Abstract
The human intestine is colonized by an estimated 100 trillion bacteria. Some of these bacteria are essential for normal physiology, whereas others have been implicated in the pathogenesis of multiple inflammatory diseases including IBD and asthma. This review examines the influence of signals from intestinal bacteria on the homeostasis of the mammalian immune system in the context of health and disease. We review the bacterial composition of the mammalian intestine, known bacterial-derived immunoregulatory molecules, and the mammalian innate immune receptors that recognize them. We discuss the influence of bacterial-derived signals on immune cell function and the mechanisms by which these signals modulate the development and progression of inflammatory disease. We conclude with an examination of successes and future challenges in using bacterial communities or their products in the prevention or treatment of human disease.
Keywords: commensal, bacteria, IBD, allergy, antibiotic, germ-free, microbiota, colitis
INTRODUCTION
Microorganisms are the most abundant life form on earth. Although many are free living, some have evolved to participate in close and often long-lasting interactions with multicellular species. Some of these relationships are pathogenic, whereas others are beneficial to the multicellular host. Such beneficial relationships have evolved to represent a conserved feature of multicellular life, important for normal development and physiology in plants (1), insects (2), nematodes (3), fish (4), birds (5), and mammals (6).
After birth, the epithelial surfaces of mammals are colonized with viruses, fungi, bacteria, protozoa, and helminths, creating complex microbial communities in multiple environmental niches. The mammalian intestine is the best studied of these microbial environments. By adulthood, the mammalian intestine is colonized by members of all three domains of life; bacteria are the most abundant, with more than 100 trillion individual organisms (7). Several terms have been used to describe the relationship between microorganisms and multicellular organisms (see sidebar), but for the purposes of this review we use “intestinal bacteria” to refer to all bacteria that inhabit the intestine and the term “beneficial” to specify those that participate in a mutualistic or commensal relationship. In some cases, millennia of evolution have resulted in mutualistic relationships between beneficial bacteria and the multicellular host (8). For example, beneficial bacteria supply essential nutrients, aid in the digestion of otherwise indigestible compounds, promote angiogenesis and enteric nerve function, defend against opportunistic pathogens, and contribute to the development and regulation of the mammalian immune system (9, 10).
Although signals derived from intestinal bacteria are important for normal mammalian development and physiology, alteration of these communities (dysbiosis) in patients or animal models is associated with multiple disease states including inflammatory bowel disease (IBD) (11), obesity (12), cancer (13), diabetes (14), and allergy (15). In many cases, altered immune responses to intestinal bacteria contribute to inflammation (16, 17), implicating dysbiosis as a biomarker and a potential trigger for disease. As such, understanding how signals derived from intestinal bacteria influence the mammalian immune system has important implications for defining the etiology of human inflammatory diseases as well as for the development of preventative or therapeutic intervention strategies.
This review focuses on the influence of intestinal bacteria on the mammalian immune system. We first give an overview of the role of intestinal bacteria in mammalian health and disease. We then review the acquisition and composition of bacterial communities in the mammalian intestine, with particular attention given to genetic and environmental factors that influence bacterial community structure. Next, we discuss immunomodulatory signals derived from intestinal bacteria, the receptors that recognize them, and their influence on innate and adaptive immune cell homeostasis. Finally, we discuss the prospects of exploiting our knowledge of signals from intestinal bacteria to prevent or treat human disease.
INTESTINAL BACTERIA IN MAMMALIAN HEALTH AND DISEASE
Although beneficial bacteria colonize all mammalian epithelial surfaces, the gastrointestinal tract has the largest bacterial burden, with more than 100 trillion individual organisms at a density of 1011 to 1014 cells per gram of luminal contents (7, 18). The bacterial communities of the mammalian intestine are also some of the best characterized; studies carried out as early as the 1960s using culture-based and microbiological identification methods began to identify the major bacterial groups present in the mammalian intestine (19). Currently, molecular advances in DNA bar coding and 454 pyrosequencing of 16S ribosomal RNA gene segments are allowing previously unattainable insights into nonculturable bacterial communities (20–23) and are placing species estimates from conservative numbers of 1000–2000 to numbers as high as 15,000–40,000 individual members (24).
Over the past century, studies in animals and humans have identified important roles for bacterial signals in promoting the optimal digestion of food (25), maintaining epithelial homeostasis (26), modulating fat metabolism (27), promoting angiogenesis (28) and enteric nerve function (29), supporting resistance to infection (30), and promoting normal development and regulation of immune cell homeostasis (6) (Figure 1). Despite these beneficial sequelae, dysbiosis may be both a biomarker and a potential contributing factor to human inflammatory diseases.
Figure 1.
Intestinal bacteria in mammalian health and disease. Schematic of the known influences of intestinal bacteria on normal mammalian physiology and inflammatory disease states.
For example, IBD is thought to result from inappropriate and ongoing mucosal immune responses to normal intestinal bacteria (31). Tolerance to intestinal bacteria is broken in IBD (32), leading to inappropriate local (33) and systemic (34, 35) immune responses to intestinal communities that may contribute to pathogenesis (16). Additionally, bacterial communities from the intestine of IBD patients have a reduced diversity compared with those from healthy individuals (11), and IBD patients display aberrant cytokine production, T cell activation, and IgG antibody responses to intestinal bacteria (32, 33). Genetic susceptibility loci have been identified for the inflammatory bowel diseases Crohn’s and ulcerative colitis, including mutations in the pattern-recognition receptor NOD2 (nucleotide-binding oligomerization domain-containing protein 2) (36, 37), a component of the innate immune system that is important for immune recognition and responses to intracellular bacteria (38) (see below). These findings implicate altered immune responses to intestinal bacteria in the pathogenesis of IBD.
Animal models of IBD have provided additional insights into the influence of intestinal bacteria on the pathogenesis of this disease. Reducing microbial stimulation in murine models of IBD, achieved by rearing animals under germ-free conditions, ameliorates intestinal disease. For example, IL-2-deficient mice spontaneously develop intestinal inflammation when raised under conventional conditions but have a delayed and milder disease course when raised under germ-free conditions (39). Similarly, both IL-10-deficient or TCRαβ-deficient mice develop spontaneous colitis associated with inappropriate inflammatory immune cell responses when maintained under conventional conditions but were protected against disease when maintained under germ-free conditions (40, 41). These findings support an essential role for microbial-derived signals in driving pathogenic inflammatory responses in these models.
Antibiotic treatment can also ameliorate disease in murine models of IBD. For example, mice deficient in the multiple drug resistance gene (mdr1aI) developed spontaneous colitis when housed under specific pathogen–free conditions but were protected from disease by oral antibiotic treatment (42). In addition, mice deficient in keratin-8, a major intermediate filament protein present in the intestinal epithelia, were protected from spontaneous colitis by oral antibiotic treatment (43), as were IL-10-deficient mice (44, 45). These findings suggest that the role of bacterial-derived signals in disease pathogenesis is not purely developmental; rather, they identify bacterial signals as important in the maintenance of intestinal inflammation.
In addition to contributing to inflammatory states, dysbiosis alone can cause disease in otherwise healthy animals. For example, mice deficient in the inflammatory transcription factor T-bet and the recombinase-activating gene RAG2 (TRUC mice) developed spontaneous colitis that was ameliorated by antibiotic treatment (46). When wild-type mice were cohoused with TRUC mice, they also developed colitis, implicating vertical and horizontal transmission of colitogenic bacterial communities as a cause of disease in immunocompetent animals (46).
Altered signals from intestinal bacteria may also influence risk of developing asthma and other systemic atopic disorders in humans (17). Atopy describes inappropriate, type 2 inflammatory responses to environmental allergens (reviewed in 47), and some patients with atopic diseases have altered intestinal bacterial communities (15). In addition, antibiotic treatment of children increases the risk of developing asthma later in life (48) (presumably as a result of altering intestinal communities), as does early colonization with the intestinal bacterium Bacteroides fragilis (49), a bacterial group that increases in frequency upon antibiotic treatment of mice (50). Similarly, colonization with Bifidobacterium, Clostridium difficile, or Escherichia coli is associated with the development of eczema in humans (15, 51, 52), an association that may be related to formula feeding (53), although this hypothesis remains to be tested directly.
Animal models have provided important insights into the influence of intestinal bacteria on systemic immune responses that may contribute to disease states. For example, outgrowths of Candida albicans after antibiotic treatment of conventional mice were associated with the development of a CD4+ T cell–mediated allergic airway disease (54). In addition, inflammatory responses following subcutaneous injections of carrageenan, lipopolysaccharide (LPS), TNF-α, IL-1β, or the chemokine CXCL1 were reduced in germ-free mice (55). These immune defects were reversed through conventionalization, or the systemic administration of LPS, implicating bacterial signals in the regulation of systemic inflammatory responses (55). Finally, intestinal bacteria may also influence the development of type 1 diabetes, as nonobese diabetic mice deficient in the Toll-like receptor (TLR) adaptor molecule MyD88 are protected against diabetes development (14). Taken together, these findings implicate signals from intestinal bacteria in the regulation of local and systemic inflammatory responses that contribute to disease pathogenesis.
BACTERIAL COMPOSITION AND COLONIZATION DYNAMICS IN THE MAMMALIAN INTESTINE
Humans and other mammals are born from a sterile environment and subsequently acquire intestinal bacteria during their first months of life (56). Early studies using culture-based and microbiological identification methods identified lactobacilli, anaerobic streptococci, and members of the Bacteroides genus as residents of the normal adult human intestine (19). However, a large percentage of intestinal bacteria are anaerobes that lack the enzymes necessary for the detoxification of oxygen. As such, even under ideal conditions, it is estimated that only half of bacteria in stool are culturable (57).
More recently, DNA bar coding and 454 pyrosequencing of 16S ribosomal RNA gene segments have provided more accurate characterization of intestinal communities. These studies have identified the Firmicutes and Bacteroidetes phyla as the major bacterial groups present in the mammalian intestine (20–23) (Figure 2). Of the Firmicutes, 95% belong to the Clostridia class, whereas large variations exist in the Bacteroidetes phylotypes among individuals (20–22, 58). Other phyla present in relatively low abundance include the Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia (20, 21, 23, 58, 59). New sequencing methods also allow for metage-nomic analysis of intestinal communities and are providing novel insights into the influence of microbial-derived genes and gene products on normal mammalian physiology (24).
Figure 2.
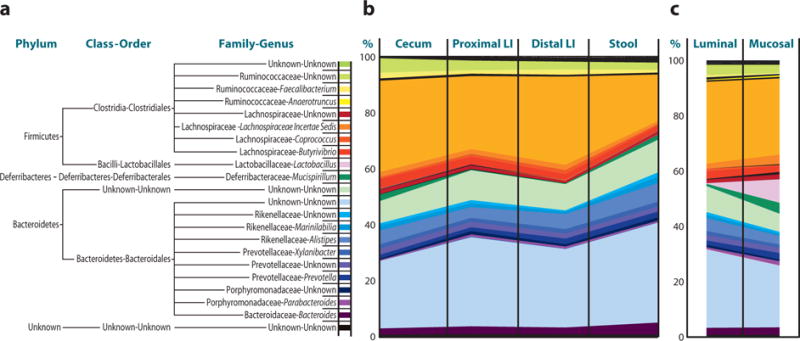
The composition of bacterial communities along the length and between luminal and mucosal compartments of the mammalian intestine. Stool pellet, luminal content, or mucosal-associated communities were sterilely collected. Total sample DNA was extracted and bacterial 16S rRNA gene fragments were PCR amplified with bar code–tagged primers and subjected to pyrosequencing, and taxonomic assignments for each sequence were obtained using RDP Classifier. (a) Commonly found bacteria in the murine colon. (b) Relative frequencies and distribution of bacteria along the length of the murine colon and in murine stool samples. (c) Relative frequencies and distribution of bacteria between luminal and mucosal-associated compartments of the murine colon. Adapted from Reference 50.
Bacterial communities exhibit differences along the length of the colon and between the luminal and mucosal-associated microenvi-ronments (20, 60, 61), suggesting that defined microbial communities at different anatomical locations may be important for normal mammalian physiology (Figure 2b and 2c). For example, although lactobacilli have been cited for potential probiotic effects (62) and can be isolated from approximately 80% of adults, they represent a relatively low proportion of luminal bacteria (58). However, these potentially beneficial microorganisms represent a much higher proportion of mucosal-associated bacteria (up to 13%) in the mammalian intestine (Figure 2) (50).
Although intestinal bacteria are likely to be continuously acquired, mammals undergo two dominant phases of intestinal colonization, the first during breast milk or formula feeding and the second upon weaning to solid foods. Mammalian breast milk is both a continuous source of defined microorganisms and an important source of passive immunity that shapes developing intestinal communities (63). As such, breast-feeding is considered important for the development and maintenance of normal bacterial communities in the intestine (64). The temporal and spatial patterns of intestinal colonization in infants are variable between individuals (65) and depend on multiple factors, including country of birth (66), prematurity (67–69), mode of delivery (67, 70), history of hospitalization (67), antibiotic use (67), feeding practices (53, 56, 67, 71–74), and other factors (Table 1). For example, vaginally born and breast-fed infants have a predominance of Bifidobacteria in their intestine, with smaller contributions of E. coli, Bacteroides, and Clostridia species (75). In comparison, infants delivered by Cesarean section have delayed colonization kinetics compared with vaginally born infants, as well as persistent changes to community compositions (70), including lower burdens of Bifidobacteria and Bacteroides and higher burdens of C. difficile (67). Although these individuals likely acquire mature adult bacterial communities upon transition to solid foods, these early alterations may not be benign, given that some associations exist between early alterations to intestinal bacteria and increased risk of atopic disease (76, 77).
Table 1.
