Abstract
The biogenesis of iron–sulfur (Fe/S) proteins in eukaryotes is a multistage, multicompartment process that is essential for a broad range of cellular functions, including genome maintenance, protein translation, energy conversion, and the antiviral response. Genetic and cell biological studies over almost 2 decades have revealed some 30 proteins involved in the synthesis of cellular [2Fe-2S] and [4Fe-4S] clusters and their incorporation into numerous apoproteins. Mechanistic aspects of Fe/S protein biogenesis continue to be elucidated by biochemical and ultrastructural investigations. Here, we review recent developments in the pursuit of constructing a comprehensive model of Fe/S protein assembly in the mitochondrion.
Keywords: acyl carrier protein (ACP), chaperone, fatty acid metabolism, frataxin, mitochondrial disease, cysteine desulfurase, ferredoxin, glutaredoxin, lipoic acid, metal biology
Ubiquitous iron–sulfur clusters and their synthesis: an overview
Iron–sulfur (Fe/S)2 clusters are inorganic cofactors that are essential for the proper functioning of virtually all biological cells (1). The chemical versatility of these clusters is utilized in fundamental life processes such as energy production, metabolic conversions, DNA maintenance, gene expression regulation, protein translation, and the antiviral response (Fig. 1) (2–4). In eukaryotes, Fe/S proteins are found in or associated with the mitochondrion, endoplasmic reticulum, cytosol, and the nucleus. These cofactors participate in electron transfer reactions, Lewis acid catalysis, transfer of sulfur atoms, or facilitating structural roles (5, 6). Although the activities of some Fe/S proteins are dispensable for cell survival under certain conditions, for example fungal Fe/S enzymes in the metabolism of amino acids, others such as Fe/S proteins involved in DNA maintenance or protein translation are essential for cell viability (Fig. 1). The growing number of diseases that implicate Fe/S proteins or their assembly factors illustrates the essentiality of the various functions of these protein cofactors (7–9). The phenotypes associated with these “Fe/S diseases” and the in vivo work in model systems such as Saccharomyces cerevisiae and human cell culture have led to a mechanistic model of eukaryotic Fe/S protein biogenesis, in which the sequence of events required for proper synthesis and trafficking of Fe/S clusters has been elucidated (2). This model has been invaluable to diagnosing new mitochondrial disorders, and its continued advancement will enhance the ability for early diagnosis (10–13). The focus of this review will be on the latest developments of the functional and mechanistic aspects that have advanced the model of mitochondrial Fe/S protein biogenesis.
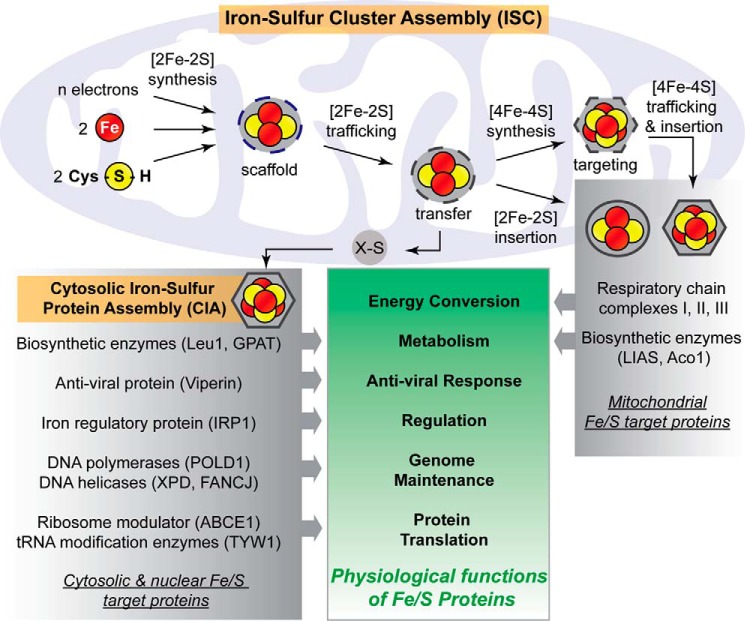
Figure 1.
Simplified overview of the assembly of eukaryotic Fe/S proteins and their cellular functions. The process begins in the mitochondrion with components of the early ISC machinery synthesizing a [2Fe-2S] cluster on a scaffold protein. This requires iron, cysteine, and electrons as the basic components. Following the de novo assembly reaction, the cluster is released from the scaffold and bound to a transfer protein, from where the transiently associated [2Fe-2S] cluster is trafficked to [2Fe-2S] targets or the late ISC machinery for [4Fe-4S] cluster synthesis. Additionally, an unknown sulfur-containing component, X-S, is generated (shown exported from the mitochondrion) and delivered to the CIA system for maturation of cytosolic and nuclear Fe/S proteins. Mitochondrial [4Fe-4S] clusters are finally trafficked to apoproteins by ISC targeting factors. Prominent examples of eukaryotic Fe/S proteins are listed with their corresponding cellular roles.
Cells typically maintain a strict balance of iron and sulfide ion concentrations due to their damaging redox reactions when present in excess (14, 15). The cell also uses a complex biosynthetic system to ensure that the sometimes redox-sensitive and labile Fe/S clusters are assembled correctly, trafficked to specific target apoproteins, and remain protected during these processes. This cellular control is exemplified by the 18 known “Fe/S cluster assembly” (ISC) proteins involved in the proper biogenesis and trafficking of clusters in mitochondria and the 11 known “cytosolic Fe/S protein assembly” (CIA) proteins responsible for synthesis, trafficking, and insertion of clusters in the cytosol and nucleus (Figs. 1 and 2) (2, 16). Mitochondria or the evolutionarily derived hydrogenosomes and mitosomes appear to be essential for biogenesis of all cellular Fe/S proteins (7, 17, 18). The only notable exception to this rule may be a newly characterized eukaryotic organism, which appears to be devoid of mitochondria (19).
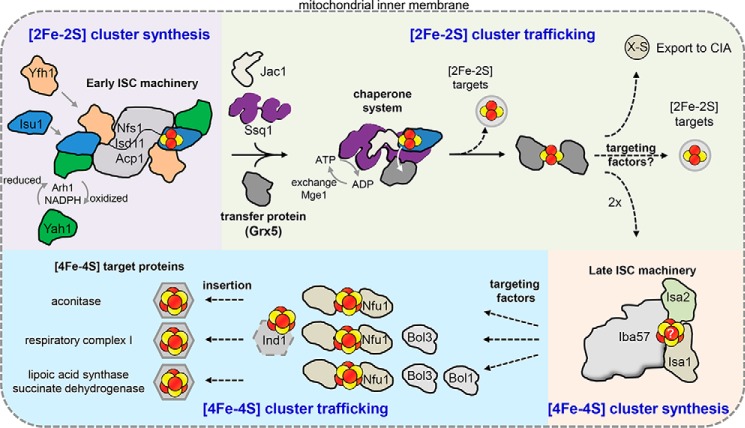
Figure 2.
Cartoon model of the mitochondrial Fe/S protein assembly process. A cascade of ISC proteins is required for the de novo synthesis of [2Fe-2S] and [4Fe-4S] clusters and their proper trafficking to target apoproteins in mitochondria. Initially, a [2Fe-2S] cluster is synthesized by the early ISC machinery, composed of the Isu1 scaffold protein requiring sulfide from the cysteine desulfurase complex Nfs1-Isd11-Acp1, electrons from the transfer chain NADPH-Arh1 and the ferredoxin Yah1, and the regulator and/or iron donor Yfh1. The Isu1-bound [2Fe-2S] cluster is then delivered to the monothiol glutaredoxin Grx5, a reaction accomplished by the Hsp70 chaperone Ssq1 with the help of the J-type co-chaperone Jac1. This reaction is dependent on ATP hydrolysis by Ssq1. The exchange factor Mge1 facilitates the exchange of ADP for ATP. The resulting bridging [2Fe-2S] cluster on a Grx5 dimer is inserted directly into [2Fe-2S] recipient apoproteins or trafficked to the late ISC machinery for [4Fe-4S] cluster biogenesis. The early ISC machinery, including the chaperones and Grx5, is also responsible for generating the component X-S for transport of sulfur out of the mitochondria to the CIA machinery for cytosolic-nuclear Fe/S protein biogenesis. The late ISC machinery consists of the yet structurally and functionally uncharacterized Isa1-Isa2-Iba57 complex and is needed for the generation of [4Fe-4S] clusters. Trafficking and insertion of the [4Fe-4S] clusters into target Fe/S proteins are facilitated by specific ISC targeting factors, such as Nfu1, the complex I-specific Ind1, and the Bol proteins. Dashed arrows indicate steps that remain poorly elucidated on the biochemical level.
