Abstract
Importance
Body mass index (BMI) and gestational weight gain are increasing globally. In 2009, the Institute of Medicine (IOM) provided specific recommendations regarding the ideal gestational weight gain. However, the association between gestational weight gain consistent with theIOM guidelines and pregnancy outcomes is unclear.
Objective
To perform a systematic review, meta-analysis, and metaregression to evaluate associations between gestational weight gain above or below the IOM guidelines (gain of 12.5-18 kg for underweight women [BMI <18.5]; 11.5-16 kg for normal-weight women [BMI 18.5-24.9]; 7-11 kg for overweight women [BMI 25-29.9]; and 5-9 kg for obese women [BMI ≥30]) and maternal and infant outcomes.
Data Sources and Study Selection
Search of EMBASE, Evidence-Based Medicine Reviews, MEDLINE, and MEDLINE In-Process between January 1, 1999, and February 7, 2017, for observational studies stratified by prepregnancy BMI category and total gestational weight gain.
Data Extraction and Synthesis
Data were extracted by 2 independent reviewers. Odds ratios (ORs) and absolute risk differences (ARDs) per live birth were calculated using a random-effects model based on a subset of studies with available data.
Main Outcomes and Measures
Primary outcomes were small for gestational age (SGA), preterm birth, and large for gestational age (LGA). Secondary outcomes were macrosomia, cesarean delivery, and gestational diabetes mellitus.
Results
Of 5354 identified studies, 23 (n = 1 309 136 women) met inclusion criteria. Gestational weight gain was below or above guidelines in 23% and 47% of pregnancies, respectively. Gestational weight gain below the recommendations was associated with higher risk of SGA (OR, 1.53 [95% CI, 1.44-1.64]; ARD, 5% [95% CI, 4%-6%]) and preterm birth (OR, 1.70 [1.32-2.20]; ARD, 5% [3%-8%]) and lower risk of LGA (OR, 0.59 [0.55-0.64]; ARD, −2% [−10% to −6%]) and macrosomia (OR, 0.60 [0.52-0.68]; ARD, −2% [−3% to −1%]); cesarean delivery showed no significant difference (OR, 0.98 [0.96-1.02]; ARD, 0% [−2% to 1%]). Gestational weight gain above the recommendations was associated with lower risk of SGA (OR, 0.66 [0.63-0.69]; ARD, −3%; [−4% to −2%]) and preterm birth (OR, 0.77 [0.69-0.86]; ARD, −2% [−2% to −1%]) and higher risk of LGA (OR, 1.85 [1.76-1.95]; ARD, 4% [2%-5%]), macrosomia (OR, 1.95 [1.79-2.11]; ARD, 6% [4%-9%]), and cesarean delivery (OR, 1.30 [1.25-1.35]; ARD, 4% [3%-6%]). Gestational diabetes mellitus could not be evaluated because of the nature of available data.
Conclusions and Relevance
In this systematic review and meta-analysis of more than 1 million pregnant women, 47% had gestational weight gain greater than IOM recommendations and 23% had gestational weight gain less than IOM recommendations. Gestational weight gain greater than or less than guideline recommendations, compared with weight gain within recommended levels, was associated with higher risk of adverse maternal and infant outcomes.
Key Points
Question
What is the association between gestational weight gain above or below the Institute of Medicine guidelines and maternal and infant outcomes?
Findings
In this systematic review and meta-analysis of 1 309 136 pregnancies, gestational weight gain below recommendations (in 23% of women) was associated with higher risk of small for gestational age (odds ratio [OR], 1.53) and preterm birth (OR, 1.70) and lower risk of large for gestational age (OR, 0.59) and macrosomia (OR, 0.60). Gestational weight gain above recommendations (47%) was associated with lower risk of small for gestational age (OR, 0.66) and preterm birth (OR, 0.77) and higher risk of large for gestational age (OR, 1.85), macrosomia (OR, 1.95), and cesarean delivery (OR, 1.30).
Meaning
Gestational weight gain below or above the Institute of Medicine guidelines was associated with higher risk of some adverse maternal and infant outcomes.
This meta-analysis evaluates associations between gestational weight gain above or below the Institute of Medicine guidelines and maternal and infant outcomes including size for gestational age, preterm birth, cesarean delivery, and gestational diabetes mellitus.
Introduction
Excessive and insufficient gestational weight gain have been associated with adverse pregnancy outcomes, including small for gestational age (SGA), large for gestational age (LGA), macrosomia, cesarean delivery, gestational diabetes mellitus (GDM), preeclampsia, postpartum weight retention, and offspring obesity. The Institute of Medicine (IOM; now known as the National Academy of Medicine) recommendations regarding gestational weight gain were developed in 1990 to guide clinical practice. These aimed to reduce the incidence of low-birth-weight babies and were based on a 1980 National Natality Survey of a largely white population. The updated IOM guidelines in 2009 incorporated World Health Organization (WHO) categories of maternal body mass index (BMI; calculated as weight in kilograms divided by height in meters squared; BMI for underweight, <18.5; normal weight, 18.5-24.9; overweight, 25-29.9; and obese, ≥30) and recommended less gestational weight gain for obese women (Table 1). The 2009 guidelines identified maternal and infant relationships with gestational weight gain but were based on lower general population BMI with limited ethnic diversity. The 2009 IOM guidelines are endorsed by the American College of Obstetricians and Gynecologists, although they are not universally implemented.
Table 1. Recommendations for Gestational Weight Gain During Pregnancya.
| Recommendation | Prepregnancy Weight | |||
|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Obese | |
| BMI | <18.5 | 18.5-24.9 | 25-29.9 | ≥30 |
| Total weight gain range, kg | 12.5-18 | 11.5-16 | 7-11.5 | 5-9 |
| Total weight gain range, lbs | 28-40 | 25-35 | 15-25 | 11-20 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Adapted from 2009 Institute of Medicine guidelines.
The prevalence of obesity and excess gestational weight gain are increasing. The US female obesity prevalence was 40% in 2013-2014. More than 50% of obese pregnant women gained gestational weight greater than the IOM gestational weight gain recommendations in a US study that collected data from 2002 through 2008.
The purpose of this review and meta-analysis was to compare gestational weight gain with IOM guidelines from diverse international cohorts and to evaluate associations between gestational weight gain above and below guidelines with maternal and infant outcomes.
Methods
This systematic review, meta-analysis, and metaregression was prospectively registered with PROSPERO International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42015023325).
Search Strategy
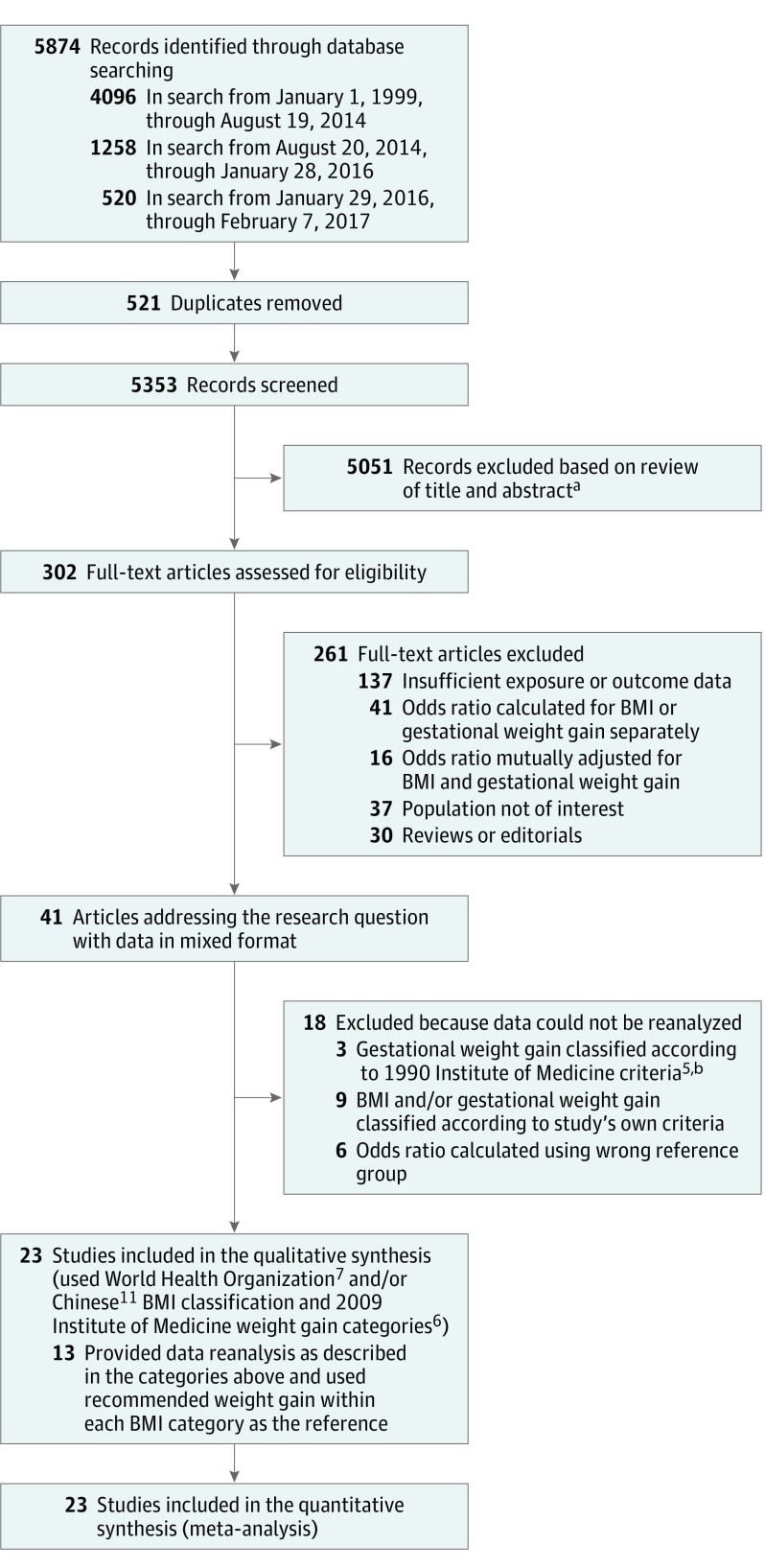
A systematic search string of relevant terms was developed (eAppendix 1 in the Supplement). Searched databases in Ovid included EMBASE, all Evidence-Based Medicine Reviews, MEDLINE, and MEDLINE In-Process from January 1, 1999, to January 28, 2016 (Figure 1). The search was limited to articles from 1999 onward to represent more current populations. The search was later updated to February 7, 2017. Of 7 newly identified studies, 4 were included in the analyses. Three studies were excluded because the data were not in the required format, and there was insufficient time to obtain data from the authors. Bibliographies of included studies were reviewed to identify additional studies. Details of the search strategy and data extraction are shown in eAppendix 2 in the Supplement.
