Abstract
Elevated dosage of the GAC1 gene from the yeast Saccharomyces cerevisiae causes hyperaccumulation of glycogen whereas a gene disruption of GAC1 results in reduced glycogen levels. Glycogen synthase is almost entirely in the active, glucose 6-phosphate-independent, form in cells with increased gene dosage of GAC1 whereas the enzyme is mostly in the inactive form in strains lacking GAC1. GAC1 encodes an 88 kDa protein that is similar to the regulatory subunit (RG1) of phosphoprotein phosphatase type 1 (PP-1) from skeletal muscle that targets PP-1 to glycogen particles. Taken together, these results suggest that GAC1 encodes a regulatory subunit of PP-1. As previously shown for glycogen phosphorylase (GPH1), GAC1 RNA accumulates concomitantly with the appearance of glycogen. A strain with a mutation in the regulatory subunit of the cAMP-dependent protein kinase (bcy1) fails to accumulate GPH1 and GAC1 RNA. These results point to coordinate regulation of enzymes involved in glycogen metabolism at the level of RNA accumulation and indicate that at least part of this control is exerted by the RAS-cAMP pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt K. T., Styles C. A., Fink G. R. A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989 Feb 24;56(4):527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Becker J. U. Mechanisms of regulation of glycogen phosphorylase activity in Saccharomyces carlsbergensis. J Gen Microbiol. 1982 Mar;128(3):447–454. doi: 10.1099/00221287-128-3-447. [DOI] [PubMed] [Google Scholar]
