Abstract
Numerous studies have investigated the association between eosinophilia and clinical outcome of patients with chronic obstructive pulmonary disease (COPD) but the evidence is conflicting. We conducted a pooled analysis of outcome measures comparing eosinophilic and non-eosinophilic COPD patients. We searched articles indexed in four databases using Medical Subject Heading or Title and Abstract words including COAD, COPD, eosinophil, eosinophilia, eosinopenia from inception to December 2016. Observational studies and randomized controlled trials with parallel groups comparing COPD patients with and without eosinophilia were included. Comparing to the non-eosinophilic group, those with eosinophilic COPD had a similar risk for exacerbation in 12 months [Odds ratio = 1.07, 95% confidence interval (CI) 0.86–1.32, P = 0.55] and in-hospital mortality [OR = 0.52, 95% CI 0.25–1.07]. Eosinophilia was associated with reduced length of hospital stay (P = 0.04). Subsequent to therapeutic interventions, eosinophilic outpatients performed better in pulmonary function tests [Mean Difference = 1.64, 95% CI 0.05–3.23, P < 0.001]. Inclusion of hospitalized patients nullified the effect. Improvement of quality of life was observed in eosinophilic subjects [Standardized Mean Difference = 1.83, 95% CI 0.02–3.64, P = 0.05], independent of hospitalization status. In conclusion, blood eosinophilia may be predictive of favorable response to steroidal and bronchodilator therapies in patients with stable COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is an obstructive airway disease with both overlapping and distinctive features as with asthma1. Asthma is characterized by eosinophilic inflammation2, whereas COPD is predominantly associated with neutrophilic inflammation in the airways3. Growing evidence suggested that neither characteristic was immutably ingrained in either disease. This difference in cellular composition of induced sputum may, if ever, be indistinguishable between these disease groups2. Increased sputum eosinophils has been reported in both stable3 and exacerbation phase4 of patients with COPD, implying the potential role of eosinophils in the pathogenesis of COPD2.
Eosinophilia is generally defined as greater or equal to 2% eosinophils in either blood or sputum3,5–7. Alternatively, an absolute blood eosinophil count of 0.34 × 109 cells per liter can be used as a threshold for risk stratification7. Peripheral blood eosinophil count is highly associated with eosinophilia of the respiratory tract5. This blood biomarker has also been shown to reflect submucosal eosinophilia of the lung and reticular basement membrane thickening8. Given this context, we considered that patients with COPD who had more than 2% of eosinophils, either in the blood or sputum, as eosinophilic COPD.
Acute exacerbation of COPD significantly increases symptoms, deteriorates pulmonary function, increases rate of hospitalization and lengthens hospital stay further impairing functional capacity and quality of life (QOL) imposing additional burden to healthcare system9–11. The in-hospital mortality can reach 30% or more12. Seeking for predictive biomarkers for clinical outcome in this population is thus of high priority.
Numerous studies have evaluated eosinophilia in relation to exacerbation risk5,7,13, length of hospital stay14–16, in-hospital mortality12,17,18, and response to steroidal and bronchodilator therapies9–11 but the evidence is conflicting. Some studies have reported a higher risk for exacerbation in patients with eosinophilic COPD13,19. Conversely, a retrospective study suggested that a higher level of eosinophils protected against disease aggravation16. Other research teams failed to detect any association5,7,20.
We conducted a systematic review and meta-analysis of clinical outcome measures comparing patients with COPD who had eosinophilia and those without eosinophilia.
Results
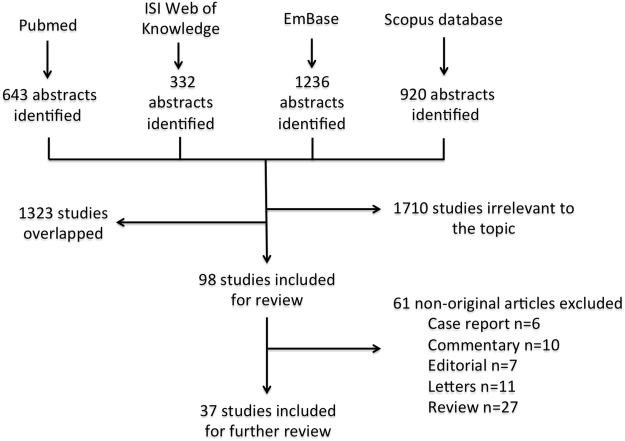
Of 3,131 abstracts identified by the initial search, 1,710 and 1,323 articles were removed, respectively, because of irrelevance or overlaps. After exclusion, 37 studies involving 99,122 patients published between 1998 and 2016 were included for qualitative synthesis (Fig. 1). Of these, 14 studies were included in meta-analysis. The number of entries derived from different search terms has been summarized in Table 1. The mean age of the subjects was 66.95 years with the proportion of male subjects ranging from 455 to 100%21. On average, each subject had a 46 pack-year smoking history. The mean forced expiratory volume in one second (FEV1) ranged from 0.96 L to 1.62 L. A total of 21 studies explored the role of blood eosinophilia in COPD. The remaining articles detected eosinophils in sputum and bronchial fluid after treatment with bronchodilators or steroidal therapy. The description of studies is summarized in Table 2. More than half of the included studies were either conducted in the United Kingdom1,9–11,13,17,18,22–27 or other European countries2–4,21,28–31. Eleven studies were originated from the Asia-Pacific region5,6,32–35 and the North America19,20,36–38. There was only a single relevant publication from the Middle East12.
