Abstract
Highly potent and broadly neutralizing anti-HIV-1 antibodies (bNAbs) have been used to prevent and treat lentivirus infections in humanized mice, macaques and humans1–12. To determine whether the administration of combination bNAbs during the acute SHIV infection of rhesus macaques might lead to long-term control of virus replication, animals challenged with SHIVAD8-EO by mucosal or intravenous routes received a single 2-week course of 2 potent passively transferred bNAbs (3BNC117 and 10-107413,14). Viremia remained undetectable for 56–177 days, depending on bNAb half-life in vivo. Moreover, in the 13 treated monkeys, plasma virus loads subsequently declined to undetectable levels in 6 controller macaques. 4 additional animals maintained their CD4+ T cell counts and very low levels of viremia persisted for over 2 years. The frequency of cells carrying replication-competent virus was less than 1 per 106 circulating CD4+ T cells in the 6 controller macaques. Infusion of a T cell depleting anti-CD8β mAb to the controller animals led to a specific decline in levels of CD8+ T cells and rapid reappearance of plasma viremia. In contrast, macaques treated for 15 weeks with combination anti-retroviral therapy (cART), beginning on day 3 after infection, experienced sustained rebound plasma viremia when treatment was interrupted. We conclude that passive immunotherapy during the acute SHIV infection differs from cART in that it facilitates the emergence of potent CD8+ T cell immunity able to durably suppress virus replication.
Following an initial and massive burst of HIV replication15, viremia is incompletely controlled by the emergence of a virus-specific CD8+ T cell response, leading to a chronic phase during which the infection is never cleared16,17. Virions that integrate their DNA into the host cell genome become a source of virus production, which persists despite anti-retroviral treatment over extended periods of time and gives rise to a recrudescent progressive infection when treatment is stopped18. This virus reservoir persists for the lifetime of the infected individual19.
Nonetheless, initiation of cART within 6 months of HIV-1 infection may limit damage to the immune system as well as the size of the reservoir, as measured by preservation of anti-virus T cell responses and lower levels of cell-associated viral DNA20. In a rare subpopulation of HIV-1 infected persons, cART initiated during the early phases of infection resulted in sustained control of viremia to very low levels, following treatment interruption21. Durable control of plasma viremia has also been reported in some SIVsmE660 infected rhesus macaques after anti-retroviral treatment, started 24 or 72h post IV inoculation, was discontinued22.
Because bNAbs suppress viremia3,23, accelerate the clearance of cell-free HIV-1 virions24, enhance clearance of infected cells6,25,26, and boost host humoral immunity23 in humans, they have the potential to mitigate deleterious events occurring during the acute infection and possibly alter the long-term clinical course. Consistent with this hypothesis, experiments in humanized mice indicate that early intervention with bNAbs may be more effective in preventing establishment of the reservoir than does cART5. However, humanized mice do not have intact immune systems, and fail to sustain infection beyond 3–4 months.
We previously reported that initiation of bNAb monotherapy, 12 weeks after SHIVAD8-EO inoculation, controls plasma viremia in macaques for 1 or 2 weeks, after which resistant viral variants emerge12. Thus, it is problematic whether even early combination bNAb immunotherapy might durably control viremia. To address this question, combination 10-107413 and 3BNC11714 immunotherapy, which targets non-overlapping epitopes on the envelope spike, was administered to rhesus monkeys at the earliest possible time (viz. 3 days post infection [PI]) when we were certain that a SHIVAD8-EO infection had been established.
SHIVAD8-EO infection resembles HIV-1 infection in a number of important ways. It is R5 tropic, generates sustained levels of plasma viremia in inoculated macaques, exhibits a Tier 2 neutralization sensitivity phenotype, produces neutralization resistant variants in bNAb and ART treated animals, and causes irreversible depletions of CD4+ T cells in infected monkeys12,27–29 (and unpublished). Infection leads to symptomatic immunodeficiency associated with opportunistic infections and a fatal clinical outcome in untreated monkeys.
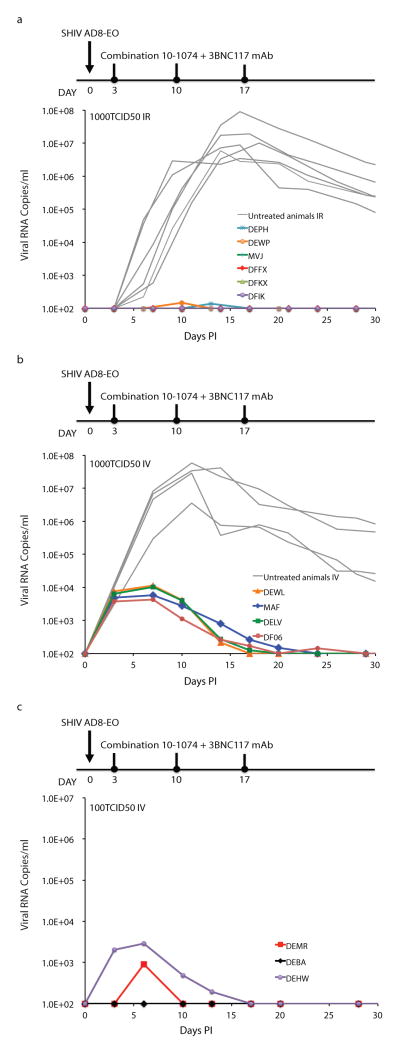
In the initial experiment to assess the potency of combination bNAbs during acute infection, 6 macaques were inoculated intrarectally with 1000 TCID50 of SHIVAD8-EO, which is sufficient to infect all animals challenged by this route11. Beginning on day 3 after inoculation, each monkey received a single course of 3 weekly intravenous (IV) bNAb infusions (on days 3, 10, and 17 PI) of 10-107413 plus 3BNC11714. Compared to untreated animals, extremely low levels of plasma viremia could be detected in 2 of the 6 bNAb recipients during the first 30 days of infection (Fig. 1a). Plasma viremia in the other 4 macaques was not measurable (<100 SHIV RNA copies/ml) using standard RT-PCR assays.
