Abstract
Despite the wide success of antibiotics in modern medicine, the treatment of bacterial infections still faces critical challenges, especially due to the rapid emergence of antibiotic resistance. As a result, local antimicrobial treatment aimed at enhancing drug concentration at the site of infection while avoiding systemic exposure is becoming increasingly attractive, as it may alleviate resistance development. Meanwhile, therapeutic nanoparticles, especially liposomes, polymeric nanoparticles, dendrimers, and inorganic nanoparticles, are gaining traction to improve the therapeutic efficacy with many applications specifically focused on local antimicrobial treatment. This review highlights topics where nanoparticle-based strategies hold significant potential to advance treatment against local bacterial infections, including (1) promoting antibiotic localization to the pathogen, (2) modulating drug-pathogen interaction against antibiotic resistance, and (3) enabling novel anti-virulence approaches for ‘drug-free’ antimicrobial activity. In each area, we highlight the innovative antimicrobial strategies tailored for local applications and review the progress made for the treatment of bacterial infections.
Keywords: Nanomedicine, nanoparticle, bacterial infection, antimicrobial treatment, local delivery
Graphic Abstract
1. Introduction
During the past decades, the use of antibiotics has achieved profound successes in combating numerous bacterial infections [1, 2]. Traditionally, antibiotics are widely administered through systemic routes for the advantage of reaching broadly distributed pathogenic bacteria. Alternatively, they have also been administered locally, especially for achieving a high drug concentration at the infection site is critical [3]. Local antimicrobial treatment has recently gained increasing attention, owing largely to its benefit of minimizing drug systemic exposure and potentially reducing resistance development [4, 5]. Accordingly, numerous biomaterials and medical devices have been tailored-made to facilitate the administration and management of antibiotics locally, making local treatment favorable for various infections [6–8]. Despite the progress that has been made, local antimicrobial delivery approach still faces various obstacles, including various drug clearance mechanisms upon administration that make local application impractical or ineffective, diffusion barriers within the local environment that prevent drug molecules from reaching bacteria, and drug resistance acquired by target bacteria that diminishes the therapeutic efficacy of antibiotics [9–11]. Collectively, these obstacles highlight the desire to continuously search for alternative and effective local antimicrobial strategies.
Recently, advances in nanotechnology, particularly the development of nanoparticles for drug delivery, have generated significant impact in medicine and healthcare [12, 13]. Nanoparticle delivery systems enhance drug solubility, offer stealth for immune evasion, modulate drug release characteristics, target drug molecules to desired sites, and deliver multiple drugs simultaneously. Due to these unique advantages, they are able to improve the pharmacokinetic profile and therapeutic index of drug payloads when compared with free drug counterparts. A number of nanoparticle-based drug delivery systems have been approved for clinical use including the treatment for infections. Meanwhile, antimicrobial nanoparticle formulations are increasingly investigated and many are under various stages of pre-clinical and clinical tests [14, 15].
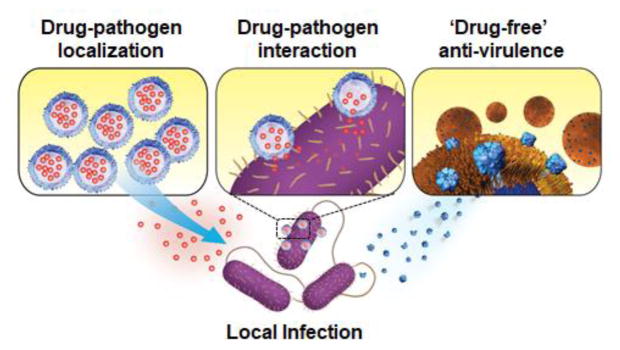
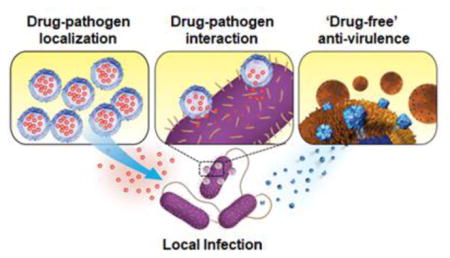
As nanomedicine continually advances, innovative approaches focused on improving local antimicrobial drug delivery are emerging. In this review article, we highlight three aspects where nanoparticle-based delivery strategies have made significant contribution to improve local antimicrobial treatment, namely: (1) promoting antibiotic localization to the pathogen, (2) modulating drug-pathogen interaction to overcome antibiotic resistance, and (3) enabling ‘drug-free’ anti-virulence therapy (Figure 1). Progresses achieved in these aspects not only facilitate the use of existing antibiotics, but also produce entirely new bactericidal mechanisms toward more effective local antimicrobial techniques. Collectively, they address the aforementioned obstacles facing local antibiotic delivery. Herein, we review each aspect with highlights of the current and forthcoming nanoparticle platforms for local antimicrobial drug delivery.
Figure 1.
Schematic summary of nanoparticle-based local antimicrobial drug delivery strategies including promoting antibiotic location to the pathogen (left), modulating drug-pathogen interaction against bacterial drug resistance (middle), and enabling anti-virulence therapy for ‘drug-free’ antimicrobial activity (right).
2. Promoting antibiotic localization to the pathogen
Local administration of antibiotics faces varying transport barriers unique to different organs, tissues, and subcellular compartments, as well as their pathophysiological states, which often prevent effective antibiotic localization to bacteria for activity. For example, at the organ level, flow conditions within the airway of the lung or the lumen of the gastrointestinal tract cause fast drug clearance [16, 17]. The flow also induces high shear forces at sites including the endocardial surface of the heart [18], the lumen of urinary tract [19], and the corneal of the eye [20], further reducing the effective drug retention. At the tissue level, the indiscriminate and uncontrolled diffusion of antibiotics within the local tissue causes off-target drug loss, limiting the effective dosage that can reach bacteria [21, 22]. At the intracellular level, various bacteria survive and multiply within cells, giving rise to a series of chronic infections [23, 24]. Additionally, most antibiotics are hydrophilic and unable to spontaneously cross the plasma membrane of the infected cells [25]. As a result, the standard treatment of intracellular infections remains ineffective. These challenges together have motivated various nanoparticle designs aiming to directly localize antimicrobial drugs onto bacteria.
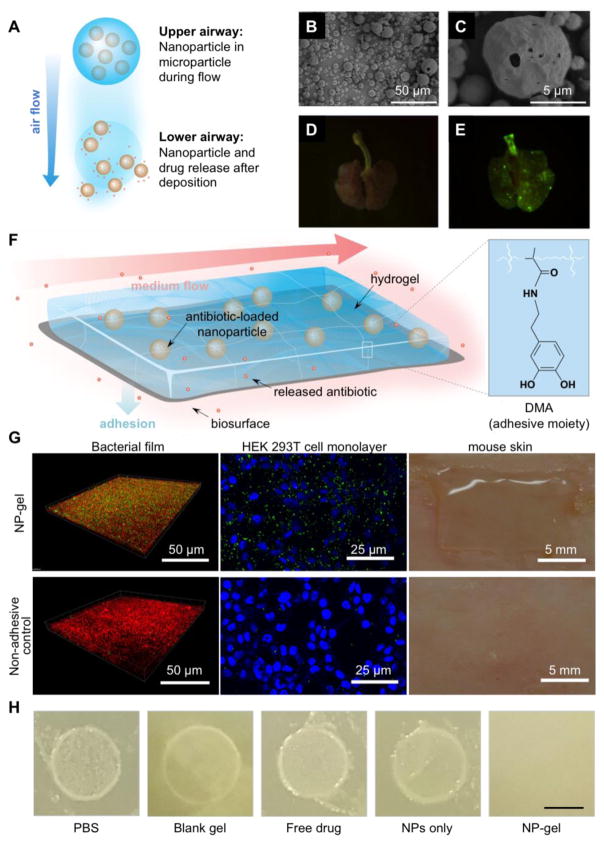
At the organ level, to overcome poor drug retention under various flow conditions, an emerging approach is to integrate nanoparticles into other biomaterial platforms for delivery. Such hybrid design becomes attractive, as it preserves the engineering flexibility and controllability of nanoparticles on drug encapsulation and release, while allowing for retention mechanisms under flow to be independently tuned and optimized. For example, porous microparticles have the features of small mass density and large size, which make them capable of effective deposition to deep lung and escape from the lung’s natural clearance mechanisms [26, 27]. To enhance antimicrobial delivery to lung infection, incorporation of drug-loaded nanoparticles into swellable and respirable microparticles is a promising approach to embracing the virtues of both nano- and micron-scale particles. Upon deposition in the lungs and exposure to the humid environment and the lung lining fluid, the matrix of the microparticles dissolves and readily releases the nanoparticles containing antimicrobial agents such as rifampicin and thymopentin for subsequent therapeutic activity, making the micro-nanoparticle hybrid attractive to treat deep lung infections such as tuberculosis (Figure 2A–E) [28, 29]. Similarly, the hybrid design has also been applied to combine self-propelled and autonomously controlled micromotors with therapeutic nanoparticles for active delivery [30]. In particular, their potential to enhance drug delivery in stomach was recently reported [31]. Zn- and Mg-based micromotors harnessing water or acid in the stomach fluid as fuel were demonstrated as motile carriers with versatile cargo-loading capabilities. In addition, they possessed convective fluid transport that enhanced their mobility and contact probability with targets compared to conventional carriers driven by passive diffusion. Intriguingly, the propulsion of micromotors was demonstrated to provide a driving force strong enough to penetrate the mucus layer and subsequently enhance the payload retention in the stomach. Recently, an enteric micromotor system capable of precise positioning and controllable retention of nanoparticle payloads in desired segments of the gastrointestinal tract was developed, which expanded the potential of nanoparticle-motor hybrid system from stomach to entire gastrointestinal tract for site-specific antibiotic delivery [32]. Meanwhile, to overcome the high shear force under flow conditions, recently, antimicrobial nanoparticles were embedded into hydrogels with a strong bioadhesive property for local drug delivery (Figure 2F–H) [33]. The adhesion was achieved by using cross-linkers functionalized with dopamine methacrylamide, a catechol moiety responsible for marine mussel attachment to a variety of surfaces [34, 35]. With this design, adhesion properties of the hydrogel can be tailored independently to fit the local environment without altering controlled antibiotic release from the nanoparticles. The resulting nanoparticle-hydrogel hybrid material (NP-gel) was able to withstand physiologically relevant shear stresses without detaching from biological surfaces. When ciprofloxacin was encapsulated into the nanoparticles, the NP-gel showed superior inhibition of Escherichia coli (E. coli) bacterial film formation under flow conditions compared to free ciprofloxacin in the same hydrogel. The catechol-based hydrogel has been explored for adhesion inside blood vessels and on atherosclerotic plaques [36]. The success suggests that NP-gel approach is promising to treat infections such as infective endocarditis [37, 38] and urinary tract infections [39], where high shear forces under flow challenge effective antibiotic retention.
