Abstract
Background
Pneumonia is one of the most common complications in children hospitalized with influenza. We describe hospitalized children with influenza-associated pneumonia and associated risk indicators.
Methods
Through Emerging Infections Program Network population-based surveillance, children aged <18 years hospitalized with laboratory-confirmed influenza with a chest radiograph during hospitalization were identified during the 2003–2008 influenza seasons. A case with radiologically confirmed influenza-associated pneumonia was defined as a child from the surveillance area hospitalized with: (1) laboratory-confirmed influenza and (2) evidence of new pneumonia on chest radiograph during hospitalization. Hospitalized children with pneumonia were compared with those without pneumonia by univariate and multivariate analysis.
Results
Overall, 2992 hospitalized children with influenza with a chest radiograph were identified; 1072 (36%) had influenza-associated pneumonia. When compared with children hospitalized with influenza without pneumonia, hospitalized children with influenza-associated pneumonia were more likely to require intensive care unit admission (21% vs. 11%, P < 0.01), develop respiratory failure (11% versus 3%, P < 0.01), and die (0.9% vs. 0.3% P = 0.01). In multivariate analysis, age 6 to 23 months (adjusted OR: 2.1, CI: 1.6 –2.8), age 2 to 4 years (adjusted OR: 1.7, CI: 1.3–2.2), and asthma (adjusted OR: 1.4, CI: 1.1–1.8) were significantly associated with influenza-associated pneumonia.
Conclusions
Hospitalized children with influenza-associated pneumonia were more likely to have a severe clinical course than other hospitalized children with influenza, and children aged 6 months to 4 years and those with asthma were more likely to have influenza-associated pneumonia. Identifying children at greater risk for influenza-associated pneumonia will inform prevention and treatment strategies targeting children at risk for influenza complications.
Keywords: influenza, pneumonia, hospitalization
Influenza causes considerable morbidity among children,1,2 and pneumonia is one of the most common complications in children hospitalized with influenza. Pneumonia has been reported in 10% to 26% of children hospitalized with influenza,2–6 and in a recent study of influenza-associated pediatric mortality, 35% of children who died had pneumonia.7 The demographic and clinical characteristics of hospitalized adults with influenza-associated pneumonia have been described,8 but there are no published studies describing the characteristics of hospitalized children with influenza-associated pneumonia in the United States. The occurrence of influenza varies considerably by season and geography, and multisite, multiyear studies are needed to identify consistent characteristics of persons with influenza. In this analysis, we used the Centers for Disease Control and Prevention’s (CDC) Emerging Infections Program Network, a population-based surveillance system that collects information about hospitalized children with laboratory-confirmed influenza, to describe the epidemiologic features of influenza-associated pneumonia in children from a geographically diverse surveillance area over 5 consecutive influenza seasons and to evaluate risk indicators of influenza-associated pneumonia.
METHODS
Analysis Setting and Population
Surveillance for influenza-associated hospitalization was conducted through the CDC’s Emerging Infections Program Network for 5 consecutive influenza seasons, from 2003 to 2008 (during the 2003–2004 season, surveillance was conducted in the following areas in 9 states: 5 Denver-area counties in Colorado; 11 towns in New Haven County, Connecticut; 8 counties in the metropolitan Atlanta area, Georgia; Baltimore City and 5 surrounding counties, Maryland; 7 counties in the Minneapolis area, Minnesota; 15 counties in the Rochester and Albany areas, New York; 2 counties in the Portland area, Oregon; 8 counties in the Nashville area, Tennessee; and Northern California Kaiser members in 3 San Francisco-area counties. During the 2004–2005 season, surveillance was expanded to surveillance areas in 10 states by including 1 Albuquerque-area county in New Mexico, and the surveillance area in Connecticut was expanded to include all of New Haven. During the 2005–2006 influenza season, surveillance in California was expanded to include all children aged less than 2 years in 3 San Francisco-area counties in addition to all Kaiser members aged less than 18 years in those counties. During the 2006–2007 season, surveillance in California was expanded to include all residents of the 3 San Francisco-area counties who were aged less than 18 years. During the 2007–2008 season, 1 Roswell-area county and 1 Santa Fe-area county were added to the surveillance area in New Mexico). During the 2007–2008 season, the surveillance area included 149 hospitals serving the pediatric population in 10 states and approximately 5.3 million children aged less than 18 years, representing 7% of the United States population of children.