Figure 1. Control of SHIVAD8-EO replication by a 2-week course of combination bNAb therapy.
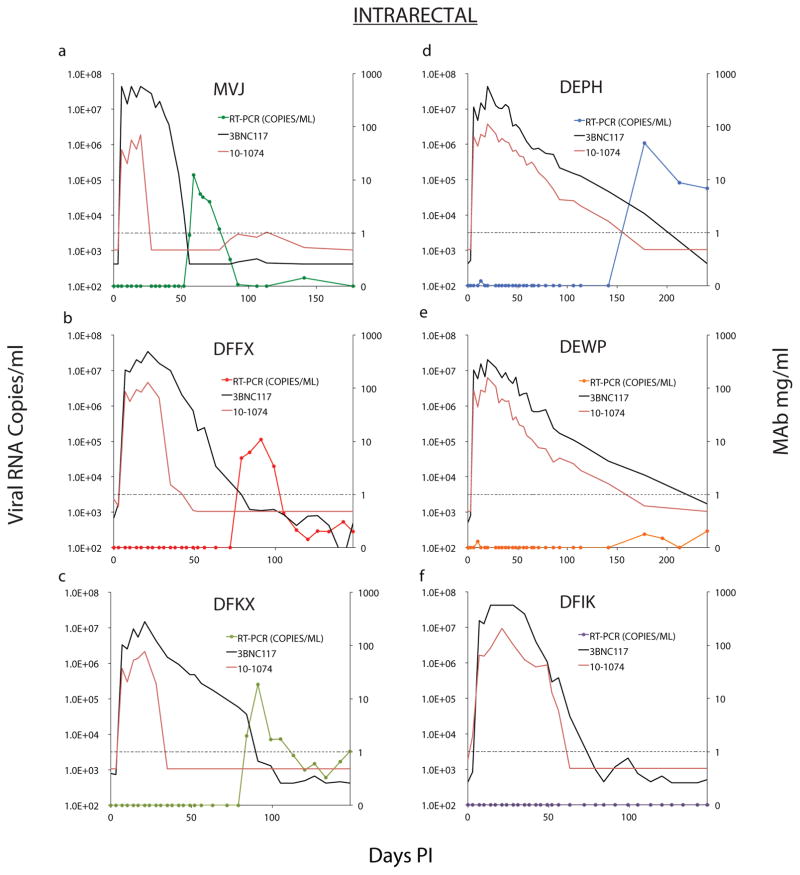
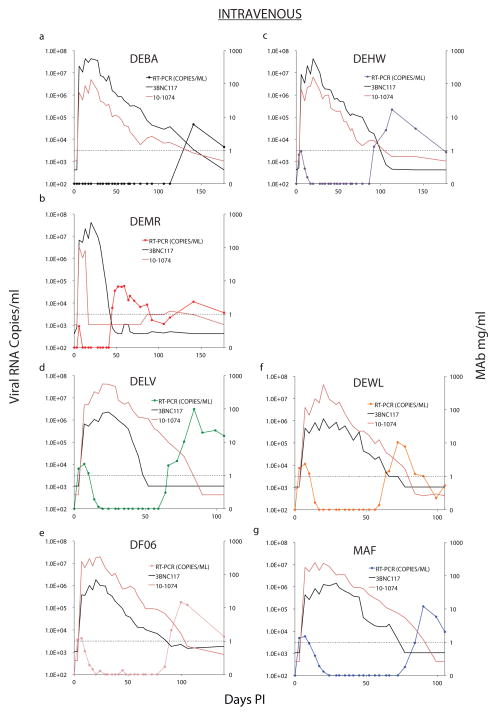
a, Six macaques were inoculated intrarectally with 1000 TCID50 of SHIVAD8-EO and were treated with 10-1074 plus 3BNC117 (10mg/kg of each) mAbs on days 3, 10, and 17 PI. Gray curves denote replication profiles of 6 similarly inoculated but untreated animals. Macaques inoculated intravenously with 1000 TCID50 (b) or 100 TCID50 (c) of SHIVAD8-EO were treated with 10-1074 plus 3BNC117 mAbs as in panel a. Gray curves in panel b indicate the replication kinetics of four untreated monkeys inoculated intravenously with 5000 or 500 TCID50 of SHIVAD8-EO. Plasma viral loads were measured by the standard RT-PCR assay (limit of detection 100 SHIV RNA copies/ml) at the indicated times.
All 6 monkeys infused with the bNAbs experienced sustained periods of virus suppression lasting 56 to 177, days at which point rebound viremia occurred in 5 of the 6 treated macaques (Extended Data Fig. 1). Plasma viremia remained below levels of detection in the sixth macaque (DFIK) during the first 150 days of infection/treatment (Extended Data Fig. 1f). As expected, the time to virus rebound was directly related to the concentration of bNAb in the plasma. For example, the SHIVAD8-EO rebound in macaque MVJ occurred on day 56 when the level of 3BNC117 decayed below 1 μg/ml in plasma, whereas monkeys DEPH and DEWP experienced prolonged suppression of viremia before rebound was observed (Extended Data Fig. 1a, d, and e). We conclude that a single 2 week course of combination antibody therapy with 3BNC117 and 10-1074, administered early after an IR infection, can control SHIVAD8-EO viremia for up to 177 days.
