Diabetes mellitus, with sustained high-sugar levels in the blood, has been steadily rising in prevalence worldwide. Around 12–14% of the US population has been diagnosed with diabetes in recent years.1 One major reason for the rise of diabetes is dietary changes. Increased consumption of processed carbohydrates with inadequate consumption of dietary fiber (DF) has been recognized as a major risk factor for diabetes, as hypothesized several decades ago by Trowell.2 Principle DFs include soluble fibers, such as pectin, inulin, arabinoxylan and hemicellulose, and insoluble fibers such as cellulose. Soluble fibers are preferentially fermented by microbiota in the colon to generate short-chain fatty acids (SCFAs) such as acetate (C2), propionate (C3) and butyrate (C4). Moreover, resistant forms of starch (RS), which are basically starch but difficult to degrade by digestive enzymes due to their conformation, can reach the colon for fermentation to increase SCFA levels. Both soluble and insoluble fibers have beneficial effects on diabetes. The beneficial effect of DF on obesity, metabolic disease, and type 2 diabetes (T2D) has long been recognized3 (Figure 1). With recent advances in our understanding of the functions of SCFAs in immune system regulation, roles of microbiota and SCFAs in suppressing type 1 diabetes (T1D) are an active area of research exemplified by a recent publication by Marino et al.4 Here, I highlight the overlapping roles of DF, SCFAs and microbiota in regulating immune cells and diabetes.
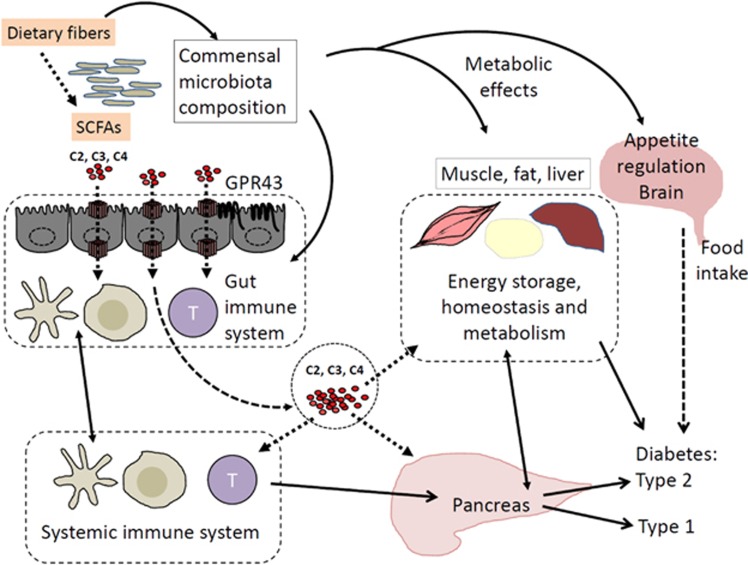
Figure 1.
Beneficial effects of DF and SCFAs on diabetes. Prebiotics, such as dietary fiber (DF) and resistant starch, are fermented into SCFAs in the colon by certain communal bacterial species. Prebiotics and SCFAs alter gut commensal bacteria, enriching certain bacteria with disease-regulatory effects. SCFAs in the gut lumen are absorbed into enterocytes, and they eventually reach the blood circulation. SCFAs in the blood circulation affect glucose storage in the muscle, liver and fat. Acetate (C2) reaches the brain and decreases appetite to decrease food consumption. All of these effects can function to decrease type 2 diabetes (T2D). SCFAs can regulate myeloid cells and lymphocytes to facilitate the generation of lymphocytes that promote immunity but prevent inflammatory diseases. SCFAs can activate GPR43 on intestinal epithelial cells to enhance gut barrier function to prevent inflammatory diseases caused by invading bacteria. These functions are likely to contribute to suppression of autoimmune lymphocytes and type 1 diabetes (T1D). Arrows indicate SCFA transport or interactive regulation.
