ABSTRACT
HIV broadly neutralizing antibodies (bnAbs) have been shown to occasionally display unusual virus neutralization profiles with nonsigmoidal slopes and plateaus at <100% neutralization against a variety of viruses. The significance of incomplete neutralization for the ability of bnAbs to mediate protective effects in vivo, however, is undetermined. In the current study, we selected two bnAbs, PGT121 and 3BNC117, as they incompletely neutralize the clade C simian-human immunodeficiency virus (SHIV) stock (SHIV-327c) at 85% and 70%, respectively, and performed a protection study in rhesus macaques. The animals were intravenously (i.v.) administered PGT121 or 3BNC117 at 10 and 2 mg/kg of body weight before being rectally challenged with a single high dose of SHIV-327c. PGT121 protected 6 out of 7 monkeys, while 6 out of 7 3BNC117-pretreated animals became infected, although with significantly delayed plasma viremia compared to the control animals. These data suggest that complete neutralization is not imperative for bnAbs to prevent infection but that with increasing levels of incomplete neutralization the sterilizing activity diminishes.
IMPORTANCE Multiple antibodies have been identified that potently neutralize a broad range of circulating HIV strains. However, not every virus-antibody combination results in complete neutralization of the input virus, suggesting that a fraction of virus particles are resistant to antibody neutralization despite high antibody concentrations. This observation of “incomplete neutralization” is associated with nonsigmoidal neutralization curves plateauing below 100% neutralization, but the significance of the phenomenon for the ability of neutralizing antibodies to mediate protective effects in vivo is undetermined. In this study, we show that the broadly neutralizing antibody PGT121, which neutralized only up to 85% of the SHIV-327c challenge stock in vitro, protected 6 out of 7 rhesus macaques against infection while the antibody 3BNC117, which neutralized up to 70% of SHIV-327c in vitro, did not prevent, though it significantly delayed, establishment of infection, suggesting that with increasing levels of incomplete neutralization the ability of a bnAb to mediate sterilizing protection diminishes.
KEYWORDS: broadly neutralizing antibodies, incomplete neutralization in vitro, passive immunization, protection against acquisition, SHIV, incomplete neutralization, monoclonal antibodies
INTRODUCTION
Human immunodeficiency virus (HIV) broadly neutralizing antibodies (bnAbs) are currently being explored for prophylactic and therapeutic interventions. In particular, neutralizing antibodies directed toward the glycan-dependent V3 region and the CD4 binding site have shown promise in preclinical studies in which single intravenous (i.v.) doses of antibodies protected rhesus macaques against single high-dose or repeated low-dose challenges with simian-human immunodeficiency virus (SHIV) (1–4). While the nonhuman primate (NHP) studies demonstrated the striking antiviral activity of bnAbs in vivo, they all used viruses that were exquisitely sensitive to neutralization when tested in a conventional TZM-bl cell assay. Recently, McCoy et al. described the phenomenon of incomplete neutralization, where particular virus-antibody combinations failed to achieve 100% neutralization of the input virus, leading to nonsigmoidal neutralization curves plateauing below 100%. While some antibodies could neutralize 50% of a given virus at low antibody concentrations, they failed to completely neutralize the same virus even at extremely high concentrations (5). The observation of incomplete neutralization occurred with all classes of antibodies tested, although some antibody targets, e.g., against the V2 apex of envelope or against gp41, demonstrated this incomplete neutralization at higher rates than, e.g., glycan or CD4 binding site-specific antibodies (5). The observation raised the question of whether incomplete neutralization in vitro would translate into the failure of an antibody to protect in vivo against a viral challenge. Recent data demonstrating that bnAbs confer protection by involving viral clearance in tissues rather than complete blockade of virus at the mucosal surface (6, 7) suggest that the inability to completely neutralize the viral inoculum could result in the establishment of small foci of infection in distal tissues, which could fuel a systemic infection as soon as the antibody levels decline.
To further explore the significance of incomplete neutralization for the ability of bnAbs to mediate protective effects in vivo, we selected the V3 glycan-dependent monoclonal antibody (MAb) PGT121 and the CD4 binding site MAb 3BNC117, as both bnAbs showed neutralization curves against the challenge stock, SHIV-327c, that saturated below 100% in both TZM-bl cell- and peripheral blood mononuclear cell (PBMC)-based neutralization assays. Furthermore, both bnAbs had previously demonstrated that they could robustly protect macaques against challenges with other commonly used SHIVs, which both antibodies neutralize to 100% in vitro (1, 2, 8). Rhesus macaques received a single infusion of either PGT121, 3BNC117, or placebo before being rectally challenged with a high dose of SHIV-327c, and we followed all the animals for the occurrence of breakthrough infection.
RESULTS
Incomplete in vitro neutralization of the challenge virus SHIV-327c by PGT121 and 3BNC117.