To determine whether low-level viral replication prior to virus rebound persisted despite apparent control of viremia, we performed ultrasensitive nested RT PCR (qRT-PCR) on plasma samples from animals with viral loads below levels of detection by standard methods (Extended Data Table 1). For example, in macaque DEPH, which did not generate a detectable virus rebound until day 177 (Extended Data Fig. 2d), the ultrasensitive assay measured less than 2 viral RNA copies/ml in plasma, between days 34 and 106 PI, and 10 RNA copies/ml on day 141 PI, a month prior to rebound. We conclude that low levels of virus are continuously being produced systemically and released into the circulation in the infected and bNAb treated macaques, even when plasma viremia is not detected by standard assays.
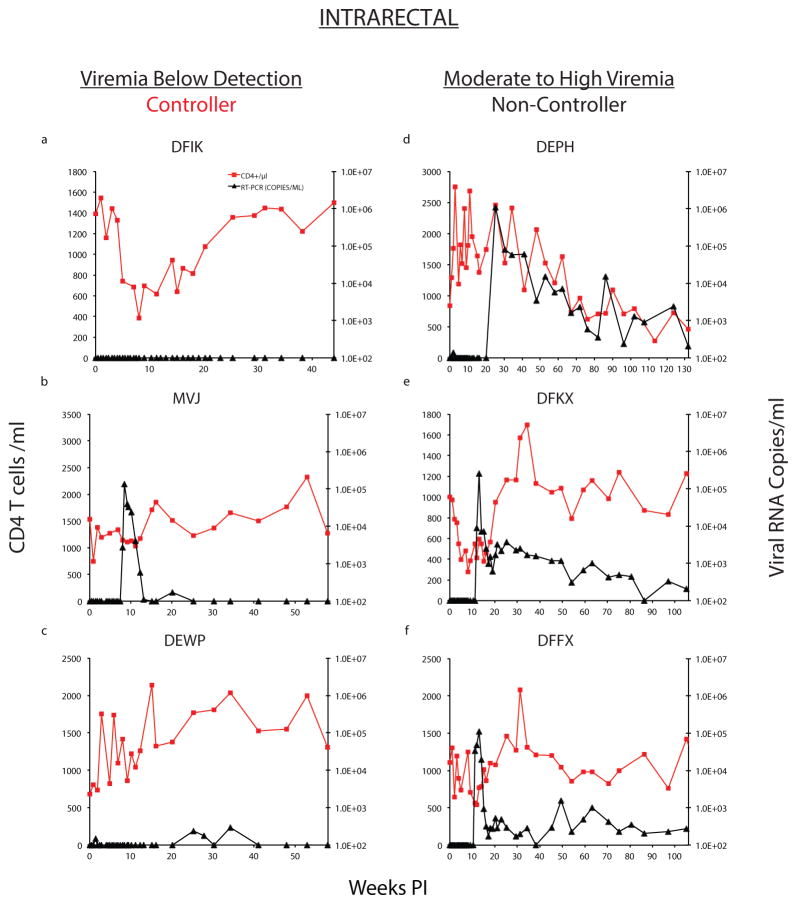
Two patterns of “post-rebound” plasma viremia were observed in the 6 bNAb treated monkeys. In the first, (DFIK, MVJ, and DEWP; Controller [Fig 2a–c]), the rebound SHIVAD8-EO RNA loads in plasma returned to undetectable levels after a maximum of 20 weeks and remained so for an additional 20 to 30 weeks of observation. The second pattern occurred in monkeys DEPH, DFKX and DFFX (Fig. 2d–f; Non-Controller). These three macaques never fully controlled their viremia after rebound and displayed viral loads of 1 × 102–103 RNA copies/ml between 110 and 130 weeks after virus inoculation.
Figure 2. Establishment of controller status in bNAb treated animals inoculated with SHIVAD8-EO by the intrarectal route.
Plasma virus loads (black) and CD4+ T cell levels (red) are shown in 3 Controller (a–c) or 3 Non-Controller (d–f) macaques.
Sustained control of plasma viremia by early bNAb therapy following a mucosal SHIVAD8-EO challenge in 3 of 6 monkeys suggested that the same intervention might also be effective against challenge by the IV route, for which a smaller virus inoculum is typically required to establish an infection. Macaques were therefore inoculated with either 100 or 1000 TCID50 of SHIVAD8-EO IV and then administered 3 weekly bNAb infusions starting on day 3 after infection. As shown in Fig. 1b, the 4 recipients of 1000 TCID50 of virus showed peak plasma virus loads ranging from 4.2 × 103 to 1.1 × 104 RNA copies/ml during the first 2 weeks of bNAb administration. Two of the 3 recipients (DEMR and DEHW) inoculated with 100 TCID50 of virus IV had lower levels of plasma viremia during the early treatment period, and in the third animal (DEBA), viral loads remained below the level of detection throughout this time (Fig. 1c). Viremia declined to undetectable levels by 30 days following bNAb administration and remained suppressed for 48 to 110 days PI in all 7 combination bNAb treated monkeys inoculated by the IV route (Extended Data, Fig. 2). As previously observed in the IR challenged animals, virus rebound in the IV inoculated monkeys was directly associated with the decline of circulating bNAb levels.
