Abstract
Coronary artery disease (CAD) is the primary critical cardiovascular event. Endothelial cell and monocyte dysfunction with subsequent extravagant inflammation are the main causes of vessel damage in CAD. Thus, strategies that repress cell death and manage unsuitable pro-inflammatory responses in CAD are potential therapeutic strategies for improving the clinical prognosis of patients with CAD. SIRT1 (Sirtuin 1) plays an important role in regulating cellular physiological processes. SIRT1 is also thought to protect the cardiovascular system by means of its antioxidant, anti-inflammation and anti-apoptosis activities. In the present study, we found that the SIRT1 expression levels were repressed and the acetylated p53 expression levels were enhanced in the monocytes of patients with CAD. LOX-1/oxidative stress was also up-regulated in the monocytes of patients with CAD, thereby increasing pro-apoptotic events and pro-inflammatory responses. We also demonstrated that monocytes from CAD patients caused endothelial adhesion molecule activation and the adherence of monocytes and endothelial cells. Our findings may explain why CAD patients remain at an increased risk of long-term recurrent ischemic events and provide new knowledge regarding the management of clinical CAD patients.
Keywords: CAD, SIRT1, Oxidative stress, LOX-1
Highlights
-
•
SIRT1 expression is repressed in monocytes from CAD subjects.
-
•
LOX-1 and oxidative stress were increased in monocytes from CAD subjects.
-
•
SIRT1 inhibition causes mitochondrial dysfunction and apoptosis in monocytes from CAD subjects.
-
•
Monocytes from CAD patients had an increased adherence to endothelial cells through SIRT1 repression.
1. Introduction
Coronary artery disease (CAD) is the primary critical cardiovascular event, causing high morbidity and mortality all over the world [1]. Coronary artery luminal obstructions and plaque cracks due to atherosclerosis are the most common causes of CAD, which is distinguished by endothelial damage, lipid aggregation and the generation of atherosclerotic plaques [2]. Apoptosis and necrosis of cardiomyocytes, endothelial cells and monocytes with subsequent extravagant inflammation are the main causes of vessel damage under CAD [3]. Thus, strategies that repress cell death and manage unsuitable pro-inflammatory responses under CAD are potential therapeutic strategies for improving the clinical prognosis of patients with CAD.
Oxidative stress is one complication that occurs when the generation of reactive oxygen species (ROS) exceeds antioxidant enzyme activity [4]. Oxidative stress is recognized as a key regulator of the progression of cardiovascular diseases. For example, previous studies have suggested that hyperlipidemia and diabetes mellitus (DM) are both associated with elevated oxidative stress, which may result in the development of atherosclerosis and CAD [5]. In addition to cardiovascular diseases, other systemic diseases, degeneration and aging are associated with oxidative stress and have been well reported by investigators [6]. In normal situations, cells are protected from ROS by antioxidant enzymes such as Superoxidase dismutase (SOD), glutathione peroxidase (GPx), and catalase [7]. SOD quickly catalyzes the chelation of O− to H2O2·H2O2 is converted to O2 and H2O by catalase or GPx [8]. Therefore, antioxidant enzyme activity is important for the normal redox balance in humans.
The chronic inflammation linked with CAD is currently well-known in clinical practice. The American Heart Association suggested that the blood C-reactive protein (CRP) level is a risk factor in coronary disease development [9]. In addition, the immune capacity in the human body plays a critical role in the induction and deterioration of atherosclerosis, with monocytes/macrophages playing critical roles in this action [10]. Monocyte participation in the progression of atherosclerotic plaques was demonstrated in the 1970s, with monocyte aggregation manifested in atherosclerotic lesions [11].
SIRT1 (Sirtuin 1) is recognized to play an important role in regulating cellular physiological processes, such as metabolism, cell degeneration, cell growth and cell survival. In human endothelial cells, SIRT1 regulates anti-aging in endothelial cells and protects against endothelial inflammation [12], [13]. Some survival genes or stress-resistance related genes are targets of SIRT1, such as mTOR, PI-3K, PPAR-γ and p53 [14]. SIRT1 has also been shown to enhance antioxidant enzyme activity and inhibit free radical-mediated oxidative injuries via decreasing NADPH oxidase activation [15]. Furthermore, a previous study reported that the expression level and activity of SIRT1 were reduced in inflammatory endothelial cells [16]. Recently, SIRT1 has been recognized as a novel target in preventing human endothelial pathology. For example, SIRT1 protects against ionomycin-induced ICAM-1 expression in endothelial cells [17] and attenuates thrombomodulin down-regulation after particulate matter exposure [18]. Activating SIRT1 function via drugs has also been reported to reduce oxidative injury-induced endothelial cell death [19]. Moreover, the expression level of SIRT1 is shown to be decreased in inflammatory human endothelial cells [16]. SIRT1 influences the biological activity and signaling transduction of a number of proteins by modulating their deacetylation or through non-deacetylating reactions. LKB1 is one of the important targets of SIRT1. A previous study suggested that SIRT1 positively regulates endothelial cell proliferation and prevents senescence by targeting LKB1 [20]. SIRT1 has been well investigated in endothelial cells. However, there is only one study that has reported on monocyte SIRT1 repression in patients with CAD [21]. This study was designed to understand whether SIRT1 inhibition causes oxidative stress and inflammation in patients with coronary artery disease.
2. Materials and methods
2.1. Study patients
The study group included 30 patients diagnosed with CAD over 60 days and 30 cases with normal coronary arteries. The two groups were recruited from the National Cheng Kung University Hospital. All patients presented with stable clinical symptoms and syndromes. The basic parameters of the studied population are presented in Table 1. In detail, the clinical diagnosis of stable CAD was made according to a clinical evaluation, echocardiography, and angiography. Patients who satisfied the following inclusion criteria were included in the study: patients diagnosed with CAD without angina or clinical presentation and syndromes that remained stable for at least 60 days with no indication of new myocardial damage. Exclusion criteria were previous coronary bypass surgery, unstable angina, and new myocardial infarction. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of National Cheng Kung University Hospital (Approval number A-ER-103-335), and each participant provided written informed consent.
Table 1.
