Abstract
Purpose of Review
Sleep habits, sleep physiology, and sleep disorders change with increasing age. However, there is a longstanding debate regarding whether older adults need sleep to maintain health and daily functioning (reduced-sleep-need view). An alternative possibility is that all older adults need sleep, but that many older adults have lost the ability to obtain restorative sleep (reduced-sleep-ability view). Prior research using behavioral and polysomnography outcomes has not definitively disentangled the reduced-sleep-need and reduced-sleep-ability views. Therefore, this review examines the neuroimaging literature to determine whether age-related changes in sleep cause—or are caused by—age-related changes in brain structure, function, and pathology.
Recent Findings
In middle-aged and older adults, poorer sleep quality, greater nighttime hypoxia, and shorter sleep duration related to cortical thinning in frontal regions implicated in slow wave generation, in frontoparietal networks implicated in cognitive control, and in hippocampal regions implicated in memory consolidation. Furthermore, poor sleep quality was associated with higher amyloid burden and decreased connectivity in the default mode network, a network that is disrupted in the pathway to Alzheimer’s disease.
Summary
All adults need sleep, but cortical thinning and amyloidal deposition with advancing age may weaken the brain’s ability to produce restorative sleep. Therefore, sleep in older adults may not always support identical functions for physical, mental, and cognitive health as in young adults.
Keywords: magnetic resonance imaging, cortical thinning, amyloid, obstructive sleep apnea, default mode network, Alzheimer’s disease, polysomnography, slow wave sleep, sleep spindles
Introduction
One of the most pronounced behavioral and physiological changes with aging is sleep [1]. With increasing age, adults report greater frequency of nighttime awakenings, waking too early without being able to go back to sleep, increased daytime napping, and advanced circadian phase [2]. The effects are most pronounced in older individuals with physical illnesses or psychiatric conditions [3]. Though the proximal causes of awakenings are varied, adults often attribute their sleep disruptions to greater need to use the bathroom during the night (nocturia): 53% of adults ages 55–84 report experiencing nocturia almost every night [4]. Frequency of nocturia confers an increased risk of mortality, though it is unclear whether the mechanism is via sleep disturbances [5].
When measuring sleep using polysomnography (PSG), there are several robust changes across the adult lifespan [6]. The Sao Paulo Epidemiologic Study (EPISONO) conducted nocturnal PSG recordings on over 1,000 adults ranging in age from 20 to 80 [7]. Nearly every PSG variable showed a significant age-related change. They observed age-related increases in N1 sleep, which were likely due to (or the cause of) increased nighttime awakenings. By contrast, chronological age was inversely related to REM duration, N2 duration, and N3 duration (slow wave sleep). Interestingly, in rodent models, older mice show greater slow wave activity than younger mice [8], and some human subjects researchers argue that N3 only declines with aging because EEG amplitude declines (guidelines dictate that slow waves be scored only if amplitude is > 75 mV) [1]. However, in studies of aging humans, quantitative EEG analyses have demonstrated that the incidence, frequency, slope, and density of slow waves are altered in older age [9, 10].
PSG variables often correlate with participants’ subjective impressions of their sleep quality, at least in young adults [11]. Surprising, such associations may not observed in older adults [12]. One potential resolution is that sleep macroarchitecture (e.g., sleep stages) is not as sensitive to biological age as is sleep microarchitecture (e.g., sleep spindles)[13]. For example, in the Sleep and Health in Women study that enrolled 211 women ages 22–71, quantitative EEG analyses of spindle density, K-complex density, and slow wave activity were more strongly associated with advancing age than typical measures of sleep stages [14].
Sleep disorders, such as obstructive sleep apnea, also increase in prevalence with age [15]. In the EPISONO study [7], the apnea-hypopnea index increased from 1.4 breathing events/hour in 20–24 year olds to 21.9 breathing events/hour in 75–80 year olds. Sleep apnea induces hypoxia and sleep fragmentation that, theoretically, should impair neural and cognitive functioning [16]. Nevertheless, some researchers report that sleep apnea incurs few or no behavioral consequences in the elderly [17, 18].
Theories of Whether Older Adults Need Sleep
A common assumption is that age-related changes in sleep have short- and long-term effects on physical, mental, and cognitive health. And, there is good reason to think so: In young adults, sleep restriction/deprivation triggers acute difficulties with cognitive functions such as attention, mood, memory, and creativity [19, 20]. Sleep loss increases susceptibility to the common cold [21], alters gene expression [22], and affects metabolism [23]. Moreover, in epidemiological studies, sleep disturbances are associated with cardiovascular conditions [24], cancer [25], Type II diabetes [26], and Alzheimer’s disease [27]. Because these conditions are more prevalent with advancing age, sleep alterations might explain many deleterious effects generally attributed to aging (hereafter, the preserved-sleep-need view).
An alternative view is that the “need” to sleep is reduced with increasing age (for reviews, see 28, 29). The reduced-sleep-need view contends that sleep is important during infancy, childhood, and young adulthood, but is less necessary when older. According to the reduced-sleep-need view, sleep restriction should have no costs and sleep extension should have no benefits to physical, mental, and cognitive health (at least relative to young adults). What is lost in the “sleep need” debate is that sleep may serve a different function in older adults than in young adults (just as there may be different functions from childhood to adulthood [30]), or that neural changes prevent older adults from obtaining restorative sleep (hereafter, the reduced-sleep-ability view; see also, functional weakening hypothesis)[28, 31].
Resolving the “sleep need” debate is important to science, but also has implications in clinical settings. According to the preserved-sleep-need view, complaints of disturbed sleep or daytime sleepiness must be addressed, rather than concluding that poor sleep is a normal part of aging. Similarly, according to the reduced-sleep-ability view, practitioners should not only treat the sleep disturbance, but other potential causes of changes in brain structure/function (e.g., cardiovascular dysfunction). By contrast, according to the reduced-sleep-need view, practitioners should not prescribe sedative hypnotics to older adults due to these medications’ dangerous side effects such as falls [32].
