Abstract
Objective
Daily stress processes have been previously linked to health-related outcomes, but implications for longevity remain unclear. The present study examined whether daily stress exposure and/or affective responses to daily stressors predicted mortality risk over a 20-year period. Based on the hypothesis that chronic illness confers vulnerability to deleterious effects of stress, we also examined whether its presence accentuated the association between daily stress processes and later mortality risk.
Methods
Participants were 1,346 middle-aged adults from the survey of Midlife Development in the United States who also completed the National Study of Daily Experiences. Participants reported on their experiences of stress and affect for eight consecutive evenings, and mortality data were collected over the next 20 years, using the National Death Index and other methods.
Results
There was a positive association between total number of stressors experienced across days and mortality risk. There was also a positive association between increases in negative affect on stressor days relative to non-stressor days and risk for mortality. The presence of a chronic illness moderated this association such that negative affective reactivity predicted mortality risk among individuals with at least one chronic illness, but not among otherwise healthy individuals. This association was independent of sociodemographic characteristics, typical levels of negative affect on non-stressor days, and total number of endorsed stressors.
Conclusion
These results suggest that greater increases in negative affect in response to stress in everyday life may have long-term consequences for longevity, particularly for individuals with chronic illness.
Keywords: daily experience, stress reactivity, negative affect, chronic disease, longevity
Acute stressful experiences, such as interpersonal conflict and work deadlines, are ubiquitous in everyday life. Research suggests that these everyday experiences with stress are consequential for physical health. For instance, individuals who report more stress in their daily lives endorse more somatic and infectious illness symptoms and have smaller antibody responses to ingested antigens (DeLongis, Folkman, & Lazarus, 1988; Stone et al., 1994; Stone, Reed, & Neale, 1987). More daily stress is also associated with alterations in biological processes thought to contribute to the development and worsening of diseases, including elevated blood pressure, stress hormones, and inflammatory biomarkers (Chiang, Eisenberger, Seeman, & Taylor, 2012; Stawski, Cichy, Piazza, & Almeida, 2013; Uchino, Berg, Smith, Pearce, & Skinner, 2006). Among individuals with existing chronic diseases, such as asthma, rheumatoid arthritis, irritable bowel syndrome, and diabetes, higher daily stress is associated with more severe illness-related symptoms (Halford, Cuddihy, & Mortimer, 1990; Levy, Cain, Jarrett, & Heitkemper, 1997; Stone, Broderick, Porter, & Kaell, 1997).
Of importance is that not all individuals confronting stress develop poor health, which has been attributed, in part, to variability in people’s affective responses to stress (Almeida, Piazza, Stawksi, & Klein, 2011; Lovallo & Gerin, 2003). Perceptions of threat elicit increases in negative affect and decreases in positive affect, which in turn, can modify patterns of cardiac, vascular, endocrine, metabolic, and immune functioning (Holmes, Krantz, Rogers, Gottdiener, & Contrada, 2006; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Lovallo & Gerin, 2003; Pressman & Cohen, 2005). Repeated and/or heightened activation of these systems over time may exact a toll, ultimately altering the function of tissues and organs in ways that contribute to morbidity and mortality (McEwen & Seeman, 1999). Thus, individuals who react strongly (i.e., greater increases in negative affect or decreases in positive affect) to stress repeatedly day after day may be more vulnerable to health problems associated with exposure to stress.
Indeed, greater stress-related increases in negative affect in daily life have been linked to smaller antibody responses to ingested antigens (Stone, Marco, Cruise, Cox, & Neale, 1996), higher cortisol levels (Jacobs et al., 2007), lower heart rate variability (Sin, Sloan, McKinley, & Almeida, 2016), and self-reports of mood problems and chronic illness (Charles, Piazza, Mogle, Sliwinski, & Almeida, 2013; Piazza, Charles, Sliwinski, Mogle, & Almeida, 2013). Moreover, larger reductions in positive affect in response to daily stress have been associated with higher levels of inflammatory biomarkers (Sin, Graham-Engeland, Ong, & Almeida, 2015), lower sleep efficiency and quality (Ong et al., 2013), and more depressive symptoms (O’Neill, Cohen, Tolpin, & Gunthert, 2004). Of particular interest, more positive affect reactivity has been linked to increased mortality risk in the VA Normative Aging Study (Mroczek et al., 2015). In this study of 181 men, larger decreases in positive affect on stressor days compared to non-stressor days were associated with greater risk for mortality 10 years later. This association was independent of men’s frequency of stress exposure and typical experiences of negative and positive affect in daily life. Interestingly, greater increases in negative affect in response to stress did not predict mortality risk.
To our knowledge, the study conducted by Mroczek et al. is the only study to date that has explored how daily stress and affective processes relate to longevity. Although provocative, findings from this initial study were based on a small, all-male sample, raising concerns regarding generalizability. Furthermore, while this study used daily reports of physical symptoms and bodily pain to adjust for pre-existing health problems, it did not explicitly consider the role of chronic diseases. The most prevalent chronic illnesses in America, like cardiovascular disease and various cancers, can alter people’s affective and biological responses to stress (e.g., Costanzo, Stawski, Ryff, Coe, & Almeida, 2012; Kop et al., 2008; Van Der Pompe, Antoni, & Heijnen, 1996), and forecast shorter lifespans even among those who are successfully treated (Hudson et al., 1998; Ronkainen et al., 2001). These observations raise the possibility that chronic diseases contributed to the mortality risks associated with greater positive affective reactivity (i.e., daily stress-related decreases in positive affect) in the Mroczek study. Alternatively, chronic disease may function as a moderator of this association, creating an underlying vulnerability that accentuates the mortality risks associated with stressor exposure and/or affective reactivity. Indeed, there is evidence to suggest that stress-evoked changes in the cardiovascular and inflammatory systems are magnified in patients with a history of coronary artery disease (Huikuri et al., 1994; Kop et al., 2008; Nijm, Kristenson, Olsson, & Jonasson, 2007).
