Abstract
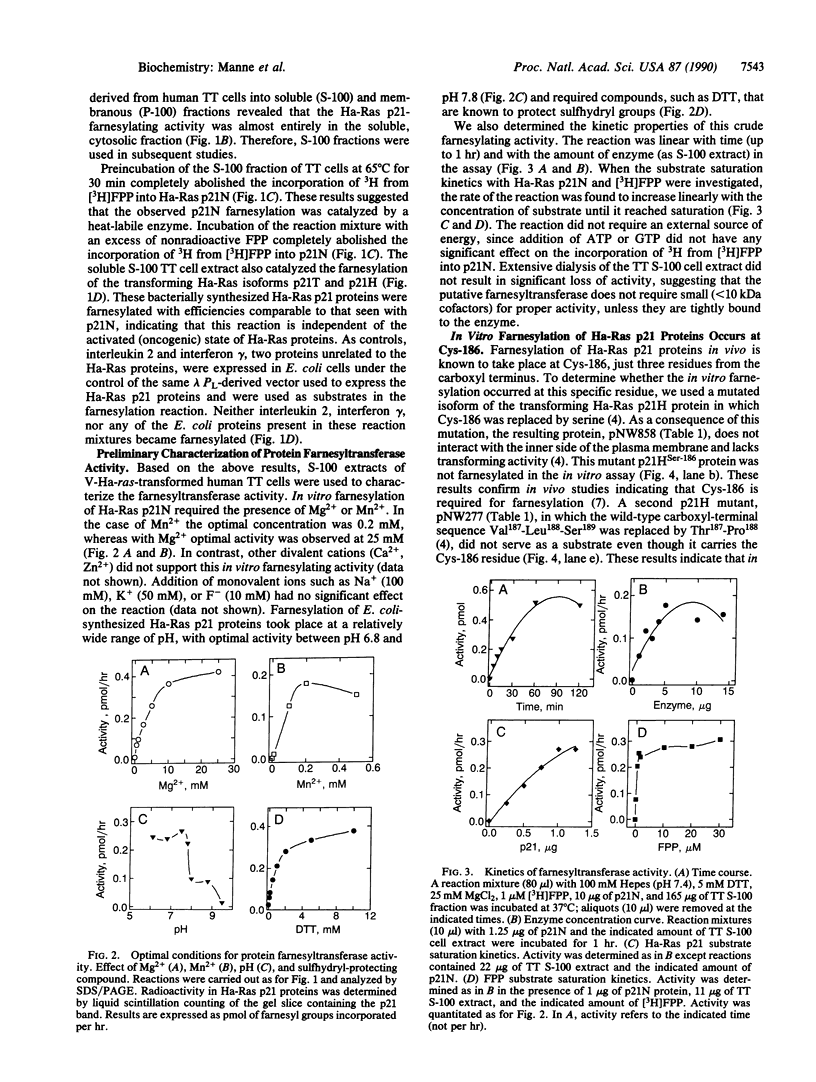
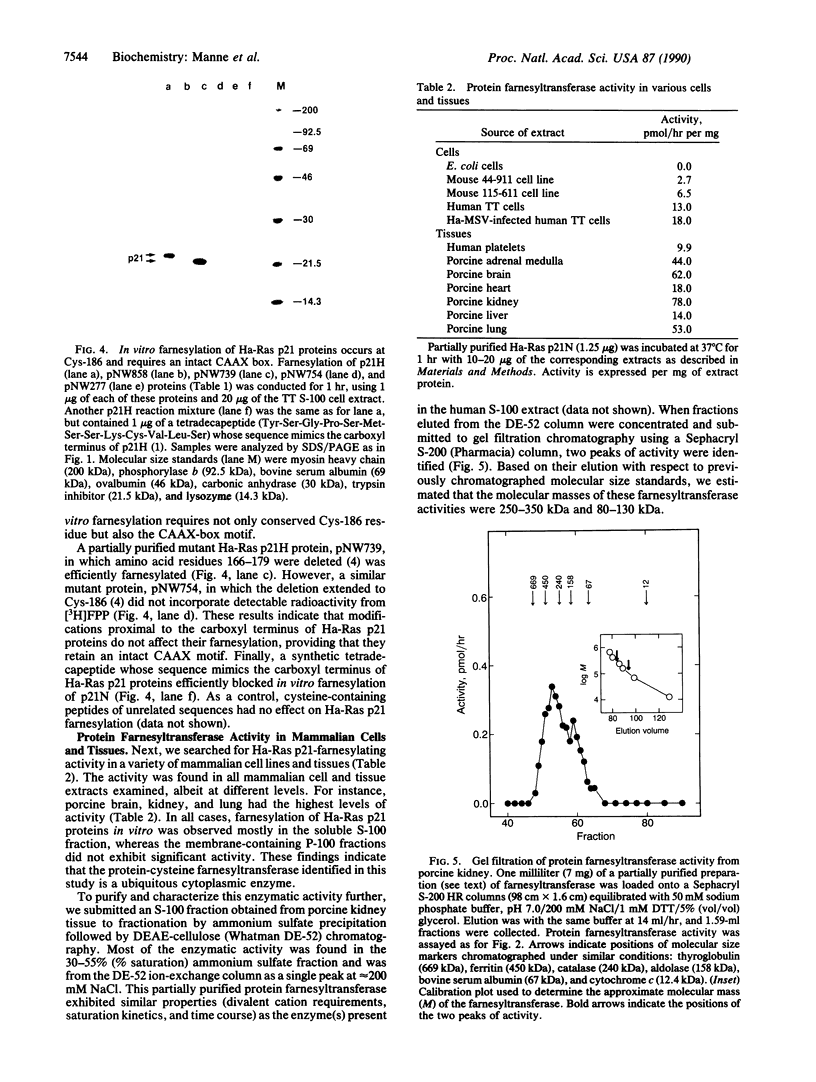
Ras proteins must be isoprenylated at a conserved cysteine residue near the carboxyl terminus (Cys-186 in mammalian Ras p21 proteins) in order to exert their biological activity. Previous studies indicate that an intermediate in the mevalonate pathway, most likely farnesyl pyrophosphate, is the donor of this isoprenyl group. Inhibition of mevalonate synthesis reverts the abnormal phenotypes induced by the mutant RAS2Val-19 gene in Saccharomyces cerevisiae and blocks the maturation of Xenopus oocytes induced by an oncogenic Ras p21 protein of human origin. These results have raised the possibility of using inhibitors of the mevalonate pathway to block the transforming properties of ras oncogenes. Unfortunately, mevalonate is a precursor of various end products essential to mammalian cells, such as dolichols, ubiquinones, heme A, and cholesterol. In this study, we describe an enzymatic activity(ies) capable of catalyzing the farnesylation of unprocessed Ras p21 proteins in vitro at the correct (Cys-186) residue. This farnesylating activity is heat-labile, requires Mg2+ or Mn2+ ions, is linear with time and with enzyme concentration, and is present in all mammalian cell lines and tissues tested. Gel filtration analysis of a partially purified preparation of protein farnesyltransferase revealed two peaks of activity at 250-350 kDa and 80-130 kDa. Availability of an in vitro protein farnesyltransferase assay should be useful in screening for potential inhibitors of ras oncogene function that will not interfere with other aspects of the mevalonate pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Direct identification of palmitic acid as the lipid attached to p21ras. Mol Cell Biol. 1986 Jan;6(1):116–122. doi: 10.1128/mcb.6.