Figure 1. Flow Diagram of Study Selection Process.
aExact breakdown for exclusion not documented.
bThe Institute of Medicine 1990 guidelines differ from the 2009 guidelines. In the 1990 guidelines, the recommended weight gain range was 12.5 to 18 kg for women with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) less than 19.8; 11.5 to 16 kg for women with a BMI of 19.8 to 26.0; 7 to 11.5 kg for women with a BMI between 26.0 and 29.0; and at least 6.8 kg for women with a BMI higher than 29.0.
Study Eligibility Criteria
Observational studies published in English and assessing singleton pregnancies in women aged 18 years or older were included. Study sample sizes larger than 500 women were required to identify outcomes present across the BMI categories. We postulated that small studies would have insufficient sample size to detect outcomes within each BMI group. Studies were included if they presented data examining women by prepregnancy BMI category, stratified by the total gestational weight gain. Studies that categorized by mean weight gain per week were excluded. Only studies presenting odds ratios (ORs) stratified by maternal BMI and gestational weight gain were included. Studies that simultaneously adjusted for categories of BMI and gestational weight gain to estimate the independent associations of weight change with outcomes were excluded because the aim of this review was to assess the association of gestational weight gain (specific for each BMI category) and outcomes.
Studies meeting these criteria used different BMI categories (eg, Metropolitan Life Insurance Tables, WHO classifications, or Chinese classifications [BMI for underweight, <18.5; normal weight, 18.5-23.9; overweight, 24-28; and obese, ≥28]) and gestational weight gain categories (eg, 1990 IOM, 2009 IOM, population-specific, or study-specific categories) to classify participants. Additionally, some studies used a reference of normal gestational weight gain within each BMI group, whereas others used a reference of normal-weight women with normal weight gain.
In this review, BMI was defined by WHO categories and/or Chinese BMI categories. Gestational weight gain was defined by 2009 IOM criteria; thus, authors of identified studies were contacted to reanalyze data using these categories. The ORs were calculated using recommended gestational weight gain within each BMI category as the reference.
Gestational weight gain was defined as the difference between the final weight and the prepregnancy weight and was classified as below, within, or above the 2009 IOM guidelines. The prepregnancy weight was either self-reported (which correlates well with measured weight) or measured at first antenatal visits. Final pregnancy weight was measured at the last antenatal visit or the time of delivery or was self-reported within 1 year of delivery.
Primary outcomes were the following: (1) SGA, indicated by birth weight less than the 10th percentile for gestational age; (2) preterm birth, indicated by spontaneous birth before 37 weeks’ gestation; and (3) LGA, indicated by birth weight greater than the 90th percentile for gestational age. Secondary outcomes were the following: (1) macrosomia, indicated by birth weight greater than 4000 g; (2) cesarean delivery; and (3) GDM. Outcomes were selected based on the original IOM studies, and end points were determined on a 2-round Delphi survey of experienced clinicians that was used to rank clinically important outcomes in a meta-analysis of lifestyle interventions to reduce weight gain in pregnancy.
Risk of Bias Appraisal
Two authors assessed risk of bias (R.F.G. and S.K.A.). Discrepancies were resolved by consensus in discussion with a third reviewer (M.M.). Methodological quality of included studies was assessed using the Monash Centre for Health Research and Implementation evidence synthesis appraisal assessment tool. Individual quality items were assessed using a descriptive approach including exposure and outcome measures, reporting bias, confounding, and conflict of interest. Each study was classified as low, medium, or high risk of bias.
Data Synthesis Strategy
Findings were synthesized by target population characteristics, study type, and outcome. Outcome measures were produced for each study by calculating ORs and 95% confidence intervals, using recommended gestational weight gain within each BMI category as the reference. When 2 or more studies assessed the same outcome, results were pooled using both fixed- and random-effects meta-analysis. There were no significant differences between fixed- and random-effects analyses. Random effects are presented given heterogeneity among studies. Extracted pooled ORs for individual outcomes were combined to construct summary pooled ORs. Crude data were used where possible, given variable control for confounding factors. However, some articles presented adjusted ORs only. Absolute risk differences (ARDs) per live birth were calculated from event rates (available for a subset of studies) using random-effects meta-analysis.
Heterogeneity was assessed using the I2 statistic, where I2 > 50% indicated substantial heterogeneity. Metaregression was performed to investigate sources of heterogeneity (percentage of smokers during pregnancy, mean age, and percentage of nulliparous women). Race/ethnicity data were not available for the metaregression. Where 5 or more studies were available, publication bias was assessed using Egger test plots. Statistical significance was defined as 2-sided P < .05. Statistical analysis used Stata software version 14 (StataCorp LP).
A subgroup analysis was performed in specific population groups identified a priori (studies using Chinese or Korean BMI categories, not presented herein). Obesity subclasses were included after reviewing studies that stratified by obesity class. Tests for trend based on the Cochran-Armitage test in Stata were used to assess trends in this subgroup analysis.
Results
Study Selection
Of 5874 studies identified by the initial search, 302 were selected for full-text review; 261 of these were excluded, leaving 41 (Figure 1). These studies grouped women by prepregnancy BMI category, stratified by total gestational weight gain. One study did not meet inclusion criteria as published; however, prior collaboration had made data available in the required format. Of 41 identified studies, 18 were excluded because data could not be obtained in the required format. Of these 18 studies, authors of 15 were contacted and unable to reanalyze and authors of 3 were not contacted from the updated search because of insufficient time prior to publication (eAppendix 2 in the Supplement). Overall, 23 cohort studies were included, involving 1 309 136 women. Of these 23 studies, 7 were included without contacting the authors because data were in the required format. Of 16 authors contacted, 13 reanalyzed data and were included; 3 provided additional information, thereby avoiding reanalysis.
Study Characteristics
Table 2 and Table 3 list characteristics of the studies (descriptive characteristics are shown in eTable 1 in the Supplement). Eighteen studies were retrospective, and 5 were prospective. Ten were from the United States, 8 were from Asia (4 from China, 2 from Korea, and 1 each from Taiwan and Japan), and 5 were from Europe (1 each from Norway, Belgium, Italy, Denmark, and Sweden). Sample sizes ranged from 1034 to 570 672 women.
Table 2. Characteristics of 19 Studies That Stratified by Prepregnancy BMI Categoriesa.
| Source | Study Period, Design | Total No. of Women | Setting | Outcomes | Prepregnancy BMI Category | Women, No. | No. of Events/No. of Live Births (%) by Gestational Weight Gain Categoryb | ||
|---|---|---|---|---|---|---|---|---|---|
| Below Guidelines | Within Guidelines | Above Guidelines | |||||||
| Durst et al, 2016 (United States) | 2000-2014, Retrospective | 5651 | University of Alabama at Birmingham | SGA, LGA, cesarean delivery, macrosomia | Overweight | 5651 | NR | NR | NR |
| Enomoto et al, 2016 (Japan) | 2013, Retrospective | 97 157 | Japan Society of Obstetrics and Gynecology Registry system with 280 participating hospitals | SGA | Underweight | 17 724 | 2032/13 529 (15.0) | 286/3783 (7.6) | 23/412 (5.6) |
| Normal weight | 69 126 | 4575/44 189 (10.4) | 1254/20 835 (6.0) | 163/4102 (4.0) | |||||
| Overweight | 7502 | 275/2990 (9.2) | 179/2810 (6.4) | 89/1702 (5.2) | |||||
| Obese | 2805 | 112/1297 (8.6) | 48/853 (5.6) | 38/655 (5.8) | |||||
| LGA | Underweight | 17 724 | 518/13 529 (3.8) | 388/3783 (10.3) | 61/412 (14.8) | ||||
| Normal weight | 69 126 | 3322/44 189 (7.5) | 2754/20 835 (13.2) | 868/4102 (21.1) | |||||
| Overweight | 7502 | 363/2990 (12.1) | 489/2810 (17.4) | 450/1702 (26.4) | |||||
| Obese | 2805 | 213/1297 (16.4) | 206/853 (24.2) | 215/655 (32.8) | |||||
| Preterm birth | Underweight | 17 724 | 1979/13 529 (14.6) | 167/3783 (4.4) | 8/412 (1.9) | ||||
| Normal weight | 69 126 | 5891/44 189 (13.3) | 994/20 835 (4.8) | 178/4102 (4.3) | |||||
| Overweight | 7502 | 508/2990 (17.0) | 240/2810 (8.5) | 125/1702 (7.3) | |||||
| Obese | 2805 | 199/1297 (15.3) | 95/853 (11.1) | 44/655 (6.7) | |||||
| Cesarean delivery | Underweight | 17 724 | 3174/13 539 (23.4) | 739/3783 (19.5) | 82/412 (19.9) | ||||
| Normal weight | 69 126 | 12 446/44 189 (28.2) | 5062/20 835 (24.3) | 1119/4102 (27.3) | |||||
| Overweight | 7502 | 1151/2990 (38.5) | 991/2810 (35.3) | 617/1702 (36.3) | |||||
| Obese | 2805 | 542/1297 (41.8) | 367/853 (43.0) | 296/655 (45.2) | |||||
| Macrosomia | Underweight | 17 724 | 16/13 529 (0.1) | 20/3783 (0.5) | 11/412 (2.7) | ||||
| Normal weight | 69 126 | 149/44 189 (0.3) | 214/20 835 (1.0) | 111/4102 (2.7) | |||||
| Overweight | 7502 | 34/2990 (1.1) | 35/2810 (1.3) | 63/1702 (3.7) | |||||
| Obese | 2805 | 21/1297 (1.6) | 37/853 (4.3) | 37/655 (5.7) | |||||
