Abstract
Synaptotagmin I is a synaptic vesicle-associated protein essential for synchronous neurotransmission. We investigated its impact on the intracellular Ca2+-dependence of large dense-core vesicle (LDCV) exocytosis by combining Ca2+-uncaging and membrane capacitance measurements in adrenal slices from mouse synaptotagmin I null mutants. Synaptotagmin I-deficient chromaffin cells displayed prolonged exocytic delays and slow, yet Ca2+-dependent fusion rates, resulting in strongly reduced LDCV release in response to short depolarizations. Vesicle recruitment, the shape of individual amperometric events, and endocytosis appeared unaffected. These findings demonstrate that synaptotagmin I is required for rapid, highly Ca2+-sensitive LDCV exocytosis and indicate that it regulates the equilibrium between a slowly releasable and a readily releasable state of the fusion machinery. Alternatively, synaptotagmin I could function as calcium sensor for the readily releasable pool, leading to the destabilization of the pool in its absence.
The release of neurotransmitters from nerve terminals and hormones from neuroendocrine cells occurs through exocytosis of secretory vesicles in response to increases in the intracellular Ca2+ concentration [Ca2+]i (1). The supralinear Ca2+ dependence of neurosecretion suggests that the binding of at least 3–5 Ca2+ ions to Ca2+-sensing entities on the fusion machinery is required to trigger the rapid fusion of secretory vesicle with the plasma membrane (2–6). At present, the exact mechanism of Ca2+-dependent exocytosis and the molecular identity of the involved Ca2+ sensor(s) remain matters of debate.
Numerous studies indicate that the synaptic vesicle protein synaptotagmin I, a brain-enriched member of the synaptotagmin family, plays a key role in Ca2+-dependent neurosecretion. Synaptotagmin I has been described to interact with several synaptic proteins including the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins syntaxin (7) and SNAP-25 (8), the assembled SNARE complex (9–11), and the clathrin assembly protein complex AP-2 (12). Through the first of its two C2 domains (C2A), synaptotagmin I binds Ca2+ and rapidly interacts with phospolipid membranes in a Ca2+-dependent manner (9, 13). Functional evidence has been presented that implicate synaptotagmin I in vesicle docking (14), fusion (15–20), and recycling (21). Most strikingly, gene mutation studies in mice (20, 22) demonstrated that synaptotagmin I is specifically required for rapid synchronous neurotransmission but not for asynchronous or Ca2+-independent release (i.e., spontaneous release and release triggered by hypertonic solutions or α-latrotoxin). This finding led to the hypothesis that synaptotagmin I is the major Ca2+ sensor for rapid exocytosis. However, other explanations for the impairment of synchronous release in synaptotagmin I mutants cannot be excluded. For example, synaptotagmin I could regulate the activity of a different Ca2+ sensor or act as a linker mediating the tight coupling between Ca2+ channels and the exocytic machinery (23).
The impact of synaptotagmin I on the intracellular Ca2+ dependence of the vesicle fusion reaction, although crucial for the understanding of its exact role, has not yet been determined in a direct and quantitative manner. To provide this information, we studied large dense-core vesicle (LDCV) exocytosis in adrenal chromaffin cells from control and synaptotagmin I null mutant mice by using flash photolysis of caged Ca2+. Because flash uncaging raises [Ca2+]i in a spatially homogenous manner, we could use fluorescent Ca2+ indicators to directly monitor the biologically relevant [Ca2+]i at the exocytic Ca2+ sensor, whereas the kinetics of exocytosis were simultaneously determined by using high time-resolution membrane capacitance (Cm) measurements. Using these methods, we demonstrate that synaptotagmin I-deficient chromaffin cells display a selective loss of functional readily releasable vesicles. The results are discussed in the framework of our current model for LDCV exocytosis in chromaffin cells.