Despite the scientific and clinical importance of whether sleep need is preserved or reduced with aging, there remains scant agreement amongst the sleep community. Consider, for example, the sleep field’s recent consensus papers on what constitutes sufficient sleep quantity and quality in adults. Sleep experts rated the appropriateness of individual sleep measures (e.g., total sleep time, N3 duration) across the lifespan [33–35]. Despite many points of consensus for children and young adults, the expert panels produced multiple “uncertain” ratings for measures of sleep quality and quantity in older adults (≥65 years). For example, the expert panelists disagreed on whether any amount of N3—as short as 5%, and as large as greater than 25%—was bad or good in older adults.
Behavioral and PSG Investigations of Poor Sleep in Older Adults
Given overwhelming changes to sleep physiology with aging, and strong associations between sleep quality and cognitive functioning in young adults, the preserved-sleep-need view predicts that age-related changes in sleep explain age-related changes in cognition. Some studies of sleep and cognitive aging converge with this prediction, but many have not [1, 36, 37]. Therefore, behavioral measures have not incontrovertibly answered whether older adults need sleep.
Some sleep researchers use PSG to examine whether older adults need sleep. These investigations typically find that following sleep deprivation, slow wave rebound is lower in older adults than in young adults [38]. Furthermore, when given 16 hours of bedrest for up to one week, older adults do not sleep as many hours as young adults [39]. Though consistent with the reduced-sleep-need view, these findings also support the reduced-sleep-ability view [13]. For example, if the neural systems supporting sleep initiation and slow wave generation are deficient in older adults, then older adults might still need restorative sleep, but lack the ability to obtain restorative sleep [40, 41].
Structural Neuroimaging Studies
A promising approach to legislating between the preserved-sleep-need, reduced-sleep-need, and reduced-sleep-ability theoretical viewpoints is to determine whether, and how, sleep correlates with cortical thinning in aging adults. Structural changes should precede behavioral outcomes (potentially explaining why sleep could change in older adults without consistent changes to cognitive ability [1]). Furthermore, the investigation of how poor sleep quantity/quality relates to brain structure should inform which types of behavioral outcomes are most likely to be impacted by poor sleep in older adults.
As detailed in Table 1, several structural neuroimaging studies in healthy older adults have been cross-sectional and used self-report measures of sleep, such as the Pittsburgh Sleep Quality Index (PSQI; [42]) and Epworth Sleepiness Scale (ESS; [43]). The primary findings are illustrated in Figure 1. An early study that used the ESS in middle-aged adults found that greater sleepiness was associated with lower ventromedial prefrontal cortex volume [44], a region that is implicated in emotional regulation and decision making (see Figure 1)[45]. In a recent, large study of older adults (N=1,374), Carvalho et al. [46] found that higher ESS scores not only related to lower cortical thickness in the frontal lobe, but also lower thickness in the parietal, temporal, and occipital lobes. An important question though is whether the daytime sleepiness (ESS) data reflected the presence of sleep apnea, general deficits in sleep quality, or the influence of non-sleep fatiguing factors.
Table 1.
Structural neuroimaging studies in relation to sleep measures in adults with a mean age ≥30 years old. Unless otherwise noted, sleep disturbances are associated with lower volumetric estimates bilaterally.
| Sample | Measurements | Brain Regions Associated with Sleep | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper | N | Age Mean s |
MRI | Sleep | Design | Frontal | Parietal | Tempor al |
Occipit al |
|||
| Killgore [44] | 36 | M=30 | VBM | ESS | Cross-Sectional | vmPFC | ||||||
| Carvalho [46] | 1,374 | M=72 | SBA | ESS | Cross-Sectional | Total Frontal | Total Parietal | HC, Total Temporal | Total Occipital | |||
| Sexton [47] | 147 | M=54 | SBA | PSQI | Cross-Sectional & Longitudinal (3.5 years) | Cluster, R-SFG | Cluster, L-PCUN | Cluster | ||||
| Branger [49] | 50 | M=64 | VBM | PSQI | Cross-Sectional | R-Insula, R-Putamen | ||||||
| Lo [55] | 66 | M=67 | SBA | PSQI, TST | Longitudinal (2 years) | Ventricle size increase | ||||||
| Yaffe [60] | 631 | M=50 | VBM, MD, FA | TST | Longitudinal (5 years) | WM-MD | WM-MD | WM-MD | ||||
| Spira [61] | 122 | M=67 | SBA | TST | Longitudinal (8 years) | SFG, L-SFS, L-IFG, L-MFG | L-STL | IOG, IOS | ||||
| Weber [64] | 55 | M=31 | VBM | TST | Cross-Sectional | vmPFC | ||||||
| Lauriola [65] | 70 | M=64 | SBA | Home PSG | Cross-Sectional | |||||||
| Sanchez-Espinosa [66] | 42 | M=68 | SBA | PSG | MCI Case Control | L-PCC, L-PCUN, post-CG | ||||||
| Fogel [67] | NYA=13, NOA=15 | MYA=24 MOA=62 | VBM | PSG | Cross-Sectional | |||||||
| Mander [69] | NYA=16 NOA = 14 | MYA=20 MOA = 72 | VBM | PSG | Cross-Sectional | mPFC | ||||||
| Dubé [71] | NYA=30, NOA =33 | MYA=23 MOA=60 | SBA | PSG | Cross-Sectional | R-MFG | R-IPL, R-SPL | R-STL, R-ITG | L-cuneus L-lingual | |||
| Weng [93] | 408 | M=47.4 | OSA Case-Control ALE meta-analysis on VBM studies | R-SFG | L-MTG, PHG | |||||||
| Baril [96] | 71 | M=65 | VBM, SBA | PSG | OSA Case - Control | ↑ R-FP, IFG, L-lateral PFC | ↑ R-IPL, R-SPL, L PCC, | ↑ amygdala | ||||
| Dusak [97] | 42 | M=45 | Manual | PSG, ESS | OSA Case - Control | HC | ||||||
| Akhan [98] | 209 | M=52 | Manual | PSG, ESS | OSA Case - Control | HC | ||||||
| Sforza [99] | 232 | M=75 | SBA | Home PSG, ESS | OSA Cross-Sectional | HC | ||||||
| Zuurbier [100] | 681 | M=62 | SBA | Home PSG | OSA Cross-Sectional | GM/WM parietal | HC | |||||
Figure 1.
