Abstract
Viral binding and entry provides the first trigger of a cell death response and thus how human cytomegalovirus (HCMV) evades this – particularly during latent infection where a very limited pattern of gene expression is observed – is less well understood. It has been demonstrated that the activation of cellular signalling pathways upon virus binding promotes the survival of latently infected cells by the activation of cell encoded anti-apoptotic responses. In CD34+ cells, a major site of HCMV latency, ERK signalling is important for survival and we now show that the activation of this pathway impacts on multiple aspects of cell death pathways. The data illustrate that HCMV infection triggers activation of pro-apoptotic Bak which is then countered through multiple ERK-dependent functions. Specifically, ERK promotes ELK1 mediated transcription of the key survival molecule MCL-1, along with a concomitant decrease of the pro-apoptotic BIM and PUMA proteins. Finally, we show that the elimination of ELK-1 from CD34+ cells results in elevated Bak activation in response to viral infection, resulting in cell death. Taken together, these data begin to shed light on the poly-functional response elicited by HCMV via ERK-MAPK to promote cell survival.
Introduction
Amongst the first host responses activated upon viral infection are cell autonomous immune responses. These functions are ubiquitous to every cell type and are normally triggered by pattern recognition receptors which detect various components of the incoming pathogen (Bieniasz 2004; Everett & Chelbi-Alix 2007; Randow et al. 2013). One aspect of the cell autonomous immune response is the activation of apoptotic and cell death pathways, which will ultimately eliminate both the pathogen and the infected cell (Clem et al. 1991; Everett & McFadden 1999; Levine et al. 1993). During lytic infection, human cytomegalovirus (HCMV) counters this with an arsenal of virally encoded anti-apoptotic functions that counter stress signals induced by viral binding and, subsequently, throughout the course of viral replication (Brune 2010; Goldmacher et al. 1999; Guo et al. 2015; Reeves et al. 2007; Skaletskaya et al. 2001; Stevenson et al. 2014; Terhune et al. 2007). However, a lack of expression of these functions in non-lytic infections raised the question of how cell survival is achieved – particularly during the initial phases of infection.
Current studies hypothesise that viral infection in cells non-permissive for lytic infection requires the generation of a pro-survival phenotype driven by virally induced up-regulation of key cellular anti-apoptotic proteins (Chan et al. 2010; Peppenelli et al. 2016; Reeves et al. 2012; Stevenson et al. 2014). This event relies on the regulation of a number of prosurvival and pro-death signals via the modulation of cellular signalling pathways initiated upon virus binding and entry (Chan et al. 2010; Reeves et al. 2012). It is the outcome of these signalling events which, ultimately, determines the fate of the cell during these very early stages of infection. Once latency is established, there are additional mechanisms activated which would be consistent with the latent virus propagating an anti-apoptotic state (Poole et al. 2011, 2015; Slobedman et al. 2004).
Apoptosis and cell death is an evolutionarily conserved process that is extensively regulated by Bcl-2 homology domain 3 (BH3) proteins (Doerflinger et al. 2015; Horvitz 1999; Puthalakath & Strasser 2002). The precise mechanism of action still remains equivocal although it is clear that the BH3 proteins trigger the activation of key apoptosis effector proteins (e.g. Bak and Bax) which exert their pro-apoptotic function predominantly at mitochondrial membranes (Wei et al. 2001; Westphal et al. 2014). A number of regulatory mechanisms have been suggested: The indirect activation model posits that Bax/Bak are required to be retained in an inactive form via direct sequestration by anti-apoptotic BCL-2 family members (Willis et al. 2005) and that BH3 proteins must engage with these to release the Bax/Bak to initiate apoptosis (Uren et al. 2007; Willis et al. 2007). The direct activation model hypothesises that BH3-only activators (e.g. PUMA and BIM) bind to Bak/Bax directly to activate them or, alternatively, other BH3 members (e.g. Bad) target BCL-2 members and inhibit their anti-apoptotic function through sequestration (Doerflinger et al. 2015; Erlacher et al. 2006; Kuwana et al. 2002; Villunger et al. 2003; Wei et al. 2000). The most recent evidence argues that these events are not mutually exclusive and that, instead, both mechanisms of regulation are likely to be active (Llambi et al. 2011; Westphal et al. 2014) although the strict delineation between activators and sensitisers in the BH3 family may not be entirely valid (Westphal et al. 2014).
Our own research interests have centred on the BCL-2-like protein myeloid cell leukaemia-1 (MCL-1). Unlike other BCL-2 proteins the regulation of MCL-1 is dynamic with a turnover of 30 minutes under certain experimental conditions (Adams & Cooper 2007; Warr & Shore 2008) and it is thus considered a highly responsive determinant of haematopoietic cell viability (Perciavalle & Opferman 2013) – the ablation of this protein from progenitor cells of the haematopoietic lineage being lethal (Opferman et al. 2005; Perciavalle & Opferman 2013). MCL-1 (along with BCL-XL) has been suggested to block Bak activity to exert an anti-apoptotic function (Willis et al. 2005) and is regulated by both the PI3K and ERK-MAPK pathways (Huang et al. 2000; Mills et al. 2008). Pertinently, MCL-1 is also regulated by HCMV through activation of the ERK and PI3K pathways in a cell type specific manner (Chan et al. 2010; Reeves et al. 2012). Depletion of MCL-1 from monocytes or THP1 cells renders them unable to prevent virally induced cell death upon infection (Chan et al. 2010; Reeves et al. 2012). In CD34+ cells, HCMV survival is also associated with MCL-1 (Reeves et al. 2012) although the absolute requirement for endogenous MCL-1 in normal CD34+ cells rendered similar genetic analysis through MCL-1 deletion intractable. The inducible survival effect in CD34+ cells required virus binding and was likely dependent on the engagement of glycoprotein B with an unknown receptor. Furthermore, the survival effect induced was transient (Reeves et al. 2012) and thus did not appear to be propagated for as long as was observed in the monocyte model of infection (Chan et al. 2010).
ELK-1 is part of a 27 member superfamily of transcription factors that dictate a diverse range of processes including haematopoiesis, differentiation and survival, oncogenesis and inflammation (Sharrocks 2001). ELK-1 is a nuclear phosphoprotein that exhibits bimodal activity: redundant promoter binding via dimers with other Ets transcription factors and specific binding to a subset of genes through an interaction with serum response factor (SRF) (Odrowaz & Sharrocks 2012; Sharrocks 2001). Amongst these genes are MCL-1 and c-Fos, which, characteristically for this subset of genes, are dynamically regulated (Boros et al. 2009; Treisman et al. 1992). The activation of ELK-1 requires phosphorylation of serine residue 383 promoting the recruitment of transactivating co-factors to promoters in order to drive transcription (Boros et al. 2009; Gille et al. 1992, 1995). Furthermore, ELK-1 mediated activation of MCL-1 gene expression has been shown to be important for survival in a number of experimental models (Booy et al. 2011; Demir et al. 2011; Sun et al. 2013; Townsend et al. 1999) and likely contributes to the role of ELK-1 in cancer.
