Abstract
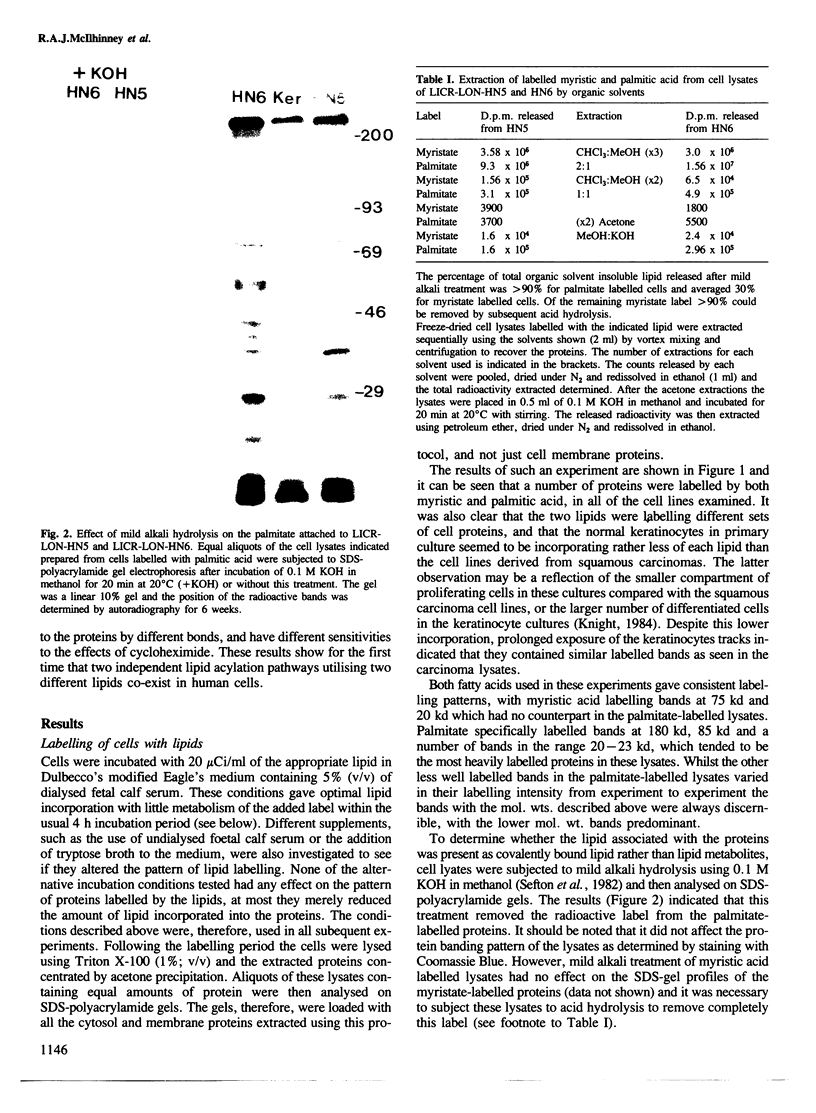
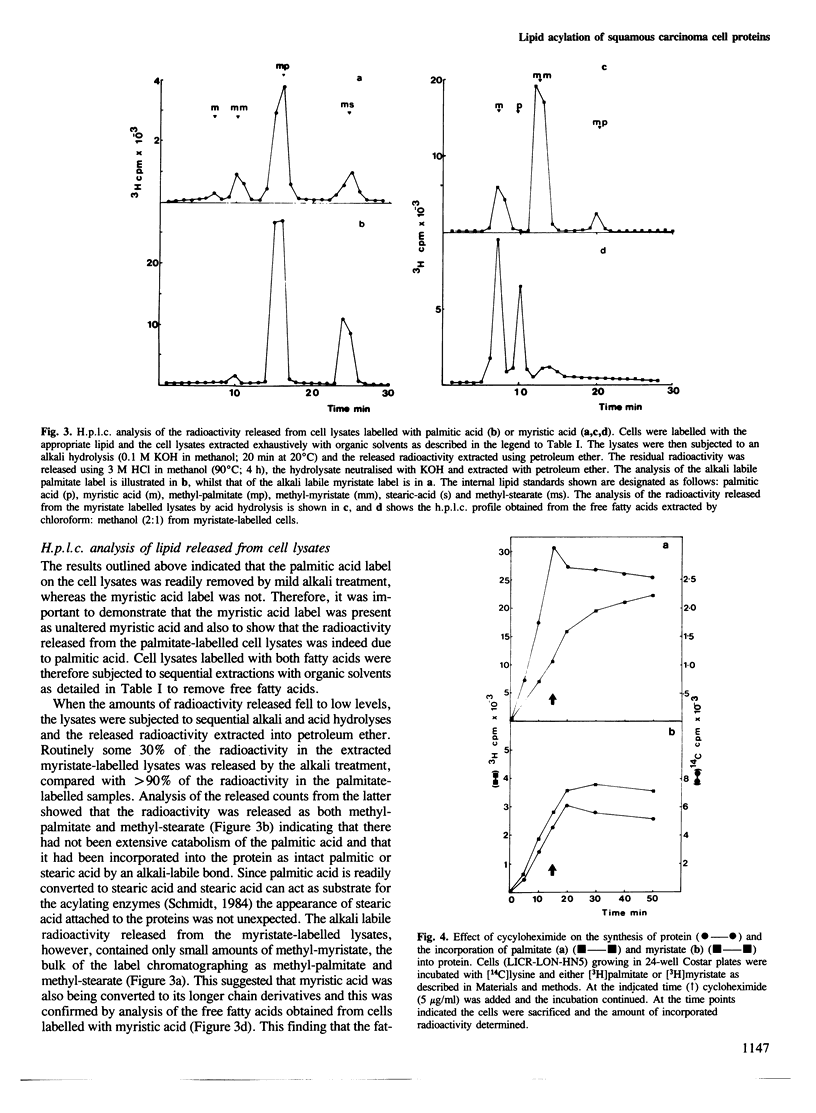
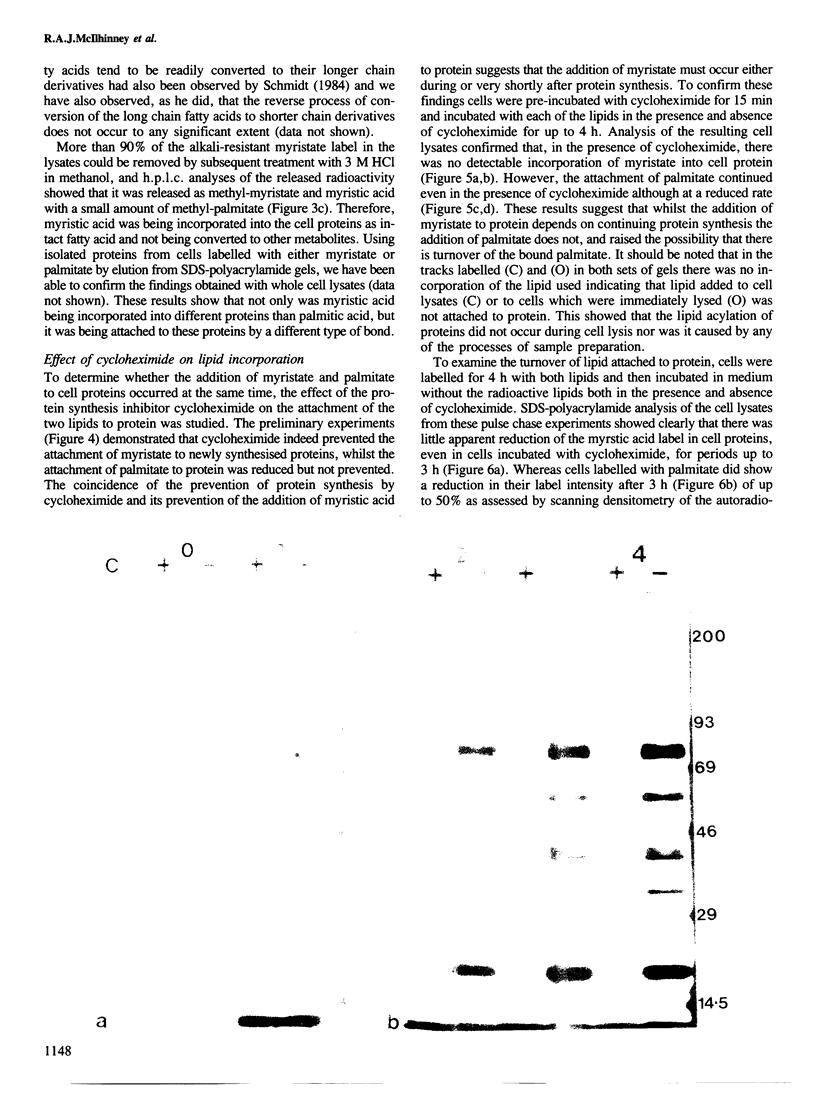
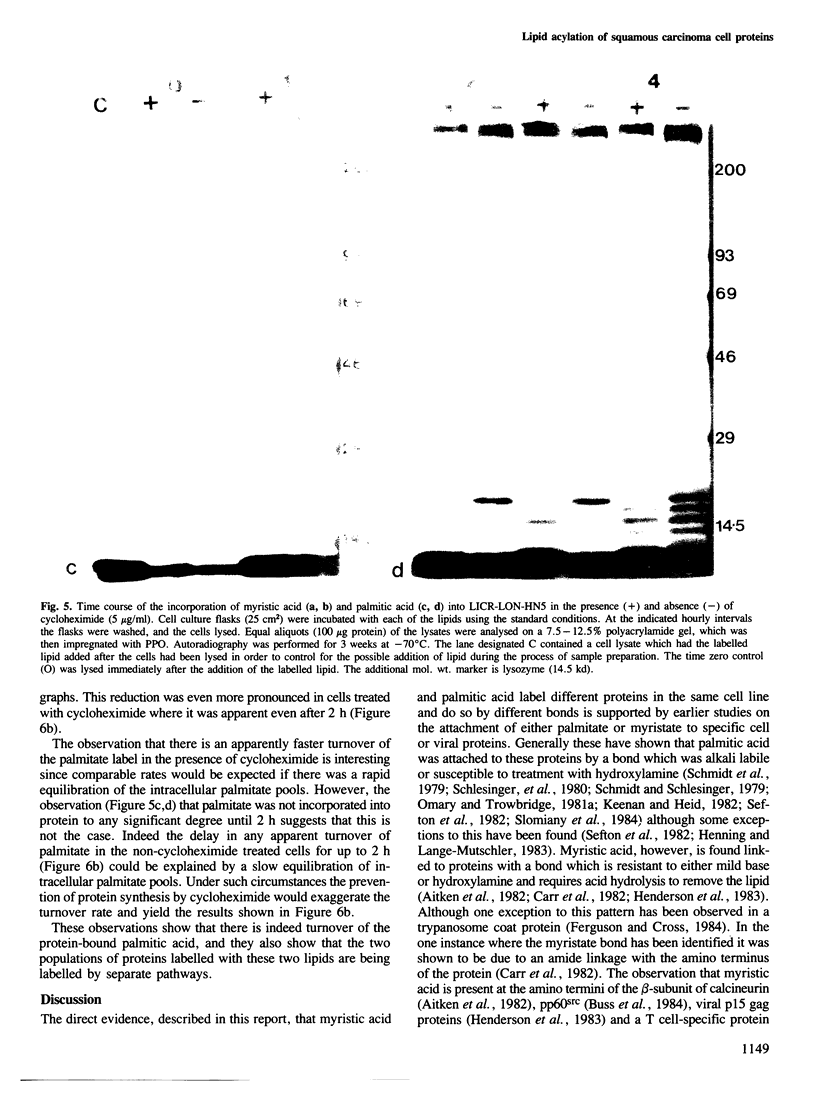
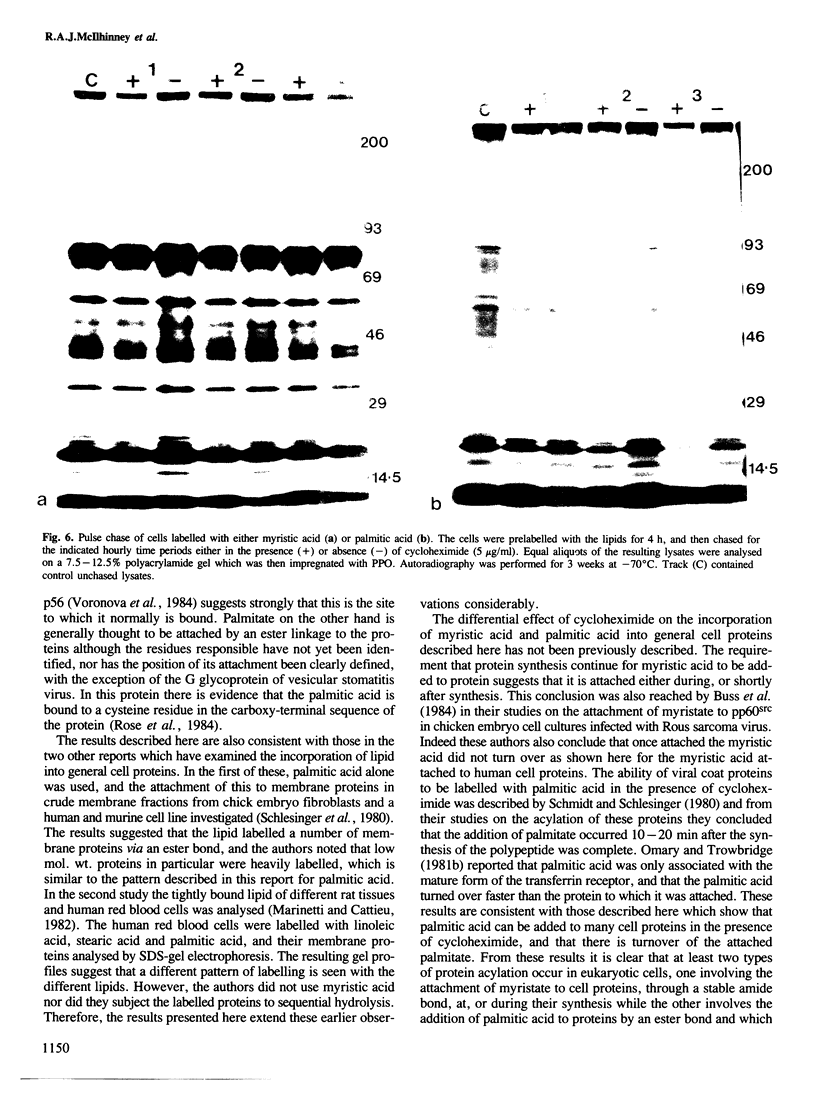
The ability of human keratinocytes and squamous carcinoma cell lines to attach lipid covalently to cell proteins has been examined using both palmitic and myristic acids. SDS-polyacrylamide gel analyses of the proteins labelled with these lipids demonstrated that each labelled a different set of proteins. Covalently protein bound palmitic acid could be removed from the proteins by mild alkali hydrolysis but the bound myristic acid required prolonged acid hydrolysis to release it from the associated proteins. H.p.l.c. analyses of the released lipid confirmed that both lipids were attached to proteins directly and that the labelling