Abstract
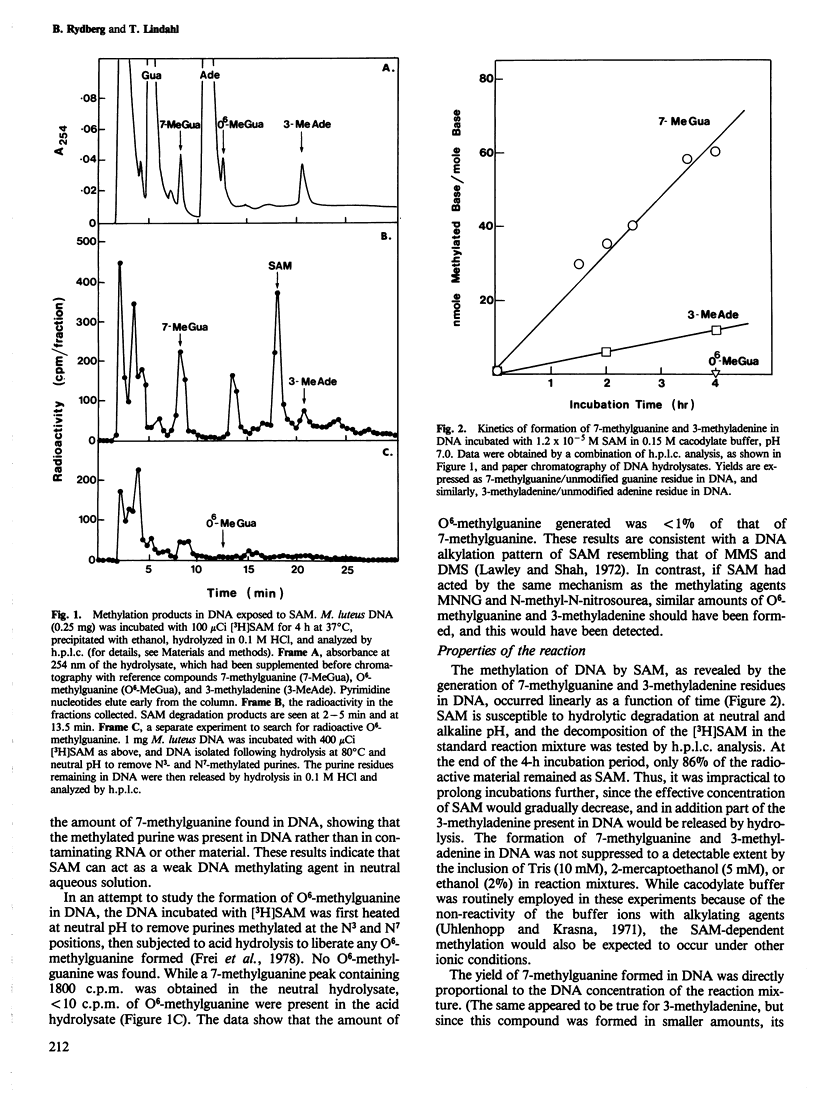
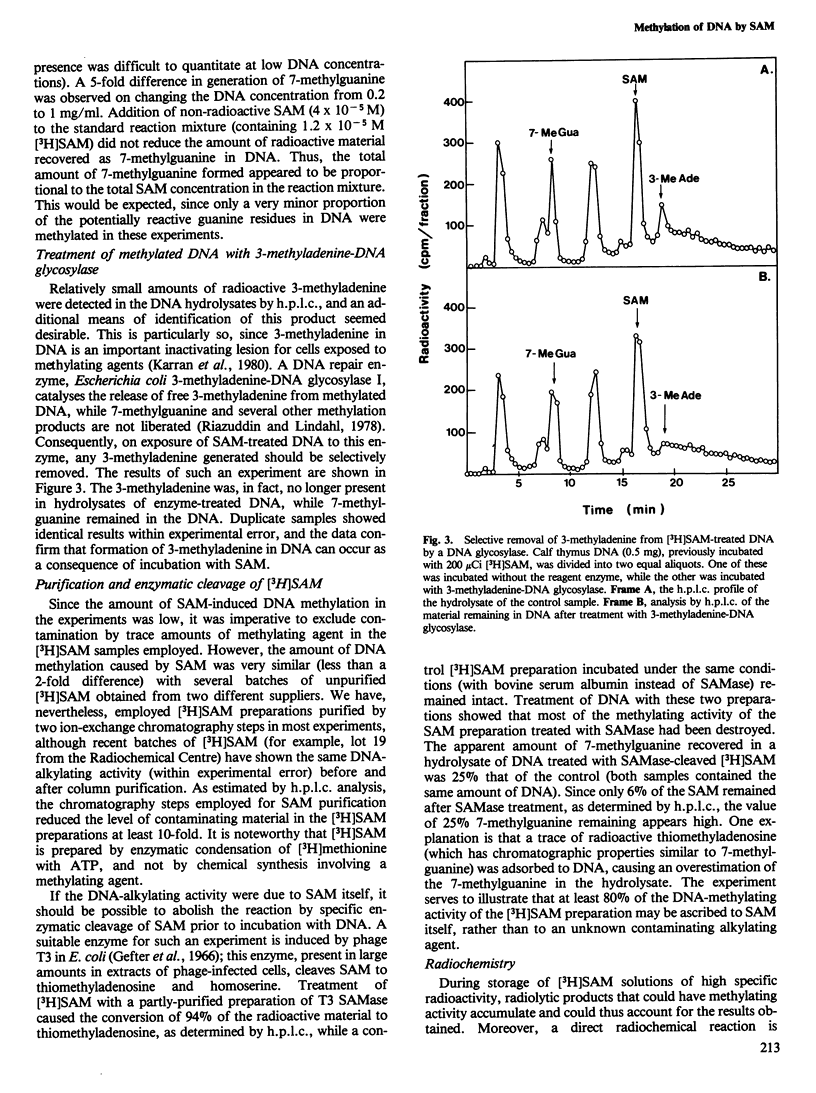
Incubation of DNA with S-adenosyl-L-methionine (SAM) in neutral aqueous solution leads to base modification, with formation of small amounts of 7-methylguanine and 3-methyladenine. The products have been identified by high performance liquid chromatography of DNA hydrolysates and by the selective release of free 3-methyladenine from SAM-treated DNA by a specific DNA glycosylase. We conclude that SAM acts as a weak DNA-alkylating agent. Several control experiments including extensive purification of [3H-methyl]SAM preparations and elimination of the alkylating activity by pretreatment of SAM with a phage T3-induced SAM cleaving enzyme, have been performed to determine that the activity observed was due to SAM itself and not to a contaminating substance. We estimate that SAM, at an intracellular concentration of 4 X 10(-5) M, causes DNA alkylation at a level similar to that expected from continuous exposure of cells to 2 X 10(-8) M methyl methane-sulphonate. This ability of SAM to act as a methyl donor in a nonenzymatic reaction could result in a background of mutagenesis and carcinogenesis. The data provide an explanation for the apparently universal occurrence of multiple DNA repair enzymes specific for methylation damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker R. A., Barrows L. R., Shank R. C. Methylation of liver DNA guanine in hydrazine hepatotoxicity: dose-response and kinetic characteristics of 7-methylguanine and O6-methylguanine formation and persistence in rats. Carcinogenesis. 1981;2(11):1181–1188. doi: 10.1093/carcin/2.11.1181. [DOI] [PubMed] [Google Scholar]
- Clapp N. K., Craig A. W., Toya R. E., Sr Oncogenicity by methyl methanesulfonate in male RF mice. Science. 1968 Aug 30;161(3844):913–914. doi: 10.1126/science.161.3844.913. [DOI] [PubMed] [Google Scholar]
