Summary
Matrix vesicle-mediated mineralization is an orchestrated sequence of ultrastructural and biochemical events that lead to crystal nucleation and growth. The influx of phosphate ions into the matrix vesicle is mediated by several proteins such as TNAP, ENPP1, Pit1, annexin and so forth. The catalytic activity of ENPP1 generates pyrophosphate (PPi) using extracellular ATPs as a substrate, and the resultant PPi prevents crystal overgrowth. However, TNAP hydrolyzes PPi into phosphate ion monomers, which are then transported into the matrix vesicle through Pit1. Accumulation of Ca2+ and PO43− inside matrix vesicles then induces crystalline nucleation, with calcium phosphate crystals budding off radially, puncturing the matrix vesicle’s membrane and finally growing out of it to form mineralized nodules.
Keywords: Matrix vesicle, Mineralization, TNAP, ENPP1, Mineralized nodule
1. Introduction
Bone is a living mineralized tissue composed of calcium phosphates and a variety of organic materials, with collagen being the most abundant. Bone mineralization has two phases: primary and secondary. Primary mineralization is orchestrated by osteoblasts; osteoblasts secret a large amount of collagen fibrils, non-collagenous proteins, and matrix vesicles, which are extracellular vesicles that trigger mineralization via membrane transporters and enzymes. The degree of the primary mineralization is controlled by fine-tuning bone formation and mineral apposition rates. In contrast, secondary mineralization is a phenomenon whereby there is a gain in bone mineral density after primary mineralization. It is hypothesized that secondary mineralization is regulated physicochemically, i.e., through crystal maturation, and by the osteocytic network inside the mineralized bone matrix. Still, the mechanisms behind secondary mineralization are relatively untapped.
In bone, primary mineralization can be divided into two phases: first, matrix vesicle-mediated mineralization, and second, collagen mineralization. During the process of the matrix vesicle-mediated mineralization, osteoblasts regulate vesicle synthesis as well as the activity of membrane transporters and enzymes with which matrix vesicles are equipped. Discovery of matrix vesicles was a breakthrough in the field of bone mineralization [1], [2], [3], [4], [5], [6], [7]. Long before that, it was proposed that alkaline phosphatase may supply phosphates by hydrolyzing phosphate substrates and then help forming crystalline calcium phosphates [8]. However, this theory is based on the physicochemical regulation of bone mineralization. In contrast, the theory behind matrix vesicle-mediated mineralization sustains that the processes are mainly under the control of osteoblasts through the regulation of membrane transporters/enzymes and surrounding extracellular organic materials. Knowledge of the ultrastructure and biological activities of membrane transporters/enzymes may clarify the cellular mechanisms of matrix vesicle-mediated mineralization and explain, for instance, why mineral appositional rate is higher in regions of accelerated bone turnover.
In this review, we will present the ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization in bone.
2. Ultrastructural aspects of matrix vesicle-mediated mineralization in bone
Matrix vesicles are extracellular vesicles enveloped by a plasma membrane (ranging 30–1000 nm in diameter) secreted by osteoblasts [2] (Fig. 1). Matrix vesicles bear several membrane transporters and enzymes on their membranes and in their interior, providing a nurturing microenvironment for calcium phosphate nucleation and subsequent crystal growth. Mineralization begins when a crystalline calcium phosphate, i.e., hydroxyapatite [Ca10(PO4)6(OH)2], appears inside the matrix vesicles, growing and eventually breaking through the vesicle’s membrane to form mineralized nodules—also known as calcifying globules (Figs. 1 & 2). Under transmission electron microscopy (TEM), each hydroxyapatite crystal has a small, ribbon-like structure profile approximately 25 nm wide, 10 nm high and 50 nm long [9], [10].
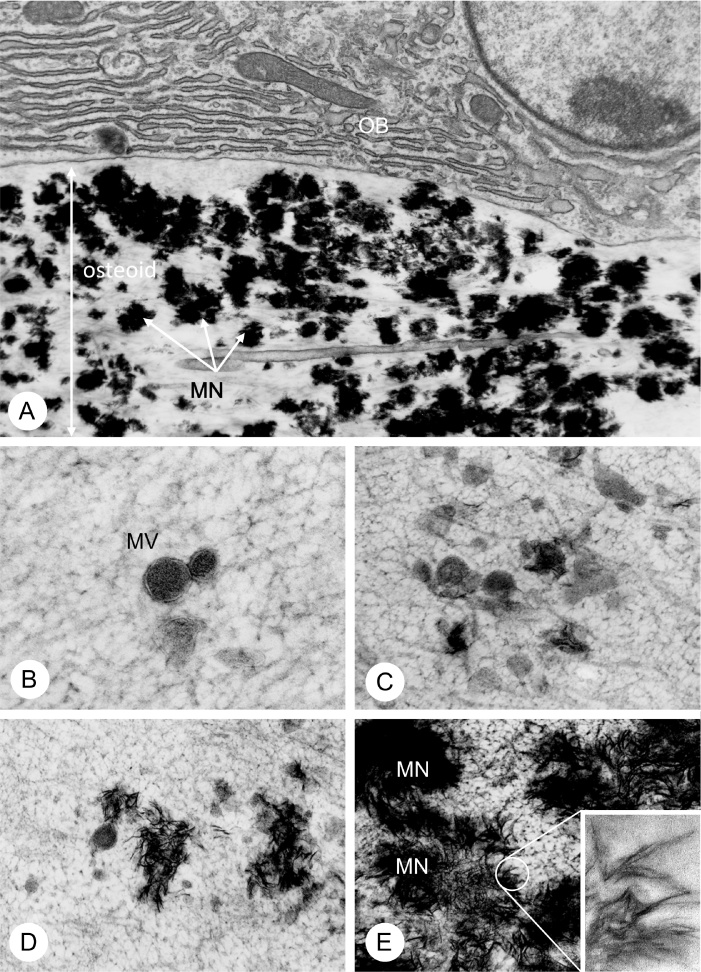
Figure 1.
TEM observation of matrix vesicles and mineralized nodules in osteoid.
(A) TEM image of ultratrsucture of osteoid underlying mature osteoblasts (OB). Notice many mineralized globular structures referred to as mineralized nodule (MN) in the osteoid. (B–E) TEM images of matrix vesicles (MV) (B), appearance of mineral crystals in the matrix vesicles (C), exposure of mineral crystals out of the matrix vesicles (D) and mineralized nodule (MN) formation (E). An inset demonstrate a highly-magnified image of needle-shape of mineral crystals.