Factors influencing the acquisition and/or composition of intestinal bacterial communities
| Factor | Reported alteration | References |
|---|---|---|
| Birth in North America | Increased Bacteroides and Bifidobacterium species, decreased Lactobacillus and Eubacterium aerofaciens | 66 |
| Hospitalization/prematurity | Delayed colonization, reduced community diversity, reduced Bifidobacterium species, higher C. difficile | 67–69 |
| Delivery by Cesarean section | Delayed colonization, lower Bifidobacterium species and Bacteroides fragilis, higher C. difficile | 67, 70 |
| Antibiotic use | Decreased Bifidobacterium and Bacteroides species, increased Campylobacter | 61, 67 |
| Formula feeding | Delayed colonization with Bifidobacterium species, more often colonized with staphylococci, E. coli, C. difficile, Bacteroides, and lactobacilli | 53, 56, 67, 72, 74 |
| Vegetarian diet | Higher Clostridium species | 71 |
| Old age | Lower Clostridium species, higher Ruminococcus obeum and gammaproteobacteria species | 377 |
| Older siblings | Higher Bifidobacterium species | 67 |
| Infectious colitis | Increased Campylobacter, decreased Lactobacillus | 61, 378 |
| Species | Bacterial communities differ between mammalian species | 61 |
| Gender | Bacterial communities differ between genders | 61 |
| Genetics | Innate immune function regulates intestinal communities in flies | 379 |
The initial immune response to colonization of the mammalian intestine is best described in mice and is characterized by a general inflammatory response that peaks within a week of birth and subsequently stabilizes over the first year of life (78). A hallmark of colonization is the production and secretion of IgA into the intestinal lumen by the host (79) in response to small numbers of intestinal bacteria that penetrate the intestinal epithelium (80, 81). Upon penetration, Peyer’s patch dendritic cells (DCs) phagocytose penetrating bacteria and initiate IgA responses through T cell–dependent and –independent mechanisms (82–85). These DCs induce IgA class switching in the mesenteric lymph nodes (mLNs), but not in systemic secondary lymphoid structures (80), indicating that induction of this initial IgA response is compartmentalized to mucosal tissues (86). The resulting secretion of IgA across the intestinal epithelium feeds back in a homeostatic mechanism to reduce epithelial penetration by intestinal bacteria (87).
In addition to IgA responses, the mammalian host mounts an innate immune response upon colonization that contributes to maintenance of the mucosal barrier (reviewed in 88). For example, intestinal Paneth cells directly sense intestinal bacteria through cell-autonomous MyD88 activation, resulting in upregulated expression of the antimicrobial peptide REGIIIγ (89). In addition, intestinal goblet cells upregulate their expression of RELMβ, but not of RELMα or RELMγ, in response to intestinal colonization (90). These early innate responses by intestinal epithelial cells (IECs) have important immunoprotective roles. For example, MyD88-deficient mice fail to upregulate REGIIIγ and are susceptible to Listeria monocytogenes infection; reconstitution of MyD88-deficient mice with recombinant REGIIIγ enhances clearance of this pathogen (91).
The systemic response to colonization, and the subsequent development of systemic tolerance, is less well described. Serum antibodies to components of intestinal bacteria are found in humans and other mammals (92), and these antibodies help contain bacteria to the intestine in the absence of innate mechanisms (93, 94). In addition, patients with IBD display systemic immune responses to intestinal bacteria (34, 35), suggesting that tolerance to intestinal bacteria is important for systemic immune cell homeostasis. Although the mechanisms by which the naive host tolerates intestinal bacteria are an ongoing field of study (see below), it will be interesting to examine whether colonization of the intestine with microorganisms early in life influences immunological thresholds from which subsequent proinflammatory or immunoregulatory responses are determined (48, 49).
INNATE RECOGNITION OF BACTERIAL-DERIVED SIGNALS IN THE INTESTINE
As discussed above, the colonization of the mammalian intestine results in rapid and dramatic responses by the mucosal immune system that are important for maintaining mucosal homeostasis and protecting against intestinal pathogens. These responses are likely mediated in part through the recognition of bacterial signals (cell wall components, DNA segments, metabolites, etc.) by innate TLRs, NOD-like receptors (NLRs), and G protein–coupled receptors (GPCRs) expressed in hematopoietic and nonhematopoietic cells of the intestine. In this section, we review the signals derived from intestinal bacteria, their innate receptors, and the role that IECs and DCs play in recognizing these signals and modulating subsequent adaptive immune responses (summarized in Table 2).
Table 2.
Bacterial signal receptors, ligands, and immunologic effects
| Ligand | Proposed receptor | Immunologic outcome | References |
|---|---|---|---|
| PSA | TLR2 | Promotes normal Th1/Th2 balance Enhances response to abscess-forming bacteria Suppresses intestinal IL-17 production Protects against colitis |
110, 113 110, 111 112 112 |
| LPS | TLR4 | Activates NF-κB Induces DC migration Activates systemic DCs Inhibits mucosal DCs Protects against colitis |
104 105 106 107 103 |
| Flagellin | TLR5 | Positively associated with Crohn’s disease Protects against chemical-, bacterial-, viral-, and radiation-induced mortality |
99 98 |
| CpG | TLR9 | Enhances intestinal IFN-γ and IL-17 production Protects against intestinal parasites Protects against systemic allergy |
109 109 108 |
| Muramyl dipeptide | NOD2 | Activates NF-κB Promotes lymphoid tissue development Regulates intestinal bacterial communities Promotes antigen-specific immune responses Protects against colitis Promotes tolerance to bacterial products Inhibits IL-12p70 production Protects against IL-12-driven experimental colitis Protects against Crohn’s disease |
114 116 116 117 127–129 130 125 126, 127 36, 37 |
| Ado | A2A | Protects against colitis Drives intestinal Th17 cell differentiation |
137 138 |
| Butyrate | GPR109A | May protect against colon cancer Induces ROS and suppresses NF-κB signaling in IECs Reduces TNF-α, TNF-β, IL-6, and IL-1β production by LPLs in IBD patients |
140–142, 380 142, 143 144, 145, 381 |
| Succinate | GPR91 | Acts on intestinal DCs to trigger intracellular calcium release, induce migration, induce proinflammatory cytokine production, and enhance antigen-specific T cell activation | 147 |
| SlpA | DC-SIGN | Induces IL-10 and IL-12p70 production by DCs | 148 |
TLR Ligands: Flagellin, Lipopolysaccharide, Polysaccharide A, and CPG Motifs
TLRs are innate pattern-recognition receptors that recognize evolutionarily conserved motifs found in bacteria and other microorganisms (95). TLRs have evolved to recognize multiple microbial-derived products including double-stranded viral RNA (TLR3), gram-negative LPS (TLR4), and gram-negative and gram-positive flagellin (TLR5) (reviewed in 96). Most TLRs are expressed on the surface of cells, with the exception of TLR3, 7, 8, and 9, which are localized to endosomal compartments (97).
Several disease models have provided insights into the role of TLR ligands in modulation of the mammalian immune system and indicate both proinflammatory and immunoregulatory functions for TLR signals in various disease settings. As discussed above, nonobese diabetic mice deficient in the TLR signaling molecule MyD88 are protected against the development of type 1 diabetes (14), suggesting that microbial-derived signals are central to disease development in this model. In contrast, treatment with flagellin protects against chemical-, bacterial-, viral-, and radiation-induced mortality in animal models (98), indicating that this molecule has important immunoregulatory roles. In humans, dominant-negative TLR5 polymorphisms reduce adaptive responses to flagellin and are negatively associated with Crohn’s disease (99), whereas TLR2 expression is higher in antigen-presenting cells (APCs) from patients with psoriatic arthritis (100). These findings have spurred interest in TLR ligands as therapeutic agents for human disease, and TLR ligands are currently being investigated as treatments for human allergy (101) and as adjuvant therapies for cancer (102).
One mechanism by which TLR ligands may influence disease states is through modulation of mucosal immune cell function. For example, LPS-induced TLR-dependent signaling protects against experimental colitis (103). Cellular studies showed that LPS elicits TLR-dependent NF-κB activation in IECs, providing a possible mechanism by which IECs monitor and initiate immune responses to intestinal bacteria (104). In addition, LPS causes differential DC migration (105) and differential DC activation, depending on anatomical location; TLR4 signaling on DCs promotes antigen-specific CD4+ T cell–mediated pulmonary inflammation (106), whereas intestinal DCs are reported to become hyporesponsive upon TLR4 ligation (107).
Other TLR ligands have proinflammatory and immunoregulatory roles depending on the anatomical location examined. Administration of CpG, a microbial DNA motif that is recognized by TLR9, reduced the susceptibility of TLR4-deficient mice to systemic allergy (108), implicating this molecule in immunoregulatory roles. However, CpG rescued defective IFN-γ and IL-17 production in the intestine of germ-free mice and protected against infection with intestinal parasites (109). In contrast, the Bacteroides fragilis cell wall component polysaccharide A (PSA) may have a predominantly immunoregulatory role, given that it promotes normal immune homeostasis (110, 111) and protects against experimental colitis through the suppression of IL-17 production (112, 113).
NLR Ligands: Peptidoglycan
NLRs, such as NOD1 and NOD2, detect intracellular ligands and are recognized as key mediators of proinflammatory and immunoregulatory responses (114). NLRs recognize several bacterial components, including peptidoglycan-containing meso-diaminopimelic acid (NOD1) and muramyl dipeptide (NOD2) (reviewed in 115). In a pathogenic setting, NOD2 ligation initiates NF-κB activation and upregulation of inflammatory cytokines, including IL-12 (114). In addition, recognition of bacterial signals through NLRs is important for the development of intestinal lymphoid tissues (116), the maintenance of normal intestinal bacterial communities (116), and the mounting of antigen-specific immunity (117).
Approximately 15% of patients with Crohn’s disease have homozygous or compound heterozygous mutations in the gene that encodes NOD2 (CARD15) (36, 37). Disease-associated alleles were shown to impair NOD2 receptor activity (118), leading to the hypothesis that impaired control of intestinal bacteria was central to disease development (119, 120). However, subsequent studies in NOD2-deficient mice, in particular the seemingly unaltered susceptibility of these mice to some experimental colitis models, suggested a more complex immunomodulatory role for NOD2 signaling (121, 122). Indeed, contrary to the prevailing dogma, it was shown that Crohn’s disease– associated NOD2 alleles potentiate rather than attenuate NF-κB signaling (123, 124).
Subsequent studies explored the complex proinflammatory and immunoregulatory roles for NOD2. NOD2 activation was shown to inhibit TLR2-dependent activation of NF-κB, suggesting one possible mechanism by which Crohn’s disease–associated NOD2 alleles could result in an inflammatory state (125). Consistent with this, NOD2-deficient mice displayed TLR2-dependent susceptibility to colitis that was characterized by antigen-specific IFN-γ-producing CD4+ T cells (126). Mu-ramyl dipeptide activation of NOD2 also protected mice from experimental colitis (127), and NOD2 transgenic mice exhibited enhanced muramyl dipeptide–mediated resistance to colitis (128). Cellular studies have indicated that administration of muramyl dipeptide decreases the production of IL-12p40, IL-6, and TNF-α by intestinal DCs (127–129). Furthermore, APCs from NOD2-deficient mice produced greater IL-12p70 when stimulated with pepti-doglycan, whereas addition of muramyl dipeptide to cultures of APCs from NOD2-sufficient mice lead to decreased IL-12p70 responses (125). While these findings suggest that NOD2 plays an important immunoregulatory role in attenuating TLR2-mediated proinflammatory responses to intestinal bacteria (130), other studies have highlighted synergistic inflammatory and immunoregulatory roles for NOD-and TLR-dependent signaling (124, 131, 132).
NOD signaling also modulates production of the immunoregulatory cytokine IL-10. Animal models have shown that TLR2 and NOD2 can act synergistically to induce IL-10 production by macrophages (133), and Crohn’s disease–associated NOD2 alleles suppress transcription of human IL10 by inhibiting the activity of the nuclear ribonucleoprotein hnRNP-A1 (134). As such, both positive and negative interactions between TLRs and NLRs potentially exist to modulate inflammatory and immunoregulatory cytokine responses. While complex, the intricacies of these interactions likely hold important potential for future preventative and therapeutic interventions for IBD and other inflammatory diseases.
G Protein–Coupled and Other Receptors: Adenosine, Short-Chain Fatty Acids, and Surface Layer A Protein
In addition to the TLR and NOD ligands, immunoregulatory GPCR ligands such as the purine adenosine (Ado) are of growing interest in the fields of IBD and other inflammatory disease research (135). Ado may function as an endogenously generated regulator of inflammation, depending on the receptor it binds. For example, an Ado A2A receptor agonist did not alter the course of dextran sodium sulfate (DSS)-induced colitis (136), whereas Ado A2B– deficient mice had increased susceptibility to DSS colitis (137). Additionally, intestinal bacteria can be a source of ATP that can drive Th17 cell differentiation in the lamina propria by inducing IL-6 and IL-23p19 production by a population of CD70high CC11clow cells (138). Consistent with this, germ-free mice exhibited lower concentrations of luminal ATP, as well as fewer numbers of Th17 cells in the lamina propria, a defect that could be reversed through the systemic or rectal administration of ATP.
The normal bacteria of the mammalian intestine also produce significant amounts of butyrate, as well as other short-chain fatty acids (SCFAs) (reviewed in 139). The receptor for butyrate, GPR109A, is expressed on IECs and is downregulated in human colon cancer, in a mouse model of intestinal/colon cancer, and in colon cancer cell lines (140, 141). Consistent with the view that GPR109A is a tumor suppressor, expressing GPR109A in colon cancer cells induces apoptosis in the presence of butyrate (142). Butyrate signals through GPR109A in IECs to suppress NF-κB signaling (142, 143) and reduces production of TNF-α, TNF-β, IL-6, and IL-1β by lamina propria lymphocytes (LPLs) in Crohn’s and ulcerative colitis patients (144, 145), implicating this microbial metabolite in the regulation of multiple cell populations. Additionally, the SCFA receptor GPR43 has recently been identified as a key mediator of microbial-derived immunomodulatory signals, as mice deficient in GPR43 show exacerbated or unresolving inflammation in models of colitis, arthritis, and asthma (146).
Succinate, a component of the citric acid cycle, modulates DC function by signaling through the extracellular GPCR GPR91. In one study, succinate signaling triggered intracellular calcium release, induced migratory responses, and acted in synergy with TLR ligands to induce the production of proinflammatory cytokines by DCs (147). In this study, GPR91−/− mice exhibited reduced Langerhans cell migration to draining lymph nodes and succinate enhanced antigen-specific activation of human and mouse CD4+ T cells (147). GPR91−/− mice displayed impaired tetanus toxoid–specific recall T cell responses, further implicating GPR91-dependent succinate signaling as a signal of immunologic danger.
Finally, bacterial adhesion via surface proteins can directly modulate immune cell function. For example, Lactobacillus acidophilus NCFM attaches to DCs and induces concentration-dependent production of IL-10 and IL-12p70 in a DC-specific, ICAM-3-grabbing nonintegrin (DC-SIGN)-specific manner (148). This immunomodulatory function may depend on the bacterial surface component surface layer protein A (SlpA) because purified SlpA protein binds directly to DC-SIGN, and T cells primed with DCs that are stimulated with L. acidophilus NCFM lacking SlpA produce less IL-4 than do those stimulated with wild-type L. acidophilus NCFM. In summary, microbial signals can have proinflammatory and immunoregulatory effects on multiple immune cell lineages. The next section examines how recognition of microbial signals by IECs and DCs can result in modulation of both local and systemic immune responses.