With these issues in mind, the overarching goal of the present study was to clarify how daily stress processes relate to overall mortality patterns, using a national sample of midlife Americans who were followed over an average of 20 years in the study of Midlife Development in the United States (MIDUS). Specifically, we examined (1) whether daily stress exposure predicted mortality risk 20 years later; (2) whether negative affective responses to daily stress predicted mortality risk; and (3) whether chronic illness operated as a confounder and/or moderator of these associations. We focused primarily on negative affective responses because the study’s assessment of positive affective responses to daily stress was quite limited in scope. Nevertheless, we use the available data to evaluate parallel hypotheses for positive affect, keeping in mind that these findings are subject to interpretive limitations.
Methods
Participants & Procedures
Data for the present study came from the first waves of MIDUS and the National Study of Daily Experiences (NSDE). MIDUS is a national survey study investigating the development of health and well-being from midlife to older adulthood. A national sample of adults was recruited via random-digit dialing and completed telephone interviews and self-administered survey measures. The NSDE is one of the in-depth projects within MIDUS that examines daily stress processes. Each night for eight consecutive evenings, participants were interviewed via telephone about stressful events they encountered, and their activities, behaviors, and emotions in the last 24 hours.
Participants from MIDUS I (1995–1996) were 7,108 non-institutionalized, English-speaking adults ages 25 to 74 years. Of these, a random subsample of 1,843 was selected to participate in NSDE I (1996–1997). The majority of selected participants (n = 1,499) agreed to participate—8% declined participation and 11% were difficult to contact. Mortality data were obtained through October 2015. Fifty-nine individuals were excluded from analyses due to missing information on demographic variables and chronic conditions collected in MIDUS I. An additional 94 individuals were excluded because the computation of affective reactivity requires having both stressor and non-stressor days and these participants reported experiencing stress either every day or none of the days. Thus, the final analytic sample was 1,346. All study procedures were approved by the institutional review boards at University of Wisconsin and Harvard Medical School.
Compared to the broader MIDUS I sample, participants in the analytic sample had slightly higher educational attainment (t(7093) = −3.08, p = .002, d = −.09) and were more likely to be female (χ2 = 24.31, p < .001). They did not differ in age (t(7047)= 1.57, p = .12, d = .05), race (t(6174) = .84, p = .40, d = .03), or total number of chronic illnesses (t(7105) = −1.41, p = .16, d = −.04). Participants in the present study were marginally more likely to be of decedent status relative to those in the original MIDUS I cohort (χ2 = 3.58; p = .06). Among participants who died, those in the present study had longer survival times (t(6313)= −3.83; p < .001, d = −.12).
Measures
Daily stress exposure
The Daily Inventory of Stressful Events (Almeida, Wethington, & Kessler, 2002) was used to assess daily stressful experiences. Participants reported on whether they experienced seven different stressors in the past 24 hours. Stressors included had an argument, avoided an argument, had a stressor at work or school, had a stressor at home, faced discrimination, had a network stressor, or experienced any other stressor. Stress exposure was operationalized in two ways. First, the total number of stressors reported across the 8-day period was summed across all days to index cumulative stressor exposure. Second, for each day of reporting, a dichotomous variable was computed such that participants who reported no stressors were assigned a value of zero, whereas those who endorsed any of the stressors were assigned a value of one. This variable indexed stressor versus non-stressor days. Recoded scores were then averaged across the 8-day period to index the proportion of stressor days. Both cumulative stressor exposure and proportion of stressor days were used in analyses focusing on mortality risk associated with daily stress exposure.
Affective reactivity to stress
Scales developed for NSDE were used to assess affect each day on a 5-point scale (0 = none of the time 4 = all of the time). Negative affect items included: so sad nothing could cheer you up, restless or fidgety, nervous, worthless, everything was an effort, hopeless, angry or irritable. Items were averaged to compute a summary negative affect score for each day. Cronbach’s α ranged from .69 to .76 for during the 8-day study period. Assessment of positive affect in NSDE was limited and included only a single item: in good spirits.
In line with previous research (Mroczek et al., 2015; Piazza et al., 2013; Sin et al., 2015), affect reactivity was operationalized as the difference in affect levels on stressor days compared to non-stressor days. Multi-level modeling was used to estimate reactivity coefficients for each individual:
Level 1 (day level):
Level 2 (person level):
These models used the computed dichotomous variable representing exposure to any one of the stressors on a given day (stressor vs. non-stressor day). Accordingly, at the day level, the intercept β0i represents levels of negative or positive affect on days when no stress was experienced. The slope β1i reflects the link between stress and affect at the day level; or more technically, the difference in affect between days when a stressor was and was not endorsed. The residual parameter eij indexes the day-to-day variability in affect for each individual. The person level of the model includes parameters representing the sample’s average levels of affect (γ00) and of affective reactivity (γ10) across the 8-day period. It also contains the variance parameters u0i and u1i, reflecting the extent of each individual’s deviation from these sample-wide averages. Affective reactivity for each participant was indexed by summing his/her estimated u1i value and the sample fixed effect for affective reactivity (γ10). Separate negative and positive reactivity scores were estimated; they were scored such that higher values signify greater increases in negative affect, and smaller decreases in positive affect, on stressor days compared to non-stressor days.
