Abstract
To monitor the health of cells, the immune system tasks antigen presenting cells with gathering antigens from other cells and reporting them to CD8 T cells in the form of peptides bound to MHC I molecules. Most cells would be unable to perform this function because they use their MHC I molecules to exclusively present peptides derived from the cell’s own proteins. However, the immune system evolved mechanisms for dendritic cells and some other phagocytes to sample and present antigens from the extracellular milieu on MHC I through a process called cross-presentation (XPT). How this important task is accomplished, its role in health and disease and its potential for exploitation are the subject of this review.
Keywords: Antigen presentation, antigen processing, dendritic cell, immune surveillance, CD8 T lymphocytes, phagosomes
Introduction
In cells, all intracellular proteins continuously turn over as part of normal catabolism. The hydrolysis of these cellular polypeptides occurs primarily through the ubiquitin-proteasome pathway (1). Proteasomes are large barrel-shaped proteolytic particles that are present in the cytosol and nucleus and are responsible for virtually all of the extralysosomal catabolism of cellular proteins. Proteasomes cleave these substrates into oligopeptides, the bulk of which are further hydrolyzed by peptidases and ultimately reduced to amino acids (2). However, a fraction of the oligopeptides escape this fate when they are transferred into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) (3). Once inside the ER, peptides of the right length (usually 8–11 amino acids long) and sequence are loaded onto MHC I molecules and are transported to the cell surface for display to CD8 T cells (Figure 1). Peptides transported into the ER that are too long for presentation can be further trimmed by the aminopeptidase ERAP1 (and ERAP2, if present) to the right size for MHC I binding (4, 5). This antigen processing and presentation process is called the classical (or endogenous) MHC I antigen presentation pathway.
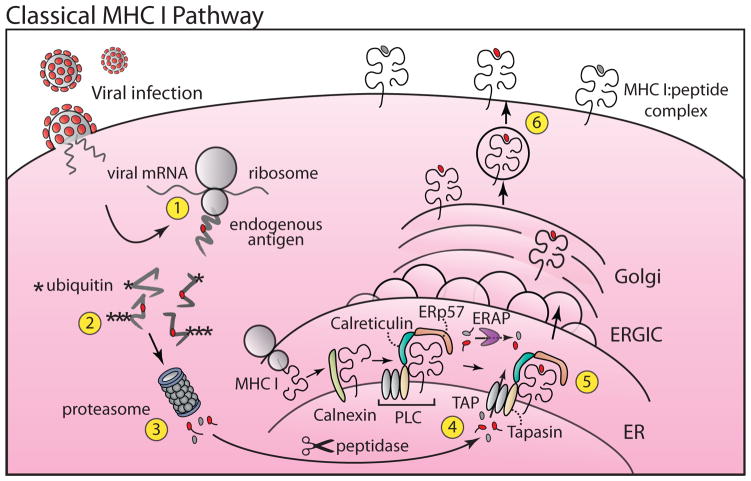
Figure 1. Classical Class I Antigen Presentation.
The classical pathway monitors the self and foreign proteins that are synthesized by cells (Step 1). Expressed proteins destined for degradation are conjugated with ubiquitin (Step 2) followed by proteasomal degradation (Step 3). Long peptides undergo trimming by cytosolic peptidases. A fraction of peptides (red) are translocated into the lumen of the ER via TAP (Step 4). Some long peptides undergo trimming in the ER by ERAP Newly synthesized MHC I molecules first associate with the chaperone calnexin and then via tapasin to TAP in the PLC. After binding TAP-transported peptide (Step 5) the MHC I: peptide complexes are transported through the secretory pathway to the plasma membrane (Step 6) where they are presented to CD8+ cytotoxic T cells. Transporter associated with antigen processing (TAP), Endoplasmic reticulum aminopeptidase (ERAP), peptide loading complex (PLC), ER-Golgi intermediate compartment (ERGIC).
In the classical pathway, the proteins that are turned over by the ubiquitin-proteasome pathway are normally all ones that were synthesized by the cell itself. Therefore, by monitoring the peptides generated during this catabolism, the MHC I antigen presentation pathway allows CD8 T cells to monitor the nature of the self-proteins made by cells. Under normal physiological conditions all of these MHC I-presented peptides will be from autologous proteins. In normal individuals, these peptide-MHC I complexes don’t provoke a response because CD8 T cells are tolerant to the autologous sequences. However, if a cell is infected with a virus or expressing mutated genes (e.g. in a cancer), or is from an allogeneic transplant, then “foreign” antigenic peptides will be displayed, allowing CD8 T cell effectors to identify such cells, and eliminate them.
In contrast, antigens that are in a cell’s external environment do not normally gain access to the subcellular compartments that are monitored by the MHC I antigen presentation pathway (6). This exclusion of external proteins from the MHC I pathway is adaptive because the presentation of external antigens on MHC I molecules could cause an immune response to otherwise healthy cells. Instead, exogenous antigens are internalized into endocytic compartments, where they are degraded into peptides (endosomes and lysosomes being the other major proteolytic compartments in cells). Such endosomal peptides are not normally presented on MHC I molecules, but instead can be bound and displayed on MHC II molecules, where they can stimulate CD4 T cell immunity (7).; in this review and more generally in the field, presentation of exogenous antigens on MHC II is not referred to as XPT.
A curious exception to these rules was first observed in 1976, when it was found that genetically polymorphic antigens from a transplant somehow stimulated CD8 T cells recognizing these antigens presented on the recipient’s rather than the transplant’s MHC I molecules (8). This phenomenon was called “cross-priming” and the response suggested that antigens from one cell could be acquired and presented by a different cell (a phenomenon called XPT). For many years, the only known mechanism by which cells could acquire and present exogenous antigens was when they were pulsed with peptides from protein antigens that bound to surface MHC I molecules (9). However, in 1990 it was discovered that some primary cells in lymphoid organs could generate and display MHC I-presented peptides from whole proteins in the extracellular milieu (10). The antigen presenting cells (APC) turned out to be dendritic cells (DCs) and macrophages (11–16). The XPT pathway in these APCs was relatively inefficient, generating many fewer peptide-MHC complexes compared to the endogenous pathway (17), but what was generated was sufficient to stimulate CD8 T cell responses.
Role of XPT: Priming CD8 T cells
XPT was originally viewed as an obscure phenomenon of little importance. However, we now appreciate that this pathway plays a key role in immune surveillance of cancers and in the immune response to certain infections and transplants. This key role is because of the way in which tissues are monitored for these conditions. Naïve CD8 T cells do not directly patrol peripheral tissues looking for APCs. In fact, there are simply too few naïve CD8 cells of any particular specificity to do so effectively. Instead, naïve CD8 T cells recirculate only through secondary lymphoid tissues (18) and thus, antigens in the peripheral tissues must be brought to CD8 T cells in lymphoid tissues to initiate immune responses and this task is the providence of DCs. To accomplish this, DCs are widely dispersed throughout tissues, where they collect exogenous antigens through phagocytosis or other endocytic mechanisms (19). These cells subsequently are directed by their chemokine receptors, such as CCR7, to migrate into the T cell zone in lymph nodes (20) where they display the tissue antigens they have acquired as peptides bound to MHC I and II molecules. Antigens that drain through lymphoid tissues can also be acquired and cross-presented by resident DCs (21). DCs can use the same MHC I presentation mechanisms as other cells to present antigens if they have been infected with a virus sometime during their sojourns (22, 23). However, the DCs require XPT mechanisms to present exogenous antigens they have collected but are not themselves synthesizing.
That such XPT is indeed necessary for inducing CD8 T cell response to exogenous antigens has been shown in experiments with mice whose DCs lack the ability to present antigens (e.g. due to TAP-deficiency). Such animals are unable to generate CD8 T cell responses to cancers (24) or to tissue-tropic viral infections that don’t infect DCs (25). In addition, a substantial component of transplant rejection to minor histocompatibility antigens (all the genetically polymorphic proteins that are encoded outside of the MHC complex) arises from XPT (26). Given this role in initiating responses to antigens in cells, it is not surprising that XPT can also contribute to the pathogenesis of autoimmune disease. The XPT of self-antigens that are released from autologous cells has been suggested to be involved in the initiation of autoimmunity (27, 28) and autoimmune epitope spreading (29). XPT is also likely important for anti-tumor immunotherapy with checkpoint inhibitors (30).
There is evidence that XPT can also contribute to initiating CD8 T cell immunity even for viruses that can infect DCs. (31–33). Presumably, XPT by uninfected DCs and direct presentation by infected ones are occurring in parallel. XPT from retained antigen may also allow for prolonged antigen presentation after an infection is cleared (34). In addition, XPT is likely to be an even more important component for priming CD8 T cells in situations where direct viral infection impairs the function of DCs (32, 35). One of the limitations in assessing the full extent of the contribution of XPT to CD8 T cell immunity is that the available experimental models do not completely eliminate all the XPT pathways. Therefore, a more complete understanding of the role of XPT awaits the generation of better experimental models wherein XPT is more effectively eliminated.
Instead of inducing immunity, cross-presenting DCs can also induce tolerance in a phenomenon called “cross-tolerance.” This is thought to occur when these cells cross-present antigen but have not been induced to express co-stimulatory molecules. Naïve CD8 T cells that are stimulated by peptide-MHC complexes (signal 1) without co-stimulation (signal 2) may be tolerized instead of being activated (36). Cross-tolerance has been shown to occur for transgenic and endogenous antigens expressed in peripheral tissues and has been proposed to be an important mechanism for inducing peripheral tolerance (28, 37, 38). It may also be a mechanism that contributes to central tolerance through negative selection in the thymus (39).
DC acquisition of antigens for XPT
Exogenous antigens that are taken up by fluid phase pinocytosis are usually cross-presented very inefficiently, with peptide-MHC I complexes only being generated at very high antigen concentrations (10, 12). This is one of the reasons that soluble antigens in the extracellular fluids usually fail to prime CD8 T cell responses (40). In contrast particulate antigens that are internalized by phagocytosis or macropinocytosis are cross-presented much more efficiently (12, 41, 42). It is thought that this is because the phagosomes and macropinosomes have the appropriate molecular machinery for XPT (see below) and perhaps also because these endocytic mechanisms internalize larger amounts of antigen. Similarly, soluble proteins that are internalized by receptor-mediated endocytosis can be cross-presented more efficiently, presumably for similar reasons (43–45).
One of the important physiological (and pathological) sources of particulate antigens comes from dying autologous cells. Reducing ingestion of such cells with a dominant negative Rac 1 transgene expressed in DCs (although this model also has some reduction in DC numbers that may contribute to the phenotype) or deletion of the C-type lectin receptor Clec9a impairs XPT as well as cross-priming of CD8 T cell responses in vivo (31, 46). Cell surface receptors such as the AXL/LRP-1/RANBP9 complex have been implicated in the ingestion and XPT of antigens from apoptotic cells (47) as has Clec9a for necrotic cells (31, 48, 49). In this process all exogenous cellular antigens, regardless of their subcellular location in the original antigen donor cell, are thought to have the potential to be cross-presented (50, 51) Additionally, there is some evidence that when antigens are in autophagosomes or bound to heat shock proteins (HSP), vesicles or complexes that may form in stressed cells), their XPT may be enhanced (52–54), presumably due to enhanced uptake into cross-presenting compartments. Since cell stress and death may be a consequence of a viral infection or malignant transformation, it makes biological sense for the immune system to focus on cross-presenting antigens from such cells.
