Summary
Objective
The objective of the study is to investigate the association of interleukin‐6 (IL6) promoter single‐nucleotide polymorphisms rs1800797 (‐597 G/A) and rs1800796 (‐572 G/C) with obesity or metabolic syndrome in Mexican‐Americans.
Methods
The rs1800797 and rs1800796 single‐nucleotide polymorphisms were genotyped in Mexican‐Americans (n = 437) from South Texas, and results were correlated with measures of obesity and metabolic syndrome including body mass index, waist circumference, blood pressure, cholesterol, triglycerides, glucose, liver enzymes, plasma IL6 and high‐sensitive C‐reactive protein (hs‐CRP).
Results
Significant associations were found for the rs1800796 variant with increased waist circumference, insulin resistance, lower IL6 levels and higher hs‐CRP levels. The rs1800797 variant showed no associations with metabolic traits but was associated with higher IL6 levels and lower hs‐CRP levels.
Conclusions
Findings in this study support the anti‐inflammatory, anti‐obesity and glucose homeostatic roles of IL6 in Mexican‐American youth.
Keywords: Genetics, inflammation, interleukin‐6, obesity
Introduction
Most genetic association studies for obesity are conducted in older populations where epigenetic effects can result in reduced heritability calculations and distract from investigating true genetic susceptibility 1. Epigenetics may be less influential in younger populations where environment has not yet confounded the genetic contribution to obesity. Studies in obesity‐related inflammation are also generally performed in older age groups, although some studies in adolescents or younger age groups show similar obesity associated elevated levels of pro‐inflammatory cytokines, such as tumour necrosis factor alpha, interleukin‐1 beta and interleukin‐6 (IL6) 2, 3.
Pro‐inflammatory cytokines (e.g. IL6) can influence adipocyte function, lipid metabolism, homeostasis, blood pressure and insulin sensitivity and thus play a major role in the development of diabetes, atherosclerosis and cardiovascular diseases 4. However, IL6 can also be anti‐inflammatory, regenerative and homeostatic by controlling acute‐phase response and modulating glucose and liver metabolism 5, 6.
Interleukin‐6 polymorphisms have been investigated in many populations for associations with various chronic diseases 4. For example, single‐nucleotide polymorphisms (SNPs) rs1800797 (‐597 G/A), rs1800796 (‐572 G/C) and rs1800795 (‐174 G/C), located in the promoter region of IL6, have been shown to be associated with obesity and metabolic traits in different ethnic groups (Table 1). The rs1800797 SNP is associated with type 2 diabetes in German 7 and metabolic syndrome in French 8 populations. The rs1800796 SNP is linked with high insulinogenic index in Danes 10, hyperglycaemia in Mexicans 11 and hypertension, obesity, type 2 diabetes and carotid intima‐medial thickness in Asians 9, 12, 13, 14. These three IL6 promoter SNPs have also been studied as haplotypes (Table 1). Haplotypes generated from the rs1800797, rs1800796 and rs1800795 combinations have been shown to be associated with type 2 diabetes in Danes 10 and hyperglycaemia and obesity in Mexicans 11. The GG haplotype for rs1800797 and rs1800795 has been shown to be associated with type 2 diabetes in Mexicans 15 and Asian Indians 16.
Table 1.
Published associations between IL6 SNPs and obesity or metabolic traits
| IL6 SNP | First author (Reference) | Ethnicity | Associated genotype | Associated phenotype |
|---|---|---|---|---|
| rs1800797 | Illig T. 7 | German | GG | Type 2 diabetes |
| Phillips C.M. 8 | French | GG | Metabolic syndrome | |
| rs1800796 | Tanaka C. 9 | Japanese | GG | Systolic blood pressure and carotid intima‐medial thickness |
| Hamid Y.H. 10 | Danes | CC | High insulinogenic index | |
| Ramirez‐Lopez G. 11 | Mexican | CC | Hyperglycaemia | |
| Yin Y.W. 12 | Asian | GG | Type 2 diabetes | |
| Yang X. 13 | Asian | GG | Hypertension and obesity | |
| Ma H. 14 | Asian | GG | Hypertension | |
| Haplotype rs1800797/796*/795† | Hamid Y.H. 10 | Danes | GCG | Type 2 diabetes |
| Ramirez‐Lopez G. 11 | Mexican | GCG | Hyperglycaemia and obesity | |
| Haplotype rs1800797/795† | Zamora‐Ginez I. 15 | Mexican | GG | Type 2 diabetes |
| Saxena M. 16 | Indian | GG | Type 2 diabetes |
796*, rs1800796; 795†, rs1800795.
IL6, interleukin‐6; SNP, single‐nucleotide polymorphism.
Epidemiological investigations in Mexican‐Americans of South Texas have observed a high prevalence of overweight and obesity (40–50%) 17, 18, 19. Non‐alcoholic fatty liver disease prevalence is also higher in this population, and it is associated with obesity and diabetes 20. Recent studies have observed elevated levels of liver enzymes in young Mexican‐Americans of South Texas with obesity and non‐alcoholic fatty liver disease 19, 20. Based on these observations and the link between IL6 and obesity‐related traits in other populations, this study hypothesized genetic associations between IL6 polymorphisms and obesity or metabolic syndrome in Mexican‐Americans. This is the first study that investigates genetic associations between the IL6 promoter SNPs, rs1800797 and rs1800796, with obesity, insulin resistance and metabolic traits including liver enzymes in a cohort of mostly young (90.2% of cohort population is below 30 years old) Mexican‐Americans of the Rio Grande Valley.
Methods
Subjects
DNA samples, anthropometric measurements and laboratory analyses were obtained from Mexican‐American adolescents and adults residing in the area of Rio Grande Valley, South Texas. Of the 437 subjects, 394 were between age of 14 and 30 years (90.2% characterized as mostly young), and 43 were older than 30 years (9.8%). Three hundred subjects were between age 14 and 19 years, 94 between age 20 and 30 years, 26 between age 30 and 40 years and 17 older than 40 years. Characteristics of the study subjects were previously described by Rentfro et al. 18 and Duran‐Gonzalez et al. 21. Studies were approved by the Institutional Review Board (University of Texas at Rio Grande Valley). The studies were performed upon receiving written informed consents from subjects and, in the case of adolescents, from their parents. No stipend was provided to any of the participants.
Anthropometric and metabolic measurements
Individuals 19 years old or younger were categorized as adolescents, and individuals 20 years old or older were categorized as adults. Based on Centers for Disease Control and Prevention growth charts, adolescents with body mass index (BMI) between 85th and 95th percentile were considered overweight, and adolescents with BMI above 95th percentile were considered obese. In adults, BMI values between 25 and 29.9 kg m−2 were considered overweight, and values equal or above 30 kg m−2 were considered obese 18, 21. Waist circumference cut‐off value was the 80th percentile in adolescents and in adults was above 88 cm in women and 102 cm in men 21. Homeostatic model assessment of insulin resistance (HOMA‐IR) values equal or above 3.16 were considered abnormal 18, 21. The obesity classifications (overweight and obese) were coded as abnormal. Blood pressure (in adolescents), glucose and triglycerides cut‐off values were defined as in Rentfro et al. 18. In adults, systolic blood pressure was considered abnormal if it was equal or above 130 mmHg, and diastolic blood pressure was considered abnormal if it was equal or above 85 mmHg 22.