Chronic conditions
In MIDUS I, participants reported whether they had experienced any of 26 chronic and acute physical health conditions in the past year. They also indicated whether they had ever been diagnosed with heart disease or cancer. For the purposes of this paper, we restricted analyses to chronic life-threatening health problems, and where patients could be expected to provide accurate self-reports of its presence. These conditions included HIV/AIDS, cancer, heart disease (stroke, heart attack, valve disease, hole in heart, blocked artery, heart failure), diabetes or high blood sugar, neurological disorders, and arthritis or bone disease. To reflect total disease burden, the number of chronic conditions was summed for each individual.
Mortality
Mortality data were gathered using several methods, namely National Death Index reports, tracing that included mortality closeout interviews, and longitudinal sample maintenance. Survival times for decedents were computed as the interval from the date of the MIDUS 1 interview to the date of death. Because only month and year of death were documented in order to protect confidentiality, the day for all deaths was set to the 15th day of each month. Survival times for participants who were still living reflected the length of follow-up censored at October 31, 2015.
Covariates
Demographic data on age, gender, race, and education level were assessed during the parent study MIDUS I and included as covariates. Participants reported their gender (0=male, 1=female) and their date of birth from which age was computed. Education was coded as less than high school, high school diploma, some college, or four-year college degree or higher. Race was coded as European-American, African-American, or other due to small numbers of other racial minorities. From this variable, two dummy variables were created with European-Americans as the reference group.
Daily stress exposure and affect on non-stressor days were also included as covariates in some analyses to ensure that any observed associations were not due to greater stress exposure and to discern between the effects of affective reactivity to stress and typical experiences of affect. In these analyses, the total number of stressors experienced across the 8-day study period was used to index cumulative stress exposure, and affect on non-stressor days computed as the average level of negative and positive affect across all non-stressor days was used to index typical levels of affect.
Analyses
Primary analyses consisted of estimating a series of Cox proportional hazard models in Stata 14 to test whether daily stress exposure and negative affective reactivity to daily stress predicted mortality risk. Unadjusted models were examined in the first step. Demographic covariates (i.e., age, gender, race, and educational attainment) and main effects of daily stress exposure or negative affective reactivity were entered in the second step. To probe the role of chronic conditions, total number of chronic conditions was added as a covariate in the third step, followed by the interaction between stress exposure or negative affective reactivity and number of chronic conditions in the final step. In subsequent models, we stratified the sample by presence of at least one chronic condition. We estimated models in each group and included additional covariates, namely negative affect on non-stressor days and total number of stressors. Separate models were estimated for each daily stress exposure variable (i.e., total number of stressors and proportion of stressor days) and negative affective reactivity. In exploratory analyses, parallel models were tested to examine whether results extended to positive affective reactivity.
Results
Sample characteristics from NSDE are presented in Table 1. At baseline, participants were approximately 46 years old and primarily from European-American backgrounds. The gender distribution was relatively balanced. Thirty percent of the sample reported having at least one chronic condition with arthritis or bone disease being the most common condition reported. Participants reported an average of nearly four stressors across the 8-day period and experienced at least one stressor on 41% of days. Of the 1,346 participants included in the present study, 210 (15.6%) died over the roughly 20-year follow-up period. The deceased subgroup lived an average survival time of nearly 12 years from the initial MIDUS I assessment (range: 1.47 – 20.13 years).
Table 1.
Participant Characteristics and Descriptive Data of Study Variables
| n (%) | Mean (SD) | |
|---|---|---|
| Age | 45.88 (12.74) | |
| Gender | ||
| Female | 733 (54.5) | |
| Male | 613 (45.5) | |
| Race | ||
| European-American | 1,221 (90.7) | |
| African-American | 81 (6.0) | |
| Other race | 44 (3.3) | |
| Education | ||
| < High school | 110 (8.2) | |
| High school | 365 (27.1) | |
| Some college | 441 (32.8) | |
| ≥ College degree | 430 (32.0) | |
| Number of Chronic Conditions | ||
| One | 327 (24.3) | |
| Two | 60 (4.5) | |
| ≥ Three | 16 (1.2) | |
| Type of Chronic Condition | ||
| HIV/AIDS | 2 (0.001) | |
| Diabetes/High blood sugar | 57 (3.9) | |
| Neurological disorder | 22 (1.6) | |
| Arthritis/Bone disease | 264 (19.6) | |
| Heart disease | 69 (5.1) | |
| Cancer | 90 (6.7) | |
| Daily Experience | ||
| Cumulative number of stressors across days | 3.85 (3.07) | |
| Proportion of stressor days | .41 (.25) | |
| Negative affective reactivity | .18 (.16) | |
| Positive affective reactivity | −.28 (.07) | |
| Negative affect on non-stressor days | 1.13 (.22) | |
| Positive affect on non-stressor days | 4.23 (.64) | |
| Mortality | ||
| Deceased | 210 (15.6) |
Bivariate correlations among study variables are displayed in Table 2. Several demographic variables were associated with the study’s primary variables of interest. Specifically, younger age, female gender, and higher educational attainment were associated with more daily stress exposure, both in terms of cumulative number of stressors across days and proportion of stressor days. Younger age was also associated with greater negative affective reactivity to stress, greater negative affect and lower positive affect on non-stressor days, fewer chronic illnesses, and lower mortality. Females tended to exhibit more positive affect reactivity, and as educational attainment increased, negative and positive affect reactivity, negative affect on non-stressor days, and mortality risk decreased.
