Abstract
Distal metastasis of luminal breast cancer is frequent and incurable, yet the metastasis mechanisms are poorly understood. Estrogen, even at postmenopausal concentrations, suppresses invasiveness of luminal breast cancer cells through the estrogen receptor (ER). Invasive tumors overexpress the short progesterone receptor A (PR-A) isoform. Even at postmenopausal concentrations, progesterone activates PR-A, inducing invasiveness by counteracting estrogen's effects, particularly when cells are hypersensitized to progesterone by PR-A overexpression. To interrogate the role of this cross-talk in metastasis, we investigated selective cross-talk mechanisms of PR-A with ER. We developed a quantitative PCR-based lymph node infiltration assay to address the slowness of metastasis of tumor xenografts. We found that 15 microRNAs (miRNAs) are regulated by progesterone via PR-A, but not the longer PR-B isoform, with increased progesterone sensitivity when PR-A was overexpressed. Two of these miRNAs whose induction (miR-92a-3p) or repression (miR-26b-5p) by estrogen was suppressed by progesterone plus PR-A were critical for the PR-A–ER cross-talk causing a gene-regulatory pattern of invasiveness and metastasis and complete rescue of invasiveness in vitro. Constitutive expression of miR-92a-3p or inhibition of miR-26b-5p profoundly suppressed metastasis. Finally, in primary breast tumors, PR-A expression was correlated negatively with miR-92a-3p expression and positively with miR-26b-5p expression. Therefore, hormonal cross-talk of PR-A with ER is probably a fundamental mechanism that enables metastasis of luminal breast cancer. Moreover, miRNA biomarkers of hyperactive PR-A may help predict metastatic potential of luminal breast tumors. Further, miR-92a-3p and miR-26b-5p may reveal target pathways for selective intervention to suppress hormone-regulated metastasis, both pre- and postmenopause.
Keywords: cell invasion, estrogen, estrogen receptor, microRNA (miRNA), progesterone, tumor metastasis, luminal breast cancer, progesterone receptor
Introduction
Breast oncogenesis may span up to several decades. Most newly diagnosed breast cancer cases (>78%) occur in women older than 50 years (1), with a median age at diagnosis of 61 years (2). Most breast tumors express the nuclear receptors for estrogen (ER)2 and progesterone (PR) (3) even through progression (4). Primary ER+ tumors are highly responsive to anti-estrogen therapy. However, ER+ breast cancer is often metastatic at the time of diagnosis, and metastatic ER+ tumors also frequently appear after many years of dormancy (5, 6). Indeed, over one-fifth of breast cancer patients harbor distally metastasized ER+ tumors (7). Unfortunately, the metastatic disease is generally incurable, and even targeted therapies are generally only palliative. Therefore, it is necessary to understand more about deregulated molecular mechanisms that confer invasive/metastatic properties on ER+ breast cancer cells. However, in contrast to more aggressive cancers, studies of metastasis of luminal breast cancer are rather sparse, due in part to the inherently slow metastatic spread of the tumors in animal models (8). This has probably lead to the use of models of metastasis of ER+ breast tumors in which the tumor cells are directly injected into the circulation, bypassing events in the initiation of metastasis at the primary tumor site (9). Therefore, it is also desirable to design an experimental strategy that can functionally link novel physiological mechanisms governing invasiveness of ER+ breast cancer cells to their ability to leave the primary tumor site.
Estrogen supports the growth of ER+ breast tumors, but it suppresses invasiveness of the tumor cells whether or not their growth is hormone-sensitive and also suppresses breast tumor progression (10). High-dose and potent synthetic forms of progestins directly support invasiveness and metastasis in ER+/PR+ breast cancer cells, demonstrated using in vivo experimental models (8). These models may be physiologically relevant in postmenopausal women on high-dose hormone replacement therapy, where the combination of estrogen and progestin has been associated with increased incidence of invasive breast cancer and breast cancer mortality compared with non-users (11); in contrast, estrogen monotherapy in women with prior hysterectomy has been associated with a persistent decrease in the onset of invasive breast cancer (12). However, in postmenopausal women who are not undergoing hormone replacement, the role of the endogenous hormones in the progression of ER+/PR+ breast tumors has not been adequately studied. Compared with the knowledge on the influence of estrogen on breast tumor physiology, much less is known about the mechanisms of progesterone action, particularly in the presence of active estrogen signaling. Moreover, although the levels of estrogen and progesterone change throughout the menstrual cycle and decrease after menopause, very little is known about the hormone actions on tumor invasiveness/progression in the context of this changing hormone status during a woman's lifetime. Our recent findings have addressed these questions by identifying a fundamental role for cross-talk between ER and PR in governing invasiveness of a variety of model luminal breast cancer cell lines in the entire range (pre- and postmenopausal) of physiological levels of estrogen and progesterone (13).
PR has two isoforms, A and B, expressed by alternative promoter usage from a single gene; PR-B is identical to PR-A except for the presence of an additional 164-amino acid amino-terminal segment that contains within it an additional activation function, AF3 (13). PR-B and PR-A induce both distinctive and overlapping patterns of agonist-induced gene activation or gene repression, depending on the variable contexts of the target promoters and the nature of the associated chromatin sites of PR binding (13–15). The heterodimer of PR-A and PR-B regulates a smaller and unique set of genes compared with the homodimers (14). Clinical studies have shown that although in normal breast PR-A and PR-B are expressed at comparable levels, this balance is commonly altered during breast oncogenesis, with an increase in PR-A in early as well as progressed lesions (16). Overexpression of PR-A is associated with increased invasiveness of clinical tumor lesions and a lower rate of disease-free survival (17).
In vitro studies in the literature originally suggested that PR-B is the principal mediator of progesterone-induced invasiveness of breast cancer cells (18, 19), at odds with the clinical observations noted above that implicate PR-A in tumor progression. However, the in vitro studies of PR-B were performed at high (luteal stage and pregnancy-associated) concentrations of progesterone and also were conducted in the absence of estrogen signaling (18–22). The plasma estrogen range in pre-menopausal women is 1.4–1.6 nm during the follicular phase and 3.6–4.2 nm during the luteal phase (23). Plasma progesterone ranges from <4 nm during follicular phase up to >50 nm during the luteal phase (24). Postmenopause, there is a marked decrease in circulating hormone levels, with median values of 0.14 nm for estrogen and 0.13 nm for progesterone, yet the breast tissue may retain up to about a 1 nm concentration of each hormone (25, 26). We have recently reported studies that were performed in the entire range of estrogen and progesterone concentrations corresponding to pre- and postmenopausal hormone status and in the presence of both estrogen and progesterone signaling (13). As the previous in vitro studies of high-dose progesterone effects on metastasis were conducted in the absence of estrogen signaling, we considered the possibility that modulation of estrogen action may comprise a distinct aspect of the regulation of invasiveness by progestins in the range of its physiological levels (13). Estrogen strongly suppressed invasiveness of ER+ breast cancer cells at concentrations below 0.01 nm.
At low (<1 nm) concentrations, progesterone/progestins completely abrogated the inhibition of invasiveness by estrogen. It was only in a higher (5–50 nm) concentration range that progestins progressively induced invasiveness in the absence of estrogen. The ability of progestins to rescue invasiveness from estrogen regulation was exclusively mediated by PR-A and was uninfluenced by PR-B. On the other hand, PR-B mediated the estrogen-independent component of progestin-induced invasiveness at either pharmacological (used in hormone replacement) progestin levels or progesterone levels associated with pregnancy. Overexpression of PR-A in PR-A+/PR-B+ cells lowered the progestin concentration needed to completely rescue invasiveness (to <0.2 nm). The studies demonstrate that progesterone influences breast cancer cell invasiveness by rescuing it from estrogen regulation, exclusively via PR-A, in the entire pre- and postmenopausal range of estrogen and progesterone concentrations (13). These findings reconcile in vitro actions of PR isoforms with the clinically observed association between PR-A and progression of luminal breast cancer.
Although progesterone, acting through PR-A, appears to be the major culprit in promoting invasiveness of luminal breast cancer cells by counteracting estrogen, directly testing the effect of this mechanism on metastasis in vivo by manipulating hormone levels is not possible because estrogen depletion would prevent tumor formation. Instead, if a critical pathway of hormonal cross-talk between PR-A and ER that regulates in vitro invasiveness could be identified, it should be possible to test the effect of disrupting this pathway on metastasis using an appropriate in vivo model. Additionally, such a cross-talk pathway(s) may reveal better therapeutic targets, as clinical interventions that broadly or systemically obstruct progesterone/PR signaling are precluded by the need for progesterone for endometrial homeostasis (27, 28) and off-target effects of progesterone antagonists (29). In luminal breast cancer cells, ER strongly regulates tumor cell characteristics by regulating micro-RNAs (miRNAs) (30–33) and is itself also regulated by miRNAs (30, 33, 34). There is less information on the regulation of PR by miRNAs or on regulation of miRNAs by progesterone/PR. Indeed, miRNAs regulate up to one-third of the human genome and have diverse roles in normal physiology as well as profound roles as tumor suppressors and oncogenes (35). This study was undertaken to explore the possibility that regulation of certain miRNAs could be critical for cross-talk between progesterone/PR-A and estrogen/ER in the specific context of hormonal control of invasion in vitro and to manipulate such a cross-talk pathway(s) as a means of establishing the role of hormonal cross-talk of PR-A with ER in metastasis of luminal breast cancer.