Herein we wanted to further explore the consequences of HCMV infection on the development of an anti-apoptotic phenotype in CD34+ cells based on our previous studies of MCL-1 and ERK-MAPK signalling. Here we show that the HCMV protection from cell death is concomitant with an inhibition of prolonged Bak activation in response to chemical and viral insult. Secondly, we show that the protective phenotype driven by virus induced ERK-MAPK signalling correlates with the down-regulation of pro-apoptotic BH3 proteins PUMA and BIM and, concomitantly, is dependent on the phosphorylation and thus activation of ELK-1 – a transcription factor important for MCL-1 expression and cell survival. Importantly, knock down of ELK-1 expression is sufficient to abrogate the protective effect elicited by HCMV infection. Taken together, these data suggest that HCMV infection drives a survival phenotype by simultaneously up-regulating MCL-1 proteins levels and reducing the levels of antagonistic interaction partners. We propose that the net effect tips the balance in favour of survival contributing to the successful establishment of latent infection.
Materials and Methods
Virus, cell lines, culture and reagents
The Merlin strain of HCMV was purified from infected human fibroblasts as previously described (Compton 2000). Primary CD34+ haematopoietic cells (Lonza, Slough, UK) were resuscitated for 24 hours prior to any studies of cell viability in X-vivo-15 (Biowhittaker, Lonza, Slough, UK) media supplemented with 10% human serum. For all subsequent studies, cells were switched into serum-free X-vivo media supplemented with 2mM L-Glutamine. For all infection experiments an MOI of 5 (based on titration in fibroblasts) was used.
Inhibition of the ERK-MAPK pathway was achieved using U0126 (final concentration 1uM; Calbiochem/Millipore, Darmstadt, Germany). The inhibitor was added directly to the culture media with 0.1% DMSO used as the solvent control 1 hour prior to virus infection.
Nucleic acid isolation, reverse transcription, PCR and Western Blot
Ten micrograms of DNase I treated RNA isolated using RNAeasy spin columns was reverse transcribed using ImpromII RT kit (Promega, Madison, WI) or Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany). Chromatin Immunoprecipitations were performed as previously described (Kew et al. 2014). Briefly, 106 cells were fixed and lysed and then subjected to sonication to shear DNA into 200-500bp fragments. DNA was then incubated with anti-ELK1 antibody (Cell Signaling, Danvers, MA; 1:100 dilution), anti-phospho ELK-1 antibody (Cell Signaling, Danvers, MA; 1:100 dilution) or an isotype matched control (mouse IgG1; SIGMA, St Louis, MO). DNA was rescued from the IP complexes and amplified with MCL-1 promoter specific primers.
Gene and promoter specific primers were then used to amplify target sequences by real time PCR using SYBR green amplification kit (Qiagen, Hilden, Germany). MCL-1 (gene): 5’-TGC AGG TGT GTG CTG GAG TAG and 5’-GCT CTT GGC CAC TTG CTT TTC’, GAPDH: 5’-GAG TCA ACG GAT TTG GTC GT and 5’-TTG ATT TTG GAG GGA TTC TCG, 18S: 5’- GTA ACC CGT TGA ACC CCA and 5’- CCA TCC AAT CGG TAG TAG CG, Bak: 5’-GCC CAG GAC ACA GAG GAG GTT TTC and 5’-AAA GTG GCC CAA CAG AAC CAC ACC, Bim: 5’-CAC AAA CCC CAA GTC CTC CTT and 5’-TTC AGC CTG CCT CAT GGA A, Puma: 5’-ACG ACC TCA ACG CAC AGT ACG and 5’-TGG GTA AGG GCA GGA GTC C, UL138: 5’- GAG CTG TAC GGG GAG TAC GA and 5’- AGC TGC ACT GGG AAG ACA CT; MCL-1 (promoter): 5′-TAG GTG CCG TGC GCA ACC CT and 5′-ACT GGA AGG AAG CGG AAG TGA GAA (Booy et al. 2011).
For Western Blot, 105 cells were lysed in Laemmli buffer and subjected to SDS-PAGE electrophoresis. Following transfer, blots were incubated with anti-MCL-1 (Cell Signaling, Danvers, MA; 1:500), anti-ELK-1 or anti-phospho-ELK-1 (phosphor-serine 383; Cell signaling, Danvers, MA; both 1:750), anti-Bak (#06-536, Millipore, Darmstadt, Germany; 1:500), anti-PUMA (Santa Cruz, CA, 1:200) anti-actin (Abcam, Cambridge, UK; 1:1000) or anti-GAPDH (Abcam, Cambridge, UK; 1:2000) for 1 hour followed by detection with the appropriate HRP-conjugated secondary antibody. Specific bands were visualized by ECL detection (Amersham, Horsham, UK).
siRNA knockdown
CD34+ cells were transfected with Silencer® ELK-1 siRNAs or Silencer® negative control (Thermofisher, Waltham, MA) using Viromer Green transfection reagent as described by the manufacturer (lipocalyx/Cambridge Biosciences, UK). CD34+ cells were transfected in bulk in X-vivo serum free media and then plated at the required density for downstream analyses at 48 hours post-transfection. Prior to plating, the viromer:siRNA mix was removed from the cell culture 4 hours post-transfection by pelleting the CD34+ cells at 300g followed by resuspension in fresh X-vivo 15 media.
Cell Death assay and Bak activation
Induction of cell death was achieved using the chemotherapeutic drug cisplatin A (Rosenberg et al. 1965). Cisplatin A (25uM-250uM; SIGMA-Aldrich, St Louis, MO) or 0.1% DMSO (mock) control was added for 4 hours to trigger the apoptotic pathway. Since the effects of cisplatin A induced cell death are not evident until at least 12-24 hours post treatment (Barry et al. 1990) cell viability was performed 21 hours after cisplatin A addition. To determine levels of cell death, cells were stained with a TUNEL detection kit (Roche, Basel, Switzerland) as described by the manufacturer and analysed for apoptotic cell death by immune-fluorescent microscopy.
Alternatively, cell death was induced using the virus as a ligand through blockade of ERK-MAPK signaling (U0126; 1uM, Calbiochem) 1 hour prior to infection with HCMV. 0.1% DMSO was used as solvent control.
Bak activation in CD34+ cells by flow cytometry was measured as previously described (Griffiths et al. 1999). Briefly, 105 cells were incubated with rabbit serum for 20 minutes and then with an anti-Bak antibody directed against the N terminal region (#06-536, Millipore, Darmstadt, Germany; 1:50 dilution in PBS) or an isotype matched control for 15 minutes at 4°C. Cells were washed, incubated with goat serum for 20 minutes and then incubated with a goat anti-rabbit FITC conjugated secondary antibody (1:100 dilution in PBS) for 15 minutes at 4°C. Unstained, isotype stained and Bak stained cells were then analysed by flow cytometry.
Assays for latent infection
CD34+ cells were infected with HCMV 48hrs post treatment with siRNAs in X-vivo-15 media for 3 hours. Cells were pelleted, supernatant removed, washed in PBS and then re-suspended in fresh X-vivo media and cultured for 3 days. RNA was then isolated, converted to cDNA and amplified in a UL138 (viral gene) and 18S (cellular gene) specific PCR. Changes in gene expression were calculated using 2-ΔΔCT method (UL138 and 18S RNA).