Figure 2.
(A) Schematic illustration of inhalable microparticles as carriers for delivery of drug-loaded nanoparticles to the deep lung. (B) Scanning microscopic images of microparticles made from mannitol and leucine using a spray-drying process. The microparticles were loaded with nanoparticles made from glyceryl monostearate and soybean phosphatidylcholine with a double emulsion process. (C) A zoomed-in image of (B). Fluorescence images of lungs from untreated rat (D) and rat after intrapulmonary delivery of microparticles fluorescein-labeled nanoparticles (E). (Reprinted with permission from Ref. 28) (F) Schematic illustration of a nanoparticle–hydrogel hybrid (NP–gel) system with tissue adhesive properties for localized antibiotic delivery under flow conditions. In this design, dopamine methacrylamide (DMA) containing catechol functional group was conjugated into gel matrix for adhesion. (G) NP–gel was tested for adhesion under a flow (shear stress = 3.2 Pa) on E. coli bacterial film (green: nanoparticles in the gel; bacteria: red), HEK 293T cell monolayer (blue: cell nuclei; green: nanoparticles in the gel), and shaved mouse skin. (H) E. coli biofilm formation when the bacteria were treated with PBS, blank gel (gel without nanoparticles or ciprofloxacin), free ciprofloxacin, ciprofloxacin-loaded nanoparticles (without hydrogel), and ciprofloxacin-loaded NP–gel (scale bar = 5 mm). (Reprinted with permission from Ref. 33).
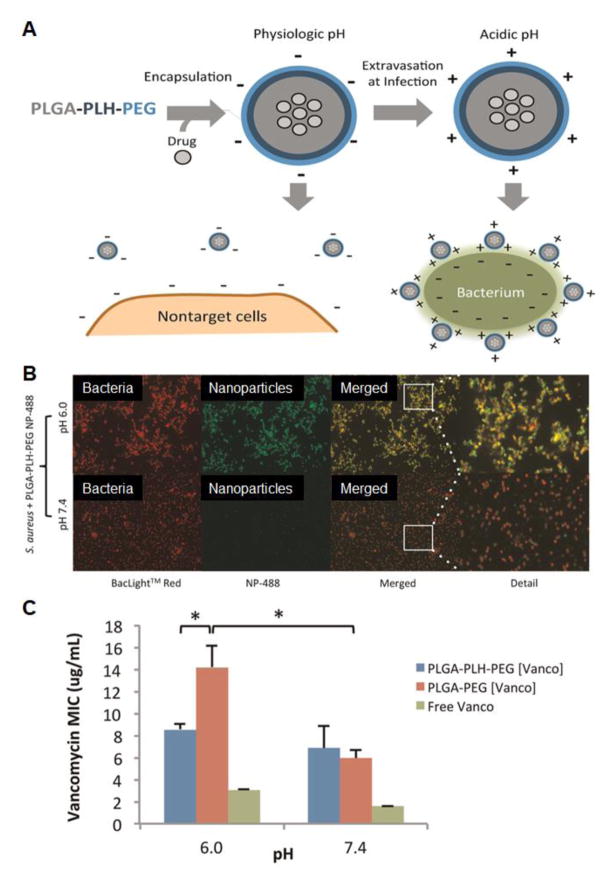
At the tissue level, efficient drug-pathogen localization hinges on a rapid permeation and minimal loss of drug molecules during their transmigration across various types of tissues [21, 22]. Upon reaching the proximity of bacteria, they need to overcome clearance by bacterial metabolism or excretion as well as physical barriers of the infected tissues [40, 41]. The cell wall of pathogenic bacteria has an overall negative charge under physiological conditions; therefore, cationic nanoparticles have been studied to target bacteria through electrostatic interactions [42, 43]. For example, biopolymers including poly(lactic-glycolic acid) (PLGA), poly histidine, and poly(ethylene glycol) (PEG) were conjugated into a tri-block copolymer and used for ‘charge-switching’ nanoparticles. They maintained a negative charge at physiological pH (7.4); however, when exposed to acidic pH levels of some infections, the imidazole groups became the protonated and switched the surface charge to positive, resulting in bacterium-nanoparticle localization and enhanced antibacterial efficacy (Figure 3) [44]. Charge-based nanoparticle-bacterium localization offers the capability of targeting polymicrobial infections, multivalent binding to the pathogen, and increased local densities of the bactericidal components, which together enhance the antimicrobial efficacy [45–47]. Furthermore, cationic peptides can spontaneously insert into and damage negatively charged bacterial cell surfaces.[48, 49] Nanoparticles self-assembled from cationic peptides were shown to cross the blood–brain barrier, hence attractive for brain inflammatory diseases such as meningitis and encephalitis primarily caused by bacteria including Bacillus anthrax, Bacillus subtilis or Staphylococcus aureus (S. aureus) [50, 51]. For more specific localization, conjugating bacterium-specific ligands onto nanoparticle surfaces for active targeting is also a popular strategy. Using this approach, various ligands including small molecules [52–54], proteins or antibodies [55–57], and aptamers [58–60] have been extensively explored and conjugated onto nanoparticles to target pathogenic bacteria. However, the ‘bottom-up’ conjugation method involves ligand identification, purification, and conjugation, all labor intensive and likely impractical. Recently, using plasma membrane derived from natural cells to coat nanoparticles offers a unique ‘top-down’ approach to functionalizing nanoparticles for drug targeting, resulting in a biomimetic technique that overcomes the disadvantages in conjugation methods [61]. In addition, cell membrane provides a natural medium that allows protein anchorage through ligand binding or transmembrane insertion while avoiding chemical conjugations that may compromise protein structural integrity and bio-functionalities [62]. Meanwhile, cellular membranes and particle cores can be independently processed prior to coating, hence offering additional engineering flexibility to assemble multiple-functionalities. Using this strategy, nanoparticles coated with the plasma membrane of human platelets (PNPs) inherited bacterial adherence properties and showed enhanced binding and targeted antibiotic delivery to platelet-adhering pathogens [63]. Although studied with systemic administration, PNPs are expected to facilitate antibiotic localization to various opportunistic bacteria in local infection settings, including strains of staphylococci and streptococci, which exploit platelets through diverse adherence mechanisms for immune evasion and colonization [64].
Figure 3.
(A) Schematic illustration of a pH-responsive anti-microbial nanoparticle design for drug targeting to bacterial cell walls. Poly(lactic-glycolic acid), poly(L-histidine), and poly(ethylene glycol) was conjugated into a triblock copolymer (PLGA-PLH-PEG) for nanoparticle formulation. Drugs are loaded with a double emulsion process. At physiologic pH (7.4), the nanoparticles maintain a slight negative charge. The surface PEGylation also prevents uptake or binding to non-targeted cells or blood components. However, at acidic pH level, the nanoparticles become positively charged and subsequently bind to negatively charged bacteria for antimicrobial activity. (B) S. aureus bacteria (red) were added with the nanoparticles (green). Fluorescence microscopy images show bacterium-nanoparticle co-localization (yellow) at pH 6.0 but not pH 7.4. (C) Different vancomycin formulations were tested against S. aureus. Minimum inhibitory concentrations (MIC) values show the loss of vancomycin activity in non-targeted control nanoparticles (denoted ‘PLGA-PEG[Vanco]’, nanoparticles made from PLGA-PEG di-block polymers without PLH) and free form, but not in PLGA-PLH-PEG nanoparticles (denoted ‘PLGA-PLH-PEG[Vanco]’). (*indicates p<0.05.) (Reprinted with permission from Ref. 44).
