In this study, Rodriguez-Barrueco et al. analyzed ∼3000 primary tumors and show that miR-424(322)/503 is commonly lost in a subset of aggressive breast cancers; they then describe the genetic aberrations that inactivate its expression. Their data show that miR-424(322)/503 is a tumor suppressor in breast cancer and provide a link between mammary epithelial involution, tumorigenesis, and the phenomenon of chemoresistance.
Keywords: chemoresistance, breast cancer, microRNA, tumor suppressor
Abstract
The female mammary gland is a very dynamic organ that undergoes continuous tissue remodeling during adulthood. Although it is well established that the number of menstrual cycles and pregnancy (in this case transiently) increase the risk of breast cancer, the reasons are unclear. Growing clinical and experimental evidence indicates that improper involution plays a role in the development of this malignancy. Recently, we described the miR-424(322)/503 cluster as an important regulator of mammary epithelial involution after pregnancy. Here, through the analysis of ∼3000 primary tumors, we show that miR-424(322)/503 is commonly lost in a subset of aggressive breast cancers and describe the genetic aberrations that inactivate its expression. Furthermore, through the use of a knockout mouse model, we demonstrate for the first time that loss of miR-424(322)/503 promotes breast tumorigenesis in vivo. Remarkably, we found that loss of miR-424(322)/503 promotes chemoresistance due to the up-regulation of two of its targets: BCL-2 and insulin-like growth factor-1 receptor (IGF1R). Importantly, targeted therapies blocking the aberrant activity of these targets restore sensitivity to chemotherapy. Overall, our studies reveal miR-424(322)/503 as a tumor suppressor in breast cancer and provide a link between mammary epithelial involution, tumorigenesis, and the phenomenon of chemoresistance.
In animals, the regulation of every cell must be exquisitely orchestrated in order to support the viability of the whole organism. Genetic alterations that accumulate in genes such as tumor suppressors and oncogenes in individual cells can lead to the disruption of normal biological processes and promote tumorigenesis.
The female mammary gland is a very dynamic organ that undergoes continuous tissue remodeling during adulthood (Howard and Gusterson 2000; Ip and Asch 2000; Macias and Hinck 2012). These processes are tightly regulated in time and space to ensure proper organ function and avoid pathological consequences. During regular ovarian cycles, there is an increase in the mammary epithelial content of the mammary gland that serves to prepare the mammary gland for a potential pregnancy. In the absence of pregnancy, the diestrus phase terminates with the regression of the mammary epithelium to its basal state. If pregnancy occurs, the mammary gland undergoes massive tissue remodeling, and lactiferous alveoli invade nearly the entire mammary fat pad. After weaning, regression of this architecture leads to alveolar and secretory duct collapse, a process known as involution (Watson 2006; Stein et al. 2007). Although it is well established that the number of menstrual cycles (Collaborative Group on Hormonal Factors in Breast Cancer 2012) and pregnancy (in this case, transiently) (Lambe et al. 1994) increase the risk of breast cancer, the reasons are unclear. Growing clinical and experimental evidence indicates that improper involution plays a role in the development of this malignancy (Watson 2006; Radisky and Hartmann 2009). Not surprisingly, some of the pathways described to have a role in this process are also involved in tumorigenesis. Two main discrete phases of involution have been described. The first phase is initiated by milk stasis and the release of soluble factors such as TGF-β (Nguyen and Pollard 2000; Bierie et al. 2009) and IL-6 cytokine family members LIF (Kritikou et al. 2003) and OSM (Tiffen et al. 2008). These activate STAT3 (Chapman et al. 1999; Kreuzaler et al. 2011; Sargeant et al. 2014), NF-κB (Baxter et al. 2006), and insulin-like growth factor (IGF) signaling, (Neuenschwander et al. 1996; Allan et al. 2004), leading to apoptotic (Jager et al. 1997; Walton et al. 2001; Rucker et al. 2011) and nonapoptotic (Kreuzaler et al. 2011) programmed cell death. During the second phase, nonepithelial cells are also critical, and macrophage function (Hutchins et al. 2012; O'Brien et al. 2012) and high protease activity from the stroma (matrix metalloproteinases) (Talhouk et al. 1992; Fata et al. 2001; Green and Lund 2005) are required. Despite the above, overall, our understanding of the molecular mechanisms involved in mammary epithelial dynamics is far from complete. Moreover, how deregulation of specific genes during involution influences tumorigenesis is poorly understood.
Recently, through the generation of a knockout mouse model, we discovered that the microRNA (miRNA) cluster miR-424(322)/503 is a TGF-β-induced regulator of involution in the mammary epithelium (Llobet-Navas et al. 2014a,b). Importantly, the miR-424(322)/503 locus is commonly lost in a subset of breast cancers that have clinicopathological characteristics of poor prognosis. This miRNA cluster contains two miRNAs, miR-424(322) and miR-503, which belong to the miR-16 family and consequently can modulate the expression of the same target genes (Liu et al. 2008; Finnerty et al. 2010). Our studies demonstrated that part of the biological activity of miR-424(322)/503 in the mammary epithelium is mediated by its ability to down-regulate the expression of CDC25A, BCL-2, and IGF-1 receptor (IGF1R). Notably, these three genes have been found to be overexpressed in human breast cancers presenting oncogenic functions (CDC25A [Cangi et al. 2000], IGF1R [Kucab and Dunn 2003], and BCL-2 [Dawson et al. 2010]). Furthermore, up-regulation of BCL-2 (Dawson et al. 2010; Colitti 2012; Vaillant et al. 2013) and IGF1R signaling (Kucab and Dunn 2003; Hendrickson and Haluska 2009; Vu and Claret 2012) is a common and well-established mechanism that cancer cells, including breast cancers, use to overcome apoptosis induced by chemotherapy. Thus, based on the above findings, we aimed to investigate a putative tumor suppressor function for the miR-424(322)/503 cluster and specifically the impact that loss of this miRNA cluster has on response to chemotherapy.
Results
The miR-424(322)/503 cluster is lost in breast cancers
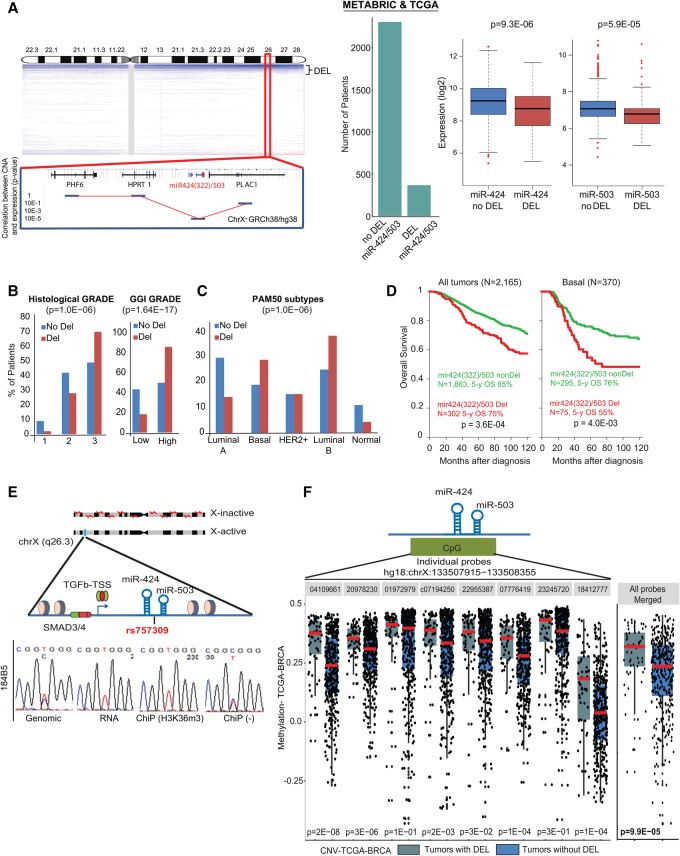
Our previous studies revealed that miR-424(322)/503−/− female mice present defects in mammary gland involution and display alveolar hyperplasia after pregnancy (Llobet-Navas et al. 2014a). Thus, we decided to investigate a putative tumor suppressor role for this miRNA cluster in breast cancer. First, we pooled the METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) (Curtis et al. 2012) and the BRCA-The Cancer Genome Atlas (TCGA) (The Cancer Genome Atlas Network 2012) data sets, which are the two largest data sets containing matched DNA copy number alteration (CNA) and expression analysis (coding genes and miRNAs) of primary breast cancers. We then analyzed the 2165 pretherapeutic samples from patients with nonmetastatic primary invasive breast cancer who underwent surgery and had available follow-up data (Supplemental Table S1). In each data set, the miR-424(322)503 locus was deleted in ∼14% of breast cancers, and its deletion correlated with lower expression levels of the mature miR-424 and miR-503 forms (Fig. 1A). Although small deletions (<2 Mb) occurred, the majority was chromosomal arm losses resulting in heterozygosis (Ht) (Fig. 1A).