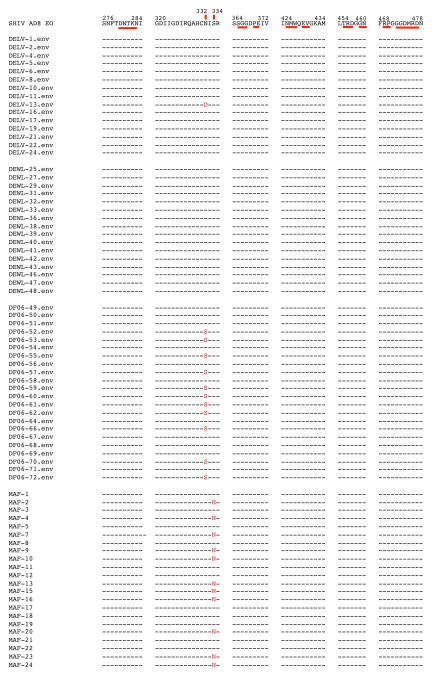
To determine whether virus rebound was due to declining levels of bNAbs in the plasma or to the emergence of mutation(s) conferring resistance, we sequenced plasma viral RNA from the four monkeys (DELV, DF06, DEWL, and MAF), generating detectable viremia at a time when the 3BNC117 mAb was not detectable, but 10-1074 mAb concentrations remained above threshold levels (see Extended Data Fig 2). When samples collected at the peak of virus rebound from these 4 animals were analyzed, no changes were observed in amplicons from macaques DELV and DEWL, but 9 of 18 amplified sequences from macaque DF06 carried the N332S change and 12 of 24 amplicons from monkey MAF had the S334N substitution, both of which eliminated the glycan at position 332 of gp120 (Extended Data Fig. 3), the epitope targeted by the 10-1074 mAb. This result indicated that resistance to the 10-1074 mAb likely occurred in these 2 animals during.
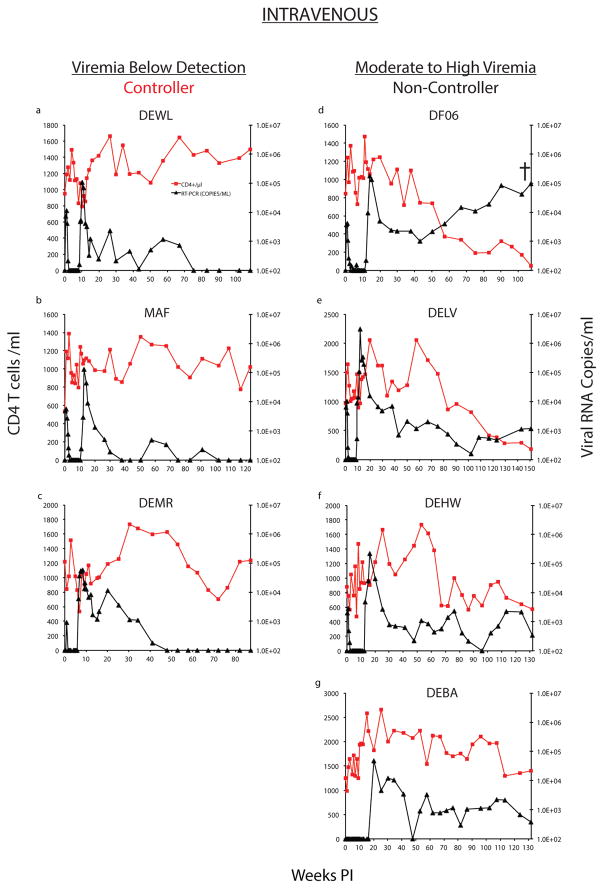
Similar to the macaques challenged by the IR route, passive immunotherapy after IV infection also led to sustained virus control following the resolution of rebound viremia in some of the challenged monkeys. In 3 macaques (DEWL, MAF, and DEMR; Controllers), rebound viremia remained detectable for 42 to 90 weeks, and was then followed by long periods (25 to 56 additional weeks) during which virus replication was stably suppressed below the limits of detection (Fig. 3a–c). CD4+ T cell counts were maintained at a level of 800 cells/μl or higher in these 3 controller animals. In the second group of IV challenged animals (monkeys DF06, DELV, DEHW, and DEBA; Non-Controllers), virus replication was never completely suppressed following rebound (Fig. 3d–g). Failure to control viremia was associated with CD4+ T cell loss and progression to AIDS in 1 (macaque DF06) of these 4 monkeys.
Figure 3. Establishment of controller status in bNAb treated animals inoculated with SHIVAD8-EO by the intravenous route.
Plasma virus loads (black) and CD4+ T cell levels (red) are shown in 3 Controller (a–c) or 4 Non-Controller (d–g) macaques.
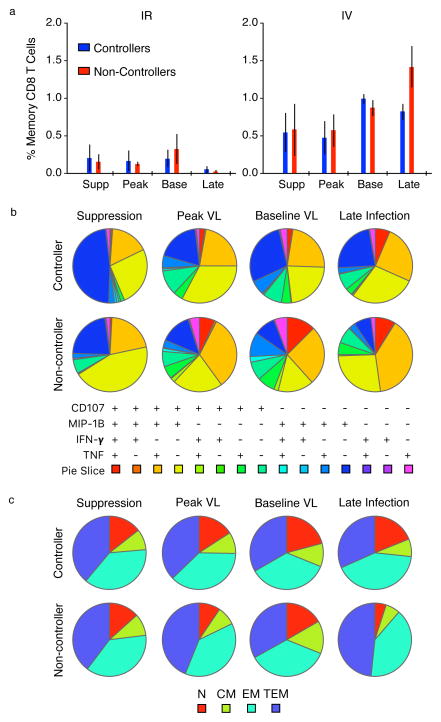
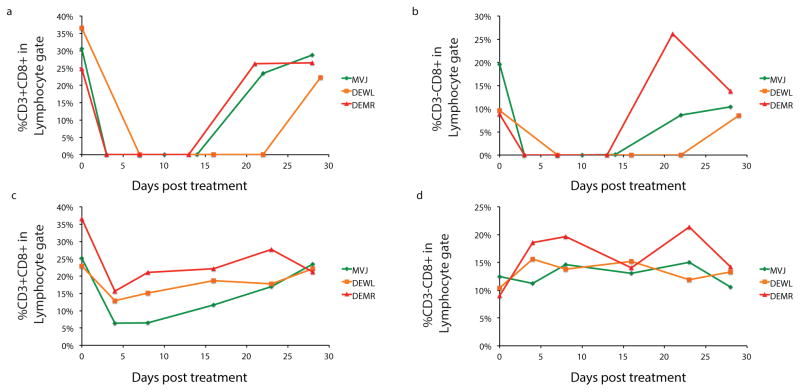
Humoral and cellular immune responses were assessed before and after SHIVAD8-EO rebound to determine whether anti-viral immunity was associated with controller status. No measurable correlation was observed between virus control and antibody levels. The controllers generated very low levels of anti-gp120 binding antibodies that were barely detectable by ELISA. Anti-viral CD8+ T cell responses were measured at 4 different times during the bNAb treatment, virus rebound, and post-rebound phases of their infections in both controllers and non-controllers. Although anti-SIVmac239 Gag CD8+ T cell responses were higher in animals challenged by the IV route, no major differences were observed between controllers and non-controllers (Extended Data, Fig. 4a). Furthermore, the polyfunctionality of the CD8+ T cell responses and distribution of CD8+ T cell memory subsets in IV inoculated controllers and non-controllers at different times following infection, treatment, and rebound were comparable (Extended Data Fig. 4b, c).