| Hung and Hsieh, 2016 (Taiwan) | 2009-2015, Retrospective | 10 973 | Taipei Chang Gung Memorial Hospital | SGA | Underweight | 1556 | 117/691 (16.9) | 63/718 (8.8) | 10/147 (6.8) |
| Normal weight | 8247 | 199/2304 (8.7) | 233/3827 (6.1) | 90/2116 (4.3) | |||||
| Overweight + obese | 1170 | 121/161 (75.2) | 23/403 (5.7) | 24/606 (4.0) | |||||
| LGA | Underweight | 1556 | 8/691 (1.2) | 34/718 (4.7) | 18/147 (12.2) | ||||
| Normal weight | 8247 | 103/2304 (4.5) | 306/3827 (8.0) | 274/2116 (12.9) | |||||
| Overweight + obese | 1170 | 18/161 (11.2) | 61/403 (15.1) | 107/606 (17.7) | |||||
| Cesarean delivery | Underweight | 1556 | 143/691 (20.7) | 151/718 (21.0) | 54/147 (36.7) | ||||
| Normal weight | 8247 | 412/2304 (17.9) | 882/3827 (23.1) | 644/2116 (30.4) | |||||
| Overweight + obese | 1170 | 34/161 (21.1) | 89/403 (22.1) | 197/606 (32.5) | |||||
| Macrosomia | Underweight | 1556 | 3/691 (0.4) | 4/718 (0.6) | 5/147 (3.4) | ||||
| Normal weight | 8247 | 17/2304 (0.7) | 63/3827 (1.7) | 74/2116 (3.5) | |||||
| Overweight + obese | 1170 | 6/161 (3.7) | 9/403 (2.2) | 30/606 (5.0) | |||||
| Xiong et al, 2016 (China)c | 2012-2013, Prospective | 57 891 | Hospitals and community centers | Cesarean delivery | Underweight | 10 121 | NR | NR | NR |
| Normal weight | 44 522 | ||||||||
| Overweight | 2877 | ||||||||
| Obese | 371 | ||||||||
| Shin and Song, 2015 (United States) | 2004-2011, Retrospective | 219 868 | Pregnancy Risk Assessment Monitoring System | SGA, preterm birth, LGA | Underweight | 11 865 | NR | NR | NR |
| Normal weight | 113 523 | ||||||||
| Overweight | 51 517 | ||||||||
| Obese | 42 963 | ||||||||
| Wen and Lv, 2015 (China)c | 2009-2013, Retrospective | 13 776 | Jishuitan Hospital | Preterm birth | Underweight | 0 | NR | NR | NR |
| Normal weight | 13 776 | ||||||||
| Overweight | 0 | ||||||||
| Obese | 0 | ||||||||
| Yang et al, 2015 (China)c | 2011-2013, Prospective | 85 765 | Wuhan Women and Children Health Care Center | Macrosomia | Underweight | 14 477 | 13/158 (8.2) | 93/4723 (2.0) | 449/7139 (6.3) |
| Normal weight | 65 536 | 361/11627 (3.1) | 623/14 103 (4.4) | 3387/33 647 (10.1) | |||||
| Overweight + obese | 5752 | 35/573 (6.1) | 79/982 (8.0) | 551/3299 (16.7) | |||||
| Badon et al, 2014 (United States) | 2000-2006, Prospective | 5297 | North American Field Centers, HAPO Study | LGA | Underweight | 179 | NR | NR | NR |
| Normal weight | 3013 | ||||||||
| Overweight | 1322 | ||||||||
| Obese | 783 | ||||||||
| Chihara et al, 2014 (United States) | 2003-2005, Retrospective | 19 130 | Hawaii’s Special Supplemental Nutrition Program for Women, Infants, and Children | Macrosomia | Underweight | 1153 | NR | NR | NR |
| Normal weight | 9291 | ||||||||
| Overweight | 4391 | ||||||||
| Obese | 4295 | ||||||||
| Haugen et al, 2014 (Norway)d | 1999-2008, Prospective | 56 082 | Norwegian Mother and Child Cohort Study | SGA | Underweight | 1610 | 143/457 (31.3) | 144/751 (19.2) | 37/402 (9.2) |
| Normal weight | 37 315 | 1327/7798 (17.0) | 1662/14 904 (11.2) | 1042/14 613 (7.1) | |||||
| Overweight | 12 181 | 156/1037 (15.0) | 281/2485 (11.3) | 557/8659 (6.4) | |||||
| Obese | 4976 | 97/878 (11.1) | 99/1054 (9.4) | 204/3044 (6.7) | |||||
| LGA | Underweight | 1610 | 4/457 (0.9) | 21/751 (2.8) | 30/402 (7.5) | ||||
| Normal weight | 37 315 | 250/7798 (3.2) | 914/14 904 (6.1) | 1796/14 613 (12.3) | |||||
| Overweight | 12 181 | 62/1037 (6.0) | 212/2485 (8.5) | 1370/8659 (15.8) | |||||
| Obese | 4976 | 90/878 (10.3) | 154/1054 (14.6) | 676/3044 (22.2) | |||||
| Cesarean delivery | Underweight | 1610 | 44/457 (9.6) | 71/751 (9.5) | 47/402 (11.7) | ||||
| Normal weight | 37 315 | 726/7798 (9.3) | 1526/14 904 (10.2) | 1836/14 613 (12.6) | |||||
| Overweight | 12 181 | 137/1037 (13.2) | 327/2485 (13.2) | 1439/8659 (16.6) | |||||
| Obese | 4976 | 173/878 (19.7) | 227/1054 (21.5) | 703/3044 (23.1) | |||||
| Macrosomia | Underweight | 1610 | 15/457 (3.3) | 68/751 (9.1) | 62/402 (15.4) | ||||
| Normal weight | 37 315 | 782/7798 (10.0) | 2573/14 904 (17.3) | 4014/14 613 (27.5) | |||||
| Overweight | 12 181 | 160/1037 (15.4) | 530/2485 (21.3) | 2472/8659 (28.5) | |||||
| Obese | 4976 | 206/878 (23.5) | 300/1054 (28.5) | 1822/3044 (59.9) | |||||
| Lee et al, 2014 (Korea)e | 2010-2012, Retrospective | 16 297 | Single medical center | LGA | Underweight | 2655 | NR | NR | NR |
| Normal weight | 12 250 | ||||||||
| Overweight | 1191 | ||||||||
| Obese | 201 | ||||||||
| Black et al, 2013 (United States) | 2005-2010, Retrospective | 9835 | Kaiser Permanente Southern California | SGA | Underweight | 179 | 11/55 (20.0) | 9/75 (12.0) | 3/51 (5.9) |
| Normal weight | 3805 | 158/1031 (15.3) | 125/1388 (9.0) | 114/1386 (8.2) | |||||
| Overweight | 3116 | 58/424 (13.7) | 70/815 (8.6) | 119/1877 (6.3) | |||||
| Obese | 2735 | 57/608 (9.4) | 54/648 (8.3) | 80/1479 (5.4) | |||||
| LGA | Underweight | 179 | 0/55 | 4/73 (5.5) | 2/51 (3.9) | ||||
| Normal weight | 3805 | 2/1031 (0.2) | 46/1388 (3.3) | 113/1386 (8.2) | |||||
| Overweight | 3116 | 15/424 (3.5) | 39/815 (4.8) | 205/1877 (10.9) | |||||
| Obese | 2735 | 32/608 (5.3) | 52/648 (8.0) | 235/1479 (15.9) | |||||
| Preterm birth | Underweight | 179 | 10/55 (18.2) | 2/73 (2.7) | 1/51 (2.0) | ||||
| Normal weight | 3805 | 127/1031 (12.3) | 77/1388 (5.5) | 45/1386 (3.2) | |||||
| Overweight | 3116 | 54/424 (12.7) | 76/815 (9.3) | 87/1877 (4.6) | |||||
| Obese | 2735 | 53/608 (8.7) | 49/648 (7.6) | 80/1479 (5.4) | |||||
| Cesarean delivery | Underweight | 179 | 6/55 (10.9) | 14/73 (19.2) | 13/51 (25.5) | ||||
| Normal weight | 3805 | 219/1031 (21.2) | 293/1388 (21.1) | 339/1386 (24.5) | |||||
| Overweight | 3116 | 105/424 (24.8) | 200/815 (24.5) | 589/1877 (31.4) | |||||
| Obese | 2735 | 184/608 (30.3) | 216/648 (33.3) | 575/1479 (38.9) | |||||
| Macrosomia | Underweight | 179 | 0/55 | 5/73 (6.8) | 3/51 (5.9) | ||||
| Normal weight | 3805 | 28/1031 (2.7) | 63/1388 (4.5) | 148/1386 (10.7) | |||||
| Overweight | 3116 | 17/424 (4.0) | 46/815 (5.6) | 225/1877 (12.0) | |||||
| Obese | 2735 | 38/608 (6.3) | 65/648 (10.0) | 265/1479 (17.9) | |||||
| Li et al, 2013 (China)f | 2009-2011, Retrospective | 33 973 | Tianjin Women’s and Children’s Health Center | SGA | Underweight | 3732 | 151/733 (20.6) | 323/1820 (17.7) | 114/1179 (9.7) |
| Normal weight | 24 262 | 383/2777 (13.8) | 817/9347 (8.7) | 935/12 138 (7.7) | |||||
| Overweight | 4998 | 6/86 (7.0) | 54/665 (8.1) | 277/4247 (6.5) | |||||
| Obese | 981 | 1/16 (6.3) | 6/54 (11.1) | 44/911 (4.8) | |||||
| LGA | Underweight | 3732 | 15/733 (2.0) | 49/1820 (2.7) | 77/1179 (6.5) | ||||
| Normal weight | 24 262 | 15/2777 (0.5) | 625/9347 (6.7) | 1549/12 138 (12.8) | |||||
| Overweight | 4998 | 32/86 (37.2) | 86/665 (12.9) | 741/4247 (17.4) | |||||
| Obese | 981 | 4/16 (25.0) | 10/54 (18.5) | 228/911 (25.0) | |||||
| Preterm birth | Underweight | 3732 | 31/733 (4.2) | 45/1820 (2.5) | 22/1179 (1.9) | ||||
| Normal weight | 24 262 | 142/2777 (5.1) | 322/9347 (3.4) | 248/12 138 (2.0) | |||||
| Overweight | 4998 | 5/86 (5.8) | 37/665 (5.6) | 134/4247 (3.2) | |||||
| Obese | 981 | 1/16 (6.3) | 2/54 (3.7) | 61/911 (6.7) | |||||
| Cesarean delivery | Underweight | 3732 | 375/733 (51.2) | 945/1820 (51.9) | 723/1179 (61.3) | ||||
| Normal weight | 24 262 | 1677/2777 (60.4) | 5645/9347 (60.4) | 8208/12 138 (67.6) | |||||
| Overweight | 4998 | 62/86 (72.1) | 480/665 (72.2) | 3348/4247 (78.8) | |||||
| Obese | 981 | 11/16 (68.8) | 50/54 (92.6) | 774/911 (85.0) | |||||
| Macrosomia | Underweight | 3732 | 141/733 (19.2) | 53/1820 (2.9) | 751/1179 (63.7) | ||||
| Normal weight | 24 262 | 145/2777 (5.2) | 581/9347 (6.2) | 1464/12 138 (12.1) | |||||
| Overweight | 4998 | 6/86 (7.0) | 71/665 (10.7) | 687/4247 (16.2) | |||||
| Obese | 981 | 4/16 (25.0) | 9/54 (16.7) | 203/911 (22.3) | |||||
| Di Benedetto et al, 2012 (Italy) | 2004-2009, Retrospective | 2225 | University hospital | Macrosomia, cesarean delivery | Underweight | 89 | NR | NR | NR |
| Normal weight | 1468 | ||||||||
| Overweight | 493 | ||||||||
| Obese | 175 | ||||||||
| Simas et al, 2012 (United States) | 2006-2010, Retrospective | 11 203 | University hospital | SGA, LGA | Underweight | 427 | NR | NR | NR |
| Normal weight | 5707 | ||||||||
| Overweight | 2756 | ||||||||
| Obese | 2313 | ||||||||
| Park et al, 2011 (Korea)e | 2005-2007, Retrospective | 2311 | University hospital | SGA, LGA, macrosomia, cesarean delivery | Underweight | 385 | NR | NR | NR |
| Normal weight | 1666 | ||||||||
| Overweight | 221 | ||||||||
| Obese | 39 | ||||||||
| Park et al, 2011 (United States) | 2004-2007, Retrospective | 560 672 | Florida birth certificate data | SGA | Underweight | 28 119 | 1987/7555 (26.3) | 1815/11 676 (15.5) | 865/8888 (9.7) |
| Normal weight | 305 295 | 11 213/71 025 (15.8) | 10 324/103 613 (10.0) | 8460/130 657 (6.5) | |||||
| Overweight | 135 668 | 2167/16 723 (13.0) | 3033/30 731 (9.9) | 5516/88 214 (6.3) | |||||
| Obese | 101 590 | 2157/19 740 (10.9) | 1501/17 350 (8.7) | 4147/64 500 (6.4) | |||||
| LGA | Underweight | 28 119 | 84/7555 (1.1) | 366/11 676 (3.1) | 669/8888 (7.5) | ||||
| Normal weight | 305 295 | 2479/71 025 (3.5) | 6183/103 613 (6.0) | 15 146/130 657 (11.6) | |||||
| Overweight | 135 668 | 851/16 723 (5.1) | 2329/30 731 (7.6) | 12 417/88 214 (14.1) | |||||
| Obese | 101 590 | 16 571/19 740 (83.9) | 1906/17 350 (11.0) | 10 794/64 500 (16.7) | |||||
| Vesco et al, 2011 (United States) | 2000-2005, Retrospective | 2080 | Kaiser Permanente group practice | SGA, LGA, macrosomia | Underweight | 0 | NR | NR | NR |
| Normal weight | 0 | ||||||||
| Overweight | 0 | ||||||||
| Obese | 2080 | ||||||||
| Rode et al, 2007 (Denmark) | 1996-1998, Prospective | 2248 | University hospital | Macrosomia | Underweight | 128 | NR | NR | NR |
| Normal weight | 1654 | ||||||||
| Overweight | 349 | ||||||||
| Obese | 117 | ||||||||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HAPO, Hyperglycemia and Adverse Pregnancy Outcome; LGA, large for gestational age; NR, not reported; SGA, small for gestational age.