Panel A is derived from Ref. [71] (Amizuka and Ozawa), while panels B–F are modified from Ref. [72] (Amizuka et al.).
2.1. Ultrastructural properties of the matrix vesicles under electron microscopy
Some of these incipient mineral crystals were initially found associated with the inner leaflet of the matrix vesicle membranes, and it seems plausible that crystal nucleation would begin at that specific site. Matrix vesicle membranes are rich in acidic phospholipids such as phosphatidylserine and phosphatidylinositol, which have high affinity for Ca2+. The affinity of phosphatidylserine for Ca2+ is particularly high and can produce a stable calcium phosphate–phospholipid complex associated with the inner leaflet of the vesicle’s membrane [7] (Fig. 2A). The possibility that such complexes may play an important role in crystal nucleation has been pointed out before [11], [12]. However, the early phases of calcium phosphate nucleation inside the matrix vesicles may be rather amorphous [13], but gradually become crystalline (hydroxyapatite) in later stages. Calcium phosphate crystals formed inside matrix vesicles can grow through the addition of Ca2+ and PO43−, which enter the vesicles through the action of membrane transporters and enzymes. Inside the matrix vesicle, “needle-shaped” crystalline calcium phosphates form a stellate assembly, bud off radially from the vesicle’s interior, and then, rip through the plasma membrane to form mineralized nodules, also referred to as calcifying globules [6] (Figs. 1E and 2D). While the needle-shaped crystals are identified as crystalline structures by electron diffraction, freeze-substitution and cytochemical calcium detection methods such as κ-pyroantimonate combined with energy-dispersive X-ray spectroscopy may show them as non-crystalline structures containing calcium and phosphate [14], [15], [16].
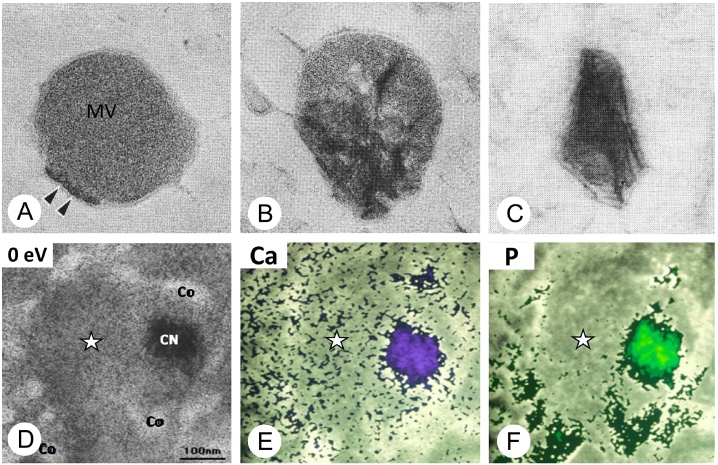
Figure 2.
Highly-magnified images of matrix vesicles and elemental mapping of calcium and phosphate ions.
(A–C) TEM images of matrix vesicles (MV). (A) amorphous electron-dense structure (double arrows, black) are shown to be associated with plasma membrane of the matrix vesicle. (B) The grown mineral crystals (black structures) are seen inside the matrix vesicles. (C) Mineral crystals are getting out of the matrix vesicles. (D–F) TEM image (D) and elemental mapping of calcium (Ca, panel E) and phosphorus (P, panel F) assessed by electron energy loss spectroscopy. Note that calcium (Ca) was evenly distributed in the peripheral region of matrix vesicles, while phosphorus (P) is predominant in collagen fibrils.
The images of A–C are modified from Ref. [73] (Ozawa et al.), and D–F are from Ref. [17] (Hoshi et al.).
According to observations of the osteoid in bone derived from the quick frozen-freeze substitution technique with electron energy loss spectroscopy, which enables elemental mapping at the molecular level, calcium was evenly distributed in the proteoglycan-rich, peripheral region of matrix vesicles, whereas phosphate was detected predominantly in collagen fibrils [17] (Fig. 2D–F). Therefore, it seems feasible that, in non-calcified sites, the extracellular meshwork of organic substances limits the production of hydroxyapatite and inhibits precipitation of calcified crystals by controlling the spatial distribution of Ca2+ and PO43−, even if the extracellular fluid is supersaturated with those ions. In addition, a biological mechanism of PO43− supplementation and subsequent transport into matrix vesicles must be in place, since PO43− is not abundant in the periphery of matrix vesicles. Later in the text, we will discuss about the biological actions of membrane transporters and enzymes to produce PO43− and to warrant the influx of Ca2+ and PO43− into the matrix vesicles.
2.2. Mineralized nodules developed from matrix vesicles
Matrix vesicles and developing mineralized nodules can be observed in the osteoid below mature osteoblasts (Fig. 1A). It seems that the osteoid provides an adequate microenvironment for the development of mineralized nodules in bone. Even though the vesicle’s membrane is ripped during the process of crystal growth, the mineralized nodules might retain the membrane-associated enzymes and transporters, which will be mentioned later in this text.
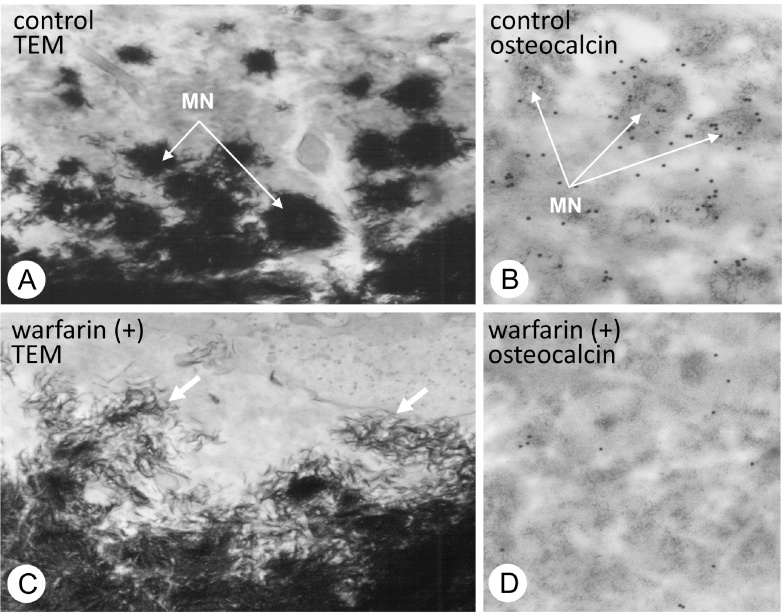
Mineralized nodule is a globular assembly of numerous needle-shaped mineral crystals that has been exposed to extracellular environment (Fig. 1E). It seems likely that the growth of mineralized nodules is regulated by a large number of extracellular organic materials in the osteoid. Among them, osteopontin is especially suited to the task of regulating mineralization, because it effectively inhibits apatite formation and growth [18], [19]. Osteopontin is localized in the periphery of mineralized nodules, where it might act as a blocker of excessive mineralization [20]. Since other organic materials can combine with osteopontin [21], they can form the so-called “crystal ghosts” [22], [23], [24]. Among these materials, osteocalcin is known for containing γ-carboxyglutamic acid and for their ability to bind to mineral crystals [25], [26], [27]. Warfarin, which is an inhibitor for γ-carboxylation of glutamine residues, induces an embryopathy consisting of nasal hypoplasia, stippled epiphyses and distal extremity hypoplasia when given to women in the first trimester of pregnancy [25], [28]. In our observations, the administration of warfarin resulted in the dispersion of numerous fragments of needle-shaped crystal minerals throughout the osteoid [29] (Fig. 3). Recently, γ-carboxylase-deficient mice revealed the same abnormality with disassembled, scattered crystal minerals in bone [30]. Therefore, osteocalcin may play an important role in the globular assembly of needle-shaped mineral crystals, probably binding the organic components of the crystal sheath together.
