and a small amplitude tremor (best seen when drawing a spiral).
Segment 9. Family F (PLA2G6) (Video of three Iranian cases). Case DP5: The patient showed stooped, slow shuffling gait with hypomimic face and drooling. Upper limb bradykinesia and poor tandem walking with a tendency to fall can also be observed. There is no speech and reduced vertical up gaze. Case DP3: The patient is confined to his bed with severe dystonic posturing of his upper and lower limbs. He has no speech and swallowing difficulties. Case DP4: The patient is confined to his bed with severe dystonic posturing of his upper and lower limbs and involuntary movements.
Acknowledgments
We are grateful to the patients and families who support the donation of tissue for research and to the following for essential grant support; The Bachmann Strauss Foundation (CPR, JH), The Medical Research Council (MRC) HH (MRC clinician scientist fellowship) and JH, The Michael J Fox Foundation (HH, CPR and JH), The Brain Research Trust (BRT) (HH, JH, SAS), Ataxia UK (HH) and The BMA Vera Down Award (HH), and the Iran National Science Foundation (EE). We thank The NICHD Brain and Tissue Bank for Developmental Disorder at the University of Maryland, Baltimore, MD. The role of NICHD Brain and Tissue Bank is to distribute tissue, and, therefore, does not endorse the studies performed or the interpretation of results. This work was supported in part by the Intramural Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project number Z01 AG000958-05. This study was clinically supported by the NIHR UCLH/UCL Comprehensive Biomedical Research Centre. None of the funders had any input into the writing of this manuscript.
Footnotes
Potential conflict of interest: Nothing to report.
Financial Disclosures: Coro Paisán-Ruiz, Rosa Guevara, Monica Federoff, Hasmet Hanagasi, Sina F, Elaha Elahe, Susanne A. Schneider, Petra Schwingenschuh, Andrew B. Singleton, Sebastian Brandner, Henry Houlden: None. Nin Bajaj received contracts. Murat Emre has received contracts and honoraria from Teva-Lundbeck, MerkSerono, Novartis, Orion, Boehringer-Ingelheim and sits on advisory boards for Teva-Lundbeck, MerckSerono, Novartis, Orion Pharmaceuticals. John Hardy has received contracts and honoraria from Merck and Boehringer-Ingelheim and sits on advisory boards for Eisai and MerckSerono. Kailash P. Bhatia has received contracts and honoraria from GSK, Boehringer-Ingelheim, Ipsen, Merz, and Orion Pharmaceuticals. Andrew J. Lees has received contracts and honoraria from Britannia, Novartis, Roche, GSK, Boehringer, Solvey, Tiva, Eli Lilly, Pfizer, Medtronic, Valeant and Orion Pharmaceuticals.
Additional Supporting Information may be found in the online version of this article.
Author Roles: Coro Paisán-Ruiz carried out and supervised the molecular work. Rosa Guevara and Monica Feder-off assisted with the molecular work. Elaha Elahe, Susanne A. Schneider, Petra Schwingenschuh, Nin Bajaj, Murat Emre, Kailash P. Bhatia, Andrew J. Lees carried out the clinical investigations, Sebastian Brandner carried out the pathological investigation. John Hardy, Henry Houlden, Coro Paisán-Ruiz and Andrew B. Singleton obtained funding to support the work, John Hardy and Coro Paisán-Ruiz drafted the manuscript which was edited by Susanne A. Schneider, Kailash P. Bhatia, Andrew J. Lees and Henry Houlden.