Figure 1.
Flow diagram of literature search and selection of studies.
Table 1.
Number of entries by different search terms.
| Keywords | PubMed | ISI | EmBase | Scopus |
|---|---|---|---|---|
| Eosinophil | 41656 | 19002 | 53271 | — |
| COPD | 66801 | 36622 | 59900 | — |
| COAD | 62620 | 406 | 650 | 737 |
| Chronic Obstructive Pulmonary Disease | 62138 | 36569 | 64583 | 62441 |
| Chronic Obstructive Airway Disease | 62957 | 9182 | 16818 | 17754 |
| COPD OR COAD OR Chronic Obstructive Pulmonary Disease OR Chronic Obstructive Airway Disease | 68033 | 53234 | 91310 | 75056 |
| (Esosinophil) AND (COPD OR COAD OR Chronic Obstructive Pulmonary Disease OR Chronic Obstructive Airway Disease) | 643 | 332 | 1236 | 920 |
| Total | 3131 | |||
Table 2.
Description of the included studies.
| First author | Year | Country | Single/Multi-center | Number of subjects | Study design | Mean age (Years) | Male (%) | Baseline FEV1 | Smoking (Pack-years) | Specimens | Eosinophil measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bafadhel | 2009 | UK | Single | 34 | Longitudinal | 68 | 82.4 | 36% Pred | 45 | Sputum | Absolute and differential count |
| Bafadhel | 2011 | UK | Single | 145 | Longitudinal | 69 | 70 | 1.33 L | 49 | Blood and Sputum | Absolute and differential count |
| Bafadhel | 2012 | UK | Single | 164 | RCT | 69 | 65.2 | 1.19 L | 54.5 | Blood and Sputum | Absolute and differential count |
| Bafadhel | 2016 | UK | Multiple | 243 | Prospective cohort | 71 | 55 | 1.05 L | 49 | Blood | Absolute and differential count |
| Balzano | 1999 | Italy | Single | 46 | Case-control | 66.3 | 100 | 46.6% Pred | ≥1 | Sputum | Differential count and ECP level |
| Barnes | 2016 | UK | Single | 751 | RCT | 63.8 | 72 | 1.32 L | 43.2 | Blood | Absolute and differential count |
| Bathoorn | 2009 | The Netherlands | Single | 45 | Longitudinal | 64 | 81.6 | 63% Pred | 40 | Blood and Sputum | Absolute and differential count |
| Brightling | 2000 | UK | Single | 67 | RCT | 68 | 59 | 1.15 | 33 | Sputum | Differential count and ECP level |
| Couilard | 2016 | USA | Single | 167 | Retrospective cohort | 71.4 | 51.5 | 52.2% Pred | NA | Blood | Differential count |
| Brightling | 2005 | UK | Single | 60 | RCT | 67 | 66 | 1.22 | 40 | Blood and Sputum | Absolute and differential count |
| D’Armiento | 2009 | USA | Single | 148 | Case-control | 65.8 | 58.1 | 41.3% Pred | 57.8 | Lung larvage and plasma | Lung lavage eotaxin-I level |
| DiSantostefano | 2016 | USA | Population-based | 948 | Cross-sectional | 59.5 | 59.7 | ≤70% Pred | ≥10 | Blood | Absolute and differential count |
| Duman | 2015 | Turkey | Single | 1704 | Retrospective cohort | 70 | 66.9 | ≤70% Pred | NA | Blood | Absolute and differential count |
| Eltobili | 2014 | USA | Single | 103 | Case-control | 66.5 | 66.9 | 51 | 48 | Blood and Sputum | Absolute and differential count |
| Fabbri | 2003 | Italy | Single | 46 | Case-control | 65.3 | 65.2 | 1.62 L | 35.8 | Sputum and bronchial biopsy | Differnetial count and histology |
| Fijimoto | 1999 | Japan | Single | 24 | Prospective cohort | 69 | 100 | 40.5% Pred | 60 | Sputum | Absolute and differential count |
| Fujimoto | 2005 | Japan | Single | 62 | Longitudinal nested case-control | 68.5 | 94 | 1.40 L | 50.5 | Sputum | Absolute and differential count |
| Gorska | 2008 | Poland | Single | 39 | Case-control | 56.8 | 58.8 | 73% Pred | 38.6 | Sputum | Absolute and differential count |