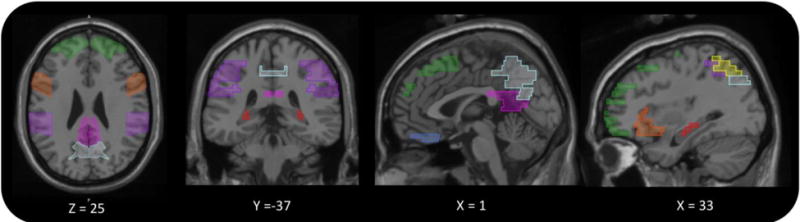
Illustration of brain regions that were associated with sleep duration or sleep quality by at least two structural neuroimaging studies. The figure was created using Mango 4.0.1 software (see original sources listed in Table 1 for specific Talairach coordinates). Regions depicted include the hippocampus (red), superior frontal gyrus (green), ventromedial prefrontal cortex (blue), inferior frontal gyrus (orange), precuneus (turquoise), posterior cingulate (pink), inferior parietal lobe (purple), and superior parietal lobe (yellow).
Higher PSQI scores—indicating lower sleep quality—have also been linked to structural neuroimaging measures. In cross-sectional studies, PSQI-defined poor sleepers showed alterations in the right superior frontal gyrus [47], the hippocampus [48], and the insula [49]. These three regions are implicated in cognitive and emotional functioning. The superior frontal gyrus supports cognitive control (including working memory and emotional inhibition)[50–52], the hippocampus facilitates memory encoding and consolidation [53]; and the insula broadly assists with emotional processing, cognitive control, interoception, time perception, and music processing [54].
PSQI scores also relate to longitudinal changes in structural MRI measures. Chee and colleagues [55] reported that, of 66 older adults, worse PSQI-defined sleep quality at baseline was associated with faster ventricular expansion over 2 years, which is interesting because ventricular expansion is associated with preclinical Alzheimer’s disease biomarkers [56]. They did not observe any impact of PSQI scores on hippocampal or frontal volume, and so it remains unclear how broadly PSQI scores predict cortical thinning. Nevertheless, it is clear that cortical thinning strongly affects PSQI scores. In 147 adults ages 20 to 84 who were followed for 3.5 years, Sexton et al. [47] reported that cortical atrophy predicted PSQI sleep quality, with significant clusters spanning the superior frontal gyrus, precuneus, retrosplenial cortex, the lateral and medial frontal cortex, and the cingulate cortex. Note that several of these brain regions compose the brain’s default mode network, a network that is active during wakeful rest and mind wandering [57]. The default mode network is disrupted in healthy young adults following acute sleep deprivation [58] and is weakened in preclinical Alzheimer’s disease (for elaboration, see amyloid neuroimaging section)[59].
The PSQI and ESS questionnaires indicate that sleep disturbances relate to cortical thinning, but do not inform which sleep disturbance is most important. A popular view is that short sleep underpins declining brain health. In the Coronary Artery Risk Development in Young Adults (CARDIA) study of 613 middle-aged adults, white matter integrity in frontal, parietal, and temporal regions declined over 5 years in participants who reported 6.1–8.0 hours of sleep versus those sleeping 6 or fewer hours at baseline [60]. In the Baltimore Study of Aging of 122 healthy older adults, faster cortical thinning in frontotemporal regions occurred over 8 years in participants reporting sleeping 5 or 6 hours/night relative to participants sleeping 7 hours/night [61]. Interestingly, they also observed faster cortical thinning in participants sleeping 8 or 9 hours/night; this U-shaped pattern is consistent with epidemiological studies in which self-reported short and long sleep duration are associated with poorer health measures [62].
Several researchers have contended that short sleep duration is not related to cortical thinning [47, 49, 55, 63]. Part of the difficulty in connecting sleep duration to cortical thinning is it remains unresolved whether self-reported sleep duration represents time asleep or time in bed. Furthermore, many demographic and psychological factors can affect how participants respond on sleep questionnaires (e.g., social desirability, mental health)[62]. One recent idea that might reconcile the literature is that reported sleep duration should be considered relative to individuals’ perceived sleep need. When Killgore and colleagues [64] subtracted participants’ reported sleep need (i.e., minimum duration needed to not be functionally impaired) from the self-reported sleep duration, they found that middle-aged adults who had more sleep than needed also had greater ventromedial prefrontal cortex volume (gyrus rectus, medial orbitofrontal gyrus).
Given the limitations of subjective sleep measures, a seemingly advantageous approach would be to use PSG (Table 1). However, several PSG studies have failed to connect variability in sleep efficiency [65], sleep stages [66], and spindle activity [67] with variability in structural neuroimaging measures in heathy older adults. For example, Cantero and colleagues [66] found that shorter REM duration was associated with cortical thinning in default mode network regions in patients with patients with amnestic mild cognitive impairment, but not in healthy controls (possibly because both REM sleep and the default mode network are impaired in the pathway to Alzheimer’s disease [59, 68]). Furthermore, Fogel et al. [67] found that sleep spindle duration and density were associated with cerebellar and hippocampal gray matter volume in young adults, but not in healthy older adults. Perhaps these PSG results indicate an age-related change in how the brain generates sleep or an age-related change in the function that sleep serves for the older brain.
Or, perhaps neuroimaging studies need to look more closely at slow wave activity [13]. Changes in slow wave activity across the adult lifespan constitute critical evidence for the purported decline in sleep need (e.g., older adults show lower slow wave rebound following sleep deprivation than young adults [38]). Walker and colleagues [69] reported that greater slow wave activity was associated with greater medial PFC gray matter volume (see also [70]). Likewise, Carrier and colleagues [71] found that slow wave density was associated with greater cortical thickness in widespread frontal, temporal, and parietal regions. They concluded that age-related cortical atrophy explains age-related changes in slow wave activity. Thus, in contrast to the reduced-sleep-need view, age-related cortical thinning reduces the brain’s ability to produce slow wave activity.