RECOGNITION OF INTESTINAL BACTERIA BY EPITHELIAL CELLS
The intestinal epithelium has a diverse set of physiologic functions, including digestion and absorption of nutrients, creating a physical barrier between the external and internal environments, and immunological surveillance of intestinal bacteria and potential pathogens. Epithelial cells are continually replaced from a pool of Lgr5+ multipotent stem cells that reside in crypts of the intestine (149, 150). These epithelial cells provide an effective physical barrier to the outside environment as intercellu-lar tight junctions prevent paracellular traffic and actin-rich microvillar extensions create an apical brush border that impedes microbial attachment and invasion (151). Uptake of macromolecules, particulate antigens, and microorganisms across the intestinal epithelia occurs only by active vesicular transport across epithelial cells, and as such is regulated by multiple mechanisms (reviewed in 152).
In addition to physical adaptations that control transport of solutes across the epithelium, biochemical adaptations have evolved, including production of heavily glycosylated, mucin-rich secretions from goblet cells that create a relatively impermeable, apically adhered glycocalyx (153). The epithelium also produces antimicrobial peptides, including defensins, cathelicidins, and calprotectins that confer broad-spectrum antimicrobial properties through the formation of pores in bacterial cell walls (154). These adaptations are consistent with the view that IECs, in addition to promoting digestion and absorption of nutrients, perform essential barrier functions that obstruct the entry of beneficial and pathogenic bacteria into the underlying lamina propria.
IECs are in continuous contact with beneficial and pathogenic bacteria and, as a result, are ideally located for immunological surveillance of the intestinal lumen. As discussed above, IECs express TLRs (155, 156), NLRs (38), and GPCRs that recognize microbial components and modulate cellular responses (Figure 3). IEC expression of innate pattern-recognition receptors is important for mounting immune responses to pathogenic microorganisms (115, 157) by promoting the expression of proinflammatory cytokines (95, 114), chemokines, and antimicrobial peptides (97) as well as the direct induction of IgA class switching by B cells (82, 158, 159). Responses by IECs to intestinal bacteria are not uniform, however: IECs selectively initiate proinflammatory responses to pathogenic bacteria while promoting tolerance to beneficial bacteria (151, 157, 160). One example of this is the gram-negative bacteria Bacteroides thetaiotaomicron, which induces IEC expression of the antimicrobial peptide REGIIIγ, whereas the gram-positive Bifidobacterium longum, a common component of intestinal communities, does not (161, 162).
Figure 3.
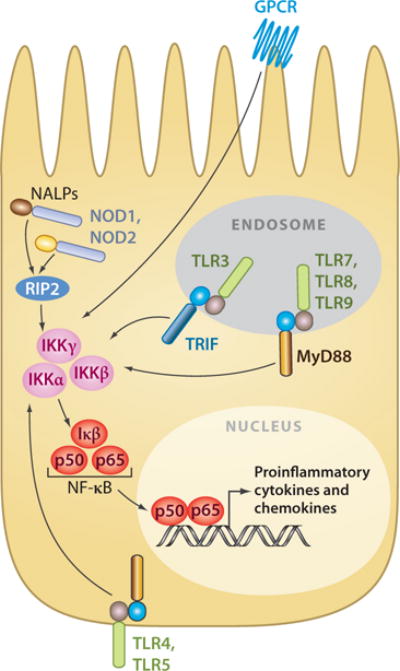
Innate receptors and signaling cascades of mammalian intestinal epithelial cells (IECs). Schematic shows the location of innate pattern-recognition receptors and their signaling cascades in mammalian IEC. Innate pattern-recognition receptors converge on a common NF-κB signaling cascade to regulate transcription of proinflammatory cytokines and chemokines.
Two mechanisms by which IECs may discriminate between beneficial and pathogenic bacteria are (a) through subcellular sequestering of pattern-recognition receptors away from luminal signals and (b) differential receptor expression. For example, TLR5, which recognizes bacterial flagellin, is expressed exclusively on the basolateral surfaces of IECs (reported in Reference 156). Additionally, TLR3, 7, 8, and 9 are reported to be expressed exclusively in intracellular endosomal organelles (97), and NLRs are localized to the cytoplasm, reducing exposure of these receptors to luminal bacteria (163, 164). In addition, under steady-state conditions, IECs express little or no TLR2, TLR4, or CD14, further minimizing stimulation by luminal bacteria (165, 166). However, subcellular localization and differential expression alone cannot account for discrimination between beneficial and pathogenic bacteria, as GPCRs that recognize and initiate responses to bacterial products are continuously expressed on the apical surface of IECs. As such, further investigations are necessary to fully understand how IECs discriminate against beneficial and pathogenic bacteria.
The recognition of bacterial signals by IECs is essential to mucosal homeostasis, implicating IECs as central modulators of inflammatory responses (103, 116, 167). One mechanism by which IECs may regulate mucosal homeostasis is by influencing DCs, macrophages, and lymphocytes through the local expression of immunoregulatory cytokines, including thymic stromal lymphopoietin (TSLP), IL-10, transforming growth factor-β (TGF-β), prostaglandin E2, retinoic acid, and IL-25 (168–174). For example, a population of intestinal DCs induced regulatory T cells (Tregs) that expressed the forkhead box P3 transcription factor (Foxp3+) in a TGF-β- and retinoic acid–dependent manner in vitro, implicating epithelial-derived signals in the conditioning of DCs and subsequent adaptive responses (175, 176). In addition, deletion of NF-κB signaling specifically in IECs resulted in the dysregulated expression of DC-derived proinflammatory cytokines and the development of spontaneous or infection-induced intestinal inflammation (177, 178). These findings provided the first in vivo evidence of a crucial role for IECs in the conditioning of intestinal DC responses. In vitro studies recapitulated the in vivo results as monocyte-derived or circulating DCs conditioned with supernatants from Caco-2 cells or IECs isolated from healthy patients induced Foxp3+ Tregs, whereas IEC supernatants from Crohn’s disease patients did not (179). This effect was dependent on the production of TGF-β and retinoic acid by IECs, but not on TSLP production, as DCs deficient in the TSLP receptor (TSLPR−/−) and wild-type DCs exhibited a similar capacity to convert naive T cells into Tregs (180).
Epithelial-derived TSLP in particular has important immunomodulatory roles. High levels of Tslp mRNA are expressed by epithelial cells at the barrier surfaces of the skin, airways, and intestine, and expression can be upregulated by infection, inflammation, and tissue injury (172, 178, 181–184) in an NF-κB-dependent manner (185). In vitro studies have shown that TSLP-conditioned human DCs can promote Th2 cell responses (172, 186–188) through the inhibition of IL-12 production and the induction of OX40L expression (172, 188). In addition, in vivo studies in the skin and lung (186, 187, 189, 190) have shown that transgenic overexpression of TSLP in cutaneous or pulmonary epithelial cells results in the onset of Th2 cytokine–mediated inflammation resembling atopic dermatitis or asthma, respectively (189, 190). This finding suggests that TSLP is necessary and sufficient for the initiation of Th2 cytokine–driven inflammation (reviewed in 191). Indeed, TLSP expression and TSLP-TSLPR interactions are important for immunity to the intestinal nematode Trichuris and for protection against experimental colitis through the in vivo inhibition of IL-12/23p40 production by DCs (173, 178, 192).
Finally, IECs are in direct contact with in-traepithelial lymphocytes (IELs) and express all the molecular machinery required for antigen processing and presentation, including proteolytically active cathepsins, the invariant chain, and MHC class II (MHCII) molecules (193). In addition, IECs isolated from patients with IBD were shown to express MHCII molecules and localize exogenous antigens to the late endosome on their basolateral surfaces (194). In vitro studies have shown that rodent IECs, although less potent than professional APCs, could process and present antigen through the MHCII pathway (195). Despite the ability of IECs to process and present antigen, they are reported to lack expression of costimulatory molecules (196), indicating that IECs cannot prime naive T cells and may instead provide tolerogenic signals. However, the intestine contains a large population of memory/activated T cells that exhibit less stringent requirements for costimulation and therefore may be influenced by IEC-intrinsic antigen presentation. Additionally, IECs may deliver inhibitory or tolerogenic signals directly to T cells, consistent with their known role in controlling B cell responses (159). Taken together, these studies highlight that IEC-mediated recognition of intestinal bacteria results in IEC-intrinsic gene expression and the production of immunoregulatory signals that can control innate and adaptive immune cell function.
RECOGNITION OF INTESTINAL BACTERIA BY DENDRITIC CELLS
DCs are perhaps the most efficient modulators of adaptive immune responses (reviewed in 197), and they represent an important link between the innate and adaptive immune systems. In the intestine, DCs take on specific phenotypic characteristics and perform distinct functions depending on their anatomical location (198). In the small intestine, DCs reside in the intestinal lamina propria (LP DCs) and in organized lymphoid structures such as Peyer’s patches (PP DCs), solitary isolated lymphoid tissue, and mLNs where they function to sample and present luminal and self antigen to T cells (199) (Figure 4). PP DCs can be divided into three groups based on chemokine receptor expression, location within the Peyer’s patch, and functional characteristics: CX3CR1+ PP DCs are found in close contact with the follicle-associated epithelium, where they participate in a close functional relationship with the epithelial M (microfold) cell (200) to sample luminal antigens in a pattern-recognition receptor–dependent manner (201); CCR6+ PP DCs are found in the subepithelial dome but can quickly migrate to the follicle-associated epithelium in response to microbial stimulation (202); and CCR7+ PP DCs are found in T cell areas (203), where they orchestrate helper T cell responses (204), T cell migration (205, 206), and IgA production (207, 208) in response to microbial signals (reviewed in 209).
Figure 4.
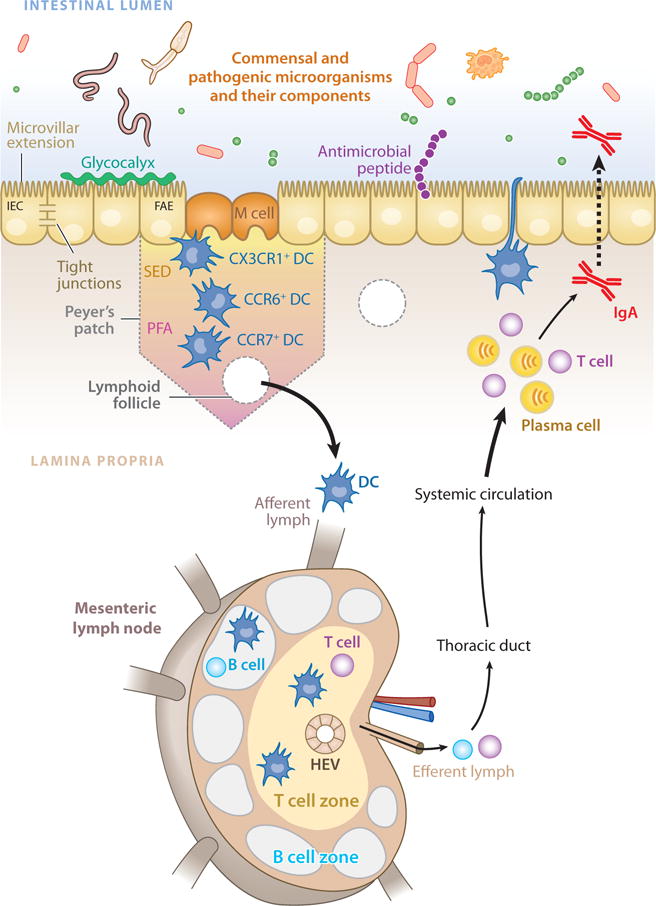
The mucosal immune system of the mammalian intestine. Innate recognition of signals from intestinal bacteria takes place at the intestinal epithelium, in the lamina propria, and in gut-associated lymphoid tissues such as Peyer’s patches and isolated lymphoid follicles. Specialized intestinal epithelial cells known as M (microfold) cells overlie Peyer’s patches and lymphoid follicles to facilitate luminal sampling and to transport microbial components to professional antigen-presenting cells present in the subepithelial dome (SED). Dendritic cells (DCs) in the SED and perifollicular area (PFA) acquire antigens and influence adaptive responses. Additionally, specialized DC subsets directly sample luminal antigens. Intestinal DCs transport antigens to mesenteric lymph nodes through the afferent lymphatic system. DCs in the mesenteric lymph node promote differentiation of regulatory and effector T lymphocytes, as well as class switching of B lymphocytes, which then exit through the efferent lymph into the systemic circulation. Some of these cells home back to the intestine, where they exert their effector functions.
Although DCs resident in the Peyer’s patches are important mediators of immune function, Peyer’s patches are relatively rare along the length of the intestinal tract. DCs also reside in the lamina propria of the small intestine, where they may be seeded from circulating precursors (210). LP DCs express tight-junction proteins that allow for direct luminal sampling through the extension of dendrites between IECs (211), a process that is reported to be dependent on the CX3C chemokine receptor 1 (CX3CR1) (212) and TLR ligation (213). Accordingly, CX3CR1−/− mice exhibit defective luminal sampling by DCs and impaired resistance to Salmonella typhimurium infection. As such, luminal sampling by LP DCs may play a role in the development of protective immune responses in the intestine (212). However, impaired protective immunity in this system may also be due to changes in other myeloid cells such as macrophages.
Intestinal DCs recognize bacteria through the expression of innate pattern-recognition receptors (97) that can modify luminal sampling (213), migration (105, 214), and the induction of T cell differentiation (215–217). Intestinal DCs are tolerogenic compared with systemically circulating DCs (175, 176), a phenotype that may contribute to the generation of oral tolerance (218, 219). For example, stimulation of intestinal DCs with the TLR4 ligand LPS resulted in elevated IL-10 production, whereas stimulation of systemic DCs resulted in proinflammatory activation (220, 221). The mechanisms by which intestinal DCs may be skewed toward a tolerogenic phenotype are still under investigation and include reduced TLR expression (220, 221), hyporesponsiveness to TLR stimulation (107), and/or negative regulation of the NF-κB pathway via NOD2 signaling (as discussed above) (125–129). Additionally, IECs may play an active role in regulating the functional capacity of intestinal DCs (see above).
Intestinal resident DCs regulate local T cell responses in part through the production of IL-12 and IL-23. IL-12 is a key regulatory cytokine that induces Th1 cell differentiation (222) and plays an important role in Th1-mediated experimental models of autoimmune diseases, including IBD (223–226). IL-23 is a heterodimer cytokine composed of a p40 subunit (that is shared with IL-12) and a unique p19 subunit (227). Although one study has implicated IL-23 as having a protective role in a murine model of colitis (228), others have shown that IL-23 drives inflammatory Th17 responses (229, 230; reviewed in 231) and is the causative agent in such inflammatory disorders as joint inflammation (232), intestinal inflammation (230, 233– 236), and psoriasis (237, 238). The recent association of IBD with the gene that encodes the IL-23R has further increased clinical interest in an IL-23 inflammatory axis (239).