Unless indicated otherwise, prepregnancy categories of BMI categories were according to World Health Organization (WHO) categories as follows: BMI for underweight, less than 18.5; normal weight, 18.5 to 24.9; overweight, 25 to 29.9; and obese, 30 or higher.
Refer to eFigure 1 in the Supplement for odds ratios.
Data according to Chinese BMI categories only (BMI for underweight, <18.5; normal weight, 18.5-23.9; overweight, 24-28; and obese, ≥28).
Sample size changed when additional data were provided.
Data according to both Korean BMI categories (BMI for underweight, <18.5; normal weight, 18.5-22.9; overweight, 23-25; and obese ≥25) and WHO BMI categories (WHO reported herein).
Data according to both Chinese and WHO BMI categories (WHO reported herein).
Table 3. Characteristics of 4 Studies That Stratified by Prepregnancy Obesity Class.
| Source | Study Period, Design | Total No. of Women | Setting | Outcomes | Prepregnancy Obesity Classa | Women, No. | No. of Events/No. of Live Births (%) by Gestational Weight Gain Category | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Loss | Gain Below Guidelines | Gain Within Guidelines | Gain Above Guidelines | |||||||
| Bogaerts et al, 2015 (Belgium) | 2009-2011, Retrospective | 18 053 | Flemish Centre for the Study of Perinatal Epidemiology | SGA | 1 | 12 994 | 52/420 (12.4) | 164/1554 (10.6) | 316/3585 (8.8) | 421/7435 (5.7) |
| 2 | 3787 | 32/263 (12.2) | 54/648 (8.3) | 80/1156 (6.9) | 95/1720 (5.5) | |||||
| 3 | 1272 | 16/171 (9.4) | 16/291 (5.5) | 22/379 (5.8) | 20/431 (4.6) | |||||
| LGA | 1 | 12 994 | 29/420 (6.9) | 152/1554 (9.8) | 474/3585 (13.2) | 1468/7438 (19.7) | ||||
| 2 | 3787 | 28/263 (10.6) | 78/648 (12.0) | 189/1156 (16.3) | 409/1720 (23.8) | |||||
| 3 | 1272 | 19/171 (11.1) | 59/291 (20.3) | 97/379 (25.6) | 122/431 (28.3) | |||||
| Cesarean delivery | 1 | 12 994 | 101/420 (24.0) | 353/1554 (22.7) | 844/3585 (23.5) | 2020/7435 (27.2) | ||||
| 2 | 3787 | 65/263 (24.7) | 153/648 (23.6) | 344/1156 (29.8) | 561/1720 (32.6) | |||||
| 3 | 1272 | 61/171 (35.7) | 98/291 (33.7) | 132/379 (34.8) | 168/431 (39.0) | |||||
| Macrosomia | 1 | 12 994 | 29/420 (6.9) | 155/1554 (10.0) | 435/3585 (12.1) | 1384/7435 (18.6) | ||||
| 2 | 3787 | 23/263 (8.7) | 76/648 (11.7) | 165/1156 (14.3) | 366/1720 (21.3) | |||||
| 3 | 1272 | 11/171 (6.4) | 49/291 (16.8) | 89/379 (23.5) | 100/431 (23.2) | |||||
| Swank et al, 2014 (United States) | 2007, Retrospective | 1034 | California birth certificate data | Cesarean delivery | 3 | 1034 | 96/170 (56.5) | 134/226 (59.3) | 139/243 (57.2) | 253/395 (64.1) |
| Macrosomia | 3 | 1034 | 14/170 (8.2) | 35/226 (15.5) | 52/243 (21.4) | 104/395 (26.3) | ||||
| Kominiarek et al, 2013 (United States)b | 2002-2008, Retrospective | 21 020 | 12 Institutions (19 hospitals) | SGA | 1 | 12 005 | 66/406 (16.3) | 135/1352 (10.0) | 187/1931 (9.7) | 549/8316 (6.6) |
| 2 | 5320 | 57/354 (16.1) | 100/918 (10.9) | 85/1018 (8.3) | 193/3030 (6.4) | |||||
| 3 | 3695 | 57/486 (11.7) | 72/748 (10.4) | 51/664 (7.7) | 119/1797 (6.6) | |||||
| LGA | 1 | 12 005 | 13/406 (3.2) | 76/1352 (5.6) | 119/1931 (6.2) | 1029/8316 (12.4) | ||||
| 2 | 5320 | 17/354 (4.8) | 67/918 (7.3) | 101/1018 (9.9) | 435/3030 (14.4) | |||||
| 3 | 3695 | 31/486 (6.4) | 77/748 (10.3) | 85/664 (12.8) | 315/1797 (17.5) | |||||
| Cesarean delivery | 1 | 12 005 | 64/406 (15.8) | 292/1352 (21.6) | 425/1931 (22.0) | 1816/8316 (21.8) | ||||
| 2 | 5320 | 86/354 (24.3) | 237/918 (25.8) | 277/1018 (27.2) | 754/3030 (24.9) | |||||
| 3 | 3695 | 144/486 (29.6) | 222/748 (29.7) | 233/664 (35.1) | 562/1797 (31.3) | |||||
| Macrosomia | 1 | 12 005 | 2/406 (0.5) | 7/1352 (0.5) | 15/1931 (0.8) | 191/8316 (2.3) | ||||
| 2 | 5320 | 1/354 (0.3) | 8/918 (0.9) | 13/1018 (1.3) | 87/3030 (2.9) | |||||
| 3 | 3695 | 7/486 (1.4) | 11/748 (1.5) | 19/664 (2.9) | 66/1797 (3.7) | |||||
| Blomberg, 2011 (Sweden) | 1993-2008, Retrospective | 46 595 | Swedish Medical Birth Register | SGA | 1 | 32 991 | 51/1341 (3.8) | 88/3105 (2.8) | 162/8807 (1.8) | 232/19 738 (1.2) |
| 2 | 10 068 | 13/798 (1.6) | 40/1466 (2.7) | 58/2927 (2.0) | 70/4877 (1.4) | |||||
| 3 | 3536 | 19/517 (3.7) | 16/616 (2.6) | 17/1002 (1.7) | 27/1401 (1.9) | |||||
| LGA | 1 | 32 991 | 87/1341 (6.5) | 228/3105 (7.3) | 757/8807 (8.6) | 2674/19 738 (13.5) | ||||
| 2 | 10 068 | 57/798 (7.1) | 142/1466 (9.7) | 361/2927 (12.3) | 853/4877 (17.5) | |||||
| 3 | 3536 | 57/517 (11.0) | 88/616 (14.3) | 155/1002 (15.5) | 278/1401 (19.8) | |||||
| Cesarean delivery | 1 | 32 991 | 206/1341 (15.4) | 554/3105 (17.8) | 1675/8807 (19.0) | 4431/19 738 (22.4) | ||||
| 2 | 10 068 | 135/798 (16.9) | 306/1466 (20.9) | 713/2927 (24.4) | 1312/4877 (26.9) | |||||
| 3 | 3536 | 125/517 (24.2) | 148/616 (24.0) | 289/1002 (28.8) | 439/1401 (31.3) | |||||
Abbreviations: LGA, large for gestational age; SGA, small for gestational age.
Class 1 indicates a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 30 to 34.9; class 2, BMI of 35 to 39.9; and class 3, BMI of 40 or higher.
Sample size changed when additional data were provided, and the odds ratios were not recalculated.
Underweight women composed 7% (n = 94 399); normal-weight women, 55% (n = 720 456); overweight women, 18% (n = 235 295); and obese women, 20% (n = 258 986). Gestational weight gain was below, within, or above guidelines in 23% (n = 300 723), 30% (n = 387 409), and 47% (n = 621 004), respectively.