| Hinds | 2016 | USA | Multiple | 3255 | RCT | 65 | 61 | ≤70% Pred | ≥10 | Blood | Absolute and differential count |
| Holland | 2010 | UK | Single | 65 | Retrospective cohort | 75.9 | NA | NA | NA | Blood | Differential count |
| Iqbal | 2015 | UK | Multiple | 4647 | Retrospective cohort | ≥40 | NA | ≤70% Pred | ≥10 | Blood | Absolute and differential count |
| Kitaguchi | 2012 | Japan | Single | 63 | Case-control | 72 | 90.5 | 47.5% Pred | 60.8 | Sputum | Absolute and differential count |
| Louis | 2002 | UK | Single | 49 | Case-control | 61 | 73.3 | 54% Pred | ≥20 | Sputum | Differential count and ECP level |
| Mercer | 2005 | UK | Single | 19 | Longitudinal | 69 | 85 | 1 L | NA | Sputum | Absolute and differential count |
| Negewo | 2016 | Australia | Multiple | 141 | Case-control | 69.8 | 63 | 57.5% Pred | 37.5 | Blood | Absolute and differential count |
| Papi | 2006 | Italy | Single | 64 | Longitudinal | 70.6 | 87.5 | 0.96 L | 48.3 | Sputum | Absolute and differential count |
| Park | 2016 | Korea | Single | 130 | Prospective cohort | 67 | 97.7 | ≤80% Pred | 46 | Blood | Absolute and differential count |
| Pavord | 2016 | UK | Multiple | 3045 | Retrospective cohort | 64.1 | 79 | ≤70% Pred | 38 | Blood | Absolute and differential count |
| Perng | 2006 | Taiwan | Single | 62 | RCT | 72 | 98.4 | 1.27 L | 48 | Sputum | Absolute and differential count |
| Pesci | 1998 | Italy | Single | 12 | Case-control | 62.6 | 91.7 | 71.1% Pred | 38.6 | Bronchial larvage | Differnetial count and ECP level |
| Rahimi-rad | 2015 | Iran | Single | 100 | Prospective cohort | 70.8 | 69 | 37.27% Pred | NA | Blood | Differential count |
| Salturk | 2015 | Turkey | Single | 647 | Retrospective cohort; Nested case-control | 68 | 80.8 | NA | 41.5 | Blood | Differential count |
| Serafino-Agrusa | 2016 | Italy | Single | 132 | Retrospective cohort; Nested case-control | 72.9 | 68.9 | 44.9% Pred | 70.3 | Blood | Absolute and differential count |
| Siva | 2007 | UK | Single | 82 | RCT | 70 | 67 | 1.02 L | 49.1 | Blood and Sputum | Absolute and differential count |
| Snoeck-Stroband | 2008 | The Netherlands | Multiple | 114 | Case-control | 60 | 86.8 | 63% Pred | 41 | Sputum and bronchial biopsy | Absolute and differential count |
| Vedel-Krogh | 2016 | Denmark | Population-based | 81668 | Prospective cohort | 58 | 45 | 78% Pred | 30 | Blood | Absolute and differential count |
| Zanini | 2015 | Italy | Single | 31 | Cross-sectional | 67 | 79.3 | 68% Pred | 51 | Sputum | Absolute and differential count |
Keys: ECP, eosinophil cationic protein; NA, not reported; Pred, predicted; RCT, Randomized controlled trial.
Overall, included studies fell into low to moderate quality (Supplementary Tables 1 and 2). Of 24 non-randomized observational studies evaluated by Newcastle-Ottawa scale, the mean score was 4.5 out of nine (range: 2–6). Five studies scored six or above in a nine-point scale, indicating high study quality6,7,11,22,30. In 13 randomized control trials assessed by Cochrane Collaboration Risk of Bias tool, seven studies were rated as low risk in terms of allocation concealment, blinding of participants and personnel, blinding of outcome assessment and incomplete outcome data9–11,20,24,26,27. Notably, two studies were ranked as high risk for randomization, blinding, and selective reporting4,32.