Intestinal DCs (240) transport self (241) and bacterial (80, 197) antigens to the mLNs where they can influence local immune responses. For example, LP and mLN DCs can promote conversion of naive CD4+ T cells into Tregs (175, 176, 199) in a retinoic acid– and TGF-β-dependent manner. Intestinal DCs in the mLNs can also target B and T lymphocytes back to the intestine by promoting the upregulation of CCR9 and α4β7 (205, 206, 242–244). Taken together, these findings suggest that the tolerogenic phenotype of intestinal DCs may be important for maintaining mucosal homeostasis. However, the role that commensal bacteria play in maintaining or promoting the tolerogenic phenotype of mucosal DCs remains to be examined.
SIGNALS DERIVED FROM INTESTINAL BACTERIA REGULATE IMMUNE CELL DEVELOPMENT AND FUNCTION
As discussed above, signals from intestinal bacteria appear to influence human and murine models of disease by modulating innate and adaptive immune responses. One model used to study the role of microbial signals in immune cell development and regulation is the germ-free animal (245). Germ-free animals are born and live in a sterile environment and are therefore free of exposure to live microbial signals (10). Additionally, animals with altered intestinal communities, primarily achieved through the administration of antibiotics (30, 50, 103, 109, 168) or selective colonization studies, have provided a complimentary approach to germ-free studies and have identified key roles for bacterial signals in the regulation of immune cell homeostasis. In this section, we review the evidence for developmental and regulatory roles for bacterial signals in the regulation of mammalian physiology as well as innate (summarized in Table 3) and adaptive (summarized in Table 4) immune cell function.
Table 3.
The role of microbial signals in the development and regulation of innate immune cell function
| Parameter | Evidence from germ-free compared with conventionally reared animals (reference) | Evidence from antibiotic-treated animals (reference) | Evidence from ex-germ-free animals (reference) |
|---|---|---|---|
| Secondary lymphoid tissue development | Lymphatic tissue and lymphatic system underdeveloped (259, 260, 382) Peyer’s patch number and cellularity reduced (261, 382) Reduced ILFs (116) mLNs smaller and less cellular, with reduced germinal centers (262) |
Subcutaneous cefmetazole decreases cellularity of Peyer’s patches (263) | Recovery of lymphatic tissue and the lymphatic system upon conventionalization of germ-free animals (259) Recovery of mLN size and cellularity upon conventionalization of germ-free animals (262) Peptidoglycan from gram-negative bacteria necessary and sufficient to induce ILF genesis via NOD1 in epithelial cells (116) |
| Macrophage development | Decreased surface expression of macrophage activation markers in the intestine (383) Intestinal macrophages present at high levels from birth (265) Reduced monocyte/macrophage numbers in the ileum and spleen (267) |
Recovery of ileum and spleen monocytes/macrophages upon monoassociation of germ-free mice with L. acidophilus and L. reuteri (267) | |
| DC development and function | Normal expression of surface markers and ability to stimulate T cell proliferation by DCs from the spleen and mLNs (266) Reduced DCs in the intestine (264, 265) |
Recruitment of DCs to the lamina propria upon monoassociation of germ-free animals with E. coli (264) Lamina propria APCs stimulated by ATP to produce IL-6, IL-23, and TGF-β, resulting in Th17 cell differentiation (138) |
|
| LTi/NK cell development | Reduced NK-22 cells (276) | LTi cells and NK-22 cells upregulate IL-22 production shortly after birth (276, 278) |
Table 4.
The role of microbial signals in the development and regulation of adaptive immune cell function
| Parameter | Evidence from germ-free compared with conventionally reared animals (reference) | Evidence from antibiotic-treated animals (reference) | Evidence from ex-germ-free animals (reference) |
|---|---|---|---|
| B cell development and function | Reduced numbers of plasma cells in small intestine (262) Decreased IgA production in small intestine (288) Reduced systemic germinal centers and plasma cells (289) Low systemic immunoglobulin levels (260, 290–293) Decreased IgM and IgG response to DNP-BSA (294) Normal IgM response to sheep red blood cells or phosphorylcholine (294, 296) Normal IgM and IgG response to DNP-lys-Ficoll (294) Delayed and reduced primary antibody titers against heat-killed E. coli (295) Increased antibody response to ferritin and DNP-Ficoll (384, 385) Increased IgE-bearing B cells in Peyer’s patches (298) |
Increased IgE responses caused by antibiotic treatment (108) | Recovery of intestinal IgA upon conventionalization of germ-free animals (288, 297) Recovery of systemic germinal centers and plasma cells upon conventionalization of germ-free animals (289) Recovery of systemic immunoglobulin levels upon conventionalization of germ-free animals (290, 293) |
| Th17/Treg cell development and function | Reduced Th17 cells in the small intestine (307) Increased Th17 cells in the large intestine (168) Reduced Foxp3 mRNA in CD4+ T cells from the mLNs (321, 322) Selective reduction in the percentage of Foxp3+CD4+CD25+ T cells in the mLN (321) CD4+ T cell proliferation not suppressed as well in vitro by Tregs from the mLN (323) Less IL-10 produced by Tregs from the mLN (322); disease not protected as well by Tregs (322) Increased frequency of CD4+Foxp3+ in the small intestine (307) Similar frequency of lamina propria CD4+Foxp3+ T cells from the colon (168) |
Fewer Th17 cells in the lamina propria of the small intestine of mice treated with vancomycin (138, 307, 313) Reduced frequency of Th17 cells in the mLN of antibiotic-treated mice (50) |
Monoassociation of germ-free mice with cytophaga-flavobacter-bacteroidetes rescues Th17 cell defect (307) |
| Th1/Th2 balance cell development and function | Decreased delayed-type hypersensitivity in response to sheep red blood cells (386) Reduced αβ TCR–bearing IELs (387) Decreased response to T cell mitogens (294, 388) Normal response to Ova but reduced oral tolerance (389–392) Reduced tolerance to Th2 antigens (393) Normal graft-versus-host reaction (394) Reduced T cell numbers in the jejunum (264) |
Long-term Th2-skewed immunological memory caused by Kanamycin treatment (330) Enhanced IgE responses caused by antibiotic treatment (108) |
Recovery of response to T cell mitogens upon conventionalization of germ-free animals (388) Recovery of αβ TCR-bearing IELs upon conventionalization of germ-free animals (387) Recovery of jujunal T cells upon mono-association with E. coli (264) Recovery of oral tolerance upon conventionalization of germ-free or antibiotic-treated animals (300, 393, 395, 396) Recovery of long-term Th2-skewed immunological memory upon Kanamycin treatment through oral inoculation with Enterococcus faecalis and Lactobacillus acidophilus (300) |
| CD8+ T cell development and function | More diverse repertoire of CD8+ IELs in germ-free rats (353), no difference in mice (397) Reduced number and cytotoxicity of CD8+ IELs (262, 350, 387, 397) Delayed development of IELs (265) |
Recovery of CD8+ IEL diversity upon conventionalization of germ-free animals (353) Recovery of number and cytotoxicity of CD8+ IELs upon conventionalization of germ-free animals (262, 350, 387, 397) Reduced numbers of naive, splenic CD8+ lymphocytes in animals colonized with nonpathogenic Clostridium sp. compared with conventionally reared controls (355) |
Intestinal Morphology and Function
Germ-free animals display morphologic defects compared with conventionally reared animals. Perhaps most striking is the dramatic enlargement of the cecum (an intestinal segment located between the distal small intestine and proximal colon) observed in germ-free animals. This enlargement is due in part to the accumulation of undegraded mucus glycoproteins (246) that are produced by the intestinal epithelium and are normally degraded by glycoside hydrolases from intestinal bacteria (namely Peptostreptococcus micros and members of the genera Ruminococcus and Bifidobacterium) (247–249). Accordingly, cecal enlargement can be rapidly reversed through selective monoassociation with Peptostreptococcus micros (247). Germ-free animals also accumulate bile acids in their cecum and large intestine that may contribute to cecal distention by causing osmotic imbalances across the epithelial wall (250).
There are also histologic alterations in the architecture of the germ-free intestine. The villi of the cecum are longer and wider in germ-free compared with conventionally reared mice (50), and morphologic studies in rats suggest that colonic crypts are shorter and contain fewer cells in germ-free compared with conventionally reared animals (251). These changes in crypt architecture could be due in part to the decreased turnover of IECs in germ-free compared with conventionally reared animals (252) or could be due to anatomical changes as a result of bacterial reduction (described above).
The intestine of conventionally reared animals undergoes waves of peristalsis that help move luminal contents. Intestinal bacteria have been shown to influence enteric nerve function, as transient manipulations of intestinal communities can lead to persistent neuromuscular dysfunction and enteritis (253). Consistent with this, germ-free animals show defects in small intestinal peristalsis characterized by slower and less frequent migrating motor complexes as a result of reduced responsiveness to enteroendocrine cell products (254). Peristaltic defects can be reversed through conventionalization of germ-free animals, suggesting that bacterial signals dynamically influence the intestinal neuromuscular function (29). Conversely, intestinal motility is important for the maintenance of normal intestinal bacterial communities as the ablation of enteric neurons specifically in the jejunum and ileum results in bacterial overgrowth and jejunoileitis (255).
Gut-Associated Lymphoid Tissues
It is estimated that the intestinal mucosa of humans contains more lymphocytes, and produces more antibodies, than any other organ in the body (256). In mice, most of these cells reside in gut-associated lymphoid tissues (GALT) (257), which include the mLN, Peyer’s patches (258), cecal patch, and isolated lymphoid follicles (ILF) that exist along the length of the gastrointestinal tract, with increasing frequency in the colon and rectum (258).
Germ-free mice display reduced intestinal lymphatic tissue and an underdeveloped lymphatic system compared with conventionally reared mice (259, 260). Specifically, Peyer’s patch numbers and cellularity are reduced (261), as are the number of ILFs (116). Defects in lymphoid tissue genesis are not limited to the immediate intestinal compartment, as mLNs are smaller, are less cellular, and have fewer germinal centers in germ-free compared with conventionally reared animals (262). These findings suggest that microbial signals are required for lymphoid tissue development and/or maintenance in the mammalian intestine.
Several studies have begun to examine how bacterial signals influence the maintenance of intestinal lymphoid tissues. Defects in intestinal lymphoid tissues observed in germ-free animals are not developmental, as selective colonization of germ-free animals with intestinal bacteria results in the recovery of intestinal lymphoid structures (259) and the recovery of mLN size and cellularity (262). Rather, bacterial signals continuously support mucosal lymphoid tissue maintenance. For example, antibiotic treatment decreases the cellularity of intestinal Peyer’s patches (263). In addition, one study showed that peptidoglycan from gram-negative bacteria is both necessary and sufficient to induce ILF formation in the mammalian intestine in a NOD1-dependent manner (116). These findings support an important role for innate recognition of bacterial-derived signals in the maintenance of adaptive lymphoid tissues.
DCs/Macrophages
Signals from intestinal bacteria have developmental and regulatory influences on intestinal APCs. Intestinal DCs are present in reduced numbers in the intestine of germ-free animals (264, 265). These defects appear to be isolated to the intestinal compartment, as DCs from the spleen and mLNs of germ-free animals had normal surface marker expression of CD86 and MHCII and induced similar levels of T cell proliferation in vitro compared with those isolated from conventionally reared animals (266). Intestinal DCs were recruited to the intestinal lamina propria upon monoassociation of germ-free animals with E. coli, further suggesting that intestinal bacteria may play an active role in the regulation of intestinal DC populations (264). Monocyte/macrophage development may also be influenced by signals from intestinal bacteria. While monocyte/macrophage numbers in the intestine were either normal (265) or reduced (267), systemic monocyte/macrophage numbers were reduced in germ-free compared with conventionally reared animals (267). Again, systemic and intestinal defects in monocyte/macrophage populations could be recovered upon monoassociation of germ-free animals with L. acidophilus and L. reuteri, implicating signals from intestinal bacteria in the regulation of these cell types (267).
As discussed previously, some intestinal APCs have a tolerogenic phenotype that promotes tolerance to oral antigens and commensal bacteria (reviewed in 97, 199, 221). However, it is important that intestinal DCs remain responsive to potential pathogens, a characteristic that may be mediated in part through differential TLR expression. For example, TLR5 recognizes bacterial flagellin, a structural protein of flagella that promotes bacterial chemotaxis, adhesion, and invasion of host tissues (268), whereas TLR4 recognizes LPS, a component of the outer membrane of most gram-negative bacteria present in the intestinal lumen. Intestinal CD11c+ lamina propria cells selectively express TLR5, but not TLR4, and produce proinflammatory cytokines in response to bacterial flagellin (216). Appropriately, these CD11c+ lamina propria cells produced proinflammatory IL-6 in response to pathogenic flagellated S. typhimurium in a TLR5-dependent manner, but produced little IL-6 in response to the nonflagellated commensal Enterobacter cloacae (216). Thus, intestinal DCs may remain unresponsive to normal intestinal bacteria while mounting proinflammatory responses to potential pathogens.
The tolerogenic nature of intestinal APCs may be imparted directly or indirectly by select intestinal bacteria. For example, Lactobacillus rhamnosus GG decreases TNF-α production in LPS-activated macrophages in a contact-independent manner (269), a phenomenon that may be important for controlling pathogenic, proinflammatory immune responses (198). The ability of intestinal bacteria to impart a tolerogenic influence may be lost in IBD, as Crohn’s disease patients have higher numbers of proinflammatory intestinal macrophages compared with healthy individuals (270). These cells express both macrophage (CD14, CD33, CD68) and DC (CD205, CD209) markers and evoke Th1 and Th17 cell differentiation (271), suggesting that intestinal APCs that lack tolerogenic properties could contribute to pathogenic states. Finally, while some intestinal DCs are thought to be specialized for induction of tolerance, evidence exists for tolerogenic DCs outside of the intestinal compartment. For example, a CD11cloCD45RBhi DC subset present in the spleen and lymph nodes of mice produces IL-10 and promotes suppressive functions of Tregs (272). However, whether or not signals from intestinal bacteria influence these systemic DCs remains to be tested directly.