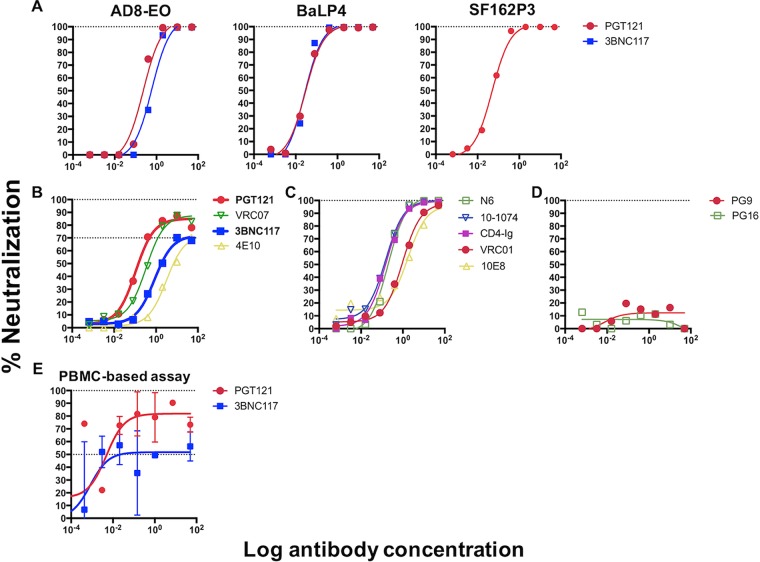
PGT121 and 3BNC117 have demonstrated robust efficacy in protecting rhesus macaques against challenge with SHIVSF162P3 (PGT121) (1), SHIVAD8EO (PGT121 and 3BNC117) (2, 8), and SHIVDH12-V3AD8 (3BNC117) (2). These and/or other commonly used SHIVs, however, are potently (to 100%) neutralized in vitro by these bnAbs (1, 2, 8) (Fig. 1A). To further explore the role of incomplete neutralization, we selected the clade C-derived SHIV-327c (9), as both antibodies failed to completely neutralize the virus preparation, with the neutralization curves plateauing at approximately 85% for PGT121 (median 50% inhibitory concentration [IC50]/IC80, 0.11 μg/ml and 0.62 μg/ml, respectively) and approximately 70% for 3BNC117 (median IC50, 0.84 μg/ml, with IC80 values of >50 μg/ml, as the curve plateaus below 80% neutralization) (Fig. 1B). Interestingly, the CD4bs antibody VRC07 and the membrane-proximal external region (MPER) antibody 4E10 also displayed similar plateaus (Fig. 1B), while other bnAbs, like VRC01, N6, 10-1074, 10E8, and CD4-Ig, neutralized this SHIV to 100% (Fig. 1C). While both V1/2 antibodies, PG9 and PG16, had no activity against SHIV-327c (Fig. 1D), there was no specific pattern that linked the epitope specificity of bnAbs to the level of neutralization, as previously described (5). Given the extensive experience with PGT121 and 3BNC117 in protecting monkeys against 100% sensitive SHIVs, we selected these bnAbs for further evaluation. We next measured the abilities of both bnAbs to neutralize SHIV-327c in a PBMC-based assay using cells from rhesus macaques. Again, we observed incomplete neutralization, with curves reaching plateaus at approximately 82% neutralization for PGT121 and approximately 52% neutralization for 3BNC117 (Fig. 1E), confirming the kinetics seen before in the TZM-bl cell assay. These data confirm that both antibodies, despite neutralizing other commonly used SHIV strains to 100%, were unable to achieve complete neutralization of our challenge stock in 2 different cell assays, thus justifying the use of this antibody-SHIV combination for further in vivo evaluation of the effects of incomplete neutralization on protection.
FIG 1.
(A) Neutralization of SHIV challenge stocks AD8-EO, BaL-P4, and SF162P3 by PGT121 and/or 3BNC117. Neutralization was measured in the TZM-bl cell assay and showed that both antibodies achieved 100% neutralization of these commonly used challenge viruses. (B to D) Neutralization of SHIV-327c by bnAbs that showed neutralization plateaus below 100%, including PGT121 and 3BNC117 (B); bnAbs that neutralized SHIV-327c to 100% (C); and bnAbs that did not neutralize the SHIV at all (D). (E) Neutralization of SHIV-327c by PGT121 and 3BNC117 in a PBMC-based assay confirming similar patterns of incomplete neutralization by the two bnAbs. The values are means and standard errors of the mean (SEM).
Efficacy against mucosal SHIV-327c challenge.
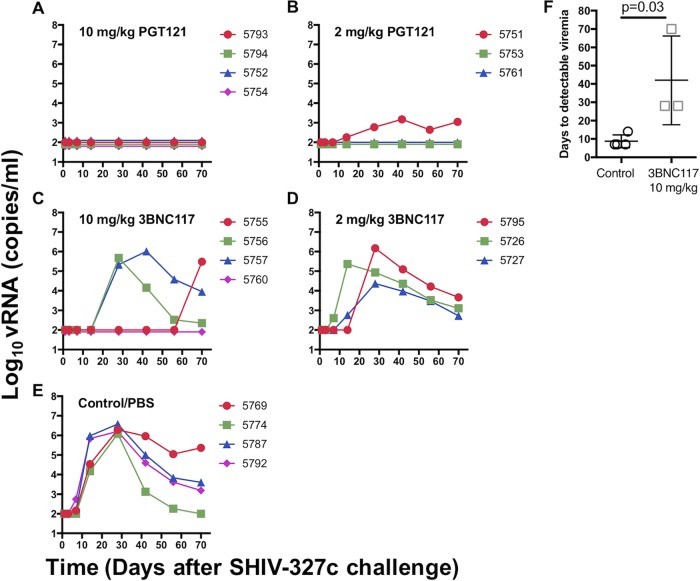
To evaluate the activities of PGT121 and 3BNC117 in vivo, we designed an antibody titration protection study using the SHIV-macaque model. A total of 18 animals were used, which were randomized to five treatment groups: one group of 4 animals received 10 mg/kg of body weight PGT121, one group of 3 animals received 2 mg/kg PGT121, one group of 4 animals received 10 mg/kg 3BNC117, one group of 3 animals received 2 mg/kg 3BNC117, and one group of 4 animals received phosphate-buffered saline (PBS) as a control. The antibodies and the control were administered i.v. 24 h before the animals were rectally challenged with a single high dose of SHIV-327c (300 50% tissue culture infective doses [TCID50]). All the animals in the control group readily became infected, with detectable viremia between days 7 and 14 and peak viremia of 106 to 107 viral copies per ml (Fig. 2E) at week 3. In contrast, no detectable viremia (the detectable limit was 150 RNA copies per ml) was observed for all the animals given 10 mg/kg of PGT121, suggesting sterilizing immunity (Fig. 2A), while 2 out of 3 animals who had received 2 mg/kg of PGT121 (Fig. 2B) were protected, as well. The animal in the latter group that became infected showed a lower level of peak viremia (103 viral copies per ml) than the control group. In the group receiving 10 mg/kg of 3BNC117, three of four animals became infected (Fig. 2C), but virus was detected only at days 28 to 70 postchallenge, demonstrating that systemic infection was significantly delayed compared to the controls (8.75 versus 42 days to detectable SHIV RNA; P < 0.03) (Fig. 2F). In the second 3BNC117 group, which had received 2 mg/kg, all 3 animals became infected, and 1 animal (number 5795) again demonstrated a delay (to day 28) in detectable viremia (Fig. 2D). Overall, the results suggest that PGT121 mediated robust protection while being relatively unaffected by its degree of incomplete neutralization. 3BNC117, with more extensive levels of incomplete neutralization, failed to prevent infection but still had a virus-inhibitory effect, delaying systemic infection.