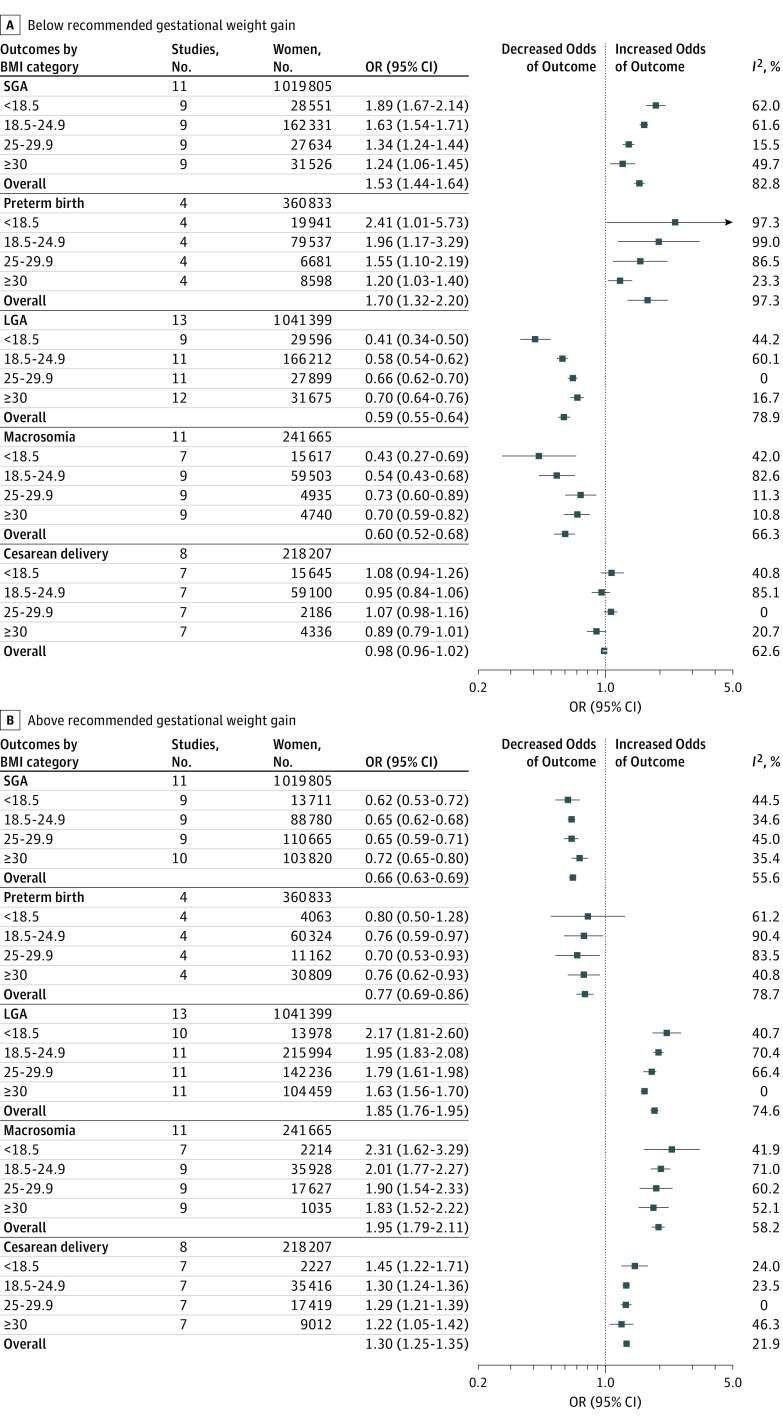
Figure 2 shows pooled ORs for primary and secondary outcomes. eFigure 1 in the Supplement shows pooled ORs for individual outcomes. eTable 2 in the Supplement reports event rates. eTable 3 and eFigure 2 in the Supplement report ARDs and P values. The ARDs are expressed as percentage difference per live birth.
Figure 2. Summary of Pooled Odds Ratios (ORs) for the Association Between Gestational Weight Gain Below and Above Guidelines With Adverse Outcomes.
Pooled ORs are shown for the association between gestational weight gain below (A) and above (B) guidelines with adverse outcomes. Reference group is women with recommended weight gain in each category of body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). For each outcome, the sample size represents the total number of women in the studies that assessed the outcome. For each BMI category, the sample size represents the total number of women with gestational weight gain below or above the guidelines. LGA indicates large for gestational age; SGA, small for gestational age.
Primary Outcomes
Small for Gestational Age
Eleven studies assessed SGA, defined as birth weight less than the 10th percentile for gestational age in 5 studies. Four studies defined SGA by additionally accounting for sex, 1 for sex and race/ethnicity, and another for sex, race, and parity.
Across BMI categories, gestational weight gain below guidelines was associated with higher risk for SGA than gestational weight gain within guidelines (OR, 1.53 [95% CI, 1.44 to 1.64]; I2 = 82.8%; ARD, 5% [95% CI, 4% to 6%]). This association was greatest in lower prepregnancy BMI (underweight: OR, 1.89 [95% CI, 1.67 to 2.14]; ARD, 8% [95% CI, 6% to 11%]; normal weight: OR, 1.63 [95% CI, 1.54 to 1.71]; ARD, 5% [95% CI, 4% to 6%]; overweight: OR, 1.34 [95% CI, 1.24 to 1.44]; ARD, 3% [95% CI, 3% to 4%]; and obese: OR, 1.24 [95% CI, 1.06 to 1.45]; ARD, 2% [95% CI, 2% to 3%]).
Compared with gestational weight gain within guidelines, gain above guidelines was associated with lower risk for SGA (OR, 0.66 [95% CI, 0.63 to 0.69]; I2 = 56%; ARD, −3% [95% CI, −4% to −2%]). The association was similar across BMI categories (underweight: OR, 0.62 [95% CI, 0.53 to 0.72]; ARD, −6% [95% CI, −8% to −3%]; normal weight: OR, 0.65 [95% CI, 0.62 to 0.68]; ARD, −2% [95% CI, −3% to−1%]; overweight: OR, 0.65 [95% CI, 0.59 to 0.71]; ARD, −3% [95% CI, −4% to −2%]; and obese: OR, 0.72 [95% CI, 0.65 to 0.80]; ARD, −2% [95% CI, −3% to −1%]).
Preterm Birth
Four studies assessed preterm birth (<37 weeks’ gestation). Of these, 3 did not specify whether the preterm birth was spontaneous or induced and 1 specified spontaneous and induced combined.
Compared with gestational weight gain within guidelines, weight gain below guidelines was associated with higher risk for preterm birth (OR, 1.70 [95% CI, 1.32 to 2.20]; I2 = 97.3%; ARD, 5% [95% CI, 3% to 8%]). This association was greatest with lower BMI (underweight: OR, 2.41 [95% CI, 1.01 to 5.73]; ARD, 8% [95% CI, 1% to 15%]; normal weight: OR, 1.96 [95% CI, 1.17 to 3.29]; ARD, 6% [95% CI, 0% to 11%]; overweight: OR, 1.55 [95% CI, 1.10 to 2.19]; ARD, 4% [95% CI, −1% to 9%]; and obese: OR, 1.20 [95% CI, 1.03 to 1.40]; ARD, 3% [95% CI, 1% to 5%]).
Gestational weight gain above guidelines was associated with lower risk for preterm birth (OR, 0.77 [95% CI, 0.69 to 0.86]; I2 = 78.7%; ARD, −2% [95% CI, −2% to −1%]). This association was significant for normal-weight and overweight women (underweight: OR, 0.80 [95% CI, 0.50 to 1.28]; ARD, −1% [95% CI, −3% to 0%]; normal weight: OR, 0.76 [95% CI, 0.59 to 0.97]; ARD, −1% [95% CI, −2% to 0%]; overweight: OR, 0.70 [95% CI, 0.53 to 0.93]; ARD, −3% [95% CI, −5% to −1%]; and obese: OR, 0.76 [95% CI, 0.62 to 0.93]; ARD, −2% [95% CI, −5% to 2%]).
Large for Gestational Age
Thirteen studies assessed LGA, defined as birth weight greater than the 90th percentile for gestational age in 6 studies. Four defined LGA by additionally accounting for infant sex, 1 for sex and race/ethnicity, 1 for sex, race, and parity, and 1 for sex, parity, and study center.
Gestational weight gain below guidelines was associated with lower risk of LGA than gestational weight gain within guidelines (OR, 0.59 [95% CI, 0.55 to 0.64]; I2 = 78.9%; ARD, −2% [95% CI, −10% to −6%]). This was significant for underweight and normal-weight women (underweight: OR, 0.41 [95% CI, 0.34 to 0.50]; ARD, −3% [95% CI, −5% to −1%]; normal weight: OR, 0.58 [95% CI, 0.54-0.62]; ARD, −3% [95% CI, −4% to −2%]; overweight: OR, 0.66 [95% CI, 0.62 to 0.70]; ARD, −11% [95% CI, −33% to 10%]; and obese: OR, 0.70 [95% CI, 0.64 to 0.76]; ARD, 13% [95% CI, −34% to 60%]).
Gestational weight gain above guidelines was associated with higher risk of LGA (OR, 1.85 [95% CI, 1.76 to 1.95]; I2 = 74.6%; ARD, 4% [95% CI, 2% to 5%]). The association increased as BMI decreased (underweight: OR, 2.17 [95% CI, 1.81 to 2.60]; ARD, 4% [95% CI, 4% to 5%]; normal weight: OR, 1.95 [95% CI, 1.83 to 2.08]; ARD, 6% [95% CI, 5% to 7%]; overweight: OR, 1.79 [95% CI, 1.61 to 1.98]; ARD, −2% [95% CI, −14% to 9%]; and obese: OR, 1.63 [95% CI, 1.56 to 1.70]; ARD, 7% [95% CI, 5% to 8%]).
Secondary Outcomes
Macrosomia
Of 11 studies assessing macrosomia, 10 defined macrosomia as birth weight greater than 4000 g, and 1 defined it as birth weight greater than 4500 g.
Gestational weight gain below guidelines was associated with lower risk of macrosomia (OR, 0.60 [95% CI, 0.52 to 0.68]; I2 = 66.3%; ARD, −2% [95% CI, −3% to −1%]). The association was strongest in underweight women (underweight: OR, 0.43 [95% CI, 0.27 to 0.69]; ARD, −1% [95% CI, −3% to 0%]; normal weight: OR, 0.54 [95% CI, 0.43 to 0.68]; ARD, −2% [95% CI, −5% to 1%]; overweight: OR, 0.73 [95% CI, 0.60 to 0.89]; ARD, −2% [95% CI, −6% to 2%]; and obese: OR, 0.70 [95% CI, 0.59 to 0.82]; ARD, −3% [−4% to −2%]).
Gestational weight gain above guidelines was associated with higher risk of macrosomia (OR, 1.95 [95% CI, 1.79 to 2.11]; I2 = 58.2%; ARD, 6% [95% CI, 4% to 9%]). This association was strongest in underweight women according to the ORs, and all associations were significant according to the ARDs (underweight: OR, 2.31 [95% CI, 1.62 to 3.29]; ARD, 3% [95% CI, 2% to 4%]; normal weight: OR, 2.01 [95% CI, 1.77 to 2.27]; ARD, 10% [95% CI, 5% to 15%]; overweight: OR, 1.90 [95% CI, 1.54 to 2.33]; ARD, 5% [95% CI, 1% to 10%]; and obese: OR, 1.83 [95% CI, 1.52 to 2.22]; ARD, 6% [95% CI, 1% to 12%]).