Materials and Methods
Adrenal glands were removed from newborn offspring of synaptotagmin I (+/−) mice (20), and 80- to 100-μm thick slices were prepared as described (6). After the electrophysiological recordings, we genotyped newborn mice independently, by using PCR and synaptotagmin I-specific primers. Because no significant differences were observed between synaptotagmin I (+/+) and (+/−) cells, data from both groups were pooled and termed control. Synaptotagmin I (−/−) cells were termed mutant.
Adrenal slices were bathed in a solution containing 125 mM NaCl, 26 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 0.2 d-tubocurarine bubbled with 5% CO2, 95% O2. Whole-cell patch-clamp recordings (24) were performed by using a pipette solution containing 110 mM Cs-glutamate, 8 mM NaCl, 3.5 mM CaCl2, 5 mM nitrophenyl-EGTA, 2 mM MgATP, 0.3 mM Na2-GTP, 0.3 mM Furaptra, 0.2 mM Fura-2, and 20 mM Cs-Hepes (pH 7.2). Measurement of intracellular calcium, flash photolysis of NP-EGTA, and Cm measurements were performed as described (6).
Carbon fiber electrodes were prepared from 10 μM diameter fibers (Amoco Performance Products, Greenville, SC) as described (25). A constant voltage of 780 mV vs. Ag/AgCl reference was applied to the electrode, the tip of which was gently pressed against the cell surface. The amperometric current was sampled at 10 kHz and digitally filtered at 1 kHz. Analysis of single spikes was as described (26).
Results and Discussion
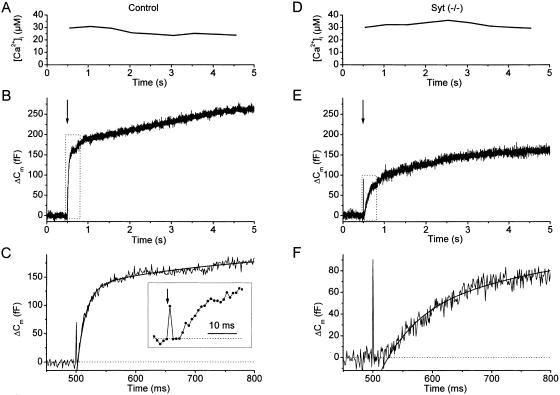
Fig. 1 A–C shows the exocytic response of a control chromaffin cell to a flash-induced elevation of [Ca2+]i to ≈30 μM (Fig. 1A). The resulting Cm increase (Fig. 1B) consisted of a fast initial phase, the exocytic burst, followed by a slower sustained phase of secretion (27). Detailed kinetic analysis of the initial phase of the flash response (Fig. 1C) revealed a short exocytic delay between the flash-induced rise in [Ca2+]i and the onset of the Cm increase (≈3 ms in this example; Fig. 1C Inset) (6). The exocytic burst could be fitted by the sum of two exponential terms (smooth line in Fig. 1C). The fast and slower component correspond to the exocytosis of vesicles from two distinct populations of fusion-competent vesicles: the readily releasable pool (RRP) and the slowly releasable pool (SRP). For this control cell, this procedure yielded an RRP size of 135 fF with a fusion rate constant of 76.5 s−1, and an SRP size of 76 fF with a fusion rate constant of 5.6 s−1. A similar experiment performed on a chromaffin cell from a synaptotagmin I (−/−) mouse is shown in Fig. 1 D–F. When compared to the results from the control cell, it can be immediately appreciated that, despite a similar postflash [Ca2+]i (Fig. 1D), the total response was smaller and the initial Cm rise was slower (Fig. 1E). Moreover, there was a much longer delay between the rise in [Ca2+]i and the onset of Cm increase (≈25 ms in this example; Fig. 1F). In contrast to controls, the exocytic burst of mutant cells could be well fitted by a single exponential with, for this example, an amplitude of 86 fF and a fusion rate constant of 6.3 s−1 (smooth line in Fig. 1F).
