Abstract
Alzheimer's disease (AD) is a chronic neurodegenerative disorder characterized by the pathological accumulation of amyloid beta (Aβ) peptides and neurofibrillary tangles containing hyperphosphorylated neuronal tau protein. AD pathology is also characterized by chronic brain inflammation, which promotes disease pathogenesis. In this context, the blood-brain barrier (BBB), a highly specialized endothelial cell membrane that lines cerebral microvessels, represents the interface between neural cells and circulating cells of the immune system. The BBB thus plays a key role in the generation and maintenance of chronic inflammation during AD. The BBB operates within the neurovascular unit (NVU), which includes clusters of glial cells, neurons and pericytes. The NVU becomes dysfunctional during AD, and each of its components may undergo functional changes that contribute to neuronal injury and cognitive deficit. In transgenic animals with AD-like pathology, recent studies have shown that circulating leukocytes migrate through the activated brain endothelium when certain adhesion molecules are expressed, penetrating into the brain parenchyma, interacting with the NVU components and potentially affecting their structural integrity and functionality. Therefore, migrating immune system cells in cerebral vessels act in concert with the modified BBB and may be integrated into the dysfunctional NVU. Notably, blocking the adhesion mechanisms controlling leukocyte–endothelial interactions inhibits both Aβ deposition and tau hyperphosphorylation, and reduces memory loss in AD models. The characterization of molecular mechanisms controlling vascular inflammation and leukocyte trafficking could therefore help to determine the basis of BBB dysfunction during AD and may lead to the development of new therapeutic approaches.
Keywords: Alzheimer's disease, Blood–brain barrier, Neurovascular unit, Vascular inflammation, Immune system cells, Leukocyte trafficking
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder with typical clinical characteristics including amnesic-type memory impairment, language deterioration, and visuospatial deficits (Cumming, 2004). AD cognitive and behavioral deficits correlate with neuronal loss and atrophy, mainly in the hippocampus and neocortex (Caselli et al., 2006). The central neuropathological hallmarks of AD are neuronal degeneration, loss of synapses, neurofibrillary tangles, gliosis and amyloid beta (Aβ) accumulation in senile plaques (Kidd, 1963, Wisniewski and Frangione, 1992, Querfurth and LaFerla, 2010). Aβ deposition is also observed in the cerebrovasculature, and is usually described as cerebral amyloid angiopathy (CAA) (Jellinger, 2002, Viswanathan and Greenberg, 2011). AD pathology is also characterized by chronic inflammation fueled by resident microglial cells and macrophages, with the contribution of circulating immune system cells (Heneka et al., 2015, Zenaro et al., 2015).
Numerous studies suggest that neurovascular dysfunction contributes to the onset and progression of AD, and propose a link between cerebrovascular changes and neurodegeneration (Kalaria, 2000, Farkas and Luiten, 2001, de la Torre, 2004, Viswanathan and Greenberg, 2011, Zlokovic, 2011, Sagare et al., 2012). Accordingly, recent data confirm age-dependent deterioration of the blood-brain barrier (BBB) during normal aging in the human hippocampus, a region involved in learning and memory, but more accelerated degradation in patients with mild cognitive impairment (MCI) compared to age-matched neurologically intact controls, suggesting this phenomenon contributes to early cognitive impairment (Montagne et al., 2015).
Aβ deposition in the vasculature leads to pro-inflammatory and cytotoxic events that contribute to the greater BBB permeability in the AD brain (Roher et al., 2003, Carrano et al., 2011, Erickson and Banks, 2013). Furthermore, CAA is associated with the degeneration of smooth muscle cells, pericytes and endothelial cells, contributing to the disruption of the BBB (Erickson and Banks, 2013). Evidence from in vitro studies and transgenic mouse tauopathy models suggests that tau may also promote BBB deterioration (Vidal et al., 2000, Forman et al., 2005, Kovac et al., 2009, Blair et al., 2015). BBB dysfunction correlates with the appearance of perivascular tau around major hippocampal blood vessels (Blair et al., 2015). Notably, when tau expression was suppressed, the integrity of the BBB was preserved, suggesting that the BBB can be stabilized in tauopathic brains by reducing tau levels (Blair et al., 2015). Both tau and Aβ may therefore promote the loss of BBB integrity, exacerbating the neurodegenerative process and associated inflammatory responses. Circulating neutrophils, which migrate in the brain of AD patients and accumulate in the central nervous system (CNS) of transgenic mice with AD-like pathology, may also contribute to vascular dysfunction by adhering and spreading on the brain endothelium and releasing inflammatory mediators and neutrophil extracellular traps (NETs) (Zenaro et al., 2015).
In this review, we discuss BBB dysfunction during AD in the context of the neurovascular unit (NVU), highlighting vascular inflammation mechanisms that contribute to disease pathogenesis. We describe the roles of the junctional complex, endothelial cells, basal lamina, pericytes and glial cells in the context of AD pathology. We also emphasize the role of cell adhesion molecules as markers of endothelial dysfunction and vascular inflammation, and discuss recent data revealing the emerging role of leukocyte trafficking in BBB and NVU dysfunction during AD.
2. Overview of the BBB and NVU
The BBB is a highly specialized endothelial cell membrane lining cerebral microvessels, which regulates the entry of plasma components, red blood cells and leukocytes into the CNS, and ensures the export of potentially neurotoxic molecules from the brain to the blood (Abbott et al., 2006, Zlokovic, 2008, Abbott et al., 2010, Zlokovic, 2011). There are two further sites in the CNS that form a barrier between the blood and cerebrospinal fluid (CSF): the arachnoid epithelium forming the middle layer of the meninges, and the choroid plexus epithelium (Abbott et al., 2006). At each site, the physical barrier is mainly determined by tight junctions that reduce the permeability of the intercellular adhesion areas (Abbott et al., 2006). These unique biological barrier structures comprise a combination of physical, transport and metabolic barriers that separate the neural milieu from the blood (Abbott et al., 2006, Zlokovic, 2008).
Brain microvessel endothelial cells (BMECs) have distinct luminal (apical) and abluminal (basolateral) membrane compartments that regulate the physical and functional integrity of the BBB (Betz and Goldstein, 1978, Daneman and Prat, 2015). BMECs thus support the three essential functions of the BBB (Daneman and Prat, 2015, Chow and Gu, 2015): (1) on the apical side, the membrane between CNS endothelial cells establishes a paracellular diffusion barrier to small hydrophilic molecules and ions (Pappenheimer et al., 1951, Brightman and Reese, 1969); (2) the passive and active receptors/channels on the luminal and/or abluminal surfaces regulate the transport of macromolecules and proteins in and out of the brain (Löscher and Potschka, 2005, Saunders et al., 2013, Xiao and Gan, 2013); and (3) generally, the BMECs serve as an interface for communication between the CNS and periphery, in particular by regulating the entry of circulating immune system cells into the brain microenvironment (Ransohoff and Engelhardt, 2012).
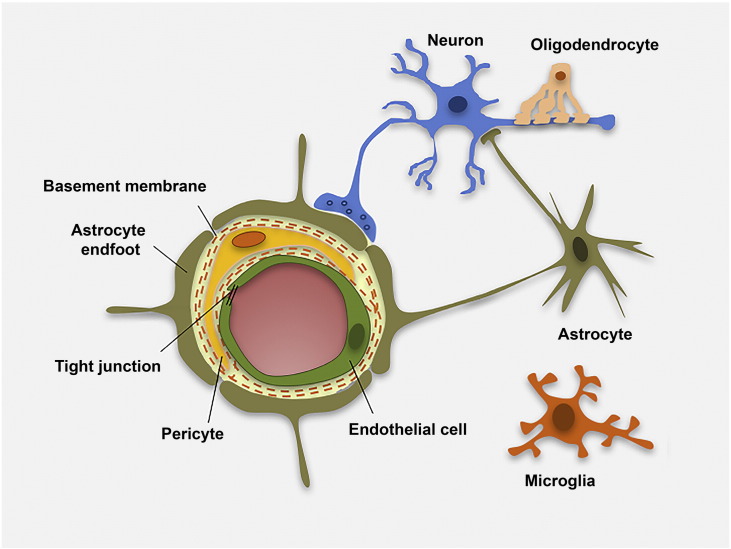
The BBB is part of the NVU, together with pericytes, vascular smooth muscle cells from the vessel wall, neurons and glial cells (Fig. 1) (Iadecola, 2004, Hawkins and Davis, 2005, Zlokovic, 2005, Abbott et al., 2010). The NVU controls BBB permeability and cerebral blood flow, and maintains the chemical composition of the brain interstitial fluid, which is required to support functional neuronal circuits (Zlokovic, 2011).
Fig. 1.
Schematic cross-sectional representation of a cerebral capillary. Brain microvascular endothelial cells are the first barrier between blood vessels and brain parenchyma. Endothelial cells are linked by tight junctions (TJs), closely surrounded by pericytes and encircled by the basal lamina, which is contiguous with the plasma membranes of astrocyte endfeet and endothelial cells. Astrocyte endfeet processes support endothelial functions and provide the cellular link to neuronal cells. Ramified microglia can sense neuronal injury and release signals that are detrimental to the BBB.
BBB/NVU dysfunction has several pathological aspects, including: (1) the leakage of circulating substances from the plasma into the CNS; (2) the modulation of transporters leading to an inadequate nutrient supply, the accumulation of toxins in the CNS, or the entry of compounds that are normally excluded; and (3) the altered expression and/or secretion of proteins by NVU cells, which can promote inflammation, oxidative stress and neuronal damage. Current evidence suggests that dysfunctional NVU cells contribute to the development of several CNS pathologies, including dementia, thus promoting neurodegeneration and cognitive decline during aging and in AD (Deane and Zlokovic, 2007, Lok et al., 2007, Neuwelt et al., 2008, Bell and Zlokovic, 2009, Bell et al., 2010, Zlokovic, 2011, Erickson and Banks, 2013).
Historically, the CNS was considered an immunoprivileged organ, lacking a lymphatic system and shielded from the peripheral circulation by the BBB. However, it is now clear that the BBB has the ability to respond to soluble factors and plasma proteins, and to communicate with peripheral immune system cells thus establishing neuro-immune system interactions, supporting the view that neuroinflammation contributes to AD pathology (Quan and Banks, 2007, Heneka et al., 2015). Therefore, the brain can no longer be viewed as an immunoprivileged organ and NVU dysfunction should be considered in a broader context, including peripheral immune cells and circulating soluble molecules that mediate immune responses (Heneka et al., 2015, Zenaro et al., 2015).
3. BBB endothelial junctions
Intercellular junctions play fundamental roles in tissue integrity but also in vascular permeability. The tightly sealed monolayer of endothelial cells forming the BBB is connected at a junctional complex by tight junctions (TJs) and adherens junctions (AJs) (Wallez and Huber, 2008, Zlokovic, 2008, Tietz and Engelhardt, 2015). Interendothelial adhesive proteins control endothelial cell–cell interactions, but some may also participate in leukocyte transendothelial migration via homophilic or heterophilic interactions (Muller, 2003, Tietz and Engelhardt, 2015).
3.1. Tight junctions
The TJ is the outermost element of the junctional complex between two endothelial cells, functioning as a boundary between the apical and basolateral plasma membrane domains (Bednarczyk and Lukasiuk, 2011). By physically sealing the paracellular space between brain capillary endothelial cells, TJs prevent the diffusion of proteins between the membrane compartments and control cell trafficking from the blood to the CNS (Zlokovic, 2008, Tietz and Engelhardt, 2015). This network of sealing proteins includes a series of transmembrane molecules embedded in the plasma membrane, such as occludin, claudins and junctional adhesion molecules (JAMs), which in turn are attached to several cytoskeleton and cytoplasmic scaffold proteins (Chiba et al., 2008, Bauer et al., 2011). The expression and activity of TJ proteins and the adaptor molecules linking the TJ to the actin cytoskeleton are affected during acute and chronic CNS diseases, suggesting that the modification of TJ components may contribute to disease pathogenesis (Petty and Lo, 2002, Persidsky et al., 2006, Bednarczyk and Lukasiuk, 2011, Gonçalves et al., 2013).
Occludin is a transmembrane protein found in TJs, which anchors the cytoplasmic zona occludens (ZO) proteins 1 and 2 and the plasma membranes of adjacent cells (Furuse et al., 1993, Schneeberger and Lynch, 2004). Claudins form the backbone of TJ strands, whereas occludin is incorporated into claudin-based strands and appears to regulate permeability (Förster, 2008). Among the 24 claudin family proteins identified in mammals, the cerebral microvascular endothelium expresses only claudins 1, 3, 5 and 12 (Tsukita and Furuse, 1999, Morita et al., 1999, Liebner et al., 2000, Nitta et al., 2003, Wolburg et al., 2003). The transmembrane proteins of the TJ associate the cytoplasm with peripheral membrane components, which form the large protein complexes of the membrane-associated guanylate kinase-like (MAGUK) family (Hawkins and Davis, 2005). ZO-1 binds directly to many TJ proteins in vitro (including occludin and claudins) and orchestrates the formation of TJ complexes (Fanning et al., 1998). Occludin, claudin-5 and ZO-1 are considered to be sensitive indicators of structural changes in the BBB during disease pathogenesis (Yang and Rosenberg, 2011, Naik et al., 2014).