Lymphoid-Tissue Inducer and Natural Killer Cells
Lymphoid-tissue inducer (LTi) cells are RORγt+ IL-7Rα+ innate leukocytes that induce lymph node development in the embryo through the production of lymphotoxin-β and TNF and the recruitment of circulating LTi cells, their precursors, and more mature lymphocytes (reviewed in 273). Clustering of LTi cells in the intestine is mediated by the chemokines CXCL13 and CCL21, and initial clustering seems to be dependent exclusively on CXCL13 expression by stromal organizer cells by retinoic acid and neuronal stimulation (274). In adult mice, clusters of LTi cells are found in the cryptopatches of the small intestine and in secondary lymphoid organs such as the spleen, where they participate in maintaining local lymphoid tissue anatomy (273). LTi cells are an innate source of IL-17 and IL-22 (an IL-10 family member that contributes to epithelial cell resistance and repair by inducing the production of antimicrobial proteins such as β-defensins, RegIIIγ, and S100 calcium-binding proteins) (275), although experimental evidence supporting a role for these cells in initiating or regulating immune responses is lacking at present.
The observation that lymphoid follicles are underdeveloped in germ-free mice has led some to speculate that LTi cells might be regulated by bacterial signals; however, one study to date indicated that LTi cell numbers and function were similar in the mucosa of germ-free compared with conventionally reared mice (276). Nevertheless, an intriguing connection exists between LTi cells and another cell population that is regulated by intestinal bacteria: IL-22-producing intestinal natural killer (NK)-like (NK-22) cells. Intestinal NK-22 cells are found in the cryptopatches and lamina propria of adult mice, where they are also an important innate source of IL-22 (276–279). Production of IL-22 by mucosal NK-22 cells may contribute to defense against the extracellular enteric pathogen Citrobacter rodentium, as the partial depletion of mucosal NK-22 cells increases the mortality of infected mice (277, 278). In addition, NK-22 cells mediate protection from experimental colitis (280), supporting the hypothesis that these cells are involved in defense against assaults to the enteric mucosa.
LTi cells differentiate into NK-like cells in vitro (281) that express both LTi and NK cell receptors (reviewed in 282). For example, human fetal LTi cells give rise to NKp44+ cells that produce IL-17 and IL-22 in vitro (283). The dependency of NK-22 cells on RORγt expression further suggests that these cells may derive from LTi cells and has led to speculation that LTi cells and NK-22 cells may be related functionally and developmentally (273). Additionally, intestinal LTi cells and NK-22 cells upregulate IL-22 production shortly after birth, suggesting that colonization of the intestine drives IL-22 production by these cell types in humans and mice (276, 278). Finally, absolute and relative numbers of NK-22 cells are reduced in germ-free compared with conventionally reared mice (276), further implicating signals from intestinal bacteria in NK-22 cell development. These findings suggest that signals from intestinal bacteria promote mucosal homeostasis by inducing IL-22-producing innate leukocytes. However, the relationship between LTi cells and NK-22 cells in vivo remains to be investigated. For example, LTi cells are required for lymphoid tissue development, whereas lymphoid tissue development and the development of IL-22-producing intestinal NK cells are both dependent on bacterial signals. However, LTi development seems to be independent of signals from intestinal bacteria (276). As such, more investigation into the relationship between LTi cells and IL-22-producing intestinal NK cells, and the role that signals from intestinal bacteria may play in the respective development of these cell populations, is needed.
B Cells
There is an intimate relationship between intestinal communities and B lymphocytes that reside in the intestine. As discussed previously, the first immunological response to bacterial signals during colonization is IgA production and secretion into the intestinal lumen (79, 284). In fact, most B cells in the intestine are IgA-producing plasma cells that produce and secrete IgA into the intestinal lumen at an estimated rate of 0.8 g per meter of intestine per day (257, 285) (Figure 4). This secretory immune response, which is characterized by class switching of B cells from IgM to IgA production, is orchestrated through an intimate functional relationship between secretory epithelia and local plasma cells and is mediated by TLRs through both T cell– dependent and –independent mechanisms (82, 158). For example, intestinal bacteria trigger T cell–independent IgA class switching in B cells through IEC secretion of cytokines such as a proliferation-inducing ligand (APRIL) (158, 159). In addition, IEC-derived TSLP and IL-10, produced in response to bacterial signals, may orchestrate local B cell responses (158, 159, 286). Secretory IgA creates a first-line defense against mucosal compromise that is lost during IBD (35, 287), implicating early recognition of bacterial signals and the subsequent modulation of B cell responses as important processes that promote normal mucosal homeostasis.
Several findings in germ-free mice support a role for bacterial signals in B cell development. There are reduced numbers of plasma cells in the germ-free small intestine (262), which correlates with reduced IgA production (288). Further, there are reduced systemic numbers of germinal centers and plasma cells in germ-free mice (289), which correlates with reduced systemic immunoglobulin levels (260, 290–293). Germ-free mice also show reduced antigen-specific immunoglobulins to some antigens (DNP-BSA, E. coli) (294, 295) but not to others (RBC, phosphorylcholine, DNP-lys-Ficoll) (294, 296). In general, these intestinal and systemic immunoglobulin defects are corrected upon conventionalization of germ-free animals, suggesting that these defects are not purely developmental (288, 290, 293, 297).
One exception to the trend of reduced immunoglobulin levels is IgE. There are increased numbers of IgE-bearing B cells in the Peyer’s patches of germ-free mice (298) and elevated serum IgE levels in germ-free and antibiotic-treated mice (108), a finding that may be linked to impaired oral tolerance to Th2 antigens in mice with reduced microbial stimulation (108, 299, 300). IgE in germ-free mice is likely composed of natural specificities and induced by a mechanism independent of MHCII cognate help (301). These findings suggest that, in general, microbial signals act as an adjuvant to immunoglobulin responses, and, with the exception of allergic IgE responses, bacterial signals may play an immunoregulatory role.
In addition, accumulating evidence suggests that B cells can play a regulatory role in many models of colitis through the production of IL-10 (reviewed in 302). For example, autoanti-bodies from B cells suppress colitis in mice deficient in TCRα chain (303) and intestinal inflammation induces a population of CD1d-expressing B cells in (GALT) that produces IL-10 and suppresses progression of colitis (304). Roles for B cell–derived IL-10 in controlling colitis in other animal models have also been reported (305). Although the influence of bacterial signals on regulatory B cell development and function remains to be examined, these findings suggest that B cells play an integral role in controlling inflammation in animal models of colitis.
Th17/Treg Cells
The differentiation of Th17 cells is characterized by RORγt expression, requires TGF-β and IL-6 or IL-21, and relies on IL-23 for Th17 cell maturation and survival (reviewed in 306). In the steady state, IL-17-producing cells are present in high numbers in the lamina propria of the small intestine (307), where the Th17-related cytokines IL-22 and IL-17 play a role in host protection against extracellular pathogens (reviewed in 308, 309).
Conventionally reared animals have TLR-independent spontaneous IL-17 production in the lamina propria of the small intestine (307). Spontaneous IL-17 production was absent in the small intestine of germ-free animals, while MyD88-deficient mice had normal numbers of Th17 cells, suggesting that intestinal bacteria signal through a TLR-independent mechanism to promote Th17 cell development (307). Specific bacteria and bacterial-derived stimuli have been identified as key regulators of Th cell responses in the mammalian intestine. For example, colonization of germ-free mice with segmented filamentous bacteria induced strong Th1, Th2, Th17, and Treg responses in the lamina propria (310, 311). Another mechanism by which intestinal bacteria may regulate Th17 cell development independent of TLR signaling is through the production of other bioactive molecules (see above). Indeed, systemic or rectal administration of ATP into germ-free mice stimulates lamina propria APCs to produce IL-6, IL-23, and TGF-β, resulting in Th17 cell differentiation (138).
Although Th17 cells are reduced in the small intestine of germ-free mice, more Th17 cells have been observed in the large intestine of germ-free compared with conventionally reared mice (168). In the large intestine, bacterial signals can regulate Th17 cell development through bacterial-dependent production of the IL-17 family cytokine IL-25 (IL-17E), which downregulates IL-17 production through the inhibition of IL-23 production by lamina propria macrophages (168). These findings suggest that regulation of Th17 cell differentiation depends on anatomical location. Indeed, the small and large intestines are micro-biologically and immunologically distinct sites. As discussed above, there are site-specific differences in intestinal bacterial communities along the length of the intestine (Figure 2). In addition, there are more IELs and LPLs per epithelial cell in the small intestine, and the lymphocyte composition and migration to the small versus large intestine is differentially regulated (307, 312). Other environmental factors, such as diet and non-live microbial signals, could also influence Th17 populations. Although more studies are required, these fundamental differences may underlie differential regulation of Th17 cells in distinct anatomical locations of the intestine.
Studies from antibiotic-treated mice mirror some of the observations made in germ-free mice and support a role for bacterial signals in influencing homeostasis of Th17 cells. For example, mice treated with vancomycin have fewer Th17 CD4+ T cells in the lamina propria of the small intestine (138, 307, 313), whereas mice treated with a complex antibiotic mixture displayed reduced Th17 CD4+ T cell frequencies in the mLNs (50). Additionally, Th17 cell differentiation in the lamina propria of the small intestine requires specific intestinal bacteria: cytophaga-flavobacter-bacteroidetes bacteria (307). This induction of Th17 cells is independent of TLR signaling, IL-21, or IL-23, but requires TGF-β activation, suggesting that specific intestinal bacteria may regulate the Th17/Treg balance in the mammalian intestine.
An intimate relationship exists in the intestinal mucosa between proinflammatory Th17 cells and CD4+ Tregs, which play an important role in controlling Th17 cell responses (reviewed in 314, 315). Tregs are characterized as CD4+ CD25+ cells that express Foxp3+ and suppress the proliferation of effector T cells in vitro and protect against autoimmune and other inflammatory diseases in several animal models (reviewed in 316). For example, mice carrying a loss-of-function mutation of Foxp3 completely lack Tregs and develop lethal autoimmune disease (317). Additionally, mice engineered to lack the expression of specific regulatory cytokines in T cells, including IL-10 or TGF-β, develop colitis when pathogenic bacteria are present in the intestine (318–320). These findings identify Tregs as an important regulatory cell type that contributes to intestinal and systemic immune homeostasis.
There are conflicting reports regarding the influence of bacterial signals on Treg development and function. Consistent with studies that showed reduced Foxp3 mRNA in CD4+ T cells from mLNs of germ-free mice (321, 322), early studies in germ-free animals showed a selective reduction in the percentage of Foxp3+CD4+CD25+ T cells in the mLN of germ-free mice (321). In addition to reduced frequencies, Tregs from the mLN do not suppress CD4+ T cell proliferation in vitro in either germ-free animals or in conventionally reared animals (323). Furthermore, Tregs from the mLN of germ-free animals produce less IL-10 and do not protect as well against disease in a transfer model of experimental colitis, compared with Tregs from conventionally reared animals (322). Consistantly, some intestinal bacteria may help promote Treg development; for example, Lactobacillus and Bifidobacterium strains caused expansion of Tregs in the intestinal intraepithelial compartment, which correlated with protection against experimental colitis (324). These results suggest that signals from intestinal bacteria are important for normal development of Treg numbers and function in the mLNs.
However, bacterial signals seem to have different effects on other Treg populations. One study reported no change in the frequency of lamina propria CD4+Foxp3+ T cells from the colon of germ-free animals (168), whereas another reported increased percentages of CD4+Foxp3+ in the germ-free small intestine (307). These differing results could be due to sampling Treg cell subsets from different anatomical locations (mLN versus intestine). Additionally, differences in experimental methods, animal housing methods, diet, nonbacterial microbial stimulation, employed assays, or Treg identification methods could explain these conflicting results.
Th1/Th2 Cells
In humans, as in mice, several distinct patterns of cytokine secretion have been defined among CD4+ helper T cell clones. Th1 cells produce IL-2, IFN-γ, and TNF-β, whereas Th2 cells produce IL-4, IL-5, IL-9, and IL-13 (reviewed in 325). These distinct immune responses are important for fighting distinct types of infection; Th1 cell responses are protective against bacterial, viral, and protozoan infections, whereas Th2 cell responses are important in mediating immunity to helminths and ectoparasites.
Inappropriate Th1 and Th2 cytokine responses result in distinct forms of human disease. For example, Crohn’s disease is characterized by exaggerated IFN-γ responses, as well as IL-23 and IL-17 responses (reviewed in 231), whereas ulcerative colitis and atopic diseases are primarily associated with elevated Th2 cytokine responses (16). As discussed above, IBD patients display altered bacterial communities in their intestine (326), and tolerance to intestinal bacteria is broken in these diseases (32, 33, 35), suggesting that a dysregulated mucosal immune response to intestinal bacteria could be linked to pathogenesis (16). Consistently, animal models of IBD exhibit CD4+ T cells specific for bacterial antigens (327) that induce colitis when adoptively transferred into naive SCID mice (35). The loss of regulatory mechanisms, such as mucosal T cells with regulatory properties that suppress inappropriate Th1 responses (328), may contribute to disease pathogenesis in these models (329).
The influence of intestinal bacteria on Th1 and Th2 cell development and regulation is not limited to the intestinal mucosa. For example, recent epidemiological studies indicate that antibiotic use in infancy may be associated with an increased risk of developing atopy (48). As discussed above, patients with atopic diseases such as asthma have altered intestinal bacterial communities (15), suggesting that bacterial signals influence systemic type 2 inflammatory responses. Consistent with this notion, treating animals with antibiotics during infancy promoted a shift in the Th1/Th2 balance toward a Th2-dominant immunity (330) that could be corrected by oral inoculation with Enterococcus faecalis or Lactobacillus acidophilus (300).
The balance of proinflammatory or regulatory immune responses to intestinal bacteria may be regulated by mucosal DC populations. For example, subsets of mucosal DCs have tolerogenic capacities that contribute to the induction of tolerance to intestinal bacteria and food antigens (97, 221). However, other nonhematopoietic cells may influence the balance of inflammatory or tolerogenic responses; IECs may deliver inhibitory or tolerogenic signals indirectly or directly to T cells (discussed above). In addition, T cells express pattern-recognition receptors under certain conditions and could be directly responsive to bacterial-derived signals (331), although this possibility has not been examined in detail.
Finally, granulocytes such as mast cells, basophils, and eosinophils are important effectors of protective and pathogenic type 2 inflammatory responses (332). In addition, granulocyte populations are important sources of type 2 cytokines (333), and basophils have recently be identified as an important cellular source of IL-4 that express surface MHCII and can prime antigen-specific Th2 cell differentiation (334– 336), suggesting that basophils may also play a role in the initiation of type 2 responses. Several lines of evidence suggest that granulocytes are dysregulated in human inflammatory disease. For example, tissue from patients with ulcerative colitis show mast cell aggregation along the line of demarcation, dividing inflamed from healthy tissue (337), and the number of NOD2+ intestinal mast cells are increased in Crohn’s disease patients compared with healthy controls (338), identifying a possible role for these cells in responding to innate, bacterial-derived signals. Mast cells are of particular interest in IBD because they are thought to modify disease progress through release of histamine (339), TNF-α (340), and the T cell chemoattractants XCL-1 (341) and IL-16 (342). Indeed, in a model of experimental allergen-induced asthma, TLR4-defective mice subjected to sensitization and pulmonary challenge with a protein allergen had reductions in airway inflammation, allergen-specific IgE levels, and Th2 cytokine production (106), although rigorous investigation of potential influences that intestinal bacteria have on gran-ulocyte development or function is lacking at present.