In addition, it is also clear that when living cells are injected in vivo, their antigens are cross-presented (33, 55, 56). This XPT does not seem to be just from the transferred cells that subsequetly die because antigens from injected living cells can be even more efficiently cross-presented than ones from equivalent numbers of dead cells (Shen & Rock, unpublished data). Moreover, there is XPT of antigens from normal cells resident in tissues that are thought to be long-lived with little turn over (e.g. beta islet cells in the pancreas) (28). This has led to the idea that DCs are able to sample antigens not only from dead cells but also from live ones (55–57). One mechanism by which this could occur is by DCs ingesting antigens that are secreted, especially in particulate form, e.g. in exosomes (57). In addition, DCs may acquire antigens from living cells by taking a bite out of them (a process that has been termed “nibbling”) (58).
Some other potential mechanisms that may also contribute to the acquisition of antigens from living, and in some cases also dying or dead cells. In one such mechanism, peptides may be transferred through GAP junctions that form between DCs and antigen donor cells (59). Another mechanism is the transfer of peptide-MHC I complexes from the antigen donor cell to DCs, a process called “cross-dressing” or “trogocytosis” (60–62). However, there is some data that suggest that GAP junctions and trogocytosis are unlikely to be the major mechanisms underlying XPT in vivo, at least in many situations. In one set of experiments, donor cells with large numbers of antigen-MHC I complexes but low levels of intact antigen failed to cross-prime responses (63–65). Therefore, peptide or peptide-MHC I complexes aren’t dominant mechanisms driving XPT in vivo, at least in these systems. Consistent with this, intact cellular proteins have been shown to be the predominant source of cross-presented antigen (51, 66). Moreover, cross-priming to viral and cancer antigens requires a functional TAP peptide transporter in bone marrow-derived cells and TAP should not be required if DCs were simply acquiring peptide-MHC I complexes by trogocytosis. In addition, antigens from cells that lack the proper MHC I molecules (e.g. antigens in allogeneic antigen donor cells) can also cross-prime responses (67). Although trogocytosis by APC may not prime CD8 T cell responses in naïve individuals, there is some evidence that it can stimulate memory CD8 responses in vivo (68).
In addition to cells, parasites and bacteria are also naturally occurring particles that are ingested by phagocytes and some may chronically infect phagocytes. Antigens from microbes can be cross-presented (41, 69–71) and this process is thought to play a role in CD8 T cell immunity, especially to intracellular pathogens, such as Mycobacterium, Leishmania, and Toxoplasma (72–74)
Pathways of XPT of antigens in phagosomes
Before beginning the discussion of the cell biology of XPT, it is important to point out some caveats about this subject. As described below, there are several different pathways that can underlie XPT and not all studies have rigorously examined whether one or more is operative in the system under investigation. A number of studies have only examined XPT for purified proteins in solution and this form of antigen is probably not physiologically relevant because injection of purified proteins in vivo generally fails to cross-prime responses. Moreover soluble antigen may be handled by APCs differently than more biologically relevant substrates such as cell-associated particulate antigens (e.g. some soluble proteins have been reported to undergo retrograde transport to the ER) (75). A number of studies have used proteins conjugated to particles as mimics of cell-associated antigens and pathogens – these are much more efficiently presented than soluble proteins and while they are thought to be handled identically to cell-associated antigens, this is not known with certainty. Other complicating factors are that different antigens or forms of an antigen may engage different receptors on cells and these receptors may target the antigen into distinct subcellular compartments or may influence the state of the APC in ways that can alter the pathway used for XPT. Similarly, what an antigen is bound to can also influence this process (e.g. an antigen attached to one kind of bead may be cross-presented differently than the same antigen bound to a different kind of bead) (33). With this complexity in mind, we will try to distil what is known about the major mechanisms underlying XPT.
A major mechanism by which DCs cross-present antigens is through a phagosome-to-cytosol (P2C) pathway (76–78) (Figure 2). In this process, a fraction of the antigens that are internalized into phagosomes and macropinosomes are transferred into the cytosol, where they become fodder for proteasome hydrolysis and MHC I presentation. This mechanism was first discovered when it was found that XPT of ovalbumin (OVA)-conjugated beads required proteasomes and TAP (76). Since the proteasome is cytosolic and TAP transports peptide from the cytosol, these findings strongly implied that the ingested antigen was being transferred to the cytosol for hydrolysis and the resulting peptides then transported by TAP. Subsequently, P2C antigen transfer has been documented in other ways, including the findings that exogenous antigens: (i) caused biological effects in the cytosol (e.g. exogenous gelonin, a ribosomal inactivating protein, inhibited protein synthesis and exogenous cytochrome c induced Apaf1-dependent apoptosis) (42, 76, 79); (ii) could be found in the cytosol by staining (HRP cytochemistry) or cell fractionation (western blot) (80, 81); or (iii) could be acted upon by cytosolic enzymes (e.g. to cleave and generate a fluorescent signal) (82).
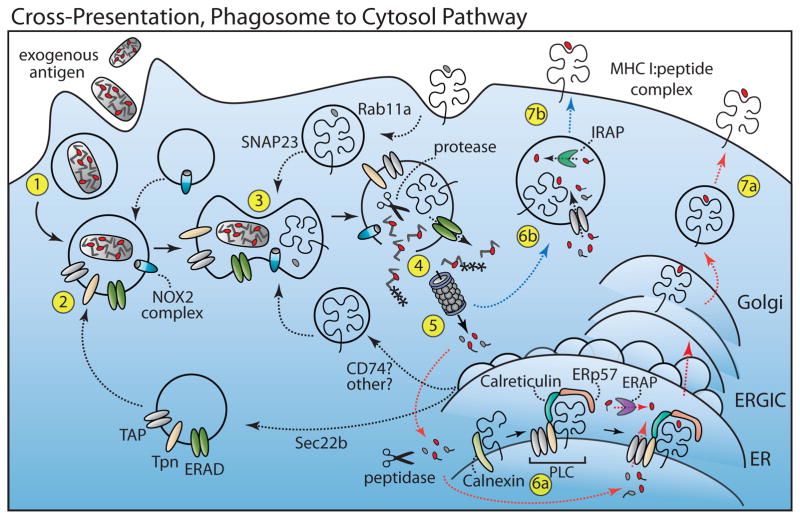
Figure 2. Cross-Presentation, Phagosome-to-Cytosol Pathway (PCP).
For XPT, exogenous antigen is internalized via pinocytosis, receptor-mediated endocytosis or phagocytosis (Step 1). Components of the PLC traffic to the endocytic compartment through a mechanism involving Sec22b (Step 2). MHC I molecules may also traffic to endosomes from the plasma membrane (in part via a Rab11a and SNAP-23 mechanism) or from the ER (potentially by way of CD74 or another mechanism) (Step 3). Antigen within the phagosome escapes to the cytosol either by membrane disruption or through an ERAD-like translocation (green) (Step 4). Antigen is then conjugated with ubiquitin and degraded by the proteasome (Step 5). Peptides (red) are then transported by TAP into the ER (red line, Step 6a) or back into the endocytic compartment (blue line, Step 6b). Long peptides in the ER can be further trimmed by ERAP (purple) while those in the endosome can be trimmed by IRAP (green) before binding MHC I. The peptide-MHC I complexes are then exported to the plasma membrane (Step 7). Dashed lines indicate steps in the pathway that are not fully understood. NADPH-oxidase 2 complex (NOX2 Complex), Tapasin (Tpn), Transporter associated with antigen processing (TAP), Endoplasmic reticulum aminopeptidase (ERAP), peptide loading complex (PLC), insulin-regulated aminopeptidase (IRAP), ER-Golgi intermediate compartment (ERGIC).
In addition to the P2C pathway there are other mechanisms that may contribute to XPT, the best characterized of which is the vacuolar pathway of XPT (Figure 3). In this pathway the presentation of exogenous antigens does not require proteasomes or TAP (33, 83, 84, 85). Instead, the presented peptides are generated by protein catabolism within the endocytic compartments and then loaded on MHC I molecules in these vesicles (33, 86–88) (Figure 3, Step 2). Cathepsin S has been implicated in this process for a couple of antigens both in vitro and in vivo (33, 86). Other lysosomal proteases may also help generate peptides or destroy them (33, 70, 89, 90).
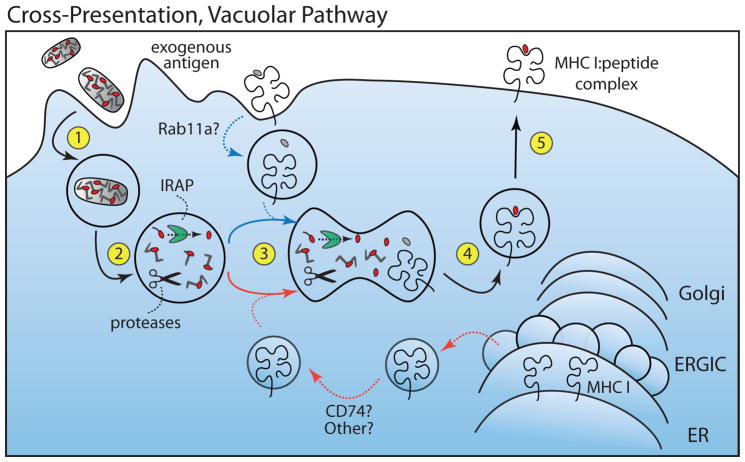
Figure 3. Cross-Presentation, Vacuolar Pathway.
Exogenous antigen is internalized via phagocytosis, pinocytosis or receptor mediated endocytosis (Step 1). Antigen is cleaved by proteases within the endocytic compartment (primarily by cathepsins) and can be further trimmed by IRAP (Step 2). MHC I molecules are recruited either from the plasma membrane (blue line, Step 3) or from ER (red line, Step 3), presumably through similar mechanisms as in the P2C pathway. Peptide is loaded into MHC I in the endosome (Step 4) and the complexes then presented at the plasma membrane (Step 5). Dashed lines indicate steps in the pathway that are not fully understood. Insulin-regulated aminopeptidase (IRAP), ER-Golgi intermediate compartment (ERGIC).