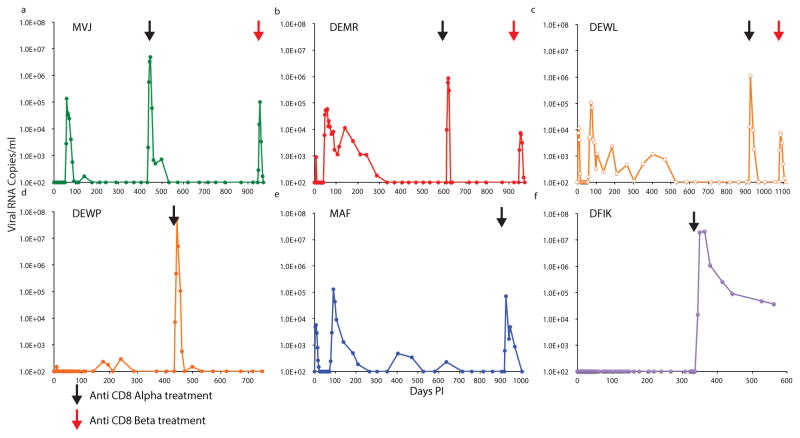
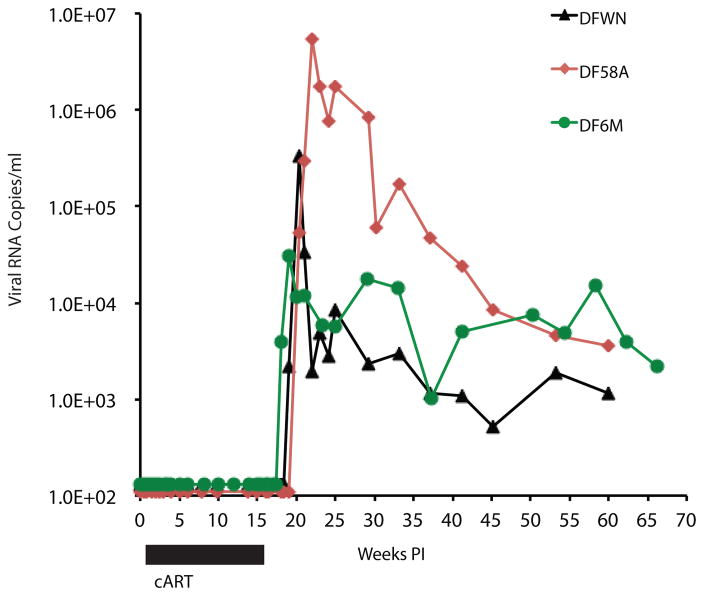
To determine whether CD8+ T cells might, in fact, be mediating the sustained suppression of virus replication, we initially administered the CD8 T cell depleting mAb MT807R1, which is specific for the CD8α chain, to all 6 controller macaques (MVJ [at day 434 PI], DEWP [at day 434 PI], DFIK [at day 336 PI], DEMR [at day 609 PI], MAF [at day 917 PI], and DEWL [at day 917 PI]) (Fig. 4a–f). All animals responded with a burst of plasma viremia, reaching levels between 105 and 107 viral RNA copies/ml, and which subsequently declined to baseline in all of the monkeys, except for DFIK.
Figure 4. Depletion of CD8+ T cells in controller macaques results in the rapid induction of plasma viremia.
Plasma SHIVAD8-EO levels prior to and following administration of anti-CD8 depleting mAbs to six controller monkeys. The black arrows in each panel indicate the time of infusion of the anti-CD8α depleting mAb MT807R1. The red arrows in panels a to c indicate infusion of the anti-CD8β depleting mAb CD8b255R1 to controller macaques MVJ, DEMR, and DEWL.
Prior to the administration of the anti-CD8α mAb, quantitative virus outgrowth assays were performed on samples collected from the 6 controllers to measure the frequency of circulating CD4+ T cells releasing replication-competent virions. As shown in Extended Data Table 2, less than 1 in 106 CD4+ T cells carrying infectious virus was detected in all of the controller monkeys immediately before infusion of the depleting anti-CD8α mAb. Interestingly, in 5 of the 6 anti-CD8α mAb recipients in which plasma viremia had returned to baseline levels following mAb infusion, the frequency of cells carrying replication-competent virus, determined by virus outgrowth assays, was also less than 1 in 106 CD4+ T cells (Extended data Table 2).