Table 2.
Bivariate correlations among primary study variables.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | .00 | −.09*** | −.04 | .36*** | −.13*** | −.18*** | −.11*** | .04 | −.09*** | .15*** | .47*** | |
| 2. Gender | .00 | −.12*** | .02 | .10** | .09** | .04 | −.05* | .02 | −.05† | −.05† | ||
| 3. Race | −.02 | −.03 | −.02 | .00 | .02 | .00 | .04 | −.04 | −.06* | |||
| 4. Education | −.08** | .18*** | .16*** | −.20*** | .09*** | −.13*** | .03 | −.08** | ||||
| 5. Number of chronic illnesses | −.02 | −.02 | .07** | −.04 | .06* | .00 | .31*** | |||||
| 6. Cumulative number of stressors across days | .80*** | .16*** | −.09*** | .11*** | −.12*** | −.04 | ||||||
| 7. Proportion of stressor days | .17*** | −.08* | .12*** | −.13*** | −.10*** | |||||||
| 8. Negative affective reactivity | −.42*** | −.67** | .43*** | −.01 | ||||||||
| 9. Positive affective reactivity | −.09** | .01 | −.04 | |||||||||
| 10. Negative affect on non-stressor days | −.56*** | −.03 | ||||||||||
| 11. Positive affect on non-stressor days | .06* | |||||||||||
| 12. Deceased |
Note.
p = .08
p < .05,
p < .01,
p < .01.
Daily experience variables were associated with one another in the expected directions. For instance, cumulative stress across days was positively correlated with proportion of stressor relative to non-stressor days. Both of these stress exposure variables were correlated positively with negative affect reactivity and negative affect on non-stressor days, and negatively with positive affect reactivity (i.e., greater decreases in positive affect) and positive affect on non-stressor days. More negative affect reactivity and negative affect on non-stressor days were both associated with chronic illness burden, which in turn was associated with higher mortality risk.
Daily stress exposure
Analyses first focused on the link between cumulative stress exposure and longevity. In an unadjusted model, the total number of stressors was unrelated to risk for mortality (HR: .96; 95% CI: .92–1.01; p = .10). However, when demographic variables were added as covariates, greater number of stressors experienced in everyday life was associated with greater mortality risk (HR: 1.05; 95% CI: 1.00–1.10; p = .04). When the presence of chronic conditions was added to the model, the magnitude and significance of the stress coefficient did not change appreciably (HR: 1.05; 95% CI: 1.00–1.10; p = .05), suggesting that chronic disease is unlikely to have a mediating role. Likewise, the non-significant interaction term indicated that chronic conditions did not moderate the association between total stress exposure and later mortality risk (HR: .99; 95% CI: .94–1.04; p = .62).
We then examined whether the proportion of days participants endorsed at least one stressor was associated with mortality risk. In the unadjusted model, there was a positive association between proportion of stressor days and mortality risk (HR: .36; 95% CI: .20–.63; p < .001). However, adding demographic characteristics to the model attenuated this association to non-significance, (HR: 1.32; 95% CI: .73–2.40; p = .36), and the same was true when chronic conditions were incorporated (HR: 1.29; 95% CI: .71–2.35; p = .40). There was also no evidence to suggest that the stress-mortality association was moderated by chronic illness (HR: .96; 95% CI: .51–1.80; p = .90).
Negative affective reactivity
Next, we examined whether negative affective reactivity to daily stressors predicted mortality risk. As shown in Table 3 (column 1), there was no significant main effect of daily negative affective reactivity to stress in predicting mortality in the unadjusted model (p = .65). However, this association became significant when adjusting for demographic characteristics (p = .03; column 2) and marginally significant when adjusting for chronic conditions (p = .08; column 3). Notably, this main effect was qualified by a significant interaction between negative affective reactivity and chronic disease burden (p = .01; column 4). To probe the interaction, we stratified participants into those without any chronic condition (n = 943) and those with at least one chronic condition (n = 403). As depicted in Figure 1, the relation between negative affective reactivity to daily stress and mortality risk was significant among individuals with at least one chronic condition, independent of demographic characteristics (HR: 2.33; 95% CI: 1.14–4.74; p = .02). This association remained significant even when controlling for negative affect on non-stressor days and cumulative stressor exposure (HR: 6.50; 95% CI: 1.66–25.50; p = .01), indicating it was not simply a reflection of individual proneness to experiencing stress and affect. By contrast, there was no relation among healthy individuals free of chronic diseases with all covariates in the model (HR: .62; 95% CI: .10–3.95; p = .61).
Table 3.
