ABSTRACT
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry has emerged as a reliable technique to identify molds involved in human diseases, including dermatophytes, provided that exhaustive reference databases are available. This study assessed an online identification application based on original algorithms and an extensive in-house reference database comprising 11,851 spectra (938 fungal species and 246 fungal genera). Validation criteria were established using an initial panel of 422 molds, including dermatophytes, previously identified via DNA sequencing (126 species). The application was further assessed using a separate panel of 501 cultured clinical isolates (88 mold taxa including dermatophytes) derived from five hospital laboratories. A total of 438 (87.35%) isolates were correctly identified at the species level, while 26 (5.22%) were assigned to the correct genus but the wrong species and 37 (7.43%) were not identified, since the defined threshold of 20 was not reached. The use of the Bruker Daltonics database included in the MALDI Biotyper software resulted in a much higher rate of unidentified isolates (39.76 and 74.30% using the score thresholds 1.7 and 2.0, respectively). Moreover, the identification delay of the online application remained compatible with real-time online queries (0.15 s per spectrum), and the application was faster than identifications using the MALDI Biotyper software. This is the first study to assess an online identification system based on MALDI-TOF spectrum analysis. We have successfully applied this approach to identify molds, including dermatophytes, for which diversity is insufficiently represented in commercial databases. This free-access application is available to medical mycologists to improve fungal identification.
KEYWORDS: MALDI-TOF mass spectrometry, dermatophytes, filamentous fungi, fungi, mass spectrometry, online identification
INTRODUCTION
The analysis of protein profiles via matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) has recently emerged as a simple and reliable method to identify yeasts and molds, including dermatophytes (1–12). However, while yeast identification via MALDI-TOF MS is now commonly used in routine laboratories, the identification of molds and dermatophytes using this technique remains reserved for a small number of highly specialized laboratories. Mold identification via MALDI-TOF technology still requires the establishment of comprehensive databases covering the vast diversity of fungi involved in human pathologies (5, 6). This diversity has expanded in recent years, as demonstrated by the Atlas of Clinical Fungi, in which 320 species were described in the first edition in 1995, and 560 species were included in the third edition in 2013 (13–17).
Establishment of an extensive online database of fungal spectra covering all species involved in human diseases could represent a major advance in this field, provided that reliable algorithms are developed that enable thousands of reference spectra to be queried in a fraction of a second. In this study, to assess the interest and feasibility of this approach, we developed a website and software that scans a fungal database of 11,851 spectra, corresponding to 938 species (pathogenic and nonpathogenic) and 246 genera encountered in medical laboratories. We present the evaluation of this identification system using an extensive panel of strains collected in five different hospitals.
RESULTS
Experiment design.
We first tested a previously published panel of 422 strains (panel 1 [Table 1]) against the online algorithm and subsequently established identification criteria and threshold, since the online software, referred to as MSI (Mass Spectrometry Identification platform; https://biological-mass-spectrometry-identification.com/msi/), has never been assessed or published. The in-house database and the MSI software were then assessed by testing 2,004 spectra derived from 501 isolates prospectively collected in five hospitals in France and Belgium (panel 2). Panel 2 was also tested in parallel against the Bruker database and the IHEM/Marseille database using the MALDI Biotyper software from Bruker.
TABLE 1.
Strain characteristics: panels, sampling institutions, and sampling periods
| Sampling institutiona | Panel | Analysis period | No. of strains |
|
|---|---|---|---|---|
| Selected | Sequenced | |||
| BCCM/IHEM (Be) | 1 | 198 | 198 | |
| Marseille (Fr) | 1 | 224 | 224 | |
| Bordeaux (Fr) | 2 | 30 March 2015 to 26 August 2015 | 343 | 103 |
| Nice (Fr) | 2 | 12 March 2015 to 30 September 2015 | 244 | 101 |
| Marseille (Fr) | 2 | 2 February 2015 to 29 May 2015 | 439 | 148 |
| Bruges (Be) (AZ Sint-Jan) | 2 | 8 December 2015 to June 2016 | 47 | 36 |
| Brussels (Be) (UZ Brussel) | 2 | 8 October 2015 to June 2016 | 196 | 113 |
Be, Belgium; Fr, France; UZ Brussel, Universitair Ziekenhuis Brussel.
Threshold establishment.
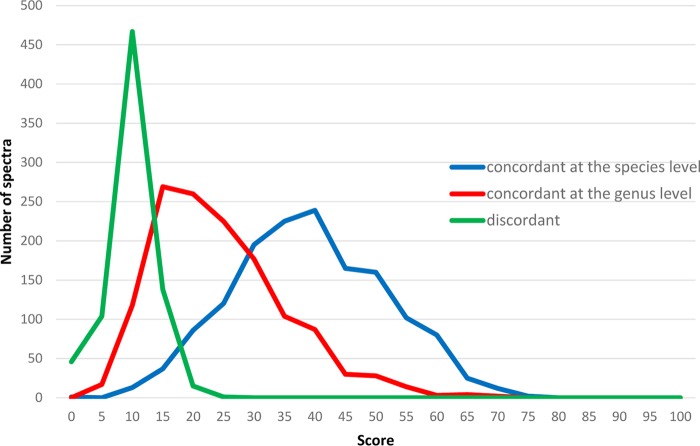
Data extracted from the 1,688 spectra of the panel 1 strains are plotted in Fig. 1. The highest scores corresponding to false genus identifications peaked around 10, and very few false genus results exceeded 20, whereas most results corresponding to correct species identification surpassed this threshold. In contrast, it was markedly more difficult to define a satisfying cutoff that differentiated between accurate species identification and accurate genus but false species identification (Fig. 1).