Mitochondrial Fe/S protein assembly can be divided into four steps (Figs. 1 and 2). In the first step, de novo [2Fe-2S] cluster synthesis is orchestrated on a scaffold protein (Isu1) by a set of essential ISC proteins (Fig. 2). In the second step, a chaperone/co-chaperone system facilitates the release of the newly synthesized [2Fe-2S] cluster from the scaffold and its binding to a downstream ISC transfer protein (Grx5). The [2Fe-2S] cluster then is inserted into [2Fe-2S] target proteins, trafficked to the late-acting ISC machinery for [4Fe-4S] cluster synthesis, or used for synthesis and mitochondrial export of a yet unknown sulfur-containing species (X-S in Fig. 1) to be utilized by the CIA system. In the third step, conversion of the [2Fe-2S] into a [4Fe-4S] cluster requires a second hub of cluster synthesis. The fourth step involves the specific insertion of the newly generated [4Fe-4S] clusters into apoproteins by dedicated ISC targeting factors (Fig. 2).
In keeping with the evolutionary origin of the mitochondrion, the eukaryotic ISC machinery is believed to have been inherited from an alphaproteobacterium (18), and hence many of the mitochondrial and bacterial ISC proteins are highly similar in both structure and function (20, 21). In fact, the functional investigation of the mitochondrial ISC system has largely benefited from the progress made in studying the related bacterial system and vice versa (6, 20, 22). Nevertheless, evolution has slightly extended the function of the eukaryotic ISC machinery in various ways, and it has modified the regulation of the biosynthetic process (23, 24). Because of its genetic tractability and rapid cell growth, S. cerevisiae has proven to be an optimal model organism for studying the essential process, yet human cell culture has also been used successfully for functional studies on the ISC system. Here, we primarily use the corresponding yeast nomenclature to describe the highly conserved protein machinery and biochemical reactions (24). Key to recent progress in the untangling of the molecular mechanisms involved has been the development of in vitro reconstitution assays that employ spectroscopic techniques to follow Fe/S cluster synthesis (25–28), labeling methods (29), or the use of isolated mitochondria (30). Structural and biophysical work on Fe/S cluster biogenesis factors from bacteria to humans has stimulated and complemented these assays, providing molecular details into the ISC proteins and the modes of transient Fe/S cluster coordination by these factors.
De novo synthesis of a [2Fe-2S] cluster on the mitochondrial scaffold Isu1
Central to the de novo synthesis of [2Fe-2S] clusters is the scaffold protein Isu1 where the cluster is initially assembled with the assistance from other ISC proteins (Figs. 2 and 3) (31). A key ISC protein is the cysteine desulfurase Nfs1, along with its partner proteins Isd11 and Acp1 (32). The Nfs1-Isd11-Acp1 complex is responsible for the generation of transient persulfides (–SSH) on the active-site cysteine of Nfs1. Two co-crystal structures of the bacterial desulfurase IscS and the scaffold IscU have depicted that the ISC scaffold protein binds near a flexible loop of the desulfurase containing the active-site cysteine (Cys loop, Fig. 3) (33, 34). The structures also indicate that Nfs1 acts as a homodimer providing two independent sites for Isu1 binding and hence two possible active sites for [2Fe-2S] cluster synthesis. Mutations of both S. cerevisiae Nfs1 and Isu1 at their putative binding interfaces show decreased interaction, supporting this mode of association for the scaffold protein (35). The small protein Isd11 contributes to the stability and possibly the regulation of the desulfurase enzyme (36–40). Isd11 (mammalian LYRM4) belongs to the leucine–tyrosine–arginine-motif (LYRM) family of proteins, members of which bind to respiratory complexes I–III and V or their assembly intermediates (41–43). Although S. cerevisiae has no complex I, that of the yeast Yarrowia lipolytica contains the LYRM6 protein, which is essential for the catalytic activity of this respiratory complex. LYRM6 and the LYR family members subunit B14 and B22 of mammalian complex I anchor the mitochondrial acyl-carrier protein (mammalian SDAP-α/β, Y. lipolytica ACPM1) to the complex, and the 3D structure of this dimer has been resolved (43) (Fig. 3). A similar interaction mode can now be expected for Isd11 attaching Acp1 to the Fe/S cluster biogenesis complex in S. cerevisiae (32, 44). The dual role of Acp1 in Fe/S protein biogenesis and mitochondrial fatty acid metabolism, including lipoic acid synthesis, may provide a regulatory device linking the biogenesis of respiratory functions and metabolic activities. Critical to the function of Acp1 in complex I, fatty acid synthesis, and the ISC complex is a 4′-phosphopantetheine moiety covalently bound via an invariant serine residue and carrying a fatty acyl chain (Fig. 3) (45). This raises the question of how Acp1 coordinates all these diverse mitochondrial functions. The interaction surface of the Isd11-Acp1 subcomplex and the Nfs1 dimer remains elusive; however, mutations in α-helix 1, α-helix 3, and the C-terminal region of Isd11 have been shown to compromise interactions with the desulfurase (40).
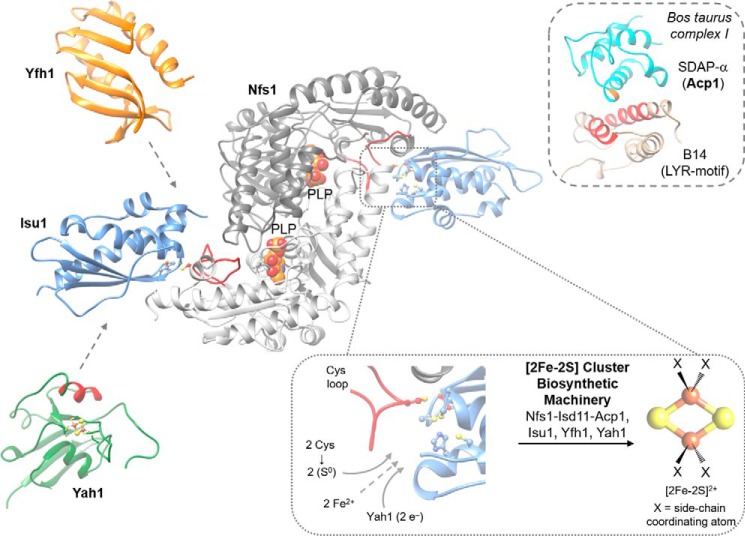
Figure 3.