Results
HCMV infection reduces the level of activated Bak in cisplatin A treated cells
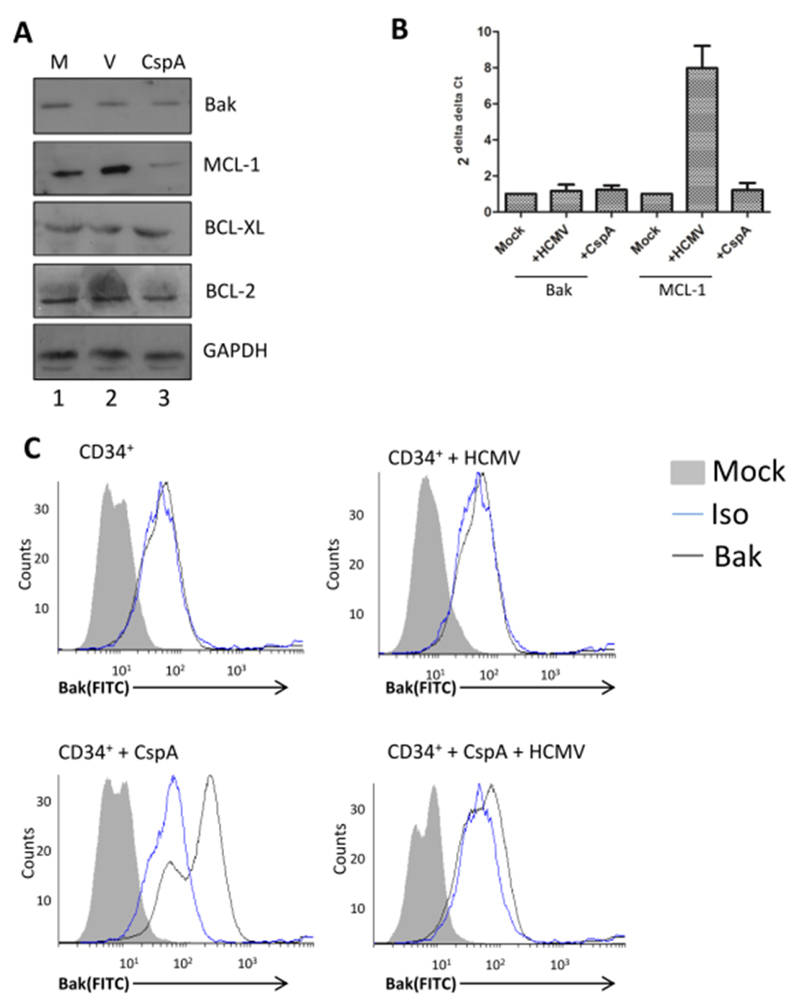
Our previous study had shown that HCMV infection could block the induction of CD34+ cell death in response to the chemotherapeutic drug, cisplatin A (Reeves et al. 2012). Cisplatin A-induced degradation of survival molecules and the triggering of cell death are functionally entwined events (Yang et al. 2007). Here we show that in CD34+ cells, cisplatin A promotes the degradation of pro-survival MCL-1, but not the related BCL-XL or Bcl-2 proteins (Figure 1A). As expected, HCMV infection promoted MCL-1 upregulation but, interestingly, no effect on BCL-2 or BCL-XL was again observed (Figure 1A). A key target of MCL-1 is Bak. However, no impact on the total levels of Bak were observed whether treated with cisplatin A or infected with HCMV (Figure 1A). Similarly, no impact on the transcription of Bak was detected at these early times post-infection (Figure 1B). In contrast, HCMV infection was a clear inducer of MCL-1 transcription (Figure 1B). We next investigated the activation of Bak and although levels in the total protein did not change, evidence of increased activation of Bak in response to cisplatin A was detected (Figure 1C). The specific activation of Bak can be detected by flow cytometry utilising the conformational change that exposes Bak epitopes when active (i.e. pro-apoptotic) Bak is released from the inactive complex it is normally sequestered in. As expected, resting CD34+ cells show very little evidence of Bak activation (Figure 1C) consistent with their viability status. In contrast, stimulation of CD34+ cells with cisplatin A resulted in a substantial increase in the level of activated Bak detectable in the cells (Figure 1C). Pertinently, minimal levels of active Bak were detectable in HCMV infected cells at 3hpi. Crucially, pre-infection with HCMV prior to cisplatin A treatment markedly reduced the detection of active Bak (Figure 1C) - consistent with the protective phenotype of HCMV against cisplatin A. Taken together, these data suggested HCMV engendered a cellular phenotype that was directly antagonistic to cisplatin A induced cell death.
Figure 1. HCMV infection blocks cisplatin A mediated activation of Bak.
(A) Western blot analysis of CD34+ cells either mock (M), HCMV infected (V) or cisplatin A treated (CspA) for Bak, MCL-1, BCL-XL, Bcl-2 or GAPDH expression 2 hours post-infection. (B) qRT-PCR analysis of RNA isolated from mock, HCMV infected or cisplatin A treated cells for Bak or MCL-1 expression 2 hours post-infection. Changes in gene expression were identified using GAPDH and 2-ΔΔCT method. n=3 (C) CD34+ cells were mock or HCMV infected and then incubated with DMSO or cisplatin A 3 hours post-infection. Cells were then permeabilised and stained with an antibody that specifically recognises an N terminal peptide exposed in activated Bak protein or with an isotype matched control. Fluorescent staining was achieved using a FITC-Goat anti-mouse antibody and then cells analysed by flow cytometry.
HCMV activation of ERK-MAPK is necessary to prevent prolonged activation of Bak upon infection
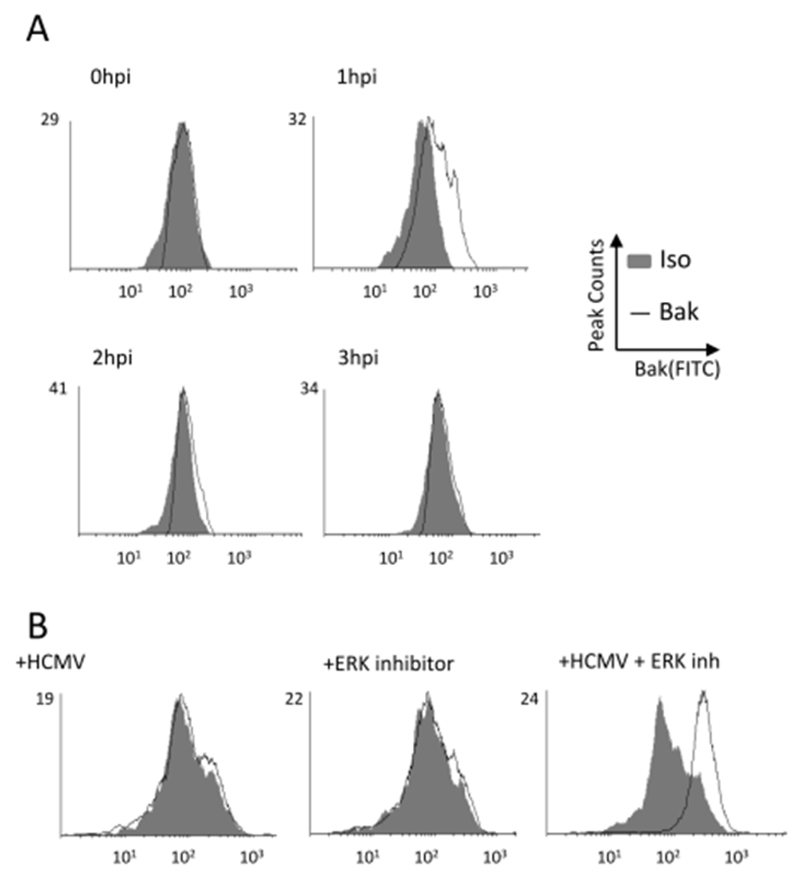
To investigate this further we first asked whether Bak activation occurred during viral infection – which would underpin the need to promote a cellular environment that antagonises Bak activation under normal infection conditions. Intriguingly, a time course analysis revealed that Bak was clearly activated in infected cells at 1hpi and that, over time, the level of Bak activation was reversed by 3hpi (Figure 2A).
