Abstract
Plasmodium parasites of mammals, including the species that cause malaria in humans, infect the liver first and develop there into clinically silent liver stages. Liver stages grow and ultimately produce thousands of first-generation merozoites, which initiate the erythrocytic cycles causing malaria pathology. Here, we present a Plasmodium protein with a critical function for complete liver stage development. UIS4 (up-regulated in infective sporozoites gene 4) is expressed exclusively in infective sporozoites and developing liver stages, where it localizes to the parasitophorous vacuole membrane. Targeted gene disruption of UIS4 in the rodent model malaria parasite Plasmodium berghei generated knockout parasites that progress through the malaria life cycle until after hepatocyte invasion but are severely impaired in further liver stage development. Immunization with UIS4 knockout sporozoites completely protects mice against subsequent infectious WT sporozoite challenge. Genetically attenuated liver stages may thus induce immune responses, which inhibit subsequent infection of the liver with WT parasites.
Keywords: attenuated parasite, malaria, parasitophorous vacuole, stage-specific gene expression
Plasmodium parasites are obligatory intracellular protozoa. They use highly specialized, motile invasive stages to traverse tissue barriers and actively invade host cells in their mosquito vector and vertebrate host. Sporozoites are the invasive stages that are transmitted by the bite of an infected mosquito. After inoculation, sporozoites enter the blood stream and are transported to the liver, where they exit the blood vessel and invade hepatocytes (1). After invasion, the parasite resides in a parasitophorous vacuole (PV), a membranous compartment that physically separates the parasite from the host cell cytoplasm, and here it transforms into the liver stage (2, 3). Within a few days, liver stages undergo a process of remarkable growth and replication (4) and finally produce thousands of red blood cell-infectious merozoites. Unfortunately, even 56 years after their discovery (5), liver stages remain the most intractable stages of the Plasmodium life cycle, mainly because of their limited experimental accessibility (6). A better understanding of liver stage biology is highly desirable, not least because it is the only stage of the malaria parasite that can be completely eliminated by sterilizing immune responses, thereby preventing malaria infection (7, 8). Hence, liver stage parasites constitute an excellent target for antimalaria vaccines (9).
Toward the identification of preerythrocytic stage-specific genes, we previously used transcription-profiling using sporozoites of the rodent model malaria parasites Plasmodium yoelii (Py) and Plasmodium berghei (Pb) (10, 11). The profiling was based on the prediction that infectious sporozoites residing in the mosquito salivary glands are uniquely equipped with transcripts that encode proteins required for hepatocyte invasion and subsequent development of liver stages. We reisolated two of the identified genes, termed UIS3 and UIS4 for “up-regulated in infective sporozoites,” in a screen for preerythrocytic stage-specific transcripts that are not expressed in blood stages (12). Together, the data indicated that UIS3 and UIS4 are transcribed exclusively in sporozoites that are programmed to infect the mammalian host.
Here, we show that depletion of UIS4 by targeted gene disruption does not affect sporozoite invasion of hepatocytes or transformation into early liver stages. However, early liver stages lacking UIS4 are severely impaired in growth and development in vitro and in vivo and, consequently, in their ability to establish infection of the mammalian host.
Materials and Methods
Pb Transfection and Genotypic Analysis. For gene targeting of PbUIS4, a 582-bp fragment was amplified by using primers UIS4INT forward (5′-CGGAATTCATCATATTACTAATTTTCGGGGG-3′) and UIS4INT reverse (5′-TCCCCGCGGTTATTCCATGTTATAAACGTTATTTCC-3′), with Pb genomic DNA as a template. Cloning into the Pb targeting vector (13) resulted in plasmid pAKM15. Parasite transformation and selection were performed as described in ref. 13. Integration-specific PCR amplification of the uis4- locus was achieved by using the following primers: test 1, Toxoplasma gondii DHFR-TS forward (5′-CCCGCACGGACGAATCCAGATGG-3′) and UIS4 test reverse (5′-CCCAAGCTTAGTTTGCATATACGGCTGCTTCC-3′); test 2, UIS4 test forward (5′-CGGAATTCTGGATTCATTTTTTGATGCATGC-3′) and T7 (5′-GTAATACGACTCACTATAGGC-3′). For replacement of PbUIS4, two fragments (1 kb and 600 bp) were amplified by using primers UIS4rep1 forward (5′-GAATTCTGGATTCATTTTTTGATGCATGC-3′) and UIS4rep2 reverse (5′-GGGGTACCTTTATTCAGACGTAATAATTATGTGC-3′) for the 1-kb fragment and UIS4rep3 forward (5′-AAAACTGCAGATAATTCATTATGAGTAGTGTAATTCAG-3′) and UIS4rep4 reverse (5′-CCCCAAGCTTAAGTTTGCATATACGGCTGCTTCC-3′) for the 600-bp fragment, with Pb genomic DNA as a template. Cloning into the hDHFR targeting vector (14) resulted in plasmid pAKM17. To detect UIS4 expression in WT and mutant Pb parasites, 1 × 105 salivary gland sporozoites were solubilized in 10 μl of SDS sample buffer. UIS4 was visualized on Western blots by using the polyclonal UIS4 antisera (12) and horseradish peroxidase-coupled anti-rabbit IgG secondary antibody (Amersham Biosciences). For RT-PCR analysis, we dissected 8 × 105 uis4-, 8 × 105 uis4REP-, and 4 × 105 WT salivary gland sporozoites and isolated poly(A)+ RNA by using oligo(dT) columns (Invitrogen). For cDNA synthesis and amplification, we performed a two-step PCR using random decamer primers (Ambion, Austin, TX) and subsequent standard PCRs.