CD8+ T Cells
The first observation that microbial signals could influence tumor immunity came in the eighteenth century when Deidier reported a correlation between patient infection and the remission of malignant disease, a finding that eventually led to the use of LPS to treat primarily inoperable sarcoma, with a cure rate of better than 10% (343). These early findings indicated that microbial signaling through TLRs is important for initiating or sustaining antitumor immune responses (reviewed in 344). This hypothesis has now been supported by several studies in animal models (345–347) and patients (102), leading to a well-accepted role for bacterial products as adjuvants that promote the recruitment and/or stimulation of CD8+ cytotoxic T lymphocytes (348).
The intestinal mucosa of conventionally reared animals normally contains CD8+ cytotoxic T lymphocytes. In the conventionally reared mouse intestine, CD4+ T cells are found primarily in the lamina propria, whereas CD8+ T cells dominate in the intraepithelial compartment. The maintenance of CD8+ IELs depends on bacterial signals, as MyD88-deficient (349) and germ-free mice (350, 351) display reduced CD8+ T cell numbers in the IEL compartment of the small intestine. The systemic distribution of CD8+ T cells also depends on bacterial signals. The liver is a site of activated CD8+ T cell sequestering and subsequent apoptosis during systemic viral immune responses, a phenomenon that is dependent on TLR4 ligands such as endotoxin from intestinal bacteria (352).
Cytotoxic activity of IELs is impaired in both germ-free (351) and antibiotic-treated mice, a phenotype that can be reversed through the administration of the TLR4 ligand LPS (345). In germ-free mice, this cytotoxic defect was isolated to αβ TCR-bearing IELs, whereas the number and cytotoxicity of γδ TCR-bearing IELs was comparable between germ-free and conventionally reared mice (351). These defects may be in part due to impaired clonal expansions of CD8αβ+ and CD8αα+ IELs in germ-free mice (353). This functional defect is not limited to intestinal compartments, as conventionally reared mice have a selective reduction in systemic plasmacytoid DCs as a result of enhanced cytotoxic T lymphocyte activity that is not observed in germ-free mice (354). Finally, animals colonized with nonpathogenic Clostridium sp. have reduced numbers of naive, splenic CD8+ lymphocytes compared with conventionally reared controls (355), further suggesting that signals from intestinal bacteria play an active role in CD8+ lymphocyte regulation.
Although the adjuvant effect of microbial signals on cytotoxic cells is well established, microbial stimulation can also result in tumorigenesis under some conditions. For example, MyD88-deficient mice are protected against the development of spontaneous intestinal tumors in a model of familial-associated polyposis (356), and germ-free animals have reduced intestinal tumorigenesis compared with conventionally reared animals following the same protocol of colorectal cancer induction (357). Similarly, hepatocellular carcinoma, the most common liver cancer, occurs mainly in males. This gender disparity is also seen in mice given the chemical carcinogen diethylnitrosamine and is dependent on MyD88-mediated increases in serum IL-6 (358). In humans, associations between intestinal bacteria and cancer risk have been explored (reviewed in 359), and studies were performed to identify fecal bacterial communities associated with high colorectal cancer risk (13, 66, 360), although they failed to provide conclusive results. In summary, clarifying the influence of bacterial signals in establishing normal cytotoxic immune capacities has led to exciting therapeutic possibilities, but more research is needed to fully understand the role that signals from intestinal bacteria play in tumorigenesis and control.
MANIPULATION OF INTESTINAL BACTERIAL COMMUNITIES: PROSPECTS FOR PREVENTION AND TREATMENT OF INFLAMMATORY DISEASES
The identification of the important roles that intestinal bacteria play in normal development and regulation of the mammalian immune system provides a rationale for using therapeutic agents based on bacterial-derived signals in preventative or therapeutic endeavors. Live bio-logics given as supplements to confer some benefit to the host are referred to as probiotics. Several studies in animal models have investigated the beneficial effects of probiotic bacteria, including Lactobacillus ssp. and others. In one study, administration of Lactobacillus ssp. protected IL-10-deficient mice from developing spontaneous colitis (361). In another, probiotic mixtures including Lactobacillus ssp. protected mice against chemically induced colitis (324). Probiotics are thought to mediate their beneficial effects in part through modulation of immune cell function. For example, probiotic bacteria including lactobacilli induce IL-10 production by DCs and inhibit subsequent generation of Th1 cells in vitro (148, 362), and Lactobacillus and Bifidobacterium strains prevent experimental colitis while expanding IEL γδ T cells and Tregs (324).
These and other studies have led to clinical trials in human patients examining the potential role for probiotic bacteria in preventing and treating human disease. These treatments have been associated with modulation in DC function (362, 363), as well as the function of other immune cells (364), but have reported mixed successes in the treatment of intestinal inflammatory and atopic diseases (62, 365, 366). One positive study showed that E. coli provided an effective probiotic therapy to ulcerative colitis patients (367). In addition, delivery of probiotics seems to be effective for the prevention and treatment of pouchitis, a complication following surgical treatment of ulcerative colitis (368–371). Despite these encouraging results, how probiotics modulate intestinal communities to modify subsequent immunoregulatory signals is unknown. A better understanding of these mechanisms is necessary to realize the full potential of probiotic therapies.
In addition to interventions with unmodified bacteria, more recent work using genetically modified bacteria that act as platforms for the delivery of drugs, antimicrobial agents, vaccines, and other biologically active molecules to physiologically relevant sites has expanded the definition of probiotic therapy. For example, Lactobacillus strains have been engineered to produce IL-10 as a therapeutic intervention in animal models of IBD (372), while Streptococcus gordonii, engineered to express an antimicrobial antibody fragment, confers a therapeutic benefit to rats with vaginal Candida albicans infections (373). In addition, genetically modified lactobacilli that express the tetanus toxin fragment C efficiently induce local and systemic antigen protection when given orally to mice (374), while those that express CD4 molecules and secrete HIV-1 fusion inhibitors have been developed as a step toward the use of topical, genetically engineered bacteria to prevent HIV transmission (375, 376).
In the development of new therapeutics, the use of specific bacterial products with immunoregulatory characteristics, as well as their molecular derivatives, could offer new therapeutic modalities in the prevention and treatment of human disease. For example, identifying specific bacterial compounds that could be administered early in life to promote tolerance could play a role in preventing the development of atopy later in life. Similarly, bacterial products that bolster mucosal barrier function or that regulate inflammatory immune responses in the intestinal mucosa may be important in the treatment of human IBD. Finally, bacterial-derived proinflammatory molecules that modulate immune responses could play a role in vaccine development, cancer treatment, or the treatment of infection. To move such therapies into the clinics, the specific microbial products and their immunoregulatory properties must be precisely defined.
CONCLUSION
Technological and conceptual advances have ushered in an exciting time in mucosal immunology and in our understanding of how bacterial-derived signals influence the immune system. New sequencing techniques are allowing previously unattainable insights into the complex bacterial communities integral to mammalian health. Additionally, established tools such as germ-free animals are being complemented with antibiotic treatment, selective colonization, and treatment with specific microbial products to probe the molecular mechanisms that facilitate cross-talk between intestinal bacteria and their mammalian hosts. It is hoped that these insights will offer new therapeutic strategies in the prevention and treatment of human diseases.
COMMON TERMS DESCRIBING INTESTINAL COMMUNITIES.
Symbiosis: close and often long-term relationship between different biological species. Symbiotic relationships can be described as
-
■
Mutualistic: both species derive a benefit from the interaction,
-
■
Parasitic: one member benefits and the other is harmed, or
-
■
Commensal: one member benefits and the other is unaffected.
Dysbiosis: the condition of having microbial imbalances on or within the body.
Probiotic: an organism given as a live supplement to confer a biological benefit to the host.
Acknowledgments
We thank Rick Bushman and members of the Artis laboratory for helpful discussions. Research in the Artis laboratory is supported by the NIH (AI61570 and AI74878, F32-AI72943, F31-GM082187, T32-AI060516 (DAH), T32-AI007532-08, T32-CA09140-30, T32-AI055438-06, T32-AI05528, and S10RR024525), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award), the Crohn’s and Colitis Foundation of America, and pilot grants from the University of Pennsylvania (CID, PGFI, and URI).
Glossary
- IBD
inflammatory bowel disease
- LPS
lipopolysaccharide
- TLR
Toll-like receptor
- DC
dendritic cell
- IEC
intestinal epithelial cell
- NLR
Nod-like receptor
- APC
antigen-presenting cell
- LPL
lamina propria lymphocyte
- IEL
intraepithelial lymphocyte
- NK cell
natural killer cell
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Paszkowski U. Mutualism and parasitism: the yin and yang of plant symbioses. Curr Opin Plant Biol. 2006;9:364–70. doi: 10.1016/j.pbi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Muyskens JB, Guillemin K. Bugs inside bugs: What the fruit fly can teach us about immune and microbial balance in the gut. Cell Host Microbe. 2008;3:117–18. doi: 10.1016/j.chom.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, et al. Isolation of naturally associated bacteria of necromenic pristionchus nematodes and fitness consequences. J Exp Biol. 2008;211:1927–36. doi: 10.1242/jeb.014944. [DOI] [PubMed] [Google Scholar]
- 4.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–82. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. 2008;9:101–10. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 6.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:S1046–51. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 7.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–83. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 9.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–18. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 10.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 11.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–75. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202–7. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollyky PL, Bice JB, Sweet IR, Falk BA, Gebe JA, et al. The Toll-like receptor signaling molecule Myd88 contributes to pancreatic beta-cell homeostasis in response to injury. PLoS ONE. 2009;4:e5063. doi: 10.1371/journal.pone.0005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penders J, Thijs C, Van Den Brandt PA, Kummeling I, Snijders B, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 2007;56:661–67. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 17.Penders J, Stobberingh EE, Van Den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 18.Eckburg PB, Lepp PW, Relman DA. Archaea and their potential role in human disease. Infect Immun. 2003;71:591–96. doi: 10.1128/IAI.71.2.591-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubos R, Schaedler RW, Costello R, Hoet P. Indigenous, normal, and autochthonous flora of the gastrointestinal tract. J Exp Med. 1965;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–38. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002;46:535–48. doi: 10.1111/j.1348-0421.2002.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 22.Hold GL, Pryde SE, Russell VJ, Furrie E, Flint HJ. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol Ecol. 2002;39:33–39. doi: 10.1111/j.1574-6941.2002.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Heazlewood SP, Krause DO, Florin TH. Molecular characterization of the microbial species that colonize human ileal and colonic mucosa by using 16S rDNA sequence analysis. J Appl Microbiol. 2003;95:508–20. doi: 10.1046/j.1365-2672.2003.02005.x. [DOI] [PubMed] [Google Scholar]
- 24.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 25.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 26.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 27.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–55. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husebye E, Hellström PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci. 1994;39:946–56. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- 30.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76:4726–36. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 32.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams RJ, Heazlewood SP, Gilshenan KS, O’Brien M, McGuckin MA, Florin TH. IgG antibodies against common gut bacteria are more diagnostic for Crohn’s disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–96. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- 35.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Investig. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 37.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 38.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 39.Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, et al. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1461–72. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 40.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-αβ-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 42.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–44. [PubMed] [Google Scholar]
- 43.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci. 2005;118:1971–80. doi: 10.1242/jcs.02316. [DOI] [PubMed] [Google Scholar]
- 44.Madsen KL, Doyle JS, Tavernini MM, Jewell LD, Rennie RP, Fedorak RN. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology. 2000;118:1094–105. doi: 10.1016/s0016-5085(00)70362-3. [DOI] [PubMed] [Google Scholar]
- 45.Hoentjen F, Harmsen HJ, Braat H, Torrice CD, Mann BA, et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–27. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–98. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 48.Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003–10. doi: 10.1542/peds.2008-1146. [DOI] [PubMed] [Google Scholar]
- 49.Vael C, Nelen V, Verhulst SL, Goossens H, Desager KN. Early intestinal Bacteroides fragilis colonization and development of asthma. BMC Pulm Med. 2008;8:19. doi: 10.1186/1471-2466-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.132. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gore C, Munro K, Lay C, Bibiloni R, Morris J, et al. Bifidobacterium pseudocatenulatum is associated with atopic eczema: a nested case-control study investigating the fecal microbiota of infants. J Allergy Clin Immunol. 2008;121:135–40. doi: 10.1016/j.jaci.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 52.Penders J, Stobberingh EE, van den Brandt PA, van Ree R, Thijs C. Toxigenic and nontoxigenic clostridium difficile: determinants of intestinal colonization and role in childhood atopic manifestations. Gut. 2008;57:1025–26. doi: 10.1136/gut.2007.143214. [DOI] [PubMed] [Google Scholar]
- 53.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243:141–47. doi: 10.1016/j.femsle.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 54.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA. 2008;105:2193–97. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15:189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- 57.Adlerberth I, Wold AE. Establishment of the gut microbiota in western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 58.Finegold SM, Sutter VL, Mathisen GE. Normal indigenous intestinal flora. In: Hentges DJ, editor. Human Intestinal Microflora in Health and Disease. London: Academic; 1983. pp. 3–31. [Google Scholar]
- 59.Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenna P, Hoffmann C, Minkah N, Aye PP, Lackner A, et al. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 2009;123:335–41. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Diaz RL, Hoang L, Wang J, Vela JL, Jenkins S, et al. Maternal adaptive immunity influences the intestinal microflora of suckling mice. J Nutr. 2004;134:2359–64. doi: 10.1093/jn/134.9.2359. [DOI] [PubMed] [Google Scholar]
- 64.Martin R, Heilig GH, Zoetendal EG, Smidt H, Rodriguez JM. Diversity of the lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol. 2007;103:2638–44. doi: 10.1111/j.1365-2672.2007.03497.x. [DOI] [PubMed] [Google Scholar]
- 65.Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benno Y, Suzuki K, Suzuki K, Narisawa K, Bruce WR, Mitsuoka T. Comparison of the fecal microflora in rural Japanese and urban Canadians. Microbiol Immunol. 1986;30:521–32. doi: 10.1111/j.1348-0421.1986.tb02978.x. [DOI] [PubMed] [Google Scholar]
- 67.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 68.Stark PL, Lee A. The bacterial colonization of the large bowel of preterm low birth weight neonates. J Hyg. 1982;89:59–67. doi: 10.1017/s0022172400070546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blakey JL, Lubitz L, Barnes GL, Bishop RF, Campbell NT, Gillam GL. Development of gut colonization in preterm neonates. J Med Microbiol. 1982;15:519–29. doi: 10.1099/00222615-15-4-519. [DOI] [PubMed] [Google Scholar]
- 70.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi H, Sakamoto M, Benno Y. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol Immunol. 2002;46:819–31. doi: 10.1111/j.1348-0421.2002.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 72.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 73.Lundequist B, Nord CE, Winberg J. The composition of the faecal microflora in breastfed and bottle fed infants from birth to eight weeks. Acta Paediatr Scand. 1985;74:45–51. doi: 10.1111/j.1651-2227.1985.tb10919.x. [DOI] [PubMed] [Google Scholar]
- 74.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–21. [PubMed] [Google Scholar]
- 75.Bullen CL, Tearle PV, Willis AT. Bifidobacteria in the intestinal tract of infants: an in-vivo study. J Med Microbiol. 1976;9:325–33. doi: 10.1099/00222615-9-3-325. [DOI] [PubMed] [Google Scholar]
- 76.Renz-Polster H, David MR, Buist AS, Vollmer WM, O’Connor EA, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35:1466–72. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 77.Salam MT, Margolis HG, McConnell R, McGregor JA, Avol EL, Gilliland FD. Mode of delivery is associated with asthma and allergy occurrences in children. Ann Epidemiol. 2006;16:341–46. doi: 10.1016/j.annepidem.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 78.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–13. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macpherson AJ. IgA adaptation to the presence of commensal bacteria in the intestine. Curr Top Microbiol Immunol. 2006;308:117–36. doi: 10.1007/3-540-30657-9_5. [DOI] [PubMed] [Google Scholar]