Several studies have shown that Aβ is responsible for changes in the TJ proteins that disrupt the physiological function of the BBB in AD patients (Marco and Skaper, 2006, Kook et al., 2012, Wan et al., 2015). When endothelial cells isolated from rat cerebral cortex microvessels were treated with Aβ1-42, occludin expression was suppressed and both claudin-5 and ZO-2 were redistributed to the cytoplasm, whereas in untreated cells occludin, claudin-1/5 and ZO-1/2 were distributed continuously along the plasma membrane at cell–cell contacts (Marco and Skaper, 2006). In other studies, Aβ1-42 oligomers were shown to disrupt TJs and increase vascular permeability by suppressing the expression of ZO-1, claudin-5 and occludin, and by inducing the expression of the matrix metalloproteases (MMP)-2 and MMP-9 in monolayer cultures of bEnd.3 brain endothelial cells (Kook et al., 2012, Wan et al., 2015). Aβ is toxic in vitro to brain endothelial cells because it binds to the receptor for advanced glycation end products (RAGE) and induces the production of reactive oxygen species (ROS), which ultimately disrupt TJs and reduce BBB integrity (Carrano et al., 2012). Consistent with these in vitro findings, cerebral capillaries were found to be disrupted near Aβ deposits together with elevated RAGE expression and enhanced MMP secretion in the microvessels of 5xFAD mice, an animal model of AD characterized by massive Aβ accumulation in the brain at early stages of the disease (Kook et al., 2012). Furthermore, the expression of occludin, claudin-5, and ZO-1 was strongly suppressed in the Aβ-laden capillaries of patients with capillary CAA, suggesting that the modulation of TJ protein expression may increase vascular permeability in the AD brain (Carrano et al., 2011, Carrano et al., 2012).
JAMs are members of the immunoglobulin (Ig) superfamily. There are three variants (JAM-A, JAM-B and JAM-C) and they primarily undergo homophilic interactions, e.g. JAM-A on the surface of one endothelial cell will bind to JAM-A on the surface of its neighbor (Garrido-Urbani et al., 2014). However, JAMs also engage leukocyte integrins in a heterophilic manner, and may participate in leukocyte extravasation during inflammatory responses (Del Maschio et al., 1999, Ostermann et al., 2002, Santoso et al., 2002, Muller, 2003). In vitro studies have shown that Aβ1-42 suppresses the expression of JAM-C in human umbilical vein endothelial cells (HUVECs), but it is unclear whether this is also the case in vivo and the involvement of JAMs in AD therefore remains to be clarified (Chao et al., 2016).
3.2. Adherens junctions
Cadherins are membrane-spanning proteins that mediate intercellular adhesion in AJs, the inner element of the junctional complex between two endothelial cells (Zlokovic, 2008, Bednarczyk and Lukasiuk, 2011, Tietz and Engelhardt, 2015). The principal cadherin is vascular endothelial (VE) cadherin, a homophilic adhesion molecule located on the lateral endothelial cell surface. VE-cadherin expression was recently shown to be reduced in brain vessels that accumulate fibrinogen-Aβ (Fg-Aβ) complexes in a model of hyperhomocysteinemia, a pathology that may accompany cognitive disorders including AD (Muradashvili et al., 2014). These data suggest that Aβ binding to fibrinogen can also exacerbate cerebrovascular permeability during AD, by modulating VE-cadherin levels in cerebral microvessels (Muradashvili et al., 2014). Real-time observation of neutrophil transmigration in vitro across endothelial cell monolayers expressing VE-cadherin fused to green fluorescent protein (GFP) revealed that these cells transmigrate at regions of low VE-cadherin expression (Shaw et al., 2001). Moreover, the in vivo administration of antibodies that block the adhesive function of VE-cadherin increases vascular permeability and accelerates the recruitment of neutrophils to inflammation sites (Gotsch et al., 1997, Corada et al., 1999). Therefore, we hypothesize that neutrophil transmigration into the CNS, as observed in mouse models of AD, may take place in brain microvessels with low levels of VE-cadherin (Zenaro et al., 2015).
4. Endothelial cells
The brain vasculature of AD patients is characterized by a greater prevalence of collapsed or degenerated endothelium, and more severe impairment of BBB transport systems, compared to age-matched control subjects (Kalaria and Hedera, 1995, Zlokovic, 2008). For example, glucose transporter GLUT1 is expressed at lower levels in the brain capillaries of AD patients and mouse models of AD than corresponding age-matched controls, suggesting that the AD brain suffers a chronic shortage of energy-rich metabolites (Kalaria and Harik, 1989, Simpson et al., 1994, Mooradian et al., 1997, Farkas and Luiten, 2001, Hooijmans et al., 2007, Merlini et al., 2011). Recently, it has been shown that GLUT1 is necessary for the maintenance of proper brain capillary networks, blood flow and BBB integrity as well as neuronal function (Winkler et al., 2015). GLUT1 deficiency in endothelial cells leads to BBB breakdown with extravascular accumulation of fibrin and IgG in brain parenchyma and reduction of TJ proteins in AD mice, suggesting a role for GLUT1 decrease on endothelial cells in AD pathogenesis (Winkler et al., 2015). Several studies using F-2-fluoro-2-deoxy-d-glucose-PET (FDG-PET) have demonstrated that MCI patients take up less glucose at the BBB, and FGD-PET may therefore be used as a marker for the preclinical diagnosis of AD (Hunt et al., 2007, Samuraki et al., 2007, Mosconi et al., 2008). Moreover, these studies suggest that reduced glucose uptake across the BBB may precede the neurodegenerative process and brain atrophy that occurs when MCI converts to AD.
Aβ peptides also cross BMECs to and from the brain by active transport, so the altered expression of Aβ peptide transporters in endothelial cells could contribute to the accumulation of Aβ (Zlokovic et al., 2010). BMECs express low density lipoprotein receptor related protein 1 (LRP1) as well as RAGE, which play opposing roles: LRP1 mediates the efflux of Aβ from the brain to the periphery, whereas RAGE is thought to promote Aβ influx back into the CNS (Deane et al., 2004, Pflanzner et al., 2010, Sagare et al., 2012). Clinical studies have demonstrated that AD pathology correlates with lower LRP1 levels and higher RAGE levels, which promotes the accumulation of Aβ peptides in the brain parenchyma (Donahue et al., 2006, Zlokovic, 2008, Deane et al., 2012). A recent study has shown that 1,25(OH)D3, the active form of vitamin D, has a neuroprotective effect during AD pathogenesis by inducing the clearance of Aβ, which is achieved by inducing LRP1 expression and reducing the expression of RAGE in brain endothelial cells (Guo et al., 2016). Accordingly, 5xFAD mice lacking the LRP1 receptor show much lower plasma levels of Aβ1-42, whereas the levels of soluble Aβ in the brain increased sharply (Storck et al., 2016). Aβ oligomers can directly upregulate the expression of RAGE in endothelial cell lines (Wan et al., 2015) and their binding to RAGE triggers multiple cellular signaling cascades in BMECs. For example, the Aβ–RAGE interaction induces MMP-2 expression and reduces P-glycoprotein (P-gp) expression in vitro (Du et al., 2012, Park et al., 2014). P-gp is an ATP-dependent efflux transporter that transports Aβ out of the brain, and its expression in the brain endothelium of 5xFAD mice is suppressed near Aβ plaques (Demeule et al., 2001, Lam et al., 2001, Kuhnke et al., 2007, Park et al., 2014). Furthermore, recent studies have shown that β-secretase 1 enzyme (BACE1) is more abundant on the BMECs of AD mice (hAPPSL model) than healthy controls, suggesting a role for BACE-1 the accumulation of Aβ in blood vessels (Devraj et al., 2015). Collectively, these results suggest that the accumulation of Aβ in blood vessels and the induction of CAA are promoted by the overexpression of Aβ by endothelial cells and the altered expression and function of Aβ transporters on BMECs.
Other vascular-specific molecular pathways may contribute to AD pathogenesis, such as homeodomain-transcription factor GAX encoded by MEOX2 (mesenchyme homeobox 2) gene. Previous studies have shown that deletion of MEOX2 leads to reduction of brain capillary density and cerebral blood flow, loss of the angiogenic response to hypoxia in the brain and reduced LRP1 expression in a mouse model of AD (Wu et al., 2005). Restoring MEOX2 gene expression in brain endothelial cells obtained from individuals with AD stimulates angiogenesis, suppresses AFX1 forkhead transcription factor–mediated apoptosis and increases the levels of LRP1 and its receptor-associated protein (RAP), further supporting a role for GAX in AD (Wu et al., 2005). Moreover, in AD subjects, vascular smooth muscle cells (VSMC) in small pial and intracerebral arteries express high levels of serum response factor (SRF) and myocardin (MYOCD), two interacting transcription factors that orchestrate a VSMC-differentiated phenotype, which in turn leads to a hypercontractile phenotype in small cerebral arteries causing reduction in the cerebral blood flow and decreased Aβ clearance (Chow et al., 2007, Bell et al., 2009).
Oxidative stress is also considered a pivotal mechanism leading to cerebrovascular dysfunction in AD, and ROS act as signaling molecules that modulate gene expression, proliferation, migration, angiogenesis, apoptosis, and senescence in endothelial cells (Frey et al., 2009). NADPH oxidases, the major source of ROS in blood vessels, are responsible for the cerebrovascular dysregulation induced by Aβ1-40 in the blood vessels of Tg2576 mice, an animal model of amyloid pathology (Park et al., 2005). Moreover, a recent investigation in 3xTg-AD mice revealed that thrombin, a mediator of cerebrovascular inflammation and oxidative stress, significantly increases the cerebrovascular expression of inflammatory proteins and ROS, and that the inhibition of thrombin blocks the generation of ROS induced by hypoxia (Tripathy et al., 2013). Therefore, ROS production by brain endothelial cells in response to hypoxia is a detrimental mechanism that probably contributes to AD pathogenesis (Feng and Wang, 2012).
Other inflammatory mediators may play a role in BBB dysfunction during the progression of AD. Brain microvessels from AD patients secrete higher levels of several inflammatory mediators (such as nitric oxide, pro-inflammatory cytokines, chemokines, prostaglandins and MMPs) and express leukocyte adhesion molecules, clearly indicating pro-inflammatory changes in the vasculature (Grammas et al., 2011). In particular, interleukin 17 (IL-17) has previously been shown to induce BBB damage in vitro, and our recent data show that neutrophils migrating into the brains of AD-like mice secrete this cytokine, suggesting it plays a role in endothelial dysfunction during AD (Kebir et al., 2007, Zenaro et al., 2015).
5. Basement membranes
Two basement membranes can be distinguished at the level of CNS microvessels: an endothelial basement membrane beneath the endothelial cells, and an astroglial basement membrane, which underlies the astrocytic endfeet (Engelhardt and Sorokin, 2009). The first and contains fibronectin, type IV collagen, perlecan, and laminins 4 and 5, whereas the second comprises fibronectin, agrin and laminins 1 and 2 (Sixt et al., 2001, Engelhardt and Sorokin, 2009). The astroglial basement membrane, together with the leptomeningeal basement membrane, constitutes the parenchymal basement membrane, which delineates the neuropil. The endothelial and parenchymal basement membranes are clearly distinguishable in the postcapillary venules, whereas in brain capillaries they fuse to form one basement membrane structure (Fig. 1, Fig. 2) (Bechmann et al., 2007, Engelhardt and Sorokin, 2009). The extracellular matrix (ECM) structural proteins of the BBB basement membranes establish the essential physical scaffolding for cells and therefore support the assembly of TJs (Tilling et al., 1998, Savettieri et al., 2000).
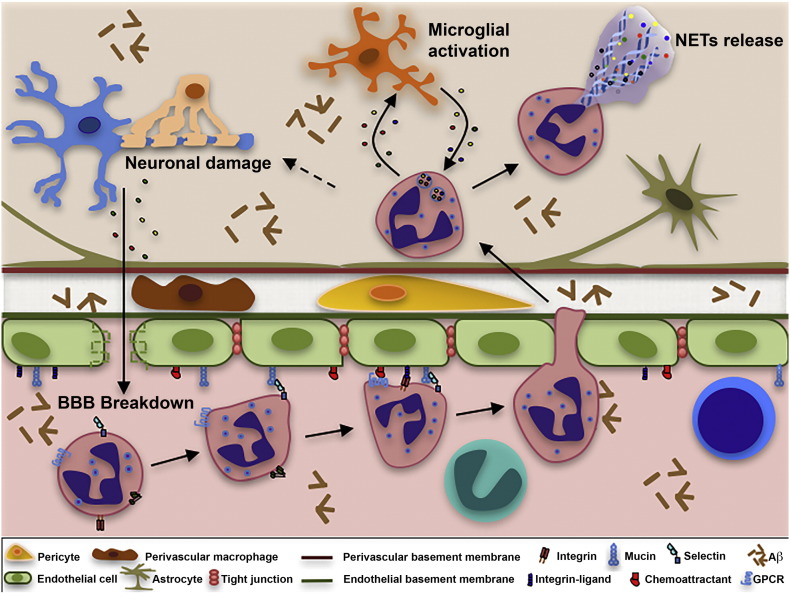
Fig. 2.
Schematic representation of neutrophil extravasation in postcapillary venules and potential CNS damage. Aβ and other inflammatory stimuli promote the activation of cerebral endothelium and immune system cells. The activated endothelium in brain venules upregulates the expression of adhesion molecules and chemoattractants supporting the adhesion of neutrophils that transmigrate into the brain parenchyma. Neutrophils adhered on activated endothelium may acquire a toxic phenotype, releasing reactive oxygen species (ROS), cytokines, chemokines and enzymes contributing to the destruction of the BBB. Neutrophils may also release neutrophil extracellular traps (NETs) comprising decondensed chromatin and active proteases that may damage the BBB. Migrated neutrophils and microglia may sustain their reciprocal activation, resulting in chronic inflammation and neuronal degeneration. Intraparenchymal neutrophils may be toxic towards neuronal and glial cells and may promote synaptic dysfunction, Aβ deposition and tau hyperphosphorylation.