Figure 2. HCMV infection induces a transient activation of Bak which is not reversed when ERK responses are inhibited.
(A) CD34+ cells were infected with HCMV and then at times 0 to 3 hours post infection cells were permeabilised and stained for evidence of Bak activation by flow cytometry. (B-C) CD34+ cells were either pre-treated with an ERK inhibitor (B) or subjected to ELK1 or control siRNA knock-down (C) and then either mock of HCMV infected. Cells were then permeabilised and stained for evidence of Bak activation by flow cytometry.
To explore this further we next exploited the knowledge that inhibition of survival pathways triggered by HCMV promoted the death phenotype (Reeves et al. 2012). CD34+ cells were pre-incubated with ERK-MAPK inhibitor for 1 hour prior to infection (Figure 2B). Cells were then stained for Bak activation. As expected, there was little evidence of Bak activation at 3hpi in infected cells or cells treated with ERK inhibitor alone. However, the infection of cells with inhibited ERK-MAPK signalling resulted in a clear Bak activation phenotype (Figure 2B) and thus clearly pheno-copied the cisplatin effect. Taken together, the data show that HCMV infection activates Bak which it then reverses via the concomitant activation of ERK-MAPK signalling.
HCMV modulates the activity of multiple Bak-interacting functions in protected cells
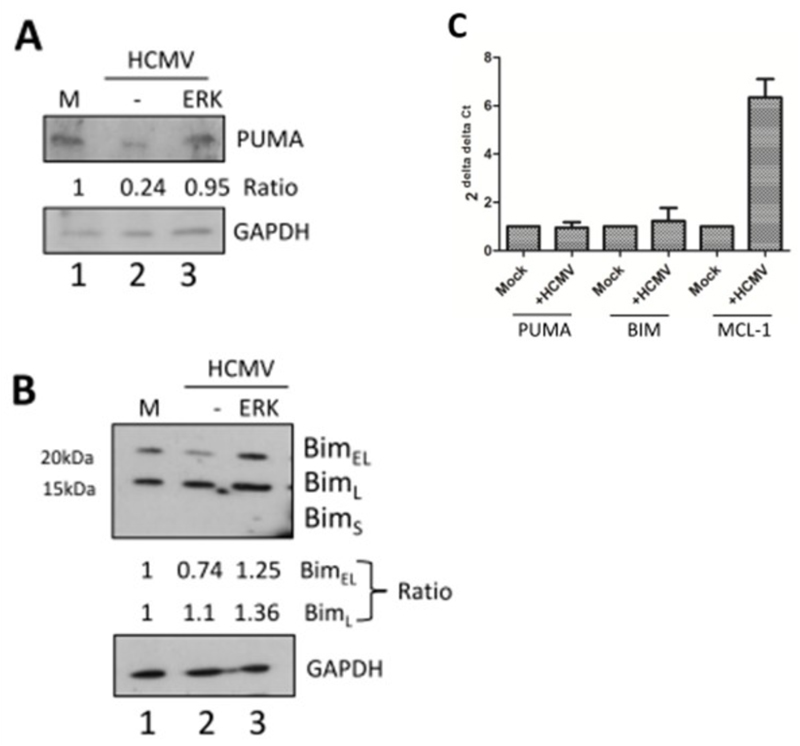
The activation of Bak in the mitochondrial membrane is elicited through a complicated interplay between effector and inhibitor mechanisms (Llambi et al. 2011). Having already observed changes to the levels of the MCL-1 regulator we next asked whether any impact on activators of Bak were triggered by HCMV. Two key pro-apoptotic molecules reported to be up-stream of Bak in this pathway are the pro-apoptotic PUMA and BIM BH3 proteins (Westphal et al. 2014). Western blot analyses indicated that basal levels of both PUMA and 2 of 3 BIM isoforms (BimEL and BimL) were detectable in uninfected cells. Furthermore, HCMV infected cells analysed at 1hpi suggested that a down-regulation of both PUMA and BimEL was evident. In contrast no changes in BimL were detected (Figure 3A, B). We also noted that the highly proapoptotic isoform BimS (Marani et al. 2002) was not detectable nor was expression induced upon infection. The down-regulation of both PUMA and BIMEL products was specific to the protein as no effect on RNA levels was observed (Figure 3C). Finally, the reduction in both PUMA and BIMEL levels by HCMV was dependent on ERK-MAPK activity (Figure 3A, B).
Figure 3. HCMV infection promotes down-regulation of pro-apoptotic proteins in an ERK dependent manner.
(A,B) Western blot analysis of CD34+ cells either mock (M), HCMV infected (HCMV) or HCMV infected after 1 hour of pre-incubation with and ERK-MAPK inhibitor (ERK) for expression of PUMA (A) or BIM isoforms. (B) protein expression was performed at 2 hours post-infection. GAPDH served as loading control. Densitometry was used to measure relative levels of protein expression (C) qRT-PCR analysis of RNA isolated from mock or HCMV infected cells for PUMA, BIM or MCL-1 expression was performed 2 hours post-infection. Changes in gene expression were identified using GAPDH and 2-ΔΔCT method, n=3.
Thus HCMV activation of ERK-MAPK was promoting several related effects that would be conducive for cell survival – BIM isoform and PUMA degradation alongside an up-regulation of MCL-1 levels. MCL-1 protein levels within the cell are regulated by multiple processes. As well as increased transcription, MCL-1 is also regulated post-translationally with ERK hypothesised to promote a stabilising phosphorylation event of MCL-1 that antagonises degradation. Thus to investigate the potential contribution of transcription and translation of MCL-1 in response to HCMV, we reasoned we were required to understand the basis of HCMV induced up-regulation of MCL-1 mRNA expression.