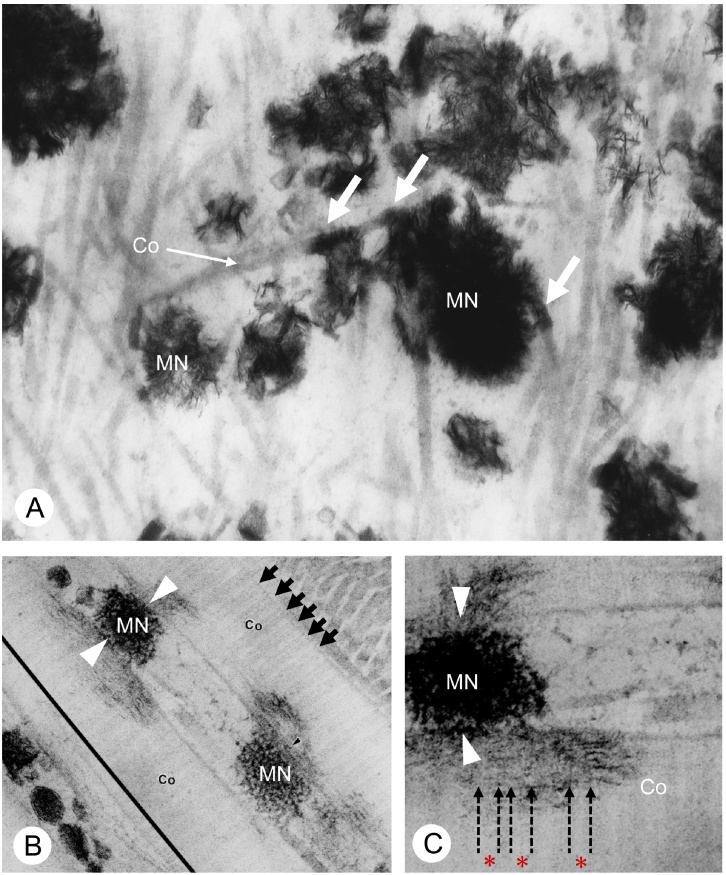
Figure 3.
TEM observation on mineralized nodules of normal rats and warfarin-treated rats.
Panels A and C demonstrate the ultrastructure of mineralized nodules in the osteoid of normal and warfarin-administered rat bones. In control group, globular mineralized nodules (MN) are shown to be composed of many needle-shaped mineral crystals (A). However, the warfarin-administered osteoid shows dispersed mineral crystals throughout the osteoid (C). (B and D) Immunoelectron microscopy for osteocalcin localization. Osteocalcin immunoreactivity (black particles) can be seen on the mineralized nodules (MN, grey colored globular structures) of the control osteoid (B), while little immunoreactivity for osteocalcin is seen in the warfarin-administered osteoid (D).
The images are derived from Ref. [29] (Amizuka et al.).
3. Enzymes and membrane transporter are necessary for matrix vesicle-mediated mineralization in bone
Several enzymes and proteins found in the matrix vesicles are involved in the metabolism of proteoglycans and pyrophosphate. Among these enzymes and membrane transporters, tissue nonspecific alkaline phosphatase (TNAP), ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), and others, e.g., annexin and ankylin protein (ANK) and sodium/phosphate co-transporter type III (Pit1) appear to a play pivotal role in matrix vesicle-mediated mineralization.
3.1. Biological function of tissue nonspecific alkaline phosphatase (TNAP) in matrix vesicle-mediated mineralization
One of the most important enzymes to initiate mineralization in bone is TNAP, a glycosylphosphatidylinositol anchor enzyme associated with cell membranes (Fig. 4). TNAP can hydrolyze various phosphate esters, especially pyrophosphate (PPi), and is responsible for the production of inorganic phosphate, i.e., TNAP serve as pyrophosphatase to generate PO43− monomer. The resultant PO43− is transported into the matrix vesicles by means of sodium/phosphate co-transporter type III (Pit1). Therefore, many believe TNAP is a potent inducer of mineralization.
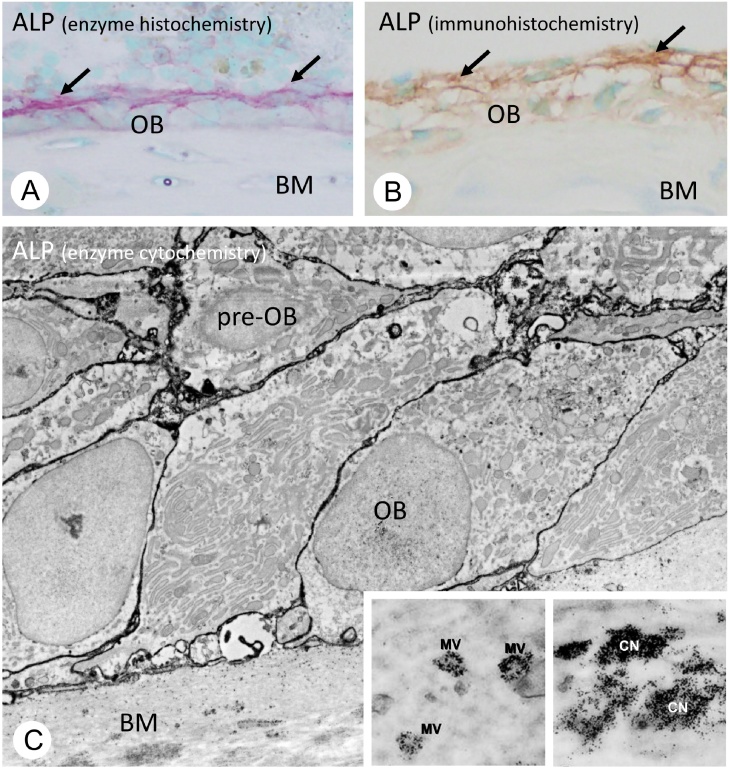
Figure 4.