Previous studies have identified thinning and discontinuities within the vascular basement membrane in AD patients, suggesting that significant ECM changes occur during AD (Zipser et al., 2007, Farrall and Wardlaw, 2009). The expression of collagen IV, perlecan and fibronectin in the basement membrane-associated ECM of subclinical and clinical AD patients increased in the frontal and temporal cortices compared to controls and positively correlated with Aβ deposition, suggesting that changes affecting the brain microvascular structure could drive disease pathogenesis (Lepelletier et al., 2015).
Alterations in the structure and organization of basement membrane ECM proteins such as laminins may favor the migration of circulating leukocytes into the brain during inflammation. After the initial penetration of the endothelial cell monolayer, extravasating leukocytes need to traverse the endothelial basement membrane. Laminin 4 is localized in all endothelial basement membranes, whereas laminin 5 is predominantly found in the walls of capillaries and postcapillary venules, the sites of leukocyte extravasation (Hallmann et al., 2005). The role of ECM proteins in leukocyte trafficking during AD is unknown, but insight gained from other CNS inflammatory diseases may provide useful information. For example, in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, T cells migrate across laminin α4 whereas laminin α5 inhibits their migration (Wu et al., 2009). Moreover, endothelial basement membranes containing laminin 8 are permissive for T cell transmigration in mice with EAE, whereas those containing laminin 10 are restrictive (Sixt et al., 2001). The subsequent penetration of the parenchymal basement membrane containing laminins 1 and 2 by activated T cells occurs only after the disruption of this outer barrier, probably via the release of MMPs (Graesser et al., 1998).
Secreted MMPs cleave protein components of the ECM and have been implicated in many brain disorders associated with BBB injury, including AD (Agrawal et al., 2006, Hu et al., 2009, Stomrud et al., 2010, Qiu et al., 2011, Yang and Rosenberg, 2011, Zhang et al., 2012, Barichello et al., 2014, Wan et al., 2015). MMP-2 and MMP-9 can digest the endothelial basal lamina and TJ scaffold proteins, which are necessary for BBB integrity (Qiu et al., 2011, Zhang et al., 2012). MMP-2 and MMP-9 have a high affinity for dystroglycan, which anchors laminins 1 and 2, and these enzymes are required for T cells to penetrate through the parenchymal basal membrane (Agrawal et al., 2006). Under neuroinflammatory conditions, MMP-3 also contributes to the disruption of the BBB by attacking TJ and basal lamina proteins, facilitating neutrophil migration into the brain (Gurney et al., 2006). MMP-3 and MMP-9 are more abundant in the CSF of AD patients than controls, and may be involved in ECM degradation and AD pathogenesis (Stomrud et al., 2010). Interestingly, neutrophils, which have recently been shown to migrate in the AD brain, contain preformed MMP-9 proteins in their granules, which can be released during transendothelial migration, leading to basement membrane damage (Opdenakker et al., 2001, Noble et al., 2002, Zenaro et al., 2015). Moreover, Aβ1-42 oligomers can trigger a significant increase in the expression of MMP-2 and MMP-9 in bEnd.3 cells in vitro (Wan et al., 2015). We therefore hypothesize that the higher levels of circulating or perivascular Aβ in AD patients and transgenic animals with AD-like disease, as well as the accumulation of leukocytes adhering to the vascular wall or transmigrating into the neuropil, may contribute to basement membrane degradation and BBB dysfunction.
6. Pericytes
Pericytes are recruited to the nascent vessels during embryogenesis more than a week before astrocytes emerge, and they are necessary for the formation of the BBB (Daneman et al., 2010). They regulate several functions of the BBB, including the formation of TJs and vesicle trafficking in the CNS, and they inhibit the accumulation of molecules that increase vascular permeability and the infiltration of the CNS by immune system cells (Daneman et al., 2010). Pericytes also maintain the structural stability of microvessels, clear the CNS of toxic cellular byproducts, and regulate the blood flow through brain capillaries by controlling cellular contraction/relaxation (Peppiatt et al., 2006, Dore-Duffy, 2008, Sagare et al., 2013).
Pericyte-deficient mice show an age-dependent reduction in cerebral microcirculation, and the BBB deteriorates leading to neurodegeneration and cognitive impairment (Bell et al., 2010). In support of the data from experimental animals, age-dependent BBB degradation was also observed in the hippocampus of MCI patients compared to control subjects, correlating with injury to BBB-associated pericytes and increased BBB permeability in the CA1 and DG sectors (Montagne et al., 2015). Interestingly, the analysis of CSF biomarkers revealed higher levels of soluble platelet-derived growth factor receptor β (sPDGFRβ), a marker of pericyte injury, in MCI patients compared to control subjects, whereas no other signs of endothelial or neural injury, or tau and Aβ pathology, were observed (Montagne et al., 2015).
Pericyte dysfunction is associated with AD neuropathology (Bell et al., 2010, Zlokovic, 2011, Bell et al., 2012, Sagare et al., 2013, Sengillo et al., 2013, Winkler et al., 2014, Montagne et al., 2015). AD patients suffer a significant loss of pericytes in the cortex and hippocampus compared to control subjects, correlating with the severity of BBB degradation (Sengillo et al., 2013). Aβ deposition in cerebral vessels is associated with pericyte and smooth muscle cell degeneration (Verbeek et al., 2000) and the direct treatment of acute hippocampal slices with Aβ42 oligomers increases the production of ROS by pericytes, which accelerates their loss (Veszelka et al., 2013). In addition, recent in vitro data indicate that Aβ deposits are associated with the shedding of PDGFRβ from primary cultures of human pericytes, further suggesting that Aβ contributes to pericyte degeneration in AD (Montagne et al., 2015). Moreover, LRP1 and other Aβ-binding receptors such as the low density lipoprotein receptor (LDLR), RAGE, and CD36 are expressed on pericytes from post-mortem AD brains associated with cerebral amyloid angiopathy (Wilhelmus et al., 2007a). The in vitro treatment of human pericytes with Aβ induces the expression of LRP-1 and LDLR, suggesting that these receptors are involved in the Aβ-mediated death of cerebral perivascular cells (Wilhelmus et al., 2007a, Wilhelmus et al., 2007b). Pericyte deficiency in APPsw/0 Pdgfrβ+/− mice accelerates the deposition of Aβ plaques in the brain parenchyma and blood vessels compared to normal amyloid precursor protein (APP) mice (Sagare et al., 2013). Notably, APPsw/0 Pdgfrβ+/− mice normally present amyloid pathology alone, but also accumulate hyperphosphorylated tau species resulting in tau aggregates and neuronal loss during the early stages of the disease, suggesting that pericyte deficiency favors the development of tau pathology (Sagare et al., 2013).
Previous studies have shown that lack of murine Apoe and expression of APOE4 gene, a major genetic risk factor for AD, but not APOE2 and APOE3, leads to BBB dysfunction by activating a proinflammatory cyclophilin A (CypA)–nuclear factor-κ B–matrix-metalloproteinase-9 pathway in mouse pericytes (Bell et al., 2012). Interestingly, Apoe−/− and APOE4 mice also show a reduction of TJ proteins and develop vascular defects before neuronal and synaptic changes occur (Bell et al., 2012). In support of these data, recent studies using postmortem human brain tissues have shown that APOE4 compared with APOE3 accelerates pericyte loss in AD, which correlates with the magnitude of BBB breakdown to plasma proteins immunoglobulin G and fibrin (Halliday et al., 2016). APOE4 compared with APOE3 leads to a higher accumulation of CypA and MMP-9 in pericytes and endothelial cells in AD suggesting a role for LRP1-dependent CypA–MMP-9 BBB degrading pathway in accelerated BBB breakdown in AD APOE4 compared with AD APOE3 carriers (Halliday et al., 2016).
Recent studies have also implicated pericytes in brain immune responses, including the regulation of leukocyte trafficking to the CNS (ElAli et al., 2014, Hill et al., 2014). Mice defective in pericyte development express higher levels of intercellular adhesion molecule 1 (ICAM-1) on the brain endothelium, and larger numbers of Gr1+ leukocytes infiltrate into the brain parenchyma, suggesting a role for pericytes in leukocyte trafficking in the CNS (Daneman et al., 2010). Accordingly, recent intravital microscopy studies in inflamed cremasteric venules have shown that neutrophils exhibit abluminal crawling along pericyte processes to gaps between adjacent pericytes with almost no crawling observed on pericyte-deficient regions (Proebstl et al., 2012). Neutrophil-pericyte adhesion interactions in these studies were mediated by ICAM-1, LFA-1 and macrophage-1 antigen (Mac-1) (Proebstl et al., 2012). Furthermore, pericytes can sense inflammation and danger-associated molecular patterns (DAMPs), and in turn upregulate adhesion molecules, which mediate adhesive interactions with cells of the innate immune system (Stark et al., 2013). In response to tumor necrosis factor (TNFα), N-formyl-methionyl-leucyl-phenylalanine (fMLP) or lysates of necrotic cells, pericytes may acquire a pro-inflammatory phenotype, characterized by higher ICAM-1 levels and the secretion of significantly greater amounts of chemoattractants such as CXCL1, CXCL8, macrophage migration inhibitory factor (MIF), CCL2 and IL-6 (Stark et al., 2013). These results suggest that pericytes may facilitate ICAM-1 dependent pericyte–leukocyte interactions, thus modulating innate immune responses during the sterile inflammation that occurs in AD. Indeed, Aβ is a clinically relevant endogenous danger signal sensed by formyl peptide receptor 1 (FPR1), LRP1, LDLR, RAGE and CD36, which are expressed on pericytes in post-mortem AD brains (Yan et al., 1996, Wilhelmus et al., 2007a, Wilhelmus et al., 2007b, Stewart et al., 2010). Based on these studies and our recent data showing neutrophil accumulation in AD, we speculate that Aβ triggers pro-inflammatory signals through these receptors in pericytes, promoting leukocyte recruitment in AD (Zenaro et al., 2015). The role of pericytes in neurovascular dysfunction is still not well understood and future studies are required to determine the molecular mechanisms underlying pericyte impairment and their influence on neighboring cells within the NVU during AD.
7. Glial cells and perivascular macrophages
Astrocytic perivascular endfeet surround ~ 98% of the parenchymal basal membrane of brain microvessels, with microglial ramifications covering the remaining surface. Astrocytes also communicate with neurons, establishing a link for endothelial–neuronal coupling (Fig. 1). Astrocytes can upregulate many BBB features, leading to tighter TJs (physical barrier), the expression and polarized localization of transporters (transport barrier), and specialized enzyme systems (metabolic barrier) (Abbott et al., 2006).
Studies of AD brain cortex biopsies have revealed specific ultrastructural changes in astrocytes near Aβ deposits both in the brain parenchyma and cerebrovasculature (Wisniewski et al., 1989). Accordingly, in transgenic mice with both arctic and Swedish APP mutations (Arc/SweAβ), which are characterized by strong CAA pathology, astrocyte endfeet surrounding vascular Aβ deposits showed morphological changes including retraction and swelling and reduction expression of GLUT1 and lactate transporters (Merlini et al., 2011). These changes occur at early stages of the disease and are consistent with NVU uncoupling, suggesting that astrocyte dysfunction can contribute to the early behavioral and cognitive impairments seen in Arc/SweAβ mice.
The uptake and degradation of Aβ by astrocytes in the NVU is mediated by the expression of aquaporin-4 (AQP4), which is abundant in astrocytic processes adjacent to cerebral microvessels (Hoshi et al., 2012, Yang et al., 2012). Animals lacking AQP4 in their astrocytes are unable to clear soluble Aβ efficiently, suggesting that this pathway may remove Aβ from the CNS (Iliff et al., 2012). In the Arc/SweAβ mouse model of AD, changes in AQP4 expression emerge just after the appearance of the first Aβ plaques (Yang et al., 2011). The reallocation of AQP4 from endfoot membranes at sites of perivascular Aβ deposits to non-endfoot membrane domains in the neuropil surrounding Aβ plaques potentially causes astrocyte depolarization (Yang et al., 2011). These data suggest that the aggregation of Aβ deposits in blood vessels disrupts the perivascular sheath of astrocyte processes and interferes with the mechanisms that are normally responsible for the anchoring of perivascular AQP4 molecules (Yang et al., 2011). Accordingly, the loss of astrocyte polarization was recently observed in 5XFAD mice, together with lower expression levels of AQP4 and the astrocyte-derived basement membrane component laminin α2 compared to wild-type controls (Park et al., 2014).