Cesarean Delivery
Eight studies assessed cesarean delivery. Seven included emergency and elective deliveries, and 1 did not specify. One study included repeated cesarean delivery (total cesarean deliveries), 1 included primary cesarean delivery only, and 6 did not distinguish these.
Gestational weight gain below guidelines was not significantly associated with cesarean delivery (OR, 0.98 [95% CI, 0.96 to 1.02]; I2 = 62.6%; ARD, 0% [−2% to 1%]).
Gestational weight gain above guidelines was associated with higher risk of cesarean delivery (OR, 1.30 [95 CI, 1.25 to 1.35]; I2 = 21.9%; ARD, 4% [95% CI, 3% to 6%]). The ARD was significant for underweight women only (underweight: OR, 1.45 [95% CI, 1.22 to 1.71]; ARD, 6% [95% CI, 1% to 12%]; normal weight: OR, 1.30 [95% CI, 1.24 to 1.36]; ARD, 0% [95% CI, −4% to 3%]; overweight: OR, 1.29 [95% CI, 1.21 to 1.39]; ARD, 1% [0% to 3%]; and obese: OR, 1.22 [95% CI, 1.05 to 1.42]; ARD, −2% [95% CI, −5% to 1%]).
Gestational Diabetes Mellitus
Six studies assessed GDM, but they did not use consistent definitions and had different findings for gestational weight gain above guidelines and GDM risk. Black et al defined GDM by International Association of Diabetes in Pregnancy Study Groups criteria and included only women not treated for hyperglycemia (the center used different criteria in clinical practice and excluded those treated). They found no association between weight gain above guidelines and GDM in the underweight, normal-weight, and obese groups but reported lower risk in overweight women. Enomoto et al used International Association of Diabetes in Pregnancy Study Groups criteria, with higher risk in normal-weight women and lower risk in overweight women. Durst et al used Carpenter-Coustan criteria and found no association. Hung and Hsieh used Carpenter-Coustan and International Association of Diabetes in Pregnancy Study Groups criteria and found an association of gestational weight gain above guidelines with lower risk of GDM in overweight and obese women. Li et al included both impaired glucose tolerance and type 2 diabetes by WHO criteria, with weight gain above guidelines associated with lower risk of GDM in all groups except obese women. Shin and Song used self-reported GDM and found an association of gestational weight gain above guidelines with lower risk in all groups except underweight women.
An intended meta-analysis of gestational weight gain and its relationship to GDM could not be completed because of inconsistent definitions and treatments.
Obese Subgroup Analysis Stratified by Obesity Class
Obesity classes include the following: class 1, BMI of 30 to 34.9; class 2, BMI of 35 to 39.9; and class 3, BMI of 40 or higher. Obese studies generally included a subgroup-defined weight loss as well as gestational weight gain below, within, or above guidelines. Three studies assessed outcomes stratified by BMI classes 1 through 3. Another study investigated only superobese women (BMI ≥50) and was included in the obesity class 3 analysis. These 4 studies were included in the subgroup analysis only (not in the overall meta-analyses). Class 1 included 67% of women; class 2, 22%; and class 3, 11%. Weight loss and gestational weight gain below, within, or above recommendations occurred in 6%, 13%, 25%, and 57% of pregnancies, respectively.
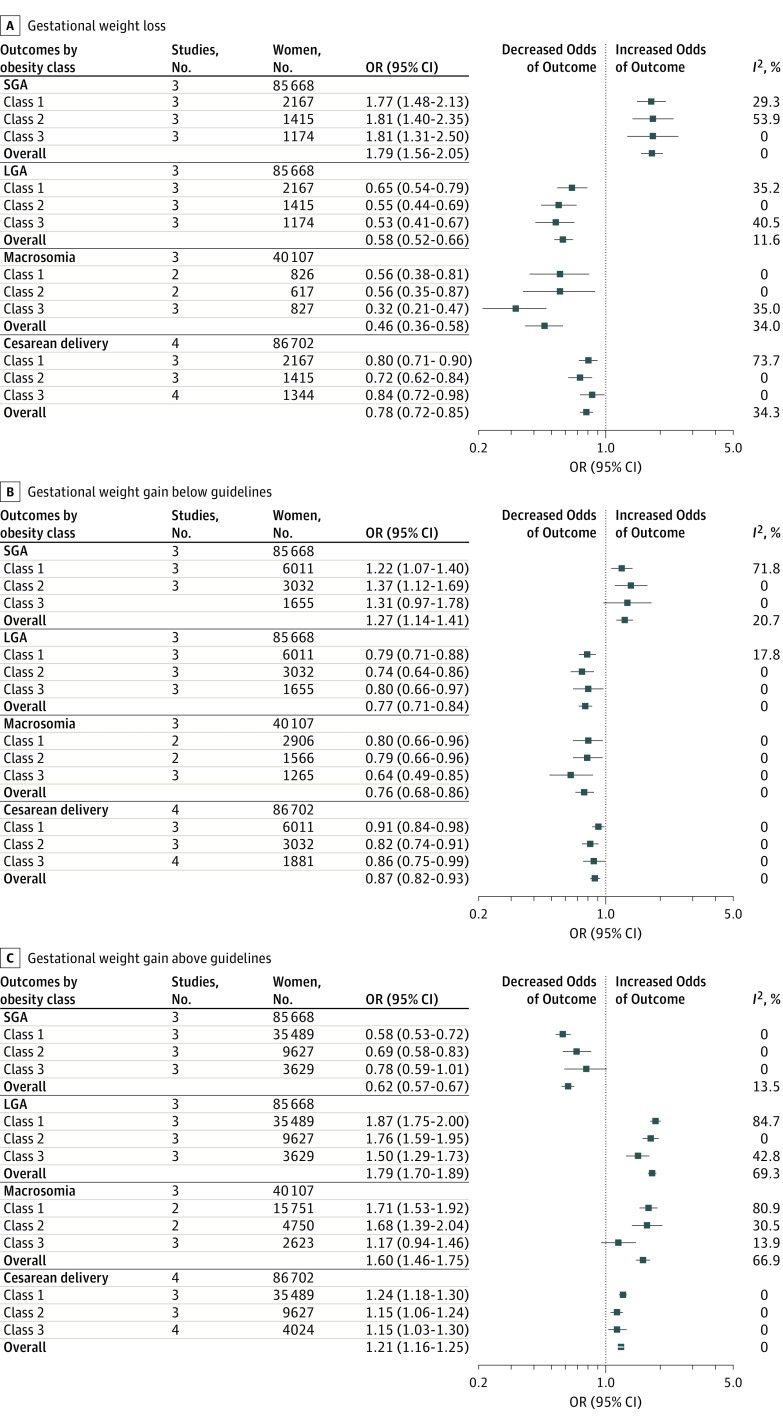
Figure 3 summarizes pooled ORs for primary (SGA and LGA) and secondary (macrosomia and cesarean delivery) outcomes. eFigure 3 in the Supplement shows pooled ORs for individual outcomes. eTable 4 in the Supplement reports ARDs and P values. Only 1 study assessed preterm birth and GDM in the obese subgroups, preventing meta-analysis. Kominiarek et al provided separate ORs for nulliparous and multiparous women (multiparous values used herein), whereas other studies combined women with different parity into 1 group.
Figure 3. Obese Subgroup Analysis With Summary of Pooled Odds Ratios (ORs) for the Association Between Gestational Weight Loss, Gain Below Guidelines, and Gain Above Guidelines With Adverse Outcomes.
Pooled ORs are shown for the association between gestational weight loss (A), gestational weight gain below guidelines (B), and gestational weight gain above guidelines (C) with adverse outcomes. Obesity classes indicate body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) as follows: class 1, BMI of 30 to 34.9; class 2, BMI of 35 to 39.9; and class 3, BMI of 40 or higher. Reference group is women with recommended weight gain in each category of BMI. For each outcome, the sample size represents the total number of women in the studies that assessed the outcome. For each obesity category, the sample size represents the total number of women with weight loss, gestational weight gain below the guidelines, or gestational weight gain above the guidelines.
SGA by Obesity Class
Three studies assessed SGA. One defined SGA as birth weight less than the 10th percentile for gestational age alone, and 2 also used sex and parity to define SGA.
Weight loss and weight gain below guidelines were associated with higher SGA risk (weight loss: OR, 1.79 [95% CI, 1.56 to 2.05]; I2 = 0%; ARD, 3% [95% CI, 1% to 5%]; weight gain below guidelines: OR, 1.27 [95% CI, 1.14 to 1.41]; I2 = 20.7%; ARD, 1% [95% CI, 1% to 1%]). Gestational weight gain above guidelines was associated with lower SGA risk (OR, 0.62 [95% CI, 0.57 to 0.67]; I2 = 13.5%; ARD, −1% [−2% to 0%]). Weight gain in class 1 had the strongest association with lower SGA risk (lowest OR, 0.58; 95% CI, 0.53 to 0.72]; P for trend < .001).
LGA by Obesity Class
Three studies assessed LGA. One defined LGA as birth weight greater than the 90th percentile for gestational age alone, and 2 also used sex and parity to define LGA.
Weight loss and gestational weight gain below guidelines were associated with lower LGA risk (weight loss: OR, 0.58 [95% CI, 0.52 to 0.66]; I2 = 11.6%; ARD, −5% [95% CI, −7% to −3%]; weight gain below guidelines: OR, 0.77 [95% CI, 0.71 to 0.84]; I2 = 0%; ARD, −2% [95% CI, −3% to −1%]). Weight loss in class 3 had the strongest association with lower LGA risk (lowest OR, 0.53 [95% CI, 0.41 to 0.67]; P for trend < .001). Weight gain above guidelines was associated with higher LGA risk (OR, 1.79 [95% CI, 1.70 to 1.89]; I2 = 69.3%; ARD, 5% [95% CI, 5% to 6%]). LGA was most strongly associated with class 1 obesity compared with the other classes (highest OR, 1.87 [95% CI, 1.75 to 2.00]; P for trend < .001).
Macrosomia by Obesity Class
Three studies assessed macrosomia, defined as birth weight greater than 4000 g in 1 study, greater than 4500 g in 1 study, and both greater than 4000 g and greater than 4500 g in 1 study. Meta-analysis used data for birth weight greater than 4000 g.
Weight loss and gestational weight gain below guidelines were associated with lower macrosomia risk (weight loss: OR, 0.46 [95% CI, 0.36 to 0.58]; I2 = 34.0%; ARD, −5% [95% CI, −9% to −2%]; weight gain below guidelines: OR, 0.76 [95% CI, 0.68 to 0.86]; I2 = 0%; ARD, −2% [95% CI, −3% to 0%]). Low weight gain in class 3 had the strongest association with lower macrosomia risk (lowest OR, 0.64 [95% CI, 0.49 to 0.85]; P for trend = .046). Gestational weight gain above guidelines was associated with higher risk of macrosomia (OR, 1.60 [95% CI, 1.46 to 1.75]; I2 = 66.9%; ARD, 3% [95% CI, 0% to 6%]).