Histochemical localization of alkaline phosphatase in bone.
(A and B) Enzyme histochemistry of alkaline phosphatase (ALPase, red color) and tissue nonspecific ALPase (TNAP, brown color) immunohistochemistry. Both histochemical technique consistently reveal an intense enzymatic activity of ALPase and immunoreactivity of TNAP in the regions of perosteoblasts (arrows, in A and B), rather than mature osteoblast (OB) located on bone matrix (BM). (C) TEM image of ALPase enzyme cytochemistry. Note the ALPase activity (black) can be seen on cell membranes of preosteoblasts (pre-OB) and mature osteoblasts (OB) on the bone surfaces. Insets demonstrate the ALPase enzyme activity on matrix vesicle (MV) and mineralized nodule referred to as calcified nodules (black, CN).
Panels A and B are derived from Ref. [72] (Amizuka et al.), while panel C is from Ref. [71] (Amizuka and Ozawa).
In bone, TNAP activity has been detected on osteoblasts and matrix vesicles [31], [32]. Interestingly, the distribution of TNAP on the cell membrane is not uniform in osteoblasts, which reflect the cell polarity with distinct basolateral and secretory (osteoidal) domains (Fig. 4). It has been reported that the plasma membrane Ca2+ transport ATPase was restricted to the osteoidal domain of the osteoblastic cell membrane, while TNAP was predominantly present on the basolateral domain [33]. Consistently, using specific antiserum to TNAP [34], we could observe relatively intense immunoreactivity and enzymatic activity for TNAP on preosteoblasts and on the basolateral aspect of mature osteoblasts’ membranes [35]. Thus, in bone, the membranes featuring intense activity of TNAP are not identical to those that serve as sites for matrix vesicle formation, for matrix vesicles derive from the secretory (osteoidal) cell membrane of osteoblasts—which show weak TNAP enzymatic activity. While the actual reason for that difference is still a matter of debate, it could be that high levels of TNAP activity in the matrix vesicles would induce mineral crystals overgrowth within the osteoid.
Mice homogyzous for Tnap gene depletion have been generated [36], [37] and mimic severe hypophosphatasia, indicating that TNAP is likely involved in mineralization. While TNAP might act as a pyrophosphatase [38], other pyrophosphatases may exist. Tnap−/− mice are born with intact bones, but gradually develop growth retardation and other skeletal deformities. Although the absence of endogenous TNAP activity did not result in complete lack of mineral uptake in skeletal tissues [37], Tnap−/− mice revealed a severe disturbance of the growth plate, suggesting abnormal endochondral ossification [39]. It was shown that depletion of Tnap gene results not only in hypomineralization of the skeleton, but also in a severe disorder of mineral crystal alignment in growing long bones with a disordered bone matrix architecture [40]. Murshed et al. have reported that transgenic mice expressing Tnap in the dermis showed extracellular mineralization consisting of hydroxyapatite crystals [41]. Given the evidence, it seems that TNAP activity is essential for mineralization in bone, but it is still unknown why the cell membranes with intense TNAP activity in bone are not identical to those forming matrix vesicles.
Recently, basic research on TNAP provided a promising tool for clinicians. The FDA approved asfotase alfa (Strensiq) in 2015 for the treatment of hypophosphatasia caused by a rare hereditary mutation in the alkaline phosphatase gene. Patients with either perinatal or infant onset of the disease who were treated with asfotase alfa showed improvement in overall survival: 97% patients receiving the drug were alive at age 1 year compared with 42% of control patients selected from a natural history study group. Patients with juvenile-onset hypophosphatasia also experienced improved growth and bone health compared with patients in a natural history database [42], [43].
3.2. Biological action of ecto-nucleotide pyrophosphatase/phosphodiesterase 1, ENPP 1 in bone mineralization
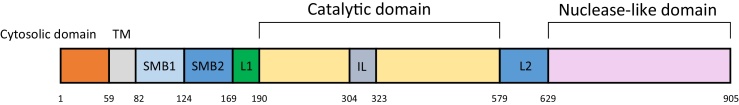
ENPP 1 is a member of the ENPP family of proteins, which are conserved in vertebrates and hydrolyze pyrophosphate or phosphodiester bonds in various extracellular compounds such as nucleotides and phospholipids. ENPP1 is composed of two N-terminal somatomedin B-like domains (SMB1 and SMB2), a catalytic domain and nuclease-like domain (Fig. 5). ENPP1 participates in different biological processes through distinct sets of domains: the catalytic and nuclease-like domains act in bone mineralization, while the SMB-like domains for insulin signaling. The crystalline structure analysis of ENPP1 suggests that the nucleotides are able to be accommodated in a pocket formed by an insertion loop in the catalytic domain, explaining why ATPs are ENPP1’s preferred substrate [44].
Figure 5.
Domain organization of mouse ENPP1.
Modified from Ref. [44] (Kato et al.).
In bone mineralization, ENPP1’s catalytic activity generates PPi – presumably using extracellular ATPs – and the resultant PPi inhibits mineralization by binding to incipient hydroxyapatite crystals and preventing their overgrowth [45], [46], [47] (Fig. 6). In our observations, ENPP1 immunoreactivity was seen throughout the cytoplasm and on the secretory (osteoidal) pole of mature osteoblasts, as well as in osteocytes (data not shown). The localization of ENPP1 predominantly on the osteoidal surface of osteoblasts may suggest that it inhibits the overgrowth of mineral crystals. The histological evidence that TNAP is not intensely localized on the osteoidal surface appears to be consistent with the distribution of ENPP1 on osteoblasts.