Figure 1.
Secretory responses to flash-induced [Ca2+]i steps in a control and a mutant cell. (A) After whole-cell dialysis of a control cell, a brief UV flash (arrow in B) caused a rapid and sustained [Ca2+]i increase to ≈30 μM. (B) The resulting Cm increase consisted of a rapid initial phase (exocytic burst) followed by a sustained phase. (C) First 300 ms of the flash response (boxed area in B) with a double-exponential fit overlaid (smooth line). (C Inset) The first 30 ms after the flash, illustrating the brief delay between the flash (arrow) and the onset of the Cm rise. (D–F) A similar experiment performed in mutant cell. Despite a [Ca2+]i increase of similar amplitude (D), the exocytic burst was smaller, had a slower time course, and a longer exocytic delay (E and F).
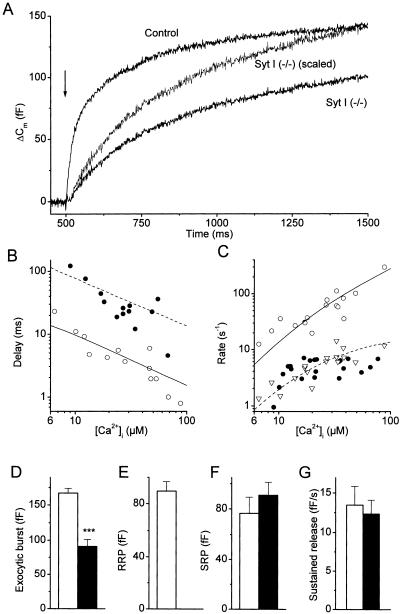
Fig. 2A compares the first second of the averaged flash responses from control and mutant cells. Although we found robust secretory responses in both cell types, it appeared that mutant cells lacked the rapid initial phase in the exocytic burst. Because these differences were observed with spatially homogenous [Ca2+]i steps of similar amplitude, we can exclude that the main function of synaptotagmin I is to link the fusion machinery to Ca2+ channels. A quantitative analysis of the intracellular Ca2+ dependence of LDCV fusion in control and mutant cells is provided in Fig. 2 B and C, showing the exocytic delays and fusion rate constants for postflash [Ca2+]i levels between 8 and 100 μM. For both control and mutant cells, the exocytic delays became shorter with increasing [Ca2+]i (Fig. 2B). Despite some scatter, it can be readily appreciated that the exocytic delays were ≈10 times longer in the mutants. These longer exocytic delays correspond to the delays that were previously determined for the fusion of vesicles from the SRP (6). Fig. 2C plots the exocytic rate constants vs. [Ca2+]i. In control cells, the rate constants of the fast and slow component of the exocytic burst increased with higher [Ca2+]i levels and differed by approximately 1 order of magnitude over the [Ca2+]i range tested. For mutant cells, the exocytic burst generally had a monoexponential time course and the corresponding fusion rate constants matched with the rate constants of the slow component in the control cells. The Ca2+ dependence of the fusion reaction for the mutant cells and for the fast kinetic component of the exocytic burst in control cells could be described by kinetic schemes in which three reversible Ca2+-binding reactions precede an irreversible fusion reaction (dashed and solid lines in Fig. 2 B and C). The Ca2+-binding and -unbinding rate constants in mutant cells were approximately 1 order of magnitude lower than for the RRP fusion in control cells but very similar to previously published values for fusion from the SRP (6).
Figure 2.