The proposed immunoactive properties of astrocytes are still a matter of debate. However, several recent reports suggest that astrocytes actively contribute to the immune responses at the NVU, especially by integrating signals from the periphery and the brain. At the site of neuroinflammation, astrocyte-derived cytokines and chemokines play both immunoregulatory and pro-inflammatory roles in brain lesions. Non-stimulated human astrocytes in culture express cytokines and chemokines such as granulocyte and granulocyte-macrophage colony stimulating factors (G-CSF, GM-CSF), CXCL1, IL-6, IL-8 (CXCL8), monocyte chemoattractant protein 1 (MCP-1/CCL2) and MIF (Choi et al., 2014). Furthermore, in response to stimulation with IL-1β and TNFα, the cytokines most frequently associated with neuroinflammation, cultured human astrocytes produce IL-1β, IL-1ra, TNFα, interferon gamma-induced protein 10 (IP-10/CXCL10), macrophage inflammatory protein 1 alpha (MIP-1α/CCL3), and regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), in addition to soluble ICAM-1 (sICAM-1) and complement component 5 (Choi et al., 2014). Together, these data indicate that soluble factors released by astrocytes may promote the trafficking of cells related to innate and acquired immunity in the CNS (Choi et al., 2014). Notably, IL-8 is a potent chemoattractant that draws neutrophils to sites of inflammation, and higher levels of this molecule are found in the CSF of MCI and AD patients compared to healthy controls (Galimberti et al., 2006, Galimberti et al., 2008). Furthermore, we have recently reported the presence of neutrophils producing IL-17 in the brain of mouse AD models at early stages of the disease (Zenaro et al., 2015) and these results, together with data showing that astrocyte stimulation with IL-17 increases the expression of pro-inflammatory molecules and neutrophil-mobilizing cytokines and chemokines including GM-CSF, CXCL1 (KC), CXCL2 (MIP-2), CCL20 and MMP9 (Kang et al., 2010), suggest that neutrophil–astrocyte interplay may promote neuroinflammation during AD (Fig. 2).
Microglial cells and perivascular macrophages are the two main immune system cell populations in the CNS. In their basal state these cells may be distinguished by their location, phenotype and morphology, but following activation it is difficult to discriminate between these cell populations (Guillemin and Brew, 2004, Hanisch, 2013, Harry, 2013, Cartier et al., 2014, Fu et al., 2014). The plasticity of these cells and their ability to transition between activation states is reflected by their complicated and heterogeneous phenotypes (Cameron and Landreth, 2010). Therefore, some functions attributed to perivascular microglia may be carried out by perivascular macrophages. Some microglial cells are found in the NVU, suggesting that microglia may influence endothelial cells and contribute to the function of the BBB (da Fonseca et al., 2014). However, blood-derived human macrophages in a BBB cell culture model also interact with the cerebral endothelium and modulate BBB-specific functions (Zenker et al., 2003). Furthermore, some studies have shown that perivascular macrophages rather than resident microglia are responsible for the physiological degradation of Aβ, a function that has been previously attributed to microglial cells (El Khoury et al., 2007, Hawkes and McLaurin, 2009, Malm et al., 2010).
Microglia are involved in the active surveillance of the CNS and continuously scan the environment to detect pathogens or tissue damage (Yang et al., 2011). The activation of microglia induces an innate immune response dominated by the release of pro-inflammatory cytokines and chemotactic factors that may act on circulating leukocytes (Heneka, 2015). Several reports indicate that microglia promote the sustained migration of lymphocyte and monocytes through the BBB into the CNS during brain inflammation (Persidsky et al., 1999, Hudson et al., 2005, Lécuyer et al., 2016).
Microglia may initially play a protective role in AD by facilitating the clearance of Aβ, whereas at later disease stages the chronic activation of microglia is accompanied by diminished phagocytosis and the more abundant secretion of pro-inflammatory cytokines, leading to the accumulation of Aβ and the amplification of neuroinflammation (El Khoury et al., 2007, Hickman et al., 2008, Krabbe et al., 2013, Heneka et al., 2015). During AD, the activation of microglia in response to neuronal damage and Aβ also becomes a chronic source of ROS, which causes neurotoxicity and the impairment of BBB function, including the reorganization of TJs and restricted transendothelial electrical resistance (Block, 2008, Sumi et al., 2010). Furthermore, IL-1β, a major pro-inflammatory cytokine released from activated microglia, increases the permeability of the BBB and abolishes the ability of astrocytes to maintain BBB integrity (Wang et al., 2014). IL-1β also promotes neutrophil transmigration in a BBB model, establishing a link between microglial activation and neutrophil trafficking through the BBB, which was recently described in AD transgenic mice and AD patients (Allen et al., 2012, Zenaro et al., 2015).
Several reports have revealed vascular fibrin and fibrinogen deposits coincident with areas of BBB permeability and Aβ deposition, suggesting that fibrinogen/fibrin-Aβ interactions may exacerbate neurovascular damage and promote cognitive impairment through perivascular microglial activation (van Oijen et al., 2005, Paul et al., 2007, Xu et al., 2008, Ryu and McLarnon, 2009, Cortes-Canteli et al., 2010). In agreement with this hypothesis, recent two-photon microscopy data in an EAE model have shown that fibrinogen leaking from the blood engages the microglial integrin receptor CD11b/CD18 and induces the rapid formation of microglial perivascular clusters on the vasculature, leading to axonal damage and neuroinflammation (Davalos et al., 2012). Fibrinogen also causes the activation of microglial cells in experimental stroke models, resulting in the upregulation of inflammatory proteins and the appearance of intracellular vesicles containing particles that express CD31+, a blood vessel marker, suggesting that perivascular microglia can internalize and break down endothelial cells (Jolivel et al., 2015). Perivascular microglia therefore promote the disintegration of the BBB and therapeutic approaches that inhibit microglial activation may have a beneficial effect in neuroinflammatory diseases.
8. Vascular adhesion molecules as markers of endothelial dysfunction
The expression of vascular adhesion molecules that mediate leukocyte trafficking is minimal or undetectable under physiological conditions, with P-selectin, E-selectin and ICAM-1 immunoreactivity detected in pial and choroid plexus venules in the normal brain (Kivisäkk et al., 2003). However, during brain inflammation, the expression of endothelial adhesion molecules increases strongly, and their soluble forms are released into the circulation to provide biomarkes of endothelial dysfunction and vascular inflammation (Bö et al., 1996, Jander et al., 1996, Stins et al., 1997, Alvarez et al., 2011, Staykova et al., 2000, Garton et al., 2006, Rossi et al., 2011).
Two main classes of adhesion molecules are associated with microvascular endothelial activation: integrin ligands from the immunoglobulin (Ig) superfamily and endothelial selectins (Rossi et al., 2011). Vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1 belong to the first class and act as ligands for the very late antigen 4 (VLA-4) and LFA-1 integrins, respectively, with VCAM-1 exclusively expressed on endothelial cells and ICAM-1 also expressed on leukocytes (Rossi et al., 2011). The second class of endothelial adhesion molecules is represented by E-selectin and P-selectin, with E-selectin exclusively present on endothelial cells and P-selectin also located together with Aβ precursor proteins in platelet α-granules (Rossi et al., 2011, Vignini et al., 2011, Järemo et al., 2013, Vignini et al., 2013). The upregulation of selectins and integrin ligands on the endothelium is regulated by cytokines, hormones, cellular stress and other factors (Carlos and Harlan, 1994, Roebuck and Finnegan, 1999, Vestweber and Blanks, 1999, Ley, 2003).
Higher levels of soluble VCAM-1, ICAM-1, E-selectin and P-selectin are found in plasma samples from AD patients compared to control subjects, suggesting that vascular inflammation occurs during AD (Rentzos et al., 2005, Nielsen et al., 2007, Zuliani et al., 2008, Huang et al., 2015, Järemo et al., 2013). Notably, higher levels of soluble VCAM-1 correlate with more advanced dementia, including poor short-term memory and visuospatial functions, as well as with changes in white matter hyperintensities observed during magnetic resonance imaging (MRI), suggesting VCAM-1 could be used as a biomarker for cognitive decline during AD (Huang et al., 2015). However, soluble E-selectin levels are inversely correlated with the tau/Aβ1-42 ratio in the CSF and E-selectin is more abundant in AD patients without the typical CSF biomarker signature, suggesting it may be useful as a biomarker for vascular dysfunction concurring with dementia (Li et al., 2015). In addition to selectins and integrin ligands, soluble platelet endothelial cell adhesion molecule 1 (PECAM-1) also accumulates to higher levels in the plasma of AD patients compared to control subjects (Nielsen et al., 2007, Vestweber, 2015). PECAM-1 is an endothelial adhesion molecule also found on platelets and leukocytes, which can interact in a homophilic manner and mediates leukocyte transmigration during inflammation (Vestweber, 2015). Soluble vascular adhesion molecules retain the ability to bind their ligands and may thus activate their counter-receptors on circulating leukocytes, increasing their adhesion capacity (Lo et al., 1991, Newman et al., 1993, Wang et al., 2007). For example, soluble P-selectin may bind and crosslink P-selectin glycoprotein ligand-1 (PSGL-1) or the glycoprotein T cell immunoglobulin and mucin domain 1 (TIM-1), triggering intracellular signaling pathways in leukocytes that promote adhesion and activate additional pro-inflammatory functions (Wang et al., 2007, Angiari et al., 2014, Angiari and Constantin, 2014). Therefore, elevated levels of soluble adhesion molecules may represent a negative predictive factor linking vascular inflammation to the progression of AD.
The role of vascular inflammation in AD is supported by our recent findings that the expression of E-selectin, P-selectin, VCAM-1 and ICAM-1 is significantly higher in 4-month-old 5xFAD mice than age-matched wild-type controls (Zenaro et al., 2015). These adhesion molecules were expressed mainly in the vessels of the meninges and cortex, but also in the choroid plexi, hippocampus and amygdala of the AD mice. Similar data were obtained in 3xTg-AD mice, which present both amyloid and tau pathology: we observed the high-level expression of all vascular adhesion molecules compared to age-matched controls, especially E-selectin and P-selectin in the hippocampus and cortex at 6 months of age. Notably, in both transgenic models of AD, adhesion molecules were expressed not only during the early phase of the disease but also in older animals, suggesting that vascular inflammation may play a continuous pathogenic role (Zenaro et al., 2015). In agreement with our results, a recent study in Arc/SweAβ mice confirmed the strong upregulation of VCAM-1 and ICAM-1 in the brains of 20–24-month-old mice compared to wild-type littermates (Ferretti et al., 2016). Interestingly, in both animal AD models and human AD patients, the expression of adhesion molecules was observed in areas burdened by Aβ plaques and rich in migrated leukocytes (Frohman et al., 1991, Zenaro et al., 2015, Ferretti et al., 2016). Accordingly, in vitro studies have demonstrated that Aβ peptides induce the expression of endothelial selectins and integrin ligands in mouse and human brain endothelial cells (Giri et al., 2000, Zenaro et al., 2015). In addition, the in vitro stimulation of human brain endothelial cells with Aβ1-40 promoted the adhesion and transendothelial migration of monocytes, but this was inhibited by an antibody to PECAM-1 (Giri et al., 2000, Giri et al., 2002). Therefore, soluble Aβ oligomers induce the expression of vascular adhesion molecules, which may promote leukocyte adhesion and transmigration during AD.
9. Leukocyte trafficking during AD
Leukocytes enter the CNS via three distinct routes: the first is from the blood to the parenchyma through the walls of parenchymal post-capillary venules; the second is from the blood to the subarachnoid space through the walls of meningeal vessels; and the third is from the blood to the CSF across the venule wall and then the stroma and epithelium of the choroid plexus (Ransohoff et al., 2003). Under inflammatory conditions, the first two routes are used by leukocytes for CNS invasion, whereas the last route is considered the major site of CNS immunosurveillance under physiological conditions (Ransohoff et al., 2003, Engelhardt and Ransohoff, 2005, Man et al., 2007, Shechter et al., 2013). Leukocyte extravasation across the inflamed endothelium is a multistep process mediated by adhesion molecules, chemoattractants and signaling molecules expressed on the surface of brain endothelial cells (Fig. 2). The migration cascade involves a sequence of adhesion and activation events including: 1) capture (tethering) and rolling, which are mediated by the interactions between selectins and mucins, and/or between integrins and their ligands from the Ig superfamily; 2) integrin activation induced by chemoattractants exposed by the endothelial cells through leukocyte G protein coupled receptors (GPCRs); 3) arrest mediated by activated integrins and their counter-ligands; 4) crawling mediated by leukocyte β1 or β2 integrins and their endothelial counter-ligands; and 5) paracellular or transcellular diapedesis, mainly mediated by VE-cadherin, JAMs, CD99 and PECAM-1 (Ley et al., 2007, Rossi et al., 2011, Vestweber, 2015). During extravasation into the inflamed CNS, leukocytes leaving the circulation in post-capillary venules also need to cross the endothelial basement membrane and glia limitans (Farach-Carson et al., 2014). Therefore, during extravasation, leukocytes need to express glycosidases and proteases allowing them to degrade the basement membrane molecules (Yadav et al., 2003, Vreys and David, 2007, Wang et al., 2005, Reichel et al., 2008, Li and Vlodavsky, 2009, Voisin et al., 2009, Vestweber, 2015).
Leukocyte–vascular interactions in CNS venules during inflammation are mediated predominantly by endothelial P-selectin and E-selectin and their mucin ligands PSGL-1 and TIM-1, as well as leukocyte integrins that include α4β1 (also known as very late antigen 4, VLA-4) which bind VCAM-1, and leukocyte integrins αLβ2 (LFA-1) and αMβ2 (Mac-1) which bind ICAM-1 and ICAM-2 (Ley et al., 2007, Rossi et al., 2011, Angiari et al., 2014, Angiari and Constantin, 2014, Vestweber, 2015). The β2 integrins are mainly involved in neutrophil extravasation across the inflamed BBB, whereas VLA-4 mainly contributes to T cell trafficking in the CNS (Ransohoff and Engelhardt, 2012, Gorina et al., 2014, Zenaro et al., 2015). The role of circulating immune system cells in AD-related brain damage is still unclear, but the migration of cells related to both innate and adaptive immunity has been observed in the AD brain (Pietronigro et al., 2016) (Fig. 3).