Meanwhile, at the intracellular level, various pathogens including Listeria monocytogenes [65, 66], Anaplasma phagocytophilum [67], Shigella [68, 69], Legionella pneumophila [70, 71], and methicillin-resistant S. aureus (MRSA) [72, 73] are also known to invade and survive inside host cells such as epithelial cells and macrophages. As a result, they evade immune clearance and further diminish the efficacy of existing antibiotic treatments [23, 74]. Incomplete clearance of intracellular infection further facilitates their dissemination and subsequent invasion of different cell types [75]. As a result, intracellular infection is often associated with a number of chronic or recurrent infections such as recurrent rhinosinusitis, pulmonary infections, osteomyelitis, and endocarditis [76]. To overcome the cellular barrier, nanoparticles are designed to target infected host cells and thus gain intracellular access for bioactivity [77]. For example, nanoparticles locally administered to the infection sites could be spontaneously taken up by macrophages infected with Mycobacterium tuberculosis (M. tuberculosis), Salmonella typhimurium, or MRSA due to the phagocytic nature of macrophages [78–80]. Enhancement of antibiotic potency with nanoparticles was also observed in human lung epithelial cells and mouse fibroblasts infected with Chlamydia trachomatis [81]. Synthetic nanoparticles made from cationic polymers such as polyethylenimine, chitosan, and polyhexamethylene biguanide, rely on strong charge interactions to enhance uptake by the host cells [82–84]. Modifying nanoparticles with targeting ligands against infected cells also enhances cell uptake [85, 86]. In this regard, various ligands, such as mannose, O-stearoyl amylopectin, maleylated bovine serum albumin, were applied to enhance macrophage uptake of nanoparticles for the treatment of intracellular infection [87, 88].
3. Modulating drug-pathogen interaction to overcome antibiotic resistance
In response to the selective pressure of antibiotics, bacteria acquire resistance through various mechanisms including the prevention of drug entry, the expulsion of drug via active efflux, mutation of targets, and enzymatic inactivation of drug function [89, 90]. By exploiting these mechanisms, resistant strains continually emerge and evolve, making standard antimicrobial treatment increasingly ineffective [91–93]. To counteract antibiotic resistance, nanoparticles are designed to modulate drug-pathogen interaction with emphasis on structural, combinatorial, and responsive dynamics, resulting in new, effective formulations to combat local infections.
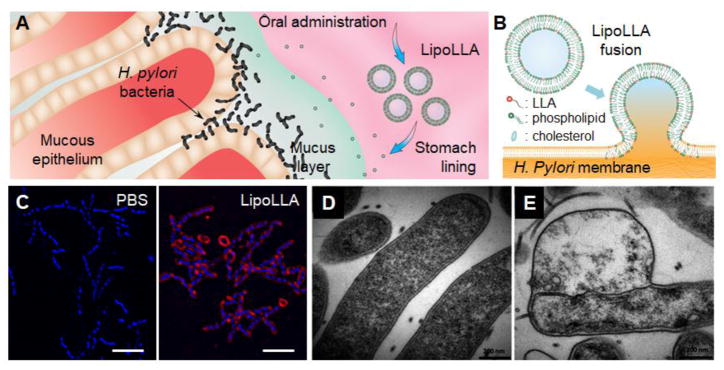
Among various nanoparticle platforms, liposomes feature a cell membrane-like bilayer structure with a unique capability of fusing directly with bacterial membranes [94, 95]. Through such fusion process, a high dose of antibiotics is burst-released into the bacteria, overwhelming the efflux pumps that otherwise cause drug resistance by preferentially pumping antibiotics out of the cells [96, 97]. Notably, the fusion process can also disturb membrane structural stability and distribute membrane-bound lipophilic molecules exclusively into the bacterial membranes [98]. This feature was recently used to develop liposomes incorporating various amphiphilic free fatty acids (FFAs) in their hydrophobic membranes for bactericidal activity, resulting in a series of liposomal formulations effective against pathogens including Gram-negative Propionibacterium acnes (P. acnes), Helicobacter pylori (H. pylori), and Gram-positive MRSA both in vitro [99–102] and in mouse models of the infections [103, 104]. Especially, linolenic acid (LLA) was incorporated into liposome formulations (LipoLLA) and inhibited H. pylori bacteria including various clinically isolated and antibiotic-resistant strains in both active spiral and dormant coccoid forms (Figure 4) [102]. Intriguingly, in these applications, the liposome formulation was found to act not only as passive vehicles to solubilize FFAs for delivery, but also as an active player that hindered the rate of resistance development in comparison to traditional antibiotics and free LLA. In-depth mechanism studies showed distinct liposome-bacterial membrane fusion and exclusive distribution of FFA molecules into the bacterial membranes [105]. Following the fusion, LipoLLA caused rapid structural changes in the bacterial membranes, compromised membrane integrity, and ultimately led to leakage of cytoplasmic contents for bacterial killing. Based on these results, it seemed that LipoLLA prevented FFAs from interacting with bacterial intracellular pathways, thus avoiding biochemical alterations on the bacteria that were prone to resistance selections. Instead, the liposomes promoted physical and non-specific structural disruption of bacterial membranes that ultimately led to cell permeation and death, a process less likely to elicit resistance development.
Figure 4.
(A) Schematic illustration showing oral administration of liposomal linolenic acid (LipoLLA) for the treatment of Helicobacter pylori (H. pylori) infection in stomach. (B) Schematic illustration of LipoLLA formulation and liposome-membrane fusion for antibacterial activity. (C) Fluorescence microscopy images show the fusion interaction between LipoLLA (red) and H. pylori (blue) (scale bars = 5 μm). (Reprinted with permission from Ref. 102). (D, E) Bacterial morphology observed under a transmission electron microscope. H. pylori bacteria show intact morphology in PBS without LipoLLA (D), but compromised bacterial membrane structure in PBS containing LipoLLA (E). (Reprinted with permission from Ref. 105).
Combinatorial antibiotic release against multiple drug targets is a common strategy to broaden the antimicrobial spectrum and generate synergy to counteract antibiotic resistance [106, 107]. However, drug compounds differ significantly with their physicochemical properties. Varying drug profiles following the administration complicate the dosing and scheduling of drug combinations and in turn compromise antibiotic synergy in vivo. To address this challenge, liposomes and polymeric nanoparticles are well-known platforms to co-encapsulate drug molecules with distinct physicochemical properties such as size, charge, and hydrophobicity for co-delivery [14]. These resulting combinatorial nanoparticles provide drug combination with unified tissue distribution and ratiometric interactions with bacterial targets. Using this approach, combinatorial nanoparticles have enhanced the antimicrobial efficacy of existing antibiotics against infections caused by bacteria including Pseudomonas aeruginosa (P. aeruginosa), S. aureus, M. tuberculosis, Burkholderia cepacia, Mycobacterium avium, and H. pylori [15]. Meanwhile, metallic nanoparticles have increasingly been exploited to complex with small antibiotics for synergistic antimicrobial activity. For example, synergy against resistant strains of E. coli was observed when silver nanoparticles chelated with amoxicillin or ampicillin [108, 109]. The mechanism was attributed to the disruption of bacterial cell wall by amoxicillin, which subsequently enhanced the diffusion of silver nanoparticles into bacteria for the inhibition of DNA synthesis. Antimicrobial synergy was also observed with ZnO nanoparticles when complexed with ciprofloxacin against S. aureus and E. coli. In this case, ZnO nanoparticles inhibited the pumping activity of NorA protein resided on the membrane of S. aureus bacteria, therefore blocking ciprofloxacin efflux from the bacteria [110]. Furthermore, vancomycin is ineffective against resistant enterococci due to the mutation of the binding sites on the bacteria. However, when complexed with gold nanoparticles, vancomycin became effective and showed superb activity [111]. In this case, gold nanoparticles, not the vancomycin, were able to bind to the amino acid residues on the bacteria through nonspecific and multivalent interactions. Therefore, they acted as anchors for vancomycin and restored its ability to inhibit cell wall synthesis.