FIG 2.
Plasma viral loads in rhesus macaques pretreated with different doses of PGT121 and 3BNC117 and challenged with SHIV-327c. SHIV RNA levels are shown for animals that received 10 mg/kg PGT121 (A), 2 mg/kg PGT121 (B), 10 mg/kg 3BNC117 (C), and 2 mg/kg 3BNC117 (D) or PBS control (E). All animals treated with PBS-control became infected. Sterilizing immunity was observed in all animals given 10 mg/kg PGT121, in two of three animals given 2 mg/kg of PGT121, and in one of four animals given 10 mg/kg 3BNC117. (F) Time in days from challenge to the first detectable SHIV RNA in plasma Shown are means ± SEM.
Pharmacokinetics of PGT121 and 3BNC117 in vivo.
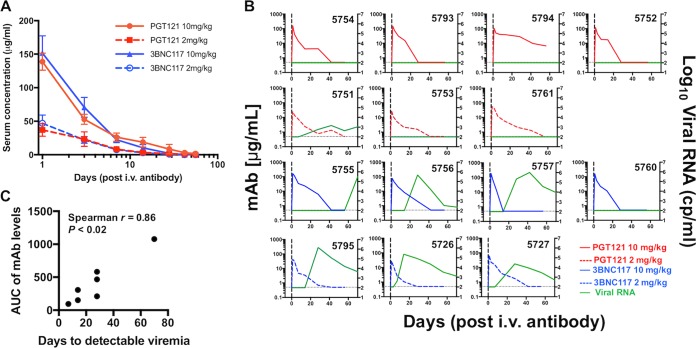
Serum samples were obtained throughout the study, and concentrations of PGT121 and 3BNC117 were determined by an HIV-1 gp140-specific enzyme-linked immunosorbent assay (ELISA). The results showed that the animals administered PGT121 at 10 mg/kg and 2 mg/kg had average serum antibody concentrations of 138.7 μg/ml and 37 μg/ml at the time of challenge (Fig. 3A). Average serum concentrations for 3BNC117 were 151.8 μg/ml and 47 μg/ml for the animals that had received 10 mg/kg and 2 mg/kg of 3BNC117, respectively. Based on the level of serum antibody decay (one-phase exponential decay), starting at day 3 to account for the initial distribution phase, the half-life of PGT121 was determined to be 5.6 days, and for 3BNC117, the half-life was 2.7 days. While there was expected heterogeneity in the MAb kinetics between the animals (Fig. 3B), overall, the serum concentrations were not significantly different between the bnAb groups. Interestingly, in the animals that experienced breakthrough infection, the area under the concentration-time curve (AUC) for each MAb correlated with the time until plasma virus was detected (Spearman r, 0.86; P < 0.02) (Fig. 3C). Specifically, animal 5755, which developed plasma viremia only on day 70 following challenge, had the highest plasma MAb levels of the 3BNC117-pretreated animals, suggesting that the bnAb controlled the initial infection, but without eradicating it.
FIG 3.
(A) Serum concentrations of PGT121 and 3BNC117 in antibody-treated animals. bnAb concentrations were determined throughout the study. The serum concentrations were not significantly different between the bnAb groups. To account for the initial distribution phase, the serum half-life for the elimination phase was calculated from day 3 onward and resulted in half-lives of 5.6 days and 2.7 days for PGT121 and 3BNC117, respectively. The values are means and SEM. (B) Kinetics of viral RNA levels and serum antibody concentrations per animal throughout the study (the top row shows all the animals that received PGT121 at 10 mg/kg, the second row shows all the animals that received PGT121 at 2 mg/kg, the third row shows the animals that received 3BNC117 at 10 mg/kg, and the fourth row shows the animals that received 3BNC117 at 2 mg/kg. cp, copies. (C) Correlation of the AUC for each MAb in each animal that experienced viral breakthrough infection and the time to detectable viremia for each animal.
Nonneutralizing activities of PGT121 and 3BNC117.