Characteristics of subjects a.
| Characteristicsb | Control (n=30) | CAD (n=30) | p valuesc |
|---|---|---|---|
| Age (years) | 52.2 ± 9.5 | 57.4± 11.2 | 0.455 |
| Male/Female | 14/16 | 17/13 | 0.075 |
| BMI (kg/m2) | 24.4 ± 3.4 | 25.6 ± 4.7 | 0.121 |
| BUN (mmol/L) | 5.9 ± 1.4 | 6.0 ± 2.1 | 0.335 |
| CK (U/dL) | 130.5 ± 18.3 | 183.3 ± 25.1 | 0.035* |
| Hs-crp (mmol/L) | 1.2 ± 0.4 | 2.8 ± 2.1 | 0.041* |
| TC (mmol/L) | 4.1 ± 1.1 | 4.9 ± 1.3 | 0.036* |
| TG (mmol/L) | 1.7 ± 0.7 | 2.0 ± 1.1 | 0.184 |
| LDL-C (mmol/L) | 2.3 ± 0.7 | 3.3 ± 0.8 | 0.042* |
| HDL-C (mmol/L) | 1.2 ± 0.4 | 1.1± 0.4 | 0.551 |
| HTN,n(%) | 7 (23.3) | 23(76.6) | 0.034* |
| DM,n(%) | 6 (20) | 19 (63.3) | 0.024* |
| Smoking habit,n(%) | 14 (46.6) | 17(63.3) | 0.412 |
BMI: body mass index; BUN: blood urea nitrogen; CK: creatine kinase; Hs-crp: high-sensitivity C-reactive protein; TC: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; HTN: hypertension; DM: Diabetes mellitus.
Parameters for age, BMI, Hs-crp, and lipid analysis are provided as mean ± SD.
Control versus CAD.
Bold indicates significant differences (p < 0.05).
2.2. Reagents
Fetal bovine serum (FBS), medium 199 (M199), and trypsin-EDTA were obtained from GIBCO (Grand Island, NY). EDTA, penicillin, and streptomycin were obtained from Sigma (St. Louis, MO). Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining kits were obtained from Boehringer Mannheim (Mannheim, Germany). The superoxide dismutase activity assay kit was purchased from Calbiochem (San Diego, CA). DCF-AM was obtained from Molecular Probes (Eugene, OR). 5,58,6,68-tetraethylbenzimidazolcarbocyanine iodide (JC-1) and anti-active caspase 3 were obtained from BioVision (Palo Alto, CA). Anti-vascular cell adhesion molecule-1 (VCAM-1), anti-intercellular adhesion molecule (ICAM), and anti-E-selectin were purchased from R&D Systems (Minneapolis, MN). SRT1720, anti-SIRT1 and anti-β-actin were obtained from Santa Cruz Biotechnology (CA, USA). Anti-acetyl-p53 and anti-p53 were obtained from Cell Signaling (MA, USA).
2.3. Laboratory data analysis and blood monocytes isolation
Blood samples were collected into vacuum tubes containing ethylenediamine tetraacetic acid (EDTA) for the measurement of blood profiles, cardiac markers and pro-inflammatory parameters. Blood was sampled for isolation of monocytes. Monocytes were isolated from heparinized blood from CAD subjects and control cases using Isopaque-Ficoll (Lymphoprep; Fresenius Kabi Norge AS, Oslo, Norway) gradient centrifugation. Monocytes were isolated for further biological tests. In some cases, isolated monocytes were cultured in RPMI with 10% FBS at a density of 5 × 106 cells/ml for transfection.
2.4. Total RNA isolation and real-time PCR reaction
Total RNA was isolated using TRIzol reagent. Reverse transcription was performed at 42 °C for 60 min, followed by incubation at 95 °C for 5 min. The reaction 20 mixture (20 μl of total volume) consisted of 2 μg of isolated total RNA, 1 mM dNTP, 1 unit/μl of recombinant RNasin ribonuclease inhibitor, 15 U/μg of avian myeloblastosis 22 virus (AMV) reverse transcriptase, 5× RT buffer, and 0.5 μg of oligo (dT)12 primer. The gene-specific primers used are listed in Table 2. Real-time PCR reactions were performed using the SYBR Green method in an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's guidelines. Primers were designed using the computer software Primer Express 2.0 (Applied Biosystems, Foster City, CA). The reactions were set by mixing 12.5 μl of the SYBR Green Master Mix (Applied Biosystems, Foster City, CA) with 1 μl of a solution containing 10-μM concentrations of both primers and 2 μl of cDNA solution. The Ct value was defined as the number of PCR cycles required for the fluorescence signal to exceed the detection threshold value. The relative amounts of mRNA for each gene were normalized based on the amount of the housekeeping gene β-actin.
Table 2.
The oligonucleotide sequences.
| Forward | Reverse | |
|---|---|---|
| SIRT1 | 5′-CGGATTAAAATTTGAGTTGTTTC-3′ | 5′-CCTTCCTCTTTATAACGAACGTA-3′ |
| iNOS | 5′-CCCTTCCGA AGT TTCTGGCAGCAG C-3′ | 5′-GGCTG CAGAGCCTCGTGGCTTTGG-3′ |
| LOX-1 | 5′-CTGGCTGCTGCCACTCTA-3′ | 5′-TTGCTTGCTCTTGTGTTAGGA-3′ |
| β-actin | 5′-GAATTCTGGCCACGGCTGCTTCCAGCT-3′ | 5′-AAGCTTTTTCGTGGATGCCACAGGACT-3′ |
2.5. Immunoblotting
Monocytes were lysed in RIPA buffer (in mM: HEPES 20, MgCl2 1.5, EDTA 2, EGTA 5, dithiothreitol 0.1, phenylmethylsulfonyl fluoride 0.1, pH 7.5). Proteins (30 µg) were separated by electrophoresis on an SDS-polyacrylamide gel. After the protein was transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), the blots were incubated with blocking buffer (1X PBS and 5% nonfat dry milk) for 1 h at room temperature and then probed with primary antibodies overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 h. To control equal loading of total protein in all lanes, blots were stained with mouse anti-β-actin antibody at a 1:50000 dilution. The bound immunoproteins were detected by an enhancer chemiluminescent assay (ECL; Amersham, Berkshire, UK). The intensities were quantified by densitometric analysis (Digital Protein DNA Imagineware, Huntington Station, NY).
2.6. Antioxidant enzyme levels measurement
To determine the antioxidant enzyme leveld, monocytes were collected from whole blood after centrifugation at 2500×g and 4 °C for 10 min. SOD (Cell Biolabs, STA-340) and catalase level (Cell Biolabs, STA-341) in monocytes was determined via an enzymatic assay method using a commercial kit according to the manufacturer's instructions. Enzyme level was converted to units per milligram of protein.