FIG 1.
Data extracted from the 1,688 spectra of the panel 1 strains using the MSI platform. The highest score value distributions of the 1,688 tested spectra were determined, considering the first concordant identification at the species level (blue line), the first concordant identification at the genus level (red line), and the first discordant identification at the genus level (green line) for each spectrum.
Among the 1,688 spectra, only 47 corresponded to a false identification as an initial result. The scores corresponding to these 47 spectra ranged from 2.31 to 16.05 (mean = 10.26; standard deviation = 3.04), which was far below the selected threshold.
Description of the species identified in panel 2.
A total of 1,270 isolates were collected in five hospitals during the study period. However, only 501 isolates were identified via DNA sequencing, whereas the other isolates, which corresponded to common and easy-to-identify species, were identified using only morphological criteria and were thus not included in the study. Among the 501 isolates that were used to assess the in-house database and the online identification system, a total of 88 microbial taxa were identified, with a repartition from 9 to 43 taxa per hospital (Table 2). The details of the DNA identification results for these 501 isolates and the targets used are indicated in Table 2.
TABLE 2.
Panel 2 species results obtained after sequencing for five participating centers
| Genus | Strain | DNA targeta | No. of isolates |
|||||
|---|---|---|---|---|---|---|---|---|
| Jette | Brugge | Bordeaux | Nice | Marseille | Total | |||
| Acremonium | Acremonium sclerotigenum | ITS | 3 | 3 | ||||
| Acremonium sp. | ITS | 2 | 2 | |||||
| Acrophialophora | Acrophialophora levis | ITS | 1 | 1 | ||||
| Alternaria | Alternaria abundans | ITS | 1 | 1 | ||||
| Alternaria alternata | ITS | 2 | 1 | 1 | 4 | |||
| Alternaria sp. | ITS | 2 | 1 | 3 | ||||
| Aphanocladium | Aphanocladium album | ITS | 1 | 1 | ||||
| Arthrinium | Arthrinium arundinis | ITS | 1 | 2 | 3 | |||
| Arthrinium sp. | ITS | 1 | 1 | |||||
| Arthroderma | Arthroderma benhamiae | ITS | 2 | 1 | 1 | 4 | ||
| Arthrographis | Arthrographis curvata | ITS | 1 | 1 | ||||
| Aspergillus | Aspergillus calidoustus | BTUB | 1 | 1 | 1 | 3 | ||
| Aspergillus flavus | BTUB | 1 | 5 | 29 | 35 | |||
| Aspergillus fumigatus | BTUB | 1 | 17 | 6 | 1 | 25 | ||
| Aspergillus hollandicus | BTUB | 1 | 1 | |||||
| Aspergillus jensenii | BTUB | 1 | 1 | |||||
| Aspergillus lentulus | BTUB | 1 | 1 | |||||
| Aspergillus nidulans | BTUB | 1 | 1 | |||||
| Aspergillus nidulantes | BTUB | 3 | 3 | |||||
| Aspergillus niger | BTUB | 2 | 2 | 8 | 12 | |||
| Aspergillus niger group | BTUB | 3 | 3 | |||||
| Aspergillus ochraceus | BTUB | 1 | 1 | |||||
| Aspergillus parasiticus | BTUB | 1 | 1 | |||||
| Aspergillus pseudoglaucus | BTUB | 1 | 1 | |||||
| Aspergillus puulaauensis | BTUB | 1 | 1 | |||||
| Aspergillus sydowii | BTUB | 4 | 4 | |||||
| Aspergillus terreus | BTUB | 3 | 3 | 12 | 18 | |||
| Aspergillus tubingensis | BTUB | 2 | 2 | |||||
| Aspergillus versicolor group | BTUB | 1 | 1 | |||||
| Aspergillus westerdijkiae | BTUB | 1 | 1 | |||||
| Bionectria | Bionectria sp. | ITS | 1 | 1 | ||||
| Bipolaris | Bipolaris hawaiiensis | ITS | 1 | 1 | ||||
| Ceriporia | Ceriporia lacerata | ITS | 3 | 3 | ||||
| Cladosporium | Cladosporium cladosporioides | ITS | 4 | 4 | ||||
| Engyodontium | Engyodontium album | ITS | 1 | 1 | ||||
| Eutypella | Eutypella scoparia | ITS | 1 | 1 | 2 | |||
| Fomes | Fomes fomentarius | ITS | 3 | 3 | ||||
| Fomitopsis | Fomitopsis pinicola | ITS | 1 | 1 | ||||
| Fusarium | Fusarium incarnatum-equiseti complex | EF | 3 | 3 | ||||
| Fusarium oxysporum | EF | 4 | 1 | 2 | 5 | 3 | 15 | |
| Fusarium proliferatum | EF | 1 | 2 | 3 | 3 | 9 | ||
| Fusarium solani | EF | 3 | 2 | 1 | 6 | |||
| Gallactomyces | Gallactomyces candidum | ITS | 1 | 1 | 2 | |||
| Gallactomyces geotrichum | ITS | 3 | 2 | 5 | ||||
| Geotrichum capitatum | ITS | 6 | 6 | |||||
| Geosmithia | Geosmithia argillacea | BTUB | 1 | 1 | ||||
| Microsporum | Microsporum audouinii | ITS | 6 | 3 | 9 | |||
| Microsporum canis | ITS | 2 | 1 | 3 | ||||
| Microsporum incurvatum | ITS | 1 | 1 | |||||