Results
Identification of microRNAs that are uniquely regulated by low-dose progesterone through PR-A
Progesterone bound to PR-A could impinge on regulation of invasiveness by E2/ER in a manner that is mediated by one or more miRNAs in the following ways (depicted as schematics in Fig. 1, A and B). First, progesterone/PR-A could either activate or repress miRNA(s) (independent of estrogen), and this would then result in rescue of invasiveness from estrogen repression (Fig. 1A). On the other hand, E2/ER could either activate or repress miRNA(s), resulting in inhibition of invasiveness; in this case, progesterone/PR-A could suppress regulation of the miRNAs by E2, resulting in rescue of invasiveness from estrogen repression (Fig. 1B). To identify these putative miRNAs, we used the following cell line models: T47D-A (ER+/PR-A+/PR-B-null), T47D-B (ER+/PR-A-null/PR-B+), parental T47D (ER+/PR-A+/PR-B+), BT474 (ER+/PR-A+/PR-B+), and ZR-75–1 (ER+/PR-A+/PR-B+).
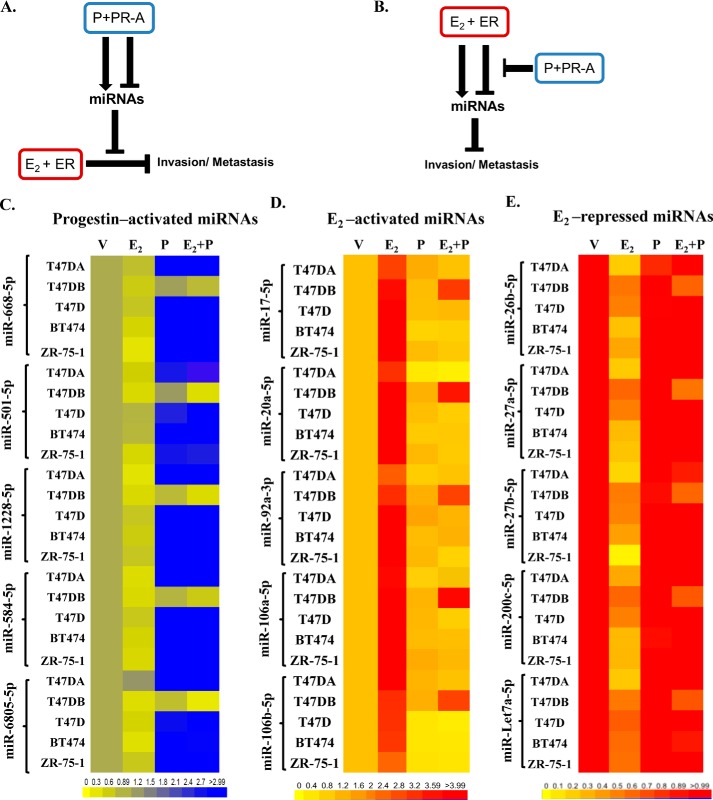
Figure 1.
Possible pathways and candidate miRNAs that could be involved in the hormone-dependent cross-talk of PR-A with ER that regulates invasiveness in breast cancer cells. A and B, schematic representations of possible pathways by which miRNAs could mediate the cross-talk of progestin (P)-bound PR-A with E2-bound ER to influence invasiveness and metastasis of luminal breast cancer cells. C–E, hormone-depleted T47D-A, T47D-B, T47D, BT474, and ZR-75–1 cells at 30% confluence were treated with vehicle, 1 nm E2, 1 nm R5020 (P), or 1 nm E2 plus 1 nm R5020 (E2 + P) for 48 h. Total RNA was then extracted, and the relative levels of each miRNA indicated were quantified in all of the samples. The miRNA profiling data are represented in the heat map in C for miRNAs activated by progestin plus PR-A (even in the absence of E2), in D for E2-activated miRNAs that were counterregulated by progestin plus PR-A, and in E for E2-repressed miRNAs that were counterregulated by progestin plus PR-A. The data represent results from experimental triplicates.
To identify candidate miRNAs in the putative pathways illustrated in Fig. 1A, we first undertook Affymetrix miRNA profiling to screen for miRNAs activated or repressed by PR-A but not PR-B at a low dose (1 nm) of R5020. This was accomplished by using Affymetrix miRNA profiling of miRNA changes in hormone-depleted T47D-A versus T47D-B cells treated with R5020 followed by data validation by quantitative RT-PCR. We found five miRNAs that were activated by low-dose progestin and PR-A (i.e. in T47D-A cells) but not by PR-B (i.e. in T47D-B cells). They are miR-6805-5p, miR-584-5p, miR-1228-5p, miR-501-5p, and miR-668-5p. These five miRNAs were also strongly up-regulated in other PR-A–positive cell lines, including parental T47D cells, BT474 cells, and ZR-75-1 cells (Fig. 1C; values in Table S1). The same Affymetrix miRNA analysis did not reveal any miRNAs that were repressed via PR-A alone.
In contrast to progestin-regulated miRNAs, there are considerable literature data on miRNAs regulated by E2 acting through ER (Table S2). All of these miRNAs that were either activated or repressed by E2 were tested by real-time RT-PCR analysis to identify miRNAs whose regulation by E2 was prevented by R5020 in T47D-A cells (i.e. via PR-A) but not in T47D-B cells (i.e. via PR-B). In this manner, 10 miRNAs were identified; five among them (miR-17-5p, miR-20a-5p, miR-92a-3p, miR-106a-5p, and miR-106b-5p) were activated by E2, and five (miR-26b-5p, miR-27a-5p, miR-27b-5p, miR-200c-5p, and Let7a-5p) were repressed by E2 in the absence of progestin/PR-A. The counterregulation of these E2-regulated miRNAs by progestin/PR-A was also confirmed in the other PR-A–positive cell lines, including parental T47D cells, BT474 cells, and ZR-75–1 cells (Fig. 1, D and E; values in Tables S3 and S4).
Finally, to ensure that higher doses of progestin did not enable PR-B to mimic regulation of the 15 miRNAs by PR-A, T47D-B cells were treated with vehicle, 1 nm E2, and 1 nm E2 plus R5020 (1–50 nm). There was no progestin dose–dependent miRNA activation or opposition to E2 regulation by PR-B (Fig. S1).
Regulation of microRNAs by PR-A is sensitized to lower doses of progestin by overexpression of PR-A to a level observed in tumors
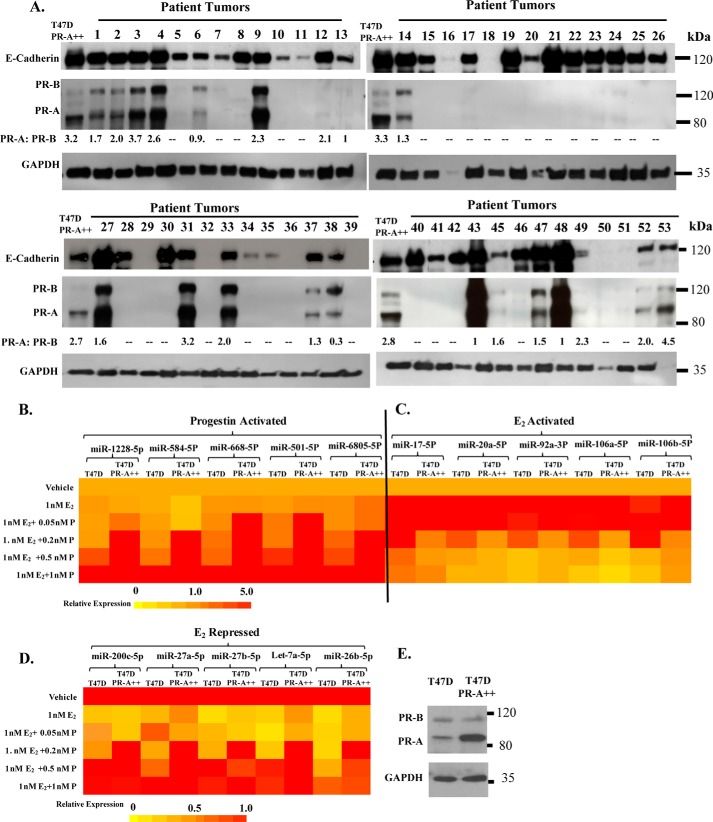
We have previously reported that progestin-induced rescue of invasiveness from estrogen repression is dependent exclusively on PR-A and is unaffected by co-expression of PR-B. We have also reported that, compared with T47D cells, in the isogenic recombinant T47D-PR-A++ cells that stably express a 3.6-fold higher level of PR-A (Fig. 2E and Fig. S2), lower doses of progesterone can rescue invasiveness from estrogen repression (13). To ensure the clinical relevance of the PR-A expression level in the T47D-PR-A++ model, we examined primary luminal breast tumor specimens by Western blotting. As the pathologists' definition of hormone receptor positivity of breast tumors may include tumors in which as few as 10% and often even 1% of the tumor cells show receptor expression, and as the distribution of the receptor-positive cells within a tumor could be heterogeneous, we tested lysates from specimens obtained from 53 patient tumors to ensure an adequate number of samples in which we could detect PR expression by Western blotting. As the tumors could also have variable ratios of epithelial to stromal tissue, we also probed the blots for the epithelial marker E-cadherin. Clearly, the tumor specimens frequently showed levels of PR-A expression that were comparable with or higher than that in T47D-PR-A++ cells (Fig. 2A), and the expression of PR-A was also frequently higher than that of PR-B in the tumors (Fig. 2A). These data confirm the clinical relevance of the isogenic T47DA++ cell model.