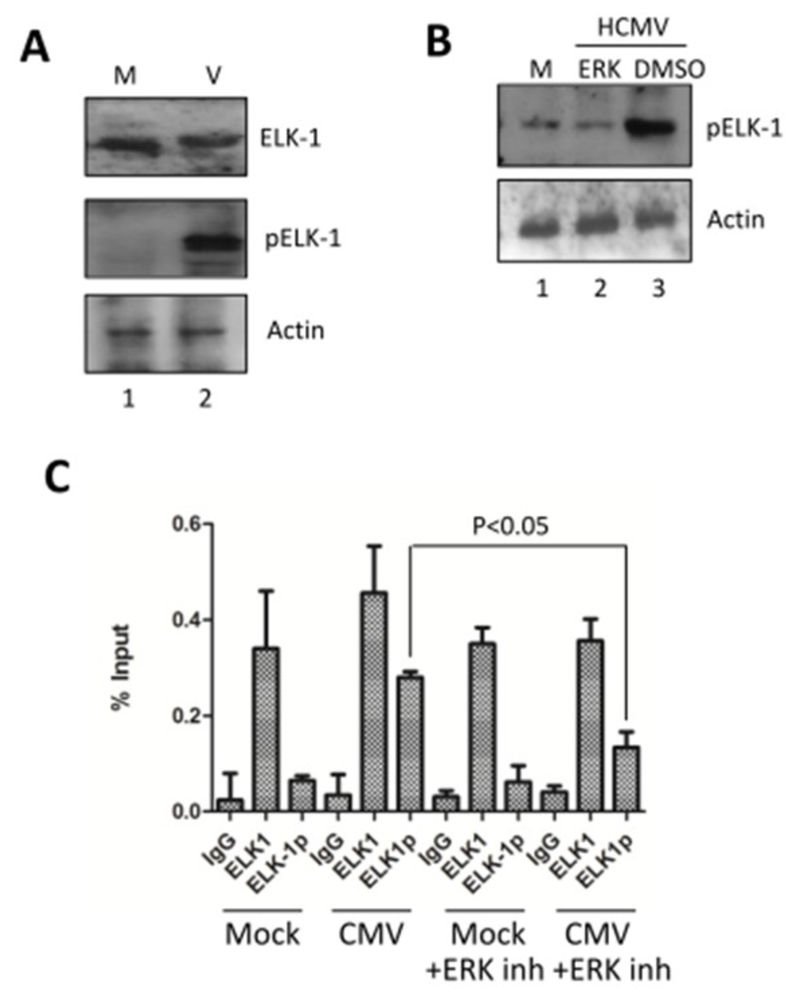
Key elements responsible for the regulation of the MCL-1 promoter include binding sites for the ELK family of proteins (Booy et al. 2011). Western blot analysis of infected cells revealed that although HCMV binding did not increase the levels of ELK-1 in the cell, there was an apparent mobility shift which would be indicative of ELK-1 phosphorylation. This was confirmed using a phosphor-ELK1 specific antibody (Figure 4A). We next showed that the phosphorylation, and thus activation of ELK-1, was dependent on ERK-MAPK signalling (Figure 4B). To link this clear activation of ELK-1 upon infection with MCL-1 expression we performed chromatin immuno-precipitation analyses. These revealed that the ELK1 protein was bound to the MCL-1 promoter in CD34+ cells irrespective of whether they were virally infected or not (Figure 4C). Although the data suggested that more ELK1 was bound in virally infected cells, it was the analysis of phosphorylated ELK1 bound to the MCL-1 promoter which exhibited the clearest phenotype. Here, viral infection promoted an increase in the detectable levels of pELK1 at the MCL-1 promoter and this event was significantly dependent on ERK signalling (Figure 4C).
Figure 4. HCMV targets ELK1 for phosphorylation in an ERK dependent manner.
(A) Western blot analysis of CD34+ cells, either mock (M) or HCMV infected (V) for ELK1 and ELK1 phosphorylation, 2 hours post-infection. Actin served as loading control. (B) Western blot analysis of CD34+ cells, either mock (M) or HCMV infected (HCMV) cells, with or without prior incubation with ERK-MAPK inhibitor (ERK) or DMSO control for 1 hour at 2 hours post-infection. Actin served as loading control. (C) Chromatin immuno-precipitation assays were performed on CD34+ cells with isotype (IgG), ELK1 or phosphor-ELK1 (ELK-1p) antibodies. ChIPs were performed on CD34+ cells, HCMV infected CD34+ cells or the equivalent but with prior incubation with U0126 ERK inhibitor for 1 hour. Cells were analysed at 2 hours post infection, n=3. Students t-test was used to test for significance at p<0.05.
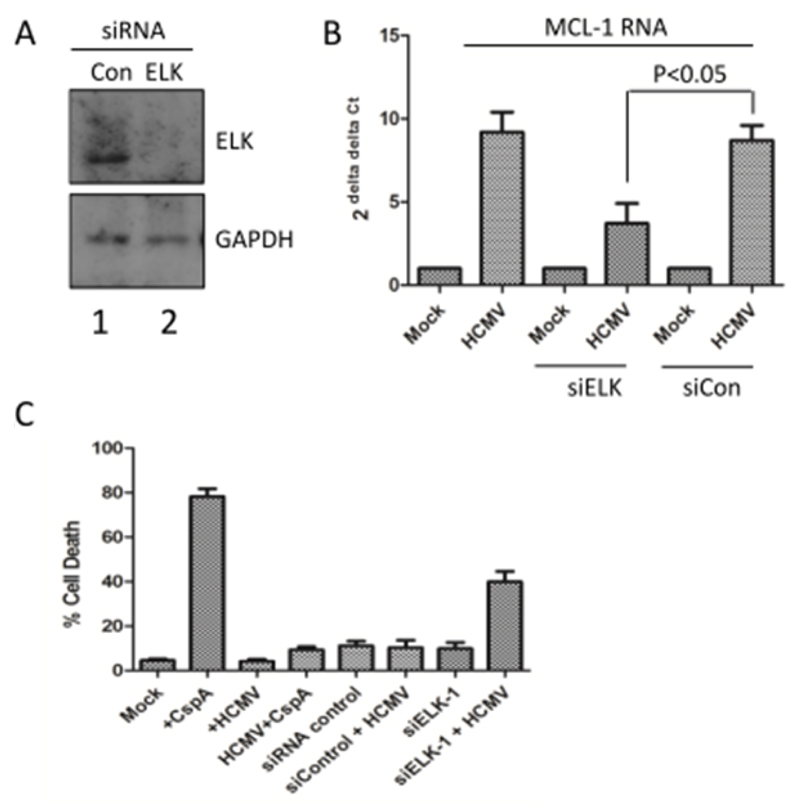
To test whether ELK-1 was directly involved in the pro-survival phenotype we used siRNA knock-down in CD34+ cells to address this. Delivery of ELK-1 siRNAs significantly reduced ELK-1 protein present in the cell (Figure 5A) but did not result in any overt effects on cell viability over the short time frame of analysis (Figure 5C). Consistent with the ChIP data (Figure 4C), ELK-1 knockdown in the CD34+ cells dramatically impacted on the ability of HCMV to up-regulate MCL-1 RNA expression (Figure 5B). Together with the phenotypic impact on MCL-1 gene expression, there was a clear abrogation of the virally induced survival response (Figure 5C). Although the delivery of ELK-1 siRNAs alone was not deleterious to the cell, viral infection triggered a marked increase in cell death (Figure 5C) indicating that the elimination of ELK-1 from CD34+ cells renders them more sensitive to HCMV induced cell death.
Figure 5. Depletion of ELK1 from CD34+ cells abrogates the HCMV survival response.
(A) Western blot analysis of CD34+ cells 48 hours post-transfection with a control (Con), ELK1-specific (ELK) siRNA for ELK1 and GAPDH expression. (B) qRT-PCR analysis of RNA isolated from mock or HCMV infected cells 2 hours post-infection that have first been treated with either mock, control (Scr KD) or ELK1 (ELK KD) siRNAs. Changes in gene expression were identified using GAPDH and 2-ΔΔCT method, n=3. Students t-test was used to test for significance at p<0.05. (C) CD34+ cells were either mock or HCMV infected and then, 3 hours post-infection, incubated with cisplatin A or DMSO control. Alternatively, CD34+ cells were transfected with control or ELK-1 specific siRNAs and then either mock or HCMV-infected. In all experiments cell viability was measured 24 hours post-infection. Graph is the average of 2 independent experiments analysed in triplicate.