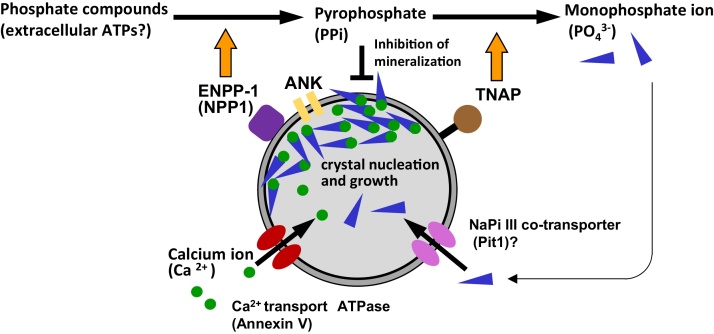
Figure 6.
Schematic design of matrix vesicle-mediated mineralization.
Matrix vesicles provide adequate micro-circumstance for initiation of mineralization. Membrane transporters and enzymes including TNAP, ENPP1, annexins, ANK and Pit1 equipped on matrix vesicles play a pivotal role in Ca2+ and PO43− transport into the vesicles. Phosphatidylserine and so forth in the plasma membrane has a high affinity to produce a stable calcium phosphate–phospholipid complex associated with the inner leaflet of the vesicle’s membrane. Thereafter, amorphous calcium phosphates develop hydroxyapatite to form needle-shaped mineral crystals. Many mineral crystals penetrate the vesicles’ membranes to form a globular assembly of numerous mineral crystals, i.e., mineralized nodules.
Severe ENPP1 deficiency was recently shown to be related to a syndrome of spontaneous infantile arterial and periarticular mineralization [48], [49]. They suggested that the PPi generated by ENPP1 activity in vascular smooth muscle cells and chondrocytes disrupts the growth of hydroxyapatite crystals. In addition, the linkage of genetic ENPP1 dysfunction to infantile arterial mineralization suggests abnormal PPi metabolism seems to be an important regulatory factor or inducer of osteoblastic differentiation in vascular smooth muscle cell.
Mice with Enpp1 gene depletion (Enpp1−/− mice), also known as tiptoe walking (ttw/ttw) mice, develop progressive ankylosing intervertebral and peripheral joint hyperostosis and articular cartilage mineralization [50], [51], [52], [53], [54]. Mackenzie et al. [54] have recently reported on the details of the skeletal deformities seen in Enpp1−/− mice: mineralization in arteries and in the kidney, ectopic formation of cartilage in the joints and spine, despite the reduced circulating calcium and phosphate levels. Also, there was hypermineralization of the talocrural joint and hypomineralization of the femur and tibia with reduced trabecular number, trabecular bone volume and trabecular/cortical thickness. Consistent with the marked increased in fibroblast growth factor (FGF) 23 mRNA, circulating FGF23 was significantly elevated in Enpp1−/− mice [54].
3.3. Putative function of ankylosis protein (ANK) in pyrophosphate transport in cells
ENPP1 can be found not only on the cell surface but also in cytoplasmic regions, generating PPi in the both regions of the cell (Fig. 6). Ankylosis protein, ANK, which is encoded by the mouse progressive ankylosis (Ank) gene, appears to function as a multiple-pass, transmembrane PPi-channelling protein, allowing PPi molecules to pass through the plasma membrane from the cytoplasm to the outside of the cell [55], [56]. In mice, Ank mRNA is expressed in several tissues including the heart, brain, liver, spleen, lung, muscle and kidney, as well as in bone and cartilage such as the articular cartilage in the shoulder, elbow, wrist and digits. Since PPi and its derivatives are naturally potent mineralization inhibitors both in vivo and in vitro, ANK-mediated regulation of PPi levels provides a room for inhibition of excessive mineralization in several tissues [55]. Considering that infants carrying Ank mutations display a three- to five-fold decrease in extracellular PPi, the Ank gene appears to regulate both intra- and extracellular levels of an important inhibitor of hydroxyapatite crystal formation [55]. Thus, local PPi production naturally inhibits hydroxyapatite deposition, blocking undesirable mineralization in articular cartilage and other tissues. With loss of ANK activity, however, extracellular PPi levels attenuate, intracellular PPi levels rise, and unregulated mineralization takes place.
3.4. Ca2+ transport through annexins
Matrix metalloproteinase (MMP)-3 [57], TNAP [5], [16], [17], [32], [57], [58], [59], [60], annexins [61], phospholipase A2, carbonic anhydrase II and lactate dehydrogenase [62] are some of the relevant enzymes and proteins involved in matrix vesicle-mediated mineralization.
Among these molecules, annexins are Ca2+- and lipid-binding proteins involved in Ca2+ transport and serving as ion channels in the matrix vesicles (Fig. 6). Three annexins were identified in the matrix vesicles: annexin A2, A5 and A6 [63], [64], [65], [66]. In the first phase of matrix vesicle-mediated mineralization, mineral complexes appears on the inner leaflet of the matrix vesicle’s membrane. The affinity of phophatidylserine for Ca2+ is quite strong in the inner leaflet of the matrix vesicle membrane, which is enriched with anionic lipids [67], [68]. The annexin A5 shows Ca2+-dependent phosphatidylserine-binding properties and may play a central role in mineralization. However, Annexin A5−/− mice did not demonstrate abnormal skeletal development; thus, other annexins could replace the biological functions of annexin A5 in knockout mice. Thus, further studies seem to be necessary to clarify the precise role of annexins during bone mineralization.
4. Ultrastructure of collagen mineralization
After the onset of matrix vesicle-mediated mineralization, mineralized nodules would contact the surrounding collagen fibrils. The collagen fibrils mineralization begins at the point of contact with mineralized nodules (Fig. 7). There are at least two theories explaining collagen mineralization: one is the hole zone theory, and another is the one supporting that mineralization takes place along the superhelix of collagen fibrils, which are arranged in parallel, but shifted at certain intervals. According to the hole zone theory, during the non-mineralizing phase, the gaps within the collagen fibrils are occupied by small proteoglycans such as decorin and biglycan. However, after elimination of these proteoglycans, extracellular Ca2+ and PO43− fill in the gap to generate calcium phosphate nuclei and mineralize the collagen fibrils. Thus, the initial mineralization begins in the collagen fibrils’ “holes”.
Figure 7.
TEM images of the contact between collagen fibrils and mineralized nodules.
(A) In osteoid, several mineralized nodules (MN) are shown to make contact with surrounding collagen fibrils (Co) (arrows). (B) In other region, mineralized nodules spread out minerals to neighboring collagen fibrils. Thus, collagen mineralization seems to be associated with mineralized nodules. In contrast, however, collagen striation (short arrows) do not show any mineral deposition. (C) At a higher magnification, laddering structures of mineral deposition are parallel to the longitudinal axis of the collagen fibrils, and the length of mineral crystal seems to be identical to that of superhelix.