Effect of synaptotagmin I deletion on the Ca2+-dependent kinetics of LDCV exocytosis. (A) Comparison of the averaged exocytic burst in control cells (n = 19 cells; N = 5 animals) and mutant cells (n = 22; N = 6). The Cm trace from the mutants also was normalized to the Cm increase in control cells 1 s after the flash, to illustrate the difference in time course between both groups. The average [Ca2+]i after the flash was almost identical in both groups (28.1 ± 4.7 μM and 28.0 ± 4.2 μM for control and null mutants, respectively). (B) Ca2+ dependence of the delays between the [Ca2+]i rise and the onset of release for control (○) and mutants (●). Data were corrected for the delay introduced by the flash time course (500 μs) and the time required for Ca2+ to be released from photolysed NP-EGTA (100 μs). (C) Ca2+ dependence of the rate constants (i.e., the reciprocal of the exponential time constants) of the fast (○) and slow (▵) kinetic component of the exocytic burst in control cells and of the single kinetic component in null mutants (●). Included in B and C are the best fits with a kinetic model in which three reversible Ca2+-binding reactions precede an irreversible fusion reaction (6). The following parameters were obtained: kon = 4.5 s−1, koff = 50 μM−1⋅s−1, γ = 1,600 s−1 for control (solid lines) and kon = 0.6 s−1, koff = 4 μM−1⋅s−1, γ = 18 s−1 for mutants (dotted lines). (D–G) Comparison of the amplitude of the exocytic burst (D), the RRP (E), and the SRP (F), and of the rate of sustained release (G) for control (hollow bars) and mutant (solid bars) cells. ***, P < 0.001 (Student's unpaired t test).
Statistical analysis revealed that the amplitude of the exocytic burst, which is equivalent to the total amount of fusion-competent vesicles, was reduced by ≈50% in the mutants (Fig. 2D). This reduction could be completely attributed to the absence of fusion of readily releasable vesicles (Fig. 2E) because there was no significant difference in the amount of slowly releasable vesicles (Fig. 2F). The rate of sustained release, determined as the slope of a line fitted to the Cm traces between 2 and 5 s after a flash, was not affected in the null mutants (Fig. 2G), indicating that vesicle supply does not critically depend on synaptotagmin I.
Lower time resolution (0.5 Hz) measurements of Cm changes after a flash revealed that the ability to restore the membrane area to the prestimulus level was not affected in mutant cells (data not shown). Endocytic membrane retrieval followed an approximately mono-exponential time course with time constants of 17.2 ± 2.0 s (n = 8) and 16.9 ± 2.7 s (n = 8) for wild-type and mutant cells, respectively. We conclude that synaptotagmin I is not required for slow, compensatory endocytosis in chromaffin cells.
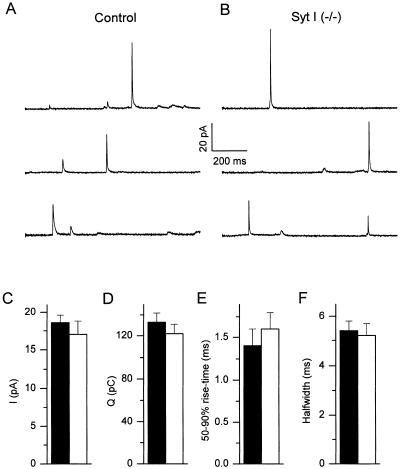
To investigate whether the absence of synaptotagmin I affects the properties of individual fusion events, we combined Cm measurements with carbon fiber amperometry on single chromaffin cells that were dialyzed with a high [Ca2+]i solution. Under these conditions, amperometric spikes could be measured from both control (Fig. 3A) and mutant cells (Fig. 3B). Analysis of single amperometric spikes, which correspond to the oxidation of catecholamines contained in individual LDCVs (28, 29), revealed no significant changes between control and mutant cells with regard to the peak current, the current integral, 50–90% rise-time and the spike half-width (Fig. 3 C–F). Moreover, averaging of Cm traces after alignment according to amperometric events (30) revealed that the mean Cm increase because of fusion of a single LDCV was not different between control and mutants, with an average Cm increase of 530 aF for control and 549 aF for mutant cells. Taken together, these results indicate that synaptotagmin I does not affect the size of individual LDCVs, the intravesicular catecholamine concentration, or the rate of catecholamine discharge once fusion has been initiated.