- Belazzi T., Wagner A., Wieser R., Schanz M., Adam G., Hartig A., Ruis H. Negative regulation of transcription of the Saccharomyces cerevisiae catalase T (CTT1) gene by cAMP is mediated by a positive control element. EMBO J. 1991 Mar;10(3):585–592. doi: 10.1002/j.1460-2075.1991.tb07985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger P. H., Wieser R., Hamilton B., Ruis H. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol Cell Biol. 1989 Mar;9(3):1309–1315. doi: 10.1128/mcb.9.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R., Beach D. Involvement of a type 1 protein phosphatase encoded by bws1+ in fission yeast mitotic control. Cell. 1989 Jun 16;57(6):1009–1016. doi: 10.1016/0092-8674(89)90339-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Gibbs J. B., Tatchell K. Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics. 1986 Jun;113(2):247–264. doi: 10.1093/genetics/113.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. F., Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1987 Aug;7(8):2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. R., Johnson T. R., Dollard C., Shuster J. R., Denis C. L. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell. 1989 Feb 10;56(3):409–419. doi: 10.1016/0092-8674(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
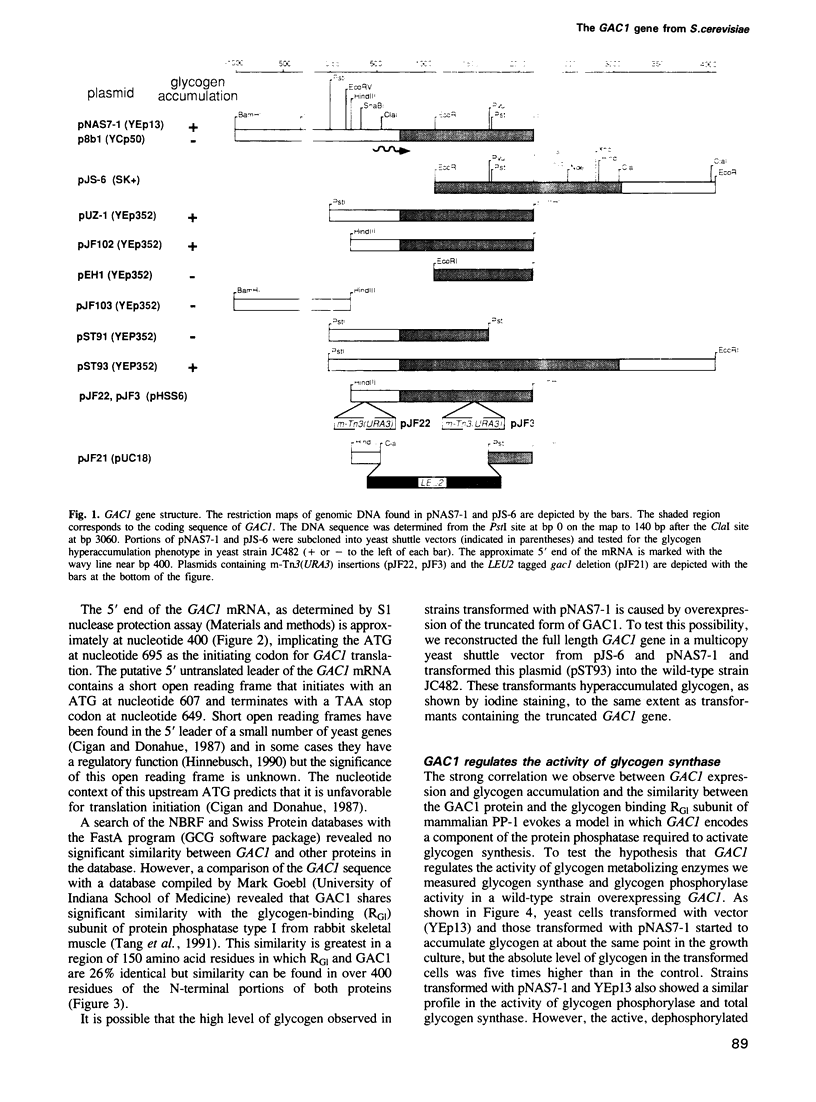
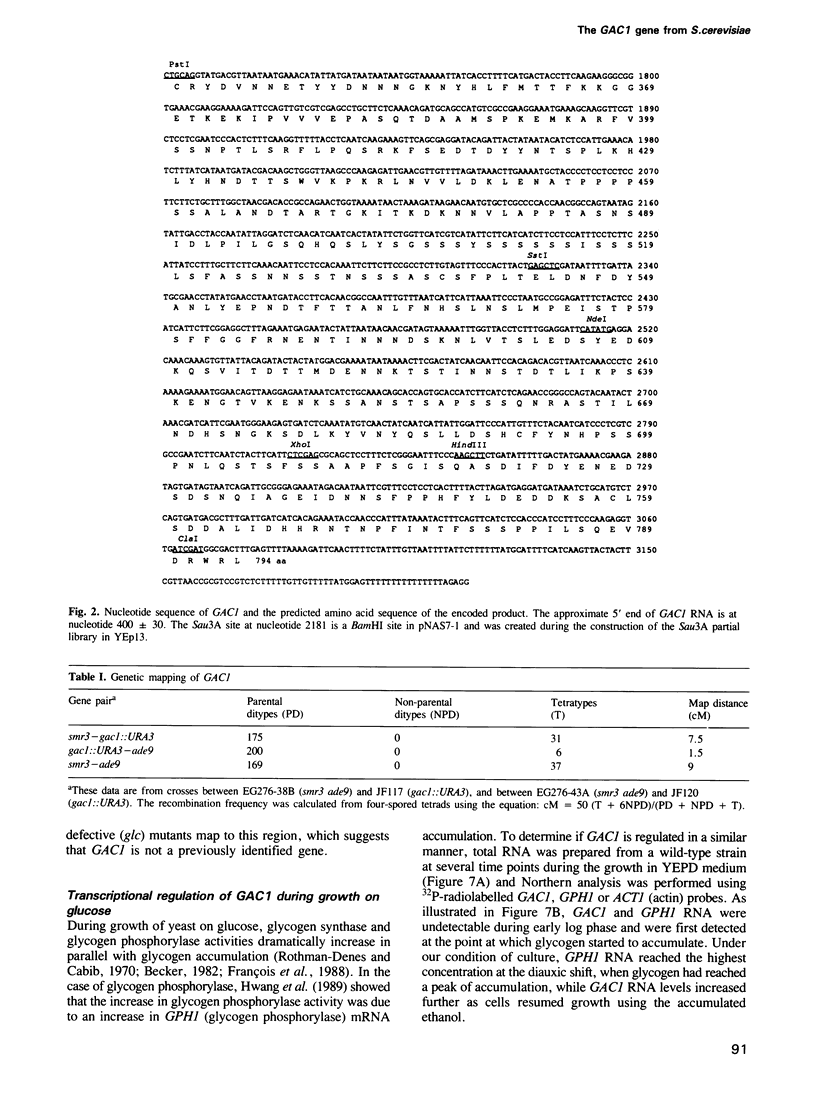