Figure 2.
PR overexpression in primary luminal breast tumor specimens and the effect of PR-A overexpression on hormone sensitivity of miRNA regulation. Whole-cell lysates from primary luminal breast tumors from 53 patients together with whole-cell lysates from T47D PR-A++ cells were analyzed on Western blots that were probed for PR, E-cadherin, and GAPDH. Ratios of PR-A to PR-B are indicated in each lane (A). Hormone-depleted T47D parental cells and T47D PR-A++ cells at 30% confluence were treated with vehicle, 1 nm E2, or 1 nm E2 plus a range (0.05–1 nm) of concentrations of R5020 (P) for 48 h. Total RNA was then extracted, and the relative levels of each miRNA indicated were quantified in all of the samples. The miRNA profiling data are represented in the heat map in B for miRNAs activated by progestin plus PR-A (even in the absence of E2), in C for E2-activated miRNAs that were counterregulated by progestin plus PR-A, and in D for E2-repressed miRNAs that were counterregulated by progestin plus PR-A. The data represent results from experimental triplicates. E, whole-cell lysates from T47D cells and T47D PR-A++ cells were probed by Western blotting for PR and GAPDH.
In T47DA++ cells, every one of the above 15 miRNAs was optimally regulated at or below 0.2 nm R5020, whereas the parental T47D cells required 0.5–1 nm R5020 for the same level of regulation (increase or decrease in the absence or presence of 1 nm E2) (Fig. 2, B–D; values in Table S5). This level of hypersensitization to progestin of miRNA regulation in T47D-PR-A++ cells corresponds to the previously reported progestin dose dependence of invasiveness of T47D-PR-A++ cells versus parental T47D cells (13).
miR-92a-3p and miR-26b-5p are functionally linked to hormonal control of invasiveness
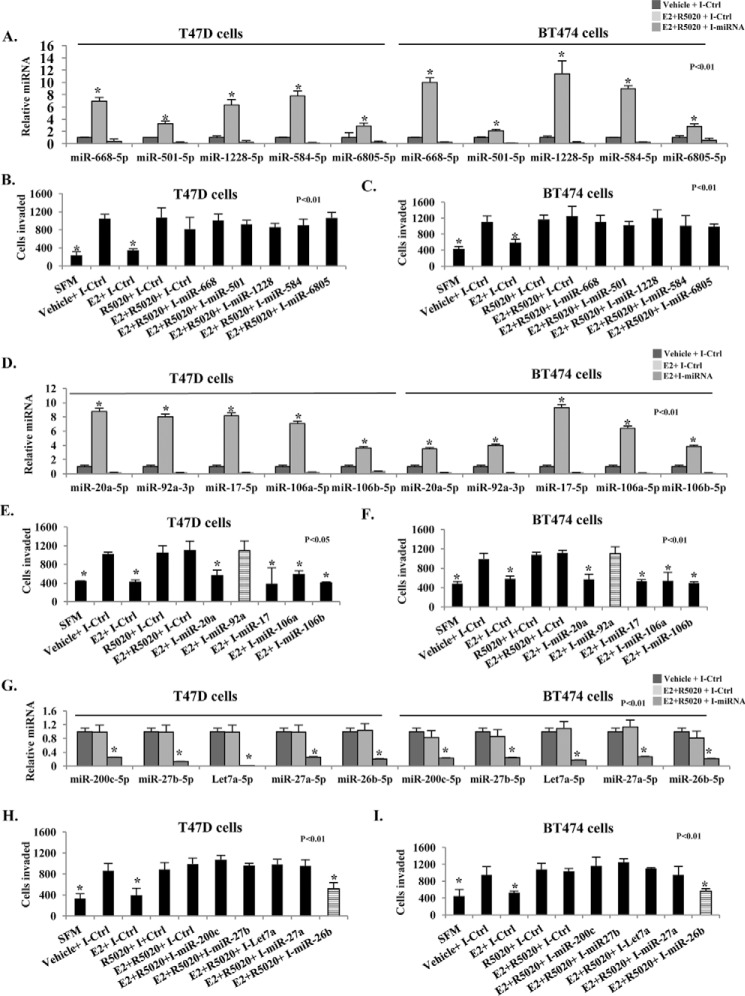
We tested the ability of E2 to repress invasiveness or of R5020 to rescue invasiveness following inhibition of each of the above 15 miRNAs in T47D and BT474 cells. Inhibition was confirmed by reduction in target miRNA 72 h post-transfection, although residual miRNA-inhibitor duplexes may also be detected.
We first inhibited each of the progestin/PR-A–activated (but not E2-regulated) miRNAs: miR-6805–5p, miR-584–5p, miR-1228-5p, miR-501-5p, and miR-668-5p (Fig. 3A). Invasiveness was unaffected in all cases in the presence of E2 and R5020 (Fig. 3, B and C), indicating that these miRNAs do not have a role in hormonal cross-talk between progestin/PR-A and E2/ER that influences invasiveness.
Figure 3.
Effect of inhibiting miRNAs hormonally regulated by PR-A and ER on invasiveness of luminal breast cancer cells. Hormone-depleted T47D and BT474 cells were transfected with either control inhibitor (I-Ctrl) or the indicated miRNA-specific inhibitor. The inhibitors are indicated in the figure by using the prefix I for the corresponding target miRNA. Twenty-four hours later, cells were treated for 48 h with vehicle, E2 (1 nm), or E2 (1 nm) + R5020 (1 nm), as indicated. Total RNA was then extracted, and the relative levels of each miRNA indicated were quantified in all of the samples. Histograms show the miRNA expression data for progestin-activated miRNAs (A), E2-activated miRNAs (D), and E2-repressed miRNAs (G). In parallel, the treated cells were subjected to a trans-well Matrigel invasion assay (B, C, E, F, H, and I). In the negative controls, serum-free medium (SFM) was used instead of the FBS chemoattractant. Values are represented as the average number of cells invaded from triplicate treatment sets, and the error bars represent S.D. One-way analysis of variance with post hoc unpaired t test was performed on triplicate treatment sets, and p values are indicated.
Next, we inhibited the five miRNAs whose activation by E2 was blocked by progestin (miR-17-5p, miR-20a-5p, miR-92a-3p, miR-106a-5p, and miR-106b-5p) (Fig. 3D). Inhibition of miR-92a-3p alone abrogated the ability of E2 to repress invasiveness in both T47D and BT474 cells (Fig. 3, E and F). Therefore, the ability of progestin/PR-A to block activation of miR-92a-3p by E2 must be critical for the functional cross-talk of progesterone via PR-A.
Finally, we inhibited the five miRNAs whose repression by E2 was blocked by progestin (miR-26b-5p, miR-27a-5p, miR-27b-5p, miR-200c-5p, and Let7a-5p) (Fig. 3G). Inhibition of miR-26b-5p alone abrogated the ability of progestin to rescue invasiveness from E2 repression in both T47D and BT474 cells (Fig. 3, H and I). Therefore, the ability of progestin/PR-A to block repression of miR-26b-5p by E2 must also be a critical mechanism of cross-talk of progesterone via PR-A in the rescue of invasiveness.