Figure 1.
miR-424(322)/503 is lost in breast cancer and is associated with poor prognosis and aggressive subtypes. (A) Copy number profile of the X chromosome in human breast cancers (METABRIC plus TCGA data sets), highlighting the chromosomal region containing the miR-424(322)/503 locus. The correlation between copy number and expression for surrounding genes is included at the bottom. The bar graph and box plots show the number of breast cancers presenting deletion (DEL) of the miR-424(322)/503 locus as well as the association between copy number and expression for miR-424(322) and miR-503. The bar graphs show the association between deletion of the miR-424(322)/503 locus with grade (B) and PAM50 molecular subtypes (C). (D) Overall survival of breast cancer patients with and without deletion of the miR-424(322)/503 locus (all patients and basal tumors are shown). (E) The miR-424(322)/503 cluster is monoallelically expressed as shown by sequencing a single-nucleotide polymorphism (SNP) located between the miRNAs in the cluster at the DNA and RNA level. Chromatin immunoprecipitation (ChIP) assays confirm that the expressed allele is associated with the active transcription elongation histone mark H3K36me3. (F) Methylation status analysis of the miR-424(322)/503 locus in breast cancers (TCGA-BRCA data set) with and without deletion of the locus. Each dot represents an individual primary tumor.
We then searched for correlations between miR-424(322)/503 status (302 cases with and 1863 cases without deletion, referred to here as miR-DEL and miR-non-DEL, respectively) and the clinicopathological and molecular characteristics of the tumors. Loss of miR-424(322)/503 was associated with poor prognosis characteristics such as high-grade (histological and genomic-grade index [GGI]) (Sotiriou et al. 2006) larger pathological tumor size, triple-negative (TN) status, strong cell proliferation (KI67 mRNA expression), and GGI signature (Fig. 1B; Supplemental Table S2). Commonly, primary breast cancers are also stratified into different molecular subtypes based on genome-wide studies of mRNA expression and CNAs (Perou et al. 2000; Curtis et al. 2012). We found that deletion of this miR cluster was more frequent in tumor subtypes with aggressive behavior such as basal and luminal B (Fig. 1C) and the integrative clusters 1, 2, 6, 9, and 10 (Supplemental Fig. S1A).
Based on its association with poor prognosis characteristics, we hypothesized that loss of the miR-424(322)/503 cluster could influence patient survival. The median follow-up was 63 mo (range 1–302), during which 1711 patients remained alive, and 454 patients died. The 5-yr overall survival was 84% (95% CI 82–86). The “miR-DEL” subgroup was associated with shorter overall survival in the whole population, with 75% 5-yr overall survival (95% CI 70–81) versus 85% (95% CI 83–87) for the “miR-non-DEL” subgroup (P = 3.60 × 10−4, log-rank test) (Fig. 1D; Supplemental Table S3). A similar result was observed in basal tumors, in which the 5-yr overall survival was 55% (95% CI 43–69) in the case of miR deletion and 76% (95% CI 71–82) in the absence of deletion (P = 4.01 × 10−3, log-rank test) (Fig. 1D; Supplemental Fig. S1B; Supplemental Table S3). No prognostic value was observed in the other molecular subtypes (Supplemental Fig. S1B; Supplemental Table S3). In multivariate analysis, the “miR-DEL” status retained unfavorable prognostic significance in the whole population (P = 2.95 × 10−2, Wald test; hazard ratio [HR] = 1.34 [95% CI 1.03–1.75]) and the basal subtype (P = 1.4 × 10−2, Wald test; HR = 1.99 [95% CI 1.30–3.04]) (Supplemental Table S3).
The miR-424(322)/503 locus is located on the X chromosome, and we hypothesized that, due to X chromosome inactivation, it may be monoallelically expressed in cells of female origin. In this case, the commonly found Ht deletion of this locus would be sufficient to abrogate the expression of the miRNA cluster if the active allele is the target of the deletion. Thus, we studied the expression of a single-nucleotide polymorphism (SNP) located between miR-424 and miR-503 in the primary miRNA (pri-miRNA) (Fig. 1E) in normal human mammary epithelial cell lines (diploid for the X chromosome). Genomic DNA and RNA extraction followed by PCR/RT–PCR and sequencing revealed that while MCF-10A cells and two different immortalized HuMEC (human mammary epithelial cell) clones were homozygous for the SNP (data not shown), 184B5 cells were heterozygous and presented the T/C polymorphism in the genomic DNA (Fig. 1E). As predicted, expression studies showed that only one of the two alleles was expressed in 184B5 cells (Fig. 1E). To further demonstrate that the expressed allele was located on the active X chromosome (Xa), we performed chromatin immunoprecipitation (ChIP) followed by sequencing (ChIP-seq) using antibodies for H3K36me3, a mark of active transcription (Bannister and Kouzarides 2011). As expected, only the expressed allele was found to be associated with this histone mark (Fig. 1E). Overall, the above confirmed that, in mammary epithelial cells of female origin, miR-424(322)/503 is monoallelically expressed.
Finally, we investigated which of the two miR-424(322)/503 alleles (active or inactive) was targeted by the deletion observed in breast cancers. DNA hypermethylation is linked with the inactive X chromosome (Xi), while hypomethylation is seen at the Xa (Heard 2004; Briggs and Reijo Pera 2014); we reasoned that deletion of the active allele would increase the level of methylation detected at the miR-424(322)/503 locus by methylation arrays. Thus, we integrated the methylation profiles and the CNA data available in the TCGA-BRCA database (The Cancer Genome Atlas Network 2012). This study revealed that breast cancers with deletions in the miR-424(322)/503 locus present a significantly higher level of methylation (Fig. 1F). This result supports the notion that the active allele is selectively deleted, whereas the methylated inactive allele remains intact.
miR-424(322)/503−/− female mice develop mammary tumors that are promoted by pregnancy
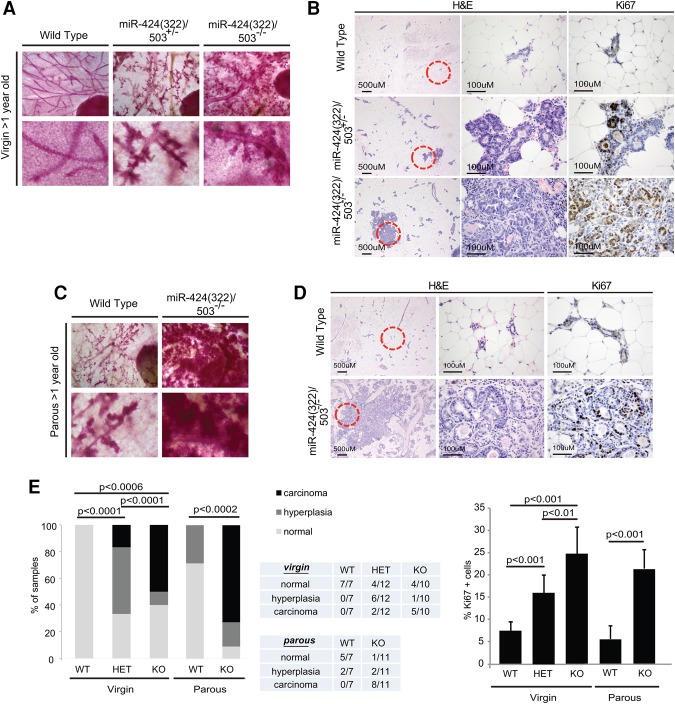
We previously described the generation of a C57BL/6/miR-424(322)/503−/− mouse model (Llobet-Navas et al. 2014a). However, the inbred FVB strain presents enhanced sensitivity to tumorigenesis and is favored for cancer-related studies (Davie et al. 2007). To further investigate the role of this miRNA cluster in carcinogenesis, we first crossed our C57BL/6/miR-424(322)/503−/− model with wild-type FVB mice for more than six generations to produce a >98.5% clean FVB/miR-424(322)/503−/− background (Supplemental Fig. S2A). All experiments in this study were done in the FVB background, and we refer to them here as the miR-424(322)/503−/− model. Next, the mammary epithelia of >1-yr-old FVB wild-type, miR-424(322)/503+/−, and miR-424(322)/503−/− virgin females were evaluated by carmine red staining (Fig. 2A) and H&E staining (Fig. 2B). Remarkably, while none of the wild-type animals presented mammary epithelial abnormalities, all of the miR-424(322)/503−/− females displayed enlargement of the terminal ductal lobular units, and we were able to detect microscopic invasive carcinomas in 50% of them (Fig. 2E). Heterozygous miR-424(322)/503+/− animals presented an intermediate phenotype where hyperplasia predominated (Fig. 2E). Ki-67 immunohistochemistry (IHC) revealed that these lesions presented a higher proliferative index than the mammary epithelia of wild-type animals (Fig. 2B,E).