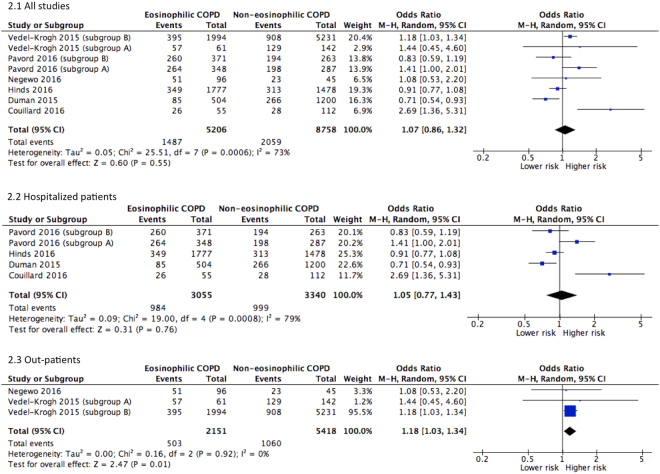
Eight populations of six studies5,7,13,16,19,20 were pooled for risk analysis. Overall, no association was observed between eosinophilia and risk for exacerbation warranting hospital admission in 12 months (OR = 1.07, 95% CI 0.86–1.32, P = 0.55, I 2 = 73%). This null effect remained in sub-group analysis of studies involving hospitalized COPD patients13,16,19,20. Interestingly, in patients with stable COPD as defined as having no hospitalization in the previous 12 months, eosinophilia appears to increase the risk for exacerbation by 18% (OR = 1.18, 95% CI 1.03–1.34, I 2 = 0%) (Fig. 2 ).
Figure 2.
Forest plots of studies comparing the risk for exacerbation in 12 months in COPD patients with or without eosinophilia. Vedel-Krogh (2015) subgroup A, clinical COPD; Vedel-Krogh (2015) subgroup B, COPD cohort in general population; Pavord (2016) subgroup A, COPD patients on fluticasone propionate and salmeterol; Pavord (2016) subgroup B, COPD patients on fluticasone propionate.
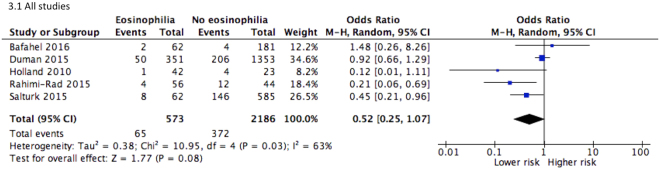
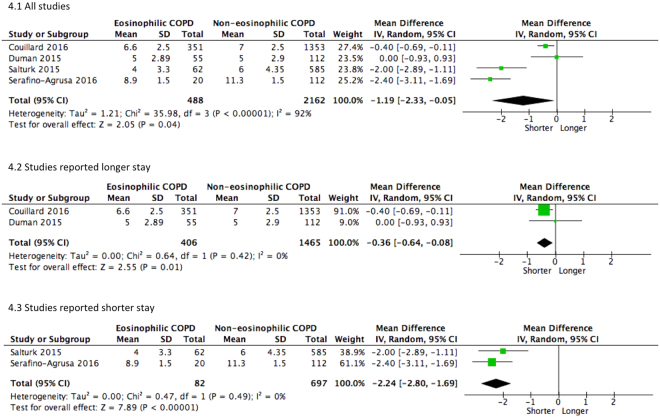
Pooled estimate of five studies12,14,16–18 did not indicate an association between eosinophilia and in-hospital mortality, though approaching statistical significance (P = 0.08). Of note, a single largest study published in the Lancet26 did not identify any association between clinical outcomes and eosinophilia. Although pooled estimate of the other studies12,14,17,18 showed that eosinophilia was a protective factor against in-hospital mortality (OR = 0.38, 95% CI 0.17–0.86, I 2 = 35%), these studies have to be interpreted with cautions due to potential risk of bias. Patients with eosinophilic COPD had 1.2 days shorter hospital stay than non-eosinophilic individuals. Given moderate to high heterogeneity of overall estimates, sensitivity analysis was performed. Except for in-hospital mortality, no single study substantially altered the pooled estimates (Figs 3 and 4).
Figure 3.
Forest plots of studies comparing the risk for in-hospital mortality in COPD patients with or without eosinophilia.
Figure 4.
Forest plots of studies comparing the mean difference of the length of hospital stay.