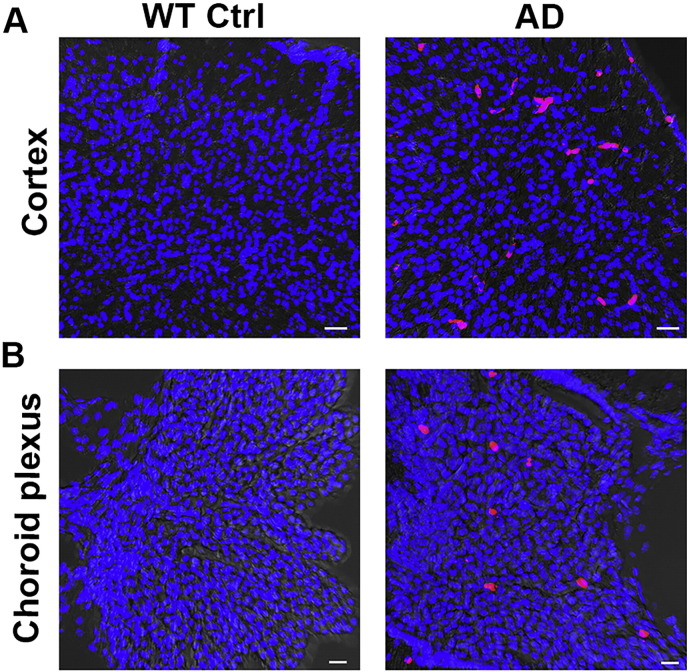
Fig. 3.
Leukocytes migrate in the brain of mice with AD-like disease. Confocal microscopy images of CD45+ leukocytes (red cells) localized in the cortex (A) and choroid plexus (B) of sex/age-matched wild-type control animals (left panels) and 3xTg-AD mice (right panels) at 6 months of age. Nuclei are stained with DAPI in blue. Scale bars = 30 μm.
9.1. Monocyte trafficking during AD
Monocytes are the most widely-studied circulating immune system cells in AD and they migrate through the BBB into the AD brain in a CCR2-dependent manner (El Khoury et al., 2007, Naert and Rivest, 2013). CCR2 is a GPCR constitutively expressed in monocytes, at high levels in the ‘pro-inflammatory’ subset, but at low or undetectable levels in ‘patrolling’ monocytes, which represent the ‘anti-inflammatory’ subset (Pimentel-Coelho et al., 2015). CCL2, the main CCR-2 ligand, is the most effective activator of the signal transduction pathway leading to monocyte transmigration and is upregulated in the microvessels of AD-like mouse brains and the postmortem brains of AD patients (Ishizuka et al., 1997, Grammas et al., 2001, Simard et al., 2006, El Khoury et al., 2007, Deshmane et al., 2009, Conductier et al., 2010). The beneficial role of monocytes in AD relies on the clearance of Aβ, thus CCR-2 deficiency in the Tg2576 and APPSwe/PS1 mouse models of AD exacerbates amyloidosis and memory deficit (El Khoury et al., 2007, Naert and Rivest, 2011). In agreement with these data, defective production of CCR2+ monocytes in APPSwe/PS1 mice is characterized by cognitive decline, accumulation of soluble Aβ and disruption of synaptic activity (Naert and Rivest, 2012, Naert and Rivest, 2013). Furthermore, intravital two-photon microscopy studies indicate that patrolling CCR2− monocytes are attracted to and crawl onto the luminal walls of Aβ+ veins and that their selective removal in APP/PS1 mice significantly increases the Aβ load in the cortex and hippocampus, suggesting that patrolling monocytes can naturally target and eliminate Aβ within the lumen of veins (Michaud et al., 2013). Aβ1-42 is an activating and chemotactic factor for monocytes that induces the transmigration of human monocytes in an in vitro BBB model (Fiala et al., 1998). Furthermore, cultured human peripheral monocytes stimulated with Aβ1-42 secrete pro-inflammatory cytokines including TNFα, IL-6, IL-1β and IL-12, as well as MCP-1, MIP-lα, MIP-1β, and IL-8, suggesting that they may have a detrimental role in AD (Fiala et al., 1998). The three-dimensional reconstruction of spatial relationships between vessels, Aβ deposits, and cells of the monocyte/microglial lineage in Tg2576 mice suggested that monocytes/macrophages recruited from the blood play a role in fibrillar Aβ deposition (Wegiel et al., 2004). Despite the evidence discussed above, two studies have recently challenged the view that circulating monocytes help to clear Aβ in AD models. The replacement of brain-resident myeloid cells with circulating peripheral monocytes in mouse models of cerebral β-amyloidosis showed that monocyte repopulation does not modify the amyloid load, arguing against a long-term role for peripheral monocytes in Aβ clearance (Prokop et al., 2015, Varvel et al., 2015).
9.2. Neutrophil migration in the AD brain
Neutrophils are highly reactive cells that release ROS, enzymes, NETs and cytokines, and can thus cause long-term collateral tissue damage even if they do not accumulate substantially within tissues during chronic sterile inflammation. Indeed, previous studies (including our own) have shown that neutrophils do not need to accumulate in high numbers in order to induce tissue damage, and that adhesion on the vessel wall without transmigration is sufficient to induce endothelial injury (Zarbock and Ley, 2008, Fabene et al., 2008, DiStasi and Ley, 2009).
Our recent data show that neutrophils play a role in the induction of neuropathological changes and memory deficit in 5xFAD and 3xTg-AD mice (Zenaro et al., 2015). We found that neutrophils adhered inside cerebral vessels or migrated in the parenchyma in higher numbers in the AD models (at the onset of memory deficit) compared to wild-type controls, suggesting these cells may play a role in AD pathogenesis (Fig. 2). Extravasated neutrophils produced NETs and IL-17, suggesting they may harm the BBB and neural cells (Zenaro et al., 2015). Two-photon laser-scanning microscopy revealed that neutrophils adhere and crawl inside blood vessels and migrate into the parenchyma in areas with Aβ deposits, further supporting a role for neutrophils in AD (Fig. 2) (Zenaro et al., 2015). Accordingly, other recent imaging studies have shown that Gr1+ cells extravasate through the BBB in the brain parenchyma of 5xFAD mice and migrate towards Aβ plaques, suggesting a role for Aβ in neutrophil recruitment during AD (Baik et al., 2014). Furthermore, we found that soluble oligomeric Aβ1-42 triggers the rapid, integrin-dependent adhesion of human and mouse neutrophils on fibrinogen and ICAM-1, which are both integrin ligands (Fig. 2). Notably, Aβ1-42 also triggers the LFA-1 integrin high-affinity state in human neutrophils, providing an explanation for the tendency of neutrophils to arrest in areas with Aβ deposits in vivo. LFA-1 integrin is necessary for neutrophil adhesion in brain vessels, and neutrophils from LFA-1 deficient mice are unable to adhere or crawl in blood vessels and thus cannot transmigrate in the brain parenchyma of AD mice. Accordingly, blocking LFA-1 integrin and neutrophil adhesion has a therapeutic effect on mouse models of AD, suggesting a prominent role for LFA-1 integrin in neutrophil trafficking and AD pathogenesis (Zenaro et al., 2015).
Neutrophils also adhere intravascularly in the brains of AD patients and migrate near areas with Aβ deposits suggesting that Aβ influences the microenvironmental positioning of neutrophils inside the AD brain (Savage et al., 1994, Zenaro et al., 2015). As shown in transgenic mice with AD-like disease, they produce intravascular NETs, which may be harmful to the endothelial cells, suggesting these cells may promote the loss of BBB integrity and thus the pathogenesis of AD in humans (Fig. 2) (Zenaro et al., 2015).
9.3. Lymphocyte accumulation in the AD brain
Lymphocytes can also cross the BBB during AD. Both CD4+ and CD8+ T cells were observed adhering to the vascular endothelium, or migrating into the parenchyma, of AD patients (Itagaki et al., 1988, Rogers et al., 1988, Togo et al., 2002, Town et al., 2005). The number of these cells was higher in AD patients than in healthy, age-matched controls, with the majority of the T cells infiltrating the hippocampus and other limbic structures, which are the most severely affected by AD (Itagaki et al., 1988, Rogers et al., 1988, Togo et al., 2002, Town et al., 2005). Furthermore, MCI and mild AD patients contained a greater number of activated CD4+ and CD8+ T cells in the CSF, with the proportion of activated CD8+ T cells showing the highest increase, supporting the hypothesis that activated T cells migrate from the blood into the brain during AD (Lueg et al., 2015).
Similarly, T cells also infiltrate into the brains of APP/PS1 mice, and may secrete interferon gamma (IFNγ) or IL-17, suggesting that the release of these cytokines could accelerate AD neuropathology (Browne et al., 2013). The infection of APP/PS1 mice with the Gram-negative respiratory pathogen Bordetella pertussis promoted infiltration by T cells and natural killer cells producing IFNγ and IL-17, accompanied by increased glial activation and Aβ deposition. This suggests that peripheral inflammation may favor the entry of circulating activated T cells into the brain, which may in turn exacerbate AD pathology (McManus et al., 2014). Brain amyloidosis promotes T cell migration into the brain and the expression of vascular adhesion molecules in brain vessels (Ferretti et al., 2016). Cells of the adaptive immune system play a negative role during AD, and APP/PS1 transgenic mice crossbred with recombination activating gene 2 (RAG2) knockout mice lacking functional B and T cells are characterized by limited brain Aβ pathology, enhanced microgliosis and the more efficient phagocytosis of Aβ peptide aggregates (Späni et al., 2015). Together, these data suggest that activated T cells play a detrimental role in AD pathology and that interfering with activated T cell development or activity may have a beneficial impact. Accordingly, recent studies suggest that regulatory T (Treg) cells, which suppress adaptive T cell responses and T cell activation, play a beneficial role in AD models during the early phase of the disease, by slowing disease progression and modulating microglial responses to Aβ deposition. (Dansokho et al., 2016). However, Treg cells play a negative role in old 5xFAD mice (Baruch et al., 2015), and the systemic inhibition of Treg cell function for one week was sufficient to significantly reduce Aβ accumulation in these mice during the later stages of AD characterized by robust cerebral Aβ plaque pathology. The role of T cell populations at different AD stages therefore remains to be determined, and future studies should aim to clarify the role of these cells in AD pathology.
The mechanisms controlling T cell recruitment in the AD brain are not well understood, but transforming growth factor β (TGF-β), endothelial activation and Aβ deposition may each play a role. The overexpression of TGF-β1 in the brains of wild-type mice or transgenic AD-like mice promotes T cell infiltration in the meninges and parenchyma after immunization with Aβ1-42 (Buckwalter et al., 2006). Moreover, in vitro studies suggest that Aβ1-42 induces the release of TNFα by microglial cells, which in turn promotes major histocompatibility complex I expression on the brain endothelium followed by the transendothelial migration of T cells (Yang et al., 2013). Accordingly, T cells together with macrophages and monocytes were observed in leptomeningeal and cortical vessels, associated with CAA, suggesting that Aβ and CAA favor T cell migration into the AD brain (Yamada et al., 1996). In agreement with these results, the injection of Aβ into the rat hippocampus activates endothelial cells through the engagement of RAGE and induces CCR5, which may in turn promote T cell migration into the brain (Li et al., 2009). Additionally, peripheral T cells in AD patients overexpress MIP-1α, which may bind to CCR5 on the surface of brain endothelial cells, potentially supporting T cell migration through endothelial tight junctions into the CNS (Man et al., 2007). MIP-1α is one the most common chemokines expressed during CNS inflammation (Xia and Hyman, 1999, Mennicken et al., 1999) and CCR5 is expressed on brain endothelial cells in aged AD patients (Xia et al., 2000, Mo et al., 2003). Notably, MIP-1α production by activated microglia promotes T cell migration in the CNS in EAE models, and we suggest this may also be the case in AD (Karpus et al., 1995). Furthermore, circulating T cells in AD patients also overexpress CXCR2, a chemokine receptor that is widely expressed on immune system cells that may promote their transendothelial migration (Liu et al., 2010). The inflammatory response stimulated by T cells that have migrated into the AD brain may activate microglia and astrocytes and may recruit other inflammatory cells that are potentially harmful to the CNS, thus exacerbating the pathogenesis of AD.
10. Concluding remarks
BBB dysfunction during AD influences Aβ clearance and endothelial transport, impairs endothelial cell and pericyte functions, affects TJ integrity, activates glial cells and facilitates the recruitment of leukocytes in the brain. However, the mechanisms that regulate BBB breakdown in the context of chronic neuroinflammation during AD still remain to be elucidated. The role of circulating immune system cells in AD-related brain damage is poorly understood, and future studies are needed to determine how specific populations of cells representing the innate and adaptive immune systems promote the cognitive deficit and neuropathological changes in AD. In this sense, advanced imaging techniques will help to provide insights into the mechanisms controlling leukocyte–endothelial interactions in AD, and may lead to the development of novel therapeutic strategies based on the inhibition of integrins, selectins, mucins and other adhesion molecules, as well as chemoattractants to delay the progression of the disease. Plasmatic adhesion molecule ectodomains may offer valuable biomarkers of neuroinflammation and endothelial dysfunction, and more studies are needed to correlate circulating soluble adhesion molecules with BBB integrity and the severity of AD.