ELK-1 knockout cells show elevated levels of Bak activation upon HCMV infection resulting in a less efficient establishment of latency
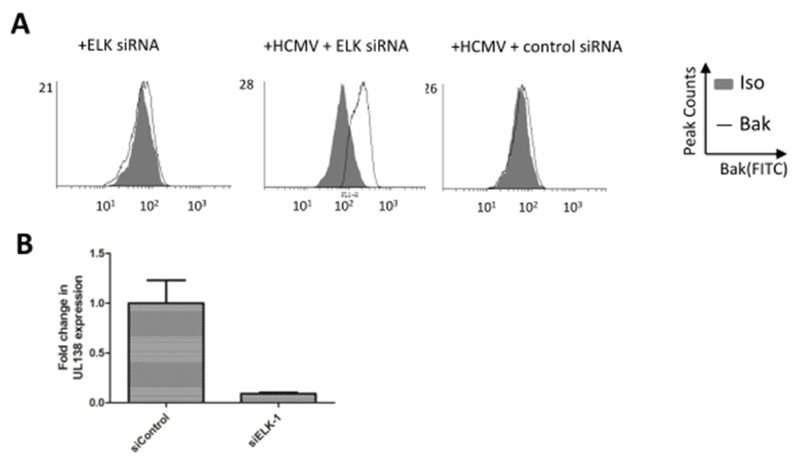
Next, we revisited our studies of Bak activation using our ELK-1 knock down cells (Figure 6A). Unsurprisingly, ELK-1 depletion from cells had little impact on levels of Bak activation. However, infection of ELK-1 knock down cells again resulted in an increased detection of activated Bak (Figure 6A). Although the analysis suggested that not all cells displayed evidence of activated Bak – which may reflect relative efficiency of siRNA knock down – the data clearly suggested that removal of ELK-1 from CD34+ cells promoted Bak activation in response to HCMV infection.
Figure 6. ELK-1 is required for the reversal of Bak activation and the establishment of HCMV latency.
(A,B) CD34+ cells were subjected to ELK1 or control siRNA knockdown and then either mock of HCMV-infected. Cells were then either permeabilised and stained for evidence of Bak activation by flow cytometry at 3hpi (A) or analysed 3 days post infection for evidence of viral latent gene expression (B).
Finally, to assess if this was having any impact on the virus we assessed the ability of HCMV to establish latency (Figure 6B). Control or ELK-1 siRNA treated cells were infected with HCMV and analysed 3 days post-infection for evidence of latent viral gene expression. It was evident that the failure to prevent virally induced cell death manifested with significantly reduced levels of UL138 expression, which would be consistent with a failure to establish a latent infection.
Discussion
In this study we have investigated the phenotype of the pro-survival environment elicited by infection of CD34+ cells with HCMV. This and previous studies provide accumulating evidence that HCMV is required to promote an anti-apoptotic environment through the activation of signalling pathways in non-permissive cells in order to ensure long term survival (Chan et al. 2010; Peppenelli et al. 2016; Reeves et al. 2012; Stevenson et al. 2014). These events are likely critical for the pathogenesis and dissemination of virus in monocytes and the establishment of latency in CD34+ cells.
The requirement for a pro-survival signalling cascade is necessitated by the evident induction of prodeath responses which likely represent anti-viral responses to infection. This induction of pro-death responses is assumed to be due to the highly proapoptotic phenotype associated with viral infection when the activity of the survival factors is impaired (Chan et al. 2010; Reeves et al. 2012). Thus HCMV cannot necessarily completely eliminate pro-death signalling but, instead, relies on the concomitant upregulation of survival factors to counter-balance this. It is of interest that a pan up-regulation of the anti-apoptotic machinery is not evident in the infected CD34+ cells. An analysis of 3 major players MCL-1, BCL-XL and BCL-2 revealed that only MCL-1 levels were affected by HCMV infection during the initial phases of latent infection in this cell type. However, we cannot dismiss a role for the other proteins completely, as the localisation or binding to target pro-apoptotic proteins of these other BCL-2 family members may be modified to augment the virally induced survival observed. Furthermore, our studies have really focussed on the role of MCL-1 as a transitory regulator during the very initial stages of infection. It is important to note that data from studies performed in the monocyte model have shown that BCL-2 activity becomes increasingly important to maintain the viability of the infected cells (Collins-McMillen et al. 2015), suggesting that different molecules are required at different stages of viral infection. Consistent with this, reports in progenitor model systems have suggested that PEA-15 up-regulation via the activity of interleukin-10 could play a role in long term latency in CD34+ cells (Poole et al. 2011, 2015; Slobedman et al. 2004). In this regard, the apparent targeting of the MCL-1 member of the BH3 family during the very early stages could be consistent with the central role that MCL-1 plays in the regulation of apoptosis in haematopoietic cells (Opferman et al. 2005) and also reflective of its more dynamic regulation in the cell (Adams & Cooper 2007). Unlike counterparts (e.g. Bcl-2, Bcl-Xl), MCL-1 has a much shorter half-life (Adams & Cooper 2007). Ablation of MCL-1 results in spontaneous cell death in a number of cell lineages, indicating that, firstly, endogenous levels of other BCL-2 proteins are not sufficient to compensate and secondly, it has a central role in haematopoietic cell survival (Dzhagalov et al. 2008; Opferman 2007; Opferman et al. 2005). This exquisite sensitivity of primary cells to MCL-1 levels may also involve the complex interaction of MCL-1 with mitochondria (Huang & Yang-Yen 2010; Perciavalle et al. 2012). As well as a classical role in apoptosis, MCL-1 has also been shown to regulate ATP biogenesis (Perciavalle et al. 2012) which we know, from studies of lytic HCMV infection, has a profound effect on the viability of infected cells (Reeves et al. 2007). Whether the impact of MCL-1 on mitochondrial bioenergetics is important here is not clear but, given the relatively short lived nature of the protection in the experimental conditions, it is perhaps unlikely. It is more plausible to have a role either in the MCL-1 mediated protection of monocytes where elevated MCL-1 expression persists for 72 hours (Chan et al. 2010) or, it could possibly augment the function of beta 2.7 during lytic HCMV infection.
Cisplatin A mediated MCL-1 degradation is a trigger for apoptosis (Yang et al. 2007) and thus tumours exhibiting resistance to cisplatin A often display elevated levels of MCL-1 that is resistant to drug induced degradation (Michels et al. 2014). It is hypothesised that the degradation of MCL-1 exposes Bak to the activity of pro-apoptotic proteins like PUMA and BIM, ultimately resulting in Bak-mediated mitochondrial dysfunction (Letai et al. 2002). The precise nature of the inhibitory effect of MCL-1 on Bak is debated: MCL-1 could bind to Bak directly to block activation or could block activation by sequestering pro-apoptotic BH3 proteins like PUMA and BIM or, in fact, perform both functions (Erlacher et al. 2006, Letai et al. 2002; Marani et al. 2002; Willis & Adams 2005).