- Cigan A. M., Donahue T. F. Sequence and structural features associated with translational initiator regions in yeast--a review. Gene. 1987;59(1):1–18. doi: 10.1016/0378-1119(87)90261-7. [DOI] [PubMed] [Google Scholar]
- Clotet J., Posas F., Casamayor A., Schaaff-Gerstenschläger I., Ariño J. The gene DIS2S1 is essential in Saccharomyces cerevisiae and is involved in glycogen phosphorylase activation. Curr Genet. 1991 May;19(5):339–342. doi: 10.1007/BF00309593. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- De Vendittis E., Vitelli A., Zahn R., Fasano O. Suppression of defective RAS1 and RAS2 functions in yeast by an adenylate cyclase activated by a single amino acid change. EMBO J. 1986 Dec 20;5(13):3657–3663. doi: 10.1002/j.1460-2075.1986.tb04696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Domdey H., Apostol B., Lin R. J., Newman A., Brody E., Abelson J. Lariat structures are in vivo intermediates in yeast pre-mRNA splicing. Cell. 1984 Dec;39(3 Pt 2):611–621. doi: 10.1016/0092-8674(84)90468-9. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Farkas I., Hardy T. A., DePaoli-Roach A. A., Roach P. J. Isolation of the GSY1 gene encoding yeast glycogen synthase and evidence for the existence of a second gene. J Biol Chem. 1990 Dec 5;265(34):20879–20886. [PubMed] [Google Scholar]
- Farkas I., Hardy T. A., Goebl M. G., Roach P. J. Two glycogen synthase isoforms in Saccharomyces cerevisiae are coded by distinct genes that are differentially controlled. J Biol Chem. 1991 Aug 25;266(24):15602–15607. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Forsberg M., Westin G. Enhancer activation by a single type of transcription factor shows cell type dependence. EMBO J. 1991 Sep;10(9):2543–2551. doi: 10.1002/j.1460-2075.1991.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Muir L. W., Nielsen L. D., Fischer E. H. Purification and properties of yeast glycogen phosphorylase a and b. Biochemistry. 1971 Oct 26;10(22):4105–4113. doi: 10.1021/bi00798a015. [DOI] [PubMed] [Google Scholar]
- François J., Eraso P., Gancedo C. Changes in the concentration of cAMP, fructose 2,6-bisphosphate and related metabolites and enzymes in Saccharomyces cerevisiae during growth on glucose. Eur J Biochem. 1987 Apr 15;164(2):369–373. doi: 10.1111/j.1432-1033.1987.tb11067.x. [DOI] [PubMed] [Google Scholar]
- François J., Higgins D. L., Chang F., Tatchell K. Inhibition of glycogen synthesis in Saccharomyces cerevisiae by the mating pheromone alpha-factor. J Biol Chem. 1991 Apr 5;266(10):6174–6180. [PubMed] [Google Scholar]
- François J., Villanueva M. E., Hers H. G. The control of glycogen metabolism in yeast. 1. Interconversion in vivo of glycogen synthase and glycogen phosphorylase induced by glucose, a nitrogen source or uncouplers. Eur J Biochem. 1988 Jun 15;174(3):551–559. doi: 10.1111/j.1432-1033.1988.tb14134.x. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S. The ras oncogene--an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989 Jun;53(2):171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Involvement of an initiation factor and protein phosphorylation in translational control of GCN4 mRNA. Trends Biochem Sci. 1990 Apr;15(4):148–152. doi: 10.1016/0968-0004(90)90215-w. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga A., Kemp B. E., Cohen P. Further studies on the structure of the glycogen-bound form of protein phosphatase-1 from rabbit skeletal muscle. Eur J Biochem. 1987 Mar 2;163(2):253–258. doi: 10.1111/j.1432-1033.1987.tb10795.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Cabib E. Yeast glycogen synthetase in the glucose 6-phosphate-dependent form. I. Purification and properties. J Biol Chem. 1974 Jun 25;249(12):3851–3857. [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. Regulation of protein phosphatase-1G from rabbit skeletal muscle. 1. Phosphorylation by cAMP-dependent protein kinase at site 2 releases catalytic subunit from the glycogen-bound holoenzyme. Eur J Biochem. 1989 Dec 22;186(3):701–709. doi: 10.1111/j.1432-1033.1989.tb15263.x. [DOI] [PubMed] [Google Scholar]
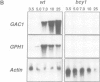
- Hwang P. K., Tugendreich S., Fletterick R. J. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Apr;9(4):1659–1666. doi: 10.1128/mcb.9.4.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
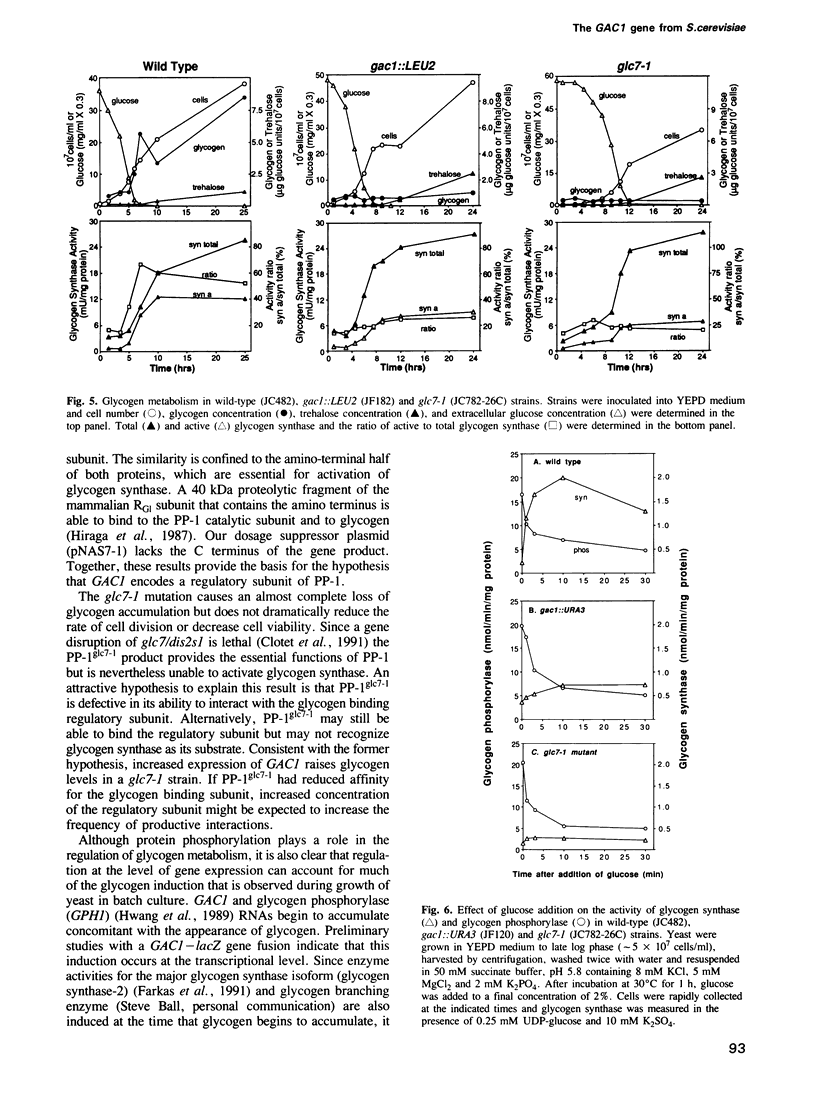
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Sass P., Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
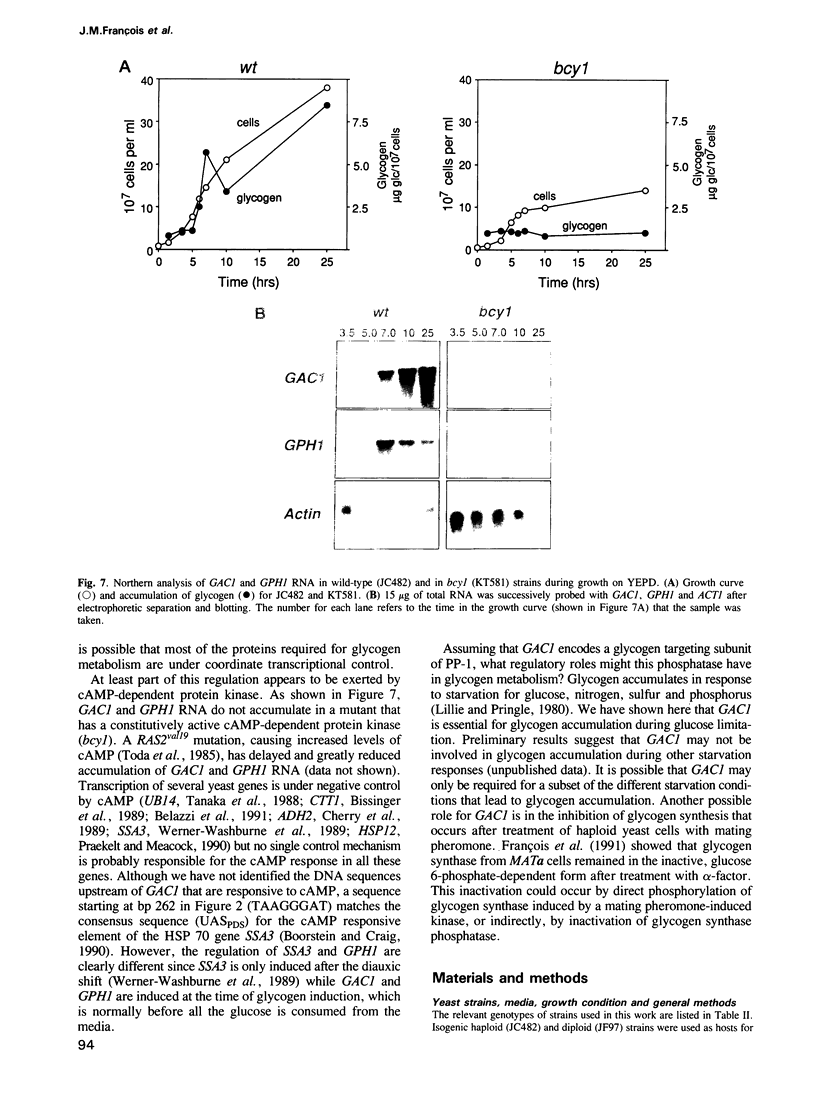