Acknowledgements
This work was supported in part by the European Research Council grants 695714 IMMUNOALZHEIMER, 261079 NEUROTRAFFICKING and 693606 IMPEDE; American Drug Discovery Foundation (ADDF), USA; National Multiple Sclerosis Society (NMSS), New York, NY, USA; Fondazione Cariverona; Italian Ministry of Health grant GR2009; Italian Ministry of Education and Research (MIUR) and by Fondazione Italiana Sclerosi Multipla (FISM) (to GC).
References
- Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G., Sorokin L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C., Thornton P., Denes A., McColl B.W., Pierozynski A., Monestier M., Pinteaux E., Rothwell N.J., Allan S.M. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J. Immunol. 2012;10:381–392. doi: 10.4049/jimmunol.1200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J.I., Cayrol R., Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Angiari S., Constantin G. Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein. Trends Mol. Med. 2014;20:675–684. doi: 10.1016/j.molmed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Angiari S., Donnarumma T., Rossi B., Dusi S., Pietronigro E., Zenaro E., Della Bianca V., Toffali L., Piacentino G., Budui S., Rennert P., Xiao S., Laudanna C., Casasnovas J.M., Kuchroo V.K., Constantin G. TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity. Immunity. 2014;40:542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik S.H., Cha M.Y., Hyun Y.M., Cho H., Hamza B., Kim D.K., Han S.H., Choi H., Kim K.H., Moon M., Lee J., Kim M., Irimia D., Mook-Jung I. Migration of neutrophils targeting amyloid plaques in Alzheimer's disease mouse model. Neurobiol. Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichello T., Generoso J.S., Michelon C.M., Simões L.R., Elias S.G., Vuolo F., Comim C.M., Dal-Pizzol F., Quevedo J. Inhibition of matrix metalloproteinases-2 and -9 prevents cognitive impairment induced by pneumococcal meningitis in Wistar rats. Exp. Biol. Med. 2014;239:225–231. doi: 10.1177/1535370213508354. [DOI] [PubMed] [Google Scholar]
- Baruch K., Rosenzweig N., Kertser A., Deczkowska A., Sharif A.M., Spinrad A., Tsitsou-Kampeli A., Sarel A., Cahalon L., Schwartz M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat. Commun. 2015;6:7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H.C., Traweger A., Zweimueller-Mayer J., Lehner C., Tempfer H., Krizbai I., Wilhelm I., Bauer H. New aspects of the molecular constituents of tissue barriers. J. Neural Transm. 2011;118:7–21. doi: 10.1007/s00702-010-0484-6. [DOI] [PubMed] [Google Scholar]
- Bechmann I., Galea I., Perry V.H. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bednarczyk J., Lukasiuk K. Tight junctions in neurological diseases. Acta Neurobiol. Exp. 2011;71:393–408. doi: 10.55782/ane-2011-1861. [DOI] [PubMed] [Google Scholar]
- Bell R.D., Zlokovic B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Deane R., Chow N., Long X., Sagare A., Singh I., Streb J.W., Guo H., Rubio A., Van Nostrand W., Miano J.M., Zlokovic B.V. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat. Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., Zlokovic B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., Zlokovic B.V. Apolipoprotein E controls cerebrovascular integrity via cyclophilin a. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A.L., Goldstein G.W. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978;202:225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- Blair L.J., Frauen H.D., Zhang B., Nordhues B.A., Bijan S., Lin Y.C., Zamudio F., Hernandez L.D., Sabbagh J.J., Selenica M.L., Dickey C.A. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 2015;3:8. doi: 10.1186/s40478-015-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M.L. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9(Suppl. 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bö L., Peterson J.W., Mørk S., Hoffman P.A., Gallatin W.M., Ransohoff R.M., Trapp B.D. Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 1996;55:1060–1072. [PubMed] [Google Scholar]
- Brightman M.W., Reese T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne T.C., McQuillan K., McManus R.M., O'Reilly J.A., Mills K.H., Lynch M.A. IFN-γ production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J. Immunol. 2013;190:2241–2251. doi: 10.4049/jimmunol.1200947. [DOI] [PubMed] [Google Scholar]
- Buckwalter M.S., Coleman B.S., Buttini M., Barbour R., Schenk D., Games D., Seubert P., Wyss-Coray T. Increased T cell recruitment to the CNS after amyloid beta 1-42 immunization in Alzheimer's mice overproducing transforming growth factor-beta 1. J. Neurosci. 2006;26:11437–11441. doi: 10.1523/JNEUROSCI.2436-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B., Landreth G.E. Inflammation, microglia, and Alzheimer's disease. Neurobiol. Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T.M., Harlan J.M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Carrano A., Hoozemans J.J., van der Vies S.M., Rozemuller A.J., van Horssen J., de Vries H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal. 2011;15:1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- Carrano A., Hoozemans J.J., van der Vies S.M., de Vries H.E., van Horssen J., Rozemuller A.J. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener. Dis. 2012;10:329–3231. doi: 10.1159/000334916. [DOI] [PubMed] [Google Scholar]
- Cartier N., Lewis C.A., Zhang R., Rossi F.M. The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol. 2014;128:363–380. doi: 10.1007/s00401-014-1330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli R.J., Beach T.G., Yaari R., Reiman E.M. Alzheimer's disease a century later. J. Clin. Psychiatry. 2006;67:1784–1800. doi: 10.4088/jcp.v67n1118. [DOI] [PubMed] [Google Scholar]
- Chao A.C., Lee T.C., Juo S.H., Yang D.I. Hyperglycemia increases the production of amyloid beta-peptide leading to decreased endothelial tight junction. CNS Neurosci. Ther. 2016;22:291–297. doi: 10.1111/cns.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Choi S.S., Lee H.J., Lim I., Satoh J., Kim S.U. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B.W., Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N., Bell R.D., Deane R., Streb J.W., Chen J., Brooks A., Van Nostrand W., Miano J.M., Zlokovic B.V. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc. Natl. Acad. Sci. U. S. A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G., Blondeau N., Guyon A., Nahon J.L., Rovère C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Corada M., Mariotti M., Thurston G., Smith K., Kunkel R., Brockhaus M., Lampugnani M.G., Martin-Padura I., Stoppacciaro A., Ruco L., McDonald D.M., Ward P.A., Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M., Paul J., Norris E.H., Bronstein R., Ahn H.J., Zamolodchikov D., Bhuvanendran S., Fenz K.M., Strickland S. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66:695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming J.L. Alzheimer's disease. N. Engl. J. Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- da Fonseca A.C., Matias D., Garcia C., Amaral R., Geraldo L.H., Freitas C., Lima F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansokho C., Ait Ahmed D., Aid S., Toly-Ndour C., Chaigneau T., Calle V., Cagnard N., Holzenberger M., Piaggio E., Aucouturier P., Dorothée G. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–1251. doi: 10.1093/brain/awv408. (Apr) [DOI] [PubMed] [Google Scholar]
- Davalos D., Ryu J.K., Merlini M., Baeten K.M., Le Moan N., Petersen M.A., Deerinck T.J., Smirnoff D.S., Bedard C., Hakozaki H., Gonias Murray S., Ling J.B., Lassmann H., Degen J.L., Ellisman M.H., Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J.C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- Deane R., Zlokovic B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- Deane R., Wu Z., Zlokovic B.V. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- Deane R., Singh I., Sagare A.P., Bell R.D., Ross N.T., LaRue B., Love R., Perry S., Paquette N., Deane R.J., Thiyagarajan M., Zarcone T., Fritz G., Friedman A.E., Miller B.L., Zlokovic B.V. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maschio A., De Luigi A., Martin-Padura I., Brockhaus M., Bartfai T., Fruscella P., Adorini L., Martino G., Furlan R., De Simoni M.G., Dejana E. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J. Exp. Med. 1999;190:1351–1356. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeule M., Labelle M., Régina A., Berthelet F., Béliveau R. Isolation of endothelial cells from brain, lung, and kidney: expression of the multidrug resistance P-glycoprotein isoforms. Biochem. Biophys. Res. Commun. 2001;281:827–834. doi: 10.1006/bbrc.2001.4312. [DOI] [PubMed] [Google Scholar]
- Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interf. Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devraj K., Poznanovic S., Spahn C., Schwall G., Harter P.N., Mittelbronn M., Antoniello K., Paganetti P., Muhs A., Heilemann M., Hawkins R.A., Schrattenholz A., Liebner S. BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease. J. Cereb. Blood Flow Metab. 2015 doi: 10.1177/0271678X15606463. (pii: 0271678X15606463. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStasi M.R., Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue J.E., Flaherty S.L., Johanson C.E., Duncan J., 3rd., Silverberg G.D., Miller M.C., Tavares R., Yang W., Wu Q., Sabo E., Hovanesian V., Stopa E.G. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Du H., Li P., Wang J., Qing X., Li W. The interaction of amyloid β and the receptor for advanced glycation endproducts induces matrix metalloproteinase-2 expression in brain endothelial cells. Cell. Mol. Neurobiol. 2012;32:141–147. doi: 10.1007/s10571-011-9744-8. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., Luster A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- ElAli A., Thériault P., Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int. J. Mol. Sci. 2014;15:6453–6574. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B., Ransohoff R.M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. (Nov) [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J. Cereb. Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabene P.F., Navarro Mora G., Martinello M., Rossi B., Merigo F., Ottoboni L., Bach S., Angiari S., Benati D., Chakir A., Zanetti L., Schio F., Osculati A., Marzola P., Nicolato E., Homeister J.W., Xia L., Lowe J.B., McEver R.P., Osculati F., Sbarbati A., Butcher E.C., Constantin G. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A.S., Jameson B.J., Jesaitis L.A., Anderson J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Farach-Carson M.C., Warren C.R., Harrington D.A., Carson D.D. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E., Luiten P.G. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Farrall A.J., Wardlaw J.M. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Feng Y., Wang X. Antioxidant therapies for Alzheimer's disease. Oxidative Med. Cell. Longev. 2012;2012:472932. doi: 10.1155/2012/472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti M.T., Merlini M., Späni C., Gericke C., Schweizer N., Enzmann G., Engelhardt B., Kulic L., Suter T., Nitsch R.M. T-cell brain infiltration and immature antigen-presenting cells in transgenic models of Alzheimer's disease-like cerebral amyloidosis. Brain Behav. Immun. 2016;54:30030–30070. doi: 10.1016/j.bbi.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Fiala M., Zhang L., Gan X., Sherry B., Taub D., Graves M.C., Hama S., Way D., Weinand M., Witte M., Lorton D., Kuo Y.M., Roher A.E. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood–brain barrier model. Mol. Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- Forman M.S., Lal D., Zhang B., Dabir D.V., Swanson E., Lee V.M., Trojanowski J.Q. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 2005;25:3539–3550. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R.S., Ushio-Fukai M., Malik A.B. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid. Redox Signal. 2009;4:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman E.M., Frohman T.C., Gupt S., de Fougerolles A., van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer's disease. J. Neurol. Sci. 1991;106:105–111. doi: 10.1016/0022-510x(91)90202-i. [DOI] [PubMed] [Google Scholar]
- Fu R., Shen Q., Xu P., Luo J.J., Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014;49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D., Schoonenboom N., Scheltens P., Fenoglio C., Bouwman F., Venturelli E., Guidi I., Blankenstein M.A., Bresolin N., Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Galimberti D., Fenoglio C., Scarpini E. Inflammation in neurodegenerative disorders: friend or foe? Curr. Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- Garrido-Urbani S., Bradfield P.F., Imhof B.A. Tight junction dynamics: the role of junctional adhesion molecules (JAMs) Cell Tissue Res. 2014;355:701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- Garton K.J., Gough P.J., Raines E.W. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukoc. Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- Giri R., Shen Y., Stins M., Du Yan S., Schmidt A.M., Stern D., Kim K.S., Zlokovic B., Kalra V.K. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 2000;279:C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- Giri R., Selvaraj S., Miller C.A., Hofman F., Yan S.D., Stern D., Zlokovic B.V., Kalra V.K. Effect of endothelial cell polarity on beta-amyloid-induced migration of monocytes across normal and AD endothelium. Am. J. Physiol. Cell Physiol. 2002;283:C895–C904. doi: 10.1152/ajpcell.00293.2001. [DOI] [PubMed] [Google Scholar]
- Gonçalves A., Ambrósio A.F., Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers. 2013;1 doi: 10.4161/tisb.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R., Lyck R., Vestweber D., Engelhardt B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 2014;192:324–337. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- Gotsch U., Borges E., Bosse R., Böggemeyer E., Simon M., Mossmann H., Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- Graesser D., Mahooti S., Haas T., Davis S., Clark R.B., Madri J.A. The interrelationship of alpha4 integrin and matrix metalloproteinase-2 in the pathogenesis of experimental autoimmune encephalomyelitis. Lab. Investig. 1998;78:1445–1458. [PubMed] [Google Scholar]