- Craddock V. M., Henderson A. R. De novo and repair replication of DNA in liver of carcinogen-treated animals. Cancer Res. 1978 Jul;38(7):2135–2143. [PubMed] [Google Scholar]
- Druckrey H., Kruse H., Preussmann R., Ivankovic S., Landschütz C. Cancerogene alkylierende Substanzen. 3. Alkyl-halogenide, -sulfate, -sulfonate und ringgespannte Heterocyclen. Z Krebsforsch. 1970;74(3):241–273. [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. Measurement of S-adenosyl-L-methionine levels by SP Sephadex chromatography. Anal Biochem. 1978 Dec;91(2):516–520. doi: 10.1016/0003-2697(78)90538-9. [DOI] [PubMed] [Google Scholar]
- Green M. H., Muriel W. J., Bridges B. A. Use of a simplified fluctuation test to detect low levels of mutagens. Mutat Res. 1976 Feb;38(1):33–42. doi: 10.1016/0165-1161(76)90077-7. [DOI] [PubMed] [Google Scholar]
- Hoppe H., 4th, Skopeck T. R., Liber H. L., Thilly W. G. Alkyl methane sulfonate mutation of diploid human lymphoblasts and Salmonella typhimurium. Cancer Res. 1978 Jun;38(6):1595–1600. [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Ofsteng I., Evensen G. B., Seeberg E. Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol. 1980 Jun 15;140(1):101–127. doi: 10.1016/0022-2836(80)90358-7. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Mende C., Reucher W. Tumours of the peripheral and central nervous system induced in BD-rats by prenatal application of methyl methanesulfonate. Eur J Cancer. 1972 Dec;8(6):641–645. doi: 10.1016/0014-2964(72)90146-6. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Brookes P. Cytotoxicity of alkylating agents towards sensitive and resistant strains of Escherichia coli in relation to extent and mode of alkylation of cellular macromolecules and repair of alkylation lesions in deoxyribonucleic acids. Biochem J. 1968 Sep;109(3):433–447. doi: 10.1042/bj1090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Reaction of alkylating mutagens and carcinogens with nucleic acids: detection and estimation of a small extent of methylation at O-6 of guanine in DNA by methyl methanesulphonate in vitro. Chem Biol Interact. 