Fasting blood samples were drawn and sent to Clinical Laboratory Improvement Amendments approved clinical laboratories for metabolic measurements including blood glucose, triglycerides, cholesterol and liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Total cholesterol values above 200 mg dL−1 were considered abnormal, low‐density lipoprotein cholesterol values greater than or equal to 100 mg dL−1 were classified as high and high‐density lipoprotein cholesterol values of less than 40 mg dL−1 were classified as low 23. The cut‐off value for abnormal liver enzymes (AST and ALT) was 40 U L−1 20. Plasma levels of IL6 (Merck Millipore, Burlington, MA, USA) and high‐sensitive C‐reactive protein (hs‐CRP) (Alpha Diagnostic International, San Antonio, TX, USA) were measured using ELISA kits as per manufacturer's instructions from fasting blood samples.
DNA isolation and genotyping
QIAmp DNA Mini Kit (Qiagen, Valencia, CA, USA) was used to extract DNA from 1 mL of white blood cells. DNA quantification was conducted using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA (50–100 ng well−1) was used in genotyping assays. TaqMan SNP Genotyping Assays were used for genotyping rs1800797 and rs1800796 in Step One Plus Real‐time PCR system (Applied Biosystems, Carlsbad, CA, USA).
Statistical analysis
All statistical analyses were performed using PLINK v.1.07 (http://pngu.mgh.harvard.edu/~purcell/plink). Hardy–Weinberg equilibrium was used to analyse genotype distribution deviations in the two IL6 SNPs. Linkage disequilibrium statistics were also performed in PLINK. Associations of SNPs with obesity were performed using logistic regression analyses assuming additive, dominant and recessive models, while associations of SNPs with metabolic traits were performed using logistic regression analyses assuming only the additive model. For the all subjects group, logistic regression analyses for obesity were adjusted for age and gender, while for metabolic traits were adjusted for age, gender and waist circumference. Fisher exact test was used to identify frequency of affected and non‐affected alleles for each SNP. Data are presented as odds ratios with 95% confidence intervals with respect to the minor alleles. P‐values were obtained from association analyses, and permutation‐P tests were performed to correct for multiple testing (P‐values <0.05 were consider significant). Plasma levels of IL6, hs‐CRP and liver enzymes (AST and ALT) in various genotypic groups were compared and statistical significance assessed using Student's t‐tests. Comparisons with all subjects for which values were available were made, as well as with outliers removed for IL6 and hs‐CRP. Values above 1.5 times the interquartile range were categorized as outliers. Table 7 shows data with outliers removed for IL6 and hs‐CRP. Table S1 shows the data including outliers.
Results
Participants consisted of 437 Mexican‐Americans: 291 were female, and 146 were male. Anthropometric and clinical characteristics of the population are described in Table 2. Population was mostly young (median age = 17 years, SD = ± 8.26) and with overweight/obesity (52.17%) (Table 4). In Mexican‐Americans, rs1800797 is in complete linkage disequilibrium with rs1800795 (D′ = 1 and r2 = 1) and in high linkage disequilibrium (D′ = 1) with rs1800796 but with different allele frequencies resulting in a low r2 (r2 = 0.08); therefore, rs1800797 and rs1800796 were selected for genotyping and subsequent association analyses. Four hundred and twenty‐nine individuals were successfully genotyped for rs1800797 and 428 for rs1800796. However, complete phenotype data on these individuals varied. Both polymorphisms were in Hardy–Weinberg equilibrium (rs1800797 P = 0.87; rs1800796 P = 0.34) (Table 3).
Table 2.
Anthropometric and clinical characteristics of Mexican‐Americans studied
| Characteristics | Subjects with phenotype data (n) | Median ± SD | Q1/Q3 | Min/Max |
|---|---|---|---|---|
| Age (years) | All subjects (437)* | 17 ± 8.26 | 15/21 | 14/60 |
| Female (291) | 17 ± 8.70 | 15/22 | 14/60 | |
| Male (146) | 17 ± 7.29 | 15/20 | 14/54 | |
| BMI (kg m−2) | All subjects (436) | 24.94 ± 6.55 | 22.29/29.87 | 15.68/55.24 |
| Female (290) | 24.56 ± 6.51 | 22.2/29.56 | 17.17/53.13 | |
| Male (146) | 25.91 ± 6.64 | 22.51/30.23 | 15.68/55.24 | |
| WC (cm) | All subjects (436) | 83 ± 16.83 | 75.5/95.63 | 30/167 |
| Female (290) | 81.75 ± 15.6 | 75/94 | 40.5/167 | |
| Male (146) | 85.3 ± 18.92 | 77.63/100.5 | 30/160 | |
| SBP (mmHg) | All subjects (437) | 110 ± 12.29 | 101/120 | 85/156 |
| Female (291) | 110 ± 11.38 | 100.5/115.5 | 85/153 | |
| Male (146) | 116 ± 13.26 | 104/123 | 85/156 | |
| DBP (mmHg) | All subjects (437) | 69 ± 9.74 | 63/76 | 40/97 |
| Female (291) | 69 ± 9.34 | 63/74 | 40/96 | |
| Male (146) | 70 ± 10.27 | 66/78.75 | 45/97 | |
| HOMA‐IR | All subjects (374) | 2.05 ± 2.87 | 1.21/3.39 | 0.02/36.89 |
| Female (246) | 2.15 ± 3.18 | 1.36/3.24 | 0.13/36.89 | |
| Male (128) | 1.86 ± 2.16 | 1.09/3.44 | 0.02/12.53 | |
| AST (U L−1) | All subjects (230) | 21 ± 16.16 | 16/28 | 7/157 |
| Female (153) | 19 ± 17.75 | 15/26.25 | 7/157 | |
| Male (77) | 25 ± 12.69 | 18/32 | 12/68 | |
| ALT (U L−1) | All subjects (230) | 33 ± 18.73 | 28/39 | 2.4/164 |
| Female (153) | 31 ± 18.42 | 28/35 | 2.4/162 | |
| Male (77) | 36 ± 18.86 | 33/44 | 21/164 | |
| Cholesterol (mg dL−1) | All subjects (350) | 149 ± 34.88 | 131/171 | 81/460 |
| Female (231) | 147 ± 37.24 | 132/171 | 89/460 | |
| Male (119) | 151 ± 29.89 | 130/171.5 | 81/230 | |
| Triglycerides (mg dL−1) | All subjects (349) | 68 ± 51.12 | 48/95 | 18/403 |
| Female (230) | 64.5 ± 43.64 | 46/92.75 | 25/281 | |
| Male (119) | 72 ± 62.16 | 52/111.5 | 18/403 | |
| HDL (mg dL−1) | All subjects (350) | 46 ± 11.85 | 40/55 | 14/97 |
| Female (231) | 47 ± 11.86 | 41/56 | 14/97 | |
| Male (119) | 44 ± 11.5 | 38/53 | 24/89 | |
| LDL (mg dL−1) | All subjects (350) | 86.5 ± 25.52 | 72/103.85 | 38/211 |
| Female (231) | 86 ± 26.53 | 72/103 | 38/211 | |
| Male (119) | 88.4 ± 23.53 | 72.8/106 | 45/163.2 | |
| Glucose (mg dL−1) | All subjects (416) | 90 ± 16.76 | 83.06/96.04 | 43.06/265.95 |
| Female (276) | 89 ± 16.07 | 83/95.25 | 43/228 | |
| Male (140) | 92 ± 18.14 | 85/97 | 65/266 | |
| Plasma IL6 (pg mL−1) | All subjects (128) | 3.68 ± 22.21 | 1.52/14.98 | 0.18/125.1 |
| Female (84) | 3.58 ± 18.83 | 1.6/10.43 | 0.2/99.19 | |
| Male (44) | 4.17 ± 27.09 | 1.46/27.01 | 0.18/125.1 | |
| hs‐CRP (mg L−1) | All subjects (267) | 0.66 ± 4.93 | 0.35/1.77 | 0.11/57.42 |
| Female (171) | 0.76 ± 5.92 | 0.35/2.23 | 0.16/57.42 | |
| Male (96) | 0.53 ± 1.95 | 0.35/1.27 | 0.11/14.8 |
Of the 437 subjects, 394 were between age 14 and 30 years (90.2% characterized as mostly young), and 43 were older than 30 years (9.8%). Three hundred subjects were between age 14 and 19 years, 94 between age 20 and 30 years, 26 between age 30 and 40 years and 17 older than 40 years.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitive C‐reactive protein; IL6, interleukin‐6; LDL, low‐density lipoprotein cholesterol; Max, maximum values; Min, minimum values; SBP, systolic blood pressure; SD, standard deviation; Q1, 1st quartile; Q3, 3rd quartile; WC, waist circumference.