Amyloid Neuroimaging Studies
Because self-reported sleep disturbances are common to, and can be predictive of, Alzheimer’s disease [72], researchers are investigating whether sleep correlates with beta-amyloid (Aβ) deposition in preclinical adults. This research is important for clinical purposes and for contrasting the reduced-sleep-need and reduced-sleep-ability theories. For example, subtle pathological processes (e.g., amyloid accumulation) may cause sleep quality to decline such that sleep is less deep and less restorative (i.e., indicating reduced sleep ability, not reduced sleep need). Existing studies on sleep and Aβ in humans have been cross-sectional in design, often using plasma [66] or cerebrospinal fluid [73] measures of amyloid. Given this review’s focus on neuroimaging, this section covers studies that used positron emission tomography (PET) to measure Aβ burden in the brain [74].
Brain regions with greater neuronal activity are most vulnerable to amyloid deposition [75]. Therefore, Aβ burden is often highest in the default mode network (precuneus, posterior cingulate, medial prefrontal cortex, lateral temporal cortex, hippocampal formation, angular gyrus, retrosplenial cortex)[57]. Interestingly, individuals who report more sleep disturbances also show greater Aβ burden in several default mode network regions [76]. Which sleep disturbance is most related to Aβ burden is still debated. One study found that greater PET Aβ burden was associated with shorter total sleep time [77], whereas two studies found that PET Aβ burden was associated with longer sleep onset latency [49, 78]. Future studies should control for sleep disorders (e.g., obstructive sleep apnea) that increase risk for Alzheimer’s disease [72] and control for psychiatric conditions that are linked to both sleep disturbances and amyloid (e.g., anxiety [79]).
Very few studies on amyloid imaging have concomitantly used PSG. Mander et al. [70] reported that increased medial PFC Aβ deposition in older adults was associated with lower slow wave activity (0.6 to 1.0 hz range) over source localized channels (Fz and Cz). The effects were specific to the medial PFC (which included the superior frontal, anterior cingulate, and medial orbitofrontal regions); slow wave activity was not related to Aβ burden in the dorsolateral PFC, temporal cortex, parietal cortex, or occipital cortex. Thus, many of the PET studies showed associations between sleep difficulties and Aβ burden in vulnerable default mode network brain regions.
Functional Neuroimaging Studies of Aging
In functional neuroimaging studies, researchers can measure regional glucose metabolism and oxygen consumption during wakeful rest or during cognitive tasks. During wakeful rest, older adults who have mild cognitive impairment show decreased connectivity in the default mode network if they have more sleep disturbances [80, 81]. During cognitive tasks, hippocampal activity has been connected to left-frontal fast sleep spindles [82] and to maintaining consistent circadian rhythm patterns [83]. A tantalizing possibility is that nighttime slow wave activity restores frontal lobe functioning. Though frontal lobe glucose metabolism decreases with aging, Buysse and colleagues [84] found that adults ages 37–61 who showed greater nighttime slow wave activity showed greater whole brain glucose metabolism the following day (adults ages 25–36, however, did not show this association). When controlling for chronological age, greater slow wave activity was associated with increased metabolism the next-day in regions associated with cognitive control (right superior frontal gyrus [50]), language processing (inferior frontal gyrus [85]), and motor functioning/learning (precentral gyrus [86]).
Functional neuroimaging is particularly helpful in addressing one of the fundamental findings used to support the reduced-sleep-need theory. In behavioral studies, experimental sleep deprivation impairs cognition and increases subjective sleepiness in young adults more than in older adults [1]. After sleep deprivation, older adults may even outperform young adults on attention-based reaction time tasks [87], an age benefit that is almost never observed when participants are not experimentally sleep deprived [88]. These findings are consistent with the reduced-sleep-need view, but have been challenged because behavioral studies have not ruled out the possibility that older adults are approaching the cognitive tasks differently than the young adults, perhaps due to greater motivation or greater lifetime experience with compensating for sleep deprivation.
Drummond and colleagues [89] tested 28 older adults (≥59 years old) and 28 younger adults (18–39 years old) on the Go/No-Go attention task while undergoing neuroimaging, once following normal sleep and once following total sleep deprivation. According to the reduced-sleep-need theory, older adults should show no functional changes following sleep deprivation. By contrast, following sleep deprivation, the older adults required significantly greater activation in the frontoparietal attention network [90] to perform the same task as after normal sleep. These patterns indicate less efficient neural processing and/or greater compensatory effort. The elderly brain needs sleep to function efficiently.
Neuroimaging Studies in Patients with Sleep Disordered Breathing
Most neuroimaging studies of patients with sleep disorders have used structural neuroimaging (for coverage of insomnia, see 91, 92). Weng et al. [93] conducted a meta-analysis on eight papers published from 2000 to 2012 comparing obstructive sleep apnea participants to age- and gender-matched participants. They reported that sleep apnea was associated with decreased gray matter in the right superior frontal gyrus (cognitive control [50]), left middle temporal gyrus (semantic processing [94]), and bilateral parahippocampal gyrus (a hub connecting the memory network to the default mode network [95]).
There have been several substantive MRI studies on sleep apnea since Weng et al.’s [93] meta-analysis. Though one study found less cortical thinning in patients with higher apnea-hypopnea index [96], most studies find the opposite pattern. Dusak et al. [97] and Akhan et al. [98] both observed that hippocampal volume was lower in sleep apnea participants than in healthy control participants. The hippocampal reductions related to a greater apnea-hypopnea index [98] and greater subjective sleepiness [46, 97, 99].
Zuurbier et al. [100] conducted the largest, population-based investigation of brain structure alterations in sleep apnea, with their study involving 681 patients who were not using CPAP (age range of 51–95). They controlled for an impressive number of covariates: age, gender, intracranial volume, body mass index, education, smoking, alcohol use, diabetes, myocardial infarction, and interval between MRI and PSG (average of 10 months). Neuroimaging measures did not significantly correlate with arousals, and correlated only weakly with apnea-hypopnea index. However, oxygen desaturations (number of drops by ≥3% per minute) correlated strongly with smaller hippocampal and parietal white matter estimates. Consistent with these desaturation findings, in the Honolulu-Asia Aging Study, lower oxygen saturation levels predicted post-mortem autopsy findings of microinfarcts, Lewy bodies, and gliosis and neuronal loss by approximately 6 years [101]. These results converge with Yaffe et al.’s [102] finding that oxygen desaturations were predictive of developing mild cognitive impairment or dementia whereas arousals and other measures of sleep fragmentation were not predictive.