2.7. Investigation of reactive oxygen and nitrogen species (RONS) production
Monocytes were incubated with 10 µM DCF-AM for 1 h. The fluorescence intensity was measured with a fluorescence microplate reader (Labsystem, CA) calibrated for excitation at 485 nm and emission at 538 nm.
2.8. Measurement of mitochondria membrane potential and O2 consumption
The lipophilic cationic probe fluorochrome 5,58,6,68-tetraethylbenzimidazol- carbocyanine iodide (JC-1) was used to explore the mitochondrial membrane potential (ΔΨm). JC-1 exists either as a green fluorescent monomer at depolarized membrane potential or as a red fluorescent J-aggregate at hyperpolarized membrane potential. JC-1 exhibits potential-dependent accumulation in mitochondria, as indicated by the fluorescence emission shift from 530 to 590 nm. Cells were rinsed with M199, and JC-1 (5 μM) was loaded. After 20 min of incubation at 37 °C, cells were examined under a fluorescence microscope. Determination of the ΔΨm was performed using a FACScan flow cytometer. O2 consumption was tested using an Oxygen Consumption Rate Assay Kit (MitoXpress® Xtra HS Method). In brief, cells were cultured on a black 96-well plate. After 24hrs, the plate was washed with the corresponding clear respiration media consisted of phenol-free RPMI with 10% FBS. A MitoXpress-Xtra-HS, phosphorescent oxygen sensitive probe, was put together to the wells to test oxygen consumption of monocytes. Oxygen consumption was evaluated by time-resolved fluorescence (TR-F) with a dual delay time of 30 μs and 70 μs. The TR-F was converted to phosphorescence lifetime values by the following equation: lifetime=Delay time 2- Delay time 2/ ( measured intensity value 1/ measured intensity value 2).
2.9. Investigation of apoptosis
Apoptotic cells were assessed by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Roche) under a fluorescence microscope or in a flow cytometer. The level of active caspase-3 was detected by flow cytometry and fluorescence microscopy using a commercial fluorescein active caspase kit (Mountain View, CA).
2.10. Transfection with small interfering RNA (siRNA)
On-target Plus SMART pool siRNAs for non-targeting control and SIRT1 was purchased from Dharmacon. A jetPRIME® was used for siRNA transfection. In brief, a final concentration of 50 nM siRNA per well was added into 200 μl of jetPRIME® buffer. For SIRT1 overexpression. And than, 4 μl jetPRIME® reagent was added and mixed for 10 s followed by 15 mins incubation. The transfection reagent was mixed with the cells in serum containing medium. 48hrs after transfection, cells were treated with reagent as indicated for further experiments. Transfection efficiencies had been confirmed by Western blotting assay.
2.11. SIRT1 overexpression
Human SIRT1 was bough from Addgene (Cambridge, MA, USA). SIRT1 was subcloned into p3XFLAG-Myc-CMV-26 expression vector. Transient transfections with 2 µg plasmid DNA were accomplished with Lipofectamine 2000 (Invitrogen) at 1: 3 ratio for 48 h. Transfection efficiencies had been confirmed by Western blotting assay.
2.12. Preparation of nuclear and cytosolic extracts
Nuclear and cytosolic extracts were isolated with a Nuclear and Cytoplasmic Extraction kit (Pierce Chemical, Rockford, IL). Monocytes were collected by centrifugation at 600g for 5 min at 4 °C. The pellets were washed twice with ice-cold PBS, followed by the addition of 0.2 ml of cytoplasmic extraction buffer A and vigorous mixing for 15 s. Ice-cold cytoplasmic extraction buffer B (11 μl) was added to the solution. After vortex mixing, nuclei and cytosolic fractions were separated by centrifugation at 16,000g for 5 min. The cytoplasmic extracts (supernatants) were stored at −80 °C. Nuclear extraction buffer was added to the nuclear fractions (pellets), which were then mixed by vortex mixing on the highest setting for 15 s. The mixture was iced, and a 15-s vortex was performed every 10 min for a total of 40 min. Nuclei were centrifuged at 16,000g for 10 min. The nuclear extracts (supernatants) were stored at −80 °C until use. NF-κB expression was measured using an NF-κB-p65 Active ELISA Kit (Imgenex, San Diego, CA, USA) according to the manufacturer's instructions. The absorbance at 405 nm was determined using a microplate reader (SpectraMax 340).
2.13. Nitric oxide (NO) accumulation
NO production in the medium and was assayed using Gries reagent. Briefly, 100 μl of Gries reagent (1% sulfanilamide-0.1% naphthylethylene diamine dihydrochloride-2.5% H3PO4) (Sigma, St. 12 Louis, MO) was added to 100 μl of each supernatant in triplicate wells of 96-well plates. The plates were read in a microplate reader (Molecular Devices, Palo Alto, CA, USA) at 550 nm against a standard curve of NO in culture medium.
2.14. Adhesion assay
HUVECs at 1 × 105 cells/ml were cultured in 96-well plates. HUVECs incubated with monocytes (prelabeled with 4-μM BCECF-AM for 30 min in RPMI at 1 × 106 cells/ml density) were added to fresh RPMI. The cells were allowed to adhere at 37 °C for 1 h in a 5% CO2 incubator. Plates were washed three times with M199 to remove non-adherent cells. The cells were then lysed with 0.1 ml 0.25% Triton X-100. Fluorescence intensity was measured with a fluorescence microplate reader (Labsystem, CA) and calibrated for excitation at 485 nm and for emission at 538 nm.
2.15. Adhesion molecule expression
HUVECs were incubated with monocytes for 24 h. Cells were harvested and incubated with fluorescence-conjugated anti-ICAM-1, anti-VCAM-1, and anti-E-selectin (R&D, Minneapolis, MN) for 45 min at room temperature. After the HUVECs had been washed three times, their immunofluorescence intensity was analyzed by flow cytometry using a Becton–Dickinson FACScan flow cytometer (Mountain View, CA).
2.16. Statistical analysis
The data distribution of each covariate between the CAD and the control subjects was examined by Kruskal-Wallis rank sum tests for continuous variables and chi-square tests for clinical parameters. One-way ANOVA was used to compare the difference in gene expression levels between CAD and control subjects. In vitro studies, inter-group differences were analyzed by One-way ANOVA and Student's t-test. A p-value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Subject characteristics
A total of 60 cases were included in the present study (30 age- and gender-matched control patients without coronary artery disease and 30 ACS subjects from National Cheng Kung University Hospital). Detailed clinical parameters of the subjects’ characteristics are shown in Table 1. The medium age was 54 years old. CAD was diagnosed based on electrocardiography findings and clinical laboratory parameters. Overall, CK, Hs-crp and TC concentrations were higher in CAD subjects. Higher LDL cholesterol concentrations were found in CAD patients compared to control subjects.