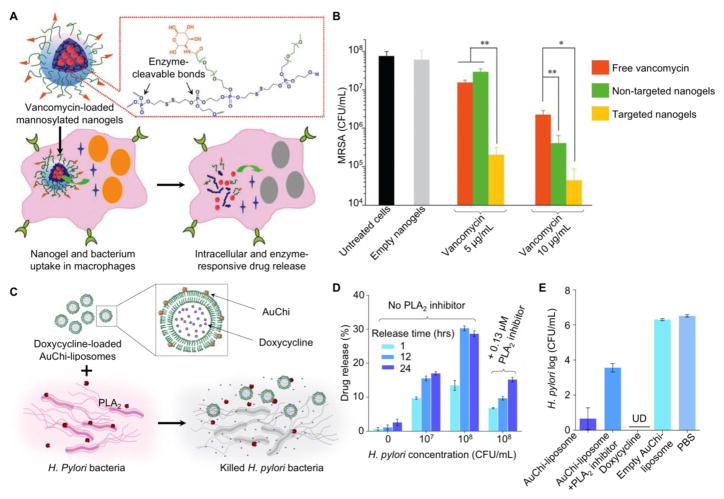
Nanoparticles can also remain inactive, but release antibiotics for activity in response to external cues at the infection sites. By modulating drug-pathogen interaction in a responsive fashion, these nanoparticles minimize drug exposure and therefore reduce resistance development. For responsive antibiotic release, polymeric nanoparticles have been made with cross-linkers prone to enzymatic degradation. For example, a nanogel formulation containing polyphosphoester cross-linked cores was stable, but degraded in response to the active phosphatase or phospholipase produced by MRSA bacteria, resulting in lesion site-specific drug release and bacterial growth inhibition (Figure 5A–B) [112]. Nanoparticles made with a polyethylene glycol (PEG) backbone were designed to undergo side chain cleavage and microstructural transformation in response to enzymes including penicillin G amidase and β-lactamase responsible for degrading antibiotic molecules for resistance [113]. The formulation showed strain-selective delivery of antibiotics to MRSA in vitro and enhanced wound healing in an in vivo murine model. Additionally, liposomes were designed with the attachment of small charged nanoparticles onto liposome surfaces for triggered antimicrobial release. In this design, charged nanoparticles were adsorbed nonspecifically onto phospholipid bilayer surfaces and subsequently provided steric repulsion and reduced surface tension for stabilization. Cationic liposomes adsorbed with negatively charged gold nanoparticles only showed fusion activity toward bacteria at an acidic pH. Such acid-triggered antimicrobial activity made them suitable against various skin pathogens such as P. acne and S. aureus that thrive in an acidic environment [114]. In contrary, anionic liposomes when stabilized with cationic gold nanoparticles were stable in gastric acid, but spontaneously fused with bacteria at physiological pH, making them appealing to treat gastric pathogens such as H. pylori [115]. Even when the changes of pH are unavailable to trigger nanoparticle detachment, these liposomes still exposed a substantial fraction of their surfaces accessible to membrane-active bacterial toxins. This property made the nanoparticle-stabilized liposomes responsive to pathogens that secret pore-forming toxins (PFTs) to damage lipid membrane and trigger drug release such as hemolytic group A streptococci and S. aureus [116]. Similarly, a liposome formulation was made with a lipid composition sensitive to bacterium-secreted phospholipase A2 (PLA2) and adsorbed chitosan-modified gold nanoparticles onto the liposome surface for stabilization (Figure 5C–E) [117]. The liposomes were stable, but rapidly released the encapsulated doxycycline in response to PLA2 secreted by H. pylori bacteria in culture and effectively inhibited bacterial growth.
Figure 5.
(A) Schematic illustration of a vancomycin-loaded mannosylated nanogels (MNG-V) for intracellular targeting and bacteria-responsive drug release. (B) Nanogel formulations were tested against Staphylococcus aureus in Raw264.7 macrophages. Infected macrophages were cultured with free vancomycin, empty targeted-nanogels, vancomycin-loaded non-targeted nanogels, and vancomycin-loaded targeted nanogels, respectively (CFU, colony-forming units, *P < 0.05 and ** P < 0.01 based on Student’s t test.) (Reprinted with permission from Ref. 112) (C) Schematic illustration of liposomes stabilized with chitosan-modified gold nanoparticles (AuChi-liposome) and phospholipase A2 (PLA2)-triggered antibiotic release to treat H. pylori (PLA2-positive) infection. Fusion of doxycycline-loaded liposomes is prevented for stability with AuChi. When H. pylori bacteria are present, PLA2 cleaves the phospholipids and releases doxycycline to kill H. pylori. (D) After incubation with H. pylori culture, doxycycline release from AuChi-liposomes were measured. Bacterial culture added with quinacrine (a PLA2 inhibitor) served as a control. Data represent mean ± SD (n = 3). (E) Bactericidal effect of doxycycline-loaded AuChi-liposome against H. pylori. The effect of PLA2 on the observed antimicrobial activity was tested with quinacrine. Empty AuChi-liposome (no encapsulated doxycycline) and PBS (pH = 6.5) were used as two negative controls while free doxycycline served as a positive control. Data represent mean ± SD (n = 3). (Reprinted with permission from Ref. 117).
4. Enabling ‘drug-free’ anti-virulence therapy
Anti-virulence therapy inhibits the expression or activity of virulence traits critical for bacterial colonization in the host [118, 119]. Without engaging direct disruption of bacterial cycles for killing, this strategy is considered less likely to develop resistance in comparison to traditional antibiotics [120, 121]. Inhibition of virulence factors is also considered as a way to facilitate the host immune system to inhibit or clear bacterial infections [122, 123]. Additionally, anti-virulence approaches have been used in combination with other compounds to generate synergistic antimicrobial activities [124]. Together, these advantages have led to the development of various anti-virulence platforms, including anti-sera, monoclonal antibodies, small-molecule inhibitors, and molecularly imprinted polymers, with success in combating various pathogenic bacteria [125, 126].
Although promising, these platforms target primarily the molecular structure of virulence factors for efficacy, therefore requiring customized design for different infections. Given the enormous diversity of bacterial virulence factors, such structure-based approaches have been challenged by an overwhelming number of distinctive molecular structures and epitopic targets [127, 128]. To address this limitation, a biomimetic nanoparticle design has recently emerged by coating polymeric nanoparticle cores with plasma membrane derived from natural red blood cells [61, 129]. Intriguingly, these cell membrane-coated nanoparticles (denoted ‘nanosponges’) harnessed the functional similarity among bacterial virulence factors, in particularly PFTs that perforate cell membranes, regardless of their molecular structures and epitopic targets. Nanosponges were demonstrated to neutralize various bacterial PFTs, including α-toxin, streptolysin-O, and melittin, and effectively protect mice from their virulence attacks.
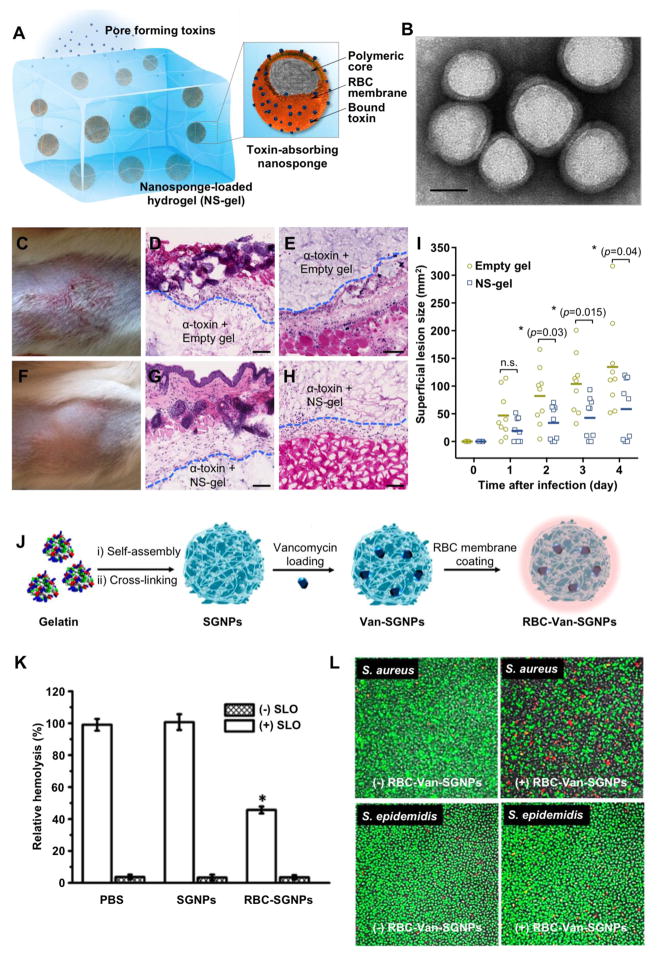
Following their initial development, nanosponges were loaded into a hydrogel to form a hybrid material (NP-gel) specifically for local treatment against MRSA infection (Figure 6A–I) [130]. In this design, nanosponges were able to spontaneously absorb PFTs for neutralization and the hydrogel facilitated the process by retaining the nanosponges within local infection sites. In this way, the hybrid design brings together the advantages of nanosponges and the hydrogel into one system toward better localized toxin neutralization and overall therapeutic outcomes [131, 132]. In this study, monomer and cross-linker compositions were tailored for effective nanosponge retention without affecting toxin transport into polymer matrix and access of nanosponges for neutralization. When the NP-gel was injected subcutaneously into mice, they effectively retained the nanosponges at the injection sites. In a mouse model of MRSA subcutaneous infection, the study demonstrated that NP-gel reduced MRSA skin lesion development in mice. Overall, such hybrid strategy seeks synergy between distinct materials and offers a new detoxification approach to treat local infections.
Figure 6.
