Abstract
Background
This study was done to characterize parameters associated with semen human immunodeficiency virus (HIV)-1 ribonucleic acid (RNA) viral load (VL) variability in HIV-infected, therapy-naive men.
Methods
Paired blood and semen samples were collected from 30 HIV-infected, therapy-naive men who have sex with men, and 13 participants were observed longitudinally for up to 1 year. Human immunodeficiency virus RNA, bacterial load by 16S RNA, herpesvirus (Epstein-Barr virus and cytomegalovirus [CMV]) shedding, and semen cytokines/chemokines were quantified, and semen T-cell subsets were assessed by multiparameter flow cytometry.
Results
Semen HIV RNA was detected at 93% of visits, with >50% of men shedding high levels of virus (defined as >5000 copies/mL). In the baseline cross-sectional analysis, an increased semen HIV VL correlated with local CMV reactivation, the semen bacterial load, and semen inflammatory cytokines, particularly interleukin (IL)-8. T cells in semen were more activated than blood, and there was an increased frequency of Th17 cells and γδ-T-cells. Subsequent prospective analysis demonstrated striking interindividual variability in HIV and CMV shedding patterns, and only semen IL-8 levels and the blood VL were independently associated with semen HIV levels.
Conclusions
Several clinical and immune parameters were associated with increased HIV semen levels in antiretroviral therapy-naive men, with induction of local proinflammatory cytokines potentially acting as a common pathway.
Keywords: cytokines, herpesviruses, HIV, microbiome, semen
Semen containing human immunodeficiency virus (HIV)-1 is the major vector worldwide for transmission from an HIV-infected, antiretroviral therapy (ART)-naive man to his sexual partner(s). Although the blood HIV ribonucleic acid (RNA) level is perhaps the best-defined predictor of transmission, with the annual transmission risk in HIV serodiscordant couples increasing from 0 to 25% as blood viral load (bVL) increases from undetectable levels to >50000 HIV-1 RNA copies/mL [1], this reflects in part the correlation between bVL and semen VL (sVL) [2]. In keeping with this, the sVL of a male partner was an even stronger independent predictor of HIV transmission than the bVL, with each log10 sVL increase being associated with a 1.8-fold increased HIV transmission risk [2].
There can be substantial variability in the sVL, independent of the bVL, and at any given time they are moderately correlated at best [3]. Genital infections are an important cause of this variability, with acute Neisseria gonorrhoeae urethritis increasing the sVL almost 10-fold without any effect on bVL [4]. However, approximately one third of ART-naive men demonstrate levels of HIV RNA in semen that are disproportionately higher than those in blood, even in the absence of symptomatic urethritis or asymptomatic classic sexually transmitted infections (STIs) [8]. Compartmentalized reactivation of asymptomatic, persistent human herpesviruses (HHVs), such as CMV and Epstein-Barr virus (EBV), have been linked with an elevated sVL [5–9], and the association of a disproportionately high sVL with increased unprotected insertive (but not receptive) sex suggests that nonclassic STI may also be involved [10]. Associations are also seen between the sVL of ART-naive men and total semen bacterial levels (one aspect of the semen microbiome) [11, 12], several semen proinflammatory cytokines [9, 13–15], and the presence of various immune cell subsets [15–17].
Effective ART dramatically reduces both the sVL and the probability of HIV transmission [18, 19]. However, many HIV-infected men in the world do not know their status and/or do not have access to ART, and so understanding the factors that cause variability in the sVL of ART-naive men remains an important priority, and this may lead to novel avenues for HIV prevention. Our goal in this study was to perform a comprehensive assessment in ART-naive men who have sex with men of several clinical and mucosal parameters that have previously been associated with HIV RNA shedding, to define independent predictors of increased HIV sVL, and to assess prospective patterns of HIV semen shedding.
METHODS
Study Participants and Design
Men who have sex with men infected with HIV for ≥1 year were recruited through the Maple Leaf Medical Clinic, Toronto between 2011 and 2013. Participants were ART naive and were screened for STIs at each study visit. All participants provided paired blood and semen samples at a single time point, and a subgroup of men who opted to defer ART provided monthly semen and blood samples for up to 1 year. All participants provided informed, written consent; ethical approval for this study was obtained through the research ethics board of the University of Toronto.
Sample Collection and Diagnostic Testing
Blood was collected into acid citrate dextran, and semen was collected by masturbation directly into a sterile container containing 10 mL of Roswell Park Memorial Institute (RPMI) medium with penicillin/streptomycin after 48 hours of abstinence [20]. Samples were processed within 2 hours of collection. Semen plasma (SP) was isolated by centrifugation at 850 × g for 10 minutes, and blood plasma (BP) was isolated after Ficoll density gradient centrifugation at 500 × g for 25 minutes. Blood plasma and SP HIV RNA VL were assayed using the Abbott RealTime HIV-1 assay using an automated m2000 system (Abbott Molecular Diagnostics). Due to occasional spillage of RPMI medium during semen collection and/or sample transport, correction for semen dilution assumed a semen volume of 2 mL, as previously validated [20]. The lower limit of detection for bVL and sVL were 50 and 300 HIV RNA copies/mL, respectively. High-level semen shedding was defined as a VL >5000 HIV RNA copes/mL [1].
Real-Time Quantification of Semen Herpesvirus Levels
Viral load quantification of herpes simplex virus (HSV)-1, HSV-2, HHV-3, EBV, CMV, HHV-7, and HHV-8 was performed by reverse transcription-polymerase chain reaction (PCR) using the genesig quantitative PCR (qPCR) detection kit (PrimerDesign Ltd); HHV-6 A-B was quantified using the RealStar PCR kit (Altona Diagnostics) [21]. In brief, deoxyribonucleic acid (DNA) was extracted from 400 μL of SP using a DNA mini extraction kit (QIAGEN, Inc., Valencia, CA). For the genesig kits, extracted SP DNA (5 μL) containing the provided internal control was combined with a PCR mix containing 10 μL 2×Precision MasterMix, 1 μL Primer/Probe mix, and 4 μL RNAse/DNAse-free water. For the RealStar PCR kit, 10 μL of extracted SP DNA was added to the mastermix containing 5 μL of master-A, 15 μL of master-B, and 1 μL of the internal control. All viruses were quantified using the Applied Biosystems 7900 HT Real-Time PCR system with the after reactions conditions: 95°C for 10 minutes followed by 50 cycles of 95°C for 10 seconds (genesig) or 15 minutes (RealStar) and 60°C for 1 minute. Results were analyzed using the Sequence Detection System, version 2.4 (Applied Biosystems). Lower limit of detection was 200 copies/mL.