Analysis Definitions, Case Identification, and Data Collection
A case was defined as a child aged less than 18 years old residing in the surveillance area who was hospitalized in a surveillance area hospital and who had laboratory confirmation of influenza infection within 14 days of admission during the 2003– 2004 through 2007–2008 influenza seasons. Laboratory testing for influenza was ordered at the discretion of clinicians providing clinical care. Laboratory confirmation was defined as a positive result from viral culture, direct or indirect fluorescent antibody staining, rapid antigen test, or reverse transcription polymerase chain reaction, or documentation of a positive test result in a patient’s medical record.
Cases were identified prospectively through state-mandated disease reporting systems or retrospectively through review of admission or discharge logs, hospital laboratory lists, or infection control logs from October 1 to April 30 of each season. For each identified case, data were collected from the case’s medical record regarding demographic characteristics, medical history, influenza vaccination status, clinical course, and treatment with antiviral medications using a standardized protocol and data collection form.
During the 2003–2004 influenza season, data were collected on the following pre-existing medical conditions: asthma, cardiovascular disease, chronic lung disease, chronic metabolic disease, cystic fibrosis, hemoglobinopathy, immunosuppressive conditions, renal disease, and seizure disorders. During subsequent influenza seasons, data were also collected on developmental delay, febrile seizures, prematurity, and neuromuscular disorders, including cerebral palsy. Prematurity was considered a pre-existing medical condition if a child was born at less than 37 weeks gestation and was aged less than 2 years. A child was considered immunosuppressed if he/she had received immunosuppressive therapy in the 2 weeks prior to admission or had immunoglobulin deficiency, leukemia, lymphoma, or HIV/AIDS. Treatment with steroids was considered an immunosuppressive therapy if a child received oral or injectable steroids for a minimum of two weeks prior to admission. A child was considered to have a neuromuscular disorder if the child had cerebral palsy or another neurologic disorder, excluding seizure disorders and febrile seizures which were considered separate pre-existing medical conditions.
To determine a patient’s influenza vaccination status, the medical record was reviewed. If influenza vaccination status was not recorded in the medical record or if influenza vaccination status was recorded as unknown in the record, the state vaccination registry was consulted. If no state registry existed or if influenza vaccination status was still unknown after consulting the registry, three attempts were made to contact the patient’s primary care provider and/or guardian to obtain information about the patient’s vaccination history. A child was considered to have been eligible for influenza vaccine if the child was aged 6 months or older at the time of hospitalization.
Data about bacterial co-infections were collected for each case when available. Decisions about whether to obtain cultures to assess for possible bacterial coinfections were made by the treating clinicians. Bacterial coinfection was defined as growth of a bacterial pathogen from a normally sterile site (eg, blood, cerebrospinal fluid, pleural fluid, or tissue specimen) during the 2003–2006 seasons and was expanded to include non-sterile sites, namely, endotracheal tube aspirate, or sputum during the 2006–2008 seasons. Staphylococcus epidermidis and Propionibacterium were considered pathogens only when isolated from a, cerebrospinal fluid culture or from a specimen from a neonate aged less than 28 days or an immunosuppressed child).
Children were included in the analyses only if they had a chest radiograph during their hospitalizations. A case with radiologically confirmed influenza-associated pneumonia was defined as a child less than 18 years of age from the surveillance area who was hospitalized with: 1) laboratory confirmed influenza; and 2) evidence of a new pneumonia on at least one chest radiograph obtained during the hospitalization. Cases were ascertained by reviewing data from all case-patients with influenza-associated hospitalization that had a chest radiograph during hospitalization. The discharge summary and/or radiology report were reviewed for each case, and documentation of a new infiltrate, consolidation, or other abnormality consistent with pneumonia on a chest radiograph was considered evidence of pneumonia. If conflicting interpretations of a chest x-ray were found in the discharge summary and radiology report, precedence was generally given to the interpretation from the radiology report. Chest radiograph interpretations including pneumonia as 1 of 2 or more potential diagnoses (eg, “pneumonia versus atelectasis”) were not included as cases of radiologically confirmed pneumonia).