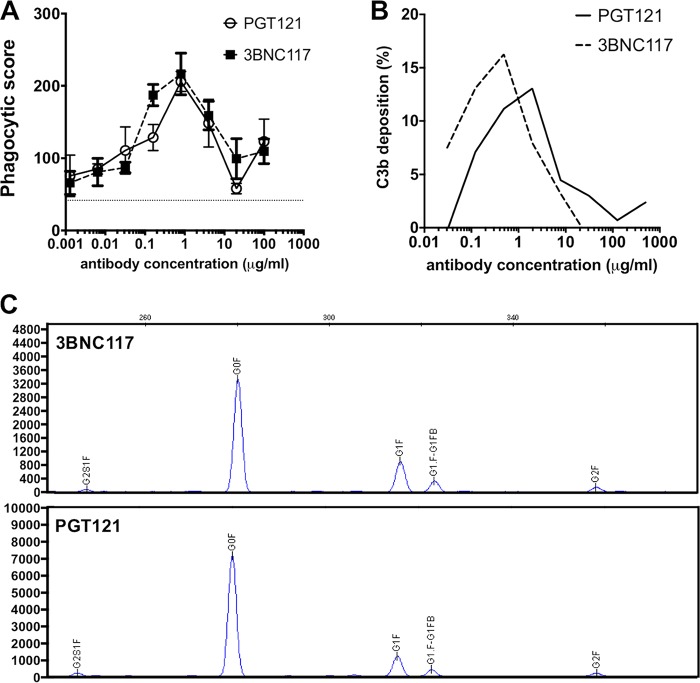
Recent data have demonstrated that low levels of viral RNA and viral DNA can be detected in distal tissues of PGT121-treated macaques for several days following SHIV challenge and that these early tissue foci of disseminated virus are subsequently cleared (6). In addition to their neutralizing capacity, antibodies are also known to display a wide variety of Fc-mediating antiviral activities, which might play a role in the ability of the antibodies to eradicate virus from tissues, thereby contributing to the protective capacity of the MAbs. We evaluated the abilities of PGT121 and 3BNC117 to mediate phagocytosis and to recruit complement. Notably, we observed that both antibodies could induce potent SHIV-327c gp120-specific antibody-dependent cellular phagocytosis (ADCP) responses (Fig. 4A) and activate complement deposition on SHIV-327c-pulsed target cell lines (Fig. 4B), with the strongest activities measured at an antibody concentration of 1 μg/ml. The two antibodies, therefore, showed comparable magnitudes and similar dose-dependent kinetics in their abilities to induce ADCP and to activate complement deposition. As Fc glycosylation plays a significant role in altering the affinity of antibodies for innate immune receptors/proteins, including Fcγ receptors and complement (10), we next compared the Fc glycan profiles of the two antibodies using capillary electrophoresis. The antibodies showed nearly identical glycan compositions, with the majority of Fcs being agalactosylated and fucosylated (3BNC117, 68%; PGT121, 77%) or monogalactosylated and fucosylated (3BNC117, 20%; PGT121, 14%) (Fig. 4C). Given these similarities, our data suggest that Fc-mediated functions were not a major determinant of the differences observed between the two bnAbs in protecting against viral challenge.
FIG 4.
(A and B) PGT121 and 3BNC117 were evaluated for the ability to induce ADCP against the challenge virus SHIV-327c (A) and for the ability to induce complement activation (B) as measured by C3b deposition on the SHIV-327c gp120-pulsed CEM cell line. Overall, the two bnAbs showed very similar activities for ADCP and complement activation. A minimum of 2 separate experiments were performed. The error bars indicate standard deviations (SD). (C) Fc glycan analysis by capillary electrophoresis for 3BNC117 and PGT121 demonstrating similar compositions of glycostructures for the two bnAbs. The structures that were observed were agalactosylated (G0), monogalactosylated (G1), digalactosylated (G2), fucosylated (F), and bisected (B). Numbers on the y axis indicate signal intensity.
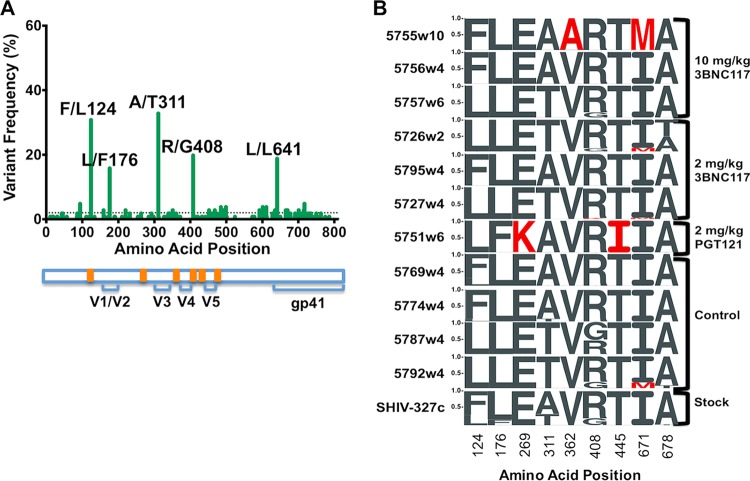
Sequence analysis of breakthrough viruses.
To examine the molecular nature of the changes in SHIV-327c in response to PGT121 and 3BNC117, we generated envelope sequences from the SHIV challenge stock and from plasma viruses at the time of peak viremia following breakthrough infection. In the challenge stock, 5 sequence variants were identified at >5% frequency: F/L124, L/F176, A/T311, R/G408, and L/L641 (Fig. 5A). For the last, the amino acid did not change despite codon modifications encoding that amino acid at position 641. While these variants could be retrieved in some of the animals, additional variants were identified (Fig. 5B). In the PGT121 group, animal 5751 showed two unique changes that differentiated it from the other animals (E/K269 and T/I445). Both changes were present at >99% frequency, and while they were in functionally interesting regions of envelope, with 269 being in the D loop and 445 in V5, the locations were outside known contact sites for PGT121. In the 3BNC117 group, variants I/M671 and A/T678 in the MPER were found in some animals but were also observed in some placebo control animals. In animal 5755, which developed very delayed plasma viremia at week 10, a unique and fixed mutation in the middle of the CD4 binding site (V/A362) was observed. This mutation was not seen in the challenge stock despite a >30,000× depth of coverage, allowing us to detect variants at a frequency of less than 0.1%, suggesting that this was not a transmitted mutation but occurred in vivo. We next tested the impact of the mutation on neutralization sensitivity in a TZM-bl cell assay using a pseudovirus typed with the animal's Env genotype. Despite the location in the CD4bs, however, the V/A362 variant did not mediate resistance to 3BNC117 (IC50, 0.145 μg/ml versus 0.2 μg/ml for the wild type [WT]) or to other CD4bs antibodies (Table 1). In summary, our data suggest that viral evolution was detectable in animals where a bnAb failed to provide sterilizing protection but that these sequence variations did not inevitably result in viral resistance.