Cytokine and Trappin-2/Elafin Levels in Semen Plasma
Cytokine levels in SP were measured using the Meso Scale Discovery multiplex System (Gaithersburg, MD). Fourteen cytokines/chemokines were measured (lower limit of quantification listed in parenthesis): interleukin (IL)-1α (3.6 pg/mL), IL-6 (3.6 pg/mL), IL-1β (3.6 pg/mL), IL-8 (1.8 pg/mL), monocyte chemoattractant protein (MCP)-1 (1.8 pg/mL), macrophage-derived chemokine ([MDC] 1.8 pg/mL), monokine induced by interferon-γ ([MIG] 1.8 pg/mL), macrophage inflammatory protein (MIP)-1β (29.28 pg/mL), MIP-3α (14.64 pg/mL), regulated on activation, normal T cells expressed and secreted ([RANTES] 3.6 pg/mL), IL-10 (1.8 pg/mL), IL-17 (3.6 pg/mL), interferon-inducible protein (IP)-10 (14.64), and tumor necrosis factor (TNF)-α (1.8 pg/mL). All samples were run in duplicate according to the manufacturer’s protocol. For cytokine levels below the lower limit of detection of the assay, values were reported as the lower limit of quantification based on the standard curve. Commercially available Trappin-2/Elafin enzyme-linked immunosorbent assay kits (Hycult Biotech) were used to measure SP levels of Elafin according to manufacturer’s instructions. The levels of many cytokines coassociate in semen, and so to reduce concerns around multiple comparisons we calculated a semen inflammation (SI) score: this was based on prior work in the female genital tract (FGT) [22], with slight modification to accommodate FGT-semen cytokine differences. Men who had 4 or more of 9 inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-17, MIP-3α, IL-1β, and MIP-1β) in the upper quartile were defined as inflamed, and the total number of cytokines in the upper quartile constituted an inflammation score (from 0 to 9).
Bacterial Load Quantification
For bacterial load quantification, 500 μL of thawed SP was lysed using a combination of chemical and mechanical methods and purified using AllPrep DNA/RNA Mini Kit (QIAGEN). Deoxyribonucleic acid was eluted in 100 μL of buffer EB, whereas RNA was eluted in 50 μL. Reverse transcription was performed using qScript cDNA SuperMix according to the manufacturer’s instructions (Quanta Biosciences, Gaithersburg, MD). Using the DNA fraction, bacterial load was quantfied, measured as bacterial 16S recombinant RNA (rRNA) gene copy/mL of SP using a broad-coverage qPCR assay, described previously [23].
T-Cell Populations in Blood and Semen
Mononuclear cells were stained for 30 minutes with fluorochrome-labeled monoclonal antibodies (CD3G-FITC, CD8-PETR, CD195-PerCPCy5.5, CD69-PECy7, β7integrin-APC, HLADR-APCH7, CD196-Pacific Blue, CD3-605NC, CD4-650NC) and a viability dye (Invitrogen) followed by fixation for 30 minutes in 1% paraformaldehyde and analyzed using BD LSR-II (BD Systems).
Statistical Analysis
Flow cytometry analysis was performed using FlowJo analytical software, version 9.4.11 (Tree Star). Non-parametric Mann-Whitney U and Wilcoxon signed-rank analysis were performed using IBM SPSS Statistics, version 20 (IBM Corporation). Backward stepwise linear regression analyses were performed for multiple independent covariants using SPSS, with P < .05 as the threshold for significance. Count and/or concentration variables were logarithmically transformed. For longitudinal multivariate analysis, a mixed model (MM) analysis was used to take into account multiple observations/measurements produced per individuals within the study. Variables associated with the outcome variable on univariate analysis (P < .1) were selected for the multivariable analysis.
RESULTS
Cross-Sectional Cohort Demographics and Viral Loads
Thirty HIV-infected, antiretroviral-naive men provided paired blood and semen samples. All participants were infected with HIV for at least 1 year (median, 3 years; range, 1–23 years). Study participants had median absolute CD4 and CD8 T-cell counts of 440 cells/mm3 (range, 190–860) and 905 cells/mm3 (range, 230–2690), respectively. The median bVL was 30544 HIV-1 RNA copies/mL (range, 824 to >500000 copies/mL), and median sVL was 5496 HIV-1 RNA copies/mL (range, 300–208152 copies/mL). All study participants were CMV seropositive, 80% (24 of 30) HSV-1 seropositive, and 33.3% (10 of 30) HSV-2 seropositive. Clinical parameters are summarized in Table 1.
Table 1.
Clinical Demographics of Study Participants
| Parameter | Cross-Sectional Cohort (n = 30) |
|---|---|
| Age | 34 (range, 24–56) |
| Years infecteda | 3 (IQR, 1–4) |
| HSV-1 | 80% (24/30) |
| HSV-2 | 33.3% (10/30) |
| HSV1+/HSV2+ | 26.7% (8/30) |
| HSV1−/HSV2− | 13.3% (4/30) |
| CMV | 100% (30/30) |
| Absolute CD4a | 440 (190–860) |
| Absolute CD8a | 905 (230–2690) |
| CD4/CD8 ratioa | 0.56 (0.17–1.97) |
| Blood viral load (copies/mL) | 30544 (824–500000) |
| Semen viral load (copies/mL) | 5496 (300–208152) |
| Semen bacterial load (16s copies/mL) | 379252 (19672–12736702) |
| Semen CMV viral load (copies/mL) | 282 (200–266761) |
| Semen EBV viral load (copies/mL) | 200 (200–409156) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; IQR, interquartile range.
aMedian values reported.