Anti-inflammatory effects of DF and RS on inflammatory diseases were identified decades ago. SCFAs, fed generally via drinking water or as starch-conjugated forms, can suppress inflammatory diseases in the intestine, lungs, joints and central nervous system (CNS). SCFAs have profound effects on intestinal epithelial cells because they are absorbed and partially consumed by enterocytes as a major energy source. SCFAs strengthen epithelial barrier function by promoting epithelial growth and innate responses to damages, as well as invading microbes. SCFAs make macrophages and dendritic cells more tolerogenic and efficient in inducing regulatory T cells.5, 6 Induction of FoxP3+ T cells by C4 has been observed in many studies. Regardless of FoxP3+ T cell induction, all major SCFAs, such as C2, C3 and C4, induce IL-10 production in T cells.7 In addition, SCFAs promote the generation of significant effector T cell subsets such as Th1 and Th17 cells. SCFAs boost Th1 and Th17 responses during infection and inflammatory processes. Moreover, SCFAs boost antibody production by promoting B cell activation and differentiation into plasma B cells.8 Unlike retinoic acid, which selectively induces IgA production, SCFAs boost most types of antibody isotypes, including IgG and IgA.8 Therefore, SCFAs not only function as anti-inflammatory microbial metabolites but also serve as immune boosters to prepare tissue and immune cells to better eliminate pathogens.
Functions of SCFAs are mediated by several different mechanisms involving G-protein-coupled receptors (GPCR) receptors, histone deacetylase (HDAC) inhibition and metabolic integration. GPR43, GPR41, GPR109A and Olfr78 have been identified as SCFA receptors.9 GPR43 and GPR41 are activated by all three major SCFAs (C2, C3, and C4), whereas GPR109A is selected activated by C4 and niacin. Olfr78 is activated by C2 and C3. Expression of these SCFA receptors is restricted to specific cell types such as intestinal epithelial cells (GPR43 and GPR41), enteroendocrine cells (GPR43 and GPR41), adipocytes (GPR41), renal endothelial cells (Olfr78) and certain myeloid cells (GPR43 for neutrophils and GPR109A for certain dendritic cells and macrophages). Activation of the intestinal epithelial cell GPR43 by SCFAs promotes the production of immunity-related cytokines such as IL-1, IL-6, IL-12 and IL-18.10 Actually, inflammasome activation is supported by GPR43 in intestinal epithelial cells.11 In addition, GPR43 functions as a neutrophil chemotactic receptor, possibly mediating neutrophil chemotaxis to SCFA-rich environments such as the gut lamina propria and lumen. GPR109A activation in macrophages and dendritic cells make them efficient inducers of Tregs, suppressing experimental colitis and colon cancer.5 While exact roles and cell types are unclear, GPR41 and GPR43 are highly expressed in the pancreas.12, 13
SCFA functions are not limited to cells that express SCFA receptors. Independent of SCFA receptors, SCFAs are readily absorbed into cells through diffusion or specific carrier proteins such as SLC16a1 and SLC5a8.9 Intracellular SCFAs suppress class I and II HDACs. HDACs play important roles in regulating proteins, particularly histones. HDAC inhibition generally promotes gene expression through histone acetylation. Therefore, SCFAs are natural HDAC inhibitors facilitating gene expression in many cell types. In lymphocytes that lack SCFA receptors, the HDAC inhibition function of SCFAs is key in regulating T and B cells by modulating gene expression. Another important function, separate from the HDAC inhibition function of SCFAs, is metabolic integration in cells to produce energy (that is, ATP). SCFAs boost glycolysis and oxidative phosphorylation, which support actively dividing lymphocytes during immune responses. In addition, SCFAs boost fatty acid biogenesis, which is important for cell proliferation and differentiation.8