Results of models predicting mortality risk from negative affective reactivity, chronic conditions, and their interaction.
| Step 1 | Step 2 | Step 3 | Step 4 | |
|---|---|---|---|---|
| Predictors | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| NA reactivity | 1.20 (0.55 – 2.62) | 2.16 (1.08 – 4.32)* | 1.90 (.93 – 3.85)† | 0.60 (0.17 – 2.08) |
| Age | 1.11 (1.1 – 1.12)*** | 1.10 (1.09 – 1.12)*** | 1.10 (1.09 – 1.12)*** | |
| Gender | 0.77 (0.59 – 1.02)† | 0.80 (0.60 – 1.05) | 0.81 (0.62 – 1.07) | |
| African American | 1.41 (0.78 – 2.54) | 1.43 (0.79 – 2.58) | 1.45 (0.80 – 2.61) | |
| Other race | 0.21 (0.03 – 1.53) | 0.20 (0.03 – 1.56) | 0.21 (0.03 – 1.51) | |
| Education | 0.89 (.78 – 1.03) | 0.90 (0.78 – 1.03) | 0.91 (0.79 – 1.05) | |
| Chronic conditions | 1.46 (1.25 – 1.71)*** | 1.22 (0.99 – 1.51)† | ||
| NA reactivity × Chronic conditions | 2.71 (1.28 – 5.77)** |
Note.
p = .07–.08
p < .05,
p ≤ .01,
p < .001.
Race was dummy-coded with European-Americans as the reference group
Figure 1.
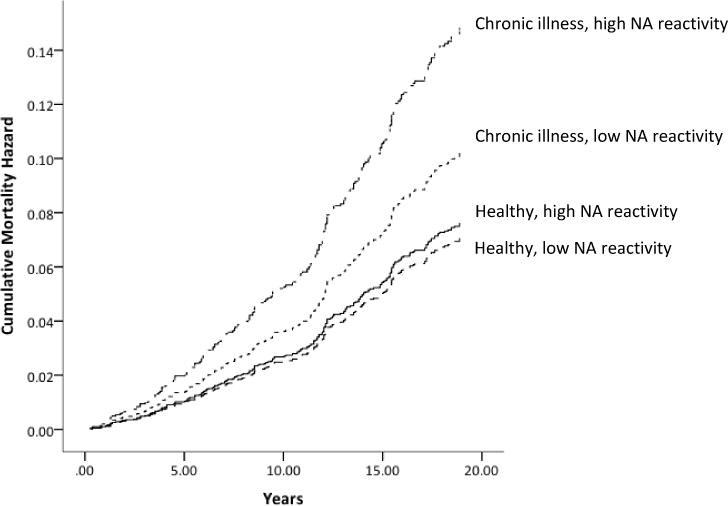
Plot of cumulative mortality hazard by years since MIDUS I study entry for individuals with and without chronic illness and high and low levels of negative affective reactivity.
Positive affective reactivity
Parallel analyses were conducted to explore whether positive affective reactivity to daily stress predicted mortality risk. In univariate analyses, positive affective reactivity was not associated with longevity (p = .11; Table 4, column 1). By contrast, positive reactivity to daily stress predicted reduced mortality risk when demographic variables were included in the model (p = .03; column 2). This association weakened somewhat when chronic conditions were added to the model (p = .05; column 3). The interaction between positive affective reactivity and chronic conditions was not significant (p = .25; column 4). Nevertheless, for exploratory purposes we stratified the sample and estimated models separately in healthy and ill persons. Positive affect reactivity to daily stress was marginally associated with mortality risk only among persons with at least one chronic conditions (HR: .10; 95% CI: .01–1.11; p = .06). This marginal association remained even when adjusting for total stress exposure and typical levels of positive affect on non-stressor days (HR: .11; 95% CI: .01–1.25; p = .08). By comparison, mortality risk did not vary as a function of positive affect reactivity independent of all covariates in otherwise healthy individuals (HR: .38; 95% CI: .01–10.46; p = .57).
Table 4.
Results of models predicting mortality risk from positive affective reactivity, chronic conditions, and their interaction.
| Step 1 | Step 2 | Step 3 | Step 4 | |
|---|---|---|---|---|
| Predictors | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| PA reactivity | 0.20 (.03 – 1.40) | 0.11 (.02 – .84)* | 0.14 (.02 – 1.03)† | 0.50 (.03 – 9.96) |
| Age | 1.11 (1.10 – 1.12)*** | 1.10 (1.08 – 1.11)*** | 1.10 (1.09 – 1.11)*** | |
| Gender | 0.76 (0.57 – 1.00)* | 0.78 (0.59 – 1.03)† | 0.79 (0.60 – 1.04) | |
| African American | 1.38 (0.76 – 2.49) | 1.41 (0.78 – 2.54) | 1.41 (0.79 – 2.55) | |
| Other race | 0.24 (0.03 – 1.69) | 0.22 (0.03 – 1.60) | 0.23 (.03 – 1.65) | |
| Education | 0.89 (0.78 – 1.03) | 0.90 (0.78 – 1.03) | 0.90 (0.78 – 1.03) | |
| Chronic conditions | 1.46 (1.25 – 1.71)*** | 0.93 (0.43 – 2.04) | ||
| PA reactivity × Chronic conditions | 0.20 (.01 – 3.07) |
Note.
p = .05–.08
p < .05,
p < .001.