Structural insights of the early ISC machinery for [2Fe-2S] cluster synthesis. Structural and functional studies of the early ISC machinery have suggested a six-membered multimeric protein complex composed of two copies of each of the following proteins. The cysteine desulfurase Nfs1 (modeled from IscS-IscU PDB code 4EB7) (100), which binds its partner proteins Isd11 and Acp1 at an unknown location; the scaffold Isu1 (modeled from IscS-IscU PDB code 3LVL); the ferredoxin Yah1 (PDB code 2MJE), and Yfh1 (PDB code 3FQL). All three proteins are expected to bind close to the Cys loop (red) of Nfs1. Note that the second set of Yah1, Yfh1, and Isd11-Acp1 components are not shown for clarity. Paramount to the synthesis of a [2Fe-2S] cluster is the scaffold protein Isu1 containing a possible cluster-binding site of three cysteines and a histidine (lower inset). The exact coordination of the [2Fe-2S] cluster is unclear throughout the biosynthetic process (designated by X). Nfs1 delivers two persulfides (–SSH) from the PLP active site (PLP represented as spheres) via the Cys loop (lower inset, red) to the active site of Isu1. Electrons are shuttled via the ferredoxin Yah1. Interaction of reduced Yah1 with Isu1 is proposed to be facilitated by α-helix 3 (red). How 2 eq of ferrous iron gain access to the active site of Fe/S cluster synthesis remains unclear. Allosteric regulation of sulfur transfer from Nfs1 to Isu1 is conferred by Yfh1. Isd11-Acp1 stabilizes the 200-kDa complex with Nfs1, yet their precise function is unknown. The Isd11-Acp1 structure may be similar to that of an LYR protein-Acp1 dimer in respiratory complex I (upper inset). The subunit B14 from Bos taurus containing an LYR motif (red) interacts with SDAP-α (Acp1), which covalently binds a 4′-phosphopantetheine moiety via a conserved serine (orange) (PDB code 5LDW) (41, 43).
Allosteric regulation of persulfide transfer from Nfs1 to the scaffold protein Isu1 is proposed to be conferred by Yfh1 (human frataxin) (29, 46). The Nfs1 desulfurase reaction takes place on a pyridoxal 5′-phosphate (PLP) cofactor that is situated at the dimer interface (Fig. 3), where free l-cysteine forms a Schiff base with PLP that is primed for sulfur release (47). The Cys loop can bind near the PLP site, which may also allosterically control access to the PLP site (48). The active-site cysteine of Nfs1 strips a sulfur atom, in the form of a persulfide, from the Schiff base and shuttles it to the active site of the scaffold protein Isu1 (34, 47). Based on small-angle X-ray scattering with Escherichia coli ISC proteins and mutational studies in S. cerevisiae, monomeric Yfh1 (or bacterial CyaY) binds to a pocket between Nfs1 and Isu1 (Fig. 3) (49, 50). This localizes Yfh1 in the vicinity of both the flexible active-site Cys loop of Nfs1 and the cluster-binding site of Isu1 suggestive of its allosteric regulatory role in persulfide transfer. A proposed second function of Yfh1 is in iron supply, which will be discussed below.
The presence of a ferredoxin in the ISC operon in prokaryotes and the requirement of Yah1 in vivo in yeast had suggested that an electron source was required for Fe/S protein biogenesis (31, 51). The dependence of this process on the [2Fe-2S] ferredoxin Yah1 or on human FDX2 (52, 53) in the early ISC machinery has now been recapitulated in vitro by reconstitution assays in both prokaryotic and eukaryotic systems (25, 54). Previous assays were independent of Yah1 because of the presence of the artificial reductant dithiothreitol (DTT), which can replace the ferredoxins as an electron source, and thus change the mechanism to a non-physiological variation. The use of DTT in reactions involving Fe/S cluster synthesis and trafficking must therefore be critically evaluated due to the ability of DTT to chemically promote the release of sulfide from the Nfs1-bound persulfide and thus chemically rather than biochemically support Fe/S cluster formation (25, 28, 54). In the mitochondrion, the ferredoxin reductase Arh1 is reduced by NADPH, and then shuttles its electrons to Yah1, which uses them for the construction of the [2Fe-2S]2+ cluster on Isu1 (Figs. 2 and 3). An interaction between Isu1 and Yah1 was observed in S. cerevisiae mitochondria and further confirmed in vitro by chemical cross-linking, gel filtration, NMR, and microscale thermophoresis (25). In contrast to Yfh1, reduced Yah1 interacts strongly only with Isu1 and not significantly with the desulfurase as in prokaryotes (Fig. 3) (25, 54, 55). As expected from a reductive role of Yah1, only its reduced form had a high affinity for Isu1 suggesting that electron transfer loosens the interaction and facilitates dissociation of the oxidized Yah1 from Isu1. At what point the electrons are transferred to the scaffold protein by Yah1 remains unclear; however, NMR studies suggest that a hydrophilic patch in α-helix-3 of Yah1 could mediate the transfer (Fig. 3) (25).
Although it is generally accepted that the newly synthesized [2Fe-2S] cluster is constructed on the scaffold protein Isu1, the mechanism of cluster synthesis remains vague (Fig. 3). The acceptor sites of the two persulfides needed for [2Fe-2S] cluster formation on the scaffold protein have yet to be determined in vivo. In vitro studies have indicated that Isu1 can indeed be persulfurated (29, 46, 56, 57), but experiments in isolated mitochondria have suggested that iron must be present before a persulfide can be transferred (30). How iron gains access to the active site remains enigmatic. Apo-Isu1 does not appear to bind iron at the active site with significant affinity (58), suggesting that iron may be actively delivered to the ISC complex. Yfh1 and its human homologue frataxin can bind ferrous iron via acidic residues on the N-terminal α-helix, but concrete in vivo evidence of Yfh1 supplying iron is still lacking (15, 59). Interestingly, frataxin-independent cluster synthesis using an Isu1 mutant protein may rule against a specific iron delivery function of frataxin (60). More detailed structural information may help resolve this still open question.
How the [2Fe-2S] cluster is coordinated on Isu1 during and shortly after its synthesis remains unclear (Fig. 3). Crystal structures with ISC proteins from Archaeoglobus fulgidus have suggested an intermediate, where the [2Fe-2S] cluster binds to Cys residues contributed from both IscS and IscU (34). Although this arrangement is highly attractive as a synthesis intermediate, it has later been noted that the IscS used in this study likely does not function as a bona fide desulfurase because it lacks both the PLP-coordinating Lys residue and desulfurase activity (61). In vitro reconstitution of Fe/S cluster synthesis on Isu1 yielded a bridging [2Fe-2S] cluster on an Isu1 dimer as a final product, but this arrangement remains to be confirmed in vivo (25).
Overall, the current model for the multimeric early-acting ISC complex includes six proteins in stoichiometric amounts, Isu1, Nfs1–Isd11–Acp1, Yfh1, and Yah1, and is required for the construction of the [2Fe-2S] cluster (25, 32). Other studies have suggested different oligomeric states of the biosynthetic machinery, yet it remains unclear how the active site of Isu1 would remain available for cluster construction and trafficking in these large complexes (62, 63).
Chaperone-facilitated [2Fe-2S] cluster release from Isu1 and trafficking by Grx5
A second multimeric protein complex is responsible for facilitating the release of the Isu1-bound [2Fe-2S] cluster for downstream trafficking. The specialized Hsp70 chaperone Ssq1 and its co-chaperone Jac1 work together in targeting the Isu1 scaffold for specific release of the bound [2Fe-2S] cluster to the monothiol glutaredoxin Grx5 (Fig. 2). Initially, Jac1 recognizes the cluster-bound Isu1 and directs it to Ssq1 (64). Jac1 either facilitates the dissociation of cluster-bound Isu1 from the early-acting ISC complex (35) or alternatively Jac1 binds to an Isu1 holo-dimer that has already dissociated from the biosynthetic machinery after Fe/S cluster synthesis (25, 65, 66). In either case, the dynamic chaperone complex is used to specifically traffic the [2Fe-2S] cluster to Grx5 or possibly directly to [2Fe-2S] target apoproteins (Fig. 2) (67–69). Grx5 is the only known [2Fe-2S] cluster-binding protein able to directly receive clusters from Isu1 by physically interacting with Ssq1 at a non-substrate-binding site (70). Notably, the suggested role of the chaperone system in [2Fe-2S] cluster trafficking has been reconstituted in vitro only in the bacterial ISC system, where the chaperones HscA and HscB stimulated up to 700-fold the [2Fe-2S] cluster transfer from the IscU scaffold to bacterial GrxD (27).