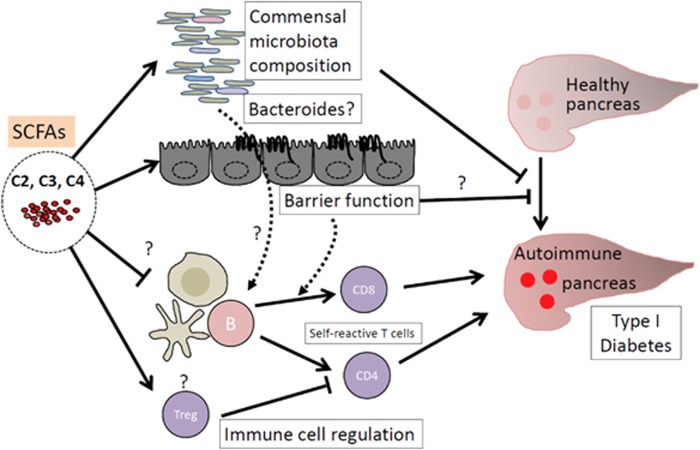
Work conducted by Marino et al. provides mechanistic insights into the protective effects of DF and SCFAs against T1D using non-obese diabetic (NOD) mice as an animal model.4 They observed increased SCFA levels in the peripheral blood in diabetes-free NOD MyD88−/− versus diabetes-prone NOD mice (Figure 2). Germ-free mice were more susceptible than conventional mice to develop T1D, indicating a positive role of microbiota in suppressing T1D. Moreover, C2-fed mice via drinking water suffered less from T1D. Compared with SCFAs administered via drinking water, SCFA (that is, C2)-conjugated starch better suppressed T1D. C2 decreased amounts of autoantigen (islet-specific glucose-6-phosphatase catalytic subunit-related protein)-specific CD8 T cells, which is associated with decreased B cell numbers and proliferation in lymphoid tissues. Because B cells could function as antigen-presenting cells to induce autoimmune T cells, the decrease in the number of B cells could partly explain the decreased number of autoimmune T cells. While C2 was more efficient than C4 in suppressing T1D, C4 was more efficient in inducing Tregs, perhaps due partly to their relatively higher HDAC inhibition activity. One interesting observation is that gut barrier function in NOD mice was improved by SCFAs as evidenced by increased expression of occludin, IL-22 and IL-21, as well as decreased bacterial lipopolysaccharide levels in the blood. This information implies the importance of gut health in suppressing T1D. DF and RS function as prebiotics to enrich certain commensal bacteria containing glycan foraging capacity.14 In addition, administration of SCFAs, versus prebiotics, can enrich characteristic commensal bacteria. Indeed, Marino et al. observed that C2 greatly expanded certain Bacteroides species. Fecal transfer of the expanded bacteria into NOD recipients suppressed T1D development, indicating that a change in bacterial composition is fundamental in SCFA-mediated protection from T1D (Figure 2).
Figure 2.
Suppression of T1D by SCFAs. Prebiotics, such as DF and resistant starch, enrich certain commensal microbiota (for example, unidentified Bacteroid species). Changes in commensal bacteria have suppressive effects on T1D; however, the mechanism remains unclear. SCFAs strengthen the gut epithelial barrier function promoting protective cytokine production and tight junction protein expression. This effect can indirectly suppress the onset of autoimmune disease responses by decreasing the threshold of the activation of antigen-presenting cells and T cells. Moreover, SCFAs can directly regulate immune cells, inducing tolerogenic macrophages and dendritic cells that preferentially generate Tregs. Tregs can suppress the generation of autoimmune T cells that migrate to the pancreas and induce tissue destruction. SCFAs additionally promote the generation of Th1 and Th17 cells, but this function is limited to sites of active immune responses rather than causing chronic inflammatory responses. Arrows denote action points and either an activation or suppression relationship between molecules and cells.