Figure 3.
Amperometric spikes in control and mutant cells. (A and B) Examples of amperometric recordings from control (A) and mutant (B) chromaffin cells. (C–F) Average values for peak current (Ipeak), current integral (Q), and 50–90% rise-time and half-width for single spikes from control (solid bars) and mutant (hollow bars) chromaffin cells. P > 0.2 (Student's unpaired t test) for all four parameters.
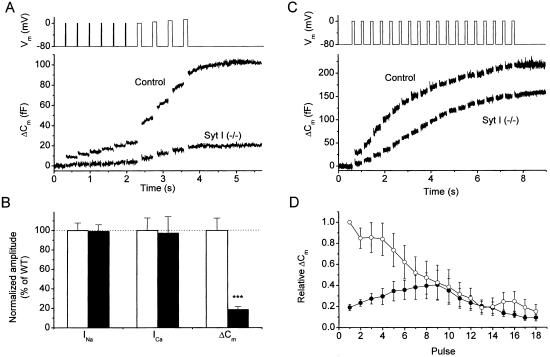
The physiological trigger for LDCV exocytosis in chromaffin cells is depolarization-induced Ca2+ influx through voltage-gated Ca2+ channels. To test the implications of synaptotagmin I on the responses to physiological stimuli, we stimulated chromaffin cells with a voltage protocol consisting of six 10-ms depolarizations followed by four 100-ms depolarizations delivered 300 ms apart (Fig. 4A). In control cells, the 10-ms stimuli caused significant Cm increases, which originate from fusion of a fraction of the RRP that is closely associated with Ca2+ channels (31). The subsequent 100-ms depolarizations resulted in a second bout of secretion, which mainly reflects the fusion of the remainder of the RRP and probably only a small fraction of the SRP (31). Depolarization-induced secretion was drastically reduced in mutant cells, with a total Cm increase amounting to <20% of control (Fig. 4 A and B). Analysis of the depolarization-induced currents revealed that the reduced secretory response was not the consequence of any changes in the amplitude of voltage-dependent inward currents (Fig. 4B). A stronger electrical stimulation protocol consisting of 18 100-ms depolarizations delivered at a frequency of 2.5 Hz elicited robust secretory responses in both control and mutant chromaffin cells (Fig. 4C). In control cells, the first depolarization of a train led to the largest Cm increase, with subsequent pulses causing progressively smaller responses (Fig. 4D). In contrast, the Cm increases became gradually larger during the first 8–10 pulses in mutant cells and progressively decreased for later stimuli (Fig. 4D). Absolute Cm increases in the mutants were smaller than control until the sixth pulse of train, and subsequent pulses caused comparable Cm responses for both control and mutant cells (Fig. 4D). We conclude that the absence of highly release-competent vesicles in the mutants leads to a strong inhibition of the secretory response to short depolarizations. The facilitating secretory response in mutant cells to a train of electrical stimuli most likely corresponds to the direct fusion of vesicles from the SRP. Indeed, we measured that the global [Ca2+]i reached values between 10 and 50 μM during such trains (data not shown), which is sufficient to trigger considerable fusion from the SRP (Fig. 2 B and C and ref. 6).
Figure 4.
Effect of synaptotagmin I deletion on depolarization-induced exocytosis. (A) Voltage protocol (Upper) and average Cm response in control (n = 16; N = 5) and mutant cells (n = 21; N = 6). (B) Comparison of the amplitude of voltage-dependent Na+ (INa) and Ca2+ currents (ICa) and of the total Cm increase in A for control (hollow bars) and mutant (solid bars) cells. ***, P < 0.001 (Student's unpaired t test). INa and ICa were determined during the first depolarization to 0 mV as the maximal inward current and the current after 10 ms, respectively. Values were normalized to the control values. (C) Average Cm increase in control (n = 9; N = 3) and mutant cells (n = 8; N = 3) in response to a 2.5-Hz train of 100-ms depolarizations (Upper). (D) Cm increases to individual depolarizations in a train for control (○) and mutants (●) are displayed after normalization to the Cm increase to the first pulse in control cells.