Phenotypic Analysis of uis4- Parasites. Anopheles stephensi mosquitoes were raised under a 14-h light/10-h dark cycle at 28°C and 75% humidity and were fed on 10% sucrose solution. Blood-feeding and mosquito dissection were performed as described in ref. 15. The number of sporozoites per infected mosquito was determined in a hemocytometer. To analyze sporozoite motility, sporozoites were deposited onto precoated glass coverslips and incubated by using primary antibody against Pb circumsporozoite protein (CSP) (16). Bound antibody was detected with Alexa Fluor 488-conjugated anti-mouse antibody (Molecular Probes). To detect liver stages in hepatoma (HepG2) cells, Pb sporozoites were added to subconfluent monolayers, incubated for 2 h at 37°C, and washed off. After 12, 24, 36, and 48 h, liver stages were revealed by using primary antibodies against parasite heat shock protein 70 (17) and a secondary antibody conjugated with Alexa Fluor 488 (Molecular Probes). To analyze sporozoite invasion, 3 × 104 salivary gland sporozoites were added to subconfluent HepG2 cells and incubated for 90 min at 37°C. The ratio of intracellular parasites to extracellular parasites was visualized by using a double staining protocol (18) with the anti-CSP antibody and confocal microscopy. To determine the infectivity of clonal sporozoite populations in vivo, C57/Bl6 mice were injected i.v. or s.c. with 100 μl of sporozoite suspension of WT parasites or knockout parasites in RPMI medium 1640. Parasitemia of the animals was checked daily by examination of a Giemsa-stained blood smear. The appearance of a single erythrocytic stage represented the first day of patency.
Immunization and Parasite Challenge Experiments. For all experiments, female C57/Bl6 mice (Charles River Laboratories) aged between 50 and 80 days were used. For immunizations, Pbuis4REP- sporozoites were extracted from the salivary glands of infected mosquitoes. Sporozoites were injected in a volume of 100 μl i.v. into the tail vein of the animals. Animals were immunized with a single dose of 10,000 or 50,000 uis4REP- sporozoites, followed by two boosts of either 10,000 or 25,000 uis4REP- sporozoites administered by i.v. injection. The first boost was given 14 days after the immunization, and a second boost was given 14 days thereafter. The animals were then monitored for parasitemia by daily blood smears. Only those animals that remained blood stage parasite negative after the first immunization and subsequent boosts (8 of 12 mice and 8 of 10 mice, respectively) were exposed to a challenge with WT sporozoites. Animals were challenged 10 days after receiving the last boost of uis4REP- sporozoites by i.v. injection. All challenges consisted of 50,000 infective Pb WT sporozoites. For both sets of experiments, five naive animals were included to verify infectivity of the sporozoite challenge dose. In each naive animal, parasitemia was readily detectable at day 3 after injection. Starting from day 3 after WT challenge, the uis4REP- sporozoite-immunized animals were examined for detectable parasitemia in Giemsa-stained blood smears. Animals did not show a detectable parasitemia within 50 days after the challenge and were considered completely protected.
Recombinant Adenovirus (Ad). The complete PyUIS4 ORF was amplified by using oligonucleotide primers (sense, 5′-ATTAGTCGACATGAAAACCACATACGTTTCTCTC-3′; antisense, 5′-ATTAGGATCCTTATATGTATGGGTCAAATGGTTTATC-3′). The resulting fragment was sequenced and was cloned into the Ad shuttle vector pΔE1Z. This vector was constructed by inserting the human CMV promoter-enhancer, intron, multiple cloning site, and bovine growth hormone polyadenylation signal sequence into the Ad E1 region of the plasmid pΔE1sp1A. Recombinant virus (Ad-UIS4) was produced by using standard procedures and was used to infect HEK 293 cells.
Immunoblotting. Sporozoites, infected mouse liver, and Ad-PyUIS4-infected HEK 293 cells were homogenized in sample buffer. For immunoblot analysis, proteins were separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad) by electroblotting. UIS4 was detected by incubation of membranes with the anti-UIS4 antiserum diluted 1:3,500 and subsequent incubation with horseradish-peroxidase-conjugated secondary antibody. Immunostained proteins were visualized with enhanced chemiluminescence detection (Pierce).