The above results implicate miR-92a-3p and miR-26b-5p as targets in the cross-talk of progestin/PR-A with E2/ER that mediate rescue of invasiveness in luminal breast cancer cells. The regulation of these two microRNAs by E2 was inhibited by the nuclear estrogen antagonist, tamoxifen (Fig. S3), consistent with genomic regulatory mechanisms.
miR-92a-3p and miR-26b-5p regulate genes associated with invasiveness and metastasis in luminal breast cancer cells
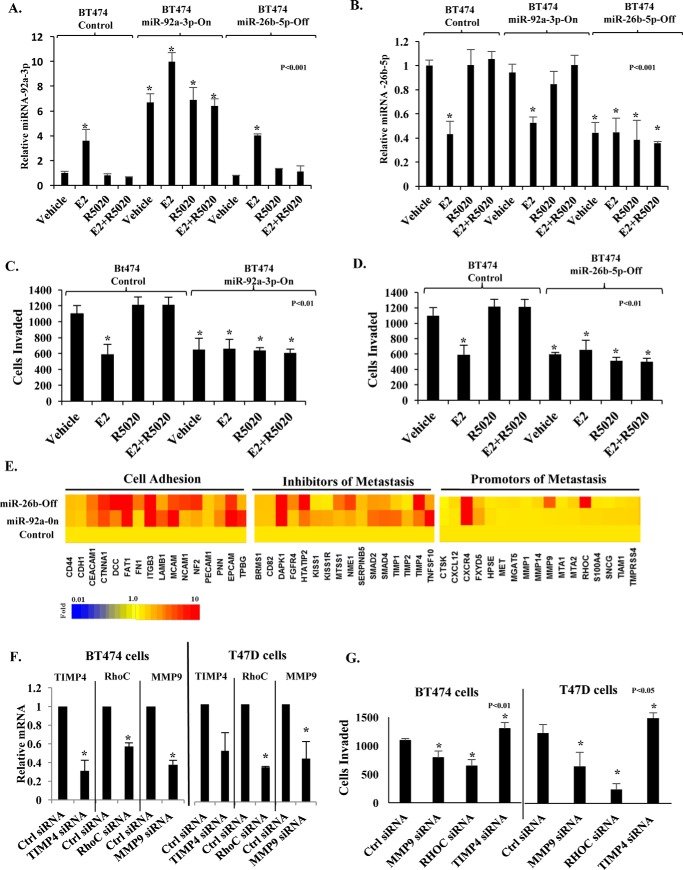
To further study the roles of miR-92a-3p and miR-26b-5p in relation to gene expression and metastasis, we generated two types of pooled (to avoid clonal bias) stable recombinant BT474 cells. In one case, miR-92a-3p (E2-activated miRNA) was constitutively expressed (miR-92a-3p-On cells in Fig. 4A). In the other case, miR-26b-5p (E2-repressed miRNA) was constitutively inhibited due to expression of a miR-26b-5p inhibitor (miR-26b-5p-Off cells in Fig. 4B). Hormonal regulation of miR-26b-5p and miR-92a-3p was unaffected in miR-92a-3p-On cells and miR-26b-5p-Off cells, respectively, indicating that these two miRNAs are regulated independently (Fig. 4, A and B). Both the miR-92a-3p-On cells (Fig. 4C) and the miR-26b-5p-Off cells (Fig. 4D) showed repressed invasiveness even in the absence of hormones, similar to the E2-treated control cells (harboring Lenti-miR-blank plasmid). Moreover, the ability of R5020 to rescue invasiveness was lost in both the miR-92a-3p-On cells (Fig. 4C) and in the miR-26b-5p-Off cells (Fig. 4D), in contrast to the control cells. The miR-92a-3p-On cells and the miR-26b-5p-Off cells showed in vitro proliferation rates similar to that of the control cells (Fig. S4).
Figure 4.
Roles of miR-92a-3p and miR-26b-5p in regulation of invasiveness and metastasis genes. Control BT474 cells (harboring Lenti-miR-Blank plasmid) and isogenic recombinant cells constitutively expressing miR-92a-3p mimic (BT474 miR-92a-3p-On cells) or stably expressing miR-26b-5p inhibitor (BT474 miR-26b-5p-Off cells) were hormone-depleted and treated with vehicle or 1 nm E2, 1 nm R5020, or a combination of E2 and R5020 for 48 h. Total RNA was then isolated, and miR-92a-3p (A) and miR-26b-5p (B) were quantified. In parallel, the trans-well invasion assay was performed on the treated BT474 miR-92a-On cells (C) and BT474 miR-26b-Off cells (D) together with the parental control cells. In E, total RNA was isolated from BT474 control, miR-92a-On, and miR-26b-Off cells treated with 1 nm E2 in combination with 1 nm R5020 for 48 h and analyzed using the Taqman metastasis transcriptome array. The transcriptome expression profile is shown in the heat map. Three genes from the transcriptome array (RhoC, MMP9, and TIMP4) were individually knocked down by transfection with siRNA together with control cells transfected with non-targeted siRNA in both BT474 and T47D cells (F and G). In all cases, there was a decrease in the targeted mRNA (F). The cellular invasiveness of the transfected cells in A was measured by the Matrigel invasion assay (G). *, p <0.05. Error bars, S.D.
Next, the miR-92a-3p-On and miR-26b-5p-Off cells, treated with both E2 and R5020, were examined for quantitative changes in the metastasis/invasion transcriptome, using a commercial pathway cDNA TaqMan PCR array. When compared with the control cells, both of the recombinant cells showed broad and partially overlapping increases in the expression of cellular adhesion molecules and inhibitors of metastasis but not a remarkable effect on the expression of genes known to promote metastasis (Fig. 4E; values in Table S6). The functionality of genes from the metastasis gene array was validated by individually knocking down three of the genes (RhoC, TIMP4, and MMP9), in BT474 cells as well as T47D cells. Both RhoC and MMP9 are known to be promoters of metastasis, and knocking down either one of these genes decreased invasive potential (Fig. 4, F and G). In contrast, TIMP4 is a known inhibitor of metastasis, and knocking down this gene increased invasiveness (Fig. 4, F and G). It may be noted, however, that our results show that the broader effects of regulating miR-92a-3p and miR-26b-5p on the transcriptome have the net effect of strongly regulating the invasive and metastatic phenotype. Therefore, miR-92a-3p and miR-26b-5p are part of independently regulated but convergent pathways through which E2 controls genes that have functional roles in metastasis. It follows that the ability of PR-A to oppose regulation of these miRNAs by E2 enables progesterone to induce a gene-regulatory pattern that supports metastasis.
An additional relevant question was whether estrogen and/or progesterone could at least partially regulate epithelial versus mesenchymal characteristics of luminal breast cancer cells, although it has been well-established that ER expression confers the epithelial phenotype. Using a commercial EMT panel antibody kit and BT474, T47D, and ZR-75-1 cell lines, we observed no change in established epithelial/mesenchymal markers when cells were treated individually or in combination with E2 and R5020 (Fig. S5, A and B) in contrast to depletion of the ER apoprotein (Fig. S5C). Therefore, it was irrelevant to explore EMT in the context of hormonal regulation of miR-92a-3p or miR-26b-5p.
miR-92a-3p and miR-26b-5p profoundly impact metastasis in vivo
Studies of hormonal regulation of metastasis of model luminal breast tumor xenografts in mice are technically challenging for the following reasons. First, estrogen needs to always be present for the tumors to grow, optimally administered to the mice through implanted slow-release, low-dose E2 pellets; therefore, the hormone levels cannot be modulated. Second, the constant exposure to even the low dose of E2 needed to support tumor growth causes urinary retention and cystitis in the mice, limiting the duration of the experiments to about 2–3 weeks. Finally, luminal breast tumors are inherently less aggressive and metastasize relatively slowly, so that it is not possible to histologically detect micrometastases within the 2–3-week duration of the experiments. We overcame these problems in the current study design that investigated whether modulation of miRNAs 92a-3p and 26b-5p could govern metastasis in mice bearing luminal breast tumor xenografts.
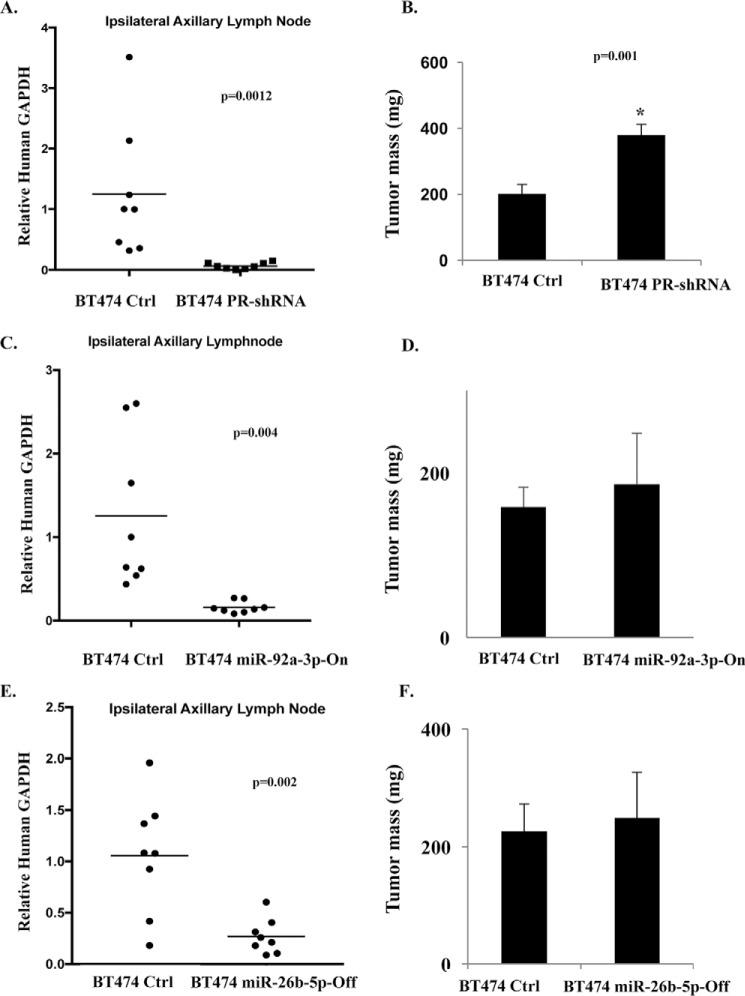
We used as xenograft models the recombinant BT474 cells in which cross-talk between E2/ER and progestin/PR-A affecting invasiveness was disrupted by constitutive expression of miR-92a-3p (miR-92a-3p-On cells discussed above) or constitutive inhibition of miR-26b-5p (miR-26b-5p-Off cells discussed above) for comparison with the control cells. As a metastasis assay validation tool, we also generated and used as xenografts pooled (to avoid clonal bias) stable recombinant BT474 cells in which PR was knocked down (BT474 PR-shRNA cells); in these cells, we confirmed that the PR knockdown resulted in loss of the ability of R5020 to rescue in vitro invasiveness from E2 regulation (Fig. S6). The previously established range of plasma estrogen in mice implanted with the slow-release E2 pellets is 1.8–4.8 nm (37, 38). The established range of plasma progesterone in mice on a commercial diet is 3.2–5.6 nm (38). To measure metastasis of subcutaneous implantation of the xenografts in the right flank, we measured tumor cell infiltration in the ipsilateral axillary lymph nodes by the sensitive assay of measuring mRNA for human GAPDH present in the lymph nodes, 14 days after implanting the xenografts. The control BT474 cells consistently infiltrated the lymph node, whereas depletion of PR in these cells showed virtually complete suppression of lymph node infiltration, validating the metastasis assay (Fig. 5, A and B). In mice bearing xenografts of miR-92a-3p-On cells (Fig. 5, C and D) or miR-26b-5p-Off cells (Fig. 5, E and F), metastasis was dramatically suppressed, demonstrating that the profound effects of changing the levels of these two miRNAs on cellular invasiveness observed above extend to their effects on metastasis in vivo.