As surmised from the biochemical assays, ATP hydrolysis on Ssq1 induces a conformational change in the peptide-binding domain of Ssq1 that leads to its stable binding to the LPPV motif of Isu1 (Fig. 2) (27, 65, 67). In turn, that reaction is believed to labilize the [2Fe-2S] cluster on Isu1 providing a facile relay to Grx5. Once the cluster release step is complete, the nucleotide exchange factor Mge1 can recycle Ssq1 by assisting ADP dissociation and allowing the rebinding of ATP (70). These concerted, regulated steps of the chaperone cycle could prevent unwanted [2Fe-2S] cluster release to the solvent, small molecules, or adventitious metal-binding sites. In support of this view, depletion of the chaperone system or of Grx5 in vivo shows an increase in Fe/S cluster binding on Isu1 (31). S. cerevisiae strains lacking Ssq1 are viable, yet JAC1 deletions are lethal indicating its indispensability for Fe/S cluster biogenesis (71). This difference for the two chaperones is explained by the presence of the second, more general Hsp70 isoform Ssc1 in S. cerevisiae partially taking over the function of Ssq1 (72).
The role of Grx5 as an Fe/S cluster transfer protein is supported by the crystal structure of the human homologue GLRX5 with a bridging [2Fe-2S] cluster (73). The definition for bacterial GrxD as a cluster “carrier protein” cannot be adopted for mitochondria to avoid confusion with “mitochondrial carrier proteins” involved in inner membrane transport of metabolites (74). The [2Fe-2S] cluster is coordinated between two Grx5 monomers using the active-site cysteine of Grx5 and the cysteine of a non-covalently Grx5-bound glutathione molecule (Fig. 4, A and B). In S. cerevisiae, the inability to immunoprecipitate Grx5 with a radiolabeled 55Fe/S cluster suggests this moiety to be bound in a labile fashion, a property that would be beneficial for trafficking the cluster to further downstream targets. Cluster binding was, however, detectable by 55Fe radiolabeling, when Schizosaccharomyces pombe or human Grx5 homologues were expressed ectopically in yeast indicating that these foreign proteins bind the cluster more stably (70). Surprisingly, deletion of S. cerevisiae GRX5 is not lethal, suggesting that the protein's function can be bypassed to some extent, despite its currently accepted central role in Fe/S cluster trafficking in mitochondria (Fig. 2). Furthermore, Grx5 function is hardly needed under anaerobic conditions suggesting that its trafficking role is particularly required under ambient or high oxygen pressure. As an alternative to Grx5-mediated trafficking, Jac1 and Ssq1 have been suggested to support the release of clusters from Isu1 directly to target proteins (Fig. 2) (68, 75). However, higher eukaryotes require efficient Grx5-dependent [2Fe-2S] trafficking reactions, because mutations in human GLRX5 are associated with human disease (7, 76). In mechanistic and molecular terms, much remains to be explored in the pathways of [2Fe-2S] cluster trafficking and insertion in the mitochondrion.
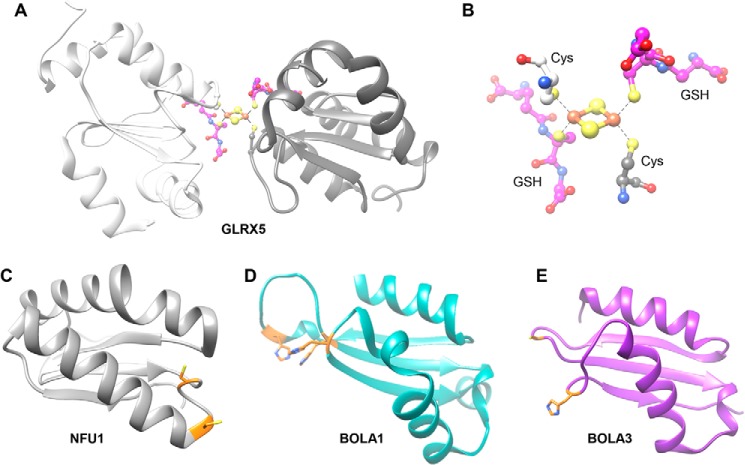
Figure 4.
ISC transfer proteins and targeting factors assisting [2Fe-2S] and [4Fe-4S] cluster trafficking. A, crystal structure of the human Grx5 homologue GLRX5 (PDB code 2WUL) as a homodimer. The different protomers are different shades of gray with the GSH carbon atoms colored in magenta (blue, nitrogen; red, oxygen; orange, iron; yellow, sulfur). B, [2Fe-2S] cluster coordination in the Grx5 homodimer involves a cysteine from each protomer and each GSH molecule, with coordination bonds shown by dotted lines. C–E, solution structures of human ISC targeting factors. C, C-terminal domain of NFU1 (PDB code 2M5O); D, BOLA1 (PDB code 5LCI); and E, BOLA3 (PDB code 2NCL). Potential Fe/S cluster coordinating residues are shown in orange as sticks (yellow, sulfur; blue, nitrogen).
Synthesis and trafficking of the [4Fe-4S] cluster in mitochondria
In vivo studies in S. cerevisiae and human cell culture have shown that a dedicated ISC complex is needed for cellular [4Fe-4S] cluster synthesis. This late-acting complex is composed of Isa1-Isa2-Iba57 and does not interact with the early ISC machinery, but it is dependent on the delivery of a [2Fe-2S] cluster species (Fig. 2) (77–79). Specifically, deficiency of any constituent of the Isa1-Isa2-Iba57 complex results in mutant yeast or human cells that lack a functional respiratory chain and lipoic acid (see below). Yeast cells additionally are lysine and glutamine auxotrophic because mitochondrial homoaconitase (Lys4) and glutamate synthase (Glt1) are not matured. The ISC proteins Isa1 and Isa2 are proposed to accept a [2Fe-2S] cluster from Grx5, connecting the [2Fe-2S] trafficking step to the late ISC machinery (Figs. 1 and 2) (78, 80–82). The two Isa proteins belong to the A-type family of ISC proteins that have been shown to be either homo- or heterodimers, which can coordinate Fe/S clusters and/or mononuclear iron via three conserved cysteine residues (78, 82–84). In vitro reconstitution of [2Fe-2S] cluster release from Grx5 to a Isa1-Isa2 heterodimer and subsequent DTT-dependent reductive coupling of two [2Fe-2S] clusters to form a [4Fe-4S] cluster have been reported based on NMR and UV-visible studies (Fig. 2) (82). This reconstitution assay, however, occurred at slow rates and did not include the third required component, Iba57, leaving its function unaccounted for. All three proteins are required for the synthesis of the [4Fe-4S] cluster in the cell, yet their stoichiometry, structure, interaction modes, and cluster-binding sites remain molecularly undefined. Depending on the oxidation states of the cluster, [2Fe-2S]1+ or [2Fe-2S]2+, a biological electron source may not or may be required for [4Fe-4S]2+ cluster synthesis, respectively.
Additional ISC targeting factors facilitate the trafficking of the [4Fe-4S] clusters from the Isa1-Isa2-Iba57 complex to dedicated apoproteins. At present, two mitochondrial [4Fe-4S] cluster-binding proteins are known, Nfu1 and Ind1 (Fig. 2) (12, 13, 85–88). Recent in vivo evidence indicates that the mitochondrial protein Nfu1 can interact with the [4Fe-4S] cluster machinery and with potential [4Fe-4S] target proteins, thus supporting its targeting function (89). These in vivo observations agree with the in vitro characterization of the C-terminal domain of Nfu1 coordinating a bridging [4Fe-4S] cluster, via a CXXC motif, between two monomers, as observed in the plant and human homologues (Fig. 4) (90–92). Furthermore, [4Fe-4S] cluster-bound Nfu1 was capable of inserting the cluster into plant target proteins in vitro (90). Yeast and human cells lacking Nfu1 contain partially defective Fe/S target proteins aconitase, succinate dehydrogenase, and lipoic acid synthase, indicating a non-essential role of Nfu1 as a [4Fe-4S]-targeting protein (Figs. 2 and 4) (12, 89, 93).