A major deficiency of using the anti-CD8α mAb to deplete CD8+ T cells is that NK, NKT and γδ T cells are also targeted for depletion, as documented by FACS analyses for monkeys MVJ, DEWL, and DEMR (Extended Data Fig 5a,b). We therefore administered the CD8b255R1 anti-CD8β mAb, which specifically targets macaque CD8+ T cells, to deplete CD8+ T cells in these same 3 controller monkeys. As shown in Fig. 4a–c (red arrows), infusion of the anti-CD8β mAb caused an immediate increase in plasma virus loads, a decline in levels of CD3+ CD8+ T cells (Extended Data Fig. 5c), and no changes in circulating CD3–CD8+ cells (Extended Data Fig. 5d). We conclude that CD8+ T cells are responsible for control of virus replication in controller macaques and depletion of this subset leads to recrudescence of viremia.
Although the non-controller monkeys failed to suppress plasma viremia to undetectable levels, 4 (DFKX, DFFX, DEHW, and DEBA) of 7 of these macaques have maintained low virus loads (105 to 385 RNA copies/ml) and did not experience significant changes in levels of circulating CD4+ T cell for 2 to 3 years after SHIVAD8-EO infection (Figs. 2 and 3). Taken together, this indicates that 10 (6 controllers and 4 non-controllers) of the 13 bNAb treated monkeys benefited from early immunotherapy.
As a control for bNAb immunotherapy during the acute SHIVAD8-EO infection, 3 macaques were inoculated by the IR route and treated daily with cART for 15 weeks, starting on day 3 after infection. This course of cART corresponded to the mean time period during which 2 weeks of bNAb therapy suppressed virus replication prior to rebound. cART therapy controlled and maintained undetectable levels of viremia for the entire 15 week treatment period (Extended Data Fig. 6). However, all 3 animals developed high sustained levels of plasma viremia following cessation of cART and none became controllers.
We speculate that the continuous production of low levels of progeny virions, as measured by ultrasensitive RT PCR (Extended Table 1) during the 50–140 day period before virus rebound in the antibody treated macaques, could drive the formation of immune complexes. Antigen-presenting dendritic cells expressing activating Fc receptors can bind to these immune complexes, leading to their activation and efficient antigen processing for presentation and cross-presentation to CD4+ and CD8+ T cells30. In contrast, the near complete inhibition of virus replication by the cART regimen employed may limit the amount of viral antigen available to induce immunity.
As proof of concept, our results demonstrate that a SHIV infection, established during the acute phase, can, in fact, be controlled. They also suggest that a delicate balance may exist between: 1) preservation of helper CD4+ T cells; 2) the size and stability of the virus reservoir; and 3) the continuous production of sufficient quantities of antigen to generate a potent and sustained CD8+ T cell response. Although rhesus macaque infections with SHIV differs from HIV-1 infections in a number of important ways, immunotherapy should be explored as a way to control systemic dissemination of virus, contain damage to the CD4+ T cell lineage, and mobilize a robust immune response that may be capable of controlling the infection in humans.
METHODS
Animal experiments
Eighteen 2 to 4 year old male and female rhesus macaques (Macaca mulatta) of Indian genetic origin were housed and cared for in accordance with Guide for Care and Use of Laboratory Animals Report number NIH 82-53 (Department of Health and Human Services, Bethesda, Maryland, 1985) in a biosafety level 2 National Institute of Allergy and Infectious Diseases (NIAID) facility. All animal procedures and experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of NIAID, NIH. Animals were not randomized and the data collected were not blinded. Phlebotomies, euthanasia, and sample collection were performed as previously described31. All of the macaques used in this study were negative for the major histocompatibility complex (MHC) class I Mamu-A*01, Mamu-B*08, and Mamu-B*17 alleles. No animals were excluded from the analysis.
Antibodies
The 3BNC117 and 10-1074 anti-HIV-1 monoclonal NAbs were isolated and produced as described elsewhere13,14. MAb 10-1074 was produced by transient transfection of IgH and IgL expression plasmids into the human embryonic kidney cells whereas 3BNC117 were produced from Chinese hamster ovary cells. All of the MAbs were IgG1. All of the monoclonal antibodies were purified by chromatography and sterile filtration and were endotoxin free. A combination of single dose (10 mg kg−1) of each MAb was administered intravenously to individual animals.
ART regimen
Three animals were treated with a three-drug ART regimen comprising two nucleoside reverse transcriptase (RT) inhibitors (tenofovir [PMPA] and emtricitabine [FTC]) and one integrase inhibitor (raltegravir [RAL]). PMPA and FTC were administered intramuscularly once a day at dosages of 20 mg/kg and 40 mg/kg, respectively. RAL was administered orally (mixed with food) at a dosage of 200 mg twice a day. The macaques received the ART regimen for 15 weeks, starting on the day 3-post virus challenge.
Virus challenge
The origin and preparation of the tissue-culture-derived SHIVAD8-EO stock have been previously described29. Animals were challenged with SHIVAD8-EO intravenously (100 or 1000 TCID50) or intrarectally (1000 TCID50). For intrarectal inoculation, a paediatric nasal speculum was used to gently open the rectum and a 1 ml suspension of virus was slowly infused into rectal cavity using a plastic tuberculin syringe as previously described5. 1000 TCID50 of SHIVAD8-EO administered intrarectally has resulted in the establishment of infections in 30 of 30 rhesus monkeys11,29 and unpublished data).
Quantification of plasma viral RNA
Viral RNA levels in plasma were determined by qRT–PCR (ABI Prism 7900HT sequence detection system; Applied Biosystems) as previously described1. Ultrasensitive measurement of plasma SIV gag RNA was performed as described previously32.
Lymphocyte immunophenotyping and intracellular-cytokine assays
EDTA-treated blood samples were stained for flow cytometric analysis for lymphocyte immunophenotyping as previously described28.