Figure 2.
miR-424(322)/503−/− female mice develop mammary tumors that are promoted by pregnancy. (A) Carmine red staining of mammary glands from >1-yr-old miR-424(322)/503−/−, miR-424(322)/503+/−, and miR-424(322)/503+/+ virgins. (B) H&E and Ki-67 immunostaining of the samples from A. (C) Carmine red staining of mammary glands from >1-yr-old miR-424(322)/503−/− and miR-424(322)/503+/+ parous females. (D) H&E and Ki-67 immunostaining of the samples from C. (E) Quantification of the premalignant and malignant mammary lesions shown in B and D. χ2 tests and t-tests (Ki-67) were used to calculate P-values in E; P < 0.05 was considered significant. All mice were processed during the estrous phase (n ≥ 7). Parous animals had one pregnancy.
Because the miR-424(322)/503 cluster has a known role in post-lactational mammary involution (Llobet-Navas et al. 2014a) and we showed previously that pregnancy promotes accumulation of abnormalities in the mammary epithelium of knockout animals, we next investigated the development of breast carcinomas in parous miR-424(322)/503−/− females. Carmine red staining (Fig. 2C) and H&E staining (Fig. 2D) of mammary glands from >1-yr-old parous mice that passed through one round of pregnancy between the ages of 4 and 7 mo showed a very enlarged mammary epithelium in miR-424(322)/503−/− animals, with a large majority of the animals (73%) presenting microscopic invasive carcinomas (Fig. 2E). Although the majority of the invasive lesions was microscopic, palpable tumors developed in a small fraction of the animals (two out of 40). Overall, these data demonstrate that loss of miR-424(322)/503 induces mammary epithelial tumorigenesis that is promoted by pregnancy (Supplemental Fig. S2B).
CDC25A, BCL-2, and IGF1R oncogenes are up-regulated in miR-424(322)/503-deficient mammary tumors
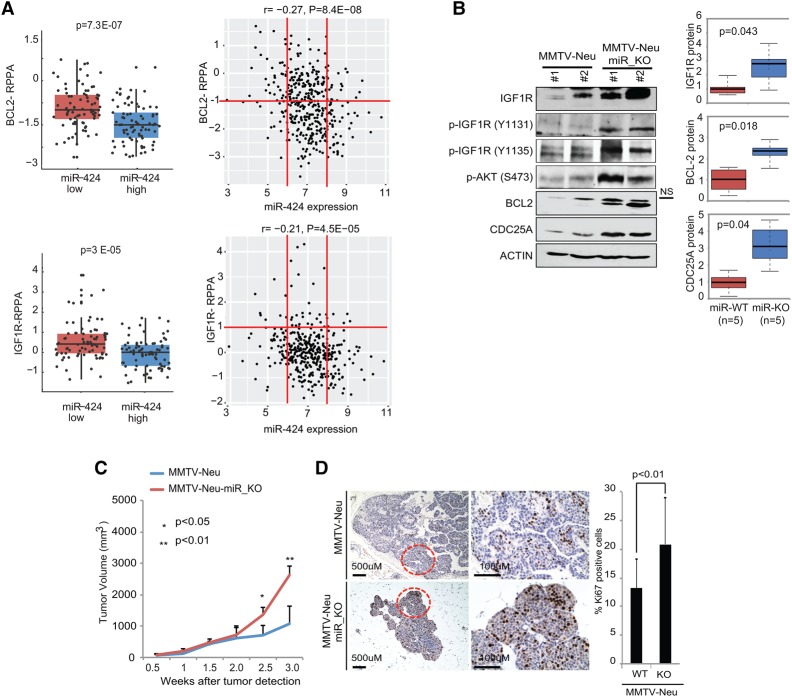
The biological functions of miRNAs are mediated by their ability to attenuate the expression of specific mRNAs. Both miR-424(322) and miR-503 belong to the miR-16 family (Liu et al. 2008; Finnerty et al. 2010), and we showed previously (Llobet-Navas et al. 2014a,b) that they target three genes that have been found to be overexpressed, presenting oncogenic functions in human breast cancers (CDC25A [Cangi et al. 2000], IGF1R [Kucab and Dunn 2003], and BCL-2 [Dawson et al. 2010]). CDC25A is a key player in the progression of the cell cycle (Nilsson and Hoffmann 2000). IGF1R is the canonical receptor for IGF-1 (Pollak 2012). IGF-1 binding leads to the activation of two main signaling pathways: the PI3K–AKT/PKB and Ras–MAPK pathways. BCL-2 is the founding member of the BCL-2 family of apoptosis regulator proteins, and its key role in the intrinsic apoptosis pathway is well established (Colitti 2012). Thus, we investigated the status of these proteins in cancer cells deficient in miR-424(322)/503. To evaluate the correlation between the expression of miR-424(322)/503 and these genes in primary tumors, we analyzed the RPPA (reverse phase protein array) data available from the TCGA-BRCA database. These data provide expression levels for ∼200 proteins, including IGF1R and BCL-2, in >400 primary breast cancers with matched information regarding miR-424(322) and miR-503 expression. Remarkably, these studies showed a significant increase of both IGF1R and BCL-2 protein in breast cancers with low expression of miR-424 (Fig. 3A) and miR-503 (Supplemental Fig. S3A).
Figure 3.
Primary miR-424(322)/503-deficient mammary tumors show aberrant up-regulation of targets CDC25A, BCL-2, and IGF1R. (A) RPPA data, available from the TCGA breast cancer database, were studied to correlate protein expression of BCL-2 and IGF1R with expression of miR-424. Two graphs are shown, comparing the 20% of samples presenting the highest miR-424 expression with the lowest 20% (left) and all samples (right). Red lines have been included as references. (B) Representative Western blots showing the expression levels of miR-424(322)/503 targets (IGF1R, BCL-2, and CDC25A) in the MMTV-Neu and MMTV-Neu-miR_KO models. The box plots show quantification of these data. The Western blots also show elevated phosphorylation (activation) of IGF1R and AKT. (C,D) Growth curves (C) as well as H&E and Ki-67 immunostaining (D) comparing tumors from MMTV-Neu and MMTV-Neu-miR_KO models. t-tests were used to calculate P-values; P < 0.05 was considered significant. For the animal studies, five to 10 animals were used per cohort.