Human Subjects Review
The nature of this data collection has been determined by CDC to be for routine public health surveillance purposes and is not subject to institutional review board approval for human research protections.
Statistical Methods
Children with pneumonia were compared with those without pneumonia by univariate and multivariate analysis. χ2 (or Fisher exact test) and odds ratios with 95% confidence intervals were calculated for categorical variables. Age was divided into 4 categories. Children aged 5 to 17 years were used as the reference group. Independent variables were considered statistically significant in all analyses if the calculated P was <0.05. Stratified analysis with the Breslow-Day test for homogeneity was used to evaluate confounding and effect modification. A logistic regression model was built with all variables statistically significant in univariate analysis. To identify effect modification, interaction terms that were statistically significant in stratified analysis also were included in the model.
All statistical analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of Hospitalized Children With Influenza-Associated Pneumonia
During the 5 influenza seasons, 4015 children were hospitalized with laboratory-confirmed influenza. Of these children, 2992 children (75%) had a chest radiograph, 1072 (36% of hospitalized children who had a chest x-ray; 27% of all children hospitalized with laboratory-confirmed influenza) of whom had radiologically confirmed influenza-associated pneumonia.
More boys than girls had influenza-associated pneumonia (overall 59%, seasonal range: 56%–66%). More than half of children with influenza-associated pneumonia were aged less than 2 years (overall 54%, seasonal range: 47%–60%), and had no pre-existing medical condition (overall 55%, seasonal range: 50%–58%). Of those who had a pre-existing medical condition, asthma was the most common condition (overall 24%, seasonal range: 21%–30%).
Of the 886 children aged 6 months or older with influenza-associated pneumonia who were eligible for influenza vaccine, fewer than half had received at least one dose of vaccine during the current season (overall 39%, seasonal range 30%–41%). Influenza vaccine status was unknown for 13% of children aged six months or older.
Of 1072 children with influenza-associated pneumonia, invasive bacterial coinfection was identified in 21 children (overall 2%, seasonal range: 1%–4%), and Staphylococcus aureus was the most commonly identified organism (overall 0.8%, seasonal range 0.5%–2%) (Table, Supplemental Digital Content 1, http://links.lww.com/INF/A383). Of the 9 children with invasive S. aureus coinfection, 7 (78%) had infection with methicillin-sensitive S. aureus, and 2 (22%) had infection with methicillin-resistant S. aureus. In addition, bacterial coinfection with S. aureus was identified in 2 additional children from specimens taken from endotracheal aspirates.
Children with influenza-associated pneumonia, when compared with those without pneumonia, had a longer median length of time between symptom onset and hospitalization (3 days vs. 2 days, P < 0.01) and a longer median length of hospitalization (4 days [range: 1–128 days] vs. 3 days [range: 1–222 days] P < 0.01) (Table 1). In addition, intensive care unit (ICU) admissions were more common among children with influenza-associated pneumonia, when compared with those without pneumonia (21% vs. 11%, P < 0.01) as were episodes of respiratory failure requiring mechanical ventilation (11% vs. 3%, P < 0.01). Among children with influenza-associated pneumonia, only 21% received antiviral treatment during their hospitalizations, and children with influenza-associated pneumonia were no more likely to have been treated with antiviral medications during their hospitalizations than children hospitalized with influenza without pneumonia (21% vs. 19%, P = 0.15). Data about the timing of initiation of antiviral treatment during the hospitalization were not collected. Overall, 10 children (0.9%) with influenza-associated pneumonia, and 5 children (0.3%) without pneumonia died during the 5 influenza seasons (P = 0.01). Of the 10 children with influenza-associated pneumonia who died, the median age was 8 years with a range of 7 months to 16 years. Of the 5 children without influenza-associated pneumonia who died, the median age was 8 years with a range of <1 month to 17 years.
TABLE 1.