Figure 5.
Roles of miR-92a-3p and miR-26b-5p in regulating metastasis in an in vivo lymph node infiltration model. BT474 control cells (A–F), BT474 PR-shRNA cells (A and B), BT474 miR-92a-3p-On cells (C and D), and BT474-miR-26b-5p-Off (E and F) were implanted subcutaneously in the right flank to form tumor xenografts in athymic female nude mice (8 mice/group) that had been implanted with slow-release, low-dose estradiol pellets. Mice were sacrificed at 2 weeks, and the proximal ipsilateral lymph node (right axillary) was harvested. Total RNA was extracted from lymph nodes, and high-efficiency species-specific Taqman probes for human and mouse GAPDH were used to measure relative degrees of lymph node infiltration by the human tumor cells. Using the Ct values for human GAPDH as a target and mouse GAPDH as an endogenous reference, the human cell infiltration into mouse lymph node was calculated by the ΔΔCt method with a calibrator ΔCt of 10.79 in all cases (A, C, and E). Tumor mass was measured on the day of sacrifice (B, D, and F). Statistical analysis using Student's unpaired t test was used for two-group comparisons, and p values are indicated within the graphs. *, p <0.05. Error bars, S.D.
The foregoing results strongly support the premise that positive (for miR-92a-3p) or negative (for miR-26b-5p) regulation of these two miRNAs by E2 causes suppression of not only invasiveness but also metastasis of luminal breast cancer cells and that, by extension of this reasoning, the restoration of these miRNAs to their original levels (as in the control tumors) by progesterone via PR-A must support metastasis.
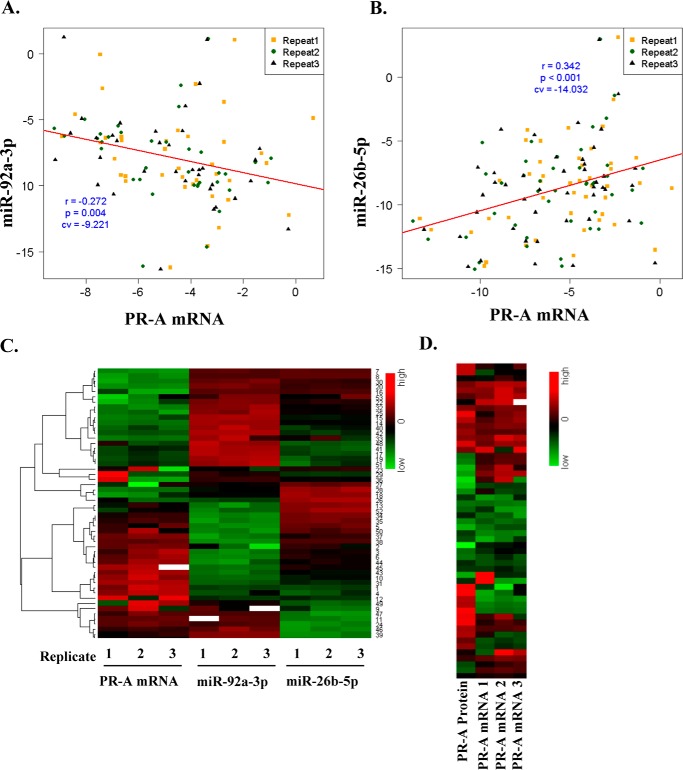
In primary human luminal breast tumors PR-A expression correlates negatively with miR-92a-3p and positively with miR-26b-5p
Using total RNA samples extracted from 53 ER+/PR+ primary luminal breast tumor specimens, the expression profile of PR-A mRNA was obtained and compared with the expression profiles of miR-92a-3p and miR-26b-5p obtained from the same RNA preparations. Despite the inherent and variable heterogeneity among the tumor specimens due to a variable ratio of tumor cells to stroma, miR-92a-3p correlated negatively with PR-A mRNA with an r = −0.272 (p = 0.004) (Fig. 6A), and miRNA-26b-5p correlated positively with PR-A mRNA with r = 0.342 (p = 0.001) (Fig. 6B). Moreover, as shown in the heat map in Fig. 6C, the inverse expression trends of the two miRNAs generally occurred within the same samples. Finally, relative PR-A protein levels among the tumors generally corresponded to the relative PR-A mRNA levels (Fig. 6D) with the caveat that the protein was measured only semiquantitatively by non-linear densitometry from Western blots (Fig. 2A) and was also necessarily extracted from a different part of each tumor specimen than RNA.
Figure 6.
Correlation analysis of PR-A, miR-92a-3p, and miR-26b-5p in primary luminal clinical breast tumor specimens. Total RNA was extracted from tumor specimens from 53 breast cancer patients. Real-time RT-PCR was used to quantify PR-A mRNA, miR-92a-3p, and miR-26b-5p. The measurements were repeated three times to generate biological replicates, resulting in 159 Ct values for each RNA. The efficiency of each primer pair (E = 2) was used to normalize the real-time RT-PCR data, and a relative gene expression value with regard to control was calculated using the equation, 2ΔCtsample = 2Ctcontrol − Ctsample, where Ctcontrol is the Ct value for a housekeeping gene (GAPDH for mRNA and SNU6 for miRNA) and Ctsample is the Ct value for each amplification. The relative gene expression value of PR-A was obtained by the equation, 2ΔCtTotal PR − 2ΔCtPR-B. In particular, if ΔCtTotal PR < ΔCtPR-B, we assigned zero for the relative expression value of PR-A. To calculate the correlation coefficients in repeated measures among RNAs, a linear mixed-effect modeling was used after each relative gene expression value was transformed with the Box–Cox transformation with two parameters due to zeros. The correlation coefficients were further investigated using log2-scaled relative gene expression values after removing zeros, and the conclusions were the same as those with the Box–Cox transformation. A negative correlation between PR-A mRNA and miR-92a-3p is shown in A, and positive correlation between PR-A mRNA and miR-26b-5p is shown in B. Expression profiles of PR-A mRNA, miR-92a-3p, and miR-26b-5p in the tumor specimens are shown in a heat map (C). Western blot densitometry–based semiquantitative measurements of relative PR-A protein expression in the tumor samples are compared with relative PR-A mRNA values for the corresponding tumors in a heat map (D).
Discussion
In aggressive cancers, including hormone receptor-negative breast cancers, at least the early stages of metastasis have probably already occurred at the time of detection of the primary tumor. In contrast, luminal breast tumors are better differentiated and relatively indolent, metastasize much slower, and offer a wider window of time for prediction of metastatic potential and for effective intervention to suppress metastasis. This study has elucidated a critical role for two miRNAs in enabling progesterone to oppose specific actions of estrogen, thus promoting invasiveness and metastasis of luminal breast cancer cells. The mechanism entails a central role for the short PR isoform A in mediating this effect of progesterone. We have previously shown that PR-A induces invasiveness in the entire range of circulating hormone levels covering pre- and postmenopausal years and that overexpression of PR-A further sensitizes the cells to progesterone levels in the low end of its postmenopausal plasma range, an observation that also held true for the response of the two miRNAs to progesterone. In this study, the discovery of miRNAs that mediate the cross-talk between PR-A and ER that results in invasiveness was crucial in developing a study design that demonstrated the profound role of this cross-talk in supporting metastasis in vivo.