Next, we tested in a mouse model of breast cancer how loss of miR-424(322)/503 impacts the expression of these targets and influences breast tumorigenesis. As shown before, loss of the miR-424(322)/503 cluster occurs in different breast cancer subtypes, including ErbB2/HER2+ tumors (see Fig. 1C; Supplemental Fig. S1D). Thus, we crossed our miRNA cluster knockout animals with the FVB-Tg(MMTV-ErbB2)NK1Mul/J mouse model (referred to here as MMTV-Neu for simplification) in which an activated oncogene HER2 drives tumor formation in the mammary gland (Supplemental Fig. S3B; Muller et al. 1988). Female MMTV-Neu mice develop multifocal palpable mammary tumors efficiently after pregnancy (Ebbesen et al. 2016). We generated FVB-Tg(MMTV-ErbB2)NK1Mul/J/miR-424(322)/503−/− animals (MMTV-Neu-miR_KO) and compared the expression of IGF1R, BCL-2, and CDC25A in size-matched tumors (0.5–0.8 cc) with MMTV-Neu animals. Here we found that the expression of all three miR-424(322)/503 targets was strongly up-regulated in mammary tumors from MMTV-Neu-miR_KO animals (Fig. 3B). As expected, the higher expression of IGF1R led to elevated levels of phospho-IGF1R and phospho-AKT (Fig. 3B). Additionally, the growth of MMTV-Neu-miR_KO tumors was accelerated (Fig. 3C), and intratumoral Ki-67 immunostaining revealed a significantly higher proliferation index (Fig. 3D), as observed in clinical samples. Despite the aforementioned differences, both animal models developed adenocarcinomas that were histologically identical.
Loss of miR-424(322)/503 induces resistance to anti-cancer therapy due to up-regulation of BCL-2 and IGF1R
Up-regulation of BCL-2 (Dawson et al. 2010; Colitti 2012) and IGF1R signaling (Kucab and Dunn 2003; Hendrickson and Haluska 2009) is a common and well-established mechanism that cancer cells, including breast cancers, use to overcome apoptosis induced by chemotherapy. As the miR-424(322)/503 cluster targets both pathways and as loss of miR-424(322)/503 in breast cancer patients is associated with higher protein levels of IGF1R and BCL-2 (Fig. 3A), we anticipated that loss of miR-424(322)/503 may induce resistance to chemotherapeutic drugs.
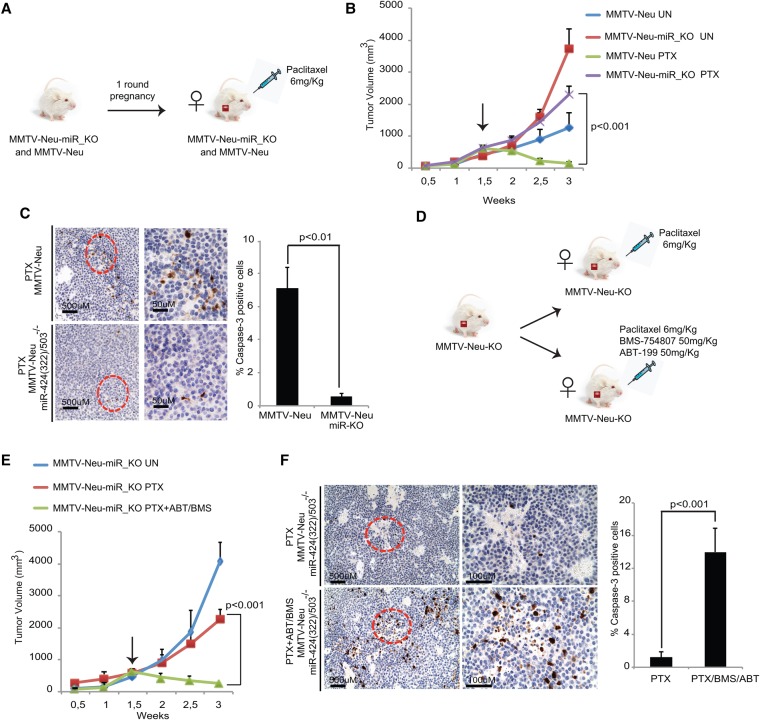
Because of the clinical relevance of treatment resistance, we decided to investigate the role of miR-424(322)/503 in this phenomenon. Thus, we compared the response to the standard chemotherapeutic drug paclitaxel between mammary tumors in MMTV-Neu and MMTV-Neu-miR_KO animals (Fig. 4A). Female mice underwent one round of pregnancy, and we subsequently followed the growth of mammary carcinomas. Once the mammary tumor mass reached ∼500 mm3, these animals were treated twice per week with an intraperitoneal dose of 6 mg/kg paclitaxel (Domingo-Domenech et al. 2012; Hannesdottir et al. 2013; Usary et al. 2013), and the response to this anti-cancer treatment was monitored (Fig. 4B). This study showed that while MMTV-Neu tumors responded well to paclitaxel, showing significant shrinkage in just 2 wk, MMTV-Neu-miR_KO tumors were refractory to this treatment and continued growing almost at the rate of the untreated tumors (Fig. 4B; Supplemental Fig. S4A). Additionally, evaluation of apoptosis by cleaved Caspase-3 immunostaining in treated samples revealed that MMTV-Neu-miR_KO tumors presented a significant reduction in the level of apoptosis compared with MMTV-Neu tumors (Fig. 4C).
Figure 4.
Loss of miR-424(322)/503 induces resistance to anti-cancer therapy due to up-regulation of BCL-2 and IGF1R. (A) Mammary tumor volumes emerging in MMTV-Neu and MMTV-Neu-miR_KO animals were monitored until they reached ∼500 mm3, and then animals were treated twice per week with paclitaxel (PTX). (B) Growth curves showing the response to paclitaxel treatment. (C) Cleaved Caspase-3 immunostaining in tumors from MMTV-Neu and MMTV-Neu-miR_KO models after treatment with paclitaxel. (D) Illustration of the strategy to evaluate the use of ABT-199 and BMS-754807 to reverse chemoresistance in MMTV-Neu-miR_KO animals. (E) Growth curves showing the response to paclitaxel alone versus paclitaxel plus BMS-754807 and ABT-199. (F) Cleaved Caspase-3 immunostaining in the MMTV-Neu-miR_KO tumors from E. For animal studies, five to eight animals were used per cohort.
Next, we reasoned that if high levels of BCL-2 and IGF1R indeed mediate resistance to chemotherapy in miR-424(322)/503-deficient breast cancers, then blocking their activity may restore sensitivity to treatment. Importantly, small molecule inhibitors that block the BCL-2 and IGF1R oncogenic pathways already exist in clinical trials. BH3 mimetics that block the anti-apoptotic activity of BCL-2 proteins have been developed and show considerable promise (Oltersdorf et al. 2005; Witham et al. 2007; Roberts et al. 2012; Vaillant et al. 2013). In patients, treatment with early-generation inhibitors induced dose-dependent thrombocytopenia due to unintended targeting of BCL-XL, leading to loss of mature platelets (Mason et al. 2007; Bajwa et al. 2012). However, new-generation inhibitors such as ABT-199/venetoclax have greater specificity for BCL-2, thus preventing this issue (Witham et al. 2007; Vaillant et al. 2013). ABT-199/venetoclax is currently under investigation in multiple phase 2 and phase 3 clinical trials, mainly for blood-related malignancies, and has been approved recently by the FDA for treatment of chronic lymphocytic leukemia (https://clinicaltrials.gov/ct2/results?term=ABT-199+&Search=Search). Multiple IGF1R inhibitors, humanized monoclonal antibodies (mAbs), and small molecule tyrosine kinase inhibitors (TKIs) have been evaluated in clinical trials (Chen and Sharon 2013; King et al. 2014). Although their efficacy in unselected patients has been limited, these targeted approaches can induce strong anti-tumor activities in selected tumors, including tumors refractory to standard therapies (Chen and Sharon 2013; King et al. 2014). Notably, multiple data from experimental models support the use of these inhibitors in cancer cells with elevated IGF1R expression and activated IGF-1 signaling (King et al. 2014). Among the inhibitors, BMS-754807 is one of the most specific TKIs for which multiple clinical trials have been completed, demonstrating safety and tolerability (https://clinicaltrials.gov/ct2/results?term=BMS-754807&Search=Search).