Clinical Course and Complications Associated With Pediatric Influenza-Associated Pneumonia
| Outcome | No. Patients With Pneumonia (%) n = 1072 |
No. Patients Without Pneumonia (%) n = 1920 |
OR (95% CI) | P |
|---|---|---|---|---|
| Median time from symptom onset to admission (d) | 3 | 2 | <0.01 | |
| Median length of stay (d) | 4 | 3 | <0.01 | |
| Antiviral treatment* | 133 (21) | 237 (19) | 0.8 (0.7–1.1) | 0.15 |
| Invasive bacterial coinfection† | 21 (2) | 32 (2) | 1.2 (0.7–2) | 0.4 |
| Staphylococcus aureus (methicillin susceptible) | 7 (33)‡ | 9 (28)‡ | ||
| Staphylococcus aureus (methicillin resistant) | 2 (10) | 0 | ||
| Streptococcus pneumoniae | 5 (24)‡ | 5 (16) | ||
| Group A streptococcus | 2 (9)‡ | 3 (9)‡ | ||
| Other | 5 (24)‡ | 15 (47)‡ | ||
| Intensive care unit hospitalization | 226 (21) | 202 (11) | 2.3 (1.8–2.8) | <0.01 |
| Mechanical ventilation | 114 (11) | 66 (3) | 3.4 (2.5–4.6) | <0.01 |
| Death | 10 (0.9) | 5 (0.3) | 3.6 (1.2–10.6) | 0.01 |
Children <1 yr and children with unknown antiviral treatment status excluded for this analysis.
Includes pathogen isolated from normally sterile site (blood, cerebrospinal fluid, pleural fluid or tissue specimen).
Percent of children with bacterial co-infection.
Factors Associated With Influenza-Associated Pneumonia Among Hospitalized Children
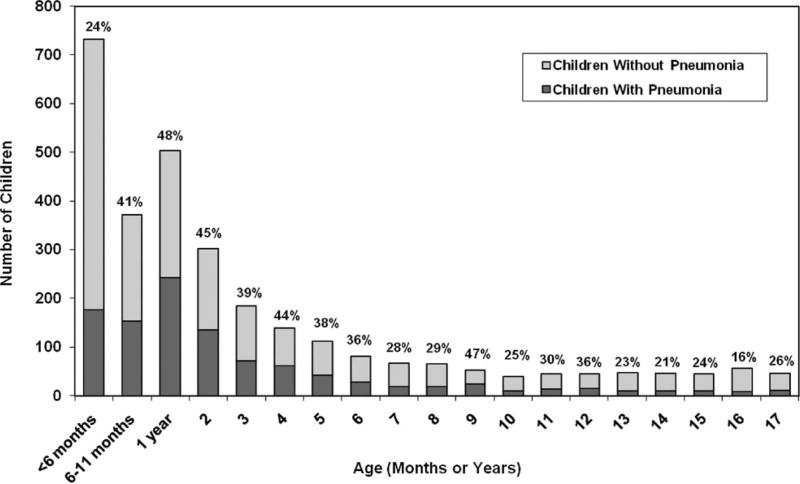
Children with influenza-associated pneumonia were compared with children hospitalized with laboratory-confirmed influenza who had a chest radiograph but did not have radiologically-confirmed pneumonia. The age distribution of hospitalized children with influenza with and without influenza-associated pneumonia is summarized in Figure 1. Children aged 6 to 23 months (OR: 1.9, CI: 1.6 –2.3) and aged 2 to 4 years (OR: 1.7, CI: 1.4 –2.2) were more likely to have pneumonia than children aged 5 to 17 years (Table 2). Children hospitalized with influenza who had asthma (OR: 1.3, CI: 1.1–1.6), cardiovascular disease (OR: 1.7, CI: 1.1–2.5), developmental delay (OR: 1.5, CI: 1.1–2.1), and neuromuscular disorders (OR: 1.7, CI: 1.1–2.5) were also significantly more likely to have pneumonia than other children hospitalized with influenza. In contrast, children hospitalized with influenza who had hemoglobinopathies (OR: 0.5, CI: 0.3– 0.8) or immunosuppressive conditions (OR: 0.5, CI: 0.3– 0.8) were less likely to have pneumonia than other children hospitalized with influenza. Hospitalized children with influenza virus type B infections were equally likely as children with influenza virus type A infections to have pneumonia (OR: 1, CI: 0.8 –1.3).