- Grammas P., Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol. Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Grammas P., Martinez J., Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev. Mol. Med. 2011;13 doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Brew B.J. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Guo Y.X., He L.Y., Zhang M., Wang F., Liu F., Peng W.X. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. doi: 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- Gurney K.J., Estrada E.Y., Rosenberg G.A. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Halliday M.R., Rege S.V., Ma Q., Zhao Z., Miller C.A., Winkler E.A., Zlokovic B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J. Cereb. Blood Flow Metab. 2016;36:216–227. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Hanisch U.K. Functional diversity of microglia - how heterogeneous are they to begin with? Front. Cell. Neurosci. 2013;7:65. doi: 10.3389/fncel.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry G.J. Microglia during development and aging. Pharmacol. Ther. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C.A., McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J. Neurosci. 2008;28:8354–8560. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Rom S., Ramirez S.H., Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J. NeuroImmune Pharmacol. 2014;9:591–605. doi: 10.1007/s11481-014-9557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans C.R., Graven C., Dederen P.J., Tanila H., van Groen T., Kiliaan A.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res. 2007;1181:93–103. doi: 10.1016/j.brainres.2007.08.063. [DOI] [PubMed] [Google Scholar]
- Hoshi A., Yamamoto T., Shimizu K., Ugawa Y., Nishizawa M., Takahashi H., Kakita A. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012;71:750–759. doi: 10.1097/NEN.0b013e3182632566. [DOI] [PubMed] [Google Scholar]
- Hu Q., Chen C., Yan J., Yang X., Shi X., Zhao J., Lei J., Yang L., Wang K., Chen L., Huang H., Han J., Zhang J.H., Zhou C. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp. Neurol. 2009;216:35–46. doi: 10.1016/j.expneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Huang C.W., Tsai M.H., Chen N.C., Chen W.H., Lu Y.T., Lui C.C., Chang Y.T., Chang W.N., Chang A.Y., Chang C.C. Clinical significance of circulating vascular cell adhesion molecule-1 to white matter disintegrity in Alzheimer's dementia. Thromb. Haemost. 2015;114:1230–1240. doi: 10.1160/TH14-11-0938. [DOI] [PubMed] [Google Scholar]
- Hudson L.C., Bragg D.C., Tompkins M.B., Meeker R.B. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res. 2005;1058:148–160. doi: 10.1016/j.brainres.2005.07.071. [DOI] [PubMed] [Google Scholar]
- Hunt A., Schönknecht P., Henze M., Seidl U., Haberkorn U., Schröder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Res. 2007;155:147–154. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin. Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P.L., Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer's disease brain tissue. Neurosci. Lett. 1988;91:259–264. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- Jander S., Pohl J., Gillen C., Schroeter M., Stoll G. Vascular cell adhesion molecule-1 mRNA is expressed in immune-mediated and ischemic injury of the rat nervous system. J. Neuroimmunol. 1996;70:75–80. doi: 10.1016/s0165-5728(96)00109-9. [DOI] [PubMed] [Google Scholar]
- Järemo P., Milovanovic M., Buller C., Nilsson S., Winblad B. P-selectin paradox and dementia of the Alzheimer type: circulating P-selectin is increased but platelet-bound P-selectin after agonist provocation is compromised. Scand. J. Clin. Lab. Invest. 2013;73:170–174. doi: 10.3109/00365513.2013.764572. [DOI] [PubMed] [Google Scholar]
- Jellinger K.A. Alzheimer disease and cerebrovascular pathology: an update. J. Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- Jolivel V., Bicker F., Binamé F., Ploen R., Keller S., Gollan R., Jurek B., Birkenstock J., Poisa-Beiro L., Bruttger J., Opitz V., Thal S.C., Waisman A., Bäuerle T., Schäfer M.K., Zipp F., Schmidt M.H. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129:279–295. doi: 10.1007/s00401-014-1372-1. [DOI] [PubMed] [Google Scholar]
- Kalaria R.N. The role of cerebral ischemia in Alzheimer's disease. Neurobiol. Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kalaria R.N., Harik S.I. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J. Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R.N., Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer's disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- Kang Z., Altuntas C.Z., Gulen M.F., Liu C., Giltiay N., Qin H., Liu L., Qian W., Ransohoff R.M., Bergmann C., Stohlman S., Tuohy V.K., Li X. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus W.J., Lukacs N.W., McRae B.L., Strieter R.M., Kunkel S.L., Miller S.D. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kivisäkk P., Mahad D.J., Callahan M.K., Trebst C., Tucky B., Wei T., Wu L., Baekkevold E.S., Lassmann H., Staugaitis S.M., Campbell J.J., Ransohoff R.M. Human cerebrospinal fluid central memory CD4 + T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S.Y., Hong H.S., Moon M., Ha C.M., Chang S., Mook-Jung I. Aβ₁₋₄₂-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca2 ⁺-calcineurin signaling. J. Neurosci. 2012;32:8845–8854. doi: 10.1523/JNEUROSCI.6102-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac A., Zilkova M., Deli M.A., Zilka N., Novak M. Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J. Alzheimers Dis. 2009;18:897–906. doi: 10.3233/JAD-2009-1197. [DOI] [PubMed] [Google Scholar]
- Krabbe G., Halle A., Matyash V., Rinnenthal J.L., Eom G.D., Bernhardt U., Miller K.R., Prokop S., Kettenmann H., Heppner F.L. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke D., Jedlitschky G., Grube M., Krohn M., Jucker M., Mosyagin I., Cascorbi I., Walker L.C., Kroemer H.K., Warzok R.W., Vogelgesang S. MDR1-P-glycoprotein (ABCB1) mediates transport of Alzheimer's amyloid-beta peptides–implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F.C., Liu R., Lu P., Shapiro A.B., Renoir J.M., Sharom F.J., Reiner P.B. Beta-amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Lécuyer M.A., Kebir H., Prat A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta. 2016;1862:472–482. doi: 10.1016/j.bbadis.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Lepelletier F.X., Mann D.M., Robinson A.C., Pinteaux E., Boutin H. Early changes in extracellular matrix in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2015 doi: 10.1111/nan.12295. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li J.-P., Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb. Haemost. 2009;102:823–828. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- Li M., Shang D.S., Zhao W.D., Tian L., Li B., Fang W.G., Zhu L., Man S.M., Chen Y.H. Amyloid beta interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood-brain barrier. J. Immunol. 2009;182:5778–5788. doi: 10.4049/jimmunol.0803013. [DOI] [PubMed] [Google Scholar]
- Li G., Xiong K., Korff A., Pan C., Quinn J.F., Galasko D.R., Liu C., Montine T.J., Peskind E.R., Zhang J. Increased CSF E-selectin in clinical Alzheimer's disease without altered CSF Aβ42 and tau. J. Alzheimers Dis. 2015;47:883–887. doi: 10.3233/JAD-150420. [DOI] [PubMed] [Google Scholar]
- Liebner S., Fischmann A., Rascher G., Duffner F., Grote E.H., Kalbacher H., Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Guo D.W., Tian L., Shang D.S., Zhao W.D., Li B., Fang W.G., Zhu L., Chen Y.H. Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging. 2010;31:175–188. doi: 10.1016/j.neurobiolaging.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Lo S.K., Lee S., Ramos R.A., Lobb R., Rosa M., Chi-Rosso G., Wright S.D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J. Exp. Med. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J., Gupta P., Guo S., Kim W.J., Whalen M.J., van Leyen K., Lo E.H. Cell-cell signaling in the neurovascular unit. Neurochem. Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Löscher W., Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lueg G., Gross C.C., Lohmann H., Johnen A., Kemmling A., Deppe M., Groger J., Minnerup J., Wiendl H., Meuth S.G., Duning T. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer's disease. Neurobiol. Aging. 2015;36:81–89. doi: 10.1016/j.neurobiolaging.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Malm T., Koistinaho M., Muona A., Magga J., Koistinaho J. The role and therapeutic potential of monocytic cells in Alzheimer's disease. Glia. 2010;58:889–900. doi: 10.1002/glia.20973. [DOI] [PubMed] [Google Scholar]
- Man S.M., Ma Y.R., Shang D.S., Zhao W.D., Li B., Guo D.W., Fang W.G., Zhu L., Chen Y.H. Peripheral T cells overexpress MIP-1alpha to enhance its transendothelial migration in Alzheimer's disease. Neurobiol. Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Marco S., Skaper S.D. Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci. Lett. 2006;401:219–224. doi: 10.1016/j.neulet.2006.03.047. [DOI] [PubMed] [Google Scholar]
- McManus R.M., Higgins S.C., Mills K.H., Lynch M.A. Respiratory infection promotes T cell infiltration and amyloid-β deposition in APP/PS1 mice. Neurobiol. Aging. 2014;35:109–121. doi: 10.1016/j.neurobiolaging.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Mennicken F., Maki R., de Souza E.B., Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol. Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- Merlini M., Meyer E.P., Ulmann-Schuler A., Nitsch R.M. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol. 2011;122:293–311. doi: 10.1007/s00401-011-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J.P., Bellavance M.A., Préfontaine P., Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013;5:646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Mo R., Chen J., Han Y., Bueno-Cannizares C., Misek D.E., Lescure P.A., Hanash S., Yung R.L. T cell chemokine receptor expression in aging. J. Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., Harrington M.G., Chui H.C., Law M., Zlokovic B.V. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian A.D., Chung H.C., Shah G.N. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol. Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Morita K., Sasaki H., Furuse M., Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999;147:1185–1194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L., Tsui W.H., Herholz K., Pupi A., Drzezga A., Lucignani G., Reiman E.M., Holthoff V., Kalbe E., Sorbi S., Diehl-Schmid J., Perneczky R., Clerici F., Caselli R., Beuthien-Baumann B., Kurz A., Minoshima S., de Leon M.J. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J. Nucl. Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Muradashvili N., Tyagi R., Metreveli N., Tyagi S.C., Lominadze D. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J. Cereb. Blood Flow Metab. 2014;34:1472–1482. doi: 10.1038/jcbfm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G., Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G., Rivest S. Age-related changes in synaptic markers and monocyte subsets link the cognitive decline of APP(Swe)/PS1 mice. Front. Cell. Neurosci. 2012;6:51. doi: 10.3389/fncel.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G., Rivest S. A deficiency in CCR2 + monocytes: the hidden side of Alzheimer's disease. J. Mol. Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- Naik P., Fofaria N., Prasad S., Sajja R.K., Weksler B., Couraud P.O., Romero I.A., Cucullo L. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E., Abbott N.J., Abrey L., Banks W.A., Blakley B., Davis T., Engelhardt B., Grammas P., Nedergaard M., Nutt J., Pardridge W., Rosenberg G.A., Smith Q., Drewes L.R. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Newman W., Beall L.D., Carson C.W., Hunder G.G., Graben N., Randhawa Z.I., Gopal T.V., Wiener-Kronish J., Matthay M.A. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J. Immunol. 1993;150:644–654. [PubMed] [Google Scholar]
- Nielsen H.M., Londos E., Minthon L., Janciauskiene S.M. Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol. Dis. 2007;26:27–35. doi: 10.1016/j.nbd.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., Furuse M., Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble L.J., Donovan F., Igarashi T., Goussev S., Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Van den Steen P.E., Dubois B., Nelissen I., Van Coillie E., Masure S., Proost P., Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Ostermann G., Weber K.S., Zernecke A., Schröder A., Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J.R., Renkin E.M., Borrero L.M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am. J. Phys. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Park L., Anrather J., Zhou P., Frys K., Pitstick R., Younkin S., Carlson G.A., Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L., Kook S.Y., Park J.C., Mook-Jung I. Aβ1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-κB signaling. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J. Exp. Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y., Ghorpade A., Rasmussen J., Limoges J., Liu X.J., Stins M., Fiala M., Way D., Kim K.S., Witte M.H., Weinand M., Carhart L., Gendelman H.E. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y., Ramirez S.H., Haorah J., Kanmogne G.D. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J. NeuroImmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Petty M.A., Lo E.H. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog. Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Pflanzner T., Kuhlmann C.R., Pietrzik C.U. Blood-brain-barrier models for the investigation of transporter- and receptor-mediated amyloid-β clearance in Alzheimer's disease. Curr. Alzheimer Res. 2010;7:578–590. doi: 10.2174/156720510793499066. [DOI] [PubMed] [Google Scholar]
- Pietronigro E., Zenaro E., Constantin G. Imaging of leukocyte trafficking in Alzheimer's disease. Front. Immunol. 2016;7:33. doi: 10.3389/fimmu.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel-Coelho P.M., Michaud J.P., Rivest S. C-C chemokine receptor type 2 (CCR2) signaling protects neonatal male mice with hypoxic-ischemic hippocampal damage from developing spatial learning deficits. Behav. Brain Res. 2015;286:146–151. doi: 10.1016/j.bbr.2015.02.053. [DOI] [PubMed] [Google Scholar]
- Proebstl D., Voisin M.B., Woodfin A., Whiteford J., D'Acquisto F., Jones G.E., Rowe D., Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S., Miller K.R., Drost N., Handrick S., Mathur V., Luo J., Wegner A., Wyss-Coray T., Heppner F.L. Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer's disease-like mice. J. Exp. Med. 2015;212:1811–1818. doi: 10.1084/jem.20150479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L.B., Zhou Y., Wang Q., Yang L.L., Liu H.Q., Xu S.L., Qi Y.H., Ding G.R., Guo G.Z. Synthetic gelatinases inhibitor attenuates electromagnetic pulse-induced blood-brain barrier disruption by inhibiting gelatinases-mediated ZO-1 degradation in rats. Toxicology. 2011;285:31–38. doi: 10.1016/j.tox.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Quan N., Banks W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Querfurth H.W., LaFerla F.M. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Kivisäkk P., Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Reichel C.A., Rehberg M., Bihari P., Moser C.M., Linder S., Khandoga A., Krombach F. Gelatinases mediate neutrophil recruitment in vivo: evidence for stimulus specificity and a critical role in collagen IV remodeling. J. Leukoc. Biol. 2008;83:864–874. doi: 10.1189/jlb.1007666. [DOI] [PubMed] [Google Scholar]