- 80.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–65. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 81.MacPherson G, Milling S, Yrlid U, Cousins L, Turnbull E, Huang FP. Uptake of antigens from the intestine by dendritic cells. Ann NY Acad Sci. 2004;1029:75–82. doi: 10.1196/annals.1309.010. [DOI] [PubMed] [Google Scholar]
- 82.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–26. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 83.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006;177:7772–83. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 84.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann NY Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 86.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–59. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 87.Macpherson AJ, Hunziker L, McCoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and nonpathogenic microorganisms. Microbes Infect. 2001;3:1021–35. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- 88.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23:115–20. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 89.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang ML, Shin ME, Knight PA, Artis D, Silberg DG, et al. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1074–83. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- 91.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berg R. Host immune response to antigens of the indigenous intestinal flora. In: Hentges D, editor. Human Intestinal Microflora in Health and Disease. New York: Academic; 1983. pp. 101–26. [Google Scholar]
- 93.Hill DA, Artis D. Maintaining diplomatic relations between mammals and beneficial microbial communities. Sci Signal. 2009;2(98):pe77. doi: 10.1126/scisignal.298pe77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–20. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–97. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 96.West AP, Koblansky AA, Ghosh S. Recognition and signaling by Toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–37. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 97.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 98.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008;180:8280–85. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 99.Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–63. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 100.Candia L, Marquez J, Hernandez C, Zea AH, Espinoza LR. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? J Rheumatol. 2007;34:374–79. [PubMed] [Google Scholar]
- 101.Racila DM, Kline JN. Perspectives in asthma: molecular use of microbial products in asthma prevention and treatment. J Allergy Clin Immunol. 2005;116:1202–5. doi: 10.1016/j.jaci.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 102.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Investig. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccha-ride activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–72. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 105.Turnbull EL, Yrlid U, Jenkins CD, MacPherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–84. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- 106.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168:4524–30. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 107.Cerovic V, Jenkins CD, Barnes AG, Milling SW, MacPherson GG, Klavinskis LS. Hyporesponsiveness of intestinal dendritic cells to TLR stimulation is limited to TLR4. J Immunol. 2009;182:2405–15. doi: 10.4049/jimmunol.0802318. [DOI] [PubMed] [Google Scholar]
- 108.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 109.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–63. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tzianabos AO, Russell PR, Onderdonk AB, Gibson FC, 3rd, Cywes C, et al. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J Immunol. 1999;163:893–97. [PubMed] [Google Scholar]
- 112.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–25. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 113.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–57. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 115.Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH. Nod-like proteins in inflammation and disease. J Pathol. 2008;214:136–48. doi: 10.1002/path.2271. [DOI] [PubMed] [Google Scholar]
- 116.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 117.Magalhaes JG, Fritz JH, Le Bourhis L, Sellge G, Travassos LH, et al. Nod2-dependent Th2 polarization of antigen-specific immunity. J Immunol. 2008;181:7925–35. doi: 10.4049/jimmunol.181.11.7925. [DOI] [PubMed] [Google Scholar]
- 118.Bonen DK, Ogura Y, Nicolae DL, Inohara N, Saab L, et al. Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124(1):140–46. doi: 10.1053/gast.2003.50019. [DOI] [PubMed] [Google Scholar]
- 119.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124(4):993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 120.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102(50):18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–34. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 122.Pauleau AL, Murray PJ. Role of Nod2 in the response of macrophages to Toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–39. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, et al. Nod2 mutation in Crohn’s disease potentiates NF-κB activity and IL-1β processing. Science. 2005;307:734–38. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 124.Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–23. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 126.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–85. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Investig. 2008;118:545–59. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Z, Fuss IJ, Watanabe T, Asano N, Davey MP, et al. NOD2 transgenic mice exhibit enhanced MDP-mediated down-regulation of TLR2 responses and resistance to colitis induction. Gastroenterology. 2007;133:1510–21. doi: 10.1053/j.gastro.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeuthen LH, Fink LN, Frokiaer H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology. 2008;124:489–502. doi: 10.1111/j.1365-2567.2007.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci USA. 2007;104:19440–45. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor ag-onists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7(1):53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 132.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with Toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73:7967–76. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moreira LO, El Kasmi KC, Smith AM, Finkelstein D, Fillon S, et al. The TLR2-MyD88-NOD2-RIPK2 signalling axis regulates a balanced pro-inflammatory and IL-10-mediated anti-inflammatory cytokine response to Gram-positive cell walls. Cell Microbiol. 2008;10:2067–77. doi: 10.1111/j.1462-5822.2008.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10(5):471–79. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selmeczy Z, Csóka B, Pacher P, Vizi ES, Hasko G. The adenosine A2A receptor agonist CGS 21680 fails to ameliorate the course of dextran sulphate-induced colitis in mice. Inflamm Res. 2007;56:204–9. doi: 10.1007/s00011-006-6150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–64. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 139.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–39. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 140.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res. 2000;12:83–95. doi: 10.3727/096504001108747558. [DOI] [PubMed] [Google Scholar]
- 141.Hague A, Elder DJ, Hicks DJ, Paraskeva C. Apoptosis in colorectal tumor cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer. 1995;60:400–6. doi: 10.1002/ijc.2910600322. [DOI] [PubMed] [Google Scholar]
- 142.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–32. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–46. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luhrs H, Gerke T, Muller JG, Melcher R, Schauber J, et al. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458–66. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 145.Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, et al. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–86. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–69. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 148.Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–79. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–65. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 150.Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 151.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–82. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 152.Neutra MR, Pringault E, Kraehenbuhl JP. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 153.Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–59. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 155.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–17. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–85. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 157.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schuller S, et al. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol. 2007;9:2404–16. doi: 10.1111/j.1462-5822.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 158.He B, Xu W, Santini PA, Polydorides AD, Chiu A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–26. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 159.Xu W, He B, Chiu A, Chadburn A, Shan M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 160.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004;41:1099–108. doi: 10.1016/j.molimm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 164.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce antimicrobial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–11. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 165.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–16. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 166.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 167.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–74. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 168.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–98. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Investig. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105:1323–32. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 171.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–26. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 172.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 173.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 178.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–56. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 179.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–89. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 180.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–50. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 181.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–58. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, et al. Cutting edge: proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–77. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 183.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–87. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11, 736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc Natl Acad Sci USA. 2007;104:914–19. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 187.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–49. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 191.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 192.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA. 2009;106:13968–73. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells— polarity and complexity. Immunol Today. 2000;21:123–28. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 194.Buning J, Hundorfean G, Schmitz M, Zimmer KP, Strobel S, et al. Antigen targeting to MHC class II-enriched late endosomes in colonic epithelial cells: trafficking of luminal antigens studied in vivo in Crohn’s colitis patients. FASEB J. 2006;20:359–61. doi: 10.1096/fj.05-4807fje. [DOI] [PubMed] [Google Scholar]
- 195.Telega GW, Baumgart DC, Carding SR. Uptake and presentation of antigen to T cells by primary colonic epithelial cells in normal and diseased states. Gastroenterology. 2000;119:1548–59. doi: 10.1053/gast.2000.20168. [DOI] [PubMed] [Google Scholar]
- 196.Sanderson IR, Ouellette AJ, Carter EA, Walker WA, Harmatz PR. Differential regulation of B7 mRNA in enterocytes and lymphoid cells. Immunology. 1993;79:434–38. [PMC free article] [PubMed] [Google Scholar]
- 197.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 198.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 199.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006;74:625–31. doi: 10.1128/IAI.74.1.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Milling SW, Cousins L, MacPherson GG. How do DCs interact with intestinal antigens? Trends Immunol. 2005;26:349–52. doi: 10.1016/j.it.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 202.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–32. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 207.George A, Cebra JJ. Responses of single germinal-center B cells in T-cell-dependent microculture. Proc Natl Acad Sci USA. 1991;88:11–15. doi: 10.1073/pnas.88.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Spalding DM, Williamson SI, Koopman WJ, McGhee JR. Preferential induction of polyclonal IgA secretion by murine Peyer’s patch dendritic cell-T cell mixtures. J Exp Med. 1984;160:941–46. doi: 10.1084/jem.160.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Powrie F. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann NY Acad Sci. 2004;1029:132–41. doi: 10.1196/annals.1309.030. [DOI] [PubMed] [Google Scholar]
- 210.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–67. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 212.Niess JH, Brand S, Gu X, Landsman L, Jung S, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–58. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 213.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yrlid U, Cerovic V, Milling S, Jenkins CD, Klavinskis LS, MacPherson GG. A distinct subset of intestinal dendritic cells responds selectively to oral TLR7/8 stimulation. Eur J Immunol. 2006;36:2639–48. doi: 10.1002/eji.200636426. [DOI] [PubMed] [Google Scholar]
- 215.Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–97. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 216.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 217.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 218.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 219.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–47. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Takenaka S, Safroneeva E, Xing Z, Gauldie J. Dendritic cells derived from murine colonic mucosa have unique functional and phenotypic characteristics. J Immunol. 2007;178:7984–93. doi: 10.4049/jimmunol.178.12.7984. [DOI] [PubMed] [Google Scholar]
- 222.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 223.Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–49. [PubMed] [Google Scholar]
- 224.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 225.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Simpson SJ, Shah S, Comiskey M, de Jong YP, Wang B, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon γ expression by T cells. J Exp Med. 1998;187:1225–34. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 228.Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–64. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 229.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–31. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Investig. 2006;116:1310–16. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–59. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 232.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–57. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–70. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, et al. IL-23 plays a key role in helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 237.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J Immunol. 2003;170:5438–44. doi: 10.4049/jimmunol.170.11.5438. [DOI] [PubMed] [Google Scholar]
- 239.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–63. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–10. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 241.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 243.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur J Immunol. 2002;32:1445–54. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 244.Svensson M, Johansson-Lindbom B, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut-tropic T cells in gut-associated lymphoid tissues: requirement for GALT dendritic cells and adjuvant. Ann NY Acad Sci. 2004;1029:405–7. doi: 10.1196/annals.1309.025. [DOI] [PubMed] [Google Scholar]
- 245.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 246.Gustafsson BE, Midtvedt T, Strandberg K. Effects of microbial contamination on the cecum enlargement of germfree rats. Scand J Gastroenterol. 1970;5:309–14. [PubMed] [Google Scholar]
- 247.Carlstedt-Duke B, Midtvedt T, Nord CE, Gustafsson BE. Isolation and characterization of a mucin-degrading strain of peptostreptococcus from rat intestinal tract. Acta Pathol Microbiol Immunol Scand B. 1986;94:293–300. doi: 10.1111/j.1699-0463.1986.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 248.Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Investig. 1985;75:944–53. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lindstedt G, Lindstedt S, Gustafsson BE. Mucus in intestinal contents of germfree rats. J Exp Med. 1965;121:201–13. doi: 10.1084/jem.121.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Eyssen HJ, Parmentier GG, Mertens JA. Sulfate bile acids in germ-free and conventional mice. Eur J Biochem. 1976;66:507–14. doi: 10.1111/j.1432-1033.1976.tb10576.x. [DOI] [PubMed] [Google Scholar]
- 251.Alam M, Midtvedt T, Uribe A. Differential cell kinetics in the ileum and colon of germfree rats. Scand J Gastroenterol. 1994;29:445–51. doi: 10.3109/00365529409096836. [DOI] [PubMed] [Google Scholar]
- 252.Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Investig. 1963;12:355–64. [PubMed] [Google Scholar]
- 253.Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224–32. doi: 10.1053/gast.1997.v113.pm9322517. [DOI] [PubMed] [Google Scholar]
- 254.Strandberg K, Sedvall G, Midtvedt T, Gustafsson B. Effect of some biologically active amines on the cecum wall of germfree rats. Proc Soc Exp Biol Med. 1966;121:699–702. doi: 10.3181/00379727-121-30864. [DOI] [PubMed] [Google Scholar]
- 255.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 256.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 257.Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, et al. Immunobiology and immunopathol-ogy of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562–84. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- 258.Langman JM, Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986;149:189–94. [PMC free article] [PubMed] [Google Scholar]