| Mucor | Mucor circinelloides | ITS | 1 | 1 | ||||
| Paecilomyces | Paecilomyces formosus | ITS | 1 | 1 | ||||
| Paecilomyces lilacinus | ITS | 1 | 1 | 2 | ||||
| Paecilomyces variotii | ITS | 1 | 1 | 2 | ||||
| Penicillium | Penicillium canescens | BTUB | 1 | 1 | ||||
| Penicillium chrysogenum | BTUB | 2 | 18 | 20 | ||||
| Penicillium citrinum | BTUB | 1 | 2 | 3 | ||||
| Penicillium corylophilum | BTUB | 1 | 1 | |||||
| Penicillium crateriforme | BTUB | 1 | 1 | |||||
| Penicillium crustosum | BTUB | 1 | 1 | |||||
| Penicillium expansum | BTUB | 1 | 1 | |||||
| Penicillium glabrum | BTUB | 11 | 8 | 19 | ||||
| Penicillium oxalicum | BTUB | 1 | 1 | |||||
| Penicillium palmense | BTUB | 1 | 1 | |||||
| Penicillium sp. | BTUB | 4 | 4 | |||||
| Talaromyces amestolkiae | BTUB | 1 | 1 | |||||
| Talaromyces diversus | BTUB | 3 | 3 | |||||
| Talaromyces minioluteus | BTUB | 1 | 1 | |||||
| Talaromyces pinophilus | BTUB | 1 | 1 | |||||
| Talaromyces rugulosus | BTUB | 1 | 1 | |||||
| Perenniporia | Perenniporia tenuis | ITS | 1 | 1 | ||||
| Phanerochaete | Phanerochaete sordida | ITS | 1 | 1 | ||||
| Phoma | Phoma cf. nebulosa | ITS | 1 | 1 | ||||
| Rhizopus | Rhizopus microsporus | ITS | 1 | 1 | ||||
| Rhizopus oryzae | ITS | 1 | 2 | 3 | ||||
| Scedosporium | Scedosporium apiospermum | BTUB | 4 | 3 | 7 | |||
| Scedosporium aurantiacum | BTUB | 2 | 2 | 4 | ||||
| Scedosporium boydii | BTUB | 1 | 3 | 4 | 8 | |||
| Scedosporium prolificans | BTUB | 1 | 1 | |||||
| Scopulariopsis | Scopulariopsis brevicaulis | ITS | 3 | 1 | 1 | 5 | ||
| Thanatephorus | Thanatephorus cucumeris | ITS | 1 | 1 | 2 | |||
| Trichoderma | Trichoderma harzianum | ITS | 1 | 1 | ||||
| Trichoderma longibrachiatum | ITS | 1 | 1 | |||||
| Trichophyton | Trichophyton equinum | ITS | 1 | 1 | ||||
| Trichophyton interdigitale | ITS | 14 | 8 | 6 | 3 | 31 | ||
| Trichophyton rubrum | ITS | 48 | 21 | 54 | 16 | 139 | ||
| Trichophyton tonsurans | ITS | 2 | 3 | 5 | ||||
| Trichophyton violaceum/soudanense | ITS | 1 | 1 | 5 | 7 | |||
| Veronaeopsis | Veronaeopsis sp. | ITS | 1 | 1 | ||||
| Total | 113 | 36 | 103 | 101 | 148 | 501 | ||
ITS, internal transcribed spacer; BTUB, beta-tubulin; EF, elongatin factor.
MALDI-TOF-based identification assessment of panel 2.
The 501 isolates included in panel 2 were identified using the MSI software with the defined threshold of 20. In parallel, the same 501 isolates were also identified using the Bruker-MALDI Biotyper and the IHEM/Marseille-MALDI Biotyper combinations. Two thresholds (1.7 as proposed by Normand et al. [18] and 2.0 as proposed by the supplier) were taken into account using the MALDI Biotyper software. For each isolate, four replicates were deposited, but only the deposit with the best identification score was taken into account, as previously proposed (18). The identifications obtained using the various algorithms were then classified into four categories (Table 3) according to the score and identification result. Regardless of the database/software combination, no false identifications were obtained. However, the percentage of isolate spectra that met the identification threshold varied greatly between the five identification systems (Table 3).
TABLE 3.
Panel 2 identification results obtained with the five identification systems
| Result for panel 2 sequenced strainsa | No. (%) of strains identified |
||||
|---|---|---|---|---|---|
| IHEM/MRS-MSI (threshold = 20) | IHEM/MRS-MBT (threshold = 1.7) | IHEM/MRS-MBT (threshold = 2.0) | Bruker-MBT (threshold = 1.7) | Bruker-MBT (threshold = 2.0) | |
| Correct at the species level | 435 (87.35) | 411 (82.53) | 312 (62.65) | 259 (52.01) | 119 (23.9) |
| Correct at the genus level | 26 (5.22) | 34 (6.83) | 12 (2.41) | 41 (8.23) | 9 (1.81) |
| False at the genus level | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Identification criteria not met | 37 (7.43) | 53 (10.64) | 174 (34.94) | 198 (39.76) | 370 (74.3) |
For each database/software combination, the number (%) of strains is specified. Correct, concordant with the molecular identification at either the species or the genus level. False, discordant with the molecular identification at the genus level. Identification criteria not met, score below the defined threshold.