- Praekelt U. M., Meacock P. A. HSP12, a new small heat shock gene of Saccharomyces cerevisiae: analysis of structure, regulation and function. Mol Gen Genet. 1990 Aug;223(1):97–106. doi: 10.1007/BF00315801. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Ruggieri R., Tanaka K., Nakafuku M., Kaziro Y., Toh-e A., Matsumoto K. MSI1, a negative regulator of the RAS-cAMP pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8778–8782. doi: 10.1073/pnas.86.22.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass P., Field J., Nikawa J., Toda T., Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon A. A., Cohen P. T., Stark M. J. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990 Dec;9(13):4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Immanuel D., Arndt K. T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991 Apr;11(4):2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
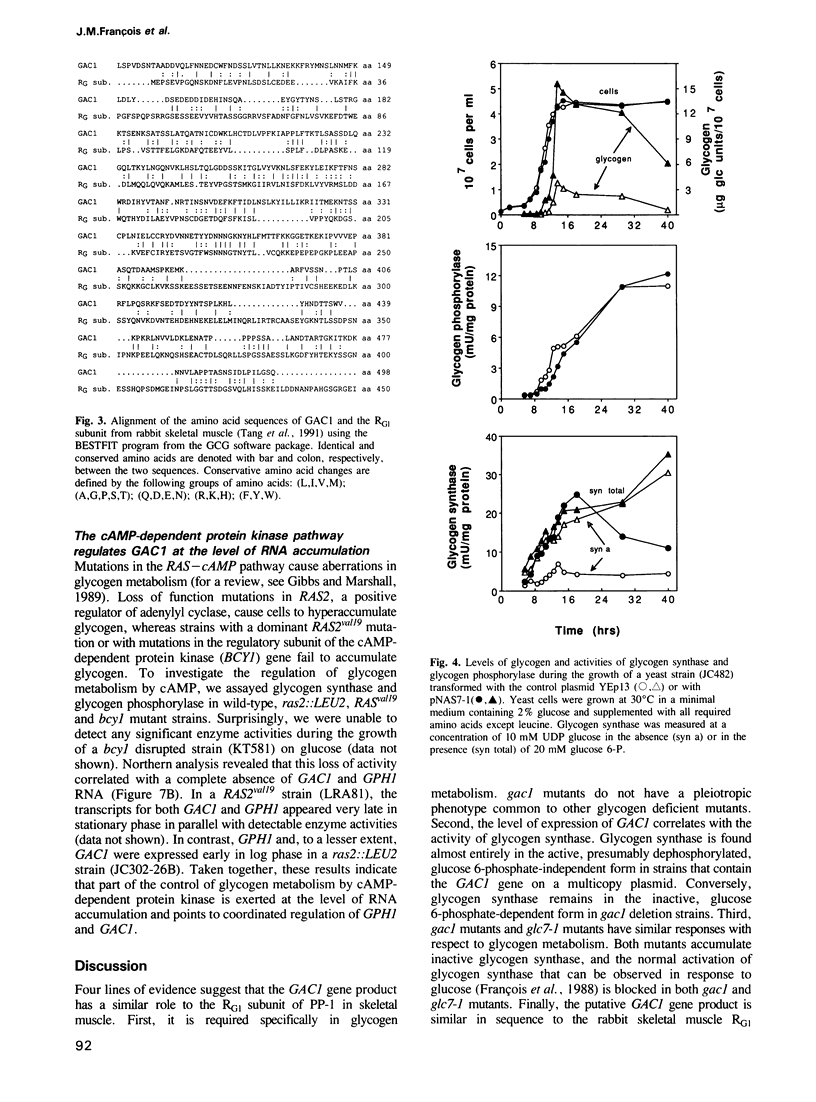
- Tang P. M., Bondor J. A., Swiderek K. M., DePaoli-Roach A. A. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J Biol Chem. 1991 Aug 25;266(24):15782–15789. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Wera S., Bollen M., Stalmans W. Purification and characterization of the glycogen-bound protein phosphatase from rat liver. J Biol Chem. 1991 Jan 5;266(1):339–345. [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. B., Tatchell K. SRA5 encodes the low-Km cyclic AMP phosphodiesterase of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):505–510. doi: 10.1128/mcb.8.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]