Table 3.
Alleles, allele frequencies and HWE calculations for rs1800797 and rs1800796
| SNP | M/m alleles | M/M (%) | M/m (%) | m/m (%) | MAF |
HWE P‐value |
|---|---|---|---|---|---|---|
| rs1800797 | G/A | 294 (68.53) | 122 (28.44) | 13 (3.03) | 0.17 | 0.87 |
| rs1800796 | G/C | 225 (52.57) | 165 (38.55) | 38 (8.88) | 0.28 | 0.34 |
HWE, Hardy–Weinberg equilibrium; M, major allele; m, minor allele; MAF, minor allele frequency; SNP, single‐nucleotide polymorphism.
Minor allele frequencies (MAFs) for both SNPs were compared between subjects with and without obesity phenotypes categorized by BMI and waist circumference. Table 4 describes association of SNPs with BMI. MAFs of rs1800797 and rs1800796 did not differ significantly between the subjects with or without obesity (rs1800797 P = 0.41; rs1800796 P = 0.22). There were no associations found between SNPs and BMI using the additive (rs1800797 P = 0.78; rs1800796 P = 0.60), dominant (rs1800797 P = 0.29; rs1800796 P = 0.09) or recessive (rs1800797 P = 0.87; rs1800796 P = 0.93) models (Table 4). Table 5 describes the association of SNPs with waist circumference. MAF of rs1800797 did not differ significantly between subjects with or without central obesity (P = 0.17), and no associations with waist circumference were found for rs1800797 using the additive (P = 0.79), dominant (P = 0.09) or recessive (P = 0.64) models (Table 5). However, frequency of the rs1800796 minor allele (C‐allele) was significantly higher in subjects with central obesity (P = 0.02; permutation P‐value = 0.04), and a significant association between rs1800796 and waist circumference under the dominant model (CC = CG vs. GG P = 0.01; permutation P ‐value = 0.03) was observed (Table 5).
Table 4.
Association of SNPs with the phenotypes of obesity categorized by body mass index
| SNP | Test | Non‐obese phenotype (n = 204) | Overweight/obese phenotype (n = 221) | OR (95% CI) | P‐value | Permutation P‐value |
|---|---|---|---|---|---|---|
| rs1800797 | MAF (A‐allele) | 0.18 | 0.16 | 0.85 | 0.41 | 0.63 |
| Additive model | 0.92 (0.51–1.67) | 0.78 | 0.95 | |||
| Dominant model AA + AG vs. GG | 69/135 | 65/156 | 0.80 (0.53–1.21) | 0.29 | 0.50 | |
| Recessive model AA vs. AG + GG | 6/198 | 6/215 | 0.90 (0.28–2.94) | 0.87 | 0.98 | |
| rs1800796 | MAF (C‐allele) | 0.26 | 0.30 | 1.21 | 0.22 | 0.38 |
| Additive model | 1.10 (0.77–1.56) | 0.60 | 0.84 | |||
| Dominant model CC + CG vs. GG | 89/115 | 113/108 | 1.41 (0.95–2.07) | 0.09 | 0.17 | |
| Recessive model CC vs. CG + GG | 18/186 | 20/201 | 1.03 (0.52–2.04) | 0.93 | 1.0 |
Associations were adjusted for age and gender.
CI, confidence interval; MAF, minor allele frequency; OR, odds ratio; SNP, single‐nucleotide polymorphism.
Table 5.
Association of SNPs with the phenotypes of obesity categorized by waist circumference
| SNP | Test | Non‐centrally obese phenotype (n = 208) | Centrally obese phenotype (n = 217) | OR (95% CI) | P‐value | Permutation P‐value |
|---|---|---|---|---|---|---|
| rs1800797 | MAF (A‐allele) | 0.19 | 0.15 | 0.78 | 0.17 | 0.31 |
| Additive model | 1.08 (0.60–1.95) | 0.79 | 0.95 | |||
| Dominant model AA + AG vs. GG | 74/134 | 60/157 | 0.70 (0.46–1.05) | 0.09 | 0.17 | |
| Recessive model AA vs. AG + GG | 5/203 | 7/210 | 1.32 (0.41–4.25) | 0.64 | 0.90 | |
| SNP | Test | Non‐centrally obese phenotype (n = 210) | Centrally obese phenotype (n = 215) | OR (95% CI) | P‐value | Permutation P‐value |
| rs1800796 | MAF (C‐allele) | 0.25 | 0.32 | 1.44 | 0.02 | 0.04 |
| Additive model | 1.29 (0.91–1.83) | 0.15 | 0.26 | |||
| Dominant model CC + CG vs. GG | 87/123 | 115/100 | 1.62 (1.11–2.39) | 0.01 | 0.03 | |
| Recessive model CC vs. CG + GG | 16/194 | 22/193 | 1.37 (0.70–2.69) | 0.36 | 0.59 |
Associations were adjusted for age and gender.
CI, confidence interval; MAF, minor allele frequency; OR, odds ratio; SNP, single‐nucleotide polymorphism. Bolded P‐values <0.05, and statistically significant.
Associations between rs1800797 and rs1800796 and several metabolic traits were investigated in all subjects, as well as in subjects separated by gender (Table 6). Metabolic traits included systolic and diastolic blood pressure, HOMA‐IR, liver enzymes (AST and ALT), total cholesterol, triglycerides, high‐density and low‐density lipoproteins and glucose. No associations were found between rs1800797 and metabolic traits in all subjects or men and women separately (Table 6). For rs1800796, there were no associations for most of the metabolic traits except for HOMA‐IR, when all subjects were considered and for female subjects separately (P = 0.009 and 0.03, respectively; Table 6). In male subjects, no associations were found between rs1800796 and any metabolic trait.