Panel 2 identification results per center.
The percentage of spectra that met the identification criteria per center and the percentage of correct identifications at the species and genus level are listed in Table 4.
TABLE 4.
Performance of the five identification systems per participating center
| City | Parameter | % Identification (threshold value) |
||||
|---|---|---|---|---|---|---|
| IHEM/MRS-MSI (20) | IHEM/MRS-MBT (1.7) | IHEM/MRS-MBT (2.0) | Bruker-MBT (1.7) | Bruker-MBT (2.0) | ||
| Bordeaux | % identification overall | 88.16 | 84.58 | 48.98 | 66.81 | 41.23 |
| % identification at the species level | 85.96 | 82.53 | 48.32 | 66.08 | 40.79 | |
| % identification at the genus level | 88.16 | 84.50 | 48.98 | 66.81 | 41.23 | |
| Bruges | % identification overall | 80.65 | 84.95 | 43.55 | 54.30 | 19.89 |
| % identification at the species level | 79.57 | 81.18 | 42.47 | 47.31 | 19.35 | |
| % identification at the genus level | 80.65 | 84.95 | 43.55 | 54.30 | 19.89 | |
| Brussels | % identification overall | 90.18 | 80.36 | 49.36 | 38.14 | 7.78 |
| % identification at the species level | 85.20 | 75.51 | 47.58 | 32.78 | 7.53 | |
| % identification at the genus level | 90.18 | 80.23 | 49.36 | 38.14 | 7.78 | |
| Marseille | % identification overall | 94.63 | 93.61 | 71.23 | 59.70 | 23.80 |
| % identification at the species level | 94.12 | 93.44 | 71.12 | 59.02 | 23.80 | |
| % identification at the genus level | 94.63 | 93.61 | 71.23 | 59.70 | 23.80 | |
| Nice | % identification overall | 82.00 | 75.51 | 50.41 | 52.26 | 18.21 |
| % identification at the species level | 80.66 | 72.53 | 49.28 | 49.49 | 17.90 | |
| % identification at the genus level | 81.89 | 75.21 | 50.41 | 51.95 | 18.21 | |
Discrepancies between DNA identification and MSI results.
Twenty-six strains were incorrectly identified at the species level, although they reached the threshold for the MSI platform. The results obtained with these 26 isolates, the obtained scores, and the percentage of homology of corresponding DNA sequences are listed in Table 5. Most of these errors (20/26) corresponded to a misidentification among closely related species of the same species complex.
TABLE 5.
List of the 26 discrepancies between DNA identification and MSI result
| Discrepancy | Strain origin | Sequencing identification |
IHEM/MRS-MSI |
NCBI sequence comparison (% similitude)a | |||
|---|---|---|---|---|---|---|---|
| Identification | NCBI reference accession no. | Identification | Score | NCBI reference accession no. used for comparison | |||
| 1 | Brussels | Alternaria abundans | FJ214898 | Chalastospora gossypii | 29 | JQ693638 | 96 (574)* |
| 2 | Brussels | Arthrographis curvata | NR_132010 | Arthrographis kalrae | 27 | NR_126108 | 98 (581)* |
| 3 | Bordeaux | Aspergillus lentulus | EF669825 | Aspergillus aureolus | 28 | EF669808 | 94 (509)* |
| 4 | Brussels | Aspergillus pseudoglaucus | EF651917 | Aspergillus rubrobrunneus | 28 | EF651920 | 94 (403)* |
| 5 | Bordeaux | Aspergillus puulaauensis | EF652266 | Aspergillus versicolor | 23 | JN853979 | 96 (374)* |
| 6 | Bordeaux | Ceriporia lacerata | KP677607 | Hexagonia hydnoides | 34 | AF163576 | 96 (530) |
| 7 | Bordeaux | Ceriporia lacerata | KP677607 | Hexagonia hydnoides | 23 | AF163576 | 96 (530) |
| 8 | Bordeaux | Ceriporia lacerata | KP677607 | Hexagonia hydnoides | 39 | AF163576 | 96 (530) |
| 9 | Brussels | Fusarium incarnatum/equiseti | KF255493 | Fusarium chlamydosporum | 21 | KR071776 | 94 (527) |
| 10 | Marseille | Fusarium oxysporum | KT794174 | Fusarium proliferatum | 32 | KT716210 | 92 (649) |
| 11 | Marseille | Fusarium oxysporum | KT794174 | Fusarium proliferatum | 27 | KT716210 | 92 (649) |
| 12 | Marseille | Fusarium solani | KM231936 | Fusarium keratoplasticum | 37 | KT716219 | 91 (641)* |
| 13 | Brussels | Microsporum incurvatum | KC784357 | Microsporum gypseum | 31 | KC784355 | 95 (666)* |
| 14 | Bordeaux | Penicillium expansum | AY674400 | Penicillium chrysogenum | 27 | AY495981 | 94 (478) |
| 15 | Bordeaux | Penicillium glabrum | GU981619 | Eupenicillium pinetorum | 27 | JX271493 | 91 (422)* |
| 16 | Bordeaux | Talaromyces diversus | KJ865723 | Talaromyces stollii | 28 | JX315633 | 83 (429)* |
| 17 | Brussels | Trichoderma longibrachiatum | AY328039 | Trichoderma pseudokoningii | 46 | NR_120296 | 98 (644)* |
| 18 | Nice | Trichophyton equinum | KT310171 | Trichophyton tonsurans | 31 | KT310172 | 100 (612)* |
| 19 | Brussels | Trichophyton interdigitale | JX122216 | Trichophyton schoenleinii | 43 | DQ860752 to DQ860817 | 98 (443)* |
| 20 | Brussels | Trichophyton interdigitale | JX122216 | Trichophyton tonsurans | 44 | KT310172 | 98 (612)* |
| 21 | Brussels | Trichophyton interdigitale | JX122216 | Trichophyton tonsurans | 40 | KT310172 | 98 (612)* |
| 22 | Marseille | Trichophyton mentagrophytes | JX122271 | Trichophyton tonsurans | 36 | KT310172 | 97 (612)* |
| 23 | Marseille | Trichophyton rubrum | JX122363 | Trichophyton violaceum | 27 | KT310173 | 99 (644)* |
| 24 | Brussels | Trichophyton rubrum | JX122363 | Trichophyton violaceum | 28 | KT310173 | 99 (644)* |
| 25 | Brussels | Trichophyton violaceum | KT310173 | Trichophyton rubrum | 52 | JX122363 | 99 (644)* |
| 26 | Marseille | Trichophyton violaceum | KT310173 | Trichophyton rubrum | 39 | JX122363 | 99 (644)* |
*, Species belonging to the same species complex. The corresponding numbers of base pairs are indicated in parentheses.