None of the five miRNAs identified in this study as being regulated by progesterone in a PR-A isoform–specific, but estrogen-independent, manner affected the ability of estrogen to suppress invasiveness. However, two of 10 miRNAs that were regulated by estrogen in a manner that was opposed by progesterone, exclusively via isoform A of PR, had profound roles in invasiveness and metastasis. They are miR-92a-3p (activated by estrogen) and miR-26b-5p (repressed by estrogen). We demonstrated that regulation of these two miRNAs enabled estrogen to suppress invasiveness, and blocking of this regulation by progesterone via PR-A restored invasiveness. Further, up-regulation of miR-92a-3p and down-regulation of miR-26b-5p induced changes in invasion and metastasis pathway genes that were similar in part but both trending toward a non-metastatic phenotype and also resulted in suppression of metastasis in vivo. Notably miR-92a-3p and miR-26b-5p mediate independent but convergent pathways of hormonal control of invasion and metastasis. The ability of estrogen to effectively suppress invasiveness even at very low concentrations (∼0.01 nm) (13) may therefore be explained by the combined effects of suboptimal regulation of these two miRNAs by estrogen.
We observed relative overexpression of PR-A in a substantial proportion of primary luminal breast tumors obtained from patients. When, in an isogenic cell line model, PR-A was overexpressed to such a level, the reduced progestin concentration required for optimal induction of invasiveness also optimally suppressed regulation of miR-92a-3p and miR-26b-5p by estrogen. The clinical relevance of this functional effect of PR-A overexpression is further supported by the negative correlation of miR-92a-3p expression and positive correlation of miR-26b-5p expression with PR-A expression in clinical tumors, notwithstanding the inherent heterogeneity in tumor versus stromal content in the specimens.
One important aspect of microRNA biology is the diversity of the physiological effects of microRNAs, despite the limited cellular repertoire of microRNAs. A single microRNA can have many molecular targets and will often show a great deal of diversity in its physiological effects in different cell types or tissue types. This is believed to be due to the functional prominence of different target proteins, pathways, and networks in different tissues (39). Consistent with our findings, miR-92a expression is inversely correlated to tumor grade, positive lymph node status, and recurrence-free survival in breast cancer (40, 41). Curiously, miR-92a is part of the miR-17∼92 cluster that supports oncogenesis and cancer progression in many other cell types (41, 42) demonstrating cell type–specific differences in the actions of miR-92a. miR-26a and miR-26b are both repressed by estrogen via stimulation of c-MYC expression, resulting in the proliferative effect of estrogen in breast cancer cells (43). However, it is only miR-26b that is of interest in our study, as the repression of miR-26a was opposed by progestin through PR-B as well as PR-A. Relatively little is known about the role of miR-26b in invasiveness and metastasis of breast cancer. In mesenchymal stem cells, miR-26b induces migration by activating focal adhesion kinase (44), whereas, in bladder cancer, miR-26b inhibits migration and invasion (45). In the case of luminal breast cancer, our studies show that the ability of progesterone plus PR-A to prevent suppression of miR-26b-5p by estrogen leads to increased invasion and metastasis, again underscoring cell type–specific differences in the actions of miR-26b-5p. We note that in a recent report (46), the authors suggest that PR may be a target of miR-26b and suggest a negative correlation between miR-26b expression and total PR mRNA in breast tumors, whereas we have observed a positive correlation between miR-26b and the PR isoform A and regulatory effects consistent with a positive correlation. The functional studies reported (46), which were only performed in MCF-7 cells, only showed a modest decrease in PR mRNA (∼20%) upon ectopic overexpression of miR-26b (at an undetermined level), and the study did not measure PR protein or function and did not examine the effect of depleting endogenous miR-26b. Additionally, the correlative analysis was performed using data from only 10 of 19 tumor specimens, and the arbitrary criteria for selecting this small sample subset were not unbiased. Therefore, we respectfully disagree with the conclusions from the part of that study that pertains to miR-26b.
PR-A has long been known to act as a strong trans-dominant repressor of ER (47), but the mode by which PR-A, but not PR-B, suppresses regulation of miRNA genes by estrogen is yet to be elucidated. This could be either indirect or direct, but in either case, the PR isoform specificity of this effect could be associated a priori with the fact that in most cellular contexts, PR-A acts as a transcriptional repressor, and PR-B acts as a transcriptional activator (48). For example, the transcriptional co-activators GRIP and SRC1 bind to PR-B but not to PR-A, which preferentially binds the co-repressor SMRT (49). This is because the first 140 amino acids of PR-A contain the inhibitory domain, which is completely exposed due to the absence of the AF-3 domain found in PR-B. Therefore, it is possible that PR-A may even directly associate with a miRNA gene target of estrogen and suppress its transcriptional activation by ER or compete with ER for tethering to the same regulatory sites, passively suppressing activation or repression by ER. Further in depth studies are warranted to explore these various mechanisms.
In conclusion, our model systems have established narrow miRNA-mediated pathways of cross-talk in hormone-dependent signaling between ER and PR-A, which may account for the variable invasive and metastatic potential of primary luminal breast tumors. The relative expression/activity of PR-A may be a particularly significant determinant of the extent of this cross-talk, especially in the context of postmenopausal plasma hormone levels. Therefore, this study may have established a fundamental physiological mechanism governing metastatic spread of luminal breast cancer. Additionally, miRNA signatures of hyperactive PR-A have the potential to serve as predictors of clinical progression of luminal breast cancer. Moreover, miRNAs identified in this study that mediate functionally relevant cross-talk between PR-A and ER may reveal target pathways for interventions to suppress progression of luminal breast cancer that would avoid disruption of hormone signaling in normal tissues.
Materials and methods
Cell line models and breast tumor specimens
BT474, T47D, and ZR-75-1 breast cancer cells were purchased from American Type Culture Collection. T47D-A (ER+/PR-A+/PR-B-null) and T47D-B (ER+/PR-B+/PR-A-null) recombinant cells isogenic with parental T47D cells were a generous gift from Dr. Katherine Horowitz (University of Colorado, Denver, CO) and were cultured as described previously (36). T47D-PR-A++ cells were previously generated in our laboratory (13). Recombinant BT474 cell lines with stable expression of PR shRNA (PR-shRNA cells), miR-92a-3p (miR-92a-3p-On cells), or miR-26b-5p inhibitor (miR-26b-5p-Off cells) and control cells harboring Lenti-miR-Blank plasmid were generated using PR shRNA lentiviral plasmid (catalog no. 0000436004, Sigma-Aldrich), hsa-miR-92a-3p miRNA Lentivector (catalog no. mh11076, ABM, Vancouver, Canada), or Lenti-miR-Off-hsa-miR-26b-5p vector (catalog no. mh30381, ABM) or control Lenti-miR-blank vector (catalog no. m007, ABM) by lentiviral transduction methods described below. All human tumor samples, classified as ER+/PR+ ductal carcinoma, were obtained from the Cooperative Human Tissue Network.
Cell culture and hormone depletion
Cell line models were cultured in DMEM supplemented with FBS (10%), penicillin (100 unit/ml), streptomycin (100 μg/ml), and l-glutamine (2 mm) at 37 °C in 5% CO2. To maintain selection pressure, the medium for the recombinant BT474 cells contained 0.5 μg/ml puromycin, and the medium for T47D-A and T47D-B cells contained 200 μg/ml Geneticin. For hormone depletion, cells were plated at 30% confluence in phenol red-free medium supplemented with charcoal-stripped FBS and incubated at 37 °C in 5% CO2 for 48 h.
Western blotting of cells and breast tumor tissues
Cell lysates were prepared as described (13). To prepare tumor tissue lysates, tissue (100 mg) suspended in 500 μl of radioimmune precipitation buffer was homogenized using the PRO200 tissue homogenizer (catalog no. 01-01200, BioGen, Cambridge, MA) for 15 s on ice and centrifuged at 15,000 × g, and supernatant was used. Western blotting was performed as described previously (13). The antibody probes include monoclonal rabbit anti-PR antibody (catalog no. 8757, Cell Signaling, Danvers, MA), EMT sampler kit antibodies (catalog no. 9782, Cell Signaling), or mouse monoclonal anti-GAPDH antibody (sc-4472, Santa Cruz Biotechnologies) and appropriate horseradish peroxidase–conjugated secondary antibodies (Vector Laboratories). Relative protein expression was determined by semiquantitative densitometry of autoradiographic film using ImageJ software (National Institutes of Health).
Boyden chamber transwell invasion assay
Cells (1 × 105) were resuspended in the appropriate culture medium devoid of serum and phenol red and added to the top chamber of the FluoroBlok inserts (catalog no. 351152, 8-μm pore membrane; BD Biosciences) coated with growth factor reduced Matrigel (0.2 mg/ml). The chemoattractant comprised phenol red-free medium supplemented with FBS (20%). The appropriate hormone treatment was included in both the top and bottom chambers. Each treatment was replicated in three wells, and the entire experiment was repeated at least three times. Cells were allowed to invade for 24 h at 37 °C with 5% CO2. Cells that invaded to the bottom surface were stained with calcein AM (4 μg/ml) in serum-free medium in the dark for 1 h at 37 °C with 5% CO2. Images were captured in an identical manner from each well in five non-overlapping fields (the middle of the well and surrounding fields) using a ×4 objective. Images were analyzed using ImageJ software (National Institutes of Health), and the number of cells invaded was quantified by brightness and pixel size.