Panel A is from Ref. [74] (Amizuka et al.), and panels B and C are modified from Ref. [73] (Ozawa et al.).
However, while decorin/biglycan-double knockout mice revealed osteopenia as a result of impaired GAG-linking to decorin and biglycan core proteins, mineralization was not stimulated [69]. Still, collagen mineralization based on the process of removal of small proteoglycans may need further investigation. On the other hand, TEM observations demonstrated that mineralization spread out from the contact point of mineralized nodules towards the periphery of collagen fibrils (Fig. 7B and C). This finding suggests that collagen mineralization is association with mineralized nodules. At a higher magnification, the spicules of calcium phosphate crystals are seen on the fibrillar structures identical to the superheix of collagen fibrils, which indicates that mineral crystals are deposited on the superhelix, which serves as a scaffold for collagen mineralization.
We also examined mineralization on osteogenic disorder shionogi (ODS) rats, which carry a hereditary defect in ascorbic acid synthesis [70]. Fragile, fibrillar collagenous structures without evident striation were found in ODS rat bones, which may be a result of misassembling of the triple helices of collagenous α-chains secondary to ascorbic acid deprivation. However, that seemed to bear no effect on mineralization: fine needle-shaped mineral crystals extended from mineralized nodules, and were apparently bound to collagenous fibrillar structures (Fig. 8).
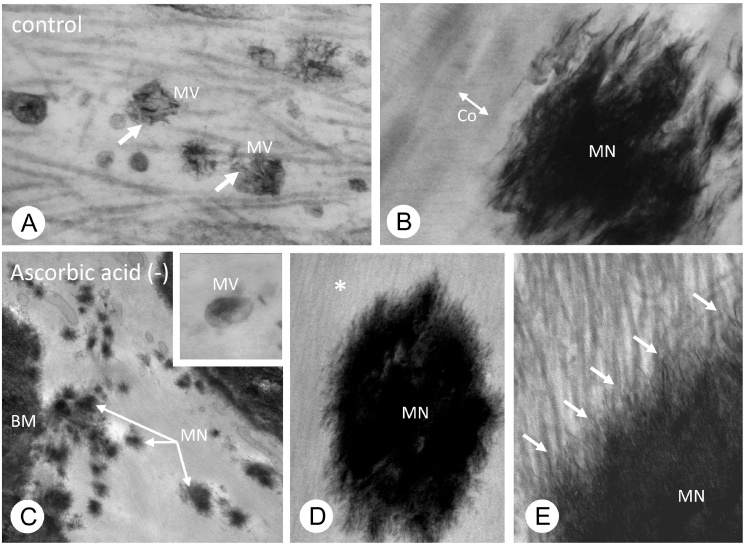
Figure 8.
Matrix vesicles and collagen mineralization in ascorbic acid insufficient circumstance.
In a normal state, TEM observations demonstrate numerous matrix vesicles (A) and mineralized nodules (B). At a higher magnification, mineral crystals extending from the mineralized nodules ran along the collagen fibrils (B). In an insufficient circumstance of ascorbic acid, which is necessary for collagen synthesis, TEM observations verified the presence of matrix vesicles and mineralized nodules (C). At a higher magnification, however, collagen fibrils are shown be very fine, but, the fine mineral crystals from the mineralized nodule extended along fine fibrillar structures of collagen fibrils (D and E).
All the images are modified from Ref. [70] (Hasegawa et al.).
Taken together, these two postulations (the hole zone theory and mineralization on the superhelix of collagen fibrils) are both without proper foundations, and therefore, need further scientific scrutiny.
5. Concluding remarks
Matrix vesicle-mediated mineralization causes a series of orchestrated ultrastructural and biochemical events in bone. To achieve proper mineralization, a variety of membrane transporters and enzymes are put at work in matrix vesicles. Of particular importance is the influx of phosphate ions into matrix vesicles, which involves a complex interplay among ENPP1, ANK, TNAP and Pit1. Mineralized nodules, the globular assembly of needle-shaped mineral crystals, are derived from matrix vesicles and may retain some activity of those transporters and enzymes. However, crystal growth is likely regulated by surrounding organic materials prior to subsequent collagen mineralization.
Conflict of interest
None declared.
Acknowledgments
This study was partially supported by the Grants-in Aid for Scientific Research (Amizuka N, Hasegawa T) and Promoting International Joint Research (Bilateral Collaborations) of JSPS and NSFC (Amizuka N, Li M).