Time required to perform online application and comparison with the Bruker software.
Identification of a full plate of 96 spectra took 8 min (5 s per spectrum) using the MALDI Biotyper software with the Bruker database (604 references) and 43 min (27 s per spectrum) with the IHEM/Marseille database (5,676 references). The MSI software performed 96 identifications in 14.50 s (0.14 s per spectrum).
Online application compatibility.
The online application was tested on Windows 7 and Windows 10. Four Web browsers were successfully tested and yielded the same identification results. The tested versions included Mozilla Firefox v35.0 and v43.0, Internet Explorer v9.0.8112.16421, Google Chrome v42.0.2311.90m, and Safari v10.0 (10602.1.50.0.10).
DISCUSSION
In this study, we confirm that the online identification of MALDI-TOF MS spectra derived from molds, including dermatophytes, is possible and that the tested identification system outperformed in situ identification using a database of nearly 6,000 references. The online application enabled us to identify up to 92.61% of 501 fungal isolates (panel 2) derived from human samples, originating from five different laboratories in two countries. Importantly, these results were obtained using a panel of strains that are particularly difficult to identify due to high species diversity and underrepresentation of relatively easy-to-identify species via MS, such as Aspergillus fumigatus, A. flavus, and A. terreus (which represent the majority of mold species identified in routine laboratories). Only 5% of the identifications were unsatisfactory (i.e., correct at the genus level but not at the species level), and none of the identifications were false at the genus level. These results are all the more encouraging considering that the laboratories used different protocols and various types of media for filamentous fungi culture. These results are also better than those usually obtained via phenotypic identification. In a study published in 2014, Gautier et al. have shown that phenotypic identification of filamentous fungi was indeed poorly reliable, with only 66.8% of strains correctly identified in a panel of 247 strains (16 species) (19). The results acquired using the online application also surpassed those obtained with the same set of strains identified against the Bruker database using the MALDI Biotyper software (only 52.01% of spectra identified and 8.23% false identifications at the species level when applying a 1.7 threshold, and a mere 23.9% identification rate when applying the recommended threshold of 2.0). Finally, the online system was rapid enough (a few seconds to identify a 96-spot assay) to enable real-time online identification. However, the identification delay may increase if a large number of users try to connect simultaneously to perform spectrum identification.
A low percentage of isolates (7.38%) corresponded to spectra that did not meet the identification threshold score. Such failures may be attributed to the lack of adequate references in the database or various issues associated with the protein extraction process (e.g., presence of agar in the fungal sample), matrix quality, and malfunction of the mass spectrometer. In this study, five different centers provided mass spectra for analysis, which involved sample preparation by at least 20 different technicians. Such user diversity probably had an effect on spectrum quality, which would explain some differences in results between the five centers (Table 4). In the future, when this identification system is more widely available, there is a risk that the percentage of spectra that fail to meet the identification criteria will increase. To minimize this problem, which could lead to discouraging results and interpretation errors, access to the site is limited only to users who are granted a login and password. To assign this login and password to a new user, we request an email contact and we will assess a set of spectra produced by the user to ensure the conformity of the sample extraction procedure.
Applying a simpler extraction method, such as depositing the sample onto the target and adding a drop of matrix, has been proposed by some authors (20–22). However, previous experiments have shown that due to the characteristics of the fungal wall, the quality of the resulting spectra is inferior to that obtained from bacterial samples (3). Therefore, we do not recommend such simplified extraction protocols. Inversely, the method recommended by Bruker, necessitating an overnight culture in liquid medium, was not used since it does not enhance the identification performance and was too complicated to implement in the routine setting (3).