Isolation and measurement of microRNA and mRNA
Total RNA was isolated from cells or tissues using the miRCURY total RNA isolation kit (Exiqon, Vedbaek, Denmark). Breast tumor tissue lysates were prepared by suspending 50 mg of tissue in 500 μl of the lysis buffer and homogenized using the PRO200 tissue homogenizer (catalog no. 01-01200, BioGen) for 15 s on ice. Homogenized solution was centrifuged at high, 15,000 × g, and supernatant was used for RNA extraction. Reverse-transcription PCRs were performed using high-capacity complementary DNA archive kit or miRNA reverse transcription kit (Life Technologies, Inc., Carlsbad, CA). cDNA was measured by quantitative real-time RT-PCR using TaqMan probes and the StepOne Plus real-time PCR system (Life Technologies). All RNA measurements were performed in biological triplicates, and all Ct values were normalized to intrasample GAPDH (mRNA) or U6 snRNA (miRNA). RNA values were represented as -fold difference, which is calculated using the expression, 2−ΔΔCt, where ΔΔCt = ΔCt sample − ΔCt calibrator.
Affymetrix profiling of microRNAs regulated by PR-A
Hormone-depleted T47D-A and T47D-B cells were treated with vehicle or R5020 (1 nm). Total RNA samples, isolated using the Exiqon miRCURY isolation kit, were analyzed at the University of Michigan Microarray Core using the Affymetrix miRNA microarray generation IV (Affymetrix, Santa Clara, CA). Expression values were normalized using quantile normalization with background subtraction. Log transformation to base 2, followed by one-way analysis of variance, was used to determine error. The differentially expressed genes were identified by comparing R5020 treatment with vehicle treatment (repressed or activated with a cutoff -fold difference of 1.5 and a p value < 0.05). Real-time RT-PCR was performed as described above to validate miRNAs that were exclusively regulated by PR-A.
Lentiviral transduction
Packaging of lentiviral particles with pCDH empty vector plasmid, pCDH-PR shRNA, hsa-miR-92a-3p miRNA Lentivector, or Lenti-miRa-Off-hsa-miR-26b-5p vector and transduction of cells were performed as described (13). The pool of recombinant cells was selected by culturing in puromycin-containing medium for 6 weeks.
Transfection of siRNA or miRNA inhibitor
Cells were plated to 30% confluence without antibiotic in phenol red–free DMEM supplemented with 10% charcoal-stripped FBS. 24 h later, cells were transfected with siRNA directed against specific gene targets, miRNA inhibitors (miRVana, Life Technologies) directed against specific miRNA targets, or appropriate non-silencing controls using Lipofectamine (Life Technologies).
Metastasis pathway gene array expression analysis
The human tumor metastasis fast TaqMan real-time PCR array (Life Technologies, Carlsbad, CA) was performed on StepOne Realtime (Applied Biosystems). cDNA samples from BT474 cells (control transduced cells), BT474 92a-3p-On cells, and BT474 miR-26b-5p-Off cells were analyzed. The ΔΔCt method was used as described above to quantify gene expression.
Mouse metastasis model
Female athymic nude mice (Envigo, Indianapolis, IN) were implanted with 0.72-mg slow-release estradiol pellets (Innovative Research of America, Sarasota, FL) on day 0. On day 3, 1 × 107 to 2 × 107 cells were suspended in 300 μl of equal parts DMEM and Matrigel and implanted subcutaneously in the right flank just below the right shoulder. Mice were sacrificed on day 17. Tumors, ipsilateral and contralateral axillary and inguinal lymph nodes, and livers were harvested from the mice. All tissues were homogenized, and total RNA was extracted as described above for human tissues. The degree of tissue infiltration by the human tumor cells (metastasis) was measured in terms of the amount of mRNA present for human GAPDH by quantitative real-time RT-PCR using human and mouse species-specific high efficiency Taqman probes. The mRNA values for human GAPDH were normalized to the mRNA for mouse GAPDH.
Author contributions
M. R., T. M., B. M., R. R., S. K., N. V.-V., and Y. H. took part in drafting experimental design, conception, and analysis of all of the data in this study. T. M., B. M., R. R., and Y. H. performed experiments. M. R. directed the entire project. T. M. and M. R. wrote the manuscript. R. R. was the major contributor of data for manuscript revision. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We acknowledge the Biostatistics Core and Animal Model and Therapeutics Evaluation Core at the Karmanos Cancer Institute, Wayne State University. The cores are supported by National Institutes of Health Grant P30CA22453.
This work was supported by National Institutes of Health R01 Grant CA 140690 (to M. R.) and NCI T-32 Fellowship CA009531 (to T. M.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Tables S1–S5 and Figs. S1–S6.
- ER
- endoplasmic reticulum
- PR
- progesterone receptor
- miRNA
- microRNA
- E2
- estradiol.
References
- 1. American Cancer Society (2008) Cancer Facts & Figures, American Cancer Society, Inc., Atlanta, GA [Google Scholar]
- 2. Ries L., Eisner M., Kosary C., Hankey B., Miller B., Clegg L., Horner M. J., Howlader N., Eisner M. P., Reichman M., and Edwards B. K. (eds) (2007) SEER Cancer Statistics Review, 1975–2004, National Cancer Institute, National Institutes of Health, Bethesda, MD [Google Scholar]
- 3. Dunnwald L. K., Rossing M. A., and Li C. I. (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 9, R6 10.1186/bcr1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomez-Fernandez C., Daneshbod Y., Nassiri M., Milikowski C., Alvarez C., and Nadji M. (2008) Immunohistochemically determined estrogen receptor phenotype remains stable in recurrent and metastatic breast cancer. Am. J. Clin. Pathol. 130, 879–882 10.1309/AJCPD1AO3YSYQYNW [DOI] [PubMed] [Google Scholar]
- 5. Cardoso F., Harbeck N., Fallowfield L., Kyriakides S., Senkus E., and ESMO Guidelines Working Group (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23, vii11–vii19 10.1093/annonc/mds232 [DOI] [PubMed] [Google Scholar]
- 6. Zhang X. H.-F., Giuliano M., Trivedi M. V., Schiff R., and Osborne C. K. (2013) Metastasis dormancy in estrogen receptor–positive breast cancer. Clin. Cancer Res. 19, 6389–6397 10.1158/1078-0432.CCR-13-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kennecke H., Yerushalmi R., Woods R., Cheang M. C. U., Voduc D., Speers C. H., Nielsen T. O., and Gelmon K. (2010) Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 8. Liang Y., Benakanakere I., Besch-Williford C., Hyder R. S., Ellersieck M. R., and Hyder S. M. (2010) Synthetic progestins induce growth and metastasis of BT-474 human breast cancer xenografts in nude mice. Menopause 17, 1040–1047 10.1097/gme.0b013e3181d3dd0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogba N., Manning N. G., Bliesner B. S., Ambler S. K., Haughian J. M., Pinto M. P., Jedlicka P., Joensuu K., Heikkilä P., and Horwitz K. B. (2014) Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 16, 489 10.1186/s13058-014-0489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Platet N., Cathiard A. M., Gleizes M., and Garcia M. (2004) Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit. Rev. Oncol. Hematol. 51, 55–67 10.1016/j.critrevonc.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 11. Chlebowski R. T., Manson J. E., Anderson G. L., Cauley J. A., Aragaki A. K., Stefanick M. L., Lane D. S., Johnson K. C., Wactawski-Wende J., Chen C., Qi L., Yasmeen S., Newcomb P. A., Prentice R. L. (2013) Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J. Natl. Cancer Inst. 105, 526–535 10.1093/jnci/djt043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaCroix A. Z., Chlebowski R. T., Manson J. E., Aragaki A. K., Johnson K. C., Martin L., Margolis K. L., Stefanick M. L., Brzyski R., Curb J. D., Howard B. V., Lewis C. E., Wactawski-Wende J., and WHI Investigators (2011) Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 305, 1305–1314 10.1001/jama.2011.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFall T., Patki M., Rosati R., and Ratnam M. (2015) Role of the short isoform of the progesterone receptor in breast cancer cell invasiveness at estrogen and progesterone levels in the pre-and post-menopausal ranges. Oncotarget 6, 33146–33164 10.18632/oncotarget.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richer J. K., Jacobsen B. M., Manning N. G., Abel M. G., Wolf D. M., and Horwitz K. B. (2002) Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277, 5209–5218 10.1074/jbc.M110090200 [DOI] [PubMed] [Google Scholar]