Semen HIV RNA was detected in 93% (28 of 30) of participants, and 50% (15 of 30) met criteria for high-level semen shedding with an sVL ≥5000 copies of HIV-1 RNA/mL. Two individuals had asymptomatic urethral infection with Chlamydia trachomatis: both had high-level semen shedding and elevated semen cytokines and T-cell populations, and these individuals were removed from subsequent analysis. In 10.7% (3 of 28) of men, the sVL exceeded the paired bVL, meeting predefined criteria for disproportionately high HIV-1 semen shedding. At baseline, there was a weak correlation between blood and semen VL (r = 0.26; P = .160). Viral load data are summarized in Table 1.
Semen Cytokines and the Semen Viral Load
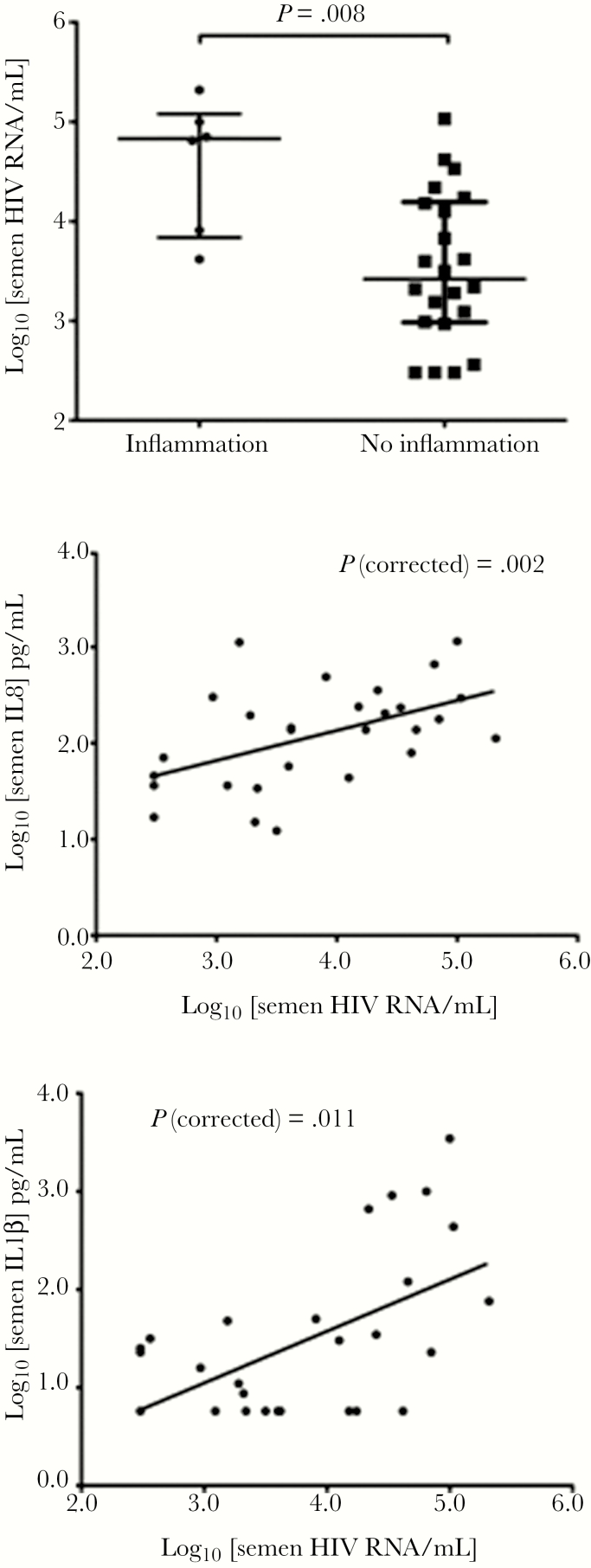
Levels of 14 cytokine/chemokines (see Methods) and of Elafin/Trappin-2 were assayed in SP (Table 2). Semen inflammation, defined as ≥4 of 9 proinflammatory cytokines in the top quartile for that cytokine (see above), was present in 6 of 28 (21%) participants, and it was strongly associated with HIV semen shedding (median sVL 4.89 log10 HIV RNA copies/mL for men with SI vs 3.42 without; MW P = .008) (Figure 1). Furthermore, an inflammation score that was calculated as the number of these cytokines in the top quartile (range, 0–9) was also strongly correlated with the sVL (Spearman correlation coefficient P = .65; P < .001). Subsequent analysis demonstrated that the sVL was positively associated with semen levels of IL-1α (P = .009), IL-8 (P = .033), IL-1β (P = .005), IL-6 (P = .037), and MIP-1β (P = .031). To determine the strongest cytokine correlates of sVL, a partial correlation analysis controlling for bVL was performed. After controlling for bVL, semen levels of IL-8 (P = .002), IL-1α (P = .024), IL-1β (P = .011), IL-6 (P = .013), and MIP-1β (P = .006) remained significantly correlated with the sVL. The independent association(s) of these individual cytokines with the sVL was then assessed in a stepwise multivariate linear regression model, with IL-8 (P < .001) emerging as the strongest independent cytokine correlate of the sVL, followed by IL-1β (P = .03) (Figure 1). Trappin-2/Elafin was readily detectable in SP, but levels did not correlate with either the sVL or semen cytokine/chemokine levels.
Table 2.
Seminal Plasma Cytokine and Chemokine Levels
| Cytokine/ Protein | Median (pg/mL) | Percent Detectable (%) | Range (pg/mL) |
|---|---|---|---|
| IL-1α | 139 | 100% | 12–1135 |
| IL-8 | 1451 | 100% | 176–180408 |
| MCP-1 | 3378 | 100% | 289–79593 |
| MDC | 26034 | 100% | 2298–127178 |
| MIG | 33511 | 100% | 2939–114179 |
| MIP-3α | 1167 | 93% | 30–3769 |
| RANTES | 373 | 100% | 25–1935 |
| IL-10 | 49 | 82% | 15–188 |
| IL-17 | 37 | 82% | 15–190 |
| IL-1β | 47 | 64% | 24–592 |
| IL-6 | 119 | 100% | 14–3866 |
| IP-10 | 35768 | 100% | 4019–222383 |
| MIP-1β | 251 | 68% | 90–7216 |
| TNF-α | 8.9 | 82% | 1.8–96 |
| Trappin-2 | 1871 | 100% | 839–7823 |
Abbreviations: IL, interleukin; IP, interferon-gamma-induced protein; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIG, monokine induced by interferon-γ; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cells expressed and secreted; TNF, tumor necrosis factor. Bolded percentage was to show cytokine/chemokine levels that were not detected in 100% of participants.