Where examined, the P2C pathway contributes more to cross-priming to cellular and viral antigens in animals than does the vacuolar pathway. The evidence for this comes from experiments where cross-priming to particles (33), viruses that don’t infect DCs (25), cancers (24) and transplants is inhibited in chimeric mice whose bone marrow lacks TAP. In contrast, CD8 T cell priming is otherwise intact in these animals in response to peptide-pulsed DCs or to an antigenic peptide that is targeted into the ER via a signal sequence. There is also data that immunoproteasomes play an important role in priming of CD8 T cells to Toxoplasma gondii (74) and since this parasite can only stimulate CD8 T cells through XPT, the participation of immunoproteasomes (which are in the cytosol of DCs) points to the P2C pathway operating in vivo. Yet another observation showing that P2C antigen transfer occurs in vivo is that cross-presenting DCs are depleted upon injection of cytochrome c into mice (this effect requires the transfer of the exogenous cytochrome c into the cytosol, where it activates Apaf1 and induces apoptosis) (79). Nevertheless, the vacuolar pathway can contribute to responses in vivo. The particles that are presented via this pathway in vitro also cross-prime responses in a TAP-independent manner in vivo (33). Similarly, priming of CD8 T cells to a viral infection can also have a TAP-independent, cathepsin S-dependent component (33), although this component contributes less than the TAP-dependent one (33), which points to the P2C pathway as being a larger contributor to these responses.
Theoretically, the P2C pathway is also better suited for stimulating T cells of the appropriate specificity for host defense because both the pathologically affected cells (infected or malignant ones) and the cross-presenting DCs use proteasomes to generate the MHC I-presented peptides (76). Therefore, the peptides that initiate CD8 T cell responses will be the same ones that the effector T cells will find on their ultimate targets. In contrast, since the vacuolar pathway uses other proteases, theoretically many of the cross-presented peptides may not match ones found in infected cells (although clearly some do).
There are yet other XPT mechanisms that have been reported to occur at least in vitro. These include ones that are proteasome-dependent, but TAP-independent (91, 92).
Recruitment of ER proteins to phagosomes
One set of findings that is relevant to bring up in advance of further discussion of XPT mechanisms is the observation that phagosomes contain a number of proteins that are normally resident in the ER. This was first discovered when the proteome of phagosomes was characterized by mass spectrometry (93, 94). Of relevance to sections below, these proteins include MHC I, TAP, and Sec61, Calnexin, Calreticulin and Tapasin, among others. How such proteins get incorporated into phagosomes was originally unclear and controversial. More recent studies have suggested that many of these molecules are delivered to phagosomes by a Sec22b-dependent transport mechanism (82) (Figure 2, Step 2). Other mechanisms deliver MHC I to phagosomes (discussed below). These various ER proteins are thought to play key roles in some of the XPT mechanisms discussed below.
Mechanism of P2C transfer of antigens
How antigens are transferred from endocytic vesicles into the cytoplasm is still not completely resolved (Figure 2, Step 4). There is a mechanism in the ER called Endoplasmic Reticulum Associated Degradation (ERAD), wherein abnormal intraluminal proteins are transported into the cytosol for degradation by the proteasome (95). With the discovery that a number of ER resident proteins were present in phagosomes, it was suggested that the ERAD pathway might also operate in phagosomes and be responsible for the P2C transfer of antigens for XPT (96). In the ERAD pathway, misfolded intraluminal and membrane proteins are tagged with ubiquitin and with the aid of the cytosolic ATPase p97 (Ye Y, Nature 2001) are then transferred across the ER membrane through a protein channel (95). This translocation was first thought to occur by retro-transport through the sec61 translocon (which normally imports proteins from ribosomes into the ER) (97), however, most now believe that such transport is not via Sec61 but through other channels, such as Derlin or Hrd1(95, 98, 99).
In support of the concept that ER proteins can be involved in P2C antigen transfer, it has been shown that blocking the delivery of ER proteins to phagosomes by silencing Sec22b reduces the P2C transfer of antigen and inhibits XPT (82). There is also some direct evidence, albeit limited, that ERAD components may participate in P2C XPT. Thus, p97 ATPase has been shown to facilitate transfer of antigens out of purified phagosomes (100) and silencing or expression of a dominant negative form of p97 inhibits XPT (101, 102). HSP90 has also been suggested to play a role in this process (80). Moreover, silencing a ubiquitin-conjugating enzyme involved in ERAD inhibits XPT (101, 103). In addition, silencing or inhibiting (with Exotoxin A, an inhibitor that is not totally selective) Sec61 in some (100, 104) but not all studies (102) or even using an intracellular antibody (intrabody) to retain Sec61 in the ER has been reported to block P2C transfer and XPT (104). However, these findings are potentially complicated by the key role of Sec61 in importing MHC I molecules and other antigen presenting machinery into the ER and therefore blocking this process could theoretically inhibit XPT. Moreover, as noted above, it is not clear that Sec61 is actually a retro-translocation channel, at least for ERAD. Silencing one of the ERAD channels Derlin, did not inhibit XPT in one report (102). The γ-interferon-inducible lysosomal thiol reductase (GILT) in phagosomes, is needed for the XPT of some antigens containing disulfide bonds and it has been suggested that GILT might be needed to reduce these proteins so that they can be unfolded for transport through a retro-translocation channel (105).
Another potential and more general mechanism for transfer of exogenous proteins into the cytosol is phagosomal disruption. Ingestion of particles can destabilize phagosomes and thereby release the contents of these vesicles into the cytosol (106). This mechanism can easily explain how intact enzymes, such as gelonin, horseradish peroxides or cytochrome c, can be transferred to the cytosol in an enzymatically active state (42, 76, 79). Such transfer is harder to explain by ERAD, because proteins that are transported through a channel must be denatured, and this would inactivate enzymes (although conceivably they could renature in the cytosol) (107). Transfer via vesicular destabilization could also more easily explain how large exogenous molecules that are not proteins, such as dextrans, (42) can be transferred into the cytosol (as these substrates can’t be ubiquitinated or likely transferred through a protein-translocation channel). However, the mechanisms that cause phagosomal destabilization aren’t understood and absent this information, it is difficult to interrogate the contribution of this process to XPT. In theory, the oxidative burst, could destabilize phagosomes via lipid peroxidation which has been suggested to contribute to XPT (108). In fact, the loss of an enzyme that generates ROS (the NADPH oxidase, NOX2) impairs XPT in some (108, 109) but not all systems (unpublished data). However, NOX2 activation has effects on pH that may explain its contribution to XPT (discussed below) (110). Nevertheless, phagosomal destabilization remains a potential mechanism for P2C transfer of antigens.
In summary, while there is some evidence that an ERAD-like mechanism may participate in P2C antigen transfer, it is still uncertain exactly how and to what extent this mechanism and other mechanisms like phagosomal destabilization contribute to this antigen transfer.
Peptide transport into the ER and phagosomes
After P2C transfer, proteins are degraded by proteasomes into oligopeptides (76, 77, 111). The XPT of these peptides then requires the TAP transporter (Figure 2, Step 6) (76, 111). It was initially assumed that TAP was transporting cross-presented peptides into the ER and, thereafter, all subsequent steps were essentially the same as those for MHC I presentation of endogenously synthesized antigens (Figure 1). However, the finding that some phagosomes also contained TAP (93) raised the possibility that after antigens are hydrolyzed in the cytosol, some of their peptides could be transported back into phagosomes and, indeed, it has been shown that this can occur in isolated phagosomes (112) (Figure 2, Step 6b).
While it initially seemed likely that peptide transport back into phagosomes might just be an epiphenomenon, there is increasing evidence that it actually may be quite important for XPT. Perhaps the strongest such evidence is that the selective inhibition of TAP in endosomes (by targeting a viral inhibitor of TAP into early endosomes) inhibits XPT of high doses of soluble OVA via the P2C pathway (81). Additional evidence comes from the discovery that peptide trimming in endosomal compartments contributes significantly to XPT (113, 114). Peptide trimming is important for MHC class I presentation because many of the peptides generated by proteasomes are too long to bind stably to MHC I molecules (115). In the classical MHC I pathway (when the antigen is endogenously expressed) (Figure 1), these long “precursor” peptides are trimmed into MHC I-presented epitopes by aminopeptidases in the cytosol and especially by ERAP1 in the ER (4, 5, 116). Inhibiting ERAP1 reduces XPT by ~50% and, since ERAP1 is not found in phagosomes, this suggests that some cross-presented peptides are being trimmed in the ER. However, silencing of a close relative of ERAP1, IRAP, also inhibits XPT of soluble and particulate antigen by ~50% (113, 114, 117). Importantly, since IRAP is not retained in the ER but rather traffics to endosomal vesicles, its participation in XPT strongly suggests that cross-presented peptides are also imported into and then trimmed in endocytic compartments. Moreover, silencing of both ERAP1 and IRAP had an additive inhibitory effect on XPT (113). Other findings support the concept that cross-presented peptides are loaded onto MHC I molecules in phagosomes (Figure 3, Step 4). The strongest evidence is that cross-presented OVA peptide-MHC I complexes have been detected in phagosomes (81, 100, 111, 118, 119). In addition and as discussed in the next section, MHC I trafficking to phagosomes is important for XPT, which also supports a pathway involving phagosomes.
Phagosomal MHC I trafficking and peptide loading
To generate peptide-MHC I complexes in the endocytic compartment, MHC I molecules must be in the phagosome and/or endosomes and this is indeed the case (118, 120). That endosomal MHC I is important for some forms of XPT (at least for soluble antigen) has been shown in experiments mutating the intracellular domain of MHC I in ways that interfere with endosomal localization (118, 121).
One of the ways that MHC I can get into endosomes and phagosomes is by trafficking from the cell surface (118, 121). MHC I trafficking from the cell surface may be particularly important for the presentation of antigens through the vacuolar pathway because vacuolar XPT in some systems is not inhibited by Brefeldin A (BFA), which blocks the transport of molecules out of the ER/Golgi complex (41, 87, 92), and is resistant to inhibition of protein synthesis (10, 41). This suggests that pre-existing, rather than newly synthesized, MHC I molecules are important for the vacuolar pathway. Surface MHC I may also contribute to the P2C pathway because blocking MHC I recycling to endosomes with the drug primaquine inhibited the XPT of soluble antigen via the P2C pathway in at least one system (81). Moreover, MHC I molecules in a Rab11a+ endosomal recycling compartment are important for XPT for antigens associated with TLR agonists. In this latter situation, intraphagosomal TLRs sense this cargo via a MyD88-dependent signaling pathway to induce SNAP23 (a vesicular transport protein)-dependent delivery of MHC I from endosomal recycling vesicles to phagosomes (122). MHC I is transported to these endosomal recycling vesicles in a Rab11a-dependent mechanism. Loss of MyD88 or Rab11a inhibits MHC I trafficking and XPT of antigens associated with TLR ligands (e.g. LPS and Pam2Csk4). However, while the MyD88 pathway can enhance XPT in some cases, it is not required and may even be detrimental for antigens that are not associated with TLR agonists both in vitro and in vivo (123, 124) (Shen&Rock unpublished).
Another way in which MHC I molecules may traffic into endosomes and phagosomes is via transport from the ER. This may be particularly important for the P2C pathway because in many systems P2C XPT is inhibited by BFA, indicating that the export of some newly synthesized proteins, most likely MHC I molecules, out of the ER/golgi is needed for this pathway (76, 77). Direct transport of MHC I from the ER to endocytic compartments may also be important to vacuolar XTP because, in at least one system, it required newly synthesized MHC I molecules and was not blocked by an inhibitor of endosomal recycling, primaquine (83).