Immunocytochemistry. Infected livers were removed from mice at 12 h, 24 h, and 44 h after i.v. injection of Py sporozoites. Infected livers were fixed in 4% paraformaldehyde overnight at 4°C and then cut into 50-μm sections by using a Vibratome (Ted Pella Inc., Redding, CA). Sections were treated with 3% hydrogen peroxide, 0.25% Triton X-100 in 1× TBS for 30 min, blocked with 5% milk-TBS for 1 h, and incubated with the anti-UIS4 antiserum (1:500) at 4°C overnight. Secondary antibody was incubated for 2 h at room temperature. Nuclei were visualized with DAPI.
Immunoelectron Microscopy. Py-infected mouse liver tissue was fixed with 4% paraformaldehyde and 0.1% glutaraldehyde (grade I; Sigma) in PBS, dehydrated, and embedded in LR White (Ted Pella, Inc., Redding, CA). Thin sections were cut with an RMC MT-7 ultramicrotome (RMC, Tucson, AZ) and labeled with a rabbit antiserum against UIS4, followed by goat anti-rabbit IgG gold (5 nm; Amersham Biosciences). Specimens were viewed with a Zeiss EM 910 electron microscope.
Results
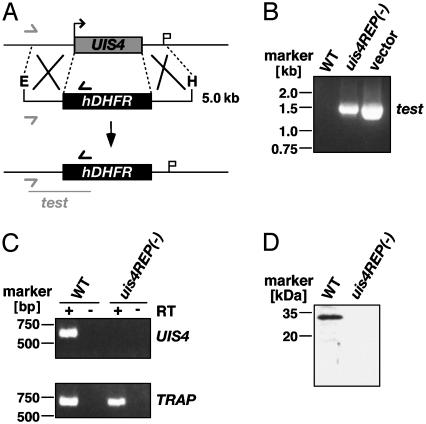
Generation of uis4- Parasites. Given that UIS4 is expressed in sporozoites but not in blood stages, we were able to pursue a targeted gene disruption at the blood stages to study the importance of UIS4 for the Plasmodium preerythrocytic life cycle stages. The endogenous PbUIS4 gene was disrupted by using an insertion strategy (data not shown) and replacement strategy (Fig. 1A). The parental blood stage population from the successful transfection was used for selection of clonal parasite lines carrying the gene disruption. We obtained insertion/disruption clones designated uis4- and replacement clones designated uis4REP- that contained exclusively the predicted mutant locus. The correct replacement event was confirmed by replacement-specific PCR (Fig. 1B). To confirm PbUIS4 deficiency of the mutant parasites, we performed RT-PCR and cDNA amplification using poly(A)+ RNA from salivary gland sporozoites as templates (Fig. 1C). Moreover, Western blot analysis of uis4REP- sporozoites did not detect PbUIS4 (Fig. 1D).
Fig. 1.
Targeted gene disruption of PbUIS4 by a replacement strategy. (A) Replacement to generate the uis4REP- parasite. The WT UIS4 genomic locus is targeted with an EcoRI/HindIII-linearized replacement fragment containing 5′ and 3′ UTRs of the UIS4 ORF and the hDHFR-positive selectable marker. Upon a double crossover event, the UIS4 ORF is replaced by the positive selectable marker. Replacement-specific test primer combinations are indicated by arrows, and expected fragment is shown as lines. (B) Replacement-specific PCR analysis. Confirmation of the predicted gene-targeting in uis4REP- parasites is achieved by primer combinations that can only amplify from the recombinant locus. Black and gray arrows shown in A indicate primers that hybridize to regions in the plasmid backbone and outside the UIS4 ORF, respectively. (C) Depletion of UIS4 transcripts in uis4REP- parasites. cDNA from WT and uis4REP- sporozoites were amplified at 35 PCR cycles. UIS4 transcript is not detectable in knockout parasites when compared with a transcript control (TRAP). (D) Western blot analysis of WT and uis4REP- sporozoites. Extracts from 100,000 WT or uis4REP- salivary gland sporozoites were separated on a 10% SDS gel and probed with the polyclonal anti-UIS4 serum.
UIS4 Is Not Necessary for Sporozoite Functions. We examined the phenotype of Pbuis4- parasites during the Plasmodium life cycle. uis4- parasites were indistinguishable from WT parasites in growth of red blood cell stages, gametocyte formation, transmission to Anopheles mosquitoes, and oocyst development (data not shown). We next analyzed sporozoite development and salivary gland invasion by comparing sporozoite numbers in infected mosquito midgut oocysts and salivary glands (Table 1). Similar sporozoite numbers were obtained for uis4-, uis4REP-, and the WT control, indicating that PbUIS4 is not involved in sporozoite development and sporozoite salivary gland invasion. This finding is in agreement with our previous observation that the gene is developmentally up-regulated only after salivary gland invasion (11). Furthermore, sporozoite gliding motility, a characteristic form of substrate-dependent locomotion of salivary gland sporozoites (15), was unaffected in uis4- parasites (Table 1). We used a two-color sporozoite invasion assay (18) with HepG2 cells to reveal a possible role for PbUIS4 during host cell invasion. No differences in proportions of intracellular parasites, and thus host cell invasion, were detected when comparing uis4- sporozoites and WT sporozoites. In contrast, control pretreatment of WT sporozoites with the actin-filament inhibitor cytochalasin D completely abolished host cell invasion (Table 1).