Previously, we showed that miR-424(322)/503−/− mouse mammary epithelial cells (MMECs) are more resistant to chemotherapeutic agents such as paclitaxel than wild-type counterparts (see also Supplemental Fig. S4B; Llobet-Navas et al. 2014a). Thus, we first tested whether treating these cells with the above-mentioned inhibitors would resensitize them. Interestingly, treating these cells with each individual inhibitor increased their sensitivity to paclitaxel, and this effect was increased when they were combined (Supplemental Fig. S4C). Based on these data, we next compared the response of MMTV-Neu-miR_KO mice treated with paclitaxel alone (as described above) versus animals treated with paclitaxel plus ABT-199 and BMS-754807 administered by gavage at 50 mg/kg three times per week (Fig. 4D; Supplemental Fig. S4D; Hou et al. 2011; Vaillant et al. 2013). Remarkably, blocking IGF1R activation and BCL-2 through the use of these inhibitors resensitized MMTV-Neu-miR_KO tumors to paclitaxel (Fig. 4E; Supplemental Fig. S4E), and the tumors showed a significant increase in treatment-induced apoptosis (Fig. 4F). No significant differences in the response to paclitaxel were observed when parental MMTV-Neu tumors were treated with these inhibitors (Supplemental Fig. S4F).
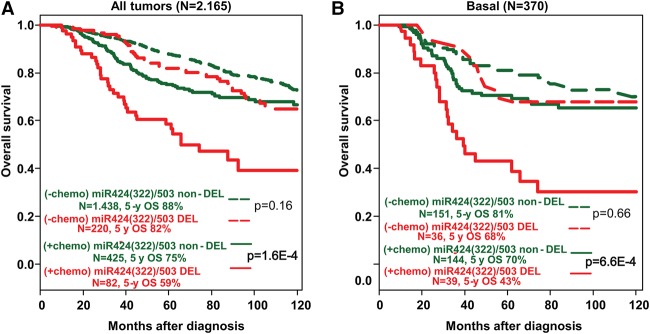
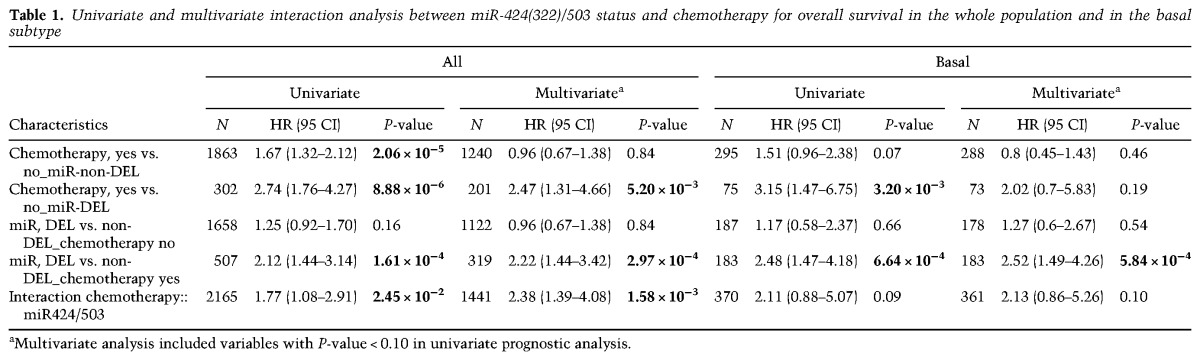
Based on the results from our animal models shown above, we postulated that the unfavorable prognosis of loss of the miR-424(322)/503 locus could be mediated at least in part by the poor response of miR-424(322)/503-deficient cells to treatment. Thus, we analyzed the prognostic value of miR-424(322)/503 loss in our data set according to the delivery or lack of adjuvant chemotherapy; that is, we searched for an association between miR-424(322)/503 status and the benefit of chemotherapy in terms of overall survival. In univariate analysis, an interaction was observed in the whole population (P = 0.02): In the chemotherapy-naïve subgroup, patients with “miR-DEL” and patients with “miR-non-DEL” tumors exhibited similar overall survival (P = 0.16, Wald test), whereas, in the chemotherapy-treated subgroup, patients with “miR-DEL” tumors exhibited shorter overall survival than patients with “miR-non-DEL” tumors (P = 1.61 × 10−4, Wald test) (Fig. 5A; Table 1). Therefore, exposure to chemotherapy was associated with a lesser risk of death and consequently a stronger benefit in patients with “miR-non-DEL” tumors (HR = 1.67 [95% CI 1.32–2.12]; P = 2.06 × 10−5) than in patients with “miR-DEL” tumors (HR = 2.74 [95% CI 1.76–4.27]). Analysis per molecular subtype revealed a similar trend in the basal subtype (P = 0.09) (Fig. 5B; Table 1), while lesser or no effect was observed in other molecular subtypes (Supplemental Table S4). Importantly, in multivariate analysis (Table 1), the interaction remained significant in the whole population (P = 1.58 × 10−3) and tended toward significance in the basal subtype (P = 0.10). Thus, these data confirm that, in breast cancer, the status of the miR-424(322)/503 cluster is associated with reduced response to chemotherapy, particularly in the basal subtype.
Figure 5.
The impact of miR-424(322)/503 loss on the overall survival of breast cancer patients depends on chemotherapy treatment. (A) Overall survival of breast cancer patients (METABRIC and TCGA data sets) with and without deletion of the miR-424(322)/503 locus and with (+chemo) and without (−chemo) delivery of adjuvant chemotherapy. (B) Similar to A, but limited to patients with basal tumors. The statistical studies shown represent univariate analysis. Values for both univariate and multivariate analysis are shown in Table 1.
Table 1.
Univariate and multivariate interaction analysis between miR-424(322)/503 status and chemotherapy for overall survival in the whole population and in the basal subtype
Discussion
Transformation of normal cells is driven by the aberrant function of genes that positively (oncogenes) and negatively (tumor suppressors) regulate the cancer phenotype (Hanahan and Weinberg 2011). Identification and characterization of those genes that provide cells with the capabilities necessary for tumor initiation and progression are critical to understanding tumorigenesis. The identification of tumor suppressors has been historically challenging due to their recessive nature and the diverse mechanisms by which they are inactivated. Here we report the tumor suppressor function of the miR-424(322)/503 cluster in breast cancers.
The miR-424(322)/503 cluster expresses miR-424(322) and miR-503, two miRNAs that belong to the miR-16 family. In humans, this family consists of at least 10 members that have been shown to regulate an array of processes such as cell division, metabolism, stress response, and angiogenesis (Finnerty et al. 2010). Two of its members, miR-15a and miR-16-1, have been associated previously with tumor suppressor functions mainly in blood cancers (Aqeilan et al. 2010; Finnerty et al. 2010). Although a role for miR-424 and miR-503 in tumorigenesis has been suggested, these studies are conflicting, and both tumor-suppressing (Xu et al. 2013a; Chong et al. 2014; Li et al. 2015) and tumor-promoting (Zhang et al. 2014; Ide et al. 2015) functions have been proposed. Arguably, a key reason for this discrepancy was that previous reports were based mainly on association studies in which miRNA expression was compared between cancerous and noncancerous tissues. Additionally, functional studies were limited to the use of cell lines in which miR-424 and miR-503 were overexpressed. Here, through the use of a knockout mouse model, we demonstrate for the first time that loss of miR-424(322)/503 promotes breast tumorigenesis in vivo and describe the genetic aberrations that inactivate its expression in human breast cancers. Notably, according to our knowledge, this is the first report of a miRNA with tumor suppressor functions that is able to generate solid tumors in mice when deleted without the use of cooperating alterations (e.g., P53-null or MYC-overexpressing mouse strains).
How does loss of miR-424(322)/503 impact mammary tumorigenesis and tumor progression? Our previous studies (Llobet-Navas et al. 2014a,b) and the new data shown here have revealed that this miRNA cluster modulates the expression of at least three well-known oncogenes (CDC25A, BCL-2, and IGF1R) in human and mouse primary breast cancers. Others have also shown that, in other cell types, this cluster targets additional genes involved in cell cycle progression (Nakashima et al. 2010; Xu et al. 2013b), angiogenesis (Ghosh et al. 2010; Nakashima et al. 2010), immune response (Xu et al. 2016), cell metabolism (Long et al. 2013), and cell adhesion (Chong et al. 2014). Thus, we propose that aberrant high levels of these targets due to loss of miR-424(322)/503 support multiple hallmarks of cancer leading to tumorigenesis (Fig. 6A).
Figure 6.