A further set of mitochondrial ISC targeting factors termed Bol1 and Bol3 are proposed to provide specificity for Fe/S cluster insertion into apoproteins. These factors are members of the Bol (BOLA) protein family, which also includes Bol2 in the cytosol (94). Two recent in vivo studies in yeast and a BOLA3 patient analysis suggested that Bol3 and Bol1 are involved in the maturation of a sub-class of mitochondrial [4Fe-4S] proteins, especially succinate dehydrogenase and lipoic acid synthase with its two [4Fe-4S] clusters, whereas several [2Fe-2S] proteins were assembled independently of the Bol proteins (Fig. 2) (13, 89, 93). Like Nfu1, the mitochondrial Bol proteins are not essential for viability of S. cerevisiae, and residual maturation of their target proteins was observed even in BOL double null mutants. Bol3 was co-immunoprecipitated with both late ISC machinery and [4Fe-4S] target proteins, a protein set overlapping with the Nfu1 interactome (89). Furthermore, deleting both BOL genes in S. cerevisiae did not show the tell-tale sign of early ISC gene disruption, i.e. a general cellular Fe/S protein defect and the activation of the iron regulon, as described for the GRX5 mutant cells, for example (31). Together, these properties place the Bol protein function in the late part of the ISC pathway after Isa1-Isa2-Iba57 function (Fig. 2).
A combination of in vitro and in vivo studies have shown that both Bol1 and Bol3 interact with Grx5, raising the question of whether Grx5 performs another function in the late ISC machinery or, alternatively, whether the Bol-Grx5 heterocomplexes could also have undisclosed functions in [2Fe-2S] cluster trafficking and/or insertion reactions (89, 93). A physiological relevance of a Grx-Bol heterodimer has been established only for the yeast cytosolic monothiol glutaredoxin Grx3 and Bol2 (formerly known as Fra2), which are involved in iron homeostasis in the yeast cytosol (94–96). Similar Fe/S cluster-containing Bol-Grx heterocomplexes have been observed in bacteria (BolA with GrxD or Grx4), plants (BolA1 and BolA2 with GrxS14 and GrxS17), and recently in humans (BOLA1 and BOLA3 with GLRX5) (93, 97, 98). Mitochondrial Bol1 proteins contain three conserved histidine residues, and Bol3 contains a histidine and a cysteine residue as candidates for coordination of the [2Fe-2S] cluster in the heterodimers with Grx5 (Fig. 4) (94, 99). In vitro characterization and NMR studies have shown that human BOLA1 and BOLA3 can specifically interact with both the apo- and holo-forms of GLRX5, yet the affinities of the respective hetero-clusters differ characteristically (89, 93). The different biochemical characteristics of the human GLRX5-BOLA complexes suggest that the two complexes may have distinct functions, e.g. different reactivities/affinities/specificities for Fe/S cluster target apoproteins.
The physical interaction between Nfu1 and Bol3 and the dramatic increase of Bol3 levels upon overexpression of Nfu1 further suggest the roles of these proteins late in the ISC pathway (89, 93). In further support of their function in the late ISC steps, deletion of all three ISC genes (bol1Δbol3Δnfu1Δ yeast mutant cells) resulted in a synergistic growth and Fe/S protein biogenesis defect approaching the phenotype observed for cells lacking the Isa1-Isa2-Iba57 proteins. However, the Bol3 and Nfu1 protein functions do not overlap, because overexpression of one factor did not ameliorate the defects arising from the deletion of the other ISC gene (93). In the triple deletion cells, severe effects were observed for α-ketoglutarate dehydrogenase and pyruvate dehydrogenase, two proteins dependent on the Fe/S protein lipoic acid synthase (Fig. 2). Intriguingly, the synthesis of lipoic acid is dependent on an Acp1-derived octanoyl fatty acyl chain, an aspect that links the early ISC machinery and the Fe/S target protein lipoic acid synthase (45). The combined defects in protein lipoylation and the respiratory chain complexes are a hallmark of Fe/S diseases, especially in the so-called multiple mitochondrial dysfunction syndromes, and are now used for diagnostic purposes (7, 9).
Conclusions and perspectives
Fe/S protein biogenesis in mitochondria continues to be an important area of active research with many key questions to be addressed. The early stage of the ISC assembly pathway is the best understood to date, yet much is to be elucidated concerning the structure of the biosynthetic ISC complex and the stepwise biochemical mechanisms of Fe/S cluster synthesis. It seems clear that the various multimeric ISC complexes play key roles in orchestrating and protecting Fe/S cluster synthesis and trafficking. Future challenges will involve understanding the associated dynamics of the individual proteins in these larger complexes. Following this, the thermodynamics and kinetics of trafficking species that connect the four ISC stages must also be clearly described, in addition to determining how the early ISC system supplies the enigmatic X-S compound for mitochondrial export.
Despite the high number of known ISC proteins, new factors may still be discovered. The non-essential function of the transfer protein Grx5 in S. cerevisiae would support this idea in addition to the newest biogenesis factor, Acp1, being added in 2016. This latest discovery also adds a new avenue of research in the mitochondrion, specifically understanding how Fe/S cluster biogenesis is regulated, e.g. by connection to mitochondrial fatty acid synthesis. Furthermore, one pressing question to address is the source and trafficker of iron to the ISC biosynthetic complex. Ferrous iron may simply diffuse to the active site of Fe/S cluster synthesis; however, the amount of energy the cell puts forth to constructing Fe/S clusters and the required metal specificity would argue against such a nonspecific mechanism. In vitro reconstitution assays will continue to be instrumental in progressing our understanding further to a complete Fe/S protein biogenesis model. However, it seems crucial to verify these findings in vivo to confirm their validity in the mitochondrial setting across various model organisms. Fruitful times are still ahead in the field of mitochondrial Fe/S protein biogenesis with many fundamental questions still to be addressed.
This work was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie agreement Grant 659325 (to J. J. B.) and generous financial support (to R. L.) from the Deutsche Forschungsgemeinschaft (Koselleck Grant, LI 415/5, SFB 987, SPP 1710, and SPP 1927), LOEWE program of State Hessen, and German-Israeli Foundation (GIF). This is the fourth article in the Thematic Minireview series “Metals in Biology 2017: Iron transport, storage, and the ramifications.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Fe/S
- iron–sulfur
- ISC
- iron–sulfur cluster assembly
- CIA
- cytosolic iron–sulfur protein assembly
- LYRM
- leucine-tyrosine-arginine motif
- PLP
- pyridoxal 5′-phosphate
- PDB
- Protein Data Bank.