Figure 1.
Association of semen human immunodeficiency virus (HIV) viral load with local inflammation and proinflammatory cytokines. Levels of semen HIV RNA in antiretroviral therapy-naive men were strongly associated with both the presence/absence of semen inflammation (top panel), based on modification of a previously defined mucosal algorithm, and with levels of individual proinflammatory cytokines (bottom 2 panels; interleukin [IL]-8 and IL-1β shown).
The Semen Viral Load and Local Herpesvirus Reactivation
Herpesvirus qPCR was performed on all semen samples; 63% (19 of 30) passed internal control standards for CMV and 67% (20 of 30) for EBV. Internal control/run failure for the remaining samples may be due to the presence of PCR inhibitors, a common issue with qPCR assays in SP [24]. Cytomegalovirus was detected in the SP of 11 of 19 (58%), and EBV was detected in 6 of 20 (30%) participants; median CMV and EBV VL were 486 copies CMV DNA/mL (range, 200–266761) and 200 copies EBV DNA/mL (range, 200–409157), respectively. Semen HSV-1, HSV-2, HHV-3, HHV-7, and HHV-8 were undetectable in all patients, and HHV6 was only detected at very low levels in the semen of 2 participants. Therefore, subsequent analysis was focused on CMV and EBV.
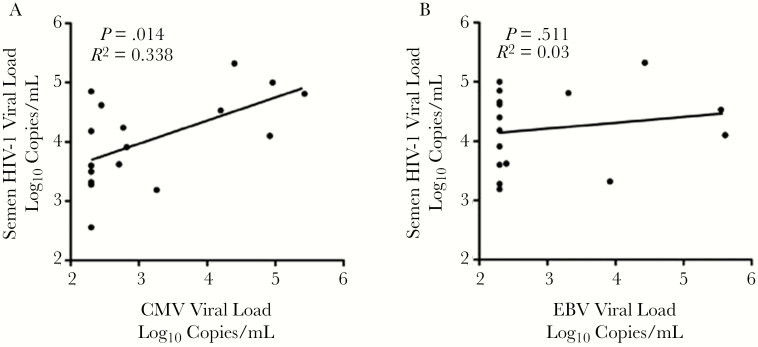
Semen levels of CMV but not EBV correlated with the HIV sVL (P = .014 and P = .511, respectively (Figure 2). Semen CMV levels tended to be higher in men with SI (median 4.40 vs 2.38 log10 DNA copies/mL; MW P = .063) and were directly correlated with the inflammation score (Spearman’s rho = 0.64; P = .006), whereas EBV levels were not related with either the presence or degree of inflammation. In particular, CMV levels correlated with semen cytokines IL-10 (0.048), IL-1β (<0.001), IL-6 (0.009), MIP-1β (0.004), and TNF-α (0.006). The independent association of these cytokines with semen CMV VL was assessed in a stepwise multivariate linear regression model with semen IL-1β level (P < .001) correlating most strongly with CMV VL.
Figure 2.
Reactivation of semen herepesviruses and the semen HIV viral load. Semen HIV RNA levels in ARV-Naive men were associated with levels of (A) semen CMV DNA (left) but not (B) semen EBV DNA (right).
Semen T-Cell Immune Parameters and the Semen Viral Load
Semen mononuclear cells (SMCs) were analyzed by flow cytometry in the subset of men (13 of 30; 43.3%) who consented to be observed longitudinally, to evaluate the prospective associations of T-cell phenotype and activation status with sVL. Ninety-three semen samples (approximately 7 samples per participant) were collected, and for practical reasons flow cytometry was performed on 76 of these samples (82%): adequate semen T-cell numbers (above our cutoff of ≥50 cell events) were present for CD4+ T-cell analysis in 69 of 76 (91%) and for CD8+ T-cell analysis in 73 of 76 (96%). Semen T cells were immunologically much more activated than those in the blood of the same participant. In particular, coexpression of CD38 and HLA-DR was higher on both semen CD4+ T cells (median, 16.25% vs 0.89%; P < .001) and CD8+ T cells (median, 13.75% vs 3.91%; P = .039), as was the early activation/TRM marker CD69 on both CD4+ (32.80% vs 3.14%; P < .001) and CD8+ (57.0 vs 5.17; P < .001) T cells. Semen CD4+ T cells also expressed higher levels of the HIV coreceptor CCR5 (24.15% vs 4.3%; P < .001) and of the Th17 marker CCR6 (28.8 vs 4.3; P = .003), whereas expression of β7, a proxy for the mucosal homing integrin α4β7+, was lower on semen CD4+ T cells (0.9% vs 2.1%; P = .013). The proportion of CD3+ T cells expressing the gamma-delta receptor (CD3G) tended to be higher in peripheral blood mononuclear cells than SMCs (2.84 vs 0.94; P = .180), although semen CD3G+ T cells expressed higher levels of CCR6 (83.7 vs 0.43; P < .001).
Overall, CD4+ and CD8+ T-cell numbers in the semen were strongly correlated with a number of immune and virologic semen parameters, including the CMV VL (Spearman rho = 0.78 and 0.89, both P < .01) and the SI score (Spearman rho = 0.77 and 0.85, both P < .001), and less strongly with the semen HIV VL (Spearman rho = 0.48 and 0.64, P = .11 and P = .02, respectively). No T-cell associations were seen with semen levels of EBV or with the semen bacterial load (see below).