Another possibility is that although PUMA and BIM are pro-apototic proteins themselves and thus viral induced degradation is a mechanism to eliminate a direct activator, another consequence of the down-regulation of these proteins could be contribute to increasing the level of ‘free’ MCL-1 in the cell. Thus the up-regulation of MCL-1 and the down-regulation of a binding partner that sequesters it increase the likelihood of an interaction with Bak. Additionally, very recent data suggests that the interaction of MCL-1 with Bak is induced under conditions that promote Bak oligomerisation/activation (Dai et al. 2015). Thus, viral infection – an apparent trigger of Bak activation – promotes a concomitant increase in MCL-1 levels which likely counters this. Indeed, it is interesting to note that when Bak activation was analysed at multiple points during the initial infection, we detected evidence of Bak activation at very early times which was quickly reversed.
Many of these observations were dependent on virally induced ERK-MAPK signalling. Viral induced activation of ERK-MAPK signalling and subsequent survival is a recurring theme suggesting that this pathway is a common target (Dai et al. 2016; Liu & Cohen 2013; Pleschka 2008; Pontes et al. 2015). The multi-factorial response driven by ERK may also partially explain the observation that ELK-1 activity, whilst important, was never as detrimental as inhibition of upstream ERK signalling. Despite this, the induction of ELK-1 phophorylation was another virally induced response that was dependent on ERK-MAPK signalling. Again, this had a pro-survival effect, whereby elimination of ELK-1 phosphorylation, and thus activation (Cruzalegui et al. 1999), either through inhibition of ERK-MAPK or via a siRNA depletion of ELK-1 was deleterious, specifically upon virus infection. We note that evidence of ELK-1 binding to the MCL-1 promoter prior to any stimulation was observed and, although viral infection provided evidence of increased occupancy of ELK-1, phosphorylation of ELK-1 was most important for MCL-1 transcription (Booy et al. 2011; Cruzalegui et al. 1999). The observed occupancy of ELK-1 in an unphosphorylated (presumably inactive) form at the promoter may suggest only a partial involvement of ELK-1 in basal MCL-1 expression in the CD34+ cells but, instead, being important for dynamic responses to further stimuli. It would also be consistent with ELK-1 activation representing a mechanism for rapidly inducing MCL-1 expression (Booy et al. 2011; Vickers et al. 2004). This stimulus-specific activity would explain the virally induced effects on MCL-1 and, also, the transient nature of the elevated MCL-1 expression (Reeves et al. 2012). The virus triggers a burst of ERK-MAPK activity upon entry promoting ELK-1 phosphorylation but once ERK signalling is down-regulated through normal feedback mechanisms (Fritsche-Guenther et al. 2011) coupled with a loss of the initial virus binding induced signal as the virus enters the cell, then MCL-1 expression returns to basal levels. The occupancy of inactive or even inhibitory (e.g. p50 homodimers) forms of transcription factors at promoters represents a mechanism for rapid induction of gene expression and has been observed at multiple promoters (Altarejos & Montminy 2011; Baer et al. 1998; Herrera et al. 1989) including in our own studies of CREB and HCMV reactivation (Kew et al. 2014). Finally, although our analyses focused on the regulation of MCL-1 we cannot preclude the possibility that ELK-1 activates multiple responses (Boros et al. 2009) that contribute to survival upon viral infection. Indeed, pathogens often utilise central components in pathways, thus it would not be unsurprising to detect further ELK-1 controlled responses that are important.
Prescient to this study is that ERK-MAPK signalling under certain conditions can, for example, be pro-apoptotic (Cagnol & Chambard 2010; Cagnol et al. 2006; Lu & Xu 2006) and thus in future studies it will be interesting to determine how HCMV directs the ERK response - possibly via the activation of concomitant pathways - to generate the pro-survival phenotype observed. Put simply, the kinase pathway that implements the final effector function (i.e. ERK-MAPK) is not dictating the outcome alone - that decision is defined by the nature of the signalling milieu activating the ERK-MAPK module upstream and the signalling and molecular context that those pathways are being activated in. Thus an aim of future studies is to address the identity of the death signals that are activated by the host cell in response to viral infection.
What this study illustrates is that viruses are the master regulators of signalling cascades and pathways with an impressive ability to hijack, re-direct or partition them to enhance infection. Understanding these events has greatly improved our understanding of cell biology and shed new light on the mechanisms that govern the activity of cellular functions required in key biological processes. Furthermore, it also illustrates how pleiotropic signalling pathways are modified to generate very specific outputs downstream. Greater understanding of these events and how they contribute to cell survival in the context of pathogen infection also has broader implications on our knowledge regarding the decision a cell makes to live or die.
Acknowledgments
Funding Information
This work was funded by a Medical Research Council Fellowship (G:0900466) awarded to MBR. MRW is funded bv the Medical Research Council Programme Grants (G:0701279 & MR/K021087/1). The funders had no role in study design, data collection and interpretation, or decision to submit the work for publication.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest associated with this work.
Authors’ contributions
VGK, MRW and MBR performed experiments and MRW and MBR analysed the data and wrote the paper.
References
- Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF. Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-kappaB p50. Mol Cell Biol. 1998;18:5678–5689. doi: 10.1128/mcb.18.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- Booy EP, Henson ES, Gibson SB. Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene. 2011;30:2367–2378. doi: 10.1038/onc.2010.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros J, Donaldson IJ, O'Donnell A, Odrowaz ZA, Zeef L, Lupien M, Meyer CA, Liu XS, Brown M, Sharrocks AD. Elucidation of the ELK1 target gene network reveals a role in the coordinate regulation of core components of the gene regulation machinery. Genome Res. 2009;19:1963–1973. doi: 10.1101/gr.093047.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune W. Inhibition of programmed cell death by cytomegaloviruses. Virus Res. 2010;157:144–150. doi: 10.1016/j.virusres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Van Obberghen-Schilling E, Chambard JC. Prolonged activation of ERK1,2 induces FADD-independent caspase 8 activation and cell death. Apoptosis. 2006;11:337–346. doi: 10.1007/s10495-006-4065-y. [DOI] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol. 2010;184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RJ, Fechheimer M, Miller LK. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Collins-McMillen D, Kim JH, Nogalski MT, Stevenson EV, Chan GC, Caskey JR, Cieply SJ, Yurochko AD. Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins. J Virol. 2015;90:2356–2371. doi: 10.1128/JVI.01994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T. Analysis of Cytomegalovirus Ligands, Receptors and the Entry Pathway. Methods Mol Med. 2000;33:53–65. doi: 10.1385/1-59259-244-9:53. [DOI] [PubMed] [Google Scholar]
- Cruzalegui FH, Cano E, Treisman R. ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene. 1999;18:7948–7957. doi: 10.1038/sj.onc.1203362. [DOI] [PubMed] [Google Scholar]