References
- 1.Davison C. Pallido-pyramidal disease. J Neuropathol Exp Neurol. 1954;13:50–59. doi: 10.1097/00005072-195401000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Panagariya A, Sharma B, Dev A. Pallido-pyramidal syndrome: a rare entity. Indian J Med Sci. 2007;61:156–157. [PubMed] [Google Scholar]
- 3.Tranchant C, Boulay C, Warter JM. Pallido-pyramidal syndrome: an unrecognized entity. Rev Neurol (Paris) 1991;147:308–310. [PubMed] [Google Scholar]
- 4.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose H, Ohye T, Takahashi E, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 6.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 7.Lucking CB, Durr A, Bonifati V, et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. New Eng J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 8.Wickremaratchi MM, Majounie E, Morris HR, et al. Parkin-related disease clinically diagnosed as a pallido-pyramidal syndrome. Mov Disord. 2009;24:138–140. doi: 10.1002/mds.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifati V, Rohé CF, Breedveld GJ, et al. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa Y, Graf WD, Wong H, et al. Dopa-responsive dystonia simulating spastic paraplegia due to tyrosine hyroxilase (TH) gene mutations. Neurology. 2001;56:260–263. doi: 10.1212/wnl.56.2.260. [DOI] [PubMed] [Google Scholar]
- 11.Clot F, Grabli D, Cazeneuve C, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with Dopa-responsive dystonia. Brain. 2009;132:1753–1763. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- 12.Tassin J, Dürr A, Bonnet AM, et al. Levodopa-responsive dystonia. GTP cyclohydrolase I or parkin mutations? Brain. 2000;123:1112–1121. doi: 10.1093/brain/123.6.1112. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez A, Heimbach A, Grundemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 14.Paisan-Ruiz C, Bhatia KP, Li A, et al. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65:19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shojaee S, Sina F, Banihosseini SS, et al. Genome-wide linkage analysis of a Parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am J Hum Genet. 2008;82:1375–1384. doi: 10.1016/j.ajhg.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anheim M, Lagier-Tourenne C, Stevanin G, et al. SPG11 spastic paraplegia. A new cause of juvenile parkinsonism. J Neurol. 2009;256:104–108. doi: 10.1007/s00415-009-0083-3. [DOI] [PubMed] [Google Scholar]
- 17.Ning YP, Kanai K, Tomiyama H, et al. PARK9-linked parkinsonism in eastern Asia: mutation detection in ATP13A2 and clinical phenotype. Neurology. 2008;70:1491–1493. doi: 10.1212/01.wnl.0000310427.72236.68. [DOI] [PubMed] [Google Scholar]
- 18.Di Fonzo A, Chien HF, Socal M, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 19.Sina F, Shojaee SE, Elahi E, Paisan-Ruiz C. R632W mutation in PLA2G6 segregates with dystonia-parkinsonism in a consanguineous Iranian family. Eur J Neurol. 2009;16:101–104. doi: 10.1111/j.1468-1331.2008.02356.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Fonzo A, Dekker MC, Montagna P, et al. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology. 2008;72:240–245. doi: 10.1212/01.wnl.0000338144.10967.2b. [DOI] [PubMed] [Google Scholar]
- 21.Najim al-Din AS, Wriekat A, Mubaidin A, et al. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol Scand. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 22.Hampshire DJ, Roberts E, Crow Y, et al. Kufor-Rakeb syndrome, pallido-pyramidal degeneration with supranuclear upgaze paresis and dementia, maps to 1p36. J Med Genet. 2001;38:680–682. doi: 10.1136/jmg.38.10.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DR, Hadeed A, al-Din AS, et al. Kufor Rakeb disease: autosomal recessive, levodopa-responsive parkinsonism with pyramidal degeneration, supranuclear gaze palsy, and dementia. Mov Disord. 2005;20:1264–1271. doi: 10.1002/mds.20511. [DOI] [PubMed] [Google Scholar]
- 24.Lees AJ, Singleton AB. Clinical heterogeneity of ATP13A2 linked disease (Kufor-Rakeb) justifies a PARK designation. Neurology. 2007;68:1553–1554. doi: 10.1212/01.wnl.0000265228.66664.f4. [DOI] [PubMed] [Google Scholar]
- 25.Khateeb S, Flusser H, Ofir R, et al. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan NV, Westaway SK, Morton JE, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartig MB, Hörtnagel K, Garavaglia B, et al. Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann Neurol. 2006;59:248–256. doi: 10.1002/ana.20771. [DOI] [PubMed] [Google Scholar]
- 28.Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009;46:73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevanin G, Santorelli FM, Azzedine H, et al. Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nat Genet. 2007;39:366–372. doi: 10.1038/ng1980. [DOI] [PubMed] [Google Scholar]
- 30.Hehr U, Bauer P, Winner B, et al. Long-term course and mutational spectrum of spatacsin-linked spastic paraplegia. Ann Neurol. 2007;62:656–665. doi: 10.1002/ana.21310. [DOI] [PubMed] [Google Scholar]