Table 6.
Association of SNPs with metabolic traits
| rs1800797 | rs1800796 | ||||||
|---|---|---|---|---|---|---|---|
| Metabolic traits (n) | OR (95% CI) | P‐value | Permutation P‐value | Metabolic traits (n) | OR (95% CI) | P‐value | Permutation P‐value |
| Systolic blood pressure (mmHg) | Systolic blood pressure (mmHg) | ||||||
| All subjects (424) | 0.89 (0.24–3.31) | 0.87 | 0.90 | All subjects (423) | 0.92 (0.42–2.04) | 0.84 | 0.87 |
| Female (279) | 0.91 (0.22–3.80) | 0.90 | 0.88 | Female (279) | 0.98 (0.30–3.26) | 0.98 | 0.92 |
| Male (145) | 0.0001 (0–*) | 1.0 | 1.0 | Male (144) | 0.73 (0.21–2.54) | 0.63 | 0.46 |
| Diastolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | ||||||
| All subjects (424) | 1.13 (0.34–3.76) | 0.85 | 0.88 | All subjects (423) | 1.31 (0.65–2.62) | 0.45 | 0.54 |
| Female (279) | 1.43 (0.43–4.73) | 0.56 | 0.56 | Female (279) | 0.75 (0.23–2.4) | 0.63 | 0.59 |
| Male (145) | 0.0001 (0–*) | 1.0 | 1.0 | Male (144) | 2.25 (0.84–5.98) | 0.10 | 0.08 |
| HOMA‐IR | HOMA‐IR | ||||||
| All subjects (363) | 0.55 (0.18–1.64) | 0.28 | 0.48 | All subjects (361) | 0.35 (0.16–0.77) | 0.009 | 0.009 |
| Female (236) | 0.61 (0.2–1.8) | 0.37 | 0.60 | Female (234) | 0.22 (0.06–0.84) | 0.03 | 0.03 |
| Male (127) | 6.1e−005 (0–*) | 1.0 | 1.0 | Male (127) | 0.68 (0.23–2.01) | 0.49 | 0.54 |
| Aspartate aminotransferase (U L−1) | Aspartate aminotransferase (U L−1) | ||||||
| All subjects (220) | 0.0001 (0–*) | 1.0 | 1.0 | All subjects (228) | 1.12 (0.33–3.87) | 0.85 | 0.66 |
| Female (144) | 0.0001 (0–*) | 1.0 | 0.99 | Female (151) | 1.31 (0.35–4.98) | 0.69 | 0.47 |
| Male (76) | NA | NA | 1.0 | Male (77) | 0.0001 (0–*) | 1.0 | 0.91 |
| Alanine aminotransferase (U L−1) | Alanine aminotransferase (U L−1) | ||||||
| All subjects (220) | 7.93e−005 (0–*) | 1.0 | 1.0 | All subjects (228) | 0.77 (0.25–2.35) | 0.65 | 0.71 |
| Female (144) | 7.78e−005 (0–*) | 1.0 | 1.0 | Female (151) | 0.83 (0.27–2.63) | 0.76 | 0.78 |
| Male (76) | NA | NA | 1.0 | Male (77) | 0.0001 (0–*) | 1.0 | 0.96 |
| Cholesterol (mg dL−1) | Cholesterol (mg dL−1) | ||||||
| All subjects (338) | 5.07e−005 (0–*) | 1.0 | 1.0 | All subjects (341) | 1.0 (0.46–2.15) | 1.0 | 0.94 |
| Female (220) | 6.03e−005 (0–*) | 1.0 | 1.0 | Female (223) | 0.79 (0.30–2.08) | 0.63 | 0.65 |
| Male (118) | NA | NA | 1.0 | Male (118) | 2.03 (0.56–7.34) | 0.28 | 0.12 |
| Triglycerides (mg dL−1) | Triglycerides (mg dL−1) | ||||||
| All subjects (337) | 0.0001 (0–*) | 1.0 | 1.0 | All subjects (340) | 0.0001 (0–*) | 1.0 | 0.83 |
| Female (219) | 0.0001 (0–*) | 0.99 | 0.98 | Female (222) | 0.0001 (0–*) | 1.0 | 0.54 |
| Male (118) | NA | NA | 1.0 | Male (118) | 0.0001 (0–*) | 1.0 | 0.40 |
| HDL (mg dL−1) | HDL (mg dL−1) | ||||||
| All subjects (338) | 7.09e−005 (0–*) | 1.0 | 1.0 | All subjects (341) | 1.16 (0.70–1.91) | 0.57 | 0.81 |
| Female (220) | 6.96e−005 (0–*) | 1.0 | 1.0 | Female (223) | 1.23 (0.66–2.32) | 0.51 | 0.64 |
| Male (118) | NA | NA | 1.0 | Male (118) | 0.86 (0.36–2.07) | 0.74 | 0.71 |
| LDL (mg dL−1) | LDL (mg dL−1) | ||||||
| All subjects (338) | 1.05 (0.49–2.28) | 0.90 | 1.0 | All subjects (341) | 1.09 (0.72–1.64) | 0.70 | 0.90 |
| Female (220) | 1.06 (0.48–2.32) | 0.89 | 0.99 | Female (223) | 1.13 (0.69–1.87) | 0.63 | 0.83 |
| Male (118) | NA | NA | 1.0 | Male (118) | 0.99 (0.45–2.19) | 0.97 | 0.95 |
| Glucose (mg dL−1) | Glucose (mg dL−1) | ||||||
| All subjects (403) | 1.63 (0.44–5.97) | 0.46 | 0.45 | All subjects (402) | 0.0001 (0–*) | 1.0 | 0.76 |
| Female (264) | 1.78 (0.48–6.61) | 0.39 | 0.40 | Female (264) | 0.0001 (0–*) | 1.0 | 0.68 |
| Male (139) | 0.0002 (0–*) | 1.0 | 0.95 | Male (138) | 0.0003 (0–*) | 1.0 | 0.89 |
P‐values for the all subjects group were adjusted for age, gender and waist circumference. P‐values for female and male subjects separately were adjusted for age and waist circumference. NA, frequency of the allele is less than 5.
Infinity.
CI, confidence interval; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein cholesterol; OR, odds ratio; SNP, single‐nucleotide polymorphism. Bolded P values are statistically significant (< 0.05).
Plasma IL6, hs‐CRP and liver enzymes (AST and ALT) levels were compared between genotypes for each SNP (Table 7 and Figures 1 and 2). Genotypes were combined because the numbers of the minor allele homozygotes for both SNPs were low compared with other genotypes. For rs1800797, plasma IL6 levels did not differ significantly between A‐allele homozygotes when compared with G‐carriers (AA vs. GG + AG P = 0.48). However, plasma IL6 levels were significantly higher in rs1800797 A‐carriers when compared with G‐allele homozygotes (AA + AG vs. GG P = 0.002) (Table 7 and Figure 1A). For rs1800796, plasma IL6 was significantly lower in C‐allele homozygotes when compared with G‐carriers (CC vs. GG + CG P = 6.6e−07), but it did not differ in C‐carriers when compared with G‐allele homozygotes (CC + CG vs. GG P = 0.33) (Table 7 and Figure 1B).