3.2. SIRT1 expression is repressed in monocytes from CAD subjects
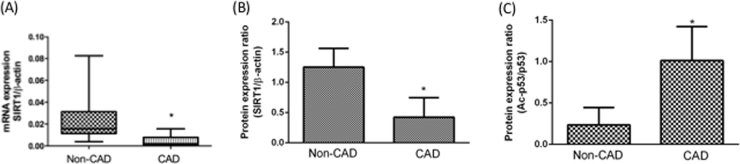
Previous reports have suggested that SIRT1 plays a critical role in CVD. These studies analyzed the potential function of SIRT1 in CVD and explained the mechanisms of pro-inflammatory response repression and protection against oxidative stress [22], [23]. Accordingly, we investigated SIRT1 expression levels in isolated monocytes using quantitative reverse transcription PCR (qPCR) analyses. As shown in Fig. 1A, SIRT1 expression was relatively lower in the monocytes of CAD patients compared to control (non-CAD) subjects. SIRT1 protein expression levels were detected using a Western blotting assay (Fig. 1B). In addition, Acetylated p53 is a surrogate marker for endogenous SIRT1 activity and promotes apoptosis. Thus, we tested acetylated p53 expression using a Western blotting assay. Our results shuuested that acetylated p53 expression was relatively higher in the monocytes of CAD patients compared to control (non-CAD) subjects (Fig. 1C).
Fig. 1.
Expression levels of SIRT1 in monocytes from clinical CAD patients. Monocytes were isolated from patients with or without CAD. SIRT1 mRNA expression levels in monocytes were investigated by real-time PCR (A). Summary data of representative Western blots shown (B) SIRT1 protein expression levels were repressed and (C) p53 acetylation were increased in CAD monocytes. *P < 0.05 compared with non-CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30.
3.3. LOX-1 and oxidative stress in patients with CAD
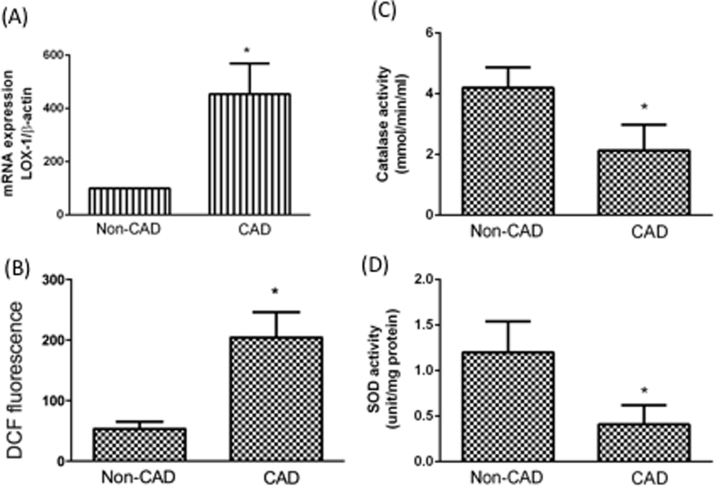
The lectin-like oxidized low-density lipoprotein receptor (LOX-1) has been traditionally described as a receptor for oxLDL [24]. LOX-1 is expressed in monocytes, endothelial cells, and smooth muscle cells [25]. In addition, LOX-1 plays critical roles in modulating the progression of atherosclerotic lesions [26]. We found that the LOX-1 expression level was higher in the monocytes of CAD patients than in control patients (Fig. 2A). The up-regulation of LOX-1 expression promptly leads to ROS production [27]. Fig. 2B shows that monocytes from CAD had a higher RONS level. In addition, we found that the antioxidative enzymes, catalase and SOD activities were higher in the monocytes of CAD patients than in control subjects (Fig. 2C and Fig. 2D). Interestingly, although the clinical presentations were stable in CAD patients, oxidative stress remained higher in CAD patients than in the control subjects.
Fig. 2.
Activation of LOX-1/oxidative stress in CAD monocytes. LOX-1 mRNA expression levels in monocytes were investigated by real-time PCR (A). The fluorescence intensity of cells was measured using a fluorescence microplate reader to measure RONS concentrations (B). Antioxidant enzymes were investigated by kits. Intracellular GSH (C) and SOD (D) levels in monocytes were detected using a GSH assay kit and a SOD activity kit. *P < 0.05 compared with non-CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30.
3.4. Monocyte mitochondrial dysfunction in CAD patients
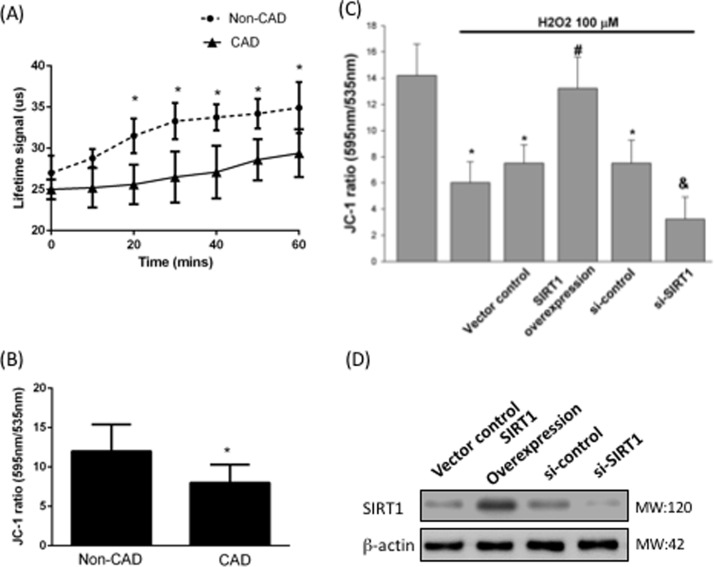
Mitochondria demand oxygen to generate ATP in sufficient quantities to drive energy-necessitating reactions. The calculation of oxygen consumption levels from isolated mitochondria in vitro is an appropriate investigation to test mitochondrial dysfunction and diseases [28]. Dual-read time-resolved fluorescence and subsequent Lifetime calculation permits estimation of the rate of fluorescence decay of the Extracellular consumption reagent, and can provide estimations of oxygen consumption that are more stable and with a wider dynamic range than evaluating signal intensity. We found that monocytes from CAD patients attenuated the rate of O2 consumption by a reduced lifetime signaling (Fig. 3A). The mitochondrial membrane potential is a key parameter of mitochondrial function and is used as an index of cell health. JC-1 was used for investigation of the mitochondrial membrane potential. The higher ratio of 535/595 indicates the higher membrane depolarization. Fig. 3B shows that monocytes from CAD patients had an impaired mitochondrial membrane potential (p=0.041). To determine whether CAD patients had an impaired mitochondrial membrane potential due to SIRT1 inhibition, monocytes from non-CAD patients were treated with SIRT1 silencing and overexpression. As expected, SIRT1 inhibition promoted H2O2-impaired mitochondrial membrane potential; however, overexpression of SIRT1 protected against H2O2-impaired mitochondrial membrane potential (Fig. 3C).