Race was dummy-coded with European-Americans as the reference group
Discussion
High levels of stress and negative affect have been linked to mortality risk (Chida & Steptoe, 2008; Kiecolt-Glaser et al., 2002; Krantz & McCeney, 2002; Schulz & Beach, 1999), but the vast majority of these studies have relied on one-time assessments of these constructs. As a consequence, we know a good deal about how global experiences of stress and affect relate to mortality, but comparatively little about the implications of day-to-day experiences in naturalistic settings. The purpose of the present investigation was to ascertain whether daily stressors and negative affective responses to them predict longevity and to elucidate the role of chronic conditions in these associations. Proportion of stressor days did not predict mortality risk. Rather, greater cumulative stressor exposure was associated with greater mortality risk. Exhibiting larger increases in negative affect on stressor days was also associated with greater mortality risk, specifically among individuals with a history of chronic illness. Notably, this association was independent of typical levels of negative affect and cumulative number of stressors endorsed, suggesting that the connection between negative affective reactivity and mortality risk may not simply be due to trait-level negative affect and stress exposure. Results from exploratory analyses revealed a similar pattern for positive affective reactivity (i.e., larger reductions in positive affect on stressor days).
The current study is one of the first to examine how exposure to naturalistic stressors and affective reactions to them in daily life relate to longevity. Only one other study that we are aware of has examined this question and found that decreases in positive affect in response to daily stress predicted longevity in a relatively small sample of adult men (Mroczek et al., 2015). Using a larger, national sample of adults, we also observed this link in the present study, although this finding remains preliminary given the limited measurement of positive affect. More importantly, the present study extended this pattern to negative affective reactivity. As noted below, it is not clear if these findings reflect a causal relationship, but if they do, it becomes important to develop plausible and testable hypotheses about how daily negative affective reactivity to stress might come to have consequences for longevity. One hypothesis is that more pronounced stress-related increases in negative affect on a day-to-day basis disrupt restorative processes like sleep, or perturb cardiac, hormonal, or immune activity, with downstream implications for morbidity and mortality (Kubzansky & Kawachi, 2000; Pressman & Cohen, 2005). Supporting this notion, experimental and diary studies have linked negative affect to alterations in cardiovascular, autonomic, endocrine, and inflammatory activity (Carroll et al., 2011; Het, Schoofs, Rohleder, & Wolf, 2012; Kibler & Ma, 2004; Sin et al., 2015; Sin et al., 2016). In turn, these alterations in biological functioning have been linked to a variety of adverse health outcomes (e.g., Ershler & Keller, 2000; Treiber et al., 2003). Daily negative affective reactivity may also influence mortality risk through disruptions in sleeping patterns. Poor sleep is known to increase risk for numerous diseases (Luyster, Strollo Jr, Zee, & Walsh, 2012), and greater negative affect in daily life has been linked to sleep problems, such as prolonged sleep latency, lower sleep quality, and shorter sleep duration (Kouros & El‐Sheikh, 2015; Tavernier, Choo, Grant, & Adam, 2016).
Building on previous research (Mroczek et al., 2015), we also considered the role of chronic conditions. There was little evidence to suggest that chronic illness operated as a confounder; the association between affective reactivity and mortality risk was only slightly attenuated when disease burden was entered into models. Rather, the results suggested a moderating effect of chronic illness. Specifically, the connection between negative affective reactivity and mortality risk were evident only among individuals with one or more chronic conditions, but not among those without any chronic illness.
Why might the mortality risks of negative affective responses to stress only be evident among those with a chronic condition? One possibility is that disease itself (or the pathogenic processes that give rise to it) may compromise homeostatic mechanisms and interfere with mounting adaptive biological responses to stress-related affect. For instance, in the context of heart disease, cardiac remodeling accompanied by a shift from parasympathetic to sympathetic dominance often follows myocardial infarction (Mostarda et al., 2014; Smith, Kukielka, & Billman, 2005). This autonomic imbalance increases risk for future cardiac events, such as plaque rupture, thrombosis, and re-infarction, by promoting endothelial shear stress, platelet activation, inflammation, and ischemia (Dakak, Quyyumi, Eisenhofer, Goldstein, & Cannon, 1995; Dutta et al., 2012; Hering, Lachowska, & Schlaich, 2015; Manuck, Olsson, Hjemdahl, & Rehnqvist, 1992; Strike et al., 2004; Wallen, Held, Rehnqvist, & Hjemdahl, 1997). Given sympathetic dominance, emotional distress related to stress may result in exaggerated and/or prolonged sympathetic activity, accelerating progression of cardiac pathophysiology and further potentiating risk for death (Dünser & Hasibeder, 2009; Hering et al., 2015). There is also evidence that after myocardial infarction, sympathetic activity triggers release of myeloid progenitor cells from the bone marrow. These cells migrate into atherosclerotic plaques, increasing their propensity to degrade and rupture (Dutta et al., 2012). If affective reactivity boosts sympathetic drive on the bone marrow, it could amplify this process or increase its frequency, and in the process, worsen underlying atherosclerosis.
Exposure to stressors, in terms of total number of stressors experienced, but not proportion of stressor days, was also related to mortality risk. This finding seems to converge with the body of work on stress and mortality. However, when the total number of stressors and affective reactivity were included in the same model, only affective reactivity significantly associated with mortality risk. Other diary studies focusing on long-term health outcomes have similarly found that affective reactivity, but not exposure to stress, predicts health and mortality a decade later (Mroczek et al., 2015; Piazza et al., 2013). Our results extend this work in a large sample followed over 20 years, and reinforce the emerging consensus that affective responses to everyday stressors may be more consequential for health than stressor exposure itself (Mroczek et al., 2015; Piazza et al., 2013; Sin et al., 2015).