Several recent studies have evaluated whether sleep apnea affects neural connectivity. Resting state neuroimaging studies have consistently pointed to disruptions in the default mode network and frontoparietal cognitive control network in sleep apnea patients (for review, see 103). In a related approach, diffusion imaging studies reveal widespread alterations in white matter integrity in sleep apnea patients relative to controls, particularly in regions implicated in sleep/wake control and cognitive functioning (bilateral prefrontal cortex, hippocampus, amygdala, insula, cerebellum, medulla, pons, basal forebrain, basal ganglia, right posterior parietal lobe, and right occipital cortex)[104]. Greater apnea-hypopnea index [105], via acute hypoxic injury [106, 107], worsens the negative effects of sleep apnea on white matter integrity.
Neuroimaging Implications for Cognitive Aging and Alzheimer’s Disease
The frontal lobe declines substantially with advancing age and this decline impacts an array of cognitive processes and behaviors [108]. Many scientists and practitioners are therefore interested in identifying modifiable factors that affect frontal functioning (e.g., blood pressure [109]). At least ten studies have reported that sleep quantity/quality relates to cortical thinning in the frontal lobe (Table 1). The frontal lobe is implicated in slow wave generation [70], and slow wave generation is implicated in restoring frontal lobe functioning [110]. Thus, regardless of whether changes in slow wave sleep temporally precede frontal lobe thinning (or vice versa), both factors should eventually be detrimentally impacted if either factor is impaired.
Sleep in advancing age is also associated with alterations in the default mode network. As illustrated in Figure 1, cortical thinning in the precuneus, posterior cingulate, inferior parietal lobe, and medial prefrontal cortex were consistently associated with poor sleep quality. These associations converge with experimental evidence that acute sleep deprivation disrupts connectivity in the default mode network. Furthermore, separate lines of research have shown that disruptions in sleep and disruptions in the default mode network are predictive of Alzheimer’s disease [59, 68]. Amyloid neuroimaging and animal model studies indicate that the sleep-amyloid association is bidirectional [27]. Greater amyloidal deposition in default mode network regions relates to poorer sleep quality and poorer sleep quality can cause greater amyloidal deposition [111, 112].
The association between sleep quality and cortical thinning informs some of the cognitive changes observed in “normal” aging (i.e., cognitive changes in the absence of dementia)[1]. For example, as shown in Figure 1, sleep quality was consistently related to thinning in the frontoparietal attention and cognitive control networks [90], including the superior frontal gyrus [50–52, 113], the inferior frontal gyrus [85, 114], and the superior parietal lobe [115, 116]. Deficits in these regions may explain why aging adults who sleep poorly tend to perform worse on some executive functioning and memory tasks [1]. Furthermore, the hippocampus, which is largely responsible for memory encoding and consolidation [53, 117], is susceptible to many health conditions (e.g., ischemia, chronic stress, seizures) [118]. In animal models, sleep loss diminishes hippocampal functioning [119], and in human studies (Table 1), this association was especially evident in patients with obstructive sleep apnea [97–100]. Thus, age-related changes in both the hippocampus and sleep physiology combine to explain why older adults show less memory consolidation than young adults [120].
Neuroimaging Implications for Whether Older Adults Need Sleep
The reduced-sleep-need view contends that neither sleep restriction nor sleep extension impair or improve physical, mental, or cognitive health in older adults (at least relative to young adults). The sleep and neuroimaging correlations reviewed herein disfavor the reduced-sleep-need view, regardless of whether sleep loss causes neural changes [60, 61] or whether neural changes cause sleep loss [47, 71]. Rather than concluding that older adults do not need sleep, if cortical thinning or amyloidal deposition are the cause of older adults’ poor sleep then a more fitting interpretation is that they have lost the ability to sleep in a restorative manner.
It is also possible that a third variable explains both poor sleep and neural changes with aging (e.g., education, depression, anxiety, cardiovascular dysfunction [121]); however, several neuroimaging studies have controlled for likely third variables and still observed correlations between sleep and cortical thinning. Future studies should disentangle causal direction by treating sleep disturbances in middle age (cognitive-behavioral therapy, continuous positive airway pressure, and/or pharmacological treatments) and assessing whether preserving sleep quality preserves brain health. But, the current literature seems sufficiently strong to produce this take-home message: The “need” for sleep persists into older age.
Conclusion: Are Sleep Changes with Aging Inevitable?
Sleep need persists into older age, but this conclusion may be irrelevant if age effects on sleep quality, quantity, and physiology are inevitable. There are three general points of evidence that adults can sleep well despite advancing age. First, self-report studies show that many older adults report their sleep quality to be good, and sometimes even better than in middle age [122]. Second, in population-based studies, many older adults remain free from sleep disorders such as obstructive sleep apnea [123, 124]. Third, in studies that followed adults aged 61–71 who had excellent physical and mental health, polysomnography features were generally stable over a three-year time period [125]. To definitively answer what percentage of adults demonstrate no marked changes in sleep physiology, a multi-decade longitudinal polysomnographic investigation would be required.
The Global Council on Brain Health recently concluded that “People, at any age, can change their behavior to improve their sleep” [126]. Positive behavior changes include obtaining more daytime light, waking up at the same time seven days a week, and consulting a sleep physician if consistently feeling sleepy during the day. Behaviors to avoid include bright light in the evening (e.g., from technology devices), caffeine use after lunch time, too much fluid intake three hours before bedtime, and unlimited use of over-the-counter or prescribed sleep medications. Beyond these recommendations for sleep hygiene, taking care of one’s body through diet [127], exercise [128], and mental engagement [129] helps preserve brain functioning, which would help to preserve sleep quality. Thus, poor sleep quality is not an inevitable consequence of aging, older adults can modify their behaviors to sleep better, and better sleep can have a positive impact on physical, mental, and cognitive health.
Acknowledgments
The author is grateful to Annie Ginty and Melanie Sekeres for their help with portions of this paper.