FIG 5.
Amino acid variation across the envelope of SHIV-327c. (A) Frequencies of all synonymous and nonsynonymous sequence variants found across the envelope sequence of the SHIV-327c challenge stock. The dotted line represents the 2% variant frequency cutoff. The variants found at >5% frequency are identified. A representation of relevant features of the SHIV-327c envelope is shown below, with CD4 binding sites highlighted in orange. (B) Sequence logograms showing variation frequencies at the nine amino acids that showed polymorphisms in the envelope consensus sequence of the virus stock (SHIV-327c) and in the breakthrough viruses following challenge. Envelope amplification for the latter was performed on plasma viral RNA using time points of peak viremia. Residues that differ from the virus stock are shown in red.
TABLE 1.
Sensitivities of pseudovirusesa
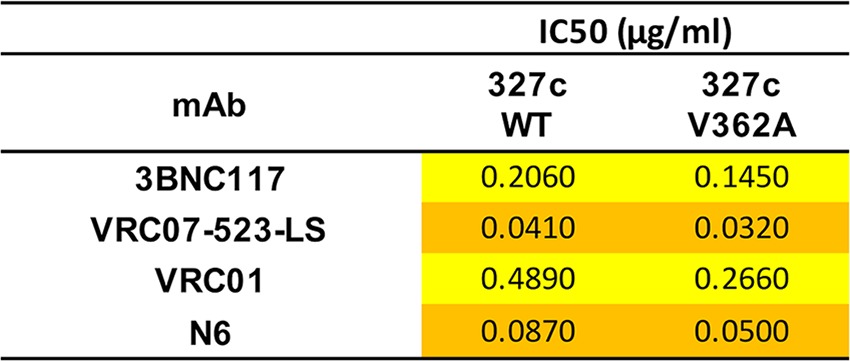
The sensitivities of pseudoviruses with the 327c envelope, WT or with the V362A mutation, to a panel of broadly neutralizing CD4 binding site-directed monoclonal antibodies are shown as IC50 neutralization. The color shading represents potency: orange, value between 0.010 and 0.100; yellow, value between 0.100 and 1.00.
DISCUSSION
We and others have previously shown that passive transfer of highly potent neutralizing anti-HIV gp120 antibodies efficiently protects against high-dose mucosal challenge with neutralization-sensitive SHIV in macaques (1, 2, 11–14). However, not all antibody-virus combinations achieved 100% neutralization in in vitro neutralization assays, raising the question of how incomplete neutralization impacts the ability of bnAbs to mediate protective effects in vivo (5, 15). In the current study, we found that PGT121, which neutralized the challenge stock up to 85% with a potent IC50 of 0.11 μg/ml, protected the majority of animals, with only one animal developing breakthrough infection. 3BNC117, which neutralized up to 70% of SHIV-327c with a less potent IC50 of 0.84 μg/ml, in contrast, did not prevent infection in the majority of animals but delayed plasma viremia significantly in a dose-dependent manner.
The difference in potency of PGT121 and 3BNC117 against our challenge virus reduces our ability to make direct associations between the levels of incomplete neutralization seen in vitro and the degree of protective effect in vivo. It is interesting, however, that in a recent study by Shingai et al., 3BNC117 was fully protective against SHIVAD8EO challenge at serum MAb levels below the ones observed in this study (2). The IC50 for SHIVAD8EO in the former study was determined to be 0.14 μg/ml (2) and therefore in a range similar to that of the IC50 for SHIV-327c. In contrast to SHIV-327c, 3BNC117 neutralized SHIVAD8EO to 100%. Our findings with both PGT121 and 3BNC117, therefore, suggest that the inability to neutralize 100% of a challenge virus does not automatically result in the failure of a bnAb to mediate antiviral activity against such a virus in vivo. Instead, robust protection can be observed if the incomplete neutralization does not drop below 85% and if the antibody has sufficient potency, as is the case for PGT121. Our results also suggest that sterilizing protection might be diminished if levels of incomplete neutralization are further reduced, e.g., down to 70%; although even in this scenario, a delay in detectable plasma viremia can still be observed, indicating some transient virus-inhibiting effect. These results are reassuring, as they imply that single bnAbs or bnAb combinations selected for passive immunization strategies might not necessarily be required to achieve complete neutralization of every potential HIV-1 strain.
While neutralizing potency certainly plays a critical role in protection against viral infection, other factors that might affect the prophylactic capacity of the bnAbs should be considered. The two antibodies showed similar pharmacokinetics, with comparable serum antibody levels on the day of challenge, and while the circulating plasma half-life of 3BNC117 was nominally shorter than that observed with PGT121, this difference was not significant across all the animals. Furthermore, nonneutralizing functions of anti-HIV antibodies need to be considered, as they might contribute to the protective potency of the antibody. A number of studies have suggested that, in addition to neutralization, interaction of IgG with Fcγ receptors may play an important role in antibody-mediated protection (16–19). We therefore determined whether both bnAbs could induce Fc-mediated phagocytosis and activation of complement deposition and found similar kinetics for both PGT121 and 3BNC117. Furthermore, the two bnAbs had nearly identical Fc glycan profiles, which is not surprising, as both antibodies had been produced in the same expression cell system. Given these highly similar functional signatures, our data suggest that Fc-mediated nonneutralizing activities were not major determinants of the different outcomes observed in the PGT121-treated versus 3BNC117-treated monkeys.