Some false identification results still occurred, especially between species belonging to the same genus. Of the 26 errors, 10 concerned dermatophyte isolates, mainly involving confusion between Trichophyton rubrum and Trichophyton violaceum. Some mycologists proposed to consider the two species as a single one, but this proposal was not retained. Nevertheless, these two closely related species are known to be very difficult to discriminate even using various DNA targets (10, 23), leading to a consequent number of misidentifications in the available DNA databases. Three of the misidentifications involved basidiomycete species that were not represented in the reference database. This error can easily be bypassed by supplementing the reference database with basidiomycete species spectra. Most of the remaining identification errors (9/13) concerned closely related species with highly similar DNA sequences. Three of the four identification errors concerning species of the Fusarium genus should be assessed in further detail, since they concern species of different species complexes that should have been differentiated using MALDI-TOF technology (11, 24, 25). However, these identification errors should have no impact on the proposed clinical therapy because these species share the same antifungal susceptibility profile (26). The fourth error, misidentification of Fusarium solani as Fusarium keratoplasticum, which are both in the same species complex, would not result in modification of the treatment. Only one Aspergillus lentulus isolate was tested in this clinical panel and was identified using the online application as Aspergillus aureolus (a species that has not been described as pathogenic and lacks MIC data). The reason that led to misidentifications for this particular species should be assessed in future studies as A. lentulus is associated with higher risk of resistance to voriconazole than A. fumigatus. Regardless of the root cause of these inaccurate identifications, expanding the reference database would indeed help to reduce the already low misidentification rate (5). In the present study, we show that the online identification system is relatively rapid, even with a reference spectrum database of more than 11,000 spectra. Thus, supplementing the database with additional spectra is feasible and would improve the accuracy and range of identification.
To ensure a reasonable level of diversity in the tested panels, we collected specimens from five laboratories located in five different cities. Nevertheless, all laboratories were situated in Western Europe. In future studies, the performance of the online identification system should be tested using a more extensive group of laboratories located throughout the world. Subsequent findings will likely highlight the need to further enhance the spectral database to address the specificities of local fungal flora. Studies are currently ongoing with laboratories in North America and French Guiana.
Finally, the online application was only tested with spectra obtained with the Microflex system, since it is the most widespread MALDI-TOF technology to date (27, 28). In future investigations, the current online application will have to be adapted to identify spectra originating from different devices, such as the bioMérieux or Andromass systems.
Widespread access to an online fungal identification application based on mass spectra would likely represent a major breakthrough for the field of medical mycology. This system will allow all laboratories to make difficult diagnostics hitherto reserved for highly specialized laboratories. This technology will also ensure more reliable diagnostics in all implicated laboratories. Furthermore, as a growing number of biologists gain access to a common identification system, this approach will enable laboratories to generate metadata useful to detect the emergence of new species and thus more rapidly detect the epidemic spread of a given strain. Finally, this application would likely facilitate interaction and collaboration between medical mycologists. For this reason, we have decided not to launch a commercial application but instead offer a free online application available to medical mycology specialists.
MATERIALS AND METHODS
Panel description.
Panel 1 comprised 422 strains selected from both the BCCM/IHEM collection and the Clinical Mycology Laboratory of Marseille Hospital. This panel has already been used to assess the identification algorithm using the MALDI Biotyper software; the description of this panel and the results of the assessment are published elsewhere (18). The details of panel 1 are found in Table 1. This panel constitutes a diverse group of mold species and genera (126 species, 38 genera), including 15 strains (13 species) for which the species was not represented in the reference spectrum database.
Panel 2 consisted of 501 isolates collected in five hospitals in France and Belgium. The isolates were collected at different time periods. Only a portion of the isolates collected in these five hospitals—those that had been sequenced using appropriate DNA targets—were included in panel 2 (501/1,269, 39.4%), while the remaining 768 isolates, which corresponded to easy-to-identify species, were identified exclusively based on phenotypic criteria. The species that were not represented in the database were intentionally retained in the panel as negative controls. Details concerning the isolates collected at each institution are provided in Table 1.
Fungal cultures.
Prior to identification, the five laboratories used different culture media: Sabouraud chloramphenicol gentamicin (Becton Dickinson [BD]) on Oxoid petri dishes or culture tubes, Sabouraud Actidione (Bio-Rad), CHROMagar Candida (BD), Sabouraud dextrose chloramphenicol (BD/Biokar), in-house made Lactrimel agar medium, Difco Sabouraud dextrose agar (BD) with 0.5 mg/liter chloramphenicol (Certa), and Sabouraud chloramphenicol 2 agar medium (bioMérieux). The laboratories also used different culture procedures, with culture temperatures ranging from 25 to 37°C and incubation times varying from 24 h to 28 days. Some strains were also received from other laboratories (mostly from bacteriology laboratories) for identification after growth on other culture media such as Can2, blood agar, chocolate agar, and Cepacia medium from various suppliers.
DNA sequencing.
All 923 strains (422 in panel 1 and 501 in panel 2) were identified via DNA sequencing in either Marseille or Brussels. The rRNA ITS2 region (primer sequences, ITS3 [GCA TCG ATG AAG AAC GCA GC] and ITS4c [TCC TCC GCT TAT TGA TAT GC]) was sequenced for all strains. When necessary, a second locus was sequenced, especially for certain relevant taxa: the partial beta-tubulin gene (primer sequences, Bt2A [GGT AAC CAA ATC GGT GCT GCT TTC] and Bt2B [ACC CTC AGT GTA GTG ACC CTT GGC]) was sequenced for Aspergillus, Penicillium, and Scedosporium species, while translation elongation factor 1-alpha (primer sequences, EF1 [ATG GGT AAG GAR GAC AAG AC] and EF2 [GGA RGT ACC AGT SAT CAT GTT]) was sequenced for Fusarium species. In all cases, identification was validated only when the obtained sequence was longer than 350 bp, with at least 99% homology using the NCBI Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) or the CBS nucleotide database (http://www.cbs.knaw.nl/Collections/BioloMICSSequences.aspx). For cases in which the DNA identification criteria mentioned above were not reached, the identification result was conserved only at the genus level. The details of the sequenced strains are listed in Table 2.