Bacterial Load in Semen and the Semen Viral Load
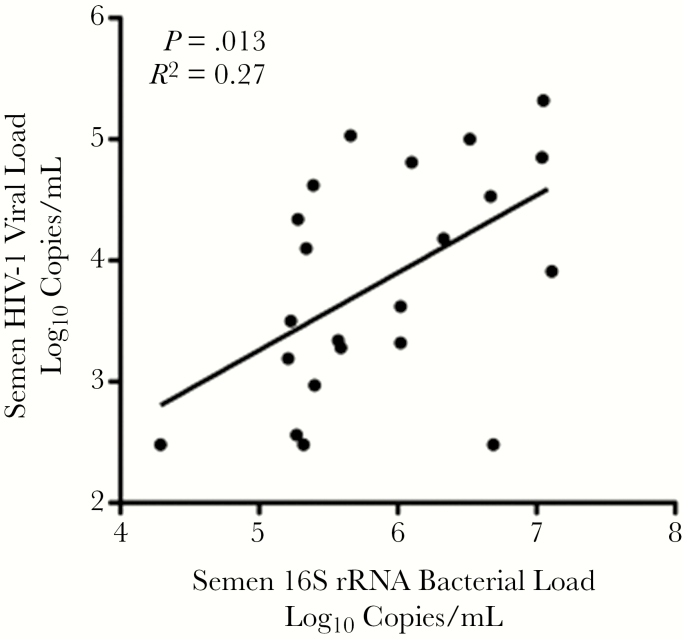
To broadly assess whether the endogenous microbiome of the semen compartment [25] impacted semen immunology and sVL, total bacterial load was quantified by 16S rRNA. In men free of bacterial STIs, the total bacterial load was positively correlated with both the HIV sVL (Spearman rho = 0.51; P = .016) (Figure 3) and the SI score (Spearman rho = 0.48; P = .024), and it was higher in participants meeting criteria for SI (median log10 bacterial load 7.03 vs 5.40; P = .003). Furthermore, the bacterial load was significantly correlated with semen levels of the individual cytokines IL-8 (P = .004), MCP-1 (P = .022), MDC (P = .017), MIP-3α (P = .008), IL-6 (P = .012), IP-10 (P = .007), and TNF-α (0.046). However, no association of the bacterial load was observed with baseline clinical parameters or the local reactivation of CMV or EBV.
Figure 3.
Association of 16s rRNA bacterial load with semen HIV-1 RNA levels. The total concentration of bacterial 16s rRNA, a crude marker of the semen microbiome, was positively correlated with semen HIV RNA levels in antiretroviral therapy-naive men.
Longitudinal Cohort Demographics
A subgroup of participants (n = 13) provided monthly, paired semen and blood samples for up to 12 months, or until initiation of ART, to permit assessment of sVL and semen immunology changes within an individual. Herpes simplex virus-1 and HSV-2 seroprevalence was 92% (12 of 13) and 46% (6 of 13), respectively, and the median duration of follow-up was 7 months (range, 2–12 months) with a total of 93 sample visits (an average of just over 7 visits per participant).
Patterns and Correlates of Human Immunodeficiency Virus Shedding in Semen
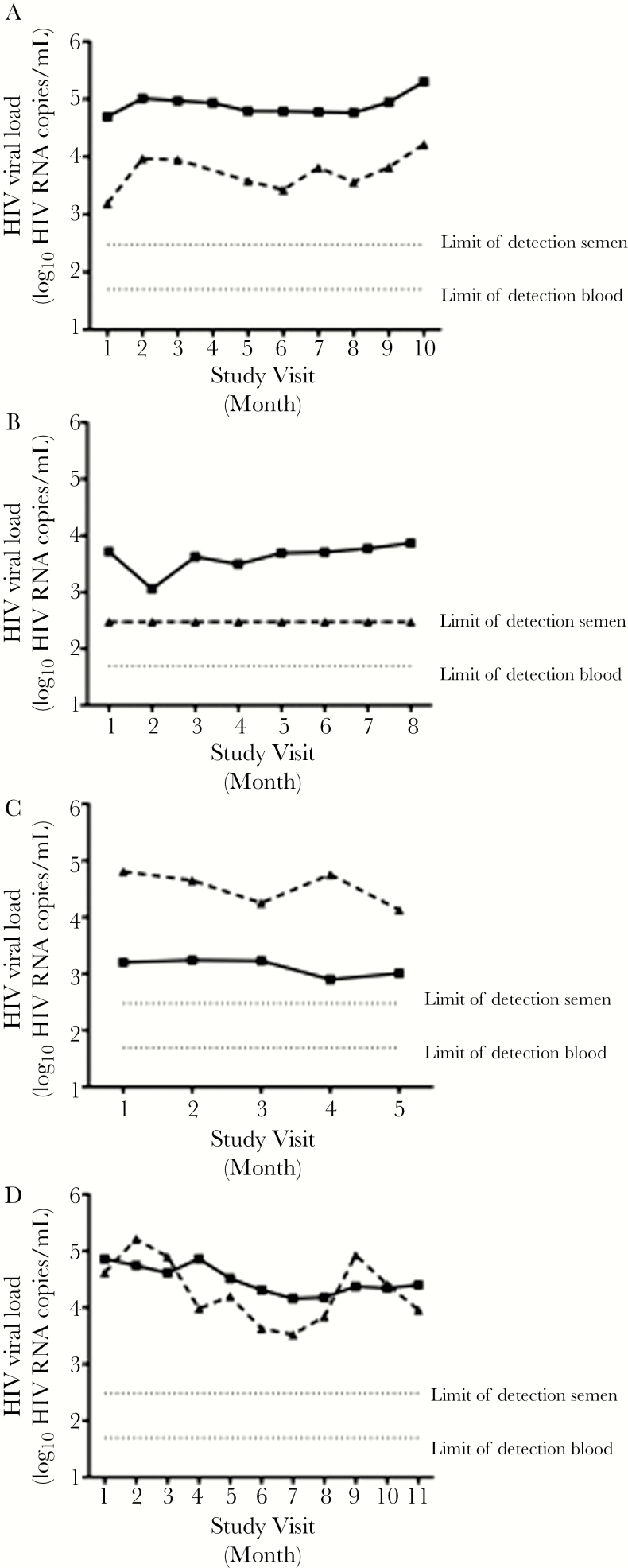
High-level semen shedding (>5000 copies/mL) had been detected at enrollment in 45.5% (5 of 11) of the STI-free prospective participants: during follow up, high-level shedding was detected at 1 study visit or more in 69% (9 of 13) and overall at 51.6% (48 of 93) of study visits. The median sVL was 0.95 log10 (0.79-fold) lower than the bVL (median bVL 4.45 log10 copies/mL, range 2.92–5.18 log10 copies/mL vs median sVL 3.50 log10 copies/mL, range 2.48–5.00 log10 copies/mL), but there was substantial inter-individual variability. In approximately half of the participants, the sVL was consistently lower than the blood (6 of 13 participants; representative example) (Figure 4A). Although only 23% (3 of 13) of study men had an undetectable sVL at any time point, 1 individual maintained an undetectable sVL at each of 9 study visits despite a median bVL of 3.69 log10 copies/mL (range, 3.06–3.87 log10 copies/mL) (Figure 4B). One participant (1 of 13) consistently demonstrated a disproportionately high sVL (Figure 4C), whereas in the remaining 6 of 13 participants, the sVL was sometimes lower than blood and sometimes disproportionately high (Figure 4D). In addition, the variability in semen versus blood HIV-1 RNA levels was assessed by calculating the ratio and variance of log10 semen/log10 blood RNA levels (Table 3). Although the median semen/blood VL ratio was 0.87, it exceeded 1 in 2 participants (15%), and there was considerable heterogeneity in the variance of this ratio, ranging from 0.002 to 0.149.