The phenomenon of incomplete in vitro neutralization has been described for several bnAbs and diverse HIV-1 isolates (3, 5, 20, 21), but the mechanism of this effect is not well understood. Prior studies have suggested that glycosylation heterogeneity might contribute to incomplete neutralization (3, 5) or that conformational heterogeneity of the envelope trimer plays a role (22). Nevertheless, it can be assumed that some antibodies can tolerate some heterogeneity in glycan usage better than others (23). The N276 glycosylation site, for example, is required for viral neutralization by CD4 binding site antibodies of the HJ16 class, and heterogeneity at this position might contribute to incomplete neutralization of certain viral strains (24). As for the VRC01 class CD4bs Abs, they must penetrate the “glycan fence” of N197, N276, N362, N386, and possibly other glycans, depending on the viral isolate. They all have slightly different angles of approach and contacts and therefore may show differences in glycan sensitivity, potentially explaining the difference in neutralization activity seen against SHIV-327c in Fig. 1. In contrast, the N332 glycan site seems to be more homogeneous, therefore potentially facilitating bnAbs that are dependent on the glycan on N332, such as 10-1074, to bind and effectively neutralize the virus (5, 25) while PGT121 is dependent on several glycans on N332, N301, N137, and N156 (20). Overall, as each trimer is going to have some differences in glycosylation processing and/or protein conformation, predicting incomplete neutralization for bnAb-virus combinations with certainty without knowledge of the complex bnAb trimer structure and the glycan composition might be difficult. Interestingly, a recent study demonstrated that the V1/V2-directed antibody PG9 that provided adequate protection in NHPs against SHIVBaLP4 challenge showed incomplete neutralization in a TZM-bl cell assay but nearly complete neutralization in a PBMC assay (3), suggesting that the observation of incomplete neutralization might be dependent on the assay used. We measured the neutralization profiles of PGT121 and 3BNC117 against our challenge virus in both cell systems (TZM-bl cell- and PBMC-based assays), and while the level of incomplete neutralization was greater in the PBMC-based assay, the difference between the bnAbs and the kinetics of incomplete neutralization were similar. While we are not aware of any published data systematically comparing antibody neutralization of replication-competent virus in TZM-bl cell- versus PBMC-based assays, recent data suggest that the overall effects are similar and not per se cell type/assay dependent (5).
The observation that plasma viremia as a correlate of systemic infection was delayed in all antibody-treated animals suggests that bnAbs, independent of the IC50 and the degree of neutralization, had an antiviral effect, inhibiting although not sterilizing. This was specifically reflected in the association between plasma MAb levels and the time to breakthrough viremia. As we did not perform any tissue studies, we can only hypothesize that circulating and mucosal bnAbs reduced the amount of transmitted virus, preventing immediate establishment of systemic infection, as observed in the control animals. This is consistent with recent data from our group demonstrating that low levels of viral RNA and viral DNA can be detected in distal tissues of PGT121-treated macaques for several days following SHIV challenge but that, in contrast to placebo-treated animals, these early tissue foci of disseminated virus are subsequently cleared (6). The occurrence of late-onset viremia in our study implies that the challenge virus was also able to establish foci of infection and that with incomplete eradication and in the presence of declining antibody levels viral replication continued. This is also supported by the finding of de novo sequence variations in functionally relevant areas of envelope in the breakthrough viruses, although these mutations did not confer resistance.
In summary, we have shown that bnAb-mediated protection against viral challenge despite incomplete neutralization in vitro can be achieved if the level of incomplete neutralization is minor and if the antibody has sufficient potency. Our results also suggest that despite the failure to confer sterilizing protection, bnAbs with more significant levels of incomplete neutralization still have an antiviral effect, e.g., substantially delaying establishment of systemic infection. These data suggest that the mechanism of antibody protection (and failure) is a dynamic relationship between the virus and the antibody in tissues over the first several days and is not an all-or-nothing phenomenon at a single point in time. These data therefore emphasize the importance of robust and broad virus-neutralizing activity of candidate bnAbs for HIV prevention studies in order to guarantee sterilizing protection. The development and implementation of bnAb cocktails, covering a broad range of viral strains, might reduce the risk that incomplete neutralizing potency of a single bnAb against a given viral strain would result in loss of protection and finally infection.
MATERIALS AND METHODS
Animals and study design.
Eighteen Indian-origin, outbred, young adult male and female, experimentally naive rhesus monkeys (Macaca mulatta) that did not express the class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17 associated with spontaneous virological control were housed at Bioqual Inc., Rockville, MD. Animals were randomly allocated to the different antibody dose and PBS control groups. The antibody dose or the PBS control was administered i.v. 24 h before the animals were atraumatically challenged rectally with 300 TCID50 of SHIV-327c. Serum samples for antibody detection and viral-load determination were obtained at days −6, 0, 1, 3, 7, 14, 28, 42, 56, and 70. This animal study was approved by the Bioqual and Beth Israel Deaconess Medical Center (BIDMC) Institutional Animal Care and Use Committees (IACUCs).
Challenge virus.
The challenge virus was SHIV-327c (9) propagated in concanavalin A-activated rhesus macaque PBMCs. Virus was quantified by simian immunodeficiency virus (SIV) p27 ELISA (Zeptometrix), and the TCID50 was determined in TZM-bl cells (9).