Caution in interpreting results is warranted in light of several limitations of the present study. First, the correlational design precludes inferences about causality, and alternative explanations, such as genetics and early experiences that co-vary with affective reactivity and/or mortality risk, are plausible. Second, we were unable to examine the effects of negative affective reactivity within specific categories of disease. This was due to the low numbers of deaths within specific conditions (e.g., 69 reported having heart disease, of whom 38 died), which would result in model overfitting. Examination of whether the present findings differ across patient groups will become more feasible as mortality increases. Third, the NSDE only included a single item to assess positive affect each day. As such, findings for positive affective reactivity remain preliminary at best, and the unique effects of negative and positive affective reactivity were not examined. Further investigation using a more comprehensive measure of positive affect, such as that in MIDUS II, are needed to confirm the link between positive affective reactivity and mortality risk and to probe the independent effects of negative and positive affective reactivity. Fourth, duration of chronic illness and specific causes of mortality were not included in MIDUS, and it will be important for future studies to elucidate whether daily affective reactivity to stress exacerbates illness to shorten the lifespan among chronically ill individuals, as proposed above. Fifth, chronic illnesses were based on self-reports, which may not be as accurate as official documentation such as hospital records. Lastly, ethnic diversity in the MIDUS study was limited, with the overwhelming majority of the sample being from European-American backgrounds. It remains to be seen whether current findings extend to other ethnic minority groups.
Our findings suggest that how people respond to the stressors they confront in their everyday lives may have long-term consequences for physical health particularly among those with a chronic illness. That is, heightened negative affect in the face of stress may further shorten the lifespans of those already afflicted with a chronic condition. For these individuals, affective responses to stressors in day to day living may be as important as global experiences of chronic or severe stress and affect for longevity. Interventions focused on building coping and emotion regulation strategies that can be practically implemented in everyday life among clinical populations may help prolong the lives of those at greatest risk for premature mortality.
Acknowledgments
The present study was supported by the National Institute on Aging (P01-AG020166; R01-AG018436) and the National Institute of Heart, Lung, and Blood (R01-HL122328; F32-HL134276).
Footnotes
Conflicts of interest: none
References
- Almeida DM, Piazza JR, Stawksi RS, Klein LC. The speedometer of life: Stress, health and aging. In: Scaie KW, Levey R, editors. The Handbook of Psychology of Aging. New York: Elsevier; 2011. pp. 191–216. [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: An interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity. 2011;25(2):232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Piazza JR, Mogle J, Sliwinski MJ, Almeida DM. The wear and tear of daily stressors on mental health. Psychological Science. 2013;24(5):733–741. doi: 10.1177/0956797612462222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proceedings of the National Academy of Sciences. 2012;109(6):1878–1882. doi: 10.1073/pnas.1120972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70(7):741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Stawski RS, Ryff CD, Coe CL, Almeida DM. Cancer survivors’ responses to daily stressors: implications for quality of life. Health Psychology. 2012;31(3):360–370. doi: 10.1037/a0027018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. The American Journal of Cardiology. 1995;76(3):125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. Journal of Personality and Social Psychology. 1988;54(3):486. doi: 10.1037//0022-3514.54.3.486. [DOI] [PubMed] [Google Scholar]
- Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. Journal of Intensive Care Medicine. 2009;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Heidt T. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51(1):245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Halford WK, Cuddihy S, Mortimer RH. Psychological stress and blood glucose regulation in Type I diabetic patients. Health Psychology. 1990;9(5):516–528. doi: 10.1037//0278-6133.9.5.516. [DOI] [PubMed] [Google Scholar]
- Hering D, Lachowska K, Schlaich M. Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Current Hypertension Reports. 2015;17(80):1–9. doi: 10.1007/s11906-015-0594-5. [DOI] [PubMed] [Google Scholar]
- Het S, Schoofs D, Rohleder N, Wolf OT. Stress-induced cortisol level elevations are associated with reduced negative affect after stress: indications for a mood-buffering cortisol effect. Psychosomatic Medicine. 2012;74(1):23–32. doi: 10.1097/PSY.0b013e31823a4a25. [DOI] [PubMed] [Google Scholar]