Figure 4.
Longitudinal patterns of HIV RNA semen shedding over time in antiretroviral therapy-naive men. Examples of semen HIV RNA semen shedding patterns over time: (a) blood viral load (VL) consistently higher than semen; (b) persistently undetectable semen HIV RNA VL; (c) sustained disproportionate HIV semen shedding; (d) intermittent disproportionate HIV semen shedding. blood plasma; semen plasma.
Table 3.
Prospective Variability in the Semen/Blood HIV RNA Viral Load Ratio
| Patient ID | Log10 sVL/Log10 bVLa | No. of Visits | Variance | Minimum | Maximum |
|---|---|---|---|---|---|
| 001 | 0.903 | 8 | 0.005 | 0.80 | 1.02 |
| 002 | 1.373 | 7 | 0.017 | 1.25 | 1.64 |
| 003 | 1.130 | 9 | 0.149 | 0.87 | 2.19 |
| 004 | 0.772 | 9 | 0.002 | 0.68 | 0.80 |
| 005 | 0.797 | 5 | 0.010 | 0.66 | 0.90 |
| 006 | 0.943 | 10 | 0.011 | 0.73 | 1.03 |
| 007 | 0.970 | 2 | 0.046 | 0.82 | 1.12 |
| 008 | 0.940 | 10 | 0.012 | 0.82 | 1.13 |
| 009 | 0.671 | 7 | 0.054 | 0.53 | 1.16 |
| 010 | 0.671 | 9 | 0.002 | 0.64 | 0.81 |
| 011 | 0.887 | 2 | 0.002 | 0.85 | 0.92 |
| 012 | 0.824 | 7 | 0.033 | 0.69 | 1.20 |
| 013 | 0.873 | 8 | 0.013 | 0.62 | 0.97 |
| All visits | 0.873 | 93 | 0.062 | 0.53 | 2.19 |
Abbreviations: bVL, blood viral load; HIV, human immunodeficiency virus; ID, identification; RNA, ribonucleic acid; sVL, semen viral load.
aMedian values reported
Longitudinal Correlates of Human Immunodeficiency Virus Shedding in Semen
Semen parameters that had been associated with the sVL in the cross-sectional analysis also varied over time. Cytomegalovirus reactivation was more common than EBV in semen, with detection in 9 of 13 vs 4 of 13 participants at any time point, and at 29.0% (27 of 93) vs 7.5% (7 of 93) of total study visits, respectively. The median semen CMV VL was 241 copies/mL (range, 200–266761 copies/mL) and exceeded 5000 copies/mL during 29.6% (8 of 27) reactivations; the median semen EBV VL was also 200 copies/mL (range, 200–353125 copies/mL) and exceeded 5000 copies/mL during 28.6% (2 of 7) reactivations. Semen flow cytometry assays were performed for 76 of 93 samples collected, and sufficient (>50 cells per sample) semen CD4+ T cells for analysis were present in 69 of 76 (90.1%) samples and CD8+ T cells in 73 of 76 (96.1%) samples. In general, the semen bacterial load, semen cytokine levels, and T-cell parameters were relatively stable over time (data not shown).
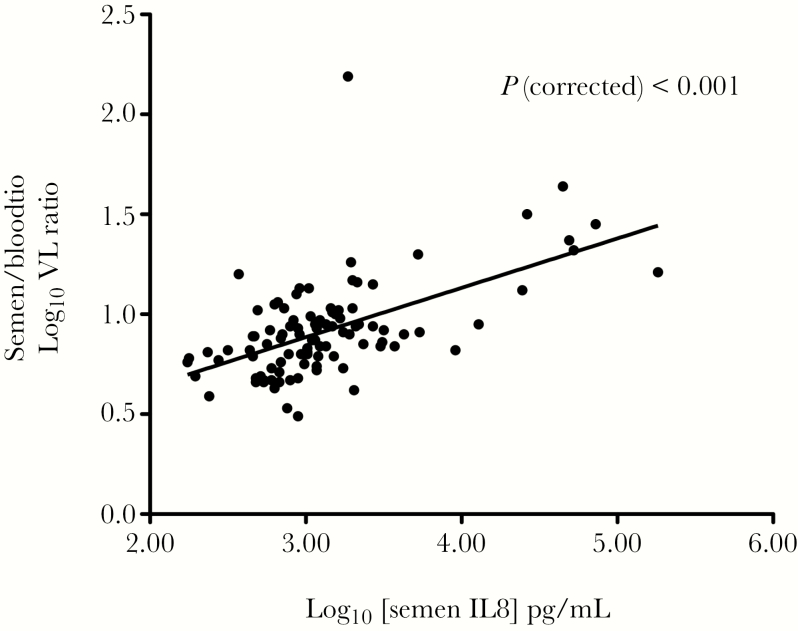
Finally, a multivariable MM that incorporated the longitudinal viral, immune, and bacterial load data was then used to assess independent associations of the HIV sVL (see Methods). In this prospective analysis, the only independent associations of HIV sVL were the bVL (P < .001) and semen levels of the proinflammatory chemokine IL-8 (P < .001). For every log10 unit increase in bVL, the sVL increased by 0.40 log10, and for every log10 increase in the IL-8, the sVL increased by 0.75 log10. In this prospective analysis, the semen to blood HIV VL ratio, which was calculated to assess the contribution of individual semen parameters to the local HIV VL, was most strongly associated with semen IL-8 levels (P < .001) (Figure 5).