One mechanism for delivering MHC I from the ER to the endocytic compartment is via CD74 (the invariant chain). CD74 is well known to bind and target MHC II molecules to these vesicles and it turns out that it can do the same thing for MHC I molecules (125). However, the role of CD74 in XPT is controversial, with one group finding that loss of CD74 impairs XPT (126) while other groups found that XPT was normal in CD74-deficient dendritic cells and mice (33, 83). Therefore, there may be additional as yet undefined mechanisms that traffic MHC I from the ER to endocytic compartments.
In summary, there are multiple mechanisms by which MHC I molecules can traffic to endosomal vesicles both from the cell surface and ER. The exact contribution of each of these mechanisms to the various XPT pathways and how it is influenced by different conditions is still not completely understood.
If the cross-presenting MHC I molecules are newly synthesized ones that are being loaded with peptides in the phagosome, then there are several interesting but unresolved questions about this process. One question is regarding how these molecules are stabilized during their transport and sojourn in the vacuole prior to acquiring cross-presented peptides. The issue here is that peptide-empty MHC I molecules are highly unstable and at physiological temperatures rapidly dissociate and denature (127). In the ER, empty MHC I molecules are stabilized by chaperones until they bind peptide. It is unknown whether chaperones stabilize empty MHC I molecules during transport and/or upon arrival in the phagosome. Peptide binding stabilizes MHC I molecules and it is possible that these stable peptide-occupied MHC I complexes are the ones transported to phagosomes. However, if this is the case, then this raises the question as to how cross-presented peptides could exchange into peptide-occupied complexes since peptides don’t easily exchange into such complexes.
Another related question is whether there is molecular machinery that promotes peptide-loading of MHC I molecules in phagosomes. In the analogous MHC II pathway of antigen presentation, HLA-DM molecules are needed in endocytic vesicles to catalyze and optimize the loading of many peptides into MHC II molecules (128). Tapasin subserves a similar function for MHC I molecules in the ER (129). Tapasin is found in phagosomes (112) and might assist in loading MHC I complexes in these vacuoles. Tapasin KO cells have been reported to have defects in XPT (130), but whether this reflects an action of Tapasin in the ER and/or phagosomes is unknown. New emerging data suggests that there may be additional mechanisms that directly or indirectly promote loading of MHC I complexes in phagosomes and this is discussed in a section below.
Phagosomal pH, proteolysis and XPT
Lysosomes fuse with phagosomes and deliver both proteases (cathepsins) and a proton pump (vacuolar ATPase). The vacuolar ATPase acidifies the phagosome, which in turn activates the cathepsins. This transforms the vacuole into a catabolic compartment, which is important for degrading the cargo that the phagocyte has ingested. This process underlies the generation of peptides for MHC II presentation and also for vacuolar XPT, as blocking vacuolar proteolysis with protease inhibitors (33) or a weak base that inhibits endosomal acidification (70) blocks such XPT.
In contrast to its effects on vacuolar XPT, blocking acidification with weak bases generally enhances P2C XPT, both in vitro and in vivo, (131). Interestingly, DCs can prevent the acidification of their phagosomes through the sustained activation of NOX2 (109, 132) (Figure 2). The loss or inhibition of NOX2 (108, 132), deficiency of Rac2 (which is needed for NOX2 assembly) (133), loss of Rab27a (which is required for NOX2 delivery to phagosomes) (134), and knock out of Vav1-3 (which are needed for linking ITAM-containing receptors to NOX2 activation) (135) all partially inhibit XPT by DCs in vitro. Similarly, loss of the transcription factor TFEB, which controls among other things lysosomal acidification, promotes XPT (136). In one study, the reduction in XPT from some of the above experimental manipulations can be reversed with protease inhibitors (134) and therefore the defect in XPT appeared to be due to excessive vacuolar proteolysis rather than other effects of low pH. Similarly, endogenous protease inhibitors, such as serpinb9, have been found to regulate XPT (137). Irgm3 (Igtp), an immune-related GTPase involved in lipid body formation, also contributes to XPT, and this may be by its limiting vacuolar acidification (138).
Nature of the cross presenting vesicles
For the MHC II antigen presentation pathway, the formation of peptide-MHC II complexes is thought to occur most efficiently in a specialized endocytic compartment called MIIC (139). It is an important but unresolved issue as to whether there are similar specialized endocytic compartments (or states of phagosomal maturation) wherein the XPT pathways operate.
The P2C transport of intact, biologically active antigens, such as peroxidases and cytochrome c, implies that this pathway may operate in early endosomes, which have little degradative capability. Consistent with this idea, targeting antigen to the mannose receptor or to Clec9a, which directs them to early endosomes (EEA1+/rab5+), promotes XPT (48, 140). Moreover, Sec61, proposed to transport antigens into the cytosol, co-localizes with the antigen in rab5+ early endocytic vesicles (104). Sec22b, which facilitates transport of ER components to the phagosomes, reaches these compartments early (within 15 mins) after phagocytosis (82). Components that mediate peptide re-entry and processing within phagosomes, such as TAP1 and IRAP, have been found in these early vesicles as well (81, 111, 113). However, this is not to say that late endosomes (LAMP1+) play no role in the P2C pathway. It has been shown that targeting antigen to DEC205, which traffics to the endolysosome, also promotes XPT (141). Moreover, in the case of soluble antigen, 25D1.16 staining of peptide-MHC I complexes has been observed both in early and late compartments (118).
The compartment wherein the vacuolar pathway occurs is even less characterized. While the P2C and vacuolar pathways share the initial step of antigen internalization, they diverge in their use of antigen processing components and in several cases, their intracellular source of MHC I. So far, there is no concrete evidence as to whether or not these pathways occur in distinct compartments. Since in the vacuolar pathway the presented peptides must be generated in the endocytic compartment, this pathway may operate in more catabolic endocytic compartments. This idea is consistent with findings that the route of antigen uptake and/or the activation state of the APC may promote vacuolar XPT, as targeting antigen to TLR2 results in peptide-loading of MHC I molecules in later, rab7+ LAMP1+ vesicles (142).
Autophagy and XPT
Autophagy is another mechanism that cells use to degrade their endogenous proteins and organelles (143). Autophagic vesicles engulf cytosolic components and these vacuoles then fuse with lysosomes. As reviewed above, autophagic vesicles from donor cells can be a source of antigen that is acquired by DCs and cross-presented (52, 53). Under normal physiological conditions, autophagy in the cross-presenting DCs may not be important to XPT (144). However, this pathway may participate in XPT in some select circumstances. Loss of several components of the autophagy pathway (Atg5, Atg7, Beclin 1) or the kinase GCN2, involved in the induction of this response in DCs, has been reported to impair XPT to some antigens under conditions where autophagy is induced (e.g. amino acid starvation) (145) or where antigens are targeted to autophagosomes (146). Atg7-deficient DCs show defective XPT of soluble but not cell-associated or DEC205-targeted antigen (147). In these settings, it is unclear how autophagy or its components are contributing to XPT. It is possible that autophagic vesicles are simply being released from one DC and acquired by another. In addition, some of the autophagic components (e.g. LC3) can interact with and influence phagosomes in ways that are independent from autophagy, including stabilizing NOX2 and promoting optimal phagosome-lysosome fusion (144, 148, 149) and perhaps some these events account for the effects on XPT.
Beyond the known XPT machinery: new potential components
An unbiased forward genetic screen in DCs for genes involved in XPT has yielded some interesting new candidates that are still undergoing characterization. Two of these that are of interest are Rab39a and H2-XP. Silencing or homozygous deletion of either of these genes in DC lines inhibits the P2C and vacuolar pathways of XPT (unpublished). The inhibition is selective for XPT because the presentation of this same antigen on MHC II or via the classical MHC I pathway (when the antigen is endogenously expressed) is not inhibited. Initial characterization suggests that these two new XPT genes are involved in somehow directly or indirectly promoting the loading of peptides onto MHC I molecules in phagosomes, although how is not yet known (unpublished data).
Rab proteins are GTPases that control the formation, content, trafficking and ultimate fusion of vesicles in cells and thereby determine the composition of endocytic compartments. Rab39a colocalizes with LAMP1 and MHC II-positive vesicles (unpublished data). Presumably, Rab39a is functioning to traffic key components of the XPT pathway into these compartments.
H2-XP is a MHC class Ib molecule that has not been previously studied. Its sequence is highly homologous to other MHC I molecules as is its predicted structure. Remarkably, when an epitope-tagged version of H2-XP was expressed in 293T cells or dendritic cells, it is not found on the plasma membrane but instead localizes in the ER and endocytic vesicles; it is also found in phagosomes in DCs (unpublished data). This distribution is unusual for an MHC I molecule and reminiscent of molecules like HLA-DM. H2-XP is also found to physically associate with MHC class Ia molecules (e.g. H2-K, H2-D and H2-L molecules) (unpublished data). Exactly what H2-XP is doing in XPT is not yet clear. However, given its binding to MHC I molecules, trafficking to phagosomes, and promotion of peptide-loading in phagosomes, one could speculate that it could be involved in stabilizing MHC I until it loads with peptides or performs an HLA-DM/Tapasin-like function, where it helps to load and/or exchange peptides - time will tell.
Types of antigen presenting cells that cross-present
When the cells in lymph nodes or spleen that are cross-presenting antigens were characterized, they turned out to be DCs (13, 150–155). Moreover, selective depletion of DCs has been shown to inhibit cross-priming (150, 151). Therefore, DCs are key cells that acquire, cross-present, and take such antigens to T cells to initiate immune responses.
There are many subsets of DCs that can be distinguished based on their origin and/or expression of various cell surface markers and many of these can cross-present antigen in vitro (13, 152–155). When the DCs that are actually cross-presenting antigens in mice have been characterized, they are most often the ones that express the chemokine receptor XCR1 (156) plus CD8 (15, 16, 157) and/or CD103 (158, 159). In the spleens of animals injected i.v. with plasmodium or OVA in soluble or cell-associated form, the cross-presenting APCs are exclusively resident CD8+ DCs. In contrast, soluble or cell associated antigen instilled into the lung is exclusively cross-presented by migratory CD103+ DCs, as is soluble and keratinocyte-expressed antigen in the skin (156, 160, 161). In the intestine, epithelial-expressed antigen is cross-presented by migratory CD103+CD8+ DCs. Moreover, mice that are deficient in the transcription factor Batf3 lack both CD8+ and CD103+ DCs and have substantial defects in XPT (150, 162), further supporting the importance of these DC subsets. (163). Antigens that are delivered to lymphoid organs can also be cross-presented by DCs that are resident in such tissues (21). Migratory DCs have also been reported to transfer antigen to lymphoid resident DCs for XPT (164). In humans the equivalent XCR1+ cross-presenting DC is thought to be BDCA3+ XCR1+ CD141+ and these cells are also efficient at XPT by the P2C in vitro. (152, 165, 166).