Table 1. Phenotypic analysis of uis4- and uis4REP- sporozoites.
| Parasite clone | Midgut sporozoites* | Salivary gland sporozoites* | Gliding motility† | Hepatocyte invasion‡ |
|---|---|---|---|---|
| WT | 23,975 ± 7,640 | 19,134 ± 4,323 | ++ | 45.0 ± 1.7 |
| uis4- | 24,054 ± 10,267 | 18,915 ± 4,156 | ++ | 43.1 ± 8.0 |
| uis4REP- | 24,428 ± 6,064 | 19,798 ± 4,608 | ++ | N/D |
| WT plus cytochalasin D§ | N/D | N/D | 0 | 2.0 ± 1.0 |
N/D, not determined.
Mean number of sporozoites per midgut or salivary gland dissected at day 14 or 17, respectively
++, vigorous continuous gliding as typically seen in salivary gland sporozoites; +, discontinuous gliding as typically seen in midgut sporozoites; 0, no gliding
Percentage of intracellular sporozoites determined by confocal microscopy (see Materials and Methods)
As a negative control for gliding and invasion, WT sporozoites were treated with 0.5 μM cytochalasin D
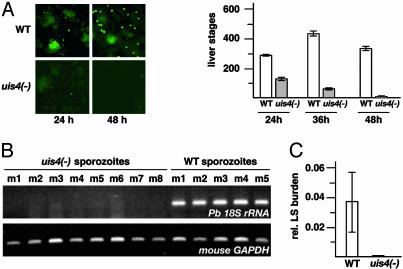
UIS4 Is Critical for Liver Stage Development. Next, we tested whether UIS4 is important for intracellular liver stage development. In HepG2 cells, which support complete Pb liver stage development (19), uis4- Pb liver stages display a dramatic developmental deficiency (Fig. 2A). Twenty-four hours after sporozoite invasion, liver stage numbers of uis4- parasites were reduced by ≈50% compared with WT parasites. At later time points, intracellular mature uis4- liver stage schizonts were not observed. However, developmentally arrested small uis4- liver stages persisted up to that time point.
Fig. 2.
uis4- parasites arrest in liver stage development. (A) UIS4 is required for liver stage development in vitro.WTand uis4 - salivary gland sporozoites were added to subconfluent hepatoma cells, and the intracellular liver stages that develop were immunostained with anti-heat shock protein 70 at the indicated time points after infection. Shown in Right are mean liver stage numbers (±SEM) from four experiments each. (B) Late liver stages are not detected in mice infected with uis4- sporozoites. Five and eight mice were injected i.v. with 10,000 WT and uis4- sporozoites, respectively. After 48 h, livers were removed and subjected to total RNA extraction and RT-PCR. cDNA amplification was done with gene-specific primers for Pb 18S rRNA and mouse GAPDH, respectively. (C) Quantitative real-time RT-PCR confirms the absence of late liver stages in mice infected with uis4- sporozoites.
Based on the dramatic in vitro results, we tested infectivity of uis4- parasites in vivo in C57/Bl6 mice that are highly susceptible to infection by Pb sporozoites (20). To monitor the presence of late Pb liver stages, we first isolated total infected mouse liver RNA taken 48 h after sporozoite injection for RT-PCR on Pb 18S rRNA. Remarkably, no parasite rRNA was detected by this assay (Fig. 2B) or by quantitative real time RT-PCR (Fig. 2C) (21). Immunostaining of uis4--infected mouse liver sections revealed morphologically similar liver stages for WT and uis4- parasites at 12 h after infection and absence of uis4- late liver stage parasites at 40 h after infection (data not shown). Taken together, the results show that uis4- parasites suffer a severe defect in liver stage development. This defect manifests itself only after sporozoite invasion and transformation into early liver stages, when the liver stages enter the growth phase.
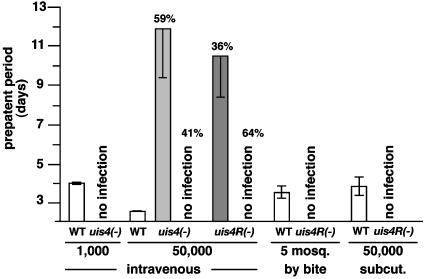
Attenuation of uis4 Knockout Parasites in Vivo. We investigated whether Pbuis4- parasites can establish blood stage infections in vivo. Intriguingly, i.v. injection of 1,000 uis4- sporozoites into each of 16 mice did not result in erythrocytic stage infection, even after 21 days, as monitored by daily blood smears (Fig. 3). i.v. injection of 1,000 WT control sporozoites reproducibly resulted in erythrocytic infections after a prepatent period of 4 days (Fig. 3). Extremely high i.v. inocula of 50,000 uis4- sporozoites caused blood stage infections in 59% of inoculated mice that were detected after an average prepatent period of 12 days (Fig. 3). PCR analysis of these blood stage parasites did not show reversion to WT (data not shown). High i.v. inocula of 50,000 uis4REP- sporozoites resulted in severely delayed blood stage infections in some mice, albeit at lower frequency when compared with uis4- parasites (Fig. 3). Importantly, however, inoculations with uis4REP- sporozoites did not lead to blood stage infections in natural transmission experiments, i.e., by mosquito bite or by s.c. injection of 50,000 uis4REP- sporozoites (Fig. 3).