Fig. 3.
Impaired mitochondria dysfunction in CAD monocytes. (A) Cellular oxygen consumption rate in monocytes were investigated in patients with or without CAD. (B) ΔΨm was inspected with the signal from monomeric and J-aggregate JC-1 fluorescence as described earlier. *P < 0.05 compared with non-CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30. (C) Monocytes from control subjects were overexpressed or silenced SIRT1 expression, JC-1 were used to investigate ΔΨm under H2O2 treatment. (D) Transfection efficiencies had been confirmed by Western blotting assay. Ѱ P < 0.05 compared with non-H2O2-treated monocytes. ϕ P < 0.05 compared with vector control monocytes. Δ P < 0.05 compared with si-control monocytes. Data represent the mean ± S.D. of five independent experiments.
3.5. Monocyte apoptosis in CAD patients
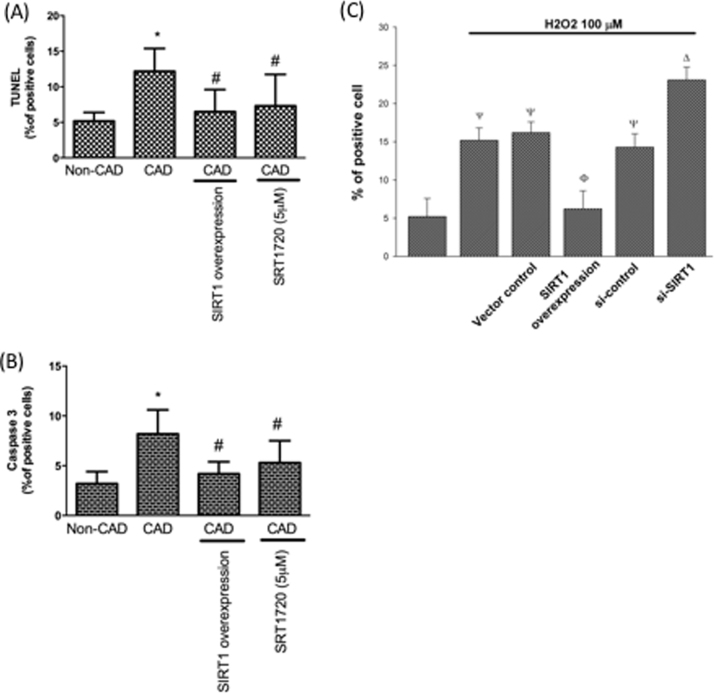
LOX-1 regulates pro-apoptotic signaling transduction and nitric oxide (NO) catabolism, thereby facilitating oxidative injuries and cell death [29]. We found more apoptotic events existed in CAD monocytes by TUNEL and caspase 3 assay. Interestingly, both SIRT1 overexpression or treatment of SIRT1 activator (SRT1720) in CAD monocytes mitigated these pro-apoptotic events (Fig. 4A and B). DNA fragmentation characterizes a representative hallmark of apoptosis. TUNEL is one well-established assay for investigating DNA fragments using flow cytometry. The fluorescence intensity increase is proportional to the increased TUNEL positive cells. We therefore used TUNEL assay to answer if SIRT1 is critical in regulation of apoptosis in CAD monocytes. In Fig. 4C, we reported that SIRT1 inhibition promoted H2O2-caused apoptosis; however, overexpression of SIRT1 protected against H2O2-caused apoptosis.
Fig. 4.
Pro-apoptotic events were up-regulated in CAD monocytes. Pro-apoptotic events were investigated by (A) TUNEL or (B) caspase 3 activity assay. *P < 0.05 compared with non-CAD monocytes. #P < 0.05 compared with CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30. (C) Monocytes from control subjects were overexpressed or silenced SIRT1 expression, TUNEL assay used to investigate apoptosis under H2O2 treatment. Ψ P < 0.05 compared with non-H2O2-treated monocytes. ϕ P < 0.05 compared with vector control monocytes. Δ P < 0.05 compared with si-control monocytes. Data represent the mean ± S.D. of five independent experiments.