Cesarean Delivery by Obesity Class
Four studies assessed cesarean delivery. They included emergency, emergency and elective, and undefined indications for cesarean delivery.
Weight loss and gestational weight gain below guidelines were associated with lower risk of cesarean delivery (weight loss: OR, 0.78 [95% CI, 0.72 to 0.85]; I2 = 34.3%; ARD, −4% [95% CI, −6% to −3%]; weight gain below guidelines: OR, 0.87 [95% CI, 0.82 to 0.93]; I2 = 0%; ARD, −2% [95% CI, −3% to −1%]). Gestational weight gain above guidelines was associated with higher risk of cesarean delivery (OR, 1.21 [95% CI, 1.16 to 1.25]; I2 = 0%; ARD, 2% [95% CI, 0% to 3%]).
Metaregression
Substantial heterogeneity (I2 > 50%) was present for gestational weight gain below and above guidelines for SGA, preterm birth, LGA, and macrosomia and for gestational weight gain above guidelines for cesarean delivery. When sufficient data were available, metaregression analysis was performed to investigate possible sources of heterogeneity: percentage of smokers during pregnancy, mean age, and percentage of nulliparous women (eTable 5 in the Supplement). The obese subgroups had insufficient studies to perform metaregression.
Gestational weight gain above guidelines and LGA demonstrated a source for heterogeneity (P = .04); specifically, there was an association between the treatment effect and the covariate smoking (P = .02). For gestational weight gain below guidelines and preterm birth, mean maternal age was the only covariate associated with outcome, where the risk for preterm birth varied by maternal age due to the heterogeneity in maternal age in included studies (P = .03); however, the overall P value was not significant (P = .09). Heterogeneity was unexplained for remaining outcomes.
Publication Bias
There was no evidence of publication bias for SGA, LGA, macrosomia or cesarean delivery (eFigure 4 in the Supplement). Assessment for publication bias was not performed for preterm births (<5 studies).
Risk of Bias
Participants were selected from maternity clinics or from large data sets (Table 4). Apart from 3 studies, inclusion and exclusion criteria were adequately described. Performance bias (a potential difference in the care provided between BMI groups) was difficult to assess. Very few studies provided information regarding diet and/or exercise advice given and whether this differed between groups. Overweight and obese women were possibly treated more intensively, which could introduce bias.
Table 4. Summary of Risk of Bias Assessment.
| Source | Selection Bias, Exposed Cohort Representative | Detection Bias | Reporting Bias, Free of Selective Outcome Reporting | Assessment of Confounding in Original Analysis | Conflict of Interest | Overall Risk of Bias | |
|---|---|---|---|---|---|---|---|
| Adequate Exposure Measures | Adequate Outcome Measures | ||||||
| Durst et al, 2016 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Enomoto et al, 2016 | Yes | NR | Yes | Yes | Yes | No | Low |
| Hung and Hsieh, 2016 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Xiong et al, 2016 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Bogaerts et al, 2015 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Shin and Song, 2015 | Yes | Yes | No (self-reported) | Yes | Partial (did not adjust for parity) | No | Moderate |
| Wen and Lv, 2015 | NR | Yes | NR | Partial (not all outcomes reported) | Partial (did not adjust for required number of confounders) | No | Moderate |
| Yang et al, 2015 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Badon et al, 2014 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Chihara et al, 2014 | Yes | Partial (self-reported final weight) | No (self-reported) | Yes | Yes | NR | Moderate |
| Haugen et al, 2014 | Yes | Partial (self-reported final weight) | Yes | Yes | Yes | No | Low |
| Lee et al, 2014 | NR | Yes | Yes | Yes | Yes | No | Low |
| Swank et al, 2014 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Black et al, 2013 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Kominiarek et al, 2013 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Li et al, 2013 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Di Benedetto et al, 2012 | Yes | Yes | Yes | Yes | Partial (did not adjust for parity) | No | Low |
| Simas et al, 2012 | Yes | Partial (some self-reported final weight) | Yes | Yes | Yes | No | Low |
| Blomberg, 2011 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Park et al, 2011 | Yes | Yes | Yes | Partial (not all outcomes reported) | Yes | NR | Low |
| Park et al, 2011 | Partial | NR | Yes | Yes | Yes | NR | Low |
| Vesco et al, 2011 | Yes | Yes | Yes | Yes | Yes | No | Low |
| Rode et al, 2007 | NR | Partial (self-reported final weight) | Yes | Yes | Partial (did not adjust for parity) | NR | Low |
Abbreviation: NR, not reported.
Three studies demonstrated moderate bias risk and 20 demonstrated low bias risk. Reasons for moderate bias risk included self-reported final weight (detection bias), self-reported outcome measures (detection bias), failure to report all outcomes (report bias), and insufficient adjustment for confounding variables (confounding bias). Nineteen studies reported no conflict of interest.
Discussion
In this analysis of 1 309 136 pregnancies from diverse international cohorts, gestational weight gain below or above 2009 IOM guidelines among women across the BMI range was associated with greater risk for maternal and infant adverse outcomes. Underweight women composed 7%; normal-weight women, 55%; overweight women, 18%; and obese women, 20%. For gestational weight gain, 23% gained below and 47% gained above guidelines. Compared with recommended gestational weight gain, gain below guidelines was associated with 5% higher risk of both SGA and preterm birth and 2% lower risk of both LGA and macrosomia. Weight gain above guidelines was associated with 3% lower risk of SGA and 2% lower risk of preterm birth and 4%, 6%, and 4% higher risk of LGA, macrosomia, and cesarean delivery, respectively.
Gestational weight gain below guidelines was associated with higher SGA risk, with greatest risk in underweight women, as shown previously. Obesity was associated with higher risk of SGA, with weight loss and gestational weight gain below guidelines increasing risks, similar to prior systematic reviews. Underweight status combined with gestational weight gain below recommendations as well as obese status combined with gestational weight loss present the highest risk groups for SGA, at 8% and 3%, respectively.
Gestational weight gain below guidelines was associated with a 5% increase in preterm birth across the included populations. With 23% having weight gain below recommendations, this could correspond to 15 000 more preterm birth events. Weight gain above guidelines was associated with lower risk of preterm birth. Prior reviews have shown similar associations, but they did not stratify by prepregnancy BMI and gestational weight gain. One small systematic review in obese women did not find associations between preterm birth and weight gain outside guidelines. With larger sample sizes and stratification by BMI and prepregnancy weight gain, the current review adds to prior work and has greater clinical applicability. Also, as maternal BMI increased, the association between gestational weight gain below guidelines and preterm birth risk was weakened, consistent with an earlier review.
Gestational weight gain below guidelines was associated with lower risks of LGA and macrosomia. This association was lowest in underweight women. Weight gain above guidelines was associated with higher risks of LGA and macrosomia, with ARDs of 4% and 6% greater risks, respectively. Underweight status was associated with the greatest risk. This is similar to the 2009 IOM report that stated, “the lower the prepregnancy BMI, the stronger the association between increased gestational weight gain and birthweight”; it may be related to higher absolute weight gain in underweight women. Animal studies suggest that baseline maternal BMI and gestational weight gain are associated with changes in the hormonal milieu, including insulin resistance. Similarly, excess weight gain in underweight women may be associated with greater changes in the hormonal milieu and placental function than in normal-weight or overweight women. Weight gain above guidelines was associated with increased risk of cesarean delivery across the BMI spectrum.
Similarly, within the obese subgroups, weight loss was associated with a 5% lower risk for both LGA and macrosomia and 4% lower risk for cesarean delivery. Weight gain below guidelines was associated with 2% lower risk across all these outcomes. Class 3 obesity combined with weight loss was associated with the greatest LGA risk reduction. Gestational weight gain above guidelines was associated with increased LGA risk. Class 1 obesity was associated with the greatest risk for LGA, which may be partly due to higher absolute weight gain in less obese women. While other systematic reviews have assessed gestational weight gain below guidelines, to our knowledge, this is the first review exploring relationships between weight gain above guidelines and outcomes within obesity classes.
While GDM has adverse maternal and infant outcomes and is related to maternal BMI and possibly to gestational weight gain, associations could not be assessed because of heterogeneity of diagnosis and treatment as well as the potential effect of GDM treatment on gestational weight gain. Prior systematic reviews have not demonstrated that healthy lifestyle and gestational weight gain reduced rates of GDM, even in high-risk populations. Consistent diagnostic criteria and reporting of gestational weight gain at GDM diagnosis are needed to study associations between gestational weight gain and GDM.
Lifestyle interventions in pregnancy can help women attain recommended gestational weight gain. Optimal interventions and effects on outcomes are currently being studied in a large-scale international individual patient data meta-analysis. The WHO has prioritized achievement of ideal BMI prior to conception and prevention of excess gestational weight gain. Identification of women prior to conception and implementing healthy lifestyle strategies before and during pregnancy have yet to be integrated into routine health care, requiring research implementation.
Strengths of this review are the inclusion of common maternal and infant risks associated with gestational weight gain below and above the 2009 IOM guidelines in women across the prepregnancy BMI spectrum and across international cohorts. Four databases were searched, a risk of bias appraisal was performed, and reanalyses were undertaken, allowing inclusion of data from more than 1.3 million pregnant women globally. Collaboration with other authors facilitated more homogeneous data, data integration, and meta-analysis.
Limitations
This study has limitations. It lacks studies from developing countries and excluded non-English-language articles. Fifteen of 31 authors contacted were unable to reanalyze data, so these studies were excluded from the meta-analysis. A meta-analysis could not be performed for GDM because of inconsistent primary data. Some outcomes were assessed in only 1 study, precluding meta-analysis. Inconsistent definitions of preterm birth, cesarean delivery, and macrosomia limited interpretation of findings. Study heterogeneity may have affected reliability of results, although the metaregression did not identify characteristics responsible for this heterogeneity. Studies published before 2009 IOM guidelines were included, and gestational weight gain targets before and after these guidelines may have differed. Preterm birth was not adjusted for gestational age, potentially resulting in less total gestational weight gain than would have been otherwise attained. Spontaneous and induced preterm birth were not clearly differentiated, and studies did not distinguish between emergency and elective or primary and repeated cesarean deliveries. These factors may limit interpretation and underscore the importance of improving outcome definition reporting. Event rates were not available for all studies, limiting interpretation of ARDs. Findings from this review are based on observational data and no causal links may be concluded. They may be applicable on a population level, but recommendations need to be individualized when applied clinically.