FIGURE 1.
Age distribution of children hospitalized with influenza with and without pneumonia, 2003–2008. Bar labels denote % of children in each age group with pneumonia.
TABLE 2.
Factors Associated With Influenza-Associated Pneumonia, 2003–2008
| Characteristic | No. Patients With Pneumonia (%) n = 1072 |
No. Patients Without Pneumonia (%) n = 1920 |
Univariate | Multivariate* Adjusted OR (95% CI) |
|
|---|---|---|---|---|---|
|
| |||||
| OR (95% CI) | P | ||||
| Sex | |||||
| Male | 630 (59) | 1085 (57) | 1.1 (1–1.3) | 0.23 | |
| Female | 442 (41) | 835 (43) | 1 (ref) | ||
| Age group | |||||
| <6 mo | 176 (16) | 556 (29) | 0.7 (0.6–0.9) | <0.01 | |
| 6–23 mo | 397 (37) | 479 (25) | 1.9 (1.6–2.3) | <0.01 | 2.1 (1.6–2.8) |
| 2–4 yr | 270 (25) | 358 (19) | 1.7 (1.4–2.2) | <0.01 | 1.7 (1.3–2.2) |
| 5–17 yr | 229 (22) | 527 (27) | 1 | ||
| Race/ethnicity | |||||
| White | 358 (33) | 718 (37) | 1 | ||
| Black | 280 (26) | 487 (25) | 1.2 (0.9–1.4) | 0.15 | |
| Asian | 37 (4) | 61 (3) | 1.2 (0.8–1.9) | 0.4 | |
| Hispanic | 242 (23) | 405 (21) | 1.2 (1–1.5) | 0.08 | |
| Viral type† | |||||
| Influenza A | 788 (85) | 1451 (85) | 1 (0.8–1.3) | 0.6 | |
| Influenza B | 134 (15) | 261 (15) | 1 (ref) | ||
| Pre-existing condition | |||||
| Asthma | 255 (24) | 361 (19) | 1.3 (1.1–1.6) | <0.01 | 1.4 (1.1–1.8) |
| Cardiovascular disease | 47 (4) | 51 (3) | 1.7 (1.1–2.5) | 0.01 | |
| Chronic lung disease | 51 (5) | 66 (3) | 1.4 (1.0–2.0) | 0.07 | |
| Chronic metabolic disease | 22 (2) | 44 (2) | 0.9 (0.5–1.5) | 0.7 | |
| Cystic fibrosis | 3 (<1) | 14 (<1) | 0.4 (0.1–1.3) | 0.1 | |
| Developmental delay‡ | 72 (7) | 91 (5) | 1.5 (1.1–2.1) | <0.01 | |
| Febrile seizures | 9 (1) | 19 (1) | 0.9 (0.4–2.0) | 0.8 | |
| Hemoglobinopathy | 26 (2) | 85 (4) | 0.5 (0.3– 0.8) | 0.03 | 0.4 (0.2– 0.7) |
| Immunosuppressive condition | 25 (2) | 90 (5) | 0.5 (0.3– 0.8) | <0.01 | |
| Neuromuscular disorder‡ | 45 (4) | 52 (3) | 1.7 (1.1–2.5) | 0.01 | |
| Renal disease | 18 (2) | 28 (1) | 1.2 (0.6–2.1) | 0.6 | |
| Seizure disorder | 52 (5) | 94 (5) | 1.0 (0.7–1.4) | 1 | |
| Influenza vaccine§ | 301 (39) | 419 (36) | 1.1 (0.9–1.4) | 0.2 | |
Multivariate analysis performed with data from 2004–2008 seasons only because all variables were not included in data collection during 2003–2004 season.
Children with unknown influenza type excluded from this analysis.
Analysis includes 2004–2008 seasons only; information not available for 2003–04 season.
Children <6 mo and with unknown vaccination status excluded for this analysis; vaccinated defined as having received 1 dose of vaccine.