Table 7.
Association of SNPs' genotypes with plasma IL6, hs‐CRP and liver enzymes levels
| Trait | SNP and genotypes | n | Mean ± SD | Genotypic combination | P‐value |
|---|---|---|---|---|---|
| Plasma IL6 (pg mL−1) | rs1800797 | ||||
| AA | 3 | 9.19 ± 5.06 |
AA vs. GG + AG AA + AG vs. GG |
0.48 0.002 |
|
| AG | 31 | 12.61 ± 15.05 | |||
| GG | 69 | 3.97 ± 4.67 | |||
| rs1800796 | |||||
| CC | 12 | 1.97 ± 1.07 |
CC vs. GG + CG CC + CG vs. GG |
6.6e−07
0.33 |
|
| CG | 45 | 6.33 ± 7.59 | |||
| GG | 48 | 6.87 ± 8.24 | |||
| hs‐CRP (mg L−1) | rs1800797 | ||||
| AA | 3 | 0.77 ± 0.01 | AA vs. GG + AG AA + AG vs. GG |
0.48 0.04 |
|
| AG | 60 | 0.67 ± 0.53 | |||
| GG | 156 | 0.86 ± 0.73 | |||
| rs1800796 | |||||
| CC | 22 | 1.34 ± 1.56 |
CC vs. GG + CG CC + CG vs. GG |
0.10 0.01 |
|
| CG | 89 | 0.86 ± 0.68 | |||
| GG | 112 | 0.69 ± 0.55 | |||
| AST (U L−1) | rs1800797 | ||||
| AA | 6 | 23.83 ± 4.31 |
AA vs. GG + AG AA + AG vs. GG |
0.71 0.91 |
|
| AG | 69 | 24.80 ± 12.86 | |||
| GG | 148 | 24.51 ± 15.04 | |||
| rs1800796 | |||||
| CC | 21 | 27.57 ± 30.59 |
CC vs. GG + CG CC + CG vs. GG |
0.63 0.40 |
|
| CG | 94 | 22.82 ± 10.12 | |||
| GG | 116 | 25.47 ± 16.30 | |||
| ALT (U L−1) | rs1800797 | ||||
| AA | 6 | 34.50 ± 5.54 |
AA vs. GG + AG AA + AG vs. GG |
0.47 0.60 |
|
| AG | 69 | 37.54 ± 20.45 | |||
| GG | 148 | 35.91 ± 15.52 | |||
| rs1800796 | |||||
| CC | 21 | 37.43 ± 24.29 |
CC vs. GG + CG CC + CG vs. GG |
0.89 0.59 |
|
| CG | 94 | 37.37 ± 19.78 | |||
| GG | 116 | 36.05 ± 16.65 | |||
Plasma levels of IL6, hs‐CRP and liver enzymes (AST and ALT) were compared between various genotype combinations for each SNP. Student's t‐tests were used to assess statistical significance. IL6 and hs‐CRP outliers were removed from the associations. Table S1 shows the data including outliers. Values above 1.5 times the interquartile range were categorized as outliers.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; hs‐CRP, high‐sensitive C‐reactive protein; IL6, interleukin‐6; SD, standard deviation; SNP, single‐nucleotide polymorphism. Bolded P values are statistically significant (< 0.05).
Figure 1.
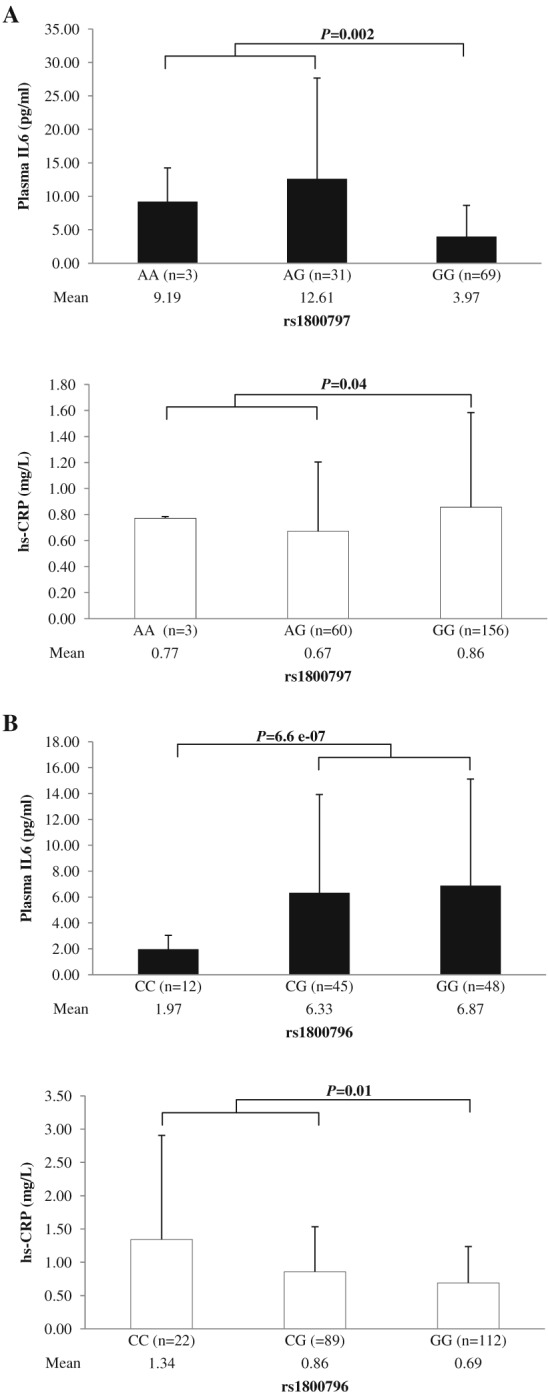
Comparison of mean plasma interleukin‐6 (IL6) and high‐sensitive C‐reactive protein (hs‐CRP) levels between genotypes for each single‐nucleotide polymorphism. A. Plasma IL6 and hs‐CRP levels were compared between rs1800797 genotypes. Plasma IL6 was significantly higher in rs1800797 A (protective allele against obesity and metabolic traits)‐carriers than in G‐homozygotes (P = 0.002), and hs‐CRP was significantly lower in rs1800797 A‐carriers than in G‐homozygotes (P = 0.04). B. Plasma IL6 and hs‐CRP levels were compared between rs1800796 genotypes. Plasma IL6 was significantly lower in rs1800796 C (risk allele for obesity and metabolic traits)‐homozygotes than in G‐carriers (P = 6.6e−07), and hs‐CRP was significantly higher in C‐carriers than in G‐homozygous (P = 0.01).
Figure 2.