Conclusions
In this systematic review and meta-analysis of more than 1 million pregnant women, 47% had gestational weight gain greater than IOM recommendations and 23% had gestational weight gain less than IOM recommendations. Gestational weight gain greater than or less than guideline recommendations, compared with gestational weight gain within recommended levels, was associated with higher risk of adverse maternal and infant outcomes.
eAppendix 1. Search Terms
eAppendix 2. Additional Methods
eTable 1. Descriptive Characteristics of 23 Included Studies
eTable 2. Range of Total Event Rates per Live Birth for Primary and Secondary Outcomes
eTable 3. Absolute Risk Difference (ARD) for Studies That Stratified by Prepregnancy Underweight (BMI <18.5 kg/m2), Normal Weight (18.5-24.9kg/m2), Overweight (25-29.9 kg/m2) and Obese (≥30 kg/m2)
eTable 4. Absolute Risk Difference (ARD) for Studies That Stratified by Prepregnancy Obesity Class 1 (30-34.9 kg/m2), Class 2 (35-39.9 kg/m2) and Class 3 (≥40 kg/m2)
eTable 5. Metaregression
eFigure 1. Pooled OR for Primary and Secondary Outcomes
eFigure 2. Absolute Risk Difference Plots (per Live Birth)
eFigure 3. Pooled OR for Primary and Secondary Outcomes for Obese Subgroup
eFigure 4. Publication Bias
eReferences
References
- 1.Nohr EA, Vaeth M, Baker JL, Sørensen TIA, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy [erratum appears in Am J Clin Nutr. 2008;88(6):1705]. Am J Clin Nutr. 2008;87(6):1750-1759. [DOI] [PubMed] [Google Scholar]
- 2.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [erratum appears in Obstet Gynecol. 2010;115(5):1092]. Obstet Gynecol. 2010;115(3):597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep). 2008;(168):1-223. [PMC free article] [PubMed] [Google Scholar]
- 4.Hrolfsdottir L, Rytter D, Olsen SF, et al. Gestational weight gain in normal weight women and offspring cardio-metabolic risk factors at 20 years of age. Int J Obes (Lond). 2015;39(4):671-676. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine Nutrition During Pregnancy: Part I: Weight Gain, Part II Nutrient Supplements. Washington, DC: National Academies Press; 1990. [PubMed] [Google Scholar]
- 6.Rasmussen K, Yaktine AL, eds; Institute of Medicine; National Research Council . Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 7.Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed] [Google Scholar]
- 8.Committee on Obstetric Practice, American College of Obstetricians and Gynecologists Weight gain during pregnancy: committee opinion 548. January 2013, reaffirmed 2015. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co548.pdf?dmc=1&ts=20160723T0702247216. Accessed July 23, 2016.
- 9.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavard JA, Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern Child Health J. 2014;18(4):1038-1047. [DOI] [PubMed] [Google Scholar]
- 11.Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83-96. [PubMed] [Google Scholar]
- 12.Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2(2):123-126. [DOI] [PubMed] [Google Scholar]
- 13.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774-777. [PubMed] [Google Scholar]
- 14.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monash Centre for Health Research and Implementation Evidence Synthesis Program Template for Critical Appraisal of a Cohort Study. Melbourne, Australia: Monash Centre for Health Research and Implementation; 2014. [Google Scholar]
- 16.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2016;22(3):dmv063. [DOI] [PubMed] [Google Scholar]
- 17.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol. 2011;117(5):1065-1070. [DOI] [PubMed] [Google Scholar]
- 18.Kominiarek MA, Seligman NS, Dolin C, et al. Gestational weight gain and obesity: is 20 pounds too much? Am J Obstet Gynecol. 2013;209(3):214.e1-214.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chihara I, Hayes DK, Chock LR, Fuddy LJ, Rosenberg DL, Handler AS. Relationship between gestational weight gain and birthweight among clients enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), Hawaii, 2003-2005. Matern Child Health J. 2014;18(5):1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badon SE, Dyer AR, Josefson JL; HAPO Study Cooperative Research Group . Gestational weight gain and neonatal adiposity in the Hyperglycemia and Adverse Pregnancy Outcome study-North American region. Obesity (Silver Spring). 2014;22(7):1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen T, Lv Y. Inadequate gestational weight gain and adverse pregnancy outcomes among normal weight women in China. Int J Clin Exp Med. 2015;8(2):2881-2886. [PMC free article] [PubMed] [Google Scholar]
- 22.Enomoto K, Aoki S, Toma R, Fujiwara K, Sakamaki K, Hirahara F. Pregnancy outcomes based on pre-pregnancy body mass index in Japanese women. PLoS One. 2016;11(6):e0157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durst JK, Sutton AL, Cliver SP, Tita AT, Biggio JR. Impact of gestational weight gain on perinatal outcomes in obese women. Am J Perinatol. 2016;33(9):849-855. [DOI] [PubMed] [Google Scholar]
- 24.Hung TH, Hsieh TT. Pregestational body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: a retrospective cohort study. Taiwan J Obstet Gynecol. 2016;55(4):575-581. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and meta-analyses of maternal and infant outcomes. PLoS One. 2015;10(7):e0132650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong C, Zhou A, Cao Z, et al. Association of pre-pregnancy body mass index, gestational weight gain with cesarean section in term deliveries of China. Sci Rep. 2016;6:37168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes [erratum appears in Obstet Gynecol. 2015;126(2):452]. Obstet Gynecol. 2015;125(3):566-575. [DOI] [PubMed] [Google Scholar]
- 31.Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. 2015;28(14):1679-1686. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Peng A, Wei S, et al. Pre-pregnancy body mass index, gestational weight gain, and birth weight: a cohort study in China. PLoS One. 2015;10(6):e0130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haugen M, Brantsæter AL, Winkvist A, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth. 2014;14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JM, Kim MJ, Kim MY, et al. Gestational weight gain is an important risk factor for excessive fetal growth. Obstet Gynecol Sci. 2014;57(6):442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swank ML, Marshall NE, Caughey AB, et al. Pregnancy outcomes in the super obese, stratified by weight gain above and below Institute of Medicine guidelines. Obstet Gynecol. 2014;124(6):1105-1110. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Liu E, Guo J, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8(12):e82310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Benedetto A, D’anna R, Cannata ML, Giordano D, Interdonato ML, Corrado F. Effects of prepregnancy body mass index and weight gain during pregnancy on perinatal outcome in glucose-tolerant women. Diabetes Metab. 2012;38(1):63-67. [DOI] [PubMed] [Google Scholar]
- 38.Simas TA, Waring ME, Liao X, et al. Prepregnancy weight, gestational weight gain, and risk of growth affected neonates. J Womens Health (Larchmt). 2012;21(4):410-417. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Lee BE, Park HS, Ha EH, Lee SW, Kim YJ. Association between pre-pregnancy body mass index and socioeconomic status and impact on pregnancy outcomes in Korea. J Obstet Gynaecol Res. 2011;37(2):138-145. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Sappenfield WM, Bish C, Salihu H, Goodman D, Bensyl DM. Assessment of the Institute of Medicine recommendations for weight gain during pregnancy: Florida, 2004-2007. Matern Child Health J. 2011;15(3):289-301. [DOI] [PubMed] [Google Scholar]
- 41.Vesco KK, Sharma AJ, Dietz PM, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol. 2011;117(4):812-818. [DOI] [PubMed] [Google Scholar]
- 42.Rode L, Hegaard HK, Kjaergaard H, Møller LF, Tabor A, Ottesen B. Association between maternal weight gain and birth weight. Obstet Gynecol. 2007;109(6):1309-1315. [DOI] [PubMed] [Google Scholar]
- 43.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93(3):269-274. [DOI] [PubMed] [Google Scholar]
- 44.Savitz DA, Stein CR, Siega-Riz AM, Herring AH. Gestational weight gain and birth outcome in relation to prepregnancy body mass index and ethnicity. Ann Epidemiol. 2011;21(2):78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? a systematic review and meta-analysis. Obes Rev. 2015;16(3):189-206. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z, Wen Z, Zhou Y, Li D, Luo Z. Inadequate weight gain in obese women and the risk of small for gestational age (SGA): a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2017;30(3):357-367. [DOI] [PubMed] [Google Scholar]
- 47.Han Z, Lutsiv O, Mulla S, Rosen A, Beyene J, McDonald SD; Knowledge Synthesis Group . Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2011;90(9):935-954. [DOI] [PubMed] [Google Scholar]
- 48.McDonald SD, Han Z, Mulla S, Lutsiv O, Lee T, Beyene J; Knowledge Synthesis Group . High gestational weight gain and the risk of preterm birth and low birth weight: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2011;33(12):1223-1233. [DOI] [PubMed] [Google Scholar]
- 49.Faucher MA, Hastings-Tolsma M, Song JJ, Willoughby DS, Bader SG. Gestational weight gain and preterm birth in obese women: a systematic review and meta-analysis. BJOG. 2016;123(2):199-206. [DOI] [PubMed] [Google Scholar]
- 50.Nicholas LM, Rattanatray L, MacLaughlin SM, et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013;27(9):3786-3796. [DOI] [PubMed] [Google Scholar]
- 51.Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity (Silver Spring). 2013;21(5):904-909. [DOI] [PubMed] [Google Scholar]
- 53.Ruifrok AE, Rogozinska E, van Poppel MNM, et al. ; i-WIP (International Weight Management in Pregnancy) Collaborative Group . Study protocol: differential effects of diet and physical activity based interventions in pregnancy on maternal and fetal outcomes—individual patient data (IPD) meta-analysis and health economic evaluation [erratum appears in Syst Rev. 2015;4:101]. Syst Rev. 2014;3:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 55.Teede H, Harrison C, Lombard C, Boyle C, East C, Brown W Case for Action proposal: obesity prevention through preventing excess weight gain during pregnancy and postpartum. September 2014. https://www.nhmrc.gov.au/research/research-translation/research-translation-faculty/ideas-research-translation-faculty-cases. Accessed August 31, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Terms
eAppendix 2. Additional Methods
eTable 1. Descriptive Characteristics of 23 Included Studies
eTable 2. Range of Total Event Rates per Live Birth for Primary and Secondary Outcomes
eTable 3. Absolute Risk Difference (ARD) for Studies That Stratified by Prepregnancy Underweight (BMI <18.5 kg/m2), Normal Weight (18.5-24.9kg/m2), Overweight (25-29.9 kg/m2) and Obese (≥30 kg/m2)
eTable 4. Absolute Risk Difference (ARD) for Studies That Stratified by Prepregnancy Obesity Class 1 (30-34.9 kg/m2), Class 2 (35-39.9 kg/m2) and Class 3 (≥40 kg/m2)
eTable 5. Metaregression
eFigure 1. Pooled OR for Primary and Secondary Outcomes
eFigure 2. Absolute Risk Difference Plots (per Live Birth)
eFigure 3. Pooled OR for Primary and Secondary Outcomes for Obese Subgroup
eFigure 4. Publication Bias
eReferences