(A) Schematic illustration of a hydrogel-nanosponge hybrid (NS-gel) designed for anti-virulence treatment of local methicillin-resistant Staphylococcus aureus (MRSA) infection. The nanosponges are made of a polymeric core wrapped with membranes of natural red blood cells (RBCs). The nanoparticles were subsequently embedded into an acrylamide hydrogel suitable for in vivo injection. (B) The spherical core–shell structure of the toxin-absorbing nanosponges was imaged with TEM (scale bar represents 50 nm). (C–E) Mice injected with α-toxin followed by the empty gel. The dashed lines indicate the approximate tissue–hydrogel boundary. (C) Skin lesions developed at 72 h after the toxin injection. Histological sections stained with hematoxylin and eosin staining show (D) edema, apoptosis, necrosis, and immune infiltration in the epidermis, and (E) muscular damage implied by interfibril edema, tears on muscle fibers, and neutrophil extravasation from surrounding vasculature. (F–H) Mice injected with α-toxin followed by NS-gel. (F) No skin lesion or skin abnormality occurred. (G) Normal epidermis and (H) muscle structure were observed. (Scale bar = 50 μm, n = 6 for each group). (I) Efficacy of NS-gel used for treating MRSA infection (n = 9 per group). Skin lesion sizes were measured. Bars represent median values. * P < 0.05, n.s.: not significant. (Reprinted with permission from Ref. 130) (J) Schematic illustration of preparing vancomycin encapsulated supramolecular gelatin nanoparticles coated with red blood cell (RBC) membranes (denoted RBC-Van-SGNPs). (K) Hemolytic activity test of RBC-Van-SGNPs shows the capability of the nanoparticles to neutralize SLO. PBS and nanoparticles without membrane coating (SGNPs) were used as two control groups. (L) Fluorescence microscopy images show the inhibition and killing of bacteria by RBC-Van-SGNPs when added to S. aureus, a gelatinase-positive strain, and Staphylococcus epidermidis (S. epidemidis), a gelatinase-negative strain, respectively. (Reprinted with permission from Ref. 133).
Meanwhile, the anti-virulence applications of membrane coating technology, especially its broad-spectrum and function-based toxin inhibition, has motivated a number of innovative designs for potential treatment of local infections. For example, RBC membranes were coated onto polymer cores composed of cross-linked gelatin (Figure 6J–L) [133]. The membrane shell absorbed the bacterial exotoxin to relieve symptoms caused by bacterial infection, while the cores released antibiotic payloads in response to gelatinase secreted by gelatinase-positive bacteria including Proteus vulgaris, S. aureus, P. aeruginosa and Serratia marcescens. Cell membrane coating onto nanoparticles was also accomplished by using a membrane vesicle-templated in situ gelation strategy [134]. In this approach, hydrogel nanoparticles coated with RBC membranes not only effectively neutralized toxins from MRSA bacteria, but also enhanced bacterial uptake by immune cells as a direct result of the toxin neutralization [135]. With disulfide bonds on the gel backbone, the nanoparticles were responsive to the intracellular reducing potential for accelerated drug release, causing more effective bacterial inhibition. When added to MRSA-infected macrophages, these nanoparticles significantly inhibited bacterial growth compared to free antibiotics and non-responsive nanoparticle counterparts.
The unique capability of cell membrane-coated nanoparticles in sequestering membrane-active toxins has further intrigued their use as vaccines against bacterial infections [136, 137]. Many bacterial toxins have been identified as the primary causative factors in various infections. Their roles in pathogenesis have in turn prompted the development of toxoid vaccines, which are inactivated toxins that can be safely administered for antimicrobial immunity. Conventional toxoid preparation techniques involve protein denaturation process to reduce toxin virulence for safety purpose. However, protein denaturation is disruptive, inevitably altering the antigenic configuration and compromising the toxin’s immune potency. As a result, toxoid vaccine development has been challenged by the trade-off between vaccine safety and efficacy [138, 139]. Cell membrane coating technology offers a new approach to address this challenge: while the toxins become detained and lose their toxicity when interacting with the nanoparticles, the sequestration preserves the intact and non-denatured configuration of the toxin for mounting a potent immune response.
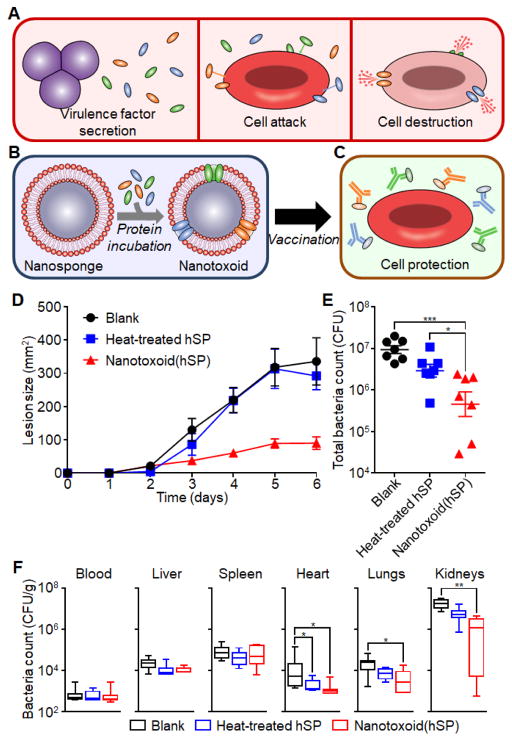
The ‘nanotoxoid’ vaccine was prepared by simply mixing nanosponges with toxins [136]. When α-hemolysin was used as a representative PFT, each nanosponge was shown to detain dozens of toxins. The resulting nanotoxoid showed no discernible toxicity when tested in mice. In addition, mice vaccinated with nanotoxoid generated higher anti-toxin immune responses when compared to those vaccinated with heat-denatured toxins, attributable to the preserved toxin structure in the nanotoxoid. Intriguingly, high doses of α-hemolysin that would have caused severe tissue damages in non-vaccinated mice did not induce the same effect in the vaccinated mice, suggesting mice vaccinated with the nanotoxoid acquired effective immunity against the toxin. Recently, the membrane-detaining strategy for ‘nanotoxoid’ design inspired in situ capture of bacterial toxins for anti-virulence vaccination, a facile approach for generating on-demand nanotoxoids from natural bacterial secretions (Figure 7) [140]. In this approach, bacteria-secreted virulent proteins were biomimetically entrapped using a membrane-coated nanosponge, which further enhanced anti-bacterial efficacy by providing multi-antigenicity while simplifying vaccine preparation through bypassing the need for identifying individual virulence factors. This new vaccine preparation does not require prior knowledge of secreted constituents and thus can be generalized to produce various pathogen-specific vaccine formulations that are safe, potentially multi-antigenic, and epitopically faithful.
Figure 7.
Schematic illustration of a nanotoxoid platform featuring on-demand loading of pathogen-specific toxins. (A) Virulence factors secreted from bacteria spontaneously insert themselves into the membrane of target cells for bioactivity. (B) Nanotoxoid was prepared by incubating culture supernatant containing hemolytic secreted protein (hSP) fraction with nanosponges coated with source cell membranes. (C) Nanotoxoid vaccination generates antibodies that protect cells against multiple virulence factors. (D-F) Protection with nanotoxoid against live bacteria. Mice were vaccinated with a blank solution, heat-treated(hSP), or nanotoxoid(hSP) on day 0 with boosts on days 7 and 14, respectively. (D) Lesion size progression after subcutaneous challenge with MRSA (USA300) on day 35 (n = 7; mean ± SEM). (E) Total bacterial load summed from major organs 3 days after intravenous challenge with MRSA USA300 on day 35 (n = 7; geometric mean ± SEM). (F) Individual, weight-normalized bacteria burdens in major organs from (E) (n = 7; min to max). *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA. (Reprinted with permission from Ref. 140).
The ‘nanotoxoid’ design further inspired the direct use of bacterial membranes to coat nanoparticles as a potential antibacterial vaccine strategy [141]. Bacterial membranes are attractive materials for developing novel vaccines owing to their unique advantages including diverse immunogenic antigens, native pathogen associated-molecular patterns, and intrinsic adjuvant properties [142, 143]. Meanwhile, physicochemical properties of the nanoparticles including particle size and geometry can be tailored through the cores to further promote antigen presentation to the immune cells [144]. By marrying the virtues of natural and synthetic materials, the bacterial membrane-coated nanoparticles were able to mount effective antibacterial immunity. Small gold nanoparticles (approximately 30 nm in diameter) coated with E. coli membrane derived from bacterial outer membrane vesicles (OMVs) induced fast dendritic cell activation and maturation in the lymph nodes of the vaccinated mice. Regarding the humoral immunity, these nanoparticles generated higher antibody titers with stronger avidity than those elicited by OMVs alone. Regarding the cellular immunity, vaccinated mice showed high levels of interferon-gamma and interleukin (IL)-17, but not IL-4, suggesting a strong Th1- and Th17-biased cell responses against the source bacteria [141].
5. Summary
The field of antimicrobial drug delivery has achieved a significant progress in recent years, especially with the rapid advance of nanomedicine in combination with the growing understandings of infectious diseases. Major efforts have been devoted to the development of nanoparticle-based strategies with a primary goal to increase therapeutic efficacy while minimizing drug resistance development. For local antimicrobial treatment, these strategies have shown promising outcomes by promoting antibiotic localization to the pathogen, modulating drug-pathogen interaction against bacterial drug resistance, and enabling novel anti-virulence therapy for ‘drug-free’ antimicrobial activity.
From a translational perspective, although clinical uses of antimicrobial nanoparticles remain scarce, their development is fast. For example, inorganic nanoparticles, such as Ag and ZnO, which have a long history of topical use against local infections, are making a remarkable comeback as effective antimicrobial agents in the era of antibiotic resistance [145, 146]. In addition, novel nanoparticle-enabled antimicrobial strategies are also emerging at a rapid pace. For example, liposomes consisting of sphingomyelin and cholesterol have been developed for toxin neutralization with the promise of treating patients who have severe pneumonia caused by Streptococcus pneumoniae [147]. The formulation has entered clinical studies. Meanwhile, a large number of nanoparticle formulations are in clinical trials and many are gaining approval for different diseases [148]. The success together suggests a promising future of antimicrobial nanoparticles for treating various infections. It is expected that nanomedicine will continue to generate innovations tailored for local antimicrobial treatment that is efficacious, patient-compliant, and cost effective.