Antibody production.
PGT121 and 3BNC117 monoclonal antibodies were generated as previously described (20) and purified by using a protein A affinity matrix (GE Healthcare). PBS was used as a control in the study. All the monoclonal antibody preparations were endotoxin free (less than 0.03 endotoxin units [EU]/mg).
ELISA.
PGT121 and 3BNC117 antibody concentrations in macaque sera were determined by ELISA using recombinant HIV-1 gp140 as described previously (26). Briefly, microtiter plates were coated with 1 μg/ml HIV-1 gp140 and incubated overnight at 4°C. The plates were washed with PBS-0.05% Tween 20 and blocked with PBS-casein (Pierce). After blocking, serial dilutions of serum samples were added to the plate and incubated for 2 h at 37°C. Binding was detected with a horseradish peroxidase (HRP)-conjugated goat anti-human IgG secondary antibody (Fisher Scientific) and visualized with SureBlue tetramethylbenzidine (TMB) microwell peroxidase (KPL Research Products).
Neutralization.
Neutralization was assessed by using TZM-bl cells as described previously (27). Briefly, SHIV-327c or other challenge SHIV stocks were preincubated with the respective antibody for 1 h at 37°C, and DEAE-dextran (Sigma-Aldrich) was added to the TZM-bl cells at a final concentration of 10 μg/ml. Luciferase expression was quantified 48 h after infection upon cell lysis and the addition of luciferase substrate (Promega). Neutralization of the SHIV-327c stock was also evaluated by using rhesus macaque PBMCs as described previously (28). Briefly, PBMCs were isolated and stimulated overnight in the presence of concanavalin A (Sigma-Aldrich) and IL-2 (Hoffmann-La Roche Inc.). Antibody and virus were preincubated for 1 h at 37°C before being added to the stimulated PBMCs in a 96-well plate. The cells were incubated for 2 days, washed three times, and incubated for an additional 5 days. The amount of SHIV was quantified in the cell supernatant by a p27-specific ELISA (Advanced BioScience Laboratories).
ADCP.
Biotinylated antigen was incubated with 1-μm yellow-green fluorescent neutravidin beads (Invitrogen) overnight. Antibodies and antigen-labeled beads were mixed and incubated for 2 h. THP-1 cells (2 × 104 cells) were then added and incubated overnight under standard tissue culture conditions. The next day, the cocultures were fixed and analyzed on a BD LSR II. For analysis, the samples were gated on live cells, and the proportion of THP-1 cells phagocytosing beads was determined. The data represent the results of two separate experiments for which a phagocytic score was calculated as follows: (percent bead positive × mean fluorescence intensity [MFI] bead positive) (29).
Antibody-dependent complement deposition assay.
The ability of PGT121 and 3BNC117 to bind and activate the complement was determined by the C3b deposition on gp120-pulsed target cells. Briefly, CEM.NKr cells were pulsed with recombinant gp120 SHIV-327c for 1 h at room temperature. Uncoated CEM.NKr cells were used as a negative control. Plasma from healthy individuals was collected and used as a source of complement for the assay. PGT121 or 3BNC117 was added to 105 CEM.NKr cells in the presence of plasma for 20 min at 37°C. The cells were stained with a fluorescein isothiocyanate (FITC)-conjugated C3b antibody and fixed. HIV Ig served as a positive control for the assay, and heat-inactivated plasma and antibodies from healthy individuals were both used as negative controls. The data are the results of two separate experiments.
Antibody glycan analysis.
Antibody Fc glycosylation was assessed as previously described (30). Briefly, whole antibodies were bound to protein G beads (Millipore) and treated with IdeZ protease (New England Biolabs) to separate the Fab from the Fc. Fc glycans were released by peptide-N-glycosidase F (PNGase F) and then labeled with the fluorescent dye 8-aminopyrene-1,3,6-trisulfonate (APTS). The labeled glycans were run on a capillary electrophoresis machine, glycan peaks were assigned using commercially available APTS-labeled standards (Prozyme), and the relative percentage of each glycan structure was determined.
Virus sequencing of breakthrough virus.
RNA extraction and envelope amplification were performed as previously described (31, 32). The primary amplification primers were SHIV327c_Nested1_FWD (5′-GGTTAATCGATAGACTAATAG-3′) and SHIV327c_Nested1_REV (5′-GAGGATAGCTCTACCAATTC-3′). The nested-amplification primers were SHIV327c_Nested2_FWD (5′-GACTAATAGAAAGAGCAGAAG-3′) and SHIV327c_Nested2_REV (5′-GTGCAAGACTTCCTAGGTAC-3′). Illumina library construction was performed using NexteraXT (Illumina) according to the manufacturer's protocol. Sequencing was performed on the Illumina MiSeq341 platform, generating 250-bp paired-end reads. Consensus sequences for each envelope were generated by de novo assembly using Vicuna software. For each sequence, the nucleic acid frequencies at each position in the viral env genome were determined with V-Phaser 2. V-Phaser 2 is a tool to identify variants and calculate their frequencies in genetically heterogeneous populations from ultradeep-sequence data. V-Phaser uses phasing (covariation) information between observed variants combined with an expectation maximization algorithm that iteratively recalibrates base quality scores to increase sensitivity and specificity, respectively. This approach allows differentiation of true low-frequency variants from sequencing errors.
Statistical analyses.
Analyses of independent data were performed by two-tailed Mann-Whitney U tests. P values of less than 0.05 were considered significant. Statistical analyses were performed using GraphPad Prism.
ACKNOWLEDGMENTS
We thank M. Lewis (Bioqual, Inc.) for assistance in the execution of the animal studies.