Because data were not collected on neuromuscular disorders and developmental delay during the 2003–2004 influenza season and these variables were significant in univariate analysis, multivariate analysis was restricted to data from the 2004–2008 influenza season. The initial multivariate model included age, asthma, cardiovascular disease, hemoglobinopathies, immunosuppressive conditions, neuromuscular disorders, and developmental delay, and the following interaction terms: antiviral treatment and hemoglobinopathies and antiviral treatment and immunosuppressive conditions. None of the interaction terms in the multivariate model was significant. Therefore, the final multivariate model included only the independent variables from the initial multivariate model. In the final multivariate model, age 6 to 23 months (adjusted OR: 2.1, CI: 1.6 –2.8), age 2 to 4 years (adjusted OR: 1.7, CI: 1.3–2.2), and asthma (adjusted OR: 1.4, CI: 1.1–1.8) remained significantly associated with pneumonia.
DISCUSSION
Influenza-associated pneumonia was documented in 27% of children hospitalized with laboratory-confirmed influenza in this analysis. In comparison to children hospitalized with influenza without pneumonia, children with influenza-associated pneumonia were more likely to require ICU admissions, develop respiratory failure requiring mechanical ventilation, and die.
In this analysis, hospitalized children aged 6 to 23 months and 2 to 4 years were approximately twice as likely to develop influenza-associated pneumonia as children aged 5 to 17 years. This finding is consistent with studies that have shown that the highest frequency of pediatric community acquired pneumonia occurs in children aged 6 months through 4 years,9 and confirms that children aged less than 5 years are at higher risk for severe influenza. Children aged 6 months through 4 years may be at higher risk for influenza-associated pneumonia due to increased exposure to new respiratory pathogens in settings such as daycare, lack of prior exposure to influenza viruses, and the presence of less existing immune memory against common bacterial pathogens than older children.
Hospitalized children with influenza aged less than 6 months were less likely to have pneumonia than children aged 6 months through 4 years, despite the fact that the highest rates of influenza-associated hospitalization occur in infants aged less than 6 months.1,10,11 The lower rate of pneumonia in young infants hospitalized with influenza, compared with children aged 6 months through 4 years hospitalized with influenza, may reflect differences in the likelihood of hospitalization with influenza as children aged less than 6 months may be more likely to be hospitalized due to their young age; differences in immune response if young infants are less able to mount an immune response sufficient to produce evidence of pneumonia on a chest radiograph; or true differences in the risk of influenza-associated pneumonia. Infants aged less than 6 months may benefit from circulating maternal antibodies which may protect against or attenuate influenza virus infection if mothers have naturally acquired influenza antibody or have received influenza vaccine during pregnancy12,13; circulating maternal antibodies may also decrease the risk of bacterial coinfection.14 Breast-fed infants may also derive protection against influenza-associated pneumonia from maternal IgA transferred in breast milk.15,16
Persons with chronic medical conditions are at higher risk for other influenza-associated complications, including clinic-visits, hospitalizations, and death.17–20 In this analysis, children hospitalized with influenza who had asthma were significantly more likely to have pneumonia than other children hospitalized with influenza. In univariate analysis, children with certain other chronic medical conditions, including cardiovascular disease, neuromuscular disorders, and developmental delay also were significantly more likely to have pneumonia. However, the effect was not significant in multivariate analysis, possibly because there were few children with these conditions in the analysis cohort.
In contrast, children with hemoglobinopathies were significantly less likely to have influenza-associated pneumonia, even after controlling for antiviral treatment as a possible confounding factor. This finding is surprising given that the occurrence of community-acquired pneumonia in children with sickle cell disease, one of the more common hemoglobinopathies, is significantly more frequent than in healthy children of the same age.21 Hospitalized children with hemoglobinopathies may appear less likely to have influenza-associated pneumonia if these children are more likely than other children to be hospitalized with mild illness due to concerns about their increased risk for bacterial infection and vaso-occlusive crisis. In addition, children with some hemoglobinopathies such as sickle cell disease are more likely to receive the pneumococcal conjugate vaccine and antibiotic prophylaxis, both of which would provide protection against secondary bacterial pneumonia caused by S. pneumoniae.