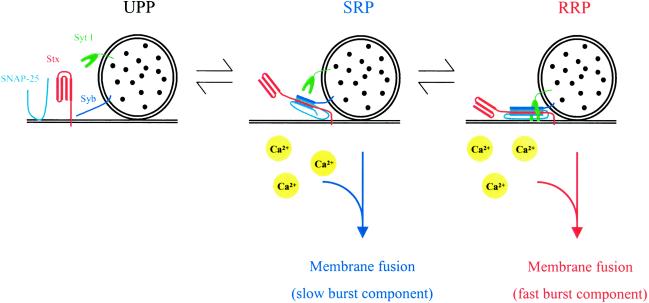
Our working hypothesis concerning the sequence of molecular events that lead to LDCV exocytosis is illustrated in Fig. 5. Fusion-competent vesicles are docked at the plasma membrane and subsequently “primed” for fusion because of the formation of trans-SNARE complexes between the vesicle SNARE synaptobrevin and the plasma membrane SNAREs syntaxin 1 and SNAP-25 (32–35). The combination of rapid Ca2+ uncaging and Cm measurements allows to distinguish two separate pools of fusion-competent vesicles in chromaffin cells, termed SRP and RRP. These two pools are represented by vesicles containing, respectively, immature (loose) and fully assembled (tight) trans-SNARE complexes (33) or oligomers of such. Vesicles residing in either SRP or RRP can undergo Ca2+-triggered exocytosis in two separate fusion reactions, involving Ca2+ sensors with distinct Ca2+-binding properties (6). Equilibrium between both pools is rapid (τ ∼ 4s) (31), Ca2+-independent (6), and can be shifted toward the SRP by manipulations that impair tightening or maturation of SNARE complexes (27, 33, 36). We now show that the fast fusion from the RRP is completely abolished in chromaffin cells from synaptotagmin I null mutants, whereas the fusion from the SRP and vesicle recruitment to the SRP are not affected. These findings clearly indicate that synaptotagmin I plays an essential role downstream of vesicle priming. In principle, the lack of a fast secretory component in the flash responses from the null mutants could have two causes, which are not necessarily mutually exclusive: (i) a defective rapid Ca2+-dependent fusion reaction from the RRP and (ii) a complete absence of an RRP (note that, for chromaffin cell exocytosis, we distinguish between SRP and RRP, two pools of vesicles, which may be quite similar to subsets of the RRP of hippocampal neurons). However, if the synaptotagmin I deletion would prevent only readily releasable vesicles from fusing through the rapid fusion reaction (without affecting the formation and stability of vesicles in the RRP), one would expect the total amount of vesicles in the SRP and RRP to be identical in control and mutant cells, provided the SRP and RRP are fully in equilibrium with each other. Then, sustained Ca2+ stimuli (e.g., a flash or a train of strong depolarizations) would cause normal amounts of secretion in the mutant cells, albeit with a slower time course because of the defective fast fusion reaction. In contrast, we found that secretory responses to such sustained stimuli were not only slower but also significantly smaller in the mutants (Figs. 2A and 4C). One straightforward explanation for these findings is that synaptotagmin I is required for formation and/or stability of the RRP, e.g., by promoting the tightening of preassembled trans-SNARE complexes or else by stabilizing such complexes (Fig. 5). An alternative explanation is that formation of the readily releasable vesicles is normal in the mutants but that these vesicles are not stimulated to fuse by calcium in the absence of synaptotagmin I, thereby secondarily destabilizing the pool. A third, least likely, possibility is that the synaptotagmin I deletion prevents these vesicles both from fusing and returning to the slowly releasable state.