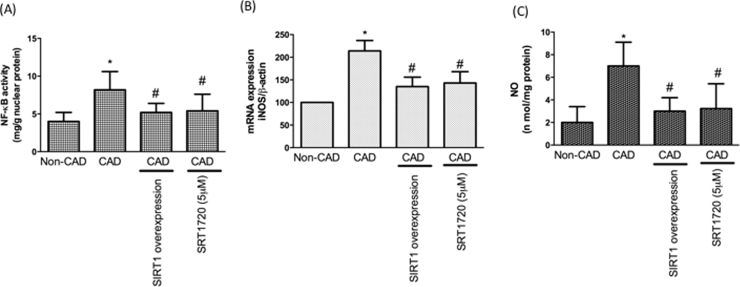
3.6. Monocyte inflammation in CAD patients
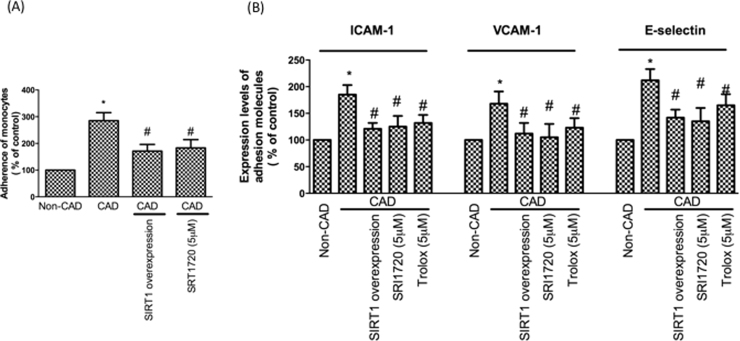
Inflammation plays a critical role in the development of CAD. Next, we focused on the occurrence of inflammatory events in CAD monocytes. Our data revealed NF-kB expression is up-regulated in CAD monocytes. We also found that iNOS expression and NO concentrations are up-regulated in CAD monocytes. As expected, overexpression or activation of SIRT1 in CAD monocytes attenuated these pro-inflammatory events (Fig. 5A–C). A previous study has shown that the retention of adhesiveness to the endothelium in human monocytes is the main process associated with the earliest stages of atherogenesis [30]. To test whether there is an increase in monocyte adhesion to HUVECs in CAD patients, confluent monocytes from control subjects or CAD patients with or without SIRT1 overexpression were co-incubated with monolayers of endothelial cells for 1 h at 37 °C. Our results demonstrated that monocytes from CAD patients increased the attachment of monocytes on endothelial cells; however, this finding was reversed by SIRT1 overexpression and activation (Fig. 6A, B). We also found that co-incubation with endothelial cell monolayers and monocytes from patients with CAD promoted endothelial adhesion molecule expression. We assumed monocytes from patients with CAD may have had a higher oxidative stress by SIRT1 inhibition, thereby causing endothelial adhesion molecule up-regulation. Therefore, we overexpressed SIRT1 or pretreated with SRT1720 or pretreated with Trolox (antioxidant) in monocytes from patients with CAD before co-culturing with endothelial cells. As anticipated, SIRT1 overexpression, SIRT1 activation and Trolox treatment reduced monocyte-derived adhesion molecule activation (Fig. 6C).
Fig. 5.
Pro-inflammatory events were promoted in CAD monocytes. (A) Pro-inflammatory event was investigated by NF-·B activity assay. (B) iNOS expression levels were tested by real-time PCR. (C) Content of NO was assayed using Griess reagent. *P < 0.05 compared with non-CAD monocytes. #P < 0.05 compared with CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30.
Fig. 6.
An increase of monocyte adhesion to endothelial cells in CAD patients. (A) Monocytes were pre-loaded with BCECF-AM were incubated with endothelial cells. The adhesiveness of HUVECs to monocytes was measured as described in the Materials and Methods. (B) CAD monocytes caused activation of adhesion molecules in endothelial cells. Adhesion molecules were investigated by flow cytometry. *P < 0.05 compared with non-CAD monocytes. #P < 0.05 compared with CAD monocytes. Data represent the mean ± S.D. Number of control subjects: 30, number of CAD subjects: 30.
4. Discussion
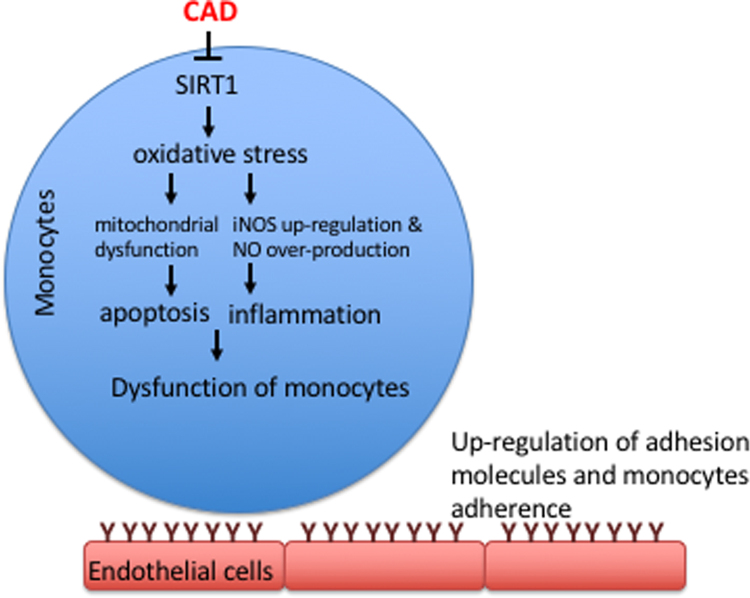
CAD is the major cause of death in both the elderly and patients with cardiovascular diseases. The protective function of SIRT1 in the cardiovascular system has been well reported. Human circulator monocytes facilitate the destabilization of the fibrous cap, causing plaque rupture. In the present study, we reported for the first time that the expression levels of SIRT1 in CAD monocytes is mitigated compared to control subjects. In addition, LOX-1/oxidative stress signaling was activated and antioxidant enzyme activities were repressed in CAD monocytes. We also found that SIRT1 inhibition impaired mitochondrial dysfunction and promoted pro-apoptotic events and pro-inflammatory responses, thereby increasing monocyte attachment to endothelial cells (Fig. 7).
Fig. 7.
Schematic diagram of the major study findings.
SIRT1 has been recognized as a new regulator of homoeostasis in the human cardiovascular system, and it has been suggested that SIRT1 exerts anti-atherosclerotic capacities against cellular dysfunction by preventing stress-facilitated senescence [31]. As an ubiquitous NAD(+) dependent deacetylase, SIRT1 plays a critical role as a modulator in physiopathology processes, metabolism, stress responses and degeneration. In addition, activation of SIRT1 is thought to be an effective approach in the management of cardiovascular diseases or metabolic diseases [32]. In contrast, SIRT1 repression has been found in some systemic disease. For example, Erion et al. suggested SIRT1 silencing in the liver represses basal hepatic glucose generation and enhances hepatic insulin responsiveness in diabetic animals [33]. In this study, we found that SIRT1 expression levels were relatively repressed in CAD monocytes compared with control subjects.
The lectin-like oxidized low-density lipoprotein receptor (LOX)-1 is a major receptor for oxLDL. OxLDL binding to LOX-1 is one important step in the development of atherosclerosis [34]. In addition, a study has shown that LOX-1 expression levels are activated in several pathological scenarios or systemic diseases, such as hypertension, diabetes, and hypercholesterolemia [24]. Activated LOX-1 facilitates the production of free radicals and impairs antioxidant level, thereby causing oxidative stress in the circulatory system [35]. SIRT1 negatively regulates LOX-1 expression by modulating the LOX-1 promoter [36]. In the present study, we found that the LOX-1 expression levels and RONS concentration are higher in monocytes with CAD. Antioxidant activities, SOD and catalase, were also reduced in CAD subjects.