This analysis had several limitations. The proportion of children hospitalized with laboratory-confirmed influenza with radiologically confirmed pneumonia may be overestimated if providers were more likely to obtain influenza diagnostic testing on children with more severe illness. In addition, the diagnosis of pneumonia in hospitalized patients is based on a combination of clinical signs and symptoms and findings on chest radiograph consistent with pneumonia. In this analysis, information about clinical signs and symptoms was not available, but it was assumed that children who had a chest radiograph had fever or other signs and symptoms suggestive of possible respiratory pathology. Thus, cases of influenza-associated pneumonia were ascertained by identifying patients who had a chest radiograph and then identifying patients in this group who had documentation in the medical chart of a new chest radiograph finding consistent with pneumonia. Chest radiographs may have been interpreted by clinicians at any level of training without a radiologist’s interpretation. By using this method, children with radiologic findings that might be confused with pneumonia, specifically atelectasis, might have been misclassified as having pneumonia if the chest radiograph was misinterpreted or if there were conflicting interpretations in the medical record. However, if a discharge diagnosis of “pneumonia” had been used to ascertain cases in this analysis, only 57% of cases with radiologic evidence of pneumonia would have been identified, and 52 cases with no radiologic evidence of pneumonia documented in the chart would have been included in the analysis (data not shown). Thus, identifying cases by discharge diagnosis was less specific for radiologically confirmed influenza-associated pneumonia than the method used in this analysis.
This analysis was also limited by the absence of complete data on full influenza vaccination status for children 6 months to 8 years of age. Children in this age group require 2 doses of influenza vaccine to be considered fully vaccinated against influenza during their first season of influenza vaccination,22 but data were missing for a large proportion of children aged 6 months to 8 years on the number of doses of influenza vaccine received during the current season and on receipt of influenza vaccine during previous influenza seasons. Thus, we were not able to assess the association between full vaccination status and influenza-associated pneumonia. Data on pneumococcal conjugate vaccination status was also incomplete. The pneumococcal conjugate vaccine (PCV-7) has been recommended for all children aged 2 to 23 months, in addition to children with sickle cell disease aged less than 5 years, since 2000,23 and has been shown to decrease invasive pneumococcal disease in healthy children and children with chronic medical conditions23–25 as well as to decrease the risk of pneumonia among young children.26 Thus, vaccination with PCV-7 would presumably be expected to reduce the risk of secondary pneumococcal pneumonia in children with influenza.
Lastly, this analysis was limited by the fact that cases of primary influenza pneumonia could not be differentiated from cases of secondary bacterial pneumonia. While 2% of children with influenza-associated pneumonia had evidence of invasive bacterial coinfection in this analysis, the frequency of secondary bacterial pneumonia in children hospitalized with influenza may be higher. Previous studies have found the frequency of bacteremia among children with any pneumonia to range from 1% to 3%, suggesting that most children with pneumonia do not develop bacteremia.27,28 Thoracentesis and bronchoscopy would likely identify more cases of bacterial pneumonia, but these procedures are used rarely and in only the sickest children due to their invasive nature. Further analysis is needed to determine the frequency of primary viral pneumonia versus secondary bacterial pneumonia and the frequency of bacterial coinfection with specific pathogens among children with influenza-associated pneumonia.