Fig. 3.
uis4- parasites are severely impaired in progression to blood stage infections in the mammalian host. WT and uis4- sporozoites were injected i.v. or s.c. or delivered by natural bite in numbers indicated. i.v. injection of 1,000 uis4- sporozoites (16 mice total) did not result in erythrocytic stage infections (patency), even after 21 days. WT-injected control mice (six mice total) become blood stage patent after 4 days (prepatent period). i.v. injection of 50,000 sporozoites resulted in delayed blood stage infections in uis4- and uis4REP- parasites at the proportion of animals indicated (56 and 75 mice total injected, respectively). Blood stage parasites from patent mice were genotyped and confirmed to be uis4- or uis4REP- mutants. Natural transmission by mosquito bite with five uis4REP- infected mosquitoes per mouse (10 mice total) or by s.c. injection of 50,000 uis4REP- sporozoites (10 mice total) did not result in blood stage infections. WT-infected control mice (10 mice total) became blood stage patent as indicated.
Immunization with uis4REP- Sporozoites Confers Sterile Protection. The fact that a large proportion of mice remained blood stage negative after inoculation with uis4REP- sporozoites allowed us to test whether immunization with these attenuated sporozoites would protect mice against WT sporozoite challenge. Therefore, we immunized C57/Bl6 mice with three doses of 50,000, 25,000, and 25,000 uis4REP- sporozoites or three doses of 10,000 uis4REP- sporozoites each and subsequently challenged the mice, which remained blood stage negative after immunization, with 50,000 infectious WT sporozoites (Table 2). None of the immunized mice developed blood stage infections after challenge and therefore enjoyed complete, sterile protection. Naive mice that were challenged with 50,000 WT sporozoites developed blood stage infections 3 days after inoculation. Mice immunized with uis4REP- sporozoites were not protected against subsequent challenge with Pb blood stages (Table 2).
Table 2. C57BI/6 mice immunized with uis4REP- sporozoites are completely protected against a challenge with WT sporozoites.
| Immunization with uis4REP- sporozoites | Boosts (days after immunization/no. of sporozoites) | No. protected/no. challenged (prepatency) |
|---|---|---|
| 50,000 | 1st (14/25,000), 2nd (28/25,000) | 8/8 (no infection)* |
| None | None | 0/5 (day 3)† |
| 10,000 | 1st (14/10,000), 2nd (28/10,000) | 8/8 (no infection)* |
| None | None | 0/5 (day 3)† |
| 10,000 | 1st (14/10,000), 2nd (29/10,000) | 0/4 (day 2)‡ |
Immunized mice were challenged with 50,000 WT Pb sporozoites at day 38 after immunization. Mice were from the same age group, and sporozoites were from the same mosquito batch. Blood smears were evaluated up to day 50 after challenge
Naive control mice were from the same age group and were challenged with 50,000 WT Pb sporozoites
Immunized mice were challenged with 10,000 blood stage parasites at day 34 after immunization
UIS4 Is Expressed Throughout Plasmodium Liver Stage Development and Localizes to the Parasitophorous Vacuole. UIS4 encodes a putative acidic transmembrane protein that is present in all Plasmodium species we examined. Synteny searches of the Plasmodium falciparum genome database (22) with the Py and Pb UIS4, which share 74% amino acid sequence identity, identified a potential orthologous protein on chromosome 10 (PF10_0164) (30% identity).
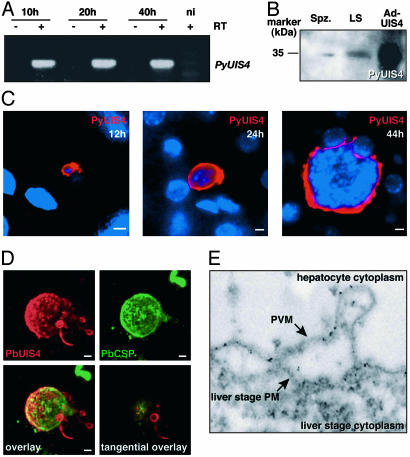
Because depletion of UIS4 leads to a severe developmental defect of liver stages, we investigated its expression and localization throughout the parasites' liver phase. PyUIS4 transcripts were detected throughout liver stage development in BALB/c mice that were infected with Py sporozoites as determined by RT-PCR (Fig. 4A). We used UIS4-specific antisera (12) and tested these by immunoblot of total protein of either salivary gland sporozoites or infected liver. In both cases, a single protein species was identified, which had a similar molecular mass when compared with Ad-expressed full-length PyUIS4 in HEK 293 cells (Fig. 4B).
Fig. 4.