Intracellular Cytokine Staining (ICS)
Cryopreserved macaque peripheral blood mononuclear cells (PBMCs) were thawed and rested overnight in a 37C/5% CO2 incubator. The next morning, cells were stimulated with HIV Clade B envelope and SIVMac239 gag peptide pools (final concentration of 2 μg/ml) in the presence of Brefeldin A, monensin, and CD107a-Cy5 PE (clone H4A3, BD Biosciences, San Jose, California) for 6 h. Negative controls received an equal concentration of DMSO instead of peptides. Intracellular cytokine staining was performed as described33 except the following monoclonal antibodies were used: CD4-Cy5.5 PE (clone S3.5; Invitrogen, Carlsbad, CA), CD8-BV570 (clone RPA-T8; BioLegend), CD45RA-Cy5 PE (clone 5H9, BD Biosciences), PD-1-BV785 (clone EH12.2H7, BioLegend), CCR7-BV650 (clone G043H7, BioLegend), CD39-PerCpeF710 (clone eBioA1, e Bioscience), MIP-1B-PE (clone D21-1351, BD Biosciences), Granzyme B-APC (clone GB12, Invitrogen), CD69-ECD (clone TP1.55.3; Beckman Coulter), CD3-Cy7APC (clone SP34.2; BD Biosciences), IFNγ-Alexa700 (clone B27; BioLegend), IL-2-BV605 (clone MQ1-17H12; BD Biosciences), IL-10-BV421 (clone JES3-9D7, BD Biosciences), and TNF-FITC (clone Mab11; BD Biosciences). Aqua LIVE/DEAD kit (Invitrogen) was used to exclude dead cells. All antibodies were previously titrated to determine the optimal concentration. Samples were acquired on an LSR II flow cytometer and analyzed using FlowJo version 9.6.3 (Treestar, Inc., Ashland, OR).
Measurement of anti-gp120 antibodies
An enzyme-linked immunosorbent assay (ELISA) to detect antibodies generated against the HIV-1 gp120 envelope protein was performed as previously described27.
MAb concentrations in plasma
Plasma concentrations of 10-1074 and 3BNC117 NAbs were separately determined against HIV-1 virus strains that are sensitive to one but not the other monoclonal antibody as previously described12.
Quantitative virus outgrowth assay
Macaque peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Hypaque density gradient centrifugation. CD4+ T cells were isolated from the PBMCs using a cell enrichment cocktail (Stemcell Technologies) and enumerated using an automatic cell counter (Muse Cell Analyzer, EMD Millipore). The frequency of circulating CD4+ T cells carrying replication-competent virus was determined using quantitative co-culture assays as previously described34. Briefly, serially diluted CD4+ T cells (five-fold dilutions from 1x106 to 320 cells per well in duplicate) as well as replicates of 1×106 cells were stimulated with irradiated PBMCs from healthy HIV-negative donors in the presence of 1μg/ml of anti-CD3 antibody (BD Pharmingen) for 24 hours. In order to further propagate the virus produced by the SHIV-infected CD4+ T cells, anti-CD3-stimulated, CD8-depleted PBMCs from healthy HIV-negative donors were added to the culture on days 1 and 7 followed by periodic removal of cell suspensions and replenishment of culture medium. Following up to 21 days of incubation periods, wells positive for SHIV-AD8 were identified by SIV p27 Antigen Capture Assay (Advanced Bioscience Laboratories) and infectious units per million cells were determined by a maximum likelihood method35.
CD8+ T lymphocyte depletion in vivo
Six controller animals were injected subcutaneously with anti-CD8 mAbα M-T807R1 (National Institutes of Health Nonhuman Primate Reagent Resource Program) (10 mg kg−1) on day 0, and intravenously (5 mg kg−1) on days 3, 7, and 1036. Three of the six controller animals were also injected intravenously with anti-CD8β mAb CD8b255R1 (National Institutes of Health Nonhuman Primate Reagent Resource Program) (50 mg kg−1).
Extended Data
Extended Data Figure 1. Sustained suppression of virus replication by a 2-week course of combination bNAb treatment following IR SHIVAD8-EO challenge.
Plasma viral RNA levels and 10-1074 or 3BNC117 mAb concentrations following bNAb therapy beginning on day 3 post IR challenge are shown (n = 6).
Extended Data Figure 2. Sustained suppression of virus replication by a 2-week course of combination bNAb treatment following intravenous SHIVAD8-EO challenge.
Plasma viral RNA levels and 10-1074 or 3BNC117 mAb concentrations following bNAb therapy beginning on day 3 post intravenous challenge are shown (n = 7).
Extended Data Figure 3. Analyses of selected SHIVAD8 gp120 sequences known to confer resistance to 10-1074 or 3BNC117 monoclonal antibodies present in virus rebounding following immunotherapy.
Nucleotide sequences present in amplicons, obtained from animals DELV (day 84 PI), DEWL (day 72 PI), DF06 (day 99 PI), or MAF (day 90 PI) during virus rebound (shown in Extended Data Fig 2d–g), were evaluated. SHIVAD8-EO gp120 sequences are shown at the top. Mutations conferring resistance to 10-1074 (vertical bars) and 3BNC117 (horizontal bars) are highlighted.
Extended Data Figure 4. CD8+ T cell responses and memory phenotype.
a) Memory CD8+ T cell responses to SIVmac239 Gag measured by ICS as a sum of IFN-γ, IL-2, TNF, CD107a, MIP-1B, and IL-10. Supp = during bNAb mediated viral suppression, Peak = during peak of virus rebound, Base = following resolution of rebound, Late = during late stage of virus infection; colored bars = mean, whiskers = SEM. b) Polyfunctionality of the CD8+ T cell responses in macaques inoculated by the intravenous route. c) CD8+ T cell memory subsets in macaques inoculated by the intravenous route. N= naïve, CM = central memory, EM = effector memory, TEM = terminal effector memory.
Extended Data Figure 5. FACS analyses of CD8+ cells in controller animals following administration of depleting anti-CD8 mAbs.