References
- 1.Ali S.Y., Sajdera S.W., Anderson H.C. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20:33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- 4.Bonucci E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103:192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa H., Yamada M., Yajima T. The ultrastructural and cytochemical aspects of matrix vesicles and calcification processes. In: Talmage R.V., Ozawa H., editors. Formation and calcification of hard tissues. Shakai Hoken Pub; Tokyo: 1978. pp. 9–57. [Google Scholar]
- 6.Ozawa H., Yamada M., Yamamoto T. Ultrastructural observations on the location of lead and calcium in the mineralizing dentine of rat incisor. In: Ascenzi A., Bonucci E., de Bernard B., editors. Matrix vesicles. Wiching Editore srl; Milano: 1981. pp. 179–187. [Google Scholar]
- 7.Wuthier R.E. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim Biophys Acta. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 8.Robison R., Soames K.M. The possible significance of hexosephosphoric esters in ossification. Biochem J. 1924;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner S. Organization of extracellularly mineralized tissues: a comparative study of biological crystal growth. CRC Crit Rev Biochem. 1986;20:365–408. doi: 10.3109/10409238609081998. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa H. Ultrastructural concepts on biological calcification; focused on matrix vesicles. J Oral Biosci. 1985;27:751–774. [Google Scholar]
- 11.Boskey A.L., Posner A.S. The role of synthetic and bone extracted Ca-phospholipid-PO4 complexes in hydroxyapatite formation. Calcif Tissue Res. 1977;23:251–258. doi: 10.1007/BF02012794. [DOI] [PubMed] [Google Scholar]
- 12.Boyan B.D., Schwartz Z., Swain L.D., Khare A. Role of lipids in calcification of cartilage. Anat Rec. 1989;224:211–219. doi: 10.1002/ar.1092240210. [DOI] [PubMed] [Google Scholar]
- 13.Mahamid J., Sharir A., Gur D., Zelzer E., Addadi L., Weiner S. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: a cryo-electron microscopy study. J Struct Biol. 2011;174:527–535. doi: 10.1016/j.jsb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa H. Current concepts of the morphophysiology of matrix vesicle calcification. Connect Tissue. 1983;15:1–12. [Google Scholar]
- 15.Ozawa H. Ultrastructural aspects on the biological calcification with special reference to freeze-substitution at liquid helium temperature. Proceedings of the XIth International Congress on Electron Microscopy; Kyoto; 1986. pp. 57–60. [Google Scholar]
- 16.Yamada M. Ultrastractural and cytochemical studies on the calcification of the tendon-bone joint. Arch Histol Jpn. 1976;39:347–378. doi: 10.1679/aohc1950.39.347. [DOI] [PubMed] [Google Scholar]
- 17.Hoshi K., Ejiri S., Ozawa H. Localizational alterations of calcium, phosphorus, and calcification-related organics such as proteoglycans and alkaline phosphatase during bone calcification. J Bone Miner Res. 2001;16:289–298. doi: 10.1359/jbmr.2001.16.2.289. [DOI] [PubMed] [Google Scholar]
- 18.Bosky A.L., Maresca M., Ullrich W., Doty S.B., Butler W.T., Prince C.W. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 19.Hunter G.K., Hauschka P.V., Poole A.R., Rosenberg L.C., Goldberg H.A. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark M.P., Butler W.T., Prince C.W., Finkleman R.D., Ruch J.-V. Developmental expression of 44-kDa phosphoprotein (osteopontin) and bone-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37:123–136. doi: 10.1111/j.1432-0436.1988.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 21.Ritter N.M., Farach-Carson M.C., Butler W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J Bone Miner Res. 1992;7:877–885. doi: 10.1002/jbmr.5650070804. [DOI] [PubMed] [Google Scholar]
- 22.Bianco P., Hayashi Y., Silvestrini G., Termine J.D., Bonucci E. Osteonectin and Gla-protein in calf bone: ultrastructural immunohistochemical localization using the Protein A-gold method. Calcif Tissue Int. 1985;37:684–686. doi: 10.1007/BF02554931. [DOI] [PubMed] [Google Scholar]
- 23.Bonucci E. The locus of initial calcification in cartilage and bone. Clin Orthop Relat Res. 1971;78:108–139. doi: 10.1097/00003086-197107000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bonucci E., Gherardi G. Histochemical and electron microscopy investigations on medullary bone. Cell Tissue Res. 1975;163:81–97. doi: 10.1007/BF00218592. [DOI] [PubMed] [Google Scholar]
- 25.Hall J.G., Pauli R.M., Wilson K.M. Maternal and fetal sequelae of anti-coagulation during pregnancy. Am J Med. 1980;68:122–140. doi: 10.1016/0002-9343(80)90181-3. [DOI] [PubMed] [Google Scholar]
- 26.Hauschka P.V., Lian J.B., Gallop P.M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975;72:3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price P.A., Otsuka A.A., Poser J.W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976;73:1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pauli R.M., Lian J.B., Mosher D.F., Suttie J.W. Association of congenital deficiency of multiple vitamin K-dependent coagulation factors and the phenotype of the warfarin embryopathy: clues to the mechanism of teratogenicity of coumarin derivatives. Am J Hum Genet. 1987;41:566–583. [PMC free article] [PubMed] [Google Scholar]
- 29.Amizuka N., Li M., Hara K., Kobayashi M., de Freitas P.H., Ubaidus S. Warfarin administration disrupts the assembly of mineralized nodules in the osteoid. J Electron Microsc. 2009;58:55–65. doi: 10.1093/jmicro/dfp008. [DOI] [PubMed] [Google Scholar]
- 30.Azuma K., Shiba S., Hasegawa T., Ikeda K., Urano T., Horie-Inoue K. Osteoblast-specific γ-glutamyl carboxylase-deficient mice display enhanced bone formation with aberrant mineralization. J Bone Miner Res. 2015;30:1245–1254. doi: 10.1002/jbmr.2463. [DOI] [PubMed] [Google Scholar]
- 31.de Bernard B., Bianco P., Bonucci E., Costantini M., Lunazzi G.C., Martinuzzi P. Biochemical and immunohistochemical evidence that in cartilage an alkaline phosphatase is a Ca2+-binding glycoprotein. J Cell Biol. 1986;103:1615–1623. doi: 10.1083/jcb.103.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzawa T., Anderson H.C. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem. 1971;19:801–808. doi: 10.1177/19.12.801. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y., Beertsen W., van den Bos T., Kawamoto T., Oda K., Takano Y. Site-specific localization of two distinct phosphatases along the osteoblast plasma membrane: tissue non-specific alkaline phosphatase and plasma membrane calcium ATPase. Bone. 2004;35:1077–1085. doi: 10.1016/j.bone.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Oda K., Amaya Y., Fukushi-Irie M., Kinameri Y., Ohsuye K., Kubota I. A general method for rapid purification of soluble versions of glycosylphosphatidylinositol-anchored proteins expressed in insect cells: an application for human tissue-nonspecific alkaline phosphatase. J Biochem. 1999;126:694–699. doi: 10.1093/oxfordjournals.jbchem.a022505. [DOI] [PubMed] [Google Scholar]
- 35.Hoshi K., Amizuka N., Oda K., Ikehara Y., Ozawa H. Immunolocalization of tissue non-specific alkaline phosphatase in mice. Histochem Cell Biol. 1997;107:183–191. doi: 10.1007/s004180050103. [DOI] [PubMed] [Google Scholar]