Acknowledgments
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-14-1-0064 and by the National Institutes of Health under Award Number R01EY025947.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aminov RI. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front Microbiol. 2010;1 doi: 10.3389/fmicb.2010.00134. article number 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willing BP, Russell SL, Finlay BB. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nature Rev Microbiol. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 3.Berdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J Antibiot (Tokyo) 2012;65:385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- 4.Andersson DI, Hughes D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nature Rev Microbiol. 2010;8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 5.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJV, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. Tackling antibiotic resistance. Nature Rev Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li LC, Deng J, Stephens D. Polyanhydride implant for antibiotic delivery - from the bench to the clinic. Adv Drug Del Rev. 2002;54:963–986. doi: 10.1016/s0169-409x(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: The past and the future. Adv Drug Del Rev. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kluin OS, van der Mei HC, Busscher HJ, Neut D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10:341–351. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 10.ter Boo G-JA, Grijpma DW, Moriarty TE, Richards RG, Eglin D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials. 2015;52:113–125. doi: 10.1016/j.biomaterials.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Inzana JA, Schwarz EM, Kates SL, Awad HA. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials. 2016;81:58–71. doi: 10.1016/j.biomaterials.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Pornpattananangkul D, Hu CMJ, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 15.Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:532–547. doi: 10.1002/wnan.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonda I. The ascent of pulmonary drug delivery. J Pharm Sci. 2000;89:940–945. doi: 10.1002/1520-6017(200007)89:7<940::AID-JPS11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Ponchel G, Irache JM. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Del Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 18.Haack T, Abdelilah-Seyfried S. The force within: Endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development. 2016;143:373–386. doi: 10.1242/dev.131425. [DOI] [PubMed] [Google Scholar]
- 19.Nielubowicz GR, Mobley HLT. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 20.Canning CR, Greaney MJ, Dewynne JN, Fitt AD. Fluid flow in the anterior chamber of a human eye. IMA J Math Appl Med Biol. 2002;19:31–60. [PubMed] [Google Scholar]
- 21.Barza M, Cuchural G. General-principles of antibiotic tissue penetration. J Antimicrob Chemother. 1985;15:59–75. doi: 10.1093/jac/15.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- 22.Lagler H, Zeitlinger M. Tissue penetration of antibiotics does the treatment reach the target site? Med Klin Intensivmed Notfmed. 2014;109:175–181. doi: 10.1007/s00063-013-0309-0. [DOI] [PubMed] [Google Scholar]
- 23.Abdullah Z, Knolle PA. Scaling of immune responses against intracellular bacterial infection. EMBO J. 2014;33:2283–2294. doi: 10.15252/embj.201489055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asner SA, Morre SA, Bochud PY, Greub G. Host factors and genetic susceptibility to infections due to intracellular bacteria and fastidious organisms. Clin Microbiol Infect. 2014;20:1246–1253. doi: 10.1111/1469-0691.12806. [DOI] [PubMed] [Google Scholar]
- 25.Baietto L, Corcione S, Pacini G, Di Perri G, D’Avolio A, De Rosa FG. A 30-years review on pharmacokinetics of antibiotics: Is the right time for pharmacogenetics? Curr Drug Metab. 2014;15:581–598. doi: 10.2174/1389200215666140605130935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards DA, Hanes J, Caponetti G, Hrkach J, BenJebria A, Eskew ML, Mintzes J, Deaver D, Lotan N, Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 27.Crowder TM, Rosati JA, Schroeter JD, Hickey AJ, Martonen TB. Fundamental effects of particle morphology on lung delivery: Predictions of stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm Res. 2002;19:239–245. doi: 10.1023/a:1014426530935. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi K, Kabasawa T, Ozeki T, Okada H. One-step preparation of rifampicin/poly(lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release. 2009;135:19–24. doi: 10.1016/j.jconrel.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Li YZ, Sun X, Gong T, Liu J, Zuo J, Zhang ZR. Inhalable microparticles as carriers for pulmonary delivery of thymopentin-loaded solid lipid nanoparticles. Pharm Res. 2010;27:1977–1986. doi: 10.1007/s11095-010-0201-z. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Esteban-Fernandez de Avila B, Gao W, Zhang L, Wang J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing and detoxification. Science Robotics. 2017;2 doi: 10.1126/scirobotics.aam6431. article number: eaam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Angsantikul P, Liu W, de Avila BEF, Thamphiwatana S, Xu M, Sandraz E, Wang X, Delezuk J, Gao W, Zhang L, Wang J. Micromotors spontaneously neutralize gastric acid for pH-responsive payload release. Angew Chem Int Ed. 2017;56:2156–2161. doi: 10.1002/anie.201611774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JX, Thamphiwatana S, Liu WJ, de Avila BEF, Angsantikul P, Sandraz E, Wang JX, Xu TL, Soto F, Ramez V, Wang XL, Gao WW, Zhang LF, Wang J. Enteric micromotor can selectively position and spontaneously propel in the gastrointestinal tract. ACS Nano. 2016;10:9536–9542. doi: 10.1021/acsnano.6b04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang JH, Chen MG, Gong H, Tharnphiwatana S, Eckmann L, Gao WW, Zhang LF. A bioadhesive nanoparticle-hydrogel hybrid system for localized antimicrobial drug delivery. ACS Appl Mater Interfaces. 2016;8:18367–18374. doi: 10.1021/acsami.6b04858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastrup CJ, Nahrendorf M, Figueiredo JL, Lee H, Kambhampati S, Lee T, Cho SW, Gorbatov R, Iwamoto Y, Dang TT, Dutta P, Yeon JH, Cheng H, Pritchard CD, Vegas AJ, Siegel CD, MacDougall S, Okonkwo M, Thai A, Stone JR, Coury AJ, Weissleder R, Langer R, Anderson DG. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc Natl Acad Sci U S A. 2012;109:21444–21449. doi: 10.1073/pnas.1217972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver B, Behrouz R, Silliman S. Bacterial endocarditis and cerebrovascular disease. Curr Neurol Neurosci Rep. 2016;16 doi: 10.1007/s11910-016-0705-y. [DOI] [PubMed] [Google Scholar]
- 38.Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, Schafers HJ, Prendergast BD. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69:325–344. doi: 10.1016/j.jacc.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 39.Cusumano CK, Pinkner JS, Han ZF, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ. Treatment and prevention of urinary tract infection with orally active fimh inhibitors. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003021. article number 109ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ. Antibiotic tissue penetration and its relevance - impact of tissue penetration on infection response. Antimicrob Agents Chemother. 1991;35:1953–1959. doi: 10.1128/aac.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner C, Sauermanna R, Joukhadar C. Principles of antibiotic penetration into abscess fluid. Pharmacology. 2006;78:1–10. doi: 10.1159/000094668. [DOI] [PubMed] [Google Scholar]
- 42.Dillen K, Bridts C, Van der Veken P, Cos P, Vandervoort J, Augustyns K, Stevens W, Ludwig A. Adhesion of plga or eudragit (r)/plga nanoparticles to staphylococcus and, pseudomonas. Int J Pharm. 2008;349:234–240. doi: 10.1016/j.ijpharm.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Sambhy V, Peterson BR, Sen A. Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew Chem Int Ed. 2008;47:1250–1254. doi: 10.1002/anie.200702287. [DOI] [PubMed] [Google Scholar]
- 44.Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenawy ER, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 46.Liu LH, Xu KJ, Wang HY, Tan PKJ, Fan WM, Venkatraman SS, Li LJ, Yang YY. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol. 2009;4:457–463. doi: 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- 47.Nederberg F, Zhang Y, Tan JPK, Xu KJ, Wang HY, Yang C, Gao SJ, Guo XD, Fukushima K, Li LJ, Hedrick JL, Yang YY. Biodegradable nanostructures with selective lysis of microbial membranes. Nat Chem. 2011;3:409–414. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- 48.Oren Z, Shai Y. Cyclization of a cytolytic amphipathic alpha-helical peptide and its diastereomer: Effect on structure, interaction with model membranes, and biological function. Biochemistry. 2000;39:6103–6114. doi: 10.1021/bi992408i. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Lopez S, Kim HS, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, Ghadiri MR. Antibacterial agents based on the cyclic d,l-alpha-peptide architecture. Nature. 2001;412:452–455. doi: 10.1038/35086601. [DOI] [PubMed] [Google Scholar]
- 50.Ewald C, Kuhn S, Kalff R. Pyogenic infections of the central nervous system secondary to dental affections - a report of six cases. Neurosurg Rev. 2006;29:163–166. doi: 10.1007/s10143-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 51.Bert F, Ouahes O, Lambertzechovsky N. Brain-abscess due to bacillus-macerans following a penetrating periorbital injury. J Clin Microbiol. 1995;33:1950–1953. doi: 10.1128/jcm.33.7.1950-1953.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi SK, Myc A, Silpe JE, Sumit M, Wong PT, McCarthy K, Desai AM, Thomas TP, Kotlyar A, Holl MMB, Orr BG, Baker JR. Dendrimer-based multivalent vancomycin nanoplatform for targeting the drug-resistant bacterial surface. ACS Nano. 2013;7:214–228. doi: 10.1021/nn3038995. [DOI] [PubMed] [Google Scholar]