Figure 5.
Association of semen interleukin (IL)-8 level with the relative abundance of HIV RNA in semen versus blood. Semen levels of the proinflammatory cytokine IL-8 were strongly correlated with relative increases in semen versus blood HIV RNA in antiretroviral therapy-naive men (random intercept linear mixed model).
DISCUSSION
The semen of an HIV-infected, ART-naive man is the vector for most global HIV transmission, with the HIV RNA VL in both blood and semen being independent predictors of HIV transmission risk [2]. Although the semen HIV RNA VL is generally lower than that in blood, virus levels in the semen compartment are more variable [26–28], and a subset of men manifest disproportionately high levels of HIV in semen [8, 10, 29] with an increased sVL linked to STIs, herpesvirus reactivation, and local immune activation [4–6, 30]. Prior studies have often been cross-sectional and focused on individual semen parameters, and it is not clear whether patterns of differential HIV shedding are stable in an individual over time. Therefore, the current study prospectively assessed the association of multiple semen parameters with the sVL in ART-naive men, to define independent associations. Factors associated with the HIV RNA sVL in univariate analysis included the blood VL, semen CMV reactivation, levels of proinflammatory cytokines and chemokines, the total bacterial load, and semen T-cell numbers. However, there was substantial inter-individual and intra-individual variability in the patterns of HIV semen shedding over time, and the only independent associations of semen HIV RNA levels were the bVL and semen levels of the proinflammatory chemokine IL-8, suggesting that the latter may constitute an inflammatory biomarker that links heterogeneous local causes of increased HIV RNA shedding in semen.
Interleukin-8 is a proinflammatory cytokine produced by a variety of cells, particularly macrophages/monocytes [31]. Macrophages constitute 20%–30% of white blood cells in semen, and they are almost 10-fold more common than T-lymphocytes (2%–5%) [32]. Not only are increased semen levels of IL-8 seen in the context of HIV infection, but IL-8 also increases HIV replication in cervical explant tissue models, and competitive inhibition of the IL-8 receptor CXCR2 decreases explant HIV susceptibility by 45%–70% [33]. Although our study demonstrates that IL-8 is an important local correlate of semen HIV levels, it is not known whether the relationship is causal or whether IL-8 serves as a biomarker for an additional, unmeasured factor associated with SI. Future studies should confirm the cellular origin of IL-8 and assess the ability of therapeutic strategies targeting IL-8 to reduce semen HIV levels.
Although several of the “upstream” mucosal parameters associated with the sVL had been previously described, others were novel. Local reactivation of CMV has been previously associated with the sVL [9, 30], and increased semen cytokines have been associated with semen levels of both CMV and HIV [8, 34, 35]. The absolute number of HIV-specific T cells in semen has also been associated with the sVL [8], although this was not seen in studies where a substantial proportion of men were on ART [34]. However, the novel finding of an association between the total semen bacterial load and the sVL in the absence of classic STIs strongly suggests that the semen microbiome plays a role in HIV transmission, an area for future research. The fact that local CMV reactivation, the semen bacterial load, and semen T-cell numbers were all associated with semen proinflammatory cytokines, and that semen IL-8 levels were the only local (semen) parameter independently associated with the sVL on multivariate analysis, suggests that IL-8 might serve as a final common pathway for an increased sVL.
There was considerable inter-individual heterogeneity in patterns of semen HIV shedding. The sVL has generally been observed to be significantly lower than the bVL [36, 37], and, on average, that was also the case in our study. However, we found that less than half of all participants consistently had an sVL lower than the bVL, whereas a slight majority (>53%) either consistently or intermittently demonstrated a disproportionately high sVL. This variability has implications for predicting HIV transmission at an individual level, and it stresses the importance of prospective studies. However, it is important to note that the early initiation of ART is now the norm in developed countries [38], both for the health benefits provided to the HIV-infected person and to reduce HIV transmission. The impact of effective ART on the sVL will supercede the parameters that we studied, because ART rapidly reduces the sVL to undetectable levels in most men [19, 27, 39–41]. However, because many people infected by HIV globally are unable to access therapy, it is still important to study HIV semen shedding in ART-naive men.
The sample size was limited in the current study, particularly for the prospective analysis of semen shedding, in large part because ART is now recommended for all HIV-infected men. Despite this, our prospective analysis of the relationship between the sVL and multiple semen parameters provides new insights of public health importance. In particular, the prospective cohort component clearly confirmed the bVL to be a strong predictor of semen HIV levels, as has been previously described, while adding semen IL-8 levels as a strong, independent mucosal (compartmentalized) predictor. Semen VLs were calculated based on an assumed volume of 2 mL, due to relatively frequent spillage of collection medium during sample collection and transport. Because there is inter-individual heterogeneity in semen volume, this will have led to some imprecision in our calculation of sVL and semen cytokine concentrations, but in prior studies this assumption did not affect the outcome of analyses based on these parameters [12, 20]. Mixed models and generalized estimating equations (GEE) are both statistical approaches to deal with correlated observations; GEE deals with marginal (ie, population level) models, and MM deals with conditional models. These approaches may provide slightly different results in nonlinear models, but for linear models (such as were used in this analysis), the 2 methods/models are practically identical for the random intercept models, and MM was used in this case to enable multilevel, random slope models.
CONCLUSIONS
In summary, our study confirms considerable variability in the sVL among HIV-infected, ART-naive men, and patterns of shedding vary considerably over time. Fluctuation in the sVL is not only dependent on the bVL, but it is also associated with multiple compartmentalized factors in semen, including T-cell influx, herpesvirus reactivation, and the microbiome, with effects on the sVL mediated through increased local levels of proinflammatory cytokines, particularly IL-8.