Why the various XCR1+ DCs are the ones preferentially cross-presenting antigens has primarily been examined for the CD8+ DC subset. One of the components that CD8+ DCs (and CD103+ DCs) uniquely express is the receptor Clec9A. Clec9A recognizes F-actin, which becomes exposed in necrotic cells (167). It is thought that this receptor promotes XPT by efficiently delivering antigens from necrotic cells into the appropriate endocytic compartment for XPT (48). Loss of Clec9A in mice impairs XPT to cell-associated antigens and in some viral infections (31, 48). In addition, CD8+ DCs express higher levels of several MHC I pathway genes (TAP, ERAP1) (168) and are the ones that regulate endosomal pH via NOX2 (133). These cells appear to have more active P2C antigen transfer as they are more sensitive to exogenous cytochrome c (which induces apoptosis when transferred to the cytosol) (79). Other components that are expressed at much higher levels in CD8+ DCs compared to other subsets are SNAP23 (122) and Rab39a (unpublished). Whether there are other specializations that make this DC subset the dominant cross-presenting APC remains to be seen.
While the XCR1+ DCs are the dominant cross-presenting cells in many systems, there is evidence that other DC subsets, particularly inflammatory/myeloid-derived ones, can also acquire and cross-present antigen in vivo. This has been observed predominantly, but not exclusively (169), in situations where inflammation is occurring, such as in viral and fungal infections, adjuvant injection, autoimmune tissue damage, and in response to cytokines (29, 170–173). The participation of other DCs in vivo has also been seen in situations where an exogenous antigen engages certain stimulatory cells receptors, such as the FcR or Dectin-1(171, 174). Why under these various circumstances these XCR1- DCs are the ones cross-presenting is not entirely clear. Certainly cytokines and/or the engagement of innate signaling receptors are known to upregulate antigen presentation, alter DC migration, and affect phagosome function in many ways (ROS/pH, P2C antigen transfer, MHC I trafficking) (122, 172) and antigen-receptor binding can influence where antigen localizes in DCs - many of these effects could promote XPT.
It is interesting that in vivo the DCs that can cross-present an exogenous antigen on MHC I are different that the ones that are presenting on MHC II, even for the same antigen (15, 16, 175). The MHC II-presenting DCs are CD8a-negative. The reason for this is unclear. Perhaps this is attributable to MHC II presentation requiring acidic vesicles for peptide generation and loading, which are conditions that are suboptimal for XPT. Indeed there can be some inverse reciprocal relationship between XPT and MHC II presentation (e.g. the transcription factor TFEB inhibits XPT, but enhances MHC II presentation) (136).
Macrophages can also cross-present exogenous antigens in vitro (12, 41). Some have suggested that the ability of macrophages to cross-present is far inferior to that of DCs and this has been attributed to the phagosomes in macrophages being more hydrolytic and destroying antigens (176). However, others find that macrophages cross-present with similar efficiency to DCs, at least in vitro (13). In a few systems, macrophages were observed to cross-present antigens in vivo, (11, 177). Moreover, most experiments analyzing the nature of the cross-presenting APC in vivo have done so by isolating the cells and assaying for the presence of cross-presented antigens ex vivo; since extraction of macrophages from tissues can be inefficient, the role of these cells could be underestimated. Monocytes may also cross-present antigens in vitro and in vivo (178). This may depend on the nature of the antigen and most of these studies have not examined macrophage XPT of the forms of antigen that are most likely to be important for these kinds of APCs – namely intracellular bacteria or parasites. These kinds of microbes chronically infect macrophages and, in order to contain or eliminate the nidus of such infections, T cells must activate or kill the infected phagocytes. In these settings, CD8+ T cells are generated, indicating that microbial antigens must be cross-presented (72–74). The initiation of such responses could be mediated solely by cross-presenting DCs. However, the contribution of CD8+ T cells to containing the infection strongly suggests that at the effector stage macrophages are cross-presenting microbial antigens. Macrophages are more hydrolytic than DCs and their XPT can be enhanced by inhibitors of acidification (92) or with antigen targeted to early, less hydrolytic endosomes (92).
It is likely that any phagocytic cell can cross-present at some level as this activity has been seen in neutrophils (88, 179), myeloid cells (180), osteoclasts (181), Kupffer cells (182), microglia (183), and even in cells of non-hematopoietic lineage, such as endothelium (182, 184), FcR-transfected 293T cells (185) and thymic epithelial cells (186). B cells don’t typically cross-present but may do so when antigen is targeted into endocytic compartments by receptor-mediated endocytosis (187) or in some other circumstances (188, 189). The biological significance of XPT by these other cells, if any, is not clear. These observations suggest that at least some of the components needed for XPT are shared by a broad array of cells, although whether all of these cells utilize the same pathways for XPT is unclear.
In summary, DCs are key cross-presenting APCs for immune surveillance of tissues and initiate CD8 T cell responses to tissue antigens. In this process, XCR1+ DCs are most often the dominant APCs for cross-presenting tissue antigens to CD8 T cells, but other DC subsets participate in some circumstances. XPT by macrophages is likely important for CD8 T cell recognition of infected phagocytes. While other cells can cross-present, the significance of their XPT is unclear.
XPT and Immune evasion
From a host’s perspective, MHC I antigen presentation of viral antigens after live infection is clearly beneficial, whereas from a virus’ perspective this pathway is a detriment to survival and propagation. Consequently, a number of viruses have evolved mechanisms to disable the MHC I pathway and thereby escape complete elimination (190).
Many of the viruses that establish lifelong infections, including CMV, HSV, and HIV, encode immune evasion molecules that inactivate key components of the MHC I pathway in the infected cells (e.g. there are viral proteins that block TAP, cause degradation of MHC I molecules, or prevent peptide-MHC complexes from reaching the cell surface) (190, 191). For these viruses, DCs that take up and cross-present the viral antigens, without themselves being infected, can prime CD8 T cell responses (the ingested immune evasion molecules are either in the wrong place and/or not in sufficient concentrations to block presentation in the DCs) (157), and this is thought to be why robust T cell responses can be generated in such infections (157, 192–194). What is the use of such effector cells if the infected cells they need to eliminate are invisible due to impairment in antigen presentation? The likely answer is that while the viral immune evasion molecules reduce MHC I presentation, they often do not completely eliminate it. Consequently, the residual antigen presentation may be sufficient for recognition by cross-primed CD8 T cell, especially because effector T lymphocytes require many fewer peptide-MHC complexes to be activated than do naïve T cells. This allows the immune system to exert some control over these infections without completely eliminating them.
On the other hand, some pathogens that reside in phagocytic cells, such as DCs and macrophages, have evolved mechanisms to impair XPT and thereby potentially impair both T cell priming and the subsequent wrath of effector cells. Leishmania does this by impairing NOX2 activity through cleavage of VAMP8 and dying Leishmania-infected neutrophils eaten by DCs somehow inhibit XPT by DCs (195, 196). Tumors also can impair XPT of their antigens (197, 198) and this, in theory, could contribute to their escape from immune surveillance.
Regulation of XPT
DCs are critical for priming CD8 T cell immunity to cross-presented (and directly presented) antigens because they can express high levels of the co-stimulatory molecules that are needed to activate naïve T cells (199). In the absence of such activation, DCs may induce tolerance instead of immunity (for cross-presented antigens, this has been referred to as “cross-tolerance”) (200). The DCs are stimulated to express the co-stimulatory molecules when they encounter microbial pathogen-associated molecular patterns (PAMPs) (201), cellular damage-associated molecular patterns (DAMPs, such as DNA and uric acid) (202, 203), or inflammatory cytokines, such as interferons (204, 205). PAMPS and DAMPS are sensed through various pattern recognition receptors. For example, TLR3, which is a nucleic acid-sensor expressed in the endosomes of XCR1+ DCs, is key in responding to some viral infections (206), and STING, which is a component of a cytosolic DNA sensing pathway (where perhaps P2C transfer of ingested DNA could be important), is a key molecule for tumor immunity (203)
DC sensing of microbes and cell injury can also influence XPT itself, sometimes in very different ways. TLR agonists (e.g. for TLR3, 4, 7 & 9) can promote XPT to soluble exogenous antigens (81, 119, 207). The TLR stimulation can increase the amount of TAP (81) and NOX2 (208) in phagosomes, promote P2C translocation of proteins (104) and restrain lysosome fusion with phagosomes (119, 209); all of these events may contribute to the enhanced XPT. Moreover, pattern recognition receptor-stimulated cytokine responses, particularly IFNs, can upregulate expression of many of the components of the MHC I pathway (e.g. MHC I molecules, TAP, Tapasin, ERAP1) (210) and affect antigen intracellular sorting and persistence in ways that increase XPT (211). In vivo, type I IFN may be critical for XPT responses (212). On the other hand, TLR4 and TLR9 agonists can reduce XPT to particulate antigens (163) and systemic administration of TLR-agonists results in a marked inhibition of XPT (213) and possibly direct presentation (214). The stimulatory vs. inhibitory effects of TLR agonists may be influenced by their purity (215). Moreover, different effects have been reported if the TLR agonist is bound to antigen particles. In this situation, TLRs in phagosomes are stimulated and result in SNAP23-dependent recruitment of MHC I into the vacuole and enhanced XPT (122).
Therapeutic potential of manipulating XPT
One of the limitations of non-living subunit vaccines is that they generally fail to prime CD8 T cell immunity (216). Among the reasons for this limitation is that such formulations are poorly acquired and cross-presented by DC because they contain soluble antigens that are poorly cross-presented. However, XPT provides a potential pathway to overcome this limitation. XPT allows for exogenous antigens to be presented on MHC I molecules and does so in the key APCs for initiating CD8 T cell responses. Importantly, this pathway is the physiological one that is used by the immune system to generate protective immunity in many situations and this biology gives “proof of concept” to its potential effectiveness. In order to exploit this pathway, antigens would need to be targeted into the XPT pathway in DCs. Some ways that this can be accomplished are by attaching antigens to phagocytic substrates (12) or targeting them to appropriate cell surface receptors (44, 217–219) such that they are efficiently acquired and cross-presented by DCs. Importantly, there are data that such methods work in vivo to prime CD8 immunity (and concomitant CD4 responses, which help CD8 responses) (12, 40, 219). Thus XPT has the potential to be exploited to develop novel vaccines that elicit CD8 T cell immunity for prophylaxis against certain viruses and intracellular bacteria or maybe even to augment responses to clear infections in chronically-infected individuals. Similarly, this approach could be used for developing novel immunotherapies for cancer. In fact, XPT likely contributes to the efficacy of checkpoint inhibitors (blockers of CTLA4 and PD1/PD1L) in cancer immunotherapy by priming responses that are amplified by these therapeutics (30). Therefore, methods to increase XPT of antigens from tumor cells or cancer vaccines could, in theory, have benefits for cancer immunotherapy.
XPT can lead to immunity or tolerance, with the specific outcome thought to be determined by the state of the DC, as discussed above. This too has the potential to be exploited for therapeutic benefit. Antigen formulations should be able to be developed with appropriate immunostimulatory components, such as DAMPs or PAMPs, which would result in more robust immunity. Antigen formulations might also be designed that would result in tolerance (220). If the latter was achieved, it could potentially be useful for preventing or treating autoimmune disease or transplantation rejection.