Mitochondria are known as the major origin of intracellular ROS under both physiological and pathological conditions. Mitochondria are important modulators of energy formation, signal transduction, inflammation and apoptosis [37]. In addition, up-regulated mitochondrial free radicals are required for the initiation and acceleration of atherosclerosis [38]. Normalization of mitochondrial function has been recognized as an effective approach in the management of atherosclerotic damage. For example, by increasing the activity of mitochondrial thioredoxin-2 (Trx2) or controlling the mitochondria-specific antioxidant mitigated atherosclerotic injuries in an animal study [39]. SIRT1 enables mitochondrial biogenesis regulation under different stress conditions [40]. SIRT1 activation protects cells from oxidative stress-caused by cellular dysfunction [36]. In addition, SIRT1 mitigated aging-caused cardiac remodeling and contractile dysfunction through increasing mitochondrial function [41]. Here, we have shown that mitochondrial function is impaired in CAD monocytes. We also found that mitochondrial dysfunction is modulated by SIRT1 inhibition.
This series of conflicting changes is also associated with pro-inflammatory events, such as the activation of NF-κB and the subsequent expression of inflammatory mediators that promote leukocyte adhesion. Considerable evidence indicates that oxLDL-induced cellular dysfunction is associated with the inhibition of eNOS and the activation of iNOS. Free radicals produced by oxLDL directly associate with NO to form peroxynitrite, a stable molecule that is toxic to cells [42]. We found that pro-inflammatory events were relatively activated in CAD monocytes compared to control samples through modulation of SIRT1.
Human monocytes increase the imbalance of the fibrous cap, leading to plaque rupture. This is primarily coordinated by matrix metalloproteinases (MMPs)[43]. A high concentration of MMPs generated by macrophages has been shown in vulnerable plaque districts, whereas up-regulated serum MMPs have been reported in patients with CAD [44]. Importantly, monocyte aggregation is up-regulated in patients with CAD and persists even after one month of the acute onset [45]. Taken together, the functional role of monocytes in CAD should be further investigated to provide a new therapeutic approach in patients with CAD. In this study, we confirmed that monocytes from CAD patients had an increased adherence to endothelial cells through SIRT1 repression. We also found that the attached monocytes caused endothelial adhesion molecule up-regulation, suggesting that up-regulated oxidative stress in monocytes in CAD may further cause endothelial dysfunction. In this present study, we used in vitro investigations to study the role of SIRT1 in CAD patients. We successfully demonstrated the SIRT1 inhibition is critical in apoptosis and oxidative stress regulation. However, there is still a gap to directly report whether SIRT1 inhibition in CAD monocytes truly increases the risk of long-term recurrence of ischemic events in CAD patients. A longitudinal study would be valuable to determine this issue.
In conclusion, this study established for the first time that oxidative stress in monocytes with CAD is significantly up-regulated by SIRT1 inhibition. In addition, pro-apoptotic events, pro-inflammatory events and mitochondrial impairment are significantly up-regulated in CAD patients, whereas activation of SIRT1 function reversed those atherosclerotic events. Results from this study might provide new knowledge with respect to the management of clinical CAD patients.
Conflict of interest
None.
Acknowledgements
This study was supported by grants from Ministry of Science and Technology (MOST 105-2311-B-006-008).
References
- 1.Ladapo J.A., Goldfeld K.S., Douglas P.S. Projected morbidity and mortality from missed diagnoses of coronary artery disease in the United States. Int. J. Cardiol. 2015;195:250–252. doi: 10.1016/j.ijcard.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yahagi K., Kolodgie F.D., Lutter C., Mori H., Romero M.E., Finn A.V., Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2016 doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orogo A.M., Gustafsson A.B. Cell death in the myocardium: my heart won't go on. IUBMB Life. 2013;65(8):651–656. doi: 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Phys. India. 2004;52:794–804. [PubMed] [Google Scholar]
- 5.Haidara M.A., Yassin H.Z., Rateb M., Ammar H., Zorkani M.A. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr. Vasc. Pharmacol. 2006;4(3):215–227. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- 6.Kayali R., Cakatay U., Tekeli F. Male rats exhibit higher oxidative protein damage than females of the same chronological age. Mech. Ageing Dev. 2007;128(5–6):365–369. doi: 10.1016/j.mad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Miller A.A., De Silva T.M., Jackman K.A., Sobey C.G. Effect of gender and sex hormones on vascular oxidative stress. Clin. Exp. Pharmacol. Physiol. 2007;34(10):1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]
- 8.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 9.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., 3rd, Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., Rifai N., Smith S.C., Jr., Taubert K., Tracy R.P., Vinicor F. C. Centers for Disease, Prevention, A. American Heart, Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 10.Oude Nijhuis M.M., van Keulen J.K., Pasterkamp G., Quax P.H., de Kleijn D.P. Activation of the innate immune system in atherosclerotic disease. Curr. Pharm. Des. 2007;13(10):983–994. doi: 10.2174/138161207780487593. [DOI] [PubMed] [Google Scholar]
- 11.Kottke B.A., Subbiah M.T. Pathogenesis of atherosclerosis. Concepts based on animal models. Mayo Clin. Proc. 1978;53(1):35–48. [PubMed] [Google Scholar]
- 12.Lei J., Gu X., Ye Z., Shi J., Zheng X. Antiaging effects of simvastatin on vascular endothelial cells. Clin. Appl. Thromb./Hemost.: Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2012 doi: 10.1177/1076029612458967. [DOI] [PubMed] [Google Scholar]
- 13.Orecchia A., Scarponi C., Di Felice F., Cesarini E., Avitabile S., Mai A., Mauro M.L., Sirri V., Zambruno G., Albanesi C., Camilloni G., Failla C.M. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PloS One. 2011;6(9):e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong Z.Z., Wang S., Shang Y.C., Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012;8(1):89–100. doi: 10.2217/fca.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarzuelo M.J., Lopez-Sepulveda R., Sanchez M., Romero M., Gomez-Guzman M., Ungvary Z., Perez-Vizcaino F., Jimenez R., Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem. Pharmacol. 2013;85(9):1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Kao C.L., Chen L.K., Chang Y.L., Yung M.C., Hsu C.C., Chen Y.C., Lo W.L., Chen S.J., Ku H.H., Hwang S.J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010;17(9):970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y., Gao P., Chen H., Wan Y., Zhang R., Zhang Z., Yang R., Wang X., Xu J., Liu D. SIRT1 suppresses PMA and ionomycin-induced ICAM-1 expression in endothelial cells. Sci. China Life Sci. 2013;56(1):19–25. doi: 10.1007/s11427-012-4407-7. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z., Liu M.C., Liang M., Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood. 2012;119(10):2422–2429. doi: 10.1182/blood-2011-04-350413. [DOI] [PubMed] [Google Scholar]