Measurement Abbreviations
- ALE
activation likelihood estimation meta-analysis
- ESS
Epworth Sleepiness Scale
- FA
fractional anisotropy
- MD
mean diffusivity
- PSQI
Pittsburgh Sleep Quality Index
- MCI
Mild Cognitive Impairment
- OA
Older adult
- OSA
Obstructive Sleep Apnea. SBM: surface-based analysis
- VBM
voxel-based morphometry
- YA
young adults
Brain Regions Abbreviations
- FP
frontal pole
- GM
gray matter
- HC
hippocampus
- IFG
inferior frontal gyrus
- IOG
inferior occipital gyrus
- IOS
inferior occipital sulcus
- IPL
inferior parietal lobe
- L
left hemisphere
- mPFC
medial prefrontal cortex
- ITG
inferior temporal gyrus
- MFG
middle frontal gyrus
- MTG
middle temporal gyrus
- OFG
orbitofrontal gyrus
- PHG
parahippocampal gyrus
- PCC
posterior cingulate cortex
- PCUN
precuneus
- post-CG
postcentral gyrus
- pre-CG
precentral gyrus
- R
right hemisphere
- SFG
superior frontal gyrus
- SFS
superior frontal sulcus
- STL
superior temporal lobe
- vmPFC
ventromedial prefrontal cortex
- WM
white matter
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Michael K. Scullin declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: Integrating a half-century of multidisciplinary research. Perspect Psychol Sci. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauderdale DS, Philip Schumm L, Kurina LM, McClintock M, Thisted RA, Chen JH, Waite L. Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(8):S125–33. doi: 10.1093/geronb/gbu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. doi: 10.1016/j.smrv.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10(5):540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endeshaw YW, Schwartz AV, Stone K, Caserotti P, Harris T, Smagula S, Satterfield S. Nocturia, insomnia symptoms and mortality among older men: The Health, Aging and Body Composition Study. J Clin Sleep Med. 2016;12(6):789–96. doi: 10.5664/jcsm.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–74. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 7.Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15(4):401–9. doi: 10.1016/j.sleep.2013.11.791. [DOI] [PubMed] [Google Scholar]
- 8.Panagiotou M, Vyazovskiy VV, Meijer JH, Deboer T. Differences in electroencephalographic non-rapid-eye movement sleep slow-wave characteristics between young and old mice. Sci Rep. 2017;7:43656. doi: 10.1038/srep43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33(4):758–66. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- 10.Chinoy ED, Frey DJ, Kaslovsky DN, Meyer FG, Wright KP. Age-related changes in slow wave activity rise time and NREM sleep EEG with and without zolpidem in healthy young and older adults. Sleep Med. 2014;15(9):1037–45. doi: 10.1016/j.sleep.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerlund A, Lagerros YT, Kecklund G, Axelsson J, Åkerstedt T. Relationships between questionnaire ratings of sleep quality and polysomnography in healthy adults. Behav Sleep Med. 2016;14(2):185–99. doi: 10.1080/15402002.2014.974181. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, et al. When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol. 2017;123:37–46. doi: 10.1016/j.biopsycho.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz JF, Åkerstedt T, Lindberg E, Gruber G, Fischer H, Theorell-Haglöw J. Age affects sleep microstructure more than sleep macrostructure. J Sleep Res. 2017 doi: 10.1111/jsr.12478. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL, Scullin MK. Normal aging. In: Kryger M, Roth T, editors. Principles and practice of sleep medicine. 6th. Elsevier; 2017. [Google Scholar]
- 16.Olaithe M, Nanthakumar S, Eastwood PR, Bucks RS. Cognitive and mood dysfunction in adult obstructive sleep apnoea (OSA): Implications for psychological research and practice. Transl Issues Psychol Sci. 2015;1(1):67–78. [Google Scholar]
- 17.Quan SF, Chan CS, Dement WC, Gevins A, Goodwin JL, Gottlieb DJ, et al. The association between obstructive sleep apnea and neurocognitive performance—the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34(3):303–314B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-García MA, Soler-Cataluna JJ, Román-Sánchez P, González V, Amorós C, et al. Obstructive sleep apnea has little impact on quality of life in the elderly. Sleep Med. 2009;10(1):104–111. doi: 10.1016/j.sleep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19•.Bennion KA, Payne JD, Kensinger EA. Selective effects of sleep on emotional memory: What mechanisms are responsible? Transl Issues Psychol Sci. 2015;1(1):79–88. ◦ An excellent review article on the mechanisms by which encoding and memory consolidation interact. [Google Scholar]
- 20.King E, Daunis M, Tami C, Scullin MK. Sleep in studio-based courses: outcomes for creativity task performance. J Interior Design. 2017 doi: 10.1111/joid.12104. [DOI] [Google Scholar]
- 21.Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–9. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110(12):E1132–E1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111(29):10761–6. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Q, Signorello LB, Brinton LA, Cohen SS, Blot WJ, Matthews CE. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016;20:25–9. doi: 10.1016/j.sleep.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bliwise DL, Greer SA, Scullin MK, Philips LS. Habitual and recent sleep durations: Graded and interactive risk for impaired glycemic control in a biracial population. Am J Med. 2017;130(5):564–71. doi: 10.1016/j.amjmed.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–9. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3(2):1–220. ◦ A 200+ page review of everything learned about sleep and aging in the first few decades of sleep science. The field has made considerable progress since 1980, but several of the challenges regarding reduced-sleep-need theory have remained. [PubMed] [Google Scholar]
- 29.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg I. Slow wave sleep and release of growth hormone. JAMA. 2000;284(21):2717–2718. [PubMed] [Google Scholar]
- 31.Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28(1):105–14. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tannenbaum C, Diaby V, Singh D, Perreault S, Luc M, Vasiliadis HM. Sedative-hypnotic medicines and falls in community-dwelling older adults: a cost-effectiveness (decision-tree) analysis from a US Medicare perspective. Drugs Aging. 2015;32(4):305–14. doi: 10.1007/s40266-015-0251-3. [DOI] [PubMed] [Google Scholar]