Mass spectrometry software.
Two software applications were used for mass spectrum identification: the MALDI Biotyper 3 by Bruker, with two thresholds (1.7 and 2.0) tested each time, and the MSI online application. Using the MSI software, identification of a given clinical sample was performed by successively aligning the spectrum with each reference spectrum and accounting for the common peaks between the two spectra. A maximum score of 100 was assigned when all peaks of the clinical spectrum were present at the same mass point (m/z) on the reference spectrum to which it was compared or when all peaks of the reference spectrum were present at the same exact mass point on the clinical spectrum. The score decreased when there was a shift, even a small one, between two peaks or when a peak found on one spectrum was lacking on the other. The MSI software not only provides the species corresponding to the reference spectrum with the highest score but also provides the best scores obtained with reference spectra corresponding to closely related species and to other species. The MSI software also provides a list of the 100 best recognized references, thus allowing the user to assess result consistency. Therefore, even with a score above 20, the user will determine whether high scores are also obtained with other species. The user also notice if the result is supported by only one or two references that are challenged by references corresponding to other species.
MS reference databases.
We used two different databases to perform the comparisons: the Bruker reference database and an in-house reference database. The Bruker database comprised 604 references of yeast and fungus (332 official references in the Bruker Taxonomy-Eukaryota and 272 unofficial references in the _DB_Content_FilFungi_111123 appendix). The in-house reference database was generated in coordination with the BCCM/IHEM culture collection and the Mycology Laboratory of Marseille Hospital using the protocol described by Normand et al. (5). The database used for this study comprised in-house references corresponding to 1,913 strains representing 938 species and 246 genera. The details concerning metaspectra construction are described elsewhere (5). Overall, a total of 5,676 references were used for identification purposes with the MALDI Biotyper. The same 1,913 strains were used to construct the MSI online reference database. No metaspectra were generated; instead, regardless of the fungus family, two spectra per culture/subculture were taken into account. Thus, a total of 11,851 spectra were included in the MSI reference database.
MS sample preparation.
As described in previous studies, all strains were subjected to protein extraction, and the extracts were deposited in quadruplicate for identification purposes (3, 9). The isolates were treated using a protocol described by Cassagne et al., which includes a formic acid and acetonitrile protein extraction step. Four aliquots of 1 μl of protein extract per isolate were deposited, and the samples were then covered with 1 μl of HCCA (α-cyano-4-hydroxycinnamic acid) matrix. MS acquisition was performed in the respective participating centers using the Bruker Microflex system.
ACKNOWLEDGMENTS
We thank the laboratory technicians who performed the cultures, mass spectrometry analyses, and sequencing analyses: Sam Roesems and Jessie Claessens at the BCCM/IHEM Institute; Johan Breynaert and Kristof Vandoorslaer at the Universitair Ziekenhuis Brussels; Nancy Ramaut in Bruges; Geraldine Desserre, Aurelie Victor, Philippe Groell, Chantal Kuleczka, Marius Verin, and Claude Hargous in Bordeaux; Elodie Chandemerle, Caroline Pacchioni-Dubois, Krystel Nerini, and Stephane Melhem in Nice; and Nathalie Berenger, Catherine Riou, Karine Bildgen, Georges Oggiano, Jeremy Duponchel, and Nicolas Aldrovandi in Marseille. We especially thank Sandra Moore for proofreading the English version of the manuscript.
This research received no specific grant from any funding agency in the public, commercial, or nonprofit sectors.