Marino et al. provides detailed information regarding the impact of SCFAs on the immune system and microbiota in NOD mice.4 However, many questions are unanswered. While SCFAs suppress T1D in NOD mice, can SCFAs also suppress diabetes in other animal models and humans? Marino et al.’s results indicate that gut microbiota change is a key mechanism for the observed protective effect of SCFAs. If microbiota change is the major protective mechanism, how can we explain the phenomenon that both DF and SCFAs suppress T1D while they enrich different groups of commensal bacteria14? Why is C2-feeding via drinking water less effective than the C2-conjugated starch diet? Is this because one is better than the other in increasing blood SCFA levels? Alternatively, it may be the starch component of the C2-conjugated starch that contributes to the luminal SCFA (beyond C2) levels because SCFA-conjugated starch can better withstand digestion in the upper alimentary tract and can more likely reach the colon compared with non-conjugated starch. A caveat of administering SCFAs via the oral route is unexpected inflammation in the renal system. We found that all major SCFAs administered at or higher than 150 mM can induce ureteritis and hydronephrosis.15 Marino et al. observed that C2 increased Treg numbers and decreased autoimmune T cell numbers and B cell proliferation. Is the effect on these lymphocytes the result of suppressed diabetes or an active cause for T1D suppression by SCFAs? The role of lymphocyte regulation by SCFAs in suppressing T1D needs further investigation. In addition, contribution of increased gut barrier function by SCFAs to the overall protective effect of gut metabolites is unclear. We additionally need to consider the possibility that the observed positive effect of SCFAs on gut epithelial barrier function is likely an event secondary to decreased autoimmune responses rather than a primary factor that prevents T1D. Therefore, researchers in the field should identify specific mechanisms of T1D suppression by SCFAs.
As we understand more concerning the beneficial functions of microbiota and SCFAs, it is necessary to understand how microbiota increase or decrease the risk of diabetes pathogenesis. Regarding the role of microbiota in regulating the immune system, refer to a recently published review article in this journal.16 The recently revealed functions of SCFAs in regulating the CNS, gut barrier function, and the immune system provide potentially important mechanisms for observed protective effects of DF and RS on diabetes pathogenesis. Marino et al.’s findings indicate that microbiota are altered by SCFAs to exert protective effects on T1D pathogenesis. This is what I like to call the ‘SCFA-microbiota conundrum’ in regulating T1D and other inflammatory diseases by DF and SCFAs. If microbiota change is the single most important factor in mediating the protective effect of SCFAs, it would be useful to isolate or selectively expand the species that exerts the effect. However, this can be a difficult task if it is a group of various species rather than a few isolatable species. Many SCFA effects on the immune system, such as inducing Tregs and suppressing antigen-presenting cells, could be indirect effects of suppressed autoimmune responses rather than a major mechanism for the protective effect. While SCFAs have various functions in preventing inflammatory diseases and regulating immune cells, we have yet to see if any of these effects on immune cells play a major role in preventing T1D and other diseases, which involves events beyond suppression of autoreactive T cells. Despite these questions, it seems apparent that it requires SCFA functions at many different levels to suppress T1D.
Acknowledgments
This study was supported partly from NIH grants (R01AI074745, R01DK076616, R01AI080769 and R01AI121302).
Footnotes
The author declares no conflict of interest.
References
- Gao HX, Regier EE, Close KL. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. J Diabetes 2016; 8: 8–9. [PubMed] [Google Scholar]
- Trowell HC. Dietary-fiber hypothesis of the etiology of diabetes mellitus. Diabetes 1975; 24: 762–765. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Bryant CA. Dietary fiber: diabetes and obesity. Am J Gastroenterol 1986; 81: 898–906. [PubMed] [Google Scholar]
- Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017; 18: 552–562. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014; 40: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 2014; 111: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015; 8: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016; 20: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 2014; 14: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013; 145: 396–406 e1-10. [DOI] [PubMed] [Google Scholar]
- Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015; 6: 6734. [DOI] [PubMed] [Google Scholar]
- Wang A, Gu Z, Heid B, Akers RM, Jiang H. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J Dairy Sci 2009; 92: 2696–2705. [DOI] [PubMed] [Google Scholar]
- Fu CY, Liu L, Gao Q, Sui XY, Li FC. Cloning, molecular characterization, and spatial and developmental expression analysis of GPR41 and GPR43 genes in New Zealand rabbits. Animal 2017, 1–9. [DOI] [PubMed]
- Louis P, Flint HJ, Michel C. How to manipulate the microbiota: prebiotics. Adv Exp Med Biol 2016; 902: 119–142. [DOI] [PubMed] [Google Scholar]
- Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J Immunol 2016; 196: 2388–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mu L. An expanding stage for commensal microbes in host immune regulation. Cell Mol Immunol 2017; 14: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]