CD8+ lymphocytes were collected from controller monkeys MVJ, DEWL, and DEMR following infusion of the MT807R1anti-CD8α mAb (a,b) or the CD8b255R1 anti-CD8β mAb (c,d) and the CD3+CD8+ and CD3–CD8+ fractions determined.
Extended Data Figure 6. Administration of cART during the acute SHIVAD8-EO infection of macaques does not result in controller status.
cART (Tenofovir, Emtricitabine, and Raltagravir) was administered daily to three animals for 15 weeks beginning on day 3 post IR challenge with 1000 TCID50 of SHIVAD8–EO.
EXTENDED DATA TABLE 1.
Plasma Viral RNA levels in macaques by ultrasensitive RT-PCR assay
| Animal | Days post infection | Plasma viral RNA (copies/ml) |
|---|---|---|
| DEPH | 10 | 10 |
| 13 | 5 | |
| 34 | <2 | |
| 48 | <2 | |
| 106 | <2 | |
| 141 | 10 | |
|
| ||
| DEWP | 10 | 5 |
| 13 | 3 | |
| 34 | <2 | |
| 48 | <2 | |
| 106 | <2 | |
| 113 | <2 | |
| 141 | 10 | |
| 177 | 260 | |
| 212 | 50 | |
|
| ||
| MVJ | 10 | 213 |
| 13 | 20 | |
| 34 | <2 | |
| 48 | <2 | |
| 106 | 30 | |
| 113 | 160 | |
| 141 | 30 | |
| 177 | 10 | |
|
| ||
| DFFX | 7 | <2 |
| 14 | <2 | |
|
| ||
| DFKX | 7 | <2 |
| 14 | <2 | |
|
| ||
| DFIK | 7 | <2 |
| 14 | <2 | |
Extended Data Table.2.
| Animal ID | Route of Virus Inoculation | Infectious SHIV (per 106 CD4+T cells) | ||
|---|---|---|---|---|
|
| ||||
| Pre-CD8 depletion | Post-CD8 depletion | |||
|
| ||||
| Viremic Phase | Controlled Phase | |||
| DEWP | IR | 0.167 | 747 | <0.100 |
| MVJ | IR | 0.566 | 2503 | <0.100 |
| DFIK | IR | <0.112 | ND | ND |
| DEMR | IV | <0.100 | ND | 0.519 |
| DEWL | IV | <0.100 | ND | <0.065 |
| MAF | IV | 0.424 | ND | 0.2245 |
Acknowledgments
We thank A. Peach and T. Lewis for determining plasma viral RNA loads and K. Rice, R. Engel, R. Petros and S. Fong for diligently assisting in the maintenance of animals and performing procedures. We thank R. Fast, Frederick National Laboratory for Cancer Research, for expert technical assistance with ultrasensitive viral load assays and J. Brenchley, Laboratory of Parasitic Diseases, NIAID for performing FACS analyses. We are indebted to Gilead Sciences for providing Tenofovir (TFV) and emtricitabine (FTC). The anti-CD8 mAbs, MT807R1 and CD8b255R1, were obtained from the NIH Nonhuman Primate Reagent Resource supported by HHSN272200900037C and OD10976. We thank NIH AIDS Research and Reference Reagent Program for TZM-bl cells. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Vaccine Research Center of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), and, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (J.D.L.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The research was also funded in part by the following grants: Collaboration for AIDS Vaccine Discovery grant OPP1033115 (M.C.N.). NIH Clinical and Translational Science Award (CTSA) program; NIH Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) 1UM1 AI100663-01 (M.C.N.); Bill and Melinda Gates Foundation grants OPP1092074 and OPP1124068 (M.C.N.); NIH HIVRAD P01 AI100148 (M.C.N.); the Robertson Foundation to M.C.N. M.C.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
AUTHOR CONTRIBUTIONS
Y.N., M.C.N., and M.A.M. designed experiments; Y.N., R.G., T-W.C., R.S., K.E.F., M.S., F.K., A.G., J.G., O.K.D., R.J.P., and M.S.S. performed experiments; Y.N., T-W.C., A.B-W., M.S.S., J.D.L., R.A.K., A.S.F., M.C.N., and M.A.M. analyzed data; Y.N., J.D.L., R.A.K., A.S.F., M.C.N., and M.A.M. wrote the manuscript.
The authors declare no competing financial interests.
Online Content
Methods, along with any additional Extended Data display items and source data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
- 1.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolton DL, et al. Human Immunodeficiency Virus Type 1 Monoclonal Antibodies Suppress Acute Simian-Human Immunodeficiency Virus Viremia and Limit Seeding of Cell-Associated Viral Reservoirs. J Virol. 2016;90:1321–1332. doi: 10.1128/JVI.02454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautam R, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessell AJ, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med. 2016;22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz JA, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch RM, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 10.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shingai M, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koup RA, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey RT, Jr, et al. T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun TW, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzon MJ, et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol. 2014;88:10056–10065. doi: 10.1128/JVI.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lifson JD, et al. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoofs T, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi T, et al. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat Med. 1999;5:211–216. doi: 10.1038/5576. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 2016;353:1045–1049. doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu CL, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautam R, et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J Virol. 2012;86:8516–8526. doi: 10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura Y, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shingai M, et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A. 2012;109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc-FcgammaR interactions in IgG-mediated microbial neutralization. J Exp Med. 2015;212:1361–1369. doi: 10.1084/jem.20151267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo Y, et al. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J Virol. 2000;74:6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foulds KE, Donaldson M, Roederer M. OMIP-005: Quality and phenotype of antigen-responsive rhesus macaque T cells. Cytometry A. 2012;81:360–361. doi: 10.1002/cyto.a.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun TW, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 35.Myers LE, McQuay LJ, Hollinger FB. Dilution assay statistics. J Clin Microbiol. 1994;32:732–739. doi: 10.1128/jcm.32.3.732-739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukazawa Y, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]