Figure 5.
Hypothetical model describing the role of SNARE complex assembly and synaptotagmin I in the last steps leading to LDCV secretion. Docked LDCVs can be subdivided in three vesicle pools. Vesicles in the UPP (unprimed pool) lack trans-SNARE complexes and, hence, are not fusion-competent. Vesicles in the SRP contain loose trans-SNARE complexes and can undergo slow Ca2+-dependent exocytosis. The RRP contains vesicles with tight trans-SNARE complexes, which can undergo rapid Ca2+-dependent exocytosis. Synaptotagmin I regulates the transition from SRP to RRP. See text for more details. Syt I, synaptotagmin I; Stx, syntaxin; Syb, synaptobrevin.
Our data demonstrate that synaptotagmin I is a necessary factor for rapid Ca2+-dependent fusion of LDCVs. However, attributing the role of the rapid Ca2+ sensor exclusively to synaptotagmin I does not explain why other manipulations, such as cleavage of SNAP-25 by botulinum toxin A (27), overexpression of a truncated form of SNAP-25 (36), or infusion of an antibody against synaptobrevin (33) cause a loss of the rapid fusion reaction similar to that observed in the synaptotagmin I null mutant. The data together indicate that rapid Ca2+ sensing requires both synaptotagmin I and intact, tightly assembled SNAREs. Interestingly, the assembled SNARE complex itself contains several binding sites for divalent cations, which could be involved in the Ca2+-sensing step (37). An intriguing possibility is that these binding sites form a slow Ca2+ sensor and that synaptotagmin I converts it into a faster Ca2+ sensor, either through an allosteric effect or by directly contributing additional ligands to a Ca2+-binding pocket. However, if the alternative explanation is correct and synaptotagmin I functions as one of several Ca2+ sensors in fast exocytosis, its Ca2+-dependent interactions with phospholipids may be the key to its effect as indicated in the studies with point mutants in mice (22).
In summary, we have presented evidence that synaptotagmin I is required after priming and is an indispensable element of rapid Ca2+-dependent fusion of LDCVs. Moreover, our data suggest that the absence of synaptotagmin I destabilizes the equilibrium between the slowly and the readily releasable state of the LDCV fusion machinery. This could occur by a function of synaptotagmin I in the maturation of the readily releasable state, or in the calcium triggering of this state, although the biochemical correlates of such functions remain elusive. In contrast to chromaffin cells, a transition between two states of secretory competence has not yet been demonstrated for small synaptic vesicles at fast synapses. However, two populations of releasable vesicles with distinct release probabilities have been described for large nerve terminals (38–40). Likewise, the releasable pool in hippocampal cells, whose size (probed by using hypertonic solutions; ref. 41) does not depend on synaptotagmin I (20), contains vesicles with both low and high release probability (42, 43). Thus, the existence of two states of the exocytic machinery with different Ca2+ sensitivity may be a more general property of neurosecretory systems. Although the regulation of the total releasable pool size is probably different at synapses with well-defined active zones, a mechanism as suggested here (Fig. 5) might be considered as a specific way to explain the impairment of synchronous synaptic transmission in synaptotagmin I mutants described so far (17, 19, 20, 22, 44). Our results do not exclude, however, that in neurons synaptotagmin I acts as a Ca2+ sensor in a more direct sense.
Acknowledgments
We thank R. Fernandez-Chacon for advice and help with the genotyping, J. Rettig, C. Rosenmund, and R. Schneggenburger for critical comments on the manuscript, and M. Pilot for technical assistance. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 523) (to T.M. and E.N.). T.V. is a postdoctoral Fellow of the Fund for Scientific Research, Flanders (Belgium).