Plasmodium UIS4 is expressed throughout liver stage development and localizes to the parasite–host membrane interface. (A) PyUIS4 transcripts are present throughout in vivo liver stage development of Py. RT-PCR detects PyUIS4 expression in the liver of mice 10, 20, and 40 h after infection with 5 × 105 sporozoites. No amplification is observed in control reactions without RT (-RT) and in an uninfected control mouse (ni). (B) Immunoblot analysis of Py salivary gland sporozoite extracts (Spz.) and extracts from Py-infected mouse livers at 44 h of liver stage development (LS). The right lane shows an extract of PyUIS4-Ad-infected HEK 293 cells (Ad-UIS4). (C) PyUIS4 is expressed in a circumferential pattern surrounding the parasite throughout liver stage development. Shown are Py liver stages in vivo at 12, 24, and 44 h of development. In all stages, PyUIS4 is localized around the parasite and in extensions that reach into the hepatocyte cytoplasm. (D) Three-dimensional serial reconstruction of a Pb liver stage (48 h) within a HepG2 cell from 25 confocal optical sections. The surface of the liver stage is visualized with anti-CSP staining, appearing uneven and patchy. PbUIS4 shows peripheral localization surrounding the liver stage and exhibits an irregular surface profile, with multiple vesicular protrusions, distinct when compared with the CSP profile. An extensive TVN is visible on one side of the liver stage, with multiple membranous vesicles and tubular structures reaching into the HepG2 cell cytoplasm, most of which maintain a connection to the PVM. Note that the CSP-positive growth-delayed small liver stage visible in the upper right corner of the micrographs does not show UIS4 expression. (Lower Right) Micrograph shows a1 μm, confocal optical section of the liver stage at a tangential plane. Vesicles of the TVN reveal a peripheral PbUIS4 localization most consistent with its localization in the vesicular membranes. (Scale bars: 5 μm.) (E) Immunoelectron microscopy confirms PyUIS4 localization to the PVM of liver stages in vivo. Five-nanometer gold particles decorate the PVM and a TVN protrusion that extends outward into the hepatocyte cytoplasm.
We then investigated the localization of PyUIS4 during liver stage development in the in vivo mouse model. In liver sections that were generated at consecutive time points after inoculation of sporozoites, PyUIS4 localized to the periphery of the developing liver stage (Fig. 4C). Twelve hours after sporozoite invasion, single-nucleated, retorte-shaped transformed liver stages displayed strong circumferential staining, with extensions reaching into the hepatocyte cytoplasm. As the liver stage grew, PyUIS4 was detected as a broad halo surrounding the parasites, a staining pattern that is indicative of UIS4 localization to the PV compartment (23). To further study the nature of UIS4 localization in the PV, we analyzed late Pb liver stages that were grown in cultured HepG2 cells by confocal microscopy (Fig. 4D). The surface of the liver stage schizont was visualized by CSP staining. PbUIS4 staining extensively overlapped with CSP staining. However, unlike CSP, PbUIS4 localized additionally to vesicular protrusions that extended from the liver stage periphery into the hepatocyte cytoplasm (Fig. 4D). We named this membranous network the “liver stage tubovesicular network,” in analogy with the tubovesicular network (TVN) that extends from the PV membrane (PVM) of intraerythrocytic stages into the cytoplasm of infected red blood cells (24). We also noticed that PbUIS4 is not expressed in growth-delayed CSP-positive liver stages, suggesting that UIS4 is a specific marker for successful liver stage development.
Immunoelectron microscopy with UIS4 antisera on sections of a Py-infected liver confirmed localization of PyUIS4 to the PV, specifically the PVM that can be clearly seen separated from the liver stage plasma membrane (Fig. 4E) where the liver stage TVN extends into the host cell cytoplasm. Together, our findings indicate that UIS4 localizes to the parasite–host interface membrane throughout Plasmodium liver stage development.
Discussion
Liver stages can be viewed as the malaria parasites' bridgehead for colonization of the mammalian host. The production of thousands of red blood cell-infectious merozoites within each liver stage over a short period (a few days to a week, depending on the Plasmodium species) allows the parasite to effectively overwhelm the host and guarantee successful blood infection. During natural malaria transmission, this strategy may well be critical for the parasites' survival, because an infectious mosquito bite likely inoculates <100 sporozoites (25–27). Currently, it remains unknown which of the thousands of parasite genes (22, 28) are specifically expressed in liver stages and could be involved in growth and development of this most elusive life cycle stage. We have shown herein that UIS4, a protein that we originally identified by gene expression analysis of liver-infectious sporozoites (11), continues to be expressed throughout liver stage development. Analysis of UIS4-depleted sporozoites shows that UIS4 has no apparent function in the sporozoites' interactions with mosquito tissues, their gliding motility, or their ability to invade hepatocytes. This finding indicates that the gene expression program, which is initiated in sporozoites residing in mosquito salivary glands, prepares the parasite for events beyond host cell invasion. In liver stages, UIS4 is positioned in the PVM, the organelle through which all interactions between the parasite and host hepatocyte occur. In addition, UIS4 also localizes to a TVN that extends from the PV into the hepatocyte cytoplasm. TVNs in intraerythrocytic stages (24) may be involved in exogenous nutrient import. Recently, a family of small blood stage PVM and TVN-resident membrane proteins termed “ETRAMPS” has been identified (29), but its importance for intraerythrocytic parasite growth and development remains to be elucidated. UIS4 function, however, is clearly restricted to intrahepatic liver stages because it is not expressed in intraerythrocytic blood stages. It could have either a structural role for the growth and maintenance of the elaborate PV/TVN membrane compartment or a role in liver stage interactions with the host hepatocyte.