- 33.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3(1):6–19. doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gui WJ, Li HJ, Guo YH, Peng P, Lei X, Yu J. Age-related differences in sleep-based memory consolidation: a meta-analysis. Neuropsychologia. 2017;97:46–55. doi: 10.1016/j.neuropsychologia.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33(2):211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep— implications for insomnia. Curr Biol. 2008;18(15):1118–23. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain. 2014;137(10):2847–61. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scullin MK, Trotti LM, Wilson AG, Greer SA, Bliwise DL. Nocturnal sleep enhances working memory training in Parkinson’s disease but not Lewy body dementia. Brain. 2012;135(9):2789–97. doi: 10.1093/brain/aws192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Killgore WD, Schwab ZJ, Kipman M, DelDonno SR, Weber M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neurosci Lett. 2012;518(1):10–3. doi: 10.1016/j.neulet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho DZ, Louis EKS, Boeve BF, Mielke MM, Przybelski SA, Knopman DS, et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 2016;32:236–43. doi: 10.1016/j.sleep.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cross NE, Lagopoulos J, Duffy SL, Cockayne NL, Hickie IB, Lewis SJ, Naismith SL. Sleep quality in healthy older people: Relationship with 1H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behav Neurosci. 2013;127(5):803–10. doi: 10.1037/a0034154. [DOI] [PubMed] [Google Scholar]
- 49.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, De Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–14. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004 Feb 13;303(5660):1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 51.Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–51. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 52.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 55.Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–8. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou YY, Leporé N, Avedissian C, Madsen SK, Parikshak N, Hua X, et al. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer’s disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46(2):394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 58.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59(2):1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 59.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yaffe K, Nasrallah I, Hoang TD, Lauderdale DS, Knutson KL, Carnethon MR, et al. Sleep duration and white matter quality in middle-aged adults. Sleep. 2016;39:1743–7. doi: 10.5665/sleep.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, et al. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep. 2016;39(5):1121–28. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bliwise DL, Young TB. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30(12):1614–5. doi: 10.1093/sleep/30.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramos AR, Dong C, Rundek T, Elkind MS, Boden-Albala B, Sacco RL, Wright CB. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res. 2014;23(5):524–30. doi: 10.1111/jsr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Weber M, Webb CA, Deldonno SR, Kipman M, Schwab ZJ, Weiner MR, Killgore WD. Habitual ‘sleep credit’ is associated with greater grey matter volume of the medial prefrontal cortex, higher emotional intelligence and better mental health. J Sleep Res. 2013;22(5):527–34. doi: 10.1111/jsr.12056. ◦ This work indicated that researchers should not only collect data on sleep duration, but participants’ perceived sleep need; sleeping fewer hours than personally needed was associated with greater frontal lobe thinning. [DOI] [PubMed] [Google Scholar]
- 65.Lauriola M, Esposito R, Pizzi SD, de Zambotti M, Londrillo F, Kramer JH, et al. Sleep changes without medial temporal lobe or brain cortical changes in community-dwelling individuals with subjective cognitive decline. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Espinosa MP, Atienza M, Cantero JL. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-β and cortical thinning. Neuroimage. 2014;98:395–404. doi: 10.1016/j.neuroimage.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Fogel S, Vien C, Karni A, Benali H, Carrier J, Doyon J. Sleep spindles: a physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol Aging. 2017;49:154–64. doi: 10.1016/j.neurobiolaging.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Haba-Rubio J, Marti-Soler H, Tobback N, Andries D, Marques-Vidal P, Waeber G, et al. Sleep characteristics and cognitive impairment in the general population: The HypnoLaus study. Neurology. 2017;88(5):463–9. doi: 10.1212/WNL.0000000000003557. [DOI] [PubMed] [Google Scholar]
- 69.Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–7. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Dubé J, Lafortune M, Bedetti C, Bouchard M, Gagnon JF, Doyon J, et al. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35(20):7795–807. doi: 10.1523/JNEUROSCI.3956-14.2015. ◦ These researchers deconstructed the qualities of slow waves (amplitude, density, slope) and demonstrated that thinning in frontal regions and along the lateral fissure explained age-dependent declines in slow wave generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, et al. Sleep, cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw032. http://dx.doi.org/10.1093/sleep/zsw032 ◦ A provocative meta-analysis paper that suggested that 15% of Alzheimer’s disease is attributable to sleep disturbances. [DOI] [PubMed] [Google Scholar]
- 73.Ju YES, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013;54(3):476–90. doi: 10.2967/jnumed.113.120618. [DOI] [PubMed] [Google Scholar]
- 75.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750–6. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568–76. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70(12):1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, et al. The relationship between sleep quality and brain amyloid burden. Sleep. 2016;39(5):1063–1068. doi: 10.5665/sleep.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pietrzak RH, Lim YY, Neumeister A, Ames D, Ellis KA, Harrington K, et al. Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry. 2015;72(3):284–91. doi: 10.1001/jamapsychiatry.2014.2476. [DOI] [PubMed] [Google Scholar]
- 80.McKinnon AC, Duffy SL, Cross NE, Terpening Z, Grunstein RR, Lagopoulos J, et al. Functional connectivity in the default mode network is reduced in association with nocturnal awakening in mild cognitive impairment. J Alzheimers Dis. 2017;56(4):1373–84. doi: 10.3233/JAD-160922. [DOI] [PubMed] [Google Scholar]
- 81.McKinnon AC, Lagopoulos J, Terpening Z, Grunstein R, Hickie IB, Batchelor J, et al. Sleep disturbance in mild cognitive impairment is associated with alterations in the brain’s default mode network. Behav Neurosci. 2016;130:305–15. doi: 10.1037/bne0000137. [DOI] [PubMed] [Google Scholar]
- 82.Mander BA, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, et al. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex. 2014;24(12):3301–9. doi: 10.1093/cercor/bht188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sherman SM, Mumford JA, Schnyer DM. Hippocampal activity mediates the relationship between circadian activity rhythms and memory in older adults. Neuropsychologia. 2015;75:617–25. doi: 10.1016/j.neuropsychologia.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 84.Wilckens KA, Aizenstein HJ, Nofzinger EA, James JA, Hasler BP, Rosario-Rivera BL, et al. The role of non-rapid eye movement slow wave activity in prefrontal metabolism across young and middle-aged adults. J Sleep Res. 2016;25:296–306. doi: 10.1111/jsr.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5(3):206–15. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 87.Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57(7):1245–51. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 89•.Almklov EL, Drummond SP, Orff H, Alhassoon The effects of sleep deprivation on brain functioning in older adults. Behav Sleep Med. 2015;13(4):324–45. doi: 10.1080/15402002.2014.905474. ◦ This was a critical study for informing whether older adults need sleep. These authors demonstrated that older adults engage compensatory mechanisms following sleep deprivation that may not be observable with behavioral measures. [DOI] [PubMed] [Google Scholar]