- 53.Choi KH, Lee HJ, Park BJ, Wang KK, Shin EP, Park JC, Kim YK, Oh MK, Kim YR. Photosensitizer and vancomycin-conjugated novel multifunctional magnetic particles as photoinactivation agents for selective killing of pathogenic bacteria. Chem Commun. 2012;48:4591–4593. doi: 10.1039/c2cc17766h. [DOI] [PubMed] [Google Scholar]
- 54.Qi GB, Li LL, Yu FQ, Wang H. Vancomycin-modified mesoporous silica nanoparticles for selective recognition and killing of pathogenic gram-positive bacteria over macrophage-like cells. ACS Appl Mater Interfaces. 2013;5:10874–10881. doi: 10.1021/am403940d. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Zhang C, Liu QF, Shao XY, Feng CC, Shen YH, Zhang QZ, Jiang XG. Solanum tuberosum lectin-conjugated plga nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J Drug Target. 2012;20:174–184. doi: 10.3109/1061186X.2011.622396. [DOI] [PubMed] [Google Scholar]
- 56.Huang PJ, Tay LL, Tanha J, Ryan S, Chau LK. Single-domain antibody-conjugated nanoaggregate-embedded beads for targeted detection of pathogenic bacteria. Chem Eur J. 2009;15:9330–9334. doi: 10.1002/chem.200901397. [DOI] [PubMed] [Google Scholar]
- 57.Tay LL, Huang PJ, Tanha J, Ryan S, Wu XH, Hulse J, Chau LK. Silica encapsulated sers nanoprobe conjugated to the bacteriophage tailspike protein for targeted detection of salmonella. Chem Commun. 2012;48:1024–1026. doi: 10.1039/c1cc16325f. [DOI] [PubMed] [Google Scholar]
- 58.Chen F, Zhou J, Luo FL, Mohammed AB, Zhang XL. Aptamer from whole-bacterium selex as new therapeutic reagent against virulent mycobacterium tuberculosis. Biochem Biophys Res Commun. 2007;357:743–748. doi: 10.1016/j.bbrc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Duan N, Wu SJ, Chen XJ, Huang YK, Xia Y, Ma XY, Wang ZP. Selection and characterization of aptamers against salmonella typhimurium using whole-bacterium systemic evolution of ligands by exponential enrichment (SELEX) J Agric Food Chem. 2013;61:3229–3234. doi: 10.1021/jf400767d. [DOI] [PubMed] [Google Scholar]
- 60.Song MY, Jurng J, Park YK, Kim BC. An aptamer cocktail-functionalized photocatalyst with enhanced antibacterial efficiency towards target bacteria. J Hazard Mater. 2016;318:247–254. doi: 10.1016/j.jhazmat.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang LF. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao W, Zhang L. Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target. 2015;23:619–626. doi: 10.3109/1061186X.2015.1052074. [DOI] [PubMed] [Google Scholar]
- 63.Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Phu N, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nature Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 65.Johnston DGW, Kearney J, Zaslona Z, Williams MA, O’Neill LAJ, Corr SC. MicroRNA-21 limits uptake of listeria monocytogenes by macrophages to reduce the intracellular niche and control infection. Front Cell Infect Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00201. article number 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witter AR, Okunnu BM, Berg RE. The essential role of neutrophils during infection with the intracellular bacterial pathogen listeria monocytogenes. J Immunol. 2016;197:1557–1565. doi: 10.4049/jimmunol.1600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rikihisa Y. Mechanisms of obligatory intracellular infection with anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24:469–489. doi: 10.1128/CMR.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kentner D, Martano G, Callon M, Chiquet P, Brodmann M, Burton O, Wahlander A, Nanni P, Delmotte N, Grossmann J, Limenitakis J, Schlapbach R, Kiefer P, Vorholt JA, Hiller S, Bumann D. Shigella reroutes host cell central metabolism to obtain high-flux nutrient supply for vigorous intracellular growth. Proc Natl Acad Sci U S A. 2014;111:9929–9934. doi: 10.1073/pnas.1406694111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mellouk N, Enninga J. Cytosolic access of intracellular bacterial pathogens: The shigella paradigm. Front Cell Infect Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartland EL. Bacterial pathogenesis: Legionella phosphoinositide tailoring. Nature Microbiology. 2017;2 doi: 10.1038/nmicrobiol.2017.13. article number 17013. [DOI] [PubMed] [Google Scholar]
- 71.Speiser Y, Zusman T, Pasechnek A, Segal G. The legionella pneumophila incomplete phosphotransferase system is required for optimal intracellular growth and maximal expression of pmra-regulated effectors. Infect Immun. 2017;85 doi: 10.1128/IAI.00121-17. article number e00121–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur S, Harjai K, Chhibber S. Bacteriophage-aided intracellular killing of engulfed methicillin-resistant Staphylococcus aureus (MRSA) by murine macrophages. Appl Microbiol Biotechnol. 2014;98:4653–4661. doi: 10.1007/s00253-014-5643-5. [DOI] [PubMed] [Google Scholar]
- 73.Pumerantz A, Muppidi K, Agnihotri S, Guerra C, Venketaraman V, Wang J, Betageri G. Preparation of liposomal vancomycin and intracellular killing of meticillin-resistant Staphylococcus aureus (MRSA) Int J Antimicrob Agents. 2011;37:140–144. doi: 10.1016/j.ijantimicag.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial subversion of host innate immune pathways. Science. 2013;340:697–701. doi: 10.1126/science.1235771. [DOI] [PubMed] [Google Scholar]
- 75.Eisenreich W, Heesemann J, Rudel T, Goebel W. Metabolic host responses to infection by intracellular bacterial pathogens. Front Cell Infect Microbiol. 2013;3 doi: 10.3389/fcimb.2013.00024. article number 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abed N, Couvreur P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents. 2014;43:485–496. doi: 10.1016/j.ijantimicag.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Kaim AH, Wischer T, O’Reilly T, Jundt G, Frohlich J, von Schulthess GK, Allegrini PR. Mr imaging with ultrasmall superparamagnetic iron oxide particles in experimental soft-tissue infections in rats. Radiology. 2002;225:808–814. doi: 10.1148/radiol.2253011485. [DOI] [PubMed] [Google Scholar]
- 79.Kisich KO, Gelperina S, Higgins MP, Wilson S, Shipulo E, Oganesyan E, Heifets L. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular mycobacterium tuberculosis. Int J Pharm. 2007;345:154–162. doi: 10.1016/j.ijpharm.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 80.Semiramoth N, Di Meo C, Zouhiri F, Said-Hassane F, Valetti S, Gorges R, Nicolas V, Poupaert JH, Chollet-Martin S, Desmaele D, Gref R, Couvreur P. Self-assembled squalenoylated penicillin bioconjugates: An original approach for the treatment of intracellular infections. ACS Nano. 2012;6:3820–3831. doi: 10.1021/nn204928v. [DOI] [PubMed] [Google Scholar]
- 81.Toti US, Guru BR, Hali M, McPharlin CM, Wykes SM, Panyam J, Whittum-Hudson JA. Targeted delivery of antibiotics to intracellular chlamydial infections using plga nanoparticles. Biomaterials. 2011;32:6606–6613. doi: 10.1016/j.biomaterials.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clemens DL, Lee BY, Xue M, Thomas CR, Meng H, Ferris D, Nel AE, Zink JI, Horwitz MA. Targeted intracellular delivery of antituberculosis drugs to mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob Agents Chemother. 2012;56:2535–2545. doi: 10.1128/AAC.06049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maya S, Indulekha S, Sukhithasri V, Smitha KT, Nair SV, Jayakumar R, Biswas R. Efficacy of tetracycline encapsulated o-carboxymethyl chitosan nanoparticles against intracellular infections of Staphylococcus aureus. Int J Biol Macromol. 2012;51:392–399. doi: 10.1016/j.ijbiomac.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Kamaruzzaman NF, Firdessa R, Good L. Bactericidal effects of polyhexamethylene biguanide against intracellular Staphylococcus aureus emrsa-15 and USA 300. J Antimicrob Chemother. 2016;71:1252–1259. doi: 10.1093/jac/dkv474. [DOI] [PubMed] [Google Scholar]
- 85.Chellat F, Merhi Y, Moreau A, Yahia L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials. 2005;26:7260–7275. doi: 10.1016/j.biomaterials.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 86.Edagwa BJ, Guo DW, Puligujja P, Chen H, McMillan J, Liu XM, Gendelman HE, Narayanasamy P. Long-acting antituberculous therapeutic nanoparticles target macrophage endosomes. FASEB J. 2014;28:5071–5082. doi: 10.1096/fj.14-255786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vyas SP, Kannan ME, Jain S, Mishra V, Singh P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int J Pharm. 2004;269:37–49. doi: 10.1016/j.ijpharm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 88.Lee WH, Loo CY, Traini D, Young PM. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin Drug Deliv. 2015;12:1009–1026. doi: 10.1517/17425247.2015.1039509. [DOI] [PubMed] [Google Scholar]