was not due to the lipids being catabolised. Cycloheximide could prevent the attachment of myristic acid to cell proteins, but only reduced the levels of palmitic acid incorporation. Pulse chase experiments indicated that there was little turnover of the attached myristic acid whereas this was significant for covalently bound palmitic acid. These observations show for the first time that two different protein populations are labelled by different lipids in eukaryotic cells, and that there appear to be two separate pathways for the acylation of proteins in such cells.
Full text
PDF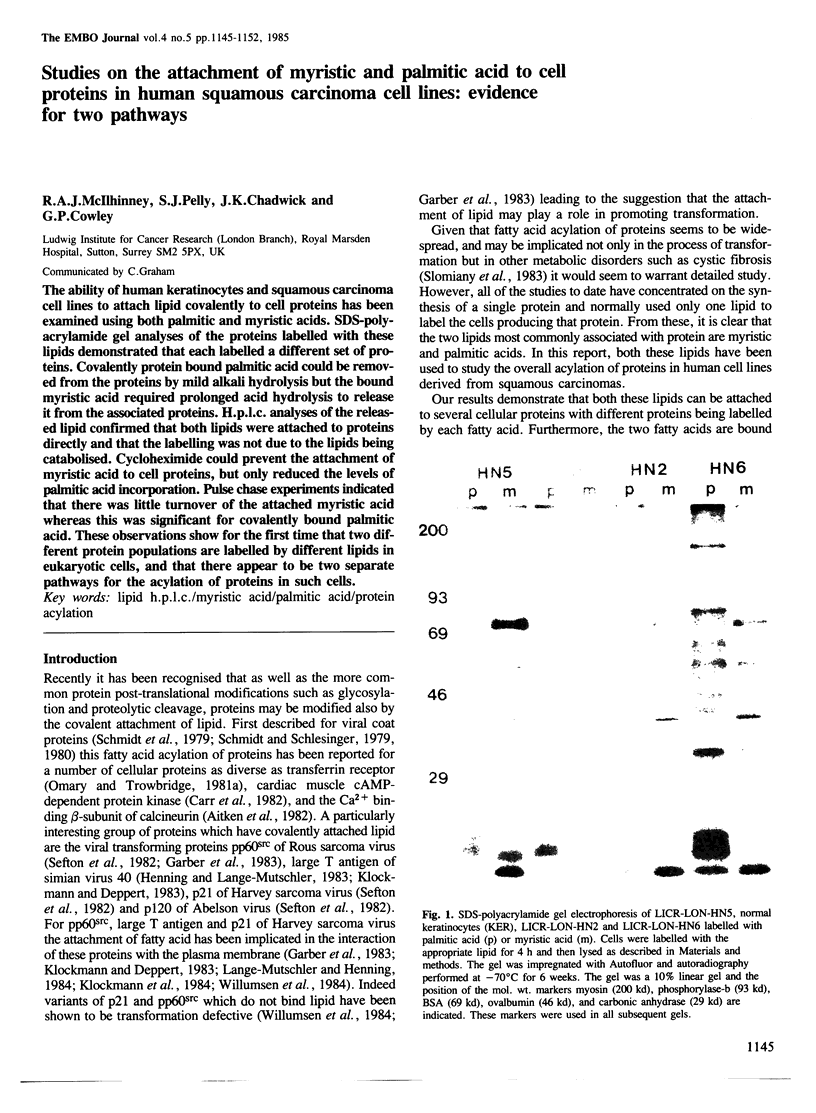


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Pittam M. R., James T. Five human tumour cell lines derived from a primary squamous carcinoma of the tongue, two subsequent local recurrences and two nodal metastases. Br J Cancer. 