1972 Sep;5(4):286–288. doi: 10.1016/0009-2797(72)90033-6. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Lee H. W., Kim S. Non-enzymatic methylation of proteins with S-adenosyl-L-methionine. FEBS Lett. 1975 Oct 15;58(1):39–42. doi: 10.1016/0014-5793(75)80220-1. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Pearson D., Lee H. W., Kim S. Nonenzymatic acetylation of histones with acetyl-CoA. Biochim Biophys Acta. 1970 Aug 8;213(2):513–522. doi: 10.1016/0005-2787(70)90058-4. [DOI] [PubMed] [Google Scholar]
- Riazuddin S., Lindahl T. Properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Biochemistry. 1978 May 30;17(11):2110–2118. doi: 10.1021/bi00604a014. [DOI] [PubMed] [Google Scholar]
- Salvatore F., Utili R., Zappia V., Shapiro S. K. Quantitative analysis of S-adenosylmethionine and S-adenosylhomocysteine in animal tissues. Anal Biochem. 1971 May;41(1):16–28. doi: 10.1016/0003-2697(71)90187-4. [DOI] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Much of spontaneous mutagenesis in Escherichia coli is due to error-prone DNA repair: implications for spontaneous carcinogenesis. Carcinogenesis. 1981;2(9):863–872. doi: 10.1093/carcin/2.9.863. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Induction of rat kidney tumours by ethyl methanesulphonate and nervous tissue tumours by methyl methanesulphonate and ethyl methanesulphonate. Nature. 1969 Aug 30;223(5209):947–949. doi: 10.1038/223947a0. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Pegg A. E., Hawks A., Farber E., Magee P. N. Evidence for ethylation of rat liver deoxyribonucleic acid after administration of ethionine. Biochem J. 1971 Jun;123(2):175–181. doi: 10.1042/bj1230175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhopp E. L., Krasna A. I. Alterations in the structure of deoxyribonucleic acid on chemical methylation. Biochemistry. 1971 Aug 17;10(17):3290–3295. doi: 10.1021/bi00793a020. [DOI] [PubMed] [Google Scholar]