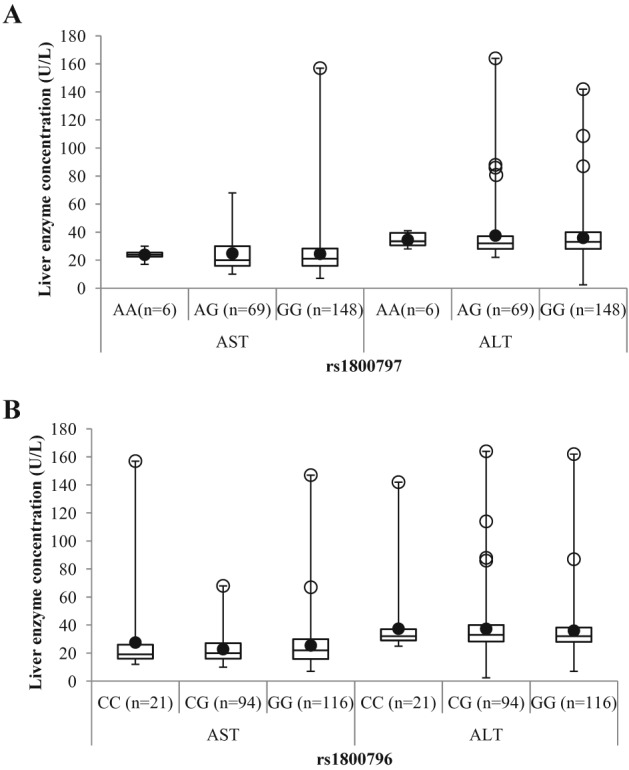
Comparison of liver enzyme (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) levels for each interleukin‐6 (IL6) single‐nucleotide polymorphism genotype. A. Liver enzymes were compared between rs1800797 genotypes. Levels of liver enzymes did not differ between rs1800797 genotypes, but a trend for higher plasma levels of liver enzymes was observed in rs1800797 GG (trend for higher hs‐CRP and lower IL6) genotypes. B. Liver enzymes were compared between rs1800796 genotypes. Plasma levels of liver enzymes did not differ between rs1800796 genotypes, but a trend of higher plasma levels of liver enzymes was observed in rs1800796 C (risk allele for waist circumference, homeostasis model assessment of insulin resistance, higher hs‐CRP and lower IL6)‐carriers. White circles refer to outliers; black circles refer to mean.
The inflammation marker, hs‐CRP, did not differ between rs1800797 A‐homozygotes and G‐carriers (AA vs. GG + AG P = 0.48), but levels were significantly lower in A‐carriers when compared with G‐homozygotes (AA + AG vs. GG P = 0.04) (Table 7 and Figure 1A). For rs1800796, hs‐CRP did not differ between C‐homozygotes and G‐carriers (CC vs. GG + CG P = 0.10), but it was significantly higher in C‐carriers when compared with G‐homozygotes (CC + CG vs. GG P = 0.01) (Table 7 and Figure 1B).
Although AST and ALT liver enzymes did not differ significantly when genotypic groups were compared, a modest increase in both enzymes was observed for each SNP's risk allele for obesity and metabolic traits (Table 7 and Figure 2). For rs1800797, higher AST and ALT values were found in G‐carriers (Table 7 and Figure 2A), whereas for rs1800796, higher values were found in C‐carriers (Table 7 and Figure 2B).
Discussion
In this study, association analyses assuming additive, dominant and recessive effects of rs1800797 G‐allele did not show any significant associations with obesity (categorized by BMI and waist circumference) and metabolic traits. The rs1800797 SNP has not been associated with metabolic syndrome and diabetes as much as the rs1800795 SNP 16; however, some studies have reported the contribution of rs1800797 G‐allele to the development of metabolic syndrome and diabetes, but not the A‐allele 7, 8. In this study, despite the lack of statistical significance, frequency of rs1800797 A‐allele was slightly higher among individuals without obesity. Plasma IL6 levels were significantly higher, and hs‐CRP levels were significantly lower in A‐allele carriers than in GG‐homozygotes, suggesting a possible protective effect of rs1800797 A‐allele against obesity and inflammation in this mostly young Mexican‐American cohort.
Findings of this study also demonstrate the possible role of rs1800796 in central obesity and insulin resistance in young Mexican‐Americans. In this study, presence of rs1800796 C‐allele was higher among subjects with increased waist circumference. This result is in accordance with a study performed in non‐Hispanic whites where waist‐to‐hip ratio was significantly higher in postmenopausal women carrying the C‐allele 24. A Caucasian study associated rs1800796 C‐allele with metabolic syndrome, although it did not find an association with waist circumference 25. Also, two studies performed in Caucasians showed associations between rs1800796 and obesity. In one of the reports, the rs1800796 C‐allele was shown to be associated with obesity in children 26, and in the other study, they observed that pregnant women who carry the rs1800796 G‐allele were protected from gestational weight gain 27. However, a study performed in a Dutch cohort observed smaller waist circumference in rs1800796‐CC individuals compared with G‐carriers 28. A Mexican study found that the rs1800797‐A/rs1800796‐G/rs1800795‐C haplotype was associated with lower prevalence of diabetes 29, which suggests that the rs1800796 G‐allele could possibly contribute to the protection against developing type 2 diabetes in Mexicans. A joint analysis including eight studies of mostly Caucasians did not find an association with the rs1800796 C‐allele and diabetes 25, where BMI was included as a covariate instead of waist circumference. The association of rs1800796 C‐allele with risk for obesity and type 2 diabetes has thus been inconsistent across ethnicities. In this study, however, increased waist circumference is associated with the rs1800796 C‐allele, and because waist circumference has been classified as a better predictor of obesity, insulin resistance and type 2 diabetes in Mexican‐Americans 30, it was included as a covariate in the analyses of IL6 SNP associations with metabolic traits. The rs1800796 C‐allele was also found to be associated with higher HOMA‐IR (a surrogate measure of insulin resistance) when analyses were conducted in all subjects (male and female) and in female subjects separately. Thus, this study correlates the rs1800796 C‐allele with increased central obesity and insulin resistance, two important indicators of type 2 diabetes and highly heritable among Mexican‐Americans 31.
Elevated levels of IL6 are typically associated with obesity‐related inflammation and type 2 diabetes in adolescents and adults with obesity 2, 3. One recent study found higher promoter activity of the rs1800796 G‐allele compared with the C‐allele 32. Interestingly, in this study, rs1800796‐CC homozygotes had lower plasma IL6 levels when compared with G‐allele carriers. Additionally, rs1800796 C‐allele carriers were found to express higher hs‐CRP levels, a marker of acute immune response (inflammation) in liver, than G‐allele homozygotes, and liver enzymes AST and ALT trended towards being elevated in rs1800796 C‐carriers. This suggests that in young Mexican‐Americans, rs1800796 C‐allele carriers with lower levels of IL6 have a higher risk for obesity associated inflammation and metabolic disturbances such as insulin resistance and liver inflammation.