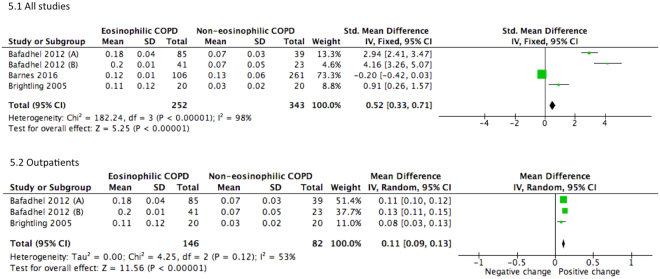
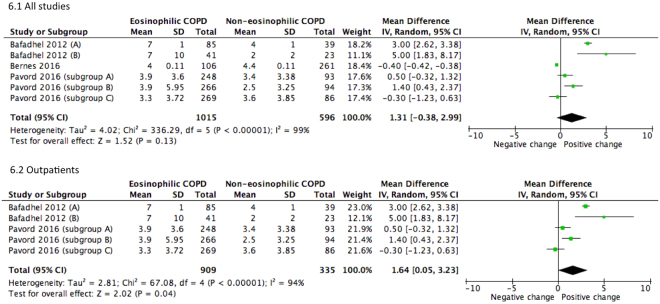
Subsequent to concurrent treatments with bronchodilators and steroids the pooled estimate revealed slight improvement in change of FEV1 (SMD = 0.52, 95% CI 0.33–0.71) (Fig. 5). Sub-group analysis has also shown that outpatients with eosinophilic COPD exhibited improvement in pulmonary function. For outpatient groups, the combined mean differences for FEV1 and percentage of predicted FEV1 were 0.11 L (95% CI 0.09–0.13, P < 0.001) and 1.64% (95% CI 0.05–3.23, P < 0.001), respectively (Figs 5 and 6).
Figure 5.
Forest plots of studies comparing the mean difference of the change of FEV1 in COPD patients after therapy. Bafadhel (2012) subgroup A, clinical outcomes in 2 weeks after therapy. Bafadhel (2012) subgroup B, clinical outcomes in 6 weeks after therapy.
Figure 6.
Forest plots of studies comparing the mean difference of the change of % FEV1 predicted in COPD patients after therapy. Bafadhel (2012) subgroup A, clinical outcomes in 2 weeks after therapy. Bafadhel (2012) subgroup B, clinical outcomes in 6 weeks after therapy. Pavord (2016) subgroup A, COPD patients on fluticasone propionate and salmeterol; Pavord (2016) subgroup B, COPD patients on fluticasone propionate; Pavord (2016) subgroup C, COPD patients on salmeterol.
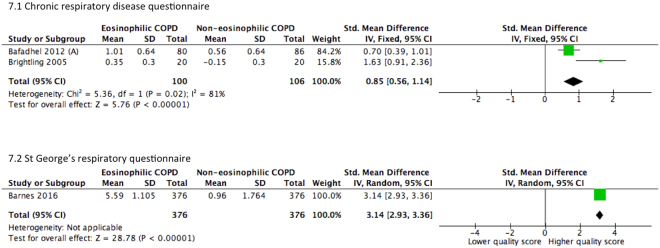
Of the three studies comparing reported QOL in patients with COPD, chronic respiratory disease questionnaire (CRQ)9,10 and St George’s respiratory questionnaire (SGRQ) were used11. The eosinophilic group consistently reported a higher QOL score subsequent to therapy. For studies using CRQ, a standardized mean difference of 0.85 (95% CI 0.56–1.14) was observed. For studies using SGRQ, an improved quality of life was also reported (SMD = 3.14, 95% CI 2.93–3.36). The pooled analysis is presented in Fig. 7.
Figure 7.
Forest plots of studies comparing the standardized mean difference of the change of quality of life scores in COPD patients after therapy. Pavord (2016) subgroup A, COPD patients on fluticasone propionate and salmeterol.
Discussion
Overall, eosinophilia in COPD patients does not contribute to exacerbation risk, in-hospital mortality, and length of hospital stay. However, higher eosinophil count in the outpatient sub-group demonstrated an increased risk of exacerbation by 18%. On the other hand, eosinophilic COPD patients appeared to be more responsive to therapeutic interventions.
In previous investigation of hospitalized COPD patients with severe exacerbation, eosinophilia lacked association with more than three-fold increased risk for re-admission in 12 months19. Retrospective analysis of COPD population with a post-bronchodilator FEV1/forced vital capacity (FVC) ratio below 0.7 did not identify significant difference in exacerbation risk amongst the eosinophil dominant group22. These were in contrast to a Turkish study in which a greater risk for re-admission was demonstrated in the eosinophilic group16. In a Dutch general population study, eosinophilia was found to increase risk for acute exacerbation of COPD7. Consistently, we found 18% increased risk for disease aggravation in outpatients. Exacerbation has been linked to airway inflammation characterized by eosinophilia4,6,24 and imbalance of metalloproteinases23. Higher level of eotaxin, an eosinophil chemotactic factor, is elevated in pulmonary lavage37. It has been suggested that frequency and severity of COPD exacerbation was a result of impaired macrophage efferocytosis of eosinophils36. Marked eosinophilia was observed in virus-induced exacerbations30.