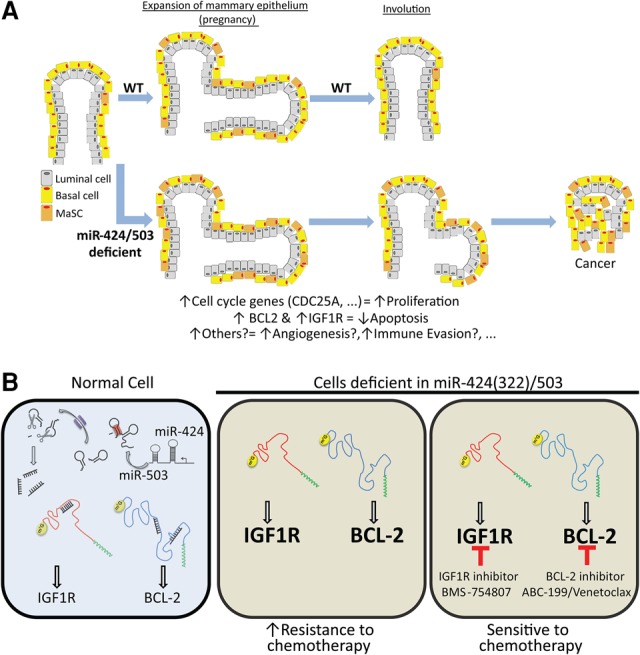
Model of transformation and induction of chemoresistance mediated by loss of miR-424(322)/503. (A) The miRNA cluster miR-424(322)/503 modulates the expression of multiples genes (including well-known oncogenes) that regulate various processes associated with tumorigenesis such as cell cycle progression, angiogenesis, immune response, cell metabolism, and cell adhesion. Note that a role for miR-424(322) and miR-503 in angiogenesis and immune evasion has been reported but was not evaluated in this study. Cells deficient in miR-424(322)/503 present aberrant high levels of these targets, leading to tumorigenesis. As some of these targets are controlled by the miR-424(322)/503 cluster during regression of the post-partum mammary epithelium, we propose that miR-424(322)/503-deficient cells are especially sensitive to transformation during pregnancy. (B) Loss of miR-424(322)/503 induces up-regulation of BCL-2 and IGF1R, which in turn promotes chemoresistance. Specific inhibitors against these proteins resensitized cancer cells to chemotherapy, representing a targeted therapeutic opportunity.
Chemoresistance is one of the most pressing problems faced by oncologists (Raguz and Yague 2008). Despite the general use of chemotherapy as the standard of care in the vast majority of anti-cancer regimens, life-threatening intrinsic and acquired resistance commonly emerges (Wilson et al. 2006). In breast cancers, this is especially problematic for patients whose tumors are of the TN and basal subtypes. As these tumors do not express steroid hormone receptors or present alterations in the HER2 oncogene, they cannot benefit from targeted therapies specifically designed against these cancer determinants. Thus, chemotherapy remains the only possible therapeutic option in the adjuvant or metastatic setting for these patients (Gluz et al. 2009; Brunello et al. 2013). The studies presented here have revealed that loss of miR-424(322)/503 occurs in ∼20% of TN and basal breast cancers and is associated with shorter overall survival independent of clinicopathological variables. Importantly, we linked the influence of miR-424(322)/503 loss on patient survival with the induction of chemoresistance due to the aberrant up-regulation of defined miR-424(322)/503 targets. Our deep understanding and characterization of the miR-424(322)/503–target axes in the normal mammary epithelium (Llobet-Navas et al. 2014a,b) as well as in breast cancers have allowed us to propose targeted therapy-based combinations to block the activity of IGF1R and BCL-2 and effectively reverse chemoresistance (Fig. 6B). Remarkably, inhibitors such as ABT-199 or BMS-754807 that are currently under investigation in clinical trials and show promising anti-cancer activity and low toxicity represent an exciting and realistic therapeutic opportunity for patients who present chemoresistance due to the loss of miR-424(322)/503. To this end, we performed toxicological studies in our preclinical animal models. This analysis revealed that addition of ABT-199 and BMS-754807 to paclitaxel treatment did not change the total white blood cell count but reduced the leucocyte fraction while increasing the percentage of neutrophils and monocytes. Chemical tests also showed a comparable pattern with a few exceptions (Supplemental Table S5).
There are some questions that are still unanswered due to limitations in our study and are worth discussing. We focused our studies on the impact that aberrant up-regulation of miR-424(322)/503 targets has on chemoresistance, but, at this point, we do not know which of the targets is responsible for the formation of mammary tumors observed in the miR-424(322)/503−/− animals. Interestingly, elevated levels of CDC25A in the MMTV-CDC25A model cause alveolar hyperplasia and reduce the latency of mammary tumorigenesis in MMTV-Ras mice (Galaktionov et al. 1996; Ray et al. 2007). Overexpression of BCL-2 in the WAP-TAg transgenic mouse model of breast cancer also accelerates tumorigenesis (Furth et al. 1999). Finally, overexpression of IGF1R induces tumor formation in a doxycycline-inducible MMTV-IGF1R model (Jones et al. 2007). Thus, it is possible that one of these oncogenes, the combination of some of them, or other unidentified targets may be involved in the observed tumorigenesis. Additional genetic studies in which these targets are abrogated individually and in combination in the miR_KO model and breast tumorigenesis is compared will be necessary to answer this question.
The miR-424(322)/503 cluster expresses two miRNAs. Is the loss of both miRNAs important for breast tumorigenesis and chemoresistance? The knockout mouse model used here presents a deletion of both miRNAs and consequently does not allow for investigation of the contribution of each miRNA independently. We generated a small cohort of knockout mice containing the deletion of only one miRNA. We found that, in these animals, the expression of the remaining mature miRNA was barely detectable (Llobet-Navas et al. 2014a). This result, which suggests that the sequence/structure of the miR-424(322)/503 primary transcript is essential for proper generation of mature miRNAs, prevents, at least in the generated models, further studies of the individual miRNAs in vivo. We and others have reported previously that miR-424(322) and miR-503 regulate a similar list of putative targets and can cooperate to down-regulate expression of their targets in vitro (Rissland et al. 2011; Llobet-Navas et al. 2014a,b). Moreover, we reported previously that individual experimental up-regulation of miR-424(322) and miR-503 is able to attenuate the expression of BCL-2 and IGF1R at a comparable level (Llobet-Navas et al. 2014b). Thus, we hypothesize that the loss of both miRNAs in the cluster contributes to the cancer phenotype. Nonetheless, the deletions observed in breast cancer involve loss of both miRNAs concurrently.
Finally, as the miR-424(322)/503 cluster is involved in involution of the mammary epithelium after pregnancy (Llobet-Navas et al. 2014a) and as our new data show that tumorigenesis in miR-424(322)/503−/− female mice is promoted by pregnancy, our results also suggest, although not fully prove, a molecular link between pregnancy and breast cancers. In this regard, it will be interesting to investigate whether breast cancers presenting loss of miR-424(322)/503 are more frequent in parous compared with nulliparous women and whether the number of pregnancies influences any potential disparity. Unfortunately, there is no reproductive history in the available data sets (including TCGA-BRCA and METABRIC), and additional studies will be necessary to answer this question.
Overall, our studies reveal the following major discoveries: First, we unambiguously demonstrated the tumor suppressor function of miR-424(322)/503 in breast cancers. Second, we uncovered the impact that loss of miR-424(322)/503 has on promoting chemoresistance. Finally, our molecular understanding of the genes regulated by the miR-424(322)/503 cluster identified therapeutic strategies to restore sensitivity to chemotherapy.
Materials and methods
Cell culture
All cell lines were obtained from American Type Culture Collection and Invitrogen and grown as recommended (detailed in the Supplemental Material).
Western blotting
Samples were lysed with EZ lysis buffer (1 M Tris at pH 7, 50% glycerol, 20% SDS, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined using the protein assay kit (Bio-Rad, 500-0006). Equal amounts of proteins were subjected to SDS-PAGE and transferred to nitrocellulose membranes (GE Healthcare, 10401197). Membranes were incubated overnight at 4°C with primary antibodies and for 1 h at room temperature with secondary HRP-conjugated antibodies. The antibodies used in this study include IGF1R, BCL-2, and CDC25A from Santa Cruz Biotechnology (sc-492, sc-713, and sc-7389, respectively); p-Akt Ser473, p-IGF1R Tyr1131, and p-IGF1R Tyr1135 from Cell Signaling (4060, 3021, and 3918, respectively); and β-Actin from US Biological (A0760-40).