Some existing therapeutics may inhibit XPT by affecting phagosomal proteolysis (221, 222). Moreover, since XPT may have some unique components that are not shared with the endogenous MHC I pathway or the MHC II pathway, it may be possible to develop other inhibitors that selectively block XPT. Such inhibitors could block sensitization of a host and conceivably be useful in some clinical situations, such as transplantation.
Conclusion
Over the past 25 years since the discovery of XPT, the field has learned much about this process, and yet there is still much that is unknown. We know that XPT plays key roles in the generation of immunity and potentially tolerance in many situations, but elucidating the full extent of its roles requires better in vivo models. Studies into the underlying mechanisms of this unique antigen presentation pathway have revealed fascinating cell biology, and yet we still don’t understand these processes fully, nor do we have a clear picture of the key XPT pathways in many situations. There is also potential to exploit this pathway for novel therapies that is as yet untapped. We should anticipate that new pieces of the puzzle will continue to be discovered and tools developed that will help us fill in the gaps in understanding and manipulating this interesting and important process.
Acknowledgments
This work was supported by grant R01 AI114495 to KLR and T32 AI095213 to BK. We thank Dr. Susan Swain and Dr. Lawrence Stern for critical reading of the manuscript.
Abbreviations
- APC
antigen presenting cell
- DC
dendritic cell
- ERAP
endoplasmic reticulum aminopeptidase
- IRAP
insulin-regulated aminopeptidase
- OVA
ovalbumin
- NOX2
NADPH oxidase 2
- P2C
phagosome-to-cytosol
- TAP
transporter associated with antigen processing
- TLR
toll-like receptor
- XPT
Cross-presentation
Literature Cited
- 1.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the Proteasome Block the Degradation of Most Cell-Proteins and the Generation of Peptides Presented on Mhc Class-I Molecules. Cell. 1994;78:761–71. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670–7. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 3.Townsend A, Trowsdale J. The transporters associated with antigen presentation. Semin Cell Biol. 1993;4:53–61. doi: 10.1006/scel.1993.1007. [DOI] [PubMed] [Google Scholar]
- 4.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–3. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 5.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat Immunol. 2002;3:1177–84. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 6.Morrison LA, Lukacher AE, Braciale VL, Fan DP, Braciale TJ. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986;163:903–21. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–16. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend AR, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–68. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 10.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–21. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 11.Grant EP, Rock KL. MHC class I-restricted presentation of exogenous antigen by thymic antigen-presenting cells in vitro and in vivo. J Immunol. 1992;148:13–8. [PubMed] [Google Scholar]
- 12.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
- 14.Rock KL, Rothstein L, Gamble S, Fleischacker C. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993;150:438–46. [PubMed] [Google Scholar]
- 15.den Haan JMM, Lehar SM, Bevan MJ. Cd8+but Not Cd8 Dendritic Cells Cross-Prime Cytotoxic T Cells in Vivo. The Journal of Experimental Medicine. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pooley JL, Heath WR, Shortman K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8(−) dendritic cells, but cross-presented to CD8 T cells by CD8(+) dendritic cells. Journal of Immunology. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 17.Sei JJ, Haskett S, Kaminsky LW, Lin E, Truckenmiller ME, Bellone CJ, Buller RM, Norbury CC. Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation. PLoS Pathog. 2015;11:e1004941. doi: 10.1371/journal.ppat.1004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–43. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–45. doi: 10.1007/s10456-013-9407-0. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell AM, Currie AJ, Brown M, Kania K, Wylie B, Cleaver A, Lake R, Robinson BW. Tumor cells, rather than dendritic cells, deliver antigen to the lymph node for cross-presentation. Oncoimmunology. 2012;1:840–6. doi: 10.4161/onci.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu RH, Remakus S, Ma X, Roscoe F, Sigal LJ. Direct presentation is sufficient for an efficient anti-viral CD8+ T cell response. PLoS Pathog. 2010;6:e1000768. doi: 10.1371/journal.ppat.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–65. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 24.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 25.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 26.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101–8. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 27.de Jersey J, Snelgrove SL, Palmer SE, Teteris SA, Mullbacher A, Miller JF, Slattery RM. Beta cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2007;104:1295–300. doi: 10.1073/pnas.0610057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderon B, Unanue ER. Antigen presentation events in autoimmune diabetes. Curr Opin Immunol. 2012;24:119–28. doi: 10.1016/j.coi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–61. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, Jure-Kunkel M, Azpilikueta A, Aznar MA, Quetglas JI, Sancho D, Melero I. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016;6:71–9. doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest. 2012;122:1628–43. doi: 10.1172/JCI60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smed-Sorensen A, Chalouni C, Chatterjee B, Cohn L, Blattmann P, Nakamura N, Delamarre L, Mellman I. Influenza A virus infection of human primary dendritic cells impairs their ability to cross-present antigen to CD8 T cells. PLoS Pathog. 2012;8:e1002572. doi: 10.1371/journal.ppat.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–65. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Heipertz EL, Davies ML, Lin E, Norbury CC. Prolonged antigen presentation following an acute virus infection requires direct and then cross-presentation. J Immunol. 2014;193:4169–77. doi: 10.4049/jimmunol.1302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleyas AG, Han YW, Patil AM, Kim SB, Kim K, Eo SK. Impaired cross-presentation of CD8alpha+ CD11c+ dendritic cells by Japanese encephalitis virus in a TLR2/MyD88 signal pathway-dependent manner. Eur J Immunol. 2012;42:2655–66. doi: 10.1002/eji.201142052. [DOI] [PubMed] [Google Scholar]
- 36.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 37.Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–32. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falo LD, Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1:649–53. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 42.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 43.van Montfoort N, Mangsbo SM, Camps MG, van Maren WW, Verhaart IE, Waisman A, Drijfhout JW, Melief CJ, Verbeek JS, Ossendorp F. Circulating specific antibodies enhance systemic cross-priming by delivery of complexed antigen to dendritic cells in vivo. Eur J Immunol. 2012;42:598–606. doi: 10.1002/eji.201141613. [DOI] [PubMed] [Google Scholar]
- 44.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 45.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–35. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerksiek KM, Niedergang F, Chavrier P, Busch DH, Brocker T. Selective Rac1 inhibition in dendritic cells diminishes apoptotic cell uptake and cross-presentation in vivo. Blood. 2005;105:742–9. doi: 10.1182/blood-2004-05-1891. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian M, Hayes CD, Thome JJ, Thorp E, Matsushima GK, Herz J, Farber DL, Liu K, Lakshmana M, Tabas I. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J Clin Invest. 2014;124:1296–308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, Schulz O, Sancho D, Reis e Sousa C. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122:1615–27. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJ. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–92. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 50.Anyaegbu CC, Lake RA, Heel K, Robinson BW, Fisher SA. Chemotherapy enhances cross-presentation of nuclear tumor antigens. PLoS One. 2014;9:e107894. doi: 10.1371/journal.pone.0107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci U S A. 2004;101:3035–40. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–95. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joubert PE, Albert ML. Antigen Cross-Priming of Cell-Associated Proteins is Enhanced by Macroautophagy within the Antigen Donor Cell. Front Immunol. 2012;3:61. doi: 10.3389/fimmu.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443–51. doi: 10.1379/1466-1268(2000)005<0443:hsptfo>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matheoud D, Perie L, Hoeffel G, Vimeux L, Parent I, Maranon C, Bourdoncle P, Renia L, Prevost-Blondel A, Lucas B, Feuillet V, Hosmalin A. Cross-presentation by dendritic cells from live cells induces protective immune responses in vivo. Blood. 2010;115:4412–20. doi: 10.1182/blood-2009-11-255935. [DOI] [PubMed] [Google Scholar]
- 56.Matheoud D, Baey C, Vimeux L, Tempez A, Valente M, Louche P, Le Bon A, Hosmalin A, Feuillet V. Dendritic cells crosspresent antigens from live B16 cells more efficiently than from apoptotic cells and protect from melanoma in a therapeutic model. PLoS One. 2011;6:e19104. doi: 10.1371/journal.pone.0019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 58.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–9. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 59.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–8. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 60.Smyth LA, Hervouet C, Hayday T, Becker PD, Ellis R, Lechler RI, Lombardi G, Klavinskis LS. Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J Immunol. 2012;189:2274–82. doi: 10.4049/jimmunol.1200664. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Kim S, Herndon JM, Goedegebuure P, Belt BA, Satpathy AT, Fleming TP, Hansen TH, Murphy KM, Gillanders WE. Cross-dressed CD8alpha+/CD103+ dendritic cells prime CD8+ T cells following vaccination. Proc Natl Acad Sci U S A. 2012;109:12716–21. doi: 10.1073/pnas.1203468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–24. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 63.Serna A, Ramirez MC, Soukhanova A, Sigal LJ. Cutting edge: Efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. Journal of Immunology. 2003;171:5668–72. doi: 10.4049/jimmunol.171.11.5668. [DOI] [PubMed] [Google Scholar]
- 64.Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, Berglund P, Gibbs J, Bennink JR, Yewdell JW. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Nanjundappa RH, Shameli A, Clemente-Casares X, Yamanouchi J, Elliott JF, Slattery R, Serra P, Santamaria P. The cross-priming capacity and direct presentation potential of an autoantigen are separable and inversely related properties. J Immunol. 2014;193:3296–307. doi: 10.4049/jimmunol.1401001. [DOI] [PubMed] [Google Scholar]