Our pooled analysis showed that eosinophilia is associated with reduced length of hospital stay. This is consistent with previous studies including severely exacerbated COPD patients14,18. Conversely, peripheral blood eosinopenia increased in-hospital mortality by up to five-fold12,17. The disparity may be attributable to the timing of blood specimen collection. For hospitalized patients, samples were collected at the time of admission12,14,16–18. The time for collection in the outpatient group varies across studies and included at the screening stage11, at exacerbation10, and at 24 h after bronchodilator therapy9. In addition, recent hospitalization histories of these outpatients were uncertain9–11. In other words, they may have never been hospitalized or had follow-up at clinics soon after discharge. It has been suggested that airway eosinophilia facilitated responsiveness to bronchodilator and steroidal therapies26,33. The better response to therapy in this patient population may explain the consistently shorter length of stay and lower mortality.
Eosinophilia has been suggested to indicate individual responsiveness to bronchodilator and steroidal therapies9–11,13,15,25,26,34. Post-hoc analysis confirmed that level of eosinophil correlates with the response to bronchodilators27. Specifically, post-bronchodilator FEV1 and sputum eosinophil level had a high correlation of 0.8231. After oral prednisolone therapy, sputum eosinophil count changed accordingly along with interleukin-525. Blood eosinophils were also found to be associated with changes in pulmonary function after inhaled corticosteroids10,11,13,20. In our meta-analysis, although the predicted %FEV1 changed by 1.64%, this may represent a substantial improvement given these subjects were considered as severe COPD with baseline predicted %FEV1 less than 50%9,10. However, the addition of hospitalized patients nullified the effect. This suggested that disease severity may be a significant confounder in the observed relationship.
The overall risk of bias in the included randomized control trials ranged from low to moderate. The inferior quality was mostly attributed to unclear sequence generation and likelihood of selective outcome reporting4,32,34,35,37. Eight of the studies applied allocation concealment, and blinding of participants and outcome assessors9–11,18,20,24,27,35. In quasi-experimental studies, the potential risks of bias included self-reporting for outcomes, insufficient follow-up period and unclear relationship between loss of follow-up and outcome of interest. In addition, appropriate adjustments were not performed for previously reported confounders associated with eosinophil level and clinical outcome of COPD38. The majority of the included population was originated from the United Kingdom and other European countries; only a few studies were conducted in the Continent of Asia and the America. This racially skewed population may preclude the generalizability of the evidence.
We performed this systematic review according to a pre-defined data abstraction form. Minor alterations were made to facilitate data pooling. There were missing data on some of the outcome measures of our interest, reducing the number of eligible studies. Given the limited number of included studies for each outcome comparison, neither funnel plot nor Doi plot were conducted to examine publication bias. Our sensitivity analysis revealed that, except for in-hospital mortality, the pooled estimates remained stable.
Given no consensus on definition of eosinophilia, there may be mixing of eosinophilic and non-eoinophilic groups of COPD patient, diluting the effect size. The estimation of eosinophil level varies with the type of specimens. Within the same patient group, bronchial biopsies yielded lower eosinophil count than induced sputum29. Importantly, the temporal variation of eosinophilia in COPD was largely ignored in the included studies. Longitudinal study of 1,483 patients with COPD revealed that 49% of the subjects had variable eosinophil counts39. Only 37% and 14% of the individuals were persistently eosinophilic and eosinopenic, respectively39. The level of this cellular marker can increase considerably soon after sputum induction40. In this connection, spotshot sampling may lead to misclassification of case and control.
The moderate to high heterogeneity of the pooled estimates suggests the presence of unknown confounders in association with eosinophilia and COPD. This may be attributed to a range of severity of COPD patients included in the studies and the timing of blood collection. Other potential confounding variables may include, but not limited to, specimen type, baseline characteristics of the study population, study quality and unknown pre-existing co-morbidities. Cross-sectional analysis of 948 COPD patients revealed that eosinophilic group was associated with lower rate of heart attack and anemia38. If these contributed to different clinical outcome of this sub-group remained equivocal. The use of steroidal therapy may interfere with the risk for exacerbation. Given the lack of accessibility to information on individual exposure, it was impossible to control for the factor of steroidal therapy in the pooled estimate of exacerbation risk.