Experimental animals
Animal maintenance and experiments were performed in accordance with the animal care guidelines and protocols approved by the Columbia University/Mount Sinai animal care units.
Mixed background miR-424(322)/503−/− females were retrocrossed for 10 generations with FvB/NJ males (Jackson, 001800) to ensure a homogeneous FvB/NJ background. Genotyping was performed on tail samples using the Direct PCR reagent (Viagen, 202-Y). Tails were incubated in 250 µL of Direct PCR reagent and 20 mg/mL Proteinase K (New England Biolabs, P8102) for 5 h at 55°C. Samples were then incubated for 45 min at 85°C, after which they were centrifuged at full speed for 5 min. One microliter of sample was used to perform genotyping PCR using the FastStart polymerase (Roche, 04738420001). Thermal conditions were as follows: predenaturing for 5 min at 94°C, 35× (denaturing for 30 sec at 94°C, annealing for 30 sec at 55°C, and elongation for 30 sec at 72°C), and final elongation for 7 min at 72°C. All experiments were performed under the Institutional Animal Care and Use Committee (IACUC) guidelines.
Primers used for genotyping were as follows: pair 1 (F, CACCAGCAGATCCTGGAAAT; R, CAAGTGAGGCGCTAACAACA) and pair 2 (F, AGTTTCGAAAAACGGACATA; R, ATTGCCTCTCATTGTACCAC).
FvB/NJ miR-424(322)/503−/− mice were crossed with MMTV-Neu mice (NK1Mul/J; Jackson, 005038) to study the effects of miR-424(322)/503 targeted deletion in mammary breast tumor growth, latency, and response to chemotherapy.
Drug administration
Paclitaxel (obtained from Columbia University Medical Center-Presbyterian Hospital) was diluted with PBS to 6 mg/mL. ABT-199 and BMS-754807 were purchased from Selleckchem. Paclitaxel (6 mg/kg) was administered intraperitoneally twice per week for 3 wk, and the combination 50 mg/kg ABT-199 + 50 mg/kg BMS-754807 was administered by gavage three times per week for 3 wk. Animals were monitored for 3 wk, and tumor volume was measured twice per week using the ellipsoid volume formula 1/2 × L × W × H.
ChIP
A standard immunoprecipitation protocol was followed. The detailed protocol is described in the Supplemental Material.
Monoallelic expression
Genomic DNA (Qiagen, 13323) and total RNA (Qiagen, 74104) were extracted from 184B5 and primary HuMECs according to the manufacturer's recommendations (Agilent, 400800). Total RNA (0.5 µg) was converted into cDNA using the high-capacity cDNA reverse transcription kit (Roche, 4368814). Genomic DNA and cDNA were amplified using specific primers (F, TTCAGTCATCCAGTCTTTATTCA; and R, GATGGCCTAAGACTTACCTGCT) at the miR-424/503 locus and flanking the SNP Rs757309. PCR products were analyzed by Sanger sequencing.
Patient samples and data analysis
Patient data were assessed from METABRIC and TCGA corresponding to breast cancer samples with available whole-genome DNA CNAs, mRNA expression data, and clinicopathological data. TCGA samples were profiled using Affymetrix SNP 6.0 arrays and Agilent G4502A custom microarrays, and METABRIC samples were profiled using Affymetrix SNP 6.0 arrays and Illumina HT-12 microarrays. These sets were collected from the TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga) and the European Genome–Phenome Archive (EGA) database (EGAS00000000083). The TCGA data set also included level 3 methylation profiling data obtained on the Illumina HumanMethylation450 platform and downloaded from the University of California at Santa Cruz Cancer Browser (https://genome-cancer.ucsc.edu) and RPPA data with expression levels for ∼200 proteins. Array-CGH data were analyzed defining breast cancers with (“miR-DEL”) versus without (“miR-non-DEL”) miR-424(322)/503 deletion. Patients’ data analysis is described in detail in the Supplemental Material.
Supplementary Material
Acknowledgments
This work was partially supported by the Agilent Technologies Leader Award (J.M.S. and C.C.-C.), the National Cancer Center (R.R.-B. and A.G.), the Irma T. Hirschl Research Award (J.M.S.), Marie Sklodowska-Curie grant number 745808 (R.R.-B.), and the J.G.W. Patterson Foundation (D.L.-N.). D.L.-N. and R.R.-B. established the animal models described. D.L.-N., R.R.-B., and E.A.N. performed the in vivo, cell, and molecular biology experiments with the assistance of T.Z.Z., A.G., and E.E. Computational analysis was performed by P.F., F.S.-G., D.B., J.Y., and F.B. Pathological analysis of mouse tumors was supervised by C.C.-C. Data analysis and interpretation were performed by D.L.-N., R.R.-B., E.N., P.F., F.B., and J.M.S. The experimental plan was conceived by J.M.S. and D.L.-N. The manuscript was written by J.M.S. with the assistance of D.L.-N. and E.N. All authors provided input and read and approved the final version of the manuscript.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.292318.116.
References
- Allan GJ, Beattie J, Flint DJ. 2004. The role of IGFBP-5 in mammary gland development and involution. Domest Anim Endocrinol 27: 257–266. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Calin GA, Croce CM. 2010. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 17: 215–220. [DOI] [PubMed] [Google Scholar]
- Bajwa N, Liao C, Nikolovska-Coleska Z. 2012. Inhibitors of the anti-apoptotic Bcl-2 proteins: a patent review. Expert Opin Ther Pat 22: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter FO, Came PJ, Abell K, Kedjouar B, Huth M, Rajewsky K, Pasparakis M, Watson CJ. 2006. IKKβ/2 induces TWEAK and apoptosis in mammary epithelial cells. Development 133: 3485–3494. [DOI] [PubMed] [Google Scholar]
- Bierie B, Gorska AE, Stover DG, Moses HL. 2009. TGF-β promotes cell death and suppresses lactation during the second stage of mammary involution. J Cell Physiol 219: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs SF, Reijo Pera RA. 2014. X chromosome inactivation: recent advances and a look forward. Curr Opin Genet Dev 28: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello A, Borgato L, Basso U, Lumachi F, Zagonel V. 2013. Targeted approaches to triple-negative breast cancer: current practice and future directions. Curr Med Chem 20: 605–612. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. 2012. Comprehensive molecular portraits of human breast tumours. Nature 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangi MG, Cukor B, Soung P, Signoretti S, Moreira G Jr, Ranashinge M, Cady B, Pagano M, Loda M. 2000. Role of the Cdc25A phosphatase in human breast cancer. J Clin Invest 106: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. 1999. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev 13: 2604–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Sharon E. 2013. IGF-1R as an anti-cancer target—trials and tribulations. Chin J Cancer 32: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y, Zhang J, Guo X, Li G, Zhang S, Li C, Jiao Z, Shao M. 2014. MicroRNA-503 acts as a tumor suppressor in osteosarcoma by targeting L1CAM. PLoS One 9: e114585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Colitti M. 2012. BCL-2 family of proteins and mammary cellular fate. Anat Histol Embryol 41: 237–247. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. 2012. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et al. 2012. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie SA, Maglione JE, Manner CK, Young D, Cardiff RD, MacLeod CL, Ellies LG. 2007. Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic Res 16: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG, McLean CA, et al. 2010. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 103: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, et al. 2012. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 22: 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbesen SH, Scaltriti M, Bialucha CU, Morse N, Kastenhuber ER, Wen HY, Dow LE, Baselga J, Lowe SW. 2016. Pten loss promotes MAPK pathway dependency in HER2/neu breast carcinomas. Proc Natl Acad Sci 113: 3030–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Voura EB, Yu HY, Waterhouse P, Murphy G, Moorehead RA, Khokha R. 2001. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest 108: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. 2010. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol 402: 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth PA, Bar-Peled U, Li M, Lewis A, Laucirica R, Jager R, Weiher H, Russell RG. 1999. Loss of anti-mitotic effects of Bcl-2 with retention of anti-apoptotic activity during tumor progression in a mouse model. Oncogene 18: 6589–6596. [DOI] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D. 1996. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 382: 511–517. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, et al. 2010. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest 120: 4141–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. 2009. Triple-negative breast cancer—current status and future directions. Ann Oncol 20: 1913–1927. [DOI] [PubMed] [Google Scholar]
- Green KA, Lund LR. 2005. ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays 27: 894–903. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hannesdottir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH, Stoitzner P, et al. 2013. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol 43: 2718–2729. [DOI] [PubMed] [Google Scholar]