- Dai H, Ding H, Meng XW, Peterson KL, Schneider PA, Karp JE, Kaufmann SH. Constitutive BAK activation as a determinant of drug sensitivity in malignant lymphohematopoietic cells. Genes Dev. 2015;29:2140–2152. doi: 10.1101/gad.267997.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Feng M, Ye Y, Wu X, Liu D, Liao M, Cao W. Exogenous avian leukosis virus-induced activation of the ERK/AP1 pathway is required for virus replication and correlates with virus-induced tumorigenesis. Sci Rep. 2016;6:19226. doi: 10.1038/srep19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir O, Aysit N, Onder Z, Turkel N, Ozturk G, Sharrocks AD, Kurnaz IA. ETS-domain transcription factor Elk-1 mediates neuronal survival: SMN as a potential target. Biochim Biophys Acta. 2011;1812:652–662. doi: 10.1016/j.bbadis.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Doerflinger M, Glab JA, Puthalakath H. BH3-only proteins: a 20-year stock-take. FEBS J. 2015;282:1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Böck G, Tzankov A, Häcker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett H, McFadden G. Apoptosis: an innate immune response to virus infection. Trends Microbiol. 1999;7:160–165. doi: 10.1016/s0966-842x(99)01487-0. [DOI] [PubMed] [Google Scholar]
- Everett RD, Chelbi-Alix MK. PML and PML nuclear bodies: implications in antiviral defence. Biochimie. 2007;89:819–830. doi: 10.1016/j.biochi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Böck G, Tzankov A, Häcker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche-Guenther R, Witzel F, Sieber A, Herr R, Schmidt N, Braun S, Brummer T, Sers C, Blüthgen N. Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol. 2011;7:489. doi: 10.1038/msb.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, Han JW, Lutz RJ, Watanabe S, Cahir McFarland ED, Kieff ED, et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the proapoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1998;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Kaiser WJ, Mocarski ES. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol. 2015;204:439–448. doi: 10.1007/s00430-015-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera RE, Shaw PE, Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989;340:68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- Huang CR, Yang-Yen HF. The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity. FEBS Lett. 2010;584:3323–3330. doi: 10.1016/j.febslet.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Huang HM, Huang CJ, Yen JJ. Mcl-1 is a common target of stem cell factor and interleukin-5 for apoptosis prevention activity via MEK/MAPK and PI-3K/Akt pathways. Blood. 2000;96:1764–1771. [PubMed] [Google Scholar]
- Kew VG, Yuan J, Meier J, Reeves MB. Mitogen and stress activated kinases act co-operatively with CREB during the induction of human cytomegalovirus immediate-early gene expression from latency. PLoS Pathog. 2014;10:e1004195. doi: 10.1371/journal.ppat.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Liu X, Cohen JI. Inhibition of Bim enhances replication of varicella-zoster virus and delays plaque formation in virus-infected cells. J Virol. 2013;88:1381–1388. doi: 10.1128/JVI.01695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels J, Obrist F, Vitale I, Lissa D, Garcia P, Behnam-Motlagh P, Kohno K, Wu GS, Brenner C, Castedo M, Kroemer G. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem Pharmacol. 2014;92:55–61. doi: 10.1016/j.bcp.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, Trojahn U, Wendel HG, Charest A, Bronson RT, Kogan SC, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odrowaz Z, Sharrocks AD. ELK1 uses different DNA binding modes to regulate functionally distinct classes of target genes. PLoS Genet. 2012;8:e1002694. doi: 10.1371/journal.pgen.1002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT. Life and death during hematopoietic differentiation. Curr Opin Immunol. 2007;19:497–502. doi: 10.1016/j.coi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Peppenelli MA, Arend KC, Cojohari O, Moorman NJ, Chan GC. Human Cytomegalovirus Stimulates the Synthesis of Select Akt-Dependent Antiapoptotic Proteins during Viral Entry To Promote Survival of Infected Monocytes. J Virol. 2016;90:3138–3147. doi: 10.1128/JVI.02879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, Youle RJ, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S. RNA viruses and the mitogenic Raf/MEK/ERK signal transduction cascade. Biol Chem. 2008;389:1273–1282. doi: 10.1515/BC.2008.145. [DOI] [PubMed] [Google Scholar]
- Pontes MS, Van Waesberghe C, Nauwynck H, Verhasselt B, Favoreel HW. Pseudorabies virus glycoprotein gE triggers ERK1/2 phosphorylation and degradation of the pro-apoptotic protein Bim in epithelial cells. Virus Res. 2016;213:214–218. doi: 10.1016/j.virusres.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Poole E, Lau JC, Sinclair J. Latent infection of myeloid progenitors by human cytomegalovirus protects cells from FAS-mediated apoptosis through the cellular IL-10/PEA-15 pathway. J Gen Virol. 2015;96:2355–2359. doi: 10.1099/vir.0.000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E, McGregor Dallas SR, Colston J, Joseph RS, Sinclair J. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34+ progenitors. J Gen Virol. 2011;92:1539–1549. doi: 10.1099/vir.0.031377-0. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science. 2013;340:701–716. doi: 10.1126/science.1233028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MB, Breidenstein A, Compton T. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proc Natl Acad Sci U S A. 2012;109:588–593. doi: 10.1073/pnas.1114966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science. 2007;316:1345–1348. doi: 10.1126/science.1142984. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobedman B, Stern JL, Cunningham AL, Abendroth A, Abate DA, Mocarski ES. Impact of human cytomegalovirus latent infection on myeloid progenitor cell gene expression. J Virol. 2004;78:4054–4062. doi: 10.1128/JVI.78.8.4054-4062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EV, Collins-McMillen D, Kim JH, Cieply SJ, Bentz GL, Yurochko AD. HCMV reprogramming of infected monocyte survival and differentiation: a Goldilocks phenomenon. Viruses. 2014;6:782–807. doi: 10.3390/v6020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun NK, Huang SL, Chang TC, Chao CC. Sorafenib induces endometrial carcinoma apoptosis by inhibiting Elk-1-dependent Mcl-1 transcription and inducing Akt/GSK3beta-dependent protein degradation. J Cell Biochem. 2013;114:1819–1831. doi: 10.1002/jcb.24530. [DOI] [PubMed] [Google Scholar]
- Terhune S, Torigoi E, Moorman N, Silva M, Qian Z, Shenk T, Yu D. Human cytomegalovirus UL38 protein blocks apoptosis. J Virol. 2007;81:3109–3123. doi: 10.1128/JVI.02124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, Craig RW. Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem. 1999;274:1801–1813. doi: 10.1074/jbc.274.3.1801. [DOI] [PubMed] [Google Scholar]
- Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992;11:4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren RT, Dewson G, Chen L, Coyne SC, Huang DC, Adams JM, Kluck RM. Mitochondrial permeabilization relies on BH3 ligands engaging multiple prosurvival Bcl-2 relatives, not Bak. J Cell Biol. 2007;177:277–287. doi: 10.1083/jcb.200606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers ER, Kasza A, Kurnaz IA, Seifert A, Zeef LA, O'donnell A, Hayes A, Sharrocks AD. Ternary complex factor-serum response factor complex-regulated gene activity is required for cellular proliferation and inhibition of apoptotic cell death. Mol Cell Biol. 2004;24:10340–10351. doi: 10.1128/MCB.24.23.10340-10351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsa-kopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21:196–205. doi: 10.1038/cdd.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Yang C, Kaushal V, Shah SV, Kaushal GP. Mcl-1 is downregulated in cisplatin-induced apoptosis, and proteasome inhibitors restore Mcl-1 and promote survival in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292:F1710–F1717. doi: 10.1152/ajprenal.00505.2006. [DOI] [PubMed] [Google Scholar]