Our gene knockout data establish that UIS4 is critical for liver stage development both in vitro and in vivo. Although UIS4 is expressed early in liver stage development, parasites lacking UIS4 are able to develop to early liver stages, indicating that UIS4 function is not essential for the transformation of sporozoites into liver stage trophozoites. The developmental deficiency of UIS4-depleted liver stages becomes apparent only during the growth and replication phase of the liver stage schizont and ultimately results in a severe impairment to produce merozoites and, thus, to initiate the blood stage phase of the life cycle. This parasite growth deficiency, although appearing similar to the growth deficiency observed with radiation-attenuated P. falciparum sporozoites (30), awaits further detailed investigation. The ability of UIS4-depleted parasites to somehow initiate blood stage infections in mice when inoculated i.v. at high sporozoite doses is clearly a rare event that is comparable to observations made with trap- sporozoites, which lack the sporozoite invasin thrombospondin-related anonymous protein (TRAP) (31). trap- sporozoites exhibit complete deficiencies in sporozoite gliding motility and hepatocyte invasion, yet they can still initiate blood stage infections when inoculated i.v. at high doses (31). Currently, it remains unknown how the extremely rare uis4- and trap- mutant parasite achieves to progress to blood stage infections with such long delay, but, because of the very scarcity of the event, this will be difficult to investigate.
The data presented here, together with our recent study of UIS3 knockout parasites (32), demonstrate that malaria parasites harbor genes that are necessary only for successful completion of the preerythrocytic mammalian infection. We have shown that deletion of such a gene effectively creates genetically attenuated malaria parasites that infect the liver of the mammalian host but are severely impaired in their ability to further progress through the life cycle and cause malaria disease. The quest for other genes in the Plasmodium genome, which are critical for liver stage development, continues, and these genes can be identified with the strategies described herein.
Finally, we have shown here that immunization with UIS4 knockout sporozoites confers complete, sterile protection against subsequent infectious sporozoite challenge in a mouse model. This, together with our recent finding that immunization of mice with UIS3- parasites can also induce protection against infectious sporozoite challenge (32), demonstrates the successful use of genetically attenuated Plasmodium parasites as live experimental vaccines.
Acknowledgments
We thank Andreas Kunze and Jack Whisler for expert technical assistance and Kirsten Heiss for help with the phenotypical analysis. We are grateful to Stefan Jentsch for valuable comments on the manuscript. This work was supported by grants from the research focus Tropical Medicine Heidelberg of the Medical Faculty of Heidelberg University, Deutsche Forschungsgemeinschaft Ma2161/3-1 (to K.M.), the National Institutes of Health, and the Bill and Melinda Gates Foundation (to S.H.I.K.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ad, adenovirus; CSP, circumsporozoite protein; PV, parasitophorous vacuole; PVM, PV membrane; Pb, Plasmodium berghei; Py, Plasmodium yoelii; TRAP, thrombospondin-related anonymous protein; TVN, tubovesicular network; UIS4, up-regulated in infective sporozoites gene 4.
References
- 1.Kappe, S. H., Kaiser, K. & Matuschewski, K. (2003) Trends Parasitol. 19, 135-143. [DOI] [PubMed] [Google Scholar]
- 2.Meis, J. F., Verhave, J. P., Jap, P. H., Sinden, R. E. & Meuwissen, J. H. (1983) Nature 302, 424-426. [DOI] [PubMed] [Google Scholar]
- 3.Meis, J. F., Verhave, J. P., Jap, P. H. & Meuwissen, J. H. (1985) Cell Tissue Res. 241, 353-360. [DOI] [PubMed] [Google Scholar]
- 4.Meis, J. F. & Verhave, J. P. (1988) Adv. Parasitol. 27, 1-61. [DOI] [PubMed] [Google Scholar]
- 5.Shortt, H. E. & Garnham, P. C. C. (1948) Nature 161, 126. [DOI] [PubMed] [Google Scholar]
- 6.Druilhe, P. L., Renia, L. & Fidock, D. A. (1998) in Malaria: Parasite Biology, Pathogenesis, and Protection, ed. Sherman, I. W. (Am. Soc. Microbiol., Washington, DC), pp. 513-543.