References
- 1. Andreini C., Rosato A., and Banci L. (2017) The relationship between environmental dioxygen and iron–sulfur proteins explored at the genome level. PLoS ONE 12, e0171279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lill R., Dutkiewicz R., Freibert S. A., Heidenreich T., Mascarenhas J., Netz D. J., Paul V. D., Pierik A. J., Richter N., Stümpfig M., Srinivasan V., Stehling O., and Mühlenhoff U. (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron–sulfur proteins. Eur. J. Cell Biol. 94, 280–291 [DOI] [PubMed] [Google Scholar]
- 3. Fuss J. O., Tsai C. L., Ishida J. P., and Tainer J. A. (2015) Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochim. Biophys. Acta 1853, 1253–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Upadhyay A. S., Vonderstein K., Pichlmair A., Stehling O., Bennett K. L., Dobler G., Guo J. T., Superti-Furga G., Lill R., Överby A. K., and Weber F. (2014) Viperin is an iron–sulfur protein that inhibits genome synthesis of tick-borne encephalitis virus via radical SAM domain activity. Cell. Microbiol. 16, 834–848 [DOI] [PubMed] [Google Scholar]
- 5. Beinert H. (2000) Iron–sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5, 2–15 [DOI] [PubMed] [Google Scholar]
- 6. Johnson D. C., Dean D. R., Smith A. D., and Johnson M. K. (2005) Structure, function and formation of biological iron–sulfur clusters. Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 7. Stehling O., Wilbrecht C., and Lill R. (2014) Mitochondrial iron–sulfur protein biogenesis and human disease. Biochimie 100, 61–77 [DOI] [PubMed] [Google Scholar]
- 8. Rouault T. A. (2015) Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat. Rev. Mol. Cell Biol. 16, 45–55 [DOI] [PubMed] [Google Scholar]
- 9. Beilschmidt L. K., and Puccio H. M. (2014) Mammalian Fe-S cluster biogenesis and its implication in disease. Biochimie 100, 48–60 [DOI] [PubMed] [Google Scholar]
- 10. Debray F. G., Stümpfig C., Vanlander A. V., Dideberg V., Josse C., Caberg J. H., Boemer F., Bours V., Stevens R., Seneca S., Smet J., Lill R., and van Coster R. (2015) Mutation of the iron–sulfur cluster assembly gene IBA57 causes fatal infantile leukodystrophy. J. Inherit. Metab. Dis. 38, 1147–1153 [DOI] [PubMed] [Google Scholar]
- 11. Lossos A., Stümpfig C., Stevanin G., Gaussen M., Zimmerman B. E., Mundwiller E., Asulin M., Chamma L., Sheffer R., Misk A., Dotan S., Gomori J. M., Ponger P., Brice A., Lerer I., et al. (2015) Fe/S protein assembly gene IBA57 mutation causes hereditary spastic paraplegia. Neurology 84, 659–667 [DOI] [PubMed] [Google Scholar]
- 12. Navarro-Sastre A., Tort F., Stehling O., Uzarska M. A., Arranz J. A., Del Toro M., Labayru M. T., Landa J., Font A., Garcia-Villoria J., Merinero B., Ugarte M., Gutierrez-Solana L. G., Campistol J., Garcia-Cazorla A., et al. (2011) A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 89, 656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cameron J. M., Janer A., Levandovskiy V., Mackay N., Rouault T. A., Tong W. H., Ogilvie I., Shoubridge E. A., and Robinson B. H. (2011) Mutations in iron–sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am. J. Hum. Genet. 89, 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabil O., Motl N., and Banerjee R. (2014) H2S and its role in redox signaling. Biochim. Biophys. Acta 1844, 1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lane D. J., Merlot A. M., Huang M. L., Bae D. H., Jansson P. J., Sahni S., Kalinowski D. S., and Richardson D. R. (2015) Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta 1853, 1130–1144 [DOI] [PubMed] [Google Scholar]
- 16. Lill R., Hoffmann B., Molik S., Pierik A. J., Rietzschel N., Stehling O., Uzarska M. A., Webert H., Wilbrecht C., and Mühlenhoff U. (2012) The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 1823, 1491–1508 [DOI] [PubMed] [Google Scholar]
- 17. Makiuchi T., and Nozaki T. (2014) Highly divergent mitochondrion-related organelles in anaerobic parasitic protozoa. Biochimie 100, 3–17 [DOI] [PubMed] [Google Scholar]
- 18. Freibert S.-A., Goldberg A. V., Hacker C., Molik S., Dean P., Williams T. A., Nakjang S., Long S., Sendra K., Bill E., Heinz E., Hirt R. P., Lucocq J. M., Embley T. M., and Lill R. (2017) Evolutionary conservation and in vitro reconstitution of microsporidian iron–sulfur cluster biosynthesis. Nat. Commun. 8, 13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karnkowska A., Vacek V., Zubáčová Z., Treitli S. C., Petrželková R., Eme L., Novák L., Žárský, Barlow V. D., Herman E. K., Soukal P., Hroudová M., Doležal P., Stairs C. W., Roger A. J., Eliáš M., Dacks J. B., Vlček Č., and Hampl V. (2016) A eukaryote without a mitochondrial organelle. Curr. Biol. 26, 1274–1284 [DOI] [PubMed] [Google Scholar]
- 20. Blanc B., Gerez C., and Ollagnier de Choudens S. (2015) Assembly of Fe/S proteins in bacterial systems: biochemistry of the bacterial ISC system. Biochim. Biophys. Acta 1853, 1436–1447 [DOI] [PubMed] [Google Scholar]
- 21. Mühlenhoff U., and Lill R. (2000) Biogenesis of iron–sulfur proteins in eukaryotes: a novel task of mitochondria that is inherited from bacteria. Biochim. Biophys. Acta 1459, 370–382 [DOI] [PubMed] [Google Scholar]
- 22. Py B., and Barras F. (2015) Genetic approaches of the Fe-S cluster biogenesis process in bacteria: historical account, methodological aspects and future challenges. Biochim. Biophys. Acta 1853, 1429–1435 [DOI] [PubMed] [Google Scholar]
- 23. Mettert E. L., and Kiley P. J. (2015) How is Fe-S cluster formation regulated? Annu. Rev. Microbiol. 69, 505–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lill R. (2009) Function and biogenesis iron-sulphur proteins. Nature 460, 831–838 [DOI] [PubMed] [Google Scholar]
- 25. Webert H., Freibert S. A., Gallo A., Heidenreich T., Linne U., Amlacher S., Hurt E., Mühlenhoff U., Banci L., and Lill R. (2014) Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 5, 5013. [DOI] [PubMed] [Google Scholar]
- 26. Fox N. G., Chakrabarti M., McCormick S. P., Lindahl P. A., and Barondeau D. P. (2015) The human iron–sulfur assembly complex catalyzes the synthesis of [2Fe-2S] clusters on ISCU2 that can be transferred to acceptor molecules. Biochemistry 54, 3871–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shakamuri P., Zhang B., and Johnson M. K. (2012) Monothiol glutaredoxins function in storing and transporting [Fe2S2] clusters assembled on IscU scaffold proteins. J. Am. Chem. Soc. 134, 15213–15216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vranish J. N., Das D., and Barondeau D. P. (2016) Real-time kinetic probes support monothiol glutaredoxins as intermediate carriers in Fe-S cluster biosynthetic pathways. ACS Chem. Biol. 11, 3114–3121 [DOI] [PubMed] [Google Scholar]
- 29. Parent A., Elduque X., Cornu D., Belot L., Le Caer J. P., Grandas A., Toledano M. B., and D'Autréaux B. (2015) Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat. Commun. 6, 5686. [DOI] [PubMed] [Google Scholar]
- 30. Pandey A., Pain J., Ghosh A. K., Dancis A., and Pain D. (2015) Fe-S cluster biogenesis in isolated mammalian mitochondria: coordinated use of persulfide sulfur and iron and requirements for GTP, NADH, and ATP. J. Biol. Chem. 290, 640–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mühlenhoff U., Gerber J., Richhardt N., and Lill R. (2003) Components involved in assembly and dislocation of iron–sulfur clusters on the scaffold protein Isu1p. EMBO J. 22, 4815–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Vranken J. G., Jeong M.-Y., Wei P., Chen Y.-C., Gygi S. P., Winge D. R., and Rutter J. (2016) The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. eLife 5, e17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi R., Proteau A., Villarroya M., Moukadiri I., Zhang L., Trempe J. F., Matte A., Armengod M. E., and Cygler M. (2010) Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 8, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marinoni E. N., de Oliveira J. S., Nicolet Y., Raulfs E. C., Amara P., Dean D. R., and Fontecilla-Camps J. C. (2012) (IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew. Chem. Int. Ed. Engl. 51, 5439–5442 [DOI] [PubMed] [Google Scholar]