- 66.Schliehe C, Bitzer A, van den Broek M, Groettrup M. Stable antigen is most effective for eliciting CD8+ T-cell responses after DNA vaccination and infection with recombinant vaccinia virus in vivo. J Virol. 2012;86:9782–93. doi: 10.1128/JVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–34. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, Houde M, Desjardins M, Sher A, Sacks D. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–33. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 71.Harriff MJ, Burgdorf S, Kurts C, Wiertz EJ, Lewinsohn DA, Lewinsohn DM. TAP mediates import of Mycobacterium tuberculosis-derived peptides into phagosomes and facilitates loading onto HLA-I. PLoS One. 2013;8:e79571. doi: 10.1371/journal.pone.0079571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, Udey MC, Sacks D. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol. 2002;168:3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- 74.Ersching J, Vasconcelos JR, Ferreira CP, Caetano BC, Machado AV, Bruna-Romero O, Baron MA, Ferreira LR, Cunha-Neto E, Rock KL, Gazzinelli RT, Rodrigues MM. The Combined Deficiency of Immunoproteasome Subunits Affects Both the Magnitude and Quality of Pathogen- and Genetic Vaccination-Induced CD8+ T Cell Responses to the Human Protozoan Parasite Trypanosoma cruzi. PLoS Pathog. 2016;12:e1005593. doi: 10.1371/journal.ppat.1005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–13. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 76.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 77.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–55. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 78.Palmowski MJ, Gileadi U, Salio M, Gallimore A, Millrain M, James E, Addey C, Scott D, Dyson J, Simpson E, Cerundolo V. Role of immunoproteasomes in cross-presentation. J Immunol. 2006;177:983–90. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- 79.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, Heath WR, Villadangos JA, Lew AM. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A. 2008;105:3029–34. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imai T, Kato Y, Kajiwara C, Mizukami S, Ishige I, Ichiyanagi T, Hikida M, Wang JY, Udono H. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci U S A. 2011;108:16363–8. doi: 10.1073/pnas.1108372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–66. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 82.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, Enninga J, Moita LF, Amigorena S, Savina A. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–68. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 83.Ma W, Zhang Y, Vigneron N, Stroobant V, Thielemans K, van der Bruggen P, Van den Eynde BJ. Long-Peptide Cross-Presentation by Human Dendritic Cells Occurs in Vacuoles by Peptide Exchange on Nascent MHC Class I Molecules. J Immunol. 2016;196:1711–20. doi: 10.4049/jimmunol.1501574. [DOI] [PubMed] [Google Scholar]
- 84.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–90. [PubMed] [Google Scholar]
- 85.Tiwari N, Garbi N, Reinheckel T, Moldenhauer G, Hammerling GJ, Momburg F. A transporter associated with antigen-processing independent vacuolar pathway for the MHC class I-mediated presentation of endogenous transmembrane proteins. J Immunol. 2007;178:7932–42. doi: 10.4049/jimmunol.178.12.7932. [DOI] [PubMed] [Google Scholar]
- 86.Hari A, Ganguly A, Mu L, Davis SP, Stenner MD, Lam R, Munro F, Namet I, Alghamdi E, Furstenhaupt T, Dong W, Detampel P, Shen LJ, Amrein MW, Yates RM, Shi Y. Redirecting soluble antigen for MHC class I cross-presentation during phagocytosis. Eur J Immunol. 2015;45:383–95. doi: 10.1002/eji.201445156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 88.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167:2538–46. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 89.Oh YK, Swanson JA. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol. 1996;132:585–93. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mant A, Chinnery F, Elliott T, Williams AP. The pathway of cross-presentation is influenced by the particle size of phagocytosed antigen. Immunology. 2012;136:163–75. doi: 10.1111/j.1365-2567.2012.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Merzougui N, Kratzer R, Saveanu L, van Endert P. A proteasome-dependent, TAP-independent pathway for cross-presentation of phagocytosed antigen. EMBO Rep. 2011;12:1257–64. doi: 10.1038/embor.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci U S A. 2009;106:17463–8. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–6. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 94.Campbell-Valois FX, Trost M, Chemali M, Dill BD, Laplante A, Duclos S, Sadeghi S, Rondeau C, Morrow IC, Bell C, Gagnon E, Hatsuzawa K, Thibault P, Desjardins M. Quantitative proteomics reveals that only a subset of the endoplasmic reticulum contributes to the phagosome. Mol Cell Proteomics. 2012;11:M111 016378. doi: 10.1074/mcp.M111.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruggiano A, Foresti O, Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204:869–79. doi: 10.1083/jcb.201312042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grotzke JE, Cresswell P. Are ERAD components involved in cross-presentation? Mol Immunol. 2015;68:112–5. doi: 10.1016/j.molimm.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–8. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 98.Mehnert M, Sommer T, Jarosch E. Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane. Nat Cell Biol. 2014;16:77–86. doi: 10.1038/ncb2882. [DOI] [PubMed] [Google Scholar]
- 99.Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell. 2010;143:579–91. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–17. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 101.Zehner M, Chasan AI, Schuette V, Embgenbroich M, Quast T, Kolanus W, Burgdorf S. Mannose receptor polyubiquitination regulates endosomal recruitment of p97 and cytosolic antigen translocation for cross-presentation. Proc Natl Acad Sci U S A. 2011;108:9933–8. doi: 10.1073/pnas.1102397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menager J, Ebstein F, Oger R, Hulin P, Nedellec S, Duverger E, Lehmann A, Kloetzel PM, Jotereau F, Guilloux Y. Cross-presentation of synthetic long peptides by human dendritic cells: a process dependent on ERAD component p97/VCP but Not sec61 and/or Derlin-1. PLoS One. 2014;9:e89897. doi: 10.1371/journal.pone.0089897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol. 2005;17:45–53. doi: 10.1093/intimm/dxh184. [DOI] [PubMed] [Google Scholar]
- 104.Zehner M, Marschall AL, Bos E, Schloetel JG, Kreer C, Fehrenschild D, Limmer A, Ossendorp F, Lang T, Koster AJ, Dubel S, Burgdorf S. The translocon protein Sec61 mediates antigen transport from endosomes in the cytosol for cross-presentation to CD8(+) T cells. Immunity. 2015;42:850–63. doi: 10.1016/j.immuni.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 105.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–8. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J. 2008;27:201–11. doi: 10.1038/sj.emboj.7601941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dingjan I, Verboogen DR, Paardekooper LM, Revelo NH, Sittig SP, Visser LJ, Mollard GF, Henriet SS, Figdor CG, Ter Beest M, van den Bogaart G. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci Rep. 2016;6:22064. doi: 10.1038/srep22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 110.Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15:527–40. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 112.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–94. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saveanu L, Carroll O, Weimershaus M, Guermonprez P, Firat E, Lindo V, Greer F, Davoust J, Kratzer R, Keller SR, Niedermann G, van Endert P. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–7. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 114.Weimershaus M, Maschalidi S, Sepulveda F, Manoury B, van Endert P, Saveanu L. Conventional dendritic cells require IRAP-Rab14 endosomes for efficient cross-presentation. J Immunol. 2012;188:1840–6. doi: 10.4049/jimmunol.1101504. [DOI] [PubMed] [Google Scholar]
- 115.Cascio P, Hilton C, Kisselev AF, Rock KL, Goldberg AL. 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. EMBO J. 2001;20:2357–66. doi: 10.1093/emboj/20.10.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saveanu L, Babdor J, Lawand M, van Endert P. Insulin-regulated aminopeptidase and its compartment in dendritic cells. Mol Immunol. 2013;55:153–5. doi: 10.1016/j.molimm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 118.Basha G, Lizee G, Reinicke AT, Seipp RP, Omilusik KD, Jefferies WA. MHC class I endosomal and lysosomal trafficking coincides with exogenous antigen loading in dendritic cells. PLoS One. 2008;3:e3247. doi: 10.1371/journal.pone.0003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crespo MI, Zacca ER, Nunez NG, Ranocchia RP, Maccioni M, Maletto BA, Pistoresi-Palencia MC, Moron G. TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8alpha+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface. J Immunol. 2013;190:948–60. doi: 10.4049/jimmunol.1102725. [DOI] [PubMed] [Google Scholar]
- 120.Ramachandra L, Sramkoski RM, Canaday DH, Boom WH, Harding CV. Flow analysis of MHC molecules and other membrane proteins in isolated phagosomes. J Immunol Methods. 1998;213:53–71. doi: 10.1016/s0022-1759(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 121.Lizee G, Basha G, Tiong J, Julien JP, Tian M, Biron KE, Jefferies WA. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 2003;4:1065–73. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 122.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–21. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Saeland E, van Kooyk Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int J Cancer. 2011;128:1371–83. doi: 10.1002/ijc.25458. [DOI] [PubMed] [Google Scholar]
- 124.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–4. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 125.Sugita M, Brenner MB. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J Biol Chem. 1995;270:1443–8. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- 126.Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, Choi KB, Jefferies WA. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol. 2012;13:237–45. doi: 10.1038/ni.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–80. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 128.Pos W, Sethi DK, Wucherpfennig KW. Mechanisms of peptide repertoire selection by HLA-DM. Trends Immunol. 2013;34:495–501. doi: 10.1016/j.it.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hulpke S, Tampe R. The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem Sci. 2013;38:412–20. doi: 10.1016/j.tibs.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 130.Chefalo PJ, Grandea AG, 3rd, Van Kaer L, Harding CV. Tapasin−/− and TAP1−/− macrophages are deficient in vacuolar alternate class I MHC (MHC-I) processing due to decreased MHC-I stability at phagolysosomal pH. J Immunol. 2003;170:5825–33. doi: 10.4049/jimmunol.170.12.5825. [DOI] [PubMed] [Google Scholar]
- 131.Accapezzato D, Visco V, Francavilla V, Molette C, Donato T, Paroli M, Mondelli MU, Doria M, Torrisi MR, Barnaba V. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817–28. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–22. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–55. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 134.Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, Amigorena S. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–78. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 135.Graham DB, Stephenson LM, Lam SK, Brim K, Lee HM, Bautista J, Gilfillan S, Akilesh S, Fujikawa K, Swat W. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J Exp Med. 2007;204:2889–97. doi: 10.1084/jem.20071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Samie M, Cresswell P. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol. 2015;16:729–36. doi: 10.1038/ni.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rizzitelli A, Meuter S, Vega Ramos J, Bird CH, Mintern JD, Mangan MS, Villadangos J, Bird PI. Serpinb9 (Spi6)-deficient mice are impaired in dendritic cell-mediated antigen cross-presentation. Immunol Cell Biol. 2012;90:841–51. doi: 10.1038/icb.2012.29. [DOI] [PubMed] [Google Scholar]
- 138.Bougneres L, Helft J, Tiwari S, Vargas P, Chang BH, Chan L, Campisi L, Lauvau G, Hugues S, Kumar P, Kamphorst AO, Dumenil AM, Nussenzweig M, MacMicking JD, Amigorena S, Guermonprez P. A role for lipid bodies in the cross-presentation of phagocytosed antigens by MHC class I in dendritic cells. Immunity. 2009;31:232–44. doi: 10.1016/j.immuni.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–83. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 140.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 141.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–20. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 142.Shen KY, Song YC, Chen IH, Leng CH, Chen HW, Li HJ, Chong P, Liu SJ. Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells. J Immunol. 2014;192:4233–41. doi: 10.4049/jimmunol.1302850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–37. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–39. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, Weiss DS, Virgin HW, Ron D, Pulendran B. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014;343:313–7. doi: 10.1126/science.1246829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol. 2011;6:645–50. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mintern JD, Macri C, Chin WJ, Panozza SE, Segura E, Patterson NL, Zeller P, Bourges D, Bedoui S, McMillan PJ, Idris A, Nowell CJ, Brown A, Radford KJ, Johnston AP, Villadangos JA. Differential use of autophagy by primary dendritic cells specialized in cross-presentation. Autophagy. 2015;11:906–17. doi: 10.1080/15548627.2015.1045178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, Kanneganti TD, Virgin HW, Green DR. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 150.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chiang MC, Tullett KM, Lee YS, Idris A, Ding Y, McDonald KJ, Kassianos A, Leal Rojas IM, Jeet V, Lahoud MH, Radford KJ. Differential uptake and cross-presentation of soluble and necrotic cell antigen by human DC subsets. Eur J Immunol. 2016;46:329–39. doi: 10.1002/eji.201546023. [DOI] [PubMed] [Google Scholar]