- 15. Shatnawi A., Tran T., and Ratnam M. (2007) R5020 and RU486 act as progesterone receptor agonists to enhance Sp1/Sp4-dependent gene transcription by an indirect mechanism. Mol. Endocrinol. 21, 635–650 10.1210/me.2006-0274 [DOI] [PubMed] [Google Scholar]
- 16. Mote P. A., Bartow S., Tran N., and Clarke C. L. (2002) Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res. Treat. 72, 163–172 10.1023/A:1014820500738 [DOI] [PubMed] [Google Scholar]
- 17. Hopp T. A., Weiss H. L., Hilsenbeck S. G., Cui Y., Allred D. C., Horwitz K. B., and Fuqua S. A. (2004) Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 10, 2751–2760 10.1158/1078-0432.CCR-03-0141 [DOI] [PubMed] [Google Scholar]
- 18. Ibrahim Y. H., Byron S. A., Cui X., Lee A. V., and Yee D. (2008) Progesterone receptor-B regulation of insulin-like growth factor–stimulated cell migration in breast cancer cells via insulin receptor substrate-2. Mol. Cancer Res. 6, 1491–1498 10.1158/1541-7786.MCR-07-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kariagina A., Xie J., Langohr I. M., Opreanu R. C., Basson M. D., and Haslam S. Z. (2013) Progesterone decreases levels of the adhesion protein E-cadherin and promotes invasiveness of steroid receptor positive breast cancers. Horm. Cancer 4, 371–380 10.1007/s12672-013-0158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin V. C., Eng A. S., Hen N. E., Ng E. H., and Chowdhury S. H. (2001) Effect of progesterone on the invasive properties and tumor growth of progesterone receptor-transfected breast cancer cells MDA-MB-231. Clin. Cancer Res. 7, 2880–2886 [PubMed] [Google Scholar]
- 21. Lin V. C., Ng E. H., Aw S. E., Tan M. G., Ng E. H., Chan V. S., and Ho G. H. (1999) Progestins inhibit the growth of MDA-MB-231 cells transfected with progesterone receptor complementary DNA. Clin. Cancer Res. 5, 395–403 [PubMed] [Google Scholar]
- 22. Sumida T., Itahana Y., Hamakawa H., and Desprez P.-Y. (2004) Reduction of human metastatic breast cancer cell aggressiveness on introduction of either form A or B of the progesterone receptor and then treatment with progestins. Cancer Res. 64, 7886–7892 10.1158/0008-5472.CAN-04-1155 [DOI] [PubMed] [Google Scholar]
- 23. Barnett J. B., Woods M. N., Lamon-Fava S., Schaefer E. J., McNamara J. R., Spiegelman D., Hertzmark E., Goldin B., Longcope C., and Gorbach S. L. (2004) Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J. Clin. Endocrinol. Metab. 89, 776–782 10.1210/jc.2003-030506 [DOI] [PubMed] [Google Scholar]
- 24. Johansson E. D. (1969) Progesterone levels in peripheral plasma during the luteal phase of the normal human menstrual cycle measured by a rapid competitive protein binding technique. Acta Endocrinol. (Copenh.) 61, 592–606 [DOI] [PubMed] [Google Scholar]
- 25. Missmer S. A., Eliassen A. H., Barbieri R. L., and Hankinson S. E. (2004) Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J. Natl. Cancer Inst. 96, 1856–1865 10.1093/jnci/djh336 [DOI] [PubMed] [Google Scholar]
- 26. Newton C. J., Samuel D. L., and James V. H. (1986) Aromatase activity and concentrations of cortisol, progesterone and testosterone in breast and abdominal adipose tissue. J. Steroid Biochem. 24(5):1033–1039 10.1016/0022-4731(86)90356-0 [DOI] [PubMed] [Google Scholar]
- 27. Li Q., Kannan A., DeMayo F. J., Lydon J. P., Cooke P. S., Yamagishi H., Srivastava D., Bagchi M. K., and Bagchi I. C. (2011) The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331, 912–916 10.1126/science.1197454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai D., Wolf D. M., Litman E. S., White M. J., and Leslie K. K. (2002) Progesterone inhibits human endometrial cancer cell growth and invasiveness. Cancer Res. 62, 881–886 [PubMed] [Google Scholar]
- 29. Bakker G. H., Setyono-Han B., Portengen H., De Jong F. H., Foekens J. A., and Klijn J. G. (1990) Treatment of breast cancer with different antiprogestins: preclinical and clinical studies. J. Steroid Biochem. Mol. Biol. 37, 789–794 10.1016/0960-0760(90)90421-G [DOI] [PubMed] [Google Scholar]
- 30. Di Leva G., Piovan C., Gasparini P., Ngankeu A., Taccioli C., Briskin D., Cheung D. G., Bolon B., Anderlucci L., Alder H., Nuovo G., Li M., Iorio M. V., Galasso M., Santhanam R., et al. (2013) Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 9, e1003311 10.1371/journal.pgen.1003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wickramasinghe N. S., Manavalan T. T., Dougherty S. M., Riggs K. A., Li Y., and Klinge C. M. (2009) Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 37, 2584–2595 10.1093/nar/gkp117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maillot G., Lacroix-Triki M., Pierredon S., Gratadou L., Schmidt S., Bénès V., Roché H., Dalenc F., Auboeuf D., Millevoi S., and Vagner S. (2009) Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 69, 8332–8340 10.1158/0008-5472.CAN-09-2206 [DOI] [PubMed] [Google Scholar]
- 33. Guttilla I. K., Adams B. D., and White B. A. (2012) ERα, microRNAs, and the epithelial–mesenchymal transition in breast cancer. Trends Endocrinol. Metab. 23, 73–82 10.1016/j.tem.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 34. Yamashita H., and Gunduz P. (2011) The role of microRNAs in estrogen receptor α-positive human breast cancer. In Breast Cancer: Carcinogenesis, Cell Growth and Signalling Pathways (Gunduz M., and Gunduz E., eds) pp. 331–338, InTech, Rijeka, Croatia [Google Scholar]
- 35. Kent O. A., and Mendell J. T. (2006) A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene 25, 6188–6196 10.1038/sj.onc.1209913 [DOI] [PubMed] [Google Scholar]
- 36. Sartorius C. A., Groshong S. D., Miller L. A., Powell R. L., Tung L., Takimoto G. S., and Horwitz K. B. (1994) New T47D breast cancer cell lines for the independent study of progesterone B-and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 54, 3868–3877 [PubMed] [Google Scholar]
- 37. Oetken K. (2013) The effects of different estradiol delivery methods on plasma estradiol levels in mice, Honors and Undergraduate Research, Texas A&M University, College Station, TX [Google Scholar]
- 38. Nakada D., Oguro H., Levi B. P., Ryan N., Kitano A., Saitoh Y., Takeichi M., Wendt G. R., and Morrison S. J. (2014) Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558 10.1038/nature12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Preusse M., Theis F. J., and Mueller N. S. (2016) miTALOS v2: analyzing tissue specific microRNA function. PLoS One 11, e0151771 10.1371/journal.pone.0151771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nilsson S., Möller C., Jirström K., Lee A., Busch S., Lamb R., and Landberg G. (2012) Downregulation of miR-92a is associated with aggressive breast cancer features and increased tumour macrophage infiltration. PLoS One 7, e36051 10.1371/journal.pone.0036051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Si H., Sun X., Chen Y., Cao Y., Chen S., Wang H., Hu C. (2013) Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J. Cancer Res. Clin. Oncol. 139, 223–229 10.1007/s00432-012-1315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mendell J. T. (2008) miRiad roles for the miR-17–92 cluster in development and disease. Cell 133, 217–222 10.1016/j.cell.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan S., Ding K., Li R., Zhang W., Li G., Kong X., Qian P., Lobie P. E., and Zhu T. (2014) Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1, and KPNA2. Breast Cancer Res. 16, R40 10.1186/bcr3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu A., Kang N., He L., Li X., Xu X., and Zhang H. (2016) MiR-221 and miR-26b regulate chemotactic migration of MSCs toward HGF through activation of Akt and FAK. J. Cell. Biochem. 117, 1370–1383 10.1002/jcb.25428 [DOI] [PubMed] [Google Scholar]
- 45. Miyamoto K., Seki N., Matsushita R., Yonemori M., Yoshino H., Nakagawa M., and Enokida H. (2016) Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br. J. Cancer 115, 354–363 10.1038/bjc.2016.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilam A., Shai A., Ashkenazi I., Sarid L. A., Drobot A., Bickel A., Shomron N. (2017) MicroRNA regulation of progesterone receptor in breast cancer. Oncotarget. 8, 25963–25976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDonnell D. P., and Goldman M. E. (1994) RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J. Biol. Chem. 269, 11945–11949 [PubMed] [Google Scholar]
- 48. Giangrande P. H., Pollio G., and McDonnell D. P. (1997) Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J. Biol. Chem. 272, 32889–32900 10.1074/jbc.272.52.32889 [DOI] [PubMed] [Google Scholar]
- 49. Giangrande P. H., Kimbrel E. A., Edwards D. P., and McDonnell D. P. (2000) The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol. Cell. Biol. 20, 3102–3115 10.1128/MCB.20.9.3102-3115.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.