In conclusion, eosinophilia is associated with a better improvement of pulmonary function and reported QOL subsequent to therapy in outpatients. Given its association with eosinophil level in the airway, blood eosinophil count may be a predictive biomarker in patients with stable COPD for response to steroidal and bronchodilator therapies.
Methods
Searching strategy
This systematic review was performed in accordance with the guidelines on Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA Statement 200941. Original articles published in PubMed (MEDLINE), ISI Web of Knowledge, EMBASE, and Scopus database were identified using Medical Subject Heading (MeSH) or Title/ Abstract keywords from inception up to December 2016. The MeSH search terms include a combination of eosinophil, blood, sputum, pulmonary disease, chronic obstructive, and/or airway disease. The number of entries retrieved from each database is summarized in Fig. 1. Two authors (JH and WH) performed the literature search and selected the relevant studies independently. Disagreements in terms of study selection were resolved by discussion with senior authors.
Inclusion and exclusion criteria
Included studies were primary research articles comparing patients with and without eosinophilic COPD in terms of exacerbation risk, mortality, morbidity, length of hospital stay, and response to corticosteroids and bronchodilators. Quasi-experimental studies and randomized controlled trials were included. Pre-clinical studies, review articles, editorials, commentaries, conference abstracts and book chapters were excluded.
Data extraction
Relevant data were extracted according to a pre-defined data abstraction form. Information on sample size, baseline characteristics, incidence of exacerbation in the past 12 months, length of hospital stay, in-hospital mortality, QOL, and pulmonary function were extracted by one researcher (JH) and verified by a second researcher (WH).
Quality assessment and statistical analysis
The methodological quality of the included randomized controlled trials and quasi-experimental studies was evaluated by the Cochrane Risk of Bias Tool42 and the Newcastle-Ottawa scale43 respectively. The former tool indicates studies with high, low or unclear risk according to five domains: selection bias, performance bias, detection bias, attrition bias, and reporting bias. The latter scale evaluates the quality of studies in three attributes, namely selection of cohort, comparability, and outcome. In this review, a high-quality study is defined as having >6 points whereas a low-quality study as having ≤5 points.
Meta-analysis compared patients with eosinophilic and non-eosinophilic COPD in terms of exacerbation risk, length of hospital stay, in-hospital mortality, and change of pulmonary function and QOL in response to medical interventions. Heterogeneity across studies was determined by the I 2 statistic using Cochrane Review Manager 5.344. An I 2 values ≥ 25, 50 and 75% were considered as mild, moderate, and high degree of heterogeneity, respectively. For pooled outcome measures with I 2 > 50%, a random-effect model was used to evaluate the overall effect of a given comparison. Studies were weighted by inverse of variance. Categorical data was presented as odds ratio (OR) in 95% confidence interval (CI). For continuous variables, the pooled estimates were compared by mean difference (MD) or standardized mean difference (SMD), as appropriate. In the occasion when the remaining studies appeared to be different from the overall estimate, sub-group analysis was performed.
Electronic supplementary material
Acknowledgements
This study was funded by Research Grants Council of the Hong Kong Special Administrative Region, China (Project no. PolyU 25103015). and departmental research grants from the Department of Anesthesia and Intensive care, The Chinese University of Hong Kong; and the Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong.
Author Contributions
J.H. and W.H. extracted data and prepared this manuscript. S.P.C.N., W.K.K.W., and B.W.M.L. critically reviewed the work. M.T.V.C., G.T., T.L., S.H.W., C.C.H.L., W.T.W., S.T., L.Z., R.Y.P.C., T.G., and J.L. assisted in editing the manuscript and approved the final version to be published, and agree to be accountable for all aspects of the work.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jeffery Ho and Wajia He contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13745-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benson W. M. Lau, Email: Benson.Lau@polyu.edu.hk
William K. K. Wu, Email: wukakei@cuhk.edu.hk
Shirley P. C. Ngai, Email: shirley.ngai@polyu.edu.hk
References
- 1.Louis RE, et al. Evidence of mast-cell activation in a subset of patients with eosinophilic chronic obstructive pulmonary disease. Eur Respir J. 2002;20:325–331. doi: 10.1183/09031936.02.00286302. [DOI] [PubMed] [Google Scholar]
- 2.Gorska K, et al. Eosinophilic airway inflammation in chronic obstructive pulmonary disease and asthma. J Physiol Pharmacol. 2008;59(Suppl 6):261–270. [PubMed] [Google Scholar]
- 3.Balzano G, et al. Eosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway function. Am J Respir Crit Care Med. 1999;160:1486–1492. doi: 10.1164/ajrccm.160.5.9810105. [DOI] [PubMed] [Google Scholar]
- 4.Bathoorn E, et al. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis. 2009;4:101–109. doi: 10.2147/COPD.S4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negewo NA, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi: 10.2147/COPD.S100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto K, et al. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25:640–646. doi: 10.1183/09031936.05.00047504. [DOI] [PubMed] [Google Scholar]