CONCLUSION
In this analysis, pneumonia was a common complication among children hospitalized with laboratory-confirmed influenza. Hospitalized children with influenza-associated pneumonia were more likely than other children hospitalized with influenza to have a severe clinical course, including intensive care unit admission, respiratory failure requiring mechanical ventilation, and death. However, only a small proportion of children with influenza-associated pneumonia were treated with antiviral medications. Identifying children who are at greater risk for influenza-associated pneumonia will help clinicians identify children who might benefit most from early antiviral treatment and inform the development of prevention strategies that target children at risk for severe influenza complications.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
References
- 1.Poehling K, Edwards K, Weinberg G, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 2.Schrag S, Shay D, Gershman K, et al. Multistate surveillance for laboratory-confirmed, influenza-associated hospitalizations in children, 2003–2004. Pediatr Infect Dis J. 2006;25:395–400. doi: 10.1097/01.inf.0000214988.81379.71. [DOI] [PubMed] [Google Scholar]
- 3.Ampofo K, Gesteland P, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118:2409–2417. doi: 10.1542/peds.2006-1475. [DOI] [PubMed] [Google Scholar]
- 4.Forster J. Influenza in children: the German perspective. Pediatr Infect Dis J. 2003;22:S215–S217. doi: 10.1097/01.inf.0000092190.43140.f7. [DOI] [PubMed] [Google Scholar]
- 5.Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infection in children. Clin Infect Dis. 2003;36:299–305. doi: 10.1086/345909. [DOI] [PubMed] [Google Scholar]
- 6.Rojo J, Ruiz-Contreras J, Fernandez M, et al. Influenza-related hospitalizations in children younger than three years of age. Pediatr Infect Dis J. 2006;25:596–601. doi: 10.1097/01.inf.0000220208.59965.95. [DOI] [PubMed] [Google Scholar]
- 7.Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus co-infection. Pediatrics. 2008;122:805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira E, Marik P, Colice G. Influenza pneumonia: a descriptive study. Chest. 2001;119:1717–1723. doi: 10.1378/chest.119.6.1717. [DOI] [PubMed] [Google Scholar]
- 9.Murphy T, Henderson F, Clyde W, et al. Pneumonia: an eleven year study in a pediatric practice. Am J Epidemiol. 1981;113:12–21. doi: 10.1093/oxfordjournals.aje.a113061. [DOI] [PubMed] [Google Scholar]
- 10.Griffin M, Walker F, Iwane M, et al. Epidemiology of respiratory infections in young children: insights from the New Vaccine Surveillance Network. Pediatr Infect Dis J. 2004;23:188–192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 11.Neuzil K, Mellen B, Wright P, et al. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 12.Reuman P, Ayoub E, Small P. Effect of passive maternal antibody on influenza illness in children: a prospective study of influenza A in mother-infant pairs. Pediatr Infect Dis J. 1987;6:398–403. doi: 10.1097/00006454-198704000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Zaman K, Roy E, Arifeen S, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 14.Zinkernagel MD. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 15.Chantry C, Howard C, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 16.Zaman K, Baqui A, Yunus M, et al. Acute respiratory infections in children: a community-based longitudinal study in rural Bangladesh. J Trop Pediatr. 1997;43:133–137. doi: 10.1093/tropej/43.3.133. [DOI] [PubMed] [Google Scholar]
- 17.Glezen P, Greenberg S, Atmar R. Impact of respiratory virus infections on persons with chronic underlying conditions. J Am Med Assoc. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 18.Mullooly J, William B. Impact of type A influenza on children: a retrospective study. Am J Public Health. 1982;72:1008–1016. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuzil K, Wright P, Mitchel E, et al. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137:856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien M, Uyeki T, Shay D, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113:585–593. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 21.De Ceulaer K, McMullen K, Maude G, et al. Pneumonia in young children with homozygous sickle cell disease: risk and clinical features. Eur J Pediatr. 1985;144:255–258. doi: 10.1007/BF00451954. [DOI] [PubMed] [Google Scholar]
- 22.Fiore A, Shay D, Penina H, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. Morb Mortal Wkly Rep. 2007;56:1–54. [PubMed] [Google Scholar]
- 23.Preventing pneumococcal disease among infants and young children: recommendations of the advisory committee on immunization practices (ACIP) Morb Mortal Wkly Rep. 2000;49:1–38. [PubMed] [Google Scholar]
- 24.Adamkiewicz T, Silk B, Howgate J, et al. Effectiveness of the 7-Valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121:562–569. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- 25.Whitney C, Pilishvili T, Farley M, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 26.Black S, Shinefield H, Ling S. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–815. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hickey R, Bowman M, Smith G. Utility of blood cultures in pediatric patients found to have pneumonia in the emergency department. Ann Emerg Med. 1996;27:721–725. doi: 10.1016/s0196-0644(96)70189-0. [DOI] [PubMed] [Google Scholar]
- 28.Shah S, Alpern E, Zwerling L, et al. Risk of bacteremia in young children with pneumonia treated as outpatients. Arch Pediatr Adolesc Med. 2003;157:389–392. doi: 10.1001/archpedi.157.4.389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.