- 35. Majewska J., Ciesielski S. J., Schilke B., Kominek J., Blenska A., Delewski W., Song J. Y., Marszalek J., Craig E. A., and Dutkiewicz R. (2013) Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron–sulfur cluster scaffold Isu protein is mutually exclusive. J. Biol. Chem. 288, 29134–29142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiedemann N., Urzica E., Guiard B., Müller H., Lohaus C., Meyer H. E., Ryan M. T., Meisinger C., Mühlenhoff U., Lill R., and Pfanner N. (2006) Essential role of Isd11 in iron–sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 25, 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandey A., Golla R., Yoon H., Dancis A., and Pain D. (2012) Persulfide formation on mitochondrial cysteine desulfurase: enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem. J. 448, 171–187 [DOI] [PubMed] [Google Scholar]
- 38. Pandey A., Gordon D. M., Pain J., Stemmler T. L., Dancis A., and Pain D. (2013) Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. J. Biol. Chem. 288, 36773–36786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adam A. C., Bornhövd C., Prokisch H., Neupert W., and Hell K. (2006) The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 25, 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saha P. P., Srivastava S., Kumar S. K. P., Sinha D., and D'Silva P. (2015) Mapping key residues of ISD11 critical for NFS1-ISD11 subcomplex stability: implications in the development of mitochondrial disorder, COXPD19. J. Biol. Chem. 290, 25876–25890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Angerer H. (2015) Eukaryotic LYR proteins interact with mitochondrial protein complexes. Biology 4, 133–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Angerer H., Radermacher M., Mańkowska M., Steger M., Zwicker K., Heide H., Wittig I., Brandt U., and Zickermann V. (2014) The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. Proc. Natl. Acad. Sci. U.S.A. 111, 5207–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu J., Vinothkumar K. R., and Hirst J. (2016) Structure of mammalian respiratory complex I. Nature 536, 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai K., Frederick R. O., Tonelli M., and Markley J. L. (2017) Mitochondrial cysteine desulfurase and ISD11 coexpressed in Escherichia coli yield complex containing acyl carrier protein. ACS Chem. Biol. 12, 918–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hiltunen J. K., Autio K. J., Schonauer M. S., Kursu V. A., Dieckmann C. L., and Kastaniotis A. J. (2010) Mitochondrial fatty acid synthesis and respiration. Biochim. Biophys. Acta 1797, 1195–1202 [DOI] [PubMed] [Google Scholar]
- 46. Bridwell-Rabb J., Fox N. G., Tsai C. L., Winn A. M., and Barondeau D. P. (2014) Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 53, 4904–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mihara H., and Esaki N. (2002) Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol. 60, 12–23 [DOI] [PubMed] [Google Scholar]
- 48. di Maio D., Chandramouli B., Yan R., Brancato G., and Pastore A. (2017) Understanding the role of dynamics in the iron sulfur cluster molecular machine. Biochim. Biophys. Acta 1861, 3154–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prischi F., Konarev P. V., Iannuzzi C., Pastore C., Adinolfi S., Martin S. R., Svergun D. I., and Pastore A. (2010) Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat. Commun. 1, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manicki M., Majewska J., Ciesielski S., Schilke B., Blenska A., Kominek J., Marszalek J., Craig E. A., and Dutkiewicz R. (2014) Overlapping binding sites of the frataxin homologue assembly factor and the heat shock protein 70 transfer factor on the Isu iron–sulfur cluster scaffold protein. J. Biol. Chem. 289, 30268–30278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng L., Cash V. L., Flint D. H., and Dean D. R. (1998) Assembly of iron–sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273, 13264–13272 [DOI] [PubMed] [Google Scholar]
- 52. Sheftel A. D., Stehling O., Pierik A. J., Elsässer H. P., Mühlenhoff U., Webert H., Hobler A., Hannemann F., Bernhardt R., and Lill R. (2010) Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 11775–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shi Y., Ghosh M., Kovtunovych G., Crooks D. R., and Rouault T. A. (2012) Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron–sulfur cluster biogenesis. Biochim. Biophys. Acta 1823, 484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yan R., Adinolfi S., and Pastore A. (2015) Ferredoxin, in conjunction with NADPH and ferredoxin-NADP reductase, transfers electrons to the IscS/IscU complex to promote iron–sulfur cluster assembly. Biochim. Biophys. Acta 1854, 1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yan R., Konarev P. V., Iannuzzi C., Adinolfi S., Roche B., Kelly G., Simon L., Martin S. R., Py B., Barras F., Svergun D. I., and Pastore A. (2013) Ferredoxin competes with bacterial frataxin in binding to the desulfurase IscS. J. Biol. Chem. 288, 24777–24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith A. D., Agar J. N., Johnson K. A., Frazzon J., Amster I. J., Dean D. R., and Johnson M. K. (2001) Sulfur transfer from IscS to IscU: the first step in iron–sulfur cluster biosynthesis. J. Am. Chem. Soc. 123, 11103–11104 [DOI] [PubMed] [Google Scholar]
- 57. Urbina H. D., Silberg J. J., Hoff K. G., and Vickery L. E. (2001) Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 276, 44521–44526 [DOI] [PubMed] [Google Scholar]
- 58. Dzul S. P., Rocha A. G., Rawat S., Kandegedara A., Kusowski A., Pain J., Murari A., Pain D., Dancis A., and Stemmler T. L. (2017) In vitro characterization of a novel Isu homologue from Drosophila melanogaster for de novo FeS-cluster formation. Metallomics 9, 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pastore A., and Puccio H. (2013) Frataxin: a protein in search for a function. J. Neurochem. 126, 43–52 [DOI] [PubMed] [Google Scholar]
- 60. Yoon H., Knight S. A., Pandey A., Pain J., Turkarslan S., Pain D., and Dancis A. (2015) Turning Saccharomyces cerevisiae into a frataxin-independent organism. PLoS Genet. 11, e1005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pagnier A., Nicolet Y., and Fontecilla-Camps J. C. (2015) IscS from Archaeoglobus fulgidus has no desulfurase activity but may provide a cysteine ligand for [Fe2S2] cluster assembly. Biochim. Biophys. Acta 1853, 1457–1463 [DOI] [PubMed] [Google Scholar]
- 62. Gakh O., Ranatunga W., Smith D. Y. 4th., Ahlgren E.-C., Al-Karadaghi S., Thompson J. R., and Isaya G. (2016) Architecture of the human mitochondrial iron–sulfur cluster assembly machinery. J. Biol. Chem. 291, 21296–21321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ranatunga W., Gakh O., Galeano B. K., Smith D. Y. 4th., Söderberg C. A., Al-Karadaghi S., Thompson J. R., and Isaya G. (2016) Architecture of the yeast mitochondrial iron–sulfur cluster assembly machinery: the sub-complex formed by the iron donor, Yfh1 protein, and the scaffold, Isu1 protein. J. Biol. Chem. 291, 10378–10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kampinga H. H., and Craig E. A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bonomi F., Iametti S., Morleo A., Ta D., and Vickery L. E. (2011) Facilitated transfer of IscU-[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry 50, 9641–9650 [DOI] [PubMed] [Google Scholar]
- 66. Iametti S., Barbiroli A., and Bonomi F. (2015) Functional implications of the interaction between HscB and IscU in the biosynthesis of FeS clusters. J. Biol. Inorg. Chem. 20, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 67. Saibil H. (2013) Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maio N., and Rouault T. A. (2016) Mammalian Fe-S proteins: definition of a consensus motif recognized by the co-chaperone HSC20. Metallomics 8, 1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vickery L. E., and Cupp-Vickery J. R. (2007) Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron–sulfur protein maturation. Crit. Rev. Biochem. Mol. Biol. 42, 95–111 [DOI] [PubMed] [Google Scholar]