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Q., Ulsh L. S., DuBois G., Shih T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J Virol. 1985 Nov;56(2):607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. H., Cochrane C. G., Bourne J. R., Solski P. A., Buss J. E., Der C. J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Santos E., Notario V., Barbacid M., Yamazaki S., Kung H., Seamans C., McAndrew S., Crowl R. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5305–5309. doi: 10.1073/pnas.81.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Yamazaki S., Kung H. F. Guanosine nucleotide binding by highly purified Ha-ras-encoded p21 protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6953–6957. doi: 10.1073/pnas.81.22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Mabry M., de Bustros A., Ihle J. N., Nelkin B. D., Baylin S. B. Introduction of v-Ha-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5923–5927. doi: 10.1073/pnas.84.16.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Robbins K. C., Barbacid M. Oncogenes in human tumor cell lines: molecular cloning of a transforming gene from human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2845–2849. doi: 10.1073/pnas.79.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Long L. K., Sorrentino V., Barbacid M. ras gene Amplification and malignant transformation. Mol Cell Biol. 1985 Oct;5(10):2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Norris K., Papageorge A. G., Hubbert N. L., Lowy D. R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984 Nov;3(11):2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Hubbert N., Bekesi E., Kung H. F., Lowy D. R. Transforming p21 ras protein: flexibility in the major variable region linking the catalytic and membrane-anchoring domains. EMBO J. 1985 Nov;4(11):2893–2896. doi: 10.1002/j.1460-2075.1985.tb04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