There is significant evidence showing that IL6 has anti‐inflammatory, anti‐obesogenic and glucose homeostatic functions. For example, the development of obesity, hepatic insulin resistance, liver inflammation and damage, insulin and glucose intolerance and mitochondrial dysfunctions was observed in high fat diet‐fed IL6‐deficient mice when compared with control mice 33. Another study observed an increase in body fat, body weight, triglycerides, very‐low‐density lipoprotein cholesterol and impaired glucose elimination in mice lacking the IL6 gene 34. In addition, body weight decreased after intraperitoneal and/or intracerebroventricular IL6 injections in IL6 knockout mice 34 and wild‐type rats 35. Disruption of the IL6 anti‐inflammatory signalling in insulin sensitive tissues was present in myeloid cells IL6 receptor inactivated mice, leading to insulin resistance 34. A human study supported the animal studies by observing higher cerebrospinal IL6 levels in subjects without obesity than in subjects with obesity 36. IL6 has been shown to play a homeostatic role in muscle through an acute exercise response. In this response, there is an increase in plasma IL6 levels, IL6 mRNA in adipose tissue and IL6 mRNA and protein content in the exercised skeletal muscle, without causing muscle damage 37. Exercise‐induced IL6 acts as an anti‐inflammatory cytokine by increasing cortisol, lipolysis, catecholamine, IL‐1 receptor antagonist, IL‐10 and CRP (which contributes to the increase of IL‐1RA) to regulate metabolic and energy homeostasis during exercise 37, 38.
A normal homeostatic response of tissues to increased inflammatory factors (caused by elevated reactive oxygen species or hypoxia in expanding adipose tissue) may be an initial acute response of elevated IL6, which in turn up‐regulates anti‐inflammatory molecules (such as cortisol, IL‐1RA, IL‐4 and IL‐10) in insulin sensitive tissues or brain regulating obesity and is associated with metabolic perturbations 36. If this normal acute response of IL6 to inflammation is suppressed or lowered (as in rs1800796‐CC genotypes) in youth, it may lead to unchecked increase in waist circumference and insulin resistance. However, in youths with the rs1800797‐GG genotype, the homeostatic response of IL6 in checking inflammation may be one of the protective factors against metabolic consequences of obesity.
However, IL6 resistance may underlie chronic obesity‐related inflammation with increased plasma levels of IL6 in adolescents and adults. O'Connor et al. 39 were the first one to demonstrate dysfunctional activity of IL‐4, an anti‐inflammatory cytokine, in type 2 diabetes conditions and suggested the development of cytokine resistance to IL‐4 in type 2 diabetes, concluding that impaired suppression of inflammation by anti‐inflammatory cytokines is present in type 2 diabetes. They observed that after IL‐4 treatment, macrophages from obese/diabetic mice failed to express anti‐inflammatory cytokines (IL‐1RA and IL‐1R2), and induction of IRS‐2, mediator of IL‐4 response, was significantly reduced in obese/diabetic macrophages when compared with controls 39. Although IL6 resistance was not studied by O'Connor et al. 39, it is entirely plausible that IL6 resistance is associated with chronic obesity and insulin resistance.
Interleukin‐6 changes its macrophage induction from anti‐inflammatory (M2) macrophages in lean individuals to pro‐inflammatory (M1) macrophages in individuals with obesity 40. In younger individuals with overweight (but not yet with obesity), the initial acute homeostatic IL6 induction of anti‐inflammatory M2 macrophages may help to prevent further increase in adiposity and/or metabolic perturbations. This initial protective M2 response may be absent or weak in rs1800796‐CC genotype carriers, with lower IL6 levels. However, in carriers of rs1800796‐GG genotype, while this phenotype may be protective initially, if obesity/morbid obesity‐associated chronic inflammation develops (with elevated plasma IL6 levels and IL6 resistance), M2 anti‐inflammatory response may no longer be induced by IL6.
In this study, although 52.17% of the subjects had obesity, only 27.6% had insulin resistance. Thus, a relatively metabolically healthy and young population (90.2% below 30 years) in which it may be easier to tease out genetic susceptibility without being influenced by environmental factors such as ageing was studied 1. The study showed that the rs1800796 C‐allele is associated with central obesity and insulin resistance, whereas rs1800797 A‐allele may be protective against obesity in a young Mexican‐American cohort from the Rio Grande Valley. Data from this study support the hypothesis that IL6 plays an anti‐inflammatory homeostatic role in obesity and metabolic diseases in a young Mexican‐American population. The limitations of the study are the small sample size, incomplete metabolic, clinical and IL6/hs‐CRP data on all genotyped samples, as well as lack of genotyping data on additional SNPs. These results will need to be replicated with more SNPs in larger sample sizes and cohorts with more comprehensive measurements to fully substantiate and support the hypothesis proposed. This is the first study investigating genetic associations of IL6 SNPs with obesity, obesity‐related inflammation and metabolic syndrome in an understudied population of Mexican‐Americans from South Texas. Because of the complexity of IL6 regulation in obesity and obesity‐related inflammation, further research needs to be conducted to investigate the myriad cellular and molecular pathways affected by the IL6 polymorphisms.
Conflict of Interest Statement
The authors declared no conflict of interest.
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) under award number R25GM065925 and by the National Institute on Minority Health and Health Disparities (NIMHD) under award number P20MD001091 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supporting information
Data S1. Supporting information
Boeta‐Lopez, K. , Duran, J. , Elizondo, D. , Gonzales, E. , Rentfro, A. , Schwarzbach, A. E. , and Nair, S. (2018) Association of interleukin‐6 polymorphisms with obesity or metabolic traits in young Mexican‐Americans. Obesity Science & Practice, 4: 85–96. doi: 10.1002/osp4.138.