REFERENCES
- 1.van Veen SQ, Claas ECJ, Kuijper EJ. 2010. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol 48:900–907. doi: 10.1128/JCM.02071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassagne C, Cella A-L, Suchon P, Normand AC, Ranque S, Piarroux R. 2012. Evaluation of four pretreatment procedures for MALDI-TOF MS yeast identification in the routine clinical laboratory. Med Mycol 51:371–377. doi: 10.3109/13693786.2012.720720. [DOI] [PubMed] [Google Scholar]
- 3.Cassagne C, Ranque S, Normand AC, Fourquet P, Thiebault S, Planard C, Hendrickx M, Piarroux R. 2011. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS One 6:e28425. doi: 10.1371/journal.pone.0028425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranque S, Normand AC, Cassagne C, Murat JB, Bourgeois N, Dalle F, Gari-Toussaint M, Fourquet P, Hendrickx M, Piarroux R. 2013. MALDI-TOF mass spectrometry identification of filamentous fungi in the clinical laboratory. Mycoses 57:135–140. doi: 10.1111/myc.12115. [DOI] [PubMed] [Google Scholar]
- 5.Normand AC, Cassagne C, Ranque S, l'Ollivier C, Fourquet P, Roesems S, Hendrickx M, Piarroux R. 2013. Assessment of various parameters to improve MALDI-TOF MS reference spectra libraries constructed for the routine identification of filamentous fungi. BMC Microbiol 13:76. doi: 10.1186/1471-2180-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau AF, Drake SK, Calhoun LB, Henderson CM, Zelazny AM. 2013. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:828–834. doi: 10.1128/JCM.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulthess B, Ledermann R, Mouttet F, Zbinden A, Bloemberg GV, Böttger EC, Hombach M. 2014. Use of the Bruker MALDI Biotyper for identification of molds in the clinical mycology laboratory. J Clin Microbiol 52:2797–2803. doi: 10.1128/JCM.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packeu A, Hendrickx M, Beguin H, Martiny D, Vandenberg O, Detandt M. 2013. Identification of the Trichophyton mentagrophytes complex species using MALDI-TOF mass spectrometry. Med Mycol 51:580–585. doi: 10.3109/13693786.2013.770605. [DOI] [PubMed] [Google Scholar]
- 9.L'Ollivier C, Cassagne C, Normand AC, Bouchara JP, Contet-Audonneau N, Hendrickx M, Fourquet P, Coulibaly O, Piarroux R, Ranque S. 2013. A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med Mycol 51:713–720. doi: 10.3109/13693786.2013.781691. [DOI] [PubMed] [Google Scholar]
- 10.Packeu A, De Bel A, l' Ollivier C, Ranque S, Detandt M, Hendrickx M. 2014. Fast and accurate identification of dermatophytes by matrix-assisted laser desorption ionization-time of flight mass spectrometry: validation in the clinical laboratory. J Clin Microbiol 52:3440–3443. doi: 10.1128/JCM.01428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triest D, Stubbe D, De Cremer K, Piérard D, Normand AC, Piarroux R, Detandt M, Hendrickx M. 2015. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of molds of the Fusarium genus. J Clin Microbiol 53:465–476. doi: 10.1128/JCM.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alanio A, Beretti JL, Dauphin B, Mellado E, Quesne G, Lacroix C, Amara A, Berche P, Nassif X, Bougnoux ME. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin Microbiol Infect 17:750–755. doi: 10.1111/j.1469-0691.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- 13.Guarro J, de Hoog GS. 1995. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Baarn, Netherlands. [Google Scholar]
- 14.De Hoog GS, Guarro J. 2000. Atlas of clinical fungi, 2nd ed American Society for Microbiology, Washington, DC. [Google Scholar]
- 15.De Hoog GS, Guarro J. 2001. Atlas of clinical fungi, 2nd ed American Society for Microbiology, Washington, DC. [Google Scholar]
- 16.De Hoog GS. 2004. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands. [Google Scholar]
- 17.De Hoog GS. 2005. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands. [Google Scholar]
- 18.Normand AC, Cassagne C, Gautier M, Becker P, Ranque S, Hendrickx M, Piarroux R. 2017. Decision criteria for MALDI-TOF MS-based identification of filamentous fungi using commercial and in-house reference databases. BMC Microbiol 17:25. doi: 10.1186/s12866-017-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautier M, Ranque S, Normand AC, Becker P, Packeu A, Cassagne C, l'Ollivier C, Hendrickx M, Piarroux R. 2014. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: revolutionizing clinical laboratory diagnosis of mould infections. Clin Microbiol Infect 20:1366–1371. doi: 10.1111/1469-0691.12750. [DOI] [PubMed] [Google Scholar]
- 20.Chen H-Y, Chen Y-C. 2005. Characterization of intact Penicillium spores by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom RCM 19:3564–3568. doi: 10.1002/rcm.2229. [DOI] [PubMed] [Google Scholar]
- 21.Erhard M, Hipler U-C, Burmester A, Brakhage AA, Wöstemeyer J. 2008. Identification of dermatophyte species causing onychomycosis and tinea pedis by MALDI-TOF mass spectrometry. Exp Dermatol 17:356–361. doi: 10.1111/j.1600-0625.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 22.De Respinis S, Vogel G, Benagli C, Tonolla M, Petrini O, Samuels G. 2010. MALDI-TOF MS of Trichoderma: a model system for the identification of microfungi. Mycol Prog 9:79–100. doi: 10.1007/s11557-009-0621-5. [DOI] [Google Scholar]
- 23.De Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y. 2017. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Hatmi AM, Mirabolfathy M, Hagen F, Normand AC, Stielow JB, Karami-Osbo R, van Diepeningen AD, Meis JF, de Hoog GS. 2016. DNA barcoding, MALDI-TOF, and AFLP data support Fusarium ficicrescens as a distinct species within the Fusarium fujikuroi species complex. Fungal Biol 120:265–278. doi: 10.1016/j.funbio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Al-Hatmi AMS, Normand AC, van Diepeningen AD, Hendrickx M, de Hoog GS, Piarroux R. 2015. Rapid identification of clinical members of Fusarium fujikuroi complex using MALDI-TOF MS. Future Microbiol 10:1939–1952. doi: 10.2217/fmb.15.108. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hatmi AM, Normand AC, Ranque S, Piarroux R, de Hoog GS, Meletiadis J, Meis JF. 2016. Comparative evaluation of Etest, EUCAST and CLSI methods for amphotericin B, voriconazole, and posaconazole against clinically relevant Fusarium species. Antimicrob Agents Chemother 61:e01671-16. doi: 10.1128/AAC.01671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassagne C, Normand AC, l'Ollivier C, Ranque S, Piarroux R. 2016. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 59:678–690. doi: 10.1111/myc.12506. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti M, Posteraro B. 2017. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 55:369–379. doi: 10.1128/JCM.01640-16. [DOI] [PMC free article] [PubMed] [Google Scholar]