- 31.Paisan-Ruiz C, Dogu O, Yilmaz A, et al. SPG11 mutations are common in familial cases of complicated hereditary spastic paraplegia. Neurology. 2008;70:1384–1389. doi: 10.1212/01.wnl.0000294327.66106.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn NP, Goadsby PJ, Lees AJ. Hereditary Juvenile parkinsonism with pyramidal signs and mental retardation. Eur J Neurol. 1995;2:23–26. doi: 10.1111/j.1468-1331.1995.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 33.Schneider SA, Paisan-Ruiz C, Quinn NP, Lees AJ, Houlden H, Hardy J, Bhatia KP. ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov Disord. 2010 Mar 22; doi: 10.1002/mds.22947. [DOI] [PubMed] [Google Scholar]
- 34.Hanagasi HA, Lees A, Johnson JO, et al. Smoking-responsive juvenile-onset Parkinsonism. Mov Disord. 2007;22:115–118. doi: 10.1002/mds.21177. [DOI] [PubMed] [Google Scholar]
- 35.Gibbs JR, Singleton A. Application of genome-wide single nucleotide polymorphism typing: simple association and beyond. PLoS Genet. 2006;2:e150. doi: 10.1371/journal.pgen.0020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camargos S, Scholz S, Simon-Sanchez J, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- 37.Hardy J, Lewis P, Revesz T, et al. The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Dogu O, Johnson J, Hernandez D, et al. A consanguineous Turkish family with early-onset Parkinson’s disease and an exon 4 parkin deletion. Mov Disord. 2004;19:812–816. doi: 10.1002/mds.20028. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Cardozo T, Lovering RC, et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Family A (PLA2G6) (Video of the pro-band). At 29 years of age she is in a wheelchair. There is a mild postural tremor of both hands and some dystonic posturing and she is slow in her movements. On testing eye movements, pursuits are broken and there is limitation of upgaze.
At age 32 years she requires nasogastric feeding and has facial hypomimia, dystonic facial grimacing and spontaneous tongue protrusion (not related to Levodopa). There is severe dystonic posturing in her upper limbs and a bilateral resting tremor, greater on the left.
Family A (PLA2G6) (Video of the cousin). The patient is now age of 33 years and in a wheelchair. This video essentially demonstrates the bilateral brisk reflexes and the Babinski signs to show the marked pyramidal component of the syndrome.
She has facial hypomimia and square wave jerks on primary gaze with slowed pursuit movements but of full range. Eyelid opening apraxia and a supranuclear vertical gaze palsy is demonstrated (although in the video the patient’s eyes were forcefully retracted which can exaggerate this appearance). She is dysarthric with slow tongue movements. There are orolingual dyskinesias probably related to Levodopa treatment. There is a pill rolling rest tremor of her left hand, mild bilateral postural arm tremor (left more than right) and bilateral bradykinesia.
Family B (ATP13A2) (Video of the proband). The initial segment shows him at the age of 16 after he had an acute hypomanic psychotic episode associated with a phenothiazine induced akinetic rigid syndrome (June 1985), this improved after a few weeks but left him with mild parkinsonian features with cog-wheel rigidity clearly demonstrated in October 1985. The second part of the video shows him at the age of 40 when his disease had progressed. He was anarthric and confined to his bed. He has facial hypomimia and risus sardonicus. There is reduced upgaze with broken pursuit and slow saccades with occasional blepharospasm. There is marked dystonic posturing and increased tone in his upper and lower limbs with little movement, there was upper limb bradykinesia and the lower limb reflexes were brisk.
Family B (ATP13A2) (Video of the cousin). The patient showed slow, stooped, dragging gait at the age of 25 years.
Segment 7. Family C (FBX07) (Video of the proband). At the age of 22 years this girl had slow monotonous speech, facial hypomia and hirsutism. During the examination of eye movements (at the start and in between) she has blinking and difficulty opening her eyes (possible apraxia of eye opening or blepharospasm). There is slowed and limited upgaze. She has bradykinesia and a postural but no rest tremor. Planters were extensor and her gait was stooped and slow with poor arm swing.
Segment 8. Family E (SPG11). There is a stiff, spastic gait with difficulty turning. When holding the arms outstretched, there is dystonic posturing of the arms. On writing, there is micrographia and a small amplitude tremor (best seen when drawing a spiral).
Segment 9. Family F (PLA2G6) (Video of three Iranian cases). Case DP5: The patient showed stooped, slow shuffling gait with hypomimic face and drooling. Upper limb bradykinesia and poor tandem walking with a tendency to fall can also be observed. There is no speech and reduced vertical up gaze. Case DP3: The patient is confined to his bed with severe dystonic posturing of his upper and lower limbs. He has no speech and swallowing difficulties. Case DP4: The patient is confined to his bed with severe dystonic posturing of his upper and lower limbs and involuntary movements.