- 153.Tel J, Sittig SP, Blom RA, Cruz LJ, Schreibelt G, Figdor CG, de Vries IJ. Targeting uptake receptors on human plasmacytoid dendritic cells triggers antigen cross-presentation and robust type I IFN secretion. J Immunol. 2013;191:5005–12. doi: 10.4049/jimmunol.1300787. [DOI] [PubMed] [Google Scholar]
- 154.Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. 2013;34:361–70. doi: 10.1016/j.it.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med. 2013;210:1035–47. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kitano M, Yamazaki C, Takumi A, Ikeno T, Hemmi H, Takahashi N, Shimizu K, Fraser SE, Hoshino K, Kaisho T, Okada T. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A. 2016;113:1044–9. doi: 10.1073/pnas.1513607113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Busche A, Jirmo AC, Welten SP, Zischke J, Noack J, Constabel H, Gatzke AK, Keyser KA, Arens R, Behrens GM, Messerle M. Priming of CD8+ T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J Immunol. 2013;190:2767–77. doi: 10.4049/jimmunol.1200966. [DOI] [PubMed] [Google Scholar]
- 158.Becker M, Guttler S, Bachem A, Hartung E, Mora A, Jakel A, Hutloff A, Henn V, Mages HW, Gurka S, Kroczek RA. Ontogenic, Phenotypic, and Functional Characterization of XCR1(+) Dendritic Cells Leads to a Consistent Classification of Intestinal Dendritic Cells Based on the Expression of XCR1 and SIRPalpha. Front Immunol. 2014;5:326. doi: 10.3389/fimmu.2014.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cerovic V, Houston SA, Westlund J, Utriainen L, Davison ES, Scott CL, Bain CC, Joeris T, Agace WW, Kroczek RA, Mowat AM, Yrlid U, Milling SW. Lymph-borne CD8alpha+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 2015;8:38–48. doi: 10.1038/mi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 161.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208:1789–97. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wagner CS, Grotzke J, Cresswell P. Intracellular regulation of cross-presentation during dendritic cell maturation. PLoS One. 2013;8:e76801. doi: 10.1371/journal.pone.0076801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 165.Cohn L, Chatterjee B, Esselborn F, Smed-Sorensen A, Nakamura N, Chalouni C, Lee BC, Vandlen R, Keler T, Lauer P, Brockstedt D, Mellman I, Delamarre L. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med. 2013;210:1049–63. doi: 10.1084/jem.20121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hanc P, Fujii T, Iborra S, Yamada Y, Huotari J, Schulz O, Ahrens S, Kjaer S, Way M, Sancho D, Namba K, Reis e Sousa C. Structure of the Complex of F-Actin and DNGR-1, a C-Type Lectin Receptor Involved in Dendritic Cell Cross-Presentation of Dead Cell-Associated Antigens. Immunity. 2015;42:839–49. doi: 10.1016/j.immuni.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 169.Nizza ST, Campbell JJ. CD11b+ migratory dendritic cells mediate CD8 T cell cross-priming and cutaneous imprinting after topical immunization. PLoS One. 2014;9:e91054. doi: 10.1371/journal.pone.0091054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–24. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Backer R, van Leeuwen F, Kraal G, den Haan JM. CD8− dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur J Immunol. 2008;38:370–80. doi: 10.1002/eji.200737647. [DOI] [PubMed] [Google Scholar]
- 172.Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, Henson PM, Jakubzick C. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun. 2014;5:4674. doi: 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A. 2009;106:20377–81. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.den Haan JMM, Bevan MJ. Constitutive versus Activation-dependent Cross-Presentation of Immune Complexes by CD8+and CD8 Dendritic Cells In Vivo. The Journal of Experimental Medicine. 2002;196:817–27. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mount AM, Smith CM, Kupresanin F, Stoermer K, Heath WR, Belz GT. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS One. 2008;3:e1691. doi: 10.1371/journal.pone.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–56. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 177.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 178.Leiriao P, del Fresno C, Ardavin C. Monocytes as effector cells: activated Ly-6C(high) mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+ T cells. Eur J Immunol. 2012;42:2042–51. doi: 10.1002/eji.201142166. [DOI] [PubMed] [Google Scholar]
- 179.Davey MS, Morgan MP, Liuzzi AR, Tyler CJ, Khan MW, Szakmany T, Hall JE, Moser B, Eberl M. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J Immunol. 2014;193:3704–16. doi: 10.4049/jimmunol.1401018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Milo I, Sapoznikov A, Kalchenko V, Tal O, Krauthgamer R, van Rooijen N, Dudziak D, Jung S, Shakhar G. Dynamic imaging reveals promiscuous crosspresentation of blood-borne antigens to naive CD8+ T cells in the bone marrow. Blood. 2013;122:193–208. doi: 10.1182/blood-2012-01-401265. [DOI] [PubMed] [Google Scholar]
- 181.Kiesel JR, Buchwald ZS, Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol. 2009;182:5477–87. doi: 10.4049/jimmunol.0803897. [DOI] [PubMed] [Google Scholar]
- 182.Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology. 2011;54:1379–87. doi: 10.1002/hep.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jarry U, Jeannin P, Pineau L, Donnou S, Delneste Y, Couez D. Efficiently stimulated adult microglia cross-prime naive CD8+ T cells injected in the brain. Eur J Immunol. 2013;43:1173–84. doi: 10.1002/eji.201243040. [DOI] [PubMed] [Google Scholar]
- 184.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol. 2014;192:5002–11. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci U S A. 2009;106:3324–9. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perchellet A, Brabb T, Goverman JM. Crosspresentation by nonhematopoietic and direct presentation by hematopoietic cells induce central tolerance to myelin basic protein. Proc Natl Acad Sci U S A. 2008;105:14040–5. doi: 10.1073/pnas.0804970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med. 1996;184:1179–84. doi: 10.1084/jem.184.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marino E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes. 2012;61:2893–905. doi: 10.2337/db12-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goodridge JP, Lee N, Burian A, Pyo CW, Tykodi SS, Warren EH, Yee C, Riddell SR, Geraghty DE. HLA-F and MHC-I open conformers cooperate in a MHC-I antigen cross-presentation pathway. J Immunol. 2013;191:1567–77. doi: 10.4049/jimmunol.1300080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.van de Weijer ML, Luteijn RD, Wiertz EJ. Viral immune evasion: Lessons in MHC class I antigen presentation. Semin Immunol. 2015;27:125–37. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 191.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–42. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 192.Nopora K, Bernhard CA, Ried C, Castello AA, Murphy KM, Marconi P, Koszinowski U, Brocker T. MHC class I cross-presentation by dendritic cells counteracts viral immune evasion. Front Immunol. 2012;3:348. doi: 10.3389/fimmu.2012.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Orr MT, Edelmann KH, Vieira J, Corey L, Raulet DH, Wilson CB. Inhibition of MHC class I is a virulence factor in herpes simplex virus infection of mice. PLoS Pathog. 2005;1:e7. doi: 10.1371/journal.ppat.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gainey MD, Rivenbark JG, Cho H, Yang L, Yokoyama WM. Viral MHC class I inhibition evades CD8+ T-cell effector responses in vivo but not CD8+ T-cell priming. Proc Natl Acad Sci U S A. 2012;109:E3260–7. doi: 10.1073/pnas.1217111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Matheoud D, Moradin N, Bellemare-Pelletier A, Shio MT, Hong WJ, Olivier M, Gagnon E, Desjardins M, Descoteaux A. Leishmania evades host immunity by inhibiting antigen cross-presentation through direct cleavage of the SNARE VAMP8. Cell Host Microbe. 2013;14:15–25. doi: 10.1016/j.chom.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 196.Ribeiro-Gomes FL, Romano A, Lee S, Roffe E, Peters NC, Debrabant A, Sacks D. Apoptotic cell clearance of Leishmania major-infected neutrophils by dendritic cells inhibits CD8(+) T-cell priming in vitro by Mer tyrosine kinase-dependent signaling. Cell Death Dis. 2015;6:e2018. doi: 10.1038/cddis.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–31. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McDonnell AM, Lesterhuis WJ, Khong A, Nowak AK, Lake RA, Currie AJ, Robinson BW. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol. 2015;45:49–59. doi: 10.1002/eji.201444722. [DOI] [PubMed] [Google Scholar]
- 199.Belz GT, Heath WR, Carbone FR. The role of dendritic cell subsets in selection between tolerance and immunity. Immunol Cell Biol. 2002;80:463–8. doi: 10.1046/j.1440-1711.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- 200.Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39:2325–30. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- 201.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 202.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 203.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–57. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kerkar SP, Chinnasamy D, Hadi N, Melenhorst J, Muranski P, Spyridonidis A, Ito S, Weber G, Yin F, Hensel N, Wang E, Marincola FM, Barrett AJ. Timing and intensity of exposure to interferon-gamma critically determines the function of monocyte-derived dendritic cells. Immunology. 2014;143:96–108. doi: 10.1111/imm.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 207.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170:4102–10. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 208.Elsen S, Doussiere J, Villiers CL, Faure M, Berthier R, Papaioannou A, Grandvaux N, Marche PN, Vignais PV. Cryptic O2- -generating NADPH oxidase in dendritic cells. J Cell Sci. 2004;117:2215–26. doi: 10.1242/jcs.01085. [DOI] [PubMed] [Google Scholar]
- 209.Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U, Joannas L, Vivar OI, Lennon-Dumenil AM, Savina A, Gevaert K, Beyaert R, Hoffmann E, Amigorena S. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity. 2015;43:1087–100. doi: 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 210.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 211.Schiavoni G, Mattei F, Gabriele L. Type I Interferons as Stimulators of DC-Mediated Cross-Priming: Impact on Anti-Tumor Response. Front Immunol. 2013;4:483. doi: 10.3389/fimmu.2013.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–72. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 214.Wong YC, Smith SA, Tscharke DC. Systemic toll-like receptor ligation and selective killing of dendritic cell subsets fail to dissect priming pathways for anti-vaccinia virus CD8(+) T cells. J Virol. 2013;87:11978–86. doi: 10.1128/JVI.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wagner CS, Cresswell P. TLR and nucleotide-binding oligomerization domain-like receptor signals differentially regulate exogenous antigen presentation. J Immunol. 2012;188:686–93. doi: 10.4049/jimmunol.1102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Raychaudhuri S, Rock KL. Fully mobilizing host defense: building better vaccines. Nat Biotechnol. 1998;16:1025–31. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 217.Platzer B, Stout M, Fiebiger E. Antigen cross-presentation of immune complexes. Front Immunol. 2014;5:140. doi: 10.3389/fimmu.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hartung E, Becker M, Bachem A, Reeg N, Jakel A, Hutloff A, Weber H, Weise C, Giesecke C, Henn V, Gurka S, Anastassiadis K, Mages HW, Kroczek RA. Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J Immunol. 2015;194:1069–79. doi: 10.4049/jimmunol.1401903. [DOI] [PubMed] [Google Scholar]
- 219.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–92. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Herve J, Dubreil L, Tardif V, Terme M, Pogu S, Anegon I, Rozec B, Gauthier C, Bach JM, Blancou P. beta2-Adrenoreceptor agonist inhibits antigen cross-presentation by dendritic cells. J Immunol. 2013;190:3163–71. doi: 10.4049/jimmunol.1201391. [DOI] [PubMed] [Google Scholar]
- 222.Hunzeker JT, Elftman MD, Mellinger JC, Princiotta MF, Bonneau RH, Truckenmiller ME, Norbury CC. A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells. J Immunol. 2011;186:183–94. doi: 10.4049/jimmunol.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]