This work was supported by NIH (K08 AI106408 to B.J.; UM1 AI126603, UMI1 AI124377, and U19 AI096040 to D.H.B.; and UM1 AI100663 to D.R.B. and D.H.B.), amfAR (109219 to D.H.B.), the Ragon Institute of MGH, MIT, and Harvard and funded in part by the intramural research program of the Vaccine Research Center, NIAID, NIH.
Project planning was performed by B. Julg, J. R. Mascola, D. R. Burton, and D. H. Barouch. Antibodies were generated by K. Le and D. Sok; viral neutralization assays were performed and analyzed by D. Sok, S. D. Schmidt, and K. Le; env sequences were generated and analyzed by R. M. Newman, M. Pack, J. Torabi, D. Su, and T. M. Allen; pseudovirus was generated by A. Pegu and S. D. Schmidt; plasma viral loads were measured by P. Abbink; PK-ELISA was performed by B. Julg and J. Nkolola; and antibody function and the Fc glycosylation pattern were measured by T. Broge and C. Linde. The manuscript was written by B. Julg, D. Sok, J. R. Mascola, D. R. Burton, and D. H. Barouch.
We declare that we have no competing interests.
REFERENCES
- 1.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd JP, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. 2014. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCoy LE, Falkowska E, Doores KJ, Le K, Sok D, van Gils MJ, Euler Z, Burger JA, Seaman MS, Sanders RW, Schuitemaker H, Poignard P, Wrin T, Burton DR. 2015. Incomplete neutralization and deviation from sigmoidal neutralization curves for HIV broadly neutralizing monoclonal antibodies. PLoS Pathog 11:e1005110. doi: 10.1371/journal.ppat.1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Jimenez J, Mondesir J, Lee B, Giglio P, Chandrashekar A, Abbink P, Colantonio A, Gittens C, Baker C, Wagner W, Lewis MG, Li W, Sekaly RP, Lifson JD, Burton DR, Barouch DH. 2016. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353:1045–1049. doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, Barnette PT, Legasse AW, Planer S, Stanton JJ, Pegu A, Chen X, Wang K, Siess D, Burke D, Park BS, Axthelm MK, Lewis A, Hirsch VM, Graham BS, Mascola JR, Sacha JB, Haigwood NL. 2016. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med 22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang HW, Tartaglia LJ, Whitney JB, Lim SY, Sanisetty S, Lavine CL, Seaman MS, Rademeyer C, Williamson C, Ellingson-Strouss K, Stamatatos L, Kublin J, Barouch DH. 2015. Generation and evaluation of clade C simian-human immunodeficiency virus challenge stocks. J Virol 89:1965–1974. doi: 10.1128/JVI.03279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subedi GP, Barb AW. 2015. The structural role of antibody N-glycosylation in receptor interactions. Structure 23:1573–1583. doi: 10.1016/j.str.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73:4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 14.Watkins JD, Siddappa NB, Lakhashe SK, Humbert M, Sholukh A, Hemashettar G, Wong YL, Yoon JK, Wang W, Novembre FJ, Villinger F, Ibegbu C, Patel K, Corti D, Agatic G, Vanzetta F, Bianchi S, Heeney JL, Sallusto F, Lanzavecchia A, Ruprecht RM. 2011. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS One 6:e18207. doi: 10.1371/journal.pone.0018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doores KJ, Burton DR. 2010. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol 84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 18.Forthal DN, Gilbert PB, Landucci G, Phan T. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol 178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 19.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol 182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poignard P, Moulard M, Golez E, Vivona V, Franti M, Venturini S, Wang M, Parren PW, Burton DR. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J Virol 77:353–365. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balla-Jhagjhoorsingh SS, Corti D, Heyndrickx L, Willems E, Vereecken K, Davis D, Vanham G. 2013. The N276 glycosylation site is required for HIV-1 neutralization by the CD4 binding site specific HJ16 monoclonal antibody. PLoS One 8:e68863. doi: 10.1371/journal.pone.0068863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard LK, Spencer DI, Royle L, Vasiljevic S, Krumm SA, Doores KJ, Crispin M. 2015. Glycan microheterogeneity at the PGT135 antibody recognition site on HIV-1 gp120 reveals a molecular mechanism for neutralization resistance. J Virol 89:6952–6959. doi: 10.1128/JVI.00230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nkolola JP, Peng H, Settembre EC, Freeman M, Grandpre LE, Devoy C, Lynch DM, La Porte A, Simmons NL, Bradley R, Montefiori DC, Seaman MS, Chen B, Barouch DH. 2010. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J Virol 84:3270–3279. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Barouch DH, Suscovich T, Ackerman M, Crispin M, Alter G. 2015. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J Immunol Methods 417:34–44. doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henn MR, Boutwell CL, Charlebois P, Lennon NJ, Power KA, Macalalad AR, Berlin AM, Malboeuf CM, Ryan EM, Gnerre S, Zody MC, Erlich RL, Green LM, Berical A, Wang Y, Casali M, Streeck H, Bloom AK, Dudek T, Tully D, Newman R, Axten KL, Gladden AD, Battis L, Kemper M, Zeng Q, Shea TP, Gujja S, Zedlack C, Gasser O, Brander C, Hess C, Gunthard HF, Brumme ZL, Brumme CJ, Bazner S, Rychert J, Tinsley JP, Mayer KH, Rosenberg E, Pereyra F, Levin JZ, Young SK, Jessen H, Altfeld M, Birren BW, Walker BD, Allen TM. 2012. Whole genome deep sequencing of HIV-1 reveals the impact of early minor variants upon immune recognition during acute infection. PLoS Pathog 8:e1002529. doi: 10.1371/journal.ppat.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]