- 7.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965–974. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 8.Eltboli O, Mistry V, Barker B, Brightling CE. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology. 2015;20:667–670. doi: 10.1111/resp.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brightling CE, et al. Sputum eosinophilia and the short-term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bafadhel M, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47:1374–1382. doi: 10.1183/13993003.01370-2015. [DOI] [PubMed] [Google Scholar]
- 12.Rahimi-Rad MH, Asgari B, Hosseinzadeh N, Eishi A. Eosinopenia as a marker of outcome in acute exacerbations of chronic obstructive pulmonary disease. Maedica (Buchar). 2015;10:10–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Pavord ID, et al. Blood eosinophils and inhaled corticosteroid/ long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71:118–125. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salturk C, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. 2015;10:1837–1846. doi: 10.2147/COPD.S88058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serafino-Agrusa L, Scichilone N, Spatafora M, Battaglia S. Blood eosinophils and treatment response in hospitalized exacerbations of chronic obstructive pulmonary disease: a case-control study. Pulm Pharmacol Ther. 2016;37:89–94. doi: 10.1016/j.pupt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Duman D, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–2478. doi: 10.2147/COPD.S90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland M, Alkhalil M, Chandromouli S, Janjua A, Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15:165–167. doi: 10.1111/j.1440-1843.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- 18.Bafadhel M, et al. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016;150:320–328. doi: 10.1016/j.chest.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in chronic obstructive pulmonary disease exacerbations are associated with increased readmissions. Chest. 2017;151:366–373. doi: 10.1016/j.chest.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Hinds DR, DiSantostefano RL, Le HV, Pascoe S. Identification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysis. BMJ Open. 2016;6:e010099. doi: 10.1136/bmjopen-2015-010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbri LM, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 22.Bafadhel M, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 23.Mercer PF, et al. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siva R, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 25.Bafadhel M, et al. Sputum IL-5 concentration is associated with a sputum eosinophilia and attenuated by corticosteroid therapy in COPD. Respiration. 2009;78:256–262. doi: 10.1159/000221902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brightling CE, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal A, Barnes NC, Brooks J. Is Blood eosinophil count a predictor of response to bronchodilators in chronic obstructive pulmonary disease? results from post-hoc subgroup analyses. Clin Drug Investig. 2015;35:685–688. doi: 10.1007/s40261-015-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesci A, et al. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J. 1998;12:380–386. doi: 10.1183/09031936.98.12020380. [DOI] [PubMed] [Google Scholar]
- 29.Snoeck-Stroband JB, et al. Chronic bronchitis sub-phenotype within COPD: inflammation in sputum and biopsies. Eur Respir J. 2008;31:70–77. doi: 10.1183/09031936.00137006. [DOI] [PubMed] [Google Scholar]
- 30.Papi A, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 31.Zanini A, et al. Bronchial hyperresponsiveness, airway inflammation, and reversibility in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1155–1161. doi: 10.2147/COPD.S80992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perng DW, et al. Inhaled fluticasone and salmeterol suppress eosinophilic airway inflammation in chronic obstructive pulmonary disease: relations with lung function and bronchodilator reversibility. Lung. 2006;184:217–222. doi: 10.1007/s00408-005-2586-8. [DOI] [PubMed] [Google Scholar]
- 33.Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2012;7:283–289. doi: 10.2147/COPD.S30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimoto K, Kubo K, Yamamoto H, Yamaguchi S, Matsuzawa Y. Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest. 1999;115:697–702. doi: 10.1378/chest.115.3.697. [DOI] [PubMed] [Google Scholar]
- 35.Park HY, et al. Association of blood eosinophils and plasma periostin with FEV1 response after 3-month inhaled corticosteroid and long-acting β2-agonist treatment in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;11:23–30. doi: 10.2147/COPD.S94797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltboli O, et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med. 2014;14:112. doi: 10.1186/1471-2466-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Armiento JM, et al. Eosinophil and T cell markers predict functional decline in COPD patients. Respir Res. 2009;10:113. doi: 10.1186/1465-9921-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88–96. doi: 10.1016/j.rmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Singh D, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 40.van der Vaart H, et al. Repeated sputum inductions induce a transient neutrophilic and eosinophilic response. Chest. 2006;130:1157–1164. doi: 10.1378/chest.130.4.1157. [DOI] [PubMed] [Google Scholar]
- 41.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 44.Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.