- 70. Uzarska M. A., Dutkiewicz R., Freibert S. A., Lill R., and Mühlenhoff U. (2013) The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol. Biol. Cell 24, 1830–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Voisine C., Cheng Y. C., Ohlson M., Schilke B., Hoff K., Beinert H., Marszalek J., and Craig E. A. (2001) Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 98, 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pukszta S., Schilke B., Dutkiewicz R., Kominek J., Moczulska K., Stepien B., Reitenga K. G., Bujnicki J. M., Williams B., Craig E. A., and Marszalek J. (2010) Co-evolution-driven switch of J-protein specificity towards an Hsp70 partner. EMBO Rep. 11, 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johansson C., Roos A. K., Montano S. J., Sengupta R., Filippakopoulos P., Guo K., von Delft F., Holmgren A., Oppermann U., and Kavanagh K. L. (2011) The crystal structure of human GLRX5: iron–sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem. J. 433, 303–311 [DOI] [PubMed] [Google Scholar]
- 74. Palmieri F., and Monné M. (2016) Discoveries, metabolic roles and diseases of mitochondrial carriers: a review. Biochim. Biophys. Acta 1863, 2362–2378 [DOI] [PubMed] [Google Scholar]
- 75. Maio N., Ghezzi D., Verrigni D., Rizza T., Bertini E., Martinelli D., Zeviani M., Singh A., Carrozzo R., and Rouault T. A. (2016) Disease-causing SDHAF1 mutations impair transfer of Fe-S clusters to SDHB. Cell Metab. 23, 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Camaschella C., Campanella A., De Falco L., Boschetto L., Merlini R., Silvestri L., Levi S., and Iolascon A. (2007) The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 110, 1353–1358 [DOI] [PubMed] [Google Scholar]
- 77. Gelling C., Dawes I. W., Richhardt N., Lill R., and Mühlenhoff U. (2008) Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes. Mol. Cell. Biol. 28, 1851–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mühlenhoff U., Richter N., Pines O., Pierik A. J., and Lill R. (2011) Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe-4S] proteins. J. Biol. Chem. 286, 41205–41216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sheftel A. D., Wilbrecht C., Stehling O., Niggemeyer B., Elsässer H. P., Mühlenhoff U., and Lill R. (2012) The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol. Biol. Cell 23, 1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim K. D., Chung W. H., Kim H. J., Lee K. C., and Roe J. H. (2010) Monothiol glutaredoxin Grx5 interacts with Fe-S scaffold proteins Isa1 and Isa2 and supports Fe-S assembly and DNA integrity in mitochondria of fission yeast. Biochem. Biophys. Res. Commun. 392, 467–472 [DOI] [PubMed] [Google Scholar]
- 81. Rodríguez-Manzaneque M. T., Tamarit J., Bellí G., Ros J., and Herrero E. (2002) Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol. Biol. Cell 13, 1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brancaccio D., Gallo A., Mikolajczyk M., Zovo K., Palumaa P., Novellino E., Piccioli M., Ciofi-Baffoni S., and Banci L. (2014) Formation of [4Fe-4S] clusters in the mitochondrial iron–sulfur cluster assembly machinery. J. Am. Chem. Soc. 136, 16240–16250 [DOI] [PubMed] [Google Scholar]
- 83. Mapolelo D. T., Zhang B., Naik S. G., Huynh B. H., and Johnson M. K. (2012) Spectroscopic and functional characterization of iron-bound forms of Azotobacter vinelandii (Nif)IscA. Biochemistry 51, 8056–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mapolelo D. T., Zhang B., Naik S. G., Huynh B. H., and Johnson M. K. (2012) Spectroscopic and functional characterization of iron–sulfur cluster-bound forms of Azotobacter vinelandii (Nif)IscA. Biochemistry 51, 8071–8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bych K., Kerscher S., Netz D. J., Pierik A. J., Zwicker K., Huynen M. A., Lill R., Brandt U., and Balk J. (2008) The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 27, 1736–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bandyopadhyay S., Naik S. G., O'Carroll I. P., Huynh B. H., Dean D. R., Johnson M. K., and Dos Santos P. C. (2008) A proposed role for the Azotobacter vinelandii NfuA protein as an intermediate iron–sulfur cluster carrier. J. Biol. Chem. 283, 14092–14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Py B., Gerez C., Angelini S., Planel R., Vinella D., Loiseau L., Talla E., Brochier-Armanet C., Garcia Serres R., Latour J. M., Ollagnier-de Choudens S., Fontecave M., and Barras F. (2012) Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol. Microbiol. 86, 155–171 [DOI] [PubMed] [Google Scholar]
- 88. Sheftel A. D., Stehling O., Pierik A. J., Netz D. J., Kerscher S., Elsässer H. P., Wittig I., Balk J., Brandt U., and Lill R. (2009) Human Ind1, an iron–sulfur cluster assembly factor for respiratory complex I. Mol. Cell. Biol. 29, 6059–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Melber A., Na U., Vashisht A., Weiler B. D., Lill R., Wohlschlegel J. A., and Winge D. R. (2016) Role of Nfu1 and Bol3 in iron–sulfur cluster transfer to mitochondrial clients. eLife 5, e15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gao H., Subramanian S., Couturier J., Naik S. G., Kim S. K., Leustek T., Knaff D. B., Wu H. C., Vignols F., Huynh B. H., Rouhier N., and Johnson M. K. (2013) Arabidopsis thaliana Nfu2 accommodates [2Fe-2S] or [4Fe-4S] clusters and is competent for in vitro maturation of chloroplast [2Fe-2S] and [4Fe-4S] cluster-containing proteins. Biochemistry 52, 6633–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tong W. H., Jameson G. N., Huynh B. H., and Rouault T. A. (2003) Subcellular compartmentalization of human Nfu, an iron–sulfur cluster scaffold protein, and its ability to assemble a [4Fe-4S] cluster. Proc. Natl. Acad. Sci. U.S.A. 100, 9762–9767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cai K., Liu G., Frederick R. O., Xiao R., Montelione G. T., and Markley J. L. (2016) Structural/functional properties of human NFU1, an intermediate [4Fe-4S] carrier in human mitochondrial iron–sulfur cluster biogenesis. Structure 24, 2080–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Uzarska M. A., Nasta V., Weiler B. D., Spantgar F., Ciofi-Baffoni S., Saviello M. R., Gonnelli L., Mühlenhoff U., Banci L., and Lill R. (2016) Mitochondrial Bol1 and Bol3 function as assembly factors for specific iron–sulfur proteins. eLife 5, e16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li H., and Outten C. E. (2012) Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis. Biochemistry 51, 4377–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mapolelo D. T., Zhang B., Randeniya S., Albetel A. N., Li H., Couturier J., Outten C. E., Rouhier N., and Johnson M. K. (2013) Monothiol glutaredoxins and A-type proteins: partners in Fe-S cluster trafficking. Dalton Trans. 42, 3107–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mühlenhoff U., Molik S., Godoy J. R., Uzarska M. A., Richter N., Seubert A., Zhang Y., Stubbe J., Pierrel F., Herrero E., Lillig C. H., and Lill R. (2010) Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron–sulfur cluster. Cell Metab. 12, 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yeung N., Gold B., Liu N. L., Prathapam R., Sterling H. J., Willams E. R., and Butland G. (2011) The E. coli monothiol glutaredoxin GrxD forms homodimeric and heterodimeric FeS cluster containing complexes. Biochemistry 50, 8957–8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roret T., Tsan P., Couturier J., Zhang B., Johnson M. K., Rouhier N., and Didierjean C. (2014) Structural and spectroscopic insights into BolA-glutaredoxin complexes. J. Biol. Chem. 289, 24588–24598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Couturier J., Przybyla-Toscano J., Roret T., Didierjean C., and Rouhier N. (2015) The roles of glutaredoxins ligating Fe-S clusters: sensing, transfer or repair functions? Biochim. Biophys. Acta 1853, 1513–1527 [DOI] [PubMed] [Google Scholar]
- 100. Bordoli L., Kiefer F., Arnold K., Benkert P., Battey J., and Schwede T. (2009) Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4, 1–13 [DOI] [PubMed] [Google Scholar]