- Holmes SD, Krantz DS, Rogers H, Gottdiener J, Contrada RJ. Mental stress and coronary artery disease: a multidisciplinary guide. Progress in Cardiovascular Diseases. 2006;49(2):106–122. doi: 10.1016/j.pcad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Poquette CA, Lee J, Greenwald CA, Shah A, Luo X, Crist WM. Increased mortality after successful treatment for Hodgkin’s disease. Journal of Clinical Oncology. 1998;16(11):3592–3600. doi: 10.1200/JCO.1998.16.11.3592. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Niemelä MJ, Ojala S, Rantala A, Ikäheimo MJ, Airaksinen KE. Circadian rhythms of frequency domain measures of heart rate variability in healthy subjects and patients with coronary artery disease. Effects of arousal and upright posture. Circulation. 1994;90(1):121–126. doi: 10.1161/01.cir.90.1.121. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, Van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology. 2007;74(1):60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Ma M. Depressive symptoms and cardiovascular reactivity to laboratory behavioral stress. International journal of behavioral medicine. 2004;11(2):81–87. doi: 10.1207/s15327558ijbm1102_3. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annual Reviews in Psychology. 2002;53(1):83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. The American Journal of Cardiology. 2008;101(6):767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kouros CD, El‐Sheikh M. Daily mood and sleep: Reciprocal relations and links with adjustment problems. Journal of Sleep Research. 2015;24(1):24–31. doi: 10.1111/jsr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Annual Review in Psychology. 2002;53(1):341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I. Going to the heart of the matter: do negative emotions cause coronary heart disease? Journal of Psychosomatic Research. 2000;48(4):323–337. doi: 10.1016/s0022-3999(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. Journal of Behavioral Medicine. 1997;20(2):177–193. doi: 10.1023/a:1025582728271. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65(1):36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35(6):727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Olsson G, Hjemdahl P, Rehnqvist N. Does cardiovascular reactivity to mental stress have prognostic value in postinfarction patients? A pilot study. Psychosomatic Medicine. 1992;54(1):102–108. doi: 10.1097/00006842-199201000-00003. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Mostarda C, Rodrigues B, Medeiros A, Moreira ED, Moraes-Silva IC, Brum PC, Irigoyen MC. Baroreflex deficiency induces additional impairment of vagal tone, diastolic function and calcium handling proteins after myocardial infarction. American Journal of Translational Research. 2014;6(3):320. [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Stawski RS, Turiano NA, Chan W, Almeida DM, Neupert SD, Spiro A. Emotional reactivity and mortality: Longitudinal findings from the VA Normative Aging Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015 doi: 10.1093/geronb/gbt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. Journal of Internal Medicine. 2007;262(3):375–384. doi: 10.1111/j.1365-2796.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- O’Neill SC, Cohen LH, Tolpin LH, Gunthert KC. Affective reactivity to daily interpersonal stressors as a prospective predictor of depressive symptoms. Journal of Social and Clinical Psychology. 2004;23(2):172–194. [Google Scholar]
- Ong AD, Exner-Cortens D, Riffin C, Steptoe A, Zautra AJ, Almeida DM. Linking stable and dynamic features of positive affect to sleep. Annals of Behavioral Medicine. 2013;46(1):52–61. doi: 10.1007/s12160-013-9484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Annals of Behavioral Medicine. 2013;45(1):110–120. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Ronkainen A, Niskanen M, Rinne J, Koivisto T, Hernesniemi J, Vapalahti M. Evidence for excess long-term mortality after treated subarachnoid hemorrhage. Stroke. 2001;32(12):2850–2853. doi: 10.1161/hs1201.099711. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychology. 2015;34(12):1154–1165. doi: 10.1037/hea0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Sloan RP, McKinley PS, Almeida DM. Linking daily stress processes and laboratory-based heart rate variability in a national sample of midlife and older adults. Psychosomatic Medicine. 2016;78(5):573–582. doi: 10.1097/PSY.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(4):H1763–H1769. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38(11):2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis & Rheumatology. 1997;10(3):185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- Stone AA, Marco CA, Cruise CE, Cox DS, Neale JM. Are stress-induced immunological changes mediated by mood? A closer look at how both desirable and undesirable daily events influence sIgA antibody. International Journal of Behavioral Medicine. 1996;3(1):1–13. doi: 10.1207/s15327558ijbm0301_1. [DOI] [PubMed] [Google Scholar]
- Stone AA, Neale JM, Cox DS, Napoli A, Valdimarsdottir H, Kennedy-Moore E. Daily events are associated with a secretory immune response to an oral antigen in men. Health Psychology. 1994;13(5):440–446. doi: 10.1037//0278-6133.13.5.440. [DOI] [PubMed] [Google Scholar]
- Stone AA, Reed BR, Neale JM. Changes in daily event frequency precede episodes of physical symptoms. Journal of Human Stress. 1987;13(2):70–74. doi: 10.1080/0097840X.1987.9936797. [DOI] [PubMed] [Google Scholar]
- Strike PC, Magid K, Brydon L, Edwards S, McEwan JR, Steptoe A. Exaggerated platelet and hemodynamic reactivity to mental stress in men with coronary artery disease. Psychosomatic Medicine. 2004;66(4):492–500. doi: 10.1097/01.psy.0000130492.03488.e7. [DOI] [PubMed] [Google Scholar]
- Tavernier R, Choo SB, Grant K, Adam EK. Daily affective experiences predict objective sleep outcomes among adolescents. Journal of Sleep Research. 2016;25(1):62–69. doi: 10.1111/jsr.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber FA, Kamarck TW, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65(1):46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Berg CA, Smith TW, Pearce G, Skinner M. Age-related differences in ambulatory blood pressure during daily stress: evidence for greater blood pressure reactivity with age. Psychology and Aging. 2006;21(2):231–239. doi: 10.1037/0882-7974.21.2.231. [DOI] [PubMed] [Google Scholar]
- Van Der Pompe G, Antoni MH, Heijnen CJ. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology. 1996;21(4):361–374. doi: 10.1016/0306-4530(96)00009-1. [DOI] [PubMed] [Google Scholar]
- Wallen NH, Held C, Rehnqvist N, Hjemdahl P. Effects of mental and physical stress on platelet function in patients with stable angina pectoris and healthy controls. European Heart Journal. 1997;18(5):807–815. doi: 10.1093/oxfordjournals.eurheartj.a015346. [DOI] [PubMed] [Google Scholar]