Abbreviations
- LDCV
large dense-core vesicle
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- Cm
membrane capacitance
- RRP
readily releasable pool
- SRP
slowly releasable pool
- [Ca2+]i
intracellular Ca2+ concentration
References
- 1.Neher E. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 2.Dodge F A J, Rahamimoff R. J Physiol (London) 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidelberger R, Heinemann C, Neher E, Matthews G. Nature (London) 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 4.Bollmann J H, Sakmann B, Borst J G. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 5.Schneggenburger R, Neher E. Nature (London) 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 6.Voets T. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Ullrich B, Zhang J Z, Anderson R G, Brose N, Sudhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 8.Schiavo G, Stenbeck G, Rothman J E, Sollner T H. Proc Natl Acad Sci USA. 1997;94:997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis A F, Bai J, Fasshauer D, Wolowick M J, Lewis J L, Chapman E R. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 10.Leveque C, Boudier J A, Takahashi M, Seagar M. J Neurochem. 2000;74:367–374. doi: 10.1046/j.1471-4159.2000.0740367.x. [DOI] [PubMed] [Google Scholar]
- 11.Gerona R R, Larsen E C, Kowalchyk J A, Martin T F. J Biol Chem. 2000;275:6328–6336. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J Z, Davletov B A, Sudhof T C, Anderson R G. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 13.Brose N, Petrenko A G, Sudhof T C, Jahn R. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 14.Reist N E, Buchanan J, Li J, DiAntonio A, Buxton E M, Schwarz T L. J Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bommert K, Charlton M P, DeBello W M, Chin G J, Betz H, Augustine G J. Nature (London) 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- 16.Nonet M L, Grundahl K, Meyer B J, Rand J B. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 17.DiAntonio A, Parfitt K D, Schwarz T L. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 18.Elferink L A, Peterson M R, Scheller R H. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 19.DiAntonio A, Schwarz T L. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 20.Geppert M, Goda Y, Hammer R E, Li C, Rosahl T W, Stevens C F, Südhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen E M, Hartwieg E, Schuske K, Nonet M L, Jin Y, Horvitz H R. Nature (London) 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Chacon R, Königstorfer A, Gerber S H, Garcia J, Matos M F, Stevens C F, Brose N, Rizo J, Rosenmund C, Südhof T C. Nature (London) 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 23.Neher E, Penner R. Nature (London) 1994;372:316–317. doi: 10.1038/372316a0. [DOI] [PubMed] [Google Scholar]
- 24.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 25.Schulte A, Chow R H. Anal Chem. 1996;68:3054–3058. doi: 10.1021/ac960210n. [DOI] [PubMed] [Google Scholar]
- 26.Voets T, Toonen R F, Brian E C, de Wit H, Moser T, Rettig J, Sudhof T C, Neher E, Verhage M. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 27.Xu T, Binz T, Niemann H, Neher E. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 28.Wightman R M, Jankowski J A, Kennedy R T, Kawagoe K T, Schroeder T J, Leszczyszyn D J, Near J A, Diliberto E J, Jr, Viveros O H. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow R H, von Ruden L, Neher E. Nature (London) 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 30.Chow R H, Klingauf J, Heinemann C, Zucker R S, Neher E. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 31.Voets T, Neher E, Moser T. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- 32.Sollner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 33.Xu T, Rammner B, Margittai M, Artalejo A R, Neher E, Jahn R. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- 34.Jahn R, Sudhof T C. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 35.Brose N, Rosenmund C, Rettig J. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 36.Wei S, Xu T, Ashery U, Kollewe A, Matti U, Antonin W, Rettig J, Neher E. EMBO J. 2000;19:1279–1289. doi: 10.1093/emboj/19.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasshauer D, Sutton R B, Brunger A T, Jahn R. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L G, Borst J G. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- 39.Burrone J, Lagnado L. J Neurosci. 2000;20:568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaba T, Neher E. J Neurosci. 2001;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 42.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 43.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 44.Littleton J T, Stern M, Schulze K, Perin M, Bellen H J. Cell. 1993;74:1125–1334. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]