Acknowledgments
Financial support. This work was funded by grants from the Canadian Institutes of Health Research ([CIHR] MOP-115020; to R. K.). B. J. W. O. and A. K. M. received studentship support from the CIHR; R. K. and K. S. M. received salary support from the Ontario HIV Treatment Network.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 2. Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis 2008; 35:55–60. [DOI] [PubMed] [Google Scholar]
- 4. Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997; 349:1868–73. [DOI] [PubMed] [Google Scholar]
- 5. Gianella S, Anderson CM, Vargas MV, et al. Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 2013; 207:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gianella S, Mehta SR, Strain MC, et al. Impact of seminal cytomegalovirus replication on HIV-1 dynamics between blood and semen. J Med Virol 2012; 84:1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lisco A, Munawwar A, Introini A, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012; 205:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheth PM, Danesh A, Sheung A, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis 2006; 193:45–8. [DOI] [PubMed] [Google Scholar]
- 9. Shin LY, Sheth PM, Persad D, et al. Impact of CMV therapy with valganciclovir on immune activation and the HIV viral load in semen and blood: an observational clinical study. J Acquir Immune Defic Syndr 2014; 65:251–8. [DOI] [PubMed] [Google Scholar]
- 10. Kalichman SC, Cage M, Barnett T, et al. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS Res Hum Retroviruses 2001; 17:1695–703. [DOI] [PubMed] [Google Scholar]
- 11. Korhonen C, Srinivasan S, Huang D, et al. Semen bacterial concentrations and HIV-1 RNA shedding among HIV-1-seropositive Kenyan men. J Acquir Immune Defic Syndr 2017; 74:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu CM, Osborne BJ, Hungate BA, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog 2014; 10:e1004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berlier W, Bourlet T, Lévy R, et al. Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol 2006; 36:204–7. [DOI] [PubMed] [Google Scholar]
- 14. Hoffman JC, Anton PA, Baldwin GC, et al. Seminal plasma HIV-1 RNA concentration is strongly associated with altered levels of seminal plasma interferon-γ, interleukin-17, and interleukin-5. AIDS Res Hum Retroviruses 2014; 30:1082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheth PM, Danesh A, Shahabi K, et al. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol 2005; 175:4789–96. [DOI] [PubMed] [Google Scholar]
- 16. Bujan L, Daudin M, Matsuda T, et al. Factors of intermittent HIV-1 excretion in semen and efficiency of sperm processing in obtaining spermatozoa without HIV-1 genomes. AIDS 2004; 18:757–66. [DOI] [PubMed] [Google Scholar]
- 17. Anderson DJ, O’Brien TR, Politch JA, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA 1992; 267:2769–74. [PubMed] [Google Scholar]
- 18. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osborne BJ, Sheth PM, Yi TJ, et al. Impact of antiretroviral therapy duration and intensification on isolated shedding of HIV-1 RNA in semen. J Infect Dis 2013; 207:1226–34. [DOI] [PubMed] [Google Scholar]
- 20. Osborne BJ, Sheth PM, Kovacs C, et al. Impact of collection method on assessment of semen HIV RNA viral load. PLoS One 2011; 6:e23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scott LE, Noble LD, Moloi J, et al. Evaluation of the Abbott m2000 RealTime human immunodeficiency virus type 1 (HIV-1) assay for HIV load monitoring in South Africa compared to the Roche Cobas AmpliPrep-Cobas Amplicor, Roche Cobas AmpliPrep-Cobas TaqMan HIV-1, and BioMerieux NucliSENS EasyQ HIV-1 assays. J Clin Microbiol 2009; 47:2209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 23. Liu CM, Aziz M, Kachur S, et al. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol 2012; 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Semprini AE, Persico T, Thiers V, et al. Absence of hepatitis C virus and detection of hepatitis G virus/GB virus C RNA sequences in the semen of infected men. J Infect Dis 1998; 177:848–54. [DOI] [PubMed] [Google Scholar]
- 25. Kiessling AA, Desmarais BM, Yin HZ, et al. Detection and identification of bacterial DNA in semen. Fertil Steril 2008; 90:1744–56. [DOI] [PubMed] [Google Scholar]
- 26. Coombs RW, Speck CE, Hughes JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis 1998; 177:320–30. [DOI] [PubMed] [Google Scholar]
- 27. Gupta P, Leroux C, Patterson BK, et al. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral Quasi species between blood and semen. J Infect Dis 2000; 182:79–87. [DOI] [PubMed] [Google Scholar]
- 28. Krieger JN, Coombs RW, Collier AC, et al. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J Urol 1995; 154:1035–40. [PubMed] [Google Scholar]
- 29. Kalichman SC, Rompa D, Cage M, et al. Sexual transmission risk perceptions and behavioural correlates of HIV concentrations in semen. AIDS Care 2002; 14:343–9. [DOI] [PubMed] [Google Scholar]
- 30. Sadiq ST, Taylor S, Copas AJ, et al. The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect 2005; 81:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsumoto T, Miike T, Nelson RP, et al. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol 1993; 93:149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolff H. The biologic significance of white blood cells in semen. Fertil Steril 1995; 63:1143–57. [DOI] [PubMed] [Google Scholar]
- 33. Narimatsu R, Wolday D, Patterson BK. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res Hum Retroviruses 2005; 21:228–33. [DOI] [PubMed] [Google Scholar]
- 34. Gianella S, Morris SR, Vargas MV, et al. Role of seminal shedding of herpesviruses in HIV Type 1 transmission. J Infect Dis 2013; 207:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olivier AJ, Masson L, Ronacher K, et al. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis 2014; 209:1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Celum CL, Buchbinder SP, Donnell D, et al. Early human immunodeficiency virus (HIV) infection in the HIV network for prevention trials vaccine preparedness cohort: risk behaviors, symptoms, and early plasma and genital tract virus load. J Infect Dis 2001; 183:23–35. [DOI] [PubMed] [Google Scholar]
- 37. Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS 2001; 15:837–45. [DOI] [PubMed] [Google Scholar]
- 38. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed June 15, 2016.
- 39. Marcelin AG, Tubiana R, Lambert-Niclot S, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS 2008; 22:1677–9. [DOI] [PubMed] [Google Scholar]
- 40. Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med 1998; 339:1803–9. [DOI] [PubMed] [Google Scholar]
- 41. Barroso PF, Schechter M, Gupta P, et al. Effect of antiretroviral therapy on HIV shedding in semen. Ann Intern Med 2000; 133:280–4. [DOI] [PubMed] [Google Scholar]