- 19.Guo H., Chen Y., Liao L., Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy Upregulation via the AMPK/SIRT1 pathway. Cardiovasc. Drugs Ther. / Spons. Int. Soc. Cardiovasc. Pharmacother. 2013;27(3):189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 20.Zu Y., Liu L., Lee M.Y., Xu C., Liang Y., Man R.Y., Vanhoutte P.M., Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 2010;106(8):1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- 21.Breitenstein A., Wyss C.A., Spescha R.D., Franzeck F.C., Hof D., Riwanto M., Hasun M., Akhmedov A., von Eckardstein A., Maier W., Landmesser U., Luscher T.F., Camici G.G. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLoS One. 2013;8(1):e53106. doi: 10.1371/journal.pone.0053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winnik S., Auwerx J., Sinclair D.A., Matter C.M. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur. Heart J. 2015;36(48):3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan S.H., Chu P.M., Kao C.L., Cheng Y.H., Hung C.H., Tsai K.L. Oleic acid activates MMPs up-regulation through SIRT1/PPAR-gamma inhibition: a probable linkage between obesity and coronary arterial disease. J. Biochem. 2016;160(4):217–225. doi: 10.1093/jb/mvw028. [DOI] [PubMed] [Google Scholar]
- 24.Mehta J.L., Chen J., Hermonat P.L., Romeo F., Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006;69(1):36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Draude G., Hrboticky N., Lorenz R.L. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem. Pharmacol. 1999;57(4):383–386. doi: 10.1016/s0006-2952(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy J.E., Tacon D., Tedbury P.R., Hadden J.M., Knowling S., Sawamura T., Peckham M., Phillips S.E., Walker J.H., Ponnambalam S. LOX-1 scavenger receptor mediates calcium-dependent recognition of phosphatidylserine and apoptotic cells. Biochem. J. 2006;393(Pt 1):107–115. doi: 10.1042/BJ20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X.P., Xun K.L., Wu Q., Zhang T.T., Shi J.S., Du G.H. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vasc. Pharmacol. 2007;47(1):1–9. doi: 10.1016/j.vph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro S.M., Gimenez-Cassina A., Danial N.N. Measurement of mitochondrial oxygen consumption rates in mouse primary neurons and astrocytes. Methods Mol. Biol. 2015;1241:59–69. doi: 10.1007/978-1-4939-1875-1_6. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto K., Ishibashi T., Sawamura T., Inoue N., Kamioka M., Uekita H., Ohkawara H., Sakamoto T., Sakamoto N., Okamoto Y., Takuwa Y., Kakino A., Fujita Y., Tanaka T., Teramoto T., Maruyama Y., Takeishi Y. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc. Res. 2009;84(1):127–136. doi: 10.1093/cvr/cvp177. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.A., Territo M.C., Wayner E., Carlos T.M., Parhami F., Smith C.W., Haberland M.E., Fogelman A.M., Berliner J.A. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler. Thromb.: J. Vasc. Biol. / Am. Heart Assoc. 1994;14(3):427–433. doi: 10.1161/01.atv.14.3.427. [DOI] [PubMed] [Google Scholar]
- 31.Ota H., Akishita M., Eto M., Iijima K., Kaneki M., Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell Cardiol. 2007;43(5):571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Chang H.C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014;25(3):138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erion D.M., Yonemitsu S., Nie Y., Nagai Y., Gillum M.P., Hsiao J.J., Iwasaki T., Stark R., Weismann D., Yu X.X., Murray S.F., Bhanot S., Monia B.P., Horvath T.L., Gao Q., Samuel V.T., Shulman G.I. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc. Natl. Acad. Sci. USA. 2009;106(27):11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyal T., Mitra S., Khaidakov M., Wang X., Singla S., Ding Z., Liu S., Mehta J.L. Current concepts of the role of oxidized LDL receptors in atherosclerosis. Curr. Atheroscler. Rep. 2012 doi: 10.1007/s11883-012-0228-1. [DOI] [PubMed] [Google Scholar]
- 35.Makino J., Asai R., Hashimoto M., Kamiya T., Hara H., Ninomiya M., Koketsu M., Adachi T. Suppression of EC-SOD by oxLDL during vascular smooth muscle cell proliferation. J. Cell Biochem. 2016;117(11):2496–2505. doi: 10.1002/jcb.25542. [DOI] [PubMed] [Google Scholar]
- 36.Hung C.H., Chan S.H., Chu P.M., Tsai K.L. Homocysteine facilitates LOX-1 activation and endothelial death through the PKCbeta and SIRT1/HSF1 mechanism: relevance to human hyperhomocysteinaemia. Clin. Sci. 2015;129(6):477–487. doi: 10.1042/CS20150127. [DOI] [PubMed] [Google Scholar]
- 37.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H., Luo Y., Zhang W., He Y., Dai S., Zhang R., Huang Y., Bernatchez P., Giordano F.J., Shadel G., Sessa W.C., Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am. J. Pathol. 2007;170(3):1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallardo-Montejano V.I., Saxena G., Kusminski C.M., Yang C., McAfee J.L., Hahner L., Hoch K., Dubinsky W., Narkar V.A., Bickel P.E. Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1alpha/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat. Commun. 2016;7:12723. doi: 10.1038/ncomms12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Mi S.L., Hu N., Doser T.A., Sun A., Ge J., Ren J. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 2014;71:208–220. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X.L., Zhang Q., Zhao R., Ding X., Tummala P.E., Medford R.M. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J. Pharmacol. Exp. Ther. 2003;305(2):573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 43.Medbury H.J., Tarran S.L., Guiffre A.K., Williams M.M., Lam T.H., Vicaretti M., Fletcher J.P. Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int. Angiol. 2008;27(2):114–123. [PubMed] [Google Scholar]
- 44.Momiyama Y., Ohmori R., Tanaka N., Kato R., Taniguchi H., Adachi T., Nakamura H., Ohsuzu F. High plasma levels of matrix metalloproteinase-8 in patients with unstable angina. Atherosclerosis. 2010;209(1):206–210. doi: 10.1016/j.atherosclerosis.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Tapp L.D., Shantsila E., Wrigley B.J., Pamukcu B., Lip G.Y. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J. Thromb. Haemost. 2012;10(7):1231–1241. doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]