- Rentzos M., Michalopoulou M., Nikolaou C., Cambouri C., Rombos A., Dimitrakopoulos A., Vassilopoulos D. The role of soluble intercellular adhesion molecules in neurodegenerative disorders. J. Neurol. Sci. 2005;228:129–135. doi: 10.1016/j.jns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Roebuck K.A., Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Rogers J., Luber-Narod J., Styren S.D., Civin W.H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol. Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Roher A.E., Kuo Y.M., Esh C., Knebel C., Weiss N., Kalback W., Luehrs D.C., Childress J.L., Beach T.G., Weller R.O., Kokjohn T.A. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol. Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- Rossi B., Angiari S., Zenaro E., Budui S.L., Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J. Leukoc. Biol. 2011;89:539–556. doi: 10.1189/jlb.0710432. [DOI] [PubMed] [Google Scholar]
- Ryu J.K., McLarnon J.G. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J. Cell. Mol. Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A.P., Bell R.D., Zlokovic B.V. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A.P., Bell R.D., Zhao Z., Ma Q., Winkler E.A., Ramanathan A., Zlokovic B.V. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Samuraki M., Matsunari I., Chen W.P., Yajima K., Yanase D., Fujikawa A., Takeda N., Nishimura S., Matsuda H., Yamada M. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1658–1669. doi: 10.1007/s00259-007-0454-x. [DOI] [PubMed] [Google Scholar]
- Santoso S., Sachs U.J., Kroll H., Linder M., Ruf A., Preissner K.T., Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N.R., Daneman R., Dziegielewska K.M., Liddelow S.A. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol. Asp. Med. 2013;34:742–752. doi: 10.1016/j.mam.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Savage M.J., Iqbal M., Loh T., Trusko S.P., Scott R., Siman R. Cathepsin G: localization in human cerebral cortex and generation of amyloidogenic fragments from the beta-amyloid precursor protein. Neuroscience. 1994;60:607–619. doi: 10.1016/0306-4522(94)90490-1. [DOI] [PubMed] [Google Scholar]
- Savettieri G., Di Liegro I., Catania C., Licata L., Pitarresi G.L., D'Agostino S., Schiera G., De Caro V., Giandalia G., Giannola L.I., Cestelli A. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286 doi: 10.1152/ajpcell.00558.2003. (C1213-C128) [DOI] [PubMed] [Google Scholar]
- Sengillo J.D., Winkler E.A., Walker C.T., Sullivan J.S., Johnson M., Zlokovic B.V. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.K., Bamba P.S., Perkins B.N., Luscinskas F.W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- Shechter R., London A., Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat. Rev. Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Simpson I.A., Chundu K.R., Davies-Hill T., Honer W.G., Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann. Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späni C., Suter T., Derungs R., Ferretti M., Welt T., Wirth F., Gericke C., Nitsch R.M., Kulic L. Reduced β-amyloid pathology in an APP transgenic mouse model of Alzheimer's disease lacking functional B and T cells. Acta Neuropathol. Commun. 2015;3:71. doi: 10.1186/s40478-015-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K., Eckart A., Haidari S., Tirniceriu A., Lorenz M., von Brühl M.L., Gärtner F., Khandoga A.G., Legate K.R., Pless R., Hepper I., Lauber K., Walzog B., Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- Staykova M., Maxwell L., Willenborg D. Kinetics and polarization of the membrane expression of cytokine-induced ICAM-1 on rat brain endothelial cells. J. Neuropathol. Exp. Neurol. 2000;59:120–128. doi: 10.1093/jnen/59.2.120. [DOI] [PubMed] [Google Scholar]
- Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., Lacy-Hulber A., El Khoury J., Golenbock D.T., Moore K.J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins M.F., Gilles F., Kim K.S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Stomrud E., Björkqvist M., Janciauskiene S., Minthon L., Hansson O. Alterations of matrix metalloproteinases in the healthy elderly with increased risk of prodromal Alzheimer's disease. Alzheimers Res. Ther. 2010;2:20. doi: 10.1186/alzrt44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck S.E., Meister S., Nahrath J., Meißner J.N., Schubert N., Di Spiezio A., Baches S., Vandenbroucke R.E., Bouter Y., Prikulis I., Korth C., Weggen S., Heimann A., Schwaninger M., Bayer T.A., Pietrzik C.U. Endothelial LRP1 transports amyloid-β1-42 across the blood-brain barrier. J. Clin. Invest. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi N., Nishioku T., Takata F., Matsumoto J., Watanabe T., Shuto H., Yamauchi A., Dohgu S., Kataoka Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell. Mol. Neurobiol. 2010;30:247–253. doi: 10.1007/s10571-009-9446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz S., Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilling T., Korte D., Hoheisel D., Galla H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M., Oda T., Tsuchiya K., Kosaka K. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J. Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Town T., Tan J., Flavell R.A., Mullan M. T-cells in Alzheimer's disease. Neruomol. Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- Tripathy D., Sanchez A., Yin X., Luo J., Martinez J., Grammas P. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front. Aging Neurosci. 2013;5:19. doi: 10.3389/fnagi.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- van Oijen M., Witteman J.C., Hofman A., Koudstaal P.J., Breteler M.M. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- Varvel N.H., Grathwohl S.A., Degenhardt K., Resch C., Bosch A., Jucker M., Neher J.J. Replacement of brain-resident myeloid cells does not alter cerebral amyloid-β deposition in mouse models of Alzheimer's disease. J. Exp. Med. 2015;212:1803–1809. doi: 10.1084/jem.20150478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek M.M., Van Nostrand W.E., Otte-Höller I., Wesseling P., De Waal R.M. Amyloid-beta-induced degeneration of human brain pericytes is dependent on the apolipoprotein E genotype. Ann. N. Y. Acad. Sci. 2000;903:187–199. doi: 10.1111/j.1749-6632.2000.tb06368.x. [DOI] [PubMed] [Google Scholar]
- Vestweber D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Blanks J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- Veszelka S., Tóth A.E., Walter F.R., Datki Z., Mózes E., Fülöp L., Bozsó Z., Hellinger E., Vastag M., Orsolits B., Környei Z., Penke B., Deli M.A. Docosahexaenoic acid reduces amyloid-β induced toxicity in cells of the neurovascular unit. J. Alzheimers Dis. 2013;36:487–501. doi: 10.3233/JAD-120163. [DOI] [PubMed] [Google Scholar]
- Vidal R., Calero M., Piccardo P., Farlow M.R., Unverzagt F.W., Méndez E., Jiménez-Huete A., Beavis R., Gallo G., Gomez-Tortosa E., Ghiso J., Hyman B.T., Frangione B., Ghetti B. Senile dementia associated with amyloid beta protein angiopathy and tau perivascular pathology but not neuritic plaques in patients homozygous for the APOE-epsilon4 allele. Acta Neuropathol. 2000;100:1–12. doi: 10.1007/s004010051186. [DOI] [PubMed] [Google Scholar]
- Vignini A., Sartini D., Morganti S., Nanetti L., Luzzi S., Provinciali L., Mazzanti L., Emanuelli M. Platelet amyloid precursor protein isoform expression in Alzheimer's disease: evidence for peripheral marker. Int. J. Immunopathol. Pharmacol. 2011;24:529–534. doi: 10.1177/039463201102400229. (SENZA DOI) [DOI] [PubMed] [Google Scholar]
- Vignini A., Morganti S., Salvolini E., Sartini D., Luzzi S., Fiorini R., Provinciali L., Di Primio R., Mazzanti L., Emanuelli M. Amyloid precursor protein expression is enhanced in human platelets from subjects with Alzheimer's disease and frontotemporal lobar degeneration: a real-time PCR study. Exp. Gerontol. 2013;48:1505–1508. [PubMed] [Google Scholar]
- Viswanathan A., Greenberg S.M. Cerebral amyloid angiopathy in the elderly. Ann. Neurol. 2011;70:871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin M.B., Woodfin A., Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler. Thromb. Vasc. Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreys V., David G. Mammalian heparanase: what is the message? J. Cell. Mol. Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wan W., Cao L., Liu L., Zhang C., Kalionis B., Tai X., Li Y., Xia S. Aβ(1-42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J. Neurochem. 2015;134:382–393. doi: 10.1111/jnc.13122. [DOI] [PubMed] [Google Scholar]
- Wang S., Dangerfield J.P., Young R.E., Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J. Cell Sci. 2005;118:2067–2076. doi: 10.1242/jcs.02340. [DOI] [PubMed] [Google Scholar]
- Wang H.B., Wang J.T., Zhang L., Geng Z.H., Xu W.L., Xu T., Huo Y., Zhu X., Plow E.F., Chen M., Geng J.G. P-selectin primes leukocyte integrin activation during inflammation. Nat. Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jin S., Sonobe Y., Cheng Y., Horiuchi H., Parajuli B., Kawanokuchi J., Mizuno T., Takeuchi H., Suzumura A. Interleukin-1β induces blood-brain barrier disruption by downregulating sonic hedgehog in astrocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J., Imaki H., Wang K.C., Wegiel J., Rubenstein R. Cells of monocyte/microglial lineage are involved in both microvessel amyloidosis and fibrillar plaque formation in APPsw tg mice. Brain Res. 2004;1022:19–29. doi: 10.1016/j.brainres.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Wilhelmus M.M., de Waal R.M., Verbeek M.M. Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer's disease. Mol. Neurobiol. 2007;35:203–216. doi: 10.1007/s12035-007-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus M.M., Otte-Höller I., van Triel J.J., Veerhuis R., Maat-Schieman M.L., Bu G., de Waal R.M., Verbeek M.M. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am. J. Pathol. 2007;171:1989–1999. doi: 10.2353/ajpath.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Sagare A.P., Zlokovic B.V. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D., Sengillo J.D., Hillman S., Kong P., Nelson A.R., Sullivan J.S., Zhao Z., Meiselman H.J., Wenby R.B., Soto J., Abel E.D., Makshanoff J., Zuniga E., De Vivo D.C., Zlokovic B.V. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski H.M., Wegiel J., Wang K.C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can. J. Neurol. Sci. 1989;16:535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Wolburg H., Wolburg-Buchholz K., Kraus J., Rascher-Eggstein G., Liebner S., Hamm S., Duffner F., Grote E.H., Risau W., Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Wu Z., Guo H., Chow N., Sallstrom J., Bell R.D., Deane R., Brooks A.I., Kanagala S., Rubio A., Sagare A., Liu D., Li F., Armstrong D., Gasiewicz T., Zidovetzki R., Song X., Hofman F., Zlokovic B.V. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- Wu C., Ivars F., Anderson P., Hallmann R., Vestweber D., Nilsson P., Robenek H., Tryggvason K., Song J., Korpos E., Loser K., Beissert S., Georges-Labouesse E., Sorokin L.M. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat. Med. 2009;15:519–527. doi: 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- Xia M.Q., Hyman B.T. Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J. Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- Xia M.Q., Bacskai B.J., Knowles R.B., Qin S.X., Hyman B.T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J. Neuroimmunol. 2000;108:227–235. doi: 10.1016/s0165-5728(00)00285-x. [DOI] [PubMed] [Google Scholar]
- Xiao G., Gan L.S. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int. J. Cell Biol. 2013;2013:703545. doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Zhang H., Zhang S., Fan X., Liu X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int. J. Clin. Pract. 2008;62:1070–1075. doi: 10.1111/j.1742-1241.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- Yadav R., Larbi K.Y., Young R.E., Noursharg S. Migration of leukocytes through the vessel wall and beyond. Thromb. Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- Yamada M., Itoh Y., Shintaku M., Kawamura J., Jensson O., Thorsteinsson L., Suematsu N., Matsushita M., Otomo E. Immune reactions associated with cerebral amyloid angiopathy. Stroke. 1996;27:1155–1162. doi: 10.1161/01.str.27.7.1155. [DOI] [PubMed] [Google Scholar]
- Yan S.D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., Migheli A., Nawroth P., Stern D., Schmidt A.M. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Rosenberg G.A. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol. Biol. 2011;762:333–345. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lunde L.K., Nuntagij P., Oguchi T., Camassa L.M., Nilsson L.N., Lannfelt L., Xu Y., Amiry-Moghaddam M., Ottersen O.P., Torp R. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer's disease. J. Alzheimers Dis. 2011;27:711–722. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- Yang W., Wu Q., Yuan C., Gao J., Xiao M., Gu M., Ding J., Hu G. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol. Cell. Neurosci. 2012;49:406–414. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Yang Y.M., Shang D.S., Zhao W.D., Fang W.G., Chen Y.H. Microglial TNF-α-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem. Res. 2013;38:2295–2304. doi: 10.1007/s11064-013-1138-5. [DOI] [PubMed] [Google Scholar]
- Zarbock A., Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am. J. Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., Montresor A., Carlucci T., Nanì S., Tosadori G., Calciano L., Catalucci D., Berton G., Bonetti B., Constantin G. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- Zenker D., Begley D., Bratzke H., Rübsamen-Waigmann H., von Briesen H. Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells. J. Physiol. 2003;551:1023–1032. doi: 10.1113/jphysiol.2003.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.M., Zhou Y., Qiu L.B., Ding G.R., Pang X.F. Altered expression of matrix metalloproteinases and tight junction proteins in rats following PEMFinduced BBB permeability change. Biomed. Environ. Sci. 2012;25:197–202. doi: 10.3967/0895-3988.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Zipser B.D., Johanson C.E., Gonzalez L., Berzin T.M., Tavares R., Hulette C.M., Vitek M.P., Hovanesian V., Stopa E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol. Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V., Deane R., Sagare A.P., Bell R.D., Winkler E.A. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain. J. Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G., Cavalieri M., Galvani M., Passaro A., Munari M.R., Bosi C., Zurlo A., Fellin R. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J. Neurol. Sci. 2008;272:164–170. doi: 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]