1981 Sep;44(3):363–370. doi: 10.1038/bjc.1981.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Cross G. A. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3011–3015. [PubMed] [Google Scholar]
- Garber E. A., Krueger J. G., Hanafusa H., Goldberg A. R. Only membrane-associated RSV src proteins have amino-terminally bound lipid. Nature. 1983 Mar 10;302(5904):161–163. doi: 10.1038/302161a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Lange-Mutschler J. Tightly associated lipids may anchor SV40 large T antigen in plasma membrane. Nature. 1983 Oct 20;305(5936):736–738. doi: 10.1038/305736a0. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Heid H. W., Stadler J., Jarasch E. d., Franke W. W. Tight attachment of fatty acids to proteins associated with milk lipid globule membrane. Eur J Cell Biol. 1982 Feb;26(2):270–276. [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Staufenbiel M., Deppert W. Membrane interactions of simian virus 40 large T-antigen: influence of protein sequences and fatty acid acylation. Mol Cell Biol. 1984 Aug;4(8):1542–1550. doi: 10.1128/mcb.4.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange-Mutschler J., Henning R. Cell surface binding simian virus 40 large T antigen becomes anchored and stably linked to lipid of the target cells. Virology. 1984 Jul 30;136(2):404–413. doi: 10.1016/0042-6822(84)90176-4. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Marinetti G. V., Cattieu K. Tightly (covalently) bound fatty acids in cell membrane proteins. Biochim Biophys Acta. 1982 Feb 23;685(2):109–116. doi: 10.1016/0005-2736(82)90086-4. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Magee A. I., Schmidt M. F. Fatty acid acylation of proteins in cultured cells. J Biol Chem. 1980 Nov 10;255(21):10021–10024. [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Schmidt M. F. The transfer of myristic and other fatty acids on lipid and viral protein acceptors in cultured cells infected with Semliki Forest and influenza virus. EMBO J. 1984 Oct;3(10):2295–2300. doi: 10.1002/j.1460-2075.1984.tb02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Witas H., Aono M., Slomiany B. L. Covalently linked fatty acids in gastric mucus glycoprotein of cystic fibrosis patients. J Biol Chem. 1983 Jul 25;258(14):8535–8538. [PubMed] [Google Scholar]
- Slomiany B. L., Takagi A., Liau Y. H., Jozwiak Z., Slomiany A. In vitro acylation of rat gastric mucus glycoprotein with [3H]palmitic acid. J Biol Chem. 1984 Oct 10;259(19):11997–12000. [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]