- Heard E. 2004. Recent advances in X-chromosome inactivation. Curr Opin Cell Biol 16: 247–255. [DOI] [PubMed] [Google Scholar]
- Hendrickson AW, Haluska P. 2009. Resistance pathways relevant to insulin-like growth factor-1 receptor-targeted therapy. Curr Opin Investig Drugs 10: 1032–1040. [PubMed] [Google Scholar]
- Hou X, Huang F, Macedo LF, Harrington SC, Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM, Carboni JM, et al. 2011. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res 71: 7597–7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BA, Gusterson BA. 2000. Human breast development. J Mammary Gland Biol Neoplasia 5: 119–137. [DOI] [PubMed] [Google Scholar]
- Hutchins AP, Poulain S, Miranda-Saavedra D. 2012. Genome-wide analysis of STAT3 binding in vivo predicts effectors of the anti-inflammatory response in macrophages. Blood 119: e110–e119. [DOI] [PubMed] [Google Scholar]
- Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y, Kusunoki M. 2015. MicroRNA-503 promotes tumor progression and acts as a novel biomarker for prognosis in oesophageal cancer. Anticancer Res 35: 1447–1451. [PubMed] [Google Scholar]
- Ip MM, Asch BB. 2000. Introduction: an histology atlas of the rodent mammary gland and human breast during normal postnatal development and in cancer. J Mammary Gland Biol Neoplasia 5: 117–118. [DOI] [PubMed] [Google Scholar]
- Jager R, Herzer U, Schenkel J, Weiher H. 1997. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene 15: 1787–1795. [DOI] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. 2007. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene 26: 1636–1644. [DOI] [PubMed] [Google Scholar]
- King H, Aleksic T, Haluska P, Macaulay VM. 2014. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev 40: 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, Poli V, Flavell RA, Clarkson RW, Watson CJ. 2011. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 13: 303–309. [DOI] [PubMed] [Google Scholar]
- Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. 2003. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130: 3459–3468. [DOI] [PubMed] [Google Scholar]
- Kucab JE, Dunn SE. 2003. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis 17: 41–47. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. 1994. Transient increase in the risk of breast cancer after giving birth. N Engl J Med 331: 5–9. [DOI] [PubMed] [Google Scholar]
- Li Q, Qiu XM, Li QH, Wang XY, Li L, Xu M, Dong M, Xiao YB. 2015. MicroRNA-424 may function as a tumor suppressor in endometrial carcinoma cells by targeting E2F7. Oncol Rep 33: 2354–2360. [DOI] [PubMed] [Google Scholar]
- Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. 2008. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res 36: 5391–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet-Navas D, Rodriguez-Barrueco R, Castro V, Ugalde AP, Sumazin P, Jacob-Sendler D, Demircan B, Castillo-Martin M, Putcha P, Marshall N, et al. 2014a. The miR-424(322)/503 cluster orchestrates remodeling of the epithelium in the involuting mammary gland. Genes Dev 28: 765–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet-Navas D, Rodriguez-Barrueco R, de la Iglesia-Vicente J, Olivan M, Castro V, Saucedo-Cuevas L, Marshall N, Putcha P, Castillo-Martin M, Bardot E, et al. 2014b. The microRNA 424/503 cluster reduces CDC25A expression during cell cycle arrest imposed by transforming growth factor β in mammary epithelial cells. Mol Cell Biol 34: 4216–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long XH, Mao JH, Peng AF, Zhou Y, Huang SH, Liu ZL. 2013. Tumor suppressive microRNA-424 inhibits osteosarcoma cell migration and invasion via targeting fatty acid synthase. Exp Ther Med 5: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias H, Hinck L. 2012. Mammary gland development. Wiley Interdiscip Rev Dev Biol 1: 533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, et al. 2007. Programmed anuclear cell death delimits platelet life span. Cell 128: 1173–1186. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. 1988. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54: 105–115. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Jinnin M, Etoh T, Fukushima S, Masuguchi S, Maruo K, Inoue Y, Ishihara T, Ihn H. 2010. Down-regulation of mir-424 contributes to the abnormal angiogenesis via MEK1 and cyclin E1 in senile hemangioma: its implications to therapy. PLoS One 5: e14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Schwartz A, Wood TL, Roberts CT Jr, Hennighausen L, LeRoith D. 1996. Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J Clin Invest 97: 2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AV, Pollard JW. 2000. Transforming growth factor β3 induces cell death during the first stage of mammary gland involution. Development 127: 3107–3118. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Hoffmann I. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res 4: 107–114. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Martinson H, Durand-Rougely C, Schedin P. 2012. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 139: 269–275. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. 2000. Molecular portraits of human breast tumours. Nature 406: 747–752. [DOI] [PubMed] [Google Scholar]
- Pollak M. 2012. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Hartmann LC. 2009. Mammary involution and breast cancer risk: transgenic models and clinical studies. J Mammary Gland Biol Neoplasia 14: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguz S, Yague E. 2008. Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer 99: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Terao Y, Fuhrken PG, Ma ZQ, DeMayo FJ, Christov K, Heerema NA, Franks R, Tsai SY, Papoutsakis ET, et al. 2007. Deregulated CDC25A expression promotes mammary tumorigenesis with genomic instability. Cancer Res 67: 984–991. [DOI] [PubMed] [Google Scholar]
- Rissland OS, Hong SJ, Bartel DP. 2011. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol Cell 43: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, et al. 2012. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker EB III, Hale AN, Durtschi DC, Sakamoto K, Wagner KU. 2011. Forced involution of the functionally differentiated mammary gland by overexpression of the pro-apoptotic protein bax. Genesis 49: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant TJ, Lloyd-Lewis B, Resemann HK, Ramos-Montoya A, Skepper J, Watson CJ. 2014. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat Cell Biol 16: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, et al. 2006. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98: 262–272. [DOI] [PubMed] [Google Scholar]
- Stein T, Salomonis N, Gusterson BA. 2007. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia 12: 25–35. [DOI] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. 1992. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol 118: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffen PG, Omidvar N, Marquez-Almuina N, Croston D, Watson CJ, Clarkson RW. 2008. A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Mol Endocrinol 22: 2677–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usary J, Zhao W, Darr D, Roberts PJ, Liu M, Balletta L, Karginova O, Jordan J, Combest A, Bridges A, et al. 2013. Predicting drug responsiveness in human cancers using genetically engineered mice. Clin Cancer Res 19: 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ, et al. 2013. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 24: 120–129. [DOI] [PubMed] [Google Scholar]
- Vu T, Claret FX. 2012. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Wagner KU, Rucker EB III, Shillingford JM, Miyoshi K, Hennighausen L. 2001. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech Dev 109: 281–293. [DOI] [PubMed] [Google Scholar]
- Watson CJ. 2006. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med 8: 1–15. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Longley DB, Johnston PG. 2006. Chemoresistance in solid tumours. Ann Oncol 17: x315–x324. [DOI] [PubMed] [Google Scholar]
- Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, Richardson A. 2007. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res 13: 7191–7198. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. 2013a. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene 32: 976–987. [DOI] [PubMed] [Google Scholar]
- Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y, Yin LR. 2013b. MicroRNA-503 suppresses proliferation and cell-cycle progression of endometrioid endometrial cancer by negatively regulating cyclin D1. FEBS J 280: 3768–3779. [DOI] [PubMed] [Google Scholar]
- Xu S, Tao Z, Hai B, Liang H, Shi Y, Wang T, Song W, Chen Y, OuYang J, Chen J, et al. 2016. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun 7: 11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li T, Guo P, Kang J, Wei Q, Jia X, Zhao W, Huai W, Qiu Y, Sun L, et al. 2014. MiR-424-5p reversed epithelial-mesenchymal transition of anchorage-independent HCC cells by directly targeting ICAT and suppressed HCC progression. Sci Rep 4: 6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.