- 36.Narisawa S., Fröhlander N., Millian J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Waymire K.G., Mahuren J.D., Jaje J.M., Guilarte T.R., Coburn S.P., MacGregor G.R. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 38.Anderson H.C., Sipe J.B., Hessle L., Dhanyamraju R., Atti E., Camacho N.P. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesch W., Vandenbos T., Roschgr P., Fratzl-Zelman N., Klaushofer K., Beertsen W. Orientation of mineral crystallites and mineral density during skeletal development in mice deficient in tissue nonspecific alkaline phosphatase. J Bone Miner Res. 2003;18:117–125. doi: 10.1359/jbmr.2003.18.1.117. [DOI] [PubMed] [Google Scholar]
- 41.Murshed M., Harmey D., Millán J.L., McKee M.D., Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whyte M.P., Greenberg C.R., Salman N.J., Bober M.B., McAlister W.H., Wenkert D. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 43.Whyte M.P., Rockman-Greenberg C., Ozono K., Riese R., Moseley S., Melian A. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab. 2016;101:334–342. doi: 10.1210/jc.2015-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato K., Nishimasu H., Okudaira S., Mihara E., Ishitani R., Takagi J. Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc Natl Acad Sci U S A. 2012;109:16876–16881. doi: 10.1073/pnas.1208017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terkeltaub R., Rosenbach M., Fong F., Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Arthritis Rheum. 1994;37:934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- 46.Johnson K., Vaingankar S., Chen Y., Moffa A., Goldring M., Sano K. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999;42:1986–1997. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 47.Johnson K., Moffa A., Chen Y., Pritzker K., Goding J., Terkeltaub R. Matrix vesicle plasma membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res. 1999;14:883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- 48.Rutsch R., Vaingankar S., Johnson K., Goldfine I., Maddux B., Schauerte P. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutsch F., Ruf N., Vaingankar S., Toliat M.R., Suk A., Höhne W. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 50.Okawa A., Nakamura I., Goto S., Moriya H., Nakamura Y., Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 51.Johnson K., Pritzker K., Goding J., Terkeltaub R. The nucleoside triphosphate pyrophosphohydrolase isozyme PC-1 directly promotes cartilage calcification through chondrocyte apoptosis and increased calcium precipitation by mineralizing vesicles. J Rheumatol. 2001;28:2681–2691. [PubMed] [Google Scholar]
- 52.Johnson K., Hashimoto S., Lotz M., Pritzker K., Goding J., Terkeltaub R. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001;44:1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Johnson K., Goding J., Van Etten D., Sali A., Hu S.I., Farley D. Linked deficiencies in extracellular inorganic pyrophosphate (PPi) and osteopontin expression mediate pathologic ossification in PC-1 null mice. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- 54.Mackenzie N.C., Zhu D., Milne E.M., van’t Hof R., Martin A., Darryl Quarles L. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS One. 2012;7:e32177. doi: 10.1371/journal.pone.0032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho A.M., Johnson M.D., Kingsley D.M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 56.Gurley K.A., Reimer R.J., Kingsley D.M. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz J.P., Schwartz Z., Sylvia V.L., Dean D.D., Calderon F., Boyan B.D. Vitamin D3 regulation of stromelysin-1 (MMP-3) in chondrocyte cultures is mediated by protein kinase C. J Cell Physiol. 1996;168:570–579. doi: 10.1002/(SICI)1097-4652(199609)168:3<570::AID-JCP9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 58.Fleish H., Neuman W.F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 59.Fleish H., Neuman W. The role of phosphatase and polyphosphates in calcification of collagen. Helv Physiol Pharmacol Acta. 1961;19:C17–C18. [PubMed] [Google Scholar]
- 60.Takano Y., Ozawa H., Crenshaw M.A. Ca-ATPase and ALPase activities at the initial calcification sites of dentine and enamel in the rat incisor. Cell Tissue Res. 1986;243:91–99. doi: 10.1007/BF00221856. [DOI] [PubMed] [Google Scholar]
- 61.Kirsch T., Nah H.D., Shapiro I.M., Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson H.C. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995;314:266–280. [PubMed] [Google Scholar]
- 63.Cao X., Genge B.R., Wu L.N., Buzzi W.R., Showman R.M., Wuthier R.E. Characterization, cloning and expression of the 67-kDA annexin from chicken growth plate cartilage matrix vesicles. Biochem Biophys Res Commun. 1993;197:556–561. doi: 10.1006/bbrc.1993.2515. [DOI] [PubMed] [Google Scholar]
- 64.Kirsch T., Nah H.D., Demuth D.R., Harrison G., Golub E.E., Adams S.L. Annexin V-mediated calcium flux across membranes is dependent on the lipid composition: implications for cartilage mineralization. Biochemistry. 1997;36:3359–3367. doi: 10.1021/bi9626867. [DOI] [PubMed] [Google Scholar]
- 65.Kirsch T., Nah H.D., Shapiro I.M., Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirsch T., Claassen H. Matrix vesicles mediate mineralization of human thyroid cartilage. Calcif Tissue Int. 2000;66:292–297. doi: 10.1007/s002230010059. [DOI] [PubMed] [Google Scholar]
- 67.Majeska R.J., Holwerda D.L., Wuthier R.E. Localization of phosphatidylserine in isolated chick epiphyseal cartilage matrix vesicles with trinitrobenzenesulfonate. Calcif Tissue Int. 1979;27:41–46. doi: 10.1007/BF02441159. [DOI] [PubMed] [Google Scholar]
- 68.Taylor M.G., Simkiss K., Simmons J., Wu L.N., Wuthier R.E. Structural studies of a phosphatidyl serine-amorphous calcium phosphate complex. Cell Mol Life Sci. 1998;54:196–202. doi: 10.1007/s000180050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corsi A., Xu T., Chen X.D., Boyde A., Liang J., Mankani M. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 70.Hasegawa T., Li M., Hara K., Sasaki M., Tabata C., de Freitas P.H. Morphological assessment of bone mineralization in tibial metaphyses of ascorbic acid-deficient ODS rats. Biomed Res. 2011;32:259–269. doi: 10.2220/biomedres.32.259. [DOI] [PubMed] [Google Scholar]
- 71.Amizuka N., Ozawa H. In: Bone and calcium, calcium—basic science, clinics, nutrition. Nishizawa Y., Shiraki M., Ezawa I., Hirota T., editors. Life Science Publishing Co. Ltd.; Tokyo: 1999. pp. 20–34. [in Japanese] [Google Scholar]
- 72.Amizuka N., Hasegawa T., Oda K., Luiz de Freitas P.H., Hoshi K., Li M. Histology of epiphyseal cartilage calcification and endochondral ossification. Front Biosci. 2012;4:2085–2100. doi: 10.2741/e526. [DOI] [PubMed] [Google Scholar]
- 73.Ozawa H., Hoshi K., Amizuka N. Current concepts of bone mineralization. J Oral Biosci. 2008;50(1):1–14. [Google Scholar]
- 74.Amizuka N., Li M., Kobayashi M., Hara K., Akahane S., Takeuchi K. Vitamin K2, a gamma-carboxylating factor of gla-proteins, normalizes the bone crystal nucleation impaired by Mg-insufficiency. Histol Histopathol. 2008;23(11):1353–1366. doi: 10.14670/HH-23.1353. [DOI] [PubMed] [Google Scholar]