References
- 1. Elks CE, den HM ZJH , Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta‐regression. Front Endocrinol (Lausanne) 2012; 3: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stelzer I, Zelzer S, Raggam RB, et al. Link between leptin and interleukin‐6 levels in the initial phase of obesity related inflammation. Transl Res 2012; 159: 118–124. [DOI] [PubMed] [Google Scholar]
- 3. El‐Wakkad A, Hassan N, Sibaii H, El‐Zayat SR. Proinflammatory, anti‐inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61: 682–687. [DOI] [PubMed] [Google Scholar]
- 4. Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006; 64: 355–365. [DOI] [PubMed] [Google Scholar]
- 5. Hunter CA, Jones SA. IL‐6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16: 448–457. [DOI] [PubMed] [Google Scholar]
- 6. Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta 2011; 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 7. Illig T, Bongardt F, Schopfer A, et al. Significant association of the interleukin‐6 gene polymorphisms C‐174G and A‐598G with type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 5053–5058. [DOI] [PubMed] [Google Scholar]
- 8. Phillips CM, Goumidi L, Bertrais S, et al. Additive effect of polymorphisms in the IL‐6, LTA, and TNF‐α genes and plasma fatty acid level modulate risk for the metabolic syndrome and its components. J Clin Endocrinol Metab 2010; 95: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka C, Mannami T, Kamide K, et al. Single nucleotide polymorphisms in the interleukin‐6 gene associated with blood pressure and atherosclerosis in a Japanese general population. Hypertens.Res. 2005; 28: 35–41. [DOI] [PubMed] [Google Scholar]
- 10. Hamid YH, Rose CS, Urhammer SA, et al. Variations of the interleukin‐6 promoter are associated with features of the metabolic syndrome in Caucasian Danes. Diabetologia 2005; 48: 251–260. [DOI] [PubMed] [Google Scholar]
- 11. Ramirez‐Lopez G, Portilla‐de BE, Sanchez‐Corona J, Salmeron‐Castro J, Mendoza‐Carrera F. Interleukin‐6 polymorphisms are associated with obesity and hyperglycemia in Mexican adolescents. Arch.Med.Res. 2013; 44: 62–68. [DOI] [PubMed] [Google Scholar]
- 12. Yin YW, Sun QQ, Zhang BB, et al. Association between the interleukin‐6 gene ‐572 C/G polymorphism and the risk of type 2 diabetes mellitus: a meta‐analysis of 11,681 subjects. Ann.Hum.Genet. 2013; 77: 106–114. [DOI] [PubMed] [Google Scholar]
- 13. Yang X, Feng L, Li C, Li Y. Association of IL‐6‐174G > C and ‐572C > G polymorphisms with risk of young ischemic stroke patients. Gene 2014; 539: 258–262. [DOI] [PubMed] [Google Scholar]
- 14. Ma H, Sun G, Wang W, et al. Association between interleukin‐6 ‐572 C>G and ‐174 G>C polymorphisms and hypertension: a meta‐analysis of case‐control studies. Medicine (Baltimore) 2016; 95 e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zamora‐Ginez I, Sánchez‐Guillén MC, Pérez‐Fuentes R, et al. Association of interleukin‐6 haplotypes, obesity, and metabolic abnormalities in a population of Central Mexico. Lab Med 2010; 41: 597. [Google Scholar]
- 16. Saxena M, Agrawal CG, Srivastava N, Banerjee M. Interleukin‐6 (IL‐6)‐597 A/G (rs1800797) & ‐174 G/C (rs1800795) gene polymorphisms in type 2 diabetes. Indian J Med Res. 2014; 140: 60–68. [PMC free article] [PubMed] [Google Scholar]
- 17. Lacar ES, Soto X, Riley WJ. Adolescent obesity in a low‐income Mexican American district in South Texas. Arch Pediatr Adolesc Med. 2000; 154: 837–840. [DOI] [PubMed] [Google Scholar]
- 18. Rentfro AR, Nino JC, Pones RM, et al. Adiposity, biological markers of disease, and insulin resistance in Mexican American adolescents, 2004–2005. Prev.Chronic.Dis. 2011; 8: A40. [PMC free article] [PubMed] [Google Scholar]
- 19. Watt GP, Vatcheva KP, Griffith DM, et al. The precarious health of young Mexican American men in South Texas, Cameron County Hispanic Cohort, 2004–2015. Prev Chronic Dis 2016; 13 E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan JJ, Qu HQ, Rentfro A, McCormick JB, Fisher‐Hoch SP, Fallon MB. Prevalence of metabolic syndrome and risks of abnormal serum alanine aminotransferase in Hispanics: a population‐based study. PLoS One 2011; 6 e21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duran‐Gonzalez J, Ortiz I, Gonzales E, et al. Association study of candidate gene polymorphisms and obesity in a young Mexican‐American population from South Texas. Arch Med Res. 2011; 42: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes 2007. Oct; 8: 299–306. [DOI] [PubMed] [Google Scholar]
- 23. National Institutes of Health . National Heart LaBI. High blood cholesterol: what you need to know. 2017.
- 24. Slattery ML, Curtin K, Sweeney C, et al. Modifying effects of IL‐6 polymorphisms on body size‐associated breast cancer risk. Obesity (Silver.Spring) 2008; 16: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huth C, Heid IM, Vollmert C, et al. IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants' data from 21 studies. Diabetes 2006; 55: 2915–2921. [DOI] [PubMed] [Google Scholar]
- 26. Oana MC, Claudia B, Carmen D, Maria PA, Septimiu V, Claudiu M. The role of IL‐6 572 C/G, 190 C/T, and 174 G/C gene polymorphisms in children's obesity. Eur J Pediatr 2014; 173: 1285–1296. [DOI] [PubMed] [Google Scholar]
- 27. Marginean C, Marginean CO, Banescu C, Melit L, Tripon F, Iancu M. Impact of demographic, genetic, and bioimpedance factors on gestational weight gain and birth weight in a Romanian population: a cross‐sectional study in mothers and their newborns: the Monebo study (STROBE‐compliant article). Medicine (Baltimore) 2016; 95 e4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Berg SW, Dolle ME, Imholz S, et al. Genetic variations in regulatory pathways of fatty acid and glucose metabolism are associated with obesity phenotypes: a population‐based cohort study. Int J Obes (Lond) 2009; 33: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 29. Zamora‐Ginez I, Garcia‐Zapien AG, Flores‐Martinez SE, et al. Low prevalence of interleukin‐6 haplotypes associated with a decreased risk of type 2 diabetes in Mexican subjects with a family history of type 2 diabetes. Arch Med Res. 2013; 44: 529–534. [DOI] [PubMed] [Google Scholar]
- 30. Mamtani M, Kulkarni H, Dyer TD, et al. Waist circumference independently associates with the risk of insulin resistance and type 2 diabetes in Mexican American families. PLoS One 2013; 8 e59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fowler SP, Puppala S, Arya R, et al. Genetic epidemiology of cardiometabolic risk factors and their clustering patterns in Mexican American children and adolescents: the SAFARI Study. Hum Genet. 2013; 132: 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang WJ, Wu LJ, Min ZC, et al. Interleukin‐6‐572G/C polymorphism and prostate cancer susceptibility. Genet Mol Res. 2016; 16: 15(3). [DOI] [PubMed] [Google Scholar]
- 33. Matthews VB, Allen TL, Risis S, et al. Interleukin‐6‐deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 2010; 53: 2431–2441. [DOI] [PubMed] [Google Scholar]
- 34. Wallenius V, Wallenius K, Ahren B, et al. Interleukin‐6‐deficient mice develop mature‐onset obesity. Nat.Med. 2002; 8: 75–79. [DOI] [PubMed] [Google Scholar]
- 35. Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin‐6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002; 293: 560–565. [DOI] [PubMed] [Google Scholar]
- 36. Stenlof K, Wernstedt I, Fjallman T, Wallenius V, Wallenius K, Jansson JO. Interleukin‐6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J Clin Endocrinol Metab 2003; 88: 4379–4383. [DOI] [PubMed] [Google Scholar]
- 37. Fischer CP. Interleukin‐6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006; 12: 6–33. [PubMed] [Google Scholar]
- 38. Steensberg A. The role of IL‐6 in exercise‐induced immune changes and metabolism. Exerc Immunol Rev. 2003; 9: 40–47. [PubMed] [Google Scholar]
- 39. O'Connor JC, Sherry CL, Guest CB, Freund GG. Type 2 diabetes impairs insulin receptor substrate‐2‐mediated phosphatidylinositol 3‐kinase activity in primary macrophages to induce a state of cytokine resistance to IL‐4 in association with overexpression of suppressor of cytokine signaling‐3. J Immunol. 2007; 178: 6886–6893. [DOI] [PubMed] [Google Scholar]
- 40. Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL‐6 promotes alternative activation of macrophages to limit endotoxemia and obesity‐associated resistance to insulin. Nat.Immunol. 2014; 15: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