- 90.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kay DB, Buysse DJ. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7(3):23. doi: 10.3390/brainsci7030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nofzinger E, Maquet P, Thorpy MJ. Neuroimaging of sleep and sleep disorders. Cambridge University Press; 2013. [Google Scholar]
- 93.Weng HH, Tsai YH, Chen CF, Lin YC, Yang CT, Tsai YH, Yang CY. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep. 2014;37(1):167–75. doi: 10.5665/sleep.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei T, Liang X, He Y, Zang Y, Han Z, Caramazza A, Bi Y. Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. J Neurosci. 2012;32(2):481–9. doi: 10.1523/JNEUROSCI.1953-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ward AM, Schultz AP, Huijbers W, Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35(3):1061–73. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201606-1271OC. [DOI] [PubMed] [Google Scholar]
- 97.Dusak A, Ursavas A, Hakyemez B, Gokalp G, Taskapilioglu O, Parlak M. Correlation between hippocampal volume and excessive daytime sleepiness in obstructive sleep apnea syndrome. Eur Rev Med Pharmacol Sci. 2013;17(9):1198–204. [PubMed] [Google Scholar]
- 98.Akhan G, Songu M, Ayik S, Altay C, Kalemci Correlation between hippocampal sulcus width and severity of obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2015;272(12):3763–8. doi: 10.1007/s00405-014-3422-7. [DOI] [PubMed] [Google Scholar]
- 99.Sforza E, Celle S, Saint-Martin M, Barthélémy JC, Roche F. Hippocampus volume and subjective sleepiness in older people with sleep-disordered breathing: a preliminary report. J Sleep Res. 2016;25:190–3. doi: 10.1111/jsr.12367. [DOI] [PubMed] [Google Scholar]
- 100.Zuurbier LA, Vernooij MW, Luik AI, Kocevska D, Hofman A, Whitmore H, et al. Apnea-hypopnea index, nocturnal arousals, oxygen desaturation and structural brain changes: A population-based study. Neurobiol Sleep Circadian Rhythms. 2016;1(1):1–7. doi: 10.1016/j.nbscr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gelber RP, Redline S, Ross GW, Petrovitch H, Sonnen JA, Zarow C, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84(3):296–303. doi: 10.1212/WNL.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khazaie H, Veronese M, Noori K, Emamian F, Zarei M, Ashkan K, et al. Functional reorganization in obstructive sleep apnoea and insomnia: A systematic review of the resting-state fMRI. Neurosci Biobehav Rev. 2017;77:219–31. doi: 10.1016/j.neubiorev.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tummala S, Palomares J, Kang DW, Park B, Woo MA, Harper RM, Kumar R. Global and regional brain non-Gaussian diffusion changes in newly diagnosed patients with obstructive sleep apnea. Sleep. 2016;39(1):51–7. doi: 10.5665/sleep.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tummala S, Roy B, Park B, Kang DW, Woo MA, Harper RM, Kumar R. Associations between brain white matter integrity and disease severity in obstructive sleep apnea. J Neurosci Res. 2016;94(10):915–923. doi: 10.1002/jnr.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palomares JA, Tummala S, Wang DJ, Park B, Woo MA, Kang DW, et al. Water exchange across the blood-brain barrier in obstructive sleep apnea: an MRI diffusion-weighted pseudo-continuous arterial spin labeling study. J Neuroimaging. 2015;25(6):900–5. doi: 10.1111/jon.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tummala S, Roy B, Vig R, Park B, Kang DW, Woo MA, et al. Non-Gaussian diffusion imaging shows brain myelin and axonal changes in obstructive sleep apnea. J Comput Assist Tomogr. 2017;41(2):181–9. doi: 10.1097/RCT.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; 2013. [Google Scholar]
- 109.Scullin MK, Gordon BA, Shelton JT, Lee JH, Head D, McDaniel MA. Evidence for a detrimental relationship between hypertension history, prospective memory, and prefrontal cortex white matter in cognitively normal older adults. Cogn Affect Behav Neurosci. 2013;13(2):405–16. doi: 10.3758/s13415-013-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural Plast. 2012 doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C. Subregions of the human superior frontal gyrus and their connections. Neuroimage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nature Neurosci. 1998;1(6):529–33. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- 117.Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–80. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- 118.Bartsch T, Wulff P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience. 2015;309:1–16. doi: 10.1016/j.neuroscience.2015.07.084. [DOI] [PubMed] [Google Scholar]
- 119.Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–90. doi: 10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 120.Scullin MK, Fairley J, Decker M, Bliwise DL. The effects of an afternoon nap on episodic memory in young and older adults. Sleep. 2017 doi: 10.1093/sleep/zsx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anujuo K, Stronks K, Snijder MB, Jean-Louis G, Rutters F, van den Born BJ, et al. Relationship between short sleep duration and cardiovascular risk factors in a multi-ethnic cohort–the helius study. Sleep Med. 2015;16(12):1482–8. doi: 10.1016/j.sleep.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Luca G, Haba Rubio J, Andries D, Tobback N, Vollenweider P, Waeber G, et al. Age and gender variations of sleep in subjects without sleep disorders. Ann Med. 2015;47(6):482–91. doi: 10.3109/07853890.2015.1074271. [DOI] [PubMed] [Google Scholar]
- 123.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59(12):2217–25. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoch CC, Dew MA, Reynolds CF, Buysse DJ, Nowell PD, Monk TH, et al. Longitudinal changes in diary-and laboratory-based sleep measures in healthy “old old” and “young old” subjects: a three-year follow-up. Sleep. 1997;20(3):192–202. doi: 10.1093/sleep/20.3.192. [DOI] [PubMed] [Google Scholar]
- 126.Global Council on Brain Health. The brain-sleep connection: GCBH recommendations on sleep and brain health. 2016 Available at www.GlobalCouncilOnBrainHealth.org.
- 127.Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29(41):12795–801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McDonough IM, Haber S, Bischof GN, Park DC. The Synapse Project: engagement in mentally challenging activities enhances neural efficiency. Restor Neurol Neurosci. 2015;33(6):865–82. doi: 10.3233/RNN-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]