- 7.Nussenzweig, R. S., Vanderberg, J., Most, H. & Orton, C. (1967) Nature 216, 160-162. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman, S. L., Goh, L. M., Luke, T. C., Schneider, I., Le, T. P., Doolan, D. L., Sacci, J., de la Vega, P., Dowler, M., Paul, C., et al. (2002) J. Infect. Dis. 185, 1155-1164. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman, S. L. & Doolan, D. L. (2000) Nat. Med. 6, 1218-1219. [DOI] [PubMed] [Google Scholar]
- 10.Kappe, S. H., Gardner, M. J., Brown, S. M., Ross, J., Matuschewski, K., Ribeiro, J. M., Adams, J. H., Quackenbush, J., Cho, J., Carucci, D. J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 9895-9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matuschewski, K., Ross, J., Brown, S. M., Kaiser, K., Nussenzweig, V. & Kappe, S. H. (2002) J. Biol. Chem. 277, 41948-41953. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser, K., Matuschewski, K., Camargo, N., Ross, J. & Kappe, S. H. (2004) Mol. Microbiol. 51, 1221-1232. [DOI] [PubMed] [Google Scholar]
- 13.Thathy, V. & Menard, R. (2002) Methods Mol. Med. 72, 317-331. [DOI] [PubMed] [Google Scholar]
- 14.de Koning-Ward, T. F., Fidock, D. A., Thathy, V., Menard, R., van Spaendonk, R. M., Waters, A. P. & Janse, C. J. (2000) Mol. Biochem. Parasitol. 106, 199-212. [DOI] [PubMed] [Google Scholar]
- 15.Vanderberg, J. P. (1974) J. Protozool. 21, 527-537. [DOI] [PubMed] [Google Scholar]
- 16.Potocnjak, P., Yoshida, N., Nussenzweig, R. S. & Nussenzweig, V. (1980) J. Exp. Med. 151, 1504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji, M., Mattei, D., Nussenzweig, R. S., Eichinger, D. & Zavala, F. (1994) Parasitol. Res. 80, 16-21. [DOI] [PubMed] [Google Scholar]
- 18.Renia, L., Miltgen, F., Charoenvit, Y., Ponnudurai, T., Verhave, J. P., Collins, W. E. & Mazier, D. (1988) J. Immunol. Methods 112, 201-205. [DOI] [PubMed] [Google Scholar]
- 19.Hollingdale, M. R., Leland, P. & Schwartz, A. L. (1983) Am. J. Trop. Med. Hyg. 32, 682-684. [DOI] [PubMed] [Google Scholar]
- 20.Scheller, L. F., Wirtz, R. A. & Azad, A. F. (1994) Infect. Immun. 62, 4844-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruna-Romero, O., Hafalla, J. C., Gonzalez-Aseguinolaza, G., Sano, G., Tsuji, M. & Zavala, F. (2001) Int. J. Parasitol. 31, 1499-1502. [DOI] [PubMed] [Google Scholar]
- 22.Gardner, M. J., Hall, N., Fung, E., White, O., Berriman, M., Hyman, R. W., Carlton, J. M., Pain, A., Nelson, K. E., Bowman, S., et al. (2002) Nature 419, 498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingelbach, K. & Joiner, K. A. (1998) J. Cell Sci. 111, 1467-1475. [DOI] [PubMed] [Google Scholar]
- 24.Haldar, K., Samuel, B. U., Mohandas, N., Harrison, T. & Hiller, N. L. (2001) Int. J. Parasitol. 31, 1393-1401. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg, R., Wirtz, R. A., Schneider, I. & Burge, R. (1990) Trans. R. Soc. Trop. Med. Hyg. 84, 209-212. [DOI] [PubMed] [Google Scholar]
- 26.Beier, J. C., Davis, J. R., Vaughan, J. A., Noden, B. H. & Beier, M. S. (1991) Am. J. Trop. Med. Hyg. 44, 564-570. [DOI] [PubMed] [Google Scholar]
- 27.Frischknecht, F., Baldacci, P., Martin, B., Zimmer, C., Thiberge, S., Olivo-Marin, J. C., Shorte, S. L. & Menard, R. (2004) Cell Microbiol. 6, 687-694. [DOI] [PubMed] [Google Scholar]
- 28.Carlton, J. M., Angiuoli, S. V., Suh, B. B., Kooij, T. W., Pertea, M., Silva, J. C., Ermolaeva, M. D., Allen, J. E., Selengut, J. D., Koo, H. L., et al. (2002) Nature 419, 512-519. [DOI] [PubMed] [Google Scholar]
- 29.Spielmann, T., Fergusen, D. J. & Beck, H. P. (2003) Mol. Biol. Cell 14, 1529-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvie, O., Semblat, J. P., Franetich, J. F., Hannoun, L., Eling, W. & Mazier, D. (2002) Parasite Immunol. (Oxford) 24, 221-223. [DOI] [PubMed] [Google Scholar]
- 31.Sultan, A. A., Thathy, V., Frevert, U., Robson, K. J., Crisanti, A., Nussenzweig, V., Nussenzweig, R. S. & Menard, R. (1997) Cell 90, 511-522. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, A. K., Labaied, M., Kappe, S. H. & Matuschewski, K. (2005) Nature 433, 164-167. [DOI] [PubMed] [Google Scholar]