- 259.Hudson JA, Luckey TD. Bacteria induced morphologic changes. Proc Soc Exp Biol Med. 1964;116:628–31. doi: 10.3181/00379727-116-29324. [DOI] [PubMed] [Google Scholar]
- 260.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–30. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 261.Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Glaister JR. Factors affecting the lymphoid cells in the small intestinal epithelium of the mouse. Int Arch Allergy Appl Immunol. 1973;45:719–30. doi: 10.1159/000231071. [DOI] [PubMed] [Google Scholar]
- 263.Yaguchi Y, Fukatsu K, Moriya T, Maeshima Y, Ikezawa F, et al. Influences of long-term antibiotic administration on Peyer’s patch lymphocytes and mucosal immunoglobulin A levels in a mouse model. JPEN J Parenter Enter Nutr. 2006;30:395–99. doi: 10.1177/0148607106030005395. [DOI] [PubMed] [Google Scholar]
- 264.Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M. Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol. 2007;119:243–53. doi: 10.1016/j.vetimm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 265.Williams AM, Probert CS, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland PW. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119:470–78. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Walton KL, He J, Kelsall BL, Sartor RB, Fisher NC. Dendritic cells in germ-free and specific pathogen-free mice have similar phenotypes and in vitro antigen presenting function. Immunol Lett. 2006;102:16–24. doi: 10.1016/j.imlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 267.Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008;121:222–31. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Macnab RM. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–58. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 269.Pena JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol. 2003;5:277–85. doi: 10.1046/j.1462-5822.2003.t01-1-00275.x. [DOI] [PubMed] [Google Scholar]
- 270.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J Clin Investig. 2008;118:2269–80. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–31. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 272.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18(5):605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 273.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 274.van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10(11):1193–99. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2008;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2008;457:722–25. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 279.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 280.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–57. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 282.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 283.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 284.Klaasen HL, Van der Heijden PJ, Stok W, Poelma FG, Koopman JP, et al. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect Immun. 1993;61:303–6. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Goldblum RM, Hannson LA, Brandtzaeg P. The mucosal defense system. In: Stiehm ER, editor. Immunologic Disorders in Infants and Children. Philadelphia, PA: Saunders; 1996. pp. 159–99. [Google Scholar]
- 286.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. 2007;8:522–31. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 287.Brandtzaeg P, Carlsen HS, Halstensen TS. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–67. doi: 10.1007/0-387-33778-4_10. [DOI] [PubMed] [Google Scholar]
- 288.Crabbe PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Investig. 1970;22:448–57. [PubMed] [Google Scholar]
- 289.Gordon HA, Bruckner-Kardoss E. Effect of the normal microbial flora on various tissue elements of the small intestine. Acta Anat. 1961;44:210–25. [Google Scholar]
- 290.Gustafsson BE, Laurell CB. Gamma globulin production in germfree rats after bacterial contamination. J Exp Med. 1959;110:675–84. doi: 10.1084/jem.110.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ikari NS. Bactericidal antibody to Escherichia coli in germ-free mice. Nature. 1964;202:879–81. doi: 10.1038/202879a0. [DOI] [PubMed] [Google Scholar]
- 292.Sell S. Immunoglobulins of the germfree guinea pig. J Immunol. 1964;93:122–31. [PubMed] [Google Scholar]
- 293.Wagner M, Wostmann BS. Serum protein fractions and antibody studies in gnotobiotic animals reared germfree or monocontaminated. Ann NY Acad Sci. 1961;94:210–17. doi: 10.1111/j.1749-6632.1961.tb35542.x. [DOI] [PubMed] [Google Scholar]
- 294.Ohwaki M, Yasutake N, Yasui H, Ogura R. A comparative study on the humoral immune responses in germ-free and conventional mice. Immunology. 1977;32:43–48. [PMC free article] [PubMed] [Google Scholar]
- 295.Horowitz RE, Bauer H, Paronetto F, Abrams GD, Watkins KC, Popper H. The response of the lymphatic tissue to bacterial antigen. Studies in germfree mice. Am J Pathol. 1964;44:747–61. [PMC free article] [PubMed] [Google Scholar]
- 296.Etlinger HM, Heusser CH. T15 dominance in BALB/c mice is not controlled by environmental factors. J Immunol. 1986;136:1988–91. [PubMed] [Google Scholar]
- 297.Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–39. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Durkin HG, Bazin H, Waksman BH. Origin and fate of IgE-bearing lymphocytes. I. Peyer’s patches as differentiation site of cells Simultaneously bearing IgA and IgE. J Exp Med. 1981;154:640–48. doi: 10.1084/jem.154.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Serebrisky D, Teper AA, Huang CK, Lee SY, Zhang TF, et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol. 2000;165:5906–12. doi: 10.4049/jimmunol.165.10.5906. [DOI] [PubMed] [Google Scholar]
- 300.Sudo N, Yu XN, Aiba Y, Oyama N, Sonoda J, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–16. doi: 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 301.McCoy KD, Harris NL, Diener P, Hatak S, Odermatt B, et al. Natural IgE production in the absence of MHC class II cognate help. Immunity. 2006;24:329–39. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 302.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–14. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 303.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J Exp Med. 1997;186(10):1749–56. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 305.Ohman L, Aström RG, Hultgren Hörnquist E. Impaired B cell responses to orally administered antigens in lamina propria but not Peyer’s patches of Gα2-deficient mice prior to colitis. Immunology. 2005;115(2):271–78. doi: 10.1111/j.1365-2567.2005.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 307.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 309.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 310.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 311.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–46. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 313.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proin-flammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–68. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 314.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 315.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-) evolutionary perspective. Nat Rev Immunol. 2009;9(12):883–89. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 316.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31(3):401–11. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 317.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 318.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 319.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–97. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 321.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–46. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 322.Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546–52. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ishikawa H, Tanaka K, Maeda Y, Aiba Y, Hata A, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153:127–35. doi: 10.1111/j.1365-2249.2008.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Roselli M, Finamore A, Nuccitelli S, Carnevali P, Brigidi P, et al. Prevention of TNBS-induced colitis by different lactobacillus and bifidobacterium strains is associated with an expansion of γδ T and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm Bowel Dis. 2009;15:1526–36. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 325.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 326.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–93. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–64. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–19. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 329.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–78. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 330.Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the TH1/TH2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol. 2001;107:153–59. doi: 10.1067/mai.2001.111142. [DOI] [PubMed] [Google Scholar]
- 331.Xu D, Komai-Koma M, Liew FY. Expression and function of Toll-like receptor on T cells. Cell Immunol. 2005;233:85–89. doi: 10.1016/j.cellimm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 332.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–61. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 333.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 334.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 337.King T, Biddle W, Bhatia P, Moore J, Miner PB., Jr Colonic mucosal mast cell distribution at line of demarcation of active ulcerative colitis. Dig Dis Sci. 1992;37:490–95. doi: 10.1007/BF01307568. [DOI] [PubMed] [Google Scholar]
- 338.Okumura S, Yuki K, Kobayashi R, Okamura S, Ohmori K, et al. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin Immunol. 2009;130:175–85. doi: 10.1016/j.clim.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 339.Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn’s disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108:127–33. doi: 10.1159/000237129. [DOI] [PubMed] [Google Scholar]
- 340.Bischoff SC, Lorentz A, Schwengberg S, Weier G, Raab R, Manns MP. Mast cells are an important cellular source of tumor necrosis factor α in human intestinal tissue. Gut. 1999;44:643–52. doi: 10.1136/gut.44.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Middel P, Thelen P, Blaschke S, Polzien F, Reich K, et al. Expression of the T-cell chemoattractant chemokine lymphotactin in Crohn’s disease. Am J Pathol. 2001;159:1751–61. doi: 10.1016/S0002-9440(10)63022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Middel P, Reich K, Polzien F, Blaschke V, Hemmerlein B, et al. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn’s disease. Gut. 2001;49:795–803. doi: 10.1136/gut.49.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Garay RP, Viens P, Bauer J, Normier G, Bardou M, et al. Cancer relapse under chemotherapy: Why TLR2/4 receptor agonists can help. Eur J Pharmacol. 2007;563:1–17. doi: 10.1016/j.ejphar.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 344.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 345.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Investig. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–59. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 347.Yusuf N, Nasti TH, Long JA, Naseemuddin M, Lucas AP, et al. Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68:615–22. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 349.Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8αα TCRαβ and TCRγδ intestinal intraepithelial lymphocytes. J Immunol. 2006;176:6180–85. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 350.Imaoka A, Matsumoto S, Setoyama H, Okada Y, Umesaki Y. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol. 1996;26:945–48. doi: 10.1002/eji.1830260434. [DOI] [PubMed] [Google Scholar]
- 351.Kawaguchi-Miyashita M, Shimizu K, Nanno M, Shimada S, Watanabe T, et al. Development and cytolytic function of intestinal intraepithelial T lymphocytes in antigen-minimized mice. Immunology. 1996;89:268–73. doi: 10.1046/j.1365-2567.1996.d01-740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.John B, Crispe IN. TLR-4 regulates CD8+ T cell trapping in the liver. J Immunol. 2005;175:1643–50. doi: 10.4049/jimmunol.175.3.1643. [DOI] [PubMed] [Google Scholar]
- 353.Helgeland L, Dissen E, Dai KZ, Midtvedt T, Brandtzaeg P, Vaage JT. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur J Immunol. 2004;34:3389–400. doi: 10.1002/eji.200425122. [DOI] [PubMed] [Google Scholar]
- 354.Fujiwara D, Wei B, Presley LL, Brewer S, McPherson M, et al. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J Immunol. 2008;180:5843–52. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol. 2005;117:221–30. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 356.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–27. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 357.Vannucci L, Stepankova R, Kozakova H, Fiserova A, Rossmann P, Tlaskalova-Hogenova H. Colorectal carcinogenesis in germ-free and conventionally reared rats: Different intestinal environments affect the systemic immunity. Int J Oncol. 2008;32:609–17. [PubMed] [Google Scholar]
- 358.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–24. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 359.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med. 2004;229:586–97. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 360.Finegold SM, Flora DJ, Attebery HR, Sutter VL. Fecal bacteriology of colonic polyp patients and control patients. Cancer Res. 1975;35:3407–17. [PubMed] [Google Scholar]
- 361.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 362.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 364.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–10. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 365.Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc. 2007;66:307–15. doi: 10.1017/S0029665107005563. [DOI] [PubMed] [Google Scholar]
- 366.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–98. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 367.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–39. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 368.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 369.Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075–84. doi: 10.1007/s10350-007-9068-4. [DOI] [PubMed] [Google Scholar]
- 370.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroen-terology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 371.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–55. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 373.Beninati C, Oggioni MR, Boccanera M, Spinosa MR, Maggi T, et al. Therapy of mucosal can-didiasis by expression of an anti-idiotype in human commensal bacteria. Nat Biotechnol. 2000;18:1060–64. doi: 10.1038/80250. [DOI] [PubMed] [Google Scholar]
- 374.Shaw DM, Gaerthe B, Leer RJ, Van Der Stap JG, Smittenaar C, et al. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100:510–18. doi: 10.1046/j.1365-2567.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Liu X, Lagenaur LA, Lee PP, Xu Q. Engineering of a human vaginal lactobacillus strain for surface expression of two-domain CD4 molecules. Appl Environ Microbiol. 2008;74:4626–35. doi: 10.1128/AEM.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. An anti-HIV mi-crobicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS. 2006;20:1917–22. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 377.Hayashi H, Sakamoto M, Kitahara M, Benno Y. Molecular analysis of fecal microbiota in elderly individuals using 16S rDNA library and T-RFLP. Microbiol Immunol. 2003;47:557–70. doi: 10.1111/j.1348-0421.2003.tb03418.x. [DOI] [PubMed] [Google Scholar]
- 378.Hoffmann C, Hill DA, Minkah N, Kirn T, Troy A, et al. Community-wide response of gut micro-biota to enteropathogenic citrobacter infection revealed by deep sequencing. Infect Immun. 2009;77:4668–78. doi: 10.1128/IAI.00493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–82. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 380.Perrin P, Pierre F, Patry Y, Champ M, Berreur M, et al. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut. 2001;48:53–61. doi: 10.1136/gut.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–98. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gordon HA. Morphological and physiological characterization of germfree life. Ann NY Acad Sci. 1959;78:208–20. doi: 10.1111/j.1749-6632.1959.tb53104.x. [DOI] [PubMed] [Google Scholar]
- 383.Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol. 2004;35:377–87. doi: 10.1023/b:hijo.0000039840.86420.b7. [DOI] [PubMed] [Google Scholar]
- 384.Bakker R, Lasonder E, Bos NA. Measurement of affinity in serum samples of antigen-free, germ-free and conventional mice after hyperimmunization with 2,4-dinitrophenyl keyhole limpet hemocyanin, using surface plasmon resonance. Eur J Immunol. 1995;25:1680–86. doi: 10.1002/eji.1830250630. [DOI] [PubMed] [Google Scholar]
- 385.Bauer H, Paronetto F, Burns WA, Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966;123:1013–24. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.MacDonald TT, Carter PB. Requirement for a bacterial flora before mice generate cells capable of mediating the delayed hypersensitivity reaction to sheep red blood cells. J Immunol. 1979;122:2624–29. [PubMed] [Google Scholar]
- 387.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of αβ T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–37. [PMC free article] [PubMed] [Google Scholar]
- 388.Wells C, Balish E. The mitogenic activity of lipopolysaccharide for spleen cells from germfree, conventional, and gnotobiotic rats. Can J Microbiol. 1979;25:1087–93. doi: 10.1139/m79-166. [DOI] [PubMed] [Google Scholar]
- 389.Walton KL, Galanko JA, Balfour Sartor R, Fisher NC. T cell-mediated oral tolerance is intact in germ-free mice. Clin Exp Immunol. 2006;143:503–12. doi: 10.1111/j.1365-2249.2006.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Moreau MC, Corthier G. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect Immun. 1988;56:2766–68. doi: 10.1128/iai.56.10.2766-2768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Moreau MC, Gaboriau-Routhiau V. The absence of gut flora, the doses of antigen ingested and aging affect the long-term peripheral tolerance induced by ovalbumin feeding in mice. Res Immunol. 1996;147:49–59. doi: 10.1016/0923-2494(96)81548-3. [DOI] [PubMed] [Google Scholar]
- 392.Maeda Y, Noda S, Tanaka K, Sawamura S, Aiba Y, et al. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer’s patches under germfree conditions. Immunobiology. 2001;204:442–57. doi: 10.1078/0171-2985-00054. [DOI] [PubMed] [Google Scholar]
- 393.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 394.Salomon JC. Induction of homologous disease in axenic (germ-free) newborn mice. C R Acad Sci D. 1965;260:4862–64. [PubMed] [Google Scholar]
- 395.Gaboriau-Routhiau V, Raibaud P, Dubuquoy C, Moreau MC. Colonization of gnotobiotic mice with human gut microflora at birth protects against Escherichia coli heat-labile enterotoxin-mediated abrogation of oral tolerance. Pediatr Res. 2003;54:739–46. doi: 10.1203/01.PDR.0000086902.52137.C9. [DOI] [PubMed] [Google Scholar]
- 396.Gaboriau-Routhiau V, Moreau MC. Gut flora allows recovery of oral tolerance to ovalbumin in mice after transient breakdown mediated by cholera toxin or Escherichia coli heat-labile enterotoxin. Pediatr Res. 1996;39:625–29. doi: 10.1203/00006450-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 397.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–11. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