- 89.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nature Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 90.Brooks BD, Brooks AE. Therapeutic strategies to combat antibiotic resistance. Adv Drug Del Rev. 2014;78:14–27. doi: 10.1016/j.addr.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 91.Karam G, Chastre J, Wilcox MH, Vincent J-L. Antibiotic strategies in the era of multidrug resistance. Critical Care. 2016;20 doi: 10.1186/s13054-016-1320-7. article 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woolhouse M, Waugh C, Perry MR, Nair H. Global disease burden due to antibiotic resistance - state of the evidence. J Glob Health. 2016;6 doi: 10.7189/jogh.06.010306. article number 010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dodds DR. Antibiotic resistance: A current epilogue. Biochem Pharmacol. 2017;134:139–146. doi: 10.1016/j.bcp.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharm. 2010;387:187–198. doi: 10.1016/j.ijpharm.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 95.Gao W, Hu CMJ, Fang RH, Zhang L. Liposome-like nanostructures for drug delivery. J Mater Chem B. 2013;1:6569–6585. doi: 10.1039/C3TB21238F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2016–2022. doi: 10.1128/AAC.01547-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma YF, Wang Z, Zhao W, Lu TL, Wang RT, Mei QB, Chen T. Enhanced bactericidal potency of nanoliposomes by modification of the fusion activity between liposomes and bacterium. Int J Nanomedicine. 2013;8:2351–2360. doi: 10.2147/IJN.S42617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilschut J, Hoekstra D. Membrane-fusion - from liposomes to biological-membranes. Trends Biochem Sci. 1984;9:479–483. [Google Scholar]
- 99.Yang DR, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, Zhang LF. The antimicrobial activity of liposomal lauric acids against propionibacterium acnes. Biomaterials. 2009;30:6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang CM, Chen CH, Pornpattananangkul D, Zhang L, Chan M, Hsieh MF, Zhang LF. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32:214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen CH, Wang YH, Nakatsuji T, Liu YT, Zouboulis CC, Gallo RL, Zhang LF, Hsieh MF, Huang CM. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J Microbiol Biotechnol. 2011;21:391–399. [PubMed] [Google Scholar]
- 102.Obonyo M, Zhang L, Thamphiwatana S, Pornpattananangkul D, Fu V, Zhang LF. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharm. 2012;9:2677–2685. doi: 10.1021/mp300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pornpattananangkul D, Fu V, Thamphiwatana S, Zhang L, Chen M, Vecchio J, Gao W, Huang CM, Zhang L. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv Healthc Mater. 2013;2:1322–1328. doi: 10.1002/adhm.201300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thamphiwatana S, Gao W, Obonyo M, Zhang L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc Natl Acad Sci U S A. 2014;111:17600–17605. doi: 10.1073/pnas.1418230111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jung SW, Thamphiwatana S, Zhang LF, Obonyo M. Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116519. article number e0116519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bayramov DF, Neff JA. Beyond conventional antibiotics - new directions for combination products to combat biofilm. Adv Drug Del Rev. 2016;112:48–60. doi: 10.1016/j.addr.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: From targets to networks. Nature Rev Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li P, Li J, Wu CZ, Wu QS, Li J. Synergistic antibacterial effects of beta-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16:1912–1917. [Google Scholar]
- 109.Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against Gram-positive and Gram-negative bacteria. Nanomed Nanotech Biol Med. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 110.Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, Moghaddam KM, Shahverdi AR. Zno nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B Appl Biomater. 2010;93:557–561. doi: 10.1002/jbm.b.31615. [DOI] [PubMed] [Google Scholar]
- 111.Gu HW, Ho PL, Tong E, Wang L, Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003;3:1261–1263. [Google Scholar]
- 112.Xiong MH, Li YJ, Bao Y, Yang XZ, Hu B, Wang J. Bacteria-responsive multifunctional nanogel for targeted antibiotic delivery. Adv Mater. 2012;24:6175–6180. doi: 10.1002/adma.201202847. [DOI] [PubMed] [Google Scholar]
- 113.Li YM, Liu GH, Wang XR, Hu JM, Liu SY. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew Chem Int Ed. 2016;55:1760–1764. doi: 10.1002/anie.201509401. [DOI] [PubMed] [Google Scholar]
- 114.Pornpattananangkul D, Olson S, Aryal S, Sartor M, Huang CM, Vecchio K, Zhang L. Stimuli-responsive liposome fusion mediated by gold nanoparticles. ACS Nano. 2010;4:1935–1942. doi: 10.1021/nn9018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thamphiwatana S, Fu V, Zhu JY, Lu DN, Gao WW, Zhang LF. Nanoparticle-stabilized liposomes for pH-responsive gastric drug delivery. Langmuir. 2013;29:12228–12233. doi: 10.1021/la402695c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang LF. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thamphiwatana S, Gao WW, Pornpattananangkul D, Zhang QZ, Fu V, Li JY, Li JM, Obonyo M, Zhang LF. Phospholipase A2-responsive antibiotic delivery via nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Mater Chem B. 2014;2:8201–8207. doi: 10.1039/C4TB01110D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 119.Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei SG, Roth M, Hughes DT, Huntley JF, Fina MW, Falck JR, Sperandio V. Targeting qSEC signaling and virulence for antibiotic development. Science. 2008;321:1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nature Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 122.Barczak AK, Hung DT. Productive steps toward an antimicrobial targeting virulence. Curr Opin Microbiol. 2009;12:490–496. doi: 10.1016/j.mib.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: Can we make evolution-proof drugs? Nature Rev Microbiol. 2014;12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 124.Paul M, Leibovici L. Combination antimicrobial treatment versus monotherapy: The contribution of meta-analyses. Infect Dis Clin North Am. 2009;23:277–293. doi: 10.1016/j.idc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 125.Beckham KSH, Roe AJ. From screen to target: Insights and approaches for the development of anti-virulence compounds. Front Cell Infect Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00139. article number 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maura D, Ballok AE, Rahme LG. Considerations and caveats in anti-virulence drug development. Curr Opin Microbiol. 2016;33:41–46. doi: 10.1016/j.mib.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schiavo G, van der Goot FG. The bacterial toxin toolkit. Nat Rev Mol Cell Biol. 2001;2:530–537. doi: 10.1038/35080089. [DOI] [PubMed] [Google Scholar]
- 128.Page R, Peti W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol. 2016;12:208–214. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 129.Hu CMJ, Fang RH, Copp J, Luk BT, Zhang LF. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8:336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang F, Gao WW, Thamphiwatana S, Luk BT, Angsantikul P, Zhang QZ, Hu CMJ, Fang RH, Copp JA, Pornpattananangkul D, Lu WY, Zhang LF. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv Mater. 2015;27:3437–3443. doi: 10.1002/adma.201501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao WW, Vecchio D, Li JM, Zhu JY, Zhang QZ, Fu V, Li JY, Thamphiwatana S, Lu DN, Zhang LF. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano. 2014;8:2900–2907. doi: 10.1021/nn500110a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao WW, Zhang Y, Zhang QZ, Zhang LF. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44:2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li LL, Xu JH, Qi GB, Zhao XZ, Yu FQ, Wang H. Core-shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano. 2014;8:4975–4983. doi: 10.1021/nn501040h. [DOI] [PubMed] [Google Scholar]
- 134.Zhang JH, Gao WW, Fang RH, Dong AJ, Zhang LF. Synthesis of nanogels via cell membrane-templated polymerization. Small. 2015;11:4309–4313. doi: 10.1002/smll.201500987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Zhang J, Chen W, Angsantikul P, Spiekermann K, Fang R, Gao W, Zhang L. Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. Journal of Controlled Release. 2017 doi: 10.1016/j.jconrel.2017.01.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu CMJ, Fang RH, Luk BT, Zhang LF. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu CMJ, Zhang LF. Nanotoxoid vaccines. Nano Today. 2014;9:401–404. doi: 10.1016/j.nantod.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kennedy AD, Wardenburg JB, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kernodle DS. Expectations regarding vaccines and immune therapies directed against Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2011;203:1692–1693. doi: 10.1093/infdis/jir141. [DOI] [PubMed] [Google Scholar]
- 140.Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou J, Gao W, Lu W, Fang RH, Zhang L. In situ capture of bacterial toxins for antivirulence vaccination. Adv Mater. 2017 doi: 10.1002/adma.201701644. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao WW, Fang RH, Thamphiwatana S, Luk BT, Li JM, Angsantikul P, Zhang QZ, Hu CMJ, Zhang LF. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Poetsch A, Wdlters D. Bacterial membrane proteomics. Proteomics. 2008;8:4100–4122. doi: 10.1002/pmic.200800273. [DOI] [PubMed] [Google Scholar]
- 143.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, Park KS, Lee JO, Kim YK, Kwon KH, Kim KP, Gho YS. Global proteomic profiling of native outer membrane vesicles derived from escherichia coli. Proteomics. 2007;7:3143–3153. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 144.Luk BT, Hu CMJ, Fang RNH, Dehaini D, Carpenter C, Gao WW, Zhang LF. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2014;6:2730–2737. doi: 10.1039/c3nr06371b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of zno nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 147.Henry BD, Neill DR, Becker KA, Gore S, Bricio-Moreno L, Ziobro R, Edwards MJ, Muhlemann K, Steinmann J, Kleuser B, Japtok L, Luginbuhl M, Wolfmeier H, Scherag A, Gulbins E, Kadioglu A, Draeger A, Babiychuk EB. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol. 2015;33:81–88. doi: 10.1038/nbt.3037. [DOI] [PubMed] [Google Scholar]
- 148.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]